Abstract
Background
Higher body mass index and waist circumference have been associated with increased risk of pancreatitis in several prospective studies; however, the results have not been entirely consistent.
Aims
We conducted a systematic review and dose-response meta-analysis of prospective studies on adiposity and risk of pancreatitis to clarify this association.
Methods
PubMed and Embase databases were searched for studies on adiposity and pancreatitis up to January 27, 2020. Prospective studies reporting adjusted relative risk (RR) estimates and 95% confidence intervals (CIs) for the association between adiposity and risk of pancreatitis were included, and summary RRs (95% CIs) were calculated using a random effects model.
Results
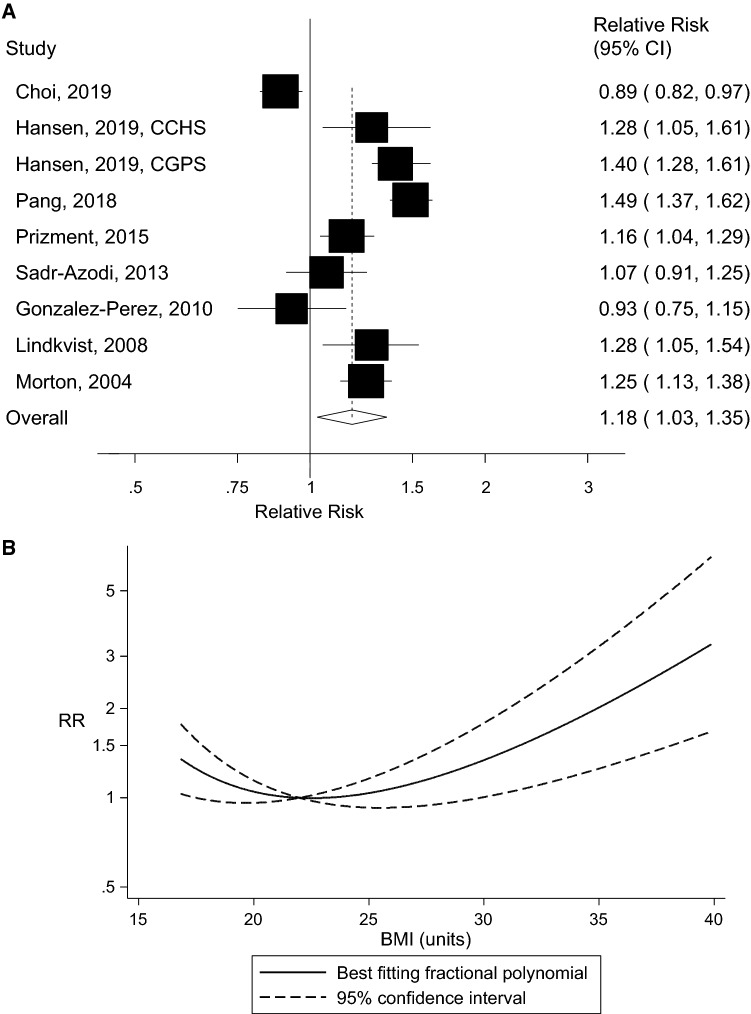
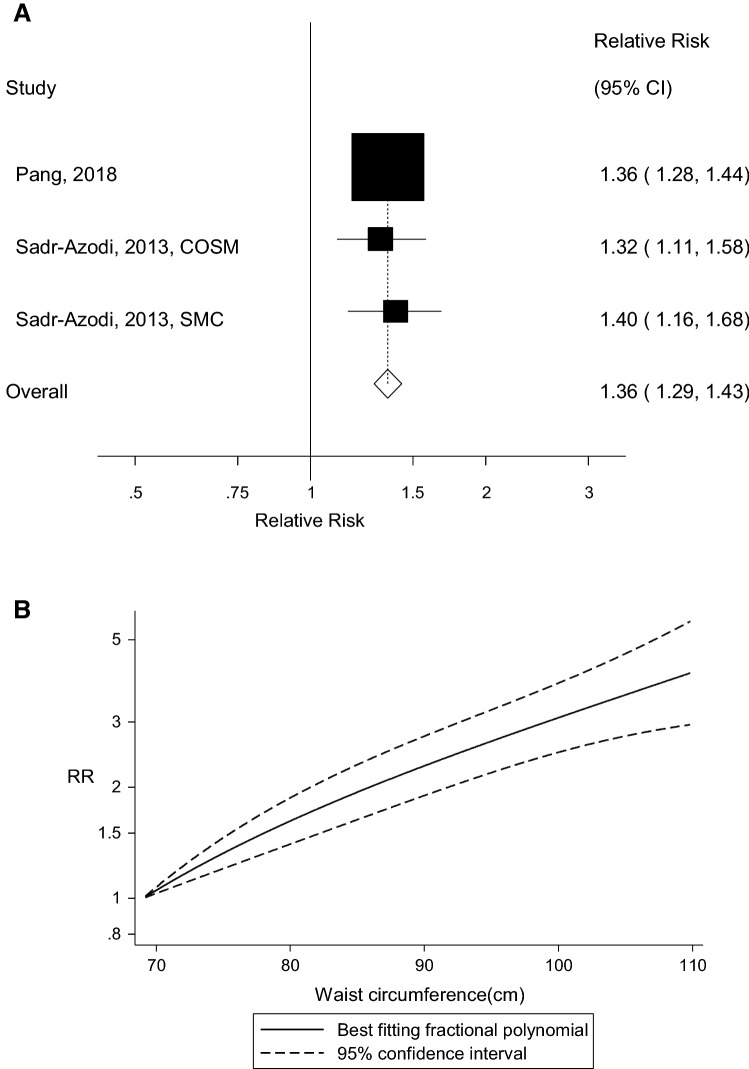
Ten prospective studies with 5129 cases and 1,693,657 participants were included. The summary RR (95% CI) of acute pancreatitis was 1.18 (95% CI: 1.03–1.35, I2 = 91%, n = 10 studies) per 5 kg/m2 increase in BMI and 1.36 (95% CI: 1.29–1.43, I2 = 0%, n = 3) per 10 cm increase in waist circumference. There was evidence of a nonlinear association between BMI and acute pancreatitis, pnonlinearity < 0.0001, with a steeper association at higher levels of BMI, but not for waist circumference, pnonlinearity = 0.19. Comparing a BMI of 35 with a BMI of 22, there was a 58% increase in the RR and there was a fourfold increase in the RR comparing a waist circumference of 110 cm with 69 cm. There was no evidence of publication bias.
Conclusions
This meta-analysis suggests that both increasing BMI and waist circumference are associated with a dose-response-related increase in the risk of acute pancreatitis.
Electronic supplementary material
The online version of this article (10.1007/s10620-020-06275-6) contains supplementary material, which is available to authorized users.
Keywords: Body mass index, Central adiposity, Waist circumference, Pancreatitis, Systematic review, Meta-analysis
Introduction
Pancreatitis is an inflammatory disorder of the pancreas characterized by symptoms such as severe abdominal pain, nausea and vomiting [1]. Pancreatitis develops when digestive enzymes produced by the pancreas are activated in the pancreas instead of in the small intestine, causing inflammation and fibrosis [2]. Pancreatitis is an important risk factor for pancreatic cancer (relative risk of 8) [3, 4] as well as for all-cause and cause-specific mortality [4, 5]. Incidence rates of pancreatitis vary from 4 up to > 100 cases per 100,000 persons per year globally, with higher rates in America for acute pancreatitis, and in Europe for chronic pancreatitis, while the lowest rates are observed in Southeast Asia [6]. In addition, the incidence of the disease has been increasing substantially over the last decades in secular trend studies in the USA and Europe [7–13]. The wide variation in the incidence of pancreatitis internationally combined with the rapid changes in the incidence of the disease over time within countries suggests that modifiable risk factors may be of major importance in the etiology of the disease. High alcohol intake [4, 14], smoking [4, 15], diabetes mellitus [4] and a history of gallstones [4, 14] are among the established or suspected risk factors for pancreatitis.
