Abstract
BACKGROUND:
Taurine is an antioxidant that is abundant in some common energy drinks. Here we hypothesized that the antioxidant activity of taurine in red blood cells (RBCs) could be leveraged to counteract storage-induced oxidant stress.
STUDY DESIGN AND METHODS:
Metabolomics analyses were performed on plasma and RBCs from healthy volunteers (n = 4) at baseline and after consumption of a whole can of a common, taurine-rich (1000 mg/serving) energy drink. Reductionistic studies were also performed by incubating human RBCs with taurine ex vivo (unlabeled or 13C15N-labeled) at increasing doses (0, 100, 500, and 1000 μmol/L) at 37°C for up to 16 hours, with and without oxidant stress challenge with hydrogen peroxide (0.1% or 0.5%). Finally, we stored human and murine RBCs under blood bank conditions in additives supplemented with 500 μmol/L taurine, before metabolomics and posttransfusion recovery studies.
RESULTS:
Consumption of energy drinks increased plasma and RBC levels of taurine, which was paralleled by increases in glycolysis and glutathione (GSH) metabolism in the RBC. These observations were recapitulated ex vivo after incubation with taurine and hydrogen peroxide. Taurine levels in the RBCs from the REDS-III RBC-Omics donor biobank were directly proportional to the total levels of GSH and glutathionylated metabolites and inversely correlated to oxidative hemolysis measurements. Storage of human RBCs in the presence of taurine improved energy and redox markers of storage quality and increased posttransfusion recoveries in FVB mice.
CONCLUSION:
Taurine modulates RBC antioxidant metabolism in vivo and ex vivo, an observation of potential relevance to transfusion medicine.
Taurine (2-aminoethanesulfonic acid) is a sulfur-amino acid that derives from cysteine that was first discovered in 1827 by German scientists Friedrich Tiedemann and Leopold Gmelin in ox bile (Taurus in Latin).1 A main constituent of human bile salts as well, it was later identified in 1846 by Ronalds; while the mechanisms are still incompletely understood, it has since been appreciated for its role as an antioxidant,2-6 osmoprotectant,7 modulator of mitochondrial calcium homeostasis,8,9 and neuro-protective agent.10 Taurine is a nonessential amino acid, the circulating levels of which are significantly impacted by dietary intake (the normal diet typically contains 40 to 400 mg per day). Being abundant in meat and fish, vegan diets often result in decreases in circulating and urinary levels of taurine (approx. 78 and 29% of normal values, respectively11). With a maximum recommended intake of 3000 mg/day,12 taurine is an abundant ingredient in energy drinks, with some containing levels as high as 1000 to 2000 mg/serving.13 However, little is known about the metabolic impact of taurine-rich energy drinks on blood metabolism.
While taurine is abundant in the tissues of many animals and has been reported to have an antioxidant capacity, the mechanisms through which taurine exerts its antioxidant function are not fully understood. It has been argued that taurine is neither a regulator of antioxidant defenses nor a classical scavenger of reactive oxygen species (ROS; e.g., superoxide anion, hydroxyl radical, and hydrogen peroxide),14 the literature is controversial on the topic.10 Indeed, numerous studies suggest that taurine is an effective inhibitor of ROS generation. For example, taurine has been shown to counteract the deleterious effects of β-alanine on the synthesis of protein components of the mitochondrial electron transport chain complexes;15 in so doing, taurine was shown to prevent electron transport chain uncoupling and subsequent generation of ROS in cardiomyocytes.15 While this mechanism may be relevant to nucleated cells with mitochondria, it is unclear whether these mechanisms could apply to cells devoid of both de novo protein synthesis and mitochondria, like red blood cells (RBCs)—and thus whether taurine can be considered an antioxidant for RBCs, the most abundant cell in the human body.16
Generation and management of oxidant stress is indeed critical to RBC biology, as the mature RBC is loaded with approximately 250 to 270 million copies of hemoglobin (Hb),17 which allow an RBC to transport up to a billion molecules of oxygen per cell at full oxygen saturation. To facilitate gas transport, the prosthetic groups of Hbs are loaded with iron (2.6 g of bodily iron, approx. 66% of the iron in the human body is in RBCs18), which results in the generation of radical species through Fenton and Haber-Weiss chemistry. While RBCs have evolved to cope with such oxidant stress, RBC aging in the bloodstream in vivo—especially under pathologic conditions (e.g., hemoglobinopathies)—results in the progressive loss of the RBC capacity to counteract oxidant stress, while being unable to synthesize new proteins to replace oxidatively damaged components. In this view, RBCs represent a unique model to investigate whether the antioxidant capacity of taurine is independent from the regulation of gene product expression in a context of exacerbated oxidant stress.
Red blood cells are equipped with a series of antioxidant systems. Metabolism of glucose through the pentose phosphate pathway contributes to the generation of reducing equivalents (the reduced form of nicotinamide adenine dinucleotide phosphate [NADPH]) that are required to recycle the main antioxidant systems in RBCs, including glutathione (GSH; through NADPH-dependent glutathione reductase) and other NADPH-dependent enzymes (e.g., glutathione peroxidase, catalase, peroxiredoxins,19 glutaredoxins, thioredoxin reductase system, biliverdin reductase B,20 and the ascorbate21–tocopherol axis). All of these systems have been shown to positively respond to taurine stimulation in a dose-dependent fashion in RBCs of cadmium-treated mice5 or RBCs exposed to hydrogen peroxide,22 nitrite,3 hypochlorous acid,23 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH24) or phenylhydrazine.25 Preliminary data in the literature suggests a protective role for taurine on murine RBCs exposed to heavy metals such as cadmium,5 although the mechanisms through which this occurs or the pathways that are activated in RBCs after targeted supplementation have not yet been elucidated.
The RBC capacity to cope with oxidant stress is also critical in the case of medically relevant iatrogenic interventions, such as RBC storage in the blood bank, which is characterized by the progressive accumulation of oxidant stress to the RBC.26 As such, here we hypothesize that interdonor heterogeneity in RBC taurine levels at the time of donation may represent an unappreciated variable impacting the antioxidant potential of the RBC facing oxidant challenge during storage in the blood bank. In this view, in this study we leveraged a combination of in vivo studies and ex vivo reductionistic approaches in humans, as well as an animal model of RBC storage and transfusion to investigate whether the supplementation of taurine would boost the antioxidant capacity of the stored RBC and improve RBC metabolic markers of hemolysis and posttransfusion recovery, the gold standards to determine the quality of end of storage RBCs as per Food and Drug Administration guidelines.
MATERIALS AND METHODS
Metabolic effect of taurine-rich beverage
Blood (1 mL) was obtained through venipuncture in EDTA tubes from four healthy male volunteers (age, 32 ± 3 years) at 8 a.m. All donors were fasting (including no coffee, tea, or caffeinated beverage) when they consumed a whole 250-mL can of a commercially available caffeine and taurine-rich energy drink (Red Bull, Red Bull GmbH). Whole blood was collected at 1 hour postconsumption and separated into RBCs and plasma via centrifugation for 10 minutes at 1500 × g at 4°C.
Ex vivo incubation of human RBCs with taurine
Red blood cells from three healthy donor volunteers were obtained from the Vitalant Research Institute in Denver, Colorado, for research purposes. Units were processed according to blood bank processing standards. Briefly, whole blood (450 mL) was collected in CPD-AS3 upon leukofiltration. RBCs were then incubated with taurine (catalogue no. T0625, Sigma Aldrich) at increasing doses (0, 50, 100, and 500 μmol/L) for 0, 15, and 30 minutes and 4 hours at 37°C. Samples were collected at each time point and RBCs and supernatants were separated via centrifugation for 10 minutes at 1500 × g at 4°C for metabolomics analyses.
Tracing experiment with stable isotope–labeled taurine
Red blood cells (n = 3) were obtained from RBC concentrates, as outlined in the previous paragraph and incubated for 15 minutes and 4 and 16 hours at 37°C in presence or absence of stable isotope-labeled 13C215N-taurine (CNLM-10253, Cambridge Isotopes). RBCs were also left untreated or oxidatively challenged with hydrogen peroxide 0.1 or 0.5%. Samples were collected for metabolomics analyses.
