Abstract
In recent years, nonalcoholic fatty liver disorders have become one of the most common liver pathologies; therefore, it is necessary to investigate the dietary compounds that may support the regulation of liver metabolism and related inflammatory processes. The present study examines the effect of raspberry polyphenolic extract (RE) combined with fructo-oligosaccharides (FOSs) or pectins (PECs) on caecal microbial fermentation, liver lipid metabolism and inflammation in rats with fatty liver induced by an obesogenic diet. The combination of RE with FOSs or PECs reduced the production of short-chain fatty acids in the caecum. RE combined with FOSs exerted the most favourable effects on liver lipid metabolism by decreasing liver fat, cholesterol, triglyceride content and hepatic steatosis. RE and FOSs reduced lobular and portal inflammatory cell infiltration and IL-6 plasma levels. These effects might be related to a decrease in the hepatic expressions of PPARγ and ANGPTL4. In conclusion, PECs and FOSs enhanced the effects of RE against disorders related to nonalcoholic fatty liver; however, the most effective dietary treatment in the regulation of liver lipid metabolism and inflammation caused by an obesogenic diet was the combination of RE with FOSs.
Keywords: ellagic acid, anthocyanin, flavanol, dietary fibre, obesity, liver disorder
1. Introduction
It is increasingly accepted that a diet rich in fats, mostly saturated fatty acids, and low in dietary fibre, increases the risk of obesity and thus development of nonalcoholic fatty liver disease (NAFLD) [1,2]. NAFLD has become the most common liver pathology worldwide, affecting an estimated 25%–30% of most populations. Several pharmacological treatments have been proposed for the treatment of NAFLD, but the reported results are inconclusive [3]. A potentially new approach in the treatment of liver metabolic disorders is the consumption of antioxidant-rich foods in general and of polyphenols or fibre–polyphenol complexes in particular. Diets enriched with polyphenols and dietary fibres may present hepatoprotective effects by increasing fatty acid oxidation and modulating oxidative stress, lipid metabolism and inflammation, which are the main pathogenetic factors linked to the progression from simple fat accumulation to NAFLD [4,5]. Most of the studies focus on the health-promoting effects of dietary fibre or polyphenols while there is a lack of information on how supplementation with dietary fibre may affect the hepatoprotective effects of polyphenols, especially against disorders related to the development of nonalcoholic fatty liver (NAFL).
A valuable source of polyphenols is raspberries. These fruits are known as a rich source of dietary antioxidants largely due to their high levels of phenolic compounds, which are primarily comprised of anthocyanins and ellagitannins [6]. In addition to strong antioxidant properties, raspberry polyphenols have also shown other beneficial bioactivities, including anti-inflammation, antimicrobial activity against pathogenic intestinal bacteria, antiproliferation of human cancer cells and improvement of the blood lipid profile [7,8,9]. A study on hepatocytes showed that polyphenols from raspberries may also regulate immunometabolic signals associated with the development of obesity [10].
An important agent that may modulate the health-promoting effects of polyphenols is the activity of the intestinal microbiota [11]. Well-known dietary compounds that considerably affect the microbiota are fructo-oligosaccharides (FOSs) and pectins (PECs). The chemical structure of these nondigestible saccharides is different, although both of them promote increased bacterial production of short-chain fatty acids (SCFAs) in the hindgut and favourably regulate the blood lipid profile [12,13]. Our previous nutritional study on healthy Wistar rats showed that the addition of polyphenolic extract containing mainly ellagitannins to the diet reduced the positive effects of FOSs in the gastrointestinal tract, while parameters of plasma lipid profile were improved [14]. In the development of NAFL, changes in the blood lipid profile play a significant role [15]; therefore, the regulation of this parameter may support preventive treatment against the development of fatty liver.
Due to previous experiments describing the beneficial action of dietary FOSs and PECs [12,16] in the rat caecum and blood lipid profile as well as the regulatory effects of raspberry polyphenols on obesogenic signals in hepatocytes [10], we hypothesised that a dietary combination of FOSs, PECs and raspberry polyphenols would increase the desired beneficial effect against NAFL-related disorders induced by an obesogenic diet in rats. Moreover, PECs and FOSs exert different effects on hindgut microbiota activity [13]; therefore, combination with raspberry polyphenols could modulate hepatic metabolic mechanisms to different extents.
2. Materials and Methods
2.1. Raspberry Polyphenolic Extract (RE) Production
The polyphenol extract was obtained from frozen raspberry pomace, which was a waste product from the production of raspberry fruit juice of the “Polana” variety. The exact method of juice production is described in Supplementary Material 1 in our earlier publication [10]. After production, the pomace was immediately frozen and stored at −18 °C for 1 month in sealed polyethylene bags.
After thawing, 20 kg of pomace was subjected to a three-stage extraction with a 30/70 (v/v) acetone–water mixture. The pomace was placed in a polypropylene tank, and 20 L of extractant (solvent/solid ratio of 1:1) was added. After thoroughly mixing the pomace with the extractant, the tank was closed, and static extraction was carried out for 24 h at 20 °C. The extraction mixture had the character of a dense fruit pulp, which after a specified time was pressed in a laboratory screw-press (capacity of 5 L). After pressing in an amount of approximately 18 L, the raw extract was cooled to 4 °C. The extraction residue was subjected to two additional extractions in the same manner as described above. The extracts obtained from the three stages were combined to obtain a total of 55 L of extract and then filtered using the Hobrafit S40N cellulose filter with 5 μm nominal retention (Hobra-Školnik S.R.O., Broumov, Czech Republic). Acetone was removed from the obtained raw extract using a Heidolph Hei-Vap evaporator equipped with an Automatic Hei-Vap Distimatic module (Heidolph, Schwabach, Germany), where a temperature of 60 °C and a pressure of 135 mbar were applied. The acetone-free extract was again filtered using a Hobrafit S40N filter. The filtered extract (approximately 36 L) was then purified on an Amberlite XAD 1600 sorption bed. This process was carried out on a 50 cm long column with an inside diameter of 7.5 cm in eight cycles. One cycle involved applying 4.5 L extract to the column, followed by washing with 10%, 20%, 30%, 40%, 50% and 60% ethanol solutions (2 L each). After the elution process, the column was conditioned with 2 L of 60% ethanol, 2 L of 30% ethanol and 2 L of water before the next cycle. All solutions used for elution were acidified with formic acid to 0.01%. The purification process was carried out by gravity, with the liquid flow in the range of 20–40 mL/min. The richest fractions in polyphenolic compounds (i.e., ellagitannins, proanthocyanidins and anthocyanins) were fractions eluted with 30% and 40% ethanol. These fractions from 8 cycles (32 L) were combined and subjected to an ethanol removal process using a Heidolph Hei-Vap Distimatic evaporator at 60 °C and 125 mbar. After removing ethanol, the solution was further concentrated (60 °C, 72 mbar) to 6 L and freeze-dried (48 h, −36 °C). Finally, 225 g of purified red powder was obtained in this way.