Overweight and obesity as measured by body mass index (BMI, kg/m2) is an established risk factor for type 2 diabetes [16] and gallstone or gallbladder disease [17], which are risk factors for pancreatitis [4], and adiposity is also an established risk factor for pancreatic cancer [18], for which pancreatitis is a risk factor [4]. High BMI has also been directly associated with increased risk of acute pancreatitis in some prospective studies [4, 19–21]; however, the results are not entirely consistent as several other studies found no clear association [22–24] and one study suggested a U-shaped association [25]. In addition, higher waist circumference has been consistently associated with increased risk of acute pancreatitis [4, 24]. Whether the association differs by subtype of pancreatitis (e.g., gallstone-related or alcohol-related pancreatitis) is not clear, and whether the association between BMI and pancreatitis is independent of diabetes or gallbladder disease is also not clear. Two Swedish studies [24] and a Chinese study [4] suggested a stronger association between waist circumference and pancreatitis than for BMI, particularly when mutually adjusted, and this finding might suggest insulin resistance may be an important contributor to pancreatitis. Although two previous meta-analyses found a 34–43% increase in risk of pancreatitis or acute pancreatitis with obesity [26, 27], they both had several limitations including the low number of studies (n = 5 and n = 2) which led to the combination of patient-based studies (type 2 diabetes, hypertension), one study on recurrent pancreatitis among pancreatitis patients, one case-control study and a population-based cohort study in one meta-analysis [26], while only two cohort studies were included in a second meta-analysis [27]. In addition, no results were reported for overweight and no dose-response analyses were conducted; thus, it is not clear if there is a dose-response relationship between increasing adiposity and pancreatitis or if there are any threshold levels. Given that several population-based cohort studies on adiposity and the risk of incident pancreatitis now have been published [4, 19–25], we therefore conducted a systematic review and dose-response meta-analysis of the available prospective studies to better define the strength and shape of the dose-response relationship between adiposity and risk of pancreatitis.
Materials and Methods
Search Strategy and Inclusion Criteria
Pubmed and Embase databases were searched for eligible studies up to January 27, 2020, as part of a larger project on risk factors for pancreatitis. The search terms used are provided in the supplementary text. We followed PRISMA criteria for reporting meta-analyses [28]. The reference lists of the identified publications were also searched for further studies.
Study Selection
We included published retrospective and prospective cohort studies and nested case-control studies within cohorts that reported adjusted relative risk (RR) estimates and 95% confidence intervals (CIs) for the association between any measure of adiposity (BMI, waist circumference, waist-to-hip ratio, weight gain, body fat) and the risk of pancreatitis. A list of the excluded studies and the exclusion reasons can be found in Supplementary Table 1. DA and YMS conducted the screening of the literature search.
Data Extraction
The following data were extracted from each study: The first author’s last name, publication year, country where the study was conducted, the name of the study, study period and duration of follow-up, sample size, sex, number of cases, measure of adiposity, subgroup, RRs and 95% CIs and variables adjusted for in the analysis. The data extraction was conducted by DA and checked for accuracy by YMS.
Statistical Methods
Random effects models were used to calculate summary RRs (95% CIs) of pancreatitis per 5 kg/m2 increase in BMI and per 10 cm of waist circumference (the two adiposity measures which had enough studies to be analyzed) [29]. The average of the natural logarithm of the RRs was estimated, and the RR from each study was weighted using random effects weights. We used the method of Greenland and Longnecker to estimate linear trends across categories of BMI and waist circumference [30]. For studies which reported adiposity measures by ranges, we estimated the midpoint for each category. When extreme categories were open-ended, we estimated an upper and lower cutoff value using the width of the adjacent category; however, when the WHO categories of BMI were used we used 18.5 as a lower cutoff if the upper cutoff was < 25. For studies in which the lowest category was not the reference category, we used the method of Hamling to convert the risk estimates so the lowest category became the reference category. We also conducted sensitivity analyses simply excluding the categories below the reference category. Fractional polynomial models were used to investigate a potential nonlinear association between BMI and waist circumference and risk of pancreatitis [31, 32]. The best-fitting second-order fractional polynomial regression model, defined as the one with the lowest deviance, was determined. A likelihood ratio test was used to assess the difference between the nonlinear and linear models to test for nonlinearity [31].
Heterogeneity between studies was evaluated using Q and I2 statistics [33]. I2 is a measure of how much of the heterogeneity that is due to between study variation rather than chance. I2-values of 25%, 50% and 75% indicate low, moderate and high heterogeneity, respectively. We conducted main analyses (all studies combined) and stratified by study characteristics such as sex, duration of follow-up, geographic location, number of cases, study quality and by adjustment for confounding factors to investigate potential sources of heterogeneity. Study quality was assessed using the Newcastle Ottawa scale which rates studies according to selection, comparability and outcome assessment with a score range from 0 to 9 [34].
Publication bias was assessed using Egger’s test [35] and Begg-Mazumdar’s test [36] and by inspection of funnel plots. The statistical analyses were conducted using the software package Stata, version 13.0 software (StataCorp, Texas, USA).
Results
We identified ten population-based prospective studies (eight publications, nine risk estimates) [4, 19–25] that were included in the meta-analysis of adiposity and risk of pancreatitis risk (Fig. 1, Table 1). Two publications included results from two studies each; the Swedish Mammography Cohort and the Cohort of Swedish Men [24] and the Copenhagen City Heart Study and the Copenhagen General Population Study [21]. Two studies were conducted in women, one in men and seven studies in men and women combined. Six studies were from Europe, two studies were from the USA and two studies were from Asia (Table 1).
Fig. 1.
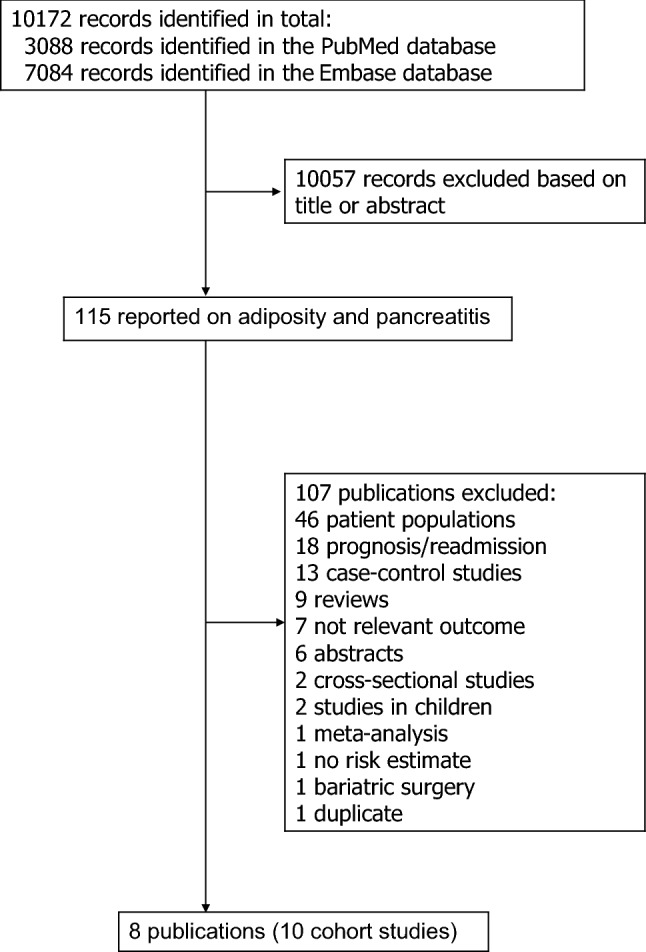
Flowchart of study selection
Table 1.
