Abstract
It is unknown how abundant extraterrestrial life is, or whether such life might be complex or intelligent. On Earth, the emergence of complex intelligent life required a preceding series of evolutionary transitions such as abiogenesis, eukaryogenesis, and the evolution of sexual reproduction, multicellularity, and intelligence itself. Some of these transitions could have been extraordinarily improbable, even in conducive environments. The emergence of intelligent life late in Earth's lifetime is thought to be evidence for a handful of rare evolutionary transitions, but the timing of other evolutionary transitions in the fossil record is yet to be analyzed in a similar framework. Using a simplified Bayesian model that combines uninformative priors and the timing of evolutionary transitions, we demonstrate that expected evolutionary transition times likely exceed the lifetime of Earth, perhaps by many orders of magnitude. Our results corroborate the original argument suggested by Brandon Carter that intelligent life in the Universe is exceptionally rare, assuming that intelligent life elsewhere requires analogous evolutionary transitions. Arriving at the opposite conclusion would require exceptionally conservative priors, evidence for much earlier transitions, multiple instances of transitions, or an alternative model that can explain why evolutionary transitions took hundreds of millions of years without appealing to rare chance events. Although the model is simple, it provides an initial basis for evaluating how varying biological assumptions and fossil record data impact the probability of evolving intelligent life, and also provides a number of testable predictions, such as that some biological paradoxes will remain unresolved and that planets orbiting M dwarf stars are uninhabitable.
Key Words: Evolutionary transitions, Observation selection effects, Bayesian analysis.
1. Introduction
Life on Earth has undergone a number of major evolutionary transitions (Smith and Szathmary, 1997). These include abiogenesis, as well as the emergence of increasingly complex forms of life such as eukaryotic, multicellular, and intelligent life. Some transitions seem to have occurred only once in Earth's history, suggesting a hypothesis reminiscent of Gould's remark that if the “tape of life” were to be rerun, “the chance becomes vanishingly small that anything like human intelligence” would occur (Gould, 1990). Here, we explore this hypothesis.
Given that we cannot rerun the “tape of life,” it is difficult to derive the probability of these major evolutionary transitions. An alternative would be to examine the timing and frequency of the transitions. The fact that eukaryotic life took over a billion years to emerge from prokaryotic precursors suggests it is a far less probable event than the development of multicellular life, which is thought to have originated independently over 40 times (Grosberg and Strathmann, 2007). The early emergence of abiogenesis is one example that is frequently cited as evidence that simple life must be fairly common throughout the Universe (Lineweaver and Davis, 2002). By using the timing of evolutionary transitions to estimate the rates of transition (probability per unit of time), we can derive information about the likelihood of a given transition even if it occurred only once in Earth's history.
However, an additional methodological challenge arises in estimating these rates from their timing in our evolutionary history, given that the timings are subject to a sample bias. In particular, we can only observe evolutionary transitions that occurred rapidly enough to fit within Earth's habitable lifetime. It is estimated that the increased luminosity of the Sun will make complex eukaryotic life impossible on Earth in about 0.8 to 1.3 billion years (Ga) (Caldeira and Kasting, 1992; Franck et al., 2006). If a long period of time is required for intelligence to evolve, any intelligent observers on an Earth-like planet may observe early evolutionary transitions occurring regardless of the true transition rate.
A previous analysis that accounted for these sample biases found that early abiogenesis was still consistent with life being rare, as long as one did not presuppose a particular order of magnitude for the transition rate (Spiegel and Turner, 2012). Here, we generalize this Bayesian analysis to a chain of multiple evolutionary transitions, drawing inspiration from the work of Carter (1983) and others (Hanson, 1998; Carter, 2008; Watson, 2008; McCabe and Lucas, 2010) that sought to explain why intelligent life emerged so late in Earth's history. The transitions we consider include abiogenesis, eukaryogenesis, the emergence of sexual reproduction, and the emergence of language and intelligence, although the model can be applied more broadly. These particular transitions are selected because large scientific uncertainty remains around how frequent such transitions are, and because they were prerequisites for the existence of intelligent life on Earth.
2. Context
The issue of how likely life and intelligence are to emerge on planets in the Universe has long been a mainstay of the SETI-related debates, both as terms in the Drake equation (Vakoch and Dowd, 2015) and whether the endeavor is even rational (Ćirković, 2013).
While some of the attempts at bounding or estimating life are based on astrophysical considerations of planet formation, habitable zones, and other abiotic properties (Kasting et al., 1993; Ward and Brownlee, 1999; Lineweaver et al., 2004; Spiegel et al., 2008; Lammer, et al., 2009; Johnson and Li, 2012; Rushby et al., 2013; Loeb et al., 2016), some of the most contested probabilities deal with abiogenesis, and the emergence of complex life and intelligence. Estimates of the probability of abiogenesis per planet in the literature range from truly microscopic (due to the need to search combinatorially vast spaces) (Hart, 1980), over the small (values giving on the order of one civilization per observable Universe) (Blum, 1965), to modest (Lineweaver et al., 2002), to so large that they predict life in nearly any habitable environment (De Duve, 1995; Halley, 2012). Similarly, the fraction of life-bearing planets with complex life (and intelligence) can be estimated to be very high (Sagan, 1963; Wallenhorst, 1981), moderate (1%) (Billingham et al., 1979; Bounama et al., 2007), or very low (Behroozi and Peeples, 2015). Indeed, for both one can find estimates in the literature and based on models spanning 100 orders of magnitude (Scharf and Cronin, 2016; Sandberg et al., 2018).
One of the oldest arguments against SETI is the biological contingency argument (Simpson, 1964): the evolution of anything similar to humans has a minuscule probability since biological evolution is dominated by contingency, is radically open-ended, and has no determinism or tendency toward intelligence. Even in similar environments, the chance of getting “humanoids” is minimal, and most environments will be vastly different. This is the same argument used by Mayr in his debate with Sagan: out of the approximately 50 billion species on Earth, only humans evolved intelligence, suggesting a low probability (Mayr, 1995a, 1995b, 1995c).
Sagan (1995) countered by noting that if there are enough possible pathways, even individually very unlikely paths can in sum give a high probability of an intelligent outcome. He also noted that extrapolating from our case is either valid, and we should expect Earth to be an average sample, or it is improper to extrapolate, in which case Mayr's argument fails. While the biological contingency argument can be attacked in other ways, for example, by emphasizing convergent evolution (Puccetti, 1968; Morris, 2003), and supported by noting the lack of convergent evolution toward human-like intelligence in the fossil record (Lineweaver, 2009), the key issue is how representative the Earth's biosphere history is (Rospars, 2013).
2.1. The Carter argument
Intelligent life emerged on a timescale similar to that of Earth's lifetime. It took 4 Ga for intelligent life to emerge, and in perhaps less than 1 Ga, the increasing luminosity of the Sun will likely destroy Earth's ability to support complex life, due to increased surface temperatures (Franck et al., 2006) and an eventual breakdown in the carbon cycle (Lenton and Bloh, 2001). Intelligent life therefore emerged on a timescale within an order of magnitude of our star's lifetime. This is puzzling, as the timescales associated with biological and stellar evolution are driven by fundamentally different processes and thus ought to be uncorrelated.
Carter (1983) noticed this coincidence and proposed a resolution to the puzzle based on observation selection effects. Letting τ⊙ denote the lifetime associated with our star, and τ be the timescale it takes for evolution to produce intelligent life, one can analyze three possibilities: τ⊙ ≫ τ, τ⊙ ≈ τ, or τ⊙ ≪ τ, denoting the situations in which the lifetime of the star either greatly exceeds the timescale associated with intelligent life, approximately equals it, or is greatly exceeded by it. Carter argues that a priori, the possibility that τ⊙ ≈ τ is exceptionally unlikely, leaving τ⊙ ≫ τ and τ⊙ ≪ τ as realistic alternatives. We can also rule out τ⊙ ≫ τ with high probability, given that intelligent life did not emerge exceptionally early when compared with the Sun's lifetime. This brings us to the possibility that τ⊙ ≪ τ. This would mean that most stars will never support intelligent life, as the star will burn out before such life emerges. However, in the rare locations in which intelligent life does emerge, it will find itself emerging within the lifetime of the star, and moreover is most likely to observe τ⊙ ≈ τ, consistent with our own observations. Observation selection effects therefore explain why we see these timescales tightly coupled, even if such an outcome is a priori unlikely.
