Abstract
Background
COVID‐19 is considered a widespread concern in global public health. Diagnoses of COVID‐19 in some cases are necessary because of severe prognosis. In this study, epidemiologies, clinical and demographic characteristics of patients with COVID‐19 were studied in Taleghani Hospital, Urmia, Iran.
Methods
This descriptive‐analytical cross‐sectional study was carried out on 215 patients with COVID‐19 during March and April 2020. Approved COVID‐19 case was considered as a person with a positive respiratory sample performed by at least one of two RT‐PCR methods or genetic sequencing. ANOVA repeated measure, independent t‐test and logistic regression were done. A P < .05 was considered significant.
Results
The mean age of patients was 50.93 ± 17.92 years. Regarding gender, there were 91 females (42.3%) and 124 males (57.7%). The mean hospital stay, the temperature at admission, and onset of symptoms were 4.91 ± 3.68 days, 37.40 ± 0.96°C and 5.88 ± 4.80 days, respectively. Close contact with suspected people was found in 10.2% of patients. Additionally, 44 patients (20.5%) were smokers. Shortness of breath and cough were found in 62.8% and 49.3% of patients. Diabetes mellitus and hypertension were the most common comorbidities of patients. Regarding lung involvement, 33 patients (33%) were normal, most of the patients (n = 71) had 5%‐25% involvement in their lung and a minority of patients (n = 13) had a severe condition of 50%‐75% lung involvement. The association between smoking and mortality was tested using chi‐square showing no significant difference (X2:2.959, P = .085). There was no significant difference between AST, ALT, ALP, total, direct Bilirubin, lung involvement and suffering from fever (P > .05). High Spo2 can increase the chance of recovery by 24% with each unit reduction. Kidney involvement increases the chance of death by about 80% (95% CI: 0.104‐0.013). The odds ratio of spo2 for recovery of COVID‐19 was 1.24 (95% CI: 1.014‐1.528; P = .037). Kaletra with odds ratio of 31.960 had the most highest effect on recovery following COVID‐19 (P = .043).
Conclusion
COVID‐19 involves different organs of the body with different severity. In the meantime, smoking was not a risk factor for the virus or associated with severe manifestations of the disease. Patients with high creatinine and CPK, pulmonary involvement above 25%, and hypoxemia had a higher mortality rate. Increase of Spo2 by 1% can improve the patients by 24%. The results indicated that Kaletra had the most highest effect on improvement following COVID‐19.
1. INTRODUCTION
Coronavirus disease (COVID‐19) is caused by SARS‐COV2, and is considered a causative agent of a potentially deadly disease resulted widespread concern in global public health. Coronavirus is one of the largest pathogens that mainly target the human respiratory tract. Previous coronavirus outbreaks have included severe acute respiratory syndrome (SARS) ‐CoV and Middle East Respiratory Syndrome (MERS) ‐CoV, which have long been known to be very threatening to public health. In late December 2019, a number of patients were admitted to hospitals whose initial diagnosis was pneumonia with unknown cause. These patients were associated with a wholesale market for seafood and wet animals in Wuhan, Hubei Province, China. 1 , 2 Preliminary reports predicted the launch of a potential COVID‐19 epidemic, based on estimates of the number of reproductions for the COVID‐19, which was calculated more than 1 (ranging from 2.24 to 3.58). 3 On February 19, 2019, 66 402 COVID‐19 infections have been confirmed in China. This number of infections has exceeded the prevalence of severe acute respiratory syndrome in China. Epidemiological studies of primary cases of COVID‐19 showed that many cases were exposed to the seafood market in Wuhan, China. 4 A study by Wang et al showed that from 10 January to 24 January, 2019, the number of people infected with the new Coronavirus‐2019 infection in China increased 31.4 times. They estimated the mortality rate for COVID‐19 to be 2.84% based on the number of patients. The researchers also found that the male‐to‐female mortality ratio was 3.25 to 1, the median age of death was 75 years, the median time from the first symptoms to death was 14 days, and the median time from the initial symptoms to death in people aged 70 years and older (11.5 days) was shorter than people under 70 years (20 days). These findings suggest that the disease may progress more rapidly in elderly than in young people especially in metropolis. 5 , 6 COVID‐19 can cause multi‐organ failure threatening the health of patients including lung and kidney involvement. 7 , 8 , 9 , 10 , 11 Nowadays there is no specific drug to treat COVID‐19 patients because of the complexity of the SARS‐COV2. 12 , 13