Prospective studies of body fatness and pancreatitis
| First author, publication year, country | Study name or description | Study period | Number of participants, number of cases | Assessment of anthropometric measures | Adiposity measure, subgroup | Comparison | Relative risk (95% confidence interval) | Adjustment for confounders |
|---|---|---|---|---|---|---|---|---|
| Morton et al. [19], USA | Kaiser Permanente Medical Care Program | 1978–1985–1998, ~16.5 years follow-up | 128934 men and women: 439 pancreatitis cases | Measured | BMI, gallstone-related pancreatitis | Per 1 kg/m2 | 1.08 (1.05–1.11) | Age, sex, race, education, alcohol |
| 168/125/110 gallstone/ alcohol/ idiopathic pancreatitis cases | BMI, alcohol-related pancreatitis | Per 1 kg/m2 | 0.96 (0.92–1.01) | |||||
| BMI, idiopathic pancreatitis | Per 1 kg/m2 | 1.04 (0.98–1.06) | ||||||
| Lindkvist et al. [22], Sweden | The Malmo Preventive Project | 1974–1992–1999, ~17.9 years follow-up | 33211 men and women, age 45.6 years: 179 acute pancreatitis cases | Measured | BMI | < 20 | 1.02 (0.56–1.88) | Age, sex, Malmo Modification of the Michigan Alcoholism Screening Test, smoking status, cigarettes per day |
| 20–25 | 1.00 | |||||||
| 25–30 | 1.15 (0.83–1.60) | |||||||
| > 30 | 1.45 (0.85–2.48) | |||||||
| Per 1 kg/m2 | 1.05 (1.01–1.09) | |||||||
| Gonzalez-Perez et al. [23], 2010, United Kingdom | The Health Improvement Network | 1996–2006, 4.0 years follow-up |
285525 men and women, age 20–79 years: 395 acute pancreatitis cases 4552 controls |
Measured | BMI |
< 20 20–24 25–29 ≥ 30 |
1.13 (0.63–2.02) 1.00 0.99 (0.75–1.31) 0.92 (0.68–1.24) |
Age, sex, Townsend index, ischemic heart disease, exposure to antibiotics, H2 blockers, proton pump inhibitors, NSAIDs (aspirin and coxibs), antihypertensive drugs, smoking, alcohol, diabetes, antidiabetic drugs, gastrointestinal disease (gallstones, biliary tract disease, cholecystitis, gastroenteritis, abdominal pain, and others), paracetamol, ACE inhibitors |
| Sadr-Azodi et al. [24], Sweden | Swedish Mammography Cohort and the Cohort of Swedish Men | 1997–2009, 12 years follow-up | 68158 men and women, age 46–84 years: 424 acute pancreatitis | Self-reported (validated) | Waist circumference, total | 72 cm | 0.55 (0.33–0.92) | Age, sex, education, diabetes, known gallstone disease, monthly alcohol consumption, smoking, fruit and vegetables, mutual adjustment between BMI and waist circumference |
| 81 | 1.00 | |||||||
| 90 | 1.31 (0.97–1.78) | |||||||
| 100 | 1.50 (1.04–2.16) | |||||||
| 110 | 2.37 (1.50–3.74) | |||||||
| Per 10 cm | 1.35 (1.19–1.53) | |||||||
| BMI | 23.0 kg/m2 | 1.00 | ||||||
| 26.8 | 1.18 (0.92–1.51) | |||||||
| 31.6 | 1.02 (0.68–1.53) | |||||||
| Waist circumference, non-gallstone related acute pancreatitis | 72 cm | 0.62 (0.30–1.30) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.44 (0.95–2.21) | |||||||
| 100 | 1.39 (0.83–2.32) | |||||||
| 110 | 2.12 (1.13–4.00) | |||||||
| Per 10 cm | 1.22 (1.03–1.45) | |||||||
| BMI | 23.0 kg/m2 | 1.00 | ||||||
| 26.8 | 1.24 (0.89–1.73) | |||||||
| 31.6 | 0.74 (0.40–1.38) | |||||||
| Waist circumference, gallstone related acute pancreatitis | 72 cm | 0.49 (0.23–1.00) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.16 (0.75–1.79) | |||||||
| 100 | 1.63 (0.96–2.75) | |||||||
| 110 | 2.79 (1.45–5.38) | |||||||
| Per 10 cm | 1.56 (1.29–1.90) | |||||||
| BMI | 23.0 kg/m2 | 1.00 | ||||||
| 26.8 | 1.12 (0.77–1.62) | |||||||
| 31.6 | 1.27 (0.73–2.24) | |||||||
| Waist circumference, total, non-severe acute pancreatitis | 72 cm | 0.46 (0.25–0.84) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.28 (0.92–1.78) | |||||||
| 100 | 1.54 (1.03–2.29) | |||||||
| 110 | 2.28 (1.37–3.78) | |||||||
| Per 10 cm | 1.38 (1.20–1.59) | |||||||
| Waist circumference, non-gallstone related non-severe acute pancreatitis | 72 cm | 0.62 (0.27–1.42) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.42 (0.88–2.29) | |||||||
| 100 | 1.45 (0.82–2.56) | |||||||
| 110 | 2.13 (1.05–4.33) | |||||||
| Per 10 cm | 1.28 (1.06–1.55) | |||||||
| Waist circumference, gallstone related non-severe acute pancreatitis | 72 cm | 0.35 (0.15–0.83) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.14 (0.71–1.81) | |||||||
| 100 | 1.64 (0.94–2.89) | |||||||
| 110 | 2.54 (1.23–5.23) | |||||||
| Per 10 cm | 1.53 (1.24–1.90) | |||||||
| Waist circumference, total, severe acute pancreatitis | 72 cm | 1.08 (0.37–3.11) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.53 (0.72–3.23) | |||||||
| 100 | 1.34 (0.53–3.36) | |||||||
| 110 | 2.88 (0.98–8.47) | |||||||
| Per 10 cm | 1.24 (0.93–1.66) | |||||||
| Waist circumference, non-gallstone related severe acute pancreatitis | 72 cm | 0.66 (0.14–3.16) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.52 (0.60–3.83) | |||||||
| 100 | 1.13 (0.35–3.64) | |||||||
| 110 | 2.06 (0.49–8.60) | |||||||
| Per 10 cm | 1.02 (0.70–1.48) | |||||||
| Waist circumference, gallstone related severe acute pancreatitis | 72 cm | 2.06 (0.44–9.57) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.45 (0.40–5.27) | |||||||
| 100 | 1.70 (0.38–7.58) | |||||||
| 110 | 4.48 (0.83–24.12) | |||||||
| Per 10 cm | 1.73 (1.08–2.80) | |||||||
| Waist circumference, total acute pancreatitis, men | 72 cm | 0.82 (0.11–6.21) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.31 (0.74–2.30) | |||||||
| 100 | 1.55 (0.85–2.82) | |||||||
| 110 | 2.25 (1.15–4.41) | |||||||
| Per 10 cm | 1.32 (1.11–1.58) | |||||||
| Waist circumference, non-gallstone related acute pancreatitis | 72 cm | 1.13 (0.15–8.78) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.22 (0.63–2.37) | |||||||
| 100 | 1.18 (0.57–2.42) | |||||||
| 110 | 1.70 (0.74–3.91) | |||||||
| Per 10 cm | 1.19 (0.95–1.48) | |||||||