In the same article, Carter proposed a simple model of evolutionary transitions to describe the process of intelligent life emerging. The model proposes that intelligent life requires n “critical steps,” each of which occurs at some rate λ. He further stipulates that λ−1 > τ⊙, so that the probability per unit time of the critical step is low enough that the time it takes for each critical step will typically exceed the lifetime of the star. A number of interesting properties follow from this model. First, the probability that the final transition occurs at time t is proportional to tn, so that the final critical step is likely to occur toward the end of habitable time remaining. Second, the amount of time remaining will be roughly equal to τ⊙/(n + 1), allowing one to estimate the number of critical steps that occurred in Earth's evolutionary history simply by knowing the amount of time left in Earth's habitable lifetime.
When Carter originally proposed the model, it was thought that the biosphere could last for another 4 Ga, which in turn suggested that there were likely only one or two critical steps in our evolutionary history. Subsequent improvements in climate models led to additional research that suggested that the time remaining is substantially shorter, on the order of 1 Ga (Caldeira and Kasting, 1992). A number of researchers have returned to Carter's critical step model and re-estimated the number of critical steps predicted by the remaining lifetime of the biosphere. Watson (2008) found that the best fit was with four critical steps, while Carter (2008) suggested between five and six. Waltham (2017) went further to demonstrate that models up to 12 critical steps still fall within a 95% confidence interval. Using the Carter model without further hard steps [e.g., just abiogenesis, as in Lineweaver et al. (2002) and Spiegel and Turner (2012)] produces significantly different estimates from including hard steps (Flambaum, 2003). The hard step model can also be combined with estimates of the window length (Lingam and Loeb, 2019), or even possible early windows for abiogenesis that later close (Lineweaver and Davis, 2003).
Here, we quantify the Carter argument in Bayesian terms. Rather than hold λ fixed and estimate n as done in past literature (i.e., estimate the number of critical steps while assuming λ−1 ≫ τ⊙), we hold n fixed and estimate λ (i.e., determine what the timing of each evolutionary transition says about its rate). This has the advantage of quantifying the data, priors, and/or assumptions that would be needed to overturn the Carter argument. Quantifying the Carter argument also helps highlight exactly how strong the argument holds. Frank and Sullivan (2016) argue that as long as the odds that intelligent life emerges on a habitable planet are >1 in 1024, we will not be alone in the observable Universe. However, we find that for reasonable priors, the Carter argument places substantial probability on the odds being <1 in 1024.
3. A Simplified Model of Evolutionary Transitions
3.1. The generalized Carter model
Let us assume that intelligent life requires a sequence of n evolutionary transitions. We assume that the transitions must occur sequentially, so that the second transition cannot occur until after the first one, the third not until after the second, and so on. Let xi be the timing of the ith transition, and let ti be the time it takes between the ith transition and the previous one, so that ti = xi − xi − 1. We set x0 = 0, representing the earliest possible time that the first transition could have occurred. We assume that once an evolutionary transition is possible (i.e., once the previous transition has occurred), it occurs at a constant average rate λi, so that each ti is exponentially distributed with an expected transition time of βi = 1/λi.
The joint probability density function for the transition times is the product of exponentials:
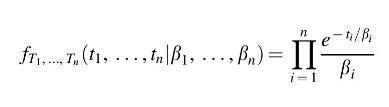
We can calculate the probability that all of the transitions successfully occur before the lifetime of Earth using the cumulative distribution function of the final transition time FXn(xn|β1,…,βn). This is done by using the properties of the hypoexponential distribution (Amari and Misra, 1997), which we describe in the Appendix.
3.2. A Bayesian analysis of transition times
Our objective is to estimate evolutionary transition rates, given how long it took to complete each transition. This can be found by using a Bayesian update as follows:
![]()
where t is the sequence of transition times t1,…,tn, β denotes our β parameters, and P(β) is a prior density over the expected transition time parameters. The term P(t|β), the probability of observing transition times t given the parameters β, is equivalent to the likelihood function as follows:
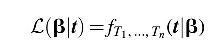
However, this likelihood function needs to be renormalized to account for the fact that we can only observe these data if all evolutionary transitions occurred before the end of Earth's lifetime. Accounting for this sample bias can be done by dividing the likelihood L(β|t) by the probability that all transitions occurred within the lifetime of Earth. If L is the lifetime of Earth, then our adjusted likelihood function is as follows:
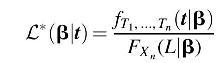
where 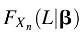 is the probability that all transitions occur before the end of Earth's lifetime.
is the probability that all transitions occur before the end of Earth's lifetime.
3.3. Limiting behavior of the likelihood
We can use limits to evaluate the likelihood of expected transition times that are arbitrarily large. Setting all rate parameters equal, so that all , one can show that for a model with n transitions, taking the limit as β → ∞ results in a likelihood of the following:
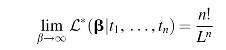
A more detailed proof of this limit is provided in the Appendix. This nonzero constant highlights our first key result, which is that the likelihood does not diminish to zero even as the expected transition times go to infinity. Extremely improbable evolutionary transitions will still be observed by intelligent life if such transitions are crucial to the existence of such intelligence, and this holds true no matter how improbable the transition event.
4. The Data and Priors
The model requires data for the starting and ending points for Earth's habitability, as well as the timing of the four transitions we evaluate (the origin of life, eukaryotes, sex, and intelligence). Earth's oceans are thought to have formed between 4.4 and 4.2 billion years ago (Gya) (Peck et al., 2001; Cavosie et al., 2005), which we take to be the earliest point at which life could form on Earth. Our earliest evidence of life dates between 4.1 and 3.5 Gya (Bell et al., 2015; Awramik, 1992), while the earliest evidence of eukaryotic life has been dated between 1.6 Gya and almost 1.9 Gya (Parfrey et al., 2011; Betts et al., 2018). Sexual reproduction has been hypothesized to have emerged concurrently with eukaryotic life, with firm evidence of its existence at 1.2 Gya (Butterfield, 2000). We date the arrival of human language, symbolic reasoning, and intelligence to be approximately present day, with the arrival of Homo sapiens at 200,000 years ago and the earliest artwork around 80,000 years ago (Pääbo, 2014). We assume the habitability of Earth will end in 0.8–1.3 Ga due to the increasing luminosity of the Sun. We summarize these data in Table 1.
Table 1.
Data for Evolutionary Transition Timing and Biosphere Start/End Dates
| Transition | Date, Gya | Source | Method |
|---|---|---|---|
| Abiogenesis | >4.10 ± 0.01 | Bell et al. (2015) | Carbon isotope ratio |
| Abiogenesis | 3.9 | Betts et al. (2018) | Molecular clock |
| Abiogenesis | >3.86 ± 0.01 | Mojzsis et al. (1996) | Carbon isotope ratio |
| Abiogenesis | >3.77–4.28 | Dodd et al. (2017) | Microfossils, isotope ratio |
| Abiogenesis | >3.5 | Awramik (1992) | Microfossils, stromatolites |
| Cyanobacteria | <10 Ma | Lazcano and Miller (1994) | Molecular evolution model |
| Eukaryotes | <1.84 | Betts et al. (2018) | Molecular clock |
| Eukaryotes | >1.87–1.68 | Parfrey et al. (2011) | Molecular clock |
| Sex | >1.2 | Butterfield (2000) | Fossils of red algae |
| Intelligence | ≈0 | Pääbo (2014) | Oldest artwork |
| Boundary | Timing | Source | Method |
|---|---|---|---|
| Oceans form |
4.40 Gya |
Peck et al. (2001) |
Oxygen isotope ratio |
| Oceans form |
4.3–4.2 Gya |
Cavosie et al. (2005) |
Oxygen isotope ratio |
| Biosphere ends |
0.8–1.2 Ga |
Franck et al. (2006) |
Climate model |
| Biosphere ends |
0.9–1.5 Ga |
Caldeira and Kasting (1992) |
Climate model |
| Biosphere ends |
≈1.0 Ga |
Kasting (1988) |
Climate model |
| Biosphere ends | 1.8–3.3 Ga | Rushby et al. (2013) | Climate model |
Biosphere end dates are given for eukaryotic life. Extremophiles may persist beyond the dates given.
4.1. The priors for transition rates
The prior distributions express subjective beliefs and uncertainties about evolutionary transition times, which are then updated to a posterior distribution based on the observed data. Given the large scientific uncertainties surrounding the “true” rate for each transition (abiogenesis, eukaryogenesis, sexual reproduction, evolution of intelligence), we begin by considering an uninformative prior: a log-uniform distribution for each transition rate. Following Spiegel and Turner (2012), this is equivalent to saying we have no prior information that informs us of even an order-of-magnitude estimate of each transition time (βi). In addition to being uninformative, the log-uniform prior is thought to be most appropriate given that it is also invariant to the choice of parameterization of event frequencies such as the mean waiting time for an event (β) or the mean number of events per unit time (β−1). In contrast, a uniform prior on mean waiting time per se would, for an interval bounded by 10−10 and 1010 Ga, imply ≈0.9 confidence in values near the upper bound, >108 Ga, while a uniform prior over the same interval expressed in frequency terms would imply ≈0.9 confidence in values near the lower bound on mean waiting times, <10−8 Ga.
