Abstract
Doxorubicin (DOX) is extensively applied in cancer therapy due to its efficacy in suppressing cancer progression and inducing apoptosis. After its discovery, this chemotherapeutic agent has been frequently used for cancer therapy, leading to chemoresistance. Due to dose-dependent toxicity, high concentrations of DOX cannot be administered to cancer patients. Therefore, experiments have been directed towards revealing underlying mechanisms responsible for DOX resistance and ameliorating its adverse effects. Nuclear factor erythroid 2-related factor 2 (Nrf2) signaling is activated to increase levels of reactive oxygen species (ROS) in cells to protect them against oxidative stress. It has been reported that Nrf2 activation is associated with drug resistance. In cells exposed to DOX, stimulation of Nrf2 signaling protects cells against cell death. Various upstream mediators regulate Nrf2 in DOX resistance. Strategies, both pharmacological and genetic interventions, have been applied for reversing DOX resistance. However, Nrf2 induction is of importance for alleviating side effects of DOX. Pharmacological agents with naturally occurring compounds as the most common have been used for inducing Nrf2 signaling in DOX amelioration. Furthermore, signaling networks in which Nrf2 is a key player for protection against DOX adverse effects have been revealed and are discussed in the current review.
Keywords: doxorubicin, chemoresistance, oxidative stress, redox signaling, nuclear factor erythroid 2-related factor 2 (Nrf2), cancer therapy
1. Introduction
Doxorubicin (DOX) is an anthracycline isolated from Streptomyces with proficiency in treatment of various cancers such as thoracic cancers, reproductive cancers, gastrointestinal and brain tumors [1]. Three major mechanisms are followed by DOX in suppressing progression and proliferation of cancer cells, including inhibiting DNA topoisomerase II activity, DNA intercalation and enhancing production of free radicals, especially reactive oxygen species (ROS) that are of importance in triggering apoptosis through mitochondrial pathway [2]. After its discovery, DOX was considered as the first option in treatment of cancer patients and showed promising clinical results. However, these ideal findings disappeared with development of DOX resistance [3,4,5,6].
Currently, two major obstacles are considered for cancer chemotherapy with DOX including A) DOX resistance, and B) dose-dependent toxicity [7,8,9]. The toxicity of DOX against normal cells has a negative impact on its efficacy in cancer therapy, since high dose of DOX cannot be administered to cancer patients to overcome resistance. In order to reverse DOX resistance, a combination of DOX with other compounds such as selenium is utilized to induce apoptosis and necrosis in cancer cells, leading to their enhanced sensitivity to DOX chemotherapy [10]. Mitochondrial transcription factor A (TFAM) stimulates mitochondrial dysfunction and AMP-activated protein kinase (AMPK) in suppressing DOX resistance [11]. That is why molecular pathways that promote cancer cell growth and viability, can induce DOX resistance. For instance, in non-small cell lung cancer, vasohibin2 (VASH2) functions as a tumor-promoting factor in enhancing proliferation that subsequently, stimulates DOX resistance [12]. Identification of such factors is of importance in suppressing DOX resistance by developing potential therapeutics for their targeting [13].
One of the processes contributing to DOX resistance is glycolysis. Cancer cells demonstrate enhanced glucose uptake and, to have enough energy, they induce glycolysis as a way of reaching a high amount of energy in a low time. In osteosarcoma cells, sphingosine kinase 1 (Sphk1) undergoes up-regulation due to hypoxia and activation of hypoxia-related molecular pathway, known as hypoxia-inducible factor 1α (HIF-1α) [14,15]. Then, an increase occurs in glycolysis, providing condition for DOX resistance [16]. Non-coding RNAs (ncRNAs) such as long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) also participate in development of DOX resistance due to their regulatory effects on biological mechanisms and molecular pathways [17,18,19,20,21]. In addition to recognition of tumor-promoting molecular pathways and using combination chemotherapy, another strategy that utilizes nanostructures for DOX delivery has been developed. This strategy is ideal for in vitro and in vivo experiments and nanoparticles can provide a platform for co-delivery of DOX with other anti-tumor agents, leading to targeted delivery at tumor site and reversing chemoresistance [22]. Therefore, the DOX resistance is an increasing challenge, and more experiments are required to find novel strategies in reversing chemoresistance.
Another obstacle in using DOX in cancer chemotherapy is its dose-dependent toxicity. Clinical studies have confirmed this issue. Cardiomyopathy, gastrointestinal (GI) side effects and hematological abnormalities result from using DOX alone or in combination with other chemotherapeutic agents in cancer therapy [23,24,25]. Increased level of oxidative stress and subsequent apoptosis induction are responsible for DOX toxicity [26]. Furthermore, DOX can stimulate matrix metalloproteinase-2 (MMP-2) for mediating cardiotoxicity. Application of MMP inhibitors is associated with inhibiting intracellular and extracellular matrix remodeling and ameliorating DOX toxicity [27]. What is noteworthy is that a number of plant-derived natural compounds such as alpha-tocopheryl succinate [28], naringenin [29] and atorvastatin [30] have been applied in DOX side effect alleviation. These compounds mainly diminish oxidative stress, inflammation and apoptosis.
These studies demonstrate that free radical generation is the most important way that DOX follows in cancer therapy. However, free radicals can negatively affect major organs in the body such as kidney, liver and brain. In the present review, we focus on a molecular pathway which involves nuclear factor erythroid 2-related factor 2 (Nrf2) as a regulator of oxidative stress in cells. Although activation of Nrf2 signaling protects cells against oxidative damage [31], it can induce chemoresistance via suppressing oxidative-mediated cell death in cancer cells [32].
2. Materials and Methods
In searching and collecting data for the current review, we used databases such as Pubmed, Google Scholar and Science Direct. Keywords such as “Nrf2 + Doxorubicin”, “Nrf2 + resistance”, and “Nrf2 + chemoprotection” were used. Furthermore, most of the experiments and articles are from 2020.
3. Nrf2 Signaling Pathway
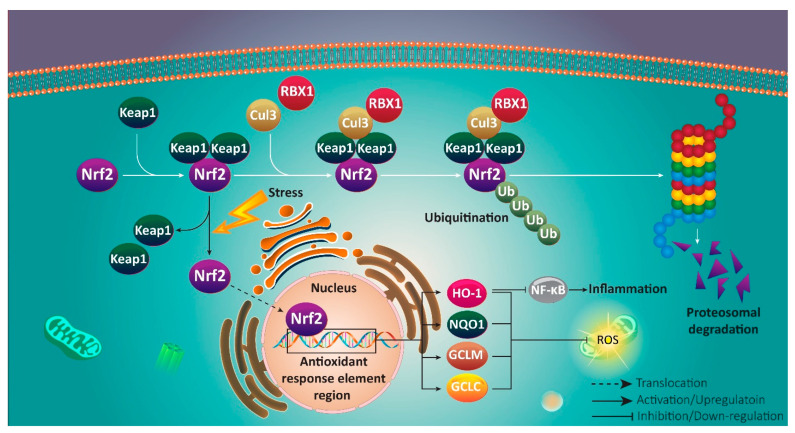
Counteracting oxidative stress and inflammation is the main aim of Nrf2 signaling in cell protection [33,34,35,36,37,38,39,40,41,42,43,44]. Sequestration of Nrf2 occurs in normal conditions by Kelch-like ECH-associated protein 1 (Keap1), when ROS and oxidative levels are at standard limit [45]. For providing proteasomal degradation of Nrf2, preventing its accumulation in cytoplasm and subsequent translocation to nucleus, Keap1 as a ubiquitin ligase adaptor protein, represents Nrf2 to Cullin-3 (Cul3)/RBX1 complex [46]. In contrast, electrophiles and oxidative stress are considered as inducers of Nrf2 signaling. In this way, Keap1 dissociation from Cul3 occurs via structural modification of Keap1 at cysteine 151 [47]. Furthermore, glycogen synthase kinase-3β (GSK-3β) prevents Nrf2 degradation by Nrf2 phosphorylation at serine 335 and 338. Then, Nrf2 polyubiquitination and its identification by β transducin repeat containing E3 ubiquitin-protein ligase (βTrCP) occur that are in favor of preventing Nrf2 degradation and providing CUL3/RBX1-induced degradation [48,49].
As a result, high levels of Nrf2 accumulate in cytoplasm that is followed by nuclear translocation and targeting genes containing antioxidant response element (ARE) region [50]. These genes include heme oxygenase-1 (HO-1), NAD(P)H dehydrogenase quinone 1 (NQO1), γ-glutamyl cysteine ligase modulatory and catalytic subunits (GCLM and GCLC, respectively), and ferritin accounting for inducing oxidant and antioxidant balance in cells [51,52,53,54,55]. Noteworthy, Nrf2 activation can be beneficial in reducing inflammation via activating HO-1, and subsequent inhibition of NF-κB signaling, which generally acts as a tumor-promoting factor [56,57,58]. Nrf2 down-regulation is associated with an increase in inflammatory response via NF-κB activation [59]. Furthermore, it has been reported that Nrf2 activation is in favor of reducing levels of pro-inflammatory cytokines in cells (Figure 1) [60,61,62,63,64,65].
Figure 1.
A schematic presentation of Nrf2 signaling pathway. Oxidative stress induces nuclear translocation of Nrf2 to promote antioxidant activity via up-regulating HO-1, NQO1, and GCLM.
4. Nrf2 in Protection and Chemoresistance
Chemoresistance remains a major challenge for cancer therapy [66,67,68,69,70]. The dual role of Nrf2 during cancer chemotherapy has been investigated in a variety of experiments. First, activation of Nrf2 signaling is advantageous in reducing side effects of chemotherapeutic agents. For instance, paclitaxel exposure is associated with induction of mechanical allodynia, while stimulation of Nrf2 signaling by oltipraz significantly reduces this adverse impact via HO-1 induction [71]. Furthermore, peroxisome proliferator-activated receptor gamma (PPARγ) can function as an upstream inducer of Nrf2 signaling in alleviation of paclitaxel-induced mechanical allodynia [72]. Exposing cells to cisplatin enhances levels of oxidant parameters such as malondialdehyde (MDA) and reduces activity and levels of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH). Nrf2, as a cytoprotective mechanism, supports kidney cells against oxidative stress and apoptosis via reinforcing antioxidant defense system [73]. Furthermore, Nrf2 activation is of importance in reducing cisplatin-mediated toxicity in reproductive system. In this way, tadalafil diminishes apoptosis and oxidative stress via Nrf2 up-regulation [74]. It has been reported that activation of Nrf2/HO-1 signaling is in favor of enhancing cell survival upon chemotherapy [75]. What is noteworthy is that phytochemicals such as curcumin [76] and formononetin [77] induce Nrf2/HO-1 signaling in reducing toxicity of oxaliplatin against liver and brain cells. These studies clearly demonstrate that Nrf2 signaling is of importance for alleviation of chemotherapy-mediated side effects.
Although Nrf2 signaling activation is of interest in reducing chemotherapy-mediated side effects, increasing evidence demonstrates association of Nrf2 activation with chemoresistance. Tumor-promoting factors such as bone morphogenetic proteins (BMP) induce Nrf2 signaling in promoting cancer cells survival and triggering chemoresistance [78]. It seems that Nrf2 can enhance tumor-initiating cell lineage that subsequently, mediates chemoresistance [79]. The p53 can provide proteasomal degradation of Keap1 via inducing Nrf2/ARE signaling to promote proliferation and apoptosis inhibition, resulting in chemoresistance [80]. Upon Nrf2 activation, glutamine metabolism increases to induce chemoresistance, and is associated with poor prognosis of cancer patients [81]. Furthermore, Nrf2 can positively interact with TAZ member of Hippo signaling in providing chemoresistance [82]. Anti-tumor compounds such as ailanthone [83] and kaempferol [84] decrease Nrf2 expression in promoting oxidative damage and ROS levels as well as triggering apoptosis, leading to enhanced cancer sensitivity to chemotherapy. Overall, studies are in agreement with the fact that Nrf2 activation induces chemoresistance [85] and its inhibition can be considered as an ideal strategy in reversing drug resistance.
5. Natural Compounds in Ameliorating Doxorubicin-Mediated Toxicity
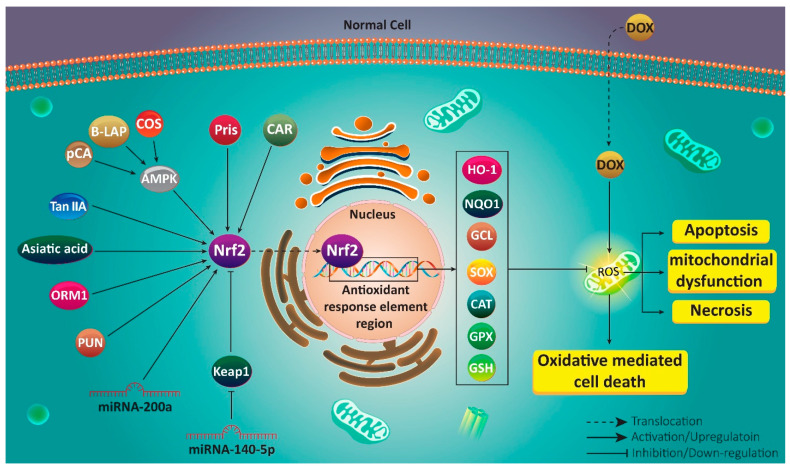
As it was discussed earlier, plant derived-natural compounds are able to regulate Nrf2 signaling in exerting their protective effect against oxidative stress-mediated diseases [86,87,88]. Pristimerin (Pris) is a natural triterpenoid compound derived from Celastraceae plant with different pharmacological activities such as anti-tumor, anti-inflammatory and antioxidant [89,90]. In respect of the potential of Pris in regulating Nrf2 signaling, it can be beneficial in ameliorating DOX-mediated cardiotoxicity that could be developed due to increased oxidative stress and ROS levels as the main risk factors. In this way, Pris enhances expression of Nrf2 at mRNA and protein levels, resulting in an increase in expression of its downstream targets including NQO1, HO-1 and GCL. Then, oxidative stress parameters undergo a decrease, while antioxidant defense system is reinforced, leading to decreased DOX-mediated cardiotoxicity [91]. In addition to heart, kidney and liver are negatively affected following DOX administration due to an increase in oxidative stress and inflammation [92,93]. Asiatic acid (AA) is another phytochemical that has been under attention due to its efficacy in preventing ageing, improving wound healing and exerting anti-tumor activity [94,95,96,97]. Recently, it has been shown that AA possesses high antioxidant potential that is of importance in reducing toxic effects of DOX against major organs of body. For this purpose, AA increases Nrf2 expression that diminishes necrosis, hyaline degeneration and congestion in heart. Hepatoprotective effects include reducing leukocyte inflammation, necrosis and apoptosis. Finally, kidney is protected against DOX toxicity via decreasing necrosis and inflammation [98]. These studies reveal that Nrf2 not only protects cells against DOX-mediated oxidative stress, but also decreases inflammation, and is therefore responsible for reducing cell death.
AMPK is considered as upstream mediator of Nrf2 signaling. It seems that AMPK activation is vital for inducing Nrf2 signaling [99]. By stimulating Nrf2 signaling, AMPK protects against oxidative stress and enhances expression of downstream targets such as HO-1 [100]. β-LAPachone (B-LAP) as a protective agent, targets AMPK/Nrf2 signaling in reducing DOX-mediated cardiotoxicity. B-LAP promotes expression of AMPK to induce Nrf2 signaling for elevating expression levels of SOX, CAT and GPX, leading to amelioration of DOX-mediated cardiotoxicity [101]. Following Nrf2 activation by phytochemicals, ROS levels decrease, preventing mitochondrial dysfunction and subsequent induction of apoptosis in cells exposed to DOX [102].
