Abstract
Endometrial stromal tumours (ESTs) are rare, intriguing uterine mesenchymal neoplasms with variegated histopathological, immunohistochemical and molecular characteristics. Morphologically, ESTs resemble endometrial stromal cells in the proliferative phase of the menstrual cycle. In 1966 Norris and Taylor classified ESTs into benign and malignant categories according to the mitotic count. In the most recent classification by the WHO (2020), ESTs have been divided into four categories: Endometrial Stromal Nodules (ESNs), Low-Grade Endometrial Stromal Sarcomas (LG-ESSs), High-Grade Endometrial Stromal Sarcomas (HG-ESSs) and Undifferentiated Uterine Sarcomas (UUSs). ESNs are clinically benign. LG-ESSs are tumours of low malignant potential, often with indolent clinical behaviour, with some cases presented with a late recurrence after hysterectomy. HG-ESSs are tumours of high malignant potential with more aggressive clinical outcome. UUSs show high-grade morphological features with very aggressive clinical behavior. With the advent of molecular techniques, the morphological classification of ESTs can be integrated with molecular findings in enhanced classification of these tumours. In the future, the morphological and immunohistochemical features correlated with molecular categorisation of ESTs, will become a robust means to plan therapeutic decisions, especially in recurrences and metastatic disease. In this review, we summarise the morphological, immunohistochemical and molecular features of ESTs with particular reference to the most recent molecular findings.
Keywords: endometrial stromal sarcoma, uterine sarcoma, ESN, ESS, EST, LG-ESS, HG-ESS, YWHAE-NUTM2, ZC3H7B-BCOR, NTRK-uterine tumours, UUS
1. Introduction
Endometrial stromal tumours (ESTs) are a rare, fascinating and complex subset of mesenchymal uterine neoplasms with heterogeneous morphological, immunohistochemical and genetic features. ESTs constitute ∼10% of uterine mesenchymal tumours [1].
Approximately 50% of endometrial stromal sarcomas (ESSs) occur in premenopausal women and the majority is detected at stage I of the International Federation of Gynecology and Obstetrics (FIGO) [2].
Morphologically, ESTs resemble endometrial stromal cells in the proliferative phase of the menstrual cycle. In 1966 Norris and Taylor attempted to classify ESTs in their seminal manuscript [3].
They divided the lesions into two groups; the first group with pushing margins was labelled as stromal nodules and the second group with infiltrating margins was defined as endolymphatic stromal myosis or stromal sarcoma according to the mitotic index: lesions with ≤ 10 mitoses per 10 HPF (high-power field) were classified as endolymphatic stromal myosis and neoplasms with ≥ 10 mitoses per 10 HPF were categorised as stromal sarcomas. In view of the clinical outcome (100% survival rate within five years), stromal nodules were considered benign. Patients with stromal sarcoma presented with 55% survival rate within five years. The authors stated that the “size of the primary tumor and presence of vein invasion showed a slight correlation with the patient’s prognosis but no correlation was found with increasing degrees of cellular atypism” [3].
In 1982Evans showed that the prognosis of ESSs is determined by nuclear atypia/pleomorphism rather than by the mitotic rate [4].
Since the fundamental description of ESTs by Norris and Taylor, the classification of ESSs has undergone several modifications [5,6].
The last classification of the World Health Organization (WHO) in 2020 sub-categorised ESTs into four groups: Endometrial Stromal Nodule (ESN), Low-Grade Endometrial Stromal Sarcoma (LG-ESS), High-Grade Endometrial Stromal Sarcoma (HG-ESS), and Undifferentiated Uterine Sarcoma (UUS) (Table 1) [7]. Molecular analysis of ESTs has resulted in better characterisation of these tumours, this, in turn, has caused the decrease in the diagnosis of UUS, which, at present, is a heterogeneous group of tumours, as well as the diagnosis of exclusion. Similarly, NTRK-sarcomas, discovered by molecular analysis, appear to fall into the HG-ESSs. However, their endometrial stromal origin has not been established with certainty and our current knowledge fails to classify these tumours appropriately.
Table 1.
The classification of Endometrial Stromal Tumours defined in the fifth edition of WHO Classification of Female Genital Tumours.
| Category | ICD-11 1 Coding (Histopathology) | ICD-O 2 Coding |
|---|---|---|
| Endometrial Stromal Nodule | XH8C13 | 8930/0 |
| Low-Grade Endometrial Stromal Sarcoma | XH1S94 | 8931/3 |
| High-Grade Endometrial Stromal Sarcoma | XH2CV3 | 8930/3 |
| Undifferentiated Uterine Sarcoma | XH6HY6 | 8805/3 |
1 ICD-11 = International Classification of Diseases, 11th revision; 2 ICD-O = International Classification of Diseases for Oncology.
The aim of this review is to shed some light on the complex classification of ESTs, highlighting the varied histopathological, immunohistochemical and molecular features for each sub-group.
2. Endometrial Stromal Nodule (ESN) and Low-Grade Endometrial Stromal Sarcoma (LG-ESS)
ESN is a benign, whereas LG-ESS is a malignant neoplasm of the uterus (affecting the body of the uterus more than the cervix) and extra-uterine sites [8,9]. The mean age for LG-ESS is 52 years, ranging between 16 and 83 years [10]. The risk factors are pelvic radiation and prolonged use of tamoxifen or oestrogen. The most common findings are abnormal uterine bleeding and pelvic pain [11,12,13]. Other symptoms of patients with LG-ESS are uterine mass and metastases to the adnexae, lymph nodes and lungs [14].
2.1. ESN Morphology
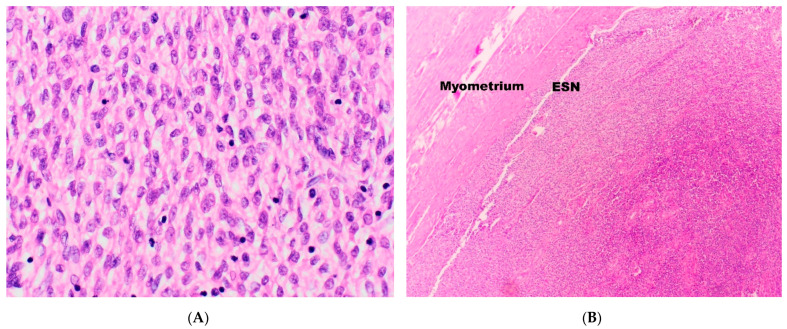
ESNs are a proliferation of bland, uniform cells with oval nuclei and scanty cytoplasm, resembling endometrial stromal cells in the proliferative phase of the menstrual cycle (Figure 1A). ESNs exhibit prominent arterioles and well-circumscribed expansive (non-infiltrative) margins (Figure 1B). It may occasionally present with infiltrative margins, however these should be 3 mm or less in maximum dimension and not exceed three foci [15,16].
Figure 1.
(A) Neoplastic cells in Endometrial Stromal Nodules (ESN) and Low-Grade Endometrial Stromal Sarcomas (LG-ESS) resemble endometrial stromal cells in the proliferative phase. (B) ESNs show a non-infiltrative margin.
The mitotic rate is not high (less than 10 × 10 HPF). Areas of coagulative necrosis and sex-cord-like differentiation may be identified, but, by definition, lympho-vascular invasion (LVIS) is not present. Areas of smooth muscle metaplasia may be present and these should not mislead to an incorrect diagnosis of myometrial invasion. The differential diagnosis includes cellular leiomyoma and LG-ESS. The vascular pattern of ESN, composed of typical arterioles, is not a prominent feature of cellular leiomyoma. The presence of large blood vessels, one of the characteristics of cellular leiomyoma, may also be detected in ESN, but is not as conspicuous as in cellular leiomyoma. In addition, ESN usually does not contain the clefts frequently seen in cellular leiomyoma [17].
ESNs can be differentiated from LG-ESS exclusively by the presence of pushing margins and lack of LVIS [3,15,16], therefore, the definitive diagnosis of ESN can be rendered on resection specimens only and cannot be confidently established on biopsy or tissue removal systems (i.e., MyoSure resections). Nevertheless, even in resection specimens, the differential diagnosis between ESNs and LG-ESS may not be straightforward and extensive sampling may be required.
2.2. LG-ESS Morphology
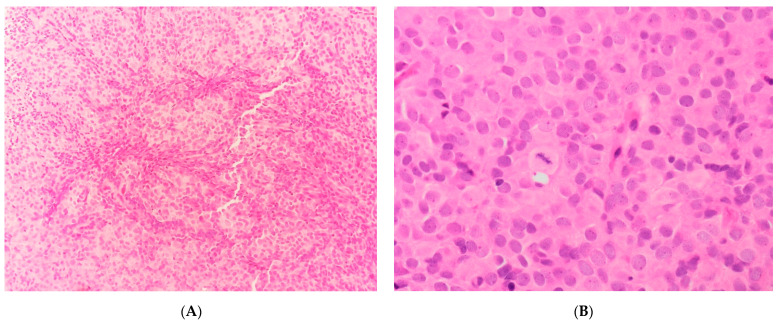
LG-ESS shows the same histopathological features as ESN, except, as has already been mentioned, for the presence of infiltrative/permeative, tongue-like margins (Figure 2A) and LVIS that may also be observed in the parametrial veins [15].
Figure 2.
(A) The infiltrative, tongue-like margin in LG-ESS. (B) Sex-cord-like structures in LG-ESS.
From the purely morphological point of view, some low-grade ESTs show “hybrid” features between ESN and LG-ESS. These lesions have been labelled as ESTs with limited infiltration (EST-LI). The extent of myometrial invasion in this entity is less than in LG-ESS but more than in ESN (occasional finger-like projections into the myometrium of up to 3 mm are allowable) [17]. Obviously, the diagnosis of this lesion is highly subjective and depends on the extent of the sampling; therefore, the diagnostic reproduceable value is limited. In addition, the clinical outcome among patients with this sub-category of morphologically low-grade EST is not clear [17,18] and a small percentage of EST-LI can show malignant behaviour with distant metastases. Based on their findings Moore & McCluggage recommended in a recent paper that these neoplasms should be regarded as LG-ESS [19].
