ABSTRACT
Epidemiological studies have established obesity as a critical risk factor for postmenopausal breast cancer (post-BC), whereas a reverse association holds prior to menopause. A significant scientific gap exists in understanding the mechanism(s) underpinning this epidemiological phenomenon, particularly the reverse association between obesity and premenopausal breast cancer (pre-BC). This study aimed to understand how folate metabolism and DNA methylation inform the association between obesity and pre-BC. Fifty normal breast tissue samples were collected from premenopausal women who underwent reduction mammoplasty. We modified the Lactobacillus Casei microbiological folate assay and measured folate levels in our breast tissue samples. The DNA methylation of LINE-1, a biomarker of genome-wide methylation, and the expression of a panel of breast cancer-related genes was measured by pyrosequencing and real-time PCR. We found that a high BMI is associated with an increase of folate levels in mammary tissue, with an increase of 2.65 ng/g of folate per every 5-unit increase of BMI (p < 0.05). LINE-1 DNA methylation was significantly associated with BMI (p < 0.05), and marginally associated with folate concentration (p = 0.087). A high expression of SFRP1 was observed in subjects with high BMI or high folate status (p < 0.05). This study demonstrated that, in premenopausal women, obesity is associated with increased mammary folate status, genome-wide DNA methylation and SFRP1 gene expression. Our findings indicated that the improved folate and epigenetic status represents a novel mechanism responsible for the reverse association between obesity and pre-BC.
KEYWORDS: Obesity, folate, DNA methylation, premenopausal breast cancer
Introduction
Breast cancer (BC) is the most prevalent cancer in women, with ~2.1 million new cases and 627, 000 deaths recorded globally in 2018. These numbers represent 24% of all cancer cases and 15% of cancer-attributed mortality in women, respectively [1]. Factors that evidently contribute to BC risk include age, physical activity, overweight/obesity, alcohol consumption, as well as factors leading to greater birthweight or greater linear growth, etc [2].
It is of particular importance to study the role of body fatness and its potential influence on the risk of BC, as obesity has increased with an alarming rate in the United States. A further increase in obesity cases from the current one-third of the US population to approximately 50% by 2030 is projected [3,4]. The association between overweight/obesity and BC is apparently contradictory – a well-established positive association exists between obesity and postmenopausal breast cancer (post-BC), whereas a reverse association holds prior to menopause [5,6]. A meta-analysis of 34 cohort studies including over 2.5 million women and nearly 24,000 post-BC subjects reported that each 5-unit increase (5 kg/m2) of BMI accompanies a relative risk of post-BC by 12% [7]. For premenopausal breast cancer (pre-BC), a meta-analysis with 37 studies and 16,371 cases performed by World Cancer Research Fund/American Institute of Cancer Research showed a 7% decreased risk per every 5-unit increase of BMI [2]. It is particularly noteworthy that greater body fatness in young adulthood (aged 18–30 years) is associated with a decrease in risk for both pre-BC (18%; with a 95% CI from 11% to 24% for every 5 kg/m2 of BMI) and post-BC (18%; with a 95% CI from 12% to 24%) [2].
A significant challenge in understanding the cellular and molecular mechanisms of action by obesity is the increased risk for post-BC, but protective impact for pre-BC. A great number of studies have been conducted to understand the positive association between obesity and post-BC. Several mechanisms governing this association have been proposed and defined, including adipose tissue-driving circulating hormones (e.g. IGF-1, oestrogens) and obesity-associated chronic low-grade inflammatory state [3,8]. In contrast, mechanistic studies to understand the negative association between obesity and pre-BC is extremely limited, and the pathways that inform this inverse relationship remain entirely elusive.
Epigenetic mechanisms have emerged as fundamental factors in breast cancer development and progression [9,10]. Over 100 genes have been reported to be hypermethylated in their promoter regions in BC [11–13], and many of those genes play important roles in DNA repair (e.g. BRCA1, MLH1), cell-cycle regulation (e.g. CCND2, SFRP1, CDKN2A), and hormone-mediated cell signalling (e.g. ESR1, PGR). To the contrary, genome-wide hypomethylation represents a feature of BC and frequently occurs in regions of segmental duplications [14,15]. Folate, an essential water-soluble vitamin, is a key factor in one-carbon metabolism, the universal source of methyl donors for DNA methylation [16]. Although epidemiological studies have shown mixed results as to the association between folate intake and the risk of BC [17–19], a study on primary breast tumours examining DNA methylation levels at 1413 sites in 733 genes demonstrated that gene-specific methylation and overall methylation patterns were significantly associated with folate intake, as well as with alcohol consumption, a known interactor of folate metabolism [20]. Our recent study [21] demonstrated that both folate levels and folate distribution were varied by BMI – overweight and obese women had lower serum folate but, surprisingly, higher levels of red blood cell folate. These associations particularly hold to be statistically significant for women under 50. In this study, we examined how obesity influences folate status, DNA methylation, and cancer-related gene expression in normal breast tissues from premenopausal women, with an effort to understand the role of folate metabolism and DNA methylation in the mediation of the protective effect of obesity on pre-BC.
