Abstract
Glucose-regulated protein 94 (GRP94) is an endoplasmic reticulum (ER)-resident member of the heat shock protein 90 (HSP90) family. In physiological conditions, it plays a vital role in regulating biological functions, including chaperoning cellular proteins in the ER lumen, maintaining calcium homeostasis, and modulating immune system function. Recently, several reports have shown the functional role and clinical relevance of GRP94 overexpression in the progression and metastasis of several cancers. Therefore, the current review highlights GRP94’s physiological and pathophysiological roles in normal and cancer cells. Additionally, the unmet medical needs of small chemical inhibitors and the current development status of monoclonal antibodies specifically targeting GRP94 will be discussed to emphasize the importance of cell surface GRP94 as an emerging therapeutic target in monoclonal antibody therapy for cancer.
Keywords: GRP94, cancer, therapeutic target, therapy, monoclonal antibody
1. Introduction
Cancer, the second leading cause of death behind cardiovascular disease, remains a major global public health problem [1]. Overall, the burden of cancer incidence and mortality has continued to increase rapidly worldwide. According to the International Agency for Research on Cancer, an estimated 19.3 million new cancer cases and 10 million cancer deaths occurred in 2020 [2]. The recent increase in cancer incidence has been associated with various factors, such as cigarette smoking, urbanization and the associated pollution, dietary changes, and the psychological stress of modern society [3,4,5]. Over the past several decades, remarkable developments in science and technology, including high-throughput screening, structure-based optimization for drug design, and state-of-the-art technology based on modern biochemistry, cell biology, and molecular biology, have improved the discovery of drugs against cancers [6,7,8,9,10,11]. As of November 2020, approximately 7000 human drug products have been approved by the United States Food and Drug Administration (USFDA) [12]. However, numerous medical unmet needs for successful cancer therapy still exist.
The traditional and most widely used approaches for treating cancer include chemotherapy, radiation therapy, and surgery [13]. Conventional chemotherapy, involving a single drug (single-agent chemotherapy) or several (combination therapy) drugs, usually targets fast-growing cancer cells [14]. Unfortunately, chemotherapy also concomitantly targets normal cells in, for example, hair follicles, bone marrow, and digestive tract, thereby resulting in severe adverse effects, including hair loss, fatigue, nausea, diarrhea, vomiting, anemia, and bone marrow suppression [15,16,17,18]. To overcome the mentioned drawbacks of traditional cancer therapies, monoclonal antibody-based targeted therapy for selective elimination of cancer cells has emerged.
Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAbs) to bind to a specific target antigen. Since the remarkable development of recombinant DNA technology, more than 570 therapeutic mAbs have been globally studied in clinical trials, and 79 therapeutic mAbs have received USFDA approval. In particular, around 30 USFDA-approved mAbs have been on the market to treat a variety of hematological and solid cancers [19]. Among these, rituximab (Mabthera; Rituxan) was the first mouse/human chimeric mAb approved by USFDA in 1997 to treat relapsed or refractory CD20-positive non-Hodgkin’s lymphoma [20]. However, despite the clinical use of numerous anticancer therapeutic antibodies, a limited number of their therapeutic targets, including CD20, vascular endothelial growth factor-A (VEGF-A), epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), programmed cell death protein-1 (PD-1), and programmed death-ligand 1 (PD-L1), have currently been identified [21,22,23,24,25]. Some examples include ofatumumab, ocrelizumab, ibritumomab tiuxetanand, and rituximab for CD20-targeted mAbs; bevacizumab, ranibizumab, and brolucizumab for VEGF-A-targeted mAbs; cetuximab, panitumumab, and necitumumab for EGFR-targeted mAbs; trastuzumab, and pertuzumab for HER2-targeted mAbs; nivolumab, pembrolizumab, and cemiplimab for PD-1-targeted mAbs; lastly, atezolizumab, avelumab, and durvalumab for PD-L1-targeted mAbs [21,22,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. As such, identifying novel therapeutic targets and understanding the molecular mechanism of metastatic cancer cells are essential for overcoming unmet medical needs in current cancer therapy.
Therefore, the current review highlights GRP94’s physiological roles in cells and its roles and relevance in cancers to clarify its pathological mechanisms in cancer progression and metastasis. Additionally, outlining the current status of GRP94-targeting inhibitors will provide insight into unmet medical needs for monoclonal antibody therapy.
2. The Structure and Physiological Roles of GRP94 in Cells
2.1. The Structure of GRP94
GRP94 is an endoplasmic reticulum (ER)-resident member of the heat shock protein 90 (HSP90) family, a paralog of cytosolic HSP90 [43]. It is also known as tumor rejection antigen 1 (TRA1), gp96, heat shock protein 90 kDa beta member 1 (HSP90B1), and endoplasmin [44,45,46]. Many different groups have discovered that GRP94, as a cell protein, is strongly induced by glucose starvation [47]. It is also a major calcium-binding protein in the ER and the most abundant ER-resident protein [48,49]. Several methods for determining the structure of GRP94, including X-ray crystallography, electron microscopy, and small-angle X-ray scattering, have revealed that it forms a “twisted V”-shaped dimeric structure in physiological conditions [50,51]. The molecular structure of GRP94 comprises four main conserved domains, including an N-terminal domain (NTD), a charged linker region (CR), a middle domain (MD), and a C-terminal domain (CTD).
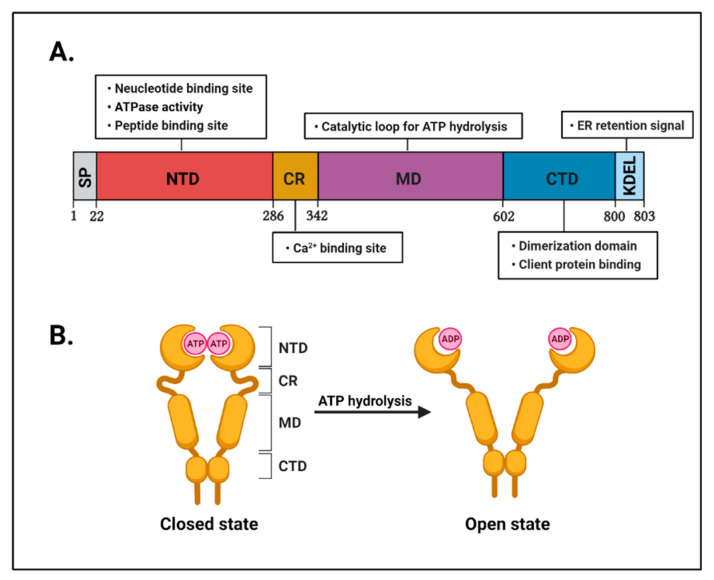
The NTD (amino acid residues 22–285) has an adenosine triphosphate (ATP)-binding pocket that is essential for ATPase activity [52]. Thus, the NTD has been used as a major target for GRP94 inhibitors such as geldanamycin (GDA) and radicicol (RDC) [53,54]. These are natural product inhibitors and ATP-competitive N-terminal inhibitors. The CR (amino acid residues 286–341) is a short dynamic region that connects the NTD and the MD. This domain is essential for controlling ATP hydrolysis activity in cooperation with the MD [55]. Furthermore, the CR also contains calcium-binding sites required for intracellular calcium homeostasis [56]. The MD (amino acid residues 342–601) contains the catalytic loop for ATPase activity and interacts with the NTD for ATP hydrolysis [57]. Lastly, the CTD (amino acid residues 602–803) is critical for not only providing a homodimer interface for the dimer formation of GRP94 but also containing a client protein-binding site for GRP94 [58]. In addition, the CTD ends with a KDEL tetrapeptide sequence that is critical for the retention of GRP94 in the ER lumen [59] (Figure 1).
Figure 1.
Structural feature of GRP94. (A) A schematic representation of the domain organization of human GRP94. The first 21 sequences are signal peptide sequences (gray) displayed as SP. Next, the N-terminal domain (NTD; red) plays an important role in ATPase activity and contains a nucleotide and peptide binding site. The charged linker region (CR) contains a calcium-binding site (orange). The middle domain (MD; purple) contains a catalytic loop for ATP hydrolysis. The C-terminal domain (CTD; blue) plays an important role in dimerization and client protein binding. KDEL (light blue), the C-terminal tetrapeptide of GRP94, serves as the endoplasmic reticulum (ER) retention/retrieval ligand for the KDEL receptor. (B) Schematic representation of the GRP94 structure. The conformational change in GRP94 occurs through ATP binding and ATP hydrolysis. GRP94 changes its state from “closed” to “open” through ATP hydrolysis.
2.2. Physiological Role of GRP94 in Cells
GRP94 was first identified as a cell protein strongly upregulated in response to glucose starvation in the ER [60]. Furthermore, in normal cells, GRP94 is also specifically upregulated by a variety of stress conditions that perturb ER functions, including oxidative stress, ER calcium depletion, accumulation of misfolded proteins, and glucose starvation [61,62]. GRP94 shares many biochemical features, such as domain structure and ATPase activity, with other HSP90 family members [63]. Thus far, several studies have suggested that GRP94 plays multifunctional roles in physiological conditions.
2.2.1. GRP94 as a Molecular Chaperone
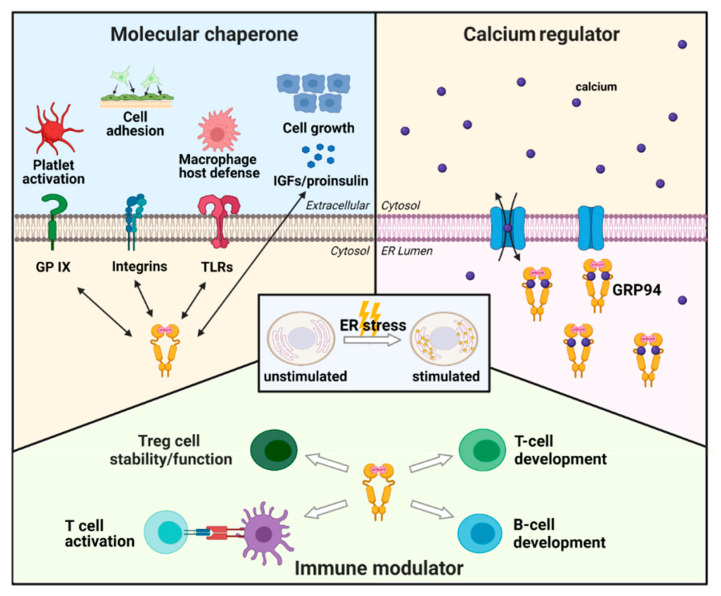
GRP94 mainly serves as an ER-resident molecular chaperone that physically interacts with and directs the folding and assembly of several secreted and membrane client proteins [64,65]. Eventually, it participates in regulating various functions, including cell growth, adhesion, and immunity (Figure 2).