Cardamonin (CAR), a flavone exclusively found in Alpinia plant, has demonstrated potential in reducing oxidative stress via regulating Nrf2 signaling. It is noteworthy that CAR activates Nrf2 signaling that is of importance in reducing Th2 cytokine generation and preventing dermatitis [103]. In vivo experiment on mice demonstrates that CAR alleviates myocardial contractile dysfunction via enhancing Nrf2 expression [104]. These studies reveal that Nrf2 is a potential target of CAR in cell protection, and similarly, CAR follows a same pathway in reducing DOX-mediated toxicity. Both inflammation and oxidative stress are inhibited by CAR administration. This is mediated by activating Nrf2 signaling and subsequent up-regulation of SOD, GSH, CAT and reduced levels of ROS [105]. Chitosan oligosaccharide (COS) is a hydrolyzed form of chitosan that is found in exoskeleton of crustaceans and walls of fungi and insects [106]. A wide variety of biological activities including immune response regulation, anti-tumor, antimicrobial and anti-apoptosis are considered for COS [107,108,109,110,111]. A recent study has shown that COS can prevent oxidative damage and induce heart growth upon exposure to DOX. COS reduces ROS levels, mitochondrial dysfunction and apoptosis in cells. Mechanistically, COS induces AMPK in triggering Nrf2/ARE axis [112]. This study demonstrates that complicated signaling networks are involved in protecting against DOX-mediated toxicity in which Nrf2 signaling is the key player. The aim of Nrf2 activation is to up-regulate expression of downstream targets such as HO-1 and NQO1 that participate in improving antioxidant/oxidant balance and ameliorating DOX-mediated toxicity [113,114].
The p-coumaric acid (pCA) is a phenolic compound that functions as a ROS scavenger in reducing oxidative stress and protecting cells against drug toxicity [115]. Different molecular pathways are affected by pCA in exerting its protective effects and Nrf2 is among them. In this way, pCA promotes Nrf2 expression to prevent ROS generation and inflammation caused by lipopolysaccharide (LPS) [116]. It seems that pCA inhibits acute lung injury via AMPK/Nrf2/HO-1 axis activation to induce antioxidant response [117]. These studies advocate the fact that pCA has potential modulatory effects on Nrf2 signaling. pCA significantly increases cell survival and prevents apoptosis via caspase-3 down-regulation. It has been reported that ROS generation inhibition and preventing mitochondrial dysfunction are major mechanisms for protecting against DOX-mediated cardiotoxicity. Consequently, pCA induces Nrf2 signaling to inhibit ROS overgeneration, preventing subsequent mechanisms that are essential for DOX-mediated cardiotoxicity [118]. Therefore, using compounds inducing Nrf2 signaling can protect against DOX-mediated cardiotoxicity [119].
Tanshinone IIA (Tan IIA) is a potent antioxidant agent exclusively found in Radix Salvia miltiorrhiza [120]. Similar to other phytochemicals with antioxidant activity, Tan IIA targets Nrf2 signaling. Tan IIA administration is associated with improvement in silica-mediated pulmonary inflammatory response, structural damage and fibrosis via Nrf2/ARE activation [121]. Furthermore, Tan IIA prevents liver injury via epigenetic activation of Nrf2 and reinforcing antioxidant defense system [122]. Hence, Tan IIA stimulates Nrf2 signaling as a way of recovering redox homeostasis and inhibiting pulmonary fibrosis [123]. In alleviation of DOX-induced cardiotoxicity, Tan IIA increases cell viability and prevents damage-associated morphological alterations in H9C2 cells. In addition, a decrease occurs in generation of ROS levels, while GSH undergoes up-regulation in activity. These protective effects of Tan IIA are mediated by activating Nrf2 signaling and its downstream targets HO-1 and NQO1 [124].
Punicalagin (PUN) is a polyphenol isolated from pomegranate and displays a variety of pharmacological activities, of which antioxidant and anti-inflammatory are the most important [125,126]. It seems that antioxidant activity of PUN is mediated via its impact on Nrf2 signaling pathway. In this way, PUN induces Nrf2/HO-1 axis in protecting DOX-mediated cardiotoxicity. The protective impacts of PUN are abolished via Nrf2 down-regulation. By Nrf2 activation, PUN not only reduces oxidative stress parameters, but also prevents loss of mitochondrial membrane potential, cytochrome C release and apoptosis induction [127]. Experiments discussed in this section demonstrate that phytochemicals can effectively induce Nrf2 signaling in protecting against DOX-mediated toxicity.
Importantly, as natural compounds suffer from poor bioavailability, using nanoparticles for their delivery can promote their therapeutic effects and impact on Nrf2 signaling that are of importance for ameliorating DOX-mediated toxicity. Future studies will shed some light on this aspect.
6. Nrf2 Modulation
MiRNAs are upstream mediators of a variety of molecular pathways due to their role in coordinating detailed biological mechanisms [128,129,130,131,132,133,134,135]. As occurs in different pathological events, miRNA dysregulation leads to alterations in normal cellular events [136,137]. Increasing evidence demonstrates that Nrf2 signaling is under surveillance of miRNAs in different pathological events for regulating response of cells to oxidative stress [138,139]. It is noteworthy that miRNA and Nrf2 interaction is of importance in DOX toxicity. It has been reported that miRNA-140-5p binds to 3/-untranslated region (3/-UTR) to reduce its expression. This leads to a reduction in activity of antioxidant enzymes such as HO-1, NQO1, and GCLM that subsequently, deteriorates DOX-mediated cardiotoxicity [140]. MiRNA-200a is another non-coding RNA that regulates Nrf2 signaling. It appears that miRNA-200a ameliorates diabetes endothelial dysfunction via reducing Keap1 expression and subsequent induction of Nrf2 signaling [141]. Furthermore, miRNA-200a inhibits apoptosis and inflammation in cardiomyocytes by regulating Keap1/Nrf2 signaling in favor of cell protection [142]. In mice exposed to DOX, miRNA-200a enhances Nrf2 expression to improve contractile function, and prevent apoptosis and oxidative stress [143].
The involvement of Nrf2 signaling in organ protection is confirmed by the study of Li and colleagues showing that Nrf2 silencing deteriorates DOX-mediated toxicity [144]. This experiment demonstrated a novel pathway in which Nrf2 follows to alleviate DOX-mediated cardiotoxicity. In this way, Nrf2 affects a mechanism known as autophagy. Primarily, autophagy is considered as a “self-digestion” mechanism that degrades toxic and aged organelles and macromolecules [145,146]. It has been reported that there is a close association between autophagy and oxidative stress in cells, so that autophagy activation can ameliorate oxidative stress [147]. This relationship is of importance in relieving oxidative damage in cells. For instance, autophagy stimulation can improve integrity of intestinal barrier against ROS and oxidative damage [148]. In reducing DOX-mediated cardiac dysfunction, Nrf2 activation can promote levels of light chain-3II (LC-3II) to induce autophagy, leading to amelioration of DOX-mediated cardiotoxicity [144]. Following Nrf2 degradation and inhibition, oxidative stress increases and apoptosis markers such as caspase-3 and caspase-9 undergo up-regulation that mediate toxic effects of DOX on organs of body [149]. An interesting point should be noted that autophagy has also interactions with apoptosis [150,151,152]. In respect to effect of Nrf2 on autophagy and also, the interaction between autophagy and apoptosis, further studies can evaluate autophagy induction by Nrf2, and its impact on incidence of apoptosis in cells exposed to DOX.
Orosomucoid 1 (ORM1) was first discovered by Tokita and Schmid in a century ago and is an acute phase protein synthesized in liver [153,154]. ORM1 has a variety of functions in cells including modulating immune system, preserving capillary barrier function, and reducing ROS levels [155,156,157]. Due to its impact of oxidative stress and ROS levels, ORM1 may be capable of regulating Nrf2 signaling. It has been reported that ORM1 overexpression is associated with activation on Nrf2 signaling and its downstream target HO-1, leading to a reduction in oxidative stress and apoptosis (caspase-3 down-regulation) that are of importance in ameliorating DOX-mediated cardiotoxicity [158].
A 3-dimensional model (3D) of cardiac system demonstrates that Nrf2 activation is a positive factor in protecting cells against DOX-mediated cardiotoxicity, further confirming role of Nrf2 in cardioprotection [159]. One of the emerging upstream mediators of Nrf2 signaling is GSK-3β that is a serine/threonine kinase with ubiquitous expression [160,161]. GSK-3β is an inhibitor of Nrf2 signaling and is correlated with development of a variety of pathological events including diabetes [162], aging [163], liver disease [164] and neurological disorders [165,166,167]. There is a reverse relationship between Nrf2 signaling and GSK-3β in cells exposed to DOX, so that GSK-3β down-regulation provides condition for Nrf2 activation and reducing DOX-mediated toxicity [168]. It is worth mentioning that chronic exposure to DOX is associated with Nrf2 inhibition that further aggravates organ toxicity [169]. Therefore, a wide variety of signaling networks, both inhibitor and inducer of Nrf2 signaling are involved in regulated DOX-mediated toxicity on organs and understanding their interactions can provide a new insight for designing novel therapeutics (Table 1, Figure 2).
Table 1.
Nrf2 signaling as a chemoprotection mechanism.
| Toxicity | Signaling Network | Compound | Nrf2 Expression | Outcomes | Refs |
|---|---|---|---|---|---|
| Cardiotoxicity | MiRNA-140-5p/Nrf2 | – | Down-regulation | Deteriorating DOX-mediated cardiotoxicity Reducing expressions of NQO1 and HO-1 Enhancing oxidative stress level |
[140] |
|
Cardiotoxicity
Hepatotoxicity Renotoxicity |
– | Asiatic acid | Up-regulation | Reducing necrosis, congestion and hyaline degeneration in heart Decreasing leukocyte inflammation, necrosis, apoptosis and fatty change in liver Decreasing necrosis and inflammation in kidney Mediating these protective effects via Nrf2 induction |
[98] |
| Cardiotoxicity | Nrf2/HO-1 Nrf2/NQO1 Nrf2/GCL |
Pristimerin | Up-regulation | Increasing expressions of Nrf2 and its downstream targets HO-1, NQO1 and GCL Reducing oxidative stress and fibrosis |
[91] |
| Cardiotoxicity | Nrf2/HO-1 Nrf2/NQO1 |
Tert-butylhydroquinone | Up-regulation | Ameliorating cardiotoxicity via induction of Nrf2 and its downstream targets | [113] |
| Cardiotoxicity | Nrf2/HO-1 | b-LAPachone | Up-regulation | Triggering nuclear translocation of Nrf2 Enhancing expressions of HO-1 and antioxidant enzymes such as SOD, CAT and GPx |
[101] |
| Cardiotoxicity | Nrf2/HO-1 Nrf2/NQO1 |
Cardamonin | Up-regulation | Protecting cells against inflammation and oxidative stress Reducing oxidative stress, apoptosis, and inflammation Inducing Nrf2 signaling and its downstream targets HO-1 and NQO1 |
[105] |
| Cardiotoxicity | Nrf2/HO-1 | Curdione | Up-regulation | Alleviating oxidative stress Preventing ROS overgeneration and mediating mitochondrial dysfunction Triggering Nrf2/HO-1 axis as an antioxidant axis |
[102] |
| Cardiotoxicity | MAPK/Nrf2/ARE | Chitosan oligosaccharide | Up-regulation | Decreasing oxidative stress and apoptosis Stimulating MAPK and subsequent induction of Nrf2/ARE axis Reinforcing antioxidant defense system |
[112] |
| Cardiotoxicity | MiRNA-200a/Nrf2 | – | Up-regulation | Improving cardiomyocyte contractile function Reducing levels of cardiac troponin I Ameliorating oxidative stress, inflammation and apoptosis Inducing Nrf2 signaling |
[143] |
| Cardiotoxicity | Nrf2/ARE | 3,3′-diindolylmethane | Up-regulation | Suppressing apoptosis Improving histopathological profile Enhancing expressions of HO-1, NQO1 and GST Reducing Bax and caspase-3 expression |
[114] |
| Cardiotoxicity | Sirt1/AMPK/Nrf2 | Acacetin | Up-regulation | Alleviation of cardiomyopathy Enhancing cell viability Preventing ROS overgeneration Activation of Sirt1/AMPK to induce Nrf2 signaling Triggering cell defense system |
[170] |
| Cardiotoxicity | Nrf2/HO-1 | Genistein | Up-regulation | Inducing Nrf2/HO-1 axis Reducing ROS levels by its scavenging feature Reducing lipid peroxidation and DNA damage |
[171] |
| Cardiotoxicity | Nrf2/LC-3II/autophagy | – | Down-regulation | Reducing oxidative stress Activating autophagy as a protective mechanism via LC-3II up-regulation Nrf2 inhibition aggravates DOX-mediated cardiotoxicity via impairing autophagy and enhancing oxidative stress |
[144] |
| Cardiotoxicity | – | p-coumaric acid | Up-regulation | Enhancing cell survival Inhibiting apoptosis and oxidative stress Providing nuclear translocation of Nrf2 |
[118] |
| Cardiotoxicity | Nrf2/NQO1 | Tanshinone IIA | Up-regulation | Enhancing cell viability and morphological profile Reducing oxidative parameters Up-regulation of NQO1 |
[124] |
| Cardiotoxicity | ORM1/Nrf2 | – | Up-regulation | ORM1 is correlated with a decrease in oxidative stress and apoptosis Up-regulation of Nrf2 and its downstream target HO-1 |
[158] |
| Testicular toxicity | – | – | Down-regulation | Inducing apoptosis and oxidative stress in testis Reducing Nrf2 expression |
[149] |
| Nephrotoxicity | – | Thymoquinone | Up-regulation | Reducing malondialdehyde and lipid peroxidation levels Enhancing SOD and GST levels Preventing necrosis and oxidative stressActivation of Nrf2 and improving antioxidant defense system |
[119] |
Figure 2.
Targeting Nrf2 signaling pathway in chemoprotection. Regulation of Nrf2 signaling by upstream mediators and protective compounds in decreasing adverse effects of doxorubicin. Apoptosis, mitochondrial dysfunction, necrosis and cell death are prevented upon Nrf2 activation.
7. Nrf2 in Doxorubicin Resistance
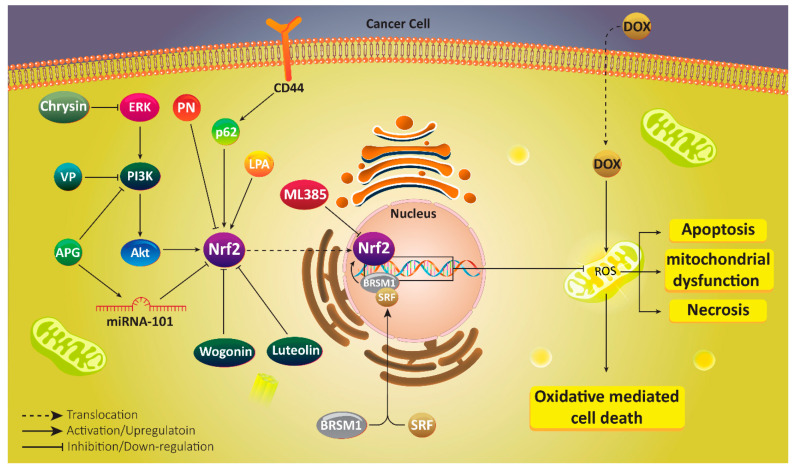
Due to the potential role of Nrf2 signaling in triggering DOX resistance, much attention has been directed towards targeting this pathway in reversing chemoresistance. For this purpose, Singh and colleagues have designed a small molecule inhibitor of Nrf2 signaling, known as ML385 that binds to Neh1 domain of Nrf2 and inhibits its DNA binding. This leads to an increase in anti-tumor activity of DOX against lung cancer cells [172]. Therefore, first strategy can be considered developing a novel inhibitor capable of binding to Nrf2 domain and suppressing its activity and nuclear translocation. The second strategy that has not been tried yet, but can be considered in next experiments, is designing molecules capable of binding to Keap1 and promoting its activity in inhibiting Nrf2. Furthermore, natural products can be utilized in targeting Nrf2 signaling for providing DOX sensitivity. Parthenolide (PN) is a sesquiterpene lactone exclusively found in Tanacetum parthenium and is famous due to its inhibitory effect on cancer progression [173,174,175]. In DOX-resistance breast cancer cells, Nrf2 undergoes up-regulation that mediates increased levels of P-glycoprotein (P-gp), Bcl-2, CAT, SOD and heat shock protein 70 (HSP70). PN administration along with DOX inhibits Nrf2 signaling and its downstream targets to promote ROS generation, leading to reversing DOX resistance [176].
