Abstract
Background: Obesity and depression co-occur in a significant proportion of the population. Mechanisms linking the two disorders include the immune and the endocrine system, psychological and social mechanisms. The aim of this systematic review was to ascertain whether weight loss through dietary interventions has the additional effect of ameliorating depressive symptoms in obese patients. Methods: We systematically searched three databases (Pubmed, Medline, Embase) for longitudinal clinical trials testing a dietary intervention in people with obesity and depression or symptoms of depression. Results: Twenty-four longitudinal clinical studies met the eligibility criteria with a total of 3244 included patients. Seventeen studies examined the effects of calorie-restricted diets and eight studies examined dietary supplements (two studies examined both). Only three studies examined people with a diagnosis of both obesity and depression. The majority of studies showed that interventions using a calorie-restricted diet resulted in decreases in depression scores, with effect sizes between ≈0.2 and ≈0.6. The results were less clear for dietary supplements. Conclusions: People with obesity and depression appear to be a specific subgroup of depressed patients in which calorie-restricted diets might constitute a promising personalized treatment approach. The reduction of depressive symptoms may be related to immunoendocrine and psychosocial mechanisms.
Keywords: obesity, depression, diet, systematic review, weight loss
1. Introduction
Both depression and obesity are major public health concerns [1,2] with high worldwide prevalence and associated increased cardiovascular risks [3,4]. Research has revealed an association between depression and obesity, with the prevalence of depression in obese individuals being twice as high as in those of normal weight [5]. The relationship between depression and obesity, although established and confirmed by numerous epidemiological studies and meta-analyses, has not yet been fully clarified. The association has been repeatedly examined with some authors asserting that depression results in weight gain and obesity and others claiming that obesity leads to depression, implying a bidirectional causality [6]. It has been suggested that both depression and obesity are due to dysregulation of stress responses, principally involving the hypothalamic–pituitary–adrenal (HPA) axis [7]. Additional mechanisms linking the two disorders are inflammation, oxidative stress, and other endocrine dysfunctions [8], as well as psychological mechanisms such as rumination, stigmatization and ostracism that contribute to and maintain the bidirectional relationship [9,10].
1.1. Diet and Depression
The typical diets of western societies have high amounts of saturated fats and refined sugars, as well as high amounts of red and processed meats, with concurrent low levels of fruit, vegetable and fiber intake. This results in a diet that is energy-dense and nutrient-poor with profound consequences for both our physical and mental health. The relationship between diet and obesity is clear; individuals consuming more calories than the recommended daily allowance, combined with consuming high amounts of foods high in fat and sugar content, are more likely to develop obesity. More recently, the impact of diet on mental health has also been revealed to be significant; for example, a recent meta-analysis found that adults following a healthy dietary pattern have fewer depressive symptoms and lower risk of developing depressive symptoms [11].
The precise etiology of depression is unknown, but many psychological, social, and biological underpinnings are thought to contribute to its development [12]. The latter includes genetic, hormonal, immunological, biochemical, and neurodegenerative factors. Concurrently, research has shown that these physiological aspects can be modulated by diet and nutrition. For example, in the case of genes, vitamin E has been shown to modulate several genes involved in neural signal transduction, inflammation and cell proliferation among others, while omega-3 polyunsaturated fatty acids (n-3 PUFAs) [13] have been shown to interact with genes that code for cytokines, cholesterol metabolizing enzymes, and growth factors [14].
1.2. Depression and Obesity
Many authors posit that depression is a heterogenous assortment of symptoms that can be divided into subtypes based on the accompanying presenting symptoms beyond low mood. Most recently, it has been subdivided into two main subtypes: type 1, which is characterized by loss of appetite and body weight, insomnia, and suicidal ideation, and type 2, also known as atypical depression, which presents with increased appetite and weight gain, leaden paralysis, hypersomnia, and a persistently poor metabolic profile [15]. Several factors are thought to moderate the relationship between obesity and depression. Stunkard et al. have reviewed the literature pertaining to what those moderators and mediators could be, and they have identified several including the severity of obesity, the severity of depression, and stress [16].
Correlations between both disorders involve disturbance of appetite regulation, changes in metabolic, hormonal and immunological parameters, and behavioral problems such as reduced physical activity [9,10,17,18,19,20]. More specifically, obesity has been shown to induce important physical, psychological, and behavioral changes in vulnerable patients, such as changes in the hormone and cytokine systems [18,21], changes in thought processes such as rumination [9], and behavioral changes such as reduced physical activity [20]. These changes are known risk factors of depression [17,20]. Thus, in obese patients, depression can be seen as a health consequence of obesity. If obesity contributes to the development and maintenance of depression, we can hypothesize that weight loss might help those depressed patients who are obese. Indeed, recent studies indicate that weight loss due to caloric restriction or gastric bypass surgery improves depressive symptoms among obese patients with depression [22,23,24,25]. Therefore, we sought to review and collate the existing research literature on the effects of diet modifications on depressive symptoms in overweight or obese individuals enrolled in dietary weight loss programs. The underlying idea was that in people with obesity and depression, depression occurs as a consequence of obesity, and therefore weight loss could not only help with regard to obesity but could also reduce depressive symptoms.
2. Materials and Methods
We conducted this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26].
2.1. Literature Search
Three electronic databases (PubMed, Medline, and EMBASE) were systematically searched from inception until 5 October 2020 using the following search terms: diet in combination with depression, in combination with obesity. Reference lists of potentially relevant papers and reviews were also scanned for potentially eligible papers.
2.2. Eligibility Criteria
Searches were limited to abstracts, studies with adult human participants, and studies written in English. Any study which assessed the effect of any dietary intervention or dietary supplementation on depressive symptoms in the context of obesity (BMI ≥ 30 kg/m2) at baseline and at least at one follow-up point was eligible for inclusion. To be eligible, at least a subgroup of study participants had to be obese. However, we did not exclude studies, when in addition to people with obesity, other study participants were overweight or of normal body weight (used as controls).
Studies were excluded if they (a) were not longitudinal clinical studies, (b) did not comment on weight/BMI change after intervention, (c) did not discuss change of depressive symptoms after intervention, or (d) were association or observational studies without a dietary intervention. Review articles, meta-analyses, case studies, conference proceedings/abstracts, book chapters, and unpublished theses were not included.
2.3. Study Selection
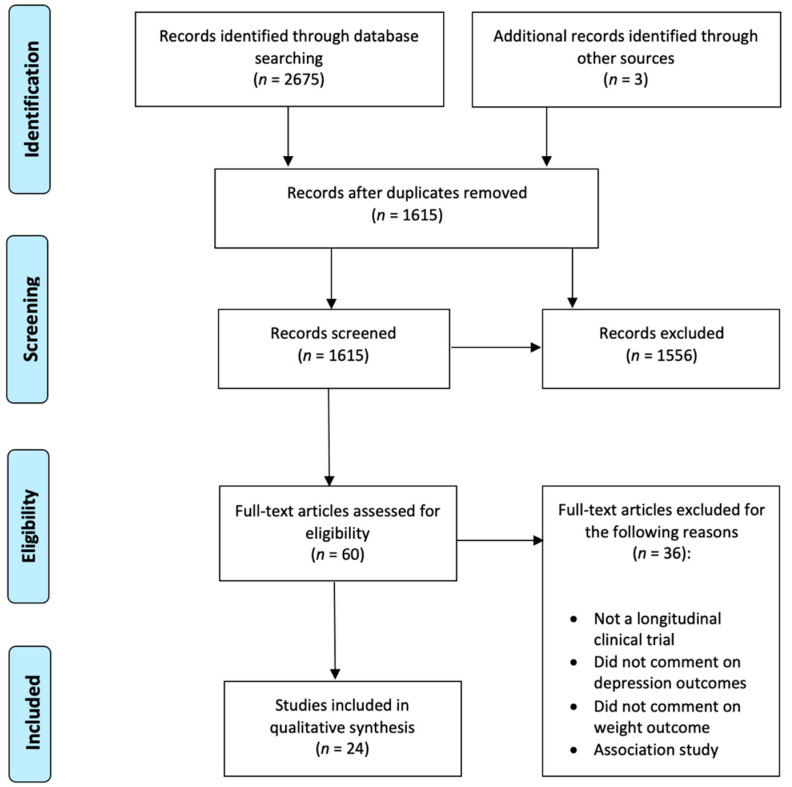
Figure 1 depicts the study selection and screening flowchart. Titles and abstracts of publications resulting from the search were imported into Mendeley and duplicates were removed. Two independent reviewers (O. P. and J. K.) performed all stages of the search, screening, and evaluation. Titles and abstracts were screened, and irrelevant articles were disregarded. Articles whose abstracts passed the first screen were read in full and assessed for eligibility based on our prespecified inclusion criteria, described above. Study quality assessment was performed using a quality assessment tool for pre-post studies from the National Heart, Lung and Blood Institute [27].
Figure 1.
PRISMA flow diagram.
3. Results
3.1. Characteristics of Included Studies
Individual study characteristics are described in Table 1. Twenty-four longitudinal clinical trials met the inclusion criteria. A total of 3244 patients participated in trials investigating the impact of diet, dietary supplements, or behavioral modification/counselling on weight and depression scores. All study participants were overweight or obese aside from some of the participants in the Breymeyer et al. [28] study which included a group of nonobese participants used as healthy controls.
