Abstract
Hypertension is the leading cause of cardiovascular disease and premature death worldwide. Owing to widespread use of antihypertensive medications, global mean blood pressure (BP) has remained constant or decreased slightly over the past four decades. By contrast, the prevalence of hypertension has increased, especially in low and middle-income countries (LMICs). Estimates suggest that in 2010, 31.1% of adults (1.39 billion) worldwide had hypertension. The prevalence of hypertension among adults was higher in LMICs (31.5%, 1.04 billion people) than in high-income countries (HICs; 28.5%, 349 million people). Variations in the levels of risk factors for hypertension, such as high sodium intake, low potassium intake, obesity, alcohol consumption, physical inactivity and unhealthy diet, may explain some of the regional heterogeneity in hypertension prevalence. Despite the increasing prevalence, the proportions of hypertension awareness, treatment and BP control are low, particularly in LMICs, and few comprehensive assessments of the economic impact of hypertension exist. Future studies are warranted to test implementation strategies for hypertension prevention and control, especially in low-income populations, and to accurately assess the prevalence and financial burden of hypertension worldwide.
INTRODUCTION
Hypertension is the leading preventable risk factor for cardiovascular disease (CVD) and all-cause mortality worldwide.1,2 In 2010, 31.1% of the global adult population (1.39 billion people) had hypertension, defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg.3 The prevalence of hypertension is rising globally owing to ageing of the population and increases in exposure to lifestyle risk factors including unhealthy diets (i.e. high sodium and low potassium intake and lack of physical activity.3 However, changes in hypertension prevalence are not uniform worldwide. In the past two decades, high-income countries (HICs) experienced a modest decrease in hypertension prevalence, while low and middle-income countries (LMICs) experienced significant increases.3 These disparities in hypertension prevalence trends suggest that health care systems in LMICs could be facing a rapidly increasing burden of hypertension and BP-related cardiovascular diseases, in some cases in addition to a substantial burden of infectious diseases.
In this Review, we discuss estimates and trends in mean BP levels and hypertension prevalence, awareness, treatment, and control worldwide. We also examine risk factors for hypertension, strategies for hypertension control, and evidence of the financial burden of hypertension. We conclude by discussing the consequences of current trends in hypertension and areas where more research is needed.
GLOBAL MEAN BLOOD PRESSURE
A study that analyzed data from 844 studies performed in 154 countries with 8.69 million participants estimated that in 2015, the global mean age-standardized systolic BP was 127.0 mmHg in men and 122.3 mmHg in women, whereas the mean age-standardized diastolic BP was 78.7 mmHg in men and 76.7 mmHg in women.4 Higher mean systolic and diastolic BPs in both men and women were found in South Asia, Sub-Saharan Africa, and Central and Eastern Europe, while lower mean BPs were found in high-income Western and high-income Asia-Pacific regions.4 Social and environmental factors, including healthcare access, availability of antihypertensive medications, and regional variations in hypertension risk factors, such as obesity, alcohol consumption, unhealthy diet and lack of physical activity, likely contribute to these regional differences3,4
This study also reported that over the past 40 years, estimated mean BP has remained constant or decreased slightly worldwide.4 Estimated global mean age-standardized systolic BP remained fairly constant in men between 1975 (126.6 mmHg) and 2015 (127.0 mmHg) but decreased slightly in women during this period (from 123.9 mmHg to 122.3 mmHg). Trends for men and women were similar for estimated global mean age-standardized diastolic BP, with very little change in men and a slight decrease in women. However, regional changes in estimated mean BP between 1975 and 2015 were more heterogeneous (Figure 1). In general, HICs experienced a significant BP decrease, while BP in LMIC regions increased.4 Substantial decreases in estimated age-standardized mean systolic and diastolic BP occurred in both men and women in high-income Western and Asia-Pacific regions between 1975 and 2015.4 Estimates suggest that the largest decrease in systolic BP was in the high-income Asia-Pacific region in which systolic BP decreased by 2.4 mmHg per decade in men and 3.2 mmHg per decade in women, whereas the largest decrease in diastolic BP was in the high-income Western region in which diastolic BP decreased by 1.5 mmHg per decade in men and 1.8 mmHg per decade in women. Decreases in mean systolic and diastolic BP were also observed in women in Central and Eastern Europe, Latin America and the Caribbean, the Middle East and North Africa, and Central Asia, while no change in BP was seen in men in these regions.4 In both men and women, systolic and diastolic BP increased in East and Southeast Asia, South Asia, Oceania, and sub-Saharan Africa.
Figure 1. Changes in mean estimated blood pressure by world region between 1975 and 2015.
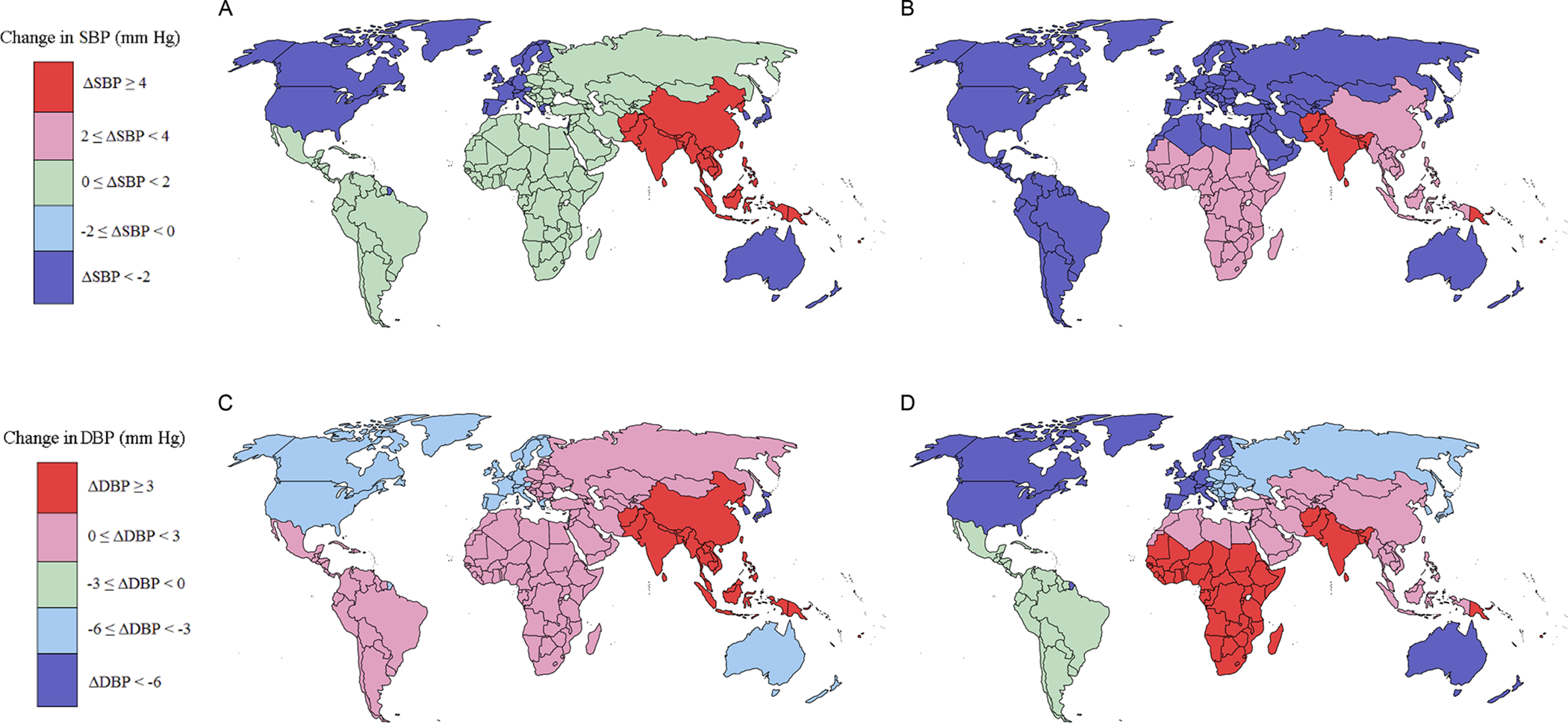
A. Change in systolic blood pressure (BP) in men. B. Change in systolic BP in women. C. Change in diastolic BP in men. D. Change in diastolic BP in Women. Data obtained from reference4.
These regional changes in mean systolic and diastolic BP have led to disparities in the burden of hypertension.3 Improvements in prevention, detection, and treatment of BP in HICs have likely contributed to lower BP levels in these regions.3–7 By contrast, limited healthcare resources together with population ageing and urbanization, which is associated with reductions in physical activity and increases in unhealthy diets, are potential drivers of BP increases in LMICs.8–10 In both HICs and LMICs, women have higher proportions of hypertension awareness, treatment, and control than men.3 These differences could contribute to the greater BP reductions observed in women than men in some regions
GLOBAL BURDEN OF HYPERTENSION
Based on an analysis of data from 135 population-based studies that included 968,419 adults from 90 countries, we estimated that in 2010 the global age-standardized prevalence of hypertension (defined as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, and/or current use of antihypertensive medication) was 31.1% (95% CI 30.0–32.2%).3 The age-standardized prevalence of hypertension was slightly higher in men (31.9%) than in women (30.1%) (Table 1)3 and was lower in HICs (28.5%) than in LMICs (31.5%) (Figure 2).3 The lowest prevalence of hypertension in men was found in South Asia (26.4%), whereas the highest prevalence was in Eastern Europe and Central Asia (39.0%). In women, the prevalence of hypertension was lowest in HICs (25.3%) and highest in Sub-Saharan Africa (36.3%). The reasons for these disparities in hypertension prevalence across regions are not fully understood but are likely influenced by differences in the prevalence of risk factors for hypertension, including unhealthy diet, lack of physical activity and obesity.3,8
Table 1.
Estimated prevalence of hypertension in high-income and low and middle-income countries in 2000 and 2010
| Population | 2000 | 2010 | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Age-standardized prevalence % (95% CIs) | ||||
| Global | 26.4 (24.6–28.2) | 25.1 (23.4–26.9) | 31.9 (30.3–33.5) | 30.1 (28.5–31.6) |
| High-income countries | 35.1 (29.8–40.3) | 26.9 (22.6–31.3) | 31.6 (29.6–33.6) | 25.3 (23.9–26.7) |
| Low and middle-income countries | 23.4 (21.6–25.2) | 24.1 (22.4–25.9) | 31.7 (29.7–33.6) | 31.2 (29.3–33.1) |
| Absolute numbers in millions (95% CIs) | ||||
| Global | 457.0 (422.9, 491.2) | 464.1 (432.0, 496.2) | 694.4 (658.7–730.1) | 693.5 (659.5–727.5) |
| High-income countries | 162.8 (140.7–184.9) | 159.5 (138.4–180.6) | 174.2 (165.3–183.2) | 174.7 (167.2–182.1) |
| Low- and middle-income countries | 294.3 (268.2–320.3) | 304.6 (280.5–328.8) | 520.1 (485.6–554.7) | 518.8 (485.7–552.0) |
Data obtained from reference3
Figure 2. Hypertension prevalence by world region in 2010.
