Abstract
Background: The present study aimed to determine the global prevalence of anosmia and dysgeusia in coronavirus disease 2019 (COVID-19) patients and to assess their association with severity and mortality of COVID-19. Moreover, this study aimed to discuss the possible pathobiological mechanisms of anosmia and dysgeusia in COVID-19.
Methods: Available articles from PubMed, Scopus, Web of Science, and preprint databases (MedRxiv, BioRxiv, and Researchsquare) were searched on November 10th, 2020. Data on the characteristics of the study (anosmia, dysgeusia, and COVID-19) were extracted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. Newcastle–Ottawa scale was used to assess research quality. Moreover, the pooled prevalence of anosmia and dysgeusia were calculated, and the association between anosmia and dysgeusia in presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was assessed using the Z test.
Results: Out of 32,142 COVID-19 patients from 107 studies, anosmia was reported in 12,038 patients with a prevalence of 38.2% (95% CI: 36.5%, 47.2%); whereas, dysgeusia was reported in 11,337 patients out of 30,901 COVID-19 patients from 101 studies, with prevalence of 36.6% (95% CI: 35.2%, 45.2%), worldwide. Furthermore, the prevalence of anosmia was 10.2-fold higher (OR: 10.21; 95% CI: 6.53, 15.96, p < 0.001) and that of dysgeusia was 8.6-fold higher (OR: 8.61; 95% CI: 5.26, 14.11, p < 0.001) in COVID-19 patients compared to those with other respiratory infections or COVID-19 like illness. To date, no study has assessed the association of anosmia and dysgeusia with severity and mortality of COVID-19.
Conclusion: Anosmia and dysgeusia are prevalent in COVID-19 patients compared to those with the other non-COVID-19 respiratory infections. Several possible mechanisms have been hypothesized; however, future studies are warranted to elucidate the definitive mechanisms of anosmia and dysgeusia in COVID-19.
Protocol registration: PROSPERO CRD42020223204.
Keywords: anosmia, COVID-19, dysgeusia, predictor, SARS-CoV-2
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was initially identified in late December 2019 in Wuhan, Hubei Province, Republic of China 1, 2. This viral pandemic rapidly spread worldwide, infecting more than 60 million people, causing more than 1 million deaths 3, and severely affecting the global healthcare system 4, 5. Several drugs have been repurposed for treating COVID-19 5- 9; however, no drug has been recommended or approved by the World Health Organization (WHO). The common symptoms of COVID-19 include dry cough, fever, dyspnea, fatigue, anorexia, diarrhea, chest pain, headache, and muscle ache 10, 11. In particular, two manifestations have been increasingly identified among asymptomatic people that later tested positive for the presence of SARS-CoV-2: anosmia and dysgeusia 12. Remarkably, previous studies reported that these olfactory issues were reported in 11.8% of COVID-19 cases before other symptoms occured 13- 15.
Anosmia, a severe condition of hyposmia, is a part of olfactory dysfunction where the person is unable to sense smell or detect odor 16. Dysgeusia is a sensory dysfunction where the individual loses the perception of taste 17. The British Association of Otorhinolaryngology reported that both dysfunctions varied from 3-20% among COVID-19 patients 18. A previous study among 42 patients revealed that more than a third presented anosmia and dysgeusia 19. A higher percentage of anosmia and dysgeusia cases were also reported 20. Furthermore, another study reported that anosmia in COVID-19 is related to the enlargement of bilateral olfactory bulb edema 21.
This evidence may be crucial in the present COVID-19 pandemic. As the real-time reverse transcriptase polymerase chain reaction (RT-PCR) test has certain limitations for screening, the manifestation of anosmia and dysgeusia could be used as an early warning for practitioners or clinicians to build a rationale to reach a firm conclusion on patients with SARS-CoV-2 infection 22, 23. Additionally, a recent study reported that anosmia and dysgeusia are among the earliest symptoms observed in COVID-19 patients 24; however, in-depth analysis of this dysfunction and its relation to the pathogenesis, severity, and mortality of COVID-19 is missing from the literature. Thus, the present study aimed to summarize the global evidence of anosmia and dysgeusia among COVID-19 patients, in order to assess their association with the severity and mortality of the disease, and provide a comprehensive review related to the possible pathogenesis of anosmia and dysgeusia in SARS-CoV-2 infection.
Methods
Registration and protocol
To comprehensively calculate the cumulative prevalence of anosmia and dysgeusia in SARS-CoV-2 infection worldwide, a systematic review was conducted following guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 25. The protocol of this systematic review has been registered at PROSPERO ( CRD42020223204).
Eligibility criteria of studies
All articles reporting anosmia and dysgeusia as the symptom of COVID-19 were included. COVID-19 case was defined by a positive RT-PCR for SARS-CoV-2 from either nasopharyngeal swab, oropharyngeal swab, bronchoalveolar lavage, or cerebrospinal fluid. All cross-sectional, retrospective, and prospective studies that randomly sampled COVID-19 cases from community or hospitals were considered eligible; whereas case reports and case series, including all editorials, reviews, and commentaries, were excluded. Studies targeting specific groups such as pregnant females, children, and other groups, were excluded. Only articles written in English during 2019-2020 were included.
Information sources and search strategy
Three bibliographical databases (PubMed, Scopus, and Web of Science) and three preprint databases (MedRxiv, BioRxiv, and Researchsquare) were used to identify the potential articles (as of November 10th, 2020). The search criteria were as follows. PubMed ([Title] “SARS-CoV-2” OR “COVID-19” OR “Wuhan coronavirus” OR “Wuhan virus” OR “novel coronavirus” OR “nCoV” OR “severe acute respiratory syndrome coronavirus 2” OR “coronavirus disease 2019 virus” OR “2019-nCoV” OR “2019 novel coronavirus” OR “severe acute respiratory syndrome coronavirus 2” OR “coronavirus” OR “coronaviruses” OR “SARS 2” OR “2019-nCoV acute respiratory disease” OR “novel coronavirus pneumonia” OR “COVID”) AND ([All] “Anosmia” OR “smell loss” OR “smell dysfunction” OR “smell impairment” OR “hyposmia” OR “dysosmia” OR “olfactory dysfunction” OR “olfactory disorder”) AND (“dysgeusia” OR “taste loss” OR “taste dysfunction” OR “taste impairment” OR “gustatory dysfunction” OR “gustatory disorder” OR “hypogeusia” OR “ageusia”). Scopus ([Title] “SARS-CoV-2” OR “COVID-19” OR “Wuhan coronavirus” OR “Wuhan virus” OR “novel coronavirus” OR “nCoV” OR “severe acute respiratory syndrome coronavirus 2” OR “coronavirus disease 2019 virus” OR “2019-nCoV” OR “2019 novel coronavirus” OR “severe acute respiratory syndrome coronavirus 2” OR “coronavirus” OR “coronaviruses” OR “SARS 2” OR “2019-nCoV acute respiratory disease” OR “novel coronavirus pneumonia” OR “COVID”) AND ([All] “Anosmia” OR “smell loss” OR “smell dysfunction” OR “smell impairment” OR “hyposmia” OR “dysosmia” OR “olfactory dysfunction” OR “olfactory disorder”) AND (“dysgeusia” OR “taste loss” OR “taste dysfunction” OR “taste impairment” OR “gustatory dysfunction” OR “gustatory disorder” OR “hypogeusia” OR “ageusia”). Web of Science ([Title] “SARS-CoV-2” OR “COVID-19” OR “Wuhan coronavirus” OR “Wuhan virus” OR “novel coronavirus” OR “nCoV” OR “severe acute respiratory syndrome coronavirus 2” OR “coronavirus disease 2019 virus” OR “2019-nCoV” OR “2019 novel coronavirus” OR “severe acute respiratory syndrome coronavirus 2” OR “coronavirus” OR “coronaviruses” OR “SARS 2” OR “2019-nCoV acute respiratory disease” OR “novel coronavirus pneumonia” OR “COVID”) AND ([All] “Anosmia” OR “smell loss” OR “smell dysfunction” OR “smell impairment” OR “hyposmia” OR “dysosmia” OR “olfactory dysfunction” OR “olfactory disorder”) AND ([All] “dysgeusia” OR “taste loss” OR “taste dysfunction” OR “taste impairment” OR “gustatory dysfunction” OR “gustatory disorder” OR “hypogeusia” OR “ageusia”).
Moreover, we searched the preprint servers MedRxiv, BioRxiv, and Researchsquare for non-peer-reviewed articles. Data were extracted from the articles as well as supplementary materials. Reference lists from the eligible articles were retrieved for further relevant studies.
Study selection and data extraction
The information of identified articles was imported into EndNote X9 (Thompson Reuters, Philadelphia, PA, USA). Duplicates between databases were removed. To identify eligible studies, the retrieved articles were screened based on title and abstract. The potentially eligible studies were then fully reviewed by two authors (MF and JKF). After reviewing the full texts, the eligibility of each study was decided.
Information of study characteristics, study site, study design, number of patients with anosmia, number of patients with dysgeusia, and COVID-19 characteristics such as number of patients, severity, and outcome were collected.
Outcomes
The primary outcomes were: (a) the global incidence of anosmia in COVID-19 patients; (b) the global incidence of dysgeusia in COVID-19 patients; (c) the association of anosmia with the severity of COVID-19; (d) the association of dysgeusia with the severity of COVID-19; (e) the association of anosmia with mortality of COVID-19; and (f) the association of dysgeusia with mortality of COVID-19. Moreover, this review was conducted to provide the possible pathogenesis of anosmia and dysgeusia in SARS-CoV-2 infection.
Data synthesis
The cumulative prevalence rate of anosmia and dysgeusia was calculated for COVID-19 cases by dividing the number of COVID-19 cases with anosmia by the total number of COVID-19 cases with and without anosmia, and was expressed as a percentage (%) with 95% confidence intervals (95% CI). Pooled odds ratios (OR) and 95% CI were calculated to assess the association of anosmia and the occurrence of SARS-CoV-2 compared to non-SARS-CoV-2 respiratory infections. The same method was used for dysgeusia. The pooled OR and 95% CI were presented in a forest plot.
Risk of bias assessment
Critical assessment was conducted for the study setting and diagnosis of SARS-CoV-2 to reduce the bias. The Newcastle-Ottawa scale (NOS) 26 was used as critical appraisals to assess the quality of eligible studies. Prior to analysis, gathered data from studies were evaluated for heterogeneity and potential publication bias.
Statistical analysis
To assess the association between anosmia or dysgeusia and the presence of SARS-CoV-2, Z test was performed ( p < 0.05 was considered statistically significant). Q test was used to evaluate the heterogeneity among studies, and the data with heterogeneity was analyzed using a random effect model. The reporting and publication bias were assessed using Egger’s test and a funnel plot ( p < 0.05 was considered having potential for publication bias). The data were analyzed using Review Manager version 5.3 27.
Results
Study eligibility results
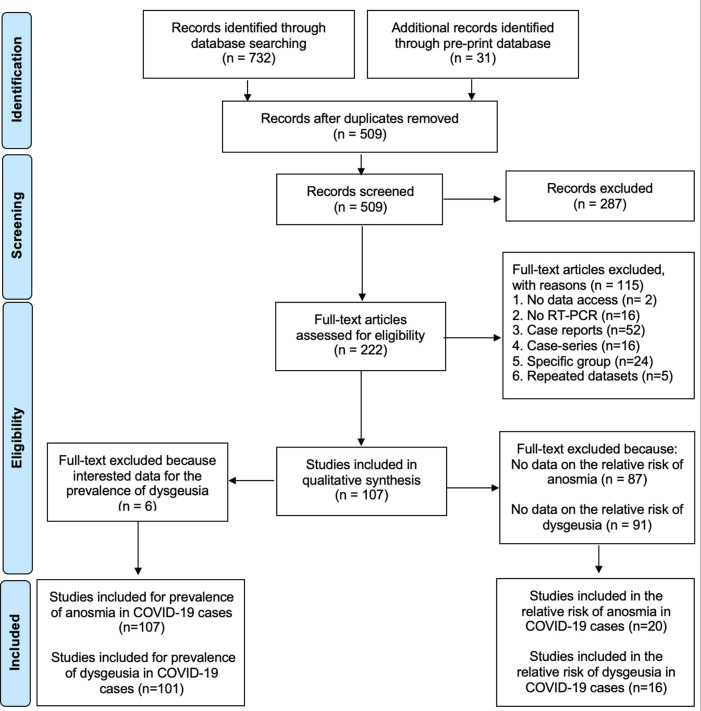
In total, 691 articles (660 reviewed articles and 31 preprint articles) were identified through the databases; of these, 182 articles were removed as duplicates. An additional 287 articles were excluded following a screening process of the titles and abstracts due to irrelevant studies, leaving 222 references ( Figure 1 ). Full-texts of the remaining 222 references were retrieved and screened for eligibility, and this process excluded an additional 115 references as the inclusion criteria was not met. This exclusion included articles with no access 28, 29, RT-PCR not clearly stated in the text 30- 45, case reports 46- 97, case seriess 98- 113, repeated datasetss 114- 118, and studies in specific groups 119- 130. A complete assessment was conducted for 107 references.
Figure 1. Flowchart of the result of literature search according to PRISMA.
The meta-analysis included 107 studies to calculate the prevalence of anosmia in COVID-19 patients. Additionally, 6 studies were excluded while calculating the prevalence of dysgeusia in COVID-19, thus leaving 101 eligible studies. In total, 20 and 16 studies were included to assess the association of anosmia and dysgeusia with the COVID-19 occurrence, respectively.
The prevalence of anosmia and dysgeusia in COVID-19
To calculate the prevalence of anosmia in COVID-19 cases, 107 studies were included comprising 32,142 COVID-19 patients, and anosmia was reported in 12,038 patients with a global pooled prevalence of 38.2% (95% CI: 36.5%, 47.2%). The list of the studies and the prevalence of anosmia in each study are presented in Table 1 .
In total, 30,901 COVID-19 patients from 101 studies were included to calculate the prevalence of dysgeusia in COVID-19. Dysgeusia was identified in 11,337 out of 30,901 COVID-19 patients resulting in a cumulative prevalence of 36.6% (95% CI: 35.2%, 45.2%). The individual studies and the prevalence of dysgeusia from each study are listed in Table 2 .
Table 1.