Figure 2.
Schematic representation of the physiological roles of GRP94. GRP94 is a molecular chaperone upregulated by ER stress. Under stress conditions, GRP94 accelerates its function as a molecular chaperone. Client proteins of GRP94 include toll-like receptors (TLRs), glycoprotein (GP) IX subunit, insulin-like growth factors (IGFs), proinsulin, and integrins. These interactions are closely associated with direct function in macrophage host defense, activation of platelets in blood clotting, cell growth, and cell adhesion. Calcium regulation is another physiological function of GRP94, one of the major calcium-binding proteins in the ER regulating calcium homeostasis. Lastly, GRP94 is a multifunctional immune modulator. As demonstrated, GRP94 is essential in early T- and B-cell development while also regulating Treg cell stability and immunosuppressive function. GRP94 activates T-cells through peptide binding.
Notably, Melnick et al. identified newly synthesized and unassembled immunoglobulin (Ig) heavy or light chains as the first GRP94 client proteins related to immunity [66]. They suggested that GRP94 assists in the correct folding and assembly of Ig subunits. Meanwhile, Randow et al. reported that GPR94 forms a complex with toll-like receptors (TLRs) [67]. These pattern recognition receptors recognize immune system pathogens and play a vital role in the folding, assembly, and export of TLRs, including TLR1, TLR2, and TLR4. After generating macrophage-specific GRP94 knockout mice and analyzing macrophage responses to TLR agonists, Yang et al. found that GRP94-deficient macrophages failed to respond to ligands for plasma membrane TLRs, suggesting the importance of GRP94 in regulating TLR responses and host defense to bacterial infection [68]. Additionally, Staron et al. revealed that the selective binding of GRP94 to the glycoprotein (GP) IX subunit plays a key role in the assembly of the platelet GP I b-IX complex, critical for platelet activation in blood clotting [69].
Apart from immune client proteins of GRP94, insulin-like growth factors (IGFs) and integrins have also been identified as major GRP94 client proteins. Ostrovsky et al. reported that GRP94 interacts physically and transiently with pro-IGF-II intermediates, while its activity is essential for the secretion of active IGF-II, thereby establishing IGF-II as a client of GRP94 [70]. By using muscle tissue-specific conditional knockout of GRP94, Barton et al. showed that GRP94 plays an important role in muscle and whole-body growth by controlling IGF-I secretion and production [71]. In addition, Ghiasi et al. reported that GRP94 participates in proinsulin production as a molecular chaperone. They demonstrated that GRP94 forms a complex with proinsulin in INS-1E rat pancreatic β-cells. Moreover, the study also revealed that GRP94 lentiviral short hairpin RNA (shRNA)-induced knockdown or CRISPR/Cas9-induced knockout significantly reduced proinsulin production in INS-1E cells [72]. Furthermore, Wu et al. reported that amino acid residues 652–678 of GRP94 and, more specifically, Met658 and Met662 are critical for binding and chaperoning integrin αL [73]. Randow et al. showed that the inducible deletion of GRP94 reveals its critical role in the expression of several integrins, including αL, α4, and αM [67]. Furthermore, Chen et al. reported that liver-specific GRP94 knockout in mice resulted in disruption of cell adhesion in liver progenitor cells due to integrin β1 loss. [74]. These studies demonstrate that the interaction between GRP94 and integrins is closely related to cell adhesion.
Lastly, thyroglobulin, bile-salt-dependent lipase, glycoprotein A repetitions predominant, and low-density lipoprotein receptor-related protein 6 were also identified as client proteins of GRP94 [75,76,77,78].
2.2.2. GRP94 as a Calcium Regulator
The calcium (Ca2+)-binding proteins in the ER not only provide a large Ca2+ storage capacity that enables the ER to accumulate high levels of free and bound Ca2+ but also regulate the concentration of intracellular Ca2+ for the activation of several signaling pathways and physiological responses in response to a variety of cellular stimuli [79,80] (Figure 2). Several lines of evidence suggest that GRP94 is one of the major Ca2+-binding proteins in the ER lumen and that it plays a key role in regulating cellular Ca2+ homeostasis [48]. Drummond et al. reported that the Ca2+ ionophore A23187 depletes intracellular Ca2+ stores and upregulates GRP94 mRNA expression in hamster fibroblasts [81]. Each GRP94 molecule has 15 putative Ca2+-binding sites, among which 4 and 11 have higher (KD ~2 μM) and lower (KD ~600 μM) affinity sites, respectively [48]. Other groups have also shown that purified GRP94 can bind 280 nmol of Ca2+ per mg protein [82]. Furthermore, Biswas et al. showed that the N-terminal portion of GRP94 contains at least one high-affinity Ca2+ binding site within its charged linker domain. The same paper also demonstrated that GRP94 knockout embryonic stem cells stopped growing in Ca2+-depleted medium, suggesting the unique role of GRP94 in Ca2+ homeostasis [56]. Additionally, Vitadello et al. reported that GRP94 overexpression promoted a significantly slower increase in intracellular Ca2+ in Ca2+ ionophore A23187-exposed muscle cell lines than in control cells, suggesting GRP94′s role as an intracellular Ca2+ regulator [83]. Similarly, Bando et al. also reported that exposure to Ca2+ ionophore A23187 increased GRP94′s expression in human neuroblastoma SH-SY5Y cells and that GRP94 could suppress A23187-induced cell death in SH-SY5Y cells, implying its Ca2+ buffering function [84].
2.2.3. GRP94 as an Immune Modulator
Apart from its chaperone function, GRP94 seems to play an important role in modulating the immune system (Figure 2), with several lines of evidence supporting this notion. Accordingly, Staron et al. reported that GRP94 knockout mice blocked T- and B-cell lymphopoiesis, indicating its central role in B- and T-cell development [85]. Zheng et al. demonstrated that GRP94 is important for stimulating dendritic cell maturation and inducing efficient T-cell priming [86]. Furthermore, Suto et al. showed direct evidence that assembled complexes of GRP94 and synthetic peptides can be presented again by an antigen-presenting cell (APC) to promote T-cell activation [87]. Biswas et al. also identified that the N-terminal fragment (amino acids 1–355) of GRP94 is responsible for peptide presentation to T-cells [56]. Thus, the studies above provide definitive evidence that GRP94 can direct peptides to MHC class I in antigen presentation and induce APC maturation and activation [88]. Tramentozzi et al. showed that purified GRP94 stimulates Ig secretion and PBMC proliferation, indicating that GRP94 can activate the humoral response via a cytokine-like, cell-mediated mechanism [89]. Lastly, Zhang et al. demonstrated that GRP94 disruption in regulatory T-cell (Treg)-specific GRP94 knockout mice impaired the suppressive function of Treg cells in vivo and promoted the development of fatal inflammatory diseases. The same study also reported that the Treg cell lineage exhibited instability and underwent conversion into IFN-γ-producing pathogenic T-cells in the absence of GRP94, suggesting that GRP94 may play a central role in Treg cell stability and immunosuppressive functions [90].
3. Role and Relevance of GRP94 in Cancer
3.1. Clinical Relevance of GRP94 in Cancer
Many studies have demonstrated that under stress conditions, GRP94 assists in the folding of newly synthesized polypeptides and prevents the aggregation of unfolded or misfolded proteins in the ER lumen [91]. Tumors particularly exhibit a wide range of stress conditions, including hypoxia, redox homeostasis changes, altered cell metabolism, acidosis, and increased cell proliferation and protein synthesis, all of which can trigger ER stress [92,93,94].
Reports have shown that GRP94 mRNA is upregulated in several types of cancer tissues, including liver cancer, breast cancer, esophageal cancer, and glioma tissues [95,96,97,98]. Furthermore, several immunohistochemical studies have revealed that GRP94 protein is highly overexpressed in various cancers, including breast, lung, colorectal, oral, esophageal, and gastric, suggesting a strong relationship with cancers [97,99,100,101,102,103]. Several among the mentioned cancers have shown an inverse correlation between GRP94 overexpression and patient survival. For instance, Liu et al. reported that patients with breast cancer tissues expressing high GRP94 had a statistically significantly shorter survival time than those with a low GRP94 expression [96]. Moreover, multiple studies have suggested that GRP94 may be a potential poor prognostic factor in various types of cancers, including lung, gastric, colorectal, and esophageal cancers [100,104,105,106]. In summary, available evidence suggests that GRP94 is closely associated with cancer progression and metastasis.
3.2. Role of GRP94 in Cancer Progression and Metastasis
During the multistep development of human tumors, cancer hallmarks include uncontrolled cell proliferation, tumor angiogenesis, invasion, and metastasis [107,108]. Accumulating evidence has revealed that GRP94 is strongly associated with increased cancer proliferation. Several in vitro experiments have demonstrated that GRP94 knockdown in cancer cells promoted growth reduction. For instance, Duan et al. reported that GRP94 knockdown in lung cancer cells inhibits its proliferation and promotes cell apoptosis by increasing caspase-7 and C/EBP homologous protein levels [100]. Moreover, Huang et al. reported that GRP94 knockdown in two different esophageal cancer cell lines using short hairpin RNA (shRNA) promoted more than 50% growth inhibition [106]. Similarly, multiple in vitro studies demonstrated that GRP94 knockdown facilitated growth inhibition in various cancer cell lines, such as gastric cancer, breast cancer, and colorectal cancer cells [99,109,110]. Another study using an in vivo xenograft mouse model showed that subcutaneous injection of GRP94-deficient hepatocellular carcinoma (HCC) cells resulted in significant tumor growth reduction [109].
Tumor angiogenesis is a vital process wherein new blood vessels are formed to properly establish a supportive microenvironment rich in oxygen and nutrients, necessary for optimal growth. Zhang et al. reported considerable growth suppression after orthotopically injecting a GRP94-knockdown melanoma cell line into mice. Further mechanistic studies demonstrated that GRP94 depletion reduced VEGF-A expression, inhibiting tumor-associated angiogenesis [110].