Materials and methods
Patients for breast tissue samples
Study subjects were women who underwent reduction mammoplasty at Baystate Medical Centre in Springfield, Massachusetts, United States. The protocol was approved by the Institutional Review Board at Baystate Medical Centre. All participants consented to donate excess breast tissue (tissue not needed for diagnostic purposes). Samples were accrued and stored in −80°C at the Biospecimen Resource and Molecular Analysis Facility in the medical centre until analysis. For this study, 50 samples from women with age ≤ 45 were collected from the tissue bank. All samples are normal mammary tissue from women with no prior history of breast cancer. As menopausal status was not recorded in these subjects, we considered women with age ≤ 45 at pre-menopausal status (>95%). Anthropometric characteristics of the subjects are shown in Table 1.
Table 1.
Anthropometric characteristics of the subjects
| BMI (kg/m2)* |
Age (year)* |
|||||
|---|---|---|---|---|---|---|
| Categories | n | Parous | Mean | Range | Mean | Range |
| Normal Weight | 8 | 4/8a | 23 ± 0.61a | 19–24 | 27 ± 3.01a | 19–36 |
| Overweight | 19 | 11/19a | 28 ± 0.32b | 25–29 | 29 ± 1.90a | 19–44 |
| Obesity | 23 | 14/23a | 35 ± 0.75 c | 30–44 | 32 ± 1.30a | 21–41 |
| <30 | 27 | 15/27a | 26 ± 0.50a | 19–29 | 28 ± 1.59a | 19–44 |
| ≥30 | 23 | 14/23a | 35 ± 0.75b | 30–44 | 32 ± 1.30a | 21–41 |
* Different letters denote significant differences among the BMI categories (p < 0.001). There are no significant differences of numbers of parous women and age among the different BMI categories (p > 0.05), with a marginal higher age for the Obesity group when compared to the Normal Weight group (p = 0.092).
Lactobacillus casei microbiological mammary tissue folate assay
For quantification of breast folate, we modified a previous folate protocol [22] and developed a Lactobacillus casei microbiological assay specifically for breast tissue folate measurement [23]. Briefly, 10–20 mg of breast tissue was homogenized with folate extraction buffer (2% sodium ascorbate, 2% Bis-Tris and 0.07% 2-mercaptoethanol), immersed in hot boiling water (20 min), cooled on ice and then centrifuged at 36,000 g (20 min; 4°C). Samples were incubated with dialysed chicken pancreas conjugase (2 h; 37°C) to de-conjugate the attached poly-glutamates. Samples were loaded into 96-well plates with serial dilutions and incubated with L. casei for 24 h. The relative turbidity caused by the extent of microbial growth is sensitive to the concentration of folate in the media. The turbidity was then measured by photospectometry at 630 nm. Breast folate concentrations were calculated based on an internal folic acid standard curve. The coefficient of variation for the breast folate assay was 12% [23].
Targeted next-generation bisulphite sequencing for DNA methylation
Genome-wide DNA methylation was measured with targeted next-generation bisulphite sequencing pyrosequencing (tNGBS) at EpigenDx, Inc. (Hopkinton, MA). LINE-1 was used as a surrogate for genome-wide DNA methylation. The sequence analysed for LINE-1 gene is: TTYGTGGTGYGTYGTTTTTTAAGTYGGTTTGAAAAG, and contains 4 CpG sites. DNA was extracted from dissected breast tissues using QiAamp DNA Mini Kit (Qiagen, Germantown, MD). DNA bisulphite modification was performed via EZ-96 DNA methylation kit (Zymo, Irvine, CA) and unmethylated cytosines were converted into uracil, whereas methylated cytosine remained unchanged. Details of the follow-up PCR amplification and pyrosequencing protocol are described in previous publications [24,25]. Methylation levels were calculated by dividing the number of methylated reads by the total number of reads.
Real-time PCR for gene expression
We performed real-time PCR for 11 genes associated with tumorigenesis and aberrant methylation in breast cancer based on prior literature [11,12]. These genes are related to DNA repair (BRCA1, MGMT, MLH1, MSH2), cell-cycle regulation (CCND2, CDKN2A, DKK1, RASSF1, SFRP1) or proliferation and apoptosis (BCL2, DAPK1). Briefly, total RNA was extracted from breast tissue with Trizol (Invitrogen, Carlsbad, CA), and cDNA synthesized with SuperScript III (Invitrogen, Carlsbad, CA, USA). Real-time PCR was performed on the ViiA™ 7 Real-Time PCR System (Applied Biosystems, Foster City, CA), utilizing the following thermal cycling conditions: 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. The cycle threshold (Ct) values were defined as the fractional cycle number at which the fluorescence passes an arbitrarily set threshold. Primer sequences are listed in supplementary Data (Supplementary Data_Table S1).