In some instances, the histological diagnosis of EST could be problematic, especially on biopsy. Some samples may contain foamy histiocytes, foci of hyalinisation [15,16,20], smooth muscle differentiation [17,21,22,23,24,25,26,27,28,29,30] where radiated collagen fibres of the smooth muscle component create a starburst appearance, commonly referred to as ‘starburst differentiation’ [31]. In addition, skeletal muscle differentiation [26,28,29], adipocytic metaplasia [26], rhabdoid changes [28,32,33], presence of osteoclast-like cells [27], cells with bizarre nuclei [26], cells with clear cytoplasm [34] and myxoid/fibro-myxoid changes may be present [17,25,32,35,36,37,38]. ESTs may show epithelioid morphology [39] with the presence of endometrial-type glands [34,40,41,42], pseudo-papillae [43] and sex-cord-like structures (Figure 2B) [17,24,28,29,32,44,45,46,47,48] that may present positive immunostaining with inhibin, calretinin, CD99, Melan-A and Wilms tumour 1 (WT1) [7,49,50,51].
The differential diagnosis of LG-ESS includes HG-ESS, gland-poor adenomyosis, cellular leiomyoma, intravascular leiomyomatosis, leiomyosarcoma with extensive intravascular component, uterine tumours resembling ovarian sex-cord tumour (UTROSCT), adenosarcoma and perivascular epithelioid cell tumour (PECOMA) [52].
2.3. Immunohistochemistry
ESNs commonly demonstrate positive immunoreactivity with CD10, oestrogen receptor (ER) (ER alpha—ERα), CD56, smooth muscle actin (SMA) and vimentin. Focal positivity is observed with progesterone receptor (PR), pan-cytokeratin (AE1/3), and desmin. ESN is usually negative with CD34, CD117 (c-kit), Cyclin D1, epithelial membrane antigen (EMA), S100, WT1 and β-catenin. p53 demonstrates a wild-type pattern of expression. The mitotic rate, evaluated by Ki67 expression, is low [53].
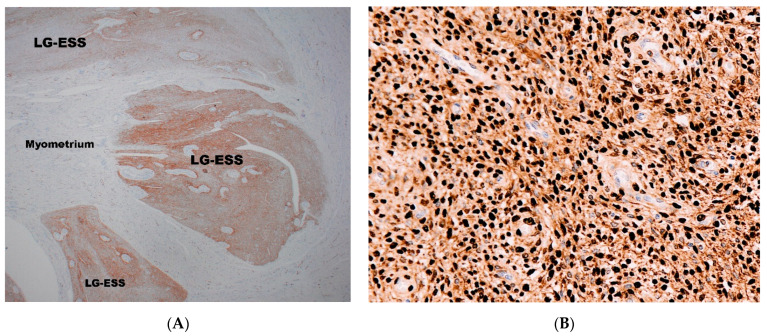
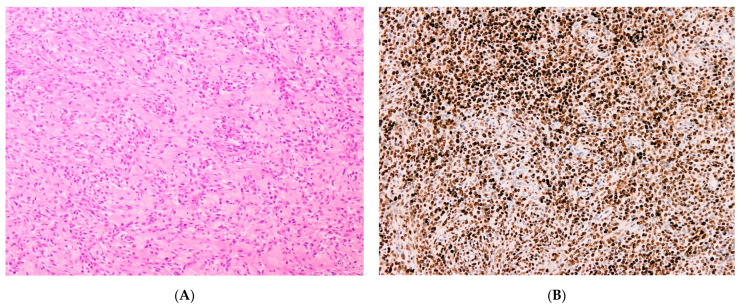
CD10 antibody is routinely used for the diagnosis of ESTs, (Figure 3A) and is the most popular antibody used to differentiate LG-ESS from HG-ESSs [54,55,56,57,58]. However, it is well known that CD10 is not specific for the diagnosis of ESTs [59,60] and some ESTs may show negative immunostaining with CD10 [61,62]. In addition, CD10 can also be strongly positive in undifferentiated uterine sarcoma [63].
Figure 3.
(A) Positive immunostaining with CD10 in LG-ESS; the surrounding myometrium is negative. (B) Diffuse strong nuclear immunoreactivity with ER in LG-ESS.
LG-ESSs demonstrate strong expression with ERα (Figure 3B) [54,56,57,58,61,64,65,66,67,68], while ER-beta (ERβ) expression is mainly negative with occasional reported cases showing weak positivity [64]. PR expression positivity has been reported in the majority of cases (>70%), with strong positivity observed in >50% of cases [58,64,69,70] and its positivity is part of the immunohistochemical confirmation of the diagnosis of LG-ESS [54,56,61,65,66,67].
Androgen receptor (AR) expression is observed in the majority of cases [58,64]. It is worth mentioning that AR-positive immunostaining has been observed in LG-ESS harbouring JAZF1-SUZ12 fusion and JAZF1 rearrangement [56,69].
A diffuse or focal positivity for SMA [56,58,66] (Figure 4A) and desmin (Figure 4B) is present [54,58,65,70]. H-caldesmon can be focally positive [56] and, typically, strong positivity is seen in areas of smooth muscle differentiation with a ‘starburst’ appearance [31]. A weak expression of gonadotropin-releasing hormone receptor (GnRH-R) has been reported in ~95% of cases [64]. Aromatase (CYP19A1) expression has been observed in ~85% of cases [64]. Interferon-induced transmembrane protein-1 (IFITM1), a novel marker for endometrial stromal cells, is positive in ~80% of cases. IFITM1 is superior to CD10 in differentiating ESTs from smooth muscle neoplasm (~30% in cases of leiomyomas and leiomyosarcomas) [71]. However, ~90% of carcinosarcomas demonstrate positivity with this marker, but in this case the differential diagnosis with ESTs would be based on pure morphological grounds.
Figure 4.
Smooth muscle actin (SMA)-positivity (A) and desmin-positivity (B) in LG-ESS.
The nuclear expression of β-catenin was reported in 50% of cases, with 90% demonstrating nuclear expression of Lymphoid Enhancer-binding Factor 1 (LEF1). This finding is suggestive of activation of the Wnt pathway in LG-ESS [72].
LG-ESSs are mainly reported to be negative with Cyclin D1 and BCOR [73] and both markers are commonly implemented in diagnostic differential panel between LG-ESS and HG-ESS.
However, in some cases, Cyclin D1 can be positive in the LG-ESS, but with concomitant strong ER and PR positivity and focal CD10 expression, which is unusual in high-grade endometrial stromal sarcoma [68,74]. The Ki67 proliferation index is usually low and has been reported to be positive in ~5–20% of the lesional cells [54,68,73,74]. It is worth mentioning that Ki67 can vary between tumours found in the same uterine specimen. Fujiishi et al. found the difference in the expression of Ki67 between the right anterior, right posterior, and fundal tumours to be 10%, 10%, and 3%, respectively [56].
Bcl-2 and vimentin may show immunopositivity [54,55,66]. CD34 is mainly negative [58], but some cases showed focal positivity [73,75]. Generally, there is positive immunoreactivity with WT1 [56,58,67,73], and Forkhead box protein L2 (FOXL2) has been reported to be positive in 87% of cases [58]. The data on expression of cytokeratins are conflictual. Positive immunoreactivity to AE1/3 and CAM5.2 has been reported [52,53], but negative cases have also been described [67,73].
INI-1 (SMARCB1, hSNF5, BAF47) expression seems to be retained [73]. S100, CD31, CD117 (c-kit), creatine kinase (CK), EMA, Melan-A, HMB-45, PAX8, inhibin, synaptophysin, chromogranin, DOG-1 and CD99 are usually negative [55,61,66,67,69,73,76,77].
2.4. Molecular Biology
LG-ESSs are genetically heterogeneous with relatively numerous identified chromosomal translocations resulting in gene fusions. However, approximately one third of these tumours do not harbour genetic fusions [7]. JAZF1-SUZ12 is the most common gene fusion, present in approximately half of the cases [31,78,79] and related to the cytogenetic hallmark of ESN and LG-ESS (Figure 5). The minority of cases of LG-ESS display other gene fusions, including EPC1-PHF1 [80], MEAF6-PHF1 [81], JAZF1-PHF1 [80], MBTD1-CXorf67 (MBTD1-EZHIP) [82], BRD8-PHF1 [83], JAZF1-BCORL1 [84], EPC2-PHF1 [85]. Recently, MEAF6-PHF1 has also been demonstrated in ESNs [59].
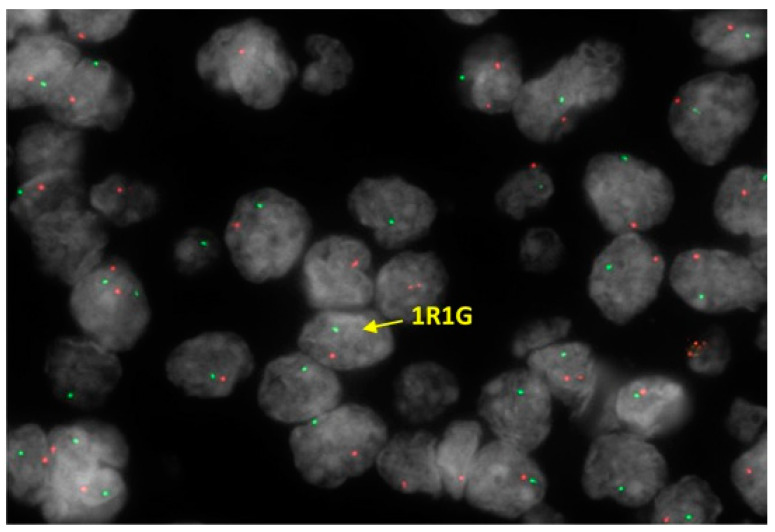
Figure 5.
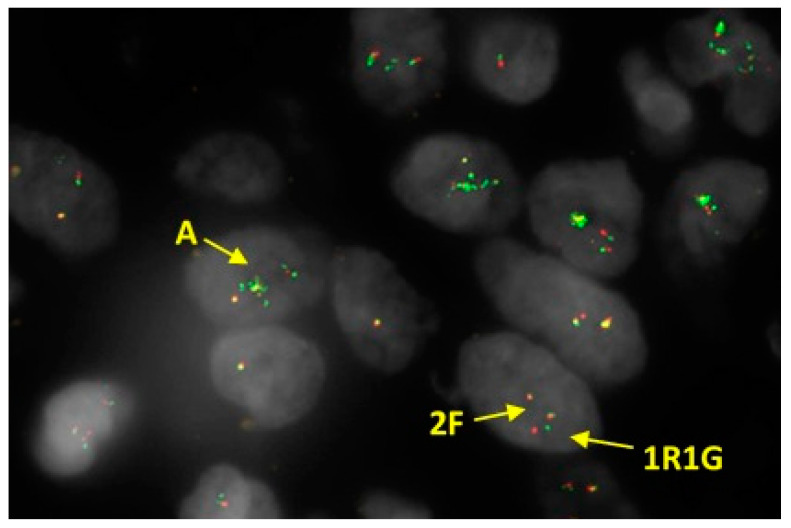
Fluorescence in situ hybridization (FISH) performed on LG-ESS, which shows JAZF1 rearrangement using dual colour break apart probe. Additionally, the other copy of JAZF1 is lost. Results are expressed as split R (separate red signal) and G (separate green signal) rearrangement.