Chrysin is a flavonoid compound that has demonstrated anti-tumor activity against different cancers via regulating molecular pathways. Increasing evidence exhibits that chrysin suppresses cancer progression and proliferation and stimulates apoptosis via down-regulating phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) axis [177,178]. Furthermore, chrysin diminishes expression level of extracellular-signal regulated kinase (ERK) in disrupting cancer progression [179,180]. In enhancing DOX sensitivity of cancer cells, chrysin affects two distinct pathways including PI3K/Akt/Nrf2 and ERK/Nrf2. In this way, chrysin reduces expression levels of ERK and PI3K/Akt to suppress Nrf2 signaling, leading to enhanced DOX sensitivity [181]. What is noteworthy is that it seems that PI3K/Nrf2 signaling has association with activity and expression of drug resistance proteins that has been evaluated. Vialenin P (VP) can suppress PI3K/Nrf2 signaling to down-regulate multidrug resistance protein 1 (MRP1), leading to enhanced accumulation of DOX in breast cancer cells, and sensitizing them to chemotherapy [182].
Apigenin (APG) is a natural bioflavonoid present in fruits and vegetables and has anti-tumor activity against different cancers. APG administration is of importance in suppressing cisplatin resistance of ovarian cancer cells via apoptosis induction and Mcl-1 down-regulation [183]. In increasing DOX cytotoxicity, APG inhibits DNA repair of breast cancer cells [184]. Furthermore, co-administration of APG with other anti-tumor agents such as sorafenib increases its efficacy in triggering apoptosis and cell cycle arrest in cancer cells [185]. In DOX-resistant hepatocellular carcinoma cells, APG enhances expression of miRNA-101 as a tumor-suppressing factor. Then, up-regulated miRNA-101 reduces Nrf2 expression to promote DOX sensitivity of cancer cells [186].
In addition to miRNAs, PI3K/Akt signaling pathway is affected by APG in suppressing DOX resistance. As it was mentioned, PI3K/Akt induction is in favor of cancer progression and its inhibition can be considered as a promising strategy in cancer therapy [187,188]. It seems that APG down-regulates PI3K/Akt signaling to inhibit Nrf2, leading to increased sensitivity to DOX chemotherapy [189]. These studies demonstrate that how molecular pathways such as Nrf2 with its main role in providing redox balance, can participate in DOX resistance [190]. Consequently, targeting Nrf2, and its upstream and downstream mediators can be considered as ideal strategies in cancer therapy and reversing DOX resistance [191].
Wogonin is a bioactive flavonoid isolated from Scutellaria baicalensis with capability of suppressing chemoresistance via down-regulating expression and activity of P-gp [192]. Wogonin functions as an enhancer of ROS levels in inducing cell proliferation inhibition [193]. Wogonin can enhance efficacy of immune system in cancer eradication and promote macrophage M1 polarization [194]. In DOX-resistant breast cancer cells, wogonin suppresses defense system mediated via Nrf2 inhibition and decreasing expressions of HO-1 and NQO1 [195]. Another experiment investigates potential of plant-derived extracts in increasing sensitivity of lung cancer cells to DOX chemotherapy. It has been reported that cinnamomic cortex extract provides Nrf2 down-regulation in promoting DOX sensitivity [196].
Similar to wogonin, luteolin also belongs to flavonoid family. Luteolin acts as a potent anti-cancer agent [197] in suppressing cancer migration and invasion via down-regulating epithelial-to-mesenchymal transition (EMT) and focal adhesion kinase [198,199]. Cancer progression occurs in hypoxic conditions, and luteolin administration is of importance in reducing HIF-1α expression and disrupting hypoxia-mediated cancer progression. Furthermore, luteolin induces apoptosis and autophagy in breast and colon cancer cells [200]. These studies advocate the fact that luteolin administration negatively affects cancer progression, and this agent is advantageous in reversing DOX resistance. In this way, luteolin reduces Nrf2 expression at mRNA level by 34% and is involved in regaining sensitivity of lung cancer cells to DOX chemotherapy [201]. Luteolin can also enhance DOX sensitivity of breast cancer cells. Luteolin dually inhibits Nrf2/HO-1 and Nrf2/MDR1 signaling pathways to remove defense system mechanism in enhancing DOX sensitivity [202].
Consequently, using anti-tumor compounds, as most of them are phytochemicals, is of importance in reversing DOX resistance via Nrf2 down-regulation [203]. However, it should be noted that anti-tumor agents, especially naturally occurring compounds suffer from poor bioavailability [204,205], and using carriers such as nanostructures for their delivery can remarkably promote their potential in down-regulating Nrf2 signaling and enhancing DOX sensitivity of cancer cells.
8. Nrf2, Upstream and Downstream Targets
In the previous section, we demonstrated that both synthesized and natural compounds can be of importance in suppressing DOX resistance via regulating Nrf2 and its downstream targets. In this section, a mechanistic discussion of signaling networks in which Nrf2 is key player and lead to DOX resistance, is provided to provide insights for developing novel therapeutics.
One of the important aspects of Nrf2 signaling is its association with drug transporters. Nrf2 can promote expression of P-gp transporter in enhancing colorectal cancer progression and triggering chemoresistance [206]. Such a relationship is found between Nrf2 and ABCB1 in DOX resistant. In hypoxic conditions, liver cancer cells increase Nrf2 expression to up-regulate activity and expression of ABCB1. Then, intracellular accumulation of DOX decreases in cancer cells that provides their resistance to apoptosis [207]. It has been reported that Nrf2 down-regulation is associated with P-gp inhibition and triggering DOX sensitivity of cancer cells [208]. Targeting Nrf2 is of importance for increasing sensitivity of cancer cells to DOX chemotherapy.
Small interfering RNA (siRNA) is a powerful genetic tool that is extensively applied in targeting molecular pathways and genes responsible for cancer progression. Recent studies have shown that siRNA can be utilized for increasing sensitivity of cancer cells to chemotherapeutic agents such as cisplatin, paclitaxel, docetaxel and so on [209,210,211]. Similarly, siRNA can be used for mediating DOX sensitivity via targeting Nrf2. Down-regulating Nrf2 expression at mRNA and protein levels is performed by siRNA, and its downstream targets such as HO-1 and NQO1 undergo down-regulation, resulting in ROS overgeneration and enhanced sensitivity to DOX chemotherapy [212]. The interesting point is that in vitro and in vivo experiments have confirmed that Nrf2 overexpression is associated with cancer proliferation, survival and chemoresistance. Abrogation of Nrf2 expression results in an increase in DOX sensitivity via enhancing ROS levels and triggering cancer cell death [213].
Cluster of differentiation 44 (CD44) is a glycoprotein and a receptor for extracellular matrix (ECM) components such as hyaluronic acid (HA). This cell-surface glycoprotein is a cancer stem cell (CSC) marker and can undergo alternative splicing and post-transcriptional modification. CD44 overexpression is an obvious finding in cancer cells and mediates their malignancy [214,215]. It has been reported that CD44 can trigger drug resistance of breast cancer stem cells. CD44 enhances p62 expression to induce Nrf2 and DOX resistance [216]. This is maybe due to increased malignancy of cancer cells, so that Keap1 down-regulation and subsequent Nrf2 induction provide conditions for cancer growth [217]. CSCs that are resistant to DOX chemotherapy, demonstrate simultaneous up-regulation of Nrf2 and ABCB1 [218]. As it was mentioned earlier, Nrf2 stimulates DOX resistance via enhancing activity and expression of drug transporters. Therefore, up-regulation of Nrf2 and ABCB1 in CSCs may have associations that should be considered in further experiments.
MRTF-A is a co-activator of serum response factor (SRF) that functions as a tumor-promoting factor in increasing proliferation, and metastasis as well as triggering drug resistance [219]. MRTF-A can cooperate with signal transducer and activator of transcription 3 (STAT3) in inducing BRSM1 hypermethylation and increasing breast cancer invasion [220]. In mediating DOX resistance, MRTF-A generates a complex containing SRF attached to CarG on promoter region of Nrf2 to stimulate its expression and reduce sensitivity of cancer cells to apoptosis [221].
Sometimes, interaction between enzymes and their product can direct conditions towards developing chemoresistance. Such association is obvious in lysophosphatidate (LPA) that is generated by autotaxin (ATX). Primarily, LPA is involved in repairing tissues by inducing proliferation, migration, angiogenesis and other important biological mechanisms. These impacts are of importance in improving pathological conditions such as arthritis, pulmonary fibrosis and inflammatory bowel disease [222,223,224,225,226]. However, it has been reported that LPA and ATX can induce cancer progression and LPA up-regulation is correlated with development of colon cancer and hepatitis [227,228,229]. In DOX-resistant breast cancer cells, LPA enhances stabilization of Nrf2 to up-regulate its expression, resulting in activation of antioxidant parameters and drug transporters that are vital for inducing DOX resistance [230]. Table 2 provides a summary of molecular pathways involved in DOX resistance, and anti-tumor agents capable of regulating Nrf2 signaling in suppressing DOX resistance (Table 2, Figure 3).
Table 2.
Activation/suppression of Nrf2 signaling and its association with DOX resistance/sensitivity.
| Cancer Type | Signaling Network | Compound/Agent | Nrf2 Expression | Remarks | Refs |
|---|---|---|---|---|---|
| Breast cancer | P62/Nrf2 | – | Up-regulation | Reducing oxidative stress Mediating DOX resistance Promoting colony formation and migration capacities Improving cancer stem cell features |
[216] |
| Breast cancer | Cul3/Nrf2 | – | Down-regulation | Association of Cul3 with Nrf2 depletion Inducing oxidative stress Increasing DOX sensitivity |
[231] |
| Breast cancer | PI3K/Nrf2/MRP1 | Vielanin P | Down-regulation | Inhibiting PI3K/Nrf2 axis Suppressing MRP1 expression Promoting DOX sensitivity |
[182] |
| Breast cancer | Nrf2/HSP70 | Parthenolide | Down-regulation | Reducing expressions of Nrf2 and HSP70 Enhancing DOX sensitivity of breast cancer cells |
[176] |
| Breast cancer | Nrf2/HO-1 Nrf2/MDR1 |
Luteolin | Down-regulation | Enhancing number of cancer cells undergoing cell death Increasing cytotoxicity of DOX Down-regulation of Nrf2 and subsequent inhibition of its downstream targets HO-1 and MDR1 |
[202] |
| Breast cancer | Nrf2/HO-1 Nrf2/NQO1 |
Wogonin | Down-regulation | Impairing cellular defense systemNrf2 signaling inhibition Down-regulation of HO-1 and NQO1 Increasing DOX cytotoxicity towards cancer cells |
[195] |
| Breast cancer | HER2/Nrf2 | – | Up-regulation | Conferring drug resistance Enhancing activities of antioxidant enzymes such as GSTA2, GSTP1 and HO-1 |
[232] |
| Breast cancer | Nrf2/p62 | Pseudomonas aeruginosa mannose-sensitive hemagglutinin | Down-regulation | Inhibiting Nrf2 signaling and its downstream target p62 Increasing DOX sensitivity Impairing cancer growth |
[190] |
| Hepatocellular carcinoma | MiRNA-101/Nrf2 | Apigenin | Down-regulation | Enhancing miRNA-101 expression Inhibiting Nrf2 signaling by binding to 3/-UTR Enhancing DOX sensitivity |
[186] |
| Hepatocellular carcinoma | PI3K/Akt/Nrf2 | Apigenin | Down-regulation | Reducing mRNA and protein levels of Nrf2 via PI3K/Akt inhibition Reducing cell proliferation Inducing apoptosis Promoting DOX sensitivity |
[189] |
| Liver cancer | Nrf2/ABCB1 | – | Up-regulation | Nrf2 overexpression occurs in hypoxic conditions Reducing apoptosis and DNA damage Inducing DOX resistance ABCB1 up-regulation |
[207] |
| Different cancers | MRTF-A/Nrf2 | – | Up-regulation | Reducing apoptosis Triggering DOX resistance |
[221] |
| Different cancers | – | – | Down-regulation | SiRNA is a powerful in Nrf2 down-regulation Inhibiting activities of ABCC3, ABCC4 and ABCG2 Enhancing DOX sensitivity |
[212] |
| Different cancers | PI3K/Akt/Nrf2 ERK/Nrf2 |
Chrysin | Down-regulation | Suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 signaling pathwaysNrf2 down-regulation and inhibiting its downstream targets HO-1 Enhancing DOX sensitivity |
[181] |
| Ovarian cancer | ALDH/Nrf2 | All-trans retinoic acid | Down-regulation | Promoting cancer stem features Enhancing colony formation capacity Mediating DOX resistance Suppressing ALDH/Nrf2 signaling by retinoic acid in reducing DOX resistance |
[191] |
| Ovarian cancer | – | – | Up-regulation | Overexpression of Nrf2 in DOX-resistant cancer cells Reducing tumor growth following Nrf2 down-regulation |
[233] |
| Ovarian cancer | – | – | Up-regulation | Obtaining DOX resistance via Nrf2 signaling and reducing cell death | [234] |
| Ovarian cancer | Nrf2/miRNA-206/c-MET/EGFR | – | Up-regulation | Reducing miRNA-206 expression Inducing expressions of c-MET and EGFR expressions Triggering DOX resistance |
[235] |
| Colorectal cancer | Nrf2/P-gp | – | Up-regulation | Enhancing P-gp expressions Reducing cell death Inducing DOX resistance |
[208] |
| Lung cancer | – | ML385 | Down-regulation | ML385 functions as an inhibitor of Nrf2 signaling Promoting DOX sensitivity |
[172] |
| Myeloid leukemia | Nrf2/HO-1 Nrf2/NQO1 |
Tritolide | Down-regulation | Enhancing drug sensitivity Apoptosis induction Suppressing Nrf2 and its downstream targets |
[203] |
Figure 3.
Nrf2 signaling in mediating DOX resistance of cancer cells. Suppressing Nrf2 signaling as a pro-survival pathway is associated with induction of apoptosis and necrosis in cancer cells, and their sensitivity to chemotherapy.
9. Room for Drug Discovery
In treatment of cancer patients, DOX is considered as a first option and is mostly preferred to surgery, as an invasive strategy. However, resistance to this well-known chemotherapeutic agent has resulted in failure in treatment of cancer patients. Nrf2 is involved in DOX resistance, and after chemotherapy, compounds activating Nrf2 signaling can be applied. As it was mentioned earlier in the main text, most of the anti-tumor compounds for providing DOX sensitivity are phytochemicals. For synthesizing new small molecule inhibitors of Nrf2 signaling, much attention should be directed towards Nrf2 and Keap1 structures. Furthermore, new chemically synthesized anti-tumor agents can also inhibit nuclear translocation of Nrf2 by binding to it and providing ubiquitination and degradation. After DOX chemotherapy, the story is completely different and if a protective agent wants to be synthesized, it should be capable of binding to Nrf2 and mediating its nuclear translocation or suppressing Keap1 activity.
10. Conclusions and Remarks
In the present review, two important aspects of Nrf2 signaling including chemoprotection and chemoresistance were discussed in view of DOX. Each section was divided into two parts describing involved molecular pathways and role of anti-tumor and protective compounds in targeting Nrf2 signaling during DOX chemotherapy. It is noteworthy that most of the compounds targeting Nrf2 signaling are phytochemicals. In the case of protecting against adverse effects of DOX, protective compounds induce Nrf2 signaling and its downstream targets such as HO-1 and NQO1 in reinforcing antioxidant defense systems and supporting against oxidative damage, while anti-tumor compounds inhibit Nrf2 signaling in promoting ROS levels and oxidative damage, resulting in cell death in cancer cells.