Statistical analyses
Data are expressed as mean ± SEM, and statistical analysis was performed using SAS (Version 9.4, SAS Institute, Cary, NC). For association analysis, linear regression analyses were performed to examine changes in breast tissue folate, gene expression, and DNA methylation in response to BMI.
For gene expression profiling, each gene was normalized to the housekeeping gene GAPDH (ΔCt = Cttarget gene-CtGAPDH). Statistical analyses were performed based on differences in ΔCt and relative expression is reported as 2−ΔΔCt (where ΔΔCt = ΔCtoverweight or obesity-ΔCtnormal weight). For the heatmap, because this population of women undergoing reduction mammoplasty typically had high BMIs, we combined normal (BMIs between 18–24.9 kg/m2) and overweight (BMIs between 25–29.9 kg/m2) women and grouped these subjects into two categories; a non-obese group with a BMI < 30 kg/m2 and an obese group with BMI ≥ 30 kg/m2. Significance was accepted with p < 0.05 and a false discovery rate (FDR) cut-off of q < 0.25 [26].
Results
Anthropometric characteristics of the subjects
As described in the Materials and Methods section, we considered women with age ≤ 45 at pre-menopausal status (>95%). In total, 50 samples were collected: 8 subjects with normal weight (BMI: 23 ± 0.61), 19 overweight (BMI: 28 ± 0.32), and 23 obese (BMI: 35 ± 0.75). No differences in parous status or age across the three BMI categories were observed (Table 1). As a majority of the subjects undergoing mastectomy have a high BMI (only 8 subjects with a BMI < 25), we categorized our subjects into two groups: non-obesity (BMI < 30) and obesity (BMI ≥ 30) in the following data analysis.
The association between BMI and breast tissue folate in premenopausal women
Based on the Lactobacillus casei microbiological tissue folate assay, a significant positive association was observed between BMI and folate level in breast tissue from premenopausal women, with an increase of 2.65 ng/g of folate per every 5-unit increase of BMI (p < 0.01) (Figure 1(a)). When the subjects were categorized into non-obese (BMI < 30) and obese (BMI ≥ 30) groups, breast tissue folate in the obese group is 27.9% higher than that in the non-obesity group (19.59 ng/g vs 15.31 ng/g, p < 0.05) (Figure 1(b)).
Figure 1.
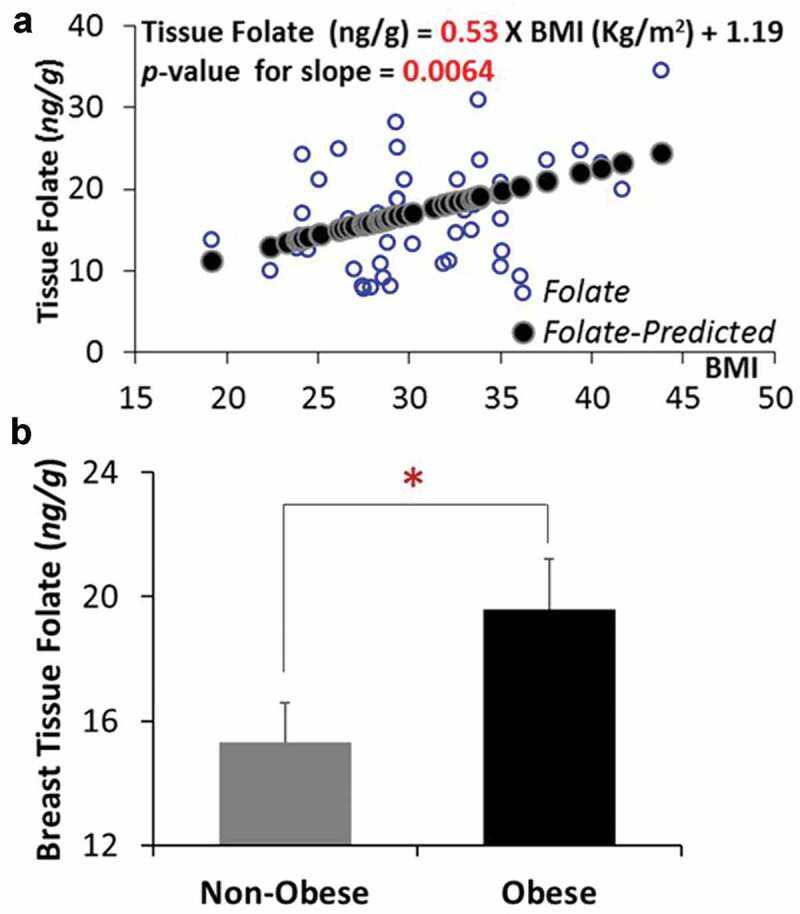
A high BMI is associated with an increased breast tissue folate in premenopausal women. (a) Linear regression between BMI and folate level in breast tissue from premenopausal women. (b) Breast tissue folate levels between non-obese and obese subjects. *p < 0.05
The association of BMI and breast tissue folate with genome-wide DNA methylation
LINE-1 gene methylation was used as a biomarker for genome-wide DNA methylation. When the study subjects were categorized by weight as normal (18.5 ≤ BMI <25), overweight (25 < BMI ≤ 30), or obese (BMI ≥ 30), the normal weight group had a significantly lower (p < 0.05) LINE-1 DNA methylation than the overweight and obesity groups (Figure 2(a)). When the normal and overweight groups were combined into non-obesity group, the difference between the non-obese and obese group was not significant (p > 0.05). The association analysis between BMI and the average LINE-1 DNA methylation indicated that a 5-unit increase of BMI is associated with a 0.52% increase (p < 0.05) in LINE-1 DNA methylation (Figure 2(b)).