Subsequently, an increasing number of reports have shown a strong relationship between GRP94 and cancer invasion and metastasis. Accordingly, Calderon et al. observed a significant decrease in invasion following GRP94 knockdown in MDA-MB-231, a highly aggressive human breast cancer cell line [111]. Wei et al. also reported that GRP94 knockdown in GRP94 shRNA-treated HCCs inhibited their invasive characteristics, including wound healing, migration, and invasion. Further analysis revealed the inhibition of the chaperonin-containing TCP1 subunit 8/c-Jun/epithelial–mesenchymal transition (CCT8/c-Jun/EMT) cascade in GRP94 shRNA-treated HCCs attenuated its invasive characteristic [112]. Moreover, the influence of GRP94 on cancer cell invasion may be explained by the fact that its client proteins include cell adhesion components, such as integrins. Recently, Hong et al. demonstrated a cell-permeable peptide that competitively inhibited the interaction between GRP94 and integrins, blocking cell invasion in leukemia [113]. Furthermore, Wang et al. reported that GRP94 expression was significantly higher in poorly differentiated colon cancers with metastasis than in well-differentiated cancers without metastasis [114]. Additionally, a statistical analysis study by Pamplona et al. demonstrated significant associations between brain metastasis progression and high GRP94 expression [115].
3.3. Role of GRP94 in Tumor Resistance
Tumor resistance to conventional therapies has remained a major challenge for successful cancer treatment [116]. Thus, discovering factors predicting cancer resistance is crucial for screening and improving adjuvant therapies for patients with cancer and preventing unnecessary treatment side effects. Multiple studies have suggested that GRP94 participates in tumor radio- and chemoresistance. Accordingly, Lin et al. observed that GRP94 was overexpressed in radioresistant head and neck cancer cells, and using siRNA against GRP94 restored radiosensitivity in the same cancer cell lines [117]. Moreover, Kubota et al. found that cervical cancer cells with increased GRP94 expression were more resistant to X-ray, while Wang et al. reported that incubation of malignant cells with chemotherapeutic agents, such as 5-fluorouracil, cisplatin, and paclitaxel, upregulated GRP94 expression [118,119]. Additionally, Zhang et al. reported that GRP94 was associated with decreased sensitivity to doxorubicin in ovarian carcinoma cell lines. Similarly, Calderon et al. reported that siRNA-induced knockdown of GRP94 expression in human breast cancer cells helped increase their sensitivity to doxorubicin [111].
4. The Development of GRP94-Specific Inhibitors
4.1. GRP94 Small Molecule Inhibitors for Cancer Therapy
Thus far, significant attention has been paid to GRP94 as a potential therapeutic target for GRP94 small molecule inhibitors [120]. It is particularly well-known that GRP94-induced protein folding depends on conformational changes in the chaperone driven by cycles of ATP binding and hydrolysis [121]. Thus, several GRP94 small molecule inhibitors have been developed to inhibit ATP binding and hydrolysis competitively [122,123]. At present, GRP94 inhibitors can be classified into three classes, namely, benzoquinone ansamycin, resorcinol, and purine [124].
4.1.1. Benzoquinone Ansamycin Class
GDA, a benzoquinone ansamycin, was originally isolated as a natural product inhibitor of both HSP90 and GRP94, with potent and broad anticancer properties [125]. Reports have shown that GDA competitively binds to the ATP-binding pocket in the NTD of GRP94 and inhibits its chaperone activity via downregulation of ATPase activity, resulting in the effective and potent killing of cancer cells [126]. However, owing to the substantial hepatotoxicity and unsatisfactory solubility of GDA, it remains a poor clinical candidate [127,128]. Nonetheless, subsequent modifications have been made to optimize GDA and improve its therapeutic index.
GDA derivative 17-allylamino-17-demethoxygeldanamycin (17-AAG) was the first HSP90 inhibitor to enter clinical trials [129,130]. 17-AAG received considerable attention given its lower toxicity than GDA and potent therapeutic efficacy for both hematologic and solid tumor animal models over multiple clinical trials. Like GDA, 17-AAG binds to HSP90 and GRP94 with similar affinities and competitively inhibits ATP binding [131]. However, this product was discontinued due to poor pharmaceutical properties, namely, water insolubility, such that it required the addition of organic additives, such as dimethyl sulfoxide and polyoxyl castor oil (cremophor), to achieve aqueous solution solubility [132]. When administered to patients, these organic solvents may cause several adverse events, including nausea, vomiting, hypersensitivity reactions, and anaphylaxis. Furthermore, clinical trials using 17-AAG together with these additives may have masked the true maximum tolerable dose of 17-AAG in patients and misled physicians when scheduling the optimal dosing required to help manage toxicities [133,134]. Therefore, more extensive effort has been directed toward developing novel, water-soluble HSP90 inhibitors.
Notably, 17-desmethoxy-17-N,N-dimethylaminoethyl amino geldanamycin (Alvespimycin; 17-DMAG) is a water-soluble GDA derivative with reduced hepatotoxicity. While 17-DMAG differs from 17-AAG in the position 17 side chain of the ansa ring, it exhibits similar therapeutic efficacy and mode of action. Surprisingly, 17-DMAG is more soluble in aqueous media, such as saline, compared to 17-AAG, resulting in greater bioavailability [135]. Unfortunately, this compound did not show sufficiently promising activity in phase II clinical testing, for which further development has been abandoned [136].
4.1.2. Resorcinol Class
RDC is a natural macrocyclic antifungal antibiotic originally isolated from Monosporium bonorden in 1953 [54,137]. RDC competitively binds to the ATP-binding site of HSP90 and GRP94 and has been found to induce apoptosis even in 17-AAG-resistant cancer cells [138]. While RDC is the most potent HSP90 inhibitor in vitro, it has failed to be effective in animal models due to its unstable epoxy group [139]. Moreover, radamide (RDA), a chimera of RDC and GDA, had been initially designed to favorably interact with a unique hydrophobic-binding pocket, exclusive to GRP94, but it did not show higher selectivity for GRP94 (Kd = 0.52 μM) over HSP90 (Kd = 0.87 μM) [140].
NVP-AUY922 is a resorcinol-derived synthetic molecule discovered using a structure-based drug designing strategy. NVP-AUY922 had an IC50 value of 535 ± 51 nM against GRP94, indicating weaker potency than HSP90 [141,142]. This molecule, developed by Novartis, reached phase II clinical trials to treat patients with refractory gastrointestinal stromal or pancreatic cancers. However, studies were discontinued after it failed to show clinically significant effectiveness at the maximum tolerable dose [143]. Several lines of evidence have led us to speculate that the insufficient response of HSP90 inhibitors in clinical trials may result from chemoresistance caused by the increased expression of HSP70. For example, multiple studies have demonstrated that HSP90 inhibitors such as 17-DMAG and NVP-AUY922 upregulate the expression of HSP70 in vitro or in vivo [144,145,146,147]. Ghoshal et al. reported that siRNA-mediated HSP70 knockdown sensitizes the apoptosis of HEL human acute myeloid leukemia cells to 17-DMAG [148]. Furthermore, Kühnel et al. also reported that siRNA-mediated downregulation of HSP70 significantly increased the potency of NVP-AUY922 to H1339 lung cancer cells. [149]. However, despite these current studies, there remains a need for more detailed studies to further investigate the molecular mechanism of HSP90 inhibitors.
4.1.3. Purine Class
PU-H71, first discovered by Memorial Sloan-Kettering Cancer Centre, has undergone a phase I clinical trial by Samus Therapeutics. However, toxicity-related issues (life-threatening grade IV hematologic toxicities) halted further clinical evaluations [150,151].
5′-N-ethylcarboxamidoadenosine (NECA) was originally identified as a GRP94-selective inhibitor. However, a recent report by Liu et al. revealed that NECA inhibits multiple HSP90 proteins, including GRP94, HSP90α, HSP82, and TRAP1 [152]. Although NECA interacts preferentially with GRP94, using the NECA scaffold for further inhibitor development has been limited because NECA is also a potent agonist of several cellular adenosine receptors [153].
BIIB021, initially developed by Conforma Therapeutics (currently Biogen Idec) through a structure-based design based on the purine scaffold, is currently undergoing a phase II clinical trial [154]. Accordingly, Ernst et al. reported that BIIB021 inhibited not only GRP94 (Kd = 143 nM) but also HSP90 (Kd = 2 nM). Thus far, known adverse effects of BIIB021 include syncope, dizziness, fatigue, hyponatremia, and hypoglycemia [155]. Nevertheless, since this agent seems to elicit therapeutically significant anticancer activity at the clinical level, clinical evaluations are underway.
4.2. GRP94 Monoclonal Antibodies for Cancer Therapy
4.2.1. Cell Surface GRP94 in Cancers
GRP94, as a molecular chaperone, promotes proper folding of unfolded or misfolded proteins and suppresses their aggregation in the ER [156]. Despite its role in the ER, multiple studies have also observed GRP94 on the surface of cancers. Accordingly, Li et al. were the first researchers to exhibit cell surface GRP94 expression through immunofluorescence staining from nonpermeabilized SK-BR-3 human breast cancer cells [157]. Over the following years, reports have shown that cell surface GRP94 is highly expressed in various human cancer cell lines, such as SLR21 renal cancer, PANC10.05 pancreatic cancer, OVCAR3 ovarian cancer, DU-145 prostate cancer, WM1158 melanoma, and HCT-116 colorectal cancer cells [110,158,159]. Moreover, Melendez et al. demonstrated that cell surface GRP94 is especially expressed in MCF-7 and AU565 malignant breast cancer cells and not in MCF-10A and HMEC nonmalignant breast cancer cells [160].