A study by Li et al reported that the mean age of 425 patients infected with COVID‐19 was 59 years, of which 56% were men. The mean incubation period was 2.5 days, and almost half of the adult patients were 60 years and older. In the early stages, the number of infected patients doubled every 7.4 days. The production rate of transmission (R0) of the disease from the infected person was 2.2. The incubation period for COVID‐19 is thought to be 14 days after exposure, with most cases occurring approximately 4 to 5 days after exposure. 14 Diagnoses of coronavirus infections in most cases are unnecessary because most patients have mild or moderate syndrome with a good prognosis. However, it may be required to identify an etiological factor in epidemiological studies, especially during the epidemic. Since the new COVID‐19 has not been found in humans before, no specific vaccine or treatment has been provided. The number of cases is rapidly increasing. Therefore, it is important to diagnose all suspected cases as soon as possible and to isolate them quickly for cutting off the source of infection. Conventional diagnostic testing methods, such as assessment to detect antiviral antibodies or viral antigens, have been clinically developed and used. New diagnostic solutions, including PCR‐RT and microscopic‐based measurements, may be effective in monitoring epidemiological measures, along with preventive measures. COVID‐19 nucleic acids can be detected in samples such as nasopharyngeal swabs, sputum, lower respiratory tract secretions, blood and faeces. 15 , 16 Clinical symptoms and graphic findings provide an acceptable diagnosis. In a study, 1099 cases of the new coronavirus infection were reported. They found that fever (78.9%) and cough (67.7%) were the most common symptoms, and diarrhoea (3.7%) and vomiting (5%) were rare. Abnormalities in CT images of the chest were observed in 96% of patients infected with COVID‐19, and in 82.1% of them lymphopenia was recorded. 17 In addition to pulmonary involvement, COVID‐19 could target other organs, including coagulation disorders in patients who died, compared with patients who survived. 18 Neurological manifestations in 36.4% and liver involvement in 14.3%‐58% of patients with COVID‐19 have been reported. 19 , 20 Acute kidney injury (AKI) risk has been reported to be 28% and at the time of hospitalisation 10% of patients have an increase in creatinine accompanied by proteinuria (60%) and urinary incontinence (48%). Also, AKI can be considered as a prognostic factor in the mortality of patients with COVID‐19, in which the mortality is 5.3 times higher in patients with AKI. 21
There are several new medical treatments that are used in COVID‐19 patients, and The United States Food and Drug Administration (FDA) has given permission to give some drugs in emergency conditions for such a situation, but the only drug with FDA approval is remedesivir, 22 other antivirals such as favipiravir, mulnopiravir (mk‐4482), lopinavir, ritonavir is used too, among them favipiravir can be helpful specially during virmic phase of covid −19 by blocking the Replication of virus genetic material 23 , 24 , 25 ; dexamethasone and corticosteroids are helpful in severe COVID‐19 patients requiring supplementary oxygen but its effect is not proved in earlier stage of disease with mild disease 26 ; recombinant ACE‐2 that can prevent COVID‐19 virus entering inside the cells, but up to now there is not any trial in animals or human, but there are some on‐going clinical trials 27 ; convalescent plasma and monoclonal antibodies in such a way that sing plasma of recovered patients from COVID‐19 or monoclonal antibodies are also be helpful in treating course for COVID‐19 patients but, large RCT is undergoing for better evaluation of the this technique effect 28 ; interfrons: inhaled form of interferon called SNG001 lowers the risk of severe COVID‐19 in infected patients in a small clinical trial 29 and also blood filtration that can omit harmful cytokines from blood in the patients with severe COVID‐19 patients to end organ damage caused by cytokines. 30
Given the emergence of this coronavirus disease, and the World Health Organization's announcement of a pandemic in February 2020, further studies are needed to identify the symptoms of the disease, prognosis, graphical and paraclinical findings for a definitive diagnosis. In this study, clinical and demographic characteristics of patients with COVID‐19 were studied in Taleghani Hospital, Urmia, Iran.