The prevalence of anosmia among COVID-19 patients around the globe.
| Study Design | Country | COVID-19 | Prevalence (%) | 95%CI | Ref | |
|---|---|---|---|---|---|---|
| Anosmia | Total | |||||
| Retrospective | Singapore | 53 | 305 | 17.38 | 13.12, 21.63 | 131 |
| Prospective | Turkey | 9 | 29 | 31.03 | 14.20, 47.87 | 119 |
| Prospective | France | 31 | 225 | 13.78 | 9.27, 18.28 | 132 |
| Case control | Spain | 25 | 79 | 31.65 | 21.39, 41.90 | 133 |
| Retrospective | Taiwan | 42 | 321 | 13.08 | 9.39, 16.77 | 134 |
| Case control | Canada | 69 | 134 | 51.49 | 43.03, 59.95 | 135 |
| Case control | US | 60 | 101 | 59.41 | 49.83, 68.98 | 136 |
| Retrospective | Italy | 13 | 213 | 6.10 | 2.89, 9.32 | 137 |
| Cross sectional | US | 40 | 59 | 67.80 | 55.87, 79.72 | 138 |
| Cross sectional | Spain | 138 | 197 | 70.05 | 63.65, 76.45 | 139 |
| Cross sectional | Brazil | 539 | 655 | 82.29 | 79.37, 85.21 | 140 |
| Retrospective | Pakistan | 4 | 30 | 13.33 | 1.17, 25.50 | 141 |
| Retrospective | Spain | 90 | 375 | 24.00 | 19.68, 28.32 | 142 |
| Observational | Europe | 997 | 1420 | 70.21 | 67.83, 72.59 | 143 |
| Prospective | South Korea | 68 | 172 | 39.53 | 32.23, 46.84 | 144 |
| Prospective | France | 62 | 197 | 31.47 | 24.99, 37.96 | 145 |
| Retrospective | USA | 45 | 251 | 17.93 | 13.18, 22.67 | 146 |
| Retrospective | Italy | 14 | 22 | 63.64 | 43.53, 83.74 | 147 |
| Cross sectional | India | 62 | 230 | 26.96 | 21.22, 32.69 | 148 |
| Cross sectional | US | 22 | 168 | 13.10 | 7.99, 18.20 | 149 |
| Cross sectional | Hongkong | 39 | 83 | 46.99 | 36.25, 57.73 | 150 |
| Retrospective | France | 54 | 114 | 47.37 | 38.20, 56.53 | 151 |
| Retrospective | Japan | 19 | 32 | 59.38 | 42.36, 76.39 | 152 |
| Prospective | Taiwan | 78 | 217 | 35.94 | 29.56, 42.33 | 153 |
| Prospective | Turkey | 18 | 172 | 10.47 | 5.89, 15.04 | 154 |
| Retrospective | Italy | 17 | 84 | 20.24 | 11.65, 28.83 | 155 |
| Prospective | Italy | 40 | 108 | 37.04 | 27.93, 46.14 | 156 |
| Cross sectional | Egypt | 80 | 96 | 83.33 | 75.88, 90.79 | 157 |
| Retrospective | Kenya | 279 | 787 | 35.45 | 32.11, 38.79 | 158 |
| Cross sectional | Germany | 29 | 73 | 39.73 | 28.50, 50.95 | 159 |
| Prospective | UK | 1 | 40 | 2.50 | 0.00, 7.34 | 160 |
| Cross sectional | India | 121 | 655 | 18.47 | 15.50, 21.45 | 161 |
| Cross sectional | France | 140 | 299 | 46.82 | 41.17, 52.48 | 162 |
| Retrospective | France | 54 | 114 | 47.37 | 38.20, 56.53 | 163 |
| Prospective | Iran | 22 | 92 | 23.91 | 15.20, 32.63 | 164 |
| Retrospective | US | 58 | 509 | 11.39 | 8.63, 14.16 | 165 |
| Cross sectional | Spain | 28 | 45 | 62.22 | 48.06, 76.39 | 166 |
| Cross sectional | Brazil | 28 | 73 | 38.36 | 27.20, 49.51 | 167 |
| Prospective | Turkey | 157 | 262 | 59.92 | 53.99, 65.86 | 168 |
| Retrospective | France | 17 | 55 | 30.91 | 18.70, 43.12 | 169 |
| Cross sectional | UK | 344 | 579 | 59.41 | 55.41, 63.41 | 170 |
| Retrospective | Somalia | 24 | 60 | 40.00 | 27.60, 52.40 | 171 |
| Prospective | US | 18 | 42 | 42.86 | 27.89, 57.82 | 172 |
| Prospective | Turkey | 33 | 143 | 23.08 | 16.17, 29.98 | 173 |
| Retrospective | China | 11 | 214 | 5.14 | 2.18, 8.10 | 174 |
| Retrospective | Brazil | 8 | 1208 | 0.66 | 0.20, 1.12 | 175 |
| Retrospective | US | 3 | 50 | 6.00 | 0.00, 12.58 | 176 |
| Cross sectional | India | 26 | 391 | 6.65 | 4.18, 9.12 | 177 |
| Retrospective | China | 34 | 86 | 39.53 | 29.20, 49.87 | 178 |
| Retrospective | France | 37 | 70 | 52.86 | 41.16, 64.55 | 179 |
| Cross sectional | Italy | 34 | 54 | 62.96 | 50.08, 75.84 | 180 |
| Retrospective | UK | 80 | 141 | 56.74 | 48.56, 64.92 | 181 |
| Prospective | Italy | 44 | 72 | 61.11 | 49.85, 72.37 | 182 |
| Retrospective | Belgium | 27 | 47 | 57.45 | 43.31, 71.58 | 183 |
| Case control | Israel | 3 | 16 | 18.75 | 0.00, 37.88 | 184 |
| Case control | Turkey | 50 | 81 | 61.73 | 51.14, 72.31 | 185 |
| Retrospective | China, France Germany | 154 | 394 | 39.09 | 34.27, 43.90 | 186 |
| Retrospective | Malaysia | 31 | 145 | 21.38 | 14.71, 28.05 | 187 |
| Retrospective | Europe | 357 | 417 | 85.61 | 82.24, 88.98 | 188 |
| Retrospective | Italy | 29 | 100 | 29.00 | 20.11, 37.89 | 189 |
| Cohort | Italy | 126 | 151 | 83.44 | 77.52, 89.37 | 190 |
| Cross sectional | Switzerland | 63 | 103 | 61.17 | 51.75, 70.58 | 191 |
| Case control | Italy | 26 | 43 | 60.47 | 45.85, 75.08 | 192 |
| Retrospective | Germany | 80 | 91 | 87.91 | 81.21, 94.61 | 193 |
| Prospective | Israel | 78 | 112 | 69.64 | 61.13, 78.16 | 194 |
| Cohort | India | 29 | 225 | 12.89 | 8.51, 17.27 | 195 |
| Case control | Turkey | 44 | 116 | 37.93 | 29.10, 46.76 | 196 |
| Retrospective | Turkey | 55 | 155 | 35.48 | 27.95, 43.02 | 197 |
| Retrospective | France | 1442 | 3737 | 38.59 | 37.03, 40.15 | 198 |
| Retrospective | South Korea | 5 | 328 | 1.52 | 0.20, 2.85 | 199 |
| Prospective | US | 23 | 46 | 50.00 | 35.55, 64.45 | 200 |
| Cross sectional | Spain | 46 | 58 | 79.31 | 68.89, 89.74 | 201 |
| Cross sectional | Germany | 22 | 34 | 64.71 | 48.64, 80.77 | 202 |
| Cohort | US | 145 | 273 | 53.11 | 47.19, 59.03 | 203 |
| Retrospective | Europe | 3 | 204 | 1.47 | 0.00, 3.12 | 204 |
| Cohort | US | 32 | 318 | 10.06 | 6.76, 13.37 | 205 |
| Prospective | South Korea | 389 | 3191 | 12.19 | 11.06, 13.33 | 206 |
| Prospective | France | 81 | 115 | 70.43 | 62.09, 78.78 | 207 |
| Retrospective | China | 30 | 196 | 15.31 | 10.27, 20.35 | 208 |
| Retrospective | Iran | 96 | 100 | 96.00 | 92.16, 99.84 | 209 |
| Retrospective | Qatar | 19 | 141 | 13.48 | 7.84, 19.11 | 210 |
| Cross sectional | 22 | 100 | 22.00 | 13.88, 30.12 | 211 | |
| Cross sectional | France | 129 | 390 | 33.08 | 28.41, 37.75 | 212 |
| Case control | India | 11 | 74 | 14.86 | 6.76, 22.97 | 213 |
| Retrospective | Turkey | 529 | 1197 | 44.19 | 41.38, 47.01 | 214 |
| Case control | Israel | 76 | 112 | 67.86 | 59.21, 76.51 | 215 |
| Cross sectional | Canada | 31 | 56 | 55.36 | 42.34, 68.38 | 216 |
| Retrospective | US | 75 | 169 | 44.38 | 36.89, 51.87 | 217 |
| Retrospective | China | 134 | 1172 | 11.43 | 9.61, 13.26 | 218 |
| Cohort | US | 15 | 177 | 8.47 | 4.37, 12.58 | 219 |
| Cross sectional | Greece | 29 | 79 | 36.71 | 26.08, 47.34 | 220 |
| Cross sectional | Saudi Arabia | 28 | 128 | 21.88 | 14.71, 29.04 | 221 |
| Cross sectional | Italy | 283 | 508 | 55.71 | 51.39, 60.03 | 222 |
| Cross sectional | Italy | 237 | 355 | 66.76 | 61.86, 71.66 | 223 |
| Cross sectional | Spain | 26 | 31 | 83.87 | 70.92, 96.82 | 224 |
| Cross sectional | Spain | 454 | 846 | 53.66 | 50.30, 57.02 | 225 |
| Prospective | Italy | 84 | 138 | 60.87 | 52.73, 69.01 | 226 |
| Case control | Brazil | 23 | 57 | 40.35 | 27.61, 53.09 | 227 |
| Case control | Iran | 59 | 60 | 98.33 | 95.09, 100.00 | 228 |
| Retrospective | US | 198 | 949 | 20.86 | 18.28, 23.45 | 229 |
| Prospective | Italy | 46 | 50 | 92.00 | 84.48, 99.52 | 230 |
| Cross sectional | Turkey | 71 | 223 | 31.84 | 25.72, 37.95 | 231 |
| Prospective | Italy | 44 | 67 | 65.67 | 54.30, 77.04 | 232 |
| Prospective | India | 62 | 76 | 81.58 | 72.86, 90.29 | 233 |
| Cross sectional | Brazil | 159 | 179 | 88.83 | 84.21, 93.44 | 234 |
| Retrospective | Global | 1324 | 1698 | 77.97 | 76.00, 79.95 | 235 |
| Retrospective | Italy | 46 | 111 | 41.44 | 32.28, 50.61 | 236 |
Table 2.
The prevalence of dysgeusia among COVID-19 patients around the globe.