These statements clearly demonstrate the dual role of Nrf2 signaling in cancer chemotherapy. In fact, the aim is determining factor for stimulating or suppressing Nrf2 signaling. The notion should be considered that chemoprotection should be performed after DOX chemotherapy, since inducing Nrf2 signaling is associated with DOX resistance. Therefore, Nrf2 inhibition should be conducted during DOX chemotherapy and Nrf2 induction after this period to prevent or ameliorate its side effects on major organs of the body. The interesting point is that Nrf2 signaling can promote stem cell population in providing DOX resistance. Hence, by targeting Nrf2 signaling, both cancer cells and CSCs are affected that are of importance in effective DOX chemotherapy.
To date, most of the studies have focused on using compounds for targeting Nrf2 signaling in chemoprotection and reversing chemoresistance. However, more progress can be performed using nanoparticles for delivery of these anti-tumor agents. Nanocarriers can significantly promote intracellular accumulation of anti-tumor agents in cancer cells and enhance their efficiency in Nrf2 inhibition and providing DOX sensitivity. Furthermore, the ability of compounds to protect during DOX chemotherapy can be improved using nanocarriers. Another important aspect is using genetic tools in targeting Nrf2 signaling. As it was mentioned, siRNA system has been applied for affecting Nrf2 signaling. Other techniques such as CRISPR/Cas9 system can also be used, and furthermore, more studies are needed to elucidate potential of siRNA for using in DOX sensitivity. Similar to compounds, nanoarchitectures can promote efficiency of genetic tools in gene silencing that should be considered in future experiments.
Abbreviations
| DOX | Doxorubicin |
| ROS | reactive oxygen species |
| TFAM | mitochondrial transcription factor A |
| AMPK | AMP-activated protein kinase |
| VASH2 | vasohibin2 |
| SpK1 | sphingosine kinase 1 |
| HIF-1α | hypoxia inducible factor-1α |
| ncRNAs | non-coding RNAs |
| lncRNAs | long non-coding RNAs |
| miRNAs | microRNAs |
| GI | gastrointestinal |
| MMP-2 | matrix metalloproteinase-2 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| Keap1 | kelch-like ECH-associated protein 1 |
| Cul3 | cullin3 |
| GSK-3β | glycogen synthase-kinase 3β |
| βTrCP | β transducing repeat containing E3 ubiquitin-protein ligase |
| HO-1 | heme oxygenase-1 |
| NQO1 | NAD(p)H dehydrogenase quinone 1 |
| ARE | antioxidant response elemento |
| PPARγ | peroxisome proliferator-activated receptor gama |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| CAT | catálase |
| GSH | glutathione |
| Pris | pristimerin |
| AA | Asiatic acid |
| B-LAP | β-LAPachone |
| CAR | cardamonin |
| COS | chitosan oligosaccharide |
| pCA | p-coumaric acid |
| LPS | lipopolysaccharide |
| Tan IIA | tanshinone IIA |
| PUN | punicalagin |
| 3/-UTR | 3/-untranslated region |
| LC-3II | light chain-3II |
| ORM1 | orosomucoid 1 |
| 3D | 3-dimensional |
| PN | parthenolide |
| P-gp | P-glycoprotein |
| HSP70 | heat shock protein 70 |
| PI3K | phosphatidylinositide 3-kinase |
| Akt | protein kinase-B |
| ERK | extracellular signal-regulated kinase |
| VP | vialenin P |
| MRP1 | multidrug resistance protein 1 |
| APG | apigenin |
| EMT | epithelial-to-mesenchymal transition |
| siRNA | small interfering RNA |
| CD44 | cluster of differentiation 44 |
| ECM | extracellular matrix |
| CSC | cancer stem cell |
| SRF | serum response factor |
| STAT3 | signal transducer and activator of transcription 3 |
| LPA | lisophosphatidate |
| ATX | autotaxin |
Author Contributions
M.A., H.K. and A.P.K. conceptualized the review, collate articles and performed critical revision of the manuscript. S.M., A.Z. (Amirhossein Zabolian), F.H., H.S., N.A., S.H., M.V.F. and K.H. contributed to preparing draft manuscript. A.Z. (Ali Zarrabi) performed software works. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Singapore Ministry of Education [T2EP30120-0042], the National Research Foundation Singapore and the Singapore Ministry of Education under its Research Centre’s of Excellence initiative to Cancer Science Institute of Singapore, National University of Singapore to A.P.K.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prathumsap N., Shinlapawittayatorn K., Chattipakorn S.C., Chattipakorn N. Effects of doxorubicin on the heart: From molecular mechanisms to intervention strategies. Eur. J. Pharmacol. 2020;866:172818. doi: 10.1016/j.ejphar.2019.172818. [DOI] [PubMed] [Google Scholar]
- 2.Denard B., Lee C., Ye J. Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. elife. 2012;1:e00090. doi: 10.7554/eLife.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu M., Xie K., Lu X., Lu L., Shi Y., Tang Y. Notoginsenoside R1 counteracts mesenchymal stem cell-evoked oncogenesis and doxorubicin resistance in osteosarcoma cells by blocking IL-6 secretion-induced JAK2/STAT3 signaling. Investig. New Drugs. 2020 doi: 10.1007/s10637-020-01027-9. [DOI] [PubMed] [Google Scholar]
- 4.Ghandhariyoun N., Jaafari M.R., Nikoofal-Sahlabadi S., Taghdisi S.M., Moosavian S.A. Reducing Doxorubicin resistance in breast cancer by liposomal FOXM1 aptamer: In vitro and in vivo. Life Sci. 2020;262:118520. doi: 10.1016/j.lfs.2020.118520. [DOI] [PubMed] [Google Scholar]
- 5.Ashrafizadeh M., Zarrabi A., Hashemi F., Zabolian A., Saleki H., Bagherian M., Azami N., Bejandi A.K., Hushmandi K., Ang H.L., et al. Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms: Enhancing Antitumor Activity. Pharmaceutics. 2020;12:1084. doi: 10.3390/pharmaceutics12111084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poh H.M., Chiou Y.S., Chong Q.Y., Chen R.M., Rangappa K.S., Ma L., Zhu T., Kumar A.P., Pandey V., Lee S.C., et al. Inhibition of TFF3 Enhances Sensitivity-and Overcomes Acquired Resistance-to Doxorubicin in Estrogen Receptor-Positive Mammary Carcinoma. Cancers. 2019;11:1528. doi: 10.3390/cancers11101528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L., Li D., Tang P., Zuo Y. Curcumin increases the sensitivity of K562/DOX cells to doxorubicin by targeting S100 calcium-binding protein A8 and P-glycoprotein. Oncol. Lett. 2020;19:83–92. doi: 10.3892/ol.2019.11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xinyong C., Zhiyi Z., Lang H., Peng Y., Xiaocheng W., Ping Z., Liang S. The role of toll-like receptors in myocardial toxicity induced by doxorubicin. Immunol. Lett. 2020;217:56–64. doi: 10.1016/j.imlet.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Wei T., Xiaojun X., Peilong C. Magnoflorine improves sensitivity to doxorubicin (DOX) of breast cancer cells via inducing apoptosis and autophagy through AKT/mTOR and p38 signaling pathways. Biomed. Pharmacother. 2020;121:109139. doi: 10.1016/j.biopha.2019.109139. [DOI] [PubMed] [Google Scholar]
- 10.Abd-Rabou A.A., Ahmed H.H., Shalby A.B. Selenium Overcomes Doxorubicin Resistance in Their Nano-platforms Against Breast and Colon Cancers. Biol. Trace Elem. Res. 2020;193:377–389. doi: 10.1007/s12011-019-01730-6. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y., Xu J., Hu W., Wang F., Zhou Y., Xu W., Gong W., Shao L. TFAM depletion overcomes hepatocellular carcinoma resistance to doxorubicin and sorafenib through AMPK activation and mitochondrial dysfunction. Gene. 2020;753:144807. doi: 10.1016/j.gene.2020.144807. [DOI] [PubMed] [Google Scholar]
- 12.Tan X., Liao Z., Zou S., Ma L., Wang A. VASH2 Promotes Cell Proliferation and Resistance to Doxorubicin in Non-Small Cell Lung Cancer via AKT Signaling. Oncol. Res. 2020;28:3–11. doi: 10.3727/096504019X15509383469698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka I., Chakraborty A., Saulnier O., Benoit-Pilven C., Vacher S., Labiod D., Lam E.W.F., Bièche I., Delattre O., Pouzoulet F., et al. ZRANB2 and SYF2-mediated splicing programs converging on ECT2 are involved in breast cancer cell resistance to doxorubicin. Nucleic Acids Res. 2020;48:2676–2693. doi: 10.1093/nar/gkz1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta A., Loo S.Y., Huang B., Wong L., Tan S.S., Tan T.Z., Lee S.C., Thiery J.P., Lim Y.C., Yong W.P., et al. SPHK1 regulates proliferation and survival responses in triple-negative breast cancer. Oncotarget. 2014;5:5920–5933. doi: 10.18632/oncotarget.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Z., Wang L.Z., Cheng J.T., Lam W.S.T., Ma X., Xiang X., Wong A.L., Goh B.C., Gong Q., Sethi G., et al. Targeting HIF-1-mediated Metastasis for Cancer Therapy. Antioxid. Redox Signal. 2020 doi: 10.1089/ars.2019.7935. [DOI] [PubMed] [Google Scholar]
- 16.Ren X., Su C. Sphingosine kinase 1 contributes to doxorubicin resistance and glycolysis in osteosarcoma. Mol. Med. Rep. 2020;22:2183–2190. doi: 10.3892/mmr.2020.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu H., Gu J., Zhou D., Cheng W., Wang Y., Wang Q., Wang X. LINC00160 mediated paclitaxel-And doxorubicin-resistance in breast cancer cells by regulating TFF3 via transcription factor C/EBPβ. J. Cell. Mol. Med. 2020;24:8589–8602. doi: 10.1111/jcmm.15487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B., Xu L., Zhang J., Cheng X., Xu Q., Wang J., Mao F. LncRNA NORAD accelerates the progression and doxorubicin resistance of neuroblastoma through up-regulating HDAC8 via sponging miR-144-3p. Biomed. Pharmacother. 2020;129:110268. doi: 10.1016/j.biopha.2020.110268. [DOI] [PubMed] [Google Scholar]
- 19.Ong M.S., Cai W., Yuan Y., Leong H.C., Tan T.Z., Mohammad A., You M.L., Arfuso F., Goh B.C., Warrier S., et al. ‘Lnc’-ing Wnt in female reproductive cancers: Therapeutic potential of long non-coding RNAs in Wnt signalling. Br. J. Pharmacol. 2017;174:4684–4700. doi: 10.1111/bph.13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra S., Verma S.S., Rai V., Awasthee N., Chava S., Hui K.M., Kumar A.P., Challagundla K.B., Sethi G., Gupta S.C. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell. Mol. Life Sci. 2019;76:1947–1966. doi: 10.1007/s00018-019-03053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashrafizadeh M., Zarrabi A., Hushmandi K., Kalantari M., Mohammadinejad R., Javaheri T., Sethi G. Association of the Epithelial-Mesenchymal Transition (EMT) with Cisplatin Resistance. Int. J. Mol. Sci. 2020;21:4002. doi: 10.3390/ijms21114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y., Li X., Lu Y., Chaurasiya B., Mi G., Shi D., Chen D., Webster T.J., Tu J., Shen Y. Co-delivery of Poria cocos extract and doxorubicin as an ‘all-in-one’ nanocarrier to combat breast cancer multidrug resistance during chemotherapy. Nanomedicine. 2020;23:102095. doi: 10.1016/j.nano.2019.102095. [DOI] [PubMed] [Google Scholar]
- 23.Pfister C., Gravis G., Fléchon A., Soulié M., Guy L., Laguerre B., Mottet N., Joly F., Allory Y., Harter V., et al. Randomized Phase III Trial of Dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients with Muscle-invasive Bladder Cancer. Analysis of the GETUG/AFU V05 VESPER Trial Secondary Endpoints: Chemotherapy Toxicity and Pathological Responses. Eur. Urol. 2020 doi: 10.1016/j.eururo.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Gadisa D.A., Assefa M., Wang S.H., Yimer G. Toxicity profile of Doxorubicin-Cyclophosphamide and Doxorubicin-Cyclophosphamide followed by Paclitaxel regimen and its associated factors among women with breast cancer in Ethiopia: A prospective cohort study. J. Oncol. Pharm. Pract. 2020;26:1912–1920. doi: 10.1177/1078155220907658. [DOI] [PubMed] [Google Scholar]
- 25.Harahap Y., Ardiningsih P., Corintias Winarti A., Purwanto D.J. Analysis of the Doxorubicin and Doxorubicinol in the Plasma of Breast Cancer Patients for Monitoring the Toxicity of Doxorubicin. Drug Des. Dev. Ther. 2020;14:3469–3475. doi: 10.2147/DDDT.S251144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X.Q., Liu Y.K., Yi J., Dong J.S., Zhang P.P., Wan L., Li K. MicroRNA-143 Increases Oxidative Stress and Myocardial Cell Apoptosis in a Mouse Model of Doxorubicin-Induced Cardiac Toxicity. Med. Sci. Monit. 2020;26:e920394. doi: 10.12659/MSM.920394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan B.Y.H., Roczkowsky A., Cho W.J., Poirier M., Sergi C., Keschrumrus V., Churko J.M., Granzier H., Schulz R. MMP inhibitors attenuate doxorubicin cardiotoxicity by preventing intracellular and extracellular matrix remodeling. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boratto F.A., Franco M.S., Barros A.L.B., Cassali G.D., Malachias A., Ferreira L.A.M., Leite E.A. Alpha-tocopheryl succinate improves encapsulation, pH-sensitivity, antitumor activity and reduces toxicity of doxorubicin-loaded liposomes. Eur. J. Pharm. Sci. 2020;144:105205. doi: 10.1016/j.ejps.2019.105205. [DOI] [PubMed] [Google Scholar]
- 29.Khan T.H., Ganaie M.A., Alharthy K.M., Madkhali H., Jan B.L., Sheikh I.A. Naringenin prevents doxorubicin-induced toxicity in kidney tissues by regulating the oxidative and inflammatory insult in Wistar rats. Arch. Physiol. Biochem. 2020;126:300–307. doi: 10.1080/13813455.2018.1529799. [DOI] [PubMed] [Google Scholar]
- 30.Oh J., Lee B.S., Lim G., Lim H., Lee C.J., Park S., Lee S.H., Chung J.H., Kang S.M. Atorvastatin protects cardiomyocyte from doxorubicin toxicity by modulating survivin expression through FOXO1 inhibition. J. Mol. Cell. Cardiol. 2020;138:244–255. doi: 10.1016/j.yjmcc.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Uruno A., Matsumaru D., Ryoke R., Saito R., Kadoguchi S., Saigusa D., Saito T., Saido T.C., Kawashima R., Yamamoto M. Nrf2 Suppresses Oxidative Stress and Inflammation in App Knock-In Alzheimer’s Disease Model Mice. Mol. Cell. Biol. 2020;40 doi: 10.1128/MCB.00467-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y., Wang K., Zhou Y., Li T., Yang M., Wang R., Chen Y., Cao M., Hu R. HEATR1 deficiency promotes pancreatic cancer proliferation and gemcitabine resistance by up-regulating Nrf2 signaling. Redox Biol. 2020;29:101390. doi: 10.1016/j.redox.2019.101390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassanein E.H., Sayed A.M., Hussein O.E., Mahmoud A.M. Coumarins as Modulators of the Keap1/Nrf2/ARE Signaling Pathway. Oxidative Med. Cell. Longev. 2020;2020:1675957. doi: 10.1155/2020/1675957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosa P.L., Bertini E.S., Piemonte F. The NRF2 Signaling Network Defines Clinical Biomarkers and Therapeutic Opportunity in Friedreich’s Ataxia. Int. J. Mol. Sci. 2020;21:916. doi: 10.3390/ijms21030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohandel Z., Farkhondeh T., Aschner M., Samarghandian S. Nrf2 a molecular therapeutic target for Astaxanthin. Biomed. Pharmacother. 2021;137:111374. doi: 10.1016/j.biopha.2021.111374. [DOI] [PubMed] [Google Scholar]