2.2. Polyphenolic Analysis
The ellagitannin and anthocyanins in the produced extract were analysed according to the same procedure and using the same apparatus as described in our previous publication [10]. Briefly, the concentrations of ellagitannin and anthocyanins in extracts diluted with methanol (40 mg/10 mL for ellagitannins and 75 mg/10 mL for anthocyanins) were measured using a Knauer Smartline HPLC system with a DAD detector. The ellagitannin separation was performed on a Gemini C18 column (250 × 4.6 mm; 5 μm, Phenomenex, Torrance, CA, USA). Anthocyanins were separated on a Gemini C18 column (150 × 4.6; 5 μm, Phenomenex). Ellagitannin detection was performed at 250 nm, and the content of individual compounds was calculated on the basis of standard curves plotted for ellagic acid (Extrasynthese, Genay, France), lambertianin C and sanguiin H-6, both produced at Lodz University of Technology according to Klewicka et al. [17]. Anthocyanin detection was performed at 520 nm, and the amount of each anthocyanin was calculated from the curve plotted for cyanidin-3-O-glucoside (Extrasynthese, Genay, France). Ellagitannin and anthocyanin identification was performed using an LC–MS apparatus (Q Exactive Orbitrap, Thermo Fisher Scientific, Waltham, MA, USA) under the same conditions as described in a previous publication [10].
The concentration of proanthocyanidins in the extract was analysed by acid-catalysed degradation of polymeric proanthocyanidins in excess of phloroglucinol, as described by Sójka et al. [18]. For phloroglucinolysis, 20 mg of freeze-dried extract was used. The same analysis conditions (column, gradient, phases, and standards) and FD (λex = 278 nm, λem = 360 nm) detector (Shimadzu RF-10Axl) were used to separate the products of these reactions, but a different chromatography system (Shimadzu, Tokyo, Japan), consisting of an LC-20AD pump, a DGU-20ASR degasser, a CTO-10AS thermostat, and an SIL-20AC autosampler, was used. Concentrations were calculated with standard curves of (−)-epicatechin and (+)-catechin for terminal units and (−)-epicatechin-phloroglucinol adduct for extender units. Free (−)-epicatechin and (+)-catechin were measured under the same HPLC conditions as used for the phenolic extracts and were prepared as above (i.e., prepared for determination of ellagitannins, etc.). The average degree of polymerisation was measured by calculating the molar ratio of all flavan-3-ol units (phloroglucinol adducts + terminal units) to (−)-epicatechin and (+)-catechin, which correspond to terminal units. The chemical characterisation of polyphenols extracted from raspberry pomace is presented in Table 1.
Table 1.
Polyphenol composition of raspberry extract.
| Compound | mg/100 g |
|---|---|
| Ellagitannins (ET) n = 3 | |
| Lambertianin C | 18,314.0 ± 1172.6 |
| Lambertianin C minus ellagic acid moiety 1 a | 764.1 ± 28.8 |
| Lambertianin C minus ellagic acid moiety 2 a | 196.4 ± 10.9 |
| Lambertianin C minus ellagic acid moiety 3 a | 426.6 ± 6.7 |
| Sanguiin H-6 | 16,975.8 ± 350.4 |
| Sanguiin H-6 minus gallic acid moiety b | 221.6 ± 9.7 |
| Sanguiin H-6 plus gallic acid moiety b | 356.3 ± 22.4 |
| Sanguiin H-10 isomer 1 b | 466.4 ± 14.0 |
| Sanguiin H-10 isomer 2 b | 533.0 ± 20.7 |
| Sanguiin H-10 isomer 3 b | 301.2 ± 10.7 |
| Ellagic acid pentose conjugate c | 171.7 ± 11.4 |
| Ellagic acid | 196.7 ± 18.1 |
| Total ET | 38,088.9 ± 1503.6 |
| Total EAC | 368.3 ± 29.4 |
| Total ET + EAC | 38,923.6 ± 1547.0 |
| Flavanols (FLAVA) n = 3 | |
| Total FLAVA | 8371.7 ± 486.5 |
| (+)-Catechin | 208.0 ± 5.4 |
| (−)-Epicatechin | 343.5 ± 5.5 |
| Proanthocyanidins | 7820.2 ± 475.7 |
| Extension units (%) | |
| (+)-Catechin | 28.5 ± 0.1 |
| (−)-Epicatechin | 3.0 ± 0.0 |
| Terminal units (%) | |
| (+)-Catechin | 13.5 ± 0.0 |
| (−)-Epicatechin | 55.0 ± 0.1 |
| mDP (°) | 1.5 ± 0.0 |
| Anthocyanins (ACY) n = 3 | |
| Cyanidin-3-O-spohoroside d | 314.6 ± 11.5 |
| Cyanidin-3-O-glucosyl-rutinoside d | 27.0 ± 0.7 |
| Cyanidin-3-O-glucoside | 152.0 ± 0.9 |
| Cyanidin-3-O-rutinoside d | 11.5 ± 0.1 |
| Pelargonidin-3-O-glucoside d | 4.5 ± 0.3 |
| Total ACY | 509.6 ± 10.9 |
| Total polyphenols (TPH) | 47,804.8 ± 1060.5 |
Values are expressed as the mean ± standard deviation (mg/100 g); n, number of measurements; mDP, mean degree of polymerisation; total EAC (total content of ellagic acid), the total content of ellagic acid and its conjugates. a The content of these substances was calculated based on the lambertianin C standard. b The content of these substances was calculated based on the sanguiin H-6 standard. c The contents of these substances were calculated based on ellagic acid standards. d The content of anthocyanins was calculated based on the cyanidin-3-O-glucoside standard.mDP: mean degree of polymerisation.
2.3. Animals and Experimental Design
The feeding experiment was conducted on 32 growing male Wistar rats aged eight weeks. The animals were allocated to four groups of eight rats each. The rats with initial body weights around 165.3 ± 1.43 g were individually housed in metabolic cages (Tecniplast Spa, Buguggiate, Italy) and a controlled environment (a 12-h light-dark cycle, a temperature of 21 ± 1 °C, relative humidity of 50–70% and 20 air changes per hour). For 12 weeks, each group had free access to tap water and was fed a modified version of the semipurified casein diet recommended for laboratory rodents by the American Institute of Nutrition [19]. With respect to the experimental diets, the control high-fat and low-fibre diet contained 23% lard fat and 3% cellulose (H), the experimental diets were a combination of the H diet with 0.64% of RE (HP diet) and the H diet with RE enriched with 3% of fructo-oligosaccharides (HPF diet) or 3% of pectins (HPP diet). The detailed composition of the diets, which were freely available to rats for the entire experimental period, is shown in Supplemental Table S1. The animal protocol was performed in accordance with European Union Directive (2010/63/EU) for animal experiments and the experiment was approved by the local Institutional Animal Care and Use Committee (Permission No. 51/2016; Olsztyn, Poland).