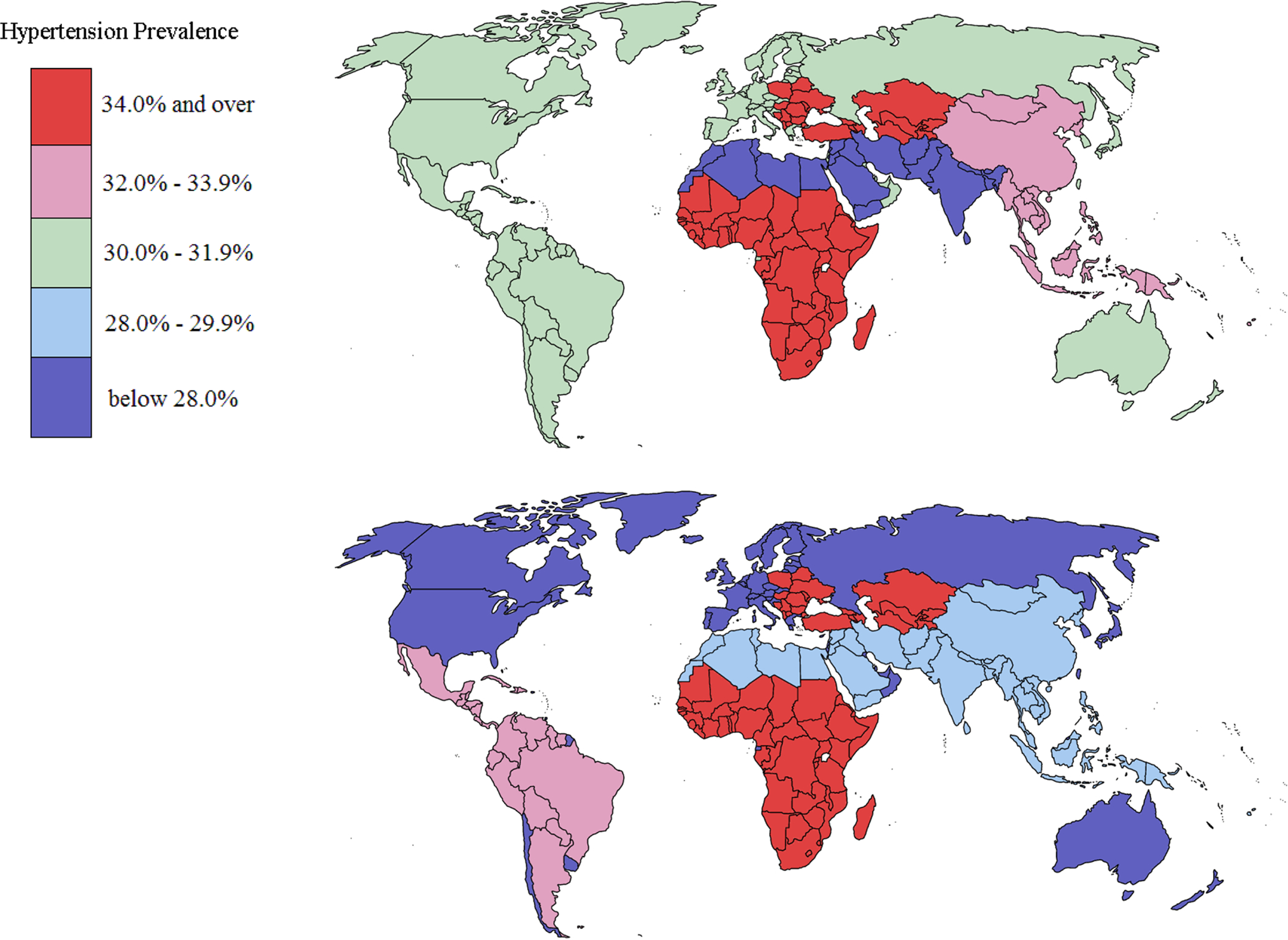
Prevalence of hypertension defined as systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg or use of antihypertensive medication in A. Men and B. Women. Data obtained from reference3.
The Prospective Urban Rural Epidemiology (PURE) study included 153,996 adults aged 35–70 years from 628 rural and urban communities in 17 geographically and economically diverse countries who were recruited between 2003 and 2009.11 This study included 142,042 participants with BP data at baseline, providing a unique opportunity to compare hypertension prevalence between rural and urban populations in different world regions. The PURE study found that 40.8% (95% CI 40.5–41.0%) of participants had hypertension with a higher prevalence in men (41.4%) than in women (37.7%).11 Residents of rural areas had a higher prevalence of hypertension than urban residents in HICs and middle-income countries (MICs), but the opposite was true in low-income countries (LICs).11
We estimated that between 2000 and 2010, the global age-standardized prevalence of hypertension in adults aged ≥20 years increased by 5.2%.3 This estimate is consistent with a 2015 Global Burden of Disease analysis that found that the prevalence of elevated systolic BP (≥140 mmHg) increased 3.2% from 17.3% in 1990 to 20.5% in 2015.12 The global increase in prevalence of hypertension was consistent by sex (5.5% in men and 5.0% in women) but varied by economic development.3 From 2000 to 2010, the prevalence of hypertension increased in LMICs and decreased in HICs (Table 1).3 LMICs experienced a sharp increase in hypertension prevalence from 23.8% in 2000 to 31.5% in 2010. By contrast, HICs experienced a decrease in hypertension prevalence from 31.1% to 28.5% in the same ten-year period.3 Hypertension prevalence was therefore higher in LMICs (31.5%) than in HICs (28.5%) in 2010. Similar to the trends observed for BP change, population ageing, urbanization and related lifestyle changes (such as unhealthy diet and lack of physical activity) might explain the increased prevalence of hypertension in LMICs.8,10
As mentioned above, an estimated 1.39 billion adults worldwide had hypertension in 2010 with an approximately even distribution between men and women (Table 1).3 Among those with hypertension, approximately 75% (1.04 billion) lived in LMICs and 25% (349 million) lived in HICs. By contrast, in 2000 an estimated 921 million people had hypertension comprising 322 million (34%) in HICs and 599 million (66%) in LMICs.3,13 Between 2000 and 2010, the absolute burden of hypertension (total number of individuals with hypertension) increased by 440 million in LMICs and only 27 million in HICs.3 In 2010, the highest absolute burden of hypertension was in East Asia and the Pacific, which had more individuals with hypertension than all HICs combined.3 The absolute burden of hypertension in men and women increased in all world regions between 2000 and 2010, except for in the Middle East and North Africa where the absolute burden in women decreased slightly.3 The Global Burden of Disease study estimated that in 2015 around 3.5 billion adults worldwide had systolic BP of at least 110–115 mmHg, a level that is associated with increased risk of ischaemic heart disease (IHD), stroke, and kidney disease.4 This prevalence represents a marked increase from 1990 when 1.87 billion people had a systolic BP of at least 110–115 mmHg.4
In 2017, the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines redefined hypertension in adults as systolic BP ≥130 mmHg and/or diastolic BP ≥80 mmHg.14 This change was based on findings from a number of large-scale, prospective observational studies that reported significant increases in risk of CVD with increasing BP even from levels as low as systolic BP 115 mmHg, as well as the results of randomized clinical trials including the SPRINT trial (discussed further below) that showed that intensive BP lowering (target systolic BP <120 mmHg) reduces CVD and all-cause mortality to an even greater extent than does standard BP lowering (target systolic BP ≤140 mmHg).15–18 When the new definition was applied to the US general population, hypertension prevalence increased from 32.0% (based on the traditional criteria) to 45.4% (Table 2).19,20 In the Chinese general population, the increase was even greater from 23.2% to 46.4%.19,20 These findings suggest that if the new criteria were applied worldwide, the difference in hypertension prevalence between LMICs and HICs would be much greater than previously reported.3 Full implementation of the new guidelines would require a greater proportion of adults to be treated with antihypertensive medications but could prevent an estimated 610,000 CVD events and 334,000 deaths per year in the US alone.19
Table 2.
Estimated prevalence of hypertension in the general populations of the US in 2013–2016 and China in 2012–2015
| Population | Age-standardized prevalence of hypertension (% (95% CI) | ||
|---|---|---|---|
| Blood pressure ≥140/90 mmHg* | Blood pressure ≥130/80 mmHg† | ||
| US 2013–2016 | Men | 32.1 (30.2–34.0) | 48.0 (45.7–50.3) |
| Women | 31.8 (29.8–33.8) | 43.1 (41.3–44.9) | |
| Total | 32.0 (30.3–33.6) | 45.4 (43.9–46.9) | |
| China 2012–2015 | Men | 24.5 (23.0–26.0) | 52.3 (50.0–54.5) |
| Women | 21.9 (20.7–23.1) | 40.4 (38.0–42.9) | |
| Total | 23.2 (21.9–24.5) | 46.4 (44.2–48.7) | |
Valid estimation of global BP levels and hypertension prevalence is highly dependent on the availability and quality of BP data from population-based studies around the world. Many factors, such as the representativeness of study populations (e.g. sampling methods and response rates), BP measurement methods (e.g. calibration of BP measuring devices, appropriate BP cuff sizes, and preparation of participants), and number of BP measurements can affect the quality of prevalence data.21 In many countries, population-based BP studies have not been conducted or BP data are not publicly available. In addition, the number of studies and the quality of the available data varies substantially between regions. As a result, BP estimates for some countries are based entirely on modelling in several pooling projects.4,12,22 This problem is particularly apparent in Sub-Saharan Africa for which BP data are sparse.23 High-quality, population-based studies that accurately measure BP in all countries worldwide, particularly in LMICs, are required to enable accurate assessment of the global burden of hypertension.
BLOOD PRESSURE, CVD, CKD AND MORTALITY
Elevated BP is associated with a large global burden of CVD and premature death. In 2015, the estimated number of all-cause deaths that were associated with systolic BP ≥110–115 mmHg was 10.7 million (19.2% of all deaths) and with systolic BP ≥140 mm Hg was 7.8 million (14.0% of all deaths).12 The largest numbers of deaths that were related to systolic BP ≥110–115 mmHg were attributed to IHD (4.9 million or 54.5% of IHD deaths), ischaemic stroke (1.5 million or 50.0% of ischaemic stroke deaths), and haemorrhagic stroke (2.0 million or 58.3% of haemorrhagic stroke deaths) (Figure 3). The corresponding numbers of deaths related to systolic BP ≥140 mmHg were 3.6 million (40.1% of IHD deaths), 1.1 million (38.1% of ischemic stroke deaths) and 1.4 million (42.5% of hemorrhagic stroke deaths).12 Consistent with trends in hypertension prevalence, the estimated numbers of BP-related all-cause and CVD deaths increased substantially from 1990 to 2015, especially in LMICs.12 Scaling up effective antihypertensive interventions to reduce BP-related morbidity and mortality should be a global public health priority.
Figure 3. Causes of deaths worldwide attributed to increased blood pressure in 2015.
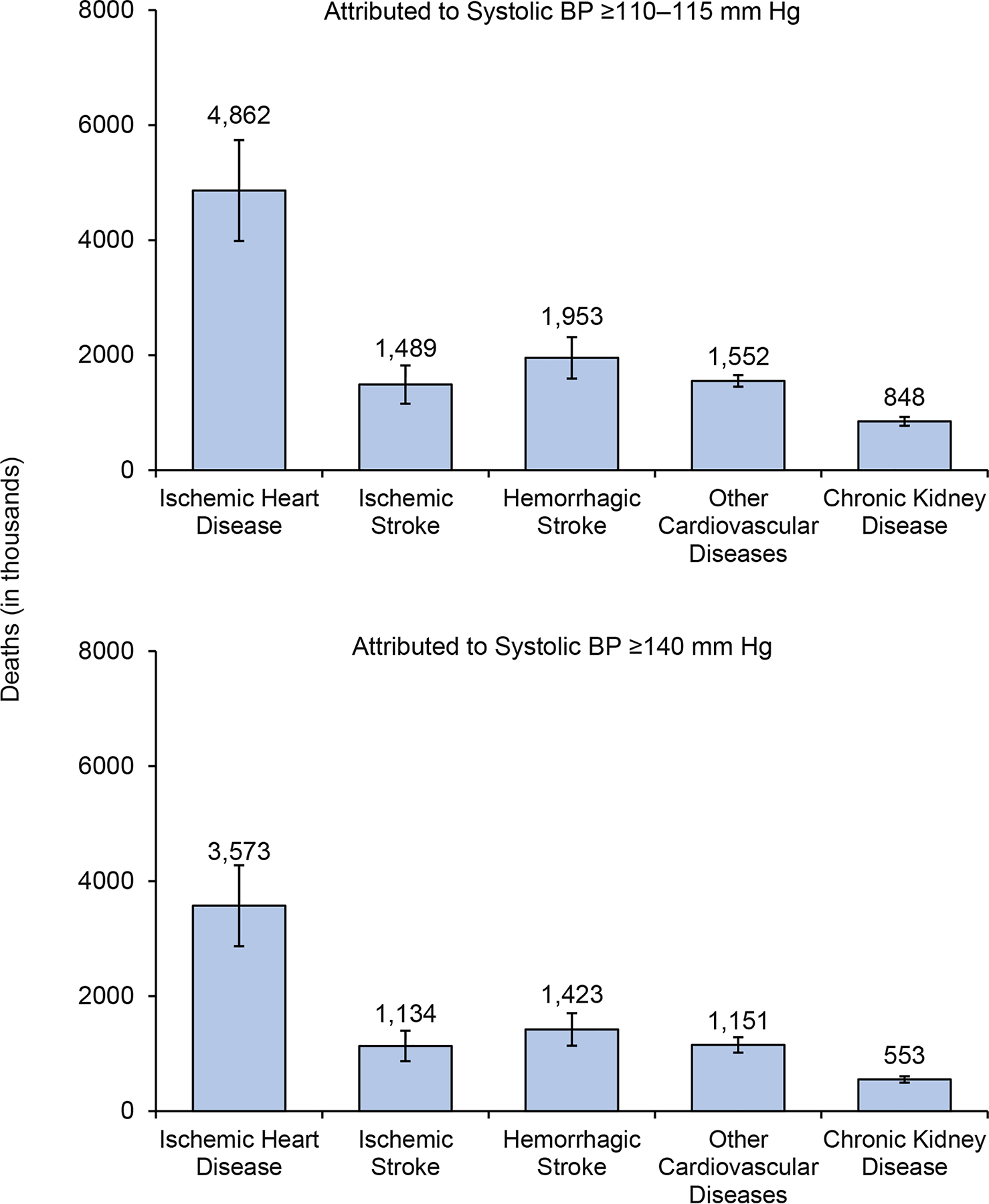
A. Estimated numbers of deaths attributed to systolic blood pressure (SBP) ≥110–115 mmHg and B. SBP ≥140 mmHg by cause of death. Data obtained from reference12.