Dickson et al. reported two cases of ESSs with clinical aggressive behaviour, showing two novel EPC1 genetic fusions: EPC1-SUZ12 and EPC1-BCOR [86]. Other novel fusions include MEAF6-SUZ12 [73], MAGED2-PLAG1 [87] and MBTD1-PHF1 [88].
Novel fusions that have been recently detected using RNA sequencing include KAT6B-KANSL1, RNF111-ARID2, ESR1-NCOA3, PTCH1-GLI1, SYNGAP1-JAZF1, PHF21A-NFIA, PHF21A-CETP, ACTB-GLI1 and GREB1-NCOA2 [89].
Some LG-ESSs harbouring YWHAE-NUTM2 fusion, which is a molecular characteristic of high-grade endometrial sarcomas, have also been reported [89,90].
3. High-Grade Endometrial Stromal Sarcoma (HG-ESS)
On gross examination, these tumours usually show haemorrhage and necrosis [5,91,92,93]. The morphological spectrum varies according to the genetic aberrations.
3.1. YWHAE-NUTM2
3.1.1. Morphology
These neoplasms [5,90,91,92,94] show necrosis with a permeative pattern of myometrium invasion. LVIS is a constant finding. The cellular population is a mixture of round and spindle cells. The round cell component displays high cellularity with a vague nested architecture (Figure 6A), composed of cells with a scanty/moderate eosinophilic cytoplasm (Figure 6B). The nuclei exhibit a finely granular to slightly vesicular chromatin with an irregular nuclear membrane, no prominent nucleoli and no significant nuclear atypia/pleomorphism. Sex-cord–like differentiation and pseudo-glandular/pseudo-papillary pattern may rarely be observed. The mitotic rate within the round cells component is high.
Figure 6.
(A) Vague nested architecture in YWHAE-NUTM2 sarcoma. (B) Cellular morphological details in YWHAE-NUTM2 sarcoma.
Approximately 50% of cases show a low-grade spindle cell component (Figure 7A) with low/intermediate cellularity, a fascicular pattern of growth consisting of a proliferation of bland spindle cells set in a fibro-collagenous to fibro-myxoid matrix, juxtaposed to the round cell component. The nuclei show even chromatin with no prominent nucleoli. The mitotic index in this component is low.
Figure 7.
(A) The fibro-collagenous low-grade spindle cell component in YWHAE-NUTM2 sarcoma. (B) The positive nuclear Cyclin D1 immunostaining in the round cell component of YWHAE-NUTM2 sarcoma.
3.1.2. Immunohistochemistry
The round cell component is positive for Cyclin D1 (Figure 7B), BCOR, CD117 (c-kit), CD56, and CD99, whereas DOG1 is negative [7,57,63,77,95,96]. Generally, ER and PR are negative, but positive staining has also been reported; CD10 shows variable expression, either positive or negative [7,56,97].
The low-grade spindle cell component may display positive staining with ER, PR, and CD10 [63,74] or show diffuse positivity with Cyclin D1 and PR and focal positivity for CD10 and p16 [74], or demonstrate positive staining with Cyclin D1 and negative staining with CD10 [96].
BCOR may show variable positivity [77]. There is no immunoreactivity with AE1/3, EMA, SMA, desmin, calretinin and inhibin [74].
3.1.3. Molecular Biology
These neoplasms demonstrate rearrangement of YWHAE (Figure 8) and commonly harbour YWHAE-NUTM2 fusion [5,7,57]. NUTM2 has been previously named as FAM22 by HUGO Gene Nomenclature Committee (HGNC) Symbols [98]. There are several members of paralog genes in this family, of which NUTM2A, NYTM2B and NUTM2E protein coding genes have been associated with HG-ESSs. Mainly NUTM2A and NUTM2B have been reported to form fusion with YWHAE as alternative gene fusion partners [7,97]. In a more recent report, NUTM2E (paralog of NUTM2B) has also been detected as an alternative gene fusion partner [89].
Figure 8.
Fluorescence In Situ Hybridisation (FISH) performed on HG-ESS, which shows YWHAE rearrangement with amplification of the fusion signal (2F) plus additional 5’ signals (A) using dual colour break apart probe. Results expressed as F (fusion signal, i.e., normal), split R (separate red signal) and G (separate green signal) rearrangement.
EPC1-BCOR, EPC1-SUZ12 and BRD8-PHF1 fusions have also been reported, but they are rare [63,86]. A few cases have demonstrated a nucleotide variation with mutation in BCOR (NM_017745 (BCOR exon 4):c.2570_2571del (p.E857fs) and in BCORL1 (NM_021946 (BCORL1 exon 7):c.A4256T (p.K1419I) [89], in addition to the presence of YWHAE-NUTM2E fusion.
WT1 gene expression is often absent or shows low expression levels [89]. Notably, it has been previously highlighted that high immunohistochemical expression of CD117 (c-kit) can be frequently found in tumours with YWHAE genetic rearrangement, but c-kit-immunoreactive YWHAE-NUTM2A/B sarcomas have not demonstrated known mutations in KIT gene [99].
3.2. ZC3H7B-BCOR
3.2.1. Morphology
These tumours often show neoplastic-type or infarct-type necrosis [92]. The pattern of myometrial invasion could be infiltrative and tongue-like, similar to LG-ESS, or may display a broad front of invasion with irregular borders. A mixed pattern may also be present. LVIS is a common finding. The neoplasm shows a fascicular pattern and is composed of cells with eosinophilic cytoplasm (scanty/moderate or abundant), and spindle/oval, occasionally round, nuclei with a finely dispersed chromatin and no discernible nucleoli. Although infrequent, severe nuclear atypia/pleomorphism has been described. The stroma is myxoid in the majority of cases and collagen plaques may be identified. The intra-tumoural vessels may be large-sized or arterioles; a hemangiopericytoma-like vascular pattern may be seen. Occasionally, tumours may contain benign-appearing endometrioid glands. The proliferation rate may be very low (1 × 10 HFP) or moderate/high (50 × 10 HFP). The accompanying LG-ESS component has not been reported.
A rare novel sub-type of HG-ESS with ZC3H7B-BCOR fusion has been described that shares significant histopathological overlap with myxoid leiomyosarcoma [100].
3.2.2. Immunohistochemistry
There is positive staining with Cyclin D1 and CD10; immunoreactivity with BCOR is seen in ~50% of cases, but in a recent paper, the BCOR positivity was up to ~80% of the lesional cells [77,101,102]. Expression of ER and PR is variable. Focal positive staining with SMA and caldesmon can be identified, but positivity with desmin is usually negative [7]. Some recent reports also demonstrated immunoreactivity with TLE1 (Transducin-like enhancer protein 1), CD99 and BCL2 [101,102,103].
Negative immunoreactivity with cytokeratins (MNF116), SMA, desmin, h-caldesmon, myogenin, myo-D1 (myoblast determination protein 1), STAT6 (signal transducer and activator of transcription 6), CD34, SOX-10 (Transcription factor SOX-10), S100, HMB45, ER, PR, CD117 (c-kit), MDM2 (E3 ubiquitin-protein ligase Mdm2) and SYT has been reported [101,102]. Nuclear staining for INI-1 is retained [102]. Ki67 proliferation index can be demonstrated between 10–25% of neoplastic cells [103]. Recently, positive staining for Pan-Trk has been reported in some cases [7,104,105].
3.2.3. Molecular Biology
ZC3H7B-BCOR and its reciprocal fusion are mainly associated with and reported in these neoplasms [77,93,105]. Recent reports showed MDM2, FRS2 and CDK4 amplification and loss of CDKN2A in some cases [89,105,106].
Yoshida et al. showed elevated expression of BCOR and significant upregulation of ZIC2, HOXA13 and NTRK3 in an extra-uterine case (chest wall) of HG-ESS with ZC3H7B-BCOR fusion [107].
3.3. BCOR Internal Tandem Duplication (ITD)
3.3.1. Immunohistochemistry
Clinical experience with regard to these tumours is very limited, with only a few reported cases. They show a different immunoprofile with respect to ZC3H7B-BCOR neoplasms. They display diffuse positive immunoreactivity with BCOR and Cyclin D1, less positive staining for CD10 and mostly negative staining with ER and PR. In addition, they may show immunoreactivity with desmin. SMA and caldesmon seem to be negative [77,93,108]. There is strong and diffuse cytoplasmic expression of pan-Trk [109].
3.3.2. Molecular Biology
Juckett et al. reported that BCOR-ITDs occurred most frequently in exon 15 and near C-terminus, and were present in 52.4% cases of uterine sarcomas. Interestingly, the tested cases did not carry any of the simultaneous gene fusions typically associated with ESSs [93].
Lin et al. reported no amplification of CDK4 and/or MDM2; however, a homozygous deletion of CDKN2A and CDKN2B was present in 20% of cases. Mutations in STAG2, PASK, SMARCB1, ATRX, CTNNB1 and ARID1A were also seen in the minority of the tested BCOR-ITDs cases [105]. Upregulation of the expression of NTRK3, FGFR3, RET, BCOR, GLI1 and PTCH1 genes has also been reported [109].
4. High-Grade Sarcomas with Uncertain Endometrial Stromal Origin
NTRK-Uterine Tumours
Morphology, Immunohistochemistry and Molecular Biology
These sarcomas show no definite endometrial stromal origin. Chiang et al. [110] describes a few cases of a sub-type of sarcomas with novel NTRK fusion. These tumours demonstrate RBPMS-NTRK3, TPR-NTRK1, LMNA-NTRK1 and TPM3-NTRK1 gene fusions and seem to affect premenopausal women with frequent cervical involvement (three in cervix uteri and one in corpus uteri). They show infiltrative or expansive myometrial invasion and are composed of fascicles of cells with spindle nuclei, small nucleoli and abundant eosinophilic cytoplasm. Severe nuclear atypia/pleomorphism and necrosis may be present. The stroma is myxoid/edematous. The vascular pattern may be either delicate with thin-walled vessels or composed of thick-walled blood vessels. LVIS is not identified. The proliferation rate is relatively high. Immunohistochemistry shows positive immunostaining with CD10 (not all cases), focal positive staining with SMA and very focal positivity (<10% of lesional cells) with S100. There is positive immunoreactivity with TrkA and pan-Trk. H3K27me3 expression is retained. AE1/3, desmin, ER, PR, CD34 and SOX-10 are negative [110].