2.4. Collection of Biological Material and Analyticla Procedures
During the experiment, rats were monitored for feed intake and body weight (BW). After 12 weeks of experimental feeding, animals were anaesthetised with a mixture of ketamine and xylazine (100 mg and 10 mg/kg BW, respectively) in physiological salt according to the recommendations for anaesthesia of experimental animals. The body fat and lean masses of anaesthetised rats were determined using time-domain NMR Minispec LF90II analyzer (Bruker). After a laparotomy, blood samples were subsequently collected from the vena cava into heparinised tubes and low-speed centrifuged for 10 min at 350× g and 4 °C. Plasma samples were stored at −80 °C until assayed. Next, the small intestine, caecum, epididymal fat and liver were removed, weighed and frozen using liquid nitrogen or were used for further treatment. Samples of fresh small intestine and caecal digesta were collected, and the pH values were measured using a microelectrode and a pH/ION metre (model 301; Hanna Instruments, Vila do Conde, Portugal). Malondialdehyde (MDA) was analysed in the liver tissue according to the procedure developed by Botsoglou et al. [20]. The MDA content was determined spectrophotometrically at 532 nm and expressed in µg of MDA per g of liver tissue. Liver lipids were extracted according to the method of Folch et al. [21]. The liver cholesterol and triglyceride concentrations were determined spectrophotometrically in the extracted lipid phase using commercial kits (Cholesterol DST, Triglycerides DST, Alpha Diagnostics, Ltd., San Antonio, TX, USA).
2.5. Caecal Microbial SCFAs
Caecal digesta samples after storage at −80 °C were subjected to SCFA analysis using a gas chromatograph (GC) (Shimadzu GC-2010, Kyoto, Japan) according to previously described method [22]. Briefly, caecal samples (0.2 g) were mixed with 0.2 mL formic acid, diluted with deionised water and centrifuged at 7211× g for 10 min. The supernatant was loaded onto a capillary column (SGE BP21, 30 m × 0.53 mm; SGE Europe Ltd., Milton Keynes, UK) using an on-column injector. The initial oven temperature was 85 °C and was raised to 180 °C by 8 °C/min and held there for 3 min. The temperatures of the flame ionisation detector was adjusted to 180 °C and the injection port 85 °C. The sample volume for GC analysis was 1 μL. Pure acetic, propionic, butyric, isobutyric, isovaleric and valeric acids were obtained from Sigma Co. (Poznan, Poland); a mixture of these acids was used to create a standard plot and then to calculate the amount of individual acids. To maintain the calibration every fifth GC run of samples contained pure acids.
2.6. Plasma Lipid Profile and Inflammatory Markers
The plasma concentration of cholesterol (total and its HDL), triglycerides and the plasma activities of aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) were determined using an automatic biochemical analyzer (Pentra C200, Horiba, Tokyo, Japan). The non-HDL fraction was calculated according to the following formula: TC-HDL-cholesterol. To measure the concentration of interleukin 6 (IL-6), interleukin 10 (IL-10) and tumour necrosis factor α (TNFα) in the plasma, validated rat ELISA kits were used (Single Analyte ELISArray Kit, Qiagen, Frederick, MD, USA).
2.7. Liver Histopathology
The liver was flushed with PBS and dried, and the tissue was immersed for 7 days in a 10% solution of buffered formalin. Liver tissues were embedded in paraffin blocks. Paraffin sections (1–2 μm) were cut with a Reichert’s microtome, and tissue fragments were passed through increasing concentrations of alcohol solutions, acetone and xylene (dewaxed). The preparations were stained with haematoxylin and eosin (H&E; Merck, Darmstadt, Germany) according to the method described by Fischer et al. [23]. The tissue sections were evaluated, and images were taken by standard light microscopy using a computer program for image analysis, B-cell and an Olympus BX50 microscope with a digital camera (Olympus Co., Tokyo, Japan). Hepatic steatosis was evaluated as follows: grade 0, no fat; grade 1, steatosis occupying less than 33% of the hepatic parenchyma; grade 2, 34%–66% of the hepatic parenchyma; grade 3, more than 66% of the hepatic parenchyma. For inflammatory cell infiltration: grade 0: none; grade 1, 1–2 foci/field; grade 2, 3–4 foci/field; grade 3, more than 4 foci/field [24]. Ballooning was graded as minimal, mild or marked [25].
2.8. Hepatic Gene Expression
qRT-PCR was performed according to the previously described method [26]. Briefly, RNA was extracted from the liver using TRI Reagent solution (Thermo Fisher Scientific) according to the manufacturer’s instructions. β-actin was selected as a reference gene. The levels of peroxisome proliferator-activated receptor gamma (PPARγ), peroxisome proliferator-activated receptor alpha (PPARα), sterol regulatory element-binding protein 1 (SREBP-1c) and angiopoietin-like 4 (ANGPTL4) mRNA expression single tube TaqMan® Gene Expression Assays (Life Technologies, Carlsbad, CA, USA) were used. Amplification was performed using a 7900HT Fast Real-Time PCR System. The mRNA expression levels of PPARγ, PPARα, SREBP-1c and ANGPTL4 were normalised to β-actin.
2.9. Statistical Analysis
The results are presented as the means and the standard errors of the means (SEMs). Statistica software (StatSoft Corp., Krakow, Poland) was used to determine whether the variables differed among the treatment groups. The statistical analysis was conducted using one-way analysis of variance (ANOVA) and Duncan’s multiple range post hoc test. If the variance was unequal, a Kruskal–Wallis ANOVA by ranks was used followed by Dunn’s post hoc test with Bonferroni correction. Differences were considered significant at p < 0.05.
3. Results
The growth parameters and basic indicators of the gastrointestinal function of rats fed the experimental diets are shown in Table 2. After 12 weeks of experimental feeding, the diet intake, body weight, and fat, lean and epididymal fat contents of rats were comparable among all groups. In the experimental groups, the tissue mass of the small intestine and the pH value of its digesta were at the same level as those of the control H diet group. However, the tissue mass of the caecum considerably increased in the HPF and HPP groups compared to the control H group. Among all experimental groups, only rats from the HP and HPP groups had reduced pH values in the caecum, but the highest acidification was in the HPP group. The combination of RE with different sources of nondigestible saccharides attenuated microbial SCFA production in the caecum. The lowest production of SCFAs in the caecum was reported in the HPF group. This group also had the lowest concentrations of acetic and butyric acid in the caecum. The concentration of caecal PSCFA was reduced in both groups, but the decrease was more pronounced in rats from the HPP group. Nevertheless, in all groups, the experimental diets did not affect the SCFA profile.
Table 2.
Growth parameters and basic indicators of gastrointestinal tract function in the rats fed the experimental diets.
