Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), causing the 2019 novel coronavirus disease (COVID-19), was introduced by WHO (World Health Organization) as “pandemic” in March 2020. According to WHO, thus far (23 November 2020) 58,425,681 infected cases including 1,385,218 deaths have been reported worldwide. In order to reduce transmission and spread of this lethal virus, attempts are globally being made to develop an appropriate vaccine. Intending to neutralize pathogens at their initial entrance site, protective mucosal immunity is inevitably required. In SARS-CoV2 infection and transmission, respiratory mucosa plays a key role; hence, apparently mucosal vaccination could be a superior approach to elicit mucosal and systemic immune responses simultaneously. In this review, the advantages of mucosal vaccination to control COVID-19 infection, limitations, and outcomes of mucosal vaccines have been highlighted. Considering the gut microbiota dysregulation in COVID-19, we further provide evidences on utilization of recombinant probiotics, particularly lactic acid bacteria (LAB) as vaccine carrier. Their intrinsic immunomodulatory features, natural adjuvanticity, and feasible expression of relevant antigen in the mucosal surface make them more appealing as live cell factory. Among all available platforms, bioengineered probiotics are considered as the most affordable, most practical, and safest vaccination approach to halt this emerging virus.
Keywords: SARS-CoV2, COVID-19, Probiotics, Lactic acid bacteria (LAB), Live mucosal vaccine, Bioengineering
Introduction
Live microorganisms which provide health advantages particularly on the gastrointestinal tract are called probiotics [1]. WHO has described probiotics as a group of bacteria which provides health advantages on the host following sufficient administration [2]. They are potent to stimulate and modulate systemic and mucosal immune functions [3, 4]. Recent advances in synthetic biology enabled us to engineer probiotics with particular therapeutic functions for several disorders that conventional medicines have failed to cure, including cancer, infections, or other metabolic diseases [5, 6]. Following the administration of engineered probiotics as vaccines, protective immune response at various mucosal membranes such as urogenital, intestinal, and aero digestive has been demonstrated [7]. There are some evidences indicating the potency of probiotic supplements on alleviation of the severity and prolongation of viral respiratory tract infection [8–10]. Moreover, proper preventive antiviral responses following probiotics application as prophylactic agents have been reported elsewhere [11]. Among probiotic bacteria, E. coli, Bacteroides, and lactic acid bacteria (LAB) are the most desirable and preferred chassis for engineered live therapeutics [12].
As the emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is spreading too fast worldwide, developing an effective vaccine is urgently required. Recently, among all available platforms, bioengineered probiotics expressing desired recombinant proteins received more attention due to their feasibility, cost effectiveness, and numerous inherent characteristics. Furthermore, the immunological coordination between the gut and lungs as two vital organs in COVID-19 infection has been demonstrated [13]. Due to the vital role of the gut microbiome in modulation of immune responses of COVID-19-infected individuals reported by Ahlawat and Sharma [13] and Tiwari et al. [11], we speculated that probiotics as vaccine carrier might be a promising approach. All abovementioned findings together prompt us in this review to provide existing advances and potential approaches for vaccine development using probiotics as vaccine carrier to elicit mucosal and systemic immunity while highlighting the superiority of their potential and inherent characteristics which could be utilized in the control and prevention of SARS-CoV2.
A Glance at Coronavirus Disease 2019 (COVID-19)
Most prevalent coronaviruses cause mild upper respiratory infections and common colds, but some pandemic outbreaks have emerged in the recent century. They include (1) the severe acute respiratory syndrome (SARS) coronavirus in 2002–2003, (2) after a decade the Middle East respiratory syndrome (MERS) coronavirus, (3) and currently the COVID-19 SARS-CoV-2 [14, 15]. According to SARS-CoV-2 genome studies, there is more correlation to SARS (80%) than MERS CoV (54%) [14]. Briefly, SARS-CoV-2 from the beta-CoV genera in the family Coronaviridae is an enveloped single-stranded positive RNA genome (29.88 kb) encoding four main structural proteins such as nucleocapsid (N) associated with the RNA, the spike (S) glycoprotein, the membrane (M) glycoprotein, the envelope (E) protein, nonstructural proteins (nsp1–16), and 5–8 accessory proteins [16]. Among all, S protein attracted the most attention in vaccine development since its receptor-binding domain (RBD) plays the main role in attachment, membrane fusion (via S1 and S2, respectively), and entrance of the virus to angiotensin-converting enzyme 2 (ACE2) receptor+ host cells [14, 16, 17]. Furthermore, S protein is capable of inducing neutralizing antibodies in patients. As blocking SARS-CoV2 RBD can prevent SARS-CoV-2 and SARS-CoV infections [18], S protein is considered as a promising candidate not only for prophylactic but also for therapeutic purposes [16]. SARS-CoV-2 could transmit via respiratory droplet, contact, and possibly through fecal-oral routes. It appears that viral replication initiates in the mucosal surface of the nasopharynx and upper respiratory tract and continues to proliferate in the lower respiratory tract and gastrointestinal mucosa, thereby causing mild viremia [19]. Infections could be controlled at this stage; some infected people might remain asymptomatic, and some may suffer from non-respiratory symptoms such as acute liver and heart injury, kidney failure, and diarrhea [20–23]. Data provided by Zou et al. [24] demonstrated the susceptibility of numerous organs such as nasal mucosa, bronchus, lung, heart, esophagus, kidney, stomach, bladder, and ileum to SARS-CoV-2 due to the prevalent expression of ACE2 [24]. Incidence of acute respiratory distress syndrome (ARDS) is associated with cytokines [25]. In this regard, an increasing body of research has shown the role of numerous genes involved in the outcome of ARDS such as ACE2, interleukin 10 (IL-10), tumor necrosis factor (TNF), and vascular endothelial growth factor (VEGF) [25]. In addition, elevated expression levels of IL-6 and IL-8 play a crucial role in adverse outcomes of ARDS [26]. Antibody-dependent enhancement (ADE) has been widely reported in viral infections. Briefly, it results in increased infection, following the interaction of antibody-bound virions to fragment the crystallizable region (Fc receptors) or other receptors [27]. Acquired knowledge from SARS demonstrated that antibodies against non-RBD regions of S protein can trigger the ADE effect, leading to further virally infected cells along with destructive immune responses [28], which has been recently proposed in COVID-19 as well [29]. Considering the findings from previous coronavirus infection, immune response can be a double-edged sword for the host to induce whether the favorable or adverse response determines disease outcome [30]. Accordingly, anti-inflammatory approaches such as various medicines, intravenous transplantation of ACE2-mesenchymal stem cells (MSCs), and intravenous immunoglobulin (IVIG) to block FcR are being applied as therapeutic strategies for severe COVID-19 [29, 31]. In COVID-19, we have basically faced two immune phases; during the first protective phase, immune responses should be boosted, while under the second inflammatory phase immune responses should be suppressed [32]. Innate immune response can induce whether the favorable or adverse response determines disease outcome [30]. Mainly, interferon (IFN) type I response at the initiation site of viral infections is the core player in proper innate immune response. Following the recognition of viral genomic RNA by pathogen-associated molecular patterns (PAMPs) such as Toll-like receptors (TLRs) 3 and 7 or RIG-I/MDA5, downstream signaling pathways such as NF-κB and IRF3 were activated. Subsequently, the expressions of pro-inflammatory cytokines and type I IFN are induced. If adequate type I IFN response was induced, replication and distribution at very early stages were inhibited, but taking into account that viruses are also able to suppress anti-viral IFN responses and replicate unlimitedly [30]. Meanwhile in anti-viral adaptive immunity, Th1 response is of importance [30]. This is the same for SARS-CoV-2, and severe outcome is the consequence of poor antibody response [33]. Generally, adaptive response including cytotoxic T cells to eliminate viral infected cells and humoral immune response to restrict infection at the later phase and prohibit reinfection is needed [30]. Although both innate and adaptive immune responses are activated in SARS-CoV-2 infection, it is worth noting that adverse local and systemic tissue damage might occur due to severe inflammatory innate or impaired adaptive immune responses. Lymphopenia as reduced numbers of CD4+, CD8+ T cells, B cells, and natural killer (NK) cells and simultaneously increased neutrophil has been reported in severe COVID-19 [18, 20]. The enhanced neutrophil-to-lymphocyte ratio and elevated levels of IL-6 usually are indicators of poor prognosis, severity of disease with pneumonia, and ARDS [14, 18]. Moreover, the so-called cytokine storm as increased serum levels of pro-inflammatory cytokines such as IL-6, IL-7, IL-1β, IL-2, IL-8, IL-17, IL-10, G-CSF, GM-CSF, IP10, MCP1, MIP1α (CCL3), and TNFα is reported in severe cases [20, 30, 34]. Shock and tissue injury in the heart, liver, and kidney and respiratory failure might occur as a consequence of these elevated levels of cytokines [14]. Worth noting is that the cytokine storm may cause more destruction than the coronavirus itself [30]. Following the cytokine storm, viral sepsis and inflammation, destruction of lung function, pneumonitis, acute respiratory distress syndrome (ARDS), respiratory failure, shock, organ failure, and potentially death might occur [30]. Considering the aspects mentioned above, SARS-CoV2 pathogenesis might result in both lymphopenia and cytokine storm. Because of repetition of the epidemic and currently pandemic emergence of coronavirus every decade in the twenty-first century, vaccine development seems to be a better strategy to control coronavirus outbreaks than therapeutics [35].
