Abstract
The rapidly advancing field of digital health technologies provides a great opportunity to radically transform the way clinical trials are conducted and to shift the clinical trial paradigm from a site‐centric to a patient‐centric model. Merck’s (Kenilworth, NJ) digitally enabled clinical trial initiative is focused on introduction of digital technologies into the clinical trial paradigm to reduce patient burden, improve drug adherence, provide a means of more closely engaging with the patient, and enable higher quality, faster, and more frequent data collection. This paper will describe the following four key areas of focus from Merck’s digitally enabled clinical trials initiative, along with corresponding enabling technologies: (i) use of technologies that can monitor and improve drug adherence (smart dosing), (ii) collection of pharmacokinetic (PK), pharmacodynamic (PD), and biomarker samples in an outpatient setting (patient‐centric sampling), (iii) use of digital devices to collect and measure physiological and behavioral data (digital biomarkers), and (iv) use of data platforms that integrate digital data streams, visualize data in real‐time, and provide a means of greater patient engagement during the trial (digital platform). Furthermore, this paper will discuss the synergistic power in implementation of these approaches jointly within a trial to enable better understanding of adherence, safety, efficacy, PK, PD, and corresponding exposure‐response relationships of investigational therapies as well as reduced patient burden for clinical trial participation. Obstacle and challenges to adoption and full realization of the vision of patient‐centric, digitally enabled trials will also be discussed.
The rapidly advancing field of digital health technologies provides an opportunity to transform the pharmaceutical industry and the way clinical trials are conducted. Although the conduct of clinical trials has evolved over the last century to improve the unbiased evaluation of new therapies, there remain several limitations in the current clinical trial paradigm. Pharmaceutical clinical trials are often site‐centric, requiring patients to come to the clinical site for sample and data collection. The need to travel to the clinical site often restricts the trial population to those that live in geographic proximity to the clinical site, and, thus, restricts who participates and limits patient diversity, leaving many patients excluded and underserved. 1 , 2 , 3 , 4 , 5 The current trial paradigm provides only static snapshots of data (corresponding to the time of the clinical visit), resulting in lost opportunity to monitor end points of disease progression, pharmacokinetics (PK), pharmacodynamics (PD), and safety and tolerability end points in between clinical visits. Additionally, clinical trial outcome measures may not be particularly meaningful to patients or their health care providers, and end points may be limited by categorical, episodic, subjective assessments that progress slowly, thus requiring large, long, expensive clinical trials to enable detection of meaningful change in the end point. Furthermore, patient medication adherence and persistence to therapy in clinical trials is often low, 6 , 7 limiting the researcher’s ability to adequately assess the drug’s safety, efficacy, and exposure‐response relationships. Lastly, patients often find the clinical trial language confusing and the trial’s expectation of what they are supposed to do intrusive into their daily lives, limiting the number of patients that participate in clinical trials and threatening the retention of those patients that do consent to participate. 1 , 2 , 3 , 4 , 5
The potential benefits of digital health and outpatient sampling technologies in clinical trials are tremendous. They can enable increased access to the appropriate patient population, reduced patient burden to participate, augmented, more informed, objective data sets (both in collecting and measuring existing end points at home and in access to new end points that would have been impossible to collect in the past), increased engagement with the patient, and better understanding of the patient experience throughout the trial. All these benefits will ultimately improve the patient experience during the trial and enable improved drug development decisions and understanding of drug and disease effects. 8
Despite all these potential improvements, the relative “explosion” in both the number of digital health technologies as well as their capabilities, and an increased adoption of consumer‐grade health‐tracking devices in the marketplace, adoption of use of such technologies in pharmaceutical trials has been lagging by comparison. 9 , 10 , 11 Some of the challenges to pharmaceutical trial adoption include questions around patient privacy, lack of sufficient validation for digital end points, lack of transparency for calculation of end points (“black box” algorithms), challenges related to patient adherence and burden of wearing and using devices, operational and data transfer challenges, and regulatory unknowns. However, use of digital end points in drug development trials, including as primary and secondary end points and to support label claims, is becoming a reality, and “pilot” trials evaluating technologies of interest, often evaluating digital end points in comparison to a traditionally accepted clinical standard end point, are being increasingly conducted. 12 , 13 , 14
The digitally enabled clinical trials initiative at Merck (Kenilworth, NJ) is aimed at using innovative, digital technologies in clinical trials both at the clinical site and in at‐home settings to reduce patient burden, collect higher quality, enrich clinical trial data sets, and ultimately enable more rapid and informed clinical decisions. We ultimately aim to shift the clinical trial paradigm from one that is site‐centric to patient‐centric. Key areas of focus include (i) collection of at‐home PK, PD, and biomarker samples (outpatient sampling), (ii) use of technologies to monitor and improve patient adherence (smart dosing), (iii) use of digital devices to collect and measure physiological and behavioral data (digital biomarkers), and (iv) development and use of data platforms that can acquire the data from digital devices, provide real‐time analytic capabilities, and maintain patient engagement throughout the trial (digital platform; Figure 1 ). Application of these components in clinical trials will lead to access to higher quality and previously unattainable data for more informed clinical decision making.
Figure 1.
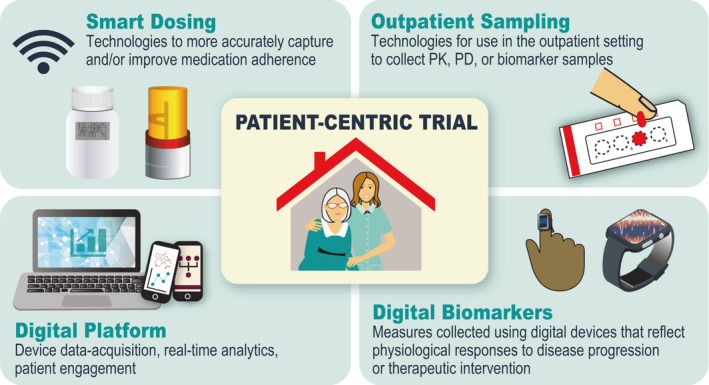
Areas of focus for digitally enabled clinical trials.
This paper describes the four key areas of focus of our digitally enabled clinical trials initiative and reviews corresponding enabling technologies. Furthermore, this paper discusses the synergistic power in implementation of these approaches jointly within a trial to enable a more accurate understanding of adherence, safety, PK, and corresponding exposure‐response relationships of investigational new drugs (INDs) as well as reduced patient burden for clinical trial participation. Obstacles and challenges to adoption and fully realizing the vision of patient‐centric, digitally enabled trials are also discussed.
SMART DOSING
Patient adherence is often less than desirable in both real‐world settings as well as in clinical trials. Adherence to long‐term therapy for chronic illnesses in developed countries averages 50%, and in developing countries, the rates are even lower. 15 Exact reasons for patient nonadherence can vary significantly across individuals, but such factors may be treatment‐related (e.g., adverse events (AEs)), disease‐related (e.g., difficulty swallowing and nausea), psychological/social (e.g., stigma of therapy), or even just human nature (e.g., forgetfulness, or discontinuation of therapy when an initial positive response is achieved). 16 , 17 , 18 , 19 , 20 Lack of medication adherence and lack of reliable medication adherence data in clinical trials impacts the ability of drug developers to understand whether INDs are safe and efficacious. The ability to deconvolute adherence from efficacy and safety is critical to inform not only the decision to progress or kill an IND, but also to define the therapeutic window of the IND and in selection of appropriate doses to progress to future trials or to market.
Accurate medication adherence data in clinical trials is important for scientific decision making; however, the current methods for determining patient adherence, which include asking clinical trial participants to self‐report their adherence via a diary and relying on returned pill counts, are severely flawed for several reasons, including:
Introduction of inadvertent inaccuracy bias (e.g., the patient forgets to record their adherence contemporaneously in their medication diary and instead records dosing after the fact);
Introduction of advertent inaccuracy bias (e.g., the patient wants to appear adherent and records their medication diary as such, even if they have not taken the drug);
Lack of contemporaneous and contextual data: a drug count of the returned package provides only a static snapshot of drug remaining, without any context on whether the drug was dosed as prescribed or whether it was dosed at all (or potentially discarded).
As a few selected case studies below indicate, there are a multitude of examples indicative of the discrepancies that exist between patient‐reported or pill count‐indicated adherence and true adherence as evidenced by PK drug exposure.
Across clinical studies reported in psychiatric indications, average nonadherence calculated from pill counts was 2.2%, however, an average of 28.9% of subjects had a PK sample below the limit of quantitation, representing a more than 10‐fold difference in nonadherence measures. 21
The TOPCAT heart failure outcome trial failed to meet its primary outcome; however, there were regional discrepancies in the data. In particular, 30% of the patients in Russia that reported taking their study medication did not, as evidenced by PK analysis. 22
In a tenofovir‐based pre‐exposure prophylaxis for HIV infection trial among African women, none of the drug regimens evaluated reduced the rates of HIV‐1 acquisition in an intention‐to‐treat analysis; however, there were large discrepancies between various measures of adherence (i.e., subject‐reported, returned product, and PK analysis). 23
Electronic‐monitoring “smart dosing” technologies provide an automated, impartial, and contemporaneously reporting observer to the dosing event, improving the accuracy and quality of adherence data, 6 and thereby enabling improved correlation of dosing to efficacy and safety. These technologies also may provide medication reminders and allow site staff to review patients’ adherence data and counsel them on their adherence patterns, improving medication adherence 24 and potentially leading to better clinical outcomes. 25 , 26
There are many different types of smart dosing technologies, each with different capabilities and considerations, as described below and in Table 1 .
Table 1.