| Parameters | Groups | ANOVA p Value | |||
|---|---|---|---|---|---|
| H | HP | HPF | HPP | ||
| Diet intake (g/day) | 25.32 ± 2.11 | 24.82 ± 1.56 | 23.92 ± 2.88 | 25.02 ± 1.31 | NS |
| Final body weight (g) | 466.64 ± 11.91 | 472.74 ± 14.22 | 465.43 ± 9.21 | 457.94 ± 15.37 | NS |
| Final body fat (g) | 137.62 ± 4.42 | 142.69 ± 9.98 | 130.05 ± 6.46 | 131.48 ± 5.44 | NS |
| Final body lean (g) | 253.43 ± 6.82 | 254.48 ± 5.53 | 258.05 ± 5.19 | 254.96 ± 6.65 | NS |
| Epididymal fat (g) | 20.54 ± 1.37 | 21.62 ± 2.17 | 18.64 ± 1.45 | 19.31 ± 1.37 | NS |
| Small intestine: | |||||
| Tissue mass (g) | 6.14 ± 0.35 | 6.62 ± 0.19 | 6.99 ± 0.27 | 6.72 ± 0.16 | NS |
| pH | 7.01 ± 0.08 | 6.83 ± 0.08 | 6.81 ± 0.078 | 6.71 ± 0.10 | NS |
| Caecum: | |||||
| Tissue mass (g) | 0.56 ± 0.03 b | 0.62 ± 0.02 ab | 0.69 ± 0.03 a | 0.66 ± 0.02 a | <0.01 |
| pH | 7.08 ± 0.08 a | 6.91 ± 0.09 ab | 7.08 ± 0.08 a | 6.70 ± 0.13 b | <0.05 |
| SCFA (µmol/g digesta) | |||||
| Acetic acid | 26.38 ± 1.85 a | 27.13 ± 4.12 a | 17.28 ± 1.71 b | 21.95 ± 1.79 ab | <0.05 |
| Propionic acid | 7.84 ± 0.34 | 8.03 ± 0.41 | 5.83 ± 0.60 | 6.95 ± 0.87 | 0.054 |
| Iso-butyric acid | 0.63 ± 0.04 a | 0.60 ± 0.07 ab | 0.44 ± 0.04 b | 0.40 ± 0.06 c | <0.05 |
| Butyric acid | 1.86 ± 0.19 a | 1.80 ± 0.24 a | 0.93 ± 0.17 b | 1.52 ± 0.12 a | <0.01 |
| Iso-valeric acid | 0.78 ± 0.05 a | 0.78 ± 0.06 a | 0.57 ± 0.05 b | 0.55 ± 0.06 b | <0.01 |
| Valeric acid | 0.62 ± 0.03 a | 0.58 ± 0.05 ab | 0.44 ± 0.05 b | 0.26 ± 0.05 c | <0.001 |
| SCFA profile (%) | |||||
| Acetic acid | 68.90 ± 0.95 | 67.92 ± 2.72 | 67.64 ± 1.33 | 69.63 ± 1.87 | NS |
| Propionic acid | 20.82 ± 0.85 | 21.79 ± 1.71 | 22.84 ± 1.19 | 21.81 ± 1.69 | NS |
| Butyric acid | 4.85 ± 0.37 | 4.66 ± 0.40 | 3.60 ± 0.54 | 4.90 ± 0.32 | NS |
| Total PSCFAs | 2.03 ± 0.10 a | 1.98 ± 0.16 a | 1.48 ± 0.15 ab | 1.22 ± 0.18 b | <0.01 |
| Total SCFAs | 38.12 ± 2.24 a | 38.94 ± 4.62 a | 25.53 ± 2.38 b | 31.64 ± 2.65 ab | <0.05 |
Values are the mean ± SEM; n = 8. a,b,c Mean values not sharing the same superscript letter within a row (a, b or c) are different at p < 0.05 in a post hoc test. (Dunn’s test). H, control high-fat diet; HP, control high-fat diet enriched with raspberry polyphenol extract; HPF, control high-fat diet enriched with raspberry polyphenol extract and fructo-oligosaccharides; HPP, control high-fat diet enriched with raspberry polyphenol extract and pectin. PSCFAs, putrefactive short-chain fatty acids (the sum of iso-butyric, iso-valeric and valeric acids); SCFAs, short-chain fatty acids.
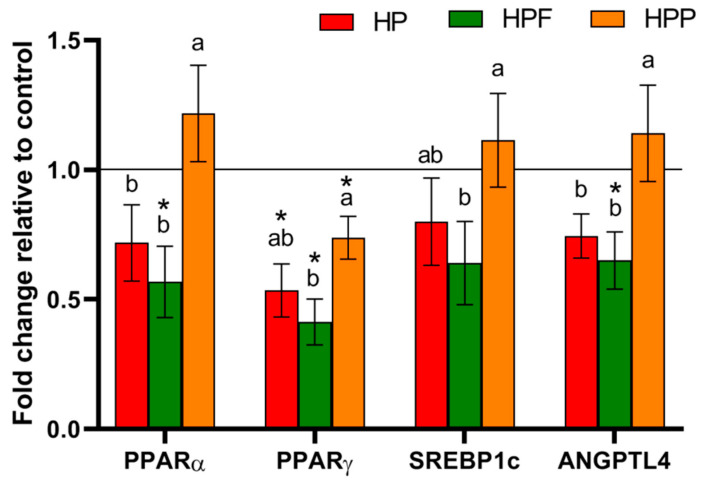
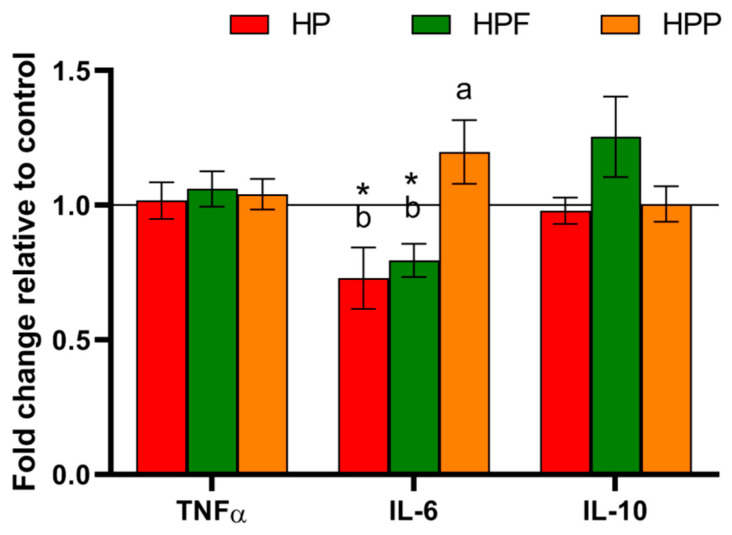
The major parameters of lipid homeostasis are shown in Table 3 and Table 4. The strongest effect on lipid metabolism was observed in rats from the HPF group. Among all experimental groups, the TGs in the plasma and liver were considerably reduced in the HPF group compared to the control H group. Additionally, the level of liver cholesterol was the lowest in the HPF group. In rats from the HPF and HPP groups, the liver fat content was considerably decreased, whereas liver mass was increased in rats from the HPF group compared to those from the control H group. The experimental diets also affected molecular factors associated with lipid metabolism in the liver (Figure 1). A comparison of all experimental groups showed that the expression of PPARα and ANGPTL4 in the liver was lower in rats from the HP and HPF groups than in rats from the HPP group. Furthermore, in the HPF group observed the lowest liver expression of the PPARγ and SREBP1c. However, when compared to the control H group, the liver expressions of PPARα and ANGPTL4 were reduced only in rats from the HPF group, while PPARγ expression was considerably reduced in all experimental groups. Disturbances in liver lipid metabolism affected by an obesogenic diet might be associated with observed changes in selected parameters of oxidative stress and inflammation (Table 4 and Figure 2). In comparison to the control H group, only the HP and HPF groups showed reduced MDA levels in the liver. Moreover, compared with rats from the control H group, rats from the HP and HPF groups showed considerable decreased AST plasma levels, but the decrease was more pronounced in the HPF group. Among all examined groups, the plasma level of IL-6 was the lowest in the HP and HPF groups.
Table 3.
