Abstract
The testis is a potential target organ for SARS‐CoV‐2 infection. Our study intended to investigate any testicular involvement in mild‐to‐moderate COVID‐19 men. We conduct a cross‐sectional study in 18 to 55‐year‐old men hospitalised for confirmed COVID‐19. A senior radiologist executed the ultrasound with multi‐frequency linear probe in all participants, regardless of any scrotal complaints. Exclusion criteria involved any situation that could impair testicular function. Statistical analysis compared independent groups, classified by any pathological change. Categorical and numerical outcome hypotheses were tested by Fisher's Exact and Mann–Whitney tests, using the Excel for Mac, version 16.29 (p < .05). The sample size was 26 men (mean 33.7 ± 6.2 years; range: 21–42 years), all without scrotal complaints. No orchitis was seen. Eleven men (32.6 ± 5.8 years) had epididymitis (42.3%), bilateral in 19.2%. More than half of men with epididymitis displayed epididymal head augmentation > 1.2 cm (p = .002). Two distinct epididymitis’ patterns were reported: (a) disseminated micro‐abscesses (n = 6) and (b) inhomogeneous echogenicity with reactional hydrocele (n = 5). Both patterns revealed increased epididymal head, augmented Doppler flow and scrotal skin thickening. The use of colour Doppler ultrasound in mild‐to‐moderate COVID‐19 men, even in the absence of testicular complaints, might be useful to diagnose epididymitis that could elicit fertility complications.
Keywords: COVID‐19, epididymitis, SARS‐CoV‐2, testis, ultrasound
1. INTRODUCTION
In December 2019, the Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) emerged from the city of Wuhan in China, massively spreading worldwide, causing the harshest pandemic in humankind in more than a century, affecting 192 countries, causing the death of over 2.4 million people, remaining still largely uncontrolled and unpredictable, due to recent variants with potentially higher virulence (Dong et al., 2020; Wu et al. 2020; Zhou et al., 2020). The coronavirus disease 2019 (COVID‐19) was initially thought to be limited to the respiratory system; however, it is increasingly revealing itself as a systemic disease with multiple organ‐damage and pathological features being unravelled at an astonishing velocity by an unprecedented global effort from scientists, medical healthcare professionals and organizations (Nunes Duarte‐Neto et al., 2020; Wiersinga et al., 2020).
The male reproductive tract is gaining increasing attention as a primary target‐organ system for infection, as the testicles widely express the angiotensin‐converting enzyme 2 (ACE2), the viral entry receptor, whose co‐expression with the viral priming transmembrane protease serine 2 (TMPRSS2) mediate SARS‐CoV‐2 cell invasion (Chen et al., 2020; Hallak et al., 2021). Scrotal clinical manifestations during the acute stage of the infection usually range from a prevalent asymptomatic scenario to an unusually mild local discomfort (Pan et al. 2020). A Turkish prospective study reported 10.98% of testicular pain in hospitalised COVID‐19 patients (Ediz et al., 2020); however, very few acute orchitis and orchiepididymitis anecdotal cases caused by confirmed SARS‐CoV‐2 infection were reported in adolescent and adult patients (Bridwell et al., 2020; Gagliardi et al., 2020; La Marca et al., 2020).
Scrotal discomfort and groin pain are common among children and adults who present to the emergency department for radiologic evaluation, often with vague symptoms and nonspecific clinical findings (Dogra et al., 2003). Frequently, imaging is required to elucidate further and pinpoint a potential cause for the scrotal pain. Generally, high‐resolution grey‐scale and colour Doppler ultrasound (US) is well tolerated, widely available and reliable, making it the ideal immediate first front‐line diagnostic tool for evaluation of scrotal pain without the use of ionising radiation (Remer et al., 2012; Sung et al., 2012).
In this context, our study intended to investigate if there were any testicular, epididymal, spermatic cord and scrotal structural involvement in hospitalised male patients with mild to moderate COVID‐19 in a tertiary care facility.
2. PATIENTS AND METHODS
This cross‐sectional quantitative study was conducted in hospitalised men 18–55 years old with a clinical scenario that demanded moderate medical attention, but not in the degree of an intensive care unit. The largest tertiary hospital in Latin America, ‘Hospital das Clinicas’, University of Sao Paulo Medical School (HC‐FMUSP), in Sao Paulo, Brazil. SARS‐CoV‐2 infection was routinely confirmed by real‐time polymerase chain reaction (RT‐PCR) positive test. All patients had mild‐to‐moderate symptoms at the time of the US examination, regardless of any scrotal symptoms. Exclusion criteria involved any past medical history that could impair testicular function or change the scrotum and testicular morphologies. They included infertility from genetic causes (Y chromosome microdeletions, karyotype abnormalities), a history of testicular dysfunction (cryptorchidism, testicular dysgenesis syndrome, and atrophy), medical background of confirmed or suggested orchitis and orchiepididymitis, previous cancer treatment (chemo or radiotherapy), sexually transmitted infections, mumps or any episode of fever in the last 6 months. Patients with a history of scrotal, inguinal or retroperitoneal surgery (such as vasectomy, varicocelectomy, testicular biopsies, hernia repair, hydrocelectomy); and one‐time or regular use of anabolic steroids, illicit drugs or patients who received any testosterone replacement therapy, were excluded (Pires et al., 2012; de Souza & Hallak, 2011).