- 36.Uddin M.S., Al Mamun A., Jakaria M., Thangapandiyan S., Ahmad J., Rahman M.A., Mathew B., Abdel-Daim M.M., Aleya L. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci. Total Environ. 2020;707:135624. doi: 10.1016/j.scitotenv.2019.135624. [DOI] [PubMed] [Google Scholar]
- 37.Raghunath A., Sundarraj K., Arfuso F., Sethi G., Perumal E. Dysregulation of Nrf2 in Hepatocellular Carcinoma: Role in Cancer Progression and Chemoresistance. Cancers. 2018;10:481. doi: 10.3390/cancers10120481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirtonia A., Sethi G., Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell. Mol. Life Sci. 2020;77:4459–4483. doi: 10.1007/s00018-020-03536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anandhan A., Dodson M., Schmidlin C.J., Liu P., Zhang D.D. Breakdown of an Ironclad Defense System: The Critical Role of NRF2 in Mediating Ferroptosis. Cell. Chem. Biol. 2020;27:436–447. doi: 10.1016/j.chembiol.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taguchi K., Yamamoto M. The KEAP1-NRF2 System as a Molecular Target of Cancer Treatment. Cancers. 2020;13:46. doi: 10.3390/cancers13010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farkhondeh T., Folgado S.L., Pourbagher-Shahri A.M., Ashrafizadeh M., Samarghandian S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020;127:110234. doi: 10.1016/j.biopha.2020.110234. [DOI] [PubMed] [Google Scholar]
- 42.Samarghandian S., Pourbagher-Shahri A.M., Ashrafizadeh M., Khan H., Forouzanfar F., Aramjoo H., Farkhondeh T. A pivotal role of the nrf2 signaling pathway in spinal cord injury: A prospective therapeutics study. CNS Neurol. Disord. Drug Targets Former. Curr. Drug Targets-CNS Neurol. Disord. 2020;19:207–219. doi: 10.2174/1871527319666200604175118. [DOI] [PubMed] [Google Scholar]
- 43.Ahmadi Z., Ashrafizadeh M. Melatonin as a potential modulator of Nrf2. Fundam. Clin. Pharmacol. 2020;34:11–19. doi: 10.1111/fcp.12498. [DOI] [PubMed] [Google Scholar]
- 44.Ashrafizadeh M., Fekri H.S., Ahmadi Z., Farkhondeh T., Samarghandian S. Therapeutic and biological activities of berberine: The involvement of Nrf2 signaling pathway. J. Cell. Biochem. 2020;121:1575–1585. doi: 10.1002/jcb.29392. [DOI] [PubMed] [Google Scholar]
- 45.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eggler A.L., Liu G., Pezzuto J.M., van Breemen R.B., Mesecar A.D. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl. Acad. Sci. USA. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw P., Chattopadhyay A. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020;235:3119–3130. doi: 10.1002/jcp.29219. [DOI] [PubMed] [Google Scholar]
- 49.Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic. Biol. Med. 2015;88:147–157. doi: 10.1016/j.freeradbiomed.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 50.Venugopal R., Jaiswal A.K. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17:3145–3156. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- 51.Saeedi B.J., Liu K.H., Owens J.A., Hunter-Chang S., Camacho M.C., Eboka R.U., Chandrasekharan B., Baker N.F., Darby T.M., Robinson B.S., et al. Gut-Resident Lactobacilli Activate Hepatic Nrf2 and Protect Against Oxidative Liver Injury. Cell. Metab. 2020;31:956–968.e955. doi: 10.1016/j.cmet.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu J., Wang W.N., Matei N., Li X., Pang J.W., Mo J., Chen S.P., Tang J.P., Yan M., Zhang J.H. Ezetimibe Attenuates Oxidative Stress and Neuroinflammation via the AMPK/Nrf2/TXNIP Pathway after MCAO in Rats. Oxidative Med. Cell. Longev. 2020;2020:4717258. doi: 10.1155/2020/4717258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alcaraz M.J., Ferrándiz M.L. Relevance of Nrf2 and heme oxygenase-1 in articular diseases. Free Radic. Biol. Med. 2020;157:83–93. doi: 10.1016/j.freeradbiomed.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Kasai S., Shimizu S., Tatara Y., Mimura J., Itoh K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules. 2020;10:320. doi: 10.3390/biom10020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahn K.S., Sethi G., Jain A.K., Jaiswal A.K., Aggarwal B.B. Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-kappaB, IkappaBalpha kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis .J. Biol. Chem. 2006;281:19798–19808. doi: 10.1074/jbc.M601162200. [DOI] [PubMed] [Google Scholar]
- 56.Soares M.P., Seldon M.P., Gregoire I.P., Vassilevskaia T., Berberat P.O., Yu J., Tsui T.Y., Bach F.H. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 57.Li F., Shanmugam M.K., Chen L., Chatterjee S., Basha J., Kumar A.P., Kundu T.K., Sethi G. Garcinol, a polyisoprenylated benzophenone modulates multiple proinflammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev. Res. 2013;6:843–854. doi: 10.1158/1940-6207.CAPR-13-0070. [DOI] [PubMed] [Google Scholar]
- 58.Shanmugam M.K., Ong T.H., Kumar A.P., Lun C.K., Ho P.C., Wong P.T., Hui K.M., Sethi G. Ursolic acid inhibits the initiation, progression of prostate cancer and prolongs the survival of TRAMP mice by modulating pro-inflammatory pathways. PLoS ONE. 2012;7:e32476. doi: 10.1371/journal.pone.0032476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan H., Wang H., Wang X., Zhu L., Mao L. The absence of Nrf2 enhances NF-κB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat. Inflamm. 2012;2012:217580. doi: 10.1155/2012/217580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abd El-Twab S.M., Hussein O.E., Hozayen W.G., Bin-Jumah M., Mahmoud A.M. Chicoric acid prevents methotrexate-induced kidney injury by suppressing NF-κB/NLRP3 inflammasome activation and up-regulating Nrf2/ARE/HO-1 signaling. Inflamm. Res. 2019;68:511–523. doi: 10.1007/s00011-019-01241-z. [DOI] [PubMed] [Google Scholar]
- 61.Mahmoud A.M., Hussein O.E., Abd El-Twab S.M., Hozayen W.G. Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food Funct. 2019;10:4593–4607. doi: 10.1039/C9FO00114J. [DOI] [PubMed] [Google Scholar]
- 62.Aladaileh S.H., Abukhalil M.H., Saghir S.A.M., Hanieh H., Alfwuaires M.A., Almaiman A.A., Bin-Jumah M., Mahmoud A.M. Galangin Activates Nrf2 Signaling and Attenuates Oxidative Damage, Inflammation, and Apoptosis in a Rat Model of Cyclophosphamide-Induced Hepatotoxicity. Biomolecules. 2019;9:346. doi: 10.3390/biom9080346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.HAS A.L., Alotaibi M.F., Bin-Jumah M., Elgebaly H., Mahmoud A.M. Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomed. Pharmacother. 2019;111:676–685. doi: 10.1016/j.biopha.2018.12.112. [DOI] [PubMed] [Google Scholar]
- 64.Ranneh Y., Akim A.M., Hamid H.A., Khazaai H., Fadel A., Mahmoud A.M. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr. Metab. 2019;16:15. doi: 10.1186/s12986-019-0341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahmoud A.M., Germoush M.O., Al-Anazi K.M., Mahmoud A.H., Farah M.A., Allam A.A. Commiphora molmol protects against methotrexate-induced nephrotoxicity by up-regulating Nrf2/ARE/HO-1 signaling. Biomed. Pharmacother. 2018;106:499–509. doi: 10.1016/j.biopha.2018.06.171. [DOI] [PubMed] [Google Scholar]
- 66.Manu K.A., Shanmugam M.K., Li F., Chen L., Siveen K.S., Ahn K.S., Kumar A.P., Sethi G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014;92:267–276. doi: 10.1007/s00109-013-1095-0. [DOI] [PubMed] [Google Scholar]
- 67.Dai X., Ahn K.S., Wang L.Z., Kim C., Deivasigamni A., Arfuso F., Um J.Y., Kumar A.P., Chang Y.C., Kumar D., et al. Ascochlorin Enhances the Sensitivity of Doxorubicin Leading to the Reversal of Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma. Mol. Cancer Ther. 2016;15:2966–2976. doi: 10.1158/1535-7163.MCT-16-0391. [DOI] [PubMed] [Google Scholar]
- 68.Manu K.A., Shanmugam M.K., Ramachandran L., Li F., Siveen K.S., Chinnathambi A., Zayed M.E., Alharbi S.A., Arfuso F., Kumar A.P., et al. Isorhamnetin augments the anti-tumor effect of capecitabine through the negative regulation of NF-κB signaling cascade in gastric cancer. Cancer lett. 2015;363:28–36. doi: 10.1016/j.canlet.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 69.Pinto V., Bergantim R., Caires H.R., Seca H., Guimarães J.E., Vasconcelos M.H. Multiple Myeloma: Available Therapies and Causes of Drug Resistance. Cancers. 2020;12:407. doi: 10.3390/cancers12020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei L., Sun J., Zhang N., Zheng Y., Wang X., Lv L., Liu J., Xu Y., Shen Y., Yang M. Noncoding RNAs in gastric cancer: Implications for drug resistance. Mol. Cancer. 2020;19:62. doi: 10.1186/s12943-020-01185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou Y.Q., Liu D.Q., Chen S.P., Chen N., Sun J., Wang X.M., Cao F., Tian Y.K., Ye D.W. Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol. Sin. 2020;41:1041–1048. doi: 10.1038/s41401-020-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Y.Q., Liu D.Q., Chen S.P., Chen N., Sun J., Wang X.M., Li D.Y., Tian Y.K., Ye D.W. PPARγ activation mitigates mechanical allodynia in paclitaxel-induced neuropathic pain via induction of Nrf2/HO-1 signaling pathway. Biomed. Pharmacother. 2020;129:110356. doi: 10.1016/j.biopha.2020.110356. [DOI] [PubMed] [Google Scholar]
- 73.Mohamed M.E., Abduldaium Y.S., Younis N.S. Ameliorative Effect of Linalool in Cisplatin-Induced Nephrotoxicity: The Role of HMGB1/TLR4/NF-κB and Nrf2/HO1 Pathways. Biomolecules. 2020;10:1488. doi: 10.3390/biom10111488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdel-Wahab B.A., Alkahtani S.A., Elagab E.A.M. Tadalafil alleviates cisplatin-induced reproductive toxicity through the activation of the Nrf2/HO-1 pathway and the inhibition of oxidative stress and apoptosis in male rats. Reprod. Toxicol. 2020;96:165–174. doi: 10.1016/j.reprotox.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 75.Azouz A.A., Abdel-Nassir Abdel-Razek E., Abo-Youssef A.M. Amlodipine alleviates cisplatin-induced nephrotoxicity in rats through gamma-glutamyl transpeptidase (GGT) enzyme inhibition, associated with regulation of Nrf2/HO-1, MAPK/NF-κB, and Bax/Bcl-2 signaling. Saudi Pharm. J. 2020;28:1317–1325. doi: 10.1016/j.jsps.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu Y., Wu S., Xiang B., Li L., Lin Y. Curcumin Attenuates Oxaliplatin-Induced Liver Injury and Oxidative Stress by Activating the Nrf2 Pathway. Drug Des. Dev. Ther. 2020;14:73–85. doi: 10.2147/DDDT.S224318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fang Y., Ye J., Zhao B., Sun J., Gu N., Chen X., Ren L., Chen J., Cai X., Zhang W., et al. Formononetin ameliorates oxaliplatin-induced peripheral neuropathy via the KEAP1-NRF2-GSTP1 axis. Redox Biol. 2020;36:101677. doi: 10.1016/j.redox.2020.101677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu Y.P., Cai L.C., Wang X.Y., Cheng S.Y., Zhang D.M., Jian W.G., Wang T.D., Yang J.K., Yang K.B., Zhang C. BMP8A promotes survival and drug resistance via Nrf2/TRIM24 signaling pathway in clear cell renal cell carcinoma. Cancer Sci. 2020;111:1555–1566. doi: 10.1111/cas.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leung H.W., Lau E.Y.T., Leung C.O.N., Lei M.M.L., Mok E.H.K., Ma V.W.S., Cho W.C.S., Ng I.O.L., Yun J.P., Cai S.H., et al. NRF2/SHH signaling cascade promotes tumor-initiating cell lineage and drug resistance in hepatocellular carcinoma. Cancer Lett. 2020;476:48–56. doi: 10.1016/j.canlet.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 80.Jiang G., Liang X., Huang Y., Lan Z., Zhang Z., Su Z., Fang Z., Lai Y., Yao W., Liu T., et al. p62 promotes proliferation, apoptosis‑resistance and invasion of prostate cancer cells through the Keap1/Nrf2/ARE axis. Oncol. Rep. 2020;43:1547–1557. doi: 10.3892/or.2020.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mukhopadhyay S., Goswami D., Adiseshaiah P.P., Burgan W., Yi M., Guerin T.M., Kozlov S.V., Nissley D.V., McCormick F. Undermining Glutaminolysis Bolsters Chemotherapy While NRF2 Promotes Chemoresistance in KRAS-Driven Pancreatic Cancers. Cancer Res. 2020;80:1630–1643. doi: 10.1158/0008-5472.CAN-19-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Escoll M., Lastra D., Pajares M., Robledinos-Antón N., Rojo A.I., Fernández-Ginés R., Mendiola M., Martínez-Marín V., Esteban I., López-Larrubia P., et al. Transcription factor NRF2 uses the Hippo pathway effector TAZ to induce tumorigenesis in glioblastomas. Redox Biol. 2020;30:101425. doi: 10.1016/j.redox.2019.101425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cucci M.A., Grattarola M., Dianzani C., Damia G., Ricci F., Roetto A., Trotta F., Barrera G., Pizzimenti S. Ailanthone increases oxidative stress in CDDP-resistant ovarian and bladder cancer cells by inhibiting of Nrf2 and YAP expression through a post-translational mechanism. Free Radic. Biol. Med. 2020;150:125–135. doi: 10.1016/j.freeradbiomed.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 84.Fouzder C., Mukhuty A., Kundu R. Kaempferol inhibits Nrf2 signalling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Arch. Biochem. Biophys. 2020;697:108700. doi: 10.1016/j.abb.2020.108700. [DOI] [PubMed] [Google Scholar]
- 85.Ma C.S., Lv Q.M., Zhang K.R., Tang Y.B., Zhang Y.F., Shen Y., Lei H.M., Zhu L. NRF2-GPX4/SOD2 axis imparts resistance to EGFR-tyrosine kinase inhibitors in non-small-cell lung cancer cells. Acta Pharmacol. Sin. 2020 doi: 10.1038/s41401-020-0443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang C., Luo P., Li X., Liu P., Li Y., Xu J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chaperones. 2020;25:395–406. doi: 10.1007/s12192-020-01079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rasheed M.S.U., Tripathi M.K., Patel D.K., Singh M.P. Resveratrol Regulates Nrf2-Mediated Expression of Antioxidant and Xenobiotic Metabolizing Enzymes in Pesticides-Induced Parkinsonism. Protein Pept. Lett. 2020;27:1038–1045. doi: 10.2174/0929866527666200403110036. [DOI] [PubMed] [Google Scholar]