COVID-19 Vaccine Requirements
Currently, the most vital challenge is to control and prevent the spread of SARS-CoV2 infection. In general, safety, efficacy, and durable immunity should be considered in vaccine development, but in pandemics such as the current situation, speed, feasibility, and time are of the essence [14]. It is worth noting that routes of entry and the target organs of virus should be considered in vaccine development. Infectious SARS-CoV-2 particles have been isolated from respiratory, fecal, and urine samples [14]. Whether SARS-CoV-2 reaches the lungs via viremia or through the upper respiratory tract, different approaches should be preferred. For instance, parenteral (IM) vaccines inducing neutralizing antibodies in serum to block viremia or intranasal vaccines, inducing mucosal immunity, and reducing nasal shedding are of interest for viremia or upper respiratory tract infection, respectively [14]. In this regard, wide-range platforms of COVID-19 candidate vaccines such as nucleic acid (DNA/RNA plasmids), virus-like particle, peptide, viral vector (replicating and non-replicating), recombinant subunit protein, live attenuated virus, and inactivated virus are under various stages of clinical trials (Table 1) [14, 36]. Among all candidate vaccines, balancing the humoral (neutralizing antibody) and T cell responses should be taken into account [37]. Mostly, their main goal is induction of anti-spike (S) protein–neutralizing antibodies to inhibit virus attachment, cell entry, and subsequent infectivity [36]. Although some approaches have not been approved for any other clinical applications, they are being evaluated in the COVID-19 pandemic for acceleration of appropriate vaccine development [36]. Among them, mRNA vaccine was the first to be evaluated in human clinical trial before others [14]. The detailed mechanism actions of immunity to COVID-19 in humans are unknown, but the importance of mucosal immunity at least to reduce nasal shedding is obvious. It should be noted that conventional vaccines are relevantly capable of prohibiting COVID-19 disease, but inadequate to prevent its transmission due to the lack of nasal shedding blockage. Reduction of disease severity and blocking viral shedding and transmission are required to control the outbreak [38]. In the current COVID-19 pandemic, a major challenge needed to be overcome is elimination of virus shedding which could be achieved through mucosal immunity. Due to both respiratory and intestinal infection and simultaneously fecal and nasal shedding, an optimal prophylactic SARS-CoV2 vaccine should be capable of preventing both enteric and respiratory infections. Also fecal and nasal shedding should be inhibited. Apparently, in the prevention of viral respiratory infection, mucosal immunity is superior [29]. The intestinal immune system is more powerful than respiratory immune systems [29]. Hence, it seems that mucosal immunity induced through the intestinal immune system might be a promising approach in current SARS-CoV2 vaccination strategies.
Table 1.
SARS-CoV-2 vaccine candidates from various platforms in clinical trials
| Type | Name | Target | Company/research group/partners | Clinical trial |
|---|---|---|---|---|
| DNA | INO-4800 | Spike | Inovio | NCT04336410 |
| bacTRLSpike | Spike | Symvivo Corporation | NCT04334980 | |
| RNA | mRNA- | Spike | Moderna | NCT04283461 |
| 1273 | ||||
| BNT162 | 3CLpro, NSP5, | BioNTech/Pfizer | NCT04380701 | |
| Mpro, | ||||
| Protein | NVXCoV2373 | Spike | Novavax; | NCT043 988 |
| SCB-2019 | Spike | Clover Biopharmaceuticals | NCT04405908 | |
| COVAX-19 | Spike | Vaxine Pty Ltd | NCT04453852 | |
| Viral vector | AZD1222 | Spike | University of Oxford (Jenner Institute)/Astra Zeneca | NCT04444674 |
| (ChAdOx1 | ||||
| nCoV-19 | ||||
| Ad5-nCoV | Spike | CanSino Biologics | NCT04313127 | |
| Ad26 | Spike | Johnson & Johnson–Janssen | NCT04436276 | |
| SARSCoV- | ||||
| 2 | ||||
| V591 | Spike | Institut Pasteur/Merck | NCT04498247 | |
| Inactivated Virus | CoronaVac(PiCoVacc) | Spike | Sinovac Biotech/ | NCT04352608 |
| COVID-19 vaccine | Spike | Beijing Institute of Biological Products/Wuhan Institute of Biological Products | ChiCTR2000031809 |
Mucosal Immunization vs Conventional Parenteral Immunization
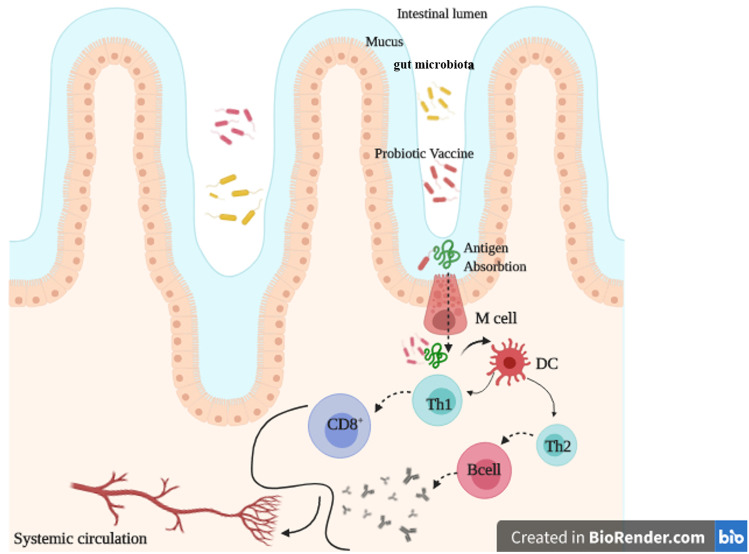
In vaccine development, induction of local immune response which neutralizes pathogens at the first entry site of infection is worthwhile [39]. For those infectious agents acquiring via mucosal routes, mucosal immunity is required [40]. Thus, mucosal vaccines eliciting sIgA antibodies at mucosal surfaces and simultaneously systemic immune responses are highly desirable [41, 42]. Since the main portal for pathogen entry is mucosal sites [43], mucosal immunization is superior to conventional parenteral immunization. Mucosal immunization is capable of eliciting not only both local and distant mucosal sites [43] but also systemic immunity [44, 45], inhibiting further entry of former pathogens [46, 47]. Mucus, peristalsis, gastric acid, bile, and antimicrobial peptides are all components of innate mucosal immune strategies whereas adaptive mucosal immune responses include antigen-specific antibodies and cell-mediated responses [43]. Both of them could induce more robustness by direct immunization at the mucosal surfaces rather than through systemic routes [43]. Current licensed vaccines are mostly administered through conventional routes such as subcutaneous or intramuscular. Despite their efficiency in providing systemic immune responses, mucosal immune responses are not simply acquirable [48]. The main shortcoming of the most commercially available vaccines is lack of suitable levels of mucosal IgA response and mucosal immunity [40, 49]. Hence, mucosal vaccines are superior to other vaccination strategies [40], which provides the active combat zone of defense for pathogens with mucosal initial site of entry (oral and nasal routes) [45, 50]. Since the composition of innate cells with particular pattern recognition receptors (PRRs) is diverse in mucosal and systemic tissues, administration of the same antigen/adjuvant or the same vaccine in various routes would lead to utterly different efficiency [51, 52]. In this regard, Pederson et al. compared the intranasal and intramuscular inoculation of a candidate influenza vaccine. Their results indicated the superiority protection of nasal approach at the virus’ entry site with robust mucosal antibodies, neutralizing serum IgG and memory T cells [53]. Mostly, oral immunization/vaccination is considered as a wise approach to elicit desired immune responses such as mucosal secretory IgA (sIgA) antibody response, neutralizing serum IgG and memory T cells [54]. Furthermore, compared with systemic vaccines, mucosal vaccines are more convenient [45]. Their advantages are simplicity of needle-free administration, feasibility of mass vaccination without demanding expert medical staff, cost effectiveness, and lower side effects [55, 56]. Orally available mucosal vaccine approaches are quite various and include micro or nanoparticles, immune-stimulating complexes, liposomes, or live viral or bacterial vectors producing antigens in vivo. In this regard, the great example of potent oral vaccine is against poliomyelitis [57, 58]. Other commercially available oral vaccines including Rotarix®, GlaxoSmithKline, RotaTeq®, and Merck against RV infection stimulate a similar immune response to natural immunity induced by typical virus [59]. Briefly, in optimal oral mucosal vaccine, activation of B cells in lymphoid tissues should occur following the attachment of antigens to intestinal mucosa, microfold (M) cells, and transmission the mucosa [60] (Fig. 1).
Fig. 1.
Probiotic-based vaccine. Following the oral administration and transmission through Mcells, desired antigens are presented by dendritic cells (DC). Subsequently, specific adaptive responses are activated. This figure has been created in BioRender.com
Mucosal Immune System
The mucosal immune system as a first barrier against pathogens consists of initiation and effector sites such as secondary lymphoid tissue known as the mucosal-associated lymphoid tissues (MALT) [61] and specific T and B cells which spread from preliminary sites via bloodstream to other mucosal effector sites [45]. Immune responses have been induced locally not only at the delivery site of antigens but also at the further distant mucosal surface [40] via lymph circulations [45, 62]. MALT consisted of epithelial cells within some specialized cells (M cells) to uptake antigens from the lumen/environment and deliver to the second layer including macrophages and dendritic cells (DCs) to process the antigen and subsequently a region full of follicular dendritic cells (FoDCs), B and T cells [50]. The hallmark of M cells such as no secretion of mucus and glycocalyx, while enabling to perform endocytosis activity, makes them suitable vehicle transferring antigens from luminal to inductive MALT sites [63]. Thus, the main entrance site of infectious agents at mucosal sites is their transportation through M cells [40, 43] (Fig. 1). At the same time, epithelial cells secrete pro-inflammatory cytokines to further provoke the mentioned immune cells [64]. Thus, delivering the antigen and simultaneously stimulating them at this region result in boosting the immune responses [50]. This considerably advanced mucosal immune system makes mucosal sites as an optimal vaccination route [65]. Briefly, following the expression of mucosal IgA from plasma cells at the mucosal surfaces, IgA binds to the immunoglobulin receptor (pIgR) on mucosal epithelial cells and subsequently transcytoses to the surface and secretes into the mucus [66]. Furthermore, mucosal T cells and epithelial cells are also involved in IgA producing B cell maturation through cytokines [67]. On the other hand, systemic IgG response is also induced via migration of IgG-producing B cells and activates DCs from mucosa to the bone marrow, lymph nodes, and spleen [68]. Cytotoxic T lymphocyte (CTL) response is also induced at mucosal sites for mucosal clearance of microbes [50]. In the current COVID-19 outbreak, adaptive responses include CTLs to eliminate viral infected cells and humoral immune response to restrict infection at the later phase and prohibit reinfection is needed [30]. All of these immune responses lead to reduction of both enteric and respiratory infections as well as viral fecal and nasal shedding of COVID-19 patients [38]. These requirements seem to be achieved through mucosal immunity. Briefly, M cells initially detect antigens, delivered through transcytosis [69] to antigen-presenting cells (APCs), then naïve T and B cells in mucosal inductive sites are activated and subsequently distributed into peripheral blood [45]. This is shown in Fig. 1.