Ultrasound was performed after an initial detailed anamnesis verifying in‐depth past medical history. A board‐certified urologist conducted a detailed physical examination addressing scrotal contents, mainly looking for any palpable alterations in the testicles or epididymis.
A senior radiologist performed a colour Doppler US of the scrotum using a high‐quality portable device (Logiq P6, General Electrics or Toshiba I800, Canon) multi‐frequency 12–15 MHz linear transducer. Scrotal skin and subcutaneous tissue were evaluated bilaterally, looking for thickness or inflammatory oedema. Testicular volume and echogenicity were assessed; the epididymis and the vas deferens were also evaluated with the patient in the supine position. For better evaluation purposes, the penis was placed in an anatomic position over the abdomen, and a towel used to elevate the scrotum. Images of each testicle and the epididymis were obtained in transverse and sagittal planes, with transverse images covering both testicles to allow for comparison. Epididymal head (Globus major) were measured in all patients in two planes. Colour Doppler US illustrated arterial‐venous blood flow in search of any signs of alteration, including potential involvement eventually caused by the SARS‐CoV‐2, as other causes were carefully discarded. Appropriate colour Doppler settings were well adjusted to optimise the representation of slow blood flow (Dudea et al., 2010; Sparano et al., 2008). The Pampiniform plexus analysis was conducted with the patient in the upright position after five minutes of standing. Vessel diameters were measured in rest and during Valsalva's manoeuvre. Colour and spectral analysis evaluated blood reflux (the cut‐off was 2 mm in diameter with 1 s or more reflux to be considered positive).
All patients were invited to participate in this study, in compliance with the Declaration of Helsinki (World Med, 2013). HC‐FMUSP Research Ethics Committee approved this investigation (registration number CAAE 30135720.8.0000.0068), and an informed consent form was given and signed by each participant.
The statistical analysis compared two independent groups, classified by a categorical variable: occurrence of COVID‐19 epididymitis. Outcomes were categorical (occurrence of hydrocele ‐ unilateral or bilateral, varicocele and epididymal cyst; enlarged epididymis > 1.2 cm; Pampiniform plexus diameter, during increased Valsalva manoeuvre, >0.2 cm) and numerical (patient's age). Categorical outcomes were expressed by frequency, while numerical were expressed by means ± standard deviation. A comparative analysis between the histogram and the normality curve was performed to assess numerical data's normality. Since data had a nonparametric distribution, categorical and numerical outcome hypotheses were tested by Fisher's Exact and Mann–Whitney tests respectively. An alpha value of 0.05 was considered statistically significant. Statistical analysis was performed using the software Excel for Mac, version 16.29.
3. RESULTS
Initially, thirty patients were enrolled in the study. Due to the absence of clinical conditions to support the physical examination, two did not have the US complete analysis during evaluation. The other two patients had previous testicular manipulations, later confirmed in their medical records. Thus, out of 30 patients formerly enlisted, the final sample size was reduced to 26 men. All patients were asymptomatic regarding any scrotal complaints and symptoms. Age ranged from 21 to 42 years (mean age ± SD 33.7 ± 6.2 years). Just one patient (3.8%) reported pain and scrotal discomfort only during the right epididymal palpation in physical examination. Scrotum colour Doppler US was performed in all patients. Eleven individuals (mean age ± SD 32.6 ± 5.8 years old) had a clear radiological finding of epididymitis (42.3%), five of them bilaterally (19.2%). More than half of the patients with epididymitis displayed significant and noticeable epididymal head augmentation >1.2 cm (p = .002), which could arguably represent an even greater involvement than those presented with clear radiological patterns of infection by the SARS‐CoV‐2. No signs of orchitis by colour Doppler US were seen in any subject (enlarged testicular volume with reduced echogenicity and increased Doppler blood flow). Table 1 summarises radiological epididymitis and epididymal head enlargement features in male COVID‐19 patients.
TABLE 1.