REDS-III RBC-Omics study participants and samples
This part of the study is an extension of the REDS III RBC-Omics study. As such, details related to donor selection and recruitment have been extensively described.27-29 Briefly, a total of 13,403 healthy blood donors were enrolled in this study. They donated a unit of leukofiltered RBCs at one of four different blood centers across the United States. The units were stored for up to 42 days (end of shelf-life), when they were tested for the propensity of stored RBCs to hemolyze, either spontaneously or after osmotic, oxidative, or mechanical insults, as previously detailed.27,28 Extreme hemolyzers (5th and 95th percentile for lowest and highest hemolysis) were asked to donate a second unit of blood, which was stored for 42 days and sampled with sterile procedures for metabolomics on Days 10, 23, and 42, as described.30 Further details about this study are detailed in prior publications.28,31
Posttransfusion recovery of mouse RBCs stored in presence of taurine
Mouse RBCs were obtained by intracardiac puncture from wild-type FVB/J mice (The Jackson Laboratory). All mice were housed in the University of Virginia vivarium, and all procedures were performed under an institutional animal care and use committee–approved protocol. RBCs were stored as previously described,32,33 in CPDA1 additive, either untreated or supplemented with 500 μmol/L taurine for up to 8 days at 4°C in at least three independent experiments (n = 3 per group—paired samples with and without taurine for each experiment). At the end of the storage period, RBCs were transfused into B6 x Ubi-GFP+ mice. Before transfusion, fresh HOD+ RBCs were added to the stored FVB RBCs and the mixture of RBCs was transfused as described.34 Posttransfusion recoveries were determined by sampling peripheral blood at 24 hours posttransfusion and enumerating stored FVB RBCs (HOD–GFP−) as a ratio of the fresh tracer population (HOD+GFP−) and correcting for pretransfusion ratios.
Human RBC storage in taurine-supplemented additives
Human RBCs (n = 3) were donated for research purposes by the Vitalant Research Institute in Denver, Colorado, and stored under standard conditions (1-6°C for 42 days) in standard additives (CPD-AS-3), either untreated or supplemented with 500 μmol/L taurine. Units were sampled at the end of their shelf life (Storage Day 42) for metabolomics analyses.
Sample processing and metabolite extraction
A volume of 50 μL of frozen RBC aliquots was extracted 1:10 in ice-cold extraction solution (methanol:acetonitrile:water 5:3:2 vol/vol) in presence of a mixture of internal standards, including 13C,15N-labeled amino acid standards at 1 μmol/L final concentration.35 Samples were vortexed and insoluble material pelleted as described.36,37
Ultra-high-pressure liquid chromatography–mass spectrometry metabolomics
Analyses were performed using a Vanquish UHPLC coupled online to a mass spectrometer (Q Exactive, Thermo Fisher). Samples were analyzed using a 3-minute isocratic condition38 or a 5-, 9-, and 17-minutes gradient as described.37,39-41 For the analysis of taurine levels, samples were analyzed using a 5-minute C18 gradient and MS-positive ion mode acquisition as described.37,39-41 MS acquisition, data analysis, and elaboration were performed as described. Solvents were supplemented with 0.1% formic acid for positive mode runs and 1 mmol/L ammonium acetate for negative mode runs. MS acquisition, data analysis, and elaboration were performed as described.37,38
Statistical analyses
Graphs and statistical analyses (repeated-measures analysis of variance [ANOVA] or paired t test, as appropriate depending on the specific portion of the study) were prepared with computer software (GraphPad Prism 8.0, GraphPad Software, Inc.). Multivariate analyses, including partial least-square–discriminant analyses (PLS-DA) and hierarchical clustering analyses were performed with computer software (MetaboAnalyst 4.0, https://www.metaboanalyst.ca/).42 The KEGG pathway-based interactome was generated with the OmicsNet tool.43
RESULTS
Taurine-rich energy drinks significantly impact plasma and RBC metabolomes
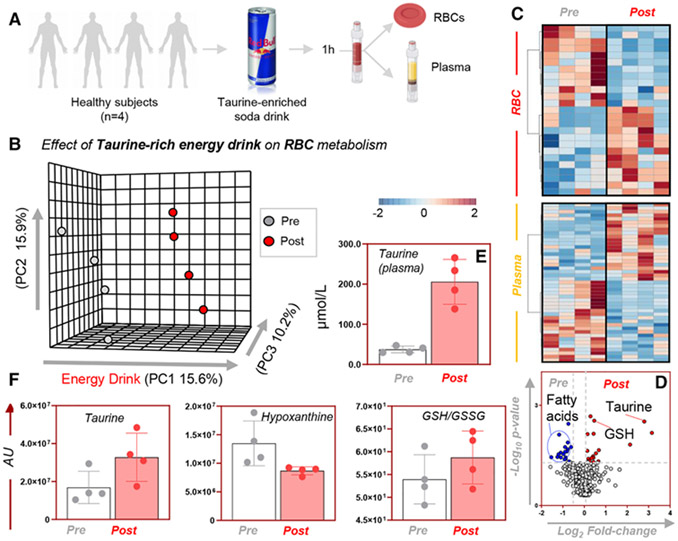
Metabolomics analyses were performed on plasma and RBCs from four healthy donor volunteers at baseline and 1 hour after consumption of a commercially available, taurine-rich popular beverage (Fig. 1A). Results are extensively reported in tabulated form in Table S1 (available as supporting information in the online version of this paper). Multivariate analyses revealed a significant impact on plasma and RBC metabolism, as gleaned by PLS-DA (15.6% of the total variance explained by Principal Component 1; Fig. 1B) and hierarchical clustering analyses (Fig. 1C). Vectorial versions of the heat maps for plasma and RBC metabolites impacted at 1 hour from the consumption of the energy drink are provided as Figs. S1 and S2, respectively (available as supporting information in the online version of this paper). A volcano plot analysis of the data (Fig. 1D) revealed that taurine was the most significantly affected metabolite with the highest fold-change in plasma (approx. 200 μmol/L; Fig. 1E), although significant increases in taurine levels were detected in RBCs as well (Fig. 1F). Additionally, taurine decreased the RBC levels of a series of oxidant stress markers, including the purine oxidation product hypoxanthine (Fig. 1F), while it increased markers of pentose phosphate pathway activation (sedoheptulose phosphate, gluconolactone phosphate; Fig. S2 [available as supporting information in the online version of this paper]) and reduced to oxidized GSH ratios (p < 0.05 in paired t test; Fig. 1F).
Fig. 1.
Metabolic impact on plasma and RBCs of taurine-rich beverage. Metabolomics analyses were performed on plasma and RBCs from four healthy donor volunteers at baseline and 1 hour upon consumption of a commercially available, taurine-rich popular beverage (A). PLS-DA (B), hierarchical clustering analysis (C), and volcano plot (D) showed distinct metabolic phenotypes between subject-matched plasma and RBC samples after consumption of the “energy drink.” Vectorial versions of the heat maps in C are provided as Figs. S1 and S2. Data are plotted as a volcano plot (combining plasma and RBC measurements) in D. Metabolites levels (y axis represents arbitrary units [AU]) affected included taurine in plasma (E) and RBCs (F), as well as metabolic pathways involved in redox homeostasis and oxidation products such as reduced GSH and hypoxanthine, respectively.