Table 1.
Characteristics of included studies.
| Study | Disease | Sample Size (Recruited) | Excluded Due to Nonadherence to Intervention | Excluded or Withdrawn for Other Reasons | Completed | Diet Intervention | Energy Restricted Diet | Nondieting Control Group | Depression Scale | Gender (M) | Age (Mean ± SD) | Summary | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bot et al. [30] | Obesity | 1025 | 779 | Multinutrient supplementation + FRBA | No | No | MINI, PHQ-9 | 772 (253) | 46.6 | No significant effect of supplements or FRBA on PHQ scores. | Good | ||
| Breymeyer et al. [28] | Overweight/ Obese vs. healthy | 82 | 82 | Isocaloric HGL and LGL (crossover) | No | No | POMS, CES-D | 41 (41) | Mood disturbance was higher on HGL diet. Significant effect of diet on CES-D score with higher depression score associated with HGL diet. | Good | |||
| Brinkwork, Buckley at al. [31] | Overweight/ Obesity | 106 | 4 | 66 | Energy restricted LCHF vs. HCLF | Yes | No | POMS, BDI | 50 ± 0.8 | Both diet groups achieved significant reduction in weight and depression scores. However, LC group rebounded to baseline levels over time whereas LF group depression scores remained low. | Good | ||
| Brinkworth, Luscombe-Marsh et al. [39] | Obesity + diabetes | 115 | 6 | 32 | 77 | Energy restricted LCHF vs. HCLF | Yes | No | POMS, BDI | 58.5 ± 7.1 | Both diet groups achieved significant decrease in weight, POMS, and BDI scores. | Good | |
| Canheta et al. [47] | Obesity | 149 | 36 | 113 | Brazilian diet vs. extra virgin olive oil vs. both | Yes | Yes | HADS | 109 (20) | 38.9 ± 8.7 | All diet groups achieved significant reduction in depression scores. | Good | |
| Coates et al. [46] | Overweight/ Obese | 151 | 2 | 20 | 128 | Isocaloric AED vs. NF | No | No | POMS | 78 (70) | 65 ± 8 | No reduction in weight or depression scores. | Good |
| Crerand et al. [43] | Obesity | 123 | Meal replacement or balanced deficit diet vs. control (nondieting group) | Yes | Yes | BDI | 123 (0) | Diet group lost significantly more weight and reported significantly greater reduction in depressive symptoms. | Good | ||||
| Fuller et al. [40] | Obesity | 70 | 60 | Diet + exercise (Korean vs. Western hypocaloric) | Yes | No | BDI-II | 44 (36) | 45.5 ± 11.1 | Significant decrease for both groups in weight and BDI scores at end of intervention. | Good | ||
| Galletly et al. [29] | Overweight + PCOS | 25 | LPHC vs. HPLC | Yes | No | HADS | 25 (0) | HPLC: 33 ± 1.2 LPHC: 32 ± 1.2 |
HPLC diet resulted in significant reduction in depression scores. No difference in weight loss between diet groups. | Good | |||
| Hadi et al. [49] | Overweight/ Obese | 60 | 0 | 1 | 59 | Synbiotics vs. placebo | No | N/A | DASS-21 | 20 (40) | Synbiotic: 34.5 ± 6 Placebo: 36.6 ± 7.3 |
Both groups showed decreased weight and depression scores, however, synbiotic group showed greater improvement compared to placebo. | Good |
| Halyburton et al. [44] | Overweight/ Obese | 121 | 5 | 21 | 95 | Energy restricted LCHF vs. HCLF | Yes | No | POMS, BDI | 95 (0) | LCHF: 50.6 ± 1.1 HCLF: 49.8 ± 1.3 |
LCHF significantly greater weight loss than HCLF. Significant reduction in POMS and BDI scores for both diet groups. | Very good |
| Hariri et al. [33] | Overweight/ Obesity | 62 | 62 | Energy restricted diet plus sumac supplement vs. energy restricted diet + placebo | Yes | No | BDI-II | 62 (0) | S: 42 ± 8.44 C: 44 ± 11.8 |
Significant reduction in weight and depression in both groups. Sumac supplement group showed significantly more reduction in weight. | Good | ||
| Lutze et al. [41] | Obesity | 117 | 8 | 43 | 66 | Isocaloric HP vs. HCLF | Yes | No | POMS, SF-36 mental health summary | 0 (66) | 49.6 ± 9.2 | No effect of HP vs. HC diet. Both diets resulted in reduced weight and reduced POMS and SF-36 scores. | Good |
| Pedersen et al. [48] | Overweight/ Obesity |
70 | 55 | AIT vs. LED | Yes | No | HADS | 12 (43) | LED mean weight loss: 9.9kg, AIT mean weight loss: 1.6%. No significant change in HADS. | Good | |||
| Raman et al. [34] | Obesity | 80 | 80 | BWL vs. BWL + CRT-O | No | N/A | DASS-21 | 69 (11) | CRT-O: 40.6 ± 7.0 C: 42.2 ± 8.8 |
BWL + CRT-O resulted in significantly more weight loss at 3-month follow-up but had no effect on depression scores | Good | ||
| Rodriguez-Lozada et al. [32] | Overweight/ Obese | 305 | 305 | MHP vs. LF | Yes | No | BDI | 213 (92) | 45.3 | Both energy intake restricted diets resulted in reduced weight and depression scores. LF diet had more pronounced effects on depression scores in women. | Good | ||
| Ruusunen et al. [35] | Overweight/ Obese + impaired glucose tolerance | 140 | 140 | Counselling on weight reduction + physical activity | No | N/A | BDI | 81 (59) | 57.7 ± 6.4 | Both groups achieved reductions in weight and depression scores. With participants showing the greatest reduction in weight also showing greater decreases in depression scores. | Good | ||
| Sanchez et al. [45] | Obesity | 105 | 104 | Moderate energy restriction + probiotic | Yes | No | BDI | 60 (45) | 35 ± 10 | Significant decrease in BDI scores in probiotic group compared to placebo. | Good | ||
| Tan et al. [50] | Overweight/ Obesity + insomnia | 73 | 2 | 6 | 49 | Energy restricted diet vs. control | Yes | Yes | Rimon’s brief depression scale | 0 (49) | D: 51 C: 52.6 |
Diet group improved sleep time and depression scores. However, depression scores reduced in both groups. | Good |
| Uemura et al. [36] | Obesity | 44 | 44 | Counselling on gut microbiota | No | N/A | CES-D | 44 (0) | I: 62 ± 8.7 C: 63.3 ± 9.1 |
BMI, body weight, and CES-D scores decreased significantly after intervention. | Good | ||
| Vaghef-Mehrabany et al. [25] | Obesity + MDD | 62 | 6 | 11 | 45 | 25% weight loss diet + probiotic vs. placebo | Yes | No | BDI-II, HDRS | 62 (0) | Regardless of supplementation group, patients who achieved >1.9kg reduction in weight, showed reduction in HDRS and borderline reduction in BDI-II. Prebiotic supplementation had no effect on depressive symptoms. | Good | |
| Vigna et al. [37] | Overweight/ Obese | 77 | 77 | LCD: Hericium erinaceus vs. control | Yes | No | Zung’s depression scale, SCL-90 | 65 (12) | 53.2 ± 0.7 | H. erinaceus supplementation decreased depression scores. | Very good | ||
| Webber et al. [38] | Overweight/ Obese | 49 | 49 | BWL vs. EBT | No | N/A | CES-D | 41 (8) | 45 ± 7.9 | Both groups showed improvements in BMI and depression scores. | Good | ||
| Wing et al. [42] | Obesity + diabetes | 33 | 2 | 31 | VLCD vs. balanced diet | Yes | No | BDI | 25 (18) | Both weight and BDI scores decreased significantly after intervention. VLCD group had more weight loss. | Fair |
Abbreviations: AED = almond-enriched diet, AIT = aerobic interval training, BDI = Beck’s depression inventory, BDI-II = Beck’s Depression Inventory-2, BWL = behavioral weight loss, CES-D = center for epidemiologic studies depression scale, CRT-O = cognitive remediation therapy for obesity, DASS-21 = depression anxiety stress scale 21 items, EBT = emotional brain training, FRBA = food-related behavioral activation, HADS = hospital anxiety and depression scale, HCLF = high carbohydrate and low fat diet, HDRS = Hamilton depression rating scale, HGL = high glycemic index, HP = high protein diet, HPLC = high protein, low carbohydrate diet, LCD = low calorie diet, LCHF = low carbohydrate, high fat diet, LED = low energy diet, LF = low fat diet, LGL = low glycemic index, LPHC = low protein, high carbohydrate diet, MHP = moderately high protein diet, MINI = mini international neuropsychiatric interview, N/A = not applicable, NF = nut-free diet, PHQ-9 = patient health questionnaire, POMS = profile of mood states, SD = standard deviation, VLCD = very low calorie diet.