To be well defined, each log-uniform distribution needs upper and lower bounds. Note that the selection of these bounds introduces an assumption that is no longer “noninformative.” To create an exceptionally conservative lower bound, we assume each expected evolutionary transition time cannot be faster than a rapid bacterial doubling time (roughly 10−14 Ga). Appropriately conservative upper bounds on expected transition times are more difficult to produce without controversy. Although some evolutionary transitions could be the result of incremental and deterministic processes, they could also require a precise combination of extremely rare events.
4.2. Combinatorial models for upper bounds
In general, if an evolutionary transition requires a specific combination of N binary elements, transition rates to any particular state decline as 2−N. Protein folding is one example that can serve as a more general analogy for why extremely long transition times should be considered. Folding of a 300 residue sequence can be naively modeled as a random search through a space of over 10285 conformational states (the bond between a given pair of residue is described by φ and ψ torsional angles, each typically regarded as occupying one of three low-energy conformations). Given this, it would take times the present age of the universe for a particular folding to occur, even assuming a sampling rate of 1 trillion conformational states per molecule per second and a volume of concentrated protein solution the size of Earth's oceans. As protein sequences have evolved to fold reliably, most proteins typically fold within seconds, driven by a so-called funnel in the free-energy landscape (Dill and Chan, 1997). However, a prebiotic world would have no evolutionary process to shape such a funnel (i.e., variation with selection), and perhaps no reliable mechanism to assemble polymeric components of the requisite kinds. If so, then expected transition times for abiogenesis could be truly immense.
The transition to eukaryotic life also involves similar “chicken and egg” difficulties, with uncertainty on how an archaeon acquired a proto-mitochondrion, since endocytosis requires complex machinery only present in eukaryotes (Lane, 2011). A second potential hurdle for eukaryogenesis was the survival of the first prokaryotic host with a bacterial symbiont. Without the protection of spliceosomes and a nucleus, the prokaryotic host would be disrupted by extensive intron transfer from the lysis of its symbionts, resulting in few functional proteins (Koonin, 2011). The chimera cell would need to evolve these complex defenses faster than the mutation ratchet effect driving the (already tiny) population to extinction, which could have also required a rare specific outcome among a vast combinatorial space.
Combinatorial models could apply to the evolution of language and intelligence as well. Human language is thought to be fundamentally different than other forms of animal communication, and fundamental to our general intelligence via the human usage of the merge operation (the ability to combine two items into an unordered set) and the resulting ability to construct hierarchal and recursive expressions (Berwick and Chomsky, 2016). If hierarchal and recursive language only results after obtaining a specific combination of neutral alleles, the probability could be extremely low that each allele would spread to fixation and combine to result in intelligence. Some argue that language acquisition was subject to selection pressure, similar to the gradual evolution of the eye (Pinker and Bloom, 1990), but others argue that language arose suddenly (Bolhuis et al., 2014), and that its origin was a consequence of biological spandrels or exaptations (Tattersall, 2008). Even more pessimistic models might incorporate fitness costs associated with large brains, both metabolically and in terms of high levels of parental care (Mayr, 1994; Lineweaver, 2009).
In the presence of this wide uncertainty, we proceed by initially setting the upper bound of each before 1010 Ga and calculating the posterior distribution. We subsequently examine what happens if we change the priors to be even more conservative and discuss whether such conservative priors are plausible.
5. Results of the Model, Data, and Priors
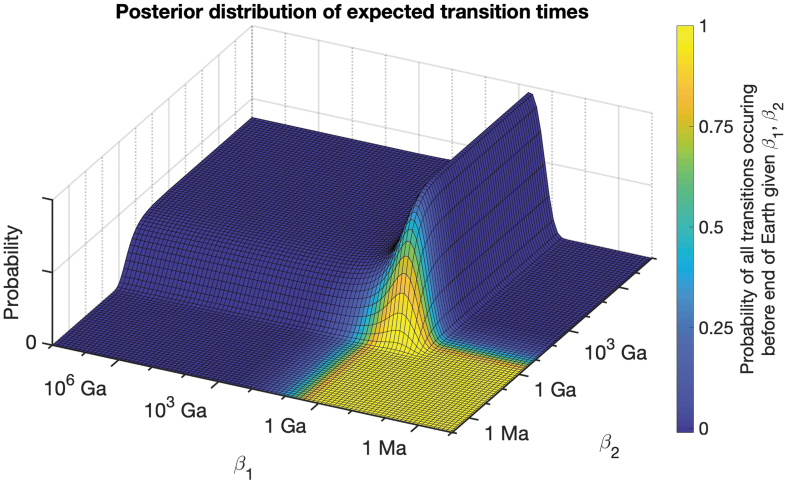
Our Bayesian calculation is repeated for a variety of combinations of evolutionary transitions (Fig. 1 and Table 2). The posterior probability of expected transition times is maximized around the transition time found in the fossil record (e.g., if abiogenesis took 1 Ga, then posterior probability is maximized along values of β1 = 1 Ga). Dramatically fast rates are assigned very low posterior probability (e.g., we can confidently rule out that an evolutionary transition that took 1 Ga does not have an expected transition time of 0.1 Ga). However, the calculation produces an interesting asymmetry, since dramatically slow rates are not assigned low probability in the same way. In fact, the fossil record data are consistent with expected transition times that exceed observed transition times, even by many orders of magnitude (with posterior probability leveling off at a nonzero value when all βi become sufficiently large). Expected transition times can become arbitrarily large while still being consistent with observed transition times, as suggested by the earlier results of nonzero likelihood in the limit when transition times went to infinity. This phenomenon is caused by observation selection effects, since even astronomically rare transitions will be observed if they were prerequisites for intelligent observers. Failing to account for this selection bias produces results that almost guarantee that all evolutionary transitions will occur within 100 Ga.
FIG. 1.
Posterior distribution of expected transition times in a two-step model (abiogenesis with expected time β1 and the emergence of human intelligence with expected time β2). Parameter combinations of rapid rates of transition, such as those resulting in expected transition times of 1 Ma, are inconsistent with the fossil record data and thus have a posterior probability close to zero. Conversely, expected transition times exceeding 106 Ga are compatible with the data, with posterior probability asymptoting along a nonzero constant as the transition times approach infinity. As a result, parameter combinations resulting in intelligent life before the end of the Earth constitute a very narrow slice of posterior probability (marked in yellow). For example, <3% of posterior probability is assigned to parameters that result in the final transition occurring before the end of Earth's lifetime with a >1% chance (second row in Table 2, which also provides the data used for this figure). Color images are available online.
Table 2.
Evolutionary Transition Times and Implied Posterior Probability of Reaching Final Transition
| Transition times, Gya |
|
|
Posterior weight |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0th | 1st | 2nd | 3rd | 4th | L | N | 10−2 | 10−12 | 10−24 |
| 4.3 | 4.2 | — | — | 0 | 5.8 | 2 | 0.068 | 0.40 | 0.83 |
| 4.2 | 3.8 | — | — | 0 | 5.2 | 2 | 0.026 | 0.25 | 0.68 |
| 4.2 | 3.8 | 1.8 | — | — | 5.2 | 2 | 0.041 | 0.27 | 0.70 |
| 4.2 | — | 1.8 | — | 0 | 5.2 | 2 | 0.012 | 0.18 | 0.61 |
| 4.3 | 4.2 | 1.8 | — | 0 | 5.8 | 3 | 0.0064 | 0.11 | 0.43 |
| 4.2 | 3.8 | 1.8 | — | 0 | 5.2 | 3 | 0.0022 | 0.059 | 0.30 |
| 4.2 | 3.8 | 1.8 | 1.2 | — | 5.2 | 3 | 0.0056 | 0.080 | 0.34 |
| 4.2 | 3.8 | 1.8 | 1.2 | 0 | 5.2 | 4 | 0.0003 | 0.014 | 0.11 |
| 4.3 | 4.2 | 1.8 | 1.7 | 0 | 5.8 | 4 | 0.0027 | 0.056 | 0.26 |
| 2 | 0.42 | 0.66 | 0.94 | ||||||
| 3 | 0.27 | 0.51 | 0.84 | ||||||
| 4 | 0.18 | 0.39 | 0.71 | ||||||
| Prior weight | |||||||||
A table of evolutionary transition combinations and for each, a proportion of posterior probability assigned to parameters that achieve the final transition with probability >10−2, 10−12, and 10−24
As the number of included transitions increases, the prior and posterior probability of reaching the final transition falls.