Critical Dilemma of Mucosal Vaccines
Despite all these advantages and the superiority of mucosal immunization, there are some critical dilemmas for oral vaccination. Mucosal/epithelial layers of the intestinal, as barriers, absorb and reduce the amounts of antigen. This reduction inhibits the effective delivery and disrupts antigen presentation leading to immune tolerance. Therefore, compared with parenteral immunizations, here more elevated antigens are required [70–73]. Not only does elicitation of mucosal immune response require the transmission of antigens through the mucosal layer [74], but also their long-lasting release to APCs is highly needed [70]. Another obstacle in mucosal vaccination is the reverse correlation between immunogenicity and solubility of antigens [45]. Moreover, presence of proteases and low pH in gastrointestinal (GI) tract [57, 75], as well as immune tolerance, are obstacles for protein vaccines that need to be conquered in oral vaccination [57].
Overcoming Obstacles of Oral Vaccination
Attachments of the desired antigen to intestinal mucosa and M cells and transmission of the mucosa ending up to B cells in lymphoid tissues should be taken place by optimal oral mucosal vaccine [60]. As mentioned above, immune tolerance is regarded as an obstacle that needed to be overcome in oral vaccine development [57]. Regulatory T cells (Tregs) play the main role in immune tolerance [76] and are considered as crucial players in both lungs and GI tract. They prevent irrelevant immune responses to environmental or self-antigens and play a role as main tools to provide tolerance to harmless antigens. Thus, there is a great possibility of T and B cell tolerance induction via mucosal vaccination [77]. Pathogenic features of attenuated bacterial and viral pathogens could assist their entry into the body and overcome to tolerance [78]. In addition, improvement of serum and mucosal antibody responses subsequent to the involvement of PRRs agonists, particularly TLRs as vaccine adjuvants, has been reported in clinic [79, 80]. Innate immune responses such as secretion of inflammatory cytokines and chemokines are results from PRR activation. Among all, the role of TLR2 in improvement of antigen uptake and DC migration has been demonstrated by Chabot et al. [81]. Mucosal vaccine efficacy could be improved through TLR-based adjuvants [50]. Thus, it is highly recommended to use the capability of PRRs to induce further immune responses and cytokine production [82]. Despite the potential of attenuated bacterial and viral pathogens to provide PAMPs which is recognized by PRRs, drawbacks of live attenuated bacterial vectors are pathogenic reversal and pre-existing immunity [83]. Hopefully, synthetic biology provided us the opportunity to genetically engineered microorganisms with any genes of interest acting as living therapeutics [84]. Furthermore, this could be extended to commensal microbes and probiotics to enhance their therapeutic effects [5, 85]. Therefore, engineered probiotics are considered as the best alternative for mucosal vaccines carrier [78] and LAB have been long investigated as live mucosal vaccine vectors [86, 87].
Probiotics, the Best Alternative as Mucosal Vaccine Vectors
It should be noted that in coronavirus studies, enterocytes are considered as a conserved cell reservoir [88]. Considering altered and dysregulated gut microbiota following the SARS-CoV-2 infection [89–92] and their gastrointestinal tendency via ACE2 receptors, the gastrointestinal tract has been proposed as a potential target in COVID-19 control and transmission [93]. Subsequent to the SARS-CoV-2 invasion into the intestinal tract, gastrointestinal disruption, inflammatory responses, and altered microbiota have occurred [94]. Inflammation leads to disruption of intestinal barrier and enhanced permeability; therefore, secondary systemic infection could occur [95]. Meanwhile, elevated concentrations of TNFα, IL-1b, and IL-6 in blood stream make systemic inflammation worse [96]. It has been shown that respiratory tract infection and intestinal interruption are frequently simultaneous. Moreover, dysregulation of gut microbiota worsens the lung injury [94]. Recently, the microbiota-lung axis as a mutual cross talk between gut microbiota and lungs has been reported by Zhang et al. [97]. Microbiota dysregulation influences the pulmonary immunity while lung inflammation disrupts the intestinal microbiota [97]. Considering the gut-lung axis (GLA) as a communicational pathway between gut and lung [98], intestinal bacteria or their metabolites could get into the lung and affect the pulmonary immune response via mesenteric lymphatic system and systemic circulation [99, 100]. Bradley et al. reported that enteritis and ventilator-associated pneumonia could be declined by modulating the gut microbiota [101]. Therefore, in order to provide the equilibrium of the gut microflora while alleviating gastrointestinal symptoms to prevent secondary bacterial infection, probiotics administration could be a beneficial approach to patients with severe COVID-19 [102]. Furthermore, the mucosal immune system and microbiome affect each other [103–106]. The detailed molecular events behind this association remained uninvestigated [45]. Nevertheless, it led to keep tolerance against harmless antigens, while providing appropriate immune response against infectious agents [107] and making homeostasis in mucosal tissue [45, 108]. Dysbiosis in content and diversity of microbiome may result in disease development [45]. In this regard, probiotics are valuable microorganisms regulating local and systemic immune responses to infections and vaccines [108]. Additionally the health and nutrition of the GI system and intestinal microbiome directly affect the immunogenicity and efficacy of vaccines [109–111].
The capability of probiotics in modification and reconstitution of microbiome has been made as appropriate therapeutic and prophylactic tools in gastrointestinal, inflammatory, and respiratory disorders [45]. They are known as generally recognized as safe (GRAS) and are not only harmless but also quite favorable. LAB, the most commonly used probiotics, have been largely applied in food industries due to their capability of increasing the acidity and antibacterial bacteriocin for food preservation and bioprocessing [112]. Their acid, bile, and salt tolerance simply enabled them to remain in an unfavorable environment of the gastrointestinal tract [113]. Gut-associated lymphoid tissues (GALT) and other cells present in the intestinal mucosa could communicate and cooperate with probiotics via PRRs and microbe-associated molecular patterns (MAMPs) expressed on microorganisms and host cells, respectively [114–118]. Moreover, gut mucous membrane health condition and distribution of lymphoid cells in GALT are maintained by LAB. The mechanism underlying these phenomena is unclear, but possibly they or their own secondary products are captured by M-cells and presented to the immune system [119]. Innate immune response triggered via the LAB cell wall peptidoglycan and lipoteichoic acid, through TLR2, nucleotide-binding oligomerization domain (NOD)–like receptor (NLR) family, and C-type lectin receptors [120, 121], and interferon responses are activated through TLR3, TLR6, and TLR9 [122, 123]. Moreover, cytokines might be elicited by LAB via presentation of antigen to T cells or subsequent interaction with immune cells directly through the receptors of LAB peptidoglycan existing on lymphocytes and macrophages [124, 125]. Their peptidoglycan induced monocytes to express IL-1, IL-6, and TNF-α leading to stimulation of T or B-cell proliferation and B-cell differentiation, respectively [125–127]. Further than their immunomodulatory features, cytokine induction, or the progression of regulatory T-cell development or their beneficial natural adjuvanticity [73, 128–130], they are internalized in the gut leading to various immune responses [73]. In addition, they could be engineered to simultaneously express and deliver the desired protein to mucosal tissues for prophylactic or therapeutic purposes (Fig. 1). These are called live cell factories which, further than their expected effect through the expression of certain gene, influence the regular health as probiotics [131]. Hence, their intrinsic characteristics for general health such as modulation of intestinal microbiome composition, host immune system, improvement of intestinal barrier function [132–134], immune stimulation via PRRs, binding to DCs, tolerance to acid and bile, and association with the mucosal epithelium via their mucus-binding protein [135], together with their experience in the food industry, facilitate their application for vaccine development [73, 136]. Iwak et al. for the first time used LAB as a vaccine vector in 1990 by eliciting specific IgG and IgA antibodies and introduced them as alternative to conventional bacterial vaccine vectors [137]. All advantages of LAB have been made as promising candidates for mucosal vaccine delivery vectors. These advantages are related to their safety, simplicity, noninvasiveness of oral or intranasal administration, feasibility of genetic modifications, cost-effectiveness, ability to elicit high levels of mucosal and systemic antibodies [87] and circumvent the cold chain, low probability of gene transfer due to their particular genetic replication system, and narrow host-range plasmids [112, 138, 139]. In some vaccine studies, the enhancement of B cell and antibody responses has been reported prior to or simultaneous with administration of probiotic with vaccination [135]. In these approaches, probiotic bacteria are appropriate candidates as adjuvants because of their surface structures [140]. Indeed, immune cells recognize their surface lipoteichoic acid (LTA), peptidoglycan (PG), and muramyl dipeptide via TLR 2/6 leading to the production of mucosal antibodies particularly IgA [141]. Thus, it would be highly desirable to use this intrinsic feature of their immunomodulatory in combination with adjuvanticity for the development of engineered probiotics as live oral/mucosal vaccine vectors [78, 87, 135, 142, 143]. Based on these properties of probiotics together with results from recent studies on the advantages of probiotics administration in patients with severe COVID-19 [102], we believe probiotics could be considered as a promising oral vaccine vector.