| Study design | Country | COVID-19 | Prevalence (%) | 95%CI | Ref | |
|---|---|---|---|---|---|---|
| Dysgeusia | Total | |||||
| Retrospective | Singapore | 53 | 305 | 17.38 | 13.12, 21.63 | 131 |
| Prospective | Turkey | 6 | 29 | 20.69 | 5.95, 35.43 | 119 |
| Case control | Spain | 29 | 79 | 36.71 | 26.08, 47.34 | 133 |
| Retrospective | Taiwan | 42 | 321 | 13.08 | 9.39, 16.77 | 134 |
| Case control | Canada | 69 | 134 | 51.49 | 43.03, 59.95 | 135 |
| Case control | US | 60 | 101 | 59.41 | 49.83, 68.98 | 136 |
| Retrospective | Italy | 6 | 213 | 2.82 | 0.59, 5.04 | 137 |
| Cross sectional | US | 42 | 59 | 71.19 | 59.63, 82.74 | 138 |
| Cross sectional | Spain | 128 | 197 | 64.97 | 58.31, 71.64 | 139 |
| Prospective | Brazil | 502 | 655 | 76.64 | 73.40, 79.88 | 140 |
| Retrospective | Pakistan | 4 | 30 | 13.33 | 1.17, 25.50 | 141 |
| Retrospective | Spain | 90 | 375 | 24.00 | 19.68, 28.32 | 142 |
| Cross sectional | Europe | 770 | 1420 | 54.23 | 51.63, 56.82 | 143 |
| Prospective | South Korea | 58 | 172 | 33.72 | 26.66, 40.79 | 144 |
| Prospective | France | 56 | 197 | 28.43 | 22.13, 34.73 | 145 |
| Retrospective | USA | 41 | 251 | 16.33 | 11.76, 20.91 | 146 |
| Retrospective | Italy | 14 | 22 | 63.64 | 43.53. 83.74 | 147 |
| Cross sectional | India | 25 | 230 | 10.87 | 6.85. 14.89 | 148 |
| Cross sectional | US | 15 | 168 | 8.93 | 4.62, 13.24 | 149 |
| Cross sectional | Hongkong | 36 | 83 | 43.37 | 32.71, 54.04 | 150 |
| Retrospective | France | 54 | 114 | 47.37 | 38.20. 56.53 | 151 |
| Retrospective | Japan | 18 | 32 | 56.25 | 39.06, 73.44 | 152 |
| Prospective | Taiwan | 78 | 217 | 35.94 | 29.56, 42.33 | 153 |
| Prospective | Turkey | 11 | 172 | 6.40 | 2.74, 10.05 | 154 |
| Retrospective | Italy | 26 | 84 | 30.95 | 21.07, 40.84 | 155 |
| Prospective | Italy | 66 | 108 | 61.11 | 51.92, 70.31 | 156 |
| Prospective | Iran | 66 | 76 | 86.84 | 79.24, 94.44 | 122 |
| Retrospective | Kenya | 279 | 787 | 35.45 | 32.11, 39.79 | 158 |
| Cross sectional | Germany | 29 | 73 | 39.73 | 28.50, 50.95 | 159 |
| Cross sectional | France | 124 | 299 | 41.47 | 35.89, 47.05 | 162 |
| Retrospective | France | 46 | 54 | 85.19 | 75.71, 94.66 | 163 |
| Prospective | Iran | 15 | 92 | 16.30 | 8.76, 23.85 | 164 |
| Retrospective | Illinois | 81 | 509 | 15.91 | 12.74, 19.09 | 165 |
| Cross sectional | Brazil | 29 | 73 | 39.73 | 28.50, 50.95 | 167 |
| Prospective | Turkey | 157 | 262 | 59.92 | 53.99, 65.86 | 168 |
| Retrospective | France | 17 | 55 | 30.91 | 18.70, 43.12 | 169 |
| Cross sectional | UK | 344 | 579 | 59.41 | 55.41, 63.41 | 170 |
| Retrospective | Somalia | 17 | 60 | 28.33 | 16.93, 39.74 | 171 |
| Prospective | US | 24 | 42 | 57.14 | 42.18, 72.11 | 172 |
| Prospective | Turkey | 51 | 143 | 35.66 | 27.81, 43.52 | 173 |
| Retrospective | China | 12 | 214 | 5.61 | 2.52, 8.69 | 174 |
| Retrospective | Brazil | 3 | 1208 | 0.25 | 0.00, 0.53 | 175 |
| Retrospective | Illinois | 5 | 50 | 10.00 | 1.68, 18.32 | 176 |
| Retrospective | China | 12 | 214 | 5.61 | 2.52, 8.69 | 237 |
| Cross sectional | India | 35 | 391 | 8.95 | 6.12, 11.78 | 177 |
| Retrospective | China | 33 | 86 | 38.37 | 28.09, 48.65 | 178 |
| Retrospective | France | 34 | 70 | 48.57 | 36.86, 60.28 | 179 |
| Cross sectional | Italy | 34 | 54 | 62.96 | 50.08, 75.84 | 115 |
| Retrospective | UK | 89 | 141 | 63.12 | 55.16, 71.08 | 181 |
| Prospective | Italy | 39 | 72 | 54.17 | 42.66, 65.68 | 182 |
| Case control | Turkey | 43 | 52 | 82.69 | 72.41, 92.97 | 125 |
| Prospective | Belgium | 37 | 86 | 43.02 | 32.56, 53.49 | 238 |
| Retrospective | Belgium | 6 | 47 | 12.77 | 3.23, 22.31 | 183 |
| Case control | Israel | 3 | 16 | 18.75 | 0.00, 37.88 | 184 |
| Case control | Turkey | 22 | 81 | 27.16 | 17.47, 36.85 | 185 |
| Retrospective | China, France Germany | 100 | 394 | 25.38 | 21.08, 29.68 | 186 |
| Retrospective | Malaysia | 34 | 145 | 23.45 | 16.55, 30.34 | 187 |
| Retrospective | Europe | 342 | 417 | 82.01 | 78.33, 85.70 | 188 |
| Retrospective | Italy | 41 | 100 | 41.00 | 31.36, 50.64 | 189 |
| Cohort | Italy | 135 | 151 | 89.40 | 84.49, 94.31 | 190 |
| Cross sectional | Switzerland | 67 | 103 | 65.05 | 55.84, 74. 26 | 191 |
| Prospective | Israel | 82 | 112 | 73.21 | 65.01, 81.42 | 194 |
| Cohort | India | 39 | 225 | 17.33 | 12.39, 22.28 | 195 |
| Case control | turkey | 48 | 116 | 41.38 | 32.42, 50.34 | 196 |
| Retrospective | turkey | 25 | 155 | 16.13 | 10.34, 21.92 | 197 |
| Retrospective | France | 1389 | 3737 | 37.17 | 35.62, 38.72 | 198 |
| Cross sectional | Iran | 37 | 49 | 75.51 | 63.47, 87.55 | 129 |
| Prospective | NA | 476 | 751 | 63.38 | 59.94, 66.83 | 239 |
| Retrospective | South Korea | 3 | 328 | 0.91 | 0.00, 19.94 | 199 |
| Cross sectional | Spain | 51 | 58 | 87.93 | 79.55, 96.31 | 201 |
| Cohort | US | 145 | 273 | 53.11 | 47.19, 59.03 | 203 |
| Retrospective | Europe | 3 | 204 | 1.47 | 0.00, 3.12 | 204 |
| Cohort | US | 24 | 318 | 7.55 | 4.64, 10.45 | 205 |
| Prospective | South Korea | 353 | 3191 | 11.06 | 9.97, 12.15 | 206 |
| Prospective | France | 81 | 115 | 70.43 | 62.09, 78.78 | 207 |
| Retrospective | China | 23 | 196 | 11.73 | 7.23, 16.24 | 208 |
| Retrospective | Qatar | 28 | 141 | 19.86 | 13.27, 26.44 | 210 |
| Cross sectional | India | 40 | 100 | 40.00 | 30.40, 49.60 | 211 |
| Cross sectional | France | 130 | 390 | 33.33 | 28.65, 38.01 | 212 |
| Retrospective | Turkey | 526 | 1197 | 43.94 | 41.13, 46.75 | 214 |
| Case control | Israel | 80 | 112 | 71.43 | 63.06, 79.80 | 215 |
| Cross sectional | Canada | 32 | 56 | 57.14 | 44.18, 70.10 | 216 |
| Retrospective | US | 70 | 169 | 41.42 | 33.99, 48.85 | 217 |
| Retrospective | China | 242 | 1172 | 20.65 | 18.33, 22.97 | 218 |
| Cohort | US | 15 | 177 | 8.47 | 4.37, 12.58 | 219 |
| Cross sectional | Greece | 22 | 79 | 27.85 | 17.96, 37.73 | 220 |
| Cross sectional | Saudi Arabia | 28 | 128 | 21.88 | 14.71, 29.04 | 221 |
| Cross sectional | Italy | 321 | 508 | 63.19 | 58.99, 67.38 | 222 |
| Cross sectional | Italy | 232 | 355 | 65.35 | 60.40, 70.30 | 223 |
| Cross sectional | Spain | 4 | 31 | 12.90 | 1.10, 24.70 | 224 |
| Cross sectional | Spain | 442 | 846 | 52.25 | 48.88, 55.61 | 225 |
| Prospective | Italy | 56 | 138 | 40.58 | 32.39, 48.77 | 226 |
| Case control | Brazil | 5 | 57 | 8.77 | 1.43, 16.12 | 227 |
| Case control | Iran | 14 | 60 | 23.33 | 12.63, 34.04 | 228 |
| Prospective | Italy | 35 | 50 | 70.00 | 57.30, 82.70 | 230 |
| Cross sectional | Turkey | 77 | 223 | 34.53 | 28.29, 40.77 | 231 |
| Prospective | Italy | 17 | 67 | 25.37 | 14.95, 35.79 | 232 |
| Prospective | India | 64 | 76 | 84.21 | 76.01, 92.41 | 233 |
| Cross sectional | Brazil | 159 | 179 | 88.83 | 84.21, 93.44 | 234 |
| Retrospective | Global | 1149 | 1687 | 68.11 | 65.89, 70.33 | 235 |
| Retrospective | Italy | 66 | 111 | 59.46 | 50.33, 68.59 | 236 |
Association of anosmia and the occurrence of COVID-19
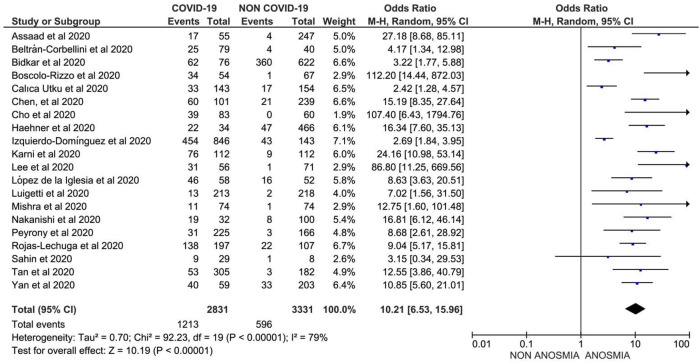
In total, 20 studies comprising 1,213 COVID-19 cases with anosmia and 2,735 non-COVID-19 patients (mostly COVID-19-like symptoms with negative RT-PCR for SARS-CoV-2) were analyzed to investigate the association between anosmia and the occurrence of COVID-19. Data suggested that anosmia was 10.2-fold more prevalent in patients with COVID-19 compared to those with COVID-19 like illness, OR 10.21 (95% CI: 6.53, 15.96) with p < 0.001 ( Figure 2 ).
Figure 2. Forest plot of the association between anosmia and the risk of COVID-19 (OR: 10.21; 95%CI: 6.53, 15.96; p<0.001; Egger’ p=0.8340; heterogeneity p<0.001; I-squared 79.33%).
Association of dysgeusia and the occurrence of COVID-19
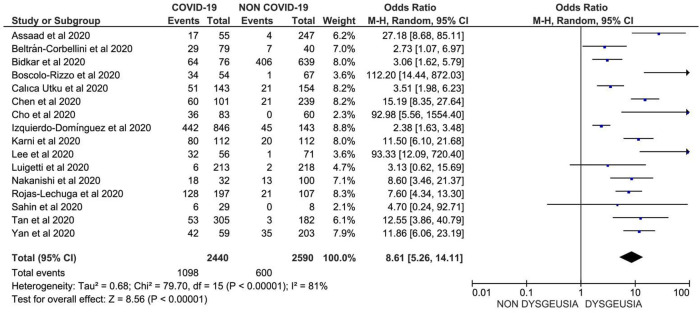
In total, 16 studies comprising 1,342 COVID-19 cases with dysgeusia and 1,990 patients with other respiratory illness (COVID-19 like illness with negative RT-PCR for SARS-CoV-2) were included to assess the association between dysgeusia and the occurrence of COVID-19. Data suggested that dysgeusia was 8.6-fold more prevalent in patients with COVID-19 compared to those with other respiratory illness, with OR 8.61 (95% CI: 5.26, 14.11) and p<0.001 ( Figure 3 ).
Figure 3. Forest plot of the association between dysgeusia and the risk of COVID-19 (OR: 8.61; 95%CI: 5.26, 14.11; p<0.001; Egger’ p=0.8220; heterogeneity p<0.001; I-squared 81.03%).
Association of anosmia and dysgeusia with COVID-19 severity and mortality
Limited studies have assessed the association between anosmia and dysgeusia and the severity and mortality of COVID-19 cases. One study linked anosmia with a lower fatality rate and a lower ICU admission 240.
Discussion
Anosmia and dysgeusia in COVID-19 patients
The pooled prevalence of anosmia in our systematic review was 38.2% of 32,142 COVID-19 cases. This result was almost thrice the initial prevalence reported from Wuhan, China 174, 208. This suggests that anosmia is a potential indicator of SARS-CoV-2 infection, and may be useful for screening and early identification of COIVD-19 patients, particularly asymptomatics 241. Some countries, such as the UK and US have used anosmia as an indicator for preventive measure, wherein COVID-19 patient with anosmia should commence self-isolation 242- 244.
Anosmia is not only present in COVID-19 patients, but also in patients with other respiratory diseases such as influenza, parainfluenza, Eipstein Barr virus, picornavirus, and rhinovirus 245- 248. However, our study demonstrated that the prevalence of anosmia was 10.2-fold higher in COVID-19 patient than that in non-COVID-19 patient. During the previous pandemics, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), anosmia was rarely reported 249. Only one study reported persistent anosmia after 2 years of recovery from SARS 250. Another study reported that anosmia in COVID-19 patients varied based on ethnicity; anosmia in Caucasian is three times more prevalent than in Asian population 251.
Dysgeusia was initially reported in 11.7% of patients who were discharged from Wuhan hospital, which persisted for at least four weeks. This result was lower than ours (36.6% out of 30,901 COVID-19 cases), which might be attributable to either lower dysgeusia prevalence in China or underestimation of this symptom itself 208. Moreover, the prevalence of dysgeusia in COVID-19 patients was 8.6-fold higher than that in non-COVID-19 like illness. Herpes zoster and HIV have also been linked to gustatory dysfunction 252, 253. Furthermore, another study reported that anosmia and dysgeusia have 82.5% predictive value for positive SARS-CoV-2 RT-PCR 254.
Possible pathogenesis of anosmia in COVID-19
Several mechanisms have been proposed to explain the emergence of anosmia in COVID-19 patients.
a. Obstruction in the nasal airway
As several viral infections in the respiratory system display blockage of nasal airway or nasal congestion, this hypothesis was initially proposed. According to this mechanism, the interaction between the odorants and olfactory receptors is inhibited by certain obstructions, thereby impairing the subsequent smelling processes 255. This condition results in anosmia. The obstruction could be caused by nasal discharge or by inflammation occurring in the nasal cavity 256; however, this hypothesis can be presumably ruled out. Moreover, several studies reported that anosmia is more prevalent than nasal congestion in COVID-19 patients 188, 256- 259. Interestingly, the incidence of rhinorrhea and nasal obstruction in SARS-CoV-2 infection is lower than other coronaviruses such as SARS-CoV and MERS-CoV 260.
Furthermore, presumably, nasal obstruction is a secondary mechanism by which anosmia is induced in COVID-19 patients as the obstruction in viral infection typically occurs as a subsequent event after damage in the mucociliary system, thereby inhibiting the nasal discharge and leading to nasal obstruction. In certain viral infections, the mucociliary system operated by ciliated cells is impaired. A previous study reported that human coronavirus (HCoV) disrupted the nasal ciliated respiratory epithelium leading to impaired mucociliary escalator system 261.
b. Damage in olfactory sensory neurons
Smelling processes commence when the odorants bind to the olfactory sensory neurons (OSNs) in the olfactory epithelium located in the nasal cavity, which subsequently transmits this information through their axons to the olfactory bulb in the brain 262. According to this concept, a viral attack on the receptor neurons eventually creates disturbances in the sense of smell; however, this hypothesis remains under debate as several recent studies reported the absence of angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS), the key factors for the virus to enter the cell 263, in the OSNs 264- 267. These findings are supported by another study carried out by Bryche et al., who demonstrated that SARS-CoV-2 was not detected in the OSNs of hamsters 266.
Moreover, after comparing the duration between anosmia incidence in COVID-19 patients and the normal cellular regeneration process, this proposed mechanism should be reconsidered. Several studies reported that COVID-19-related anosmia disappeared within 1-2 weeks, whereas regeneration of dead OSNs requires more than 2 week time period 188, 206, 255, 262, 268. This discrepancy results in a temporary conclusion that COVID-19-related anosmia is not directly associated with the impairment of the OSNs.
c. Olfactory center damage in the brain
The aforementioned dysfunction of OSNs and the mechanism by which SARS-CoV-2 directly affects the olfactory center via axonal transport of the neuron remains unclear, as the OSN lacks ACE2 and TMPRSS2 which hinders viral entry into the cell 264- 267. Nevertheless, the possibility of olfactory center disruption caused by SARS-CoV-2 should not be overlooked as the cause of anosmia, since a previous study concluded that human ACE2 (hACE2)-transgenic mice suffered from brain infection after intranasal inoculation with SARS-CoV 269. The study found that the brain infection commenced from the olfactory bulb, which is the axonal trajectory pathway of the OSNs 269. This finding suggests that SARS-CoV-2 might also first utilize another structure in the nasal cavity before it is transported into the OSNs.
d. Olfactory supporting cells dysfunction
As OSN does not express ACE2 and TMPRSS2, the virus should use another pathway to infect the olfactory system. Numerous studies have established the expression of these SARS-CoV-2 entry proteins in several supporting cells in olfactory epithelium, that is, Bowman’s gland cells, horizontal basal cells, olfactory bulb pericytes, mitral cells, sustentacular cells, and microvillar cells 264- 267. Of these supporting cells, the sustentacular cells have gained immense attention as the initial site of SARS-CoV-2 infection in the olfactory epithelium. In addition to their higher expression of ACE2 and TMPRSS2 than the others, sustentacular cells are located on the surface of the nasal cavity making them vulnerable to exposure to the external environment 264, 267.
