Abstract
It is unclear if genetic variants affect smoking cessation treatment response. This study tested whether variants in the cholinergic receptor nicotinic alpha 5 subunit (CHRNA5) predict response to smoking cessation medication by directly comparing two most effective smoking cessation pharmacotherapies. In this genotype-stratified randomized, double-blind, placebo-controlled clinical trial (5/2015–8/2019 in St. Louis, Missouri), smokers were randomized by genotype in blocks of 6 (1:1:1 ratio) to 3 conditions: 12 weeks of placebo (n=273), combination nicotine patch and lozenge (cNRT, n=275), or varenicline (n=274). All participants received counseling and were followed for 12 months. The primary endpoint was biochemically verified 7-day point prevalence abstinence at the end of treatment (EOT, week 12). Trial registration and eligibility criteria are on clinicEiltrials.gov (NCT02351167). We conducted the genetic analyses separately for 516 European American (EA) smokers and 306 non-EA smokers (including 270 African American smokers). In African American smokers, there was a genotype-by-treatment interaction for EOT abstinence (X2=10.7, df=2. P=0.0049): specifically, cNRT was more effective in smokers with rsl6969968 GG genotypes than was placebo, while varenicline was more effective in smokers of GA/AA genotypes. In EA ancestry smokers, there was no significant genotype-by-treatment interaction. In the whole sample, only varenicline, and not cNRT, produced higher abstinence at 6-month follow-up. In the whole sample, although both were effective at EOT, only varenicline, and not cNRT, was significantly effective relative to placebo at 6-month follow-up. Importantly, this study suggests that genetic information can further enhance their effectiveness.
Keywords: Smoking, Pharmacotherapy, Pharmacogenetics, Precision medicine, Addiction, Biomarkers, Clinical Trials, Genotype, Nicotine, Personalized Medicine
INTRODUCTION
Tobacco smoking reduces life expectancy by at least ten years on average with many smoking-related deaths (1). Such premature deaths are preventable; quitting at any age can lead to numerous health improvements and improve life expectancy by up to ten years. Unfortunately, most smoking cessation attempts are unsuccessful, even when evidence based treatments such as FDA approved medications are used (2–5).
Two smoking cessation medications have been shown to be especially effective; multiple studies have identified varenicline and combination nicotine replacement therapy (cNRT) as the two most efficacious smoking cessation medications (4–7). Although both medications are effective, cNRT is more accessible due to availability over the counter and generally lower cost. Varenicline is less accessible due to its requirement of a physician prescription, cost, and a previous black box warning (2012–2016) on neuropsychiatric adverse effects (2). Efficacy of even these two medications are limited in the majority of smokers (4–6).
Many factors may limit the effectiveness of smoking medications: e.g., adverse effects that limit use and inadequate adherence (3–5). Precision medicine initiatives have the goal of using individual differences such as genotypes to guide treatment so as to improve health outcomes (8). One approach would be to identify genetic moderators of smoking treatment effectiveness or adverse effects and then use such information to inform treatment application (3, 9, 10).
Multiple large meta-analyses, including our own, have identified genetic markers that predict heaviness of smoking and smoking cessation, most notably near the cholinergic receptor nicotinic alpha 5 subunit (CHRNA5) (11–14). In particular, the coding variant rs16969968 changes an amino acid (aspartic acid to asparagine) in CHRNA5. Individuals with the A allele: 1) Eire more likely to smoke heavily, 2) have increased risk for lung cancer, 3) develop lung cancer nearly four years earlier, 4) delay their quitting age by four years, 5) are less successful in unassisted quit attempts, and 6) may be more likely to benefit from pharmacotherapy for smoking cessation (15–21).
A 2017 Cochrane review examined different biomarkers and smoking cessation medication efficacy (22). This review and other evidence, including our own, (23) reported inconsistent data on the relation of CHRNA5 with medication efficacy for individuals of European Ancestry (EA) (15, 17, 22). Specifically, the Cochrane review offers little evidence that NRT efficacy differs for EA smokers across rsl6969968 genotypes (22).
The pharmacogenetic evidence is more convergent for smokers of African American Ancestry than it is for EA smokers. The Cochrane review and other evidence showed that rsl6969968-GG was associated with higher abstinence at end of treatment (EOT)/12 weeks and 6-month amongst African American smokers who received NRT versus placebo (17, 22). However, while the data suggest that NRT may be the treatment of choice for rsl6969968-GG smokers, the extant data do not indicate how to treat smokers with the GA/AA genotypes. Previous pharmacogenetic studies have focused on how the efficacy of NRT varies with CHRNA5 genotype, but there is limited research in this area on varenicline. What is needed is a prospective, genetically stratified trial to determine the relation of CHRNA5 genotypes with the two most effective cessation treatments (combination NRT & varenicline), which have not yet been directly compared with a placebo control in the same genetically informed trial.
This is the first, prospective, genotype-based stratified randomization trial to compare the two most effective smoking cessation medications, combination NRT (patch and lozenge: cNRT) and varenicline, versus placebo, in smokers of European Ancestry and smokers of non-European Ancestry. This study used a stratified randomized trial design that ensured a balanced design based on a participant’s pertinent CHRNA5 rsl6969968 genotype. We examine these hypotheses: 1) whether medication effects on abstinence (cNRT vs. varenicline vs. placebo) vary with CHRNA5 rsl6969968 status (i.e. a genotype-by-medication interaction) and 2) whether the probability of adverse events varies with genotype within the medication groups (cNRT vs. varenicline vs. placebo).