The importance of this result is that the vast majority of the parameter range consistent with the fossil record is inconsistent with expecting the final transition to occur within the window of Earth's habitability, suggesting that intelligent life is highly improbable. For a model with two transitions, over 90% of the posterior probability is assigned to rate parameters that would result in less than a 1% chance of achieving the final transition within Earth's lifetime (Table 2). This proportion of parameter space increases to 99% in a three-step model and a four-step model (Table 2). A substantial amount of posterior probability is even assigned to combinations of transition rates that have a less than 10−12 or 10−24 chance of reaching the final transition within the time that Earth is habitable (corresponding to on the order of 10−12 stars in our galaxy or 10−24 stars in the observable universe). For example, a three-transition model has over 90% of posterior probability assigned to rates that have less than a 10−12 chance of reaching the final transition (Table 2). If complex or intelligent life beyond Earth requires analogous evolutionary transitions, then the fossil record combined with uninformative priors suggests that such life is exceptionally rare.
5.1. Priors required to change the result
Our posterior estimates favored the hypothesis that the selected evolutionary transitions were exceptionally rare. However, the strength of this result will change depending on the Bayesian prior. Increasing (or decreasing) the amount of prior probability assigned to long expected transition times (e.g., by increasing or decreasing the upper bounds on the prior) will increase (or decrease, respectively) the amount of posterior probability assigned to long transition times. This is because the fossil record data are equally consistent with long transition times or extremely long transition times, and thus, only the prior rather than the data determines to what extent such extremely long transition times should be considered. Bayesian priors are meant to capture wide scientific uncertainty and a priori assumptions, so any upper bound, including our arbitrary selection of 1010 Ga, will be subject to controversy. It is perhaps more instructive to determine how conservative the priors would need to be to reach the conclusion that the evolutionary transitions should be expected within Earth's lifetime, and then ask whether limiting the prior in such a way would be reasonable.
We can calculate how low the upper bound of each prior would need to be to produce 10% or 50% of posterior weight on parameter values that predict intelligent life within Earth's lifetime with a greater than 1% or 10% chance (Table 3). To get such a result, the bounds need to be unrealistically small (e.g., setting a maximum transition time of 40 Ga for a three-step model to get 10% weight with a greater than 10% chance of life). Given the enormous uncertainties around the processes underpinning these evolutionary transitions, it seems excessively conservative to claim we should limit our priors in such a way.
Table 3.
Upper Bound Needed on the Log Uniform Before Getting a High Probability of Intelligent Life
| 10% of posterior weight such that |
50% of posterior weight such that |
|||
|---|---|---|---|---|
| n | P(life) >1% | P(life) >10% | P(life) >1% | P(life) >10% |
| 2 | 104.8 Ga | 103.1 Ga | 101.8 Ga | 101.2 Ga |
| 3 | 102.4 Ga | 101.6 Ga | 101.2 Ga | 100.8 Ga |
| 4 | 101.8 Ga | 101.2 Ga | 101.0 Ga | 100.6 Ga |
Data used for transition dates were 4.2 Gya for start of habitability window, 3.8 Gya for abiogenesis (used in all three models), 1.8 Gya for eukaryotes (used when n = 3 and n = 4), 1.2 Gya for sexual reproduction (used when n = 4), and a total habitability window of 5.2 Ga. The other transition in all models was considered to be the evolution of intelligence close to present day.
5.2. Data required to change the result
Discovering a second independent instance of an evolutionary transition (e.g., a branch of life unrelated to our universal common ancestor) would be a dramatic development that would change our estimates of transition rates. If we assume the transition rate remains constant over time, we can include such information in a Bayesian update, multiplying the previous posterior distribution by a likelihood function that incorporates the new data. This likelihood function is L(βi) = (1 − e−1/βi)τ, the probability that one or more additional transitions occurred in time τ, where τ is the time window in which the transitions could have occurred (specifically, after the prerequisite transition but before present day).
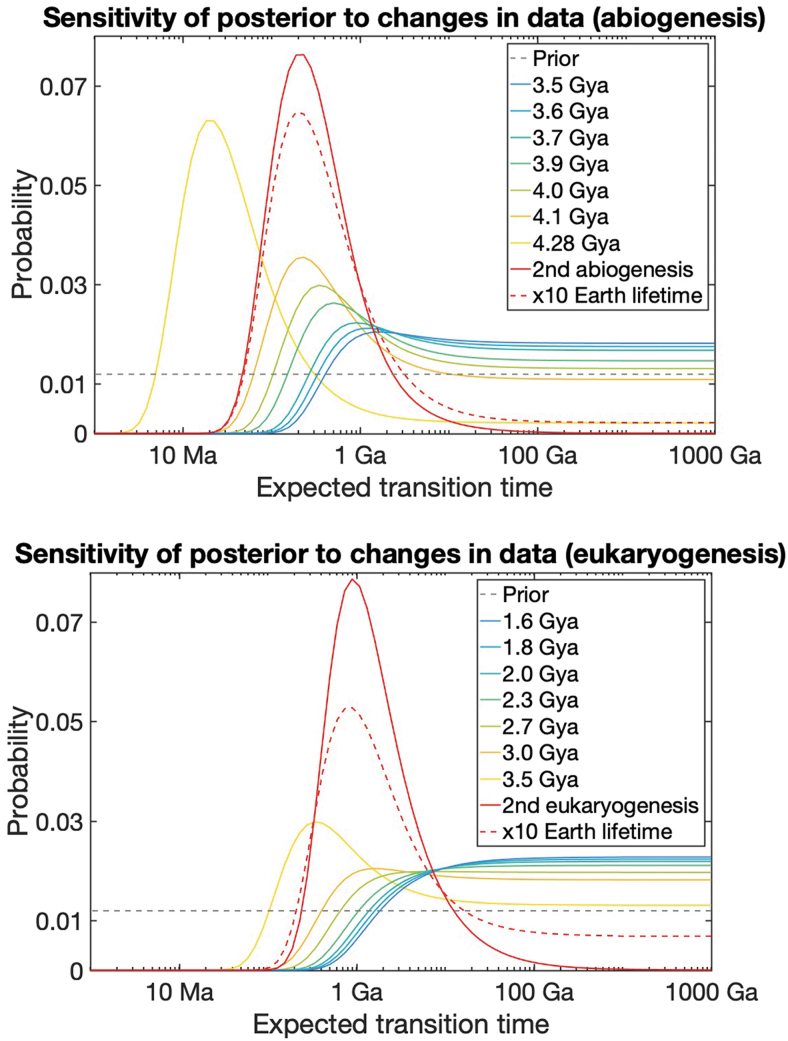
Notably, the likelihood of extremely large βi goes to zero with evidence of additional transitions (Fig. 2, red solid line). Discovering additional independent instances of a transition therefore rules out the possibility that a particular evolutionary transition is exceptionally unlikely. Indeed, multicellular life is thought to have emerged over 40 times, demonstrating that it is not an astronomically rare evolutionary transition. Finding even earlier evidence of a successful evolutionary transition could also change our estimates. We conduct a sensitivity analysis of our posterior estimates to changes in the fossil record data for abiogenesis and eukaryogenesis (Fig. 2). The primary effect of earlier evidence is to adjust the maximum likelihood peak (Fig. 2, yellow to blue spectrum). However, excluding the possibility of extremely long expected transition times requires an exceptionally rapid transition (on the order of 10s of millions of years). For example, finding evidence of eukaryotic life 3 billion years ago would still be insufficient to rule out expected transition times exceeding 1000 Ga (Fig. 2). The reason for this is that the conclusion holds so long as the habitable lifetime of Earth is roughly within the same order of magnitude as the evolutionary transition time. If it turned out that Earth will naturally remain habitable far longer than current science predicts, this would also be sufficient to overturn the conclusion that any of the transitions are rare (Fig. 2, red dotted line).
FIG. 2.
Posterior distributions of abiogenesis (top) and eukaryogenesis (bottom) transition rates for different timings of abiogenesis and eukaryogenesis. Earlier evidence of transitions pushes some posterior mass to faster transition rates but excluding exceptionally long expected transition times requires finding a second independent instance of the transition or the discovery that Earth will remain habitable for much longer than expected. If we were to find evidence of life occurring within 5 Myr of the start of Earth's habitability, this would also be enough to conclude that abiogenesis is not extremely rare. Color images are available online.