Figure 2.
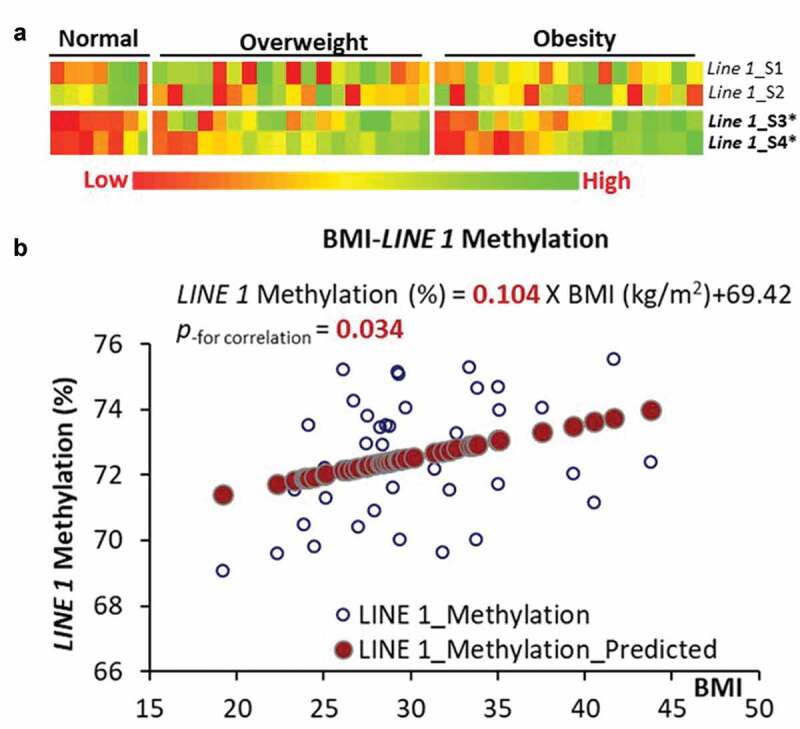
LINE-1 DNA methylation and its association with BMI. (a) DNA methylation among 4 CpG sites in LINE-1 gene categorized by normal weight, overweight and obesity. (b) Linear regression between BMI and average DNA methylation in breast tissue from premenopausal women
The heatmaps based on folate status are shown in Figure 3(a). The association analysis between breast tissue folate and the average LINE-1 DNA methylation showed that every 1 ng/g increase of breast tissue folate contributed to a marginal increase (p = 0.089) of average LINE-1 DNA methylation (Figure 3(b)). When the subjects were categorized into low and high folate groups based on the median value of breast tissue folate, the high folate group had a significantly higher level (p < 0.01) of LINE-1 gene methylation than the low folate group (p < 0.01) (Figure 3(c)).
Figure 3.