METHODS
Study design and participants
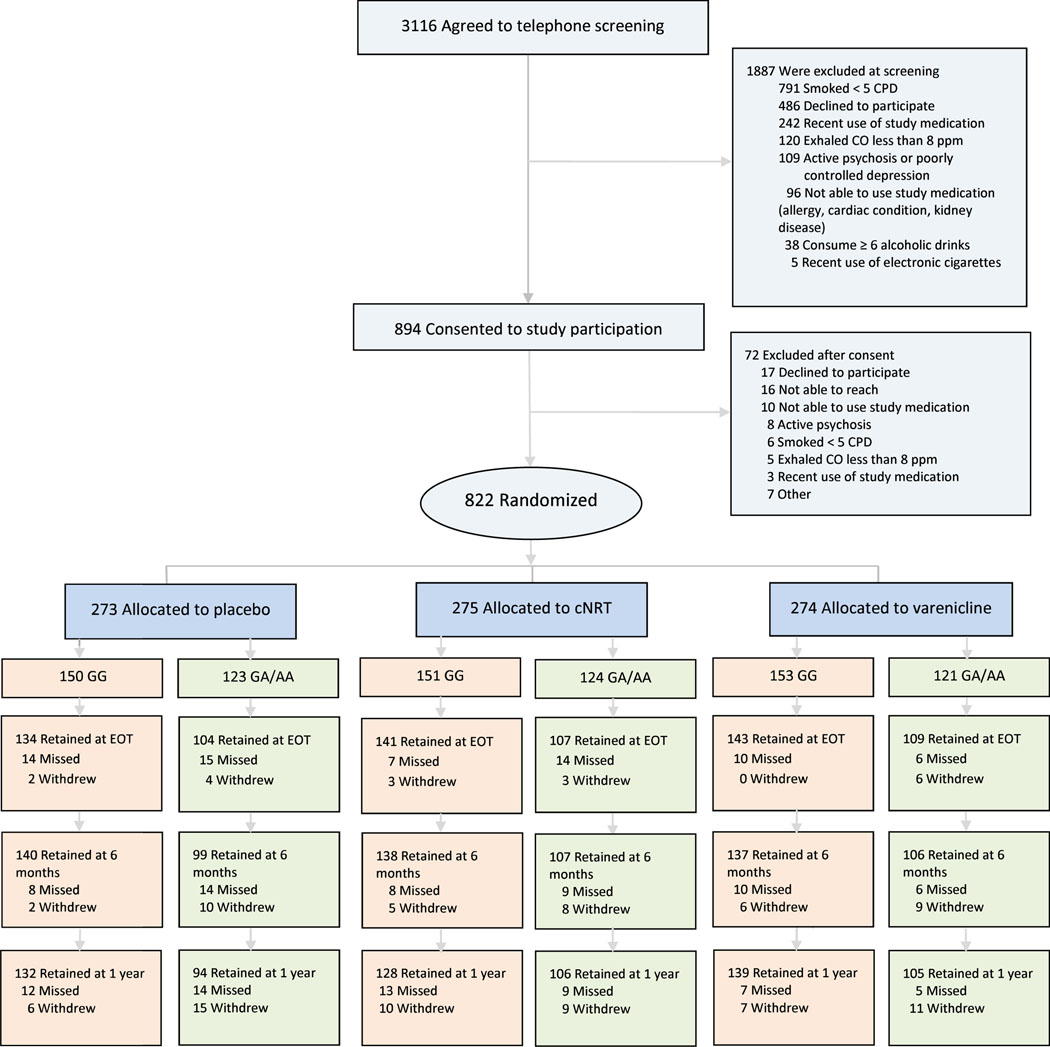
This is a prospective, genotype-based, stratified randomization trial. We randomly assigned participants by CHRNA5 rsl6969968 genotypes to one of three treatments for 12 weeks: (1) varenicline tartrate, (2) nicotine patches and nicotine lozenges, or (3) placebo varenicline tartrate or placebo nicotine patches/lozenges. Participants receiving active/placebo nicotine patches/lozenges initiated lozenge use one week pre-quit and initiated patch use on the quit date. Participants assigned to active/placebo varenicline started 1-week titration pre-quit. All treatment conditions received cessation counseling (Figure 1). Our primary aim was to compare the efficacies of cNRT and varenicline with one another and with placebo as a function of rsl6969968 genotype. Full eligibility criteria are available at clinicaltrials.gov, NCT02351167. All patients provided written, informed consent, as approved by the Institutional Review Board at Washington University in St. Louis. See Supplementary Text 1 for additional information.
Figure 1 –
Consort Diagram.
*full exclusion criteria are available at clinicaltrials.gov, identifier NCT02351167
Randomization and masking
We randomly assigned participants to three treatments in a 1:1:1 ratio. Randomization was stratified by rsl6969968 genotype in blocks of 6 patients (2 per treatment per block) to ensure approximate balance. Participants, investigators, and personnel (except for the biostatistician and senior data manager) were masked to treatment group allocation and genotype status.
Procedures
Participants provided a blood sample for genetic analysis. The survey and blood sample collection were completed at baseline prior to randomization (stratified by CHRNA5 rsl6969968 genotypes). Genotyping for rsl 6969968 was obtained with TaqMan® assay or as part of the GWAS Illumina Omni 2.5 microarray (www.illumina.com) before enrollment.
Participation in this randomized cessation trial involved follow-up assessments for up to 12 months after the scheduled quit date. Self-reported smoking status was assessed using a standard timeline follow-back procedure, and biochemically verified. See Supplementary Text 1 for additional information.
Outcomes
The primary outcome was 7-day point prevalence abstinence at EOT (week 12) to estimate the medication by genotype interaction during the medication period. Abstinence was defined as no self-reported smoking (not even a puff) for at least 7 days before the assessment with biochemical verification for those self-reporting abstinence (CO < 8 ppm). During an in person visit, research assistants assessed tobacco smoke exposure by expired CO levels using the Micro+™pro Smokerlyzer® (coVita, Haddonfield, NJ). Participants lost to follow-up were considered smokers.
Secondary endpoints were 7-day point prevalence abstinence with CO verification at 6 months, 7-day point prevalence abstinence at 1 year by self report, adverse effects, and adherence. Adverse effects were assessed with a detailed inquiry of common adverse effects at multiple time points (pre-quit, quit, post-quit, weeks 1, 2, 4, 12) and a general inquiry when participants were off medication (6 months, 1 year). The adverse effects during the medication phase (pre-quit, quit, post-quit, weeks 1, 2, 4, 12) were summed to create an overall adverse effect severity score reflecting both frequency and severity. Medication adherence was assessed by self report and verified with collection of unused medication (or pill count for phone visits) at EOT.
Statistical analysis
All participants enrolled in the trial (N=822: genotypes = 454 GG, 368 GA/AA) were included in the analyses including 516 smokers of European ancestry (EA), and 306 non-European ancestry (non-EA) (270 smokers of African American ancestry, and 36 smokers of other ancestry). With pre-specified analytic plan, we conducted the genetic analyses separately for EA smokers and African American smokers. We also conducted separate analyses for smokers of non-EA ancestry. Minor allele frequency and linkage disequilibrium patterns differ across ancestry group for rs16969968 and its region.
To test the main effects of medication, genotype, and a genotype-by-treatment interaction on the primary and secondary outcomes, we used generalized linear models with PROC GENMOD using SAS software. We also tested multivariate models controlling for age and sex. Adverse effects and adherence were examined for each active treatment (vs. placebo) within each genotype group using generalized linear models. To correct for multiple comparisons, the adjusted p value threshold was 0.00625 with 8 tests across ancestry groups and key outcomes.
RESULTS
Retention rates at EOT exceeded 87% and retention did not vary across racial groups, treatment groups, or CHRNA5 rs16969968 genotypes (consort diagram in Figure 1). The treatment groups did not differ on demographic and smoking history variables (Table 1). CHRNA5 rsl6969968 GA/AA genotypes were more common in EA smokers than in non-EA smokers as expected because the minor allele A is less common in non-EA individuals than EA individuals (24).
Table 1.