Epididymitis features in male COVID‐19 patients (n = 26)
| Radiological characteristic | Epididymitis | Total | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (mean ± SD [years]) | 32.6 ± 5.8 | 33,7 ± 6.2 | .40 a | ||
| Epididymitis | 11 | 100 | 11 | 42.3 | |
| Enlarged epididymal head (>1.2cm) | 6 | 54.5 | 6 | 23 | .002 b |
| Micro‐abscess areas | 6 | 54.5 | 6 | 23 | |
| Inhomogeneous echogenicity + reactional hydrocele | 5 | 45.4 | 5 | 19.2 | |
| Unilateral | 6 | 54.5 | 6 | 23 | |
| Bilateral | 5 | 45.4 | 5 | 19.2 | |
| Association with varicocele | 5 | 45.4 | 7 | 26.9 | .08 b |
| Scrotal pain during physical examination | 1 | 9.0 | 1 | 3.8 | |
Abbreviations: SD, Standard deviation; P, Statistical significance (p < .05).
Estimated by Mann–Whitney test.
Estimated by Fisher Exact Test.
During the radiologic evaluation, we described two distinct and different viral epididymitis’ patterns. In six patients, we identified disseminated micro‐abscesses areas as the leading US characteristic, seen as marked hypoechogenic small areas with peripherical Doppler vascularization (Figure 1). In contrast, in the other five men, the inhomogeneous echogenicity with a reactional hydrocele was the principal finding (Figure 2). Features revealed in both patterns were increased epididymal head size with augmented Doppler blood flow and scrotal skin thickening (Figures 1 and 2). Interestingly, the vas deferens, epididymal bodies and tails were not affected in any subject. Six patients had sonographic varicocele (Pampiniform plexus diameter above 2 mm with more than 1‐s blood flow reflux after Valsalva manoeuvre), one right‐sided, four left‐sided and one bilateral. None of them had signs of clinical varicocele in the physical examination. Two individuals had isolated small hydroceles, and just one had bilateral hydroceles. Almost half of the men with epididymitis (45.4%) had subclinical varicocele (p = .08); however, the varicocele side was not associated with the epididymitis itself (Table 1).
FIGURE 1.
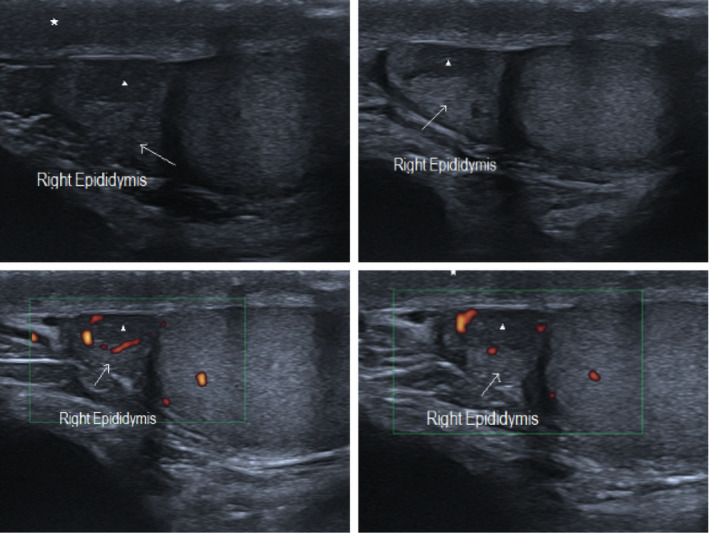
SARS‐COV 2 subclinical epididymitis pattern #1: Epididymal head enlargement (arrow) with inhomogeneous echogenicity and increased colour Doppler blood flow. Marked hypoechogenic small areas with peripherical Doppler vascularization indicating micro‐abscess (arrowhead). Reactional oedema and thickness of the scrotum wall as well (asterisk)
FIGURE 2.