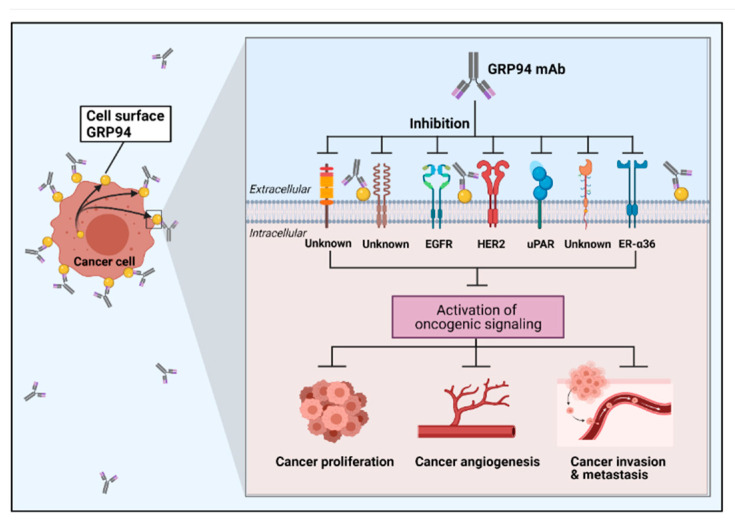
Studies have shown that cell surface GRP94 in cancer is closely associated with the promotion of cancer cell proliferation, invasion, and metastasis. Accordingly, Li et al. reported that GRP94 specifically interacts with HER2 at the plasma membrane of SK-BR-3 human breast cancer cells. The same paper also showed that overexpression of cell membrane GRP94 promotes cell proliferation and tumor growth by enhancing HER2 dimerization and the downstream signaling pathway. Furthermore, immunohistochemical analysis revealed that HER2 activation correlates with plasma membrane GRP94 expression in patients with HER2-positive primary breast cancer [157]. Hou et al. also reported that elevated cell surface GRP94 was associated with tumor metastasis and recurrence in patients with primary liver tumors. Furthermore, the same paper revealed that GRP94 interacts with urokinase-type plasminogen activator receptor (uPAR) in SK-HEP-1 human hepatoma cells, enhancing cancer cell stability, proliferation, survival, and invasion [161]. Additionally, Yan et al. also demonstrated that GRP94 present at the plasma membrane has a higher N-glycan content than ER-resident GRP94. The cell surface GRP94 forms a complex with HER2 and EGFR in breast cancer cells [158] (Figure 3).
Figure 3.
Schematic representation of cancer cell surface GRP94-targeting antibody, with its pathological role in the cancer microenvironment. GRP94 is highly expressed on the cancer cell surface. Several receptors are known for interacting with cell surface GRP94, and they facilitate cancer development by activating oncogenic signaling. So far, EGFR, HER2, uPAR, and ER-α36 are known transmembrane receptors that require GRP94 for cancer development and activation. However, still undiscovered receptors that interact with cell surface GRP94 to promote cancer development may exist. Targeting cell surface GRP94 in cancer inhibits its interactions, resulting in the reduction of oncogenic signaling transduction, which leads to the inhibition of cancer development.
4.2.2. Mouse Monoclonal Antibody
Monoclonal antibodies can be a powerful tool for validating a target protein’s potential therapeutic role for antibody therapy. Regarding GRP94-specific mouse monoclonal antibodies, the following evidence highlights the key role of GRP94 as a potential therapeutic target in cancers. Accordingly, Li et al. reported that cell surface GRP94 interacts with HER2, facilitates HER2 dimerization, and promotes cell proliferation. The same authors further demonstrated that cell surface GRP94-specific mouse antibody blocks GRP94-dependent HER2 dimerization and phosphorylation in SK-BR-3 breast cancer cells. It also suppresses HER2-driven breast cancer cell growth and induces apoptosis in a xenograft animal model [157]. Moreover, a study by Hou et al. reported that GRP94-targeting mouse mAb blocks the interaction between GRP94 and estrogen receptor-α36 (ER-α36) in the plasma membrane of MDA-MB-231 breast cancer cells, which play an important role in breast cancer growth and development. The same study showed that antibody treatment significantly inhibits breast cancer cell growth and invasion in vitro as well as tumor growth in a mouse xenograft model [159]. Another study also found that GRP94 mouse mAb specifically blocks the GRP94–uPAR interaction and inhibits uPAR-driven liver cancer cell growth, survival, and invasion, both in vitro and in vivo [161]. Collectively, these studies suggest that cell surface GRP94 could be a potential therapeutic antibody therapy target for cancers (Figure 3).
4.2.3. Human Monoclonal Antibody
Mouse monoclonal antibodies are often confronted with immunogenicity concerns caused by mouse-derived protein sequences [162,163]. Recent advancements in recombinant DNA technology have facilitated the remarkable development of therapeutic antibodies, including humanized and fully-human antibodies, which may open new avenues for improving patient survival and quality of life [19,164,165]. Sabbatino et al. were the first researchers to develop a human monoclonal antibody-targeting GRP94 named W9. More interestingly, they found that the antibody specifically recognizes the extracellular epitope of GRP94 on the membrane surface of malignant cells but not on normal cells. Furthermore, the W9 antibody could increase and restore sensitivity to v-raf murine sarcoma viral oncogene homolog B1 (BRAF) inhibitors in BRAFV600E melanoma cells that have acquired BRAF inhibitor resistance due to PDGFRα upregulation [166]. The same group also reported that treatment with the W9 antibody significantly suppressed MV3 human melanoma cell line growth in vitro. This effect also reflects the induction of apoptosis and the inhibition of several signaling pathways, including extracellular signal-regulated kinase, protein kinase B, and focal adhesion kinase pathways. Furthermore, they demonstrated that the W9 antibody induced the regression of experimental lung metastasis established in immunodeficient mice via intravenous injection of M21 melanoma cells [167].
Cetuximab is a recombinant mouse/human chimeric monoclonal antibody that targets EGFR to treat patients with EGFR- and wild-type Kirsten rat sarcoma 2 viral oncogene homolog (KRAS)-expressing colorectal cancer [168]. However, it is only effective in approximately 10–20% of patients with colorectal cancer, with other patients showing cetuximab resistance due to gene mutations in downstream EGFR effectors, including phosphoinositide-3-kinase catalytic subunit alpha (PI3KCA), phosphatase and tensin homolog (PTEN), KRAS, and BRAF [164,169]. Another interesting study by our group aimed to isolate antibodies tightly bound to the surface of HCT-116 cetuximab-resistant human colorectal cancer cells. To do so, we intensively performed several rounds of cell panning using phage display technology for antibody selection, followed by the isolation of a single-chain fragment variable (scFv) clone that strongly binds to the surface of HCT-116 cells from the combinatorial human antibody library. After converting the selected scFv clone to immunoglobulin G (IgG) and using peptide mass fingerprinting, we finally identified a 100-kDa target human GRP94 antigen. Furthermore, we demonstrated that the selected IgG antibody potently inhibited the growth of various HT-29, LoVo, HCT-8, and HCT-116 cells and significantly suppressed colorectal cancer growth without severe toxicities in an HCT-116 xenograft mouse model [165]. In the study above, we suggested that cell surface GRP94 may be a potential and novel therapeutic target in cetuximab-resistant colorectal cancers. Although further studies are needed to elucidate our findings, this study also provides substantial evidence that antibody-based targeting of cell surface GRP94 can be effective against GRP94-expressing colorectal cancers.
5. Conclusions
Cancer consists of a mixture of multiple and heterogeneous clones of tumor cells with different characteristics. Despite the numerous therapeutic agents developed for cancer therapy over several decades, such as small chemical inhibitors and mAbs, severe adverse effects and drug resistance have remained major obstacles toward better clinical outcomes. Thus, identifying a novel potential therapeutic target that is closely associated with the pathogenesis of cancer progression and metastasis is still an important challenge for new drug discovery and development.
Thus far, several reports have identified GRP94 as a prognostic marker that plays a critical role in cancer cell progression and metastasis. Although there has been considerable effort to develop small molecules that inhibit GRP94 in cancers selectively, several studies were discontinued due to severe adverse effects, such as hepatotoxicity caused by off-target effects, in some clinical trials. At the same time, several murine and human monoclonal antibodies have been generated to evaluate the role of cell surface GRP94 in antibody therapy for cancers. Compared to membrane-permeable small molecules that simultaneously inhibit both GRP94 in the ER lumen and cell surface GRP94, antibody targeting of cell surface GRP94 is speculated to be much safer, considering that high molecular weight antibodies (150 kDa) do not penetrate the plasma membrane. Thus, based on currently available evidence, we suggest that cell surface GRP94 may be a novel potential therapeutic target in antibody therapy for cancers. Furthermore, antibody-based targeting of cell surface GRP94 may be an effective strategy for suppressing tumor progression and metastasis.
Acknowledgments
The authors acknowledge the contribution of the investigators of the experimental work cited in this article. We also acknowledge the images created with Biorender.com.
Abbreviations
| 17-AAG | 17-Allylamino-17-demethoxygeldanamycin |
| 17-DMAG | 17-Desmethoxy-17-N,N-dimethylaminoethyl amino geldanamycin |
| AKT | Protein kinase B |
| APC | Antigen-presenting cell |
| ATP | Adenosine triphosphate |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| BSDL | Bile-salt-dependent lipase |
| CHOP | C/EBP homologous protein |
| CR | Charged linker region |
| CRC | Colorectal cancer |
| CTD | C-terminal domain |
| EGFR | Epidermal growth factor receptor |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| ER-α36 | Estrogen receptor-α36 |
| FAK | Focal adhesion kinase |
| GARP | Glycoprotein A repetitions predominant |
| GDA | Geldanamycin |
| GRP94 | Glucose-regulated protein 94 |
| GP | Glycoprotein |
| HCC | Hepatocellular carcinoma |
| HER2 | Human epidermal growth factor receptor 2 |
| HSP90 | Heat shock protein 90 |
| HSP90B1 | Heat shock protein 90 kDa beta member 1 |
| IGFs | Insulin-like growth factors |
| IgG | Immunoglobulin G |
| LRP6 | Lipoprotein receptor-related protein 6 |
| KRAS | Kirsten rat sarcoma 2 viral oncogene homolog |
| mAbs | Monoclonal antibodies |
| MD | Middle domain |
| NECA | 5′-N-ethylcarboxamidoadenosine |
| NTD | N-terminal domain |
| PD-1 | Programmed cell death protein-1 |
| PD-L1 | Programmed death-ligand 1 |
| PI3KCA | Phosphoinositide-3-kinase catalytic subunit alpha |
| PTEN | Phosphatase and tensin homolog |
| RDA | Radamide |
| RDC | Radicicol |
| scFv | Single-chain variable fragment |
| shRNA | Short hairpin RNA |
| TLRs | Toll-like receptors |
| TRA1 | Tumor rejection antigen 1 |
| uPAR | Urokinase-type plasminogen activator receptor |
| USFDA | The United States Food and Drug Administration |
| VEGF-A | Vascular endothelial growth factor-A |
Author Contributions
J.W.K., Y.B.C., and S.L. collected and analyzed the information, discussed and commented on the manuscript, and wrote the paper. S.L. supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Bio & Medical Technology Development Program of the National Research Foundation, funded by the Korean government (2019M3E5D 506584421, 2020M3A9I210709312).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders have no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Manisalidis I., Stavropoulou E., Stavropoulos A., Bezirtzoglou E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health. 2020;8:14. doi: 10.3389/fpubh.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.You W., Henneberg M. Cancer incidence increasing globally: The role of relaxed natural selection. Evol. Appl. 2017;11:140–152. doi: 10.1111/eva.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spring B., King A.C., Pagoto S.L., Van Horn L., Fisher J.D. Fostering multiple healthy lifestyle behaviors for primary prevention of cancer. Am. Psychol. 2015;70:75–90. doi: 10.1037/a0038806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carnero A. High throughput screening in drug discovery. Clin. Transl. Oncol. 2006;8:482–490. doi: 10.1007/s12094-006-0048-2. [DOI] [PubMed] [Google Scholar]
- 7.Albanese S.K., Chodera J.D., Volkamer A., Keng S., Abel R., Wang L. Is Structure-Based Drug Design Ready for Selectivity Optimization? J. Chem. Inf. Modeling. 2020;60:6211–6227. doi: 10.1021/acs.jcim.0c00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazanetz M.P., Goode C.H.F., Chudyk E.I. Ligand- and Structure-Based Drug Design and Optimization using KNIME. Curr. Med. Chem. 2020;27:6458–6479. doi: 10.2174/0929867326666190409141016. [DOI] [PubMed] [Google Scholar]
- 9.Downey G.P., Waddell T.K., Fukushima T., Sue A.Q.A. Current techniques in cell and molecular biology. J. Crit. Care. 1995;10:136–149. doi: 10.1016/0883-9441(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 10.Pickar-Oliver A., Gersbach C.A. The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 2019;20:490–507. doi: 10.1038/s41580-019-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohs R.C., Greig N.H. Drug discovery and development: Role of basic biological research. Alzheimers Dement. 2017;3:651–657. doi: 10.1016/j.trci.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Food & Drug Administration Fact Sheet: FDA at a Glance. [(accessed on 28 November 2020)]; Available online: https://www.fda.gov/about-fda/fda-basics/fact-sheet-fda-glance.