The sample sizes of included studies ranged between n = 25 [29] and n = 1025 [30] participants, and adherence and completion rates varied between ≈60% [31] and 100% [28,32,33,34,35,36,37,38] (see Table 1). Mean age of patients was reported in 19 studies, with a combined mean of 47.1 years. Gender was reported in 21 studies with a total of 2041 females and 863 males. The mean BMI was reported in 15 studies and pooling those means gave a mean of 33.9 kg/m2. The shortest intervention duration was 28 days [28] whilst the longest was 52 weeks [31,39,40,41]. One study did not explicitly state the data collection end point [35].
The most frequently used depression scale was Beck’s Depression Inventory (BDI) used by 11 studies [25,31,32,33,35,39,40,42,43,44,45], followed by the Profile of Mood States (POMS) used in six [28,31,39,41,44,46], and the Centre for Epidemiologic Studies Depression Scale (CES-D) [28,36,38] and the Hospital Anxiety and Depression Scale (HADS) used in three [29,47,48]. Two studies used the 21-item Depression Anxiety Stress Scale (DASS-21) [34,49] A further seven scales were used by some studies, either in conjunction with the aforementioned, or on their own (see Table 1). The majority of studies compared different dieting therapy groups to each other, with only three studies comparing an energy-restricted dieting group to a nondieting control group [43,47,50].
3.2. Study Findings
A summary of the findings of each of the included studies can be found in Table 2. Not all studies provided all values for every outcome measure but all of them commented on the desired outcomes, i.e., the effects of diet interventions on depressive symptoms in obese or overweight participants. Overall, the majority of studies concluded that weight loss, whether through calorie restriction, dietary supplements, or behavioral training, resulted in a reduction of depressive symptoms, with reported values of effect sizes on depression and depressive symptoms varying between a Cohen’s d of 0.16 [42] and 0.64 [49], while effect sizes of weight change ranged from a Cohen’s d of 0.0 [31] to 0.45 [39].
Table 2.
Findings of included studies.
| Study | Weight kg (Mean ± SD) | BMI kg/m2 (Mean ± SD) | Depression | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post | p-Value | Baseline | Post | p-Value | Baseline | Post | p-Value | |
| Bot et al. [30] | P: 31.4 P + FRBA: 31.2 S: 31.3 S + FRBA: 31.7 |
P: 7.3 (4.1) P + FRBA: 7.4 (4.4) S: 7.9 (4.4) S + FRBA: 7.1 (4) |
|||||||
| Breymeyer et al. [28] | HGL: 2.80 LGL: 2.03 |
p = 0.002 | |||||||
| Brinkworth, Buckley et al. [31] | LCHF: 96 ± 1.6 HCLF: 97.6 ± 1.6 |
LCHF: 82.3 ± 2.1 HCLF: 83.9 ± 1.9 | BDI: p
= 0.05 POMS: p = 0.05 |
||||||
| Brinkworth, Luscombe-Marsh et al. [39] | LC: 101.8 ± 2 HCLF: 101.1 ± 2 |
LC: 92.6 ± 2 HCLF: 91 ± 2 | |||||||
| Canheta et al. [47] | 46.3 ± 6.5 | p < 0.001 | |||||||
| Coates et al. [46] | AED: 84.4 ± 12 NF: 85.4 ± 14 |
AED: 84.8 ± 1.38 NF: 85.6 ± 1.36 |
p > 0.05 | AED: 30.2 ± 0.44 NF: 30.6 ± 0.43 |
AED: 30.5 ± 0.44 NF: 30.3 ± 0.43 |
p > 0.05 | AED: 0.89 ± 1.9 NF: –3.74 ± 1.88 |
AED: 1.11 ± 2.2 NF: –2.22 ± 2.17 |
POMS: p > 0.05 |
| Crerand et al. [43] | D: 97.8 ± 13.5 C: 96.1 ± 12.1 |
D: 36.2 ± 4.5 C: 35.3 ± 4.3 |
D vs. C: p < 0.001 | D: 7.7 ± 5.5 C: 7.4 ± 5.9 |
p < 0.001 | ||||
| Fuller et al. [40] | D: 90.9 ± 12.2 C: 93.8 ± 12.7 |
D: –7.9 ± 2.1 C: 0.1 |
D: 34.1 ± 4.3 C: 35.2 ± 4.8 |
D: 22.1 ± 8.1 C: 23.7 ± 11.1 |
D: 19.3 ± 6 C: 25.3 ± 12.7 |
POMS: time x group p < 0.001 | |||
| Galletly et al. [29] | HPLC: 104.2 ± 5.3 LPHC: 98.6 ± 4.6 | HPLC: –6.9 ± 0.8 LPHC: –8.5 ± 6.3 | HPLC: 37.6 ± 6.4 LPHC: 37.2 ± 6.9 | HPLC: 34.5 ± 5.7 LPHC: 34.5 ± 6.3 | HPLC: 5.6 ± 3.2 LPHC: 4.8 ± 3.4 | HPLC: 3.6 ± 2.8 LPHC: 3.4 ± 3.3 | HPLC: p < 0.001 LPHC: NS |
||
| Hadi et al. [49] | 89.4 ± 16.1 | –5.2% ± 4.3% | p < 0.001 | 31.1 ± 3.9 | 5 ± 4.6 | 2 ± 4.1 | p < 0.001 | ||
| Halyburton et al. [44] | LCHF: 93.6 ± 2.1 HCLF: 97 ± 2.1 |
LCHF: 33.3 ± 0.6 HCLF: 33.8 ± 0.6 |
p < 0.001 | ||||||
| Hariri et al. [33] | Su: 84.3 ± 11.7 P: 79.3 ± 11.4 |
Su: 78.96 ± 10.84 P: 76.89 ± 11.35 |
p < 0.001 | Su: 32.4 ± 3.73 P: 31.2 ± 3.87 |
S: 30.4 ± 3.55 P: 30.3 ± 3.89 |
p < 0.001 | Su: 25.4 ± 9.42 P: 26.17 ± 11.21 |
Su: 25.4 ± 9.42 P: 26.17 ± 11.21 |
p < 0.001 |
| Lutze et al. [41] | HP: 100.5 ± 1.8 HC: 102.6 ± 1.8 |
HP: –12.3 ± 1.4 HC: –10.9 ± 1.4 |
HP: 23.4 ± 1.09 HC: 23.04 ± 1.05 |
HP: 20.77 ± 0.97 HC: 20.19 ± 0.94 | POMS: p < 0.001 SF-36 subscales vitality and mental health: p < 0.001 |
||||
| Pedersen et al. [48] | Median: 92.8 |
LED: p < 0.001 | Median: 31.4 | ||||||
| Raman et al. [34] | CRT-O: 40.3 ± 7.8 C: 39.2 ± 7.4 |
CRT-O: 38.9 ± 7.6 C: 39.7 ± 8.4 |
CRT-O:19.1 ± 11.2 C: 13.3 ± 12.2 |
CRT-O: 4.5 ± 5.1 C: 15.4 ± 12.2 |
|||||
| Rodriguez-Lozada et al. [32] | 87.7 | –8.6 | p < 0.001 | 31.6 | –3.1 | p < 0.001 | 6.6 | –2.7 | p < 0.001 |
| Ruusunen et al. [35] | –3.14 ± 4.5 | 30.5 ± 3.4 | –1.16 ± 1.74 | I vs. C: p = 0.024 | I: 6.8 ± 5.6 | I: –0.9 ± 4.5 | I: p = 0.03 | ||
| Sanchez et al. [45] | Pro: 95.1 ± 13.9 | Pro: –5.3 ± 4.3 | Pro: 33.8 ± 3.3 | Pro: 4.4 ± 4.1 | Pro: –1.5 ± 3 | p < 0.05 | |||
| Uemura et al. [36] | I: 66.3 ± 8.74 | I: 64.6 ± 8.07 | p < 0.001 | I: 27.8 ± 3.1 | I: 27.1 ± 2.82 | p < 0.001 | I: 17.64 ± 13.58 | I: 10.05 ± 7.4 | p < 0.001 |
| Tan et al. [50] | D: 93.8 C: 93.1 |
D: 92.7 C: 94.4 |
D: p < 0.05 | D: 29.4 C: 29.2 |
D: 5.0 C: 4.0 |
D: 4.0 C: 3.0 |
p < 0.05 | ||
| Vaghef-Mehrabany et al. [25] | For >1.9kg weight loss: HDRS: 13.2 BDI: 19.5 |
For >1.9kg weight loss: HDRS: 9.1 BDI: 14.7 |
For >1.9kg weight loss: HDRS: p < 0.001 BDI: p = 0.006 |
||||||
| Vigna et al. [37] | HE: 33.1 ± 0.84 C: 33.4 ± 0.83 |
HE: 32.01 ± 0.82 C: 32.08 ± 0.88 |
HE: 48.8 ± 1.03 | HE: 43.2 ± 2.38 | HE: p < 0.05 | ||||
| Webber et al. [38] | BWL: 99 ± 16.7 EBT: 101 ± 10.8 |
BWL: 36 ± 4.3 EBT: 37 ± 4.9 |
BWL: –1.3 EBT: –0.6 |
BWL: p < 0.001 EBT: p = 0.032 BWL vs. EBT: p < 0.03 |
BWL: 7.5 ± 6.4 EBT: 10.4 ± 9.8 |
BWL: –2.9 EBT: –3.1 |
BWL: p = 0.012 EBT: p = 0.006 |
||
| Wing et al. [42] | 103.2 ± 16.9 | VLCD: 14.6 ± 9.4 BD: 11.4 ± 7.2 |
VLCD: 5 ± 6.3 BD: 2.9 ± 2.8 |
VLCD: p
< 0.001 BD: p < 0.001 |
|||||
Abbreviations: AED = almond-enriched diet, BD = balanced diet, BDI = Beck’s depression inventory, BDI-II = Beck’s Depression Inventory-2, BWL = behavioral weight loss, C = control, CES-D = center for epidemiologic studies depression scale, CRT-O = cognitive remediation therapy for obesity, D = diet, EBT = emotional brain training, FRBA = food-related behavioral activation, HCLF = high carbohydrate and low fat diet, HDRS = Hamilton depression rating scale, HE = H. erinaceus supplement, HGL = high glycemic index, HP = high protein diet, HPLC = high protein, low carbohydrate diet, I = intervention group, LCHF = low carbohydrate, high fat diet, LED = low energy diet, LGL = low glycemic index, LPHC = low protein, high carbohydrate diet, NF = nut-free diet, P = placebo, Pro = probiotic group, POMS = profile of mood states, S = supplements, Su = sumac supplement group, SD = standard deviation, SF-36 = short form health status survey, VLCD = very low calorie diet.