| Waist circumference, gallstone related acute pancreatitis | 72 cm | – | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.52 (0.52–4.47) | |||||||
| 100 | 2.60 (0.86–7.87) | |||||||
| 110 | 3.80 (1.14–12.69) | |||||||
| Per 10 cm | 1.63 (1.20–2.24) | |||||||
| Waist circumference, total acute pancreatitis, women | 72 cm | 0.55 (0.32–0.94) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.31 (0.90–1.92) | |||||||
| 100 | 1.37 (0.80–2.37) | |||||||
| 110 | 3.02 (1.43–6.37) | |||||||
| Per 10 cm | 1.40 (1.16–1.68) | |||||||
| Waist circumference, non-gallstone related acute pancreatitis | 72 cm | 0.68 (0.30–1.52) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.42 (0.80–2.51) | |||||||
| 100 | 1.54 (0.70–3.42) | |||||||
| 110 | 3.67 (1.19–11.33) | |||||||
| Per 10 cm | 1.27 (0.96–1.67) | |||||||
| Waist circumference, gallstone related acute pancreatitis | 72 cm | 0.47 (0.22–0.98) | ||||||
| 81 | 1.00 | |||||||
| 90 | 1.24 (0.74–2.07) | |||||||
| 100 | 1.24 (0.58–2.62) | |||||||
| 110 | 2.63 (0.97–7.11) | |||||||
| Per 10 cm | 1.53 (1.19–1.96) | |||||||
| Prizment et al. [20], USA | Iowa Women’s Health Study | 1986–2004, ~18 years follow-up |
36436 women, age ≥ 65 years: 511 acute pancreatitis cases 149 chronic pancreatitis cases |
Self-reported (validated) |
BMI, acute pancreatitis BMI, chronic pancreatitis |
< 25.0 | 1.00 | Age, time of Medicare enrolment, smoking status, pack-years of smoking |
| 25.0–29.9 | 1.29 (1.05–1.60) | |||||||
| ≥ 30.0 | 1.35 (1.07–1.70) | |||||||
| < 25.0 | 1.00 | |||||||
| 25.0–29.9 | 0.67 (0.46–0.97) | |||||||
| ≥ 30.0 | 0.59 (0.37–0.94) | |||||||
| Pang et al. [4], China | China Kadoorie Biobank Study | 2004–2008–2015, 9.2 years follow-up |
510314 men and women, age 30–79 years: 1079 acute pancreatitis cases 683 other pancreatitis cases 113 chronic pancreatitis cases |
Measured | BMI, acute pancreatitis | < 20.0 | 0.91 (0.75–1.10) | Age, sex, region, education, smoking, alcohol, medication (aspirin, ACE-I, beta blockers, statins, diuretics, Calcium antagonists, metformin, insulin), diabetes, physical inactivity, gallbladder disease |
| 20.0–< 22.5 | 1.00 (0.87–1.14) | |||||||
| 22.5–< 25.0 | 1.27 (1.13–1.42) | |||||||
| 25.0–< 27.0 | 1.61 (1.41–1.85) | |||||||
| ≥ 27.0 | 1.96 (1.72–2.24) | |||||||
| Per 3.4 kg/m2 | 1.31 (1.24–1.39) | |||||||
| Per 3.4 kg/m2 | 1.09 (0.97–1.27) | +waist circumference | ||||||
| Waist circumference | < 71.7 cm | 0.73 (0.62–0.87) | ||||||
| 71.7–< 77.0 | 1.00 (0.86–1.16) | |||||||
| 77.0–< 82.2 | 1.17 (1.02–1.33) | |||||||
| 82.2–< 88.5 | 1.33 (1.17–1.51) | |||||||
| ≥ 88.5 | 1.87 (1.66–2.11) | |||||||
| Per 9.8 cm | 1.35 (1.27–1.43) | |||||||
| Per 9.8 cm | 1.25 (1.11–1.41) | +BMI | ||||||
| BMI, other pancreatitis | Per 3.4 kg/m2 | 1.24 (1.15–1.34) | +waist circumference | |||||
| Waist circumference | Per 9.8 cm | 1.30 (1.20–1.40) | +BMI | |||||
| BMI, chronic pancreatitis | Per 3.4 kg/m2 | 0.86 (0.70–1.06) | ||||||
| Waist circumference | Per 9.8 cm | 1.00 (0.82–1.22) | ||||||
| Choi et al. [25], Korea | Korean National Screening Cohort | 2002–2003–2013, 10.5 years follow-up | 512928 men and women, age 40–79 years: 1656 acute pancreatitis cases | Measured | BMI, acute pancreatitis | 12.0–18.4 | 1.72 (1.32–2.23) | Age, sex, smoking status, alcohol use, physical activity, comorbid diabetes, comorbid gallstone disease at baseline |
| 18.5–20.9 | 1.44 (1.23–1.68) | |||||||
| 21.0–22.9 | 1.16 (1.01–1.34) | |||||||
| 23.0–24.9 | 1.00 | |||||||
| 25.0–27.4 | 1.00 (0.87–1.16) | |||||||
| 27.5–29.9 | 1.23 (1.02–1.49) | |||||||
| 30.0–50.0 | 1.55 (1.18–2.04) | |||||||
| Per 5 kg/m2 | 0.89 (0.82–0.97) | |||||||
| BMI, severe acute pancreatitis | 12.0–18.4 | 2.24 (1.32–3.80) | ||||||
| 18.5–20.9 | 1.69 (1.20–2.40) | |||||||
| 21.0–22.9 | 1.51 (1.10–2.08) | |||||||
| 23.0–24.9 | 1.00 | |||||||
| 25.0–27.4 | 1.26 (0.91–1.75) | |||||||
| 27.5–29.9 | 1.51 (0.99–2.31) | |||||||
| 30.0–50.0 | 1.98 (1.09–3.59) | |||||||
| Per 5 kg/m2 | 0.86 (0.72–1.04) | |||||||
| BMI, non-severe acute pancreatitis | 12.0–18.4 | 1.60 (1.18–3.59) | ||||||
| 18.5–20.9 | 1.39 (1.17–1.65) | |||||||
| 21.0–22.9 | 1.09 (0.93–1.27) | |||||||
| 23.0–24.9 | 1.00 | |||||||
| 25.0–27.4 | 0.95 (0.81–1.12) | |||||||
| 27.5–29.9 | 1.17 (0.94–1.45) | |||||||
| 30.0–50.0 | 1.46 (1.07–1.99) | |||||||
| Per 5 kg/m2 | 0.90 (0.82–0.99) | |||||||
| BMI, gallstone-related acute pancreatitis | 12.0–18.4 | 0.90 (0.43–1.88) | ||||||
| 18.5–20.9 | 1.01 (0.69–1.47) | |||||||
| 21.0–22.9 | 1.04 (0.75–1.43) | |||||||
| 23.0–24.9 | 1.00 | |||||||
| 25.0–27.4 | 1.11 (0.82–1.52) | |||||||
| 27.5–29.9 | 1.63 (1.12–2.37) | |||||||
| 30.0–50.0 | 2.04 (1.21–3.46) | |||||||
| Per 5 kg/m2 | 1.38 (1.16–1.66) | |||||||
| BMI, non-gallstone-related acute pancreatitis | 12.0–18.4 | 1.95 (1.48–2.59) | ||||||
| 18.5–20.9 | 1.56 (1.31–1.85) | |||||||
| 21.0–22.9 | 1.20 (1.02–1.40) | |||||||
| 23.0–24.9 | 1.00 | |||||||
| 25.0–27.4 | 0.97 (0.83–1.15) | |||||||
| 27.5–29.9 | 1.12 (0.90–1.40) | |||||||
| 30.0–50.0 | 1.41 (1.02–1.95) | |||||||
| Per 5 kg/m2 | 0.79 (0.72–0.87) | |||||||
| BMI and acute pancreatitis, men | Per 5 kg/m2 | 0.77 (0.69–0.86) | ||||||
| BMI and gallstone-related acute pancreatitis | Per 5 kg/m2 | 1.31 (1.02–1.68) | ||||||
| BMI and non-gallstone-related acute pancreatitis | Per 5 kg/m2 | 0.69 (0.61–0.77) | ||||||
| BMI and acute pancreatitis, women | Per 5 kg/m2 | 1.12 (0.97–1.28) | ||||||
| BMI and gallstone-related acute pancreatitis | Per 5 kg/m2 | 1.47 (1.14–1.91) | ||||||
| BMI and non-gallstone-related acute pancreatitis | Per 5 kg/m2 | 1.01 (0.85–1.18) | ||||||
| BMI and acute pancreatitis, current drinker | Per 5 kg/m2 | 0.74 (0.65–0.83) | ||||||
| BMI and gallstone-related acute pancreatitis | Per 5 kg/m2 | 1.30 (0.97–1.74) | ||||||
| BMI and non-gallstone-related acute pancreatitis | Per 5 kg/m2 | 0.66 (0.57–0.75) | ||||||