- 13.Chen H.H.W., Kuo M.T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget. 2017;8:62742–62758. doi: 10.18632/oncotarget.18409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayat Mokhtari R., Homayouni T.S., Baluch N., Morgatskaya E., Kumar S., Das B., Yeger H. Combination therapy in combating cancer. Oncotarget. 2017;8:38022–38043. doi: 10.18632/oncotarget.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chabner B.A., Roberts T.G., Jr. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 16.Carelle N., Piotto E., Bellanger A., Germanaud J., Thuillier A., Khayat D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer. 2002;95:155–163. doi: 10.1002/cncr.10630. [DOI] [PubMed] [Google Scholar]
- 17.Coates A., Abraham S., Kaye S.B., Sowerbutts T., Frewin C., Fox R.M., Tattersall M.H. On the receiving end--patient perception of the side-effects of cancer chemotherapy. Eur. J. Cancer Clin. Oncol. 1983;19:203–208. doi: 10.1016/0277-5379(83)90418-2. [DOI] [PubMed] [Google Scholar]
- 18.Zitvogel L., Apetoh L., Ghiringhelli F., Kroemer G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 19.Lu R.-M., Hwang Y.-C., Liu I.J., Lee C.-C., Tsai H.-Z., Li H.-J., Wu H.-C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020;27:1. doi: 10.1186/s12929-019-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammed R., Milne A., Kayani K., Ojha U. How the discovery of rituximab impacted the treatment of B-cell non-Hodgkin’s lymphomas. J. Blood Med. 2019;10:71–84. doi: 10.2147/JBM.S190784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranieri G., Patruno R., Ruggieri E., Montemurro S., Valerio P., Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: From the biology to the clinic. Curr. Med. Chem. 2006;13:1845–1857. doi: 10.2174/092986706777585059. [DOI] [PubMed] [Google Scholar]
- 22.Baselga J. The EGFR as a target for anticancer therapy—Focus on cetuximab. Eur. J. Cancer. 2001;37(Suppl. 4):S16–S22. doi: 10.1016/S0959-8049(01)00233-7. [DOI] [PubMed] [Google Scholar]
- 23.Harbeck N., Beckmann M.W., Rody A., Schneeweiss A., Müller V., Fehm T., Marschner N., Gluz O., Schrader I., Heinrich G., et al. HER2 Dimerization Inhibitor Pertuzumab—Mode of Action and Clinical Data in Breast Cancer. Breast Care. 2013;8:49–55. doi: 10.1159/000346837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis A.A., Patel V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer. 2019;7:278. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akinleye A., Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 2019;12:92. doi: 10.1186/s13045-019-0779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemery S.J., Zhang J., Rothmann M.D., Yang J., Earp J., Zhao H., McDougal A., Pilaro A., Chiang R., Gootenberg J.E., et al. U.S. Food and Drug Administration approval: Ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab. Clin. Cancer Res. 2010;16:4331–4338. doi: 10.1158/1078-0432.CCR-10-0570. [DOI] [PubMed] [Google Scholar]
- 27.Morschhauser F., Marlton P., Vitolo U., Lindén O., Seymour J.F., Crump M., Coiffier B., Foà R., Wassner E., Burger H.U., et al. Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann. Oncol. 2010;21:1870–1876. doi: 10.1093/annonc/mdq027. [DOI] [PubMed] [Google Scholar]
- 28.Gordon L.I., Molina A., Witzig T., Emmanouilides C., Raubtischek A., Darif M., Schilder R.J., Wiseman G., White C.A. Durable responses after ibritumomab tiuxetan radioimmunotherapy for CD20+ B-cell lymphoma: Long-term follow-up of a phase 1/2 study. Blood. 2004;103:4429–4431. doi: 10.1182/blood-2003-11-3883. [DOI] [PubMed] [Google Scholar]
- 29.Wiseman G.A., Gordon L.I., Multani P.S., Witzig T.E., Spies S., Bartlett N.L., Schilder R.J., Murray J.L., Saleh M., Allen R.S., et al. Ibritumomab tiuxetan radioimmunotherapy for patients with relapsed or refractory non-Hodgkin lymphoma and mild thrombocytopenia: A phase II multicenter trial. Blood. 2002;99:4336–4342. doi: 10.1182/blood.V99.12.4336. [DOI] [PubMed] [Google Scholar]
- 30.Maloney D.G., Grillo-López A.J., White C.A., Bodkin D., Schilder R.J., Neidhart J.A., Janakiraman N., Foon K.A., Liles T.M., Dallaire B.K., et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–2195. doi: 10.1182/blood.V90.6.2188. [DOI] [PubMed] [Google Scholar]
- 31.Ferrara N., Damico L., Shams N., Lowman H., Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 32.Yannuzzi N.A., Freund K.B. Brolucizumab: Evidence to date in the treatment of neovascular age-related macular degeneration. Clin. Ophthalmol. 2019;13:1323–1329. doi: 10.2147/OPTH.S184706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messersmith W.A., Hidalgo M. Panitumumab, a monoclonal anti epidermal growth factor receptor antibody in colorectal cancer: Another one or the one? Clin. Cancer Res. 2007;13:4664–4666. doi: 10.1158/1078-0432.CCR-07-0065. [DOI] [PubMed] [Google Scholar]
- 34.Bagchi A., Haidar J.N., Eastman S.W., Vieth M., Topper M., Iacolina M.D., Walker J.M., Forest A., Shen Y., Novosiadly R.D., et al. Molecular Basis for Necitumumab Inhibition of EGFR Variants Associated with Acquired Cetuximab Resistance. Mol. Cancer Ther. 2018;17:521. doi: 10.1158/1535-7163.MCT-17-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Minckwitz G., Huang C.S., Mano M.S., Loibl S., Mamounas E.P., Untch M., Wolmark N., Rastogi P., Schneeweiss A., Redondo A., et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 36.von Minckwitz G., Procter M., de Azambuja E., Zardavas D., Benyunes M., Viale G., Suter T., Arahmani A., Rouchet N., Clark E., et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah N.J., Kelly W.J., Liu S.V., Choquette K., Spira A. Product review on the Anti-PD-L1 antibody atezolizumab. Hum. Vaccin Immunother. 2018;14:269–276. doi: 10.1080/21645515.2017.1403694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins J.M., Gulley J.L. Product review: Avelumab, an anti-PD-L1 antibody. Hum. Vaccin Immunother. 2019;15:891–908. doi: 10.1080/21645515.2018.1551671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faiena I., Cummings A.L., Crosetti A.M., Pantuck A.J., Chamie K., Drakaki A. Durvalumab: An investigational anti-PD-L1 monoclonal antibody for the treatment of urothelial carcinoma. Drug Des. Dev. Ther. 2018;12:209–215. doi: 10.2147/DDDT.S141491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grosso J., Horak C.E., Inzunza D., Cardona D.M., Simon J.S., Gupta A.K., Sankar V., Park J.-S., Kollia G., Taube J.M., et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538) J. Clin. Oncol. 2013;31:3016. doi: 10.1200/jco.2013.31.15_suppl.3016. [DOI] [Google Scholar]
- 41.Hui R., Garon E.B., Goldman J.W., Leighl N.B., Hellmann M.D., Patnaik A., Gandhi L., Eder J.P., Ahn M.J., Horn L., et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: A phase 1 trial. Ann. Oncol. 2017;28:874–881. doi: 10.1093/annonc/mdx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Migden M.R., Rischin D., Schmults C.D., Guminski A., Hauschild A., Lewis K.D., Chung C.H., Hernandez-Aya L., Lim A.M., Chang A.L.S., et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018;379:341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 43.Marzec M., Eletto D., Argon Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta. 2012;1823:774–787. doi: 10.1016/j.bbamcr.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maki R.G., Old L.J., Srivastava P.K. Human homologue of murine tumor rejection antigen gp96: 5’-regulatory and coding regions and relationship to stress-induced proteins. Proc. Natl. Acad. Sci. USA. 1990;87:5658–5662. doi: 10.1073/pnas.87.15.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z., Srivastava P.K. Tumor rejection antigen gp96/grp94 is an ATPase: Implications for protein folding and antigen presentation. EMBO J. 1993;12:3143–3151. doi: 10.1002/j.1460-2075.1993.tb05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen B., Piel W.H., Gui L., Bruford E., Monteiro A. The HSP90 family of genes in the human genome: Insights into their divergence and evolution. Genomics. 2005;86:627–637. doi: 10.1016/j.ygeno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Lee A.S., Bell J., Ting J. Biochemical characterization of the 94- and 78-kilodalton glucose-regulated proteins in hamster fibroblasts. J. Biol. Chem. 1984;259:4616–4621. doi: 10.1016/S0021-9258(17)43091-2. [DOI] [PubMed] [Google Scholar]
- 48.Van P.N., Peter F., Söling H.D. Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium. No indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J. Biol. Chem. 1989;264:17494–17501. doi: 10.1016/S0021-9258(18)71521-4. [DOI] [PubMed] [Google Scholar]
- 49.Lee A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci. 2001;26:504–510. doi: 10.1016/S0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 50.Dollins D.E., Warren J.J., Immormino R.M., Gewirth D.T. Structures of GRP94-nucleotide complexes reveal mechanistic differences between the hsp90 chaperones. Mol. Cell. 2007;28:41–56. doi: 10.1016/j.molcel.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krukenberg K.A., Böttcher U.M., Southworth D.R., Agard D.A. Grp94, the endoplasmic reticulum Hsp90, has a similar solution conformation to cytosolic Hsp90 in the absence of nucleotide. Protein Sci. 2009;18:1815–1827. doi: 10.1002/pro.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frey S., Leskovar A., Reinstein J., Buchner J. The ATPase cycle of the endoplasmic chaperone Grp94. J. Biol. Chem. 2007;282:35612–35620. doi: 10.1074/jbc.M704647200. [DOI] [PubMed] [Google Scholar]