Categories of smart dosing technologies
| Category | |||
|---|---|---|---|
| Smart packaging | Photographic documentation via app | IST | |
| Description | Micro‐circuitry capturing time/date of patient package interaction (e.g., package/cap opening, drug removal, actuation) | Use of video (with facial and drug identification features) to record and visually confirm the drug being taken (e.g., ingestion, inhalation, etc.) | Microcircuit co‐formulated with drug that is activated after ingestion and transmits signal of ingestion event to an external source |
| Example technologies |
|
|
|
| Pros |
|
|
|
| Cons |
|
|
|
IND, investigational new drug; IST, ingestible sensor technology.
Smart packaging
The smart packaging category of smart dosing solutions encompasses packaging that has embedded micro‐circuitry that records a time/date stamp when the patient/user interacts with the package or device. For solid oral dose drugs, this date/time stamp could be when a bottle cap is opened or the foil is pierced on a blister. A smart package sensor could also be added to enable automated adherence tracking on a drug delivery device (e.g., when an inhaler is actuated or when a syringe is removed from the packaging). Data are then either transmitted automatically from the package (if so, equipped with this capability) or an NFC/RFID device extracts the data from the package. Smart packaging may also have a companion application (app) that sends reminders to either the patient or the site. The app could also be used to facilitate telemedicine visits or deliver supplemental electronic clinical outcomes assessments (eCOAs) to the patient, as dictated by the protocol.
Smart packaging is a nonintrusive way to automatically capture adherence data, as the package often appears similar to a standard package and is used by the patient in the same way. Tracking adherence in this way is most valuable in populations with limited faculties, those with aversion to technology use, and in cases where it is desired to collect more accurate adherence information without influencing adherence (i.e., passive adherence monitoring). Smart packaging has been used extensively in clinical trials 6 , 27 and is referenced in recent U.S Food and Drug Administration (FDA) guidance on enrichment strategies for clinical trials as a method that has become standard for ensuring adherence. 28 In addition, to use in the pharmaceutical research and development space, there are several examples of FDA approved smart packaging products for use in health care, such as InPen’s smart insulin pen system and Propeller Health’s inhaler‐compatible sensors.
Photographic documentation via an app
Photographic documentation is an emerging category of smart dosing and represents app‐based technologies that use a smartphone camera to confirm the oral dose image, confirm patient identity, and film the dosing event. These apps can also capture dosing of nonoral dose medications (e.g., injector pen or inhaler) and could be used to prompt the patient on how to dose using drug delivery devices if special instructions are required. These apps can send reminders via push notification or text message that a dose is upcoming or that a dose has been missed. Data (either the dosing event or notification of a missed dose) is transmitted in real‐time to the clinical site to enable proactive follow‐up and engagement with the patient. The app could also be used to send other types of reminders to the patient (e.g., reminders for visits, active remote assessments, etc.), to enable telemedicine visits, and/or to deliver supplemental eCOAs to the patient.
There are several considerations when deciding whether this category of smart dosing technology is right for a clinical trial. The patient must be capable of using a smartphone, following on‐screen instructions, and either holding or positioning the smartphone such that it is steady and enabling the right field of view during the dosing event. As images of patients are being recorded, maximum care must be taken with regard to data privacy and security.
Photographic documentation has been proven successful in several indications, particularly schizophrenia and stroke. 29 , 30 , 31 , 32 This technology is particularly valuable in populations that are on multiple therapies, those that benefit from close or real‐time patient/site engagement, where dosing is complex (e.g., requires multiple steps or use of a dose delivery device), or where the drug product relies on existing packaging for environmental (e.g., moisture/light/oxygen) protection.
Ingestible sensor technology
In ingestible sensor technologies (ISTs), a sensor is co‐formulated or otherwise combined with the drug of interest. The sensor activates on contact with stomach acid and transmits a signal. The patient wears a receiver (e.g., in the form of a skin patch or jewelry) that receives this signal and transmits it to another device with long‐range communication capability (often a cell phone or cell‐enabled tablet), which sends notification to the cloud that ingestion has occurred. Similar to smart packaging and photographic documentation, ISTs can also have a companion app that can send reminders to the patient or site, enable telemedicine, and/or deliver supplemental eCOAs. This type of smart dosing technology is particularly useful when it is important to confirm the ingestion event. An example of an FDA‐approved IST drug‐device combination product is aripiprazole tablets with sensor (Abilify MyCite).
Other smart dosing technologies of interest
There are also several newer categories of smart dosing technology available for tracking patient adherence:
Tracer‐based biomarkers, co‐formulated with the drug. A metabolite of the tracer is measured from the patient via secretion (e.g., blood, saliva, urine, or breath). Examples include Xhale Smart and nGageIT.
Home health hubs that combine social robotics and telemedicine with medication dispensing. These act as a one‐stop‐shop for patient convenience and can provide social engagement for the patient as well. Examples include Pillo Health and Medacube.
Mobile diagnostics tools, such as at‐home test‐strips that can use patient secretion (e.g., blood, saliva, urine, and sweat/skin oils) to rapidly measure presence or absence of the analyte of interest.
More clinical study is necessary to better determine the relative risk/benefit profiles of these modalities of adherence measurement.
Summary and implications of smart dosing technologies
Smart dosing technologies provide an improved and potentially real‐time representation of patient adherence that is not possible to obtain through other means. This is especially critical when there are factors that predispose the patient to nonadherence, such as nonstandard dosing regimens, medication adverse effects, and patient populations with historically low adherence rates. Some of these technologies also facilitate engagement with and rescue nonadherent patients to improve their future adherence.
Having more accurate adherence data through smart dosing technologies will allow researchers and drug developers to deconvolute adherence from efficacy and safety, resulting in less variability in exposure‐response analyses and improving decision making for therapies. Furthermore, some of these technologies also provide the opportunity to improve patient adherence to therapy in clinical trials through medication reminders and real‐time data access‐enabled follow‐up with patients, which may ultimately lead to better outcomes for patients 25 , 26 and truer signal of efficacy and safety of INDs in clinical trials. In the event of an IND failing to demonstrate efficacy in a clinical trial, this technology can help mitigate questions as to whether this was due to lack of efficacy at the selected dose(s) or a result of medication nonadherence within the trial population. Finally, adherence has a substantial impact on study power; 33 , 34 therefore, a truer signal of adherence may lead to reduction in clinical study size. 35
At Merck, we have performed clinical pilot testing with several smart dosing technologies of interest 36 , 37 and are proceeding with use of some of these technologies in IND trials, including trials in neuroscience, infectious disease, and cardiovascular disease therapeutic areas.
OUTPATIENT SAMPLING
The current gold standard for blood sample collection in clinical trials is the collection of venous blood by a trained phlebotomist. This generally requires participants in clinical trials to travel to a site to have a phlebotomist perform the blood draw. Once collected, plasma and/or serum is separated from the whole blood and transferred to a labeled tube and stored using a controlled‐temperature unit. Samples are then shipped using dry ice to a central laboratory for further processing and analysis. This approach provides several advantages, including well‐defined sample quality, chain of custody, and compatibility with established workflows. These workflows include procurement of supplies, shipping logistics, central laboratory sample handling, laboratory information management systems, and liquid handling automation systems. This is a well‐established paradigm for ensuring quality, consistency, and regulatory acceptance of PK and blood‐based biomarker data from clinical trials. However, this approach often puts burden on patients and their caregivers to visit the clinical site at specified times for sample collection. The consequence of this is that compromises are made to reduce the number of samples collected in the trial, potentially limiting understanding of the drug and its corresponding PK and/or PD time course. Furthermore, patients may experience pain during venous sample collection, and some populations (e.g., pediatric and elderly) have reduced blood volume that limits sample collection.
The goal of outpatient sampling is to enable access to samples outside of clinical visits to enhance and/or replace data collected in the clinic, and, therefore, improve understanding of the drug and the associated biology, safety, and efficacy. Outpatient sampling can enhance the researchers’ ability to arrive at the right answer through access to data from samples that otherwise would be impractical or impossible to collect in the clinic. The ability to access patient‐derived samples, such as blood, outside of clinical visits in a convenient and patient‐friendly manner has the potential to be a disruptive influence on the conduct of clinical trials. 8 Several potential benefits include reduced burden on the trial participant and/or caregiver through fewer visits to the clinical site, enhanced/faster and more diverse enrollment by attracting subjects from geographies not proximal to clinical sites, retention of patients by making it easier for them to participate in the trial, added flexibility in collection of PK/PD data, particularly in late stage trials, and, in the case of microsampling approaches, reduced blood volume requirements and potential for improved logistical feasibility. 36 , 38 , 39
At‐home collection would provide particular benefit for:
disease areas associated with episodic events (e.g., asthma, migraine, influenza, etc.), providing the ability to collect samples proximal to the time of the event;
vulnerable and at‐risk populations (e.g., elderly, immunocompromised, contagious, etc.) in which there may be concerns of potentially endangering themselves or others by being in public;
long half‐life compounds, enabling collection of PK samples during the elimination phase without necessitating the need for additional clinical visits;
developing understanding of adherence patterns for unique or new dosing regimens (e.g., dosing every few days, weekly, or monthly);
compounds with known safety concerns, enabling exposure‐response assessments of AEs;
more frequent assessment of biomarkers of efficacy and toxicity, especially for markers that have a temporal component (e.g., viral shedding, ctDNA, and viral load).