- 88.Yu X., Li Y., Mu X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ (25-35) and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. Biomed. Res. Int. 2020;2020:8210578. doi: 10.1155/2020/8210578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Costa P.M., Ferreira P.M., Bolzani Vda S., Furlan M., de Freitas Formenton Macedo Dos Santos V.A., Corsino J., de Moraes M.O., Costa-Lotufo L.V., Montenegro R.C., Pessoa C. Antiproliferative activity of pristimerin isolated from Maytenus ilicifolia (Celastraceae) in human HL-60 cells. Toxicol. In Vitro. 2008;22:854–863. doi: 10.1016/j.tiv.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 90.Tong L., Nanjundaiah S.M., Venkatesha S.H., Astry B., Yu H., Moudgil K.D. Pristimerin, a naturally occurring triterpenoid, protects against autoimmune arthritis by modulating the cellular and soluble immune mediators of inflammation and tissue damage. Clin. Immunol. 2014;155:220–230. doi: 10.1016/j.clim.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.El-Agamy D.S., El-Harbi K.M., Khoshhal S., Ahmed N., Elkablawy M.A., Shaaban A.A., Abo-Haded H.M. Pristimerin protects against doxorubicin-induced cardiotoxicity and fibrosis through modulation of Nrf2 and MAPK/NF-kB signaling pathways. Cancer Manag. Res. 2019;11:47–61. doi: 10.2147/CMAR.S186696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aktaş I., Özmen Ö., Tutun H., Yalçın A., Türk A. Artemisinin attenuates doxorubicin induced cardiotoxicity and hepatotoxicity in rats. Biotech. Histochem. 2020;95:121–128. doi: 10.1080/10520295.2019.1647457. [DOI] [PubMed] [Google Scholar]
- 93.Prospero A.G., Fidelis-de-Oliveira P., Soares G.A., Miranda M.F., Pinto L.A., Dos Santos D.C., Silva V.D.S., Zufelato N., Bakuzis A.F., Miranda J.R. AC biosusceptometry and magnetic nanoparticles to assess doxorubicin-induced kidney injury in rats. Nanomedicine. 2020;15:511–525. doi: 10.2217/nnm-2019-0300. [DOI] [PubMed] [Google Scholar]
- 94.Oh S.-K., Rha C., Kadir A., Ng T. Use of Asiatic Acid or Asiaticoside for Treatment of Cancer. Application No. 10/362,720. U.S. Patent. 2004 May 20;
- 95.Han Y., Jiang Y., Li Y., Wang M., Fan T., Liu M., Ke Q., Xu H., Yi Z. An aligned porous electrospun fibrous scaffold with embedded asiatic acid for accelerating diabetic wound healing. J. Mater. Chem. B. 2019;7:6125–6138. doi: 10.1039/C9TB01327J. [DOI] [PubMed] [Google Scholar]
- 96.Hu W.Y., Li X.X., Diao Y.F., Qi J.J., Wang D.L., Zhang J.B., Sun B.X., Liang S. Asiatic acid protects oocytes against in vitro aging-induced deterioration and improves subsequent embryonic development in pigs. Aging. 2020;12 doi: 10.18632/aging.202184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lian G.Y., Wang Q.M., Tang P.M., Zhou S., Huang X.R., Lan H.Y. Combination of Asiatic Acid and Naringenin Modulates NK Cell Anti-cancer Immunity by Rebalancing Smad3/Smad7 Signaling. Mol. Ther. 2018;26:2255–2266. doi: 10.1016/j.ymthe.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kamble S.M., Patil C.R. Asiatic Acid Ameliorates Doxorubicin-Induced Cardiac and Hepato-Renal Toxicities with Nrf2 Transcriptional Factor Activation in Rats. Cardiovasc. Toxicol. 2018;18:131–141. doi: 10.1007/s12012-017-9424-0. [DOI] [PubMed] [Google Scholar]
- 99.Song C., Heping H., Shen Y., Jin S., Li D., Zhang A., Ren X., Wang K., Zhang L., Wang J., et al. AMPK/p38/Nrf2 activation as a protective feedback to restrain oxidative stress and inflammation in microglia stimulated with sodium fluoride. Chemosphere. 2020;244:125495. doi: 10.1016/j.chemosphere.2019.125495. [DOI] [PubMed] [Google Scholar]
- 100.Lee E.H., Baek S.Y., Park J.Y., Kim Y.W. Rifampicin activates AMPK and alleviates oxidative stress in the liver as mediated with Nrf2 signaling. Chem. Biol. Interact. 2020;315:108889. doi: 10.1016/j.cbi.2019.108889. [DOI] [PubMed] [Google Scholar]
- 101.Nazari Soltan Ahmad S., Sanajou D., Kalantary-Charvadeh A., Hosseini V., Roshangar L., Khojastehfard M., Haiaty S., Mesgari-Abbasi M. β-LAPachone ameliorates doxorubicin-induced cardiotoxicity via regulating autophagy and Nrf2 signalling pathways in mice. Basic Clin. Pharmacol. Toxicol. 2020;126:364–373. doi: 10.1111/bcpt.13340. [DOI] [PubMed] [Google Scholar]
- 102.Wu Z., Zai W., Chen W., Han Y., Jin X., Liu H. Curdione Ameliorated Doxorubicin-Induced Cardiotoxicity Through Suppressing Oxidative Stress and Activating Nrf2/HO-1 Pathway. J. Cardiovasc. Pharmacol. 2019;74:118–127. doi: 10.1097/FJC.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 103.Yoo O.K., Choi W.J., Keum Y.S. Cardamonin Inhibits Oxazolone-Induced Atopic Dermatitis by the Induction of NRF2 and the Inhibition of Th2 Cytokine Production. Antioxidants. 2020;9:834. doi: 10.3390/antiox9090834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tan Y., Wan H.H., Sun M.M., Zhang W.J., Dong M., Ge W., Ren J., Peng H. Cardamonin protects against lipopolysaccharide-induced myocardial contractile dysfunction in mice through Nrf2-regulated mechanism. Acta Pharmacol. Sin. 2020 doi: 10.1038/s41401-020-0397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qi W., Boliang W., Xiaoxi T., Guoqiang F., Jianbo X., Gang W. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed. Pharmacother. 2020;122:109547. doi: 10.1016/j.biopha.2019.109547. [DOI] [PubMed] [Google Scholar]
- 106.Wang W., Meng Q., Li Q., Liu J., Zhou M., Jin Z., Zhao K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020;21:487. doi: 10.3390/ijms21020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Y., Liu H., Xu Q.-S., Du Y.-G., Xu J. Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of NF-κB and endothelial inflammatory response. Carbohydr. Polym. 2014;99:568–578. doi: 10.1016/j.carbpol.2013.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fernandes J.C., Sereno J., Garrido P., Parada B., Cunha M.F., Reis F., Pintado M.E., Santos-Silva A. Inhibition of bladder tumor growth by chitooligosaccharides in an experimental carcinogenesis model. Marine Drugs. 2012;10:2661–2675. doi: 10.3390/md10122661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Muanprasat C., Chatsudthipong V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017;170:80–97. doi: 10.1016/j.pharmthera.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 110.Zou P., Yang X., Wang J., Li Y., Yu H., Zhang Y., Liu G. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016;190:1174–1181. doi: 10.1016/j.foodchem.2015.06.076. [DOI] [PubMed] [Google Scholar]
- 111.Fang I.-M., Yang C.-M., Yang C.-H. Chitosan oligosaccharides prevented retinal ischemia and reperfusion injury via reduced oxidative stress and inflammation in rats. Exp. Eye Res. 2015;130:38–50. doi: 10.1016/j.exer.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y., Ahmad K.A., Khan F.U., Yan S., Ihsan A.U., Ding Q. Chitosan oligosaccharides prevent doxorubicin-induced oxidative stress and cardiac apoptosis through activating p38 and JNK MAPK mediated Nrf2/ARE pathway. Chem. Biol. Interact. 2019;305:54–65. doi: 10.1016/j.cbi.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 113.Wang L.F., Su S.W., Wang L., Zhang G.Q., Zhang R., Niu Y.J., Guo Y.S., Li C.Y., Jiang W.B., Liu Y., et al. Tert-butylhydroquinone ameliorates doxorubicin-induced cardiotoxicity by activating Nrf2 and inducing the expression of its target genes. Am. J. Transl. Res. 2015;7:1724–1735. [PMC free article] [PubMed] [Google Scholar]
- 114.Hajra S., Basu A., Singha Roy S., Patra A.R., Bhattacharya S. Attenuation of doxorubicin-induced cardiotoxicity and genotoxicity by an indole-based natural compound 3,3′-diindolylmethane (DIM) through activation of Nrf2/ARE signaling pathways and inhibiting apoptosis. Free Radic. Res. 2017;51:812–827. doi: 10.1080/10715762.2017.1381694. [DOI] [PubMed] [Google Scholar]
- 115.Kanski J., Aksenova M., Stoyanova A., Butterfield D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J. Nutr. Biochem. 2002;13:273–281. doi: 10.1016/S0955-2863(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 116.Kheiry M., Dianat M., Badavi M., Mard S.A., Bayati V. p-Coumaric Acid Attenuates Lipopolysaccharide-Induced Lung Inflammation in Rats by Scavenging ROS Production: An In Vivo and In Vitro Study. Inflammation. 2019;42:1939–1950. doi: 10.1007/s10753-019-01054-6. [DOI] [PubMed] [Google Scholar]
- 117.Chen J.J., Deng J.S., Huang C.C., Li P.Y., Liang Y.C., Chou C.Y., Huang G.J. p-Coumaric-Acid-Containing Adenostemma lavenia Ameliorates Acute Lung Injury by Activating AMPK/Nrf2/HO-1 Signaling and Improving the Anti-oxidant Response. Am. J. Chin. Med. 2019;47:1483–1506. doi: 10.1142/S0192415X19500769. [DOI] [PubMed] [Google Scholar]
- 118.Sunitha M.C., Dhanyakrishnan R., PrakashKumar B., Nevin K.G. p-Coumaric acid mediated protection of H9c2 cells from Doxorubicin-induced cardiotoxicity: Involvement of augmented Nrf2 and autophagy. Biomed. Pharmacother. 2018;102:823–832. doi: 10.1016/j.biopha.2018.03.089. [DOI] [PubMed] [Google Scholar]
- 119.Elsherbiny N.M., El-Sherbiny M. Thymoquinone attenuates Doxorubicin-induced nephrotoxicity in rats: Role of Nrf2 and NOX4. Chem. Biol. Interact. 2014;223:102–108. doi: 10.1016/j.cbi.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 120.Xu S., Liu P. Tanshinone II-A: New perspectives for old remedies. Expert Opin. Ther. Pat. 2013;23:149–153. doi: 10.1517/13543776.2013.743995. [DOI] [PubMed] [Google Scholar]
- 121.Feng F., Cheng P., Zhang H., Li N., Qi Y., Wang H., Wang Y., Wang W. The Protective Role of Tanshinone IIA in Silicosis Rat Model via TGF-β1/Smad Signaling Suppression, NOX4 Inhibition and Nrf2/ARE Signaling Activation. Drug Des. Dev. Ther. 2019;13:4275–4290. doi: 10.2147/DDDT.S230572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang Y., Liu L., Zhang X., Jiang X., Wang L. Tanshinone IIA prevents rifampicin-induced liver injury by regulating BSEP/NTCP expression via epigenetic activation of NRF2. Liver Int. 2020;40:141–154. doi: 10.1111/liv.14262. [DOI] [PubMed] [Google Scholar]
- 123.An L., Peng L.Y., Sun N.Y., Yang Y.L., Zhang X.W., Li B., Liu B.L., Li P., Chen J. Tanshinone IIA Activates Nuclear Factor-Erythroid 2-Related Factor 2 to Restrain Pulmonary Fibrosis via Regulation of Redox Homeostasis and Glutaminolysis. Antioxid. Redox Signal. 2019;30:1831–1848. doi: 10.1089/ars.2018.7569. [DOI] [PubMed] [Google Scholar]
- 124.Guo Z., Yan M., Chen L., Fang P., Li Z., Wan Z., Cao S., Hou Z., Wei S., Li W., et al. Nrf2-dependent antioxidant response mediated the protective effect of tanshinone IIA on doxorubicin-induced cardiotoxicity. Exp. Ther. Med. 2018;16:3333–3344. doi: 10.3892/etm.2018.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feng X., Yang Q., Wang C., Tong W., Xu W. Punicalagin Exerts Protective Effects against Ankylosing Spondylitis by Regulating NF-κB-TH17/JAK2/STAT3 Signaling and Oxidative Stress. Biomed. Res. Int. 2020;2020:4918239. doi: 10.1155/2020/4918239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang W., Bai J., Zhang W., Ge G., Wang Q., Liang X., Li N., Gu Y., Li M., Xu W., et al. Protective Effects of Punicalagin on Osteoporosis by Inhibiting Osteoclastogenesis and Inflammation via the NF-κB and MAPK Pathways. Front. Pharmacol. 2020;11:696. doi: 10.3389/fphar.2020.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ye M., Zhang L., Yan Y., Lin H. Punicalagin protects H9c2 cardiomyocytes from doxorubicin-induced toxicity through activation of Nrf2/HO-1 signaling. Biosci. Rep. 2019;39 doi: 10.1042/BSR20190229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arnold J., Engelmann J.C., Schneider N., Bosserhoff A.K., Kuphal S. miR-488-5p and its role in melanoma. Exp. Mol. Pathol. 2020;112:104348. doi: 10.1016/j.yexmp.2019.104348. [DOI] [PubMed] [Google Scholar]
- 129.Kolenda T., Guglas K., Kopczyńska M., Sobocińska J., Teresiak A., Bliźniak R., Lamperska K. Good or not good: Role of miR-18a in cancer biology. Rep. Pract. Oncol. Radiother. 2020;25:808–819. doi: 10.1016/j.rpor.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ashrafizadeh M., Hushmandi K., Hashemi M., Akbari M.E., Kubatka P., Raei M., Koklesova L., Shahinozzaman M., Mohammadinejad R., Najafi M., et al. Role of microRNA/Epithelial-to-Mesenchymal Transition Axis in the Metastasis of Bladder Cancer. Biomolecules. 2020;10:1159. doi: 10.3390/biom10081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aggarwal V., Tuli H.S., Varol A., Thakral F., Yerer M.B., Sak K., Varol M., Jain A., Khan M.A., Sethi G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules. 2019;9:735. doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dastmalchi N., Hosseinpourfeizi M.A., Khojasteh S.M.B., Baradaran B., Safaralizadeh R. Tumor suppressive activity of miR-424-5p in breast cancer cells through targeting PD-L1 and modulating PTEN/PI3K/AKT/mTOR signaling pathway. Life Sci. 2020;259:118239. doi: 10.1016/j.lfs.2020.118239. [DOI] [PubMed] [Google Scholar]
- 133.Aslan C., Maralbashi S., Kahroba H., Asadi M., Soltani-Zangbar M.S., Javadian M., Shanehbandi D., Baradaran B., Darabi M., Kazemi T. Docosahexaenoic acid (DHA) inhibits pro-angiogenic effects of breast cancer cells via down-regulating cellular and exosomal expression of angiogenic genes and microRNAs. Life Sci. 2020;258:118094. doi: 10.1016/j.lfs.2020.118094. [DOI] [PubMed] [Google Scholar]
- 134.Khalili N., Nouri-Vaskeh M., Hasanpour Segherlou Z., Baghbanzadeh A., Halimi M., Rezaee H., Baradaran B. Diagnostic, prognostic, and therapeutic significance of miR-139-5p in cancers. Life Sci. 2020;256:117865. doi: 10.1016/j.lfs.2020.117865. [DOI] [PubMed] [Google Scholar]
- 135.Azarbarzin S., Hosseinpour-Feizi M.A., Banan Khojasteh S.M., Baradaran B., Safaralizadeh R. MicroRNA -383-5p restrains the proliferation and migration of breast cancer cells and promotes apoptosis via inhibition of PD-L1. Life Sci. 2021;267:118939. doi: 10.1016/j.lfs.2020.118939. [DOI] [PubMed] [Google Scholar]