- 53.Schulte T.W., Akinaga S., Soga S., Sullivan W., Stensgard B., Toft D., Neckers L.M. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3:100–108. doi: 10.1379/1466-1268(1998)003<0100:ARBTTN>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulte T.W., Akinaga S., Murakata T., Agatsuma T., Sugimoto S., Nakano H., Lee Y.S., Simen B.B., Argon Y., Felts S., et al. Interaction of radicicol with members of the heat shock protein 90 family of molecular chaperones. Mol. Endocrinol. 1999;13:1435–1448. doi: 10.1210/mend.13.9.0339. [DOI] [PubMed] [Google Scholar]
- 55.Vogen S., Gidalevitz T., Biswas C., Simen B.B., Stein E., Gulmen F., Argon Y. Radicicol-sensitive peptide binding to the N-terminal portion of GRP94. J. Biol. Chem. 2002;277:40742–40750. doi: 10.1074/jbc.M205323200. [DOI] [PubMed] [Google Scholar]
- 56.Biswas C., Ostrovsky O., Makarewich C.A., Wanderling S., Gidalevitz T., Argon Y. The peptide-binding activity of GRP94 is regulated by calcium. Biochem. J. 2007;405:233–241. doi: 10.1042/BJ20061867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dutta R., Inouye M. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem. Sci. 2000;25:24–28. doi: 10.1016/S0968-0004(99)01503-0. [DOI] [PubMed] [Google Scholar]
- 58.Yamada S., Ono T., Mizuno A., Nemoto T.K. A hydrophobic segment within the C-terminal domain is essential for both client-binding and dimer formation of the HSP90-family molecular chaperone. Eur. J. Biochem. 2003;270:146–154. doi: 10.1046/j.1432-1033.2003.03375.x. [DOI] [PubMed] [Google Scholar]
- 59.Munro S., Pelham H.R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 60.Joslin G., Hafeez W., Perlmutter D.H. Expression of stress proteins in human mononuclear phagocytes. J. Immunol. 1991;147:1614. [PubMed] [Google Scholar]
- 61.Yang Y., Li Z. Roles of heat shock protein gp96 in the ER quality control: Redundant or unique function? Mol. Cells. 2005;20:173–182. [PubMed] [Google Scholar]
- 62.Lee A.S. Mammalian stress response: Induction of the glucose-regulated protein family. Curr. Opin. Cell Biol. 1992;4:267–273. doi: 10.1016/0955-0674(92)90042-B. [DOI] [PubMed] [Google Scholar]
- 63.Hoter A., El-Sabban M.E., Naim H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018;19:2560. doi: 10.3390/ijms19092560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melnick J., Dul J.L., Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370:373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- 65.Randow F., Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 2001;3:891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 66.Yang Y., Liu B., Dai J., Srivastava P.K., Zammit D.J., Lefrançois L., Li Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Staron M., Wu S., Hong F., Stojanovic A., Du X., Bona R., Liu B., Li Z. Heat-shock protein gp96/grp94 is an essential chaperone for the platelet glycoprotein Ib-IX-V complex. Blood. 2011;117:7136–7144. doi: 10.1182/blood-2011-01-330464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ostrovsky O., Ahmed N.T., Argon Y. The chaperone activity of GRP94 toward insulin-like growth factor II is necessary for the stress response to serum deprivation. Mol. Biol. Cell. 2009;20:1855–1864. doi: 10.1091/mbc.e08-04-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barton E.R., Park S., James J.K., Makarewich C.A., Philippou A., Eletto D., Lei H., Brisson B., Ostrovsky O., Li Z., et al. Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production. FASEB J. 2012;26:3691–3702. doi: 10.1096/fj.11-203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghiasi S.M., Dahlby T., Hede Andersen C., Haataja L., Petersen S., Omar-Hmeadi M., Yang M., Pihl C., Bresson S.E., Khilji M.S., et al. Endoplasmic Reticulum Chaperone Glucose-Regulated Protein 94 Is Essential for Proinsulin Handling. Diabetes. 2019;68:747–760. doi: 10.2337/db18-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu S., Hong F., Gewirth D., Guo B., Liu B., Li Z. The molecular chaperone gp96/GRP94 interacts with Toll-like receptors and integrins via its C-terminal hydrophobic domain. J. Biol. Chem. 2012;287:6735–6742. doi: 10.1074/jbc.M111.309526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen W.T., Tseng C.C., Pfaffenbach K., Kanel G., Luo B., Stiles B.L., Lee A.S. Liver-specific knockout of GRP94 in mice disrupts cell adhesion, activates liver progenitor cells, and accelerates liver tumorigenesis. Hepatology. 2014;59:947–957. doi: 10.1002/hep.26711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muresan Z., Arvan P. Thyroglobulin Transport along the Secretory Pathway: Investigation of the role of molecular chaperone, grp94, in protein export from the endoplasmic reticulum. J. Biol. Chem. 1997;272:26095–26102. doi: 10.1074/jbc.272.42.26095. [DOI] [PubMed] [Google Scholar]
- 74.Bruneau N., Lombardo D., Bendayan M. Participation of GRP94-related protein in secretion of pancreatic bile salt-dependent lipase and in its internalization by the intestinal epithelium. Pt 17J. Cell Sci. 1998;111:2665–2679. doi: 10.1242/jcs.111.17.2665. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y., Wu B.X., Metelli A., Thaxton J.E., Hong F., Rachidi S., Ansa-Addo E., Sun S., Vasu C., Yang Y., et al. GP96 is a GARP chaperone and controls regulatory T cell functions. J. Clin. Investig. 2015;125:859–869. doi: 10.1172/JCI79014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu B., Staron M., Hong F., Wu B.X., Sun S., Morales C., Crosson C.E., Tomlinson S., Kim I., Wu D., et al. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc. Natl. Acad. Sci. USA. 2013;110:6877–6882. doi: 10.1073/pnas.1302933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bagur R., Hajnóczky G. Intracellular Ca(2+) Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell. 2017;66:780–788. doi: 10.1016/j.molcel.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Izquierdo J.H., Bonilla-Abadía F., Cañas C.A., Tobón G.J. Calcium, channels, intracellular signaling and autoimmunity. Reumatol. Clin. 2014;10:43–47. doi: 10.1016/j.reuma.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 79.Drummond I.A., Lee A.S., Resendez E., Jr., Steinhardt R.A. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J. Biol. Chem. 1987;262:12801–12805. doi: 10.1016/S0021-9258(18)45277-5. [DOI] [PubMed] [Google Scholar]
- 80.Macer D.R., Koch G.L. Identification of a set of calcium-binding proteins in reticuloplasm, the luminal content of the endoplasmic reticulum. Pt 1J. Cell Sci. 1988;91:61–70. doi: 10.1242/jcs.91.1.61. [DOI] [PubMed] [Google Scholar]
- 81.Vitadello M., Penzo D., Petronilli V., Michieli G., Gomirato S., Menabò R., Di Lisa F., Gorza L. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J. 2003;17:923–925. doi: 10.1096/fj.02-0644fje. [DOI] [PubMed] [Google Scholar]
- 82.Bando Y., Katayama T., Aleshin A.N., Manabe T., Tohyama M. GRP94 reduces cell death in SH-SY5Y cells perturbated calcium homeostasis. Apoptosis. 2004;9:501–508. doi: 10.1023/B:APPT.0000031446.95532.ad. [DOI] [PubMed] [Google Scholar]
- 83.Staron M., Yang Y., Liu B., Li J., Shen Y., Zúñiga-Pflücker J.C., Aguila H.L., Goldschneider I., Li Z. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis. Blood. 2010;115:2380–2390. doi: 10.1182/blood-2009-07-233031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng H., Dai J., Stoilova D., Li Z. Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J. Immunol. 2001;167:6731–6735. doi: 10.4049/jimmunol.167.12.6731. [DOI] [PubMed] [Google Scholar]
- 85.Suto R., Srivastava P.K. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 86.Reed R.C., Nicchitta C.V. Chaperone-mediated cross-priming: A hitchhiker’s guide to vesicle transport (review) Int. J. Mol. Med. 2000;6:259–264. doi: 10.3892/ijmm.6.3.259. [DOI] [PubMed] [Google Scholar]
- 87.Tramentozzi E., Zamarchi R., Pagetta A., Brunati A.M., Rossi E., Tibaldi E., Finotti P. Effects of glucose-regulated protein94 (Grp94) on Ig secretion from human blood mononuclear cells. Cell Stress Chaperones. 2011;16:329–338. doi: 10.1007/s12192-010-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y., Wu B., Alessandra M., Hong F., Ansa-Addo E., Sun S., Liu B., Li Z. Molecular chaperone gp96/grp94 is critical for immunosuppressive functions of regulatory T cells (IRM15P.458) J. Immunol. 2015;194(Suppl. 1):199.6. [Google Scholar]
- 89.Lee A.S. Glucose-regulated proteins in cancer: Molecular mechanisms and therapeutic potential. Nat. Rev. Cancer. 2014;14:263–276. doi: 10.1038/nrc3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chipurupalli S., Kannan E., Tergaonkar V., D’Andrea R., Robinson N. Hypoxia Induced ER Stress Response as an Adaptive Mechanism in Cancer. Int. J. Mol. Sci. 2019;20:749. doi: 10.3390/ijms20030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rozpedek W., Pytel D., Mucha B., Leszczynska H., Diehl J.A., Majsterek I. The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr. Mol. Med. 2016;16:533–544. doi: 10.2174/1566524016666160523143937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whiteside T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rachidi S., Sun S., Wu B.X., Jones E., Drake R.R., Ogretmen B., Cowart L.A., Clarke C.J., Hannun Y.A., Chiosis G., et al. Endoplasmic reticulum heat shock protein gp96 maintains liver homeostasis and promotes hepatocellular carcinogenesis. J. Hepatol. 2015;62:879–888. doi: 10.1016/j.jhep.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu S., Li R., Zuo S., Luo R., Fang W., Xie Y. GRP94 overexpression as an indicator of unfavorable outcomes in breast cancer patients. Int. J. Clin. Exp. Pathol. 2018;11:3061–3067. [PMC free article] [PubMed] [Google Scholar]