Observational epidemiological studies have shown a strong, independent and linear association between BP and the risk of CVD without any evidence of a BP threshold.15,24–26 For example, the Prospective Studies Collaboration examined the association between BP and cause-specific mortality in approximately 1 million adults aged 40–89 years with no previous history of CVD at baseline using data from 61 prospective observational studies.15 During 12.7 million person-years of follow-up, around 56,000 CVD deaths (12,000 stroke, 34,000 IHD, and 10,000 other CVD) and 66,000 deaths owing to other causes were reported. Meta-analyses that corrected for regression dilution showed that the proportional difference in risk of CVD death that was associated with a given absolute difference in usual BP for each decade of age was similar for increases in BP from levels as low as 115 mmHg systolic and 75 mmHg diastolic.15 In those aged 40–69 years, a difference in usual systolic BP of 20 mmHg or usual diastolic BP of 10 mmHg was associated with a more than twofold difference in the rate of stroke deaths and a twofold difference in the rate of death owing to IHD and other CVD causes. The proportional difference in CVD mortality that was associated with a given absolute difference in usual blood pressure in adults aged 80–89 years was about half that of adults aged 40–49 years, but the annual absolute differences in risk were greater in old age.15
In adults who are middle aged or older (≥35 years), systolic BP is a more important determinant of CVD risk than diastolic BP.24–26 Among 347,978 men aged 35 to 57 years who were screened in the US for entry into the Multiple Risk Factor Intervention Trial (MRFIT) and had not previously been hospitalized for CVD, 7,150 deaths from IHD and 733 deaths from stroke were identified during an average of 11.6 years of follow-up.25 In every decile of BP, systolic BP was more strongly associated with risk of IHD and stroke than was diastolic BP. When the highest deciles were compared with the lowest deciles, the relative risks associated with increased systolic and diastolic BP were 3.7 and 2.8, respectively, for IHD, and 8.2 and 4.4, respectively, for stroke.
Several large prospective cohort studies have reported that elevated BP is also a strong independent risk factor for chronic kidney disease (CKD) and end-stage renal disease (ESRD).27–29 The increase in risk that was associated with higher BP was dose-response and continuous throughout the distribution of BP levels above 120 mmHg.27–29 Among 332,544 men aged 35 to 57 years who were screened for entry into the MRFIT trial and did not have ESRD at baseline, a strong independent linear relationship between both systolic and diastolic BP and incidence of ESRD was identified during an average of 16 years of follow-up.28 Compared with normotensive men who had a systolic BP <120 mmHg and diastolic BP <80 mmHg, the relative risk of ESRD for men with hypertension who had a systolic BP >210 mmHg or diastolic BP >120 mmHg was 22.1 (P<0.001). A similar association between BP and risk of ESRD was reported in a cohort of 158,365 Chinese men and women aged ≥40 years.29 In addition, BP was significantly and independently associated with progression to ESRD among patients with CKD.27 Among 3,708 patients with CKD in the Chronic Renal Insufficiency Cohort Study in the US, multivariable-adjusted relative risk (95% CI) for ESRD was 2.37 (CI, 1.48 to 3.80) and 3.37 (CI, 2.26 to 5.03) among those with systolic BP of 130–139 and ≥140 mmHg, respectively, compared with systolic BP <120 mmHg.27
Randomized clinical trials have demonstrated that BP lowering with commonly used regimens, such as diuretics, angiotensin-converting-enzyme inhibitors, angiotensin-receptor blockers, and calcium channel blockers reduces the risk of CVD and all-cause mortality.30,31 A large meta-analysis of 123 clinical trials with 613,815 participants showed that relative risk reductions of CVD and all-cause mortality were proportional to the magnitude of achieved BP reductions.31 For example, every 10 mmHg reduction in systolic BP significantly reduced the risk of major CVD events by 20% (relative risk 0.80, 95% CI 0.77–0.83), IHD by 17% (relative risk 0.83, 0.78–0.88), stroke by 27% (relative risk 0.73, 0.68–0.77), heart failure by 28% (relative risk 0.72, 0.67–0.78), and all-cause mortality by 13% (0.87, 0.84–0.91).31 This study provided no clear evidence that proportional risk reductions in major CVD events differed by baseline BP levels or comorbidities, except for patients with diabetes and CKD, for whom the risk reductions were smaller but still statistically significant.
The Systolic Blood Pressure Intervention Trial (SPRINT) randomly assigned 9,361 patients with systolic BP ≥130 mmHg and increased CVD risk to a systolic BP target of <120 mmHg (intensive treatment) or <140 mmHg (standard treatment).17 Increased CVD risk was defined as clinical or subclinical CVD, CKD, a 10-year risk of CVD of ≥15% according to the Framingham risk score, or an age of ≥75 years. At 1 year, mean systolic BP was 121.4 mmHg in the intensive treatment group and 136.2 mmHg in the standard-treatment group. Moreover, intensive treatment was associated with a 25% reduction in CVD events (hazard ratio 0.75, 95% CI 0.64–0.89, P<0.001) and a 27% reduction in all-cause mortality (hazard ratio 0.73, 95% CI 0.60–0.90, P=0.003) compared to standard treatment over a median follow-up of 3.26 years. The number needed to treat (NNT) to prevent one CVD event during the follow-up period was 61, and the NNT to prevent one death from any cause was 90.17 The SPRINT trial demonstrates that intensive BP lowering treatment with a systolic BP target of <120 mmHg is beneficial for patients with hypertension who are at high risk of CVD. The SPRINT trial prompted the revision of the definition of hypertension and BP antihypertensive treatment goals.14
The results of meta-analyses of randomized controlled trials that compared the efficacy of different BP targets or treatment intensities to reduce the risk of CVD and mortality in patients with hypertension are consistent with the SPRINT findings.18,31,32 For example, a 2015 network meta-analysis of 42 trials that included 144,220 patients with hypertension, reported linear associations between mean achieved systolic BP and risk of CVD and mortality, with the lowest risk among those who achieved a systolic BP of 120–124 mmHg.18 In these trials, study groups that achieved mean systolic BPs of 120–124 mm Hg had hazard ratios for major CVD of 0.71 (95% CI, 0.60–0.83), 0.58 (95% CI, 0.48–0.72), 0.46 (95% CI, 0.34–0.63), and 0.36 (95% CI, 0.26–0.51), compared with study groups that achieved systolic BPs of 130–134 mmHg, 140–144 mmHg, 150–154 mmHg and ≥160 mmHg, respectively.18 Network meta-analyses offer a unique advantage over traditional meta-regression methods by enabling the simultaneous comparison of the effects of multiple interventions or treatment targets on clinical outcomes while preserving trial-level treatment randomization and its associated protection against bias.