Notably, sustentacular cells act as supporting cells and promote olfactory neuron in the olfactory system. These cells detoxify harmful odorants, promote odorant-receptor binding, and provide nutritional substances to support the action of olfactory receptor neurons 255, 264. Considerably, it is plausible to suggest that any damage occurring in sustentacular cells will in turn affect the olfactory epithelium and produce anosmia.
The corresponding regeneration time to the recovery of anosmia also supports the notion that sustentacular cell damage relates to anosmia caused by SARS-CoV-2. As the replenishment of dead OSNs does not correspond to the duration of COVID-19-related anosmia within 1-2 weeks, the regeneration of sustentacular cells seems to be in line with that time frame 264, 266, 268.
Furthermore, this hypothesis is supported by a recent study conducted by Bryche et al., who reported that SARS-CoV-2 was accumulated in sustentacular cells but not in the OSNs 266. The olfactory epithelial damage and sustentacular cell loss occurred 2 days after instilling SARS-CoV-2 intranasally in golden Syrian hamsters 266.
e. Inflammation-related olfactory epithelium dysfunction
It is worth noting that the cytokine storm in COVID-19 is strongly associated with organ dysfunctions, including OSNs 232. The dysfunction in this structure can lead to disturbance in the sense of smell 270. Torabi et al. suggested that proinflammatory cytokines, particularly tumor necrosis factor a (TNF-α), may lead to COVID-19-induced anosmia 271. Another proinflammatory cytokine, interleukin-6 (IL-6), increased in cases presenting with anosmia 232 , 272.
The mechanism used by these cytokines, in particular IL-6, to produce anosmia is not fully understood. Cazzolla et al. suggested that this effect can be caused by either peripheral or central action of the cytokines 232. In the periphery, IL-6 may induce apoptosis of ciliary neuronal cells in the olfactory epithelium 272, whereas in its central action, the olfactory center in the brain is attacked by the cytokine as a result of virus infection 232.
Possible pathogenesis of dysgeusia in COVID-19
Although gustatory impairment is always displayed concomitantly with olfactory dysfunction, this symptom has a relatively different mechanism and is often distantly linked to the latter symptom. Several hypotheses have been proposed to explain the mechanism behind the emergence of dysgeusia in COVID-19 patients.
a. The subsequent effect of cranial nerves dysfunction
Considering the close relationship between the olfactory and gustatory system both peripherally and centrally, smell and taste dysfunction in COVID-19 often occurs concomitantly 256, 273. This hypothesis describes dysgeusia as a secondary event of olfactory dysfunction 274; however, several studies revealed that the percentage of dysgeusia in COVID-19 patients is higher than symptoms related to olfactory dysfunction 188, 275. Based on this finding, another mechanism may be involved in inducing SARS-CoV-2-related dysgeusia. Furthermore, COVID-19-induced dysgeusia could also occur when there is certain damage in the cranial nerves responsible for gustatory transmission (cranial nerve VII, IX, and X) 276. Among these nerves, SARS-CoV-2 exposure to cranial nerve VII has gained immense attention. Based on this hypothesis, the virus initially colonizes the nasopharynx structure, then moves to the Eustachian tube, and eventually reaches the middle ear where the virus gets access to chorda tympani and causes dysgeusia 276.
b. Zinc deficiency
Another interesting hypothesis underlying dysgeusia in COVID-19 is related to zinc deficiency 276. This hypothesis was developed as zinc is an important mineral in carbonic anhydrase, which is pivotal in maintaining taste sensation 277. Interestingly, one study reported that zinc level in patients with SARS-CoV-2 infection was significantly lower compared to that in the healthy control groups 278. Alterations in the sense of taste after being treated with certain treatments, such as irradiation in cancer patients 279, 280, could be prevented by zinc supplementation. Moreover, dengue fever virus and human immunodeficiency virus replication could be inhibited by zinc chelation 281, 282. Furthermore, pharmacological agents influencing ACE2 activity are associated with taste disturbances 283, 284.
Nevertheless, this effect does not relate to zinc deficiency as these drugs do not influence both serum and salivary zinc concentrations 284. Further investigation needs to be carried out to reveal the role of zinc in dysgeusia associated with COVID-19.
c. SARS-CoV-2-bound sialic acid
SARS-CoV-2 may produce dysgeusia via interaction with sialic acid receptors 232, 274, 285. Sialic acid plays a pivotal role in the taste processing pathway as it is a component of the normal salivary composition 286. Moreover, reduced amount of sialic acid impairs the ability to taste 287. An in silico study revealed that SARS-CoV-2 could interact with the sialic acid receptor through its spike protein 288. Previously, MERS-CoV was also reported to interact with this receptor 289. Following this occupancy, the gustatory threshold increases, while gustatory particles degrade at a higher rate 274, 287.
d. Direct attack on several oral sites
A previous study investigated the expression of ACE2 in various tissues in the oral cavity and found that the tongue had higher ACE2 expression in comparison to other tissues, such as buccal and gingival tissues 290. This finding raised a hypothesis that SARS-CoV-2 could directly attack the taste buds in the tongue, initiating inflammatory responses, and would eventually alter the sense of taste 276. It is proposed that the Toll-like receptor-mediated cascade and apoptosis are the subsequent events that could lead to taste dysfunction 276, 291.
A previous study investigating SARS-CoV infection in rhesus macaques revealed that, initially, the salivary gland was attacked by the virus 292. As the human salivary gland expresses a high level of ACE2 293, it is reasonable to pay more attention to the vulnerability of this gland against SARS-CoV-2 exposure. Disruption in the activity of the salivary gland would produce either imbalance in salivary composition or impairment of salivary flow, which could ultimately result in dysgeusia 276.
Conclusions
Out of 32,142 and 30,901 COVID-19 cases studied for anosmia and dysgeusia, respectively, the prevalence of anosmia was approximately 38.2%, whereas that of dysgeusia was 36.6%. Both of these symptoms were more common in COVID-19 compared to other respiratory infections (approximately 10 and 9 times, respectively). Several mechanisms have been proposed to explain the emergence of anosmia in COVID-19 patients including nasal airway obstruction, damage in OSNs, olfactory center damage in the brain, dysfunction of olfactory supporting cells, and inflammation-related olfactory epithelium dysfunction. Furthermore, some possible pathogenesis of dysgeusia in SARS-CoV-2 infection has been proposed including cranial nerve dysfunction, zinc deficiency, virion interaction, and direct attack of the virus to several oral sites.
Data availability
Undelying data
All data underlying the results are available as part of the article and no additional source data are required.
Reporting guidelines
Figshare: PRISMA checklist for ‘Anosmia and dysgeusia in SARS-CoV-2 infection: Incidence, effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms - A systematic review and meta-analysis’, https://doi.org/10.6084/m9.figshare.13323080.v1 294.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgement
Authors would like to thank HT Editorial Services in assisting the writing process.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 2 approved
References
- 1. Dhama K, Patel SK, Pathak M, et al. : An update on SARS-CoV-2/COVID-19 with particular reference to its clinical pathology, pathogenesis, immunopathology and mitigation strategies. Travel Med Infect Dis 2020 Sep - Oct;37:101755. Epub 2020/06/02. 10.1016/j.tmaid.2020.101755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dhama K, Khan S, Tiwari R, et al. : Coronavirus Disease 2019-COVID-19. Clin Microbiol Rev 2020 Sep 16;33(4). Epub 2020/06/26. 10.1128/CMR.00028-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Worldometers: COVID-19 coronavirus pandemic. Reference Source[cited 2020 November 14]
- 4. Greene CJ, Burleson SL, Crosby JC, et al. : Coronavirus disease 2019: International public health considerations. JACEP Open 2020;1(2):70–77. 10.1002/emp2.12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frediansyah A, Tiwari R, Sharun K, et al. : Antivirals for COVID-19: a critical review. Clin Epidemiol Glob Health 2020. 10.1016/j.cegh.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frediansyah A, Nainu F, Dhama K, et al. : Remdesivir and its antiviral activity against COVID-19: A systematic review. Clin Epidemiol Glob Health 2020. 10.1016/j.cegh.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mudatsir M, Yufika A, Nainu F, et al. : Antiviral Activity of Ivermectin Against SARS-CoV-2: An Old-Fashioned Dog with a New Trick—A Literature Review. Scientia Pharmaceutica 2020;88(3):36. 10.3390/scipharm88030036 [DOI] [Google Scholar]
- 8. Rabaan AA, Al-Ahmed SH, Sah R, et al. : Recent advances in vaccine and immunotherapy for COVID-19. Hum Vaccin Immunother 2020 Nov 6:1–12. Epub 2020/11/07 10.1080/21645515.2020.1825896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keam S, Megawati D, Patel SK, et al. : Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev Med Virol 2020 Sep;30(5):e2123. Epub 2020/07/11 10.1002/rmv.2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Wang Y, Chen Y, et al. : Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. New Microbiol 2020;92(6):568–576. 10.1002/jmv.25748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. : Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020:101623. 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marinosci A, Landis BN, Calmy A: Possible link between anosmia and COVID-19: sniffing out the truth. Eur Arch Otorhinolaryngol Suppl 2020:1–2. 10.1007/s00405-020-05966-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaye R, Chang CD, Kazahaya K, et al. : COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg 2020:0194599820922992. 10.1177/0194599820922992 [DOI] [PubMed] [Google Scholar]
- 14. Boscolo-Rizzo P, Borsetto D, Spinato G, et al. : New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2-positive subjects. Eur Arch Otorhinolaryngol Suppl 2020:1–4. 10.1007/s00405-020-06066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paderno A, Schreiber A, Grammatica A, et al. : Smell and taste alterations in Covid-19: a cross-sectional analysis of different cohorts Int Forum Allergy Rhinol Wiley Online Library;2020. 10.1002/alr.22610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huynh PP, Ishii LE, Ishii M: What is anosmia? Jama 2020;324(2):206. 10.1001/jama.2020.10966 [DOI] [PubMed] [Google Scholar]
- 17. Samaranayake L, Fakhruddin KS, Panduwawala C: Loss of taste and smell. Br Dent J 2020;228(11):813. 10.1038/s41415-020-1732-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pallanti S: Importance of SARs-Cov-2 anosmia: From phenomenology to neurobiology. Comp Psych 2020:152184. 10.1016/j.comppsych.2020.152184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levinson R, Elbaz M, Ben-Ami R, et al. : Anosmia and dysgeusia in patients with mild SARS-CoV-2 infection. medRxiv 2020. 10.1080/23744235.2020.1772992 [DOI] [PubMed] [Google Scholar]
- 20. Klopfenstein T, Toko L, Royer PY, et al. : Features of anosmia in COVID-19. Med Mal Infect 2020. 10.1016/j.medmal.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laurendon T, Radulesco T, Mugnier J, et al. : Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology 2020;95(5):224–225. 10.1212/WNL.0000000000009850 [DOI] [PubMed] [Google Scholar]
- 22. Lee DJ, Lockwood J, Das P, et al. : Self-reported anosmia and dysgeusia as key symptoms of COVID-19. CJEM 2020:1–19. 10.1017/cem.2020.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zayet S, Klopfenstein T, Mercier J, et al. : Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection 2020:1. 10.1007/s15010-020-01442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hjelmesæth J, Skaare D: Loss of smell or taste as the only symptom of COVID-19. Tidsskr Nor Laegeforen 2020. 10.4045/tidsskr.20.0287 [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Plos Med 2009 Jul;6(7):e1000097. ISI:000268452400005. English. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stang A: Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010 Sep;25(9):603–5. Epub 2010/07/24 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 27. Cochrane T: Review Manager (RevMan) 5.3. Copenhagen: The Nordic Cochrane Centre. 2008:373.