Given the wide uncertainty in the fossil record for when certain evolutionary transitions occurred, we can also examine what happens if we update our priors based on an interval of possible transition times rather than a specific transition time. Let ai be the lower end of the range of possible transition times for the ith transition (e.g., the fastest plausible transition time perhaps only tentatively supported by the fossil record), and bi be the upper end of the range (e.g., a conservative estimate where fossil evidence is clear). To update on these intervals, we use the following likelihood function:
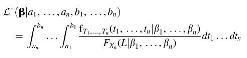
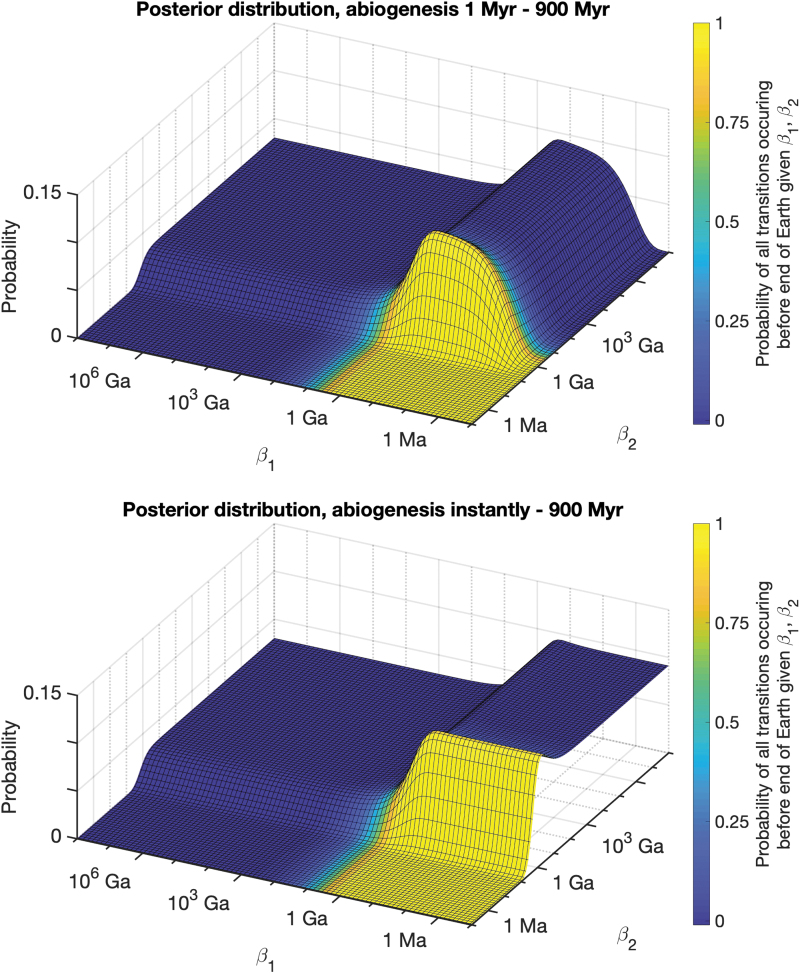
In cases where the fossil record cannot definitively rule out an exceptionally short (or even instantaneous) transition time [e.g., the origin of life or sexual reproduction, see Pearce et al. (2018)], this can cause the posterior estimates to include more weight on rapid transition rates (Fig. 3, top). When abiogenesis could take between 0 and 900 Myr, our posterior is consistent with abiogenesis being common or rare, replicating the results of Spiegel and Turner (2012). However, even when abiogenesis is common, our posterior still suggests that intelligent life is rare, supporting the Rare Earth Hypothesis (Ward and Brownlee, 1999). For a two-step model with abiogenesis taking between 0 and 900 Myr and eukaryotes taking between 800 and 2800 Myr, only 16% and 54% of posterior probability weight are assigned to parameters that would result in the transitions occurring successfully within the lifetime of Earth with >1% and 10−12 chance, respectively.
FIG. 3.
Posterior distributions updated on an interval of possible timings for the origin of life and eukaryotic life. On the top, we assume abiogenesis took between 1 and 900 Myr to occur, and on the bottom we assume that abiogenesis may have taken an amount of time ranging from instantaneously to 900 Myr. Although more posterior probability mass is assigned to rates that have a high probability of intelligent life emerging (denoted in yellow), the majority of posterior mass is still on rates that are incompatible with intelligent life occurring within the habitable lifetime of Earth (denoted in blue). Color images are available online.
5.3. Assumptions required to change the result
Our model makes a number of simplifying assumptions, primarily that each evolutionary transition has a constant probability of happening per unit time throughout Earth's history. Although this is crude, we believe this is the best model because it requires the fewest biological assumptions. Still, it is worth discussing where the constant probability assumption is likely to fall short and to what extent our results rely on this assumption.
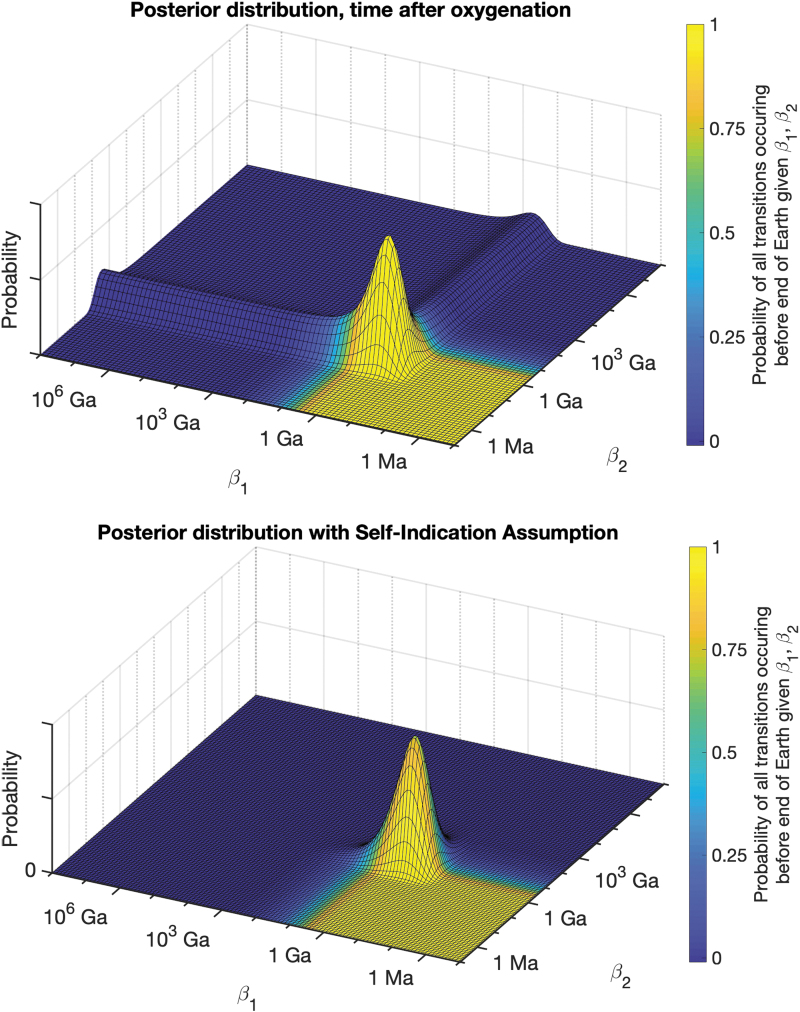
There are a number of reasons why the probability of an evolutionary transition could change over time. Perhaps most importantly, some evolutionary transitions may have required high oxygen concentrations as a source of energy, and oxygen concentrations have changed dramatically over Earth's history (Holland, 2006). The fact that oxygen concentrations have became high enough to support humans only in the past 800 Myr or so has led to some speculation that a planetary oxygenation time is the primary rate-limiting step to intelligent life (Catling et al., 2005). Relatedly, complex life on land requires shielding from ultraviolet radiation, and the emergence of an ozone layer has also been hypothesized to be a rate-limiting step that is correlated with stellar evolution, undermining Carter's original argument (Livio, 1999).
To test this, we adjust our model so that the transition rates change over time. The most dramatic example of this is a model in which the final evolutionary transition to intelligent life has a probability of zero until vertebrates on land emerge (0.34 Gya), and that transition has probability zero until Phanerozoic oxygen concentrations are reached (0.8 Gya). This model essentially tells us that these transitions occurred fairly rapidly once oxygen concentrations were high enough, and the results show a much larger peak around fast rates, suggesting a higher probability of intelligent life emerging in the right conditions (Fig. 4, top). However, even these faster transition times are not enough to exclude extremely slow rates. For example, using the previous log-uniform prior with a lower bound of 1010 Ga still results in over 60% of the posterior parameter weight on rates that result in intelligent life with probability less than a 1 in 1012. Overall, accounting for a changing environment in terms of oxygen concentrations does not seem to be sufficient to overturn our key results.
FIG. 4.