Despite the findings from SPRINT and related meta-analyses, some concerns remain about the appropriateness and effectiveness of intensive BP reduction in certain subgroups, including patients with CKD, diabetes or stroke and older adults (≥65 years). A pre-specified subgroup analysis of outcomes in SPRINT trial participants with CKD found that intensive BP treatment resulted in significant reductions in the risk of CVD and death but no significant increase in the incidence of serious adverse events compared to standard BP treatment.33 The rate of continuous eGFR decline was higher in the intensive BP treatment group but there was no significant difference in risk of CKD progression (defined as incident ESRD or halving of eGFR) between the two groups.33 In addition, a subgroup analysis of 978 SPRINT participants (519 in the intensive and 459 in the standard treatment group) showed that while eGFR was lower in the intensive treatment compared to standard treatment group, urine biomarkers of tubule function, injury, inflammation, and repair were not different over 4 years of follow-up. These findings suggest that observed eGFR declines in the intensive treatment group predominantly reflect hemodynamic changes rather than intrinsic damage to kidney tubule cells.34 An additional SPRINT subgroup analysis in patients aged ≥75 years reported that intensive BP treatment significantly reduced the incidence of CVD and mortality with no increase in serious adverse events compared with standard BP-lowering treatment.35
As patients with diabetes or stroke were not included in the SPRINT trial, questions regarding the risks and benefits of intensive BP lowering remain in these subgroups. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study reported that intensive BP treatment aimed at achieving a systolic BP target of <120 mmHg did not significantly reduce the risk of CVD events compared with standard BP treatment (systolic BP target of <140 mmHg) in patients with type 2 diabetes (hazard ratio 0.88; 95% CI 0.73–1.06).36 However, a subgroup analysis suggested a significant reduction in CVD events in the intensive BP treatment group compared to standard treatment group (hazard ratio 0.74, 95% CI 0.55 to 1.00, p=0.049) among participants who received standard glycaemic treatment.37 In addition, a meta-analysis of four randomized clinical trials with 4,895 participants reported that intensive BP control reduced the risk of recurrent stroke compared with standard BP control (relative risk 0.78; 95% CI 0.64–0.96; P = 0.02).38 On-going clinical trials (such as the OPTIMAL-DIABETES trial [NCT04040634], the OPTIMAL Stroke trial [NCT04036409], the BPROAD trial [NCT03808311], and the IBIS Trial [NCT03585595]) will likely provide definite answers regarding optimum BP treatment goals for patients with diabetes or stroke. However, the currently available evidence supports a more intensive BP treatment target.14
RISK FACTORS FOR HYPERTENSION
BP levels and hypertension prevalence increase with age in both sexes.39 Men have higher BP at younger ages than women, but BP increase per decade is higher in women than in men. By the age of 60 years, women have a higher mean BP and hypertension prevalence than men. Race and ethnicity is also a significant risk factor for hypertension. For example, a study that analyzed National Health and Nutrition Examination Survey (NHANES) 2015–2016 data for 4,821 US adults aged ≥20 years, reported a significantly higher age-standardized prevalence of hypertension in non-Hispanic Black individuals (57.3%) than in non-Hispanic White individuals (43.8%) and Hispanic-Americans (44.7%).40 There is no evidence that racial and ethnic disparities in risk of hypertension are explained by genetic factors.41,42 Sociodemographic, environmental and behavioral factors are therefore likely to be the main contributors to racial and ethnic differences in mean BP and prevalence of hypertension.42 In addition, several modifiable risk factors, including high sodium intake, low potassium intake, alcohol consumption, obesity, lack of physical activity, and unhealthy diet are associated with increased risk of hypertension
High sodium intake
The PURE study estimated that in 2010, global sodium intake was 3,950 mg per day, which is considerably higher than the recommended amounts of 2,300 mg or less per day in all published guidelines.44,45 In addition, sodium consumption varied considerably by world region from >4,200 mg per day in East Asia, Central Asia, and Eastern Europe to <3,300 mg per day in Latin America and Sub-Saharan Africa.44
Animal experiments, observational epidemiologic studies, and randomized clinical trials have demonstrated a causal relationship between sodium intake and elevated BP.46 The majority of the observational studies were cross-sectional and reported a positive, linear, and significant association of either dietary intake or 24-hour urinary excretion of sodium with BP or hypertension.47,48 For example, the Intersalt study investigated the association between 24-hour urinary sodium excretion and BP among 10,074 men and women aged 20–59 from 52 population samples in 32 countries.47 In within population analyses, a 100 mmol higher individual 24-hour urinary sodium excretion was associated with a 6.0 mmHg higher average systolic BP and a 2.5 mmHg higher average diastolic BP. In across population analyses, a 100 mmol higher median 24-hour sodium excretion was associated with a median 4.5 mmHg higher systolic and 2.3 mmHg higher diastolic BP.47 When four remote populations with extremely low urinary sodium excretion were excluded from the across population analyses, a 100 mmol increase in median 24-hour sodium excretion was associated with a 2.5 mmHg increase in systolic BP.47 The Intersalt study showed consistent patterns of regional differences in salt intake and BP levels. A positive and significant association between spot urinary sodium excretion and BP was also observed in the PURE study.49
In the DASH-Sodium trial, 412 people with an average systolic BP of 120–159 mmHg and diastolic BP of 80–95 mmHg were randomly assigned to high sodium (mean urinary excretion of 142 mmol per day), intermediate sodium (mean urinary excretion of 107 mmol per day) and lower sodium diets (mean urinary excretion of 65 mmol per day) for 30 days in a cross-over design.50 The results showed that reducing sodium intake from a high to an intermediate level lowered systolic BP by 2.1 mmHg (P<.001) during consumption of a usual American control diet and by 1.3 mm Hg (P = 0.03) during consumption of a Dietary Approaches to Stop Hypertension (DASH) diet. Further reducing sodium intake from an intermediate to a lower level resulted in an additional reduction in systolic BP of 4.6 mmHg during consumption of the control diet (P<0.001) and a 1.7 mmHg reduction during consumption of the DASH diet (P<0.01).50
Several meta-analyses of clinical trials have shown that sodium reduction significantly lowers BP in hypertensive and normotensive individuals.51–55 An Agency for Healthcare Research and Quality (AHRQ) meta-analysis that included 48 randomized control trials found a significant BP-lowering effect of dietary sodium reduction in adults both with and without hypertension.55 In this study, a 42 mmol decrease in the weighted mean sodium intake was associated with a 3.23 mmHg (95% CI 2.41–4.06) reduction in systolic BP and a 2.24 mmHg (95% CI 1.61–2.96) reduction in diastolic BP.55
Despite the strong positive association between dietary sodium intake, BP and risk of hypertension, the associations of sodium intake with risk of CVD, CKD, and mortality are inconsistent.55 Some studies have found a positive association between dietary sodium intake and these clinical outcomes, whereas others have found inverse, J-shaped or U-shaped associations.56–65 These conflicting findings can likely be partly explained by methodological limitations, such as systematic and random error in sodium measurements, reverse causality, insufficient statistical power, residual confounding, and inadequate follow-up duration.55,64,66 In summary, dietary sodium reduction should be recommended to lower population BP levels and risk of hypertension. More research is needed, however, to determine optimal dietary sodium intake for the prevention of CVD, CKD and mortality.
Low potassium intake
Similar to sodium, considerable regional variation exists in 24-hour urinary potassium excretion (the best available measure of potassium intake) with the highest levels in Europe (e.g., Finland 2,995 mg, Netherlands 2,835 mg, Germany 2,825 mg, Belgium 2,618 mg, and Spain 2,629 mg) and South America (e.g., Brazil 2,940 mg, and Colombia 2,803 mg), and the lowest levels in Asia (e.g., China 1,249 mg and Japan 1,792 mg) and Africa (e.g., Kenya 1,306 mg and Zimbabwe 1,466 mg).67,68 Observational epidemiological studies have reported an inverse association of dietary potassium intake with BP levels and hypertension. In the Intersalt study, a 50 mmol per day higher level of urinary potassium excretion was associated with a 3.4 mmHg (95% CI 1.5–5.2) lower level of systolic BP and 1.9 mmHg (95% CI 0.7–3.0) lower level of diastolic BP after adjustment for important confounding factors, including age, sex, BMI, alcohol consumption, and urinary sodium excretion, and correction for regression dilution bias.69
Randomized clinical trials have shown that potassium supplementation lowers BP in hypertensive and normotensive individuals.70–73 In a meta-analysis of 33 randomized controlled trials with 2,609 participants, potassium supplementation was associated with significant reductions in mean systolic and diastolic BP of 3.11 mmHg (95% CI 1.91–4.31) and 1.97 mmHg (95% CI 0.52–3.42), respectively.71 In 31 trials with urinary potassium measurements, the median net increase in potassium excretion in the potassium supplementation group versus the control group was 50 mmol per day; in 21 (68%) of these trials, the net difference in potassium excretion was ≥40 mmol per day.71 The effects of potassium supplementation seemed to be greater in black individuals and in those eating a high sodium diet. An AHRQ meta-analysis that included 18 randomized controlled trials also reported that potassium supplementation was associated with a significant reduction in systolic (6.43 mmHg; 95% CI 1.80–11.1) and diastolic BP (3.50 mmHg; 95% CI 0.89–6.10).55 Increasing potassium intake, especially from fruits and vegetables, is therefore recommended for the prevention and treatment of hypertension.14,43
Alcohol consumption
Globally, alcohol consumption varies considerably, with the lowest consumption in North African and the Middle East (most countries <1 L per-capita annually), and the highest consumption in Central and Eastern Europe (many countries >12 L per-capita annually). Global annual alcohol consumption increased from 5.9 L per-capita in 1990 to 6.5 L in 2017.74 Numerous observational epidemiologic studies have reported that high alcohol consumption is a risk factor for elevated BP.75,76 The Atherosclerosis Risk in Communities Study reported a J-shaped association between alcohol consumption and risk of hypertension.76 Light to moderate alcohol drinkers were at lower risk of incident hypertension than nondrinkers, whereas heavy drinkers were at higher risk than both light to moderate drinkers and nondrinkers. This pattern was observed in all race and gender groups except for black men, for whom risk was similarly elevated in light to moderate and heavy drinkers compared to nondrinkers.76 In a large prospective cohort study of over 500,000 Chinese adults, however, self-reported usual alcohol intake and genotype-predicted alcohol intake were both positively and linearly associated with BP. Genetic epidemiological data showed that the observed protective effects of moderate alcohol intake against hypertension and CVD were largely non-causal.77
A meta-analysis of 15 randomized control trials with a total of 2,234 participants reported that a reduction in alcohol intake (median: 76%, range: 16%–100%) was associated with significant reductions in mean systolic BP (3.31 mmHg, 95% CI 2.52–4.10) and diastolic BP (2.04 mmHg, 95% CI 1.49–2.58).78 A dose-response relationship was observed between mean percentage reduction in alcohol intake and mean reduction in BP. Moreover, the effects of reducing alcohol intake on BP were enhanced in those with higher BP at baseline. A recent meta-analysis of 36 trials with 2,865 participants showed that a reduction in alcohol intake was not associated with a significant reduction in BP in individuals who drank two or fewer drinks per day. However, a 50% reduction in alcohol intake was significantly associated with a 5.50 (95% CI 4.30 to 6.70) mmHg lower systolic BP and a 3.97 (3.25 to 4·70) mmHg lower diastolic BP in participants who drank six or more drinks per day.79 These findings suggest that reducing alcohol intake should be recommended as an important component of lifestyle modification for the prevention and treatment of hypertension in people who are heavy drinkers.
Lack of physical activity
Global age-adjusted prevalence of insufficient physical activity (less than 150 min of moderate-intensity, or 75 min of vigorous-intensity physical activity per week, or any equivalent combination of the two) was 27.5% with a higher prevalence in women (31.7%) than in men (23.4%). The highest levels were in women in Latin America and the Caribbean (43.7%), south Asia (43.0%), and high-income Western countries (42.3%), whereas the lowest levels were in men from Oceania (12.3%), east and southeast Asia (17.6%), and sub-Saharan Africa (17.9%). Prevalence of insufficient physical activity was much higher in urbanized populations in high-income countries.80
Epidemiological studies have reported an inverse relationship between physical activity, BP and hypertension.81 Even modest levels of physical activity (such as walking to work) are associated with a decrease in the risk of incident hypertension.82 Randomized controlled trials and meta-analyses have demonstrated that physical activity lowers BP in hypertensive and normotensive individuals.83–85 For example, a meta-analysis of 54 randomized controlled trials with 2,419 participants reported that aerobic exercise was associated with a significant reduction in mean systolic BP of 3.84 mmHg (95% CI 2.72–4.97) and diastolic BP of 2.58 mmHg (95% CI 1.81–3.35).83 Aerobic exercise-induced BP reductions were consistent in hypertensive and normotensive participants and in overweight and normal-weight participants. These findings indicate that physical activity is an effective lifestyle intervention for the prevention and treatment of hypertension.
Overweight and obesity
The prevalence of obesity has increased rapidly worldwide over the past several decades. From 1975 to 2014, global age-standardized prevalence of obesity increased from 3.2% to 10.8% in men, and from 6.4% to 14.9% in women. During the same period, global age-standardized mean body mass index (BMI) increased from 21.7 kg/m2 to 24.2 kg/m2 in men and from 22.1 kg/m2 in 1975 to 24.4 kg/m2 in women. BMI varied significantly by world region, from 21.4 kg/m2 in central Africa and south Asia to 29.2 kg/m2 in the Pacific Islands for men and from 21.8 kg/m2 in south Asia to 32.2 kg/m2 in the Pacific Islands for women in 2014.86
Epidemiological studies have consistently identified a direct relationship between BMI and BP that is continuous and almost linear, with no evidence of a threshold.87,88 The relationship between BP and waist-to-hip ratio or computed tomographic measures of central fat distribution is even stronger than the relationship between BP and BMI.89 Attributable risk estimates from the Nurses’ Health Study suggest that obesity is responsible for about 40% of hypertension80, whereas the Framingham Offspring Study suggested that obesity is responsible for 78% of hypertension in men and 65% of hypertension in women.90,91 In a meta-analysis of 25 randomized controlled trials with 4,874 participants, a net reduction in body weight of 5.1 kg (95% CI 4.25–6.03) owing to calorie restriction, increased physical activity, or both, reduced systolic BP by 4.44 mmHg (95% CI 2.95–5.93) and diastolic BP by 3.57 mmHg (95% CI 2.25–4.88). BP reductions were 1.05 mmHg (95% CI 0.66–1.43) systolic and 0.92 mmHg (95% CI 0.55–1.28) diastolic when expressed per kilogram of weight loss.92
Unhealthy diet
In addition to sodium and potassium, several macronutrients are associated with BP, including dietary fiber,93,94 protein,95,96 and fat.97,98 The Global Burden of Diseases Nutrition and Chronic Diseases Expert Group examined two different dietary patterns worldwide: one based on relatively high consumption of ten healthy items (fruits, vegetables, beans and legumes, nuts and seeds, whole grains, milk, total polyunsaturated fatty acids, fish, plant omega-3s, and dietary fiber); and another based on relatively low consumption of seven unhealthy items (unprocessed red meats, processed meats, sugar sweetened beverages, saturated fat, trans fat, dietary cholesterol, and sodium). Diets and their trends were very heterogeneous across world regions. For example, both types of dietary patterns improved in high-income countries but worsened in some low-income countries in Africa and Asia. Middle-income countries showed the largest improvement in dietary patterns based on healthy items but the largest deterioration in dietary patterns based on unhealthy items.99
The efficacy of dietary interventions for blood pressure lowering has been investigated in randomized controlled trials. For example, in the DASH trial, 459 adults with systolic BP <160 mmHg and diastolic BP of 80–95 mm Hg were randomly assigned to 8 weeks of a control diet low in fruits, vegetables, and dairy products with a fat content typical of the average diet in the US, a diet rich in fruits and vegetables or a DASH diet rich in fruit, vegetables and low-fat dairy products with a total fat and saturated fat content lower than the typical US diet.100 Sodium intake and body weight were maintained at constant levels. Compared to the control diet, the DASH diet significantly reduced systolic and diastolic BP by 5.5 mmHg and 3.0 mmHg (both P<0.001), respectively, whereas the fruit and vegetable diet reduced systolic and diastolic BP by 2.8 mmHg (P<0.001) and 1.1 mm Hg (P=0.07), respectively. In participants with hypertension, the DASH diet reduced systolic and diastolic BP by 11.4 mmHg and 5.5 mmHg compared with the control diet (both P<0.001). The DASH diet was also effective in reducing BP in normotensive participants, but the BP reductions were smaller than those seen in hypertensive participants [(3.5 mmHg (P<0.001) and 2.1 mmHg (P=0.003) compared with the control diet].100 These data indicate that the DASH diet provides an effective nutritional approach to prevent and treat hypertension.