The technology to collect samples outside of clinical visits has been around for over 50 years, but the last decade has seen an increase in efforts that make sample collection more patient‐friendly and improve the overall quality of the sampling process. The original approach used for sample collection consisted of a lancet and a cellulose paper card for collection of dried blood spots (DBS). 38 , 40 , 41 Newer approaches have focused on improving the quality of the sample collected to enable absolute quantitative analysis methods and making sample collection less painful and more reliable. The use of dried blood‐based approaches reduces some of the logistical hurdles associated with venous collection (e.g., refrigerated centrifuges and cold chain shipping). Shipping dried blood is possible using standard postal services when using standard precautions and harmonized guidance are available from national and international agencies. 42 Table 2 provides details on current approaches for outpatient sample collection.
Table 2.
Current technologies for outpatient sample collection
| Technology | Description | Pros | Cons |
|---|---|---|---|
| DBS on paper | Fingerstick‐based approach to collect blood on a paper substrate. A variety of paper types are available. | Simple and inexpensive approach, well established workflows for many analytes especially for newborn screening applications | Not a volumetric collection that can impact quantitative measurements, Repeated sampling via finger sticks can be painful, requires manual capture of meta data such as time of sampling |
| Volumetric absorptive microsampling (Neoteryx Mitra) | Fingerstick‐based approach to collect samples on a polymer matrix of defined wicking volume | Simple approach with the benefit of collecting a well‐defined volume, Automation friendly format provided by the vendor | Fingerstick is required, no automated capture of meta data, cost vs. DBS paper‐based approach |
| Volumetric absorptive microsampling (Capitainer) | Fingerstick‐based approach to collect samples using a channel of defined volume | Simple approach with the benefit of collecting a well‐defined volume | Fingerstick is required |
| HemaXis | Fingerstick‐based approach to collect samples using a microfluidic channel of defined volume | Simple approach with the benefit of collecting a well‐defined volume | Fingerstick is required |
| Trajan HemaPen | Fingerstick‐based approach to collect four simultaneous samples using capillaries of defined volume | Volumetric collection, designed to be patient friendly, integrated desiccant to preserve sample | Fingerstick is required, lack of automation for device handling to remove sample |
| SeventhSense TAP | Microneedle‐based blood collection device that is used on the arm and collects liquid blood | Pain free approach that is simple to use | Blood is collected inside the device and requires transfer to secondary container/matrix, cost relative to other approaches |
| Tasso OnDemand | Lancet‐based device that collects blood onto a cartridge | Pain free approach that is simple to use, cartridge collects dried blood | Automation of cartridge handling not available, cost relative to paper‐based methods |
DBS, dried blood spot.
Experience with at‐home sampling
Currently, the use of at‐home sampling has been limited, but as experience with this approach and technology evolves, adoption is expected to increase. A Merck program on the treatment of migraine provided the opportunity to use at‐home blood collection in a clinical trial. 39 Given the episodic nature of migraine, it was not feasible to collect PK samples in the clinic proximal to when the acute migraine events occurred, and these PK data were needed to enable exposure‐response modeling to guide dose selection. 43 A DBS assay was developed, and DBS sampling was included alongside plasma sampling in a phase I clinical trial in healthy volunteers to ensure that the drug concentration from DBS samples were well‐correlated to that from plasma samples and reliable quantitative measurement of drug exposure could be obtained from self‐collected samples. Although data from a trial participant survey indicated that some found the fingerstick samples painful, with > 40% of participants finding the experience somewhat unpleasant, 39 the pilot trial was successful. Drug concentrations from DBS and plasma samples were well‐correlated and PK variability was reduced in the DBS samples. 39 At‐home and in‐clinic fingerstick DBS sampling, along with in‐clinic plasma sampling, were then included in the phase II clinical trial. Patients were trained on sample collection in‐clinic and given a paper diary to record the date/time of at‐home sample collections. The results indicated much higher PK variability for at‐home vs. in‐clinic DBS samples (113% vs. 37% residual PK variability) 39 and revealed fundamental flaws in at‐home sample collection that need to be addressed for this approach to be useful for the assessment of PK in future clinical trials. It is well‐known that patient compliance with paper diaries can be highly variable, 44 and it is likely that errors in paper diary‐reported DBS sample collection time contributed to the results seen in this trial. 39
The experience with this trial prompted research into finding solutions that were more patient‐friendly and pain‐free and spurred the formation of a dedicated team to bring patient‐centric approaches to clinical trials. The learnings from internal efforts as well as research shared by other companies has shaped the direction of at‐home sampling efforts at Merck. To address the potential for bias from the sample collection process onto paper, a volumetric‐based approach was adopted. 45 Collection of a defined volume of blood, when combined with appropriate extraction methodology, has proven to be a reliable approach for consistent data quality. 46 However, surveys of trial participants on the use of fingerstick‐based sampling across several studies have shown that participants are less likely to accept this method if frequently collected. 36 , 37 , 47 , 48 In addition, fingerstick‐based sampling, even with a volumetric approach, has resulted in incomplete or under‐sampling in some cases. This has led to exploration of alternative methods for blood collection that are less painful, more reliable, and simpler to perform. In addition, the ability to automatically collect the date and time of sample collection is highly desired to avoid potential missing or inaccurate data when this information is patient‐reported 37 as well as recording of information, such as temperature during the shipping process to ensure sample quality is maintained. Such a capability, although not yet standard practice, is in development for a few of the technologies listed in Table 2 .
As the technology evolves and the adoption of at‐home sampling increases, the pathway to implementation of this new approach will become more defined and robust. The use of outpatient sampling approaches, especially dried microsampling, requires separate assay validation from traditional plasma or serum assay validation, and not all molecules will be amenable to this approach, depending on assay requirements and compound properties; however, bioanalytical aspects have been well‐studied, and unless the assay requires exceptionally low limits of quantitation (e.g., compounds/delivery routes with very low systemic exposure), most molecules are amenable to microsampling‐based outpatient sample collection. A retrospective analysis of Merck’s small molecule clinical programs since 2016 showed that a majority of compounds were amenable to dried blood microsample collection for drug concentration measurement, based on either direct assay experience (67%) or required assay criteria (92%). To date, we have completed full assay validation with in vivo clinical bridging (dried blood:plasma or serum) for 14 programs, including for a therapeutic monoclonal antibody program, showing that dried blood sampling is also applicable for biologics. Of these, five studies used at‐home sample collection as part of the study. The strategic integration of dried blood sampling has previously been published as well as feedback from regulatory interactions. 38 The most recent FDA guidance on bioanalytical method validation specifically calls out dried blood sampling, highlighting some of the benefits of this approach. 49 Feedback from regulatory agencies should be requested as part of the development plan when using outpatient sampling. It has been our experience that regulators see the value in the approach and are accepting of the data when supported by appropriate validation and bridging experiments.
Beyond the assessment of drug exposure in clinical trials, the use of outpatient sampling is suitable for a wide range of endogenous molecules. 50 The ability to conveniently obtain repeat or longitudinal samples from subjects in clinical trials or for general health monitoring is becoming feasible and will impact how health care is delivered. 51 , 52 Viral load measurements from dried blood is one approach that could be adopted to at‐home sampling to enable infectious disease and vaccine research and improve outcomes. 53 Measuring antibody‐mediated immunity is critical to evaluate vaccine efficacy and immunity to seasonal and emerging influenza viruses and could be greatly facilitated by at‐home sampling. 54 In oncology, the monitoring of tumor‐derived cell‐free DNA is a promising approach to diagnose, characterize, and monitor disease in patients with cancer. 55 Recent work has shown that it is possible to detect and monitor ctDNA from DBS. 56 These are but a few examples of where outpatient sampling could be applied to improve drug development and advance our understanding of human biology.
The current challenges associated with implementation of outpatient sampling in clinical trials are mainly related to logistical and operational aspects. These include things such as ensuring proper training materials for trial participants and clinical sites on how to collect, store, and ship the samples (including translation to other languages if needed), different shipping requirements and providers for different countries, labeling of collection devices/samples with required barcodes to assign to patients, tracking supplies, and expiration dates. Sample stability is often highly dependent on temperature and humidity conditions, and, therefore, it is critical that the proper sample handling and shipping procedures are followed (e.g., inclusion of desiccant), particularly when samples are being shipped/stored at room temperature. 57 Furthermore, having some means of confirming patient identity of the sample (e.g., DNA fingerprinting) may be important, particularly in the event of unexpected results. 36 , 37 Overcoming these challenges requires good communication across functional areas and groups, including bioanalytics, clinical operations, PK/PD, regulatory, clinical trial sites, and central laboratories. The goal is to develop this approach to the state where implementation becomes routine and is driven by the strategic need of the program for accessing data through patient‐centric at‐home sampling.
As the technology becomes more established, it is anticipated that it will be used more broadly to augment PK and PD data sets and provide additional data to inform medication adherence. In the future, knowing if a subject was taking their drug vs. relying on antiquated and unreliable techniques, such as pill counts, will be commonplace, 35 and the ability to conveniently obtain samples from patients as enabled by at‐home sampling will be a standard approach to reduce the incidence of false‐negative trial results. 58 The FDA has published draft guidance on enhancing diversity in clinical trials where they specifically call out the need to make participation less burdensome and to adopt retention practices that enhance inclusiveness. 59 At‐home sampling is an obvious way of making participation in trials more patient‐friendly.