Taurine improves energy and redox metabolism of human RBCs in a dose-dependent fashion
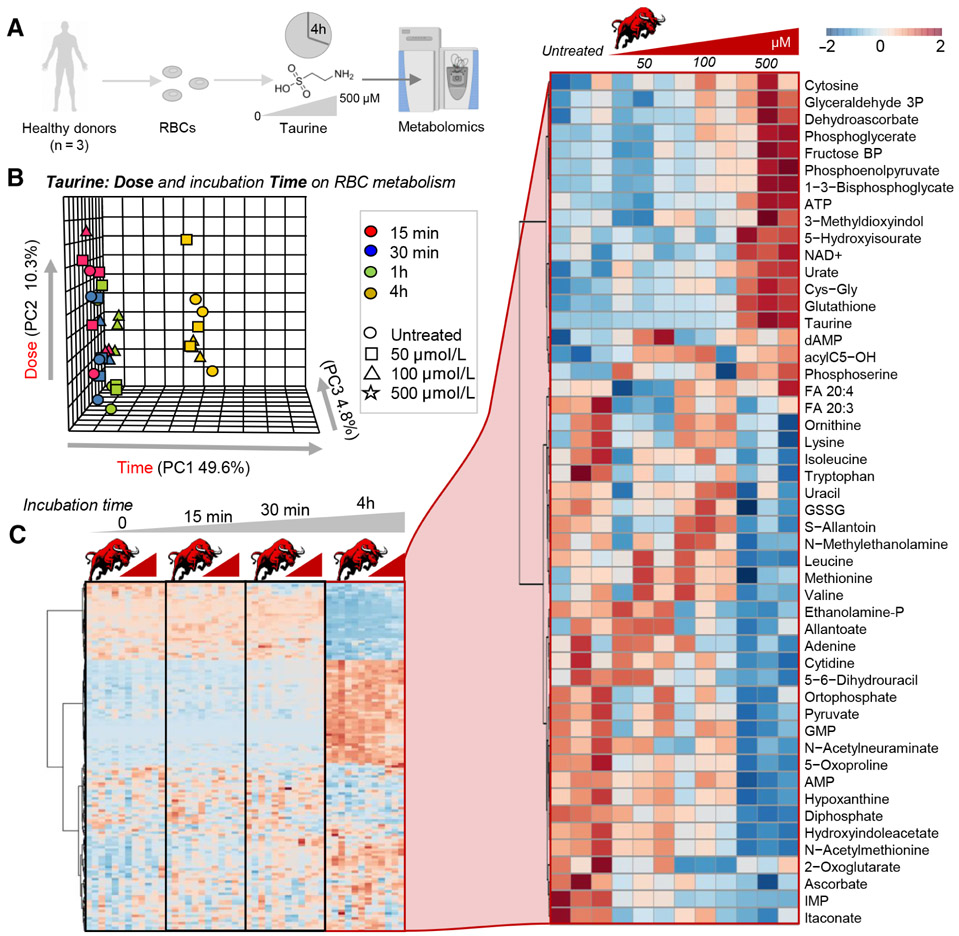
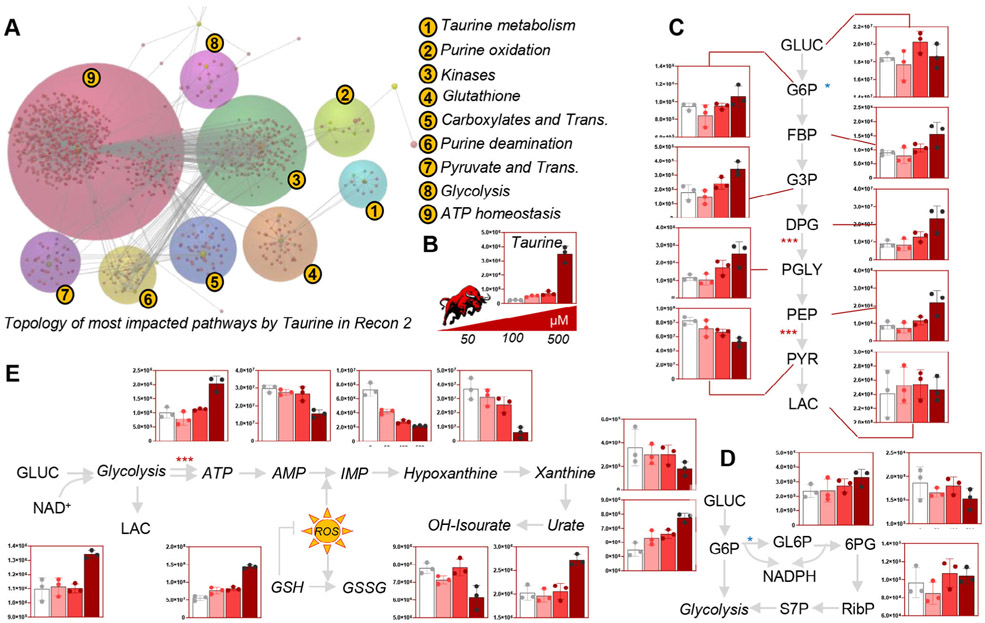
Given the significant impact of taurine-rich energy drinks on plasma and RBC metabolism, we tested whether the observed improvements in RBC antioxidant metabolism were attributable to taurine or other components. While in parallel studies we have focused on the impact of caffeine on RBC metabolism (also found in the energy drink tested; D’Alessandro et al., under review), in this study we decided to focus on the role of taurine. As such, metabolomics analyses were performed on human RBCs (n = 3) incubated with pure taurine at different doses (0, 50, 100, and 500 μmol/L) for 0, 15, and 30 minutes, up to 4 hours at 37°C (Fig. 2A). Of note, reported blood levels of taurine in the literature range from 67 ± 20 μmol/L in adults with schizophrenia, to 117 ± 7 μmol/L in adults with heart failure and 150.9 to 226.9 μmol/L in children with environmental enteric dysfunction (data from the Human Metabolome Database, http://www.hmdb.ca/metabolites/HMDB0000251#concentrations). Storage time had the strongest impact on the RBC metabolic phenotype, with the 4-hour time point clustering apart from the rest of the samples in multivariate analyses (PLS-DA and hierarchical clustering; Figs. 2B and 2C). The top 50 significant metabolites by repeated measures ANOVA are highlighted as a function of taurine dose at the 4hour time point are higlighted in the right hand panel of Fig. 2C. A pathway analysis based on these metabolites revealed a significant impact of taurine—in a dose-dependent fashion, with the strongest phenotype at the highest dose of 500 μmol/L—on sulfur metabolism, purine oxidation, GSH homeostasis, glycolysis, and energy metabolism (homeostasis of high-energy phosphate compounds; Fig. 3A). An overview of key metabolic intermediates of these pathways is provided in Figs. 3C to 3E for glycolysis, pentose phosphate pathway, purine oxidation, and GSH homeostasis, respectively. In particular, taurine levels (Fig. 3B) were positively correlated with glycolytic metabolites glucose 6-phosphate, glyceraldehyde 3-phosphate, diphosphoglycerate (including the Rapoport-Luebering shunt isobaric intermediate, 2,3-diphosphoglycerate), phosphoglycerate, and phosphoenolpyruvate but not pyruvate and lactate (Fig. 3C). Taurine levels positively correlated with the levels of 6-phosphogluconate and sedoheptulose phosphate (Fig. 3D) as well as reduced GSH and urate (Fig. 3E). On the other hand, increasing doses of taurine were accompanied by lower doses of purine breakdown products (AMP) and deaminated purines (IMP, hypoxanthine, xanthine), consistent with an improved preservation of adenosine triphosphate (ATP; Fig. 3E).
Fig. 2.
Metabolic impact of taurine at different concentrations on human RBCs. Metabolomics analyses were performed on human RBCs (n = 3) incubated with taurine at different doses (0, 50, 100, and 500 μmol/L) for 0, 15, and 30 minutes up to 4 hours at 37°C (A). In B and C, results from the PLS-DA and hierarchical clustering analysis of the significant metabolites by repeated measures Two-way ANOVA (Z-score normalized ranges are color-coded consistently with the legend in the top right corner of the figure). The small bull (in Latin taurus—is representative of taurine doses—increasing from left to right on top of each subgroups in the heat maps in C). On the right-hand panel, metabolites highlighted are the significant metabolites impacted by increasing doses of taurine at the 4-hour incubation time point.