Demographic Characteristics and Baseline Smoking-Related Variables
| Placebo | cNRT | Varenicline | All | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | GG (n=150) | GA/AA (n=123) | All (n=273) | GG (n=151) | GA/AA (n=124) | All (n=275) | GG (n=153) | GA/AA (n=121) | All (n=274) | (n=822) |
| Female, No (%) | 90 (60.0) | 71 (57.7) | 161 (59.0) | 81 (53.6) | 63 (50.8) | 144 (52.4) | 74 (48.4) | 70 (57.9) | 144 (52.6) | 449 (54.6) |
| Male, No (%) | 60 (40.0) | 52 (42.3) | 112 (41.0) | 70 (46.4) | 61 (49.2) | 131 (47.6) | 79 (51.6) | 51 (42.2) | 130 (47.5) | 373 (45.4) |
| Race | ||||||||||
| White | 71 (47.3) | 108 (87.8) | 179 (65.6) | 59 (39.1) | 109 (87.9) | 168 (61.1) | 65 (42.5) | 104 (86.0) | 169 (61.7) | 516 (62.8) |
| Black/African American | 70 (46.7) | 12 (9.8) | 82 (30.0) | 84 (55.6) | 13 (10.6) | 97 (35.3) | 81 (52.9) | 10 (8.2) | 91 (33.2) | 270 (32.9) |
| Other | 9 (6.0) | 3 (2.4) | 12 (4.4) | 8 (5.5) | 2 (1.6) | 10 (3.6) | 7 (4.6) | 7 (5.8) | 14 (5.1) | 36 (4.4) |
| Age, Mean (SD),y | 46.3 (11.4) | 46.9 (11.7) | 46.6 (11.5) | 47.1 (10.9) | 45.4 (11.6) | 46.3 (11.2) | 46.8 (10.9) | 46.3 (11.2) | 46.6 (11.0) | 46.5 (11.2) |
| Income >= $30,000/y, No. (%) | 53 (35.3) | 83 (67.5) | 136 (49.8) | 63 (41.7) | 76 (61.3) | 139 (50.6) | 73 (47.7) | 65 (53.7) | 138 (50.2) | 413 (50.2) |
| Cigarettes/d, mean (SD) | 17.4 (7.2) | 19.1 (7.8) | 18.2 (7.5) | 15.5 (7.9) | 18.9 (8.0) | 17. 0 (8.1) | 17.1 (8.1) | 18.0 (7.1) | 17. 5 (7.7) | 17.6 (7.8) |
| FTND score, mean (SD) | 4.9 (2.0) | 4.7 (2.1) | 4.8 (2.1) | 4.6 (2.1) | 4.9 (2.0) | 4.8 (2.0) | 4.9 (2.1) | 5.0 (2.0) | 4.9 (2.0) | 4.8 (2.1) |
| Exhaled Carbon monoxide, mean (SD), PPM | 26.5 (12.3) | 29.6 (14.1) | 27.9 (13.2) | 25.5 (11.4) | 28.9 (11.9) | 27.0 (11.7) | 24.5 (11.3) | 29.4 (13.5) | 26.7 (12.5) | 27.2 (12.5) |
The treatment groups did not differ on demographic and smoking history variables. cNRT, combination nicotine replacement therapy; FTND, Fagerstrom Test for Nicotine Dependence; GA/AA, rs16969968; GA/AA genotypes; GG, rs16969968; GG genotype; PPM, parts per million.
Genotype and Smoking Behaviors
Smokers with CHRNA5 rsl6969968 GA/AA genotypes smoked more cigarettes per day (β=2.75, 95%CI=1.63–3.88, p=1.0×l0−6), versus smokers with CHRNA5 rsl6969968 GG, as expected since risk genotypes of rsl6969968 (GA/AA) have an established association with increased smoking quantity (12). There was a nonsignificant trend that smokers in the placebo arm with CHRNA5 rsl6969968 GA/AA vs. GG genotypes were associated with lower abstinence at end of treatment (7.3% vs. 10.0%, X2=0.80, df=l, p=0.44) and lower abstinence at 6 months (5.7% vs. 12.0%, X2=3.10, df=l, p=0.078).
Pharmacotherapy Effectiveness of cNRT and Varenicline vs. Placebo
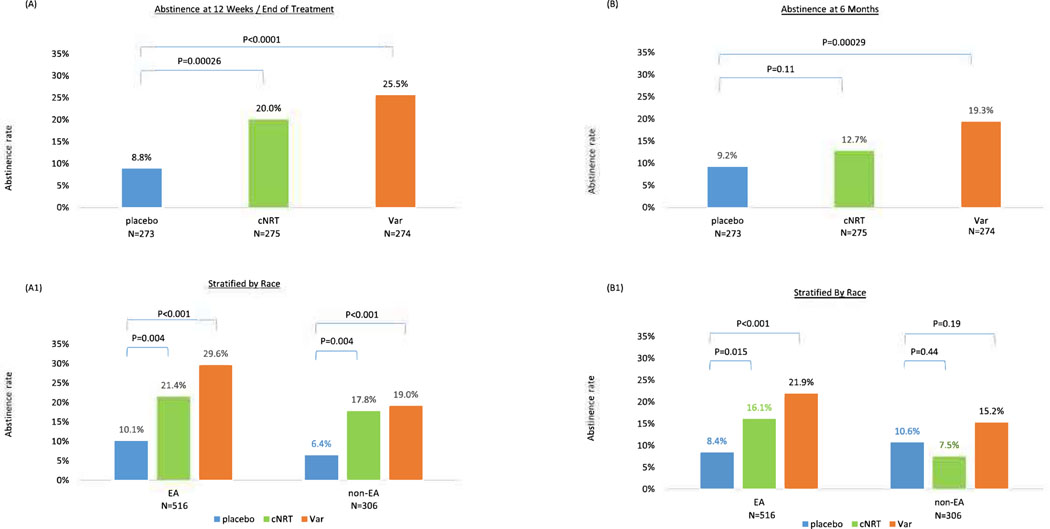
For the primary outcome 7-day point prevalence abstinence at EOT, both cNRT and varenicline vs. placebo were effective (20.0% vs. 8.8%, X2=13.3, df=l, p=0.00026 for cNRT vs. placebo; 25.5% vs. 8.8%, X2=24.9, df=l, p<0.0001 for varenicline vs. placebo, Figure 2A). For the secondary outcome, 7-day point prevalence abstinence at 6 months, cNRT vs. placebo was no longer significant (13.5% vs. 9.2%, X2=2.49, df=l, p=0.11), and varenicline vs. placebo remained significant (20.4% vs. 9.2%, X2=13.2, df=l, p=0.00029, Figure 2B). In pairwise comparison, varenicline was associated with significantly higher abstinence at 6 months compared to cNRT (20.4% vs. 13.5%, X2=4.70, p=0.030). Age and sex were not associated with the primary outcome, abstinence at EOT (X2=0.049, p=0.12 for age; X2=2.37, p=0.83 for sex).