DIGITAL BIOMARKERS
A biomarker is defined as a characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions. 60 , 61 Recent advances in digital technologies have enabled a new class of biomarkers to emerge (i.e., digital biomarkers). Digital biomarkers are objective measures of biology or health collected using a digital device that can reflect physiological responses to disease progression or therapeutic intervention. 62 In the context of drug development, these types of measures have the potential to provide valuable data as to the safety and efficacy of INDs. Digital biomarkers can be novel end points (those not typically measured in our current clinical trial paradigm; e.g., activity counts as measured by an accelerometer, or existing endpoints measured in a new, digitally enabled way; e.g., heart rate as measured by a wearable sensor). Furthermore, many of these end points are amenable to at‐home measurement and can be measured on a near‐continuous basis, enabling the potential for more frequent, objective, and sensitive measures of disease progression and of the ability of INDs to treat or modify the course of the disease. 63 , 64 Types of technologies that can enable digital biomarkers are described in Table 3 and include wearable devices (e.g., wrist‐worn accelerometers, finger‐worn sensors, and biometric skin patches), smartphone apps (e.g., voice/speech analysis or typing behavior), and “invisibles” (i.e., technologies that passively measure without requiring the patient to wear any sensors). Examples of technologies falling into the “invisibles” category include sensor‐enabled homes 65 and Emerald, a device that uses radio wave reflective signals paired with machine learning algorithms to determine, location, activity, and sleep staging. 66 , 67
Table 3.
Digital Biomarker‐enabling technology categories
| Category | |||
|---|---|---|---|
| Wearables | Smartphone/applications | Invisibles | |
| Description | Sensor‐based technologies that require the patients to wear them for measurement. Can be used for active or passive assessments. | Smartphone usage data and/or mobile apps that enable active or passive digital biomarker collection | Technologies that passively measure without requiring the patient to wear any sensors |
| Example technologies | Wrist‐worn accelerometers (Apple Watch, Fitbit, Actigraph), biometric skin patches (MC10), finger/wrist/ankle‐worn sensors (Kinesia One, APDM) smart rings (Oura) | Apps for IOS and Android devices (e.g., Sonde Health, Aural Analytics, Cambridge Cognition, nQ Medical) | Emerald, Nyce Sensors, Azure Kinect DK, Care.ai, sensor‐enabled homes |
| Example end points/assessments | Activity/sleep metrics, stride velocity, gait, finger tapping tests, heart rate, heart rate variability, SPO2, skin temperature | Voice/speech metrics, cognitive assessments, typing behavior | Sleep stages, joint position and rotation, posture, movement speed, fall detection, time spent in a certain location |
| Considerations | Commercial vs. clinical‐grade devices, battery usage, and sampling rate trade‐offs, patient comfort, adherence, and wear time | Bring your own device model, version control, privacy concerns | Location‐specific, Wi‐Fi and data transfer, privacy concerns |
Digital biomarkers can be either actively or passively collected. Active collection requires the patient to perform a specific task (e.g., sit‐to‐stand test while wearing a sensor), whereas passive collection involves the collection of data without interrupting a patient’s normal activities, other than potentially wearing a sensor (e.g., physical activity or sleep time as measured by an accelerometer). Although passive collection may provide more continuous data and be less burdensome for the patient, this type of data may be particularly difficult to interpret without context. For example, if a patient’s activity levels are higher on a given day vs. prior days, the increased activity levels could be due to the patient feeling better or there could be some other cause, such as the patient working longer hours on that day. Thus, patient diaries or annotations may provide useful information to contextualize the digital biomarker data obtained. Furthermore, electronic patient‐reported outcomes (ePROs; i.e., patient‐reported outcomes that are collected via electronic means, such as on smartphones), are related to digital biomarkers but are not necessarily objective measures (can be subjective). These ePRO assessments require active collection and can be contextualized and/or timed based on sensor data. For example, sensor data capturing whether the patient is active or sedentary proximal to a patient completing a pain score ePRO can help contextualize the pain score reported. Furthermore, through closed‐loop feedback of accelerometer data and the ePRO assessment tool, the timing of the request for the patient to assess their pain level could be triggered based on the patient’s accelerometer data.
Digital biomarkers have the potential to profoundly impact drug development. Digital biomarker‐enabling technologies give pharmaceutical researchers access to more continuous, objective data sets that were previously not attainable, including outside the clinic and during activities that are more meaningful to patient’s daily lives. They offer the promise of earlier, more sensitive, and less variable indicators of safety and efficacy, which may result in smaller, shorter duration clinical trials and faster go/no go decisions. Additionally, in late‐stage development, digital biomarkers have the potential to be used not only for internal decision making but as primary or secondary registration end points and in support of label claims. Whereas there continues to be a significant delay for the pharmaceutical industry to broadly use digital biomarker‐enabling technology across clinical trials and several barriers to adoption to overcome, and the majority of use‐cases to date have been either in pilot trials or as exploratory end points, there are some examples of regulatory acceptance of digital biomarkers as primary or secondary end points in late‐stage clinical trials. Moderate‐to‐vigorous physical activity (as measured by a wrist‐worn actigraphy device) has been approved by the FDA for use as a primary end point in a phase III study evaluating an investigational treatment in patients with pulmonary hypertension associated with interstitial lung disease. 68 Additionally, the European Medicines Agency (EMA) has published a qualification opinion that the 95th percentile of stride velocity as measured by a valid and suitable wearable device to quantify a patient’s ambulation ability in a continuous manner in an at‐home environment is an acceptable secondary end point in pivotal and exploratory drug trials for Duchenne’s muscular dystrophy. 69 , 70
There are several examples of pilot studies and exploratory trials to develop digital biomarkers with promising results. Recently, wrist‐worn accelerometers have been shown to accurately detect and measure movements associated with itching in patients with atopic dermatitis 71 and have also been shown to differentiate narcolepsy and idiopathic hypersomnia patients. 72 Other examples of such efforts include smartphone motor‐sensing for detection of early signs of Lyme disease 73 , 74 and investigation of wearable devices for cardiac monitoring. 73 , 75 , 76 Merck also conducted a study in collaboration with Koneksa Health that explored use of mobile health approaches to measure changes in heart rate and blood pressure, which found that mobile health approaches were comparable to standard in‐clinic measures and sufficiently sensitive to detect treatment differences, demonstrating potential to capture rich hemodynamic data in early clinical trials to aid decision making. 77
Digital biomarkers may be particularly useful in disease areas that are hindered by a lack of validated biomarkers to objectively measure disease onset and progression, such as neurodegenerative diseases. Clinical trial research has observed that subtle changes in cognition, sensory, and motor function precede clinical manifestations of neurodegenerative disease by several years, 78 and there are a number of trials exploring feasibility of digital biomarkers for Parkinson’s 79 , 80 , 81 , 82 , 83 and Alzheimer’s disease. 84 , 85
Although digital health tools offer the promise of remote, high‐resolution, and high‐frequency clinical observations, there continues to be a significant delay in the pharmaceutical industry to embrace technology. This trend is captured in Martec’s Law, 86 which states that whereas technology is changing very rapidly, and those changes seem to be accelerating, changing an organization—how it thinks and behaves—is still hard and slow. However, over the past 2 years, investments in digital health startups and efforts aimed at identifying digital biomarkers exceeded US $10 billion. 87 Furthermore, since 2000, the annual growth rate for including a connected digital product in a clinical trial, across all clinical trial phases, is 34%. 13 The key factors driving the area of digital biomarkers is the ubiquity of rapidly advancing technology combined with sophisticated analytics to derive clinical meaning. Recent advances in digital technologies offer the opportunity to collect more objective, sensitive measures of disease state, disease progression, and response to therapy, in both in‐clinic and at‐home settings, and have less reliance on subjective, infrequent assessments.
As part of our digitally enabled clinical trials initiative, we are committed to identifying and using technologies that can provide digital biomarkers to better assess the safety and efficacy of our compounds earlier in the drug development process. To that end, we continue to actively assess digital technologies for this purpose in clinical pilot trials, have included wearable activity monitors for exploratory end points in clinical studies, and are investing in studies to enable the identification of digital biomarkers, including in the areas of Parkinson’s and Alzheimer’s disease.
DIGITAL PLATFORM
Data emanating from sensor‐based digital technologies that can continuously measure physiological parameters tend to be high volume, high density, and high speed. Disparate data being collected from these technologies pose an interesting challenge to ensure that the data are meaningfully integrated and interpreted, especially when collected from multiple devices. Many digital devices have their own platforms for managing their data; however, if a trial contains multiple devices, then having data flow through a common platform may be advantageous for trial data management, real‐time analytics, and reduced burden for site staff. Our previous work with deploying multiple technologies in a clinical pilot study indicated that the full value of a digitally enabled trial will only be realized if there are foundational capabilities that enable acquisition and integration of data from digital devices in near real‐time and provide a means of quickly visualizing and analyzing the resultant data while minimizing any errors in the process as well as burden for clinical trial operations personnel. 36 Thus, use of platforms and tools that enable these capabilities is part of our digitally enabled clinical trials strategy. Although real‐time analyses during study conduct may not be feasible in some cases because of protocol‐dependent blinding, the use of such a platform is anticipated to reduce errors and operational overhead associated with transcription of data records, provide ability for real‐time data query generation, and support conditional triggers to enable near real‐time follow‐up with trial participants, such as in the event of missed samples/tasks, medication nonadherence, emerging AEs, or study stopping rules being met.
Such a platform needs to adhere to best practices for internet of things (IOT) data handling (e.g., security and privacy) as well as follow findable, accessible, interoperable, and reusable standards of ensuring data are available for future purposes using both human‐driven and machine‐driven approaches. 88 Key platform provider capabilities include service‐oriented cloud‐based architecture, mobile app development capability, and device provisioning. Figure 2 depicts a high‐level conceptual platform architecture with the following major components:
Figure 2.