Fig. 3.
Taurine improved energy and redox metabolism in human RBCs. Pathway analysis of metabolic changes induced by 4-hour incubation with taurine at 37°C highlight a protective role of taurine (B) with respect to energy (glycolysis, C) and redox metabolism, with a special emphasis on the pentose phosphate pathway (D), purine oxidation, and GSH homeostasis (E). Bar graphs are plotted as arbitrary units (y axis throughout C–E).
Taurine protects metabolism from hydrogen peroxide–induced oxidant stress
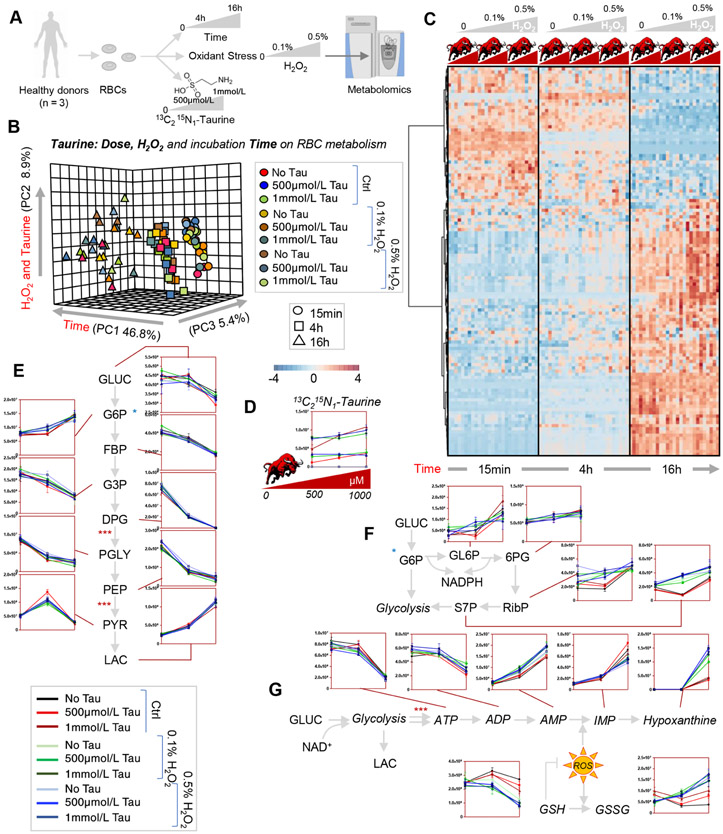
In the light of an apparent beneficial effect of taurine on energy and antioxidant metabolism, we incubated human RBCs (n = 3) with 500 μmol/L or 1 mmol/L taurine for 15 minutes and 4 and 16 hours, in the absence of stress (other than incubation itself) or in presence of added oxidant stress with hydrogen peroxide (either 0.1% or 0.5%; Fig. 4A). Data are reported extensively in Table S2 (available as supporting information in the online version of this paper). These doses and time points were chosen to center around the dose and time points with the highest impact on RBC metabolism in the experiment described above. In this experiment, we also used stable isotope-labeled 13C215N-taurine to determine potential catabolic routes for taurine in the mature RBC. Unfortunately, in this study we were unable to detect 13C- or 15N-labeled metabolites of taurine, except for negligible levels (i.e., within 10 standard deviations of the median measurement for background noise) of 13C2-sulfoacetaldehyde (data not shown). However, no increases in 15N-alanine were detected. These results are suggestive that taurine could either represent a metabolic bottleneck in the mature RBC or rather represent fuel pathways that were not amenable to the present analytical setup (e.g., acetyl-CoA). Despite these caveats, multivariate analyses revealed a significant impact of taurine on RBC metabolism as a function of taurine dose, incubation time and H2O2 challenge (Figs. 4B and 4C). Taurine levels were significantly impacted by hydrogen peroxide treatments. No significant differences were noted between the 500 μmol/L and 1 mmol/L taurine doses. However, taurine decreased proportionally to the increases in hydrogen peroxide (lowest at 0.5%; Fig. 4D). Of note, incubation time longer than 4 hours had a stronger effect than taurine and hydrogen peroxide doses on glycolysis (Fig. 4E). However, significant increases in pentose phosphate pathway activation were observed proportionally to hydrogen peroxide levels (the highest levels of ribose phosphate and sedoheptulose phosphate were observed in the 0.5% H2O2 samples; Fig. 4F). Consistently, GSH) levels decreased whereas oxidized glutathione (GSSG) levels increased proportionally to hydrogen peroxide levels (Fig. 4G). On the other hand, decreases in ATP levels, increases in ATP breakdown markers ADP and AMP, and purine oxidation markers (IMP, hypoxanthine) were proportional to oxidant stress with hydrogen peroxide, an effect that was partially counteracted by taurine supplementation—to an equal extent at 500 μmol/L and 1 mmol/L concentrations.
Fig. 4.
Tracing experiments with 13C15N-taurine in human RBCs further confirm a role in protection from H2O2-induced oxidant stress to GSH and purines. Human RBCs (n = 3) were incubated with 500 μmol/L or 1 mmol/L for 15 minutes and 4 and 16 hours, in the absence of stress or in presence of oxidant stress with hydrogen peroxide (either 0.1% or 0.5%, A). In B and C, results from the PLS-DA and hierarchical clustering analysis of the significant metabolites by repeated-measures two-way ANOVA. While no labeled catabolites of stable isotope-labeled taurine were detected in this study (D), and time was the major factor contributing to the observed alteration in glycolysis (E), this analysis confirmed a protective role of higher concentrations of taurine against oxidant stress, resulting in decreased activation of the pentose phosphate pathway (F) and better preservation of the GSH system and nonoxidized purines (G). Y axes in line plots are shown as arbitrary units (E-G).
Taurine levels in stored RBCs correlate with altered antioxidant capacity and GSH metabolism
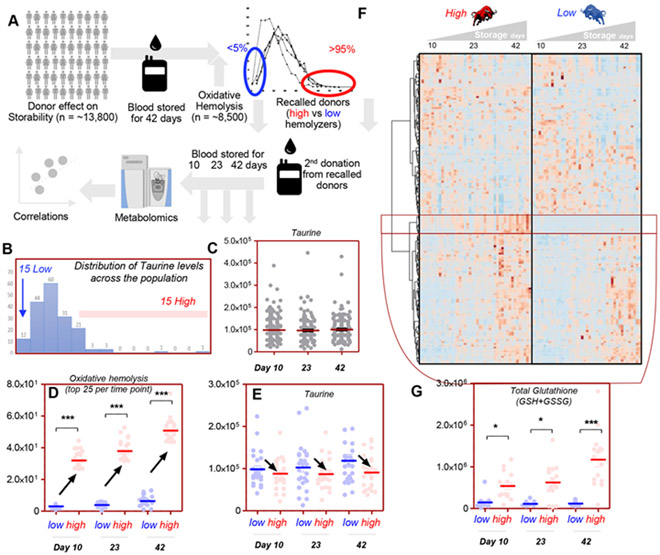
To determine the potential translational relevance of these findings, we set out to determine whether taurine levels in stored RBCs from healthy donor volunteers would correlate to RBC storage quality. To test this specific hypothesis, we leveraged the extensive cohort of healthy donor volunteers enrolled in the REDS-III RBC-Omics study. Within the framework of this study, 13,400 donors were selected based on the propensity of the RBCs they donated to hemolyze after oxidant insults with AAPH27 at the end of the shelf-life of the blood product (i.e., Storage Day 42; Fig. 5A). The donors ranking in the 5th and 95th percentiles based on these criteria were asked to donate a second unit of blood, which was stored again for 42 days, while being sterilely sampled on Storage Days 10, 23, and 42 to perform metabolomics measurements, along with new tests to assess the RBC propensity to hemolyze after oxidant insults. Within the framework of these analyses we could determine that the levels of taurine in donated blood units followed a non-normal distribution in the total of 599 samples assessed in this study (Fig. 5B), with no significant impact of storage duration on taurine levels (Fig. 5C). Interestingly, taurine negatively correlated to oxidative hemolysis measurements and metabolic markers of hemolysis (e.g., bilirubin; Table S3, available as supporting information in the online version of this paper). When focusing post hoc analyses on the 15 donors with the lowest and highest oxidative hemolysis measurements (Fig. 5D), we observed lower taurine levels in those donors with the highest hemolytic propensity (Fig. 5E). Vice versa, when focusing the analyses on the 15 donors with the lowest and highest levels of taurine as a function of storage duration, we could observe significant taurine-associated alterations in RBC metabolism (Fig. 5F; also in vectorial form with metabolite names in Fig. S3, available as supporting information in the online version of this paper). A cluster of metabolites was significantly higher through the whole storage period in those donors with the highest taurine levels (highlighted in Fig. 5F). Focusing in on that cluster of metabolites, we noticed a series of GSH metabolites (including glutathionylated hydroxynonenal metabolites and short odd-chain fatty acid products of lipid peroxidation cascades; Fig. 3). A combined representation of glutathionylated metabolites (as a proxy for the total levels of cell GSH) indicated significantly higher levels of this reducing equivalent and its metabolites in those donors with the highest levels of taurine (Fig. 5G).