2. METHODS
The present study was a descriptive‐analytical cross‐sectional that has been performed on 215 patients with COVID‐19 during March and April 2020 in Taleghani Hospital, Urmia, West Azerbaijan, Iran. After receiving the approval of the ethics committee from Urmia University of Medical Sciences, this study was performed on the existing files. In this case, the existing scans of the patient with COVID‐19 were evaluated and the variables of age, gender, body mass index, comorbidities, symptoms, liver profile, lipid profile, haemoglobin, platelet, WBC, lymphocyte, neutrophil, C‐reaction protein (CRP), Polymerase chain reaction (PCR), Creatine phosphokinase (CPK), creatinine, urea, potassium, magnesium, sodium and medications were recorded. The sampling method in this study was census (all patients in March and April). Unreadable files were removed from the study. Clinical features of suspected cases were considered to have at least two criteria of the following conditions: fever and/or respiratory symptoms; radiographic evidence of pneumonia and low or normal white blood cell counts or low lymphocyte levels. Also, epidemiological history was at least one criterion of the following conditions: travel history to epidemic areas within 14 days of the onset of symptoms; a history of contact with a patient with fever or respiratory symptoms in the epidemic areas within 14 days of the onset of symptoms; a history of close contact with approved COVID‐19. With a strict definition, all cases that met the criteria of clinical characteristics but do not comply with epidemiological history was considered as suspicious cases. Approved COVID‐19 case was considered as a person with a positive respiratory sample performed by at least one of two Reverse transcription polymerase chain reaction (RT‐PCR) methods or genetic sequencing. After entering the required information, the data were provided to the statistical analyst to be analysed based on the studied objectives.
2.1. Ethical issues
This research was performed according to the Declaration of Helsinki. Informed written consent was obtained from the patients, and the study was approved by the Ethics Committee of Urmia University of Medical Sciences (ethical code: IR.UMSU.REC.1399.017) available at https://ethics.research.ac.ir/ProposalCertificateEn.php?id=126928&Print=true&NoPrintHeader=true&NoPrintFooter=true&NoPrintPageBorder=true&LetterPrint=true.
2.2. Statistical analysis
After entering the required data from the patient's records, the data were analysed using SPSS version 18. Besides, descriptive data for continuous variables and qualitative statistics were used as bar charts and tables. Mean difference tests (independent t‐test) and ANOVA repeated measure was used to compare the patients as well as logistic regression was applied to predict the factors affecting the mortality of COVID‐19. Logistic regression gives us Odds Ratio (OR), a measure of association between exposure and an outcome. The OR represents the odds that an outcome will occur given a particular exposure, compared to the odds of the outcome occurring in the absence of that exposure.
3. RESULTS
The mean age of patients was 50.93 ± 17.92 years. Regarding gender, there were 91 females (42.3) and 124 males (57.7). The mean hospital stay, the temperature at admission, and onset of symptoms were 4.91 ± 3.68 days, 37.40 ± 0.96°C, and 5.88 ± 4.80 days, respectively. Close contact with suspected people was found in 10.2% of patients. Additionally, 44 patients (20.5) were smokers. Paraclinical characteristics of patients with COVID‐19 were presented by the mean and standard deviation. Also, hemodynamic and respiratory features of patients with COVID‐19 were presented by the mean and standard deviation (Table 1). Creatinine at admission, on the first day and the third day significantly declined (Table 2). Repeated measure analysis showed a decreasing trend after admission between two groups of cured patients and died patients (Figure 1). Also, there is another analysis on the creatinine level in three points by gender (Table 3 and Figure 2).
TABLE 1.