As we obtained studies in people with obesity and diagnosed depression and with obesity and depressive symptoms without the clinical diagnosis of depression, we will report on these two types of studies in separate sections. In people with obesity and depressive symptoms, but no diagnosis of depression, authors used different treatment approaches: energy restricted diets, energy restricted diets plus pre/probiotic supplementation, diet combined with an exercise intervention, and counselling. Thus, we dedicated one paragraph to each of these approaches. However, as these are not disjointed categories, some studies fell into multiple categories.
3.2.1. Effects of Diet Interventions on Obesity and Clinically Diagnosed Depression
Of the included studies only three were conducted in participants with concurrent obesity and clinically established depressive disorder. Participants in these three studies were on an energy restricted diet plus an additional supplement or placebo. Hariri et al. reported all relevant values for weight, BMI, and depression scores and demonstrated a decrease in weight and depression scores for both groups (sumac vs. placebo) [33]. Vaghef-Mehrabany et al. [25] and Vigna et al. [37] did not provide values for all groups at all time points but nonetheless commented on the outcomes. Vaghef-Mahrabany et al. did not find any difference between the group receiving supplementation and the placebo group, however, they did note that regardless of group classification, participants that lost more than 1.9 kg of weight showed significantly improved depression scores. In contrast, Vigna et al. reported significant reductions in depression scores for the group receiving the H. erinaceus supplement.
3.2.2. Effects of Diet Interventions on Obesity and Depressive Symptoms
Studies of Energy Restricted Diets
The majority of studies (n = 16) investigated the effects of specific calorie restricted diets on weight and depressive symptoms in overweight or obese participants without an established current clinical diagnosis of depression. None of the studies included here reported full datasets with values at each time point and corresponding significance values. Most authors reported a decrease in depressive symptoms following a calorie restricted diet, aside from one study [48] that reported no change in depression scores. Three studies compared a calorie restricted diet with a noncalorie restricted control group, and all three found a reduction in both weight and depression scores in the intervention group [40,43,50]. However, most studies compared different calorie reduced diets with each other, i.e., four studies compared a low carbohydrate, high fat (LCHF) diet with a high fat, low carbohydrate (HCLF) diet [29,31,39,44], one study compared a high protein diet with a high carbohydrate diet [41], one study compared a high protein diet with low fat diet [32], one compared a very low calorie diet with an energy reduced balanced diet [42], and one study compared the traditional Brazilian diet with olive oil supplementation [47]. The remaining studies on energy restricted diets included the use of dietary supplements and will thus be discussed separately in the next section.
Studies on Energy Restricted Diets Plus Pre/Probiotic Supplementation
Three studies reported on the impact of calorie restriction with additional pre/probiotic supplementation [25,45,49]. Hadi et al. [49] and Sanchez et al. [45] found a significant decrease in depression scores for the groups receiving pro/prebiotics whereas Vaghef-Mehrabany et al. [25] reported a decrease in depression scores for participants who achieved a weight loss greater than 1.9kg regardless of prebiotic supplementation.
Studies on Diet Combined with Exercise Intervention
Four studies investigated the impact of diet and exercise/lifestyle interventions on depressive symptoms [35,39,40,48]. Three of them reported significant reductions in depression scores accompanying reductions in weight, except from Pedersen et al. [48] who reported no differences in depression scores between the two groups (aerobic interval training (AIT) vs. low energy diet (LED)) even though the LED group achieved a 10.4% decrease in body weight.
Studies on Counselling (Not Explicitly Calorie Restricted)
We found seven studies that reported on the effects of supplements without calorie restriction [46] or on the effects of behavioral modifications/counselling on depression scores [28,30,34,35,36,38]. These studies were not specifically prescribing calorie restricted diets but were rather providing additional supplements and/or counselling on healthy lifestyle modifications, such as dietary and exercise recommendations. The exception to this was the Breymeyer et al. study, which did not include any training or calorie restriction but was comparing the effects of a high glycemic (HG) diet (vs. a low glycemic (LG) diet) on depression scores [28]. The authors concluded that mood disturbance was higher for the group on the HG diet, with higher depression scores associated with higher glycemic load. Coates et al. investigated the effects of an almond-enriched diet compared to a nut-free diet and found no differences in depression scores between the two groups [46]. Three studies [35,36,38] investigated what effect counselling or behavioral training has on depression scores and all three found improvements in depressive symptoms following the intervention. One study investigated the effects of multinutrient supplementation and/or food-related behavioral activation therapy (in a 2 × 2 design) on depressive symptoms and did not find any significant effect of either on depression scores [30]. Lastly, one study compared the effect of behavioral weight loss with or without cognitive remediation therapy on body weight and depression scores and found no changes in depression scores between the two groups [34].
4. Discussion
4.1. Summary of the Main Findings
This systematic review summarizes the existing data on the effects of diet on depressive symptoms in overweight or obese patients. Findings from the included studies were mixed, with the majority of studies reporting significant improvements in depression scores after diet and weight loss, and the remaining studies reporting no differences between depression scores between pre- and postintervention [34,46,48]. No studies reported deterioration of depressive symptoms aside from one that reported increased mood disturbance in participants on a high glycemic load diet [28]. Importantly, the majority of authors reported high adherence to the intervention, whether those were hypocaloric diets or supplements. The trend of obese individuals experiencing an improvement in their depressive symptoms after diet and weight loss is in line with previous research. Dietary interventions using a calorie-restricted diet (e.g., [25,29,44]) resulted in decreases in depressive symptoms. However, the results are less clear for dietary supplements (e.g., [33,37,46]). Overall, the dietary approaches were heterogenous in that the diets investigated were calorie reduced, traditional, high/low in protein, high/low in carbohydrates, with/or without pre-/probiotic, vitamins, or naturopathic supplements.
4.2. Possible Mechanisms for Improved Mood after Weight Loss
It is well established that depression and obesity co-occur to a high degree [5,6,51,52,53,54], however the relationship between the two disorders is complex and currently of ambiguous directionality. Stunkard et al. presented a summary on the existing data using a moderator/mediator framework in which they classified eating and physical activity as an important mediator of obesity and comorbid depression [16]. Some authors consider depression as a consequence of obesity resulting from societal stigmatization, dissatisfaction with one’s appearance, and low self-esteem [55,56,57]. Others consider obesity as resulting from decreased physical activity, excessive ‘comfort’ eating, and antidepressant medication use that often accompanies depression [58,59,60,61,62].
Several epidemiological studies have found associations between mood and diet. Particularly, a western-style diet high in processed foods and sugar content and low in fruits and vegetables, is associated with worsening of mood states. Indeed, one of our included studies found increases in depression scores in participants on a high glycemic load diet [28]. Diets that are high in carbohydrates but low in fat and protein have also been associated with lower mood scores in cross-sectional studies [63,64], whereas an abundance of research extols the beneficial effects of Mediterranean-style diets [65] which are high in fruit, vegetables, nuts, pulses and wholegrains, low in fat and carbohydrate, with very little processed foods. The differences in mood scores between these two types of diets are thought to be partly due to the increased systemic inflammation and oxidative processes that often accompanies a western-style diet [66,67,68,69].