Fig. 5.
Taurine negatively correlates with the RBC propensity to hemolyze after oxidant stress in the REDS-III RBC-Omics recalled donor population. Within the framework of the REDS-III RBC-Omics study, a large cohort (13,400) of healthy volunteers donated a unit of blood (A). Units were stored until the end of their shelf life (42 days) and then tested for the propensity to hemolyze after oxidant insult with AAPH. Donors with the lowest (5th) and highest (95th) oxidative hemolysis variables were asked to donate a second unit of blood, which was stored for up to 42 days and sampled sterilely on Days 10, 23, and 42 for taurine measurements based on chromatographic retention times and high-resolution intact mass spectra via UHPLC-MS. Taurine levels are not normally distributed across the population (B) and are not impacted by storage duration (C). Sorting of the extreme 25 high (red) and low (blue) oxidative hemolysis donors in the recalled donor population (D) highlighted a negative correlation between taurine levels and oxidative hemolysis (E). In F, a hierarchical clustering analysis was performed on the metabolites significant by ANOVA (time series, one-variable) from the metabolomics data of stored RBCs (Days 10, 23, and 42) from REDS-III RBC-Omics donors with the 15 highest and lowest levels of taurine. A vectorial version of this heat map, including metabolite names is provided in Fig. S3. In particular, taurine levels were positively associated with increases in several glutathionylated metabolites, with total GSH (reduced glutathione + GSSG) levels being significantly higher in the high-taurine group at each one of the tested time points (G).
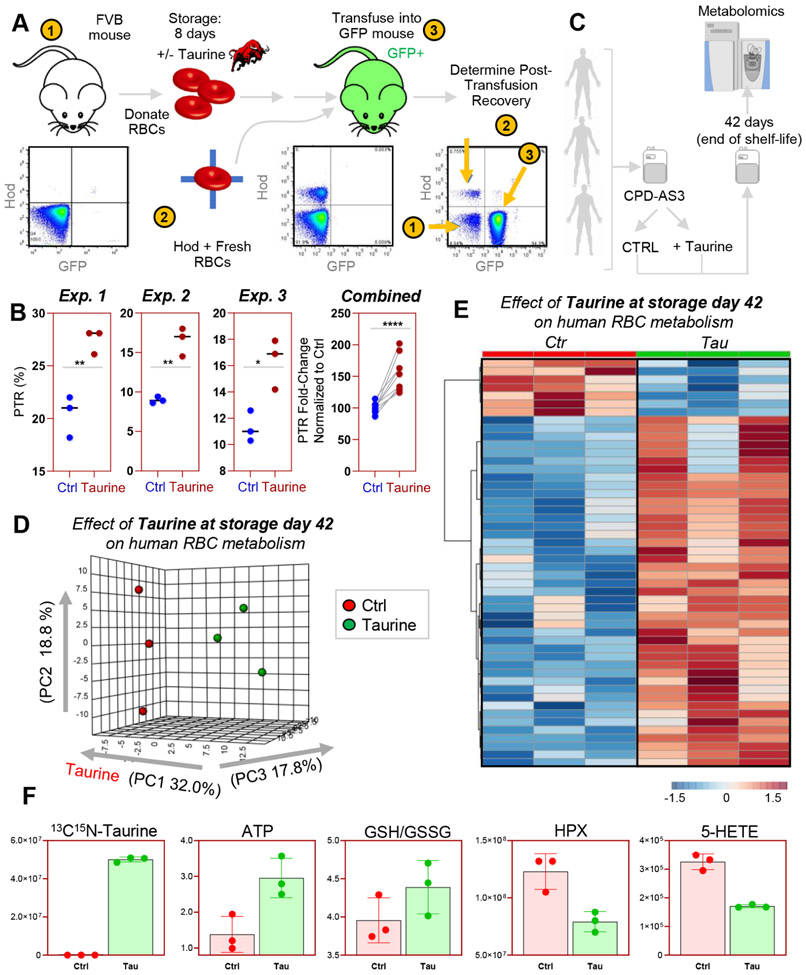
Taurine supplementation to RBC storage additives improves end of storage energy and redox metabolism in humans and improves posttransfusion recovery in FVB mice
In light of the results described above, we questioned whether the observations suggestive of a potential association between taurine levels and redox metabolism of stored RBCs were merely correlative or causative. As such, we incubated RBCs from FVB mice—extensively characterized in prior work32,34,44 as a mouse strain with a poor posttransfusion recovery in CPDA1 with or without added 500 μmol/L taurine, the lowest effective dose from the preliminary experiments described above (Fig. 6A). RBCs were stored for up to 8 days under conditions simulating human blood storage in the blood bank, before transfusion into B6 x Ubi-GFP+ mice, as previously described.32,34,44 As a result we noted a significant (p < 0.005) improvement in posttransfusion recoveries in FVB mouse RBCs stored in presence of taurine compared to those stored using regular CPDA1, in three independent experiments (Fig. 6B). While significant and reproducible in terms of paired posttransfusion recovery increment (combined fold-changes vs. paired controls is reported in the right-most panel in Fig. 6B), taurine supplementation did not improve PTR dramatically (still below <30% in all the tested samples).
Fig. 6.
Taurine increases posttransfusion recovery of RBCs from FVB mice (poor storers) and improves end of storage metabolism of human RBCs. RBCs were collected from FVB mice (previously identified as poor storers). RBCs were stored in CPDA1, in the presence or absence of 500 μmol/L taurine. RBCs were stored for up to 8 days, before transfusion into Ubi-GFP+ mice. Posttransfusion recoveries were determined via flow cytometry at 24 hours after transfusion (A), showing a significant increment in PTR in the taurine arm of the study in three independent experiments (n = 3 each—the right-most panel combines all measurements as a fold change to the median of paired control from each separate experiment) (B). Human RBCs were thus obtained from three healthy donor volunteers, leukofiltered, and stored in CPD-AS-3 in presence or absence of 500 μmol/L taurine, before storage for up to 42 days (end of the shelf life; C). In D and E, results from the PLS-DA and hierarchical clustering analysis of the significant metabolites by paired t test. A vectorial version of the heat map in E is provided as Fig. S4. In F, bar plots with superimposed dot plots highlight a few representative metabolites, including taurine, ATP, reduced to oxidized GSH ratios and markers of purine and lipid oxidation hypoxanthine and 5-HETE.