- 95.Chen X., Ding Y., Liu C.G., Mikhail S., Yang C.S. Overexpression of glucose-regulated protein 94 (Grp94) in esophageal adenocarcinomas of a rat surgical model and humans. Carcinogenesis. 2002;23:123–130. doi: 10.1093/carcin/23.1.123. [DOI] [PubMed] [Google Scholar]
- 96.Hu T., Xie N., Qin C., Wang J., You Y. Glucose-regulated protein 94 is a novel glioma biomarker and promotes the aggressiveness of glioma via Wnt/β-catenin signaling pathway. Tumor Biol. 2015;36:9357–9364. doi: 10.1007/s13277-015-3635-4. [DOI] [PubMed] [Google Scholar]
- 97.Dejeans N., Glorieux C., Guenin S., Beck R., Sid B., Rousseau R., Bisig B., Delvenne P., Buc Calderon P., Verrax J. Overexpression of GRP94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: Implications for tumor recurrence. Free Radic. Biol. Med. 2012;52:993–1002. doi: 10.1016/j.freeradbiomed.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 98.Duan X.F., Xin Y.W. Overexpression of molecule GRP94 favors tumor progression in lung adenocarcinoma by interaction with regulatory T cells. Thorac. Cancer. 2020;11:704–712. doi: 10.1111/1759-7714.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim K., Lee H.W., Lee E.H., Park M.I., Lee J.S., Kim M.S., Kim K., Roh M.S., Pak M.G., Oh J.E., et al. Differential expression of HSP90 isoforms and their correlations with clinicopathologic factors in patients with colorectal cancer. Int. J. Clin. Exp. Pathol. 2019;12:978–986. [PMC free article] [PubMed] [Google Scholar]
- 100.Nomura H., Uzawa K., Yamano Y., Fushimi K., Ishigami T., Kato Y., Saito K., Nakashima D., Higo M., Kouzu Y., et al. Network-based analysis of calcium-binding protein genes identifies Grp94 as a target in human oral carcinogenesis. Br. J. Cancer. 2007;97:792–801. doi: 10.1038/sj.bjc.6603948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fu Z., Zhen H., Zou F., Wang X., Chen Y., Liu L. Involvement of the Akt signaling pathway in ER-α36/GRP94-mediated signaling in gastric cancer. Oncol. Lett. 2014;8:2077–2080. doi: 10.3892/ol.2014.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng H.C., Takahashi H., Li X.H., Hara T., Masuda S., Guan Y.F., Takano Y. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum. Pathol. 2008;39:1042–1049. doi: 10.1016/j.humpath.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 103.Brzozowa-Zasada M., Kurek J., Piecuch A., Wyrobiec G. The clinical and prognostic evaluation of GRP94 immunoexpression in Caucasian patients with colorectal adenocarcinoma. Prz. Gastroenterol. 2019;14:140–147. doi: 10.5114/pg.2019.85898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang C.Y., Lee C.H., Tu C.C., Wu C.H., Huang M.T., Wei P.L., Chang Y.J. Glucose-regulated protein 94 mediates progression and metastasis of esophageal squamous cell carcinoma via mitochondrial function and the NF-kB/COX-2/VEGF axis. Oncotarget. 2018;9:9425–9441. doi: 10.18632/oncotarget.24114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 106.Fouad Y.A., Aanei C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017;7:1016–1036. [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang X., Zhang L., Wang S., Wu D., Yang W. Decreased functional expression of Grp78 and Grp94 inhibits proliferation and attenuates apoptosis in a human gastric cancer cell line in vitro. Oncol. Lett. 2015;9:1181–1186. doi: 10.3892/ol.2014.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jeoung M.H., Kim T.K., Kim J.W., Cho Y.B., Na H.J., Yoo B.C., Shim H., Song D.K., Heo K., Lee S. Antibody-Based Targeting of Cell Surface GRP94 Specifically Inhibits Cetuximab-Resistant Colorectal Cancer Growth. Biomolecules. 2019;9:681. doi: 10.3390/biom9110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang C.Y., Batzorig U., Cheng W.L., Huang M.T., Chen W., Wei P.L., Chang Y.J. Glucose-regulated protein 94 mediates cancer progression via AKT and eNOS in hepatocellular carcinoma. Tumour Biol. 2016;37:4295–4304. doi: 10.1007/s13277-015-4254-9. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y., Liu B., Sun S., Li Z. Essential roles of heat shock protein gp96 (Hsp90b1, grp94) in melanoma growth and melanosome development (46.1) J. Immunol. 2012;188(Suppl. 1):46.1. [Google Scholar]
- 111.Buc Calderon P., Sennesael A.L., Glorieux C. Glucose-regulated protein of 94 kDa contributes to the development of an aggressive phenotype in breast cancer cells. Biomed. Pharmacother. Biomed. Pharmacother. 2018;105:115–120. doi: 10.1016/j.biopha.2018.05.106. [DOI] [PubMed] [Google Scholar]
- 112.Wei P.L., Huang C.Y., Tai C.J., Batzorig U., Cheng W.L., Hunag M.T., Chang Y.J. Glucose-regulated protein 94 mediates metastasis by CCT8 and the JNK pathway in hepatocellular carcinoma. Tumor Biol. 2016;37:8219–8227. doi: 10.1007/s13277-015-4669-3. [DOI] [PubMed] [Google Scholar]
- 113.Hong F., Liu B., Chiosis G., Gewirth D.T., Li Z. α7 helix region of αI domain is crucial for integrin binding to endoplasmic reticulum chaperone gp96: A potential therapeutic target for cancer metastasis. J. Biol. Chem. 2013;288:18243–18248. doi: 10.1074/jbc.M113.468850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang X.P., Qiu F.R., Liu G.Z., Chen R.F. Correlation between clinicopathology and expression of heat shock protein 70 and glucose-regulated protein 94 in human colonic adenocarcinoma. World J. Gastroenterol. 2005;11:1056–1059. doi: 10.3748/wjg.v11.i7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sanz-Pamplona R., Aragüés R., Driouch K., Martín B., Oliva B., Gil M., Boluda S., Fernández P.L., Martínez A., Moreno V., et al. Expression of endoplasmic reticulum stress proteins is a candidate marker of brain metastasis in both ErbB-2+ and ErbB-2- primary breast tumors. Am. J. Pathol. 2011;179:564–579. doi: 10.1016/j.ajpath.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gatenby R., Brown J. The Evolution and Ecology of Resistance in Cancer Therapy. Cold Spring Harb. Perspect. Med. 2018;8:a033415. doi: 10.1101/cshperspect.a033415. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Lin T.Y., Chang J.T., Wang H.M., Chan S.H., Chiu C.C., Lin C.Y., Fan K.H., Liao C.T., Chen I.H., Liu T.Z., et al. Proteomics of the radioresistant phenotype in head-and-neck cancer: Gp96 as a novel prediction marker and sensitizing target for radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010;78:246–256. doi: 10.1016/j.ijrobp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 118.Kubota H., Suzuki T., Lu J., Takahashi S., Sugita K., Sekiya S., Suzuki N. Increased expression of GRP94 protein is associated with decreased sensitivity to X-rays in cervical cancer cell lines. Int. J. Radiat. Biol. 2005;81:701–709. doi: 10.1080/09553000500434727. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y., Wang X., Ferrone C.R., Schwab J.H., Ferrone S. Intracellular antigens as targets for antibody based immunotherapy of malignant diseases. Mol. Oncol. 2015;9:1982–1993. doi: 10.1016/j.molonc.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McLaughlin M., Vandenbroeck K. The endoplasmic reticulum protein folding factory and its chaperones: New targets for drug discovery? Br. J. Pharm. 2011;162:328–345. doi: 10.1111/j.1476-5381.2010.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huck J.D., Que N.L., Hong F., Li Z., Gewirth D.T. Structural and Functional Analysis of GRP94 in the Closed State Reveals an Essential Role for the Pre-N Domain and a Potential Client-Binding Site. Cell Rep. 2017;20:2800–2809. doi: 10.1016/j.celrep.2017.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karagöz G.E., Rüdiger S.G.D. Hsp90 interaction with clients. Trends Biochem. Sci. 2015;40:117–125. doi: 10.1016/j.tibs.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 123.Khandelwal A., Crowley V.M., Blagg B.S.J. Resorcinol-Based Grp94-Selective Inhibitors. ACS Med. Chem. Lett. 2017;8:1013–1018. doi: 10.1021/acsmedchemlett.7b00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang L., Xu X., Jiang Z., You Q. Modulation of protein fate decision by small molecules: Targeting molecular chaperone machinery. Acta Pharm. Sin. B. 2020;10:1904–1925. doi: 10.1016/j.apsb.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Harvey A.L. Natural products in drug discovery. Drug Discov. Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 126.Miyata Y. Hsp90 inhibitor geldanamycin and its derivatives as novel cancer chemotherapeutic agents. Curr. Pharm. Des. 2005;11:1131–1138. doi: 10.2174/1381612053507585. [DOI] [PubMed] [Google Scholar]