4.2.1. Physiological Mechanisms
Research has exposed metabolic and inflammatory dysregulation as a common denominator in depression and obesity [70,71]. Additionally, both depressed and obese patients exhibit dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis [72,73] and consequently chronic elevations in cortisol [74,75]. Increases in cortisol levels have been reported as having a causal role in depression, as well as leading to weight gain, specifically in abdominal adiposity. Recently, white adipose tissue (WAT) has been conceptualized as an endocrine organ, as opposed to how it was previously thought of—as an inert storage tissue—due to its ability to produce cytokines and other related molecules. Among these are interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α [76,77,78], which are known proinflammatory cytokines, as well as chemokines, including monocyte chemoattractant protein (MCP)-1 [79,80]. The ensuing signaling cascade leads to immune activation and white blood cell accumulation, and an overall increased inflammatory response. This immune activation has various downstream effects. For example, IL-2 reduces tryptophan plasma levels [81], possibly by activating tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenase (IDO). Tryptophan is an essential amino acid necessary for 5-HT synthesis. Low levels of tryptophan could lead to lower levels of serotonin and thus affect mood. Another example is the accumulation of peripheral monocytes in the brain as a result of systemic inflammation [82], and specifically the increased production of MCP-1 in hypothalamic neurons. This monocyte migration has been associated with increased anxiety and depression [83]. Minimization of accumulated adipose tissue through weight loss could attenuate this inflammatory process, leading to improved mood.
Another molecule implicated in both obesity and depression is leptin. Leptin is a peptide hormone released by adipocytes and crosses the blood–brain barrier via a saturable transport mechanism. Low plasma levels of leptin have been observed in depressed patients [84,85]. In the case of obesity however, plasma leptin levels have been found to be elevated [86,87]. This contradictory finding can be explained by leptin resistance (as in the case of type 2 diabetic patients being resistant to insulin) and could be a result of impaired transport across the blood–brain barrier, of reduced function of the leptin receptor, or errors in signal transduction [88,89]. Similar to cortisol and inflammatory molecules described previously, leptin modulates HPA axis function [90,91]. Leptin also interacts with monoamines and although its effect on monoamine neurotransmission remains unclear, there is evidence for leptin’s involvement in the 5-HT system [92] and in the activation of STAT3 in dopamine neurons of the ventral tegmental area (VTA) [93]. Reducing the amount of adipose tissue through diet and subsequent weight loss could ameliorate leptin resistance, reinstate leptin function, and relieve low mood.
4.2.2. Psychosocial Mechanisms
It should be borne in mind that psychosocial attributes may affect physiology, and the distinction between the two mechanisms here is for ease of discussion. A good example of this environment x biology interaction is the finding that weight discrimination, often experienced by obese individuals, increases cortisol levels [94]. Additionally, repeated discrimination can lead to lower self-esteem and increased negative affect [95]. Many studies have reported on the negative attitudes of employers, peers, and even clinicians towards obese persons [96,97]. Continued maltreatment can impact obese persons’ mood and self-concept, both of which can contribute to depression.
Even if obese individuals do not experience weight discrimination or stigma by others, their self-esteem could be impacted by their own body image dissatisfaction (BID). Some research has found correlations between BID and depressive symptoms and suggested that obesity confers risk for developing depression through increased BID [98,99]. Therefore, it is possible that losing weight improves body image satisfaction and low mood. For a more thorough discussion see Markowitz et al. [100].
It is important to note that some researchers posit that while obese individuals experiencing weight loss also experience an improvement in mood, this improvement does not seem to be mediated by the weight loss itself but is rather related to active participation in treatment [101,102,103,104].
4.3. Clinical Implications
Given the high prevalence of obesity and depression and the strain exerted on healthcare systems it would be of great value if prescribing dietary modifications for the amelioration of obesity had the additional consequence of improving depressive symptoms. Our findings suggest that dietary interventions leading to weight loss improve mood scores in both clinically and subclinically depressed obese individuals. Importantly, adherence to intervention seemed to be high in our included studies, which provides clinicians reason for optimism.
People with obesity and depression or depressive symptoms are a particularly vulnerable group who are at risk of worsening of depressive symptoms (e.g., [105]), switching from depression to mania (e.g., [106]), and of the appearance of eating disorder symptoms (e.g., [107]). Thus, further studies in obese and depressed patients should focus on the safety of diets regarding the reoccurrence of depressive symptoms, the switch from depression to mania, and the appearance of eating disorder symptoms.
4.4. Strengths and Limitations
This is the first review to systematically collate research on the effects of dietary interventions on depression and depressive symptoms in overweight/obese patients. Our strict inclusion of longitudinal clinical trials strengthens the validity of our findings. Additionally, the quality of most of the studies was good, and only one was deemed fair (see Table 1). However, the respective study quality was deemed good according to each study’s specific research question which is not the same as being of good quality to answer the research question of this review. Therefore, our finding of weight loss ameliorating depression scores in obese individuals is based on limited and heterogeneous data.
Furthermore, even though a meta-analytic approach would have provided more quantifiable evidence, such an approach would have been inappropriate based on the heterogeneity of the studies. This heterogeneity emerged from both the plethora of dietary approaches investigated as well as the varied comparison groups, and the lack of data. However, future meta-analytic research could investigate well-defined dietary categories by being less stringent with inclusion criteria, for example by including all studies in depressed patients regardless of the weight status.
A further limitation of our review is the inclusion of only three studies that compared the depression scores of participants in an energy-restricted diet group to a non-dieting control group. The lack of well-defined randomized controlled trials (RCTs) with this specific research question limits the validity and generalizability of our conclusion. Further RCTs are necessary to confirm the trend we have noted in this review.
Our study focused on the use of diet in people with both, obesity and depression. We did not include studies if obesity was not an important aspect of the study design, e.g., the SMILES trial [108] and the HELFIMED study [109], both of which showed that dietary improvement is associated with a reduction in depression scores. However, this systematic review focused on people with both, obesity and depression, because we wanted to investigate whether dietary modifications would help people who suffer from both disorders.
5. Conclusions
The findings of the current review provide preliminary evidence for the importance of weight loss in obese individuals experiencing low mood. The majority of studies included showed decreases in depression scores following dietary interventions, specifically through calorie-restricted diets. This is in line with a large body of research reporting amelioration of depressive symptoms in obese patients after weight loss. It is plausible that pursuing dietary interventions for obese patients with comorbid depression could have the additional benefit of relieving some of their depressive symptoms as well as improving their metabolic profile and cardiovascular risk. Therefore, a restricted diet might specifically help people with type 2 depression which is characterized by an increased appetite and weight gain, leaden paralysis, hypersomnia, and a persistently poor metabolic profile [13].
In summary, people with obesity and depression appear to be a specific subgroup of depressed patients. In this subgroup, calorie-restricted diets could constitute a promising personalized treatment approach which might lead to a reduction of depressive symptoms. The underlying mechanisms at play may be related to the immune and endocrine systems and to psychosocial aspects obesity.
Author Contributions
Original idea by H.H.; H.H., A.H.Y., U.S. and B.W.J.H.P. developed the scientific concept of the manuscript; O.P. and J.K. performed the systematic review; O.P. drafted the manuscript, and all authors provided feedback; all authors have read and agreed to the published version of the manuscript.