Vegetarian and Mediterranean dietary patterns are also associated with BP reduction.101,102 A meta-analysis of 7 randomized controlled trials with a total of 311 participants reported that vegetarian diets (defined as diets that never or rarely included meat) were associated with a mean reduction in systolic BP of 4.8 mmHg (95% CI 3.1–6.6) and diastolic BP of 2.2 mmHg (95% CI 1.1–3.5).101 Mediterranean diets are characterized by moderate fat intake (primarily from olive oil and nuts), low consumption of red meat, and high consumption of vegetables.102 A meta-analysis of 6 trials with a total of 2,650 participants reported a modest but significant reduction in systolic BP of 1.7 mmHg (95% CI 0.1–3.4) and diastolic BP of 1.5 mmHg (95% CI 0.8–2.1) in Mediterranean diets compared to low-fat diets.102
In summary, numerous lifestyle risk factors are associated with BP levels and hypertension. These risk factors vary by world region and this heterogeneity likely underlies some of the disparities in hypertension trends and prevalence worldwide. Lifestyle interventions that target these risk factors could therefore play an important role in reducing global disparities in hypertension.22 Implementing lifestyle interventions in HICs has contributed to reductions in hypertension prevalence and BP levels.43 However, there is limited data on implementing lifestyle intervention programs in LMIC communities.
Other potential risk factors
Several other potential risk factors for hypertension have been proposed, including cigarette smoking, air pollution, psychological stress, sleep disorders and noise exposure.103–114 Cigarette smoking has been shown to be associated with an immediate acute increase in BP, mainly through stimulation of the sympathetic nervous system.103,104,107 However, its long-term effects on BP and incidence of hypertension are inconclusive.107,108,115 Several prospective cohort studies have reported a weak positive association between cigarette smoking and risk of hypertension. For example, in 13,529 men from the Physicians’ Health Study, former and current smoking were significantly associated with an 8% (RR 1.08, 95% CI 1.01 to 1.15) and 15% (RR 1.15, 95% CI 1.03 to 1.27) increase in the risk of incident hypertension, respectively, compared with never smoking over a median follow-up of 14.5 years.107 In a prospective cohort study of 28,236 women from the Women’s Health Study, multiple-adjusted hazard ratios of developing hypertension among former smokers and current smokers of 1–14 and ≥15 cigarettes per day were 1.03 (95% CI 0.98 to 1.08), 1.02 (95% CI 0.92 to 1.13), and 1.11 (95% CI 1.03 to 1.21), respectively, compared to never smokers.108 However, direct causality between cigarette smoking and hypertension cannot be established because cessation of chronic smoking does not lower BP.116
The association between exposure to ambient air pollutants and risk of hypertension has been investigated in epidemiological studies.109,110 A meta-analysis that examined the effects of a 10 μg/m3 increase in exposure to air pollutants on hypertension, reported that short-term exposure over several days to sulfur dioxide (OR=1.046, 95% CI 1.012–1.081), particulate matter with a diameter of ≤2.5 μm (PM2.5) (OR=1.069, 95% CI 1.003–1.141), and particulate matter with a diameter of ≤10 μm (PM10) (OR=1.024, 95% CI 1.016–1.032) were all significantly associated with hypertension (based on data from 7 studies).109 Long-term exposure over years to nitrogen oxide (OR=1.034, 95% CI 1.005–1.063) and PM10 (OR=1.054, 95% CI 1.036–1.072) were also significantly associated with hypertension (based on data from 11 studies). These results suggest that short-term and long-term exposure to air pollutants might increase the risk of hypertension. Future studies are needed to assess the impacts of chronic exposure to air pollution on global disparities of hypertension.117,118
Psychosocial stress and rotating shift work have also been reported to be associated with risk of hypertension.111,112 In a meta-analysis of two observational studies with 622 participants, mental stress was associated with an increased risk of hypertension (OR 2.40, 95% CI 1.65–3.49, p < 0.001).111 However, a meta-analyses of 15 trials with 902 participants testing the effects of different stress-reduction techniques, such as biofeedback, relaxation or combined interventions, on BP concluded that the benefit of stress reduction on BP remains unproven.119 The meta-analysis identified methodological shortcomings in these trials and significant heterogeneity of BP changes: from −12 to 10 mmHg for systolic and from −10 to 1 mmHg for diastolic. In addition, a meta-analysis of 9 cohort studies with a total of 172,824 individuals reported a pooled OR of hypertension in shift workers of 1.31 (95% CI, 1.07–1.60).112 In addition, ambient noise from road, rail, and air traffic has a modest association with hypertension prevalence but not incidence in cohort studies.113,114 An inconsistent relationship between sleep duration and risk of hypertension has also been reported.105 Obstructive sleep apnea is a very common risk factor for hypertension, and continuous positive airway pressure was shown to reduce systolic BP by 2.46 mm Hg (95% CI 0.62–4.31) and diastolic BP by 1.83 mm Hg (95% CI 0.61–3.05) in a meta-analysis of 16 randomized clinical trials with 818 participants.106
In summary, observational studies have reported weak or moderate associations between these potential risk factors (i.e., cigarette smoking, air pollution, psychological stress, sleep disorders and noise exposure) and risk of hypertension. However, there is insufficient evidence from randomized clinical trials to support causal relationships between these potential risk factors and risk of hypertension. Overall, the currently available data suggest that these potential risk factors have limited effect on BP in the general population.
HYPERTENSION CONTROL IN THE COMMUNITY
As discussed above, antihypertensive treatment and lifestyle modifications have been shown to lower BP and CVD risk in randomized clinical trials.30,31,120,121 Despite these effective interventions, hypertension control remains unacceptably low, particularly in LMICs.3 The most recent global estimates suggest that in 2010, only 45.6% of people with hypertension were aware of their condition, only 36.9% were receiving treatment, and only 13.8% had achieved BP control (defined as systolic BP <140 mmHg and diastolic BP <90 mmHg.3 Moreover, HICs had approximately twice the proportion of hypertension awareness and treatment and four times the proportion of hypertension control than that of LMICs, in which the proportion of BP control was only 7.7% in 2010.3 Between 2000 and 2010, HICs experienced substantial improvements in the proportions of hypertension awareness, treatment, and control. In the same period, awareness and treatment increased much more modestly in LMICs and the proportion of patients with hypertension and controlled BP decreased slightly (Table 3).3 Similar patterns were observed in the baseline data from the PURE study in which hypertension awareness, treatment, and control were lower in communities in low-income countries (LICs) than in those in HICs.11
Table 3.
Hypertension control in high income and low and middle income countries in 2000 and 2010
| Population | 2000 | 2010 | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Age-standardized proportion of hypertension control in all patients with hypertension (% (95% CI)) | ||||
| Global | 10.0 (4.0–15.9) | 13.4 (6.5–20.2) | 10.9 (7.7–14.2) | 16.8 (13.1–20.5) |
| High-income countries | 15.5 (2.3–28.7) | 20.3 (4.8–35.8) | 24.6 (16.0–33.2) | 32.2 (23.6–40.8) |
| Low and middle-income countries | 6.9 (1.3–12.6) | 9.7 (3.2–16.3) | 5.2 (2.3–8.1) | 10.2 (6.4–14.0) |
| Age-standardized proportion of hypertension control in patients with treated hypertension (% (95% CI)) | ||||
| Global | 34.2 (24.1–44.2) | 33.7 (23.2–44.2) | 35.8 (30.8–40.7) | 38.0 (33.2–42.8) |
| High-income countries | 38.6 (21.1–56.0) | 38.6 (19.8–57.4) | 49.1 (40.7–57.4) | 51.5 (43.1–59.9) |
| Low and middle-income countries | 29.5 (19.9–39.2) | 29.2 (18.9–39.6) | 23.4 (17.9–28.9) | 28.1 (22.6–33.7) |
Data obtained from reference3
Large-scale screening programs in communities or health facilities could be an effective way to address the lack of BP awareness in LMICs. For example, May Measurement Month (MMM) 2017 was a BP screening program aimed at raising global awareness of hypertension.122 The MMM 2017 program screened over 1.2 million individuals in 80 countries who had not had their BP measured in the past year and found that 34.9% had hypertension, 17.3% of those with hypertension were not receiving treatment, and 46.3% of those with hypertension who were receiving treatment did not have controlled BP (systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg). This program demonstrated that BP screening in convenience samples can be cost-effective and can identify large numbers of individuals who could benefit from initiation or enhancement of antihypertensive treatment.122
Barriers to hypertension control exist at multiple levels: patients, healthcare providers, health systems, and communities.123–125 A meta-analysis of randomized controlled trials that assessed the comparative effectiveness of 8 implementation strategies for BP control in patients with hypertension found that multilevel, multicomponent strategies were most effective for systolic BP reduction.125 These multicomponent strategies included team-based care with medication titration by a nonphysician mean change −7.1 mmHg, 95% CI −8.9 to −5.2 mmHg]), team-based care with medication titration by a physician (−6.2 mmHg 95% CI, −8.1 to −4.2 mmHg), and multilevel strategies without team-based care (−5.0 mmHg, 95% CI, −8.0 to −2.0 mmHg). Patient-level strategies resulted in systolic BP reductions of −3.9 mmHg (95% CI, −5.4 to −2.3 mm Hg) for health coaching and −2.7 mmHg (95% CI, −3.6 to −1.7 mm Hg) for home BP monitoring. Similar trends were reported for diastolic BP reductions. Strategies that targeted provider-level barriers only, including provider training, audit and feedback, and electronic decision support systems, did not result in significant BP reductions.
Most trials of implementation strategies to overcome barriers to BP control have been conducted in HICs. Whether the same strategies will be effective in LMICs is unclear. A cluster-randomized trial published in 2017 showed that a multifaceted intervention, which included a community health worker-led home intervention (health coaching, home BP monitoring, and BP audit and feedback), a physician intervention, and a text-messaging intervention over 18 months, significantly lowered systolic BP by 6.6 mmHg (95% CI, 4.6–8.6; P <0.001) and diastolic BP by 5.4 mmHg (95% CI, 4.0–6.8 mmHg; P <0.001) in low-income patients in Argentina.126 This trial indicated that a community health worker-led multifaceted intervention was effective for improving patients’ adherence to antihypertensive medication and physicians’ adherence to clinical guidelines resulting in a significant reduction in BP and an increase in hypertension control among low-income patients with hypertension. Widespread scale-up of this proven effective intervention in LMICs should result in a substantial reduction in uncontrolled hypertension and related CVD and premature death.