Choosing the Relevant Strains
According to phylogenetic classification, LAB include six families: Aerococcaceae, Carnobacteriaceae, Enterococcaceae, Lactobacillaceae, Leuconostocaceae, and Streptococcaceae (phylum Firmicutes) with an exception of Bifidobacterium (phylum Actinobacteria). Although Bifidobacterium are phylogenetically irrelevant, they are considered as LAB [112]. Some microorganisms from various genera such as Leuconostoc, Lactococcus, Lactobacillus, Pediococcus, and Streptococcus are more widespread [11]. The challenge needed to be overcome is selecting the proper vector among all LAB [144]. Choosing the relevant strains as vaccine carrier is a critical step. For instance, Lactobacillus casei, Lactobacillus delbrueckii ssp. bulgaricus, and Lactobacillus acidophilus influence the systemic humoral response [73], whereas elevated expression levels of IL-10 and IL-4 leading to type 2 T helper cell (Th2) activation following the administration of Lact. delbrueckii ssp. Bulgaricus or Lact. Casei have been reported. IL-2 and IL-12 directing Th1 subsequent to the Lact. Acidophilus administration have been shown [73, 145]. De Moreno de LeBlanc et al. reported that not only mucosal intestinal but also systemic humoral/cellular immune responses were elicited following the oral vaccination of Lactobacillus casei [146]. Moreover, attachment and colonization of some LAB to the mucosal intestinal epithelium and M cells lead to enhanced expression and transportation of the desired antigen into Peyer’s patches where the response initiates [144]. The efficacy of mucosal immunity could be improved by their colonization [147, 148]. Colonizing strains considerably stimulate the immune system through continuous expression of antigen and durable existence in host leading to prolonged presentation of antigens to the immune system. However, non-colonizing strains are similar to particles carrying and releasing the antigen [73]. Although colonizing or non-colonizing strains have their own advantages, it is unlikely to precisely state which is preferable for vaccine. Immunogenicity is under the influence of optimal antigen presentation and is the most pivotal parameter of vaccine [73]. Thus, attachment to the mucosal membrane seems to be superior to induce potent immune response [149] and those with colonizing capability are preferable for recombinant LAB-based vaccines [73]. Furthermore, depending on the LAB strain, the interaction between LAB, APCs, and subsequent immune responses are thoroughly varied and strain-specific [150–152]. Furthermore, their survival rate in the ileum (location of Peyer’s patches) is different from each other. According to the results from the first European project, Lactobacillus plantarum NCIMB 8826 and Lactobacillus salivarius UCC 433118 are more durable than L. lactis MG1363 and Lactobacillus fermentum KLD [153]. Thus, the success of vaccine developed from one member of LAB as antigen carrier does not ensure the equivalent potency for the others [2]. Some strains play a vital role in homeostasis maintenance, reduction risk of allergic reactions, protection against pathogens, and stimulation of the mucosal epithelial cells to secrete mucus or other antimicrobial peptides (AMP) [154]. Among all LAB, the most frequently used bacteria for expression of the desired protein due to the wide range of genetic toolbox [155] and commercially available cloning/expression systems [131] is Lactococcus lactis [11], and its superiority over Lactobacilli has been reported [156]. In this regard, L. lactis, i.e., MG1363, which is a plasmid-free strain without any extracellular protease with the compatible expression system, NICE, is considered as the most commonly used strain in research and development [156]. Additionally, Bifidobacterium are anaerobic nonpathogenic bacteria existing in the human intestine. Their health-improving features such as ability to enhance the immune response, protection against viral infection [157], cost-effectiveness, non-invasive route of administration, absence of antibiotic resistance, and high safety levels have made it a favorable delivery vaccine vector [158]. Using this platform, vaccines for hepatitis C virus and enterovirus have been developed [159, 160]. A growing number of studies have reported numerous antigens expressed in engineered LAB as candidate vaccines such as urease, LcrV antigen, EP7 antigen, hemagglutinin (HA) from Helicobacter pylori, Yersinia pseudotuberculosis, human type 16 papillomavirus, and avian influenza virus, respectively [161–163], and also against viruses such as HIV [164, 165].
LAB Vaccine Development for Respiratory Pathogens
Considering the fact that most viruses transmit through the mucosal barrier and develop systemic infection, an optimal vaccine should induce both systemic and mucosal immunity [142]. As previously mentioned, it is worth noting that adequate immune responses including antigen-specific IgG, mucosal IgA, and Th1/Th17 responses are induced by proper oral vaccines [44, 166, 167]. Thus, oral vaccines are rather appropriate for respiratory tract and intestinal mucosal infections [168]. Probiotics have antiviral potentials and protective efficiency particularly against respiratory viral infections such as influenza virus, respiratory syncytial virus, dengue virus, rhinovirus, respiratory syncytial virus, adenovirus, and Ebola virus [9, 98, 169–178]. Hence, the severity of COVID-19-related symptoms could be equally reduced [171]. D'Ettorre et al. reported the considerable effect of oral administration of probiotics on outcomes of COVID-19 patients [175]. They further confirmed the previously mentioned relevance of gut-lung axis for the COVID-19 management [179, 180]. These inherent characteristics could be applied in vaccine development. Furthermore, the adjuvanticity of probiotics [109, 135, 181] could be applied in vaccine development of SARS-CoV2 [89]. Above all, the efficiency of nasal immunizations with some recombinant LAB particularly over respiratory pathogens such as tuberculosis, coronavirus, influenza, and respiratory syncytial virus has been reported [182–186]. In a study, subsequent to the oral administration of engineered Lactococcus lactis expressing avian influenza H5N1 hemagglutinin, appropriate immune responses in mice have been reported [187]. Similarly in another study performed by Shi et al., administration of engineered L. plantarum expressing H9N2 hemagglutinin (HA) induced the production of fecal and bronchiolar IgA, serum IgG, elevated B cell levels in the secondary lymphoid organ, CD8 T cell proliferation, and IFN secretion [188]. Ho et al. for the first time developed a recombinant Lactobacillus casei strain Shirota expressing corona viral TGEV S protein as live mucosal vaccines against coronavirus. After the oral immunization in mice, both mucosal and systemic humoral responses were induced and persisted in intestine for a week due to adhesion to the jejunum and ileum [142]. In this regard, COVID-19 is not an exception and similar to other respiratory and intestinal mucosa infections, an oral/mucosal vaccine is an urgent global need [168]. Moreover, according to the role of mucosa in SARS transmission and infection, working on mucosal immunization to induce systemic and mucosal immunity is favorable [183]. Recently, a genetically engineered Lactobacillus acidophilus expressing S protein of SARS-CoV2 is being investigated [168]. Likewise as recombinant probiotic vaccine, Symvivo Corporation (Vancouver-based Biotech Company) lately introduced an oral vaccine candidate, engineered Bifidobacteria longum expressing Spike protein, called bacTRL-Spike. Following its administration, both cellular and humoral immunity against Spike protein has been induced to prevent COVID-19 infection. Currently, it is under investigation in Phase I clinical trials (NCT04334980). The advantages of these two recombinant probiotic vaccines during our current pandemic situation include circumvention of injection by professional administration, cost-effectiveness, convenient storage, and being bio-friendly [168, 189–191].