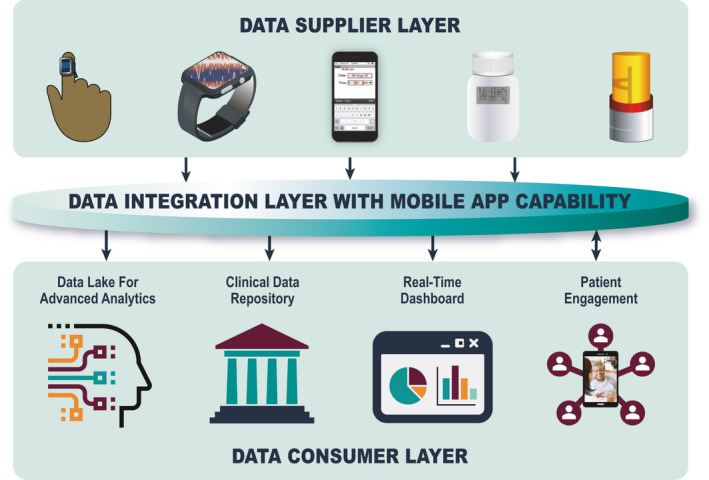
High‐level conceptual digital platform architecture.
Data supplier layer
This layer is focused on an architecture to enable acquiring data from multiple types of devices, mobile apps and sensors using multiple modes of transmission (e.g., Bluetooth, Near Field Communication, etc.) and with appropriate data encryption and security measures in place to protect data privacy. For mobile apps, support for multiple operating systems (e.g., iOS, Android) is preferable, particularly to support a “bring your own device” capability where subjects use their own device for data collection. 89
Data integration layer with mobile app capability
This layer is focused on the ability to integrate data from multiple devices in such a way that the data are interoperable and reusable for future use. If multiple streams of data collect similar information (e.g., time of dose via eDiary and also via a smart package), this layer should ensure there is a clear distinction between primary and secondary data sources as predetermined by study requirements. This layer can also serve data to the consumer layer using an application programming interface to ensure a flexible and extensible architecture and should be tightly integrated with any mobile app(s) used for data collection, such that there is no time lag between data being fetched via the mobile app and data visibility in the platform. Mobile apps can integrate with this layer both to collect data as well as to provide a mechanism for two‐way communication between the patient and clinical site staff. If desired, this layer should also allow for easy plug‐and‐play of interactive/gaming apps as tools for increased patient engagement and motivation to complete all assigned tasks per protocol requirements.
Data consumer layer
This layer is focused on redirecting incoming high‐volume digital data into the following: (i) dashboard enabling rapid decision making through quick visualization and analytics during the study, (ii) clinical data repository with processed data focused on standard reporting and data that will be reported to regulatory agencies (i.e., official data repository), (iii) data lake for storing raw data for exploratory analyses; efficiently combining, finding, and reusing data will be possible only if this component captures metadata, adhering to standard ontologies, 90 and (iv) interactive apps focused on improving patient engagement and adherence.
Patient engagement and ensuring patient compliance with use of these devices to provide data is key to implementation, and it is equally critical to reduce burden on clinicians and contract research organizations operating these technologies. Hence, there should be focus on both patient and clinician user experiences while using these tools to reduce any added burden. Furthermore, we envision that such a platform can be utilized as a tool for delivering digital content to patients (e.g., instructional materials, ePROs, and study‐specific apps), for eConsent, patient reminders (e.g., to take study medication, for study‐related tasks, or for upcoming clinical visits) as a tool to more closely engage with the patient throughout the course of the trial (e.g., through mobile apps, secure messaging, and/or videoconferencing), and for telehealth visits, depending on the needs of the study. There are several platform providers that offer such capabilities, 77 , 91 , 92 and we have tested several of these capabilities from various providers and are introducing these approaches into trials dependent on study need.
DISCUSSION
There is profound potential for innovative technologies to address limitations and gaps in the current clinical trial paradigm. The digitally enabled clinical trials initiative at Merck is aimed at increasing patient‐centricity, reducing patient burden, enriching clinical trial data sets, and augmenting decision making through the use of digital health technologies, outpatient sampling, and real‐time data access. Areas of focus include (i) use of technologies that can provide greater confidence in outpatient dosing data and improve adherence to therapy, (ii) user‐friendly methods of outpatient PK, PD, or biomarker sampling coupled with automated date/time stamps, (iii) use of technologies that can provide more objective, sensitive, and/or higher resolution safety and efficacy data, and (iv) use of digital platform solutions that can provide opportunities for real‐time data access, closer engagement with the patient during the trial, and opportunities for virtual visits. Although the inclusion of any one of these components in clinical trials can be beneficial for clinical research, there is synergistic power in implementation of these enabling technologies jointly within a trial for further improved elucidation and understanding of the PK profile, safety, and efficacy of the IND and the corresponding exposure‐response relationships. For example, inclusion of at‐home PK samples can augment PK datasets, enhance understanding of an IND’s PK profile, and enable collection of data at critical points in the PK profile that may have otherwise not been possible. However, pairing outpatient PK sampling with smart dosing approaches can be even more powerful, as population PK analyses rely on not only drug concentration measurements and time of PK sampling, but also knowledge of the dose administered, adherence patterns, and if/when doses have been taken. Without accurate understanding of adherence, pharmacometricians need to rely on either assumed or (often inaccurate) patient‐reported information on time of dosing in between visits, which contributes to inflated residual (unexplained) variability in population PK analysis. Although inclusion of smart dosing or outpatient PK sampling approaches can reduce variability in PK analyses, implementing these approaches together within a trial can do so to an even greater degree, therein enabling improved understanding of the true pharmacokinetic profile of the drug. Similarly, whereas inclusion of digital biomarker‐enabling technologies can facilitate collection of more frequent, objective, and sensitive data, including in between clinical visits, and tremendously increase understanding of the drug’s safety and efficacy, these data will remain difficult to decouple from adherence if the patient’s true adherence patterns are not known. Thus, it is our vision that clinical trials shift toward a digitally enabled, patient‐centric paradigm where smart dosing approaches, outpatient sampling, digital biomarker‐enabling technologies, and digital platforms are utilized jointly within clinical trials with emphasis on true transformation and augmentation of trials vs. sparse inclusion of “add‐ons” or digital substitutes. In addition to enabling increased understanding of the course of disease and properties of the IND, such an approach may also reduce the frequency of clinical visits and patient burden, improve patient recruitment and retention, offer less painful blood sampling options for patients, and provide increased study support and communication options with clinical trial sites (Figure 3 ).
Figure 3.

Digitally enabled clinical trials and potential benefits.
Merck has previously reported our experiences with implementation of dried blood microsampling in in‐clinic and at‐home settings 36 , 37 , 38 , 39 , 47 , 48 as well as results of phase I studies intended to evaluate technologies of interest. 36 , 37 , 77 Such technology evaluation trials are an important piece of enabling adoption of new technologies into the clinical trial paradigm and realizing the vision of patient‐centric trials. These types of trials provide the ability to rapidly sift through emerging technologies to identify which are useful for clinical research, enable rapid “learn and confirm” cycles for emerging technologies to become “ready” for use in portfolio‐facing pharmaceutical clinical trials, and provide insights into patient preferences and ease of use. It is imperative that experience with digital technologies of interest is gained well in advance of portfolio‐facing trial inclusion to ensure selection and inclusion of the appropriate technologies to address study needs, the appropriate level of validation/qualification for the context of use (e.g., internal decision making vs. registration), and that these tools can be easily deployed and used without delaying, hindering, or compromising the trial. 93 , 94 Furthering our efforts in digitally enabled trials, we have continued to pilot technologies in areas of interest, and plan for use of smart dosing, outpatient sampling, and digital biomarker‐enabling technologies in upcoming late‐phase clinical trials.
Although there is a multitude of benefits for use of digital and outpatient sampling technologies in clinical trials, there are also several obstacles and challenges to adoption and fully realizing the vision of patient‐centric, digitally enabled trials. It is not a simple endeavor to utilize any of the aforementioned technologies in a clinical trial setting—significant upfront investment is required. Not only must one ensure that the technology performs as expected, but that its use will not delay or otherwise adversely affect the planned trial. Risk mitigation efforts for technology use requires resources as well. Furthermore, it takes a long time to generate data from clinical trials, and those data may not always be successful in proving the intended hypothesis. This can result in a near‐term negative return on investment, which can be difficult for senior company leadership to buy into. 93 Other challenges to adoption include questions around ensuring patient privacy, added operational and logistical complexities, lack of prior experience, software version changes during use, and regulatory unknowns. Additionally, many emerging technologies currently lack sufficient clinical validation for reliance on these tools as primary source data. Furthermore, while attempting to minimize patient burden and reduce clinical trial visits, there is potential to add burden if at‐home assessments are too frequent or overly complex, if there are too many devices provided, or if the selected technologies are ones that patients are not amenable to using. Thus, user experience for potential digital health tools, as well as the clinical trial as a whole, must be considered when selecting technologies for inclusion in a trial. Whereas there are substantial challenges to fully realizing the opportunity of these emerging technologies, the benefits could be profound and transformational. Our progress and success to date has been enabled by a culture that is comfortable embracing innovation, coupled with senior leadership that believes in the vision of what digital health technologies can offer our patients and our trials.
There have been a growing number of precompetitive consortiums and scientific societies with dedicated efforts focused on moving the needle and taking on challenges related to the use of digital technologies in both drug development and for patient care (Table 4 ). Collaboration in a precompetitive fashion, including among pharmaceutical companies, regulators, academia, and technology companies, is what will be needed to truly shift toward digitally enabled, patient‐centric trials. Areas of potential collaboration include sharing of experiences, joint development of white papers and best practice documents, precompetitive technology development, verification and validation experimentation, disease area‐specific development of end points, and dialogues on clarity of regulatory and payer expectations. 8 , 64 , 95
Table 4.