Increased use of out-of-office BP measurements for confirmation of hypertension diagnosis and titration of antihypertensive medications could also play an important role in improving BP control. A meta-analysis of 11 prospective cohort studies showed that ambulatory BP measurements (ABPM) predicted long-term CVD outcomes independently of office BP (hazard ratio range, 1.28 to 1.40).127 An international pooling project with 11,135 participants reported that higher 24-hour and night-time BP measurements using ABPM were significantly associated with greater risks of death and CVD outcomes after adjustment for office BP.128 However, the incremental improvement in predictive values of clinical outcomes with these measurements compared with office BP was small. Adding 24-hour or nighttime systolic BP to base models that included other BP measures resulted in incremental improvements in the area under the curve (AUC) of 0.0013 to 0.0027 for mortality and 0.0031 to 0.0075 for CVD.128 However, base models that included single systolic BP measure already had an AUC of 0.83 for mortality and 0.84 for CVD. Home BP measurements (HBPM) are a more economical option than ABPM and are a useful strategy to overcome patient-level barriers to BP control by empowering patients.129 Use of self HBPM resulted in a significant reduction in systolic and diastolic BP among patients with hypertension in randomized clinical trials.125,130 Out-of-office BP measurements are recommended for diagnostic confirmation to avoid white coat hypertension and masked hypertension and for optimal titration of medications to overcome therapeutic inertia.130
The prevention and control of hypertension in communities requires a life-course approach.131 Epidemiological studies have shown that elevated BP in childhood promotes subclinical organ damage (i.e. early vascular ageing) and leads to increased risk of hypertension, CVD, and CKD later in life.132–134 To reduce BP-related risks of CVD, CKD, and premature death over the lifespan, preventive efforts should start in early childhood. Innovative strategies for early detection and optimal treatment of hypertension, such as a multifaceted intervention approach, should be implemented in communities to reduce the burden of this condition.
THE FINANCIAL BURDEN OF HYPERTENSION
Hypertension is associated with a substantial financial burden. Costs include direct healthcare expenditures associated with BP management, such as medications, laboratory tests, and clinic visits, as well as costs associated with hospitalizations for BP-related complications and indirect costs associated with lost productivity resulting from premature mortality and disability owing to hypertension-related cardiovascular and kidney diseases.135 The global financial burden of high BP in 2001 was estimated to be around 370 billion US dollars or about 10% of the world’s overall healthcare expenditure.135 However, large regional variations in healthcare costs were observed. For example, high BP accounted for 22.6% of all healthcare expenditures in Eastern Europe and Central Asia but only 7.2% in East Asia and Pacific.135 To the best of our knowledge, no more recent estimates of the global financial burden of hypertension are available.
More recent national and regional estimates of the financial burden of hypertension are available for some HICs, although they are not always consistent owing to methodologic differences.136–139 A study that used a nationally representative database, the Medical Expenditure Panel Survey, estimated that the average annual adjusted incremental expenditure for patients with hypertension was $1,920 higher compared to individuals without hypertension in the US between 2003 and 2014. Based on an average annual number of individuals with hypertension in the US (n= 68,420,747) from NHANES during this period, the estimated adjusted annual incremental cost is $131 billion higher for the hypertensive adult population compared with the non-hypertensive population..136 Using the same database, another study analyzed changes in medical expenditures associated with hypertension between 2000 and 2013. Estimated annual medical expenditures per person associated with hypertension in 2000–2001 ($1,399) were not significantly different from those in 2012–2013 ($1,494), but annual national spending for patients with hypertension increased significantly from US$58.7 billion in 2000–2001 to US$109.1 billion in 2012–2013, mainly owing to an increase in the number of treated patients.137 In addition to lower estimates of medical costs per patient, the underestimated prevalence of hypertension in the Medical Expenditure Panel Survey which used self-reported history of hypertension to define hypertension might also contribute to the conservative estimates of medical expenditure for hypertension in this study.121
Payments for prescription medications account for a large proportion of the medical expenditures associated with hypertension. The cost of hypertension medication expenditures in the US in 2007 was estimated to be $68 billion based on an analysis of data for 21,782 adults (aged ≥18 years) who participated in the Medical Expenditure Panel Survey.139 A study that used data from the National Hospital Discharge Survey reported that annual costs for hypertension-related hospitalizations in the US increased from $40 billion (5.1% of total hospital costs) during 1979–1982 to $113 billion (15.1% of total hospital costs) during 2003–2006.138 The American Heart Association projected that by 2030, the direct costs of hypertension in the US population would increase to $200 billion and the indirect costs to $40 billion.140
In a study of 314, 622 beneficiaries of the medical insurance system in Japan, inpatient medical expenditure attributable to hypertension represented 7.2% and 6.9% of the total medical expenditure for men and 2.8% and 3.8% of the total medical expenditure for women aged 40–54 years and 55–69 years, respectively.141 Estimates from Mexico suggest that combined direct and indirect costs attributable to hypertension amounted to nearly $2.5 billion in 2007, which was between 6% and 8% of the healthcare budget.122 The economic burden of hypertension in Mexico grew by 24% between 2010 and 2012, and the total direct and indirect costs of hypertension in 2011 were estimated to exceed $5.7 billion.123
The financial implications of hypertension in LICs are of particular concern because of the rapid increase in the prevalence and absolute burden of hypertension and the low current and projected healthcare spending in these regions.4,124 In 2016, only 0.4% of global health spending was in LICs even though these countries comprise 10% of the global population.124 Projections suggest that by 2050, only 0.6% of health spending will occur in countries that are currently classed as LICs although these countries will comprise 15.7% of the global population.124 Many LICs currently rely on development assistance for health spending from international agencies and private philanthropy, which is primarily focused on infectious diseases and could be less sustainable for chronic disease control.124 Large-scale studies using standardized methods are needed to improve understanding of the economic costs of hypertension, particularly in LMICs.
In general, interventions are considered to be reasonably cost-effective if they cost less than US$50,000–75,000 per quality-adjusted life year (QALY); however, payers routinely cover treatments that cost >$100,000 per QALY.145 Several analyses have concluded that all antihypertensive medications are cost effective compared with no treatment (placebo).146 In a meta-analysis of 14 studies, the incremental cost-effectiveness ratio (ICER) of all antihypertensive medications adjusted to 2015 US dollars was $19,945 per QALY gained.146 The SPRINT trial reported an ICER of $28,000 per QALY gained for the intensive BP intervention compared to the standard intervention if the treatment effects persisted for the remaining lifetime of the patient.147 Antihypertensive treatment might be even more cost effective in LMICs than in HICs. For example, treating all adults with hypertension and prior CVD for secondary prevention was projected to be cost saving in China with an ICER in 2015 of Int$9,000 per QALY gained in those with BP ≥160/100 mmHg and Int$13,000 per QALY gained in those with BP ≥140/90 mm Hg.148 Moreover, patient-centered values, such as health-related quality of life, productivity, severity of disease, and equity, are important to factor into analyses when determining the financial burden associated with hypertension and weighing the costs of prevention and treatment versus the costs associated with BP-related CVD.149,150
FUTURE PERSPECTIVES
Without intervention, the prevalence and absolute burden of hypertension is expected to continue to increase, particularly in LMICs.3,4,12,13 Consequentially, BP-related cardiovascular and kidney disease will become even greater health and financial burdens worldwide.2 Economic development and urbanization have resulted in rapid epidemiological and nutrition transitions, i.e., decreasing infant mortality and infectious diseases and increasing life expectancy and chronic diseases, in many LMICs, including China and India.151–153 The prevalence of hypertension is high and increasing in these countries, whereas the rates of awareness, treatment and control are low.3,20 Lifestyle risk factors for hypertension, such as obesity, high dietary sodium intake, low dietary potassium intake, alcohol consumption, lack of physical activity and unhealthy diet have reached epidemic proportions in LMICs.154,155 National programs for the prevention, detection, and treatment of hypertension in LMICs are needed to slow and reverse the current trends in the hypertension epidemic. In addition, community-based intervention programs are needed to reduce health disparities and improve hypertension prevention and control in low-income and ethnic minority populations in HICs.42 To accurately estimate the global burden of hypertension, worldwide prevalence surveys using standardized methodology for sampling of participants, BP measurement, and data quality control and analysis are needed, especially in under-represented regions.3 Research to develop and test the effective, equitable, and sustainable interventions for implementing evidence-based clinical guidelines and public health policies worldwide are critically important for reducing the global burden of hypertension.3
CONCLUSIONS
Although the estimated global mean BP appears to be fairly stable, the prevalence and absolute burden of hypertension is increasing globally, especially in LMICs. In 2010, an estimated 1.39 billion adults worldwide had hypertension of whom 1.04 billion were in LMICs and 349 million in HICs.3 Elevated BP is strongly, independently, and linearly associated with the risk of CVD, CKD, and all-cause mortality. Although effective lifestyle modifications and pharmaceutical treatments are available, proportion of hypertension awareness, treatment, and control are low. Hypertension, therefore, remains a global public health challenge. Few comprehensive assessments of the economic impact of hypertension in global populations exist. It was estimated that the global financial burden of hypertension was about 10% of the world’s overall healthcare expenditures, varying from about 22.6% of all healthcare expenditures in Eastern Europe and Central Asia to 7.2% in East Asia and Pacific. Future studies are warranted to develop multifaceted implementation strategies for hypertension prevention and control, especially in LICs and low-income populations, and to accurately assess the financial burden of hypertension worldwide.
KEY POINTS.
Hypertension is the leading modifiable risk factor for cardiovascular disease and premature death worldwide.
The prevalence and absolute burden of hypertension is rising globally, especially in low and middle-income countries (LMICs).
Awareness, treatment, and control of hypertension are unacceptably low worldwide, particularly in LMICs.
Reductions in risk factors, including high sodium intake, low potassium intake, obesity, alcohol consumption, physical inactivity and unhealthy diet, are recommended for the prevention and control of hypertension.
Multifaceted implementation strategies for hypertension prevention and control are needed to address barriers at the patient, provider, system, and community levels.
Comprehensive assessments are needed to evaluate the economic impact of hypertension worldwide.
Acknowledgements
The authors’ work is supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under Award Number P20GM109036 and by the National Heart, Lung, and Blood Institute of NIH under Award Number R01HL133790. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Glossary of Terms
- Pooling analysis
A type of meta-analysis in which investigators have access to and analyze the original individual level data from participating study
- Regression dilution (bias)
In an epidemiological study the regression slope between a response and predictor variable is underestimated when the predictor variable is measured imprecisely
- Network meta-analysis
A type of meta-analysis in which multiple treatments are being compared using both direct comparisons of interventions within randomized controlled trials and indirect comparisons across trials based on a common comparator
- Cross-over design
A type of clinical trial in which each participant is randomized to a sequence of two or more treatments; therefore, the participant is used as his or her own control
- Dietary Approaches to Stop Hypertension (DASH) diet
A diet emphasizing fruits, vegetables, and low-fat dairy foods; including whole grains, poultry, fish, and nuts; and limiting red meat, sweets, and sugar-containing beverages
- Weighted mean
A type of average that instead of each of the data points contributing equally to the final average, some data points contribute more than others
- Dose-response relationship
A relationship in which a change in amount, intensity, or duration of exposure is associated with either increasing or decreasing risk of the outcome
- Convenience samples
A type of non-probability sample in which the study participants are taken from a group of people easy to contact or to reach
- Quality-adjusted life year (QALY)
A measure of the burden of disease on a defined population which equals the sum of years of life lost (YLLs) and years lived with disability (YLDs). One DALY equals one lost year of healthy life
- Incremental cost-effectiveness ratio (ICER)
A measure of the cost-effectiveness of new health care interventions defined as the ratio of the difference in cost between two possible interventions divided by the difference in their effect
Footnotes
Competing interest statement
The authors declare no competing interests.