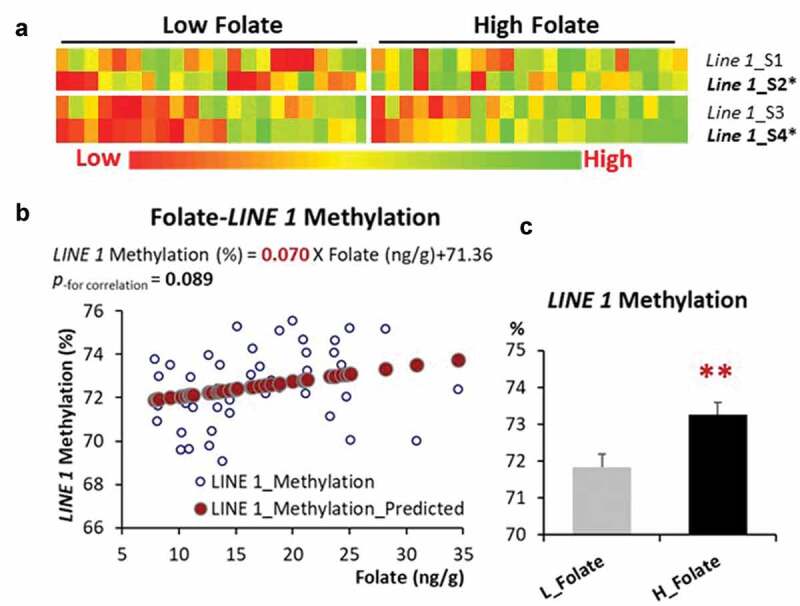
LINE-1 DNA methylation and its association with breast tissue folate. (a) DNA methylation among 4 CpG sites in the LINE-1 gene categorized by low vs high breast tissue folate status. (b) Linear regression between breast tissue folate and average DNA methylation in breast tissue from premenopausal women. (c) LINE-1 DNA methylation categorized by low vs high breast tissue folate. **p < 0.01
The influence of BMI on cancer-related gene expression in premenopausal women
As we are focused on the influence of BMI on folate metabolism and DNA methylation, we performed a real-time PCR for a group of genes (11 genes) related to breast tumorigenesis that are shown to have a gene-specific aberrant methylation profile in breast cancer [11,12]. An apparently different pattern of expression was observed between non-obese and obese groups (Figure 4(a)). The association analysis of the two genes with significantly different expressions, SFRP1, a Wnt antagonist gene within the tumorigenic Wnt-signalling pathway, and MLH1, a DNA repair gene, showed that the expression levels of both genes were positively associated with BMI (p < 0.05) (Figure 4(b)). When the subjects were categorized into non-obese vs obese groups or low vs high breast tissue folate groups, the expression of SFRP1 was higher in both the obese group (p < 0.01) and high breast tissue folate group (p < 0.05), compared to their counterpart groups. The expression of MLH1 was significantly higher in the obese group (p < 0.05) and marginally higher in the high tissue folate group (p = 0.063), when compared to the non-obese or low tissue folate group, respectively (Figure 5 and Table S1).
Figure 4.
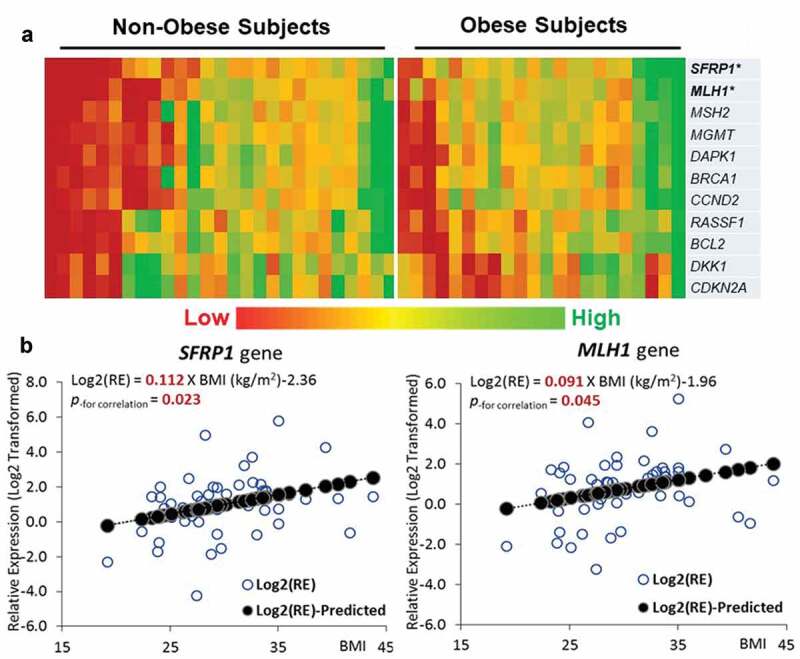
Expressions of genes related to tumorigenesis and aberrant methylation in breast cancer. (a) Heatmap of gene expressions for 11 genes selected. (b) Linear regression between BMI and the expression of SFRP1 and MLH1 gene
Figure 5.