| BMI and acute pancreatitis, non-drinkers | Per 5 kg/m2 | 1.03 (0.91–1.16) | ||||||
| BMI and gallstone-related acute pancreatitis | Per 5 kg/m2 | 1.38 (1.09–1.74) | ||||||
| BMI and non-gallstone-related acute pancreatitis | Per 5 kg/m2 | 0.93 (0.81–1.07) | ||||||
| BMI and acute pancreatitis, ever smokers | Per 5 kg/m2 | 0.67 (0.59–0.76) | ||||||
| BMI and gallstone-related acute pancreatitis | Per 5 kg/m2 | 1.42 (1.04–1.95) | ||||||
| BMI and non-gallstone-related acute pancreatitis | Per 5 kg/m2 | 0.57 (0.50–0.66) | ||||||
| BMI and acute pancreatitis, never smokers | Per 5 kg/m2 | 1.04 (0.93–1.17) | ||||||
| BMI and gallstone-related acute pancreatitis | Per 5 kg/m2 | 1.29 (1.02–1.63) | ||||||
| BMI and non-gallstone-related acute pancreatitis | Per 5 kg/m2 | 0.97 (0.85–1.11) | ||||||
| Hansen et al. [21], Denmark | Copenhagen City Heart Study and Copenhagen General Population Study | 1994–1994–2001–2003–2017 | 117531 men and women, age 20–≥ 100 years: 458 acute pancreatitis cases | Measured | BMI | < 18.5 | 1.6 (0.6–4.2) | Age, sex, smoking, alcohol, lipid-lowering therapy, physical activity, diabetes, gallstone disease |
| 18.5–24.9 | 1.0 | |||||||
| 25.0–29.9 | 1.4 (1.1–1.8) | |||||||
| 30.0–34.9 | 2.1 (1.6–2.9) | |||||||
| ≥ 35.0 | 2.8 (1.8–4.3) | |||||||
| 2003–2015–2017, 8 years follow-up | BMI, men | Per 1 kg/m2 | 1.09 (1.05–1.12) | |||||
| BMI, women | Per 1 kg/m2 | 1.05 (1.03–1.09) | ||||||
| BMI, CCHS | Per 1 kg/m2 | 1.05 (1.01–1.10) | ||||||
| BMI, CGPS | Per 1 kg/m2 | 1.07 (1.05–1.10) |
ACE angiotensin converting enzyme-inhibitor, BMI Body mass index, NSAID non-steroidal anti-inflammatory drugs
Ten prospective studies (eight publications, nine risk estimates) [4, 19–25] with 1,693,657 participants and 5129 cases were included in the analysis of BMI and the risk of acute pancreatitis. The summary RR was 1.18 (95% CI: 1.03–1.35, I2 = 91.1%, pheterogeneity < 0.0001) per 5 kg/m2 increase in BMI (Fig. 2a). There was no evidence of publication bias with Egger’s test (p = 0.93) or with Begg’s test (p = 0.35) (Supplementary Figure 1). The summary RR ranged from 1.14 (95% CI: 1.00–1.30) when excluding the study by Pang et al. [4] to 1.24 (95% CI: 1.12–1.37) when excluding the study by Choi et al. [25] (Supplementary Figure 2, Supplementary Figure 3a). One large Korean study found a L-shaped or U-shaped association between BMI and pancreatitis, which was more pronounced in ever smokers, and alcohol drinkers than in never smokers and nondrinkers, which could suggest potential residual confounding [25]. When data for never smokers were used instead of the total population for this study, the summary RR was 1.21 (95% CI: 1.09–1.34, I2 = 81.5%, pheterogeneity < 0.0001) per 5 kg/m2 increase in BMI (Supplementary Figure 4). Alternatively, when excluding the categories below the reference category when the lowest category was not the reference category, the summary RR was 1.25 (95% CI: 1.12–1.40, I2 = 85.2%, pheterogeneity < 0.0001) per 5 kg/m2 increase in BMI (Supplementary Figure 5).
Fig. 2.
Body mass index and acute pancreatitis, linear dose-response analysis per 5 kg/m2 (a) and nonlinear dose-response analysis (b)
There was evidence of a nonlinear association between BMI and acute pancreatitis (pnonlinearity < 0.0001), and there was a steeper association at higher levels of BMI than at lower levels (Fig. 2b, Supplementary Table 2). Exclusion of the Korean study led to a more linear dose-response relationship between BMI and acute pancreatitis, although the test for nonlinearity was still significant (pnonlinearity = 0.001) (Supplementary Figure 3b, Supplementary Table 2).
Only four studies (three publications, three risk estimates) were included in the analysis of BMI and gallstone-related pancreatitis [19, 24, 25] and non-gallstone-related pancreatitis [19, 24, 25], and two studies were included in the analysis of BMI and chronic pancreatitis [4, 20], and the summary RRs were 1.34 (95% CI: 1.16–1.56, I2 = 52%, pheterogeneity = 0.13) (Supplementary Figure 6), 0.93 (95% CI: 0.76–1.14, I2 = 82%, pheterogeneity = 0.004) (Supplementary Figure 7), and 0.78 (95% CI: 0.65–0.92, I2 = 0%, pheterogeneity = 0.80) (Supplementary Figure 8), respectively.
Three cohort studies (two publications) [4, 24] were included in the analysis of waist circumference and risk of acute pancreatitis (1503 cases, 578,472 participants). The summary RR was 1.36 (95% CI: 1.29–1.43, I2 = 0%, pheterogeneity = 0.90) per 10 cm increase in waist circumference (Fig. 3a). There was no evidence of a nonlinear association between waist circumference and risk of acute pancreatitis (pnonlinearity = 0.19), and there was a fourfold increase in risk when comparing a waist circumference of 110 cm with one of 69 cm (Fig. 3b, Supplementary Table 3).
Fig. 3.
Waist circumference and acute pancreatitis, linear dose-response analysis per 10 cm (a) and nonlinear dose-response analysis (b)
Subgroup and Sensitivity Analyses
There was a positive association between BMI and acute pancreatitis in most subgroup analyses stratified by sex, duration of follow-up, number of cases and adjustment for confounding factors (including age, education, alcohol, smoking, waist circumference and physical activity) or potentially mediating factors (diabetes mellitus, gallstones/gallbladder disease and triglycerides), although in some subgroups the association was not statistically significant (Table 2). With meta-regression analyses, there was no evidence that the results differed between these subgroups; however, within-subgroup heterogeneity was lower among studies in women (I2 = 0%) and among the American studies (I2 = 0.8%) (Table 2). The mean (median) study quality score was 8.4 (8.0) out of 9.0.
Table 2.