Croce et al. [111] reported a group of cervical, uterine and vaginal spindle-cell sarcomas displaying morphological resemblance with fibro-sarcomas. The authors divided these neoplasms into three categories:
-
-
Cervical NTRK fusion-positive (TPM3-NTRK1 and EML4-NTRK3) sarcomas with diffuse immunostaining with Trk;
-
-
COL1A1-PDGFB fusion-positive sarcomas with diffuse positive staining with CD34;
-
-
S100 immunoreactive sarcomas with no genetic rearrangement.
The fascinating finding is that COL1A1-PDGFB rearrangement is a characteristic of dermatofibrosarcoma protuberans (DFS) and has not been reported in uterine sarcomas. The authors classified the S100-positive sarcomas as malignant peripheral nerve sheath tumours. This group showed diffuse positive staining with Cyclin D1 and focal positivity with BCOR. CD34, HMB45, Melan A, ER, PR, desmin, Trk and H3K27me3 were negative [111].
Grindstaff et al. recently described an additional COL1A1-PDGFB fusion-positive uterine case with diffuse CD34 immunopositivity. There was focal positive staining with CD10, SMA and positive aberrant expression of p53. The Ki67 proliferation index was high. Desmin, caldesmon and p16 were negative [112].
In a uterine sarcoma with TMP3-NTRK1 rearrangement, described by Boyle et al., cytokeratins, SMA, desmin, ER, PR, SOX-10, caldesmon, ALK1, CD117 (c-kit), DOG1, CD21 and CD23 were tested and were all negative. CD10 and Pan-Trk were positive. CD34 displayed a weak cytoplasmic staining and Cyclin D1 was focally positive [113].
Michal et al. described a STRN-NTRK3-rearranged uterine sarcoma with a peculiar morphology. The neoplasm contained bland epithelioid/plasmacytoid cells embedded in a myxoid stroma with a complex, arborising vascular pattern and focal peri-vascular hyalinisation. There was infarct-type necrosis but frank tumoural necrosis, significant nuclear pleomorphism and mitoses were not seen. The neoplastic cells displayed strong positive immunostaining with S100, CD34 and nuclear and cytoplasmic Pan-Trk. The extensive immunohistochemistry with keratins, CD10, myogenic, peri-neural/neural, melanocytic, neuroendocrine and vascular markers showed negative staining. The Ki67 proliferation rate was low [114].
5. High-Grade Sarcoma Not Otherwise Specified (NOS)
The 2020 WHO classification of EST contains a vague sub-category that seems to be associated with a LG-ESS component [7].
6. Undifferentiated Uterine Sarcomas (UUS)
UUS
Morphology, Immunohistochemistry and Molecular Biology
UUS is a rare uterine sarcoma [115,116,117] that includes a variegated group of neoplasms with no specific line of differentiation and, by definition, is a diagnosis of exclusion. The patients with UUS are postmenopausal and present with uterine bleeding and pelvic pain. Macroscopically, UUS usually displays necrosis and haemorrhage. Morphologically, UUS can be sub-divided into monomorphic and pleomorphic sub-types [118]. It consists of epithelioid and/or spindled cells with high mitotic rate and a destructive pattern of myometrial invasion. LVIS and necrosis are common findings [119]. When UUS consists of a uniform cellular population, HG-ESS harbouring YWHAE-NUTM2 (FAM22) fusion should be ruled out. Conversely, some pleomorphic sarcomas may exhibit YWHAE, JAZF1 and NTRK rearrangements. Therefore, these neoplasms should not be classified as UUS [77,78,110,111]. Tumours displaying high-grade nuclear atypia/pleomorphism, but associated with a LG-ESS component, should be classified under endometrial stromal sarcoma NOS category [118].
UUS usually shows positive staining with p16, with positive aberrant p53 expression. Focal positive immunostaining with CD10, PR, Cyclin D1 and β-catenin may be present [74,119].
Cotzia et al. showed that some UUS could be under recognised HG-ESSs because of positive immunostaining with BCOR, Cyclin D1, CD10, ER and PR [63].
GREB1-NCOA2-uterine tumour is a high-grade endometrial sarcoma with a novel GREB1-NCOA2 fusion, consisting of spindle/polygonal cells with high-grade nuclear atypia. The neoplasm shows a high mitotic rate, necrosis, LVIS and invasion of the cervix and both parametria [120]. Immunohistochemistry shows that the lesional cells positive for vimentin, AE1/3, ER and PR and express desmin weakly. CK5/6, CK7, CK14, CD10, CD45, CD117, chromogranin A, synaptophysin, actin, SMA, Myf-4, HMB-45, TTF1, CD10, Ber-EP4 and PAX8 were negative. However, in view of the immunoprofile of this case, diagnosis of carcinosarcoma cannot be excluded.
UUSs can harbour simultaneous numerous gene fusions. Brahmi et al. [89] have reported cases with multiple detected (>3) in-frame fusions and a case with novel translocation CREBBP-BCOR. Moreover, UUS cases demonstrated complex genomic profiles with numerous gene fusions and/or had mutations involving TP53, KRAS, NRAS or BRAF genes.
Novel YWHAE gene rearrangement with no known partner has been reported in two UUSs with marked nuclear pleomorphism. This novel rearrangement may represent novel fusions in this sarcoma subtype [63]. A single case (1/23) harbouring HMGA2-RAD51B fusion has been described, which also demonstrated high expression of NTRK3, FGFR3, RET, BCOR, GLI1 and PTCH1, and low expression of ESR1. [109]. Other cases have demonstrated low expression of these genes along with low ESR1 (17/23) expression; however, no fusions have been observed [109].
7. Conclusions
In the era of Next Generation Sequencing (NGS) there is a huge effort to integrate the morphological and immunohistochemical classification of ESTs with molecular sub-categorisation. The aim of the molecular classification of ESTs, as in other neoplasms, is not a purely academic exercise, but rather an understanding of the molecular bases in order to develop a specific target therapy for each sub-category. This will be of paramount importance for planning a therapeutic strategy in the metastatic disease. Therefore, in the future, we will be witnessing discoveries of new entities and, gradually, the histopathological classification will be replaced by molecular classification. Genetic profile of ESSs with the most frequently reported and novel molecular alterations are presented in Table 2.
Table 2.
Shows molecular alterations most frequently reported in ESS.
| Type | Genes Involved | Most Frequent Reported Fusions/Gene Rearrangements/Alterations |
Translocations | References |
|---|---|---|---|---|
| LG-ESS | JAZF1 | MEAF6-SUZ12 | t(1;17)(p34;q11) | [73] |
| SUZ12 | JAZF1-SUZ12 | t(7;17)(p15;q11) | [31,78,79] | |
| PHF1 | JAZF1-PHF1 | t(6;7)(p21;p15) | [80] | |
| BCORL1 | EPC1-PHF1 | t(10;6)(p11;p21) | [80] | |
| EPC1 | MEAF6-PHF1 | t(1;6)(p34;p21) | [81] | |
| EPC2 | MBTD1-CXorf67 (MBTD1-EZHIP) | t(X;17)(p11.2;q21.33) | [82] | |
| MEAF6 | BRD8-PHF1 | t(5;6)(q31;p21) | [83] | |
| MBTD1 | JAZF1-BCORL1 | t(7;X)(p15;q26.1) | [84] | |
| EZHIP | EPC2-PHF1 | t(2;6)(q23;p21) | [85] | |
| BRD8 | EPC1-SUZ12 | t(10;17)(p11;q11) | [86] | |
| BCOR | EPC1-BCOR | t(10;X)(p11;p11) | [86] | |
| MAGED2 | MAGED2-PLAG1 | t(X;8)(p11.21;q12.1) | [87] | |
| PLAG1 | MBTD1-PHF1 | t(X;6)(p11.2;p21) | [88] | |
| YWHAE | YWHAE/NUTM2 | t(10;17)(q22;p13) | [89,90] | |
| NUTM2 | MEAF6-SUZ12 | t(5;6)(q31;p21) | [83] | |
| HG-ESS | YWHAE | YWHAE/NUTM2 | t(10;17)(q22;p13) | [5,7,57] |
| NUTM2A/B/E | EPC1-BCOR | t(10;X)(p11;p11) | [63,86] | |
| EPC1 | EPC1-SUZ12 | t(10;17)(p11;q11) | [63,86] | |
| SUZ12 | BRD8-PHF1 | t(5;6)(q31;p21 | [63,86] | |
| BCOR | BCOR alteration | none | [89] | |
| BRD8 | ||||
| PHF1 | ||||
| ZC3H7B-BCOR HG-ESS | ZC3H7B | ZC3H7B-BCOR | t(22;X)(q13;p11) | [77,93,105] |
| BCOR | BCOR-ZC3H7B | t(X;22)(p11;q13) | ||
| BCOR ITD HG-ESS | BCOR | BCOR ITD | none | [93] |
| NTRK-uterine sarcomas HG-ESS | TPR | TPR-NTRK1 | 1q31.1-1q23.1 | [110] |
| NTRK1 | LMNA-NTRK1 | 1q22-1q23.1 | [110] | |
| LMNA | TPM3-NTRK1 | 1q21.3-1q23.1 | [110,111] | |
| TPM3 | RBPMS-NTRK3 | t(8;15)(p12;q25.3) | [110] | |
| RBPMS | EML4-NTRK3 | t(2;15)(p21;q25.3) | [111] | |
| NTRK3 | COL1A1-PDGFB | t(17;22)(q21.33;q13.1) | [111,112] | |
| EML4 | STRN-NTRK3 | t(2;15)(p22.2;q25.3) | [114] | |
| COL1A1 | TPR-NTRK1 | 1q31.1-1q23.1 | [110] | |
| PDGFB | LMNA-NTRK1 | 1q22-1q23.1 | [110] | |
| STRN | TPM3-NTRK1 | 1q21.3-1q23.1 | [110,111] | |
| UUS | YWHAE | YWHAE gene rearrangement | unknown fusion partner | [63] |
| CREBBP | CREBBP-BCOR | t(16; X)(p13;p11) | [89] | |
| BCOR | HMGA2-RAD51B | t(12;14)(q14.3;q24.1) | [109] | |
| HMGA2 | GREB1-NCOA2 | t(2;8)(p25.1;q13.3) | [120] | |
| RAD51B | ||||
| GREB1 | ||||
| NCOA2 |
Acknowledgments
The authors would like to thank Paul Roberts for providing the photo in Figure 5 and Figure 8.