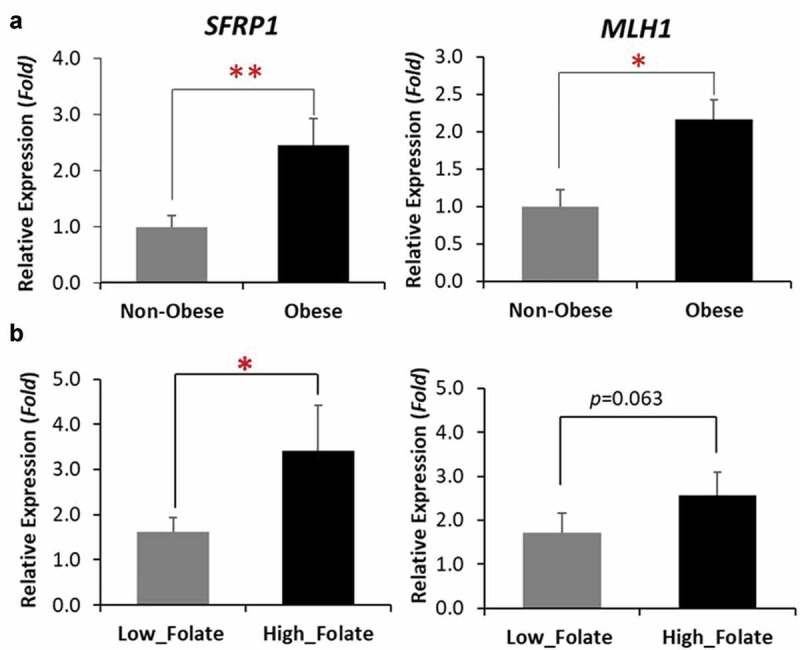
Expressions of SFRP1 and MLH1 gene categorized by BMI (a), and folate status (b). *p < 0.05, **p < 0.01
Discussion
Overweight and obesity have reached an epidemic level in the US [27,28] and worldwide [29,30]. Epidemiological studies have reported paradoxical associations between obesity and pre-BC vs post-BC: a positive association exists between obesity as indicated by BMI and post-BC, whereas a negative association apparently occurs between obesity and pre-BC [2,5,6]. A number of cellular mechanisms responsible for the increased risk of post-BC associated with obesity have been well-studied and established [3,8], whereas the molecular bases that inform the likely beneficial effect of obesity on pre-BC are entirely unknown.
Our current study demonstrated a potential mechanism by which obesity exerts protective action against pre-BC, via improving mammary tissue folate status. This discovery, to the best of our knowledge, has not been previously reported. By examining breast tissue samples from healthy premenopausal women, we observed that a high BMI is interestingly associated with an increased folate status in breast tissue in premenopausal women. This novel finding is in agreement to the association observed in our previous study between obesity and red blood cell folate [21], though obesity is generally associated with low serum folate levels [21,31–34]. Parallel with an improvement in folate status in breast tissue is the increase in genome-wide DNA methylation, as indicated by LINE-1 DNA methylation. These results implicate that a BMI status above normal might play a protective role against breast tumorigenesis by improving both mammary folate and epigenetic status in premenopausal women.
Positive associations were also observed among BMI, folate status and expression levels of a tumour suppressor (SFRP1) and a DNA repair gene (MLH1). SFRP1 is a critical negative regulator of Wnt-signalling, and it is reported that Wnt-signalling abnormalities are associated with 60% of all breast cancers [35,36]. MLH1 is one of seven DNA mismatch repair genes that work coordinately to repair DNA mismatch errors [37]. Pyrosequencing of the promoter methylation of these two genes did not reveal differences among BMI categories (data not shown). As folate metabolism may alter methylation status in elements other than the promoter region, e.g. exons [38], we speculate that changes of methylation may exist in other components, which warrants future investigation by high throughput DNA methylation sequencing.
Based on results from epidemiological studies, it is apparent that obesity has a differentiated pathological effect on pre-BC compared to that on post-BC. Historically, the adverse effect of obesity on post-BC has thought to be mediated by elevated oestrogen levels due to greater aromatase activity in the excess adipose tissue [39]. However, even though an adverse effect on pre-BC from obesity might not be seen due to the already relatively high levels of ovarian oestrogen production in premenopausal women, it could not explain the apparent protective effect of obesity on pre-BC. As described in the Introduction section, epigenetic regulation is recently demonstrated as a critical determinant in breast cancer development [9,10]. DNA methylation may be of particular importance in the development of pre-BC compared to post-BC. A general global hypomethylation profile exists in BC from young women but the degree is lesser for BC from older women [40]. Our current study shows that obesity in women improved genome-wide DNA methylation via improving folate status specifically in breast tissue, and indicates a novel mechanism for the protective effect of obesity on pre-BC.