Subgroup analyses of body mass index and acute pancreatitis
| Body mass index and acute pancreatitis | |||||
|---|---|---|---|---|---|
| n | Relative risk (95% CI) | I2 (%) | P1h | P2h | |
| All studies | 9 | 1.18 (1.03–1.35) | 91.1 | < 0.0001 | |
| Sex | |||||
| Men | 2 | 1.09 (0.55–2.14) | 97.9 | < 0.0001 | 0.60/0.753 |
| Women | 3 | 1.18 (1.09–1.27) | 0 | 0.40 | |
| Men, women | 5 | 1.20 (1.03–1.41) | 84.5 | < 0.0001 | |
| Follow-up | |||||
| < 10 years | 4 | 1.28 (1.09–1.52) | 82.0 | 0.001 | 0.26 |
| ≥ 10 years | 5 | 1.11 (0.96–1.29) | 87.7 | < 0.0001 | |
| Assessment of weight/height | |||||
| Measured | 7 | 1.20 (1.01–1.43) | 93.1 | < 0.0001 | 0.63 |
| Self-reported (validated) | 2 | 1.13 (1.03–1.23) | 0 | 0.41 | |
| Geographic location | |||||
| Europe | 5 | 1.19 (1.02–1.38) | 72.9 | 0.005 | 0.88 |
| America | 2 | 1.20 (1.12–1.30) | 0.8 | 0.32 | |
| Asia | 2 | 1.15 (0.70–1.90) | 98.6 | < 0.0001 | |
| Number of cases | |||||
| Cases < 250 | 2 | 1.28 (1.11–1.47) | 0 | 0.99 | 0.62 |
| Cases 250– < 500 | 4 | 1.17 (1.00–1.36) | 79.4 | 0.002 | |
| Cases ≥ 500 | 3 | 1.15 (0.84–1.58) | 97.2 | < 0.0001 | |
| Study quality | |||||
| 0–3 stars | 0 | NC | |||
| 4–6 stars | 0 | ||||
| 7–9 stars | 9 | 1.18 (1.03–1.35) | 91.1 | < 0.0001 | |
| Adjustment for confounding factors | |||||
| Age | |||||
| Yes | 9 | 1.18 (1.03–1.35) | 91.1 | < 0.0001 | NC |
| No | 0 | ||||
| Education | |||||
| Yes | 3 | 1.27 (1.06–1.52) | 87.2 | < 0.0001 | 0.43 |
| No | 6 | 1.14 (0.96–1.35) | 89.7 | < 0.0001 | |
| Alcohol | |||||
| Yes | 8 | 1.18 (1.01–1.39) | 92.2 | < 0.0001 | 0.91 |
| No | 1 | 1.16 (1.04–1.29) | |||
| Smoking | |||||
| Yes | 8 | 1.17 (1.00–1.37) | 92.1 | < 0.0001 | 0.76 |
| No | 1 | 1.25 (1.13–1.38) | |||
| Waist circumference | |||||
| Yes | 1 | 1.07 (0.91–1.25) | 0.59 | ||
| No | 8 | 1.19 (1.03–1.39) | 92.0 | < 0.0001 | |
| Physical activity | |||||
| Yes | 4 | 1.24 (0.93–1.64) | 96.3 | < 0.0001 | 0.52 |
| No | 5 | 1.15 (1.05–1.26) | 50.3 | 0.09 | |
| Adjustment for potential intermediate factors | |||||
| Diabetes | |||||
| Yes | 6 | 1.16 (0.93–1.43) | 94.3 | < 0.0001 | 0.70 |
| No | 3 | 1.21 (1.13–1.30) | 0 | 0.52 | |
| Gallstones or gallbladder disease | |||||
| Yes | 6 | 1.16 (0.93–1.43) | 94.3 | < 0.0001 | 0.70 |
| No | 3 | 1.21 (1.13–1.30) | 0 | 0.52 | |
| Triglycerides | |||||
| Yes | 0 | NC | |||
| No | 9 | 1.18 (1.03–1.35) | 91.1 | < 0.0001 | |
n denotes the number of risk estimates (one publication reported the results for two studies combined)
1P for heterogeneity within each subgroup
2P for heterogeneity between subgroups with meta-regression analysis
3P for heterogeneity between men and women, excluding studies of men and women combined
NC, not calculable because no studies were present in one of the subgroups
Discussion
This meta-analysis of ten prospective studies suggests that a 5 kg/m2 increase in BMI is associated with an 18% increase in the relative risk of acute pancreatitis, and a 10 cm increase in waist circumference is associated with a 36% increase in the relative risk. There was some evidence of nonlinearity in the analysis of BMI, with a steeper increase in risk at higher levels of BMI than at lower levels and with the increased risk being most pronounced in the obese and severely obese range. When comparing a BMI of 30, 35 and 40 with a BMI of 22, there was a 34%, 102% and 235% increase in the relative risk of acute pancreatitis. There was no evidence of nonlinearity in the analysis of waist circumference and acute pancreatitis, and there was a fourfold increase in risk when comparing the highest vs. the lowest level of waist circumference. There was a positive association between BMI and gallstone-related pancreatitis, but not with non-gallstone-related pancreatitis, while an inverse association was observed between BMI and chronic pancreatitis; however, these results were based on only 2–4 studies; thus, caution is needed in the interpretation of these findings and further studies are needed before firm conclusions can be drawn. Only two studies investigated waist circumference and different types of pancreatitis and found a slightly stronger association with gallstone-related pancreatitis, than for non-gallstone-related pancreatitis [24]; however, there was still a strong and significant association even for the latter. The current findings are consistent with a previous meta-analysis on BMI and acute pancreatitis which found a 43% increase in the RR among obese compared to normal weight subjects [27]; however, only three studies (two publications) with 603 cases and 101,369 participants were included in the analysis [27], while the current analysis included > 5000 cases and > 1.69 million participants and therefore provides a more comprehensive assessment of the available evidence.
Several biological pathways could explain why higher BMI or waist circumference is associated with increased risk of pancreatitis. Excess weight is a strong risk factor for type 2 diabetes [37], gallstones [17] and elevated levels of triglycerides [38], which again are strongly associated with increased risk of pancreatitis [14, 39–42]. The observation in the current analysis that there was a positive association between BMI and gallstone-related pancreatitis, but not for non-gallstone-related pancreatitis (RR = 1.34 vs. 0.93 per 5 kg/m2) might suggest that increased risk of gallstones may account for much of the increased risk of pancreatitis with overweight and obesity as measured by BMI; however, given that there were only four studies in each of these analyses, further studies are needed before firm conclusions can be made. In contrast, the association between waist circumference and pancreatitis was strong both for gallstone-related pancreatitis (RR = 2.5) and for non-gallstone-related pancreatitis (RR = 2.1) in two Swedish cohorts even after adjustment for BMI [24], suggesting that other mechanisms could contribute as well. A combined analysis of two Danish studies suggested recently that elevated triglycerides appeared to mediate 22% of the excess risk of the association between BMI and acute pancreatitis [21]. Higher BMI is associated with more adipocytes within the pancreas which are dispersed adjacent to the exocrine pancreatic acinar cells [43]. In acute pancreatitis, these adipocytes are damaged by the release of lipases which hydrolyze their triglycerides into unsaturated fatty acids, which in turn cause necrosis of the acinar cells [43]. In chronic pancreatitis, adipocyte mass is unrelated to BMI, and adipocytes are surrounded by fibrosis, which prevents the lipolytic flux between the two compartments [43]. This could be part of the explanation for the difference in the results for BMI and acute and chronic pancreatitis. Adiposity could also impact pancreatitis indirectly because certain obesity treatments such as bariatric surgery, duodeno-jejunal bypass liner and gastric balloons in some cases can cause acute pancreatitis; however, this would most likely explain only a small percentage of cases [43]. Adiposity is associated with low-grade inflammation and with lower levels of adiponectin, a cytokine with anti-inflammatory properties and which reduces insulin resistance [44]. It has been shown that adiponectin reduced the development of acute pancreatitis in mice fed a high-fat diet [45].