Clinical and paraclinical characteristic of patients with COVID‐19
| Variables | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|
| WBC (109/L) | 1400.00 | 131 000.00 | 10 079.24 | 14 235.73 |
| NEUT (%) | 8.00 | 100.00 | 71.63 | 14.82 |
| LYMPH (%) | 1.50 | 80.00 | 22.42 | 13.07 |
| HB (g/dL) | 5.00 | 21.00 | 13.96 | 2.22 |
| Platelet (109/L) | 75.00 | 760.00 | 227.88 | 105.75 |
| CRP (mg/L) | 1.00 | 2.00 | 1.64 | 0.47 |
| ESR (mm/h) | 0.90 | 215.00 | 35.12 | 34.39 |
| CPK (U/L) | 2.40 | 1081.00 | 163.18 | 192.77 |
| LDH (U/L) | 5.20 | 3173.00 | 527.22 | 402.56 |
| ALP (U/L) | 11.00 | 1633.00 | 190.46 | 150.91 |
| ALT (U/L) | 11.00 | 2435.00 | 51.61 | 196.28 |
| AST (U/L) | 11.00 | 4892.00 | 88.82 | 475.62 |
| BILIT (mg/dL) | 0.20 | 6.20 | 0.84 | 0.94 |
| BILID (mg/dL) | 0.10 | 2.90 | 0.31 | 0.41 |
| Spo2 (%) | 55.00 | 99.00 | 91.01 | 7.40 |
| Respiratory rate (bpm) | 3.00 | 32.00 | 19.68 | 3.89 |
| SBP (mm Hg) | 85.00 | 180.00 | 116.55 | 13.63 |
| DBP (mm Hg) | 50.00 | 110.00 | 75.41 | 9.03 |
TABLE 2.
Comparison of creatinine levels between two groups of cured patients and died patients
| Variable | Expired | Mean | SD | F | P value |
|---|---|---|---|---|---|
| Creatinine at admission (mg/dL) | Yes | 3.39 | 3.98 | 4.833 | .039 |
| No | 1.60 | 0.62 | |||
| Creatinine at first day (mg/dL) | Yes | 3.12 | 3.46 | ||
| No | 1.28 | 0.54 | |||
| Creatinine at third day (mg/dL) | Yes | 2.72 | 1.95 | ||
| No | 1.14 | 0.50 |
FIGURE 1.
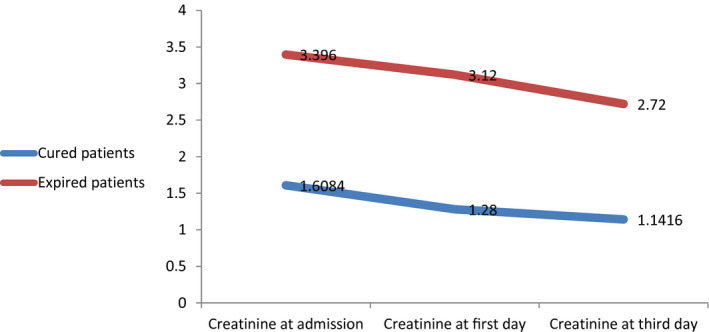
Comparison of creatinine levels between two groups of cured patients and died patients
TABLE 3.
Comparison of creatinine levels between two groups of men and women patients
| Variable | Gender | Mean | Std. Deviation | F | P value |
|---|---|---|---|---|---|
| Creatinine at admission (mg/dL) | Female | 1.55 | 0.59 | 7.350 | .013 |
| Male | 2.57 | 2.83 | |||
| Creatinine at first day (mg/dL) | Female | 1.30 | 0.59 | ||
| Male | 2.16 | 2.53 | |||
| Creatinine at third day (mg/dL) | Female | 1.28 | 0.59 | ||
| Male | 1.73 | 1.63 |
FIGURE 2.

Comparison of creatinine levels between two groups of men and women patients
Common symptoms, comorbidities, lung and renal involvements were investigated and reported as frequency and percent. Shortness of breath and cough were found in 62.8% and 49.3% of patients. Diabetes mellitus and hypertension were the most common comorbidities of patients, in which 18.1% and 20.5% reported, respectively. Regarding lung involvement, 33 patients (33%) were normal, most of the patients (n = 71) had 5%‐25% involvement in their lung and a minority of patients (n = 13) had a severe condition of 50%‐75% lung involvement. Additionally, 32 patients (14.9%) had creatinine more than 1.5 mg/dL (Table 4).
TABLE 4.