Figure 2 –
Abstinence by treatment group at 12 weeks (end of treatment) and 6 months for the entire sample, and stratified by race
N=822, 516 smokers of European Ancestry, 306 smokers of non-European Ancestry. (A) Abstinence at end of treatment. (A1) Abstinence at end of treatment stratified by race. (B) Abstinence at 6 months. (B1) Abstinence at 6 month stratified by race.
Pharmacotherapy Effectiveness and Race
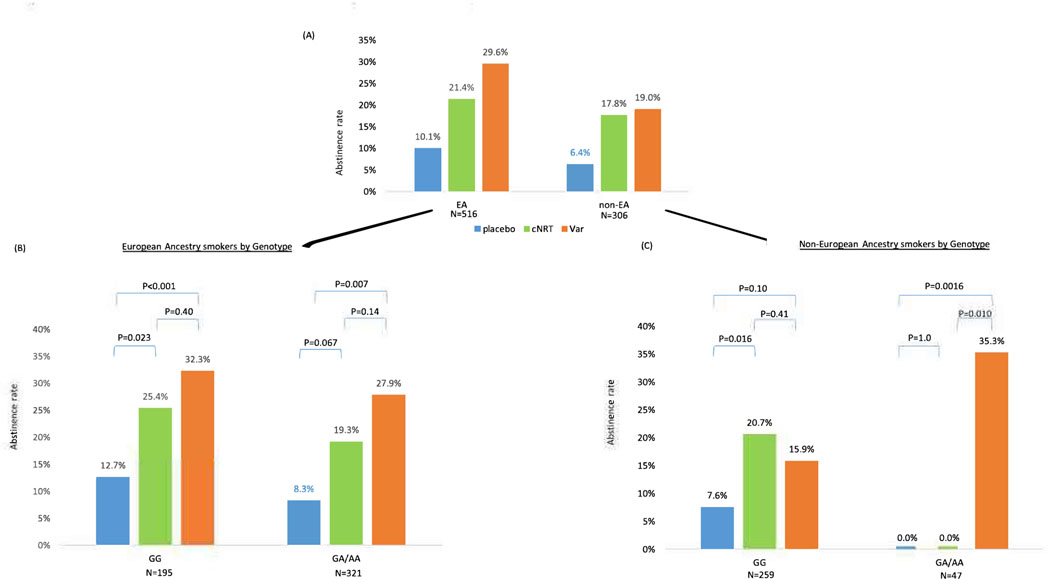
Race significantly predicted the primary outcome abstinence at EOT as abstinence rates were lower in non-EA smokers than EA smokers as shown in Figure 2A1 (OR=0.65, 95% 0=0.44–0.96, p=0.030). Within each racial group, both cNRT and varenicline were effective compared to placebo at EOT: In EA, cNRT vs. placebo abstinence 21.4% vs. 10.1%, X2=8.19, p=0.004, and varenicline vs. placebo abstinence 29.6% vs. 10.1%, X2=19.4, p<0.001. In non-EA, cNRT vs. placebo abstinence 17.8% vs. 6.4%, X2=5.49, p=0.019, and varenicline vs. placebo abstinence 19.0% vs. 6.4%, X2=6.40, p=0.021. There was no race-by-treatment interaction (interaction X2=0.65, df=2, p=0.72).
For the secondary outcome, abstinence at 6-month, race was no longer a significant predictor as shown in Figure 2B1 (OR=0.69, 95%CI=0.45–1.05, p=0.080). In EA, both cNRT and varenicline were effective compared to placebo at 6 months (17.3% vs. 8.4%, X2=5.94, p=0.015 for NRT vs. placebo; 22.5% vs. 8.4%, X2=12.5, p<0.001 for varenicline vs. placebo). In non-EA, neither cNRT nor varenicline were effective compared to placebo at 6 months (7.5% vs. 10.6%, X2=0.61, p=0.44 for cNRT vs. placebo; 17.1% vs. 10.6%, X2=1.71, p=0.19 for varenicline vs. placebo). There was no race-by-treatment interaction (interaction X2=4.07, df=2, p=0.13).
The Interplay of Genotype and Pharmacotherapy on Smoking Cessation Outcomes
Smokers of European Ancestry (EA)
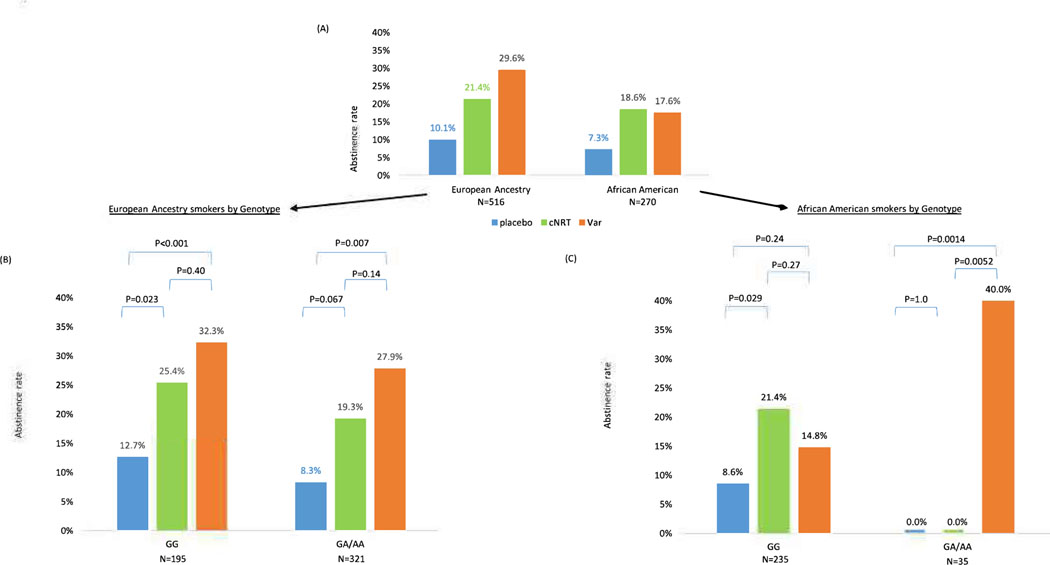
There were 516 EA smokers. For the primary outcome of abstinence at EOT, both cNRT and varenicline vs. placebo were efficacious regardless of rs16969968 genotypes (Figure 3B). There was no genotype-by-treatment interaction for the primary outcome, abstinence at EOT (Interaction X2=0.20, df=2, p=0.91). Medication efficacy is similar for smokers with rsl6969968 GG vs. GA/AA genotypes (abstinence 25.4%, 32.3% vs. 12.7% for cNRT, varenicline vs. placebo in GG genotypes; abstinence 19.3%, 27.9% vs. 8.3% for cNRT, varenicline vs. placebo in GA/AA genotypes). We confirmed similar results of no genotype-by-treatment interaction after adjusting for age and sex (interaction X2=0.24, df=2, p=0.89). While adjusting for age, sex, and cigarettes per day, we found no significant association between baseline cigarettes per day and abstinence (OR=0.97, X2=3.15, df=l, p=0.076), and reached a similar result of no genotype-by-treatment interaction (interaction X2=0.32. df=2, p=0.85).