(Top) Posterior distribution if we assume two transitions that were made possible only after high oxygenation levels. Given the late oxygenation of Earth's atmosphere, these transition times are short, resulting in higher posterior probability on faster rates. However, arbitrarily slow rates are still not excluded. (Bottom) Posterior distribution if we adopt the self-indication assumption, and weight all parameter combinations by their probability of obtaining intelligent life. Only parameters that are consistent with intelligent life are assigned high probability, and extremely slow rates are ruled out entirely. Color images are available online.
There are numerous other ways in which the evolutionary transition rates could change over time. For example, the total biomass or the number of lineages relevant for a particular evolutionary transition could change over time (e.g., the concentration of potential symbionts for eukaryotic life or the number of candidate animal lineages that could evolve intelligence), as could the rate of catastrophes that could act as evolutionary setbacks (e.g., asteroid impacts during the Late Heavy Bombardment or the rate of gamma ray bursts) (Ćirković et al., 2009). Although these examples make it clear that the assumption of a constant transition rate over time is oversimplified, creating a comprehensive model that incorporates all factors into the evolutionary transition rates would require many more assumptions. We can say in general that any alternative model still needs to explain why certain evolutionary transitions took such a long period of time.
To conclude that intelligent life is common, an alternative model would not only need to explain why intelligence emerged on roughly the same timescale of Earth's habitable lifetime, but also why eukaryotic life and other evolutionary transitions did so. Certainly with enough assumptions one could create a model that guarantees that each transition will occur at roughly the same time that it did on Earth, and then conclude that any Earth-like habitat lasting 5 Ga will have a high probability of hosting intelligent life. However, we ultimately think that the most parsimonious model is the one in which the long transition times are a byproduct of contingency.
In addition to assumptions about how evolutionary transitions occur, we also consider assumptions around how to use the information that we exist as observers. So far, we have assumed that we can derive no information on the probability of intelligent life from our own existence, since any intelligent observer will inevitably find themself in a location where intelligent life successfully emerged regardless of the probability. Another line of reasoning, known as the “Self-Indication Assumption” (SIA), suggests that if there are different possible worlds with differing numbers of observers, we should weigh those possibilities in proportion to the number of observers (Bostrom, 2013). For example, if we posit only two possible universes, one with 10 human-like civilizations and one with 10 billion, SIA implies that all else being equal we should be 1 billion times more likely to live in the universe with 10 billion civilizations. If SIA is correct, this could greatly undermine the premises argued here, and under our simple model it would produce high probability of fast rates that reliably lead to intelligent life (Fig. 4, bottom). However, embracing SIA leads to a number of other very counterintuitive results, such as essentially guaranteeing that the universe is exceptionally large or infinite even without accounting for cosmological evidence (Bostrom and Ćirković, 2003), or giving substantial probability to any bizarre theory that proposes a large enough population of observers to overwhelm the a priori implausibility of the theory (e.g., a theory that each planet has 10^10^100 copies of itself on “other planes” would seem hard to refute if one adopted SIA) (Olum, 2002). Adopting SIA thus will undermine our results, but also undermine any other scientific result that would suggest a lower number of observers in the Universe. The plausibility and implications of SIA remain poorly understood and outside the scope of our present work. We proceed by proposing a set of testable predictions.
6. Testable Predictions
The model offers a number of testable predictions. First, we conclude that intelligent life is exceptionally rare and that we may possibly be the only intelligent civilization within the observable universe, so long as we assume that intelligent life elsewhere requires similar evolutionary transitions. Although this may seem like a large assumption, there are good reasons to believe that many evolutionary transitions have universal properties (Levin et al., 2017). It also follows if we reason that our civilization is typical. If there were substantially easier evolutionary pathways to intelligent life that did not require such evolutionary transitions, we should expect to observe this easier evolutionary history instead. Although it is hard to show beyond doubt the absence of extraterrestrial intelligence, so far all of our astronomical data are consistent with being alone (Tipler, 1980). A handful of other testable predictions follow from the model as well.
6.1. Exceptionally rare transitions
The unlikeliness of different evolutionary transitions can also be tested more directly as we learn more about the underlying physical and biological processes of different evolutionary transitions or find evidence that a transition occurred more than once. Abiogenesis and eukaryogenesis both involve unexplained or partially unexplained paradoxes which could be resolved through further research. Examples include Eigen's paradox, describing the mystery of how systems for error correction evolved in the absence of error correction, with similar “chicken and egg” problems in the case of eukaryogenesis. Our model predicts that these paradoxes and similar ones may only be resolved by allowing for exceptionally rare chance events. If subsequent research discovers relatively easy pathways for each of these transitions (as it has done for multicellular life, for example), this would falsify our prediction (Chen and Kipping, 2018).
6.2. Habitability of red dwarf stars
There is an interesting connection between the evolutionary transitions model and arguments for the habitability of planets orbiting red dwarf stars (Rushby et al., 2013; Waltham, 2017).
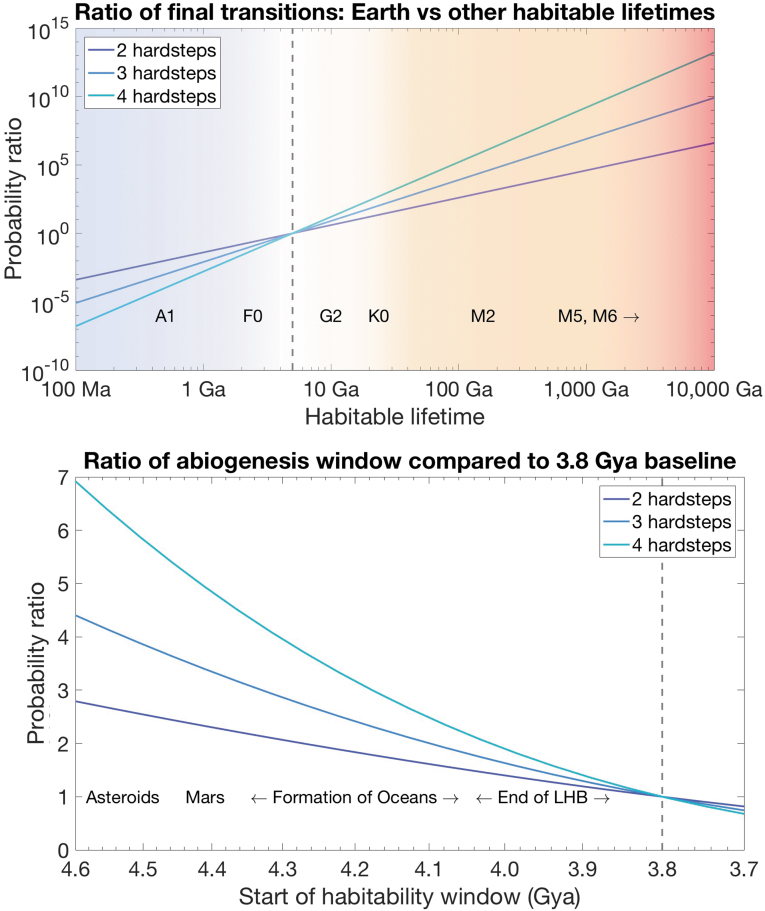
A typical M dwarf star will last 1 trillion years, roughly two orders of magnitude longer than our Sun, and could also be host to habitable exoplanets (Haqq-Misra et al., 2017). However, the habitability of planets orbiting M dwarf stars remains an open question, as tidal locking, solar flares, or other harsh conditions could make these planets inimical to complex life (Zendejas et al., 2010). If we start with the assumption that environments around dwarf stars are just as habitable as our solar system, such that the transition parameters are the same, we can calculate the probability ratio of final evolutionary transitions occurring around such dwarf stars as opposed to a habitat lasting as long as Earth. These probability ratios are heavily skewed in favor of longer lasting environments, by between 4 and 10 orders of magnitude depending on the number of transitions (Fig. 5, top). Assuming we are typical for intelligent life and finding ourselves not orbiting a red dwarf star, our model brings us to a testable prediction that the transition parameters are not similar, and some other factor is reducing the habitability of dwarf star environments by a factor of over 10,000 when compared with the Earth. The strength of this predicted factor is great enough that the prediction ought to be testable when using climate models or other tools currently accessible, and coincide with eventual scientific consensus that dwarf stars are inimical for complex life.
FIG. 5.
(Top) The ratio of final transitions that occur within a 5 Ga habitability window as opposed to a habitable window of another length of time, all else being equal. Habitable environments that last longer are more likely to support the final transition by many orders of magnitude, suggesting that other factors must reduce the habitability of red dwarf stars by many orders of magnitude. (Bottom) Probability ratios of earliest time abiogenesis could have occurred compared with a 3.8 Gya baseline. All else being equal, longer habitability windows have slightly higher probabilities, increasing the credence that the solar system was habitable from an early date (including an extraterrestrial origin of life). Color images are available online.