Conclusion
Currently, during the COVID-19 pandemic crisis, which is threatening the human health and equally global financial and social life, the feasibility and accuracy of diagnostic and therapeutic approaches or vaccine development are pivotal. It has been long demonstrated that providing mucosal immunity for prevention of viral respiratory infection is superior to other approaches. Among all mucosal vaccines, recombinant LAB are considered as one of the most appealing approaches. It is evident that dysbiosis makes people more susceptible to infectious diseases. Moreover, the potential of probiotics for modulation of gut microbiota in respiratory infections suggests their possible applications in treating or preventing COVID-19. Indeed, intrinsic immune regulatory properties of LAB in combination with in vivo production of appropriate antigen in the mucosal surface have been made as a promising vector for live oral vaccines. In the future, their prophylactic and therapeutic effects against SARS-CoV-2 infection need to be clinically examined. Despite all these advantages, there is a limitation that should be noted. Due to the risk of septicemia, application of probiotics as prophylactic agents should be thoroughly considered particularly in immune-compromised individuals. We believe that discussions provided here prompt others for further research to develop unparalleled probiotic-based vaccine approaches to control COVID-19.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jayawardena R, Sooriyaarachchi P, Chourdakis M, Jeewandara C, Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab Syndr. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyszynska A, Kobierecka P, Bardowski J, Jagusztyn-Krynicka EK. Lactic acid bacteria–20 years exploring their potential as live vectors for mucosal vaccination. Appl Microbiol Biotechnol. 2015;99(7):2967–2977. doi: 10.1007/s00253-015-6498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanauchi O, Andoh A, AbuBakar S, Yamamoto N. Probiotics and paraprobiotics in viral infection: clinical application and effects on the innate and acquired immune systems. Curr Pharm Des. 2018;24(6):710–717. doi: 10.2174/1381612824666180116163411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang EJ, Kim SY, Hwang IH, Ji YJ. The effect of probiotics on prevention of common cold: a meta-analysis of randomized controlled trial studies. Korean J Fam Med. 2013;34(1):2–10. doi: 10.4082/kjfm.2013.34.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedrolli DB, Ribeiro NV, Squizato PN, Cozetto DA, de Jesus VN, Freire PJC, Lins MRCR, Correa GG (2020) Chapter 8 - Engineering microbial living therapeutics. In: Faintuch J, Faintuch S (eds) Precision medicine for investigators, practitioners and providers. Academic Press, pp 71–82. 10.1016/B978-0-12-819178-1.00008-3
- 6.Chua KJ, Kwok WC, Aggarwal N, Sun T, Chang MW. Designer probiotics for the prevention and treatment of human diseases. Current Opinion in Chemical Biology. 2017;40:8–16. doi: 10.1016/j.cbpa.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Guardiola FA, Bahi A, Jimenez-Monreal AM, Martinez-Tome M, Murcia MA, Esteban MA. Dietary administration effects of fenugreek seeds on skin mucosal antioxidant and immunity status of gilthead seabream (Sparus aurata L.) Fish Shellfish Immunol. 2018;75:357–364. doi: 10.1016/j.fsi.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Berggren A, Lazou Ahren I, Larsson N, Onning G. Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. Eur J Nutr. 2011;50(3):203–210. doi: 10.1007/s00394-010-0127-6. [DOI] [PubMed] [Google Scholar]
- 9.de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, Ott S, Hampe J, Schreiber S, Heller K, Schrezenmeir J. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine. 2006;24(44–46):6670–6674. doi: 10.1016/j.vaccine.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 10.Boge T, Remigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R, van der Werf S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009;27(41):5677–5684. doi: 10.1016/j.vaccine.2009.06.094. [DOI] [PubMed] [Google Scholar]
- 11.Tiwari SK, Dicks LMT, Popov IV, Karaseva A, Ermakov AM, Suvorov A, Tagg JR, Weeks R, Chikindas ML. Probiotics at war against viruses: what is missingfrom the picture? Front Microbiol. 2020;11(1877):1877. doi: 10.3389/fmicb.2020.01877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claesen J, Fischbach MA. Synthetic microbes as drug delivery systems. ACS Synth Biol. 2015;4(4):358–364. doi: 10.1021/sb500258b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahlawat S, Asha Sharma KK. Immunological co-ordination between gut and lungs in SARS-CoV-2 infection. Virus Res. 2020;286:198103. doi: 10.1016/j.virusres.2020.198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen WH, Strych U, Hotez PJ, Bottazzi ME (2020) The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep:1–4. 10.1007/s40475-020-00201-6 [DOI] [PMC free article] [PubMed]
- 16.Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human Coronaviruses. Trends Immunol. 2020;41(5):355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H (2020) Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 158 (6):1831–1833 e1833. 10.1053/j.gastro.2020.02.055 [DOI] [PMC free article] [PubMed]
- 20.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan GW, Gao L, Wang JW, Wen XJ, Mao TH, Peng SW, Zhang T, Chen XM, Lu FM. Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus-infected pneumonia. Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):100–106. doi: 10.3760/cma.j.issn.1007-3418.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-Infected pneumonia in Wuhan. China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer NJ, Christie JD (2013) Genetic heterogeneity and risk of acute respiratory distress syndrome. In: Semin Respir Crit Care Med, 2013. vol 04. Thieme Medical Publishers, pp 459–474. 10.1055/s-0033-1351121 [DOI] [PubMed]
- 26.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 27.Takada A, Kawaoka Y. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev Med Virol. 2003;13(6):387–398. doi: 10.1002/rmv.405. [DOI] [PubMed] [Google Scholar]
- 28.Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35(3):266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 31.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 Pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/ad.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu WJ, Zhao M, Liu K, Xu K, Wong G, Tan W, Gao GF. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antiviral Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan M, Liu Y, Zhou R, Deng X, Li F, Liang K, Shi Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou. China. Immunology. 2020;160(3):261–268. doi: 10.1111/imm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalita P, Padhi AK, Zhang KYJ, Tripathi T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb Pathog. 2020;145:104236. doi: 10.1016/j.micpath.2020.104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Thanh T, Andreadakis Z, Kumar A, Gomez Roman R, Tollefsen S, Saville M, Mayhew S. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. Vaccine designers take first shots at COVID-19. Science. 2020;368(6486):14–16. doi: 10.1126/science.368.6486.14. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomar J, Patil HP, Bracho G, Tonnis WF, Frijlink HW, Petrovsky N, Vanbever R, Huckriede A, Hinrichs WLJ. Advax augments B and T cell responses upon influenza vaccination via the respiratory tract and enables complete protection of mice against lethal influenza virus challenge. J Control Release. 2018;288:199–211. doi: 10.1016/j.jconrel.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Islam MA, Firdous J, Badruddoza AZM, Reesor E, Azad M, Hasan A, Lim M, Cao W, Guillemette S, Cho CS. M cell targeting engineered biomaterials for effective vaccination. Biomaterials. 2019;192:75–94. doi: 10.1016/j.biomaterials.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 41.Jackson RJ, Fujihashi K, Xu-Amano J, Kiyono H, Elson CO, McGhee JR. Optimizing oral vaccines: induction of systemic and mucosal B-cell and antibody responses to tetanus toxoid by use of cholera toxin as an adjuvant. Infect Immun. 1993;61(10):4272–4279. doi: 10.1128/IAI.61.10.4272-4279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lavelle EC, O'Hagan DT. Delivery systems and adjuvants for oral vaccines. Expert Opin Drug Deliv. 2006;3(6):747–762. doi: 10.1517/17425247.3.6.747. [DOI] [PubMed] [Google Scholar]
- 43.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(4 Suppl):S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Geng N, Zhou D, Qu Y, Shi M, Xu Y, Liu K, Liu Y, Liu J. Oral immunization of chickens with recombinant Lactobacillus plantarum vaccine against early ALV-J infection. Front Immunol. 2019;10:2299. doi: 10.3389/fimmu.2019.02299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Wang Y, Sun Y, Cui H, Zhu SJ, Qiu HJ. Mucosal vaccines: strategies and challenges. Immunol Lett. 2020;217:116–125. doi: 10.1016/j.imlet.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010;33(4):479–491. doi: 10.1016/j.immuni.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HB, Yoon SY, Singh B, Oh SH, Cui L, Yan C, Kang SK, Choi YJ, Cho CS. Oral immunization of FMDV vaccine using pH-sensitive and mucoadhesive thiolated cellulose acetate phthalate microparticles. Tissue Eng Regen Med. 2018;15(1):1–11. doi: 10.1007/s13770-017-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 49.Lee WJ, Cha S, Shin M, Jung M, Islam MA, Cho CS, Yoo HS. Efficacy of thiolated eudragit microspheres as an oral vaccine delivery system to induce mucosal immunity against enterotoxigenic Escherichia coli in mice. Eur J Pharm Biopharm. 2012;81(1):43–48. doi: 10.1016/j.ejpb.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Shakya AK, Chowdhury MYE, Tao W, Gill HS. Mucosal vaccine delivery: current state and a pediatric perspective. J Control Release. 2016;240:394–413. doi: 10.1016/j.jconrel.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozlowski PA, Aldovini A. Mucosal vaccine approaches for prevention of HIV and SIV transmission. Curr Immunol Rev. 2019;15(1):102–122. doi: 10.2174/1573395514666180605092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foged C. Subunit vaccines of the future: the need for safe, customized and optimized particulate delivery systems. Ther Deliv. 2011;2(8):1057–1077. doi: 10.4155/tde.11.68. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen G, Cox R (2012) The mucosal vaccine quandary: intranasal vs. sublingual immunization against influenza. Hum Vaccin Immunother 8 (5):689–693. 10.4161/hv.19568 [DOI] [PubMed]