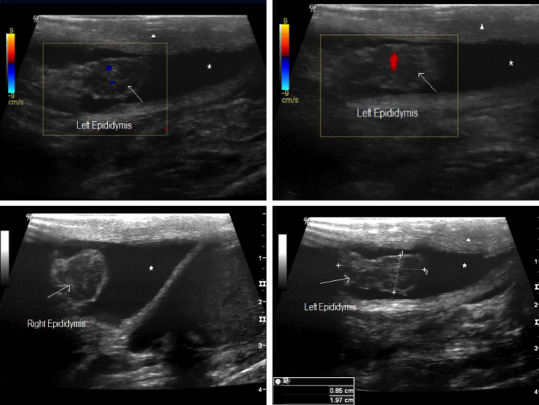
SARS‐COV 2 subclinical epididymitis pattern #2: Epididymal head enlargement (arrow) with marked inhomogeneous echogenicity and increased colour Doppler blood flow. Reactional hydroceles are associated (asterisk), oedema and thickness of the scrotum wall as well (arrowhead)
4. DISCUSSION
An ongoing debate regarding the potential deleterious SARS‐CoV‐2 effects on the male reproductive tract had encouraged the intensive investigation of the viral action upon testicular and epididymal physiology (Hallak et al., 2021), since this organ precisely displays the highest ACE2 expression, according to data gathered from the Genotype‐Tissue Expression (GTEx) project (Baughn et al., 2020). Therefore, subclinical or even asymptomatic testicular manifestations are potential and realistic concerns as scientific and medical communities have not yet had the time to gather more information on the consequences of the reproductive, sexual and hormonal status of mildly affected and under‐evaluated individuals. Naturally, the primary concern and main focus are understandably being driven to the most severe and life‐threatening scenarios amidst the panic, and even chaos established almost worldwide (Pan et al. 2020). Our study evaluated by colour Doppler US in mild‐to‐moderately symptomatic COVID‐19 men who did not present with scrotal symptoms. Surprisingly, we found 42.3% of men with colour Doppler US signs of epididymitis, with 54.5% of them presenting with enlarged epididymal head, and 19.2% bilateralism (Table 1). All epididymal changes reported were restricted to the head portion (Globus major), with alterations in echogenicity, increased Doppler blood flow, skin thickening, and the majority with significant and identifiable volumetric augmentations of the structure.
A variety of affections, including inflammatory, immunologic and other medical conditions, target the epididymis, including bacterial, viral and fungal infections, besides idiopathic inflammation and diseases linked to the immune system (Silva et al., 2009; Soares et al., 2007). Clinically symptomatic acute epididymitis is characterised by inflammation of the epididymis, presenting typically as pain and swelling, generally occurring on one side and developing over several days, causing discomfort that is likely to need medical attention (Silva et al., 2018). Up to 80% of all epididymitis cases may be bacterial in origin (Silva et al., 2018; Tracy et al., 2008). Escherichia coli and Chlamydia trachomatis are the most common agents reported but the literature lack investigations on the subclinical infections (Jantos et al., 1992; Ludwig et al., 2002).
Viral epididymitis remains mostly undetected and, consequently, underdiagnosed. Therefore, it remains a rarely cited condition, frequently noticed exclusively in the post‐infection scenario, mainly in children. Enterovirus and adenovirus are the most common associated germs (Lau et al., 1997; Somekh et al., 2004). Nevertheless, we propose that the medical community pay more attention to underlying conditions involving the reproductive tract as we have demonstrated that the epididymis is a primary target‐structure for subclinical infections.
Usually, viral infections reach the epididymis, and less frequently, the testis through the bloodstream, causing direct cellular damage (Bhushan et al., 2009) or even acting indirectly through the cytokine system, inducing a local inflammatory response (Guazzone et al., 2009). Nonetheless, a viral translocation between the testis and epididymis has been detailed in very few experimental animal models, such as a micro‐RNA targeted Zika virus clones in mice (Tsetsarkin et al., 2018) and simian immunodeficiency virus (SIV) infected monkeys (Houzet et al., 2018). For SARS‐CoV‐2 infection, probably an indirect deleterious effect mediated by cytokines could act on the epididymis, similarly as it has been hypothesised for the testis (Hallak et al., 2021).
Regular asymptomatic patients US anatomy of the epididymis is characterised by the triangular small echogenic homogeneous head (less than 1.0 cm), virtual body and tail followed by vas deferens visualized as a tubular hypoechogenic structure (Figure 3). In symptomatic patients, the US viral epididymitis pattern is similar to the one found in bacterial epididymitis, but the former usually presents clinically with concomitant orchitis (Başekim et al., 2000). Isolated viral epididymitis is relatively rare or, most likely, underdiagnosed, and the testis is often the first to call for the patient's attention and the attending physician with signs of inflammation, such as an inhomogeneous enlargement, with colour Doppler blood flow enhancement. Subsequently, a slight enlargement of all portions of epididymis with increased Doppler blood flow, reactional hydrocele, and an adjacent scrotum thickness can also be found (Başekim et al., 2000).
FIGURE 3.