Abbreviations
| AE1/3 | Pancytokeratin |
| AR | Androgen receptor |
| CC.Y. | Chit Cheng Yeoh |
| CK | Creatine kinase |
| CRS | Endometrial Stromal Sarcoma |
| DFS | Dermatofibrosarcoma protuberans |
| EMA | Epithelial membrane antigen |
| ER | Oestrogen receptor |
| ERα | Oestrogen receptor alpha |
| ERβ | Oestrogen receptor beta |
| ESN | Endometrial Stromal Nodule |
| EST | Endometrial stromal tumours |
| EST-LI | EST with limited infiltration |
| FIGO | International Federation of Gynecology and Obstetrics |
| FOXL2 | Forkhead box protein L2 |
| GnRH-R | Gonadotropin-releasing hormone receptor |
| HG-ESS | High-Grade Endometrial Stromal Sarcoma |
| HPF | High-power field |
| I.A. | Iolia Akaev |
| IFITM1 | Interferon-induced transmembrane protein-1 |
| INI-1 | SMARCB1, hSNF5, BAF47 |
| ITD | Internal Tandem Duplication |
| LEF1 | Lymphoid Enhancer-binding Factor 1 |
| LG-ESS | Low-Grade Endometrial Stromal Sarcoma |
| LVIS | Lymphovascular invasion |
| MDM2 | E3 ubiquitin-protein ligase Mdm2 |
| myo-D1 | Myoblast determination protein 1 |
| NGS | Next Generation Sequencing |
| NOS | Not Otherwise Specified |
| NTRK | Neurotrophic receptor tyrosine kinase |
| PECOMA | Perivascular epithelioid cell tumour |
| PR | Progesterone receptor |
| RNA | Ribonucleic acid |
| S.R. | Siavash Rahimi |
| SMA | Smooth muscle actin |
| SOX-10 | Transcription factor SOX-10 |
| STAT6 | Signal transducer and activator of transcription 6 |
| TLE1 | Transducin-like enhancer protein 1 |
| UTROSCT | Uterine tumours resembling ovarian sex-cord tumour |
| UUS | Undifferentiated Uterine Sarcoma |
| WHO | World Health Organisation |
| WT1 | Wilms tumour 1 |
Author Contributions
Data curation, I.A. and S.R.; writing—original draft preparation, I.A.; writing—review and editing, I.A. and S.R.; visualization, I.A.; supervision, C.C.Y. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Silverberg S.G., Kurman R.J., editors. Atlas of Tumor Pathology. Armed Forces Institute of Pathology; Washington, DC, USA: 1992. Tumors of the Uterine Corpus and Gestational Trophoblastic Disease; pp. 91–110. (3rd Series, Fascicle 3). [Google Scholar]
- 2.Felix A.S., Cook L.S., Gaudet M.M., Rohan T.E., Schouten L.J., Setiawan V.W., Wise L.A., Anderson K.E., Bernstein L., De Vivo I., et al. The etiology of uterine sarcomas: A pooled analysis of the epidemiology of endometrial cancer consortium. Br. J. Cancer. 2013;19:727–734. doi: 10.1038/bjc.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norris H.J., Taylor H.B. Mesenchymal tumors of the uterus. I. A clinical and pathological study of 53 endometrial stromal tumors. Cancer. 1966;19:755–766. doi: 10.1002/1097-0142(196606)19:6<755::AID-CNCR2820190604>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 4.Evans H.L. Endometrial stromal sarcoma and poorly differentiated endometrial sarcoma. Cancer. 1982;50:2170–2182. doi: 10.1002/1097-0142(19821115)50:10<2170::AID-CNCR2820501033>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 5.Lee C.H., Nucci M.R. Endometrial stromal sarcoma--the new genetic paradigm. Histopathology. 2015;67:1–19. doi: 10.1111/his.12594. [DOI] [PubMed] [Google Scholar]
- 6.Ali R.H., Rouzbahman M. Endometrial stromal tumours revisited: An update based on the 2014 WHO classification. J. Clin. Pathol. 2015;68:325–332. doi: 10.1136/jclinpath-2014-202829. [DOI] [PubMed] [Google Scholar]
- 7.Female Genital Tumours: WHO Classification of Tumours. 5th ed. IARC Publications; Lyon, France: 2020. [Google Scholar]
- 8.Masand R.P., Euscher E.D., Deavers M.T., Malpica A. Endometrioid stromal sarcoma: A clinicopathologic study of 63 cases. Am. J. Surg. Pathol. 2013;37:1635–1647. doi: 10.1097/PAS.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 9.Chang K.L., Crabtree G.S., Lim-Tan S.K., Kempson R.L., Hendrickson M.R. Primary extrauterine endometrial stromal neoplasms: A clinicopathologic study of 20 cases and a review of the literature. Int. J. Gynecol. Pathol. 1993;12:282–296. doi: 10.1097/00004347-199310000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Chan J.K., Kawar N.M., Shin J.Y., Osann K., Chen L.-M., Powell C.B., Kapp D.S. Endometrial stromal sarcoma: A population-based analysis. Br. J. Cancer. 2008;99:1210–1215. doi: 10.1038/sj.bjc.6604527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beer T.W., Buchanan R., Buckley C.H. Uterine stromal sarcoma following tamoxifen treatment. J. Clin. Pathol. 1995;48:596. doi: 10.1136/jcp.48.6.596-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Press M.F., Scully R.E. Endometrial “sarcomas” complicating ovarian thecoma, polycystic ovarian disease and estrogen therapy. Gynecol. Oncol. 1985;21:135–154. doi: 10.1016/0090-8258(85)90246-X. [DOI] [PubMed] [Google Scholar]
- 13.Meredith R.F., Eisert D.R., Kaka Z., Hodgson S.E., Johnston G.A., Jr., Boutselis J.G. An excess of uterine sarcomas after pelvic irradiation. Cancer. 1986;58:2003–2007. doi: 10.1002/1097-0142(19861101)58:9<2003::AID-CNCR2820580908>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Dos Santos L.A.D., Garg K., Diaz J.P., Soslow R.A., Hensley M.L., Alektiar K.M., Barakat R.R., Leitao M.M., Jr. Incidence of lymph node and adnexal metastasis in endometrial stromal sarcoma. Gynecol. Oncol. 2011;121:319–322. doi: 10.1016/j.ygyno.2010.12.363. [DOI] [PubMed] [Google Scholar]
- 15.Kurman R.J., Carcangiu M.L., Herrington C.S., Young R.H., editors. WHO Classification of Female Reproductive Organs. 4th ed. IARC; Lyon, France: 2014. [Google Scholar]
- 16.Tavassoli F.A., Norris H.J. Mesenchymal tumours of the uterus. VII. A clinicopathological study of 60 endometrial stromal nodules. Histopathology. 1981;5:1–10. doi: 10.1111/j.1365-2559.1981.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 17.Dionigi A., Oliva E., Clement P.B., Young R.H. Endometrial stromal nodules and endometrial stromal tumors with limited infiltration: A clinic pathologic study of 50 cases. Am. J. Surg. Pathol. 2002;6:567–581. doi: 10.1097/00000478-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Su T.-F., Chao T.-K., Lee H.-S., Perng C.-L., Nieh S. Malignant potential of an Endometrial stromal tumor with limited infiltration: A case report. Int. J. Surg. Pathol. 2014;22:559–563. doi: 10.1177/1066896913506934. [DOI] [PubMed] [Google Scholar]
- 19.Moore M., McCluggage W.G. Uterine endometrial stromal tumors with limited infiltration: First report of a case series indicating potential for malignant behavior. Int. J. Gynecol. Pathol. 2020;39:221–226. doi: 10.1097/PGP.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 20.Chang K.L., Crabtree G.S., Lim-Tan S.K., Kempson R.L., Hendrickson M.R. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am. J. Surg. Pathol. 1990;14:415–438. doi: 10.1097/00000478-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Oliva E., Clement P.B., Young R.H., Scully R.E. Mixed endometrial stromal and smooth muscle tumors of the uterus: A clinic-pathologic study of 15 cases. Am. J. Surg. Pathol. 1998;22:997–1005. doi: 10.1097/00000478-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Franquemont D.W., Frierson H.F., Mills S.E. An immunohistochemical study of normal endometrial stroma and endometrial stromal neoplasms. Evidence for smooth muscle differentiation. Am. J. Surg. Pathol. 1991;15:861–870. doi: 10.1097/00000478-199109000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Khalifa M.A., Hansen C.H., Moore J.L., Rusnock E.J., Lage J.M. Endometrial stromal sarcoma with focal smooth muscle differentiation: Recurrence after 17years: A follow-up report with discussion of the nomenclature. Int. J. Gynecol. Pathol. 1996;15:171–176. doi: 10.1097/00004347-199604000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Lillemoe T.J., Perrone T., Norris H.J., Dehner L.P. Myogenous phenotype of epithelial-like areas in endometrial stromal sarcomas. Arch. Pathol. Lab. Med. 1991;115:215–219. [PubMed] [Google Scholar]
- 25.Yilmaz A., Rush D.S., Soslow R.A. Endometrial stromal sarcomas with unusual histologic features: A report of 24 primary and metastatic tumors emphasizing fibroblastic and smooth muscle differentiation. Am. J. Surg. Pathol. 2002;26:1142–1150. doi: 10.1097/00000478-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Baker P.M., Moch H., Oliva E. Unusual morphologic features of endometrial stromal tumors. Am. J. Surg. Pathol. 2005;29:1394–1398. doi: 10.1097/01.pas.0000172295.05527.28. [DOI] [PubMed] [Google Scholar]
- 27.Fadare O., McCalip B., Mariappan M.R., Hileeto D., Parkash V. An endometrial stromal tumor with osteoclast-like giant cells:expanding the morphological spectrum. Ann. Diagn. Pathol. 2005;9:160–165. doi: 10.1016/j.anndiagpath.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Kim Y.H., Cho H., Kyeom-Kim H., Kim I. Uterine endometrial stromal sarcoma with rhabdoid and smooth muscle differentiation. J. Korean Med. Sci. 1996;11:88–93. doi: 10.3346/jkms.1996.11.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloreta J., Prat J. Endometrial stromal nodule with smooth and skeletal muscle components simulating stromal sarcoma. Int. J. Gynecol. Pathol. 1992;11:293–298. doi: 10.1097/00004347-199210000-00008. [DOI] [PubMed] [Google Scholar]
- 30.McCluggage W.G., Cromie A.J., Bryson C., Traub A.L. Uterine endometrial stromal sarcoma with smooth muscle and glandular differentiation. J. Clin. Pathol. 2001;54:481–483. doi: 10.1136/jcp.54.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliva E., de Leval L., Soslow R.A., Herens C. High frequency of JAZF1-JJAZ1 gene fusion in endometrial stromal tumors with smooth muscle differentiation by interphase fish detection. Am. J. Surg. Pathol. 2007;31:1277–1284. doi: 10.1097/PAS.0b013e318031f012. [DOI] [PubMed] [Google Scholar]