We then stored RBCs from three healthy human donor volunteers in the common additive CPD-AS-3, in presence or absence of 500 μmol/L taurine, and performed metabolomics analyses on RBCs at the end of the shelf life of the unit on Storage Day 42 (Fig. 6C). Multivariate analyses revealed a significant impact of taurine on stored human RBC metabolism (Figs. 6D and 6E). An overview of the top 50 metabolites impacted by the presence of taurine in storage additive is provided in Fig. 6F (a vectorial version of this figure, also including metabolite names is provided as Fig. S4, available as supporting information in the online version of this paper). Of note, taurine (Fig. 6F) significantly (p < 0.05 paired t test) increased RBC levels of ATP (marker of energy metabolism) and reduced/oxidized GSH levels (antioxidant potential), while significantly decreasing the levels of purine and lipid oxidation markers hypoxanthine and 5-HETE (Fig. 6F), both previously associated with posttransfusion recovery measurements in humans and mice.32,34,45
DISCUSSION
In this study, we showed that consumption of a common taurine-rich energy drink increases circulating levels of taurine and impacts RBC energy and redox metabolism. To further confirm that these effects were attributable to taurine rather than other ingredients in the drink (e.g., caffeine—investigated on a parallel independent study—D’Alessandro et al., under review), we performed follow-up reductionistic studies on human RBCs ex vivo. Specifically, we noted that human RBCs respond to increasing doses of taurine by boosting energy and redox metabolism. After oxidant challenge with hydrogen peroxide, we confirmed and expanded on previous observations on a protective role of taurine in RBCs incubated with H2O222 by providing an overview of how RBC metabolism responds to taurine stimulation after oxidative challenges. In particular, we noted that taurine did not significantly impact glycolysis per se; rather, it promoted the activation of the pentose phosphate pathway, the prevention of ATP breakdown and deamination, and the overall levels of GSH metabolites in a dose-dependent fashion, with a plateau at 500 μmol/L taurine. Experiments with stable isotope-labeled taurine did not reveal the presence of labeled catabolic products of taurine in RBCs, at least within the chemical space of the small molecule metabolites assayed in this study. Future studies will be necessary to expand on orthogonal classes of compounds that were not amenable to the present analyses, such as CoAs, which require a totally different analytical workflow from extraction to detection at the mass spectrometer level.
In the light of these observations, we hypothesized that taurine could represent an unappreciated player in the regulation of the RBC storage lesion. Technologies that enabled the possibility to age RBCs ex vivo have made it logistically possible to collect blood from healthy donor volunteers, store it under refrigerated conditions for up to 42 days before transfusion into hypoxic, hypovolemic recipients. Blood transfusion is a lifesaving intervention for approximately 5 million Americans every year. Indeed, it is perhaps one of the most critical advancements in modern medicine over the past century and still represent the most common in hospital medical procedure worldwide. However, refrigerated storage under blood bank conditions comes with a caveat, in that the stored RBC progressively undergoes a series of biochemical and morphological modifications collectively referred to as the storage lesion.26 Most of these lesions are triggered by alterations of energy and redox metabolism: depletion of high-energy phosphate compounds—ATP and 2,3-diphosphoglycerate (DPG)—begins early during storage, the latter being almost entirely depleted after Storage Day 14.46 This early metabolic lesion can be in part counteracted or delayed by the adoption of different storage additives that finely tune the intracellular pH of the RBC through strategies that promote its alkalinization (low chloride, no chloride, high bicarbonate, removal of both oxygen, and carbon dioxide from the unit47-49). However, as DPG is consumed, Hb oxygen saturation increases (up to 95% by Storage Week 3 in some donors), which is paralleled by increases in the levels of RBC ROS.50,51 However, the activation rate of the pentose phosphate pathway is limited by the activity of glucose 6-phosphate dehydrogenase (G6PD). This enzyme is the most commonly mutated in humans, with different variants impacting G6PD activity to a variable extent in up to 400 million people around the world, with higher incidence in regions and ethnicities that have been historically exposed to/at risk for malaria infections.52 Alterations of energy and redox metabolism is indeed exacerbated in G6PD-deficient donors,53 which are routinely accepted donors and characterize approximately 13% of the African American donor population in some of the largest metropolitan areas in the United States.54 Both in G6PD-deficient and -sufficient donors, storage progression is characterized by the progressive loss of the capacity to counteract oxidant stress through NADPH-dependent systems.55 Alternative mechanisms do exist in RBCs to repair the oxidant damage that inevitably arises upon failure of the aforementioned systems, such as methionine-fueled methylation of isoaspartyl moieties resulting from oxidation of asparaginyl or aspartyl residues that abound in the active sites of glycolytic and antioxidant enzymes in the mature RBC.35 Of note, methionine is a sulfur-containing metabolite which is upstream to cysteine, homocysteine, and cystathionine—all precursors for taurine synthesis in vivo in most mammals, including humans and excluding cats. These systems are however constrained by limited availability of methyl group donors in storage additives. In parallel, purine salvage reactions are only partially active in the mature RBC, ultimately resulting in the accumulation of deaminated purines such as hypoxanthine, a marker of the metabolic age56 of stored RBCs57,58 and a predictor of post-transfusion performances of the stored RBC upon transfusion into recipients in healthy autologous humans or heterologous mice.45 Fatty acid oxidation and the accumulation of oxidized lipids ultimately compromises RBC membrane stability and negatively impacts the capacity of the stored RBC to circulate upon transfusion in the recipient.32,34,59 Of note, taurine has been previously shown to prevent lipid peroxidation in other cell types, especially in the face of hyperglycemia (diabetes60), a condition that is brought to its extreme during storage in a blood bag, since most additives have glucose levels up to 20-fold higher than physiological values (~30 mmol/L upon introduction of RBCs at approx. 70% hematocrit). It is worth noting that some of the metabolic lesions described above are partially reversible, for example, by washing (which eliminates the irreversibly modified RBCs) and resuspension in additives that correct the metabolic defects of the metabolically impaired RBCs. These “rejuvenation” solutions are loaded with substrates to fuel glycolysis and synthesis of high-energy phosphate compounds, although none of them targets taurine metabolism. In light of the above, to further understand the potential translational relevance of our findings, we leveraged the REDS-III RBC-Omics recalled donor biobank to test whether the RBC levels of taurine correlated with the hemolytic propensity of the stored RBCs. Results highlighted a non-normal distribution of taurine levels across the healthy donor population enrolled in this study across four different US blood centers. Data also indicated a negative correlation between markers of RBC hemolysis (bilirubin) and the propensity to hemolyze after AAPH, in line with prior non-metabolomics studies on ex vivo stressed RBCs.24 Vice versa, taurine levels positively correlated with the total GSH levels (including glutathionylated metabolic intermediates of the detoxification pathways for oxidized lipids). Although correlative and confounded by covariates to taurine (e.g., caffeine), these observations informed reductionistic interventional studies in murine models of blood storage and posttransfusion efficacy. We thus leveraged a previously described and well characterized mouse model32,34,44 to investigate the impact of taurine supplementation on stored RBCs from mice that had been previously characterized as poor storers (i.e., with low posttransfusion recovery upon storage and transfusion). Results showed a significant improvement in PTR (almost doubled) in paired FVB mouse RBCs. However, it is worthwhile to point out that end of storage PTR in taurine-supplemented FVB RBCs were still well below the 75% threshold, suggesting that the benefits associated with taurine supplementation in RBCs from this mouse strain, although robust and reproducible over multiple independent experiments, are not sufficient to normalize (compared to other mouse strains) the redox metabolism of stored RBCs from these mice. These results were accompanied by the observation of a beneficial impact on metabolic markers of PTR (ATP, GSH,61 hypoxanthine,45 5-HETE32,34,59) in stored human RBCs when taurine was supplemented to storage additives. To this end, it is worth noting that PTR has been recently critiqued owing to several limitations associated with this assay,62 among which is the washing steps to remove the excess 51Cr that may untimely remove the most damaged RBCs (a step that is not necessary in the mouse studies described in the present paper) or the questionable relevance of an assay that relies on healthy autologous volunteers instead of sick heterologous recipients. To this end, other variables of extravascular hemolysis have been identified as potentially more meaningful predictors of transfusion efficacy.63 In this view, it is worth noting that taurine levels negatively correlated with marker of hemolysis such as bilirubin in the REDS portion of the data set.