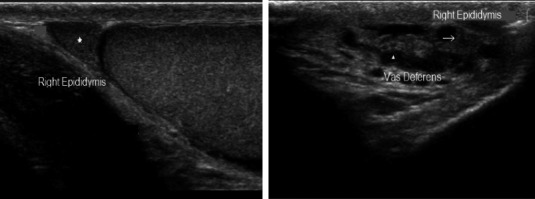
Normal epidydimal pattern: Small triangular epididymal head (asterisk), virtual body, and the tail (arrow) followed by vas deferens (arrowhead)
Currently, just a few cases of symptomatic COVID‐19 orchiepididymitis have been reported in this pandemic. A case of orchiepididymitis in a 14‐year‐old boy with COVID‐19, who had pain, fever and right scrotum's swelling for two days. Radiological features displayed inhomogeneous enlargement of the testes and epididymis with increased blood flow and reactional hydroceles (Gagliardi et al., 2020). Another reported orchiepididymitis case was in a 43‐year‐old COVID‐19 diabetic patient who complained about testicular pain and had bilateral inhomogeneous enlargement of the testis and the epididymis, and soft augmentation of colour Doppler blood flow (La Marca et al., 2020). In another report, a young adult with COVID‐19 developed bilateral orchitis but without sonographic epididymal changes (Bridwell et al., 2020). A single‐centre‐based Chinese study reported orchiepididymitis in symptomatic and nonsymptomatic patients regarding scrotum symptoms. They studied 142 mild‐to‐severe COVID‐19 patients with scrotum US evaluation and found 32 (22.5%) patients with US signs of orchitis, epididymitis or both. Seven patients had just epididymitis (4.9%) being three of than with nonsevere COVID‐19 symptoms. Thirteen individuals had scrotum symptoms by the time of the US scanning (Chen, Huang, et al., 2020; Chen, Guo, et al., 2020). The present study enrolled just asymptomatic patients with mild‐to‐moderate COVID‐19 symptoms and found 42.3% of the patients with US signs of epididymitis. The criteria for diagnosing epididymitis were the same. We did not find orchitis or orchiepididymitis in our sample, showing that those infections are probably more prevalent in severe COVID‐19 individuals. Our hitherto radiological epididymal findings shed light on the possibility of asymptomatic injury that might go unnoticed in SARS‐CoV‐2 infected men's clinical evaluation. Potential harmful deleterious effects could theoretically be expected on seminal parameters. The epididymis is the site for many essential biochemical reactions in the spermatozoa, leading to capacitation for the acrosome reaction, which is crucial to fertilizing the oocyte and sperm progressive motility. They could later correlate with seminal parameters changes that may influence male fertility status (Athayde et al., 2007; Hallak, 2017; Hallak et al., 2001).
We hypothesise that SARS‐CoV‐2 epididymitis patterns, described here for the first time, are distinct from the classical bacterial epididymitis that commonly start affecting the organ tail and then progress to the body and eventually to the head, concomitant with augmented Doppler blood flow (Brown et al., 1995). A bacterial infection usually originates in the bladder or the prostate and spreads through the vas deferens and lymphatics of the spermatic cord to the epididymis, finally reaching the testis to cause an orchiepididymitis. Due to this retrograde dissemination, the local infection begins in the epididymal tail, the most affected region in US exams, with reactive hydrocele and scrotal wall thickening (Brown et al., 1995). Also, increased epididymis size and inhomogeneous echogenicity according to evolution time are usually observed (Brown et al., 1995; Ma et al., 2013). Inflammation can produce increased colour Doppler blood flow within the epididymis, testis or both (Figure 4) (Dudea et al., 2010; Sparano et al., 2008).
FIGURE 4.
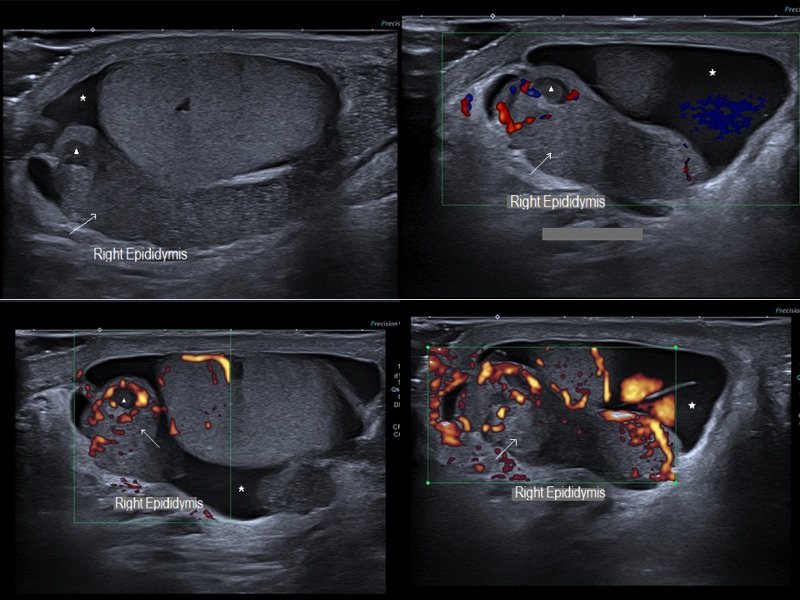
Bacterial classic epididymitis pattern: Epididymal head, body and tail enlargement (arrow) with inhomogeneous echogenicity and increased colour Doppler blood flow. Small marked hypoechogenic areas with peripherical Doppler vascularization indicating micro‐abscess (arrowhead). Reactional hydroceles associated (asterisk)
Just one patient complained about epididymal pain during a physical examination by the time of the US analysis. Therefore, even a careful physical examination, conducted by an experienced board‐certified urologist, does not substitute US evaluation to find subclinical epididymitis, that can potentially lead to deleterious effects, as symptoms might be vague, and critical diagnosis could be missed (Remer et al., 2012).