Figure 3 –
Quit rate at 12 weeks (end of treatment) in each genotype group (CHRNA5 rsl6969968) in European and African American Ancestry smokers
(A) All smokers stratified by race. (B) EA: N=516. Gene by Treatment Interaction Wald X2=0.20, df=2, p=0.91. (C) AA: N=270. Gene by Treatment Interaction X2=10.7, df=2, p=0.0049
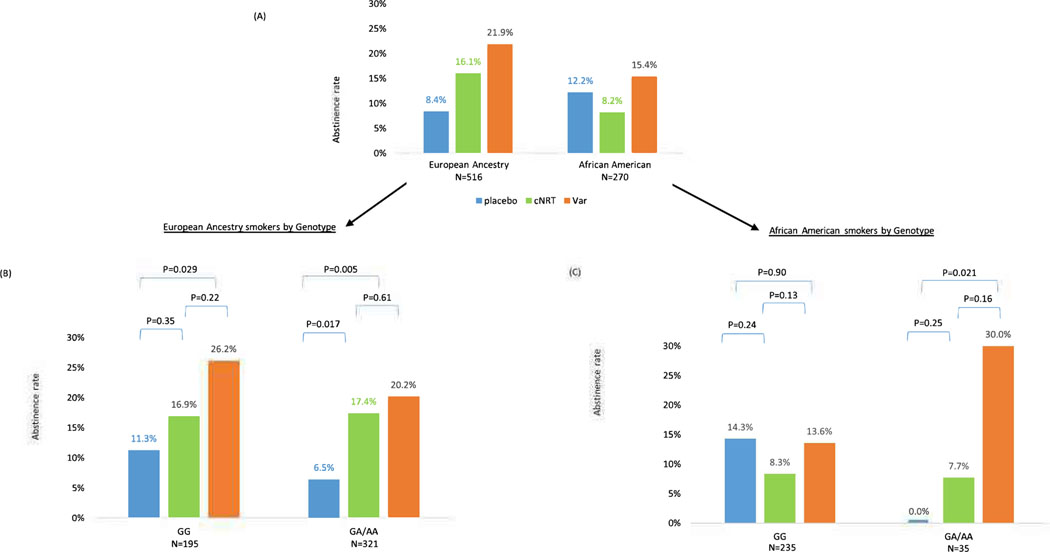
For the secondary outcome, abstinence at 6 months, we found similar results of no genotype-by-treatment interaction (Interaction X2=0.93, df=2, p=0.63, Figure 4B). For abstinence at 1 year we found no genotype-by-treatment interaction (Interaction X2=1.04, df=2, p=0.59; Figure SIB).
Figure 4 –
Abstinence at 6 months in each genotype group (CHRNA5 rsl6969968) in European and African American Ancestry smokers
(A) All smokers stratified by race. (B) All smokers of European Ancestry N-516. No gene-by-treatment interaction X2=0.93, df=2, p=0.63. (C) All smokers of African American Ancestry, N=270. Gene by treatment interaction X2=4.96, df=2, p=0.084
We also tested the interaction of each rs16969968 genotypes (GA vs. GA vs. AA) and medication and found no significant interactions for both abstinence at EOT (X2=4.56, df=4, p=0.34) and abstinence at 6 months (X2=1.32, df=4, p=0.86), as shown in Figure S2.
Smokers of African American Ancestry
There were 270 African American smokers within a total of 306 non-European Ancestry smokers. Figure 3C shows the genotype-by-treatment interaction which was significant for the primary outcome, abstinence at EOT (X2=10.7, df=2, p=0.0049). For smokers with rsl6969968 GG, cNRT vs. placebo was efficacious (abstinence 21.4% vs. 8.6%, X2=4.51, df=l, p=0.029), but varenicline vs. placebo was not (abstinence 14.8% vs. 8.6%, X2=1.36, df=l, p=0.24). The paired cNRT vs. varenicline comparison was not significant (abstinence 21.4% vs. 14.8%, X2=1.2, df=l, p=0.27). In contrast, for smokers with rs16969968 GA/AA, varenicline vs. placebo was efficacious (abstinence 40.0% vs. 0%, X2=7.40, df=l, p=0.0014), but cNRT vs. placebo was not (abstinence 0% vs. 0%). The pairwise varenicline vs. cNRT comparison was significant (abstinence 40.0% vs. 0%, X2=7.79, df=l, p=0.0052). After adjusting for age and sex, we confirmed similar results of the genotype-by-treatment interaction (interaction X2=l 1.3, df=2, p=0.0035). While adjusting for age, sex, and cigarettes per day, we found no significant association of baseline cigarettes per day (OR=0.99, X2= 0.09, df=l, p=0.76), and reached a similar significant result of a genotype-by-treatment interaction (Interaction X2=10.9. df=2, p=0.0043).
For the secondary outcome, abstinence at 6 months, the genotype-by-treatment interaction was trending, but no longer significant for abstinence at 6 months (Interaction X2=4.96, df=2, p=0.084, Figure 4C). Specifically, for smokers with rsl6969968 GG, neither cNRT nor varenicline vs. placebo was significant (cNRT vs. placebo, abstinence 8.3% vs. 14.3%, X2=1.35, df=l, p=0.24; varenicline vs. placebo, abstinence 13.6% vs. 14.3%, X2=0.02, df=l, p=0.90). In contrast, for smokers with rsl6969968 GA/AA, varenicline vs. placebo was efficacious (abstinence 30.0% vs. 0%, X2=5.31, df=l, p=0.021), but not cNRT vs. placebo (abstinence 7.7% vs. 0%, X2=1.35, df=l, p=0.25). The paired varenicline vs. cNRT comparison was no longer significant (abstinence 30.0% vs. 7.7%, X2=1.99, df=l, p=0.16). The 6-month outcome occurred 12 weeks after medication was discontinued. For abstinence at 1 year, the genotype-by-treatment interaction for rs16969968 GA/AA smokers was no longer significant (interaction X2=0.78, df=2, p=0.68, Figure SIC).
In addition, we reached similar results when examining all 306 non-European Ancestry smokers including 270 African American smokers. For abstinence at 12 weeks/end of treatment, there was a gene-by-treatment interaction (X2=l 1.4, df=2, p=0.0033, Figure 5C). For abstinence at 6 months, there was a trending but no longer significant gene-by-treatment interaction (Interaction X2=5.59, df=2, p=0.061, Figure S3C).