- 32.Stewart C.J.R., Leung Y.C., Murch A., Peverall J. Evaluation of fluorescence in-situ hybridization in monomorphic endometrial stromal neoplasms and their histological mimics: A review of 49 cases. Histopathology. 2014;65:473–482. doi: 10.1111/his.12406. [DOI] [PubMed] [Google Scholar]
- 33.Tanimoto A., Sasaguri T., Arima N., Hashimoto H., Hamada T., Sasaguri Y. Endometrial stromal sarcoma of the uterus with rhabdoid features. Pathol. Int. 1996;46:231–237. doi: 10.1111/j.1440-1827.1996.tb03604.x. [DOI] [PubMed] [Google Scholar]
- 34.Lifschitz-Mercer B., Czernobilsky B., Dgani R., Dallenbach-Hellweg G., Moll R., Franke W.W. Immunocytochemical study of an endometrial diffuse clear cell stromal sarcoma and other Endometrial stromal sarcomas. Cancer. 1987;59:1494–1499. doi: 10.1002/1097-0142(19870415)59:8<1494::AID-CNCR2820590817>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 35.Oliva E., Young R.H., Clement P.B., Scully R.E. Myxoid and fibrous endometrial stromal tumors of the uterus: A report of 10 cases. Int. J. Gynecol. Pathol. 1999;18:310–319. doi: 10.1097/00004347-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Kibar Y., Aydin A., Deniz H., Balat O., Cebesoy B., Al-Nafussi A. A rare case of low-grade endometrial stromal sarcoma with myxoid differentiation and atypical bizarre cells. Eur. J. Gynaecol. Oncol. 2008;29:397–398. [PubMed] [Google Scholar]
- 37.Kim H.-S., Yoon G., Jung Y.Y., Lee Y.-Y., Song S.Y. Fibromyxoid variant of endometrial stromal sarcoma with atypical bizarre nuclei. Int. J. Clin. Exp. Pathol. 2015;8:3316–3321. [PMC free article] [PubMed] [Google Scholar]
- 38.Schoolmeester J.K., Sciallis A.P., Greipp P.T., Hodge J.C., Dal Cin P., Keeney G.L., Nucci M.R. Analysis of MDM2 amplification in 43 Endometrial stromal tumors. Int. J. Gynecol. Pathol. 2015;34:576–583. doi: 10.1097/PGP.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 39.Oliva E., Clement P.B., Young R.H. Epithelioid endometrial and endometrioid stromal tumors: A report of four cases emphasizing their distinction from epithelioid smooth muscle tumors and other oxyphilic uterine and extrauterine tumors. Int. J. Gynecol. Pathol. 2002;21:48–55. doi: 10.1097/00004347-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Clement P.B., Scully R.E. Endometrial stromal sarcomas of the uterus with extensive endometrioid glandular differentiation: A report of three cases that caused problems in differential diagnosis. Int. J. Gynecol. Pathol. 1992;11:163–173. doi: 10.1097/00004347-199207000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Levine P.H., Abou-Nassar S., Mittal K. Extrauterine low-grade endometrial stromal sarcoma with florid endometrioid glandular differentiation. Int. J. Gynecol. Pathol. 2001;20:395–398. doi: 10.1097/00004347-200110000-00014. [DOI] [PubMed] [Google Scholar]
- 42.McCluggage W.G., Ganesan R., Herrington C.S. Endometrial stromal sarcomas with extensive endometrioid glandular differentiation: Report of a series with emphasis on the potential for misdiagnosis and discussion of the differential diagnosis. Histopathology. 2009;54:365–373. doi: 10.1111/j.1365-2559.2009.03230.x. [DOI] [PubMed] [Google Scholar]
- 43.McCluggage W.G., Young R.H. Endometrial stromal sarcomas with true papillae and pseudopapillae. Int. J. Gynecol. Pathol. 2008;27:555–561. doi: 10.1097/PGP.0b013e31817a82f9. [DOI] [PubMed] [Google Scholar]
- 44.Clement P.B., Scully R.E. Uterine tumors resembling ovarian sex-cord tumors. A clinicopathologic analysis of fourteen cases. Am. J. Clin. Pathol. 1976;66:512–525. doi: 10.1093/ajcp/66.3.512. [DOI] [PubMed] [Google Scholar]
- 45.McCluggage W.G., Date A., Bharucha H., Toner P.G. Endometrial stromal sarcoma with sex cord-like areas and focal rhabdoid differentiation. Histopathology. 1996;29:369–374. doi: 10.1111/j.1365-2559.1996.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 46.Fukunaga M., Miyazawa Y., Ushigome S. Endometrial low-grade stromal sarcoma with ovarian sex cord-like differentiation: Report of two cases with an immunohistochemical and flow cytometric study. Pathol. Int. 1997;47:412–415. doi: 10.1111/j.1440-1827.1997.tb04516.x. [DOI] [PubMed] [Google Scholar]
- 47.Richmond A., Rohrer A., Davidson S.A., Post M.D. Low-grade Endometrial stromal sarcoma with extensive sex cord differentiation, heterologous elements, and complex atypical hyperplasia: Case report and review of the literature. Gynecol. Oncol. Rep. 2017;19:34–38. doi: 10.1016/j.gore.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fekete P.S., Vellios F. The clinical and histologic spectrum of endometrial stromal neoplasms: A report of 41 cases. Int. J. Gynecol. Pathol. 1984;3:198–212. doi: 10.1097/00004347-198402000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Irving J.A., Carinelli S., Prat J. Uterine tumors resembling ovarian sex cord tumors are polyphenotypic neoplasms with true sex cord differentiation. Mod. Pathol. 2006;19:17–24. doi: 10.1038/modpathol.3800475. [DOI] [PubMed] [Google Scholar]
- 50.Baker R.J., Hildebrandt R.H., Rouse R.V., Hendrickson M.R., Longacre T.A. Inhibin and CD99 (MIC2) expression in uterine stromal neoplasms with sex-cord-like elements. Hum. Pathol. 1999;30:671–679. doi: 10.1016/S0046-8177(99)90093-X. [DOI] [PubMed] [Google Scholar]
- 51.Sumathi V.P., Al-Hussaini M., Connolly L.E., Fullerton L., McCluggage W.G. Endometrial stromal neoplasms are immunoreactive with WT-1 antibody. Int. J. Gynecol. Pathol. 2004;23:241–247. doi: 10.1097/01.pgp.0000130051.04396.13. [DOI] [PubMed] [Google Scholar]
- 52.Rahimi S., Akaev I., Marani C., Chopra M., Yeoh C.C. Immunohistochemical expression of different subtypes of cytokeratins by endometrial stromal sarcoma. Appl. Immunohistochem. Mol. Morphol. 2019;27:466–470. doi: 10.1097/PAI.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 53.Nomura Y., Tamura D., Horie M., Sato M., Sasaki S., Yamamoto Y., Kudo-Asabe Y., Umakoshi M., Koyama K., Makino K., et al. Detection of MEAF6-PHF1 translocation in an endometrial stromal nodule. Genes Chromosomes Cancer. 2020;59:702–708. doi: 10.1002/gcc.22892. [DOI] [PubMed] [Google Scholar]
- 54.Alan S., Yilmaz E., Tecellioglu F., Akatli A.N., Inci Coskun E., Gokce H. Multifocal low-grade endometrial stromal sarcoma arising from pre-existing endometriosis in a hysterectomised patient: A case report. J. Obstet. Gynaecol. 2019;39:1177–1180. doi: 10.1080/01443615.2019.1587599. [DOI] [PubMed] [Google Scholar]
- 55.Xu Y., Liang Z.X., Guo J.T., Su X., Lu Y.L., Guan X.Z. Cystic and solitary nodular pulmonary metastases in a patient with low-grade endometrial stromal sarcoma: A case report and literature review. Oncol. Lett. 2019;18:1133–1144. doi: 10.3892/ol.2019.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujiishi K., Nagata S., Kano R., Kubo C., Shirayanagi M., Ozaki M., Yamamoto T., Nakanishi K., Kamiura S., Nakatsuka S.I. JAZF1-SUZ12 endometrial stromal sarcoma forming subserosal masses with extraordinary uptake of fluorodeoxyglucose on positron emission tomography: A case report. Diagn. Pathol. 2019;14 doi: 10.1186/s13000-019-0897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subbaraya S., Murthy S.S., Devi G.S. Immunohistochemical and Molecular Characterization of Endometrial Stromal Sarcomas. Clin. Pathol. 2020;13 doi: 10.1177/2632010X20916736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fels Elliott D.R., Pekmezci M., Geiersbach K.B., Krings G., Rabban J.T., Zaloudek C., Chen Y.Y. Low-grade endometrial stromal sarcoma metastatic to the breast: Immunohistochemical and molecular characterization of an unusual mimic of mammary myofibroblastoma. Hum. Pathol. 2020;22 doi: 10.1016/j.ehpc.2020.200447. [DOI] [Google Scholar]
- 59.Abeler V.M., Nenodovic M. Diagnostic immunohistochemistry in uterine sarcomas: A study of 397 cases. Int. J. Gynecol. Pathol. 2011;30:236–243. doi: 10.1097/PGP.0b013e318200caff. [DOI] [PubMed] [Google Scholar]
- 60.McCluggage W.G., Sumathi V.P., Maxwell P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology. 2001;39:273–278. doi: 10.1046/j.1365-2559.2001.01215.x. [DOI] [PubMed] [Google Scholar]
- 61.Tang Y., Chen Y., Tian L., Chen J., Yang P., Zhang D., Cui Q., Zhao L., Li L. Low-Grade Vaginal Endometrial Stromal Sarcoma. Int. J. Gynecol. Pathol. 2019;1 doi: 10.1097/PGP.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 62.Bhargava R., Shia J., Hummer A.J., Thaler H.T., Tornos C., Soslow R.A. Distinction of endometrial stromal sarcomas from hemangiopericytomatous’ tumors using a panel of immunohistochemical stains. Mod. Pathol. 2005;18:40–47. doi: 10.1038/modpathol.3800248. [DOI] [PubMed] [Google Scholar]
- 63.Cotzia P., Benayed R., Mullaney K., Oliva E., Felix A., Ferreira J., Soslow R.A., Antonescu C.R., Ladanyi M., Chiang S. Undifferentiated Uterine Sarcomas Represent Under-Recognized High-grade Endometrial Stromal Sarcomas. Am. J. Surg. Pathol. 2019;43:662–669. doi: 10.1097/PAS.0000000000001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park J.Y., Baek M.H., Park Y., Kim Y.T., Nam J.H. Investigation of hormone receptor expression and its prognostic value in endometrial stromal sarcoma. Virchows Arch. 2018;473:61–69. doi: 10.1007/s00428-018-2358-5. [DOI] [PubMed] [Google Scholar]
- 65.Baniak N., Adams S., Chibbar R., Lee C.H., Kanthan R. Hepatic endometrial stromal sarcoma. Pathol. Res. Pract. 2018;214:1726–1731. doi: 10.1016/j.prp.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 66.Kirmizi S., Ucar B.I., Yilmaz S., Aydogan Kirmizi D. Stomach metastasis of low grade endometrial stromal sarcoma. Dicle Tıp Dergisi. 2019:609–613. doi: 10.5798/dicletip.545723. [DOI] [Google Scholar]