Plasma lipid profile of rats fed the experimental diets.
| Parameters | Groups | ANOVA p Value | |||
|---|---|---|---|---|---|
| H | HP | HPF | HPP | ||
| TG (mmol/L) | 1.05 ± 0.10 a | 0.89 ± 0.11 a | 0.64 ± 0.05 b | 0.70 ± 0.06 a | <0.05 |
| TC (mmol/L) | 2.99 ± 0.24 | 2.69 ± 0.20 | 2.97 ± 0.21 | 2.40 ± 0.30 | NS |
| HDL (mmol/L) | 0.42 ± 0.02 | 0.44 ± 0.02 | 0.39 ± 0.03 | 0.36 ± 0.03 | NS |
| non-HDL (mmol/L) | 2.58 ± 0.25 | 2.25 ± 0.20 | 2.59 ± 0.22 | 2.04 ± 0.31 | NS |
Values are the mean ± SEM; n = 8. HDL, HDL-cholesterol, LDL, LDL-cholesterol; TC, total cholesterol; TG, triacylglycerols. a,b Mean values not sharing the same superscript letter within a row (a or b) are different at p < 0.05 in a post hoc test. (Dunn’s test). H, control high-fat diet; HP, control high-fat diet enriched with raspberry polyphenol extract; HPF, control high-fat diet enriched with raspberry polyphenol extract and fructo-oligosaccharides; HPP, control high-fat diet enriched with raspberry polyphenol extract and pectin.
Table 4.
Liver indicators of inflammation, oxidative stress and lipid metabolism in rats fed the experimental diets.
| Parameters | Groups | ANOVA p Value | |||
|---|---|---|---|---|---|
| H | HP | HPF | HPP | ||
| Liver (g) | 15.93 ± 0.50 b | 17.10 ± 0.75 ab | 18.71 ± 0.51 a | 16.21 ± 0.71 b | <0.05 |
| Fat content (%) | 34.45 ± 1.47 a | 31.13 ± 1.87 a | 27.25 ± 1.24 b | 27.57 ± 1.27 b | <0.01 |
| Cholesterol (mg/g liver) | 10.38 ± 0.41 a | 8.73 ± 1.02 a | 7.63 ± 0.76 b | 8.54 ± 0.99 a | <0.05 |
| TG (mg/g liver) | 19.42 ± 0.85 a | 16.70 ± 1.80 ab | 14.81 ± 0.48 b | 17.75 ± 1.03 ab | <0.05 |
| MDA (ng/g liver) | 781.1 ± 25.4 a | 680.6 ± 21.0 b | 642.5 ± 16.9 b | 752.1 ± 35.9 a | <0.01 |
| Plasma indicators (U/L) | |||||
| AST | 204.1 ± 12.7 a | 141.9 ± 18.9 bc | 134.6 ± 8.9 c | 167.6 ± 25.2 ab | <0.05 |
| ALT | 122.2 ± 45.2 | 121.7 ± 34.1 | 108.7 ± 27.0 | 97.1 ± 29.5 | NS |
| ALP | 92.8 ± 8.8 | 85.6 ± 10.5 | 104.2 ± 3.9 | 89.7 ± 5.2 | NS |
Values are the mean ± SEM; n = 8. AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; MDA, malondialdehyde; TG, triglyceride. a,b,c Mean values not sharing the same superscript letter within a row (a, b or c) are different at p < 0.05 in a post hoc test. (Dunn’s test). H, control high-fat diet; HP, control high-fat diet enriched with raspberry polyphenol extract; HPF, control high-fat diet enriched with raspberry polyphenol extract and fructo-oligosaccharides; HPP, control high-fat diet enriched with raspberry polyphenol extract and pectin.
Figure 1.
mRNA expression of selected factors associated with lipid metabolism in the livers of rats fed experimental diets. The values are the means ± SEMs. a,b Mean values not sharing the same superscript letter (a or b) are different at p < 0.05 in a post hoc test (Dunn’s test). * Mean values are significantly different in comparison to the control H group, fed a high-fat diet. HP, control high-fat diet enriched with raspberry polyphenol extract; HPF, control high-fat diet enriched with raspberry polyphenol extract and fructo-oligosaccharides; HPP, control high-fat diet enriched with raspberry polyphenol extract and pectin. PPARα, peroxisome proliferator-activated receptor alpha; PPARγ, peroxisome proliferator-activated receptor gamma; SREBP-1c, sterol regulatory element-binding protein 1; ANGPTL4, angiopoietin-like 4.
Figure 2.
Inflammatory cytokines in the plasma of rats fed the experimental diets. The values are the means ± SEMs. a,b Mean values not sharing the same superscript letter (a or b) are different at p < 0.05 in a post hoc test (Dunn’s test). * Mean values are significantly different in comparison to the control H group, fed a high-fat diet. HP, control high-fat diet enriched with raspberry polyphenol extract; HPF, control high-fat diet enriched with raspberry polyphenol extract and fructo-oligosaccharides; HPP, control high-fat diet enriched with raspberry polyphenol extract and pectin. TNFα, tumour necrosis factor α; IL-6, interleukin 6; IL-10, interleukin 10.
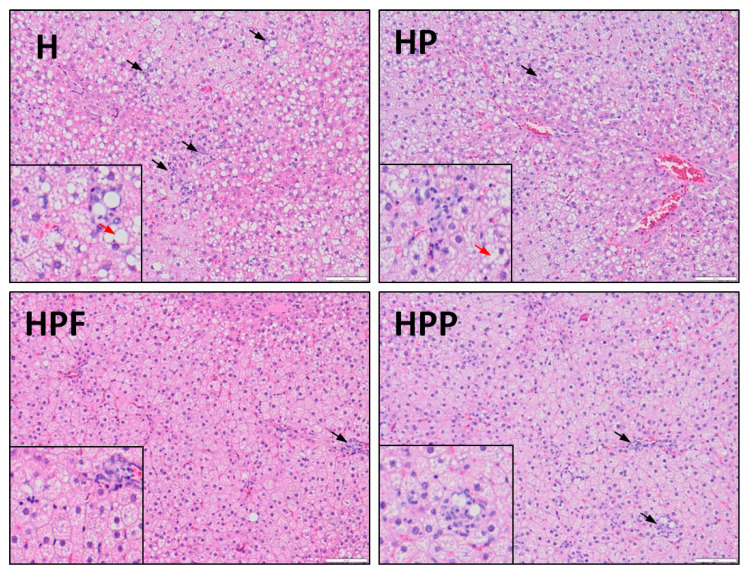
Changes in lipid metabolism and inflammatory factors were also observed in the analyses of liver histological characteristics (Table 5, Figure 3). The liver histological preparations from the control H group showed that an obesogenic diet promoted the development of NAFLD by increasing steatosis, hepatocyte ballooning, lobular and portal inflammation. All experimental groups with RE showed mitigated NAFL-related disorders to different extents. In the HP group, hepatocyte ballooning and lobular and portal inflammation were considerably reduced, but the decrease was more pronounced in the HPF and HPP groups. In these groups, the stages of steatosis were also significantly lower than those in the H group. Among the experimental groups, the most favourable effects on liver lobular and portal inflammation were reported in the HPF group.
Table 5.