References
- 1.Stanaway JD et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Stu. Lancet 392, 1923–1994 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Causes of Death Collaborators, G. A. et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 392, 1736–1788 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills KT et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 134, 441–450 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou B et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 389, 37–55 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falaschetti E, Mindell J, Knott C & Poulter N Hypertension management in England: a serial cross-sectional study from 1994 to 2011. Lancet (London, England) 383, 1912–9 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Ezzati M et al. Contributions of risk factors and medical care to cardiovascular mortality trends. Nat. Rev. Cardiol 12, 508–530 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laatikainen T, Nissinen A, Kastarinen M, Jula A & Tuomilehto J Blood Pressure, Sodium Intake, and Hypertension Control: Lessons From the North Karelia Project. Glob. Heart 11, 191–9 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim MM & Damasceno A Hypertension in developing countries. Lancet 380, 611–619 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Hogerzeil HV et al. Promotion of access to essential medicines for non-communicable diseases: practical implications of the UN political declaration. Lancet (London, England) 381, 680–9 (2013). [DOI] [PubMed] [Google Scholar]
- 10.BeLue R et al. An overview of cardiovascular risk factor burden in sub-Saharan African countries: a socio-cultural perspective. Global. Health 5, 10 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow CK et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 310, 959–68 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Forouzanfar MH et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 317, 165–182 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Kearney PM et al. Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Whelton PK et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138, e484–e594 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Lewington S, Clarke R, Qizilbash N, Peto R & Collins R Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360, 1903–1913 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Guo X et al. Association Between Pre-hypertension and Cardiovascular Outcomes: A Systematic Review and Meta-analysis of Prospective Studies. Curr. Hypertens. Rep 15, 703–716 (2013). [DOI] [PubMed] [Google Scholar]
- 17.SPRINT Research Group et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med 373, 2103–2116 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bundy JD et al. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiol 2, 775–781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bundy JD et al. Estimating the Association of the 2017 and 2014 Hypertension Guidelines With Cardiovascular Events and Deaths in US Adults: An Analysis of National Data. JAMA Cardiol 3, 572–581 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z et al. Status of Hypertension in China. Circulation 137, 2344–2356 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Muntner P et al. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertens. (Dallas, Tex. 1979) 73, e35–e66 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills KT et al. Global Disparities of Hypertension Prevalence and Control. Circulation 134, 441–450 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ataklte F et al. Burden of Undiagnosed Hypertension in Sub-Saharan Africa. Hypertension 65, 291–298 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Martin MJ, Hulley SB, Browner WS, Kuller LH & Wentworth D Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 men. Lancet (London, England) 2, 933–6 (1986). [DOI] [PubMed] [Google Scholar]
- 25.Stamler J, Stamler R & Neaton JD Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch. Intern. Med 153, 598–615 (1993). [DOI] [PubMed] [Google Scholar]
- 26.Gu D et al. Blood Pressure and Risk of Cardiovascular Disease in Chinese Men and Women. Am. J. Hypertens 21, 265–272 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Anderson AH et al. Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann. Intern. Med 162, 258–65 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klag MJ et al. Blood pressure and end-stage renal disease in men. N. Engl. J. Med 334, 13–18 (1996). [DOI] [PubMed] [Google Scholar]
- 29.Reynolds K et al. A population-based, prospective study of blood pressure and risk for end-stage renal disease in China. J. Am. Soc. Nephrol 18, 1928–35 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Turnbull F & Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet (London, England) 362, 1527–35 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Ettehad D et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet (London, England) 387, 957–967 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Xie X et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 387, 435–443 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Cheung AK et al. Effects of Intensive BP Control in CKD. J. Am. Soc. Nephrol 28, 2812–2823 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhotra R et al. Effects of Intensive Blood Pressure Lowering on Kidney Tubule Injury in CKD: A Longitudinal Subgroup Analysis in SPRINT. Am. J. Kidney Dis 73, 21–30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williamson JD et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years. JAMA 315, 2673 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ACCORD Study Group et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N. Engl. J. Med 362, 1575–1585 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Margolis KL et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care 37, 1721–1728 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitagawa K et al. Effect of Standard vs Intensive Blood Pressure Control on the Risk of Recurrent Stroke: A Randomized Clinical Trial and Meta-analysis. JAMA Neurol 76, 1309 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh GM et al. The age associations of blood pressure, cholesterol, and glucose: analysis of health examination surveys from international populations. Circulation 125, 2204–2211 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorans KS, Mills KT, Liu Y & He J Trends in Prevalence and Control of Hypertension According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline. J. Am. Heart Assoc 7, 10.1161/JAHA.118.008888 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman JS, Dolman L, Rushani D & Cooper RS The contribution of genomic research to explaining racial disparities in cardiovascular disease: a systematic review. Am. J. Epidemiol 181, 464–72 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Whelton PK et al. Research Needs to Improve Hypertension Treatment and Control in African Americans. Hypertension 68, 1066–1072 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whelton PK et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. Jama 288, 1882–1888 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Powles J et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 3, e003733 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Academies of Sciences, Engineering, and Medicine, 2019. Dietary Reference Intakes for Sodium and Potassium (2019). doi: 10.17226/25353 [DOI] [PubMed] [Google Scholar]
- 46.He J & Whelton PK Salt intake, hypertension and risk of cardiovascular disease: an important public health challenge. Int. J. Epidemiol 31, 322–327 (2002). [PubMed] [Google Scholar]
- 47.Elliott P et al. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. BMJ 312, 1249–1253 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He J, Tell GS, Tang YC, Mo PS & He GQ Relation of electrolytes to blood pressure in men. The Yi people study. Hypertens. (Dallas, Tex. 1979) 17, 378–385 (1991). [DOI] [PubMed] [Google Scholar]
- 49.Mente A et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community-level prospective epidemiological cohort study. Lancet 392, 496–506 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Sacks FM et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med 344, 3–10 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Midgley JP, Matthew AG, Greenwood CM & Logan AG Effect of reduced dietary sodium on blood pressure: a meta-analysis of randomized controlled trials. JAMA (Chicago, Ill.) 275, 1590–1597 (1996). [DOI] [PubMed] [Google Scholar]
- 52.Cutler JA, Follmann D & Allender PS Randomized trials of sodium reduction: an overview. Am. J. Clin. Nutr 65, 643S–651S (1997). [DOI] [PubMed] [Google Scholar]
- 53.Graudal NA, Galloe AM, Garred P, Galløe AM & Garred P Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta-analysis. JAMA 279, 1383–1391 (1998). [DOI] [PubMed] [Google Scholar]
- 54.He FJ, Li J & MacGregor GA Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 346, f1325 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Newberry SJ et al. Sodium and Potassium Intake: Effects on Chronic Disease Outcomes and Risks. Sodium and Potassium Intake: Effects on Chronic Disease Outcomes and Risks (Agency for Healthcare Research and Quality; (US: ), 2018). [PubMed] [Google Scholar]
- 56.Cook NR, Appel LJ & Whelton PK Lower levels of sodium intake and reduced cardiovascular risk. Circulation 129, 981–989 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He J et al. Urinary Sodium and Potassium Excretion and CKD Progression. J. Am. Soc. Nephrol 27, 1202–1212 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mills KT et al. Sodium Excretion and the Risk of Cardiovascular Disease in Patients With Chronic Kidney Disease. Jama 315, 2200–2210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stolarz-Skrzypek K et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. Jama 305, 1777–1785 (2011). [DOI] [PubMed] [Google Scholar]
- 60.O’Donnell MJ et al. Urinary sodium and potassium excretion and risk of cardiovascular events. Jama 306, 2229–2238 (2011). [DOI] [PubMed] [Google Scholar]
- 61.O’Donnell M et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N. Engl. J. Med 371, 612–623 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Mente A et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet (London, England) 388, 465–475 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Pfister R et al. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur. J. Heart Fail 16, 394–402 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Olde Engberink RHGG et al. Use of a Single Baseline Versus Multiyear 24-Hour Urine Collection for Estimation of Long-Term Sodium Intake and Associated Cardiovascular and Renal Risk. Circulation 136, 917–926 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Nomura K, Asayama K, Jacobs L, Thijs L & Staessen JA Renal function in relation to sodium intake: a quantitative review of the literature. Kidney Int 92, 67–78 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Cobb LK et al. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation 129, 1173–1186 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Iwahori T et al. Urinary sodium-to-potassium ratio and intake of sodium and potassium among men and women from multiethnic general populations: the INTERSALT Study. Hypertens. Res 42, 1590–1598 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Weaver CM, Stone MS, Lobene AJ, Cladis DP & Hodges JK What Is the Evidence Base for a Potassium Requirement? Nutr. Today 53, 1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ 297, 319–328 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cappuccio FP & MacGregor GA Does potassium supplementation lower blood pressure? A meta-analysis of published trials. J. Hypertens 9, 465–73 (1991). [DOI] [PubMed] [Google Scholar]
- 71.Whelton PK et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 277, 1624–1632 (1997). [DOI] [PubMed] [Google Scholar]
- 72.Poorolajal J et al. Oral potassium supplementation for management of essential hypertension: A meta-analysis of randomized controlled trials. PLoS One 12, e0174967 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Filippini T, Violi F, D’Amico R & Vinceti M The effect of potassium supplementation on blood pressure in hypertensive subjects: A systematic review and meta-analysis. Int. J. Cardiol 230, 127–135 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Manthey J et al. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet (London, England) 393, 2493–2502 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Klatsky AL, Friedman GD, Siegelaub AB & Gérard MJ Alcohol consumption and blood pressure. Kaiser-Permanente Multiphasic Health Examination data. N. Engl. J. Med 296, 1194–200 (1977). [DOI] [PubMed] [Google Scholar]
- 76.Fuchs FD, Chambless LE, Whelton PK, Nieto FJ & Heiss G Alcohol consumption and the incidence of hypertension: The Atherosclerosis Risk in Communities Study. Hypertens. (Dallas, Tex. 1979) 37, 1242–50 (2001). [DOI] [PubMed] [Google Scholar]
- 77.Millwood IY et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet (London, England) 393, 1831–1842 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xin X et al. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 38, 1112–1117 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Roerecke M et al. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta-analysis. Lancet Public Heal 2, e108–e120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guthold R, Stevens GA, Riley LM & Bull FC Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob. Heal 6, e1077–e1086 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Lesniak KT & Dubbert PM Exercise and hypertension. Curr. Opin. Cardiol 16, 356–9 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Hayashi T et al. Walking to work and the risk for hypertension in men: the Osaka Health Survey. Ann. Intern. Med 131, 21–6 (1999). [DOI] [PubMed] [Google Scholar]