- 67.McCarthy A.J., Clarke B.A., McGilvray I., Dickson B.C., Khalili K., Chetty R. Metastatic low-grade endometrial stromal sarcoma of uterus presenting as a primary pancreatic tumor: Case presentation and literature review. Diagn. Pathol. 2019;14:30. doi: 10.1186/s13000-019-0807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Madhumalarmaran P., Ramachandran A., Ahamedkhan N.B., Pillay R., Nair A., Subramaniam S., Guhan B. Endometrial Stromal Sarcoma: An Atypical Presentation. Indian J. Gynecol. Oncol. 2020;18 doi: 10.1007/s40944-020-00388-6. [DOI] [Google Scholar]
- 69.Yadav S., Santosh M., Bakshi G., Sangeeta D. Extra-Uterine Low-Grade Endometrial Stromal Sarcoma Presenting as a Urinary Bladder Mass: A Case Report with Review of the Literature. Indian J. Surg. Oncol. 2019;11(Suppl. S1):20–23. doi: 10.1007/s13193-019-00952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sato S., Ojima Y., Kanda M., Kizaki T., Ohara N. Endometrial stromal sarcoma arising from endometrial polyp: A case report. Kobe J. Med. Sci. 2018;64:E36–E42. [PMC free article] [PubMed] [Google Scholar]
- 71.Busca A., Gulavita P., Parra-Herran C., Islam S. IFITM1 Outperforms CD10 in Differentiating Low-grade Endometrial Stromal Sarcomas from Smooth Muscle Neoplasms of the Uterus. Int. J. Gynecol. Pathol. 2018;37:372–378. doi: 10.1097/PGP.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 72.Przybyl J., Kidzinski L., Hastie T., Debiec-Rychter M., Nusse R., van de Rijn M. Gene expression profiling of low-grade endometrial stromal sarcoma indicates fusion protein-mediated activation of the Wnt signaling pathway. Gynecol. Oncol. 2018;149:388–393. doi: 10.1016/j.ygyno.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 73.Makise N., Sekimizu M., Kobayashi E., Yoshida H., Fukayama M., Kato T., Kawai A., Ichikawa H., Yoshida A. Low-grade endometrial stromal sarcoma with a novel MEAF6-SUZ12 fusion. Virchows Arch. 2019;475:527–531. doi: 10.1007/s00428-019-02588-8. [DOI] [PubMed] [Google Scholar]
- 74.Verma A., Menon S., Rekhi B., Pai T., Maheshwari A., Ghosh J., Gupta S., Deodhar K. Utility of YWHAE fluorescent in-situ hybridisation in mesenchymal tumors of uterus- An initial experience from tertiary oncology centre in India. Indian J. Cancer. 2019;56:335–340. doi: 10.4103/ijc.IJC_722_18. [DOI] [PubMed] [Google Scholar]
- 75.Carbone F., Kaur M.M., Chok A.Y., Kontovounisios C., Ind T., Rasheed S. Endometrial stromal sarcoma arising from polypoid endometriosis: Case report and literature review. Int. J. Surg. Case Rep. 2020;72:537–540. doi: 10.1016/j.ijscr.2020.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rivera G., Niu S., Chen H., Fahim D., Peng Y. Collision Tumor of Endometrial Large Cell Neuroendocrine Carcinoma and Low-Grade Endometrial Stromal Sarcoma: A Case Report and Review of the Literature. Int. J. Surg. Pathol. 2020;28:569–573. doi: 10.1177/1066896920901764. [DOI] [PubMed] [Google Scholar]
- 77.Chiang S., Lee C.H., Stewart C.J.R., Oliva E., Hoang L.N., Ali R.H., Hensley M.L., Arias-Stella J.A., Frosina D., Jungbluth A.A., et al. BCOR is a robust diagnostic immunohistochemical marker of genetically diverse high-grade endometrial stromal sarcoma, including tumors exhibiting variant morphology. Mod. Pathol. 2017;30:1251–1261. doi: 10.1038/modpathol.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koontz J.I., Soreng A.L., Nucci M., Kuo F.C., Pauwels P., van Den Berghe H., Dal Cin P., Fletcher J.A., Sklar J. Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc. Natl. Acad. Sci. USA. 2001;98:6348–6353. doi: 10.1073/pnas.101132598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nucci M.R., Harburger D., Koontz J., Dal Cin P., Sklar J. Molecular analysis of the JAZF1-JJAZ1 gene fusion by RT-PCR and fluorescence in situ hybridization in endometrial stromal neoplasms. Am. J. Surg. Pathol. 2007;31:65–70. doi: 10.1097/01.pas.0000213327.86992.d1. [DOI] [PubMed] [Google Scholar]
- 80.Micci F., Panagopoulos I., Bjerkehagen B., Heim S. Consistent rearrangement of chromosomal band 6p21 with generation of fusion genes JAZF1/PHF1 and EPC1/PHF1 in endometrial stromal sarcoma. Cancer Res. 2006;66:107–112. doi: 10.1158/0008-5472.CAN-05-2485. [DOI] [PubMed] [Google Scholar]
- 81.Panagopoulos I., Micci F., Thorsen J., Gorunova L., Eibak A.M., Bjerkehagen B., Davidson B., Heim S. Novel fusion of MYST/Esa1-associated factor 6 and PHF1 in endometrial stromal sarcoma. PLoS ONE. 2012;7:e39354. doi: 10.1371/journal.pone.0039354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dewaele B., Przybyl J., Quattrone A., Finalet Ferreiro J., Vanspauwen V., Geerdens E., Gianfelici V., Kalender Z., Wozniak A., Moerman P., et al. Identification of a novel, recurrent MBTD1-CXorf67 fusion in low-grade endometrial stromal sarcoma. Int. J. Cancer. 2014;134:1112–1122. doi: 10.1002/ijc.28440. [DOI] [PubMed] [Google Scholar]
- 83.Micci F., Brunetti M., Dal Cin P., Nucci M.R., Gorunova L., Heim S., Panagopoulos I. Fusion of the genes BRD8 and PHF1 in endometrial stromal sarcoma. Genes Chromosomes Cancer. 2017;56:841–845. doi: 10.1002/gcc.22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Allen A.J., Ali S.M., Gowen K., Elvin J.A., Pejovic T. A recurrent endometrial stromal sarcoma harbors the novel fusion JAZF1-BCORL1. Gynecol. Oncol. Rep. 2017;20:51–53. doi: 10.1016/j.gore.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brunetti M., Gorunova L., Davidson B., Heim S., Panagopoulos I., Micci F. Identification of an EPC2-PHF1 fusion transcript in low-grade endometrial stromal sarcoma. Oncotarget. 2018;9:19203–19208. doi: 10.18632/oncotarget.24969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dickson B.C., Lum A., Swanson D., Bernardini M.Q., Colgan T.J., Shaw P.A., Yip S., Lee C.-H. Novel EPC1 gene fusions in endometrial stromal sarcoma. Genes Chromosomes Cancer. 2018;57:598–603. doi: 10.1002/gcc.22649. [DOI] [PubMed] [Google Scholar]
- 87.Mansor S., Kuick C.H., Lim-Tan S.K., Leong M.Y., Lim T.Y.K., Chang K.T.E. Novel fusion MAGED2-PLAG1 in endometrial stromal sarcoma. Pathology. 2020;52:S142–S143. doi: 10.1016/j.pathol.2020.01.029. [DOI] [Google Scholar]
- 88.Han L., Liu Y.J., Ricciotti R.W., Mantilla J.G. A novel MBTD1-PHF1 gene fusion in endometrial stromal sarcoma: A case report and literature review. Genes Chromosomes Cancer. 2020 doi: 10.1002/gcc.22845. [DOI] [PubMed] [Google Scholar]
- 89.Brahmi M., Franceschi T., Treilleux I., Pissaloux D., Ray-Coquard I., Dufresne A., Vanacker H., Carbonnaux M., Meeus P., Sunyach M.P., et al. Molecular classification of endometrial stromal sarcomas using RNA sequencing defines nosological and prognostic subgroups with different natural history. Cancers. 2020;12:2604. doi: 10.3390/cancers12092604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aisagbonhi O., Harrison B., Zhao L., Osgood R., Chebib I., Oliva E. YWHAE Rearrangement in a Purely Conventional Low-grade Endometrial Stromal Sarcoma that Transformed over Time to High-grade Sarcoma: Importance of Molecular Testing. Int. J. Gynecol. Pathol. 2018;37:441–447. doi: 10.1097/PGP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 91.Lee C.-H., Mariño-Enriquez A., Ou W., Zhu M., Ali R.H., Chiang S., Amant F., Gilks C.B., van de Rijn M., Oliva E., et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: A histologically high-grade and clinically aggressive tumor. Am. J. Surg. Pathol. 2012;36:641–653. doi: 10.1097/PAS.0b013e31824a7b1a. [DOI] [PubMed] [Google Scholar]
- 92.Lewis L., Soslow R.A., Delair D.F., Park K.J., Murali R., Hollmann T.J., Davidson B., Micci F., Panagopoulos I., Hoang L.N., et al. ZC3H7B-BCOR high-grade endometrial stromal sarcomas: A report of 17 cases of a newly defined entity. Mod. Pathol. 2018;31:674–684. doi: 10.1038/modpathol.2017.162. [DOI] [PubMed] [Google Scholar]
- 93.Juckett L.T., Lin D.I., Madison R., Ross J.S., Schrock A.B., Ali S.A. Pan-Cancer Landscape Analysis Reveals a Subset of Endometrial Stromal and Pediatric Tumors Defined by Internal Tandem Duplications of BCOR. Oncology. 2019;96:101–109. doi: 10.1159/000493322. [DOI] [PubMed] [Google Scholar]
- 94.Croce S., Hostein I., Ribeiro A., Garbay D., Velasco V., Stoeckle E., Guyon F., Floquet A., Neuville A., Coindre J.M., et al. YWHAE rearrangement identified by FISH and RT-PCR in endometrial stromal sarcomas: Genetic and pathological correlations. Mod. Pathol. 2013;26:1390–1400. doi: 10.1038/modpathol.2013.69. [DOI] [PubMed] [Google Scholar]
- 95.McCluggage W.G., Lee C.-H. YWHAE-NUTM2A/B Translocated High-grade Endometrial Stromal Sarcoma Commonly Expresses CD56 and CD99. Int. J. Gynecol. Pathol. 2019;38:528–532. doi: 10.1097/PGP.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 96.Verschoor A.J., Warmerdam F.A.R.M., Bosse T., Bovée J.V.M.G., Gelderblom H.A. remarkable response to pazopanib, despite recurrent liver toxicity, in a patient with a high grade endometrial stromal sarcoma, a case report. BMC Cancer. 2018:18. doi: 10.1186/s12885-018-3999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baniak N., Adams S., Lee C.H., Chibbar R., Kanthan R. Extrapelvic Metastases in Endometrial Stromal Sarcomas: A Clinicopathological Review with Immunohistochemical and Molecular Characterization. Int. J. Surg. Pathol. 2019;27:208–215. doi: 10.1177/1066896918794278. [DOI] [PubMed] [Google Scholar]
- 98.HUGO Gene Nomenclature Committee. [(accessed on 20 November 2020)]; Available online: https://www.genenames.org.