While our previous study [21] and others [34] have reported that overweight/obesity is paradoxically associated with decreased serum folate but increased red blood cell folate levels, the present study showed for the first time that a high BMI is associated with an increase in breast tissue folate. As to the mechanism(s) responsible for this surprising improved folate status in breast tissue from overweight/obese women, although the understanding is very limited, a few speculated mechanisms has been described in our previous paper [21]. For instance, it is possible that low folate status in the microenvironment surrounding the cells of certain organs may lead to the upregulation of folate carrier and transport genes [21].
In addition to serving as a key factor in DNA methylation via one-carbon metabolism, folate also plays a critical role in nucleotide synthesis, thereby contributing to cell proliferation and growth [41]. These two functions provide the molecular basis for the controversy regarding the influence of folate on tumorigenesis. Folate, particularly in the supplementation form (folic acid), may prevent initiation and early promotion of cancer development via maintaining proper DNA methylation and integrity, but as folate may also provide nucleotide blocks for DNA synthesis, it may also promote the growth of established precancerous and cancer cells [42,43]. Animal studies raised warnings after showing that folic acid supplementation promoted expansion after tumour establishment [44,45]. It is a striking question to investigate whether obesity-related high folate status primarily prevents the initiation of breast cancer in premenopausal women via the epigenetic mechanism, whereas it would promote tumour growth via DNA synthesis in postmenopausal women as they may harbour more precancerous cells. Nevertheless, general consensus from a portfolio of evidence from epidemiologic and animal studies is that a sufficient folate status is associated with reduced risk of certain cancers [43,46].
In conclusion, findings from the current study implicate a novel mechanism that mediates the observed protective role of obesity on premenopausal breast cancer; obesity is associated with improved mammary folate and epigenetic status in premenopausal women. This study provides important experimental data for future revision of dietary folate intake recommendations and folate fortification mandates.
Supplementary Material
Acknowledgments
This work was supported by Rays of Hope Center for Breast Cancer Research, Baystate Medical Center under grant [#113-1535, ZL]; and the US Department of Agriculture under a Hatch grant [MAS00514-1013548, ZL].
Disclosure statement
The authors report no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- [2].World Cancer Research Fund International/American Institute for Cancer Research. continuous update project report: diet, nutrition, physical activity and breast cancer. 2017. Available from: wcrf.org/breast-cancer-2017
- [3].Calle EE, Kaaks R.. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. [DOI] [PubMed] [Google Scholar]
- [4].Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563–570. [DOI] [PubMed] [Google Scholar]
- [5].Ursin G, Longnecker MP, Haik RW, et al. A meta-analysis of body mass index and risk of premenopausal breast cancer. Epidemiology. 1995;6(2):137–141. [DOI] [PubMed] [Google Scholar]
- [6].van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527. [DOI] [PubMed] [Google Scholar]
- [7].Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. [DOI] [PubMed] [Google Scholar]
- [8].De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hinshelwood RA, Clark SJ. Breast cancer epigenetics: normal human mammary epithelial cells as a model system. J Mol Med (Berl). 2008;86(12):1315–1328. [DOI] [PubMed] [Google Scholar]
- [10].Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jovanovic J, Ronneberg JA, Tost J, et al. The epigenetics of breast cancer. Mol Oncol. 2010;4(3):242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ordway JM, Budiman MA, Korshunova Y, et al. Identification of novel high-frequency DNA methylation changes in breast cancer. PLoS One. 2007;2(12):e1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li Z, Guo X, Wu Y, et al. Methylation profiling of 48 candidate genes in tumor and matched normal tissues from breast cancer patients. Breast Cancer Res Treat. 2015;149(3):767–779. [DOI] [PubMed] [Google Scholar]
- [14].Novak P, Jensen T, Oshiro MM, et al. Agglomerative epigenetic aberrations are a common event in human breast cancer. Cancer Res. 2008;68(20):8616–8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park SY, Seo AN, Jung HY, et al. Alu and LINE-1 hypomethylation is associated with HER2 enriched subtype of breast cancer. PLoS One. 2014;9(6):e100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Crider KS, Yang TP, Berry RJ, et al. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012;3:21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen P, Li C, Li X, et al. Higher dietary folate intake reduces the breast cancer risk: a systematic review and meta-analysis. Br J Cancer. 2014;110(9):2327–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang YF, Shi -W-W, Gao H-F, et al. Folate intake and the risk of breast cancer: a dose-response meta-analysis of prospective studies. PLoS One. 2014;9:e100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang S, Hunter DJ, Hankinson SE, et al. A prospective study of folate intake and the risk of breast cancer. Jama. 1999;281(17):1632–1637. [DOI] [PubMed] [Google Scholar]