- 28. Iran-Pour E, Tavabi AA, Seifi A: Presentation with Anosmia and Ageusia: Possible Hidden Carriers of COVID-19. South Med J 2020 Aug;113(8):399–400. Epub 2020/08/05. eng. 10.14423/SMJ.0000000000001123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanchana A, Varuvel VN: A study of COVID-19 symptoms using fuzzy cognitive map. Int J Pharm Res 2020;12(4):1068–1072. [Google Scholar]
- 30. Liu H, Li W, Zhang L, et al. : Clinical and CT manifestations of coronavirus disease 2019 (COVID-19): comparison of suspected cases of COVID-19 in isolation and non-COVID-19 pneumonia in a single-center study conducted in Beijing, China. Res Sq 2020 2020/11/19. 10.21203/rs.3.rs-27288/v1 [DOI]
- 31. Bagheri SH, Asghari A, Farhadi M, et al. : Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med J Islam Repub Iran 2020;34(1). 10.34171/mjiri.34.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tung-Chen Y, Martí de Gracia M, Díez-Tascón A, et al. : Correlation between Chest Computed Tomography and Lung Ultrasonography in Patients with Coronavirus Disease 2019 (COVID-19). Ultrasound Med Biol 2020;46(11):2918–2926. 10.1016/j.ultrasmedbio.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gilani S, Roditi R, Naraghi M: COVID-19 and anosmia in Tehran, Iran. Med Hypotheses 2020:141. 10.1016/j.mehy.2020.109757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brisca G, Ferretti M, Sartoris G, et al. : The early experiences of a single tertiary Italian emergency department treating COVID-19 in children. Acta Paediatr 2020;109(10):2155–2156. 10.1111/apa.15451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Denis F, Galmiche S, Dinh A, et al. : Epidemiological Observations on the Association between Anosmia and COVID-19 Infection: Analysis of Data from a Self-Assessment Web Application. J Med Internet Res 2020;22(6). 10.2196/19855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahmed MAM, Colebunders R, Siewe Fodjo JN: Evidence for significant COVID-19 community transmission in Somalia using a clinical case definition. Int J Infect Dis 2020;98:206–207. 10.1016/j.ijid.2020.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poncet-Megemont L, Paris P, Tronchere A, et al. : High Prevalence of Headaches During Covid-19 Infection: A Retrospective Cohort Study. Headache 2020. 10.1111/head.13923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lindahl JF, Hoffman T, Esmaeilzadeh M, et al. : High seroprevalence of SARS-CoV-2 in elderly care employees in Sweden. Infect Ecol Epidemiol 2020;10(1). 10.1080/20008686.2020.1789036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gerbaud L, Guiguet-Auclair C, Breysse F, et al. : Hospital and population-based evidence for covid-19 early circulation in the east of france. Int J Environ Res Public Health 2020;17(19):1–17. 10.3390/ijerph17197175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fernandes DE, Ferreira PRA, Kirsztajn GM: Pre-exposure prophylaxis during the SARS-CoV-2 pandemic: can PrEP prevent COVID-19-related symptoms? Epidemiol. Infect. 2020. 10.1017/S0950268820002253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fjaeldstad AW: Prolonged complaints of chemosensory loss after covid-19. Dan. Med. J. 2020;67(8):1–11. [PubMed] [Google Scholar]
- 42. Makaronidis J, Mok J, Balogun N, et al. : Seroprevalence of SARS-CoV-2 antibodies in people with an acute loss in their sense of smell and/or taste in a community-based population in London, UK: An observational cohort study. PLoS Med 2020 Oct;17(10):e1003358. Epub 2020/10/02. eng. 10.1371/journal.pmed.1003358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coelho DH, Kons ZA, Costanzo RM, et al. : Subjective Changes in Smell and Taste During the COVID-19 Pandemic: A National Survey—Preliminary Results. Otolaryngol Head Neck Surg 2020;163(2):302–306. 10.1177/0194599820929957 [DOI] [PubMed] [Google Scholar]
- 44. Reiter ER, Coelho DH, Kons ZA, et al. : Subjective smell and taste changes during the COVID-19 pandemic: Short term recovery. Am J Otolaryngol Head Neck Med Surg 2020;41(6). 10.1016/j.amjoto.2020.102639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spadera L, Viola P, Pisani D, et al. : Sudden olfactory loss as an early marker of COVID-19: a nationwide Italian survey. Eur. Arch. Otorhinolaryngol. 2020. 10.1007/s00405-020-06252-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aguila-Gordo D, Flores-Barragan JM, Ferragut-Lloret F, et al. : Acute myelitis and SARS-CoV-2 infection. A new etiology of myelitis? J. Clin. Neurosci. 2020 Oct;80:280–281. 10.1016/j.jocn.2020.07.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Selvaraj V, Sacchetti D, Finn A, et al. : Acute Vision Loss in a Patient with COVID-19. R I Med J (2013) 2020 Jun 10;103(6):37–8. Epub 2020/06/18. eng. [PubMed] [Google Scholar]
- 48. Mak PQ, Chung KS, Wong JSC, et al. : Anosmia and ageusia: Not an uncommon presentation of COVID-19 infection in children and adolescents. Pediatr. Infect. Dis. J. 2020;39(8):E199–E200. 10.1097/INF.0000000000002718 [DOI] [PubMed] [Google Scholar]
- 49. Li CW, Syue LS, Tsai YS, et al. : Anosmia and olfactory tract neuropathy in a case of COVID-19. J. Microbiol. Immunol. Infect. 2020. 10.1016/j.jmii.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aragão MFVV, Leal MC, Cartaxo Filho OQ, et al. : Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. Am. J. Neuroradiol. 2020;41(9):1703–1706. 10.3174/ajnr.A6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Laurendon T, Radulesco T, Mugnier J, et al. : Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology 2020;95(5):224–225. 10.1212/WNL.0000000000009850 [DOI] [PubMed] [Google Scholar]
- 52. Kadono Y, Nakamura Y, Ogawa Y, et al. : A case of COVID-19 infection presenting with a seizure following severe brain edema. Seizure 2020;80:53–55. 10.1016/j.seizure.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maniaci A, Iannella G, Vicini C, et al. : A case of covid-19 with late-onset rash and transient loss of taste and smell in a 15-year-old boy. Am J Case Rep 2020;21:1–6. 10.12659/AJCR.925813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ho BE, Ho AP, Ho MA, et al. : Case report of familial COVID-19 cluster associated with High prevalence of anosmia, ageusia, and gastrointestinal symptoms. IDCases 2020:22. 10.1016/j.idcr.2020.e00975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Caviezel C, Weiss L, Haessig G, et al. : Case report of sequential bilateral spontaneous pneumothorax in a never-ventilated, lung-healthy COVID-19-patient. Int. J. Surg. Case Rep. 2020;75:441–445. 10.1016/j.ijscr.2020.09.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Papa A, Di Dato MT, Buonavolonta P, et al. : Clinical management of il-6 driven cytokine storm related to covid-19 in a patient with recent spinal cord stimulator implants: A case report. Reg. Anesth. Pain Med. 2020;10(4):1–3. 10.5812/aapm.104151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lockey RF, Hudey SN: Coronavirus disease 2019-associated urticaria with angioedema in a morbidly obese man successfully treated with glucocorticoids. Reg. Anesth. Pain Med. 2020;125(3):359–360. 10.1016/j.anai.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zito A, Alfonsi E, Franciotta D, et al. : COVID-19 and Guillain-Barré Syndrome: A Case Report and Review of Literature. Front. Neurol. 2020:11. 10.3389/fneur.2020.00909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Elzein F, Alsherbeeni N, Almatrafi K, et al. : COVID-19 co-infection in a patient with brucella bacteremia. Respir. Med. Case. Rep. 2020:31. 10.1016/j.rmcr.2020.101183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fitsiori A, Pugin D, Thieffry C, et al. : COVID-19 is Associated with an Unusual Pattern of Brain Microbleeds in Critically Ill Patients. J. Neuroimaging 2020;30(5):593–597. 10.1111/jon.12755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mauro DD, Md, Smimmo A, Meschini C, et al. : COVID-19 Pandemic: management of patients affected by SARSCoV-2 in Rome Covid Hospital 2 Trauma Center and safety of our surgical equipe. Res Squ 2020. 2020/11/19. 10.21203/rs.3.rs-36524/v1 [DOI] [PMC free article] [PubMed]
- 62. Aksan F, Nelson EA, Swedish KA: A COVID-19 patient with intense burning pain. J Neurovirol. 2020;26(5):800–801. 10.1007/s13365-020-00887-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Al-olama M, Rashid A, Garozzo D: COVID-19-associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Acta Neurochir 2020;162(7):1495–1499. 10.1007/s00701-020-04402-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Haroon A, Alnassani M, Aljurf M, et al. : COVID-19 post hematopoietic cell transplant, a report of 11 cases from a single center. Mediterr J Hematol Infect Dis 2020;12(1). 10.4084/MJHID.2020.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coco D, Leanza S: CT scan bilateral interstitial pneumonia caused by SARS-CoV 2. Pan Afr. Med. J. 2020;35(2):1. 10.11604/pamj.2020.35.2.23114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tran TA, Cezar R, Frandon J, et al. : CT scan does not make a diagnosis of Covid-19: A cautionary case report. Int. J. Infect. Dis. 2020;100:182–183. 10.1016/j.ijid.2020.08.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rein N, Haham N, Orenbuch-Harroch E, et al. : Description of 3 patients with myasthenia gravis and COVID-19. J. Neurol. Sci. 2020:417. 10.1016/j.jns.2020.117053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rubin ES, Sansone SA, Hirshberg A, et al. : Detection of COVID-19 in a Vulvar Lesion. Am. J. Perinatol. 2020;37(11):1183–1184. 10.1055/s-0040-1713665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ortac EE: Determination of diagnosis and disease severity, hospital and intensive care unit admission criteria in COVID-19. J Crit Intensive Care 2020;11:4–7. 10.37678/dcybd.2020.2376 [DOI] [Google Scholar]
- 70. Horowitz RI, Freeman PR, Bruzzese J: Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: A report of 2 cases. Respir. Med. Case. Rep. 2020;30. 10.1016/j.rmcr.2020.101063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Demirci Otluoglu G, Yener U, Demir MK, et al. : Encephalomyelitis associated with Covid-19 infection: case report. Br. J. Neurosurg. 2020:1–3. 10.1080/02688697.2020.1787342 [DOI] [PubMed] [Google Scholar]
- 72. Hernandez A, Muñoz P, Rojas JC, et al. : Epidemiological Chronicle of the First Recovered Coronavirus Disease Patient From Panama: Evidence of Early Cluster Transmission in a High School of Panama City. Front. Public Health 2020:8. 10.3389/fpubh.2020.553730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Valencia-Sanchez C, Wingerchuk DM: A fine balance: Immunosuppression and immunotherapy in a patient with multiple sclerosis and COVID-19. Mult. Scler. Relat. Disord. 2020:42. 10.1016/j.msard.2020.102182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Scheidl E, Canseco DD, Hadji-Naumov A, et al. : Guillain-Barré syndrome during SARS-CoV-2 pandemic: A case report and review of recent literature. J. Peripher. Nerv. Syst. 2020;25(2):204–207. 10.1111/jns.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dalakas MC: Guillain-Barre syndrome: The first documented COVID-19-triggered autoimmune neurologic disease: More to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm 2020 Sep;7(5). 10.1212/NXI.0000000000000781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. de Oliveira FAA, Palmeira DCC, Rocha-Filho PAS: Headache and pleocytosis in CSF associated with COVID-19: case report. Neurol. Sci. 2020;41(11):3021–2. 10.1007/s10072-020-04694-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Melley LE, Bress E, Polan E: Hypogeusia as the initial presenting symptom of COVID-19. BMJ Case Reports 2020;13(5). 10.1136/bcr-2020-236080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pallanti S: Importance of SARs-Cov-2 anosmia: From phenomenology to neurobiology. Compr Psychiatry 2020 Jul;100:152184. Epub 2020/05/19. eng. 10.1016/j.comppsych.2020.152184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Naz S, Hanif M, Haider MA, et al. : Meningitis as an Initial Presentation of COVID-19: A Case Report. Front. Public Health 2020:8. 10.3389/fpubh.2020.00474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ray A: Miller Fisher syndrome and COVID-19: Is there a link. BMJ Case Reports 2020;13(8). 10.1136/bcr-2020-236419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. : Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 2020;95(5):e601–e605. 10.1212/WNL.0000000000009619 [DOI] [PubMed] [Google Scholar]
- 82. Palao M, Fernández-Díaz E, Gracia-Gil J, et al. : Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord 2020 Oct;45:102377. Epub 2020/07/23. eng. 10.1016/j.msard.2020.102377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lim WS, Liang CK, Assantachai P, et al. : COVID-19 and Older People in Asia: AWGS Calls to Actions. Geriatr Gerontol Int 2020 May 4. Epub 2020/05/05. 10.1111/ggi.13939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hatipoglu N, Mine Yazici Z, Palabiyik F, et al. : Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia in pediatric cases. Int. J. Pediatr. Otorhinolaryngol. 2020:139. 10.1016/j.ijporl.2020.110469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sinadinos A, Shelswell J: Oral ulceration and blistering in patients with COVID-19. Evid. Based Dent. 2020;21(2):49. 10.1038/s41432-020-0100-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Huber M, Rogozinski S, Puppe W, et al. : Postinfectious Onset of Myasthenia Gravis in a COVID-19 Patient. Front. Neurol. 2020:11. 10.3389/fneur.2020.576153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Baba H, Kanamori H, Oshima K, et al. : Prolonged presence of SARS-CoV-2 in a COVID-19 case with rheumatoid arthritis taking iguratimod treated with ciclesonide. J. Infect. Chemother. 2020;26(10):1100–1103. 10.1016/j.jiac.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zanin L, Saraceno G, Panciani PP, et al. : SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochirurgica 2020;162(7):1491–1494. 10.1007/s00701-020-04374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. de Brito CAA, Lima PMA, de Brito MCM, et al. : Second episode of COVID-19 in health professionals: Report of two cases. International Medical Case Reports Journal 2020;13:471–5. 10.2147/IMCRJ.S277882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kasuga Y, Nishimura K, Go H, et al. : Severe olfactory and gustatory dysfunctions in a Japanese pediatric patient with coronavirus disease (COVID-19). J. Infect. Chemother. 2020. 10.1016/j.jiac.2020.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen C, Chen M, Cheng C, et al. : A special symptom of olfactory dysfunction in coronavirus disease 2019: report of three cases. J Neurovirol. 2020;26(3):456–458. 10.1007/s13365-020-00849-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Paoli D, Pallotti F, Colangelo S, et al. : Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J. Endocrinol. Investig. 2020. 10.1007/s40618-020-01261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Giné C, Laín A, García L, et al. : Thoracoscopic bullectomy for persistent air leak in a 14-year-old child with COVID-19 bilateral pulmonary disease. J. Laparoendosc. Adv. Surg. Tech. A 2020;30(8):935–938. 10.1089/lap.2020.0289 [DOI] [PubMed] [Google Scholar]
- 94. Rivas-Pollmar MI, Álvarez-Román MT, Butta-Coll NV, et al. : Thromboprophylaxis in a patient with COVID-19 and severe hemophilia A on emicizumab prophylaxis. J. Thromb. Haemost. 2020;18(9):2202–2204. 10.1111/jth.14954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Marchand L, Pecquet M, Luyton C: Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020;57(10):1265–1266. 10.1007/s00592-020-01570-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Glick LR, Fogel AL, Ramachandran S, et al. : Unilateral laterothoracic exanthem in association with coronavirus disease 2019. JAAD Case Rep 2020;6(9):900–901. 10.1016/j.jdcr.2020.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. de Sousa GC, de Sousa TC, Sakiyama MAK, et al. : Vasculitis-related stroke in young as a presenting feature of novel coronavirus disease (COVID19) - Case report. J. Clin. Neurosci. 2020;79:169–71. 10.1016/j.jocn.2020.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vargas-Gandica J, Winter D, Schnippe R, et al. : Ageusia and anosmia, a common sign of COVID-19? A case series from four countries. J Neurovirol. 2020;26(5):785–789. 10.1007/s13365-020-00875-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lechner M, Chandrasekharan D, Jumani K, et al. : Anosmia as a presenting symptom of SARS-CoV-2 infection in healthcare workers - A systematic review of the literature, case series, and recommendations for clinical assessment and management. Rhinology 2020;58(4):1–9. 10.4193/Rhin20.189 [DOI] [PubMed] [Google Scholar]