By the time of this manuscript conception, we did not know any other testicular sonographic evaluation study in mild‐to‐moderate symptomatic COVID‐19 patients with no scrotum symptoms in the current pandemic of SARS‐CoV‐2. Since we found a very significant percentage of individuals with epididymal inflammatory changes, further studies should fully comprehend the epididymis’ role in systemic SARS‐CoV‐2 infection. We also suggest performing a colour Doppler ultrasound testicular evaluation in COVID‐19 reproductive‐aged men with mild to moderate symptoms and who desire to father their genetic offspring in the future.
ACKNOWLEDGEMENT
None.
Carneiro F, Teixeira TA, Bernardes FS, et al. Radiological patterns of incidental epididymitis in mild‐to‐moderate COVID‐19 patients revealed by colour Doppler ultrasound. Andrologia. 2021;53:e13973. 10.1111/and.13973
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on the request from the corresponding author. The data are not publicity available due to privacy or ethical restrictions.
REFERENCES
- Athayde, K. S. , Cocuzza, M. , Agarwal, A. , Krajcir, N. , Lucon, A. M. , Srougi, M. , & Hallak, J. (2007). Development of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. Journal of Andrology, 28(4), 613–620. 10.2164/jandrol.106.001966 [DOI] [PubMed] [Google Scholar]
- Başekim, C. , Kizilkaya, E. , Pekkafali, Z. , Baykal, K. , & Karsli, A. (2000). Mumps epididymo‐orchitis: Sonography and color Doppler sonographic findings. Abdominal Imaging, 25(3), 322–325. 10.1007/s002610000039 [DOI] [PubMed] [Google Scholar]
- Baughn, L. B. , Sharma, N. , Elhaik, E. , Sekulic, A. , Bryce, A. H. , & Fonseca, R. (2020). Targeting TMPRSS2 in SARS‐CoV‐2 infection. Mayo Clinic Proceedings, 95(9), 1989–1999. 10.1016/j.mayocp.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan, S. , Schuppe, H. C. , Fijak, M. , & Meinhardt, A. (2009). Testicular infection: Microorganisms, clinical implications and host‐pathogen interaction. Journal of Reproductive Immunology, 83(1–2), 164–167. 10.1016/j.jri.2009.07.007 [DOI] [PubMed] [Google Scholar]
- Bridwell, R. E. , Merrill, D. R. , Griffith, S. A. , Wray, J. , & Oliver, J. J. (2020). A coronavirus disease 2019 (COVID‐19) patient with bilateral orchitis: A case report. American Journal of Emergency Medicine, 10.1016/j.ajem.2020.08.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. M. , Hammers, L. W. , Barton, J. W. , Holland, C. K. , Scoutt, L. M. , Pellerito, J. S. , & Taylor, K. (1995). Quantitative Doppler assessment of acute scrotal inflammation. Radiology, 197(2), 427–431. 10.1148/radiology.197.2.7480687 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Huang, X. , Yi, Z. , Deng, Q. , Jiang, N. , Feng, C. , … Guo, R. (2020). Ultrasound Imaging Findings of Acute Testicular Infection in Patients With Coronavirus Disease 2019. Journal of Ultrasound in Medicine. 10.1002/jum.15558 [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Guo, Y. , Pan, Y. , & Zhao, Z. J. (2020). Structure analysis of the receptor binding of 2019‐nCoV. Biochemical and Biophysical Research Communications, 525(1), 135–140. 10.1016/j.bbrc.2020.02.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza, G. L. , & Hallak, J. (2011). Anabolic steroids and male infertility: A comprehensive review. BJU International, 108(11), 1860–1865. 10.1111/j.1464-410X.2011.10131.x [DOI] [PubMed] [Google Scholar]
- Dogra, V. S. , Gottlieb, R. H. , Oka, M. , & Rubens, D. J. (2003). Sonography of the scrotum. Radiology, 227(1), 18–36. 10.1148/radiol.2271001744 [DOI] [PubMed] [Google Scholar]
- Dong, E. , Du, H. , & Gardner, L. (2020). An interactive web‐based dashboard to track COVID‐19 in real time. The Lancet Infectious Diseases 20(5), 533–534. 10.1016/s1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudea, S. M. , Ciurea, A. , Chiorean, A. , & Botar‐Jid, C. (2010). Doppler applications in testicular and scrotal disease. Medical Ultrasonography, 12(1), 43–51. [PubMed] [Google Scholar]