- 83.Whelton SP, Chin A, Xin X & He J Effect of Aerobic Exercise on Blood Pressure. Ann. Intern. Med 136, 493 (2002). [DOI] [PubMed] [Google Scholar]
- 84.Ishikawa K, Ohta T, Zhang J, Hashimoto S & Tanaka H Influence of age and gender on exercise training-induced blood pressure reduction in systemic hypertension. Am. J. Cardiol 84, 192–196 (1999). [DOI] [PubMed] [Google Scholar]
- 85.Kelley GA & Kelley KS Progressive resistance exercise and resting blood pressure : A meta-analysis of randomized controlled trials. Hypertens. (Dallas, Tex. 1979) 35, 838–43 (2000). [DOI] [PubMed] [Google Scholar]
- 86.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 387, 1377–1396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang Z et al. Body Weight, Weight Change, and Risk for Hypertension in Women. Ann. Intern. Med 128, 81 (1998). [DOI] [PubMed] [Google Scholar]
- 88.He J et al. Body mass and blood pressure in a lean population in southwestern China. Am. J. Epidemiol 139, 380–9 (1994). [DOI] [PubMed] [Google Scholar]
- 89.Mertens IL & van Gaal LF Overweight, Obesity, and Blood Pressure: The Effects of Modest Weight Reduction. Obes. Res 8, 270–278 (2000). [DOI] [PubMed] [Google Scholar]
- 90.Forman JP, Stampfer MJ & Curhan GC Diet and Lifestyle Risk Factors Associated With Incident Hypertension in Women. JAMA 302, 401 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garrison RJ, Kannel WB, Stokes J & Castelli WP Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev. Med. (Baltim) 16, 235–51 (1987). [DOI] [PubMed] [Google Scholar]
- 92.Neter JE, Stam BE, Kok FJ, Grobbee DE & Geleijnse JM Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 42, 878–884 (2003). [DOI] [PubMed] [Google Scholar]
- 93.Whelton SP et al. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J. Hypertens 23, 475–481 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Streppel MT, Arends LR, van ‘t Veer P, Grobbee DE & Geleijnse JM Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch. Intern. Med 165, 150–6 (2005). [DOI] [PubMed] [Google Scholar]
- 95.Liu L, Ikeda K, Sullivan DH, Ling W & Yamori Y Epidemiological evidence of the association between dietary protein intake and blood pressure: a meta-analysis of published data. Hypertens. Res 25, 689–95 (2002). [DOI] [PubMed] [Google Scholar]
- 96.Rebholz CM et al. Dietary Protein Intake and Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am. J. Epidemiol 176, S27–S43 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Margetts BM, Beilin LJ, Armstrong BK, Vandongen R & Croft KD Dietary fat intake and blood pressure: a double blind controlled trial of changing polyunsaturated to saturated fat ratio. J. Hypertens. Suppl 2, S201–3 (1984). [PubMed] [Google Scholar]
- 98.Puska P et al. Controlled, randomised trial of the effect of dietary fat on blood pressure. Lancet (London, England) 1, 1–5 (1983). [DOI] [PubMed] [Google Scholar]
- 99.Imamura F et al. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet. Glob. Heal 3, e132–42 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Appel LJ et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med 336, 1117–1124 (1997). [DOI] [PubMed] [Google Scholar]
- 101.Yokoyama Y et al. Vegetarian Diets and Blood Pressure. JAMA Intern. Med 174, 577 (2014). [DOI] [PubMed] [Google Scholar]
- 102.Nordmann AJ et al. Meta-Analysis Comparing Mediterranean to Low-Fat Diets for Modification of Cardiovascular Risk Factors. Am. J. Med 124, 841–851. e2 (2011). [DOI] [PubMed] [Google Scholar]
- 103.Baer L & Radichevich I Cigarette smoking in hypertensive patients. Blood pressure and endocrine responses. Am. J. Med 78, 564–568 (1985). [DOI] [PubMed] [Google Scholar]
- 104.Rhee M-Y, Na S-H, Kim Y-K, Lee M-M & Kim H-Y Acute effects of cigarette smoking on arterial stiffness and blood pressure in male smokers with hypertension. Am. J. Hypertens 20, 637–41 (2007). [DOI] [PubMed] [Google Scholar]
- 105.Wang Y et al. Relationship between Duration of Sleep and Hypertension in Adults: A Meta-Analysis. J. Clin. Sleep Med 11, 1047–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bazzano LA, Khan Z, Reynolds K & He J Effect of Nocturnal Nasal Continuous Positive Airway Pressure on Blood Pressure in Obstructive Sleep Apnea. Hypertension 50, 417–423 (2007). [DOI] [PubMed] [Google Scholar]
- 107.Virdis A, Giannarelli C, Fritsch Neves M, Taddei S & Ghiadoni L Cigarette Smoking and Hypertension. Curr. Pharm. Des 16, 2518–2525 (2010). [DOI] [PubMed] [Google Scholar]
- 108.Halperin RO, Michael Gaziano J & Sesso HD Smoking and the Risk of Incident Hypertension in Middle-aged and Older Men. Am. J. Hypertens 21, 148–152 (2008). [DOI] [PubMed] [Google Scholar]
- 109.Cai Y et al. Associations of Short-Term and Long-Term Exposure to Ambient Air Pollutants With Hypertension. Hypertension 68, 62–70 (2016). [DOI] [PubMed] [Google Scholar]
- 110.Yang B-Y et al. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ. Pollut 235, 576–588 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Liu M-Y, Li N, Li WA & Khan H Association between psychosocial stress and hypertension: a systematic review and meta-analysis. Neurol. Res 39, 573–580 (2017). [DOI] [PubMed] [Google Scholar]
- 112.Manohar S, Thongprayoon C, Cheungpasitporn W, Mao MA & Herrmann SM Associations of rotational shift work and night shift status with hypertension: a systematic review and meta-analysis. J. Hypertens 35, 1929–1937 (2017). [DOI] [PubMed] [Google Scholar]
- 113.van Kempen E, Casas M, Pershagen G & Foraster M WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Cardiovascular and Metabolic Effects: A Summary. Int. J. Environ. Res. Public Health 15, 379 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dzhambov AM & Dimitrova DD Residential road traffic noise as a risk factor for hypertension in adults: Systematic review and meta-analysis of analytic studies published in the period 2011–2017. Environ. Pollut 240, 306–318 (2018). [DOI] [PubMed] [Google Scholar]
- 115.Bowman TS, Gaziano JM, Buring JE & Sesso HD A prospective study of cigarette smoking and risk of incident hypertension in women. J. Am. Coll. Cardiol 50, 2085–92 (2007). [DOI] [PubMed] [Google Scholar]
- 116.Gerace TA, Hollis J, Ockene JK & Svendsen K Smoking cessation and change in diastolic blood pressure, body weight, and plasma lipids. MRFIT Research Group. Prev. Med. (Baltim) 20, 602–20 (1991). [DOI] [PubMed] [Google Scholar]
- 117.Huang K et al. Long-Term Exposure to Fine Particulate Matter and Hypertension Incidence in China. Hypertension 73, 1195–1201 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yusuf S et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet (2019). doi: 10.1016/S0140-6736(19)32008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nagele E et al. Clinical effectiveness of stress-reduction techniques in patients with hypertension: systematic review and meta-analysis. J. Hypertens 32, 1936–44; discussion 1944 (2014). [DOI] [PubMed] [Google Scholar]
- 120.Whelton PK et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. Jama 279, 839–846 (1998). [DOI] [PubMed] [Google Scholar]
- 121.Cook NR et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 334, 885–888 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beaney T et al. May Measurement Month 2017: an analysis of blood pressure screening results worldwide. Lancet Glob. Heal 6, e736–e743 (2018). [DOI] [PubMed] [Google Scholar]
- 123.Borzecki AM, Oliveria SA & Berlowitz DR Barriers to hypertension control. Am. Heart J 149, 785–794 (2005). [DOI] [PubMed] [Google Scholar]
- 124.Khatib R et al. Patient and healthcare provider barriers to hypertension awareness, treatment and follow up: a systematic review and meta-analysis of qualitative and quantitative studies. PLoS One 9, e84238 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mills KT et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: A systematic review and meta-analysis. Ann. Intern. Med 168, 110–120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.He J et al. Effect of a Community Health Worker-Led Multicomponent Intervention on Blood Pressure Control in Low-Income Patients in Argentina: A Randomized Clinical Trial. JAMA 318, 1016–1025 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Piper MA et al. Diagnostic and Predictive Accuracy of Blood Pressure Screening Methods With Consideration of Rescreening Intervals: A Systematic Review for the U.S. Preventive Services Task Force. Ann. Intern. Med 162, 192 (2015). [DOI] [PubMed] [Google Scholar]
- 128.Yang W-Y et al. Association of Office and Ambulatory Blood Pressure With Mortality and Cardiovascular Outcomes. JAMA 322, 409 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siu AL Screening for High Blood Pressure in Adults: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med 163, 778 (2015). [DOI] [PubMed] [Google Scholar]
- 130.Agarwal R, Bills JE, Hecht TJW & Light RP Role of Home Blood Pressure Monitoring in Overcoming Therapeutic Inertia and Improving Hypertension Control. Hypertension 57, 29–38 (2011). [DOI] [PubMed] [Google Scholar]
- 131.Olsen MH et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet (London, England) 388, 2665–2712 (2016). [DOI] [PubMed] [Google Scholar]
- 132.Bao W, Threefoot SA, Srinivasan SR & Berenson GS Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am. J. Hypertens 8, 657–65 (1995). [DOI] [PubMed] [Google Scholar]
- 133.Zhang T et al. Trajectories of Childhood Blood Pressure and Adult Left Ventricular Hypertrophy. Hypertension 72, 93–101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li S, Chen W, Srinivasan SR & Berenson GS Childhood Blood Pressure as a Predictor of Arterial Stiffness in Young Adults. Hypertension 43, 541–546 (2004). [DOI] [PubMed] [Google Scholar]
- 135.Gaziano TA, Bitton A, Anand S, Weinstein MC & International Society of Hypertension. The global cost of nonoptimal blood pressure. J. Hypertens 27, 1472–1477 (2009). [DOI] [PubMed] [Google Scholar]
- 136.Kirkland EB et al. Trends in Healthcare Expenditures Among US Adults With Hypertension: National Estimates, 2003–2014. J. Am. Heart Assoc 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang D, Wang G, Zhang P, Fang J & Ayala C Medical Expenditures Associated With Hypertension in the U.S., 2000–2013. Am. J. Prev. Med 53, S164–S171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang G, Fang J & Ayala C Hypertension-associated hospitalizations and costs in the United States, 1979–2006. Blood Press 23, 126–33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang G, Yan L, Ayala C, George MG & Fang J Hypertension-associated expenditures for medication among US adults. Am. J. Hypertens 26, 1295–302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heidenreich PA et al. Forecasting the Future of Cardiovascular Disease in the United States. Circulation 123, 933–944 (2011). [DOI] [PubMed] [Google Scholar]
- 141.Nakamura K et al. Treated and untreated hypertension, hospitalization, and medical expenditure: an epidemiological study in 314622 beneficiaries of the medical insurance system in Japan. J. Hypertens 31, 1032–42 (2013). [DOI] [PubMed] [Google Scholar]
- 142.ARREDONDO A & ZUNIGA A Epidemiologic Changes and Economic Burden of Hypertension in Latin AmericaEvidence from Mexico. Am. J. Hypertens 19, 553–559 (2006). [DOI] [PubMed] [Google Scholar]
- 143.Arredondo A & Aviles R Hypertension and Its Effects on the Economy of the Health System for Patients and Society: Suggestions for Developing Countries. Am. J. Hypertens 27, 635–636 (2014). [DOI] [PubMed] [Google Scholar]
- 144.Global Burden of Disease Health Financing Collaborator Network, A. Y. et al. Past, present, and future of global health financing: a review of development assistance, government, out-of-pocket, and other private spending on health for 195 countries, 1995–2050. Lancet (London, England) 393, 2233–2260 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Woolf SH A closer look at the economic argument for disease prevention. JAMA 301, 536–8 (2009). [DOI] [PubMed] [Google Scholar]
- 146.Park M et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J. Am. Soc. Nephrol 23, 1725–1734 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bress AP et al. Cost-Effectiveness of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med 377, 745–755 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gu D et al. The Cost-Effectiveness of Low-Cost Essential Antihypertensive Medicines for Hypertension Control in China: A Modelling Study. PLoS Med 12, e1001860 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lakdawalla DN et al. Defining Elements of Value in Health Care-A Health Economics Approach: An ISPOR Special Task Force Report [3]. Value Health 21, 131–139 (2018). [DOI] [PubMed] [Google Scholar]
- 150.Marzorati C & Pravettoni G Value as the key concept in the health care system: how it has influenced medical practice and clinical decision-making processes. J. Multidiscip. Healthc 10, 101–106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.GBD 2013 DALYs and HALE Collaborators, C. J. L. et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet (London, England) 386, 2145–91 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Blacher J, Levy BI, Mourad J-J, Safar ME & Bakris G From epidemiological transition to modern cardiovascular epidemiology: hypertension in the 21st century. Lancet (London, England) 388, 530–2 (2016). [DOI] [PubMed] [Google Scholar]
- 153.He J et al. Major Causes of Death among Men and Women in China. N. Engl. J. Med 353, 1124–1134 (2005). [DOI] [PubMed] [Google Scholar]
- 154.Bi Y et al. Status of Cardiovascular Health in Chinese Adults. J. Am. Coll. Cardiol 65, 1013–1025 (2015). [DOI] [PubMed] [Google Scholar]
- 155.Yang W et al. Prevalence of Diabetes among Men and Women in China. N. Engl. J. Med 362, 1090–1101 (2010). [DOI] [PubMed] [Google Scholar]