6.3. Extraterrestrial origin of life?
When was the earliest that life could have emerged? Given our model, we can evaluate this by taking the likelihood ratio between the hypothesis of an early conducive environment (say, with the formation of the oceans at 4.3 Gya) and the hypothesis that life was only possible relatively late in Earth's beginning (say, with the end of the Late Heavy Bombardment at 3.9 Gya). It has also been suggested that Mars was habitable 100 million years before Earth's oceans formed (Sleep and Zahnle, 1998), and that asteroids could have been habitable yet another 100 million years earlier (Abramov and Mojzsis, 2011). If the rate of material transfer between Mars and Earth is high enough to consider the two planets a single environment, we can compare the likelihood ratio between an extraterrestrial origin of life and origin of life on Earth as well. In general, the model favors hypotheses with earlier possible starting dates for the first transition (Fig. 5, bottom), with the strength increasing with the number of transitions included (Davies, 2003; McCabe and Lucas, 2010). The effect is very modest though. For example, the likelihood ratio for a 4.4 Gya starting point versus a 3.8 Gya starting point differs by only a factor of about two to seven. Perhaps more relevant is a prediction that if we were to find life on Mars, it would have emerged extremely early and have a common ancestor with life on Earth.
7. Conclusions
It took approximately 4.5 billion years for a series of evolutionary transitions resulting in intelligent life to unfold on Earth. In another billion years, the increasing luminosity of the Sun will make Earth uninhabitable for complex life. Intelligence therefore emerged late in Earth's lifetime. Together with the dispersed timing of key evolutionary transitions and plausible priors, one can conclude that the expected transition times likely exceed the lifetime of Earth, perhaps by many orders of magnitude. In turn, this suggests that intelligent life is likely to be exceptionally rare. Arriving at an alternative conclusion would require either exceptionally conservative priors, finding additional instances of evolutionary transitions, or adopting an alternative model that can explain why evolutionary transitions took so long on Earth without appealing to rare stochastic occurrences. The model provides a number of other testable predictions, including that M dwarf stars are uninhabitable, that many biological paradoxes will remain unsolved without allowing for extremely unlikely events, and that, counterintuitively, we might be slightly more likely to find simple life on Mars.
Abbreviation Used
- SIA
Self Indication Assumption
- SETI
Search for extraterrestrial intelligence
- LHB
Late Heavy Bombardment
- Ma
million years
- Ga
billion years
- Mya
million years ago
- Gya
billion years ago
Appendix
The Generalized Carter Model
Let us assume that intelligent life requires a sequence of n evolutionary transitions. We assume that the transitions must occur sequentially, so that the second transition cannot occur until after the first one, the third not until after the second, and so on. Let xi be the timing of the ith transition, and let ti be the time it takes between the ith transition and the previous one, so that ti = xi − xi − 1. We set x0 = 0, representing the earliest possible time that the first transition could have occurred. We assume that once an evolutionary transition is possible (i.e., once the previous transition has occurred), it occurs at a constant average rate λi, so that each ti is exponentially distributed with an expected transition time of βi = 1/λi. The joint probability density function for the transition times is the product of exponentials:
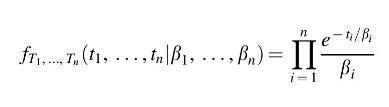
The probability that n transitions successfully occur before a certain point in time (e.g., the lifetime of Earth) can be found by computing the cumulative distribution function
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This project received funding from Jaan Tallinn, Open Philanthropy, and from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No. 669751). This paper reflects only the view of the authors: the ERCEA is not responsible for any use that may be made of the information it contains.
References
- Abramov O and Mojzsis SJ (2011) Abodes for life in carbonaceous asteroids? Icarus 213:273–279
- Amari SV and Misra RB (1997) Closed-form expressions for distribution of sum of exponential random variables. IEEE Trans Reliab 46:519–522 [Google Scholar]
- Awramik SM (1992) The oldest records of photosynthesis. Photosynth Res 33:75–89 [PubMed] [Google Scholar]
- Behroozi P and Peeples MS (2015) On the history and future of cosmic planet formation. Mon Notices Royal Astronom Soc 454:1811–1817 [Google Scholar]
- Bell EA, Boehnke P, Harrison TM, et al. (2015) Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proc Natl Acad Sci U S A 112:14518–14521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick RC and Chomsky N (2016) Why Only Us: Language and Evolution. MIT Press, Cambridge MA [Google Scholar]
- Betts HC, Puttick MN, Clark JW, et al. (2018) Integrated genomic and fossil evidence illuminates life's early evolution and eukaryote origin. Nat Ecol Evol 2:1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham J, Oliver BM, and Wolfe JH (1979) A review of the theory of interstellar communication. In Communication with Extraterrestrial Intelligence, edited by J. Billingham and R. Pešek. Elsevier, Oxford, UK, pp. 47–57 [Google Scholar]
- Blum HF (1965) Dimensions and probability of life. Nature 206:131–132 [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Tattersall I, Chomsky N, et al. (2014) How could language have evolved? PLoS Biol 12:e1001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom N (2013) Anthropic Bias: observation Selection Effects in Science and Philosophy. London, Routledge [Google Scholar]
- Bostrom N and Ćirković MM (2003) The doomsday argument and the self-indication assumption: reply to Olum. Philos Q 53:83–91 [Google Scholar]
- Bounama C, Von Bloh W, and Franck S (2007) How rare is complex life in the Milky Way? Astrobiology 7:745–756 [DOI] [PubMed] [Google Scholar]
- Brocks JJ, Logan GA, Buick R, et al. (1999) Archean molecular fossils and the early rise of eukaryotes. Science 285:1033–1036 [DOI] [PubMed] [Google Scholar]
- Butterfield NJ (2000) Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology 26:386–404 [Google Scholar]
- Caldeira K and Kasting JF (1992) The life span of the biosphere revisited. Nature 360:721. [DOI] [PubMed] [Google Scholar]
- Carter B (1983) The anthropic principle and its implications for biological evolution. Philos Trans R Soc Lond A Math Phys Sci 310:347–363 [Google Scholar]
- Carter B (2008) Five-or six-step scenario for evolution? Int J Astrobiol 7:177–182 [Google Scholar]
- Catling DC, Glein CR, Zahnle KJ, et al. (2005) Why O2 is required by complex life on habitable planets and the concept of planetary “oxygenation time.” Astrobiology 5:415–438 [DOI] [PubMed] [Google Scholar]
- Cavosie AJ, Valley JW, Wilde SA, et al. (2005) Magmatic δ18O in 4400—3900 Ma detrital zircons: a record of the alteration and recycling of crust in the Early Archean. Earth Planet Sci Lett 235:663–681 [Google Scholar]
- Chen J and Kipping D (2018) On the rate of abiogenesis from a Bayesian informatics perspective. arXiv Preprint arXiv:1806.08033 [DOI] [PubMed] [Google Scholar]
- Ćirković MM (2013) Who are the SETI sceptics? Acta Astronaut 89:38–45 [Google Scholar]
- Ćirković MM, Vukotić B, and Dragićević I (2009) Galactic punctuated equilibrium: how to undermine Carter's anthropic argument in astrobiology. Astrobiology 9:491–501 [DOI] [PubMed] [Google Scholar]
- Davies PC (2003) Does life's rapid appearance imply a Martian origin? Astrobiology 3:673–679 [DOI] [PubMed] [Google Scholar]
- De Duve C (1995) Vital Dust: Life as a Cosmic Imperative. Basic Books, New York [Google Scholar]
- Dill KA and Chan HS (1997) From Levinthal to pathways to funnels. Nat Struct Mol Biol 4:10. [DOI] [PubMed] [Google Scholar]
- Dodd MS, Papineau D, Grenne T, et al. (2017) Evidence for early life in Earth's oldest hydrothermal vent precipitates. Nature 543:60–64 [DOI] [PubMed] [Google Scholar]