- 100. Toptan T, Aktan Ç, Basari A, et al. : Case Series of Headache Characteristics in COVID-19: Headache Can Be an Isolated Symptom. Headache 2020;60(8):1788–1792. 10.1111/head.13940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kilic O, Kalcioglu MT, Cag Y, et al. : Could sudden sensorineural hearing loss be the sole manifestation of COVID-19? An investigation into SARS-COV-2 in the etiology of sudden sensorineural hearing loss. Int. J. Infect. Dis. 2020;97:208–211. 10.1016/j.ijid.2020.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Beach SR, Praschan NC, Hogan C, et al. : Delirium in COVID-19: A case series and exploration of potential mechanisms for central nervous system involvement. Gen. Hosp. Psychiatry 2020;65:47–53. 10.1016/j.genhosppsych.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Richtmann R, Torloni MR, Oyamada Otani AR, et al. : Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: A case series. Case Rep Womens Health 2020:27. 10.1016/j.crwh.2020.e00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lechien JR, Cabaraux P, Chiesa-Estomba CM, et al. : Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck 2020;42(7):1583–1590. 10.1002/hed.26279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Brandão TB, Gueiros LA, Melo TS, et al. : Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2020. 10.1016/j.oooo.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ferrero P, Piazza I, Bonino C, et al. : Patterns of myocardial involvement in children during COVID-19 pandemic: Early experience from northern Italy. Ann. Pediatr. Cardiol. 2020;13(3):230–3. 10.4103/apc.APC_77_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Liu PY, Jiang RS: Prognosis of olfactory and gustatory dysfunctions in COVID-19 patients: A case series. Clin Case Rep 2020. 10.1002/ccr3.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sisó-Almirall A, Kostov B, Mas-Heredia M, et al. : Prognostic factors in Spanish COVID-19 patients: A case series from Barcelona. PLoS ONE 2020;15(8 August 2020). 10.1371/journal.pone.0237960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rudberg AS, Havervall S, Månberg A, et al. : SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun 2020 Oct 8;11(1):5064. Epub 2020/10/10. eng. 10.1038/s41467-020-18848-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zurita MF, Iglesias Arreaga A, Luzuriaga Chavez AA, et al. : SARS-CoV-2 Infection and COVID-19 in 5 Patients in Ecuador After Prior Treatment with Hydroxychloroquine for Systemic Lupus Erythematosus. Am J Case Rep 2020 Sep 26;21:e927304. Epub 2020/09/27. eng. 10.12659/AJCR.927304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Maggiolo F, Zoboli F, Arosio M, et al. : SARS-CoV-2 infection in persons living with HIV: A single center prospective cohort. J. Med. Virol. 2020. 10.1002/jmv.26352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Seo MY, Seok H, Hwang SJ, et al. : Trend of Olfactory and Gustatory Dysfunction in COVID-19 Patients in a Quarantine Facility. J. Korean Med. Sci. 2020;35(41):e375. 10.3346/jkms.2020.35.e375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Marzano AV, Genovese G, Fabbrocini G, et al. : Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J. Am. Acad. Dermatol. 2020;83(1):280–285. 10.1016/j.jaad.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Vaira LA, Lechien JR, Salzano G, et al. : Gustatory Dysfunction: A Highly Specific and Smell-Independent Symptom of COVID-19. Indian J Otolaryngol Head Neck Surg 2020. 10.1007/s12070-020-02182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Boscolo-Rizzo P, Borsetto D, Spinato G, et al. : New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2 positive subjects. Res Sq 2020. 2020/11/19. 10.1007/s00405-020-06066-9 [DOI] [PMC free article] [PubMed]
- 116. Vaira LA, Hopkins C, Salzano G, et al. : Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 2020 Jul;42(7):1560–9. WOS:000534373200001 10.1002/hed.26269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Petrocelli M, Ruggiero F, Baietti AM, et al. : Remote psychophysical evaluation of olfactory and gustatory functions in early-stage coronavirus disease 2019 patients: The Bologna experience of 300 cases. J. Laryngol. Otol. 2020;134(7):571–576. 10.1017/S0022215120001358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Vaira LA, Salzano G, Petrocelli M, et al. : Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck 2020;42(7):1570–1576. 10.1002/hed.26228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sahin D, Tanacan A, Erol SA, et al. : A pandemic center’s experience of managing pregnant women with COVID-19 infection in Turkey: A prospective cohort study. Int. J. Gynaecol. Obstet. 2020;151(1):74–82. 10.1002/ijgo.13318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pouletty M, Borocco C, Ouldali N, et al. : Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): A multicentre cohort. Ann. Rheum. Dis. 2020;79(8):999–1006. 10.1136/annrheumdis-2020-217960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Paolo G: Does COVID-19 cause permanent damage to olfactory and gustatory function? Med Hypotheses 2020 Oct;143:110086. Epub 2020/07/30. eng. 10.1016/j.mehy.2020.110086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Karimi-Galougahi M, Safavi Naini A, Ghorbani J, et al. : Emergence and Evolution of Olfactory and Gustatory Symptoms in Patients with COVID-19 in the Outpatient Setting. Indian J Otolaryngol Head Neck Surg 2020. 10.1007/s12070-020-02166-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Papa ND, Sambataro G, Minniti A, et al. : Impact of COVID-19 outbreak in an Italian cohort of patients with systemic sclerosis. Ther Adv Musculoskelet Dis 2020:12. 10.1177/1759720X20953356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kosugi EM, Lavinsky J, Romano FR, et al. : Incomplete and late recovery of sudden olfactory dysfunction in COVID-19. Braz. J. Otorhinolaryngol. 2020;86(4):490–496. 10.1016/j.bjorl.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Islamoglu Y, Gemcioglu E, Ates I: Objective evaluation of the nasal mucosal secretion in COVID-19 patients with anosmia. Ir. J. Med. Sci. 2020. 10.1007/s11845-020-02405-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lechien J, Cabaraux P, Chiesa-Estomba C, et al. : Objective olfactory testing in patients presenting with sudden onset olfactory dysfunction as the first manifestation of confirmed COVID-19 infection. medRxiv 2020: 2020.04.15.20066472. 10.1101/2020.04.15.20066472 [DOI]
- 127. Kandemirli SG, Altundag A, Yildirim D, et al. : Olfactory Bulb MRI and Paranasal Sinus CT Findings in Persistent COVID-19 Anosmia. Acad. Radiol. 2020. 10.1016/j.acra.2020.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Strauss SB, Lantos JE, Heier LA, et al. : Olfactory bulb signal abnormality in patients with COVID-19 who present with neurologic symptoms. Am. J. Neuroradiol. 2020;41(10):1882–1887. 10.3174/ajnr.A6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Naeini AS, Karimi-Galougahi M, Raad N, et al. : Paranasal sinuses computed tomography findings in anosmia of COVID-19. Am J Otolaryngol Head Neck Med Surg 2020;41(6). 10.1016/j.amjoto.2020.102636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pieruzzini R, Ayala C, Navas J, et al. : PREDICTIVE VALUE OF SMELL AND TASTE TEST VS PCR-RT SARS-COV-2 AND RAPID DIAGNOSTIC TESTS IN THE DIAGNOSIS OF INFECTION BY COVID-19. A PROSPECTIVE MULTI-CENTRIC STUDY. medRxiv 2020: 2020.08.31.20185298. 10.1101/2020.08.31.20185298 [DOI]
- 131. Tan JY, Sim XYJ, Wee LE, et al. : A comparative study on the clinical features of COVID-19 with non-SARS-CoV-2 respiratory viral infections. J. Med. Virol. 2020. 10.1002/jmv.26486 [DOI] [PubMed] [Google Scholar]
- 132. Peyrony O, Marbeuf-Gueye C, Truong V, et al. : Accuracy of Emergency Department Clinical Findings for Diagnosis of Coronavirus Disease 2019. Ann. Emerg. Med. 2020;76(4):405–412. 10.1016/j.annemergmed.2020.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, et al. : Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur. J. Neurol. 2020;27(9):1738–1741. 10.1111/ene.14273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Liu JY, Chen TJ, Hwang SJ: Analysis of imported cases of covid-19 in taiwan: A nationwide study. Int. J. Environ. Res. Public Health 2020;17(9). 10.3390/ijerph17093311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Carignan A, Valiquette L, Grenier C, et al. : Anosmia and dysgeusia associated with SARS-CoV-2 infection: an age-matched case-control study. Cmaj 2020 Jun 29;192(26):E702–e7. Epub 2020/05/29. eng. 10.1503/cmaj.200869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chen A, Agarwal A, Ravindran N, et al. : Are Gastrointestinal Symptoms Specific for Coronavirus 2019 Infection? A Prospective Case-Control Study From the United States. Gastroenterology 2020;159(3):1161–3.e2. 10.1053/j.gastro.2020.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Luigetti M, Iorio R, Bentivoglio AR, et al. : Assessment of neurological manifestations in hospitalized patients with COVID-19. Eur. J. Neurol. 2020;27(11):2322–2328. 10.1111/ene.14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yan CH, Faraji F, Prajapati DP, et al. : Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol 2020;10(7):806–813. 10.1002/alr.22579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rojas-Lechuga MJ, Izquierdo-Domínguez A, Chiesa-Estomba C, et al. : Chemosensory dysfunction in COVID-19 out-patients. Eur. Arch. Otorhinolaryngol. 2020. 10.1007/s00405-020-06266-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Neto DB, Fornazieri MA, Dib C, et al. : Chemosensory Dysfunction in COVID-19: Prevalences, Recovery Rates, and Clinical Associations on a Large Brazilian Sample. Otolaryngol. Head Neck Surg.WOS:000565269500001. 10.1177/0194599820954825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Durrani M, Haq IU, Kalsoom U, et al. : Chest x-rays findings in covid 19 patients at a university teaching hospital-a descriptive study. Pak J Med Sci 2020;36(COVID19-S4):S22–S6. 10.12669/pjms.36.COVID19-S4.2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Galván Casas C, Català A, Carretero Hernández G, et al. : Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020;183(1):71–77. 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lechien JR, Chiesa-Estomba CM, Place S, et al. : Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J. Intern. Med. 2020;288(3):335–344. 10.1111/joim.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kim GU, Kim MJ, Ra SH, et al. : Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin. Microbiol. Infect. 2020 Jul;26(7). WOS:000544273300033. 10.1016/j.cmi.2020.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lapostolle F, Schneider E, Vianu I, et al. : Clinical features of 1487 COVID-19 patients with outpatient management in the Greater Paris: the COVID-call study. Intern. Emerg. Med. 2020;15(5):813–817. 10.1007/s11739-020-02379-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Corsini Campioli C, Cano Cevallos E, Assi M, et al. : Clinical predictors and timing of cessation of viral RNA shedding in patients with COVID-19. J Clin Virol 2020 Sep;130:104577. Epub 2020/08/11. eng. 10.1016/j.jcv.2020.104577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ferreli F, Gaino F, Russo E, et al. : Clinical presentation at the onset of COVID-19 and allergic rhinoconjunctivitis. J Allergy Clin Immunol Pract 2020;8(10):3587–3589. 10.1016/j.jaip.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rajkumar I, Anand KH, Revathishree K, et al. : Contemporary Analysis of Olfactory Dysfunction in Mild to Moderate Covid 19 Patients in A Tertiary Health Care Centre. Indian J Otolaryngol Head Neck Surg 2020. 10.1007/s12070-020-02175-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wang TZ, Sell J, Weiss D, et al. : COVID-19 presenting as anosmia and dysgeusia in New York City emergency departments, March - April, 2020. medRxiv 2020:2020.07.06.20147751. 10.1101/2020.07.06.201477 [DOI]
- 150. Cho RHW, To ZWH, Yeung ZWC, et al. : COVID-19 Viral Load in the Severity of and Recovery From Olfactory and Gustatory Dysfunction. Laryngoscope 2020;130(11):2680–2685. 10.1002/lary.29056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kadiane-Oussou NJ, Klopfenstein T, Royer PY, et al. : COVID-19: comparative clinical features and outcome in 114 patients with or without pneumonia (Nord Franche-Comte Hospital, France). Microbes Infect. 2020. 10.1016/j.micinf.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nakanishi H, Suzuki M, Maeda H, et al. : Differential Diagnosis of COVID-19: Importance of Measuring Blood Lymphocytes, Serum Electrolytes, and Olfactory and Taste Functions. Tohoku J. Exp. Med. 2020;252(2):109–119. 10.1620/tjem.252.109 [DOI] [PubMed] [Google Scholar]
- 153. Sheng WH, Liu WD, Wang JT, et al. : Dysosmia and dysgeusia in patients with COVID-19 in northern Taiwan. J. Formos. Med. Assoc. 2020. 10.1016/j.jfma.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sakalli E, Temirbekov D, Bayri E, et al. : Ear nose throat-related symptoms with a focus on loss of smell and/or taste in COVID-19 patients. Am J Otolaryngol Head Neck Med Surg 2020;41(6). 10.1016/j.amjoto.2020.102622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lagi F, Piccica M, Graziani L, et al. : Early experience of an infectious and tropical diseases unit during the coronavirus disease (COVID-19) pandemic, Florence, Italy, February to March 2020. Euro Surveill. 2020;25(17). 10.2807/1560-7917.ES.2020.25.17.2000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Vacchiano V, Riguzzi P, Volpi L, et al. : Early neurological manifestations of hospitalized COVID-19 patients. Neurol. Sci. 2020;41(8):2029–2031. 10.1007/s10072-020-04525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Amer MA, Elsherif HS, Abdel-Hamid AS, et al. : Early recovery patterns of olfactory disorders in COVID-19 patients; a clinical cohort study. Am J Otolaryngol Head Neck Med Surg 2020;41(6). 10.1016/j.amjoto.2020.102725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ombajo LA, Mutono N, Sudi P, et al. : Epidemiological And Clinical Characteristics Of Covid-19 Patients In Kenya. medRxiv 2020:2020.11.09.20228106. 10.1101/2020.11.09.20228106 [DOI]
- 159. Maechler F, Gertler M, Hermes J, et al. : Epidemiological and clinical characteristics of SARS-CoV-2 infections at a testing site in Berlin, Germany, March and April 2020—a cross-sectional study. Clin. Microbiol. Infect. 2020. 10.1016/j.cmi.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hussain MH, Mair M, Rea P: Epistaxis as a marker for severe acute respiratory syndrome coronavirus-2 status - A prospective study. J. Laryngol. Otol. 2020;134(8):717–720. 10.1017/S0022215120001863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Shah NN, Hussain RT, Mustafa H, et al. : Evaluation of Olfactory Acuity in Patients with Coronavirus Disease 2019 (COVID-19). Indian J of Otolaryngol Head Neck Surg 2020. 10.1007/s12070-020-02241-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Gorzkowski V, Bevilacqua S, Charmillon A, et al. : Evolution of Olfactory Disorders in COVID-19 Patients. Laryngoscope 2020;130(11):2667–2673. 10.1002/lary.28957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Klopfenstein T, Kadiane-Oussou NJ, Toko L, et al. : Features of anosmia in COVID-19. Med. Mal. Infect. 2020;50(5):436–439. 10.1016/j.medmal.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Jalessi M, Barati M, Rohani M, et al. : Frequency and outcome of olfactory impairment and sinonasal involvement in hospitalized patients with COVID-19. Neurol. Sci. 2020;41(9):2331–2338. 10.1007/s10072-020-04590-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Liotta EM, Batra A, Clark JR, et al. : Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann. Clin. Transl. Neurol. 2020. 10.1002/acn3.51210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Membrilla JA, de Lorenzo Í, Sastre M: Headache as a Cardinal Symptom of Coronavirus Disease 2019: A Cross-Sectional Study. Headache 2020. 10.1111/head.13967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Rocha-Filho PAS, Magalhães JE: Headache associated with COVID-19: Frequency, characteristics and association with anosmia and ageusia. Cephalalgia 2020;40(13):1443–1451. 10.1177/0333102420966770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Uygun O, Ertas M, Ekizoglu E, et al. : Headache characteristics in COVID-19 pandemic-a survey study. J. Headache Pain 2020 Oct;21(1). WOS:000579589000001. 10.1186/s10194-020-01188-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Assaad S, Avrillon V, Fournier ML, et al. : High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur. J. Cancer 2020;135:251–259. 10.1016/j.ejca.2020.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Menni C, Valdes A, Freydin MB, et al. : Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. medRxiv 2020:2020.04.05.20048421. 10.1038/s41591-020-0916-2 [DOI]
- 171. Mohamud MFY, Mohamed YG, Ali AM, et al. : Loss of taste and smell are common clinical characteristics of patients with COVID-19 in somalia: A retrospective double centre study. Infect Drug Resist 2020;13:2631–2635. 10.2147/IDR.S263632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Dawson P, Rabold EM, Laws RL, et al. : Loss of Taste and Smell as Distinguishing Symptoms of COVID-19. medRxiv 2020:2020.05.13.20101006. 10.1093/cid/ciaa799 [DOI] [PMC free article] [PubMed]