Funding
O.P., U.S., H.H., and A.H.Y. received salary support from the National Institute for Health Research (NIHR) Biomedical Research Centre for Mental Health, South London and Maudsley NHS Foundation Trust, and Institute of Psychiatry, Psychology and Neuroscience, KCL. U.S. and A.H.Y. are also supported by NIHR Senior Investigator Awards. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agha M., Agha R. The rising prevalence of obesity. Int. J. Surg. Oncol. 2017;2:e17. doi: 10.1097/IJ9.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Q., He H., Yang J., Feng X., Zhao F., Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020;126:134–140. doi: 10.1016/j.jpsychires.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Kotsis V., Tsioufis K., Antza C., Seravalle G., Coca A., Sierra C., Lurbe E., Stabouli S., Jelakovic B., Redon J., et al. Obesity and cardiovascular risk: A call for action from the European Society of Hypertension Working Group of Obesity, Diabetes and the High-risk Patient and European Association for the Study of Obesity: Part B: Obesity-induced cardiovascular disease, early prevention strategies and future research directions. J. Hypertens. 2018;36:1441–1455. doi: 10.1097/HJH.0000000000001731. [DOI] [PubMed] [Google Scholar]
- 4.Penninx B.W.J.H., Beekman A.T.F., Honig A., Deeg D.J.H., Schoevers R.A., Van Eijk J.T.M., van Tilburg W. Depression and cardiac mortality: Results from a community-based longitudinal study. Arch. Gen. Psychiatry. 2001;58:221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 5.Pereira-Miranda E., Costa P.R.F., Queiroz V.A.O., Pereira-Santos M., Santana M.L.P. Overweight and Obesity Associated with Higher Depression Prevalence in Adults: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2017;36:223–233. doi: 10.1080/07315724.2016.1261053. [DOI] [PubMed] [Google Scholar]
- 6.Luppino F.S., de Wit L.M., Bouvy P.F., Stijnen T., Cuijpers P., Penninx B.W.J.H., Zitman F.G. Overweight, Obesity, and Depression. Arch. Gen. Psychiatry. 2010;67:220. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 7.Bornstein S.R., Schuppenies A., Wong M.L., Licinio J. Approaching the shared biology of obesity and depression: The stress axis as the locus of gene-environment interactions. Mol. Psychiatry. 2006;11:892–902. doi: 10.1038/sj.mp.4001873. [DOI] [PubMed] [Google Scholar]
- 8.Hryhorczuk C., Sharma S. Metabolic disturbances connecting obesity and depression. Front. Neurosci. 2013;7:177. doi: 10.3389/fnins.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minkwitz J., Scheipl F., Cartwright L., Campbell I.C., Chittka T., Thormann J., Hegerl U., Sander C., Himmerich H. Why some obese people become depressed whilst others do not: Exploring links between cognitive reactivity, depression and obesity. Psychol. Health Med. 2019;24:362–373. doi: 10.1080/13548506.2018.1524153. [DOI] [PubMed] [Google Scholar]
- 10.Thormann J., Chittka T., Minkwitz J., Kluge M., Himmerich H. Obesity and depression: An overview on the complex interactions of two diseases. Fortschr. Neurol. Psychiatr. 2013;81:145–153. doi: 10.1055/s-0032-1330351. [DOI] [PubMed] [Google Scholar]
- 11.Nicolaou M., Colpo M., Vermeulen E., Elstgeest L.E.M., Cabout M., Gibson-Smith D., Knuppel A., Sini G., Schoenaker D.A.J.M., Mishra G.D., et al. Association of a priori dietary patterns with depressive symptoms: A harmonised meta-analysis of observational studies. Psychol. Med. 2020;50:1872–1883. doi: 10.1017/S0033291719001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Himmerich H., Kohls E., Hegerl U., Rummel-Kluge C. Predictive factors of depression and its therapy. Nervenarzt. 2014;85:1249–1254. doi: 10.1007/s00115-014-4048-0. [DOI] [PubMed] [Google Scholar]
- 13.Azzi A., Gysin R., Kempná P., Munteanu A., Villacorta L., Visarius T., Zingg J.M. Regulation of gene expression by α-tocopherol. Biol. Chem. 2004;385:585–591. doi: 10.1515/BC.2004.072. [DOI] [PubMed] [Google Scholar]
- 14.Kornman K.S., Martha P.M., Duff G.W. Genetic variations and inflammation: A practical nutrigenomics opportunity. Nutrition. 2004;20:44–49. doi: 10.1016/j.nut.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Lamers F., Beekman A.T.F., Van Hemert A.M., Schoevers R.A., Penninx B.W.J.H. Six-year longitudinal course and outcomes of subtypes of depression. Br. J. Psychiatry. 2016;208:62–68. doi: 10.1192/bjp.bp.114.153098. [DOI] [PubMed] [Google Scholar]
- 16.Stunkard A.J., Faith M.S., Allison K.C. Depression and obesity. Biol. Psychiatry. 2003;54:330–337. doi: 10.1016/S0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt F.M., Lichtblau N., Minkwitz J., Chittka T., Thormann J., Kirkby K.C., Sander C., Mergl R., Faßhauer M., Stumvoll M., et al. Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity. J. Psychiatr. Res. 2014;55:29–34. doi: 10.1016/j.jpsychires.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt F.M., Weschenfelder J., Sander C., Minkwitz J., Thormann J., Chittka T., Mergl R., Kirkby K.C., Faßhauer M., Stumvoll M., et al. Inflammatory Cytokines in General and Central Obesity and Modulating Effects of Physical Activity. PLoS ONE. 2015;10:e0121971. doi: 10.1371/journal.pone.0121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt F.M., Mergl R., Minkwitz J., Holdt L.M., Teupser D., Hegerl U., Himmerich H., Sander C. Is There an Association or Not?—Investigating the Association of Depressiveness, Physical Activity, Body Composition and Sleep with Mediators of Inflammation. Front. Psychiatry. 2020;11:563. doi: 10.3389/fpsyt.2020.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sander C., Ueck P., Mergl R., Gordon G., Hegerl U., Himmerich H. Physical activity in depressed and non-depressed patients with obesity. Eat. Weight Disord. 2018;23:195–203. doi: 10.1007/s40519-016-0347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zorena K., Jachimowicz-Duda O., Ślęzak D., Robakowska M., Mrugacz M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020;21:3570. doi: 10.3390/ijms21103570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waters G.S., Pories W.J., Swanson M.S., Meelheim H.D., Flickinger E.G., May H.J. Long-term studies of mental health after the greenville gastric bypass operation for morbid obesity. Am. J. Surg. 1991;161:154–158. doi: 10.1016/0002-9610(91)90377-P. [DOI] [PubMed] [Google Scholar]
- 23.Dymek M.P., Le Grange D., Neven K., Alverdy J. Quality of life and psychosocial adjustment in patients after Roux-en-Y gastric Bypass: A brief report. Obes. Surg. 2001;11:32–39. doi: 10.1381/096089201321454088. [DOI] [PubMed] [Google Scholar]
- 24.Emery C.F., Fondow M.D.M., Schneider C.M., Christofi F.L., Hunt C., Busby A.K., Needleman B.J., Melvin W.S., Elsayed-Awad H.M. Gastric bypass surgery is associated with reduced inflammation and less depression: A preliminary investigation. Obes. Surg. 2007;17:759–763. doi: 10.1007/s11695-007-9140-0. [DOI] [PubMed] [Google Scholar]
- 25.Vaghef-Mehrabany E., Ranjbar F., Asghari-Jafarabadi M., Hosseinpour-Arjmand S. Calorie restriction in combination with prebiotic supplementation in obese women with depression: Effects on metabolic and clinical response. Nutr. Neurosci. 2019;25:1–15. doi: 10.1080/1028415X.2019.1630985. [DOI] [PubMed] [Google Scholar]
- 26.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Heart, Lung, and Blood Institute (NHLBI) Study Quality Assessment Tools. [(accessed on 27 December 2020)]; Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 28.Breymeyer K.L., Lampe J.W., McGregor B.A., Neuhouser M.L. Subjective mood and energy levels of healthy weight and overweight/obese healthy adults on high-and low-glycemic load experimental diets. Appetite. 2016;107:253–359. doi: 10.1016/j.appet.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galletly C., Moran L., Noakes M., Clifton P., Tomlinson L., Norman R. Psychological benefits of a high-protein, low-carbohydrate diet in obese women with polycystic ovary syndrome-A pilot study. Appetite. 2007;49:590–593. doi: 10.1016/j.appet.2007.03.222. [DOI] [PubMed] [Google Scholar]
- 30.Bot M., Brouwer I.A., Roca M., Kohls E., Penninx B.W.J.H., Watkins E., van Grootheest G., Cabout M., Hegerl U., Gili M., et al. MooDFOOD Prevention Trial Investigators. Effect of Multinutrient Supplementation and Food-Related Behavioral Activation Therapy on Prevention of Major Depressive Disorder Among Overweight or Obese Adults With Subsyndromal Depressive Symptoms. JAMA. 2019;321:858. doi: 10.1001/jama.2019.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinkworth G.D., Buckley J.D., Noakes M., Clifton P.M., Wilson C.J. Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch. Int. Med. 2009;169:1873–1880. doi: 10.1001/archinternmed.2009.329. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Lozada C., Cuervo M., Cuevas-Sierra A., Goni L., Riezu-Boj J.I., Navas-Carretero S., Milagro F.I., Martinez J.A. Changes in anxiety and depression traits induced by energy restriction: Predictive value of the baseline status. Nutrients. 2019;11:1206. doi: 10.3390/nu11061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hariri N., Ghahroudi S.D., Jahangiri S., Borumandnia N., Narmaki E., Saidpour A. The beneficial effects of sumac (Rhus coriaria L.) supplementation along with restricted calorie diet on anthropometric indices, oxidative stress, and inflammation in overweight or obese women with depression: A randomized clinical trial. Phyther. Res. 2020;34:3041–3051. doi: 10.1002/ptr.6737. [DOI] [PubMed] [Google Scholar]