- 54.Czerkinsky C, Holmgren J (2012) Mucosal delivery routes for optimal immunization: targeting immunity to the right tissues. In: Kozlowski PA (ed) Mucosal vaccines: modern concepts, strategies, and challenges. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 1–18. 10.1007/82_2010 [DOI] [PubMed]
- 55.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 56.Emmer KL, Ertl HCJ (2020) Chapter 24 - Recombinant adenovirus vectors as mucosal vaccines. In: Kiyono H, Pascual DW (eds) Mucosal vaccines (Second Edition). Academic Press, pp 419–444. 10.1016/B978-0-12-811924-2.00024-9
- 57.Vela Ramirez JE, Sharpe LA, Peppas NA. Current state and challenges in developing oral vaccines. Adv Drug Deliv Rev. 2017;114:116–131. doi: 10.1016/j.addr.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Payne AM. Oral immunization against poliomyelitis. Bull World Health Organ. 1960;23(6):695–703. [PMC free article] [PubMed] [Google Scholar]
- 59.Bahamondez-Canas TF, Cui Z. Intranasal immunization with dry powder vaccines. Eur J Pharm Biopharm. 2018;122:167–175. doi: 10.1016/j.ejpb.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim SH, Jang YS. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Exp Mol Med. 2014;46(3):e85. doi: 10.1038/emm.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Czerkinsky C, Holmgren J. Topical immunization strategies. Mucosal Immunol. 2010;3(6):545–555. doi: 10.1038/mi.2010.55. [DOI] [PubMed] [Google Scholar]
- 62.Quiding M, Nordstrom I, Kilander A, Andersson G, Hanson LA, Holmgren J, Czerkinsky C (1991) Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-gamma production and evokes local immunological memory. J Clin Invest 88 (1):143–148. 10.1172/JCI115270 [DOI] [PMC free article] [PubMed]
- 63.Sansonetti PJ, Phalipon A. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin Immunol. 1999;11(3):193–203. doi: 10.1006/smim.1999.0175. [DOI] [PubMed] [Google Scholar]
- 64.Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005;23(15):1804–1813. doi: 10.1016/j.vaccine.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 65.Kiyono H, Azegami T. The mucosal immune system: from dentistry to vaccine development. Proc Jpn Acad Ser B Phys Biol Sci. 2015;91(8):423–439. doi: 10.2183/pjab.91.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8(9):656–667. doi: 10.1038/nrmicro2384. [DOI] [PubMed] [Google Scholar]
- 67.Goodrich ME, McGee DW. Regulation of mucosal B cell immunoglobulin secretion by intestinal epithelial cell-derived cytokines. Cytokine. 1998;10(12):948–955. doi: 10.1006/cyto.1998.0385. [DOI] [PubMed] [Google Scholar]
- 68.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3(10):822–829. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 69.Longet S, Lundahl MLE, Lavelle EC. Targeted strategies for mucosal vaccination. Bioconjug Chem. 2018;29(3):613–623. doi: 10.1021/acs.bioconjchem.7b00738. [DOI] [PubMed] [Google Scholar]
- 70.Correia-Pinto JF, Csaba N, Alonso MJ. Vaccine delivery carriers: insights and future perspectives. Int J Pharm. 2013;440(1):27–38. doi: 10.1016/j.ijpharm.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 71.Dupont A, Heinbockel L, Brandenburg K, Hornef MW. Antimicrobial peptides and the enteric mucus layer act in concert to protect the intestinal mucosa. Gut Microbes. 2014;5(6):761–765. doi: 10.4161/19490976.2014.972238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McEntee C, Lavelle EC, O’Hagan DT (2015) Chapter 63 - Antigen delivery systems I: nonliving microparticles, liposomes, and immune-stimulating complexes (ISCOMs). In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroutre H, Lambrecht BN (eds) Mucosal Immunology (Fourth Edition). Academic Press, Boston, pp 1211–1231. 10.1016/B978-0-12-415847-4.00063-X
- 73.Szatraj K, Szczepankowska AK, Chmielewska-Jeznach M. Lactic acid bacteria - promising vaccine vectors: possibilities, limitations, doubts. J Appl Microbiol. 2017;123(2):325–339. doi: 10.1111/jam.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nochi T, Yuki Y, Takahashi H, Sawada S, Mejima M, Kohda T, Harada N, Kong IG, Sato A, Kataoka N, Tokuhara D, Kurokawa S, Takahashi Y, Tsukada H, Kozaki S, Akiyoshi K, Kiyono H. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat Mater. 2010;9(7):572–578. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 75.Davitt CJ, Lavelle EC. Delivery strategies to enhance oral vaccination against enteric infections. Adv Drug Deliv Rev. 2015;91:52–69. doi: 10.1016/j.addr.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 76.Calzada D, Baos S, Cremades-Jimeno L, Cardaba B. Immunological mechanisms in allergic diseases and allergen tolerance: the role of Treg cells. J Immunol Res. 2018;2018:6012053. doi: 10.1155/2018/6012053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Savelkoul HF, Ferro VA, Strioga MM, Schijns VE. Choice and design of adjuvants for parenteral and mucosal vaccines. Vaccines (Basel) 2015;3(1):148–171. doi: 10.3390/vaccines3010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wells JM, Mercenier A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol. 2008;6(5):349–362. doi: 10.1038/nrmicro1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Apostolico Jde S, Lunardelli VA, Coirada FC, Boscardin SB, Rosa DS. Adjuvants: classification, modus operandi, and licensing. J Immunol Res. 2016;2016:1459394. doi: 10.1155/2016/1459394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hedayat M, Netea MG, Rezaei N. Targeting of Toll-like receptors: a decade of progress in combating infectious diseases. Lancet Infect Dis. 2011;11(9):702–712. doi: 10.1016/S1473-3099(11)70099-8. [DOI] [PubMed] [Google Scholar]
- 81.Chabot S, Wagner JS, Farrant S, Neutra MR. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol. 2006;176(7):4275–4283. doi: 10.4049/jimmunol.176.7.4275. [DOI] [PubMed] [Google Scholar]
- 82.Wang X, Meng D. Innate endogenous adjuvants prime to desirable immune responses via mucosal routes. Protein Cell. 2015;6(3):170–184. doi: 10.1007/s13238-014-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carleton HA. Pathogenic bacteria as vaccine vectors: teaching old bugs new tricks. Yale J Biol Med. 2010;83(4):217–222. [PMC free article] [PubMed] [Google Scholar]
- 84.Mays ZJ, Nair NU. Synthetic biology in probiotic lactic acid bacteria: at the frontier of living therapeutics. Curr Opin Biotechnol. 2018;53:224–231. doi: 10.1016/j.copbio.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yadav M, Shukla P. Efficient engineered probiotics using synthetic biology approaches: a review. Biotechnol Appl Biochem. 2020;67(1):22–29. doi: 10.1002/bab.1822. [DOI] [PubMed] [Google Scholar]
- 86.Diaz-Dinamarca DA, Hernandez C, Escobar DF, Soto DA, Munoz GA, Badilla JF, Manzo RA, Carrion F, Kalergis AM, Vasquez AE. mucosal vaccination with Lactococcus lactis-secreting surface immunological protein induces humoral and cellular immune protection against group B Streptococcus in a murine model. Vaccines (Basel) 2020;8(2):146. doi: 10.3390/vaccines8020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LeCureux JS, Dean GA (2018) Lactobacillus mucosal vaccine vectors: immune responses against bacterial and viral antigens. Msphere 3 (3). 10.1128/mSphere.00061-18 [DOI] [PMC free article] [PubMed]
- 88.Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369(6499):50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Konturek PC, Harsch IA, Neurath MF, Zopf Y (2020) COVID-19 - more than respiratory disease: a gastroenterologist’s perspective. J Physiol Pharmacol 71 (2). 10.26402/jpp.2020.2.02 [DOI] [PubMed]
- 90.Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC (2020) Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 159 (3):944–955 e948. 10.1053/j.gastro.2020.05.048 [DOI] [PMC free article] [PubMed]
- 91.Xu K, Cai H, Shen Y, Ni Q, Chen Y, Hu S, Li J, Wang H, Yu L, Huang H, Qiu Y, Wei G, Fang Q, Zhou J, Sheng J, Liang T, Li L. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(1):147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morais AHA, Passos TS, Maciel BLL, da Silva-Maia JK (2020) Can probiotics and diet promote beneficial immune modulation and purine control in coronavirus infection? Nutrients 12 (6). 10.3390/nu12061737 [DOI] [PMC free article] [PubMed]
- 93.Feng Z, Wang Y, Qi W (2020) The small intestine, an underestimated site of SARS-CoV-2 infection: from red queen effect to probiotics. Preprints.org. 10.20944/preprints202003.0161.v1
- 94.Gao QY, Chen YX, Fang JY. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21(3):125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ghosh SS, Wang J, Yannie PJ, Ghosh S (2020) Intestinal barrier dysfunction, LPS translocation, and disease development. J Endocr Soc 4 (2):bvz039. 10.1210/jendso/bvz039 [DOI] [PMC free article] [PubMed]
- 97.Zhang D, Li S, Wang N, Tan HY, Zhang Z, Feng Y. The cross-talk between gut microbiota and lungs in common lung diseases. Front Microbiol. 2020;11:301. doi: 10.3389/fmicb.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li N, Ma WT, Pang M, Fan QL, Hua JL. The commensal microbiota and viral infection: a comprehensive review. Front Immunol. 2019;10:1551. doi: 10.3389/fimmu.2019.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bingula R, Filaire M, Radosevic-Robin N, Bey M, Berthon JY, Bernalier-Donadille A, Vasson MP, Filaire E. Desired turbulence? Gut-lung axis, immunity, and lung cancer. J Oncol. 2017;2017:5035371. doi: 10.1155/2017/5035371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McAleer JP, Kolls JK. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol. 2018;48(1):39–49. doi: 10.1002/eji.201646721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bradley KC, Finsterbusch K, Schnepf D, Crotta S, Llorian M, Davidson S, Fuchs SY, Staeheli P, Wack A (2019) Microbiota-driven tonic interferon signals in lung stromal cells protect from Influenza virus infection. Cell Rep 28 (1):245-256 e244. 10.1016/j.celrep.2019.05.105 [DOI] [PubMed]