Liver histological characteristics of rats fed the experimental diets.
| Parameters | Stage 1 | ANOVA p Value | ||
|---|---|---|---|---|
| Group | Low | High | ||
| Steatosis, n (%) | H a | 0 (0) | 8 (100) | <0.01 |
| HP a | 2 (25) | 6 (75) | ||
| HPF b | 7 (87.5) | 1 (12.5) | ||
| HPP b | 6 (75) | 2 (25) | ||
| Ballooning, n (%) | H a | 0 (0) | 8 (100) | <0.01 |
| HP b | 4 (50) | 4 (50) | ||
| HPF c | 8 (100) | 0 (0) | ||
| HPP c | 8 (100) | 0 (0) | ||
| Lobular inflammation, n (%) | H a | 0 (0) | 8 (100) | <0.05 |
| HP b | 3 (37.5) | 5 (62.5) | ||
| HPF c | 7 (87.5) | 1 (12.5) | ||
| HPP ab | 2 (25) | 6 (75) | ||
| Portal inflammation, n (%) | H a | 1 (12.5) | 7 (87.5) | <0.05 |
| HP b | 3 (37.5) | 5 (62.5) | ||
| HPF c | 6 (75) | 2 (25) | ||
| HPP bc | 4 (50) | 4 (50) | ||
n = 8 in each group. a,b,c Mean values not sharing the same superscript letter (a, b or c) are different at p < 0.05 in a post hoc test (Dunn’s test). H, control high-fat diet; HP, control high-fat diet enriched with raspberry polyphenol extract; HPF, control high-fat diet enriched with raspberry polyphenol extract and fructo-oligosaccharides; HPP, control high-fat diet enriched with raspberry polyphenol extract and pectin. 1 Low = 0 or 1 stage; High = 2 or 3 stage.
Figure 3.
Hepatic histology in rats fed the experimental diets. H, control high-fat diet; HP, control high-fat diet enriched with raspberry polyphenol extract; HPF, control high-fat diet enriched with raspberry polyphenol extract and fructo-oligosaccharides; HPP, control high-fat diet enriched with raspberry polyphenol extract and pectin. (H) Liver with steatosis. Several inflammatory foci within the hepatic lobule (black arrow) and enhanced hepatocyte ballooning (red arrow). (HP) Reduced amount of inflammatory cells (black arrow) and mild ballooning of cells. Hepatocytes have rounded contours with clear reticular cytoplasm (red arrow). (HPF) There is one area of inflammatory cells (black arrow), the cytoplasm is pink and granular, and liver cells have sharp angles. (HPP) There are several inflammatory foci within the hepatic lobule (black arrow), and the shape of liver cells is quite similar to that observed in the HPF group. The liver samples were stained with haematoxylin and eosin at 20× and 40× (small image) magnification.
4. Discussion
The results of the present study indicated that RE obtained from raspberry pomace was a valuable source of polyphenols and consisted of 47.8 g/100 g phenolic compounds, most of which were sanguiin H-6, lambertianin C (ellagitannins), proanthocyanidins (flavanols), cyanidin-3-o-spohoroside and cyaniding-3-o-glucoside (anthocyanins). Other studies on polyphenolic raspberry extracts have reported similar polyphenol profiles [6]; however, the range of the total phenolic contents is very wide, from 37.6 to 63.3 g/100 g [9,27,28]. The profiles and contents of these bioactive substances in extracts from raspberries are strongly influenced by genotypic and extrinsic factors, such as agricultural practices, seasonal differences, developmental stage and extraction methods [27].
Supplementation with raspberry polyphenols may play important roles in the regulation of bacterial fermentation processes in the hindgut and liver lipid metabolism [10,28,29]. These polyphenols interact with intestinal microbiota [30] and thus affect their activity and the production of SCFAs. The SCFAs produced by intestinal microbiota exert protective effects on metabolic disorders [31]; however, some studies also report that these fatty acids might promote the development of obesity and thus NAFL-related disorders [32,33]. Current nutritional studies show that obesity might be associated with high levels of SCFAs but not gut microbiota richness at the phylum level [34]. In this study, the addition of the RE to the obesogenic diet had no effect on the production of bacterial SCFAs; however, when polyphenolic extract was added together with the examined nondigestible saccharides, especially FOSs, the concentration of SCFAs was considerably reduced in the caecum. Our previous study on rats [14] showed that the combination of strawberry polyphenols and FOSs increased the metabolism of ellagitannins and thus affected the activity of the microbiota by lowering the production of SCFAs in the caecum. Furthermore, the mechanisms affecting the bacterial fermentation process in the gastrointestinal tract might be associated with the absorbed polyphenols, which might be secreted together with bile acids into the intestine [11]. Some studies have reported that ellagitannins may decrease enterobacterial growth or exert antimicrobial effects against probiotics [30,35]. Additionally, anthocyanins might have antibacterial properties against bacteria living in the gastrointestinal tract [29,36]. Moreover, the combination of RE with both of the nondigestible saccharides favourably reduced the bacterial production of PSCFAs in the caecum and increased the mass of caecal tissue. This effect might be related to decreased anaerobic bacterial polypeptide and amino acid fermentation [37]. In addition to regulatory effects of raspberry polyphenols, the supplementation with FOSs and PECs might also exert effects on the intestinal microbiota activity and lipid metabolism. These nondigestible saccharides have different chemical structures and thus can affect the balance of gut microbiota to a different extent. The feeding experiment on mice fed with different mixtures of nondigestible saccharides showed that FOSs increased the SCFA production more effectively than PECs [13]. In the present study, however, an opposite effect was observed, therefore it might be assumed that the raspberry polyphenols considerably modulated effect of examined nondigestible saccharides. Nakamura et al. [38] in their study on mice showed that the dietary addition of FOSs (2.5%) either inhibited the uptake of monoglycerides or nonesterified fatty acids in the small intestine, or altered gastrointestinal function related to fat absorption, thus reducing the plasma TGs levels and affected lipid metabolism in the liver. Additionally, dietary supplementation with PECs (10%) can regulate lipid metabolism through gel formation and binding the bile acids and micelle components in the gut, such as monoglycerides, free fatty acids and cholesterol [39]. In this experiment, only supplementation with FOSs and raspberry polyphenols considerably reduced the plasma TGs. Such an effect might be associated with the different dietary amount of nondigestible saccharides used in the present study (3%). Apart from local intestinal effects, supplementation with raspberry polyphenols and examined nondigestible saccharides favourably mitigated the liver metabolic disorders caused by an obesogenic diet. Currently, the accepted pathophysiological model for NAFLD is the “two hits” model. The first stage is associated with the accumulation of fat and TGs in the liver as a result of changes in the influx, synthesis, oxidation and transport of fatty acids. The second stage, triggered by the first hit, includes oxidative imbalance and induction of proinflammatory cytokines as a result of mitochondrial dysfunction, lipid peroxidation and activation of inflammatory pathways [40,41]. In this study, the combination of FOSs and RE exerted the most favourable effect on NAFL-related disorders. This diet considerably reduced plasma TGs, liver cholesterol, TGs and fat content. Moreover, histological analyses showed that this dietary combination exerted the most favourable effect on liver through the reduction of liver steatosis and hepatocyte ballooning. The observed effects might be partially related to considerably decreased expression of ANGPTL4 and PPARγ in the liver. ANGPTL4 is responsible for the inhibition of lipoprotein lipase (LPL), thereby regulating the hydrolysis of lipoprotein and TGs and the uptake of fatty acids into tissue [42], whereas hepatic PPARγ expression promotes lipid uptake, triacylglycerol storage, and lipid droplet formation [43]. A similar effect was observed in a nutritional study on mice fed a high-fat diet [28], where daily oral administration of raspberry polyphenols upregulated hepatic LPL and reduced fat accumulation. Another gene that is also important in the regulation of liver lipid metabolism is PPARα. This gene is linked with mechanisms of liver fatty acid catabolism [43]. However, supplementation with raspberry polyphenols and FOSs reduced the hepatic expression of PPARα; therefore, it might be assumed that the main mechanisms responsible for the regulation of NAFL-related disorders in rats from HPF group are associated with the downregulation of PPARγ and ANGPTL4.