Precompetitive consortiums, scientific societies, and nonprofit organizations related to digital health
| Group/initiative | Description |
|---|---|
| Digital Medicine Society (DiMe) | Professional society for the digital medicine community, aimed at driving scientific progress and broad acceptance of digital medicine to enhance public health |
| TransCelerate BioPharma Patient Technology Initiative | TransCelerate BioPharma Inc. is a nonprofit organization with a mission to collaborate across the global biopharmaceutical research and development community to identify, prioritize, design and facilitate implementation of solutions designed to drive the efficient, effective, and high‐quality delivery of new medicines. The Patient Technology Initiative strives to enable and accelerate patient‐facing technology in support of an improved patient experience and richer data collection in clinical trials. |
| Patient Centric Sampling Interest Group | Collaborative group across organizations in non‐competitive mutual areas of interest related to patient‐centric sampling |
| IQ Consortium Patient‐Centric Sampling Working Group |
The International Consortium for Innovation and Quality in Pharmaceutical Development is a technically‐focused organization of pharmaceutical and biotechnology companies with a mission of advancing science and technology to augment the capability of member companies to develop transformational solutions that benefit patients, regulators, and the broader research and development community. The goal of the patient centric sampling working group is to encourage the implementation of patient centric sampling within the industry to provide richer data sets and gain wider acceptance with regulators |
| Open Wearables Initiative (OWEAR) | Collaboration designed to promote the effective use of high‐quality, sensor‐generated measures of health in clinical research through the open sharing of algorithms and data sets |
| Adherence Measurement Institute | Nonprofit organization that is passionate about the need of more precise patient drug dosing data |
| Critical Path for Parkinson’s (CPP) Digital Drug Development Tools (3DT) Initiative | Precompetitive collaboration amongst a subset of CPP member organizations with the goal of optimizing the efficiency of paths for developing digital tools for Parkinson's disease drug development |
| Innovative Medicines Initiative (IMI) Projects | IMI is a European initiative public‐private partnership in the field of pharmaceutical research. There are several IMI projects related to digital health with specific areas of focus, including projects related to developing new ways of remotely monitoring disease and relapse in Alzheimer’s disease (RADAR‐AD) and in central nervous system disorders (RADAR‐CNS), exploring the potential of digital technologies for use in decentralized trials (Trials@Home), and aimed at connecting digital mobility assessments to clinical outcomes for regulatory and clinical endorsement (Mobilise‐D) |
| Clinical Trials Transformation Initiative (CTTI) | Comprises organizations from across the clinical trial enterprise, with a mission of developing and driving adoption of practices that will increase the quality and efficiency of clinical trials. CTTI develops recommendations and resources related to digital health technologies and trials |
As the world becomes increasingly digital, it is a matter of when, not if, such approaches are adopted more standardly in the clinical trial paradigm. Furthermore, increased adoption of digital approaches, remote monitoring, and virtual visits has become a necessity due to changing circumstances, such as social distancing requirements from the coronavirus disease 2019 (COVID‐19) pandemic. This unprecedented crisis also presents an opportunity to further reduce patient burden, gain acceptance for telemedicine, and enhance already existing infrastructure of connectivity by enabling logistics and regulations that are needed to make these work, 96 and in fact, there has been regulatory support for expansion of the availability of digital health technologies during the COVID‐19 pandemic as well as encouragement to evaluate alternate methods for in‐person visits (including virtual visits). 97 , 98 , 99
Digital health and outpatient sampling technologies have the potential to shift the paradigm toward more efficient, informative, and patient‐centric clinical trials, and we expect that the industry is headed toward a future where use of such tools is expected and required in drug development trials vs. “nice to have” inclusions. Merck’s digitally enabled trials initiative seeks to shift clinical trials toward this future and is open to sharing our experiences in these areas so that the broader community can benefit, and wider adoption and regulatory acceptance will ensue.
Funding
This review article was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Conflict of Interest
All authors are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock/stock options in Merck & Co., Inc., Kenilworth, NJ, USA.
Acknowledgment
The authors thank Sheila Erespe (Merck & Co., Inc., Kenilworth, NJ, USA) for editorial assistance.
References
- 1. Unger, J.M. , Vaidya, R. , Hershman, D.L. , Minasian, L.M. & Fleury, M.E. Systematic review and meta‐analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J. Natl. Cancer Inst. 111, 245–255 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson, A. , Borfitz, D. & Getz, K. Global public attitudes about clinical research and patient experiences with clinical trials. JAMA Netw. Open 1, e182969 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Memorial Sloan Kettering Cancer Center . Despite pressing need, survey finds most Americans unlikely to enroll in clinical trials. ScienceDaily <https://www.sciencedaily.com/releases/2016/05/160523105038.htm> (2016). Accessed April 12, 2020.
- 4. Brundisini, F. , Giacomini, M. , DeJean, D. , Vanstone, M. , Winsor, S. & Smith, A. Chronic disease patients’ experiences with accessing health care in rural and remote areas: a systematic review and qualitative meta‐synthesis. Ont. Health Technol. Assess. Serv. 13, 1–33 (2013). [PMC free article] [PubMed] [Google Scholar]
- 5. Clark, L.T. et al. Increasing diversity in clinical trials: overcoming critical barriers. Curr. Problems Cardiol. 44, 148–172 (2019). [DOI] [PubMed] [Google Scholar]
- 6. Blaschke, T.F. , Osterberg, L. , Vrijens, B. & Urquhart, J. Adherence to medications: Insight arising from studies on the unreliable link between prescribed and actual drug dosing histories. Ann. Rev. Pharmacol. Toxicol. 52, 275–301 (2012). [DOI] [PubMed] [Google Scholar]
- 7. Breckenridge, A. , Aronson, J.K. , Blaschke, T.F. , Hartman, D. , Peck, C.C. & Vrijens, B. Poor medication adherence in clinical trials: consequences and solutions. Nat. Rev. Drug Discov. 16, 149–150 (2017). [DOI] [PubMed] [Google Scholar]
- 8. Kothare, P.A. , Jadhav, P.R. , Gupta, P. , Harrelson, J.C. & Dickmann, L. Harnessing the potential of emerging digital health and biological sampling technologies for clinical drug development: promise to reality. Clin. Pharmacol. Ther. 104, 1125–1135 (2018). [DOI] [PubMed] [Google Scholar]
- 9. Rosa, C. , Campbell, A.N. , Miele, G.M. , Brunner, M. & Winstanley, E.L. Using e‐technologies in clinical trials. Contemp. Clin. Trials. 45(Pt A), 41–54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moyer, M. Patient technology adoption in clinical trials: 4 barriers holding us back. Clinical Leader <https://www.clinicalleader.com/doc/patient‐technology‐adoption‐in‐clinical‐trials‐barriers‐holding‐us‐back‐0001> (2019). Accessed April 13, 2020.
- 11. Beyor, N. , Close, K. , Hakim, N. , Shapiro, M. , Ringel, M. & Smith, B. A Digital Redesign for Clinical Trials. BCG<https://www.bcg.com/publications/2019/digital‐redesign‐clinical‐trials.aspx> (2019). Accessed April 12, 2020.
- 12. Digital Medicine Society . DiME’s Library of Digital Endpoints. DiME endpoint library <https://www.Dimesociety.org/index.php/knowledge‐center/library‐of‐digital‐endpoints> (2019). Accessed April 15, 2020.
- 13. Marra, C. et al. Quantifying the use of connected digital products in clinical research. NPJ Digit. Med. 3, 50 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clinical Trial Transformation Initiative . CTTI feasibility Studies Database <https://feasibility‐studies.ctti‐clinicaltrials.org/> (2020). Accessed August 6, 2020.
- 15. World Health Organization . Adherence to Long‐Term Therapies. Evidence for action <https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1> (2003). Accessed April 13, 2020.
- 16. Singh, A.C. , Massey, A.J. , Thompson, M.D. , Rappa, L.R. & Honeywell, M.S. Addressing nonadherence in the schizophrenic population. J. Pharm. Pract. 19, 361–368 (2006). [Google Scholar]
- 17. Kane, J.M. Review of treatments that can ameliorate nonadherence in patients with schizophrenia. J. Clin. Psychiatry 67(suppl. 5), 9–14 (2006). [PubMed] [Google Scholar]
- 18. Borras, L. , Mohr, S. , Brandt, P.Y. , Gillieron, C. , Eytan, A. & Huguelet, P. Religious beliefs in schizophrenia: their relevance for adherence to treatment. Schizophr. Bull. 33, 1238–1246 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heerey, E.A. & Gold, J.M. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J. Abnorm. Psychol. 116, 268–278 (2007). [DOI] [PubMed] [Google Scholar]
- 20. Turner, M. , Masand, P. , Roca, M. & Kane, J. Results of a study on psychiatrists’ perceptions of adherence to medication among patients with schizophrenia: the ADHES survey. Eur. Neuropsychopharmacol. 17 (suppl. 4), s455–s456 (2007). Poster Presented at ECNP. October 13–17 2007. Vienna, Austria. [Google Scholar]
- 21. McCann, D.J. , Petry, N.M. , Bresell, A. , Isacsson, E. , Wilson, E. & Alexander, R.C. Medication nonadherence, “professional subjects”, and apparent placebo responders: overlapping challenges for medications development. J. Clin. Psychopharmacol. 35, 566–573 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Denus, S. et al. Spironolactone metabolites in TOPCAT – new insights into regional variation. N. Engl. J. Med. 376, 1690–1692 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marrazzo, J.M. et al. Tenofovir‐based preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 372, 509–518 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stirratt, M.J. et al. Advancing the science and practice of medication adherence. J. Gen. Intern. Med. 33, 216–222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merchant, R.K. et al. Effectiveness of population health management using the propeller health asthma platform: a randomized clinical trial. J. Allergy Clin. Immunol. Pract. 4, 455–463 (2016). [DOI] [PubMed] [Google Scholar]
- 26. Merchant, R.K. et al. Impact of a digital health intervention on asthma resource utilization. World Allergy Organ. J. 11, 28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mulleners, W.M. , Whitmarsh, T.E. & Steiner, T.J. Noncompliance may render migraine prophylaxis useless, but once‐daily regimens are better. Cephalgia 18, 52–56 (1998). [DOI] [PubMed] [Google Scholar]
- 28. U.S. Food &, Drug Administration . Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biologic Products, Guidance for Industry, FDA, March 15, 2019 <https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/enrichment‐strategies‐clinical‐trials‐support‐approval‐human‐drugs‐and‐biological‐products> (2019). Accessed April 13, 2020.