Figure 5 –
Quit rate at 12 weeks (end of treatment) in each genotype group (CHRNA5 rsl6969968) in European and non-European Ancestry smokers
(A) All smokers stratified by race. (B) EA: N=516. Gene by Treatment Interaction Wald X2=0.20, df=2, p=0.91. (C) Non-EA: N=306 Gene by Treatment Interaction X2=11.4, df=2, p=0.0033
Adverse Effects
The frequencies of adverse effects are listed for each treatment group in Table 2. In EA smokers, there was no significant difference in overall adverse effect severity score across medication groups of placebo, cNRT vs. varenicline (score means 4.16, 4.07, 4.97, X2=4.45, df=2, p=0.11) or genotype groups of GG vs. GA/AA (4.31, 4.94, X2=0.31, df=l, p=0.58). Table S1 shows overall adverse effect severity score stratified by both medication and genotype groups and there was no significant genotype-by-treatment interaction (X2=4.26, df=2, p=0.12).
Table 2.
Adverse effect by treatment groups in the entire sample
| Adverse effects among patients treated with placebo, varenicline, or C-NRT | |||
|---|---|---|---|
| Adverse Effect | Placebo (n=273) | cNRT (n=275) | Varenicline (n=274) |
| Section A. Agonist AE Symptoms | No. (%) with event | No. (%) with event | No. (%) with event |
| Nausea | 59(21.6) | 53(19.3) | 92 (33.6) |
| Vomiting | 27(9.9) | 28(10.2) | 36 (13.1) |
| Headache | 71 (26) | 81 (29.5) | 81 (29.6) |
| Rapid, slow, pounding, or irregular heartbeat, | 16(5.9) | 27 (9.8) | 24(8.8) |
| Insomnia | 42 (15.4) | 49(17.8) | 55(20.1) |
| Vivid dreams | 60(22.0) | 63(22.9) | 100(36.5) |
| Section B. Other Adverse Events | |||
| Dizziness | 39 (14.3) | 41 (14.9) | 52 (19.0) |
| Weakness | 32 (11.7) | 25(9.1) | 32 (11.7) |
| Sweating | 29 (10.6) | 46 (16.7) | 40 (14.6) |
| Itching/hives | 16(5.9) | 35(12.7) | 29 (10.6) |
| Rash | 22(8.1) | 33(12.0) | 18(6.6) |
| Swelling in your face or hands | 16(5.9) | 15(5.5) | 10(3.6) |
| Swelling or tingling in your mouth or throat | 11 (4.0) | 20 (7.3) | 8 (2.9) |
| Mouth problems | 21 (7.7) | 21 (7.6) | 20(7.3) |
| Indigestion | 36 (13.2) | 41 (14.9) | 51 (18.6) |
| Hiccups | 22(8.1) | 34(12.4) | 43 (15.7) |
| Chest tightness | 16(5.9) | 24 (8.7) | 24(8.8) |
| Trouble breathing | 22(8.1) | 16(5.8) | 21 (7.7) |
| Feeling worried, nervous, scared or anxious | 58(21.2) | 50 (18.2) | 64 (23.4) |
| Feeling panicky or having panic attacks | 22(8.1) | 22 (8.0) | 23(8.4) |
| Feeling agitated and restless | 43 (15.8) | 47 (17.1) | 56 (20.4) |
| Feeling hostile or angry towards others | 22(8.1) | 15(5.5) | 32 (11.7) |
| feeling significantly down, depressed or hopeless | 44(16.1) | 47 (17.1) | 58(21.2) |
In African American smokers, there was no significant difference in overall adverse effect severity score across medication groups of placebo, cNRT vs. varenicline (score means 4.15, 4.14, 4.99, X2=1.14, df=2, p=0.57) or across genotype groups of GG vs. GA/AA (4.31, 5.23, X2=0.71, df=l, p=0.40). Table S1 shows overall adverse effect scores stratified by both medication and genotype groups and there was no significant genotype-by-treatment interaction (X2=4.9, df=2, p=0.086). In addition, we reached similar results when examining all 306 non-EA smokers including the majority of 270 African American smokers (Table S2).
Serious adverse events (SAEs), defined as any adverse event irrespective of causality that resulted in death, was life threatening, required hospitalization, or resulted in disability or incapacity, were determined by the principal investigator physician and adjudicated by the DSMB. There were 27 (10%), 23 (8%), and 17 (6%) participants affected by SAEs on placebo, cNRT, and varenicline, respectively. See additional information on clinicaltrials.gov (identifier NCT02351167). Treatment effects or genotype-by-treatment interactions on SAE counts were not significant.
Adherence
Medication adherence was 64% (95% Cl 61%−67%) for EA smokers and 64% (95% Cl 60%−68%) for African American smokers. Medication adherence was 62% (95% Cl 58%−67%) for placebo, 65% (95% Cl 61%−69%) for cNRT, and 66% (95% Cl 61%−70%) for varenicline. Medication adherence was 65% (95% Cl 61%−68%) for rsl6969968 GG genotypes and 64% (95% Cl 60%−67%) for GA/AA genotypes. Adherence was similar across racial groups, treatment arms and genotype groups.
DISCUSSION
This genotype-stratified randomized clinical trial yielded evidence of differential treatment response for African American Ancestry smokers with different CHRNA5 rs16969968 genotypes, but not European Ancestry (EA) smokers. Both cNRT and varenicline were effective at end of treatment amongst all smokers. Among African American smokers when both medication and genotype are considered, cNRT is more effective in smokers with GG genotypes, whereas varenicline is more effective for smokers with GA/AA genotypes. Thus, this study supports existing pharmacogenetic evidence from independent trials (17, 22, 25) that NRT vs. placebo may be more effective in African American smokers with rs 16969968 GG than in those with GA/AA genotypes. Beyond this, this study suggests that varenicline enhances end of treatment abstinence in African American Ancestry GA/AA smokers while cNRT does not.
This differential effectiveness of medications in African American smokers with different CHRNA5 genotypes does not appear to be due to differences in trial retention, medication adherence, or adverse effects across genotypes or medications. For example, medication adherence did not vary meaningfully with medication type and genotype. This is consistent with existing findings in another trial (10).
Though replication is needed, it is important to note that the medication X gene interaction amongst African American smokers in this study may be clinically meaningful. The efficacy of varenicline is much higher (40%) than that of cNRT (0%) for smokers with GA/AA genotypes. The base rate of GA/AA genotypes in the population must be considered in evaluating the clinical relevance of these results. GA/AA genotypes occur in about 15% of African Americans while GG genotypes occur in about 85% of the population. Although the percentage of African American smokers with GA/AA is relatively small, there should be well over a million such smokers in the US given the number of African American smokers. Cost-effectiveness analysis will be needed to evaluate the cost of genotyping, treatment, continued smoking, and related health outcomes between precision vs. protocol treatment regardless of genotypes.