The disorders caused by an obesogenic diet affect numerous hepatic lipid pathways, consequently increasing hepatic inflammation, oxidative stress and fibrosis [44]. Favourable effects of RE on liver lipid metabolism may also explain mitigation of the liver inflammatory processes and lipid peroxidation caused by an obesogenic diet. Diets with RE alone or with FOSs lowered the level of plasma IL-6, the activity of AST and the level of MDA in the liver. Furthermore, the most effective reductions in liver lobular and portal inflammation were observed when the diet was enriched with RE and FOSs. Kang et al. [45], in a nutritional study on C57BL/6 male mice fed a high-fat diet, also showed that the addition of ellagitannins from raspberry seed flour to the diet significantly decreased hepatic oxidative stress and thus reduced proinflammatory gene expression and macrophage infiltration.
5. Conclusions
This nutritional study showed that the effect of raspberry polyphenols against NAFL-related disorders might be enhanced with FOSs and PECs. It was reported that this dietary combination considerably reduced microbial fermentation in the caecum. Among the examined nondigestible saccharides, the combination of FOSs with RE exerted the most favourable effects on lipid metabolism and thus inflammatory processes and lipid peroxidation in the liver. The observed effects might be partially related to decreased hepatic expressions of PPARγ and ANGPTL4. Surprisingly, in the rats fed the diet with PECs and the examined extract, the expression levels of all examined genes regulating liver lipid metabolism were higher than in rats fed diet with FOSs and RE. These data suggest that the activity of raspberry polyphenols against liver disorders caused by an obesogenic diet might be driven by supplementation with specific dietary fibres. Collectively, the results indicated that the dietary combination of RE with FOSs exerted more favourable effects than the combination with PECs; therefore, dietary addition of RE together with FOSs might be considered a dietary compound that effectively mitigates NAFL-related disorders induced by an obesogenic diet.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/13/3/833/s1, Table S1: Composition of the group-specific diets.
Author Contributions
Conceptualisation, B.F.; methodology, B.F., J.J., A.J. and M.S.; software, B.F.; validation, B.F. and J.J.; formal analysis, B.F.; investigation, B.F.; resources, B.F.; data curation, B.F., J.J. and A.J.; writing—original draft preparation, B.F.; writing—review and editing, B.F.; visualisation, B.F.; supervision, B.F.; project administration, B.F.; funding acquisition, B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science Center, Poland (decision UMO-2018/31/N/NZ9/02196).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Local Ethics Committee in Olsztyn (51/2016) on 21 December 2016.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astrup A., Dyerberg J., Elwood P., Hermansen K., Hu F.B., Jakobsen M.U., Kok F.J., Krauss R.M., Lecerf J.M., LeGrand P., et al. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: Where does the evidence stand in 2010? Am. J. Clin. Nutr. 2011;93:684–688. doi: 10.3945/ajcn.110.004622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Italian Association for the Study of the Liver (AISF) AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig. Liver Dis. 2017;49:471–483. doi: 10.1016/j.dld.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 4.Abenavoli L., Milic N., Luzza F., Boccuto L., De Lorenzo A. Polyphenols treatment in patients with nonalcoholic fatty liver disease. J. Transl. Int. Med. 2017;5:144–147. doi: 10.1515/jtim-2017-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez-Montes de Oca A., Julián M.T., Ramos A., Puig-Domingo M., Alonso N. Microbiota, fiber, and NAFLD: Is there any connection? Nutrients. 2020;12:3100. doi: 10.3390/nu12103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X., Sandhu A., Edirisinghe I., Burton-Freeman B. An exploratory study of red raspberry (Rubus idaeus L.) (poly)phenols/metabolites in human biological samples. Food Funct. 2018;21:806–818. doi: 10.1039/C7FO00893G. [DOI] [PubMed] [Google Scholar]
- 7.Liu M., Li Q., Weber C., Lee C., Brown J., Liu R. Antioxidant and antiproliferative activities of raspberries. J. Agric. Food Chem. 2002;50:2926–2930. doi: 10.1021/jf0111209. [DOI] [PubMed] [Google Scholar]
- 8.Bobinaitė R., Viškelis P., Šarkinas A., Venskutonis P.R. Phytochemical composition, antioxidant and antimicrobial properties of raspberry fruit, pulp, and marc extracts. CyTA J. Food. 2013;11:334–342. doi: 10.1080/19476337.2013.766265. [DOI] [Google Scholar]
- 9.Fotschki B., Juśkiewicz J., Sójka M., Jurgoński A., Zduńczyk Z. Ellagitannins and flavan-3-ols from raspberry pomace modulate caecal fermentation processes and plasma lipid parameters in rats. Molecules. 2015;21:22848–22862. doi: 10.3390/molecules201219878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fotschki B., Laparra J.M., Sójka M. Raspberry polyphenolic extract regulates obesogenic signals in hepatocytes. Molecules. 2018;23:2103. doi: 10.3390/molecules23092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barberan F.A., Garcia-Conesa M.T., Larrosa M., Cerdá B., Gonzalez-Barrio R., Bermudez-Soto M.J., González-Sarrías A., Espín J.C. Bioavailability, metabolism, and bioactivity of food ellagic acid and related polyphenols. Recent Adv. Polyphenol. Res. 2008;1:263–277. [Google Scholar]
- 12.Jurgoński A., Juśkiewicz J., Zduńczyk Z. Comparative effects of different dietary levels of cellulose and fructooligosaccharides on fermentative processes in the caecum of rats. J. Anim. Feed Sci. 2008;17:88–99. doi: 10.22358/jafs/66473/2008. [DOI] [Google Scholar]
- 13.Peng X., Li S., Luo J., Wu X., Liu L. Effects of dietary fibers and their mixtures on short chain fatty acids and microbiota in mice guts. Food Funct. 2013;4:932–938. doi: 10.1039/c3fo60052a. [DOI] [PubMed] [Google Scholar]
- 14.Fotschki B., Milala J., Jurgoński A., Karlińska E., Zduńczyk Z., Juśkiewicz J. Strawberry ellagitannins thwarted the positive effects of dietary fructooligosaccharides in rat cecum. J. Agric. Food Chem. 2014;62:5871–5880. doi: 10.1021/jf405612a. [DOI] [PubMed] [Google Scholar]
- 15.Nobili V., Alkhouri N., Bartuli A., Manco M., Lopez R., Alisi A., Feldstein A.E. Severity of liver injury and atherogenic lipid profile in children with nonalcoholic fatty liver disease. Pediatr. Res. 2010;67:665–670. doi: 10.1203/PDR.0b013e3181da4798. [DOI] [PubMed] [Google Scholar]