- 102.Xie P, Ma W, Tang H, Liu D. Severe COVID-19: a review of recent progress with a look toward the future. Front Public Health. 2020;8:189. doi: 10.3389/fpubh.2020.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lei YM, Nair L, Alegre ML. The interplay between the intestinal microbiota and the immune system. Clin Res Hepatol Gastroenterol. 2015;39(1):9–19. doi: 10.1016/j.clinre.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vanhoutvin SA, Troost FJ, Hamer HM, Lindsey PJ, Koek GH, Jonkers DM, Kodde A, Venema K, Brummer RJ. Butyrate-induced transcriptional changes in human colonic mucosa. PLoS One. 2009;4(8):e6759. doi: 10.1371/journal.pone.0006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kato LM, Kawamoto S, Maruya M, Fagarasan S. The role of the adaptive immune system in regulation of gut microbiota. Immunol Rev. 2014;260(1):67–75. doi: 10.1111/imr.12185. [DOI] [PubMed] [Google Scholar]
- 107.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chattha KS, Roth JA, Saif LJ. Strategies for design and application of enteric viral vaccines. Annu Rev Anim Biosci. 2015;3(1):375–395. doi: 10.1146/annurev-animal-022114-111038. [DOI] [PubMed] [Google Scholar]
- 109.Kandasamy S, Chattha KS, Vlasova AN, Rajashekara G, Saif LJ. Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model. Gut Microbes. 2014;5(5):639–651. doi: 10.4161/19490976.2014.969972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harris VC, Armah G, Fuentes S, Korpela KE, Parashar U, Victor JC, Tate J, de Weerth C, Giaquinto C, Wiersinga WJ, Lewis KD, de Vos WM. Significant correlation between the infant gut microbiome and Rotavirus vaccine response in rural Ghana. J Infect Dis. 2017;215(1):34–41. doi: 10.1093/infdis/jiw518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kraan H, Vrieling H, Czerkinsky C, Jiskoot W, Kersten G, Amorij JP. Buccal and sublingual vaccine delivery. J Control Release. 2014;190:580–592. doi: 10.1016/j.jconrel.2014.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cho SW, Yim J, Seo SW. Engineering tools for the development of recombinant lactic acid bacteria. Biotechnol J. 2020;15(6):e1900344. doi: 10.1002/biot.201900344. [DOI] [PubMed] [Google Scholar]
- 113.Ruiz L, Margolles A, Sanchez B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol. 2013;4(396):396. doi: 10.3389/fmicb.2013.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee IC, Tomita S, Kleerebezem M, Bron PA. The quest for probiotic effector molecules unraveling strain specificity at the molecular level. Pharmacol Res. 2013;69(1):61–74. doi: 10.1016/j.phrs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 115.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8(3):171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 116.Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4607–4614. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Green-Johnson JM. Immunological responses to gut bacteria. J AOAC Int. 2012;95(1):35–49. doi: 10.5740/jaoacint.sge_green-johnson. [DOI] [PubMed] [Google Scholar]
- 118.Bron PA, van Baarlen P, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol. 2011;10(1):66–78. doi: 10.1038/nrmicro2690. [DOI] [PubMed] [Google Scholar]
- 119.Gibson GR, Wang X. Regulatory effects of Bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994;77(4):412–420. doi: 10.1111/j.1365-2672.1994.tb03443.x. [DOI] [PubMed] [Google Scholar]
- 120.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 121.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278(11):8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 122.Kawashima T, Ikari N, Watanabe Y, Kubota Y, Yoshio S, Kanto T, Motohashi S, Shimojo N, Tsuji NM. Double-stranded RNA derived from lactic acid bacteria augments Th1 immunity via interferon-beta from human dendritic cells. Front Immunol. 2018;9:27. doi: 10.3389/fimmu.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jounai K, Ikado K, Sugimura T, Ano Y, Braun J, Fujiwara D. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS One. 2012;7(4):e32588. doi: 10.1371/journal.pone.0032588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fuller R (1997) Probiotics 2: applications and practical aspects, vol 2. Springer Science & Business Media
- 125.Dziarski R. Demonstration of peptidoglycan-binding sites on lymphocytes and macrophages by photoaffinity cross-linking. J Biol Chem. 1991;266(8):4713–4718. doi: 10.1016/S0021-9258(19)67707-0. [DOI] [PubMed] [Google Scholar]
- 126.Fernandes CF, Shahani KM. Anticarcinogenic and immunological properties of dietary Lactobacilli. J Food Prot. 1990;53(8):704–710. doi: 10.4315/0362-028X-53.8.704. [DOI] [PubMed] [Google Scholar]
- 127.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55(4):733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dubois B, Chapat L, Goubier A, Papiernik M, Nicolas JF, Kaiserlian D. Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood. 2003;102(9):3295–3301. doi: 10.1182/blood-2003-03-0727. [DOI] [PubMed] [Google Scholar]
- 129.Rigaux P, Daniel C, Hisbergues M, Muraille E, Hols P, Pot B, Pestel J, Jacquet A. Immunomodulatory properties of Lactobacillus plantarum and its use as a recombinant vaccine against mite allergy. Allergy. 2009;64(3):406–414. doi: 10.1111/j.1398-9995.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 130.You J, Dong H, Mann ER, Knight SC, Yaqoob P. Probiotic modulation of dendritic cell function is influenced by ageing. Immunobiology. 2014;219(2):138–148. doi: 10.1016/j.imbio.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cano-Garrido O, Seras-Franzoso J, Garcia-Fruitos E. Lactic acid bacteria: reviewing the potential of a promising delivery live vector for biomedical purposes. Microb Cell Fact. 2015;14(1):137. doi: 10.1186/s12934-015-0313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Campo Alagon Fernandez Del, P, De Orta Pando A, Straface JI, Lopez Vega JR, Toledo Plata D, Niezen Lugo SF, Alvarez Hernandez D, Barrientos Fortes T, Gutierrez-Kobeh L, Solano-Galvez SG, Vazquez-Lopez R, The use of probiotic therapy to modulate the gut microbiota and dendritic cell responses in inflammatory bowel diseases. Med Sci (Basel) 2019;7(2):33. doi: 10.3390/medsci7020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gallo A, Passaro G, Gasbarrini A, Landolfi R, Montalto M. Modulation of microbiota as treatment for intestinal inflammatory disorders: an uptodate. World J Gastroenterol. 2016;22(32):7186–7202. doi: 10.3748/wjg.v22.i32.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boirivant M, Strober W. The mechanism of action of probiotics. Curr Opin Gastroenterol. 2007;23(6):679–692. doi: 10.1097/MOG.0b013e3282f0cffc. [DOI] [PubMed] [Google Scholar]
- 135.Abdo Z, LeCureux J, LaVoy A, Eklund B, Ryan EP, Dean GA. Impact of oral probiotic Lactobacillus acidophilus vaccine strains on the immune response and gut microbiome of mice. PLoS One. 2019;14(12):e0225842. doi: 10.1371/journal.pone.0225842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Holzapfel WH, Haberer P, Snel J, Schillinger U, Huis in't Veld JH, Overview of gut flora and probiotics. Int J Food Microbiol. 1998;41(2):85–101. doi: 10.1016/s0168-1605(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 137.Iwaki M, Okahashi N, Takahashi I, Kanamoto T, Sugita-Konishi Y, Aibara K, Koga T. Oral immunization with recombinant Streptococcus lactis carrying the Streptococcus mutans surface protein antigen gene. Infect Immun. 1990;58(9):2929–2934. doi: 10.1128/IAI.58.9.2929-2934.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gunasekaran B, Gothandam KM. A review on edible vaccines and their prospects. Braz J Med Biol Res. 2020;53(2):e8749. doi: 10.1590/1414-431X20198749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.del Rio B, Redruello B, Fernandez M, Martin MC, Ladero V, Alvarez MA (2019) Lactic acid bacteria as a live delivery system for the in situ production of nanobodies in the human gastrointestinal tract. Front Microbiol 9 (3179). 10.3389/fmicb.2018.03179
- 140.Valdez Y, Brown EM, Finlay BB. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35(11):526–537. doi: 10.1016/j.it.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 141.Chorny A, Puga I, Cerutti A (2010) Chapter 2 - innate signaling networks in mucosal IgA class switching. In: Fagarasan S, Cerutti A (eds) Advances in immunology, vol 107. Academic Press, pp 31-69. 10.1016/B978-0-12-381300-8.00002-2 [DOI] [PMC free article] [PubMed]
- 142.Ho PS, Kwang J, Lee YK. Intragastric administration of Lactobacillus casei expressing transmissible gastroentritis coronavirus spike glycoprotein induced specific antibody production. Vaccine. 2005;23(11):1335–1342. doi: 10.1016/j.vaccine.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wells JM (2011) Immunomodulatory mechanisms of lactobacilli. Microb Cell Fact 10 Suppl 1 (Suppl 1):S17. 10.1186/1475-2859-10-S1-S17 [DOI] [PMC free article] [PubMed]
- 144.Vilander AC, Dean GA. Adjuvant strategies for lactic acid bacterial mucosal vaccines. Vaccines (Basel) 2019;7(4):150. doi: 10.3390/vaccines7040150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells - polarity and complexity. Immunol Today. 2000;21(3):123–128. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- 146.de Moreno de LeBlanc A, Galdeano CM, Chaves S, Perdigón G (2016) Oral administration of L. Casei CRL 431 increases immunity in bronchus and mammary glands. Eur J Inflamm 3 (1):23-28. 10.1177/1721727x0500300105
- 147.Holscher HD, Czerkies LA, Cekola P, Litov R, Benbow M, Santema S, Alexander DD, Perez V, Sun S, Saavedra JM, Tappenden KA. Bifidobacterium lactis Bb12 enhances intestinal antibody response in formula-fed infants: a randomized, double-blind, controlled trial. JPEN J Parenter Enteral Nutr. 2012;36(1 Suppl):106S–117S. doi: 10.1177/0148607111430817. [DOI] [PubMed] [Google Scholar]
- 148.Mullie C, Yazourh A, Thibault H, Odou MF, Singer E, Kalach N, Kremp O, Romond MB. Increased poliovirus-specific intestinal antibody response coincides with promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in infants: a randomized, double-blind, placebo-controlled trial. Pediatr Res. 2004;56(5):791–795. doi: 10.1203/01.PDR.0000141955.47550.A0. [DOI] [PubMed] [Google Scholar]
- 149.Perdigón G, Alvarez S (1992) Probiotics and the immune state. In: Fuller R (ed) Probiotics: the scientific basis. Springer Netherlands, Dordrecht, pp 145-180. 10.1007/978-94-011-2364-8_7
- 150.Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168(1):171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 151.Macho Fernandez E, Pot B, Grangette C. Beneficial effect of probiotics in IBD: are peptidogycan and NOD2 the molecular key effectors? Gut Microbes. 2011;2(5):280–286. doi: 10.4161/gmic.2.5.18255. [DOI] [PubMed] [Google Scholar]