Genotype did not interact with medication in EA smokers, which is in contrast with what we have found in a previous study (16), but consistent with another study we conducted (23). With the sample size of 516 EA smokers and 270 African American smokers, we had 0.8 power (2-sided α of 0.01) to detect an interaction effect size of 2.5 in EA smokers and 5.5 in African American smokers due to the smaller sample size and lower minor allele frequency in African Americans. Our evidence supports a significant interaction in African Americans but cannot rule out the possibility of an interaction with a smaller effect size in EAs. In this study, we tested the hypothesis regarding the effect of the most robust signal, rs16969968 in CHRNA5, on medication efficacy across diverse ancestry groups. The discrepant findings across ancestry groups not only highlight the need for replication, but also suggest an underlying complex biology involving additional genetic markers or interactions.
CHRNA5 clearly plays a role in heavy smoking, nicotine dependence, and delayed smoking cessation as shown in large meta-analyses in EA individuals (12, 26). Our finding supports the overall evidence that the high-risk genotypes, CHRNA5 GA/AA are associated with reduced abstinence. Whether CHRNA5 serves as a marker for medication choice among EA smokers is unclear. The previous Cochrane review on this gene-by-treatment interaction among EA smokers yielded equivocal results (15,16, 22, 27). Some trials did not participate in the Cochrane review, so a larger meta-analysis, including more studies, will be needed to evaluate this issue. On the other hand, it is possible that this pharmacogenetic interaction is observed in one ancestry group, but not the other, consistent with the Cochrane review, due to the different genetic backgrounds across ancestry groups and interactions with other unobserved factors such as nicotine metabolism, a critical factor that differs between ancestry groups.
This study contributes to our understanding of medication effectiveness in two ways. First, against a strong prior record of effectiveness for varenicline and cNRT, two recent studies showed relatively weak effects of these two agents (28, 29). The current study confirms that both cNRT and varenicline are effective, at least at end of treatment. The second contribution is that this study suggests a relative long-term superiority of varenicline over cNRT when both are compared with placebo. Much of prior evidence (4, 28) suggests near equivalence of the two agents. However, in the current research only varenicline produced significantly higher abstinence rates at 6-months, than did placebo. In addition, varenicline produced significantly higher abstinence rates than cNRT at both end of treatment and 6 months. In the field of smoking cessation, the effect of treatment often decreases over time after the medication is stopped and long-term abstinence remains an important challenge (4–6).
Our study had strengths and limitations. One strength is that it is the first smoking cessation trial to use prospective stratification based on genotypes. Also, the rate of loss to follow up did not differ by treatment or genotype group. Further, this trial occurred during a time when electronic cigarettes increasingly became popular among smokers for cessation and recreational purposes. However, the use of electronic cigarettes was infrequent and similar across the treatment arms and genotypes, and unlikely to explain our findings. Also, the size of the study and the mixed ethnicity of participants allowed for investigation of medication effects in both EA and non-EA smokers. However, there is a clear need to replicate these findings in independent studies. Our finding regarding higher efficacy of cNRT compared to placebo in CHRNA5 rsl6969968 GG non-EA smokers is consistent with findings reported in previous trials including a Cochrane review (17, 22). However, our findings regarding medication effects in GA/AA non-EA smokers should be viewed with caution given the novelty of the findings and the small size of this subsample. Finally, we examined variants in only one gene (CHRNA5). It would be important to study the combined effects of additional biomarkers such as nicotine metabolite ratio (NMR). Genome wide polygenic scores have become an increasingly useful tool in predicting disease risk, prognosis, and medication response (12, 30).
The understanding that certain genetic groups are at higher risk for failed smoking cessation is important. Most quit attempts are undertaken without any pharmacologic aid, while cNRT and varenicline can improve successful smoking cessation. The prospect of improving treatment effectiveness for African American smokers is of substantial public health importance given the racial disparities in smoking-related health outcomes in the US. The risk, prevalence, and mortality from smoking-related diseases are disproportionately higher for African American smokers than for European Ancestry smokers, (31, 32) despite African American smokers consuming fewer cigarettes and initiating smoking at a later age (33). Moreover, African American smokers are less likely to quit smoking successfully either with or without treatment (1, 34).
Our data show that in the whole sample of smokers, varenicline tended to produce higher abstinence than did cNRT, and only varenicline produced significantly higher abstinence than did placebo at 6-month follow-up. However, interactions between medication and genotype amongst African American smokers in this study and existing evidence (17, 22, 25) suggest the potential for more precise treatment to further enhance the effectiveness of cessation medication for this group of individuals who face disproportionate risks of smoking related harm. Since genotyping is rapidly accepted by the general public via consumer genomics and increasingly incorporated into electronic health records, it may serve as a clinical decision aid as we enter the era of precision medicine. Our study identifies the need for more genetically informed medication choice for all smokers, and in particular those involving under-represented participants. Our research underscores the notion that nicotine dependence may be a heterogeneous condition and pharmacotherapies are not equally effective for all smokers. Continued genome wide research will pave the way for a future comprehensive treatment algorithm including multiple genetic, non-genetic, and clinical markers.
Supplementary Material
STUDY HIGHLIGHTS.
What is the current knowledge on the topic?
Smoking cessation medication efficacy may vary according to CHRNA5 genotypes. Prior research highlights differential response to pharmacotherapy rs16969968, but there is still insufficient evidence indicating how to treat smokers of diverse ancestry background.
What question did this study address?
This study examined the relation of CHRNA5 genotypes with the two most effective cessation treatments (combination NRT and varenicline) against a placebo, including a comprehensive pharmacogenetic model of both efficacy and adverse effects/adherence.
What does this study add to our knowledge?
Our findings indicate differential treatment response by CHRNA5 rsl 6969968 genotypes for smokers of African American Ancestry, but not for smokers of European Ancestry. Among African American smokers, cNRT is more effective in smokers with GG genotypes, whereas varenicline is more effective for smokers with GA/AA genotypes.
How might this change clinical pharmacology or translational science?
The risk, prevalence, and mortality from smoking-related diseases are disproportionately higher for African American smokers than for European Ancestry smokers, and these findings enhance our ability to improve smoking treatment effectiveness for African American smokers.
ACKNOWLEDGEMENTS
The authors thank Mario Castro, Brian Gage, Eric Lenze, and Sharon Cresci for their contributions to data and safety monitoring. The authors also thank the staff and students at Washington University Health and Behavior Research Center for their help with this research.