The frequency of symptoms, comorbidity, renal and lung involvements in patients with COVID‐19
| Symptoms | Frequency | Percent |
|---|---|---|
| Shivering | 33 | 15.3 |
| Lethargy | 3 | 1.4 |
| Pharyngitis | 10 | 4.7 |
| Shortness of breath | 135 | 62.8 |
| Cough | 106 | 49.3 |
| Dry cough | 33 | 15.3 |
| Diarrhoea | 25 | 11.6 |
| Myalgia | 58 | 27 |
| Weakness | 45 | 20.9 |
| Lung involvement | ||
| Normal | 33 | 16.1 |
| Less than 5% | 64 | 31.2 |
| 5%‐25% | 71 | 34.6 |
| 25%‐50% | 24 | 11.7 |
| 50%‐75% | 13 | 6.3 |
| Comorbidity | ||
| Asthma | 19 | 8.8 |
| Heart disease | 11 | 5.1 |
| Rheumatism | 4 | 1.9 |
| DM | 39 | 18.1 |
| HTN | 44 | 20.5 |
| Autoimmune disease | 2 | 0.9 |
| Lupus | 1 | 5 |
| Renal involvement | ||
| Positive | 32 | 14.9 |
| Negative | 183 | 85.1 |
Regarding liver profile, 88.4% of patients had ALP more than 115 U/L; 12% of patients had ALT more than 56 U/L; 23.2% of patients had AST more than 40 U/L; 95.9% of patients had LDH more than 222 U/L. Platelet count in 17.3% of patients was below 150 000 per µL; ESR above 20 mm/h was observed in 58.7% of patients. Total bilirubin among 9% was high and 91% had normal range (0.2‐1.2 mg/dL). Direct bilirubin among 8% was high and 92% had normal range (0‐0.4 mg/dL). In overall, nine patients received IVIG and two of nine patients died (22.2%). Among 19 patients who passed away, 15 patients were hospitalised in ICU, so a total of 175 patients (81.4%) had no history of hospitalisation in ICU (Table 5). The association between smoking and mortality was tested using chi‐square showing no significant difference (X2:2.959, P = .085). There was no significant difference between AST, ALT, ALP, total, direct Bilirubin, lung involvement and suffering from fever (P > .05) (Table 6).
TABLE 5.
Abnormality of paraclinical tests in patients with COVID‐19
| Abnormality | Frequency | Percent | |
|---|---|---|---|
| ALP (U/L) | Yes | 114 | 88.4 |
| No | 15 | 11.6 | |
| ALT (U/L) | Yes | 20 | 12.0 |
| No | 147 | 88.0 | |
| AST (U/L) | Yes | 39 | 23.2 |
| No | 129 | 76.8 | |
| LDH (U/L) | Yes | 165 | 95.9 |
| No | 7 | 4.1 | |
| Platelet (109/L) | Yes | 35 | 17.3 |
| No | 167 | 82.7 | |
| ESR (mm/h) | Yes | 98 | 58.7 |
| No | 69 | 41.3 | |
| Direct bilirubin (mg/dL) | Yes | 9 | 8.0 |
| No | 103 | 92.0 | |
| Total bilirubin (mg/dL) | Yes | 10 | 9.0 |
| No | 101 | 91.0 | |
| Fever (°C) | Yes | 92 | 43.6 |
| No | 119 | 56.4 | |
TABLE 6.
Association of paraclinical abnormality and lung involvement with fever in patients with COVID‐19
| Abnormality | Fever | X2, P value | ||
|---|---|---|---|---|
| Yes | N | |||
| AST (U/L) | Yes | 14 | 24 | 0.927, .336 |
| No | 58 | 69 | ||
| ALT (U/L) | Yes | 7 | 13 | 0.733, .392 |
| No | 65 | 79 | ||
| ALP (U/L) | Yes | 50 | 62 | 0.689, .406 |
| No | 5 | 10 | ||
| Total bilirubin (mg/dL) | Yes | 2 | 8 | 1.772, .183 |
| No | 42 | 59 | ||
| Direct bilirubin (mg/dL) | Yes | 2 | 7 | 1.195, .274 |
| No | 42 | 61 | ||
| Lung involvement (%) | Normal | 13 | 20 | 1.797, .773 |
| Less than 5% | 26 | 36 | ||
| 5%‐25% | 35 | 34 | ||
| 25%‐50% | 11 | 13 | ||
| 50%‐75% | 5 | 8 | ||
To prevent estimation bias, variables with a significance level less than of 0.2 were entered in the logistic model. Link function was logit with significant fitting (P < .05) and large goodness of fit (P > .05). In the binary logistic regression model, high Spo2 can increase the chance of recovery by 24% with each unit reduction. Kidney involvement increases the chance of death by about 80% (95% CI: 0.104‐0.013). The odds ratio of spo2 for recovery of COVID‐19 was 1.24 (95% CI: 1.014‐1.528; P = .037) (Table 7).