- 173. Çalica Utku A, Budak G, Karabay O, et al. : Main symptoms in patients presenting in the COVID-19 period. Scott. Med. J. 2020;65(4):127–132. 10.1177/0036933020949253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Mao L, Jin HJ, Wang MD, et al. : Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020 Jun;77(6):683–90. WOS:000542138800006. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Studart-Neto A, Guedes BF, de Luca e Tuma R, et al. : Neurological consultations and diagnoses in a large, dedicated COVID-19 university hospital. Arq. Neuropsiquiatr. 2020;78(8):494–500. 10.1590/0004-282x20200089 [DOI] [PubMed] [Google Scholar]
- 176. Pinna P, Grewal P, Hall JP, et al. : Neurological manifestations and COVID-19: Experiences from a tertiary care center at the Frontline. J. Neurol. Sci. 2020:415. 10.1016/j.jns.2020.116969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Garg R, Jain R, Sodani A, et al. : Neurological symptoms as initial manifestation of Covid-19-An observational study. Ann. Indian Acad. Neurol. 2020;23(4):482–486. 10.4103/aian.AIAN_560_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Liang YJ, Xu JB, Chu M, et al. : Neurosensory dysfunction: A diagnostic marker of early COVID-19. Int. J. Infect. Dis. 2020 Sep;98:347–52. WOS:000569065400020. 10.1016/j.ijid.2020.06.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Klopfenstein T, Zahra H, Kadiane-Oussou NJ, et al. : New loss of smell and taste: Uncommon symptoms in COVID-19 patients in Nord Franche-Comte cluster, France. Int J Infect Dis 2020;100:117–122. 10.1016/j.ijid.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Boscolo-Rizzo P, Borsetto D, Spinato G, et al. : New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2-positive subjects. Eur. Arch. Otorhinolaryngol. 2020;277(9):2637–2640. 10.1007/s00405-020-06066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Patel A, Charani E, Ariyanayagam D, et al. : New-onset anosmia and ageusia in adult patients diagnosed with SARS-CoV-2 infection. Clin. Microbiol. Infect. 2020;26(9):1236–1241. 10.1016/j.cmi.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Vaira LA, Deiana G, Fois AG, et al. : Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck 2020 Jun;42(6):1252–8. WOS:000529255000001. 10.1002/hed.26204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Lechien JR, Ducarme M, Place S, et al. : Objective olfactory findings in hospitalized severe COVID-19 patients. Pathogens 2020;9(8):1–6. 10.3390/pathogens9080627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Shemer A, Einan-Lifshitz A, Itah A, et al. : Ocular involvement in coronavirus disease 2019 (COVID-19): a clinical and molecular analysis. Int. Ophthalmol. 2020. 10.1007/s10792-020-01592-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Altin F, Cingi C, Uzun T, et al. : Olfactory and gustatory abnormalities in COVID-19 cases. Eur. Arch. Otorhinolaryngol. 2020;277(10):2775–2781. 10.1007/s00405-020-06155-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Qiu CH, Cui C, Hautefort C, et al. : Olfactory and Gustatory Dysfunction as an Early Identifier of COVID-19 in Adults and Children: An International Multicenter Study. Otolaryngol. Head Neck Surg. 2020 Oct;163(4):714–21. WOS:000542267700001. 10.1177/0194599820934376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ramasamy K, Saniasiaya J, Abdul GN: Olfactory and Gustatory Dysfunctions as a Clinical Manifestation of Coronavirus Disease 2019 in a Malaysian Tertiary Center Ann. Otol. Rhinol. Laryngol. 2020. 10.1177/0003489420963165 [DOI] [PubMed]
- 188. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. : Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020 2020/08/01;277(8):2251–61. 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Meini S, Suardi LR, Busoni M, et al. : Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: sex differences and recovery time in real-life. Eur. Arch. Otorhinolaryngol. 2020. 10.1007/s00405-020-06102-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Paderno A, Mattavelli D, Rampinelli V, et al. : Olfactory and Gustatory Outcomes in COVID-19: A Prospective Evaluation in Nonhospitalized Subjects. Otolaryngol. Head Neck Surg. 2020. 10.1177/0194599820939538 [DOI] [PMC free article] [PubMed]
- 191. Speth MM, Singer-Cornelius T, Oberle M, et al. : Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: Prevalence, Severity, Timing, and Associated Characteristics. Otolaryngol. Head Neck Surg. 2020;163(1):114–120. 10.1177/0194599820929185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. D’Ascanio L, Pandolfini M, Cingolani C, et al. : Olfactory Dysfunction in COVID-19 Patients: Prevalence and Prognosis for Recovering Sense of Smell. Otolaryngol. Head Neck Surg. 2020. 10.1177/0194599820943530 [DOI] [PubMed]
- 193. Otte MS, Eckel HNC, Poluschkin L, et al. : Olfactory dysfunction in patients after recovering from COVID-19. Acta Otolaryngol. 2020. 10.1080/00016489.2020.1811999 [DOI] [PubMed] [Google Scholar]
- 194. Klein H, Asseo K, Karni N, et al. : Onset, duration, and persistence of taste and smell changes and other COVID-19 symptoms: longitudinal study in Israeli patients. medRxiv 2020:2020.09.25.20201343.
- 195. Panda S, Mohamed A, Sikka K, et al. : Otolaryngologic Manifestation and Long-Term Outcome in Mild COVID-19: Experience from a Tertiary Care Centre in India. Indian J Otolaryngol Head Neck Surg 2020. 10.1007/s12070-020-02217-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Özçelik Korkmaz M, Egilmez OK, Özçelik MA, et al. : Otolaryngological manifestations of hospitalised patients with confirmed COVID-19 infection. Eur. Arch. Otorhinolaryngol. 2020. 10.1007/s00405-020-06396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Elibol E: Otolaryngological symptoms in COVID-19. Eur. Arch. Otorhinolaryngol. 2020. 10.1007/s00405-020-06319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Lagier JC, Million M, Gautret P, et al. : Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med. Infect. Dis. 2020:36. 10.1016/j.tmaid.2020.101791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Uhm JS, Ahn JY, Hyun J, et al. : Patterns of viral clearance in the natural course of asymptomatic COVID-19: Comparison with symptomatic non-severe COVID-19. Int. J. Infect. Dis. 2020;99:279–285. 10.1016/j.ijid.2020.07.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Yan CH, Prajapati DP, Ritter ML, et al. : Persistent Smell Loss Following Undetectable SARS-CoV-2. Otolaryngol. Head Neck Surg. 2020;163(5):923–925. 10.1177/0194599820934769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. López de la Iglesia J, Fernández-Villa T, Rivero A, et al. : Predictive factors of COVID-19 in patients with negative RT-qPCR. Semergen 2020;46:6–11. 10.1016/j.semerg.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Haehner A, Draf J, Dräger S, et al. : Predictive Value of Sudden Olfactory Loss in the Diagnosis of COVID-19. ORL 2020. 10.1159/000509143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. O’Keefe JB, Tong EJ, Datoo O’Keefe GA, et al. : Predictors of disease duration and symptom course of outpatients with acute covid-19: a retrospective cohort study. medRxiv 2020:2020.06.05.20123471. 10.1101/2020.06.05.20123471 [DOI]
- 204. Pinato DJ, Lee AJX, Biello F, et al. : Presenting features and early mortality from SARS-CoV-2 infection in cancer patients during the initial stage of the COVID-19 pandemic in Europe. Cancers 2020;12(7):1–13. 10.3390/cancers12071841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Redd WD, Zhou JC, Hathorn KE, et al. : Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology 2020;159(2):765–7.e2. 10.1053/j.gastro.2020.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Lee Y, Min P, Lee S, et al. : Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J Korean Med Sci 2020 May 11;35(18):e174. Epub 2020/05/10. eng. 10.3346/jkms.2020.35.e174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Chary E, Carsuzaa F, Trijolet JP, et al. : Prevalence and Recovery From Olfactory and Gustatory Dysfunctions in Covid-19 Infection: A Prospective Multicenter Study. Am. J. Rhinol. Allergy 2020;34(5):686–693. 10.1177/1945892420930954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Lv H, Zhang W, Zhu Z, et al. : Prevalence and recovery time of olfactory and gustatory dysfunction in hospitalized patients with COVID-19 in Wuhan. China. Int. J. Infect. Dis. 2020;100:507–512. 10.1016/j.ijid.2020.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Moein ST, Hashemian SM, Tabarsi P, et al. : Prevalence and reversibility of smell dysfunction measured psychophysically in a cohort of COVID-19 patients. Int Forum Allergy Rhinol 2020;10(10):1127–1135. 10.1002/alr.22680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Al-Ani RM, Acharya D: Prevalence of Anosmia and Ageusia in Patients with COVID-19 at a Primary Health Center, Doha, Qatar. Indian J Otolaryngol Head Neck Surg 2020. 10.1007/s12070-020-02064-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Vivek Kumar P, Rohit S, Pradeepti N, et al. : Prevalence Of Anosmia And Dysgeusia In Patients Of COVID-19 In A Dedicated Covid Hospital. Res Sq 2020. 2020/11/19. 10.21203/rs.3.rs-65011/v1 [DOI] [Google Scholar]
- 212. Nouchi A, Chastang J, Miyara M, et al. : Prevalence of hyposmia and hypogeusia in 390 COVID-19 hospitalized patients and outpatients: a cross-sectional study. Eur. J. Clin. Microbiol. Infect. Dis. 2020. 10.1007/s10096-020-04056-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Mishra P, Gowda V, Dixit S, et al. : Prevalence of New Onset Anosmia in COVID-19 Patients: Is The Trend Different Between European and Indian Population? Indian J Otolaryngol Head Neck Surg 2020:72(4):484–487. 10.1007/s12070-020-01986-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Avcl H, Karabulut B, Farasoglu A, et al. : Relationship between anosmia and hospitalisation in patients with coronavirus disease 2019: An otolaryngological perspective. J. Laryngol. Otol. 2020;134(8):710–716. 10.1017/S0022215120001851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Karni N, Klein H, Asseo K, et al. : Self-rated smell ability enables highly specific predictors of COVID-19 status: a case control study in Israel. medRxiv 2020:2020.07.30.20164327. 10.1101/2020.07.30.20164327 [DOI] [PMC free article] [PubMed]
- 216. Lee DJ, Lockwood J, Das P, et al. : Self-reported anosmia and dysgeusia as key symptoms of coronavirus disease 2019. Cjem 2020 Sep;22(5):595–602. Epub 2020/06/09. eng. 10.1017/cem.2020.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Yan CRH, Faraji F, Prajapati DP, et al. : Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol 2020 Jul;10(7):821–31. 10.1002/alr.22592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Song J, Deng Y-K, Wang H, et al. : Self-reported taste and smell disorders in patients with COVID-19: distinct features in China. medRxiv 2020:2020.06.12.20128298. 10.1101/2020.06.12.20128298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. DeBiasi RL, Song X, Delaney M, et al. : Severe Coronavirus Disease-2019 in Children and Young Adults in the Washington, DC, Metropolitan Region. J. Pediatr. 2020;223:199–203.e 1. 10.1016/j.jpeds.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Konstantinidis I, Delides A, Tsakiropoulou E, et al. : Short-term follow-up of self-isolated covid-19 patients with smell and taste dysfunction in Greece: Two phenotypes of recovery. ORL 2020. 10.1159/000511436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Alshami A, Alattas R, Anan H, et al. : Silent disease and loss of taste and smell are common manifestations of SARS-COV-2 infection in a quarantine facility: Saudi Arabia. PLoS One 2020;15(10):e0241258. Epub 2020/10/31. 10.1371/journal.pone.0241258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Paderno A, Schreiber A, Grammatica A, et al. : Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol 2020;10(8):955–962. 10.1002/alr.22610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Dell’Era V, Farri F, Garzaro G, et al. : Smell and taste disorders during COVID-19 outbreak: Cross-sectional study on 355 patients. Head Neck 2020;42(7):1591–1596. 10.1002/hed.26288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Barón-Sánchez J, Santiago C, Goizueta-San Martín G, et al. : Smell and taste disorders in Spanish patients with mild COVID-19. Neurologia 2020. 10.1016/j.nrl.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Izquierdo-Domínguez A, Rojas-Lechuga MJ, Chiesa-Estomba C, et al. : Smell and taste dysfunction in covid-19 is associated with younger age in ambulatory settings: A multicenter cross-sectional study. J. Investig. Allergol. Clin. Immunol. 2020;30(5):346–357. 10.18176/jiaci.0595 [DOI] [PubMed] [Google Scholar]
- 226. Vaira LA, Hopkins C, Petrocelli M, et al. : Smell and taste recovery in coronavirus disease 2019 patients: A 60-day objective and prospective study. J. Laryngol. Otol. 2020;134(8):703–709. 10.1017/S0022215120001826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Lima MA, Silva MTT, Oliveira RV, et al. : Smell dysfunction in COVID-19 patients: More than a yes-no question. J. Neurosurg. Sci. 2020:418. 10.1016/j.jns.2020.117107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Moein ST, Hashemian SM, Mansourafshar B, et al. : Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol 2020;10(8):944–950. 10.1002/alr.22587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Foster KJ, Jauregui E, Tajudeen B, et al. : Smell loss is a prognostic factor for lower severity of coronavirus disease 2019. Ann. Allergy Asthma Immunol. 2020;125(4):481–483. 10.1016/j.anai.2020.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Freni F, Meduri A, Gazia F, et al. : Symptomatology in head and neck district in coronavirus disease (COVID-19): A possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol 2020 Sep-Oct;41(5):102612. Epub 2020/06/24. eng. 10.1016/j.amjoto.2020.102612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Salepci E, Turk B, Ozcan SN, et al. : Symptomatology of COVID-19 from the otorhinolaryngology perspective: a survey of 223 SARS-CoV-2 RNA-positive patients. Eur. Arch. Otorhinolaryngol. 2020. 10.1007/s00405-020-06284-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Cazzolla AP, Lovero R, Lo Muzio L, et al. : Taste and Smell Disorders in COVID-19 Patients: Role of Interleukin-6. ACS Chem Neurosci 2020;11(17):2774–81. Epub 2020/08/19. eng. 10.1021/acschemneuro.0c00447 [DOI] [PubMed] [Google Scholar]
- 233. Bidkar V, Mishra M, Selvaraj K, et al. : Testing Olfactory and Gustatory Dysfunctions among Quarantine COVID-19 Suspects. Indian J Otolaryngol Head Neck Surg 2020. 10.1007/s12070-020-02210-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Joffily L, Ungierowicz A, David AG, et al. : The close relationship between sudden loss of smell and COVID-19. Braz. J. Otorhinolaryngol. 2020;86(5):632–638. 10.1016/j.bjorl.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Moro E, Priori A, Beghi E, et al. : The international European Academy of Neurology survey on neurological symptoms in patients with COVID-19 infection. Eur. J. Neurol. 2020;27(9):1727–1737. 10.1111/ene.14407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Fantozzi PJ, Pampena E, Di Vanna D, et al. : Xerostomia, gustatory and olfactory dysfunctions in patients with COVID-19. Am J Otolaryngol Head Neck Med Surg 2020;41(6). 10.1016/j.amjoto.2020.102721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Mao L, Wang M, Chen S, et al. : Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study. medRxiv 2020:2020.02.22.20026500. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Cabaraux P, Lechien JR, Saussez S, et al. : Objective Olfactory Evaluation of Self-reported Olfactory Dysfunction in a Case Series of 86 COVID-19 Patients. medRxiv 2020:2020.05.03.20088526. 10.1002/hed.26279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Chiesa-Estomba CM, Lechien JR, Radulesco T, et al. : Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. Eur. J. Neurol. 2020;27(11):2318–2321. 10.1111/ene.14440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Talavera B, García-Azorín D, Martínez-Pías E, et al. : Anosmia is associated with lower in-hospital mortality in COVID-19. J. Neurol. Sci. 2020:419. . 10.1016/j.jns.2020.117163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Giorli A, Ferretti F, Biagini C, et al. : A Literature Systematic Review with Meta-Analysis of Symptoms Prevalence in Covid-19: the Relevance of Olfactory Symptoms in Infection Not Requiring Hospitalization. Curr. Treat. Options Neurol. 2020;22(10):36. Epub 2020/08/28. eng. 10.1007/s11940-020-00641-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Department of Health and Social Care : Statement from the UK Chief Medical Officers on an update to coronavirus symptoms: 18 May 2020. 2020.