Limitations that may have affected the results of the current meta-analysis include potential confounding, heterogeneity, measurement errors in the assessment of anthropometric measures and publication bias. We cannot exclude the possibility of residual confounding as persons with overweight and obesity tend to have a generally less healthy lifestyle including lower physical activity, unhealthier diets and higher rates of type 2 diabetes compared to normal weight individuals, while persons with a low BMI more often are smokers than persons with a high BMI. Several of the studies adjusted for a range of confounding factors and the results persisted across most subgroups with adjustment for confounding factors. In addition, there was little evidence of heterogeneity between these subgroup analyses. However, one study from Korea suggested an L-shaped or U-shaped association between BMI and acute pancreatitis, with an inverse association overall and among men, ever smokers and alcohol drinkers when the association was analyzed on a continuous scale, but not among women, never smokers and nondrinkers [25]. This might suggest that confounding by smoking and alcohol could have affected the results of that study and is also likely to explain the difference in the association by sex as smoking prevalence is much higher in males than among females in this population [46]. Any further studies could conduct analyses stratified by other risk factors to further explore the impact of residual confounding by smoking and alcohol or other factors. We also cannot exclude residual confounding from non-established risk factors such as diet or other unknown factors. There was high heterogeneity in the overall analysis of BMI and acute pancreatitis; however, heterogeneity was low among studies in women and among the American studies.
There was little differences in the results when subgroups were stratified by adjustment for various confounding factors including alcohol, smoking and physical activity. There was also little heterogeneity between subgroups of studies that adjusted for potential intermediate risk factors including diabetes and gallstones. We can also not exclude the possibility of residual confounding by other potential risk factors, and few studies have adjusted for dietary risk factors to date. However, the confounder would have to be relatively strong to fully account for the threefold–fourfold difference in risk observed for extremes in BMI and waist circumference.
Anthropometry was assessed by self-report in a few studies and this may have led to measurement errors in the exposure assessment; however, this was the case for only three studies which also validated the self-reports. Several studies have shown relatively high correlations between measured and self-reported anthropometric variables with correlation coefficients of around 0.95–0.97, suggesting that self-report of adiposity is relatively accurate [47–49]. Because of the prospective design of the included studies, any measurement errors in the assessment of anthropometric measures would most likely have biased the results toward the null. In addition, the positive associations between BMI and acute pancreatitis persisted both among the seven studies that used measured weight and height for the anthropometric assessment and among the three studies with self-reported weight and height. Although there was no between subgroup heterogeneity the association was slightly stronger in the studies that measured weight and height compared with those that used self-reported anthropometric measures (RR = 1.20 vs. 1.13). In the China Kadoorie Biobank Study, more or less similar associations were observed between different adiposity measures such as BMI, waist circumference, waist-to-hip ratio, hip circumference, body fat percentage, height-adjusted weight and weight change and risk of acute pancreatitis [4]. Because anthropometric variables usually were measured only at baseline, it was not possible to take into account changes in adiposity in this analysis and it is therefore possible that weight gain over time may account for part of the association observed between adiposity and the risk of developing pancreatitis. The China Kadoorie Biobank Study found a positive association between both BMI at age 25 and weight change between age 25 and baseline and the risk of acute pancreatitis [4]. Because of limited data on adiposity and severity of pancreatitis, we were not able to analyze this association; however, some previous studies suggested obesity increases the risk of severe pancreatitis, complications and mortality in pancreatitis patients [50–52].
Although publication bias can affect the results of meta-analyses of published studies, we found no evidence of publication bias in the analysis of BMI and acute pancreatitis. There were too few studies to conduct subgroup analyses or test for publication bias in the analysis of waist circumference and acute pancreatitis.
This meta-analysis has several strengths including 1) the prospective design of the included studies which avoids problems with recall bias and reduces the potential for selection bias, 2) the robustness of the findings in multiple subgroup and sensitivity analyses and 3) with > 5,100 cases and ~ 1.7 million participants, we had sufficient statistical power to detect even a moderate association between adiposity and acute pancreatitis. Our findings have important clinical and public health implications as the number of persons who are overweight and obese has increased considerably over the last decades in all areas of the world [53]. If this trend continues it may contribute to additional cases of pancreatitis and related consequences such as increased incidence of pancreatic cancer and premature mortality, but it could be halted with widespread adoption of healthier diets and increased levels of physical activity [54].
Conclusion
This meta-analysis suggests that higher BMI and waist circumference are associated with increased risk of acute pancreatitis. Further studies are needed on different measures of adiposity and the risk of different subtypes of pancreatitis (acute vs. chronic and gallstone-related vs. non-gallstone-related) and to clarify the underlying mechanisms, but the findings underscore the importance of weight control for the prevention of acute pancreatitis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Open Access funding provided by University of Oslo (incl Oslo University Hospital).
Author's contributions
DA designed the research, conducted the literature search and analyses and wrote the first draft of the paper. DA and YMS conducted the literature screening. DA, YMS, TN and ER interpreted the data, revised the subsequent drafts for important intellectual content, read and approved the final manuscript. DA takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was funded by the Imperial College School of Public Health and the South-East Regional Health Authority of Norway.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no duality of interest associated with this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dagfinn Aune, Email: d.aune@imperial.ac.uk.
Yahya Mahamat-Saleh, Email: Yahya.MAHAMAT-SALEH@gustaveroussy.fr.
Teresa Norat, Email: t.norat@imperial.ac.uk.
Elio Riboli, Email: e.riboli@imperial.ac.uk.
References
- 1.Hammad AY, Ditillo M, Castanon L. Pancreatitis. Surg Clin North Am. 2018;98:895–913. doi: 10.1016/j.suc.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: Epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon. 2014;60:530–550. doi: 10.1016/j.disamonth.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Kirkegard J, Mortensen FV, Cronin-Fenton D. Chronic pancreatitis and pancreatic cancer risk: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1366–1372. doi: 10.1038/ajg.2017.218. [DOI] [PubMed] [Google Scholar]
- 4.Pang Y, Kartsonaki C, Turnbull I, Guo Y, Yang L, Bian Z, Chen Y, Millwood IY, Bragg F, Gong W, Xu Q, Kang Q, et al. Metabolic and lifestyle risk factors for acute pancreatitis in Chinese adults: a prospective cohort study of 0.5 million people. PLoS Med. 2018;15:e1002618. doi: 10.1371/journal.pmed.1002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bang UC, Benfield T, Hyldstrup L, Bendtsen F, Beck Jensen JE. Mortality, cancer, and comorbidities associated with chronic pancreatitis: a Danish nationwide matched-cohort study. Gastroenterology. 2014;146:989–994. doi: 10.1053/j.gastro.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 6.Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55. doi: 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 7.McKay CJ, Evans S, Sinclair M, Carter CR, Imrie CW. High early mortality rate from acute pancreatitis in Scotland, 1984–1995. Br J Surg. 1999;86:1302–1305. doi: 10.1046/j.1365-2168.1999.01246.x. [DOI] [PubMed] [Google Scholar]