- Ediz, C. , Tavukcu, H. H. , Akan, S. , Kizilkan, Y. E. , Alcin, A. , Oz, K. , & Yilmaz, O. (2020). Is there any association of COVID‐19 with testicular pain and epididymo‐orchitis? International Journal of Clinical Practice, 12, e13753. 10.1111/ijcp.13753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi, L. , Bertacca, C. , Centenari, C. , Merusi, I. , Parolo, E. , Ragazzo, V. , & Tarabella, V. (2020). Orchiepididymitis in a boy with COVID‐19. Pediatric Infectious Disease Journal, 39(8), E200–E202. 10.1097/inf.0000000000002769 [DOI] [PubMed] [Google Scholar]
- Guazzone, V. A. , Jacobo, P. , Theas, M. S. , & Lustig, L. (2009). Cytokines and chemokines in testicular inflammation: A brief review. Microscopy Research and Technique, 72(8), 620–628. 10.1002/jemt.20704 [DOI] [PubMed] [Google Scholar]
- Hallak, J. (2017). Utility of sperm DNA fragmentation testing in different clinical scenarios of male reproductive abnormalities and its influence in natural and assisted reproduction. Translational Andrology and Urology, 6, S509–S512. 10.21037/tau.2017.06.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak, J. , Sharma, R. K. , Pasqualotto, F. F. , Ranganathan, P. , Thomas, A. J. , & Agarwal, A. (2001). Creatine kinase as an indicator of sperm quality and maturity in men with oligospermia. Urology, 58(3), 446–451. 10.1016/s0090-4295(01)01224-9 [DOI] [PubMed] [Google Scholar]
- Hallak, J. , Teixeira, T. A. , Bernardes, F. S. , Carneiro, F. , Duarte, S. A. D. , Pariz, J. R. , & Saldiva, P. H. N. (2021). SARS‐CoV‐2 and its relationship with the genitourinary tract: Implications for male reproductive health in the context of COVID‐19 pandemic. Andrology, 9(1), 73–79. 10.1111/andr.12896 [DOI] [PubMed] [Google Scholar]
- Houzet, L. , Pérez‐Losada, M. , Matusali, G. , Deleage, C. , Dereuddre‐Bosquet, N. , Satie, A. P. , & Dejucq‐Rainsford, N. (2018). Seminal simian immunodeficiency virus in chronically infected cynomolgus macaques is dominated by virus originating from multiple genital organs. Journal of Virology, 92(14), e00133–18. 10.1128/jvi.00133-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantos, C. , Baumgärtner, W. , Durchfeld, B. , & Schiefer, H. (1992). Experimental epididymitis due to Chlamydia trachomatis in rats. Infection and Immunity, 60(6), 2324–2328. 10.1128/IAI.60.6.2324-2328.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Marca, A. , Busani, S. , Donno, V. , Guaraldi, G. , Ligabue, G. , & Girardis, M. (2020). Testicular pain as an unusual presentation of COVID‐19: A brief review of SARS‐CoV‐2 and the testis. Reprod Biomed Online, 41(5), 903–906. 10.1016/j.rbmo.2020.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, P. , Anderson, P. , Giacomantonio, J. , & Schwarz, R. (1997). Acute epididymitis in boys: Are antibiotics indicated? British Journal of Urology, 79(5), 797–800. 10.1046/j.1464-410X.1997.00129.x [DOI] [PubMed] [Google Scholar]
- Ludwig, M. , Johannes, S. , Bergmann, M. , Failing, K. , Schiefer, H. , & Weidner, W. (2002). Experimental Escherichia coli epididymitis in rats: A model to assess the outcome of antibiotic treatment. BJU International, 90(9), 933–938. [DOI] [PubMed] [Google Scholar]
- Ma, O. J. , Mateer, J. R. , Reardon, R. F. , & Joing, S. A. (2013). Ma and Mateer's Emergency Ultrasound: McGraw Hill Professional. [Google Scholar]
- Nunes Duarte‐Neto, A. , de Almeida Monteiro, R. A. , da Silva, L. F. F. , Malheiros, D. , de Oliveira, E. P. , Theodoro Filho, J. , & Dolhnikoff, M. (2020). Pulmonary and systemic involvement of COVID‐19 assessed by ultrasound‐guided minimally invasive autopsy. Histopathology, 77(2), 186–197. 10.1111/his.14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, F. , Xiao, X. , Guo, J. , Song, Y. , Li, H. , Patel, D. P. , Hotaling, J. M. (2020). No evidence of SARS‐CoV‐2 in semen of males recovering from COVID‐19. Fertility and Sterility, 113(6), 1135–1139. 10.1016/j.fertnstert.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires, A. , Pieri, P. , Hage, M. , Santos, A. B. G. , Medeiros, M. C. R. , Garcia, R. C. T. , & Bueno, H. M. S. (2012). Repeated inhalation of crack‐cocaine affects spermatogenesis in young and adult mice. Inhalation Toxicology, 24(7), 439–446. 10.3109/08958378.2012.684450 [DOI] [PubMed] [Google Scholar]