- 34.Raman J., Hay P., Tchanturia K., Smith E. A randomised controlled trial of manualized cognitive remediation therapy in adult obesity. Appetite. 2018;123:269–279. doi: 10.1016/j.appet.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Ruusunen A., Voutilainen S., Karhunen L., Lehto S.M., Tolmunen T., Keinänen-Kiukaanniemi S., Eriksson J., Tuomilehto J., Uusitupa M., Lindström J. How does lifestyle intervention affect depressive symptoms? Results from the Finnish Diabetes Prevention Study. Diabet. Med. 2012;29:e126–e132. doi: 10.1111/j.1464-5491.2012.03602.x. [DOI] [PubMed] [Google Scholar]
- 36.Uemura M., Hayashi F., Ishioka K., Ihara K., Yasuda K., Okazaki K., Omata J., Suzutani T., Hirakawa Y., Chiang C., et al. Obesity and mental health improvement following nutritional education focusing on gut microbiota composition in Japanese women: A randomised controlled trial. Eur. J. Nutr. 2019;58:3291–3302. doi: 10.1007/s00394-018-1873-0. [DOI] [PubMed] [Google Scholar]
- 37.Vigna L., Morelli F., Agnelli G.M., Napolitano F., Ratto D., Occhinegro A., Di Iorio C., Savino E., Girometta C., Brandalise F., et al. Hericium erinaceus Improves Mood and Sleep Disorders in Patients Affected by Overweight or Obesity: Could Circulating Pro-BDNF and BDNF Be Potential Biomarkers? Evid. Based Complement. Alternat. Med. 2019;2019:7861297. doi: 10.1155/2019/7861297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webber K.H., Casey E.M., Mayes L., Katsumata Y., Mellin L. A comparison of a behavioral weight loss program to a stress management program: A pilot randomized controlled trial. Nutrition. 2016;32:904–909. doi: 10.1016/j.nut.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brinkworth G.D., Luscombe-Marsh N.D., Thompson C.H., Noakes M., Buckley J.D., Wittert G. Long-term effects of very low-carbohydrate and high-carbohydrate weight-loss diets on psychological health in obese adults with type 2 diabetes: Randomized controlled trial. J. Int. Med. 2016;280:388–397. doi: 10.1111/joim.12501. [DOI] [PubMed] [Google Scholar]
- 40.Fuller N.R., Burns J., Sainsbury A., Horsfield S., da Luz F., Zhang S., Denyer G., Markovic T.P., Caterson I.D. Examining the association between depression and obesity during a weight management programme. Clin. Obes. 2017;7:354–359. doi: 10.1111/cob.12208. [DOI] [PubMed] [Google Scholar]
- 41.Lutze J., Taylor P., Brinkworth G.D., Wyld B., Syrette J., Wilson C.J., Clifton P.M., Noakes M. Psychological well-being response to high protein and high carbohydrate weight loss diets in overweight and obese men: A randomised trial. ESPEN J. 2013;8:e235–e240. doi: 10.1016/j.clnme.2013.08.002. [DOI] [Google Scholar]
- 42.Wing R.R., Marcus M.D., Blair E.H., Burton L.R. Psychological responses of obese type II diabetic subjects to very-low-calorie diet. Diabetes Care. 1991;14:596–599. doi: 10.2337/diacare.14.7.596. [DOI] [PubMed] [Google Scholar]
- 43.Crerand C.E., Wadden T.A., Foster G.D., Sarwer D.B., Paster L.M., Berkowitz R.I. Changes in Obesity-related Attitudes in Women Seeking Weight Reduction. Obesity. 2007;15:740–747. doi: 10.1038/oby.2007.590. [DOI] [PubMed] [Google Scholar]
- 44.Halyburton A.K., Brinkworth G.D., Wilson C.J., Noakes M., Buckley J.D., Keogh J.B., Clifton P.M. Low- and high-carbohydrate weight-loss diets have similar effects on mood but not cognitive performance. Am. J. Clin. Nutr. 2007;86:580–587. doi: 10.1093/ajcn/86.3.580. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez M., Darimont C., Panahi S., Drapeau V., Marette A., Taylor V.H., Doré J., Tremblay A. Effects of a Diet-Based Weight-Reducing Program with Probiotic Supplementation on Satiety Efficiency, Eating Behaviour Traits, and Psychosocial Behaviours in Obese Individuals. Nutrients. 2017;9:284. doi: 10.3390/nu9030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coates A.M., Morgillo S., Yandell C., Scholey A., Buckley J.D., Dyer K.A., Alison M.H. Effect of a 12-Week Almond-Enriched Diet on Biomarkers of Cognitive Performance, Mood, and Cardiometabolic Health in Older Overweight Adults. Nutrients. 2020;12:1180. doi: 10.3390/nu12041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canheta A., Santos A.S., Souza J., Silveira E.A. Traditional Brazilian diet and extra virgin olive oil reduce symptoms of anxiety and depression in individuals with severe obesity: Randomized clinical trial. Clin. Nutr. 2020;40:404–411. doi: 10.1016/j.clnu.2020.05.046. [DOI] [PubMed] [Google Scholar]
- 48.Pedersen L.R., Olsen R.H., Jurs A., Astrup A., Chabanova E., Simonsen L., Wisløff U., Haugaard S.B., Prescott E. A randomised trial comparing weight loss with aerobic exercise in overweight individuals with coronary artery disease: The CUT-IT trial. Eur. J. Prev. Cardiol. 2015;22:1009–1017. doi: 10.1177/2047487314545280. [DOI] [PubMed] [Google Scholar]
- 49.Hadi A., Sepandi M., Marx W., Moradi S. Clinical and psychological responses to synbiotic supplementation in obese or overweight adults: A randomized clinical trial. Complement. Ther. Med. 2019;47:102216. doi: 10.1016/j.ctim.2019.102216. [DOI] [PubMed] [Google Scholar]
- 50.Tan X., Alén M., Wang K., Tenhunen J., Wiklund P., Partinen M., Cheng S. Effect of Six-Month Diet Intervention on Sleep among Overweight and Obese Men with Chronic Insomnia Symptoms: A Randomized Controlled Trial. Nutrients. 2016;8:751. doi: 10.3390/nu8110751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carey M., Small H., Yoong S.L., Boyes A., Bisquera A., Sanson-Fisher R. Prevalence of comorbid depression and obesity in general practice: A cross-sectional survey. Br. J. Gen. Pract. 2014;64:e122. doi: 10.3399/bjgp14X677482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levitan R.D., Davis C., Kaplan A.S., Arenovich T., Phillips D.I.W., Ravindran A.V. Obesity Comorbidity in Unipolar Major Depressive Disorder. J. Clin. Psychiatry. 2012;73:1119–1124. doi: 10.4088/JCP.11m07394. [DOI] [PubMed] [Google Scholar]
- 53.Wadden T.A., Steen S.N., Wingate B.J., Foster G.D. Psychosocial consequences of weight reduction: How much weight loss is enough? Am. J. Clin. Nutr. 1996;63(Suppl. 3):461–465. doi: 10.1093/ajcn/63.3.461. [DOI] [PubMed] [Google Scholar]
- 54.Quinn D.M., Puhl R.M., Reinka M.A. Trying again (and again): Weight cycling and depressive symptoms in U.S. adults. PLoS ONE. 2020;15:e0239004. doi: 10.1371/journal.pone.0239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brownell K.D., Puhl R.M., Schwartz M.B., Rudd L. Weight Bias: Nature, Consequences, and Remedies. Guilford Publications; New York, NY, USA: 2005. [Google Scholar]
- 56.Kasen S., Cohen P., Chen H., Must A. Obesity and psychopathology in women: A three decade prospective study. Int. J. Obes. 2008;32:558–566. doi: 10.1038/sj.ijo.0803736. [DOI] [PubMed] [Google Scholar]
- 57.Roberts R., Deleger S., Strawbridge W.J., Kaplan G.A. Prospective association between obesity and depression: Evidence from the Alameda County Study. Int. J. Obes. 2003;27:514–521. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- 58.Wise L.A., Adams-Campbell L.L., Palmer J.R., Rosenberg L. Leisure time physical activity in relation to depressive symptoms in the Black Women’s Health Study. Ann. Behav. Med. 2006;32:68–76. doi: 10.1207/s15324796abm3201_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oliver G., Wardle J. Perceived effects of stress on food choice. Physiol. Behav. 1999;66:511–515. doi: 10.1016/S0031-9384(98)00322-9. [DOI] [PubMed] [Google Scholar]
- 60.Dallman M.F., Pecoraro N.C., La Fleur S.E. Chronic stress and comfort foods: Self-medication and abdominal obesity. Brain Behav. Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Himmerich H., Minkwitz J., Kirkby K.C. Weight Gain and Metabolic Changes During Treatment with Antipsychotics and Antidepressants. Endocr. Metab. Immune Disord. Drug Targets. 2015;15:252–260. doi: 10.2174/1871530315666150623092031. [DOI] [PubMed] [Google Scholar]
- 62.Serretti A., Mandelli L. Antidepressants and Body Weight. J. Clin. Psychiatry. 2010;71:1259–1272. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 63.Pellegrin K.L., O’Neil P.M., Stellefson E.J., Fossey M.D., Ballenger J.C., Cochrane C.E., Currey H.S. Average daily nutrient intake and mood among obese women. Nutr. Res. 1998;18:1103–1112. doi: 10.1016/S0271-5317(98)00092-X. [DOI] [Google Scholar]
- 64.de Castro J.M. Macronutrient relationships with meal patterns and mood in the spontaneous feeding behavior of humans. Physiol. Behav. 1987;39:561–569. doi: 10.1016/0031-9384(87)90154-5. [DOI] [PubMed] [Google Scholar]
- 65.Muñoz M.A., Fíto M., Marrugat J., Covas M.I., Schröder H. Adherence to the Mediterranean diet is associated with better mental and physical health. Br. J. Nutr. 2009;101:1821–1827. doi: 10.1017/S0007114508143598. [DOI] [PubMed] [Google Scholar]