- [20].Christensen BC, Kelsey KT, Zheng S, et al. Breast cancer DNA methylation profiles are associated with tumor size and alcohol and folate intake. PLoS Genet. 2010;6(7):e1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bird JK, Ronnenberg AG, Choi S-W, et al. Obesity is associated with increased red blood cell folate despite lower dietary intakes and serum concentrations. J Nutr. 2015;145(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Horne DW, Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin Chem. 1988;34:2357–2359. [PubMed] [Google Scholar]
- [23].Llanos AA, Dumitrescu RG, Brasky TM, et al. Relationships among folate, alcohol consumption, gene variants in one-carbon metabolism and p16INK4a methylation and expression in healthy breast tissues. Carcinogenesis. 2015;36(1):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen Y, Armstrong DA, Salas LA, et al. Genome-wide DNA methylation profiling shows a distinct epigenetic signature associated with lung macrophages in cystic fibrosis. Clin Epigenetics. 2018;10(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bendre M, Granholm L, Drennan R, et al. Early life stress and voluntary alcohol consumption in relation to Maoa methylation in male rats. Alcohol. 2019;79:7–16. [DOI] [PubMed] [Google Scholar]
- [26].Roubert A, Gregory K, Li Y, et al. The influence of tumor necrosis factor-α on the tumorigenic Wnt -signaling pathway in human mammary tissue from obese women. Oncotarget. 2017;8:36127–36136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. Jama. 2006;295(13):1549–1555. [DOI] [PubMed] [Google Scholar]
- [28].Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. Jama. 2010;303:235–241. [DOI] [PubMed] [Google Scholar]
- [29].Silventoinen K, Sans S, Tolonen H, et al. Trends in obesity and energy supply in the WHO MONICA Project. Int J Obes Relat Metab Disord. 2004;28(5):710–718. [DOI] [PubMed] [Google Scholar]
- [30].Wang Y, Monteiro C, Popkin BM. Trends of obesity and underweight in older children and adolescents in the United States, Brazil, China, and Russia. Am J Clin Nutr. 2002;75(6):971–977. [DOI] [PubMed] [Google Scholar]
- [31].Bradbury KE, Williams SM, Mann JI, et al. Estimation of serum and erythrocyte folate concentrations in the New Zealand adult population within a background of voluntary folic acid fortification. J Nutr. 2013;144:68–74. [DOI] [PubMed] [Google Scholar]
- [32].Kim H, Hwang J-Y, Kim K-N, et al. Relationship between body-mass index and serum folate concentrations in pregnant women. Eur J Clin Nutr. 2012;66(1):136–138. [DOI] [PubMed] [Google Scholar]
- [33].Kimmons JE, Blanck HM, Tohill BC, et al. Associations between body mass index and the prevalence of low micronutrient levels among US adults. MedGenMed. 2006;8:59. [PMC free article] [PubMed] [Google Scholar]
- [34].da Silva VR, Hausman DB, Kauwell GPA, et al. Obesity affects short-term folate pharmacokinetics in women of childbearing age. Int J Obes (Lond). 2013;37(12):1608–1610. [DOI] [PubMed] [Google Scholar]
- [35].Lin SY, Xia W, Wang JC, et al. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A. 2000;97:4262–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wulf GM, Ryo A, Wulf GG, et al. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. Embo J. 2001;20(13):3459–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pal T, Permuth-Wey J, Sellers TA. A review of the clinical relevance of mismatch-repair deficiency in ovarian cancer. Cancer. 2008;113(4):733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim YI. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr. 2005;135:2703–2709. [DOI] [PubMed] [Google Scholar]
- [39].McTiernan A, Rajan KB, Tworoger SS, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003;21(10):1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oltra SS, Peña-Chilet M, Flower K, et al. Acceleration in the DNA methylation age in breast cancer tumours from very young women. Sci Rep. 2019;9(1):14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shane B. Folate chemistry and metabolism. In: Baile LB, editor. Folate in Health and Disease. New York: Marcel Dekker; 1995. p. 1–22. [Google Scholar]
- [42].Kotsopoulos J, Kim YI, Narod SA. Folate and breast cancer: what about high-risk women? Cancer Causes Control. 2012;23:1405–1420. [DOI] [PubMed] [Google Scholar]
- [43].Kim YI. Folate and cancer: a tale of Dr. Jekyll and Mr. Hyde? Am J Clin Nutr. 2018;107:139–142. [DOI] [PubMed] [Google Scholar]
- [44].Deghan Manshadi S, Ishiguro L, Sohn KJ, et al. Folic acid supplementation promotes mammary tumor progression in a rat model. PLoS One. 2014;9:e84635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hansen MF, Jensen SO, Fuchtbauer EM, et al. High folic acid diet enhances tumour growth in PyMT-induced breast cancer. Br J Cancer. 2017;116:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen J, Xu X, Liu A, et al. Folate and cancer: epidemiological perspective. In: Bailey LB, editor. Folate in health and disease. Boca Raton, FL: CRC Press; 2010. p. 205–234. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