- Remer, E. M. , Casalino, D. D. , Arellano, R. S. , Bishoff, J. T. , Coursey, C. A. , Dighe, M. , & Leyendecker, J. R. (2012). ACR Appropriateness Criteria® acute onset of scrotal pain—without trauma, without antecedent mass. Ultrasound Quarterly, 28(1), 47–51. 10.1097/RUQ.0b013e3182493c97 [DOI] [PubMed] [Google Scholar]
- Silva, C. A. A. D. , Moraes, A. J. P. , Leal, M. M. , Sallum, A. M. E. , Bonfá, E. , Borges, C. T. L. , Hilário, M. O. E. , Terreri, M. T. , Ronchezel, M. , Saito, O. , & Hallak, J. (2009). Aspectos da saúde reprodutiva em homens com miopatia inflamatória idiopática: Um estudo multicêntrico. Revista Brasileira De Reumatologia, 49(6), 677–689. 10.1590/S0482-50042009000600005 [DOI] [Google Scholar]
- Silva, E. J. R. , Ribeiro, C. M. , Mirim, A. F. M. , Silva, A. A. S. , Romano, R. M. , Hallak, J. , & Avellar, M. C. W. (2018). Lipopolysaccharide and lipotheicoic acid differentially modulate epididymal cytokine and chemokine profiles and sperm parameters in experimental acute epididymitis. Scientific Reports, 8(1), 103. 10.1038/s41598-017-17944-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, P. M. F. , Borba, E. F. , Bonfa, E. , Hallak, J. , Correa, A. L. , & Silva, C. A. A. (2007). Gonad evaluation in male systemic lupus erythematosus. Arthritis and Rheumatism, 56(7), 2352–2361. 10.1002/art.22660 [DOI] [PubMed] [Google Scholar]
- Somekh, E. , Gorenstein, A. , & Serour, F. (2004). Acute epididymitis in boys: Evidence of a post‐infectious etiology. The Journal of Urology, 171(1), 391–394. 10.1097/01.ju.0000102160.55494.1f [DOI] [PubMed] [Google Scholar]
- Sparano, A. , Acampora, C. , Scaglione, M. , & Romano, L. (2008). Using color power Doppler ultrasound imaging to diagnose the acute scrotum. A pictorial essay. Emergency Radiology, 15(5), 289–294. 10.1007/s10140-008-0710-9 [DOI] [PubMed] [Google Scholar]
- Sung, E. K. , Setty, B. N. , & Castro‐Aragon, I. (2012). Sonography of the pediatric scrotum: Emphasis on the Ts‐torsion, trauma, and tumors. American Journal of Roentgenology, 198(5), 996–1003. 10.2214/ajr.11.8034 [DOI] [PubMed] [Google Scholar]
- Tracy, C. R. , Steers, W. D. , & Costabile, R. (2008). Diagnosis and management of epididymitis. Urologic Clinics of North America, 35(1), 101–108. 10.1016/j.ucl.2007.09.013 [DOI] [PubMed] [Google Scholar]
- Tsetsarkin, K. A. , Maximova, O. A. , Liu, G. P. , Kenney, H. , Teterina, N. , Bloom, M. E. , & Pletnev, A. G. (2018). Routes of Zika virus dissemination in the testis and epididymis of immunodeficient mice. Nature Communications, 9, 13. 10.1038/s41467-018-07782-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga, W. J. , Rhodes, A. , Cheng, A. C. , Peacock, S. J. , & Prescott, H. C. (2020). Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): A review. JAMA, 324(8), 782. 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- World Med, A (2013). World Medical Association Declaration of Helsinki ethical principles for medical research involving human subjects. JAMA‐Journal of the American Medical Association, 310(20), 2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- Wu, F. , Zhao, S. , Yu, B. , Chen, Y. M. , Wang, W. , Song, Z. G. , Zhang, Y. Z. (2020). A new coronavirus associated with human respiratory disease in China. Nature, 1. 579, 265, 10.1038/s41586-020-2202-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X. L. , Wang, X. G. , Hu, B. , Zhang, L. , Zhang, W. , & Shi, Z. L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270‐+. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on the request from the corresponding author. The data are not publicity available due to privacy or ethical restrictions.