- 29. Bain, E. et al. Use of a novel artificial intelligence platform on mobile devices to assess dosing compliance in a phase 2 clinical trial in subjects with Schizophrenia. JMIR Mhealth Uhealth. 5, e18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goeldner, C. , Shafner, L. & Umbricht, D. Assessing the Value of an IA Platform During the Screening Period to Evaluate and Predict Adherence to Study Drug Intake During Treatment in an Ongoing Proof Of‐Concept Phase 2 Study in Schizophrenia. Poster presented at 14th Annual Scientific Meeting of International Society for CNS Clinical Trials and Methodology (ISCTM). February 20–22, 2018. Washington, DC, USA (2018).
- 31. Shafner, L. , Abt, M. , Kinch, R. , Tamburri, P. , Umbricht, D. & Hanina, A. Using an artifiical intelligence platform on mobile devices to monitor and increase adherene in subjects with schizophrenia. Poster presented at ASCP. May 29‐June 2, 2017. Miami, FL. W68. (2017).
- 32. Labovitz, D.L. et al. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke 48, 1416–1419 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eliasson, L. et al. How the EMERGE guideline on medication adherence can improve the quality of clinical trials. Br. J. Clin. Pharmacol. 86, 687–697 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mallayasamy, S. et al. A systematic evaluation of effect of adherence patterns on the sample size and power of a clinical study. CPT Pharmacometrics Syst. Pharmacol. 7, 818–828 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shiovitz, T.M. et al. Mitigating the effects of nonadherence in clinical trials. J. Clin. Pharmacol. 56, 1151–1164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dockendorf, M.F. et al. Leveraging digital health technologies and outpatient sampling in clinical drug development: a phase I exploratory study. Clin. Pharmacol. Ther. 105, 168–176 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dockendorf, M.F. et al. Smart trials: assessment of at‐home sampling and digital health technologies in a clinical pilot trial. Presentation and poster presented at annual ASCPT meeting. March 23, 2018. Orlando, FL <https://www.ascpt.org/Meetings/Past‐Future‐Annual‐Meetings/ASCPT‐2018/Friday‐March‐23> (2018). Accessed April 21, 2020.
- 38. Kothare, P.A. et al. An integrated strategy for implementation of dried blood spots in clinical development programs. AAPS J. 18, 519–527 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li, C.‐C. et al. Population PK analyses of Ubrogepant (MK‐1602), a CGRP receptor antagonist: enriching in‐clinic plasma PK sampling with outpatient dried blood spot sampling. J. Clin. Pharmacol. 58, 294–303 (2018). [DOI] [PubMed] [Google Scholar]
- 40. Guthrie, R. & Susi, A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 32, 338–343 (1963). [PubMed] [Google Scholar]
- 41. Capiau, S. et al. Official international association for therapeutic drug monitoring and clinical toxicology guideline: development and validation of dried blood spot‐based methods for therapeutic drug monitoring. Ther. Drug Monit. 41, 409–430 (2019). [DOI] [PubMed] [Google Scholar]
- 42. Centers for Disease Control and Prevention . Shipping Guidelines for Dried‐Blood Spot Specimens. Updated October 24, 2017 <https://www.cdc.gov/labstandards/pdf/nsqap/Bloodspot_Transportation_Guidelines.pdf> (2017). Accessed April 15, 2020.
- 43. Cámara‐Lemarroy, C.R. , Rodriguez‐Gutierrez, R. , Monreal‐Robles, R. & Marfil‐Rivera, A. Gastrointestinal disorders associated with migraine: a comprehensive review. World J. Gastroenterol. 22, 8149–8160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stone, A.A. , Shiffman, S. , Schwartz, J.E. , Broderick, J.E. & Hufford, M.R. Patient compliance with paper and electronic diaries. Control Clin. Trials 24, 182–199 (2003). [DOI] [PubMed] [Google Scholar]
- 45. Denniff, P. & Spooner, N. Volumetric absorptive microsampling: a dried sample collection technique for quantitative bioanalysis. Anal. Chem. 86, 8489–8495 (2014). [DOI] [PubMed] [Google Scholar]
- 46. Xie, I. et al. Extractability‐mediated stability bias and hematocrit impact: High extraction recovery is critical to feasibility of volumetric adsorptive microsampling (VAMS) in regulated bioanalysis. J. Pharm. Biomed. Anal. 156, 58–66 (2018). [DOI] [PubMed] [Google Scholar]
- 47. Anderson, M. , Dockendorf, M.F. , Xue, L. , Dreyer, D. , Xie, I. & Bateman, K.P. Enabling patient centricity in clinical development through at‐home sample collection. Poster presented at 4th FDA/PQRI Conference on Advancing Product Quality: Patient‐Centric Product Design, Drug Development, and Manufacturing. April 9–11, 2019 <https://pqri.org/4th‐pqri‐fda‐conference‐on‐advancing‐product‐quality‐posters/> (2019). Accessed April 21, 2020.
- 48. Yee, K.L. et al. Pharmacokinetics, safety, and tolerability of long‐acting parenteral intramuscular injection formulations of doravirine. J. Clin. Pharm. Therapeut. 45, 1098–1105 (2020). [DOI] [PubMed] [Google Scholar]
- 49. U.S. Food & Drug Administration . Bioanalytical Method Validation Guidance for Industry, May 2018 <https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/bioanalytical‐method‐validation‐guidance‐industry> (2018). Accessed April 15, 2020.
- 50. Freeman, J.D. , Rosman, L.M. , Ratcliff, J.D. , Strickland, P.T. , Graham, D.R. & Silbergeld, E.K. State of the science in dried blood spots. Clin. Chem. 64, 656–679 (2018). [DOI] [PubMed] [Google Scholar]
- 51. Lim, M.D. Dried blood spots for global health diagnostics and surveillance: opportunities and challenges. Am. J. Trop. Med. Hyg. 99, 256–265 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Razavi, M. , Anderson, N.L. , Yip, R. , Pope, M.E. & Pearson, T.W. Multiplexed longitudinal measurement of protein biomarkers in DBS using an automated SISCAPA workflow. Bioanalysis 8, 1597–1609 (2016). [DOI] [PubMed] [Google Scholar]
- 53. Barnabas, R.V. , Revill, P. , Tan, N. & Phillips, A. Cost‐effectiveness of routine viral load monitoring in low‐ and middle‐income countries: a systematic review. J. Int. AIDS Soc. 20 (suppl. 7), e25006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang, J. , Li, D. , Wiltse, A. , Emo, J. , Hilchey, S.P. & Zand, M.S. Application of volumetric absorptive microsampling (VAMS) to measure multidimensional anti‐influenza IgG antibodies by the mPlex‐Flu assay. J. Clin. Transl. Sci. 3, 332–343 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Snyder, A. , Morrissey, M.P. & Hellmann, M.D. Use of circulating tumor DNA for cancer immunotherapy. Clin. Cancer Res. 25, 6909–6915 (2019). [DOI] [PubMed] [Google Scholar]
- 56. Heider, K. et al. Detection of ctDNA from dried blood spots after DNA size selection. Clin. Chem. 66, 697–705 (2019). [DOI] [PubMed] [Google Scholar]
- 57. Dockendorf, M.F. et al. A model‐based approach to bridging plasma and dried blood spot concentration data for phase 3 verubecestat trials. Presented at American Conference on Pharmacometrics (ACoP) meeting. October 7–10, 2018. San Diego, CA <https://www.simulations‐plus.com/assets/2018‐BACE‐ACoP‐Poster_FINAL‐1.pdf> (2018). Accessed April 21, 2020. [DOI] [PubMed]
- 58. Tomko, R.L. et al. Methods to reduce the incidence of false negative trial results in substance use treatment research. Curr. Opin. Psychol. 30, 35–41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. U.S. Food & Drug Administration . Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry <https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/enhancing‐diversity‐clinical‐trial‐populations‐eligibility‐criteria‐enrollment‐practices‐and‐trial> (2019). Accessed April 15, 2020.
- 60. U.S. Food &, Drug Administration . About Biomarkers and Qualification <https://www.fda.gov/drugs/cder‐biomarker‐qualification‐program/about‐biomarkers‐and‐qualification> (2019). Accessed April 15, 2020.
- 61. FDA‐NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet]. Silver Spring (MD): Food and Drug Administration (US); co‐published by National Institutes of Health (US), Bethesda (MD) <https://www.ncbi.nlm.nih.gov/books/NBK326791> (2016). Accessed April 20, 2020. [PubMed] [Google Scholar]
- 62. European Medicines Agency . Questions and answers: qualification of digital technology‐based methodologies to support approval of medicinal products. June 1, 2020. EMA/219860/2020 <https://www.ema.europa.eu/en/documents/other/questions‐answers‐qualification‐digital‐technology‐based‐methodologies‐support‐approval‐medicinal_en.pdf> Accessed August 7, 2020.