- 136.Godínez-Rubí M., Ortuño-Sahagún D. miR-615 Fine-Tunes Growth and Development and Has a Role in Cancer and in Neural Repair. Cells. 2020;9:1566. doi: 10.3390/cells9071566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ferrari E., Gandellini P. Unveiling the ups and downs of miR-205 in physiology and cancer: Transcriptional and post-transcriptional mechanisms. Cell Death Dis. 2020;11:980. doi: 10.1038/s41419-020-03192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mu Z., Zhang H., Lei P. Piceatannol inhibits pyroptosis and suppresses oxLDL-induced lipid storage in macrophages by regulating miR-200a/Nrf2/GSDMD axis. Biosci. Rep. 2020;40 doi: 10.1042/BSR20201366. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139.Wang X., Ye L., Zhang K., Gao L., Xiao J., Zhang Y. Upregulation of microRNA-200a in bone marrow mesenchymal stem cells enhances the repair of spinal cord injury in rats by reducing oxidative stress and regulating Keap1/Nrf2 pathway. Artif. Organs. 2020;44:744–752. doi: 10.1111/aor.13656. [DOI] [PubMed] [Google Scholar]
- 140.Zhao L., Qi Y., Xu L., Tao X., Han X., Yin L., Peng J. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol. 2018;15:284–296. doi: 10.1016/j.redox.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang Z., Wu J., Ma F., Jiang J., Xu L., Du L., Huang W., Wang Z., Jia Y., Lu L., et al. MicroRNA-200a improves diabetic endothelial dysfunction by targeting KEAP1/NRF2. J. Endocrinol. 2020;245:129–140. doi: 10.1530/JOE-19-0414. [DOI] [PubMed] [Google Scholar]
- 142.Ma Y., Pan C., Tang X., Zhang M., Shi H., Wang T., Zhang Y. MicroRNA-200a represses myocardial infarction-related cell death and inflammation by targeting the Keap1/Nrf2 and β-catenin pathways. Hellenic. J. Cardiol. 2020 doi: 10.1016/j.hjc.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 143.Hu X., Liu H., Wang Z., Hu Z., Li L. miR-200a Attenuated Doxorubicin-Induced Cardiotoxicity through Upregulation of Nrf2 in Mice. Oxidative Med. Cell. Longev. 2019;2019:1512326. doi: 10.1155/2019/1512326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li S., Wang W., Niu T., Wang H., Li B., Shao L., Lai Y., Li H., Janicki J.S., Wang X.L., et al. Nrf2 deficiency exaggerates doxorubicin-induced cardiotoxicity and cardiac dysfunction. Oxidative Med. Cell. Longev. 2014;2014:748524. doi: 10.1155/2014/748524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Singh S.S., Vats S., Chia A.Y., Tan T.Z., Deng S., Ong M.S., Arfuso F., Yap C.T., Goh B.C., Sethi G., et al. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37:1142–1158. doi: 10.1038/s41388-017-0046-6. [DOI] [PubMed] [Google Scholar]
- 146.Patra S., Mishra S.R., Behera B.P., Mahapatra K.K., Panigrahi D.P., Bhol C.S., Praharaj P.P., Sethi G., Patra S.K., Bhutia S.K. Autophagy-modulating phytochemicals in cancer therapeutics: Current evidences and future perspectives. Semin. Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 147.Ren H., Shao Y., Wu C., Ma X., Lv C., Wang Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol. Cell. Endocrinol. 2020;500:110628. doi: 10.1016/j.mce.2019.110628. [DOI] [PubMed] [Google Scholar]
- 148.Liang D., Zhuo Y., Guo Z., He L., Wang X., He Y., Li L., Dai H. SIRT1/PGC-1 pathway activation triggers autophagy/mitophagy and attenuates oxidative damage in intestinal epithelial cells. Biochimie. 2020;170:10–20. doi: 10.1016/j.biochi.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 149.Renu K., Valsala Gopalakrishnan A. Deciphering the molecular mechanism during doxorubicin-mediated oxidative stress, apoptosis through Nrf2 and PGC-1α in a rat testicular milieu. Reprod. Biol. 2019;19:22–37. doi: 10.1016/j.repbio.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 150.Zhou B., Liu J., Kang R., Klionsky D.J., Kroemer G., Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin. Cancer Biol. 2020;66:89–100. doi: 10.1016/j.semcancer.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 151.Liu Q., Zhao M., Chen W., Xu K., Huang F., Qu J., Xu Z., Wang X., Wang Y., Zhu Y., et al. Mainstream cigarette smoke induces autophagy and promotes apoptosis in oral mucosal epithelial cells. Arch. Oral Biol. 2020;111:104646. doi: 10.1016/j.archoralbio.2019.104646. [DOI] [PubMed] [Google Scholar]
- 152.Deng S., Shanmugam M.K., Kumar A.P., Yap C.T., Sethi G., Bishayee A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer. 2019;125:1228–1246. doi: 10.1002/cncr.31978. [DOI] [PubMed] [Google Scholar]
- 153.Chihara D., Westin J.R., Oki Y., Ahmed M.A., Do B., Fayad L.E., Hagemeister F.B., Romaguera J.E., Fanale M.A., Lee H.J., et al. Management strategies and outcomes for very elderly patients with diffuse large B-cell lymphoma. Cancer. 2016;122:3145–3151. doi: 10.1002/cncr.30173. [DOI] [PubMed] [Google Scholar]
- 154.McGuckin M.M., Giesy S.L., Davis A.N., Abyeta M.A., Horst E.A., Saed Samii S., Zang Y., Butler W.R., Baumgard L.H., McFadden J.W., et al. The acute phase protein orosomucoid 1 is upregulated in early lactation but does not trigger appetite-suppressing STAT3 signaling via the leptin receptor. J. Dairy Sci. 2020;103:4765–4776. doi: 10.3168/jds.2019-18094. [DOI] [PubMed] [Google Scholar]
- 155.Fandiño-Vaquero R., Fernández-Trasancos A., Alvarez E., Ahmad S., Batista-Oliveira A.L., Adrio B., Fernández A.L., González-Juanatey J.R., Eiras S. Orosomucoid secretion levels by epicardial adipose tissue as possible indicator of endothelial dysfunction in diabetes mellitus or inflammation in coronary artery disease. Atherosclerosis. 2014;235:281–288. doi: 10.1016/j.atherosclerosis.2014.05.921. [DOI] [PubMed] [Google Scholar]
- 156.Ren F., Chen Y., Wang Y., Yan Y., Zhao J., Ding M., Zhang J., Jiang Y., Zhai Y., Duan Z. Comparative serum proteomic analysis of patients with acute-on-chronic liver failure: Alpha-1-acid glycoprotein maybe a candidate marker for prognosis of hepatitis B virus infection. J. Viral. Hepat. 2010;17:816–824. doi: 10.1111/j.1365-2893.2009.01242.x. [DOI] [PubMed] [Google Scholar]
- 157.Zhu H.Z., Zhou W.J., Wan Y.F., Ge K., Lu J., Jia C.K. Downregulation of orosomucoid 2 acts as a prognostic factor associated with cancer-promoting pathways in liver cancer. World J. Gastroenterol. 2020;26:804–817. doi: 10.3748/wjg.v26.i8.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cheng X., Liu D., Xing R., Song H., Tian X., Yan C., Han Y. Orosomucoid 1 Attenuates Doxorubicin-Induced Oxidative Stress and Apoptosis in Cardiomyocytes via Nrf2 Signaling. Biomed. Res. Int. 2020;2020:5923572. doi: 10.1155/2020/5923572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tomlinson L., Lu Z.Q., Bentley R.A., Colley H.E., Murdoch C., Webb S.D., Cross M.J., Copple I.M., Sharma P. Attenuation of doxorubicin-induced cardiotoxicity in a human in vitro cardiac model by the induction of the NRF-2 pathway. Biomed. Pharmacother. 2019;112:108637. doi: 10.1016/j.biopha.2019.108637. [DOI] [PubMed] [Google Scholar]
- 160.Wang H., Brown J., Martin M. Glycogen synthase kinase 3: A point of convergence for the host inflammatory response. Cytokine. 2011;53:130–140. doi: 10.1016/j.cyto.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cohen P., Frame S. The renaissance of GSK3. Nat. Rev. Mol. Cell. Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 162.Bitar M.S., Al-Mulla F. A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011;301:E1119–E1129. doi: 10.1152/ajpendo.00047.2011. [DOI] [PubMed] [Google Scholar]
- 163.Tomobe K., Shinozuka T., Kuroiwa M., Nomura Y. Age-related changes of Nrf2 and phosphorylated GSK-3β in a mouse model of accelerated aging (SAMP8) Arch. Gerontol. Geriatr. 2012;54:e1–e7. doi: 10.1016/j.archger.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 164.Jiang Y., Bao H., Ge Y., Tang W., Cheng D., Luo K., Gong G., Gong R. Therapeutic targeting of GSK3β enhances the Nrf2 antioxidant response and confers hepatic cytoprotection in hepatitis C. Gut. 2015;64:168–179. doi: 10.1136/gutjnl-2013-306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rojo A.I., Rada P., Egea J., Rosa A.O., López M.G., Cuadrado A. Functional interference between glycogen synthase kinase-3 beta and the transcription factor Nrf2 in protection against kainate-induced hippocampal cell death. Mol. Cell. Neurosci. 2008;39:125–132. doi: 10.1016/j.mcn.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 166.Espada S., Rojo A.I., Salinas M., Cuadrado A. The muscarinic M1 receptor activates Nrf2 through a signaling cascade that involves protein kinase C and inhibition of GSK-3beta: Connecting neurotransmission with neuroprotection. J. Neurochem. 2009;110:1107–1119. doi: 10.1111/j.1471-4159.2009.06208.x. [DOI] [PubMed] [Google Scholar]
- 167.Correa F., Mallard C., Nilsson M., Sandberg M. Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: Restoring effects of inhibitors of HDACs, p38 MAPK and GSK3β. Neurobiol. Dis. 2011;44:142–151. doi: 10.1016/j.nbd.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou S., Wang P., Qiao Y., Ge Y., Wang Y., Quan S., Yao R., Zhuang S., Wang L.J., Du Y., et al. Genetic and Pharmacologic Targeting of Glycogen Synthase Kinase 3β Reinforces the Nrf2 Antioxidant Defense against Podocytopathy. J. Am. Soc. Nephrol. 2016;27:2289–2308. doi: 10.1681/ASN.2015050565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nordgren K.K.S., Wallace K.B. Disruption of the Keap1/Nrf2-Antioxidant Response System After Chronic Doxorubicin Exposure In Vivo. Cardiovasc. Toxicol. 2020;20:557–570. doi: 10.1007/s12012-020-09581-7. [DOI] [PubMed] [Google Scholar]
- 170.Wu W.Y., Cui Y.K., Hong Y.X., Li Y.D., Wu Y., Li G., Li G.R., Wang Y. Doxorubicin cardiomyopathy is ameliorated by acacetin via Sirt1-mediated activation of AMPK/Nrf2 signal molecules. J. Cell. Mol. Med. 2020;24:12141–12153. doi: 10.1111/jcmm.15859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen M., Samuel V.P., Wu Y., Dang M., Lin Y., Sriramaneni R., Sah S.K., Chinnaboina G.K., Zhang G. Nrf2/HO-1 Mediated Protective Activity of Genistein Against Doxorubicin-Induced Cardiac Toxicity. J. Environ. Pathol. Toxicol. Oncol. 2019;38:143–152. doi: 10.1615/JEnvironPatholToxicolOncol.2019029341. [DOI] [PubMed] [Google Scholar]
- 172.Singh A., Venkannagari S., Oh K.H., Zhang Y.Q., Rohde J.M., Liu L., Nimmagadda S., Sudini K., Brimacombe K.R., Gajghate S., et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem. Biol. 2016;11:3214–3225. doi: 10.1021/acschembio.6b00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li X., Kong L., Yang Q., Duan A., Ju X., Cai B., Chen L., An T., Li Y. Parthenolide inhibits ubiquitin-specific peptidase 7 (USP7), Wnt signaling, and colorectal cancer cell growth. J. Biol. Chem. 2020;295:3576–3589. doi: 10.1074/jbc.RA119.011396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sztiller-Sikorska M., Czyz M. Parthenolide as Cooperating Agent for Anti-Cancer Treatment of Various Malignancies. Pharmaceuticals. 2020;13:194. doi: 10.3390/ph13080194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jin X., Yang Q., Cai N., Zhang Z. A cocktail of betulinic acid, parthenolide, honokiol and ginsenoside Rh2 in liposome systems for lung cancer treatment. Nanomedicine. 2020;15:41–54. doi: 10.2217/nnm-2018-0479. [DOI] [PubMed] [Google Scholar]
- 176.Carlisi D., De Blasio A., Drago-Ferrante R., Di Fiore R., Buttitta G., Morreale M., Scerri C., Vento R., Tesoriere G. Parthenolide prevents resistance of MDA-MB231 cells to doxorubicin and mitoxantrone: The role of Nrf2. Cell Death Discov. 2017;3:17078. doi: 10.1038/cddiscovery.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang B., Huang J., Xiang T., Yin X., Luo X., Huang J., Luo F., Li H., Li H., Ren G. Chrysin inhibits metastatic potential of human triple-negative breast cancer cells by modulating matrix metalloproteinase-10, epithelial to mesenchymal transition, and PI3K/Akt signaling pathway. J. Appl. Toxicol. 2014;34:105–112. doi: 10.1002/jat.2941. [DOI] [PubMed] [Google Scholar]
- 178.Woo K.J., Jeong Y.J., Park J.W., Kwon T.K. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem. Biophys. Res. Commun. 2004;325:1215–1222. doi: 10.1016/j.bbrc.2004.09.225. [DOI] [PubMed] [Google Scholar]
- 179.Wang J., Wang H., Sun K., Wang X., Pan H., Zhu J., Ji X., Li X. Chrysin suppresses proliferation, migration, and invasion in glioblastoma cell lines via mediating the ERK/Nrf2 signaling pathway. Drug Des. Dev. Ther. 2018;12:721–733. doi: 10.2147/DDDT.S160020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xia Y., Lian S., Khoi P.N., Yoon H.J., Joo Y.E., Chay K.O., Kim K.K., Do Jung Y. Chrysin inhibits tumor promoter-induced MMP-9 expression by blocking AP-1 via suppression of ERK and JNK pathways in gastric cancer cells. PLoS ONE. 2015;10:e0124007. doi: 10.1371/journal.pone.0124007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gao A.M., Ke Z.P., Shi F., Sun G.C., Chen H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem. Biol. Interact. 2013;206:100–108. doi: 10.1016/j.cbi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 182.Gao H.L., Xia Y.Z., Zhang Y.L., Yang L., Kong L.Y. Vielanin P enhances the cytotoxicity of doxorubicin via the inhibition of PI3K/Nrf2-stimulated MRP1 expression in MCF-7 and K562 DOX-resistant cell lines. Phytomedicine. 2019;58:152885. doi: 10.1016/j.phymed.2019.152885. [DOI] [PubMed] [Google Scholar]
- 183.Qi Y., Ding Z., Yao Y., Ren F., Yin M., Yang S., Chen A. Apigenin induces apoptosis and counteracts cisplatin-induced chemoresistance via Mcl-1 in ovarian cancer cells. Exp. Ther. Med. 2020;20:1329–1336. doi: 10.3892/etm.2020.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Korga-Plewko A., Michalczyk M., Adamczuk G., Humeniuk E., Ostrowska-Lesko M., Jozefczyk A., Iwan M., Wojcik M., Dudka J. Apigenin and Hesperidin Downregulate DNA Repair Genes in MCF-7 Breast Cancer Cells and Augment Doxorubicin Toxicity. Molecules. 2020;25:4421. doi: 10.3390/molecules25194421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Şirin N., Elmas L., Seçme M., Dodurga Y. Investigation of possible effects of apigenin, sorafenib and combined applications on apoptosis and cell cycle in hepatocellular cancer cells. Gene. 2020;737:144428. doi: 10.1016/j.gene.2020.144428. [DOI] [PubMed] [Google Scholar]