TABLE 7.
Logistic regression to predict the binary outcome (recovery following COVID‐19)
| Variable | B | SE | Wald | P value | Odds ratio | 95% C I | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Spo2 (%) | 0.219 | 0.105 | 4.365 | 0.037 | 1.244 | 1.014 | 1.528 |
| HB (g/dL) | −0.094 | 0.244 | 0.148 | 0.700 | 0.910 | 0.564 | 1.470 |
| Age (y) | 0.006 | 0.038 | 0.024 | 0.877 | 1.006 | 0.934 | 1.083 |
| Positive chest‐X ray | 1.747 | 2.065 | 0.716 | 0.398 | 5.736 | 0.100 | 328.26 |
| Positive HRCT | −0.335 | 2.269 | 0.022 | 0.883 | 0.715 | 0.008 | 61.062 |
| LDH (U/L) | −0.002 | 0.001 | 3.387 | 0.066 | 0.998 | 0.996 | 1.000 |
| ICU | 1.472 | 1.248 | 1.391 | 0.238 | 4.358 | 0.377 | 50.338 |
| Kaletra | 3.464 | 1.713 | 4.090 | 0.043 | 31.960 | 1.113 | 917.83 |
| Neut (%) | −0.121 | 0.167 | 0.524 | 0.469 | 0.886 | 0.639 | 1.230 |
| Lymph (%) | −0.075 | 0.186 | 0.163 | 0.686 | 0.928 | 0.644 | 1.336 |
| Cough | 0.154 | 1.097 | 0.020 | 0.888 | 1.166 | 0.136 | 10.024 |
| Kidney involvement | −2.262 | 1.045 | 4.682 | 0.030 | 0.104 | 0.013 | 0.808 |
| Lung involvement | −0.347 | 0.419 | 0.687 | 0.407 | 0.707 | 0.311 | 1.606 |
4. DISCUSSION
In our study, the creatinine levels of in the patients with COVID‐19 decreased during hospitalisation, 18 patients who were admitted to the ICU had a creatinine level above 1.5 mg/dL, and 12 patients who died had a high creatinine level, according to a systematic review, 193 patients had high creatinine levels at the time of hospitalisation in 10% of patients, and 22% of patients experienced an increase in creatinine levels within 5 days of hospitalisation. 31 In various studies, an increase in creatinine in patients with COVID‐19 has been reported from 0.5% to 19%. 32 Huang et al found that 98% of patients with COVID‐19 had fever, of which 78% had a temperature above 38°C. They observed 76% of patients with cough, 44% of patients with fatigue and myalgia and 55% of patients with dyspnoea. In addition, the patients had sputum expectoration (28%), headache (8%), hemoptysis (5%) and diarrhoea (3%). Laboratory tests showed that 25% of infected patients had leukopenia and 64% had lymphopenia. Aspartate aminotransferase levels are elevated in 37% of patients. Myocarditis was diagnosed in 12% of patients, and very sensitive troponin I levels was significantly increased in these patients. Abnormalities in chest CT images were observed in 100% of patients. Grinding glass‐like and consolidation were found in 37% of the lungs on both sides of infected patients. 33
Zhao et al stated ACE2 as a receptor for the COVID‐19. In the normal human lung, ACE2 is expressed in type 2 and 1 alveolar epithelial cells, of which 83% of type 2 alveolar cells express ACE2. Men have higher levels of ACE2 in their alveolar cells than women. Asians have higher levels of ACE2 expression in their alveolar cells than in white and African‐American populations. Connection of new coronavirus‐2019 to ACE2 increases the expression of ACE2, which can lead to damage to alveolar cells. Injury to alveolar cells can in turn cause a series of systemic reactions and even death. They also confirmed that Asian men were more prone to COVID‐19. 34 Studies to find acceptable medication is ongoing and many drug are tested in clinical trials such as bromhexine effect on COVID‐19. 35 A review of Rahmat et al on the new coronavirus included a total of 50 studies. According to the World Health Organization, it is necessary to examine all people with acute respiratory diseases with a history of travel to Saudi Arabia or countries with epidemics of this new virus. 36 , 37 These results show the importance of knowledge about the epidemiological aspect of this disease. Guan et al reported 1099 cases of the new coronavirus infection. They found that fever (78.9%) and cough (67.7%) were the most common symptoms. Diarrhoea (3.7%) and vomiting (5%) were rare. Abnormalities in CT images of the chest were observed in 96% of the patients infected with the new Coronavirus‐2019, and lymphocytes were recorded in 82.1% of them. 17 In the study of Besharat et al in Tehran, the clinical and demographic characteristics of patients who died following COVID‐19 development were consistent with this study. 38 In this study, we showed that COVID‐19 can involve multiple organs such as liver and kidney. Samimi Ardestani et al 39 , showed that olfactory dysfunction is one of the other complications following COVID‐19 and about 86% of the patients were recovered from olfactory dysfunction approximately a month after the onset of olfactory dysfunction.