- 8.Kingsnorth A, O’Reilly D. Acute pancreatitis. BMJ. 2006;332:1072–1076. doi: 10.1136/bmj.332.7549.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaakkola M, Nordback I. Pancreatitis in Finland between 1970 and 1989. Gut. 1993;34:1255–1260. doi: 10.1136/gut.34.9.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spanier BW, Dijkgraaf MG, Bruno MJ. Trends and forecasts of hospital admissions for acute and chronic pancreatitis in the Netherlands. Eur J Gastroenterol Hepatol. 2008;20:653–658. doi: 10.1097/MEG.0b013e3282f52f83. [DOI] [PubMed] [Google Scholar]
- 11.Tinto A, Lloyd DA, Kang JY, Majeed A, Ellis C, Williamson RC, Maxwell JD. Acute and chronic pancreatitis–diseases on the rise: a study of hospital admissions in England 1989/90–1999/2000. Aliment Pharmacol Ther. 2002;16:2097–2105. doi: 10.1046/j.1365-2036.2002.01367.x. [DOI] [PubMed] [Google Scholar]
- 12.Fagenholz PJ, Castillo CF, Harris NS, Pelletier AJ, Camargo CA., Jr Increasing United States hospital admissions for acute pancreatitis, 1988-2003. Ann Epidemiol. 2007;17:491–497. doi: 10.1016/j.annepidem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 2017;17:155–165. doi: 10.1016/j.pan.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Lin HH, Chang HY, Chiang YT, Wu MS, Lin JT, Liao WC. Smoking, drinking, and pancreatitis: a population-based cohort study in Taiwan. Pancreas. 2014;43:1117–1122. doi: 10.1097/MPA.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 15.Majumder S, Gierisch JM, Bastian LA. The association of smoking and acute pancreatitis: a systematic review and meta-analysis. Pancreas. 2015;44:540–546. doi: 10.1097/MPA.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 16.Abdullah A, Peeters A, de Court, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89:309–319. doi: 10.1016/j.diabres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Aune D, Norat T, Vatten LJ. Body mass index, abdominal fatness and the risk of gallbladder disease. Eur J Epidemiol. 2015;30:1009–1019. doi: 10.1007/s10654-015-0081-y. [DOI] [PubMed] [Google Scholar]
- 18.Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, Cade JE, Burley VJ, Norat T. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 19.Morton C, Klatsky AL, Udaltsova N. Smoking, coffee, and pancreatitis. Am J Gastroenterol. 2004;99:731–738. doi: 10.1111/j.1572-0241.2004.04143.x. [DOI] [PubMed] [Google Scholar]
- 20.Prizment AE, Jensen EH, Hopper AM, Virnig BA, Anderson KE. Risk factors for pancreatitis in older women: the Iowa Women’s Health Study. Ann Epidemiol. 2015;25:544–548. doi: 10.1016/j.annepidem.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen SEJ, Madsen CM, Varbo A, Nordestgaard BG. Body mass index, triglycerides, and risk of acute pancreatitis: a population-based study of 118,000 individuals. J Clin Endocrinol Metab. 2020;105:2020. doi: 10.1210/clinem/dgz059. [DOI] [PubMed] [Google Scholar]
- 22.Lindkvist B, Appelros S, Manjer J, Berglund G, Borgstrom A. A prospective cohort study of smoking in acute pancreatitis. Pancreatology. 2008;8:63–70. doi: 10.1159/000114868. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Perez A, Schlienger RG, Rodriguez LA. Acute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort study. Diabetes Care. 2010;33:2580–2585. doi: 10.2337/dc10-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadr-Azodi O, Orsini N, Andren-Sandberg A, Wolk A. Abdominal and total adiposity and the risk of acute pancreatitis: a population-based prospective cohort study. Am J Gastroenterol. 2013;108:133–139. doi: 10.1038/ajg.2012.381. [DOI] [PubMed] [Google Scholar]
- 25.Choi JS, Yi SW, Park JW, Lee S, Jeong SH, Yi JJ, Han KJ. Body mass index and the risk of acute pancreatitis by etiology: a prospective analysis of Korean National Screening Cohort. J Gastroenterol Hepatol. 2019;34:603–611. doi: 10.1111/jgh.14570. [DOI] [PubMed] [Google Scholar]
- 26.Hong S, Qiwen B, Ying J, Wei A, Chaoyang T. Body mass index and the risk and prognosis of acute pancreatitis: a meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:1136–1143. doi: 10.1097/MEG.0b013e32834b0e0e. [DOI] [PubMed] [Google Scholar]
- 27.Alsamarrai A, Das SL, Windsor JA, Petrov MS. Factors that affect risk for pancreatic disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2014;12:1635–1644. doi: 10.1016/j.cgh.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 31.Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol. 2004;159:1077–1086. doi: 10.1093/aje/kwh142. [DOI] [PubMed] [Google Scholar]
- 32.Royston P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med. 2000;19:1831–1847. doi: 10.1002/1097-0258(20000730)19:14<1831::aid-sim502>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 34.Wells G, Shea B, O’Connell D., Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 09.08.2018.
- 35.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 37.Langenberg C, Sharp SJ, Schulze MB, Rolandsson O, Overvad K, Forouhi NG, Spranger J, Drogan D, Huerta JM, Arriola L, de Lauzon-Guillan B, Tormo MJ, et al. Long-term risk of incident type 2 diabetes and measures of overall and regional obesity: the EPIC-InterAct case-cohort study. PLoS Med. 2012;9:e1001230. doi: 10.1371/journal.pmed.1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bays HE, Toth PP, Kris-Etherton PM, Abate N, Aronne LJ, Brown WV, Gonzalez-Campoy JM, Jones SR, Kumar R, La FR, Samuel VT. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:304–383. doi: 10.1016/j.jacl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Girman CJ, Kou TD, Cai B, Alexander CM, O’Neill EA, Williams-Herman DE, Katz L. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab. 2010;12:766–771. doi: 10.1111/j.1463-1326.2010.01231.x. [DOI] [PubMed] [Google Scholar]
- 40.Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care. 2010;33:2349–2354. doi: 10.2337/dc10-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urushihara H, Taketsuna M, Liu Y, Oda E, Nakamura M, Nishiuma S, Maeda R. Increased risk of acute pancreatitis in patients with type 2 diabetes: an observational study using a Japanese hospital database. PLoS One. 2012;7:e53224. doi: 10.1371/journal.pone.0053224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen SB, Langsted A, Nordestgaard BG. Nonfasting mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis. JAMA Intern Med. 2016;176:1834–1842. doi: 10.1001/jamainternmed.2016.6875. [DOI] [PubMed] [Google Scholar]
- 43.Khatua B, El-Kurdi B, Singh VP. Obesity and pancreatitis. Curr Opin Gastroenterol. 2017;33:374–382. doi: 10.1097/MOG.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr Pharm Des. 2010;16:1896–1901. doi: 10.2174/138161210791208893. [DOI] [PubMed] [Google Scholar]
- 45.Araki H, Nishihara T, Matsuda M, Fukuhara A, Kihara S, Funahashi T, Kataoka TR, Kamada Y, Kiyohara T, Tamura S, Hayashi N, Shimomura I. Adiponectin plays a protective role in caerulein-induced acute pancreatitis in mice fed a high-fat diet. Gut. 2008;57:1431–1440. doi: 10.1136/gut.2007.135665. [DOI] [PubMed] [Google Scholar]
- 46.Jee SH, Samet JM, Ohrr H, Kim JH, Kim IS. Smoking and cancer risk in Korean men and women. Cancer Causes Control. 2004;15:341–348. doi: 10.1023/B:CACO.0000027481.48153.97. [DOI] [PubMed] [Google Scholar]
- 47.Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5:561–565. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 48.Bes-Rastrollo M, Sabate J, Jaceldo-Siegl K, Fraser GE. Validation of self-reported anthropometrics in the Adventist Health Study 2. BMC Public Health. 2011;11:213. doi: 10.1186/1471-2458-11-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee DH, Shin A, Kim J, Yoo KY, Sung J. Validity of self-reported height and weight in a Korean population. J Epidemiol. 2011;21:30–36. doi: 10.2188/jea.JE20100058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papachristou GI, Papachristou DJ, Avula H, Slivka A, Whitcomb DC. Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6:279–285. doi: 10.1159/000092689. [DOI] [PubMed] [Google Scholar]
- 51.Chen SM, Xiong GS, Wu SM. Is obesity an indicator of complications and mortality in acute pancreatitis? An updated meta-analysis. J Dig Dis. 2012;13:244–251. doi: 10.1111/j.1751-2980.2012.00587.x. [DOI] [PubMed] [Google Scholar]
- 52.Dobszai D, Matrai P, Gyongyi Z, Csupor D, Bajor J, Eross B, Miko A, Szako L, Meczker A, Hagendorn R, Marta K, Szentesi A, et al. Body-mass index correlates with severity and mortality in acute pancreatitis: a meta-analysis. World J Gastroenterol. 2019;25:729–743. doi: 10.3748/wjg.v25.i6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.