- Flambaum VV (2003) Comment on” Does the rapid appearance of life on Earth suggest that life is common in the universe?” Astrobiology 3:237–239 [DOI] [PubMed] [Google Scholar]
- Franck S, Bounama C, and Von Bloh W (2006) Causes and timing of future biosphere extinctions. Biogeosciences 3:85–92 [Google Scholar]
- Frank A and Sullivan III WT (2016) A new empirical constraint on the prevalence of technological species in the universe. Astrobiology 16:359–362 [DOI] [PubMed] [Google Scholar]
- Gould SJ (1990) Wonderful Life: The Burgess Shale and the Nature of History. New York, WW Norton & Company [Google Scholar]
- Grosberg RK and Strathmann RR (2007) The evolution of multicellularity: a minor major transition? Annu Rev Ecol Evol Syst 38:621–654 [Google Scholar]
- Halley JW (2012) How Likely is Extraterrestrial Life? Heidelberg, Dordrecht, London, and New York, Springer Science & Business Media
- Hanson R (1998) Must early life be easy? The rhythm of major evolutionary transitions. Unpublished manuscript
- Haqq-Misra J, Kopparapu RK, and Wolf ET (2017) Why do we find ourselves around a yellow star instead of a red star? Int J Astrobiol 17:77–86
- Hart MH (1980) N is very small. In Strategies for the Search for Life in the Universe, edited by Papagiannis, Springer, Dordrecht, pp. 19–25 [Google Scholar]
- Holland HD (2006) The oxygenation of the atmosphere and oceans. Philos Trans R Soc B Biol Sci 361:903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL and Li H (2012) The first planets: the critical metallicity for planet formation. Astrophys J 751:81 [Google Scholar]
- Kasting JF (1988) Runaway and moist greenhouse atmospheres and the evolution of Earth and Venus. Icarus 74:472–494 [DOI] [PubMed] [Google Scholar]
- Kasting JF, Whitmire DP, and Reynolds RT (1993) Habitable zones around main sequence stars. Icarus 101:108–128 [DOI] [PubMed] [Google Scholar]
- Koonin EV (2011) The Logic of Chance: The Nature and Origin of Biological Evolution. FT Press, New Jersey [Google Scholar]
- Lammer H, Bredehöft JH, Coustenis A, et al. (2009) What makes a planet habitable? Astron Astrophys Rev 17:181–249 [Google Scholar]
- Lane N (2011) Energetics and genetics across the prokaryote-eukaryote divide. Biol Direct 6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazcano A and Miller SL (1994) How long did it take for life to begin and evolve to cyanobacteria? J Mol Evol 39:546–554 [DOI] [PubMed] [Google Scholar]
- Lenton TM and Bloh W (2001) Biotic feedback extends the life span of the biosphere. Geophys Res Lett 28:1715–1718 [Google Scholar]
- Levin SR, Scott TW, Cooper HS, et al. (2017) Darwin's aliens. Int J Astrobiol 18:1–9 [Google Scholar]
- Lineweaver CH (2009) Paleontological tests: human-like intelligence is not a convergent feature of evolution. In From fossils to astrobiology, edited by J Seckbach and M Walsh. Springer, Dordrecht, pp. 353–368 [Google Scholar]
- Lineweaver CH and Davis TM (2002) Does the rapid appearance of life on Earth suggest that life is common in the universe? Astrobiology 2:293–304 [DOI] [PubMed] [Google Scholar]
- Lineweaver CH and Davis TM (2003) On the nonobservability of recent biogenesis. Astrobiology 3:241–243 [Google Scholar]
- Lineweaver CH, Fenner Y, and Gibson BK (2004) The galactic habitable zone and the age distribution of complex life in the Milky Way. Science 303:59–62 [DOI] [PubMed] [Google Scholar]
- Lingam M and Loeb A (2019) Role of stellar physics in regulating the critical steps for life. Int J Astrobiol 18:527–546 [Google Scholar]
- Livio M (1999) How rare are extraterrestrial civilizations, and when did they emerge? Astrophys J 511:429 [Google Scholar]
- Loeb A, Batista RA, and Sloan D (2016) Relative likelihood for life as a function of cosmic time. J Cosmol Astropart Phys 2016. DOI: 10.1088/1475-7516/2016/08/040 [DOI] [Google Scholar]
- Mayr E (1994) Does it pay to acquire high intelligence? Perspect Biol Med 37:337–338 [Google Scholar]
- Mayr E (1995a) Can SETI succeed? Not likely. Bioastron News 7 [Google Scholar]
- Mayr E (1995b) A critique of the search for extraterrestrial intelligence. Bioastron News 7:2–4, 7, 2–4 [Google Scholar]
- Mayr E (1995c) Answer to __The abundance of life-bearing planets__. Bioastronomy News 7 [Google Scholar]
- McCabe M and Lucas H (2010) On the origin and evolution of life in the Galaxy. Int J Astrobiol 9:217–226 [Google Scholar]
- Mojzsis SJ, Arrhenius G, McKeegan KD, et al. (1996) Evidence for life on Earth before 3,800 million years ago. Nature 384:55. [DOI] [PubMed] [Google Scholar]
- Morris SC (2003) Life's Solution: Inevitable Humans in a Lonely Universe. Cambridge University Press, Cambridge, UK [Google Scholar]
- Olum KD (2002) The doomsday argument and the number of possible observers. Philos Q 52:164–184 [Google Scholar]
- Pääbo S (2014) Neanderthal Man: In Search of Lost Genomes. Hachette, United Kingdom [Google Scholar]
- Parfrey LW, Lahr DJ, Knoll AH, et al. (2011) Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc Natl Acad Sci U S A 108:13624–13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce BK, Tupper AS, Pudritz RE, et al. (2018) Constraining the time interval for the origin of life on Earth. Astrobiology 18:343–364 [DOI] [PubMed] [Google Scholar]
- Peck WH, Valley JW, Wilde SA, et al. (2001) Oxygen isotope ratios and rare earth elements in 3.3 to 4.4 Ga zircons: ion microprobe evidence for high δ18O continental crust and oceans in the Early Archean. Geochim Cosmochim Acta 65:4215–4229 [Google Scholar]
- Pinker S and Bloom P (1990) Natural language and natural selection. Behav Brain Sci 13:707–727 [Google Scholar]
- Puccetti R (1968) Persons: A Study of Possible Moral Agents in the Universe. Macmillan, London, UK [Google Scholar]
- Rospars J-P (2013) Trends in the evolution of life, brains and intelligence. Int J Astrobiol 12:186–207 [Google Scholar]
- Rushby AJ, Claire MW, Osborn H, et al. (2013) Habitable zone lifetimes of exoplanets around main sequence stars. Astrobiology 13:833–849 [DOI] [PubMed] [Google Scholar]
- Sagan C (1963) Direct contact among galactic civilizations by relativistic interstellar spaceflight. Planet Space Sci 11:485–498 [Google Scholar]
- Sagan C (1995) The abundance of life-bearing planets. Bioastron News 7:1–4 [Google Scholar]
- Sandberg A, Drexler E, and Ord T (2018) Dissolving the Fermi paradox. arXiv Preprint arXiv:1806.02404 [Google Scholar]
- Scharf C and Cronin L (2016) Quantifying the origins of life on a planetary scale. Proc Natl Acad Sc U S A 113:8127–8132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG (1964) The nonprevalence of humanoids. Science 143:769–775 [DOI] [PubMed] [Google Scholar]
- Sleep NH and Zahnle K (1998) Refugia from asteroid impacts on early Mars and the early Earth. J Geophys Res Planets 103:28529–28544 [Google Scholar]
- Smith JM and Szathmary E (1997) The Major Transitions in Evolution. Oxford University Press, Oxford, UK [Google Scholar]
- Spiegel DS and Turner EL (2012) Bayesian analysis of the astrobiological implications of life's early emergence on Earth. Proc Natl Acad Sci U S A 109:395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel DS, Menou K, and Scharf CA (2008) Habitable climates. Astrophys J 681:1609 [Google Scholar]
- Tattersall I (2008) An evolutionary framework for the acquisition of symbolic cognition by Homo sapiens. Comp Cogn Behav Rev 3:99–114 [Google Scholar]
- Tipler FJ (1980) Extraterrestrial intelligent beings do not exist. Q J R Astronom Soc 21:267–281 [Google Scholar]
- Vakoch DA and Dowd MF (2015) The Drake Equation: Estimating the Prevalence of Extraterrestrial Life Through the Ages Vol. 8. Cambridge University Press, Cambridge, UK [Google Scholar]
- Wallenhorst SG (1981) The Drake equation reexamined. Q J R Astronom Soc 22:380 [Google Scholar]
- Waltham D (2017) Star masses and star-planet distances for Earth-like habitability. Astrobiology 17:61–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P and Brownlee D (1999) Rare earth. In Copernicus, Springer-Verlag, New York, NY [Google Scholar]
- Watson AJ (2008) Implications of an anthropic model of evolution for emergence of complex life and intelligence. Astrobiology 8:175–185 [DOI] [PubMed] [Google Scholar]
- Zendejas J, Segura A, and Raga AC (2010) Atmospheric mass loss by stellar wind from planets around main sequence M stars. Icarus 210:539–544 [Google Scholar]