- 243. Ear N Throat Society of the United Kingdom (ENTUK) : Loss of sense of smell as marker of COVID-19 infection. 2020.
- 244. Kaye R, Chang CWD, Kazahaya K, et al. : COVID-19 Anosmia Reporting Tool: Initial Findings. Otolaryngol. Head Neck Surg. 2020 2020/07/01;163(1):132–4. 10.1177/0194599820922992 [DOI] [PubMed] [Google Scholar]
- 245. Suzuki M, Saito K, Min W-P, et al. : Identification of Viruses in Patients With Postviral Olfactory Dysfunction. Laryngoscope 2007 2007/02/01;117(2):272–7. 10.1097/01.mlg.0000249922.37381.1e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kondo Y, Miyazaki S, Yamashita R, et al. : Coinfection with SARS-CoV-2 and influenza A virus. BMJ Case Rep 2020;13(7). 10.1016/j.idcr.2020.e00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Langerbeins P, Fürstenau M, Gruell H, et al. : COVID-19 complicated by parainfluenza co-infection in a patient with chronic lymphocytic leukemia. Eur. J. Haematol. 2020;105(4):508–511. 10.1111/ejh.13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Zayet S, Kadiane-Oussou NJ, Lepiller Q, et al. : Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microbes Infect. 2020;22(9):481–488. 10.1016/j.micinf.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Alshebri MS, Alshouimi RA, Alhumidi HA, et al. : Neurological Complications of SARS-CoV, MERS-CoV, and COVID-19. SN Compr Clin Med 2020 2020/11/01;2(11):2037–47. 10.1007/s42399-020-00589-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Hwang CS: Olfactory neuropathy in severe acute respiratory syndrome: report of A case. Acta Neurol. Taiwan. 2006 Mar;15(1):26–8. Epub 2006/04/08. eng. [PubMed] [Google Scholar]
- 251. von Bartheld CS, Hagen MM, Butowt R: Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis Reveals Significant Ethnic Differences. ACS Chem. Neurosci. 2020 2020/10/07;11(19):2944–61. 10.1101/2020.06.15.20132134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Heymans F, Lacroix J-S, Terzic A, et al. : Gustatory dysfunction after mandibular zoster. Neurol. Sci. 2011 2011/06/01;32(3):461–4. 10.1007/s10072-010-0421-3 [DOI] [PubMed] [Google Scholar]
- 253. Graham CS, Graham BG, Bartlett JA, et al. : Taste and smell losses in HIV infected patients. Physiol. Behav. 1995 1995/08/01/;58(2):287–93. 10.1016/0031-9384(95)00049-o [DOI] [PubMed] [Google Scholar]
- 254. Zayet S, Klopfenstein T, Mercier J, et al. : Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection 2020:1–5. eng. 10.1007/s15010-020-01442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Butowt R, von Bartheld CS: Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2020 Sep 11:1073858420956905. 10.1177/1073858420956905 [DOI] [PMC free article] [PubMed]
- 256. Altin F, Cingi C, Uzun T, et al. : Olfactory and gustatory abnormalities in COVID-19 cases. Eur. Arch. Otorhinolaryngol. 2020;277(10):2775–81. Epub 2020/06/23. eng. 10.1007/s00405-020-06155-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. El-Anwar MW, Elzayat S, Fouad YA: ENT manifestation in COVID-19 patients. Auris Nasus Larynx 2020;47(4):559–64. Epub 2020/06/15. eng. 10.1016/j.anl.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Govindarajalu P, Shah R, Parsana M: Otorhinolaryngological manifestations of COVID-19-A systematic review. Res Sq 2020.
- 259. Tong JY, Wong A, Zhu D, et al. : The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2020 Jul;163(1):3–11. Epub 2020/05/06. eng. 10.1177/0194599820926473 [DOI] [PubMed] [Google Scholar]
- 260. Lehrich BM, Goshtasbi K, Raad RA, et al. : Aggregate Prevalence of Chemosensory and Sinonasal Dysfunction in SARS-CoV-2 and Related Coronaviruses. Otolaryngol Head Neck Surg 2020 Jul;163(1):156–61. Epub 2020/05/20. eng. [DOI] [PubMed] [Google Scholar]
- 261. Chilvers MA, McKean M, Rutman A, et al. : The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J 2001 Dec;18(6):965–70. Epub 2002/02/07. eng. 10.1183/09031936.01.00093001 [DOI] [PubMed] [Google Scholar]
- 262. Brann JH, Firestein SJ: A lifetime of neurogenesis in the olfactory system. Front Neurosci 2014;8:182. eng. 10.3389/fnins.2014.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Hoffmann M, Kleine-Weber H, Schroeder S, et al. : SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020;181(2):271–80. e8. Epub 2020/03/05. eng. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Bilinska K, Jakubowska P, Von Bartheld CS, et al. : Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem Neurosci 2020;11(11):1555–62. Epub 2020/05/19. eng. 10.1021/acschemneuro.0c00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Brann DH, Tsukahara T, Weinreb C, et al. : Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. bioRxiv 2020:2020.03.25.009084. 10.1101/2020.03.25.009084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Bryche B, St Albin A, Murri S, et al. : Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun 2020;89:579–86. Epub 2020/07/03. eng. 10.1016/j.bbi.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Fodoulian L, Tuberosa J, Rossier D, et al. : SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. bioRxiv 2020. 10.1016/j.isci.2020.101839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Schwob JE: Neural regeneration and the peripheral olfactory system. Anat Rec 2002 Feb 15;269(1):33–49. Epub 2002/03/14. eng. 10.1002/ar.10047 [DOI] [PubMed] [Google Scholar]
- 269. Netland J, Meyerholz DK, Moore S, et al. : Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008 Aug;82(15):7264–75. Epub 2008/05/23. eng. 10.1128/JVI.00737-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Rodriguez S, Cao L, Rickenbacher GT, et al. : Innate immune signaling in the olfactory epithelium reduces odorant receptor levels: modeling transient smell loss in COVID-19 patients. medRxiv 2020:2020.06.14.20131128. eng. 10.1101/2020.06.14.20131128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Torabi A, Mohammadbagheri E, Akbari Dilmaghani N, et al. : Proinflammatory Cytokines in the Olfactory Mucosa Result in COVID-19 Induced Anosmia. ACS Chem Neurosci 2020;11(13):1909–13. Epub 2020/06/11. eng. 10.1021/acschemneuro.0c00249 [DOI] [PubMed] [Google Scholar]
- 272. Henkin RI, Schmidt L, Velicu I: Interleukin 6 in hyposmia. JAMA Otolaryngol Head Neck Surg 2013 Jul;139(7):728–34. Epub 2013/07/23. eng. 10.1001/jamaoto.2013.3392 [DOI] [PubMed] [Google Scholar]
- 273. Small DM, Prescott J: Odor/taste integration and the perception of flavor. Exp Brain Res 2005 Oct;166(3–4):345–57. Epub 2005/07/20. eng. 10.1007/s00221-005-2376-9 [DOI] [PubMed] [Google Scholar]
- 274. Vaira LA, Salzano G, Fois AG, et al. : Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int Forum Allergy Rhinol 2020 Sep;10(9):1103–4. Epub 2020/04/29. eng. 10.1002/alr.22593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Giacomelli A, Pezzati L, Conti F, et al. : Self-reported Olfactory and Taste Disorders in Patients With Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Clin Infect Dis 2020 Jul 28;71(15):889–90. eng. 10.1093/cid/ciaa330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Lozada-Nur F, Chainani-Wu N, Fortuna G, et al. : Dysgeusia in COVID-19: Possible Mechanisms and Implications. Oral Surg Oral Med Oral Pathol Oral Radiol 2020;130(3):344–6. Epub 2020/06/27. eng. 10.1016/j.oooo.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Komai M, Goto T, Suzuki H, et al. : Zinc deficiency and taste dysfunction; contribution of carbonic anhydrase, a zinc-metalloenzyme, to normal taste sensation. Biofactors 2000;12(1-4):65–70. Epub 2001/02/24. eng. 10.1002/biof.5520120111 [DOI] [PubMed] [Google Scholar]
- 278. Jothimani D, Kailasam E, Danielraj S, et al. : COVID-19: Poor outcomes in patients with zinc deficiency. International Journal of Infectious Diseases 2020 2020/11/01/;100:343–9. 10.1016/j.ijid.2020.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Najafizade N, Hemati S, Gookizade A, et al. : Preventive effects of zinc sulfate on taste alterations in patients under irradiation for head and neck cancers: A randomized placebo-controlled trial. J Res Med Sci 2013 Feb;18(2):123–6. Epub 2013/08/06. eng. [PMC free article] [PubMed] [Google Scholar]
- 280. Ripamonti C, Zecca E, Brunelli C, et al. : A randomized, controlled clinical trial to evaluate the effects of zinc sulfate on cancer patients with taste alterations caused by head and neck irradiation. Cancer 1998 May 15;82(10):1938–45. Epub 1998/05/20. eng. [DOI] [PubMed] [Google Scholar]
- 281. Kar M, Khan NA, Panwar A, et al. : Zinc Chelation Specifically Inhibits Early Stages of Dengue Virus Replication by Activation of NF-κB and Induction of Antiviral Response in Epithelial Cells. Front Immunol 2019;10:2347. Epub 2019/10/22. eng. 10.3389/fimmu.2019.02347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Xiao Z, Ehrlich E, Luo K, et al. : Zinc chelation inhibits HIV Vif activity and liberates antiviral function of the cytidine deaminase APOBEC3G. Faseb J 2007 Jan;21(1):217–22. Epub 2006/12/01. eng. 10.1096/fj.06-6773com [DOI] [PubMed] [Google Scholar]
- 283. Suliburska J, Duda G, Pupek-Musialik D: The influence of hypotensive drugs on the taste sensitivity in patients with primary hypertension. Acta Pol Pharm 2012 Jan-Feb;69(1):121–7. Epub 2012/05/12. eng. [PubMed] [Google Scholar]
- 284. Tsuruoka S, Wakaumi M, Araki N, et al. : Comparative study of taste disturbance by losartan and perindopril in healthy volunteers. J Clin Pharmacol 2005 Nov;45(11):1319–23. Epub 2005/10/22. eng. 10.1177/0091270005280445 [DOI] [PubMed] [Google Scholar]
- 285. Tanasa IA, Manciuc C, Carauleanu A, et al. : Anosmia and ageusia associated with coronavirus infection (COVID-19) - what is known? Exp Ther Med 2020 2020/09/01;20(3):2344–7. 10.3892/etm.2020.8808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Witt M, Miller IJ, Jr: Comparative lectin histochemistry on taste buds in foliate, circumvallate and fungiform papillae of the rabbit tongue. Histochemistry 1992 Oct;98(3):173–82. Epub 1992/10/11. eng. [DOI] [PubMed] [Google Scholar]
- 287. Pushpass RG, Pellicciotta N, Kelly C, et al. : Reduced Salivary Mucin Binding and Glycosylation in Older Adults Influences Taste in an in vitro Cell Model. Nutrients 2019 Sep 24;11(10). Epub 2019/09/27. eng. 10.3390/nu11102280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Milanetti E, Miotto M, Rienzo LD, et al. : In-Silico evidence for two receptors based strategy of SARS-CoV-2. bioRxiv 2020:2020.03.24.006197. 10.1101/2020.03.24.006197 [DOI] [Google Scholar]
- 289. Park Y-J, Walls AC, Wang Z, et al. : Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors. Nat. Struct. Mol. Biol. 2019 2019/12/01;26(12):1151–7. 10.1038/s41594-019-0334-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Xu H, Zhong L, Deng J, et al. : High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020 2020/02/24;12(1):8. 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Wang H, Zhou M, Brand J, et al. : Inflammation and taste disorders: mechanisms in taste buds. Ann N Y Acad Sci 2009;1170:596–603. eng. 10.1111/j.1749-6632.2009.04480.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Liu L, Wei Q, Alvarez X, et al. : Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol 2011 Apr;85(8):4025–30. Epub 2011/02/04. eng. 10.1128/JVI.02292-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Xu J, Li Y, Gan F, et al. : Salivary Glands: Potential Reservoirs for COVID-19 Asymptomatic Infection. J. Dent. Res. 2020 2020/07/01;99(8):989. 10.1177/0022034520918518 [DOI] [PubMed] [Google Scholar]
- 294. Harapan H: Anosmia and dysgeusia in SARS-CoV-2 infection: Incidence, effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms - A systematic review and meta-analysis. figshare 2020. Journal contribution. 10.6084/m9.figshare.13323080.v1 [DOI] [PMC free article] [PubMed]