- 63. Coravos, A. et al. Digital medicine: a primer on measurement. Digit. Biomark 3, 31–71 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Coravos, A. , Khozin, S. & Mandl, K.D. Developing and adopting safe and effective digital biomarkers to improve patient outcomes. NPJ Digit. Med. 2, 14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. The SPHERE Parkinson’s Measuring Project . The Cure Parkinson’s Trust website <https://www.cureparkinsons.org.uk/news/sphere‐pd‐measuring> Accessed May 20, 2020.
- 66. Zhao, M. , Yue, S. , Katabi, D. , Jaakkola, T. & Bianchi, M. Learning Sleep Stages from Radio Signals: A Conditional Adversarial Architecture. Poster presented at 34th International Conference on Machine Learning (ICML). August 7–9, 2017. Sydney, Australia. (2017).
- 67. Kabelac, Z. et al. Passive monitoring at home: a pilot study in Parkinson disease. Digit. Biomarkers 3, 22–30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bellerophon Announces Agreement with FDA on Regulatory Approval Pathway for INOpulse for Treatment of Pulmonary Hypertension Associated with Interstitial Lung Disease. Bellerophon. Press release <http://investors.bellerophon.com/news‐releases/news‐release‐details/bellerophon‐announces‐agreement‐fda‐regulatory‐approval‐pathway> (2019). Accessed April 15, 2020.
- 69. Haberkamp, M. et al. European regulators' views on a wearable‐derived performance measurement of ambulation for Duchenne muscular dystrophy regulatory trials. Neuromuscul. Disord. 29, 514–516 (2019). [DOI] [PubMed] [Google Scholar]
- 70. European Medicines Agency . Qualification opinion on stride velocity 95th centile as a secondary endpoint in Duchenne Muscular Dystrophy measured by a valid and suitable wearable device. April 26, 2019 <https://www.ema.europa.eu/en/documents/scientific‐guideline/qualification‐opinion‐stride‐velocity‐95th‐centile‐secondary‐endpoint‐duchenne‐muscular‐dystrophy_en.pdf> (2019). Accessed April 15, 2020.
- 71. Smith, M.P. et al. Emerging methods to objectively assess pruritus in atopic dermatitis. Dermatol. Ther. 9, 407–420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Filardi, M. , Pizza, F. , Martoni, M. , Vandi, S. , Plazzi, G. & Natale, V. Actigraphic assessment of sleep/wake behavior in central disorders of hypersomnolence. Sleep Med. 16, 126–130 (2015). [DOI] [PubMed] [Google Scholar]
- 73. Izmailova, E.S. , Wagner, J.A. & Perakslis, E.D. Wearable devices in clinical trials: hype and hypothesis. Clin. Pharmacol. Ther. 104, 42–52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li, X. et al. Digital health: tracking physiomes and activity using wearable biosensors reveals useful health‐related information. PLoS Biol. 15, e2001402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Barrett, P.M. et al. Comparison of 24‐hour Holter monitoring with 14‐ day novel adhesive patch electrocardiographic monitoring. Am. J. Med. 127, e11–e17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bent, B. , Goldstein, B.A. , Kibbe, W.A. & Dunn, J.P. Investigating sources of inaccuracy in wearable optical heart rate sensors. NPJ Digit. Med. 3, 18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huang, Q. et al. “In‐House” data on the outside—a mobile health approach. Clin. Pharmacol. Ther. 107, 948–956 (2020). [DOI] [PubMed] [Google Scholar]
- 78. Kourtis, L.C. , Regele, O.B. , Wright, J.M. & Jones, G.B. Digital biomarkers for Alzheimer’s disease: the mobile/wearable devices opportunity. NPJ Digit. Med. 2, 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pissadaki, E.K. et al. Decomposition of complex movements into primitives for Parkinson's disease assessment. IBM J. Res. Develop. 62, 5:1–5:11 (2018). [Google Scholar]
- 80. Erb, M.K. et al. mHealth and wearable technology should replace motor diaries to track motor fluctuations in Parkinson’s disease. NPJ Digit. Med. 3, 6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Silva de Lima, A.L. et al. Large‐scale wearable sensor deployment in Parkinson’s patients: the Parkinson@Home Study Protocol. JMIR Res. Protoc. 5, e172 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cedarbaum, J.M. et al. Enabling efficient use of digital health technologies to support Parkinson's disease drug development through precompetitive collaboration. Presented at the American Society for Clinical Pharmacology & Therapeutics (ASCPT) Meeting. March 13–16, 2019. Washington, DC <https://c‐path.org/wp‐content/uploads/2019/09/cpp_mds_2019_final_revised.pdf> (2019). Accessed April 21, 2020.
- 83. Silva de Lima, A.L. et al. Feasibility of large‐scale deployment of multiple wearable sensors in Parkinson’s disease. PLoS One 12, e0189161 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chen, R. et al. Developing Measures of Cognitive Impairment in the Real World from Consumer‐Grade Multimodal Sensor Streams. In Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining (KDD ’19). Association for Computing Machinery, New York, NY, USA, 2145–2155. 10.1145/3292500.3330690 (2019). Accessed April 15, 2020. [DOI] [Google Scholar]
- 85. Berisha, V. et al. Tracking Discourse Complexity Preceding Alzheimer’s Disease Diagnosis: A Case Study Comparing the Press Conferences of Presidents Ronald Reagan and George Herbert Walker Bush. J. Alzheimers Dis. 45, 959–963. 10.3233/JAD-142763 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Brinker, S. Martec’s Law: Technology changes exponentially, organizations change logarithmically. Chiefmartec Website <https://chiefmartec.com/2013/06/martecs‐law‐technology‐changes‐exponentially‐organizations‐change‐logarithmically/> (2013). Accessed April 15, 2020.
- 87. Tabatabai, A. Where top VCs are investing in digital health. Techcrunch Website <https://techcrunch.com/2019/12/16/where‐top‐vcs‐are‐investing‐in‐digital‐health/> (2019). Accessed April 15, 2020.
- 88. Wilkinson, M.D. et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 3, 160018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Faulds, M.C. et al. The feasibility of using ‘bring your own device’ (BYOD) technology for electronic data capture in multicenter medical audit and research. Anaesthesia 71, 58–66 (2016). [DOI] [PubMed] [Google Scholar]
- 90. Badawy, R. et al. Metadata concepts for advancing the use of digital health technologies in clinical research. Digit. Biomark. 3, 116–132 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Saxena, A. & Ramaswamy, N. Digital Reimagination of Clinical Trials. (2020) p. 1–7 <https://www.tcs.com/content/dam/tcs/pdf/Industries/life‐sciences‐and‐healthcare/Digital%20Reimagination%20of%20Clinical%20Trials.pdf>. Accessed August 7, 2020.
- 92. Medable, Inc. The Centricity of Decentralizing: Breaking Down the Basics of Decentralized Clinical Trials. p. 1–6 (2020) <https://f.hubspotusercontent10.net/hubfs/7482664/Centricity%20of%20Decentralizing%20Whitepaper%20061520%2003.pdf> Accessed August 7, 2020.
- 93. Polhemus, A.M. et al. Accelerating adoption of patient‐facing technologies in clinical trials: a pharmaceutical industry perspective on opportunities and challenges. Ther. Innov. Regul. Sci. 53, 8–24 (2019). [DOI] [PubMed] [Google Scholar]
- 94. Clinical Trials Transformation Initiative . CTTI Recommendations: Optimizing Mobile Clinical Trials by Engaging Patients and Sites. February 21, 2019 <https://www.ctti‐clinicaltrials.org/sites/www.ctti‐clinicaltrials.org/files/ctti_recommendations_‐_mct_engaging_patients_and_sites_final.pdf> (2019). Accessed May 20, 2020.
- 95. Deore, U. et al. A Shared Perspective of Patient Technology Implementation in Clinical Trials <http://transceleratebiopharmainc.com/wp‐content/uploads/2020/03/TransCelerate_PT_SIK_Concluding‐Insights‐Paper_March‐2020.pdf> (2020). Accessed May 20, 2020.
- 96. Bashshur, R. , Doarn, C.R. , Frenk, J.M. , Kvedar, J.C. & Woolliscroft, J.O. Telemedicine and the COVID‐19 pandemic, lessons for the future. Telemed. e‐Health 26, 571–573 (2020). [DOI] [PubMed] [Google Scholar]
- 97. U.S. Food & Drug Administration . Enforcement Policy for Health Devices for Treating Psychiatric Disorders During the Coronavirus Disease 2019 (COVID‐19) Public Health Emergency. Guidance for Industry and Food and Drug Administration Staff. April 2020 <https://www.fda.gov/media/136939/download> (2020). Accessed April 21, 2020.
- 98. U.S. Food &, Drug Administration . FDA’s Digital Health Policies Allow Innovators to Create COVID‐19Related Public Health Solutions. Digital Health Policies and Public Health Solutions for COVID‐19 <https://www.fda.gov/medical‐devices/digital‐health/digital‐health‐policies‐and‐public‐health‐solutions‐covid‐19> (2020). Accessed April 21, 2020.
- 99. U.S. Food & Drug Administration . FDA guidance on conduct of clinical trials of medicinal products during trials of medicinal products during COVID19 Public Health Emergency. Guidance for Industry, Investigators and Institutional Review Boards. March 2020. Updated July 2, 2020 <https://www.fda.gov/media/136238/download> (2020). Accessed August 7, 2020.