In conclusion, in this study we provide an overview of the impact of taurine-rich energy drinks on plasma and RBC metabolism. Through a series of interventional studies, we define a beneficial role for taurine in boosting RBC energy and, above all, antioxidant metabolism—with a special emphasis on GSH homeostasis. These observations suggest the potential translational relevance of the antioxidant taurine in the context of blood storage, an iatrogenic intervention that exacerbates oxidant stress to the RBC. By leveraging a large clinical cohort and interventional studies in humans and mice, we confirm a beneficial impact of taurine on stored RBC metabolism, including on metabolic markers of posttransfusion performances—such as the capacity to circulate at 24 hours posttransfusion; however, the effect on the latter variable was reproducible albeit insufficient to normalize posttransfusion recovery measurements in FVB mice back to the greater than 75% values reported for other mouse strains.32,34,44 This study falls short of recommending that routine blood donors should increase their taurine intake before donation or that donors with lower blood levels of taurine (e.g., vegan diets) may be more susceptible to the storage lesion—and prospective human studies will be necessary to test these specific hypotheses. However, the study suggests that taurine is an unappreciated regulator of RBC antioxidant metabolism and, by highlighting donor-to-donor variability in taurine levels, it further compounds with the growing body of literature on the interdonor heterogeneity with respect to RBC antioxidant capacity.
Supplementary Material
Figure S1. Impact of Taurine Drink on Plasma Metabolism.
Figure S2. Impact of Taurine Drink on RBC Metabolism.
Figure S3. Taurine levels and stored RBC metabolism in REDS.
Figure S4. Impact of Taurine on human RBC storage d42.
Table S1. Taurine dose response and time course.
Table S2. Labeled Taurine and H2O2 Time series – balanced.
Table S3. Taurine in REDS.
ACKNOWLEDGMENTS
The authors express their gratitude Dr. Simone Glynn of NHLBI for her support throughout this study, to the RBC-Omics research staff at all participating blood centers and testing labs for their contribution to this project, and to all blood donors who agreed to participate in this study.
Research reported in this publication was funded by the NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III), which was supported by NHLBI contracts NHLBI HHSN2682011-00001I, −00002I, −00003I, −00004I, −00005I, −00006I, −00007I, −00008I, and −00009I, as well as funds from the National Institute of General and Medical Sciences (RM1GM131968 to ADA), NHLBI R01HL146442 (ADA) and R01HL148151 (ADA, JCZ), the Boettcher Webb-Waring Investigator Award (ADA), and a shared instrument grant by the National Institutes of Health (S10OD021641). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS:
- AAPH
2,2′-azobis(2-amidinopropane) dihydrochloride
- GSH
glutathione
- GSSG
oxidized glutathione
- PLS-DA
partial least-square–discriminant analyses
- ROS
reactive oxygen species
Appendix
RBC-OMICS STUDY GROUP MEMBERS
The NHLBI Recipient Epidemiology Donor Evaluation Study-III (REDS-III), Red Blood Cell (RBC)-Omics Study, is the responsibility of the following persons:
Hub
A.E. Mast, J.L. Gottschall, W. Bialkowski, L. Anderson, J. Miller, A. Hall, Z. Udee, and V. Johnson, BloodCenter of Wisconsin, Milwaukee, WI
D.J. Triulzi, J.E. Kiss, and P.A. D’Andrea, The Institute for Transfusion Medicine (ITXM), Pittsburgh, PA
E.L. Murphy and A.M. Guiltinan, University of California at San Francisco, San Francisco, CA
R.G. Cable, B.R. Spencer, and S.T. Johnson, American Red Cross Blood Services, Farmington, CT
Data coordinating center
D.J. Brambilla, M.T. Sullivan, S.M. Endres, G.P. Page, Y. Guo, N. Haywood, D. Ringer, and B.C. Siege, RTI International, Rockville, MD
Central and testing laboratories
M.P. Busch, M.C. Lanteri, M. Stone, and S. Keating, Blood Systems Research Institute, San Francisco, CA
T. Kanias and M. Gladwin, Pittsburgh Heart, Lung, Blood, and Vascular Medicine Institute, Division of Pulmonary, Allergy and Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA
Steering committee chairman
S. H. Kleinman, University of British Columbia, Victoria, BC, Canada
National Heart, Lung, and Blood Institute, National Institutes of Health
S.A. Glynn, K.B. Malkin, and A.M. Cristman.
Footnotes
CONFLICT OF INTEREST
Though unrelated to the contents of this manuscripts, the authors declare that AD and TN are founders of Omix Technologies Inc and Altis Biosciencens LLC. James C Zimring serves as a consultant for Rubius Therapeutics. The other authors have disclosed no conflicts of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Tiedemann F, Gmelin L. Einige neue Bestandtheile der Galle des Ochsen. Ann Phys 1827;85:326–37. [Google Scholar]
- 2.Adedara IA, Ojuade TJD, Olabiyi BF, et al. Taurine ameliorates renal oxidative damage and thyroid dysfunction in rats chronically exposed to fluoride. Biol Trace Elem Res 2017;175:388–95. [DOI] [PubMed] [Google Scholar]
- 3.Ansari FA, Ali SN, Mahmood R. Taurine mitigates nitrite-induced methemoglobin formation and oxidative damage in human erythrocytes. Environ Sci Pollut Res Int 2017;24:19086–97. [DOI] [PubMed] [Google Scholar]
- 4.Roy A, Sil PC. Tertiary butyl hydroperoxide induced oxidative damage in mice erythrocytes: Protection by taurine. Pathophysiology 2012;19:137–48. [DOI] [PubMed] [Google Scholar]
- 5.Sinha M, Manna P, Sil PC. Taurine protects the antioxidant defense system in the erythrocytes of cadmium treated mice. BMB Rep 2008;41:657–63. [DOI] [PubMed] [Google Scholar]
- 6.Selvaraj N, Bobby Z, Sathiyapriya V. Effect of lipid peroxides and antioxidants on glycation of hemoglobin: an in vitro study on human erythrocytes. Clin Chim Acta Int J Clin Chem 2006;366:190–5. [DOI] [PubMed] [Google Scholar]
- 7.Trachtman H, Del Pizzo R, Sturman JA, et al. Administration of taurine analogues affords cerebral osmoprotection during chronic hypernatremic dehydration. Am J Dis Child 1988;142:1194–8. [DOI] [PubMed] [Google Scholar]
- 8.El Idrissi A, Trenkner E. Taurine regulates mitochondrial calcium homeostasis. Adv Exp Med Biol 2003;526:527–36. [DOI] [PubMed] [Google Scholar]
- 9.El Idrissi A Taurine increases mitochondrial buffering of calcium: role in neuroprotection. Amino Acids 2008;34:321–8. [DOI] [PubMed] [Google Scholar]
- 10.Ripps H, Shen W. Review: taurine: a “very essential” amino acid. Mol Vis 2012;18:2673–86. [PMC free article] [PubMed] [Google Scholar]
- 11.Laidlaw SA, Shultz TD, Cecchino JT, et al. Plasma and urine taurine levels in vegans. Am J Clin Nutr 1988;47:660–3. [DOI] [PubMed] [Google Scholar]
- 12.Shao A, Hathcock JN. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul Toxicol Pharmacol 2008;50:376–99. [DOI] [PubMed] [Google Scholar]
- 13.Curran CP, Marczinski CA. Taurine, caffeine, and energy drinks: reviewing the risks to the adolescent brain. Birth Defects Res 2017;109:1640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaffer SW, Azuma J, Mozaffari M. Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol 2009;87:91–9. [DOI] [PubMed] [Google Scholar]
- 15.Jong CJ, Azuma J, Schaffer S. Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids 2012. June;42:2223–32. [DOI] [PubMed] [Google Scholar]
- 16.Nemkov T, Reisz JA, Xia Y, et al. Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport. Expert Rev Proteomics 2018;15:855–64. [DOI] [PubMed] [Google Scholar]
- 17.Bryk AH, Wiśniewski JR. Quantitative analysis of human red blood cell proteome. J Proteome Res 2017;16:2752–61. [DOI] [PubMed] [Google Scholar]
- 18.D’Alessandro A, Hansen KC, Eisenmesser EZ, et al. Protect, repair, destroy or sacrifice: a role of oxidative stress biology in inter-donor variability of blood storage? Blood Transfus 2019;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion 2011;51:1439–49. [DOI] [PubMed] [Google Scholar]