- 16.Juśkiewicz J., Milala J., Jurgoński A., Król B., Zduńczyk Z. Consumption of polyphenol concentrate with dietary fructo-oligosaccharides enhances cecal metabolism of quercetin glycosides in rats. Nutrition. 2010;27:351–357. doi: 10.1016/j.nut.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Klewicka E., Sójka M., Klewicki R., Kołodziejczyk K., Lipińska L., Nowak A. Ellagitannins from raspberry (Rubus idaeus L.) fruit as natural inhibitors of Geotrichum candidum. Molecules. 2016;21:908. doi: 10.3390/molecules21070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sójka M., Klimczak E., Macierzyński J., Kołodziejczyk K. Nutrient and polyphenolic composition of industrial strawberry press cake. Eur. Food Res. Technol. 2013;237:995–1007. doi: 10.1007/s00217-013-2070-2. [DOI] [Google Scholar]
- 19.Reeves P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997;127:838–841. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 20.Botsoglou N.A., Fletouris D.J., Papageorgiou G.E., Vassilopoulos V.N., Mantis A.J., Trakatellis A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994;42:1931–1937. doi: 10.1021/jf00045a019. [DOI] [Google Scholar]
- 21.Folch J., Lees M., Sloane-Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 22.Jurgoński A., Juśkiewicz J., Fotschki B., Kołodziejczyk K., Milala J., Kosmala M., Grzelak-Błaszczyk K., Markiewicz L. Metabolism of strawberry mono- and dimeric ellagitannins in rats fed a diet containing fructo-oligosaccharides. Eur. J. Nutr. 2017;56:853–864. doi: 10.1007/s00394-015-1133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer A.H., Jacobson K.A., Rose J., Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008:pdb.prot4986. doi: 10.1101/pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 24.Xu Z.J., Fan J.G., Ding X.D., Qiao L., Wang G.L. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig. Dis. Sci. 2010;55:931–940. doi: 10.1007/s10620-009-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunt E.M., Janney C.G., Di Bisceglie A.M., Neuschwander-Tetri B.A., Bacon B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 26.Opyd P.M., Jurgoński A., Juśkiewicz J., Fotschki B., Koza J. Comparative effects of native and defatted flaxseeds on intestinal enzyme activity and lipid metabolism in rats fed a high-fat diet containing cholic acid. Nutrients. 2018;10:1181. doi: 10.3390/nu10091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton-Freeman B.M., Sandhu A.K., Edirisinghe I. Red raspberries and their bioactive polyphenols: Cardiometabolic and neuronal health links. Adv. Nutr. 2016;1:44–65. doi: 10.3945/an.115.009639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kshatriya D., Li X., Giunta G.M., Yuan B., Zhao D., Simon J.E., Wu Q., Bello N.T. Phenolic-enriched raspberry fruit extract (Rubus idaeus) resulted in lower weight gain, increased ambulatory activity, and elevated hepatic lipoprotein lipase and heme oxygenase-1 expression in male mice fed a high-fat diet. Nutr. Res. 2019;68:19–33. doi: 10.1016/j.nutres.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duda-Chodak A., Tarko T., Satora P., Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015;54:325–341. doi: 10.1007/s00394-015-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puljula E., Walton G., Woodward M.J., Karonen M. Antimicrobial activities of ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules. 2020;25:3714. doi: 10.3390/molecules25163714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin H.V., Frassetto A., Kowalik E.J., Jr., Nawrocki A.R., Lu M.M., Kosinski J.R., Hubert J.A., Szeto D., Yao X., Forrest G., et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry R., Peng L., Barry N., Cline G., Zhang D., Cardone R., Petersen K.F., Kibbey R.G., Goodman A.L., Shulman G.I. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tirosh A., Calay E.S., Tuncman G., Claiborn K.C., Inouye K.E., Eguchi K., Alcala M., Rathaus M., Hollander K.S., Ron I., et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci. Transl. Med. 2019;11:eaav0120. doi: 10.1126/scitranslmed.aav0120. [DOI] [PubMed] [Google Scholar]
- 34.Larrosa M., González-Sarrías A., Yáñez-Gascón M.J., Selma M.V., Azorín-Ortuño M., Toti S., Tomás-Barberán F., Dolara P., Espín J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Kim K.N., Yao Y., Ju S.Y. Short chain fatty acids and fecal microbiota abundance in humans with obesity: A systematic review and meta-Analysis. Nutrients. 2019;11:2512. doi: 10.3390/nu11102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandes I., Faria A., Calhau C., de Freitas V., Mateus N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods. 2014;7:54–66. doi: 10.1016/j.jff.2013.05.010. [DOI] [Google Scholar]
- 37.Juśkiewicz J., Zduńczyk Z., Żary-Sikorska E., Król B., Milala J., Jurgoński A. Effect of the dietary polyphenolic fraction of chicory root, peel, seed and leaf extracts on caecal fermentation and blood parameters in rats fed diets containing prebiotic fructans. Br. J. Nutr. 2011;105:710–720. doi: 10.1017/S0007114510004344. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura Y., Natsume M., Yasuda A., Ishizaka M., Kawahata K., Koga J. Fructooligosaccharides suppress high-fat diet-induced fat accumulation in C57BL/6J mice. Biofactors. 2017;43:145–151. doi: 10.1002/biof.147. [DOI] [PubMed] [Google Scholar]
- 39.Bray J.K., Chiu G.S., McNeil L.K., Moon M.L., Wall R., Towers A.E., Freund G.G. Switching from a high-fat cellulose diet to a high-fat pectin diet reverses certain obesity-related morbidities. Nutr. Metab. 2018;15:55. doi: 10.1186/s12986-018-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rolo A.P., Teodoro J.S., Palmeira C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Than N.N., Newsome P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis. 2015;239:192–202. doi: 10.1016/j.atherosclerosis.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Ito Y., Nagaike H. Increased angiopoietin like protein 4 (Angptl4) is associated with higher concentration of LDL-triglycerides in type 2 Diabetes. Atherosclerosis. 2019;287:80–81. doi: 10.1016/j.atherosclerosis.2019.06.232. [DOI] [Google Scholar]
- 43.Wang Y., Nakajima T., Gonzalez F.J., Tanaka N. PPARs as metabolic regulators in the liver: Lessons from liver-specific PPAR-null mice. Int. J. Mol. Sci. 2020;21:2061. doi: 10.3390/ijms21062061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byrne C.D., Targher G. NAFLD: A multisystem disease. J. Hepatol. 2015;62:47–64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Kanga I., Espín J.C., Carra T.P., Tomás-Barberán F.A., Chunga S. Raspberry seed flour attenuates high-sucrose diet-mediated hepatic stress and adipose tissue inflammation. J. Biol. Chem. 2016;32:64–72. doi: 10.1016/j.jnutbio.2016.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from the corresponding authors upon reasonable request.