- 152.Zeuthen LH, Fink LN, Frokiaer H. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and Bifidobacteria in dendritic cells. Immunology. 2008;124(4):489–502. doi: 10.1111/j.1365-2567.2007.02800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mercenier A, Muller-Alouf H, Grangette C. Lactic acid bacteria as live vaccines. Curr Issues Mol Biol. 2000;2(1):17–25. [PubMed] [Google Scholar]
- 154.Isolauri E, Salminen S, Ouwehand AC. Microbial-gut interactions in health and disease. Probiotics. Best Pract Res Clin Gastroenterol. 2004;18(2):299–313. doi: 10.1016/j.bpg.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 155.Bron PA, Kleerebezem M. Lactic acid bacteria for delivery of endogenous or engineered therapeutic molecules. Front Microbiol. 2018;9:1821. doi: 10.3389/fmicb.2018.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Israr B, Kim J, Anam S, Anjum F. Lactic acid bacteria as vectors: a novel approach for mucosal vaccine delivery. J Clin Cell. 2018 doi: 10.4172/2155-9899.1000548. [DOI] [Google Scholar]
- 157.Saavedra JM, Bauman NA, Oung I, Perman JA, Yolken RH. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. 1994;344(8929):1046–1049. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- 158.Shih HI, Wu CJ, Tu YF, Chi CY. Fighting COVID-19: a quick review of diagnoses, therapies, and vaccines. Biomed J. 2020;43(4):341–354. doi: 10.1016/j.bj.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takei S, Omoto C, Kitagawa K, Morishita N, Katayama T, Shigemura K, Fujisawa M, Kawabata M, Hotta H, Shirakawa T. Oral administration of genetically modified Bifidobacterium displaying HCV-NS3 multi-epitope fusion protein could induce an HCV-NS3-specific systemic immune response in mice. Vaccine. 2014;32(25):3066–3074. doi: 10.1016/j.vaccine.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 160.Yu Z, Huang Z, Sao C, Huang Y, Zhang F, Ma G, Chen Z, Zeng Z, Qiwen D, Zeng W. Oral immunization of mice using Bifidobacterium longum expressing VP1 protein from enterovirus 71. Arch Virol. 2013;158(5):1071–1077. doi: 10.1007/s00705-012-1589-z. [DOI] [PubMed] [Google Scholar]
- 161.Bermudez-Humaran LG, Langella P, Miyoshi A, Gruss A, Guerra RT, Montes de Oca-Luna R, Le Loir Y. Production of human papillomavirus type 16 E7 protein in Lactococcus lactis. Appl Environ Microbiol. 2002;68(2):917–922. doi: 10.1128/aem.68.2.917-922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Szatraj K, Szczepankowska AK, Saczynska V, Florys K, Gromadzka B, Lepek K, Plucienniczak G, Szewczyk B, Zagorski-Ostoja W, Bardowski J. Expression of avian influenza haemagglutinin (H5) and chicken interleukin 2 (chIL-2) under control of the ptcB promoter in Lactococcus lactis. Acta Biochim Pol. 2014;61(3):609–614. doi: 10.18388/abp.2014_1884. [DOI] [PubMed] [Google Scholar]
- 163.Zhou Z, Gong S, Li XM, Yang Y, Guan R, Zhou S, Yao S, Xie Y, Ou Z, Zhao J, Liu Z. Expression of Helicobacter pylori urease B on the surface of Bacillus subtilis spores. J Med Microbiol. 2015;64(Pt 1):104–110. doi: 10.1099/jmm.0.076430-0. [DOI] [PubMed] [Google Scholar]
- 164.Kuczkowska K, Mathiesen G, Eijsink VG, Oynebraten I. Lactobacillus plantarum displaying CCL3 chemokine in fusion with HIV-1 Gag derived antigen causes increased recruitment of T cells. Microb Cell Fact. 2015;14(1):169. doi: 10.1186/s12934-015-0360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chamcha V, Jones A, Quigley BR, Scott JR, Amara RR. Oral immunization with a recombinant Lactococcus lactis-expressing HIV-1 antigen on group A Streptococcus Pilus induces strong mucosal immunity in the gut. J Immunol. 2015;195(10):5025–5034. doi: 10.4049/jimmunol.1501243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu M, Zhong Y, Chen J, Liu Y, Tang C, Wang X, Zhang Y, Wang P, Logan SM, Chen W, Wei B. Oral immunization of mice with a multivalent therapeutic subunit vaccine protects against Helicobacter pylori infection. Vaccine. 2020;38(14):3031–3041. doi: 10.1016/j.vaccine.2020.02.036. [DOI] [PubMed] [Google Scholar]
- 167.Mustafa AD, Kalyanasundram J, Sabidi S, Song AA, Abdullah M, Abdul Rahim R, Yusoff K. Proof of concept in utilizing in-trans surface display system of Lactobacillus plantarum as mucosal tuberculosis vaccine via oral administration in mice. BMC Biotechnol. 2018;18(1):63. doi: 10.1186/s12896-018-0461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang M, Fu T, Hao J, Li L, Tian M, Jin N, Ren L, Li C. A recombinant Lactobacillus plantarum strain expressing the spike protein of SARS-CoV-2. Int J Biol Macromol. 2020;160:736–740. doi: 10.1016/j.ijbiomac.2020.05.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nakayama Y, Moriya T, Sakai F, Ikeda N, Shiozaki T, Hosoya T, Nakagawa H, Miyazaki T. Oral administration of Lactobacillus gasseri SBT2055 is effective for preventing influenza in mice. Sci Rep. 2014;4:4638. doi: 10.1038/srep04638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Eguchi K, Fujitani N, Nakagawa H, Miyazaki T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci Rep. 2019;9(1):4812. doi: 10.1038/s41598-019-39602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Akour A. Probiotics and COVID-19: is there any link? Lett Appl Microbiol. 2020;71(3):229–234. doi: 10.1111/lam.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Luoto R, Ruuskanen O, Waris M, Kalliomaki M, Salminen S, Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2014;133(2):405–413. doi: 10.1016/j.jaci.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Starosila D, Rybalko S, Varbanetz L, Ivanskaya N, Sorokulova I (2017) Anti-influenza activity of a Bacillus subtilis probiotic strain. Antimicrob Agents Chemother 61 (7). 10.1128/AAC.00539-17 [DOI] [PMC free article] [PubMed]
- 174.Kiousi DE, Karapetsas A, Karolidou K, Panayiotidis MI, Pappa A, Galanis A. Probiotics in extraintestinal diseases: current trends and new directions. Nutrients. 2019;11(4):788. doi: 10.3390/nu11040788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.d'Ettorre G, Ceccarelli G, Marazzato M, Campagna G, Pinacchio C, Alessandri F, Ruberto F, Rossi G, Celani L, Scagnolari C, Mastropietro C, Trinchieri V, Recchia GE, Mauro V, Antonelli G, Pugliese F, Mastroianni CM. Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front Med (Lausanne) 2020;7(389):389. doi: 10.3389/fmed.2020.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Clua P, Kanmani P, Zelaya H, Tada A, Kober A, Salva S, Alvarez S, Kitazawa H, Villena J. Peptidoglycan from Immunobiotic Lactobacillus rhamnosus improves resistance of infant mice to respiratory syncytial viral infection and secondary Pneumococcal Pneumonia. Front Immunol. 2017;8:948. doi: 10.3389/fimmu.2017.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, Ott S, Hampe J, Schreiber S, Heller K, Schrezenmeir J (2005) Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clin Nutr 24 (4):481–491. 10.1016/j.clnu.2005.02.006 [DOI] [PubMed]
- 178.Olivares M, Diaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonolla J, Navas M, Rodriguez JM, Xaus J. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007;23(3):254–260. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 179.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieers G, Guery B, Delhaes L. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018;20(12):e12966. doi: 10.1111/cmi.12966. [DOI] [PubMed] [Google Scholar]
- 181.Wang Y, Pan CQ, Xing H. Advances in gut microbiota of viral hepatitis cirrhosis. Biomed Res Int. 2019;2019:9726786. doi: 10.1155/2019/9726786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Falcone V, Mihm D, Neumann-Haefelin D, Costa C, Nguyen T, Pozzi G, Ricci S. Systemic and mucosal immunity to respiratory syncytial virus induced by recombinant Streptococcus gordonii surface-displaying a domain of viral glycoprotein G. FEMS Immunol Med Microbiol. 2006;48(1):116–122. doi: 10.1111/j.1574-695X.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- 183.Lee JS, Poo H, Han DP, Hong SP, Kim K, Cho MW, Kim E, Sung MH, Kim CJ. Mucosal immunization with surface-displayed severe acute respiratory syndrome coronavirus spike protein on Lactobacillus casei induces neutralizing antibodies in mice. J Virol. 2006;80(8):4079–4087. doi: 10.1128/JVI.80.8.4079-4087.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Oliveira ML, Areas AP, Campos IB, Monedero V, Perez-Martinez G, Miyaji EN, Leite LC, Aires KA, Lee Ho P. Induction of systemic and mucosal immune response and decrease in Streptococcus pneumoniae colonization by nasal inoculation of mice with recombinant lactic acid bacteria expressing pneumococcal surface antigen A. Microbes Infect. 2006;8(4):1016–1024. doi: 10.1016/j.micinf.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hanniffy SB, Carter AT, Hitchin E, Wells JM. Mucosal delivery of a pneumococcal vaccine using Lactococcus lactis affords protection against respiratory infection. J Infect Dis. 2007;195(2):185–193. doi: 10.1086/509807. [DOI] [PubMed] [Google Scholar]
- 186.Campos IB, Darrieux M, Ferreira DM, Miyaji EN, Silva DA, Areas AP, Aires KA, Leite LC, Ho PL, Oliveira ML. Nasal immunization of mice with Lactobacillus casei expressing the pneumococcal surface protein A: induction of antibodies, complement deposition and partial protection against Streptococcus pneumoniae challenge. Microbes Infect. 2008;10(5):481–488. doi: 10.1016/j.micinf.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 187.Lei H, Xu Y, Chen J, Wei X, Lam DM. Immunoprotection against influenza H5N1 virus by oral administration of enteric-coated recombinant Lactococcus lactis mini-capsules. Virology. 2010;407(2):319–324. doi: 10.1016/j.virol.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 188.Shi SH, Yang WT, Yang GL, Cong YL, Huang HB, Wang Q, Cai RP, Ye LP, Hu JT, Zhou JY, Wang CF, Li Y. Immunoprotection against influenza virus H9N2 by the oral administration of recombinant Lactobacillus plantarumNC8 expressing hemagglutinin in BALB/c mice. Virology. 2014;464–465:166–176. doi: 10.1016/j.virol.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 189.Shakoor S, Rao AQ, Shahid N, Yaqoob A, Samiullah TR, Shakoor S, Latif A, Tabassum B, Khan MAU, Shahid AA, Husnain T. Role of oral vaccines as an edible tool to prevent infectious diseases. Acta Virol. 2019;63(3):245–252. doi: 10.4149/av_2019_301. [DOI] [PubMed] [Google Scholar]
- 190.Savoie K. Edible vaccine success. Nat Biotechnol. 2000;18(4):367. doi: 10.1038/74356. [DOI] [PubMed] [Google Scholar]
- 191.Lal P, Ramachandran VG, Goyal R, Sharma R. Edible vaccines: current status and future. Indian J Med Microbiol. 2007;25(2):93–102. doi: 10.4103/0255-0857.32713. [DOI] [PubMed] [Google Scholar]