- 66.Akbaraly T.N., Brunner E.J., Ferrie J.E., Marmot M.G., Kivimaki M., Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br. J. Psychiatry. 2009;195:408–413. doi: 10.1192/bjp.bp.108.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacka F.N., Pasco J.A., Mykletun A., Williams L.J., Hodge A.M., O’Reilly S.L., Nicholson G.C., Kotowicz M.A., Berk M. Association of western and traditional diets with depression and anxiety in women. Am. J. Psychiatry. 2010;167:305–311. doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- 68.Kiecolt-Glaser J.K. Stress, food, and inflammation: Psychoneuroimmunology and nutrition at the cutting edge. Psychosom. Med. 2010;72:365–369. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shelton R.C., Miller A.H. Eating ourselves to death (and despair): The contribution of adiposity and inflammation to depression. Prog. Neurobiol. 2010;91:275–299. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raison C., Miller A. Is depression an inflammatory disorder? Curr. Psychiatry Rep. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milaneschi Y., Simmons W.K., van Rossum E.F.C., Penninx B.W.J.H. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry. 2019;24:18–33. doi: 10.1038/s41380-018-0017-5. [DOI] [PubMed] [Google Scholar]
- 72.De Bellis M.D., Gold P.W., Geracioti T.D., Listwak S.J., Kling M.A. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am. J. Psychiatry. 1993;150:656–657. doi: 10.1176/ajp.150.4.656. [DOI] [PubMed] [Google Scholar]
- 73.De Mello A.F., De Mello M.F., Carpenter L.L., Price L.H. Update on stress and depression: The role of the hypothalamic-pituitary-adrenal (HPA) axis. Braz. J. Psychiatry. 2003;25:231–238. doi: 10.1590/S1516-44462003000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gibbons J.L., McHugh P.R. Plasma cortisol in depressive illness. J. Psychiatr. Res. 1962;1:162–171. doi: 10.1016/0022-3956(62)90006-7. [DOI] [PubMed] [Google Scholar]
- 75.Sachar E.J., Hellman L., Fukushima D.K., Gallagher T.F. Cortisol Production in Depressive Illness: A Clinical and Biochemical Clarification. Arch. Gen. Psychiatry. 1970;23:289–298. doi: 10.1001/archpsyc.1970.01750040001001. [DOI] [PubMed] [Google Scholar]
- 76.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 77.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Makki K., Froguel P., Wolowczuk I. Adipose Tissue in Obesity-Related Inflammation and Insulin Resistance: Cells, Cytokines, and Chemokines. ISRN Inflamm. 2013;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K.I., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., et al. MCP–1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kamei N., Tobe K., Suzuki R., Ohsugi M., Watanabe T., Kubota N., Ohtsuka-Kowatari N., Kumagai K., Sakamoto K., Kobayashi M., et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 81.Capuron L., Ravaud A., Neveu P.J., Miller A.H., Maes M., Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol. Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 82.Wohleb E.S., McKim D.B., Sheridan J.F., Godbout J.P. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 2015;8:447. doi: 10.3389/fnins.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ataka K., Asakawa A., Nagaishi K., Kaimoto K., Sawada A., Sawada A., Hayakawa Y., Tatezawa R., Inui A., Fujimiya M. Bone Marrow-Derived Microglia Infiltrate into the Paraventricular Nucleus of Chronic Psychological Stress-Loaded Mice. Harrison JK, editor. PLoS ONE. 2013;8:e81744. doi: 10.1371/journal.pone.0081744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kraus T., Haack M., Schuld A., Hinze-Selch D., Pollmächer T. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology. 2001;73:243–247. doi: 10.1159/000054641. [DOI] [PubMed] [Google Scholar]
- 85.Jow G.M., Yang T.T., Chen C.L. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J. Affect. Disord. 2006;90:21–27. doi: 10.1016/j.jad.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 86.Maffei M., Halaas J., Ravussin E., Pratley R., Lee G.H., Zhang Y., Fei H., Kim S., Lallone R., Ranganathan S., et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1997;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 87.Silha J.V., Krsek M., Skrha J.V., Sucharda P., Nyomba L.G., Murphy L.J. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: Correlations with insulin resistance. Eur. J. Endocrinol. 2003;149:331–335. doi: 10.1530/eje.0.1490331. [DOI] [PubMed] [Google Scholar]
- 88.Münzberg H., Björnholm M., Bates S.H., Myers M.G. Leptin receptor action and mechanisms of leptin resistance. Cell. Mol. Life Sci. 2005;62:642–652. doi: 10.1007/s00018-004-4432-1. [DOI] [PubMed] [Google Scholar]
- 89.Münzberg H., Myers M.G. Molecular and anatomical determinants of central leptin resistance. Nat. Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 90.Licinio J., Mantzoros C., Negrao A.B., Cizza G., Wong M.L., Bongiorno P.B., Chrousos G.P., Karp B., Allen C., Flier J.S., et al. Human leptin levels are pulsatile and inversely related to pituitary–ardenal function. Nat. Med. 1997;3:575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 91.Chen H., Charlat O., Tartaglia L.A., Woolf E.A., Weng X., Ellis S.J., Lakey N.D., Culpepper J., Moore K.J., Breitbart R.E., et al. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/S0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 92.Calapai G., Corica F., Corsonello A., Sautebin L., Di Rosa M., Campo G.M., Buemi M., Mauro V.N., Caputi A.P. Leptin increases serotonin turnover by inhibition of brain nitric oxide synthesis. J. Clin. Investig. 1999;104:975–982. doi: 10.1172/JCI5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fulton S., Pissios P., Manchon R.P., Stiles L., Frank L., Pothos E.N., Maratos-Flier E., Flier J.S. Leptin Regulation of the Mesoaccumbens Dopamine Pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 94.Jackson S.E., Kirschbaum C., Steptoe A. Perceived weight discrimination and chronic biochemical stress: A population-based study using cortisol in scalp hair. Obesity. 2016;24:2515–2521. doi: 10.1002/oby.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kessler R.C., Mickelson K.D., Williams D.R. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J. Health Soc. Behav. 1999;40:208–230. doi: 10.2307/2676349. [DOI] [PubMed] [Google Scholar]
- 96.Carr D., Friedman M.A. Is obesity stigmatizing? Body weight, perceived discrimination, and psychological well-being in the United States. J. Health. Soc. Behav. 2005;46:244–259. doi: 10.1177/002214650504600303. [DOI] [PubMed] [Google Scholar]
- 97.Schwartz M.B., Chambliss H.O.N., Brownell K.D., Blair S.N., Billington C. Weight Bias among Health Professionals Specializing in Obesity. Obes. Res. 2003;11:1033–1039. doi: 10.1038/oby.2003.142. [DOI] [PubMed] [Google Scholar]
- 98.Friedman M.A., Brownell K.D. Psychological Correlates of Obesity: Moving to the Next Research Generation. Psychol. Bull. 1995;117:3–20. doi: 10.1037/0033-2909.117.1.3. [DOI] [PubMed] [Google Scholar]
- 99.Friedman K.E., Reichmann S.K., Costanzo P.R., Musante G.J. Body image partially mediates the relationship between obesity and psychological distress. Obes. Res. 2002;10:33–41. doi: 10.1038/oby.2002.5. [DOI] [PubMed] [Google Scholar]
- 100.Markowitz S., Friedman M.A., Arent S.M. Understanding the Relation Between Obesity and Depression: Causal Mechanisms and Implications for Treatment. Clin. Psychol. Sci. Pract. 2008;15:1–20. doi: 10.1111/j.1468-2850.2008.00106.x. [DOI] [Google Scholar]
- 101.Wing R.R., Epstein L.H., Marcus M.D., Kupfer D.J. Mood changes in behavioral weight loss programs. J. Psychosom. Res. 1984;28:189–196. doi: 10.1016/0022-3999(84)90019-9. [DOI] [PubMed] [Google Scholar]
- 102.Karlsson J., Hallgren P., Kral J., Lindroos A.K., Sjöström L., Sullivan M. Predictors and effects of long-term dieting on mental well-being and weight loss in obese women. Appetite. 1994;23:15–26. doi: 10.1006/appe.1994.1031. [DOI] [PubMed] [Google Scholar]
- 103.Bryan J., Tiggemann M. The effect of weight-loss dieting on cognitive performance and psychological well-being in overweight women. Appetite. 2001;36:147–156. doi: 10.1006/appe.2000.0389. [DOI] [PubMed] [Google Scholar]
- 104.Nauta H., Hospers H., Jansen A. One-year follow-up effects of two obesity treatments on psychological well-being and weight. Br. J. Health Psychol. 2001;6:271–284. doi: 10.1348/135910701169205. [DOI] [PubMed] [Google Scholar]
- 105.Kraft A.M. Weight loss and depression. Am. J. Psychiatry. 1991;148:947–948. doi: 10.1176/ajp.148.7.947c. [DOI] [PubMed] [Google Scholar]
- 106.Junig J.T., Lehrmann J.A. A psychotic episode associated with the Atkins Diet in a patient with bipolar disorder. Bipolar. Disord. 2005;7:305–306. doi: 10.1111/j.1399-5618.2005.00195.x. [DOI] [PubMed] [Google Scholar]
- 107.Drapeau V., Jacob R., Panahi S., Tremblay A. Effect of energy restriction on eating behavior traits and psychobehavioral factors in the low satiety phenotype. Nutrients. 2019;11:245. doi: 10.3390/nu11020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jacka F.N., O’Neil A., Opie R., Itsiopoulos C., Cotton S., Mohebbi M., Castle D., Dash S., Mihalopoulos C., Chatterton M.L., et al. A randomised controlled trial of dietary improvement for adults with major depression (the “SMILES” trial) BMC Med. 2017;15:23. doi: 10.1186/s12916-017-0791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parletta N., Zarnowiecki D., Cho J., Wilson A., Bogomolova S., Villani A., Itsiopoulos C., Niyonsenga T., Blunden S., Meyer B., et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED) Nutr. Neurosci. 2019;22:474–487. doi: 10.1080/1028415X.2017.1411320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.