- 20.Paukovich N, Xue M, Elder JR, et al. Biliverdin reductase B dynamics are coupled to coenzyme binding. J Mol Biol 2018;430(18 Pt B):3234–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pallotta V, Gevi F, D’alessandro A, et al. Storing red blood cells with vitamin C and N-acetylcysteine prevents oxidative stress-related lesions: a metabolomics overview. Blood Transfus 2014;12:376–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pokhrel PK, Lau-Cam CA. Protection by taurine and structurally related sulfur-containing compounds against erythrocyte membrane damage by hydrogen peroxide. Adv Exp Med Biol 2000;483:411–29. [DOI] [PubMed] [Google Scholar]
- 23.Vissers MC, Stern A, Kuypers F, et al. Membrane changes associated with lysis of red blood cells by hypochlorous acid. Free Radic Biol Med 1994;16:703–12. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T, Ogasawara M, Koyama I, et al. The protective effect of taurine on the biomembrane against damage produced by oxygen radicals. Biol Pharm Bull 1993;16:970–2. [DOI] [PubMed] [Google Scholar]
- 25.Pokhrel PK, Lau-Cam CA. In vitro and in vivo effects of taurine and structurally related sulfur-containing compounds against phenylhydrazine-induced oxidative damage to erythrocytes. Adv Exp Med Biol 2000;483:503–22. [DOI] [PubMed] [Google Scholar]
- 26.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 2015;55:205–19. [DOI] [PubMed] [Google Scholar]
- 27.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv 2017;1:1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanteri MC, Kanias T, Keating S, et al. Intradonor reproducibility and changes in hemolytic variables during red blood cell storage: results of recall phase of the REDS-III RBC-Omics study. Transfusion 2019;59(1):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone M, Keating SM, Kanias T, et al. Piloting and implementation of quality assessment and quality control procedures in RBC-Omics: a large multi-center study of red blood cell hemolysis during storage. Transfusion 2019;59:57–66. [DOI] [PubMed] [Google Scholar]
- 30.D’Alessandro A, Culp-Hill R, Reisz JA, et al. Heterogeneity of blood processing and storage additives in different centers impacts stored Red Blood Cell metabolism as much as storage time: lessons from REDS III – Omics. Transfusion 2019;59(1):89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endres-Dighe SM, Guo Y, Kanias T, et al. Blood, sweat, and tears: Red Blood Cell-Omics study objectives, design, and recruitment activities. Transfusion 2019;59:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howie HL, Hay AM, de Wolski K, et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv 2019;3:2272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waterman HR, Kapp LM, Howie HL, et al. Analysis of 24-h recovery of transfused stored RBCs in recipient mice of distinct genetic backgrounds. Vox Sang 2015;109:148–54. [DOI] [PubMed] [Google Scholar]
- 34.de Wolski K, Fu X, Dumont LJ, et al. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica 2016;101:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reisz JA, Nemkov T, Dzieciatkowska M, et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion 2018;58:2978–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemkov T, Hansen KC, Dumont LJ, et al. Metabolomics in transfusion medicine. Transfusion 2016;56:980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 2017;57:325–36. [DOI] [PubMed] [Google Scholar]
- 38.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom 2017;31:663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu X, Felcyn JR, Odem-Davis K, et al. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion 2016;56:2560–70. [DOI] [PubMed] [Google Scholar]
- 40.Reisz JA, Zheng C, D’Alessandro A, et al. Untargeted and semi-targeted lipid analysis of biological samples using mass spectrometry-based metabolomics. Methods Mol Biol 1978;2019:121–35. [DOI] [PubMed] [Google Scholar]
- 41.Nemkov T, Reisz JA, Gehrke S, et al. High-throughput metabolomics: isocratic and gradient mass spectrometry-based methods. Methods Mol Biol 1978;2019:13–26. [DOI] [PubMed] [Google Scholar]
- 42.Chong J, Soufan O, Li C, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 2018;46(W1):W486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou G, Xia J. OmicsNet: a web-based tool for creation and visual analysis of biological networks in 3D space. Nucleic Acids Res 2018;46(W1):W514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimring JC, Smith N, Stowell SR, et al. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion 2014;54:137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nemkov T, Sun K, Reisz JA, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica 2018;103:361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Alessandro A, Nemkov T, Kelher M, et al. Routine Storage of Red Blood Cell Units in Additive Solution-3: a comprehensive investigation of the RBC metabolome. Transfusion 2015;55:1155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Alessandro A, Reisz JA, Culp-Hill R, et al. Metabolic effect of alkaline additives and guanosine/gluconate in storage solutions for red blood cells. Transfusion 2018;58:1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dumont LJ, D’Alessandro A, Szczepiorkowski ZM, et al. CO2-dependent metabolic modulation in red blood cells stored under anaerobic conditions. Transfusion 2016;56:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hess JR, Hill HR, Oliver CK, et al. Alkaline CPD and the preservation of RBC 2,3-DPG. Transfusion 2002;42:747–52. [DOI] [PubMed] [Google Scholar]
- 50.D’Alessandro A, D’Amici GM, Vaglio S, et al. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica 2012;97:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. J Cell Mol Med 2007;11:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters AL, Van Noorden CJF. Glucose-6-phosphate dehydrogenase deficiency and malaria: cytochemical detection of heterozygous G6PD deficiency in women. J Histochem Cytochem 2009;57:1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tzounakas VL, Kriebardis AG, Georgatzakou HT, et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic Biol Med 2016;96:152–65. [DOI] [PubMed] [Google Scholar]
- 54.Francis RO, Jhang JS, Pham HP, et al. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sang 2013;105:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood 2016;128:e32–42. [DOI] [PubMed] [Google Scholar]
- 56.D’Alessandro A, Zimring JC, Busch M. Chronological storage age and metabolic age of stored red blood cells: are they the same? Transfusion 2019;59:1620–3. [DOI] [PubMed] [Google Scholar]
- 57.Paglia G, D’Alessandro A, Rolfsson Ó, et al. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood 2016;128:e43–50. [DOI] [PubMed] [Google Scholar]
- 58.Bordbar A, Johansson PI, Paglia G, et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion 2016;56:852–62. [DOI] [PubMed] [Google Scholar]
- 59.Hay A, Howie HL, Waterman HR, et al. Murine red blood cells from genetically distinct donors cross-regulate when stored together. Transfusion 2017;57:2657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.You JS, Chang KJ. Effects of taurine supplementation on lipid peroxidation, blood glucose and blood lipid metabolism in streptozotocin-induced diabetic rats. Adv Exp Med Biol 1998;442:163–8. [DOI] [PubMed] [Google Scholar]
- 61.Roussel C, Buffet PA, Amireault P. Measuring post-transfusion recovery and survival of red blood cells: strengths and weaknesses of chromium-51 labeling and alternative methods. Front Med 2018;5:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Francis RO, Mahajan S, Rapido F, et al. Reexamination of the chromium-51-labeled posttransfusion red blood cell recovery method. Transfusion 2019;59:2264–75. [DOI] [PubMed] [Google Scholar]
- 63.Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J Clin Invest 2017;127:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Impact of Taurine Drink on Plasma Metabolism.
Figure S2. Impact of Taurine Drink on RBC Metabolism.
Figure S3. Taurine levels and stored RBC metabolism in REDS.
Figure S4. Impact of Taurine on human RBC storage d42.
Table S1. Taurine dose response and time course.
Table S2. Labeled Taurine and H2O2 Time series – balanced.
Table S3. Taurine in REDS.