FUNDING
LC was supported by the National Institute on Drug Abuse grant R01 DA038076, Siteman Cancer Center and NCI Cancer Center Support Grant P30 CA091842. TBB’s involvement was supported in part by R01 HL109031. LJB was supported by National Center for Advancing Translation Sciences grant UL1TR002345. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. LC obtained free supply of study medication (varenicline and placebo) for this trial from Pfizer with an investigator-initiated research agreement (IIR). This free Pfizer product constitutes the support for this study.
Role of the funding source
The funding source had no role in the study design, collection, analysis and interpretation of data, in writing of the report, or in the decision to submit the paper for publication. The corresponding author had full access to the data in the trial and had final responsibility for the decision to submit for publication.
LC received free supply of study medication (varenicline and placebo) for this research project via an investigator-initiated research agreement (IIR) from Pfizer. This free Pfizer product constitutes the support for this study. Pfizer supports the Principal Investigator to exercise the academic freedom and encourages publication of study results whether or not they are favorable for the Pfizer Product. RMC or a member of his family owns stock in Pfizer Inc. LJB is listed as an inventor on Issued U.S. Patent 8,080,371 “Markers for Addiction” covering the use of certain single nucleotide polymorphisms (SNPs) in determining the diagnosis, prognosis, and treatment of addiction, and served as a consultant for the pharmaceutical company Pfizer Inc. (New York City, New York, USA) in 2008. The spouse of NLS is also listed as an inventor on Issued U.S. Patent 8,080,371 “Markers for Addiction.”
Footnotes
CONFLICT OF INTEREST
All other authors declared no competing interests for this work.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi:10.1002/cpt.l971
REFERENCES
- (1).U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. (U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, 2014). [Google Scholar]
- (2).Anthenelli RM et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 387, 2507–20 (2016). [DOI] [PubMed] [Google Scholar]
- (3).Lerman C. et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir. Med 3,131–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Cahill K, Stevens S, Perera R & Lancaster T Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst. Rev 5, CD009329 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C & Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst. Rev 5, CD000146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lindson N, Chepkin SC, Ye W, Fanshawe TR, Bullen C & Hartmann-Boyce J. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev 4, CD013308 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ahluwalia JS et al. The effects of nicotine gum and counseling among African American light smokers: a 2 × 2 factorial design. Addiction 101, 883–91 (2006). [DOI] [PubMed] [Google Scholar]
- (8).NIH. All of Us Research Program, <https://allofus.nih.gov/> (2019). Accessed November 6 2019.
- (9).Rose JE, Behm FM, Drgon T, Johnson C & Uhl GR Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score. Mol. Med 16, 247–53 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Culverhouse RC et al. Variants in the CHRNA5-CHRNA3-CHRNB4 Region of Chromosome 15 Predict Gastrointestinal Adverse Events in the Transdisciplinary Tobacco Use Research Center Smoking Cessation Trial. Nicotine Tob. Res 22, 248–55 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bierut LJ et al. Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatry 165, 1163–71 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Liu M. et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet 51, 237–44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).David SP et al. Genome-wide meta-analyses of smoking behaviors in African Americans. Transl. Psychiatry 2, el 19 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Minicã CC, Mbarek H, Pool R, Dolan CV, Boomsma DI & Vink JM Pathways to smoking behaviours: biological insights from the Tobacco and Genetics Consortium meta-analysis. Mol. Psychiatry 22, 82–8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Bergen AW et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet. Genomics 23,94–103 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chen LS et al. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. Am. J. Psychiatry 169, 735–42 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Zhu AZ et al. Association of CHRNA5-A3-B4 SNP rs2036527 with smoking cessation therapy response in African-American smokers. Clin. Pharmacol. Ther 96, 256–65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chenoweth MJ & Tyndale RF Pharmacogenetic Optimization of Smoking Cessation Treatment. Trends Pharmacol. Sci 38, 55–66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Korhonen T & Kaprio J [Genetic aspects in nicotine dependence]. Duodecim 128,1065–71 (2012). [PubMed] [Google Scholar]
- (20).Tomaz PRX et al. Cholinergic receptor nicotinic alpha 5 subunit polymorphisms are associated with smoking cessation success in women. BMC Med. Genet 19, 55 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Sarginson JE et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am. J. Med. Genet. B Neuropsychiatr. Genet 156B, 275–84 (2011) [DOI] [PubMed] [Google Scholar]
- (22).Schuit E, Panagiotou OA, Munafo MR, Bennett DA, Bergen AW & David SP Pharmacotherapy for smoking cessation: effects by subgroup defined by genetically informed biomarkers. Cochrane Database Syst. Rev 9, CD011823 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (23).Chen LS et al. Genetic variation (CHRNA5), medication (combination nicotine replacement therapy vs. varenicline), and smoking cessation. Drug Alcohol. Depend 154, 278–82 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Database of Single Nucleotide Polymorphisms (dbSNP). dbSNP accession: rs16969968, (dbSNP Build ID: 153). <https://www.ncbi.nlm.nih.gov/snp/rsl6969968> (2019). Accessed November 25 2019.
- (25).Panagiotou OA, Schuit E, Munafo MR, Bennett DA, Bergen AW & David SP Smoking Cessation Pharmacotherapy Based on Genetically-Informed Biomarkers: What is the Evidence? Nicotine Tob. Res 21, 1289–93 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Chen LS et al. CHRNA5 risk variant predicts delayed smoking cessation and earlier lung cancer diagnosis—a meta-analysis. J. Natl. Cancer Inst 107, djvlOO (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Tyndale RF et al. Lack of Associations of CHRNA5-A3-B4 Genetic Variants with Smoking Cessation Treatment Outcomes in Caucasian Smokers despite Associations with Baseline Smoking. PLOS One 10, e0128109 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Baker TB et al. Effects of Nicotine Patch vs Varenicline vs Combination Nicotine Replacement Therapy on Smoking Cessation at 26 Weeks: A Randomized Clinical Trial. JAMA 315,371–9(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Tulloch HE, Pipe AL, Els C, Clyde MJ & Reid RD Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial. BMC Med. 14, 80 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Maas P. et al. Breast Cancer Risk From Modifiable and Nonmodifiable Risk Factors Among White Women in the United States. JAMA Oncol. 2,1295–302 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Etzel CJ et al. Development and validation of a lung cancer risk prediction model for African-Americans. Cancer Prev. Res 1, 255–65 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Jemal A, Siegel R, Ward E, Murray T, Xu J & Thun MJ Cancer Statistics, 2007. CA Cancer J. Clin 57,43–66(2007). [DOI] [PubMed] [Google Scholar]
- (33).Benowitz NL, Bemert JT, Caraballo RS, Holiday DB & Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am. J. Epidemiol 169, 236–48 (2009). [DOI] [PubMed] [Google Scholar]
- (34).West R. et al. Factors associated with the efficacy of smoking cessation treatments and predictors of smoking abstinence in EAGLES. Addiction 113,1507–16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.