5. CONCLUSION
COVID‐19, like other viral diseases, can involve different organs of the body with different severity. In the meantime, it has a lot in common with other viral diseases and there are some common points. Its differences include its high infectivity. Also, in this study, smoking was not a risk factor for the virus or associated with severe manifestations of the disease. Early lymphopenia and high CRP disease were common manifestations of the disease, but in this study a significant number of patients were at first without lymphopenia and CRP high. The patients who were worse at the onset of symptoms had worse prognosis than patients with mild symptoms. The main symptoms of the patients were fever and cough and most of the patients with fever had a low‐grade one. It is important to note that patients should be hospitalised until the improvement of the severe symptoms and treatment should be continued on an outpatient basis at home. This study revealed that at the time of referral, creatinine, CPK, pulmonary involvement above 25%, and hypoxemia had a higher mortality rate. Also, increase of Spo2 can improve the patients following COVID‐19. The results indicated that Kaletra had the highest effect on recovery following COVID‐19.
Gharebaghi N, Farshid S, Boroofeh B, et al. Evaluation of epidemiology, clinical features, prognosis, diagnosis and treatment outcomes of patients with COVID‐19 in West Azerbaijan Province. Int J Clin Pract. 2021;75:e14108. 10.1111/ijcp.14108
REFERENCES
- 1. Bogoch A, Watts A, Thomas‐Bachli C, Huber MUG, Kraemer K. Pneumonia of unknown etiology in wuhan, China: potential for international spread via commercial air travel. J Trav Med. 2020;27:taaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in wuhan China: the mystery and the miracle. J Med Virol. 2020;92:401‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao S, Lin Q, Ran J, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019‐nCoV) in China, from2019 to 2020: a data‐driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu FZS, Bin Y, Chen YM, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019‐nCoV) in Wuhan, China. J Med Virol. 2020;92:441‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daneshfar M, Dadashzadeh N, Ahmadpour M, et al. Lessons of mortality following COVID‐19 epidemic in the United States especially in the geriatrics. J Nephropharmacol. 2021;10:e06. [Google Scholar]
- 7. Aleebrahim‐Dehkordy E, Reyhanian A, Saberianpour S, Hassanpour‐ DH. Acute kidney injury in COVID19. J Nephropathol. 2020;9:e31. [Google Scholar]
- 8. Lotfi B, Farshid S, Dadashzadeh N, Valizadeh R, Rahimi MM. Is coronavirus disease 2019 (COVID‐19) associated with renal involvement? A review of century infection. Jundishapur J Microbiol. 2020;13:e102899. [Google Scholar]
- 9. Valizadeh R, Baradaran A, Mirzazadeh A, Bhaskar LVKS. Coronavirus‐nephropathy; renal involvement in COVID‐19. J Renal Inj Prev. 2020;9:e18. [Google Scholar]
- 10. Mubarak M, Nasri N. COVID‐19 nephropathy; an emerging condition caused by novel coronavirus infection. J Nephropathol. 2020;9:e21. [Google Scholar]
- 11. Dadashzadeh N, Farshid S, Valizadeh R, Rahimi MM. Acute respiratory distress syndrome in COVID‐19 disease. Immunopathol Persa. 2020;6:e16. [Google Scholar]
- 12. Forouzesh M, Rahimi A, Valizadeh R, Dadashzadeh N, Mirzazadeh A. Clinical display, diagnostics and genetic implication of novel Coronavirus (COVID‐19) epidemic. Eur Rev Med Pharmacol Sci. 2020;24:4607‐4615. [DOI] [PubMed] [Google Scholar]
- 13. Valizadeh R, Dadashzadeh N, Zakeri R, James Kelllner S, Rahimi MM. Drug therapy in hospitalized patients with very severe symptoms following COVID‐19. J Nephropharmacol. 2020;9:e21. [Google Scholar]