- 186.Gao A.M., Zhang X.Y., Ke Z.P. Apigenin sensitizes BEL-7402/ADM cells to doxorubicin through inhibiting miR-101/Nrf2 pathway. Oncotarget. 2017;8:82085–82091. doi: 10.18632/oncotarget.18294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mohan C.D., Srinivasa V., Rangappa S., Mervin L., Mohan S., Paricharak S., Baday S., Li F., Shanmugam M.K., Chinnathambi A., et al. Trisubstituted-Imidazoles Induce Apoptosis in Human Breast Cancer Cells by Targeting the Oncogenic PI3K/Akt/mTOR Signaling Pathway. PLoS ONE. 2016;11:e0153155. doi: 10.1371/journal.pone.0153155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ong P.S., Wang L.Z., Dai X., Tseng S.H., Loo S.J., Sethi G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016;7:395. doi: 10.3389/fphar.2016.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gao A.M., Ke Z.P., Wang J.N., Yang J.Y., Chen S.Y., Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013;34:1806–1814. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- 190.Wei Y., Liu D., Jin X., Gao P., Wang Q., Zhang J., Zhang N. PA-MSHA inhibits the growth of doxorubicin-resistant MCF-7/ADR human breast cancer cells by downregulating Nrf2/p62. Cancer Med. 2016;5:3520–3531. doi: 10.1002/cam4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim D., Choi B.H., Ryoo I.G., Kwak M.K. High NRF2 level mediates cancer stem cell-like properties of aldehyde dehydrogenase (ALDH)-high ovarian cancer cells: Inhibitory role of all-trans retinoic acid in ALDH/NRF2 signaling. Cell Death Dis. 2018;9:896. doi: 10.1038/s41419-018-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang H.Y., Zhao L., Yang Z., Zhao Q., Qiang L., Ha J., Li Z.Y., You Q.D., Guo Q.L. Oroxylin a reverses multi-drug resistance of human hepatoma BEL7402/5¯FU cells via downregulation of P¯glycoprotein expression by inhibiting NF-κB signaling pathway. Mol. Carcinog. 2012;51:185–195. doi: 10.1002/mc.20789. [DOI] [PubMed] [Google Scholar]
- 193.Koh H., Sun H.N., Xing Z., Liu R., Chandimali N., Kwon T., Lee D.S. Wogonin Influences Osteosarcoma Stem Cell Stemness Through ROS-dependent Signaling. In Vivo. 2020;34:1077–1084. doi: 10.21873/invivo.11878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang D., Guo Q., Liang Y., Zhao Y., Tian X., Ye Y., Tian J., Wu T., Lu N. Wogonin induces cellular senescence in breast cancer via suppressing TXNRD2 expression. Arch. Toxicol. 2020;94:3433–3447. doi: 10.1007/s00204-020-02842-y. [DOI] [PubMed] [Google Scholar]
- 195.Zhong Y., Zhang F., Sun Z., Zhou W., Li Z.Y., You Q.D., Guo Q.L., Hu R. Drug resistance associates with activation of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by down-regulating Nrf2-mediated cellular defense response. Mol. Carcinog. 2013;52:824–834. doi: 10.1002/mc.21921. [DOI] [PubMed] [Google Scholar]
- 196.Ohnuma T., Matsumoto T., Itoi A., Kawana A., Nishiyama T., Ogura K., Hiratsuka A. Enhanced sensitivity of A549 cells to the cytotoxic action of anticancer drugs via suppression of Nrf2 by procyanidins from Cinnamomi Cortex extract. Biochem. Biophys. Res. Commun. 2011;413:623–629. doi: 10.1016/j.bbrc.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 197.Selvi R.B., Swaminathan A., Chatterjee S., Shanmugam M.K., Li F., Ramakrishnan G.B., Siveen K.S., Chinnathambi A., Zayed M.E., Alharbi S.A., et al. Inhibition of p300 lysine acetyltransferase activity by luteolin reduces tumor growth in head and neck squamous cell carcinoma (HNSCC) xenograft mouse model. Oncotarget. 2015;6:43806–43818. doi: 10.18632/oncotarget.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Masraksa W., Tanasawet S., Hutamekalin P., Wongtawatchai T., Sukketsiri W. Luteolin attenuates migration and invasion of lung cancer cells via suppressing focal adhesion kinase and non-receptor tyrosine kinase signaling pathway. Nutr. Res. Pract. 2020;14:127–133. doi: 10.4162/nrp.2020.14.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cao D., Zhu G.Y., Lu Y., Yang A., Chen D., Huang H.J., Peng S.X., Chen L.W., Li Y.W. Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed. Pharmacother. 2020;129:110462. doi: 10.1016/j.biopha.2020.110462. [DOI] [PubMed] [Google Scholar]
- 200.Monti E., Marras E., Prini P., Gariboldi M.B. Luteolin impairs hypoxia adaptation and progression in human breast and colon cancer cells. Eur. J. Pharmacol. 2020;881:173210. doi: 10.1016/j.ejphar.2020.173210. [DOI] [PubMed] [Google Scholar]
- 201.Tang X., Wang H., Fan L., Wu X., Xin A., Ren H., Wang X.J. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic. Biol. Med. 2011;50:1599–1609. doi: 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 202.Sabzichi M., Hamishehkar H., Ramezani F., Sharifi S., Tabasinezhad M., Pirouzpanah M., Ghanbari P., Samadi N. Luteolin-loaded phytosomes sensitize human breast carcinoma MDA-MB 231 cells to doxorubicin by suppressing Nrf2 mediated signalling. Asian Pac. J. Cancer Prev. 2014;15:5311–5316. doi: 10.7314/APJCP.2014.15.13.5311. [DOI] [PubMed] [Google Scholar]
- 203.Chen F., Liu Y., Wang S., Guo X., Shi P., Wang W., Xu B. Triptolide, a Chinese herbal extract, enhances drug sensitivity of resistant myeloid leukemia cell lines through downregulation of HIF-1α and Nrf2. Pharmacogenomics. 2013;14:1305–1317. doi: 10.2217/pgs.13.122. [DOI] [PubMed] [Google Scholar]
- 204.Shanmugam M.K., Warrier S., Kumar A.P., Sethi G., Arfuso F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017;15:503–519. doi: 10.2174/1570161115666170713094319. [DOI] [PubMed] [Google Scholar]
- 205.Kashyap D., Tuli H.S., Yerer M.B., Sharma A., Sak K., Srivastava S., Pandey A., Garg V.K., Sethi G., Bishayee A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2019 doi: 10.1016/j.semcancer.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 206.Sadeghi M.R., Jeddi F., Soozangar N., Somi M.H., Shirmohamadi M., Khaze V., Samadi N. Nrf2/P-glycoprotein axis is associated with clinicopathological characteristics in colorectal cancer. Biomed. Pharmacother. 2018;104:458–464. doi: 10.1016/j.biopha.2018.05.062. [DOI] [PubMed] [Google Scholar]
- 207.Xia X., Wang Q., Ye T., Liu Y., Liu D., Song S., Zheng C. NRF2/ABCB1-mediated efflux and PARP1-mediated dampening of DNA damage contribute to doxorubicin resistance in chronic hypoxic HepG2 cells. Fundam. Clin. Pharmacol. 2020;34:41–50. doi: 10.1111/fcp.12505. [DOI] [PubMed] [Google Scholar]
- 208.Ryoo I.G., Kim G., Choi B.H., Lee S.H., Kwak M.K. Involvement of NRF2 Signaling in Doxorubicin Resistance of Cancer Stem Cell-Enriched Colonospheres. Biomol. Ther. 2016;24:482–488. doi: 10.4062/biomolther.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang J., Zhang Q., Liu Y., Zhang X., Shan W., Ye S., Zhou X., Ge Y., Wang X., Ren L. Nanoparticle-based co-delivery of siRNA and paclitaxel for dual-targeting of glioblastoma. Nanomedicine. 2020;15:1391–1409. doi: 10.2217/nnm-2020-0066. [DOI] [PubMed] [Google Scholar]
- 210.Wu B., Yuan Y., Han X., Wang Q., Shang H., Liang X., Jing H., Cheng W. Structure of LINC00511-siRNA-conjugated nanobubbles and improvement of cisplatin sensitivity on triple negative breast cancer. FASEB J. 2020;34:9713–9726. doi: 10.1096/fj.202000481R. [DOI] [PubMed] [Google Scholar]
- 211.Chen J., Wu Z., Ding W., Xiao C., Zhang Y., Gao S., Gao Y., Cai W. SREBP1 siRNA enhance the docetaxel effect based on a bone-cancer dual-targeting biomimetic nanosystem against bone metastatic castration-resistant prostate cancer. Theranostics. 2020;10:1619–1632. doi: 10.7150/thno.40489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li P.C., Tu M.J., Ho P.Y., Jilek J.L., Duan Z., Zhang Q.Y., Yu A.X., Yu A.M. Bioengineered NRF2-siRNA Is Effective to Interfere with NRF2 Pathways and Improve Chemosensitivity of Human Cancer Cells. Drug Metab. Dispos. 2018;46:2–10. doi: 10.1124/dmd.117.078741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shao J., Glorieux C., Liao J., Chen P., Lu W., Liang Z., Wen S., Hu Y., Huang P. Impact of Nrf2 on tumour growth and drug sensitivity in oncogenic K-ras-transformed cells in vitro and in vivo. Free Radic. Res. 2018;52:661–671. doi: 10.1080/10715762.2018.1462494. [DOI] [PubMed] [Google Scholar]
- 214.Yan Y., Zuo X., Wei D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem. Cells Transl. Med. 2015;4:1033–1043. doi: 10.5966/sctm.2015-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zöller M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 216.Ryoo I.G., Choi B.H., Ku S.K., Kwak M.K. High CD44 expression mediates p62-associated NFE2L2/NRF2 activation in breast cancer stem cell-like cells: Implications for cancer stem cell resistance. Redox Biol. 2018;17:246–258. doi: 10.1016/j.redox.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Probst B.L., McCauley L., Trevino I., Wigley W.C., Ferguson D.A. Cancer Cell Growth Is Differentially Affected by Constitutive Activation of NRF2 by KEAP1 Deletion and Pharmacological Activation of NRF2 by the Synthetic Triterpenoid, RTA 405. PLoS ONE. 2015;10:e0135257. doi: 10.1371/journal.pone.0135257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Goto S., Kawabata T., Li T.S. Enhanced Expression of ABCB1 and Nrf2 in CD133-Positive Cancer Stem Cells Associates with Doxorubicin Resistance. Stem. Cells Int. 2020;2020:8868849. doi: 10.1155/2020/8868849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Song Z., Liu Z., Sun J., Sun F.L., Li C.Z., Sun J.Z., Xu L.Y. The MRTF-A/B function as oncogenes in pancreatic cancer. Oncol. Rep. 2016;35:127–138. doi: 10.3892/or.2015.4329. [DOI] [PubMed] [Google Scholar]
- 220.Xing W.J., Liao X.H., Wang N., Zhao D.W., Zheng L., Zheng D.L., Dong J., Zhang T.C. MRTF-A and STAT3 promote MDA-MB-231 cell migration via hypermethylating BRSM1. IUBMB Life. 2015;67:202–217. doi: 10.1002/iub.1362. [DOI] [PubMed] [Google Scholar]
- 221.Xu Y., Luo Y., Wang Z.Y., Li X., Zheng P., Zhang T.C. MRTF-A can activate Nrf2 to increase the resistance to doxorubicin. Oncotarget. 2017;8:8436–8446. doi: 10.18632/oncotarget.14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Brindley D.N., Lin F.-T., Tigyi G.J. Role of the autotaxin–lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids. 2013;1831:74–85. doi: 10.1016/j.bbalip.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kehlen A., Lauterbach R., Santos A., Thiele K., Kabisch U., Weber E., Riemann D., Langner J. IL-1β-and IL-4-induced down-regulation of autotaxin mRNA and PC-1 in fibroblast-like synoviocytes of patients with rheumatoid arthritis (RA) Clin. Exp. Immunol. 2001;123:147–154. doi: 10.1046/j.1365-2249.2001.01432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nikitopoulou I., Oikonomou N., Karouzakis E., Sevastou I., Nikolaidou-Katsaridou N., Zhao Z., Mersinias V., Armaka M., Xu Y., Masu M. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J. Exp. Med. 2012;209:925–933. doi: 10.1084/jem.20112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tager A.M., LaCamera P., Shea B.S., Campanella G.S., Selman M., Zhao Z., Polosukhin V., Wain J., Karimi-Shah B.A., Kim N.D. The lysophosphatidic acid receptor LPA 1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 226.Hozumi H., Hokari R., Kurihara C., Narimatsu K., Sato H., Sato S., Ueda T., Higashiyama M., Okada Y., Watanabe C. Involvement of autotaxin/lysophospholipase D expression in intestinal vessels in aggravation of intestinal damage through lymphocyte migration. Lab. Investig. 2013;93:508–519. doi: 10.1038/labinvest.2013.45. [DOI] [PubMed] [Google Scholar]
- 227.Euer N., Schwirzke M., Evtimova V., Burtscher H., Jarsch M., Tarin D., Weidle U.H. Identification of genes associated with metastasis of mammary carcinoma in metastatic versus non-metastatic cell lines. Anticancer Res. 2002;22:733–740. [PubMed] [Google Scholar]
- 228.Lin S., Wang D., Iyer S., Ghaleb A.M., Shim H., Yang V.W., Chun J., Yun C.C. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology. 2009;136:1711–1720. doi: 10.1053/j.gastro.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cooper A.B., Wu J., Lu D., Maluccio M.A. Is autotaxin (ENPP2) the link between hepatitis C and hepatocellular cancer? J. Gastrointest. Surg. 2007;11 doi: 10.1007/s11605-007-0322-9. [DOI] [PubMed] [Google Scholar]
- 230.Venkatraman G., Benesch M.G., Tang X., Dewald J., McMullen T.P., Brindley D.N. Lysophosphatidate signaling stabilizes Nrf2 and increases the expression of genes involved in drug resistance and oxidative stress responses: Implications for cancer treatment. FASEB J. 2015;29:772–785. doi: 10.1096/fj.14-262659. [DOI] [PubMed] [Google Scholar]
- 231.Loignon M., Miao W., Hu L., Bier A., Bismar T.A., Scrivens P.J., Mann K., Basik M., Bouchard A., Fiset P.O., et al. Cul3 overexpression depletes Nrf2 in breast cancer and is associated with sensitivity to carcinogens, to oxidative stress, and to chemotherapy. Mol. Cancer Ther. 2009;8:2432–2440. doi: 10.1158/1535-7163.MCT-08-1186. [DOI] [PubMed] [Google Scholar]
- 232.Kang H.J., Yi Y.W., Hong Y.B., Kim H.J., Jang Y.J., Seong Y.S., Bae I. HER2 confers drug resistance of human breast cancer cells through activation of NRF2 by direct interaction. Sci. Rep. 2014;4:7201. doi: 10.1038/srep07201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Manandhar S., Lee S., Kwak M.K. Effect of stable inhibition of NRF2 on doxorubicin sensitivity in human ovarian carcinoma OV90 cells. Arch. Pharm. Res. 2010;33:717–726. doi: 10.1007/s12272-010-0511-z. [DOI] [PubMed] [Google Scholar]
- 234.Shim G.S., Manandhar S., Shin D.H., Kim T.H., Kwak M.K. Acquisition of doxorubicin resistance in ovarian carcinoma cells accompanies activation of the NRF2 pathway. Free Radic. Biol. Med. 2009;47:1619–1631. doi: 10.1016/j.freeradbiomed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 235.Choi B.H., Ryu D.Y., Ryoo I.G., Kwak M.K. NFE2L2/NRF2 silencing-inducible miR-206 targets c-MET/EGFR and suppresses BCRP/ABCG2 in cancer cells. Oncotarget. 2017;8:107188–107205. doi: 10.18632/oncotarget.22513. [DOI] [PMC free article] [PubMed] [Google Scholar]