- 99.Lee C.H., Hoang L.N., Yip S., Reyes C., Marino-Enriquez A., Eilers G., Tao D., Chiang S., Fletcher J.A., Soslow R.A., et al. Frequent expression of KIT in endometrial stromal sarcoma with YWHAE genetic rearrangement. Mod. Pathol. 2014;27:751–757. doi: 10.1038/modpathol.2013.199. [DOI] [PubMed] [Google Scholar]
- 100.Hoang L.N., Aneja A., Conlon N., Delair D.F., Middha S., Benayed R., Hensley M.L., Park K.J., Hollmann T.J., Hameed M.R., et al. Novel High-Grade Endometrial Stromal Sarcoma: A Morphologic Mimicker of Myxoid Leiomyosarcoma. Am. J. Surg. Pathol. 2017;41:12–24. doi: 10.1097/PAS.0000000000000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ondič O., Bednářová B., Ptáková N., Hájková V., Šteiner P., Šidlová H., Presl J., Bouda J., Alaghehbandan R., Michal M. ZC3H7B–BCOR high-grade endometrial stromal sarcoma may present as myoma nascens with cytoplasmic signet ring cell change. Virchows Arch. 2020;476:615–619. doi: 10.1007/s00428-020-02744-5. [DOI] [PubMed] [Google Scholar]
- 102.Nagaputra J.C., Goh R.C.H., Kuick C.H., Chang K.T.E., Sittampalam K. ZC3H7B-BCOR high-grade endometrial stromal sarcoma with osseous metaplasia: Unique feature in a recently defined entity. Hum. Pathol. 2019;15:54–58. doi: 10.1016/j.ehpc.2018.11.002. [DOI] [Google Scholar]
- 103.Lu B., Chen J., Shao Y., Shi H. Two cases of ZC3H7B-BCOR high grade endometrial stromal sarcoma with an extension on its morphological features. Pathology. 2020 doi: 10.1016/j.pathol.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 104.Solomon J.P., Linkov I., Rosado A., Mullaney K., Rosen E.Y., Frosina D., Jungbluth A.A., Zehir A., Benayed R., Drilon A., et al. NTRK fusion detection across multiple assays and 33,997 cases: Diagnostic implications and pitfalls. Mod. Pathol. 2020;33:38–46. doi: 10.1038/s41379-019-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin D.I., Hemmerich A., Edgerly C., Duncan D., Severson E.A., Huang R.S.P., Ramkissoon S.H., Connor Y.D., Shea M., Hecht J.L., et al. Genomic profiling of BCOR-rearranged uterine sarcomas reveals novel gene fusion partners, frequent CDK4 amplification and CDKN2A loss. Gynecol. Oncol. 2020;157:357–366. doi: 10.1016/j.ygyno.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 106.Kommoss F.K.F., Chang K.T.E., Stichel D., Banito A., Jones D.T.W., Heilig C.E., Fröhling S., Sahm F., Stenzinger A., Hartmann W., et al. Endometrial stromal sarcomas with BCOR-rearrangement harbor MDM2 amplifications. J. Pathol. Clin. Res. 2020;6:178–184. doi: 10.1002/cjp2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoshida A., Arai Y., Hama N., Chikuta H., Bando Y., Nakano S., Kobayashi E., Shibahara J., Fukuhara H., Komiyama M., et al. Expanding the clinicopathologic and molecular spectrum of BCOR -associated sarcomas in adults. Histopathology. 2020;76:509–520. doi: 10.1111/his.14023. [DOI] [PubMed] [Google Scholar]
- 108.Marinõ-Enriquez A., Lauria A., Przybyl J., Ng T.L., Kowalewska M., Debiec-Rychter M., Ganesan R., Ganesan R., Sumathi V., George S., et al. BCOR Internal Tandem Duplication in High-grade Uterine Sarcomas. Am. J. Surg. Pathol. 2018;42:335–341. doi: 10.1097/PAS.0000000000000993. [DOI] [PubMed] [Google Scholar]
- 109.Momeni-Boroujeni A., Mohammad N., Wolber R., Yip S., Köbel M., Dickson B.C., Hensley M.L., Leitao M.M., Antonescu C.R., Benayed R., et al. Targeted RNA expression profiling identifies high-grade endometrial stromal sarcoma as a clinically relevant molecular subtype of uterine sarcoma. Mod. Pathol. 2020:1–9. doi: 10.1038/s41379-020-00705-6. [DOI] [PubMed] [Google Scholar]
- 110.Chiang S., Cotzia P., Hyman D.M., Drilon A., Tap W.D., Zhang L., Hechtman J.F., Frosina D., Jungbluth A.A., Murali R., et al. NTRK Fusions Define a Novel Uterine Sarcoma Subtype with Features of Fibrosarcoma. Am. J. Surg. Pathol. 2018;42:791–798. doi: 10.1097/PAS.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Croce S., Hostein I., Longacre T.A., Mills A.M., Pérot G., Devouassoux-Shisheboran M., Velasco V., Floquet A., Guyon F., Chakiba C., et al. Uterine and vaginal sarcomas resembling fibrosarcoma: A clinicopathological and molecular analysis of 13 cases showing common NTRK-rearrangements and the description of a COL1A1-PDGFB fusion novel to uterine neoplasms. Mod. Pathol. 2019;32:1008–1022. doi: 10.1038/s41379-018-0184-6. [DOI] [PubMed] [Google Scholar]
- 112.Grindstaff S.L., Di Silvestro J., Hansen K., Di Silvestro P., Sung C.J., Quddus M.R. COL1A1-PDGFB fusion uterine fibrosarcoma: A case report with treatment implication. Gynecol. Oncol. Rep. 2020;31:100523. doi: 10.1016/j.gore.2019.100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boyle W., Williams A., Sundar S., Yap J., Taniere P., Rehal P., Ganesan R. TMP3-NTRK1 rearranged uterine sarcoma: A case report. Case Rep. Womens Health. 2020;28:e00246. doi: 10.1016/j.crwh.2020.e00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Michal M., Hájková V., Skálová A., Michal M. STRN-NTRK3-rearranged Mesenchymal Tumor of the Uterus. Am. J. Surg. Pathol. 2019;43:1152–1154. doi: 10.1097/PAS.0000000000001292. [DOI] [PubMed] [Google Scholar]
- 115.Pautier P., Nam E.J., Provencher D.M., Hamilton A.L., Mangili G., Siddiqui N.A., Westermann A.M., Reed N.S., Harter P., Ray-Coquard I. Gynecologic Cancer InterGroup (GCIG) consensus review for high-grade undifferentiated sarcomas of the uterus. Int. J. Gynecol. Cancer. 2014;24(Suppl. S3):S73–S77. doi: 10.1097/IGC.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 116.Ducimetière F., Lurkin A., Ranchère-Vince D., Decouvelaere A.V., Péoc’h M., Istier L., Chalabreysse P., Muller C., Alberti L., Bringuier P.P., et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS ONE. 2011;6:e20294. doi: 10.1371/journal.pone.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kyriazoglou A., Liontos M., Ziogas D.C., Zagouri F., Koutsoukos K., Tsironis G., Tsiara A., Kaparelou M., Zakopoulou R., Thomakos N., et al. Management of uterine sarcomas and prognostic indicators: Real world data from a single-institution. BMC Cancer. 2018;18:1247. doi: 10.1186/s12885-018-5156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kurihara S., Oda Y., Ohishi Y., Iwasa A., Takahira T., Kaneki E., Kobayashi H., Wake N., Tsuneyoshi M. Endometrial stromal sarcomas and related high-grade sarcomas: Immunohistochemical and molecular genetic study of 31 cases. Am. J. Surg. Pathol. 2008;32:1228–1238. doi: 10.1097/PAS.0b013e31816a3b42. [DOI] [PubMed] [Google Scholar]
- 119.Kurihara S., Oda Y., Ohishi Y., Kaneki E., Kobayashi H., Wake N., Tsuneyoshi M. Coincident expression of beta-catenin and cyclin D1 in endometrial stromal tumors and related high-grade sarcomas. Mod. Pathol. 2010;23:225–234. doi: 10.1038/modpathol.2009.162. [DOI] [PubMed] [Google Scholar]
- 120.Brunetti M., Panagopoulos I., Gorunova L., Davidson B., Heim S., Micci F. RNA-sequencing identifies novel GREB1-NCOA2 fusion gene in a uterine sarcoma with the chromosomal translocation t(2;8)(p25;q13) Genes Chromosomes Cancer. 2018;57:176–181. doi: 10.1002/gcc.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]