- 14. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emery SL, Erdman DD, Bowen MD, et al. Real‐time reverse transcription–polymerase chain reaction assay for SARS‐associated coronavirus. Emerg Infect Dis. 2004;10:311‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaunt ER, Hardie A, Claas EC, Simmonds P, Templeton KE. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real‐time PCR method. J Clin Microbiol. 2010;48:2940‐2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mao L, Jin H, Wang M. Neurological Manifestation of Hospitalized Patients with COVID‐19 in Wuhan, China: a retrospective case series study. JAMA Neurol. 2020;77:683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428‐430. 10.1016/S2468-1253(20)30057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zaim S, Chong JH, Sankaranayanan V, Harky A. COVID 19 multi organ response. Curr Probl Cardiol. 2020;45:100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19—preliminary report. N Engl J Med. 2020;383:1813‐1826. [DOI] [PubMed] [Google Scholar]
- 23. Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID‐19: an open‐label control study. Engineering. 2020;6:1192‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rahimi MM, Jahantabi E, Lotfi B, Forouzesh M, Valizadeh R, Farshid S. Renal and liver injury following the treatment of COVID‐19 by remdesivir. J Nephropathol. 2021;10:e10. [Google Scholar]
- 25. Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta‐1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID‐19: an open‐label, randomised, phase 2 trial. Lancet. 2020;395:1695‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID‐19‐preliminary report. N Engl J Med. 2020;6:1192‐1198. [Google Scholar]
- 27. Hasan A, Paray BA, Hussain A, et al. A review on the cleavage priming of the spike protein on coronavirus by angiotensin‐converting enzyme‐2 and furin. J Biomol Struct Dyn. 2020;21:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid‐19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davoudi‐Monfared E, Rahmani H, Khalili H, et al. A randomized clinical trial of the efficacy and safety of interferon β‐1a in treatment of severe COVID‐19. Antimicrob Agents Chemother. 2020;64:e01061‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clark EG, Hiremath S, McIntyre L, Wald R, Hundemer GL, Joannidis M. Haemoperfusion should only be used for COVID‐19 in the context of randomized trials. Nat Rev Nephrol. 2020;8:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Z, Wu M, Yao J, et al. Caution on kidney dysfunctions of COVID‐19 patients. SSRN Electron J. 2020. 10.2139/ssrn.3559601. [DOI] [Google Scholar]
- 32. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID‐19 and multiorgan response. Curr Probl Cardiol. 2020;45:100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single‐cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019‐nCov. Am J Respir Crit Care Med. 2020;202:756‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barzegar A, Ghadipasha M, Rezaei N, Forouzesh M, Valizadeh R. New hope for treatment of respiratory involvement following COVID‐19 by bromhexine. J Nephropharmacol. 2021;10:e11. [Google Scholar]
- 36. Vahdat K, Amini A, Najafi A, Haerinejad MJ. A review of novel coronavirus, cause of middle east respiratory syndrome. Iran South Med J. 2014;16:486‐492. [Google Scholar]
- 37. Tavakoli A, Karbalaie Niya MH, Keshavarz M, Safarnezhad Tameshkel F, Monavari SH. Middle east respiratory syndrome coronavirus (MERS‐CoV). Iran J Med Microbiol. 2017;11:1‐8. [Google Scholar]
- 38. Afaghi S, Tarki FE, Rahimi FS, Irvani SS, Besharat S, Alamdari NM, Bagheri L. Therapeutic options and critical care strategies in COVID‐19 patients; where do we stand in this battle? School Med Stud J. 2020;2:3‐12. [Google Scholar]
- 39. Samimi Ardestani SH, Mohammadi Ardehali M, Rabbani Anari M, et al. the prevalence, prognosis, and recovery from olfactory dysfunction (OD). Acta Otolaryngol. 2019;2020:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]