- 127.Supko J.G., Hickman R.L., Grever M.R., Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother. Pharmacol. 1995;36:305–315. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- 128.Samuni Y., Ishii H., Hyodo F., Samuni U., Krishna M.C., Goldstein S., Mitchell J.B. Reactive oxygen species mediate hepatotoxicity induced by the Hsp90 inhibitor geldanamycin and its analogs. Free Radic. Biol. Med. 2010;48:1559–1563. doi: 10.1016/j.freeradbiomed.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Price J.T., Quinn J.M.W., Sims N.A., Vieusseux J., Waldeck K., Docherty S.E., Myers D., Nakamura A., Waltham M.C., Gillespie M.T., et al. The Heat Shock Protein 90 Inhibitor, 17-Allylamino-17-demethoxygeldanamycin, Enhances Osteoclast Formation and Potentiates Bone Metastasis of a Human Breast Cancer Cell Line. Cancer Res. 2005;65:4929. doi: 10.1158/0008-5472.CAN-04-4458. [DOI] [PubMed] [Google Scholar]
- 130.Talaei S., Mellatyar H., Asadi A., Akbarzadeh A., Sheervalilou R., Zarghami N. Spotlight on 17-AAG as an Hsp90 inhibitor for molecular targeted cancer treatment. Chem. Biol. Drug Des. 2019;93:760–786. doi: 10.1111/cbdd.13486. [DOI] [PubMed] [Google Scholar]
- 131.Schulte T.W., Neckers L.M. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother. Pharmacol. 1998;42:273–279. doi: 10.1007/s002800050817. [DOI] [PubMed] [Google Scholar]
- 132.Schnur R.C., Corman M.L., Gallaschun R.J., Cooper B.A., Dee M.F., Doty J.L., Muzzi M.L., DiOrio C.I., Barbacci E.G., Miller P.E., et al. erbB-2 oncogene inhibition by geldanamycin derivatives: Synthesis, mechanism of action, and structure-activity relationships. J. Med. Chem. 1995;38:3813–3820. doi: 10.1021/jm00019a011. [DOI] [PubMed] [Google Scholar]
- 133.Pacey S., Gore M., Chao D., Banerji U., Larkin J., Sarker S., Owen K., Asad Y., Raynaud F., Walton M., et al. A Phase II trial of 17-allylamino, 17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Investig. New Drugs. 2012;30:341–349. doi: 10.1007/s10637-010-9493-4. [DOI] [PubMed] [Google Scholar]
- 134.Goetz M.P., Toft D., Reid J., Ames M., Stensgard B., Safgren S., Adjei A.A., Sloan J., Atherton P., Vasile V., et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J. Clin. Oncol. 2005;23:1078–1087. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 135.Smith V., Sausville E.A., Camalier R.F., Fiebig H.H., Burger A.M. Comparison of 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17DMAG) and 17-allylamino-17-demethoxygeldanamycin (17AAG) in vitro: Effects on Hsp90 and client proteins in melanoma models. Cancer Chemother. Pharmacol. 2005;56:126–137. doi: 10.1007/s00280-004-0947-2. [DOI] [PubMed] [Google Scholar]
- 136.Pacey S., Wilson R.H., Walton M., Eatock M.M., Hardcastle A., Zetterlund A., Arkenau H.-T., Moreno-Farre J., Banerji U., Roels B., et al. A Phase I Study of the Heat Shock Protein 90 Inhibitor Alvespimycin (17-DMAG) Given Intravenously to Patients with Advanced Solid Tumors. Clin. Cancer Res. 2011;17:1561. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Delmotte P., Delmotte-Plaque J. A new antifungal substance of fungal origin. Nature. 1953;171:344. doi: 10.1038/171344a0. [DOI] [PubMed] [Google Scholar]
- 138.Amolins M.W., Blagg B.S.J. Natural product inhibitors of Hsp90: Potential leads for drug discovery. Mini-Rev. Med. Chem. 2009;9:140–152. doi: 10.2174/138955709787316056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Soga S., Shiotsu Y., Akinaga S., Sharma S.V. Development of radicicol analogues. Curr. Cancer Drug Targets. 2003;3:359–369. doi: 10.2174/1568009033481859. [DOI] [PubMed] [Google Scholar]
- 140.Crowley V.M., Khandelwal A., Mishra S., Stothert A.R., Huard D.J.E., Zhao J., Muth A., Duerfeldt A.S., Kizziah J.L., Lieberman R.L., et al. Development of Glucose Regulated Protein 94-Selective Inhibitors Based on the BnIm and Radamide Scaffold. J. Med. Chem. 2016;59:3471–3488. doi: 10.1021/acs.jmedchem.6b00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eccles S.A., Massey A., Raynaud F.I., Sharp S.Y., Box G., Valenti M., Patterson L., de Haven Brandon A., Gowan S., Boxall F., et al. NVP-AUY922: A novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008;68:2850–2860. doi: 10.1158/0008-5472.CAN-07-5256. [DOI] [PubMed] [Google Scholar]
- 142.Sharp S.Y., Roe S.M., Kazlauskas E., Čikotienė I., Workman P., Matulis D., Prodromou C. Co-Crystalization and In Vitro Biological Characterization of 5-Aryl-4-(5-Substituted-2-4-Dihydroxyphenyl)-1,2,3-Thiadiazole Hsp90 Inhibitors. PLoS ONE. 2012;7:e44642. doi: 10.1371/journal.pone.0044642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seggewiss-Bernhardt R., Bargou R.C., Goh Y.T., Stewart A.K., Spencer A., Alegre A., Bladé J., Ottmann O.G., Fernandez-Ibarra C., Lu H., et al. Phase 1/1B trial of the heat shock protein 90 inhibitor NVP-AUY922 as monotherapy or in combination with bortezomib in patients with relapsed or refractory multiple myeloma. Cancer. 2015;121:2185–2192. doi: 10.1002/cncr.29339. [DOI] [PubMed] [Google Scholar]
- 144.Kudryavtsev V.A., Khokhlova A.V., Mosina V.A., Selivanova E.I., Kabakov A.E. Induction of Hsp70 in tumor cells treated with inhibitors of the Hsp90 activity: A predictive marker and promising target for radiosensitization. PLoS ONE. 2017;12:e0173640. doi: 10.1371/journal.pone.0173640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu Y., Yu Y., Chu H., Bing D., Wang S., Zhou L., Chen J., Chen Q., Pan C., Sun Y., et al. 17-DMAG induces Hsp70 and protects the auditory hair cells from kanamycin ototoxicity in vitro. Neurosci. Lett. 2015;588:72–77. doi: 10.1016/j.neulet.2014.12.060. [DOI] [PubMed] [Google Scholar]
- 146.Drysdale M.J., Brough P.A., Massey A., Jensen M.R., Schoepfer J. Targeting Hsp90 for the treatment of cancer. Curr. Opin. Drug Discov. Dev. 2006;9:483–495. [PubMed] [Google Scholar]
- 147.Dakappagari N., Neely L., Tangri S., Lundgren K., Hipolito L., Estrellado A., Burrows F., Zhang H. An investigation into the potential use of serum Hsp70 as a novel tumour biomarker for Hsp90 inhibitors. Biomarkers. 2010;15:31–38. doi: 10.3109/13547500903261347. [DOI] [PubMed] [Google Scholar]
- 148.Ghoshal S., Rao I., Earp J.C., Jusko W.J., Wetzler M. Down-regulation of heat shock protein 70 improves arsenic trioxide and 17-DMAG effects on constitutive signal transducer and activator of transcription 3 activity. Cancer Chemother. Pharmacol. 2010;66:681–689. doi: 10.1007/s00280-009-1210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kühnel A., Schilling D., Combs S.E., Haller B., Schwab M., Multhoff G. Radiosensitization of HSF-1 Knockdown Lung Cancer Cells by Low Concentrations of Hsp90 Inhibitor NVP-AUY922. Cells. 2019;8:1166. doi: 10.3390/cells8101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Usmani S.Z., Bona R.D., Chiosis G., Li Z. The anti-myeloma activity of a novel purine scaffold HSP90 inhibitor PU-H71 is via inhibition of both HSP90A and HSP90B1. J. Hematol. Oncol. 2010;3:40. doi: 10.1186/1756-8722-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Patel H.J., Patel P.D., Ochiana S.O., Yan P., Sun W., Patel M.R., Shah S.K., Tramentozzi E., Brooks J., Bolaender A., et al. Structure–Activity Relationship in a Purine-Scaffold Compound Series with Selectivity for the Endoplasmic Reticulum Hsp90 Paralog Grp94. J. Med. Chem. 2015;58:3922–3943. doi: 10.1021/acs.jmedchem.5b00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu S., Street T.O. 5’-N-ethylcarboxamidoadenosine is not a paralog-specific Hsp90 inhibitor. Protein Sci. 2016;25:2209–2215. doi: 10.1002/pro.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cheng C.-C., Yang Y.L., Liao K.H., Lai T.W. Adenosine receptor agonist NECA increases cerebral extravasation of fluorescein and low molecular weight dextran independent of blood-brain barrier modulation. Sci. Rep. 2016;6:23882. doi: 10.1038/srep23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dickson M.A., Okuno S.H., Keohan M.L., Maki R.G., D’Adamo D.R., Akhurst T.J., Antonescu C.R., Schwartz G.K. Phase II study of the HSP90-inhibitor BIIB021 in gastrointestinal stromal tumors. Ann. Oncol. 2013;24:252–257. doi: 10.1093/annonc/mds275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ernst J.T., Liu M., Zuccola H., Neubert T., Beaumont K., Turnbull A., Kallel A., Vought B., Stamos D. Correlation between chemotype-dependent binding conformations of HSP90α/β and isoform selectivity-Implications for the structure-based design of HSP90α/β selective inhibitors for treating neurodegenerative diseases. Bioorganic Med. Chem. Lett. 2014;24:204–208. doi: 10.1016/j.bmcl.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 156.Eletto D., Dersh D., Argon Y. GRP94 in ER quality control and stress responses. Semin. Cell Dev. Biol. 2010;21:479–485. doi: 10.1016/j.semcdb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li X., Sun L., Hou J., Gui M., Ying J., Zhao H., Lv N., Meng S. Cell membrane gp96 facilitates HER2 dimerization and serves as a novel target in breast cancer. Int. J. Cancer. 2015;137:512–524. doi: 10.1002/ijc.29405. [DOI] [PubMed] [Google Scholar]
- 158.Hou J., Li X., Li C., Sun L., Zhao Y., Zhao J., Meng S. Plasma membrane gp96 enhances invasion and metastatic potential of liver cancer via regulation of uPAR. Mol. Oncol. 2015;9:1312–1323. doi: 10.1016/j.molonc.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yan P., Patel H.J., Sharma S., Corben A., Wang T., Panchal P., Yang C., Sun W., Araujo T.L., Rodina A., et al. Molecular Stressors Engender Protein Connectivity Dysfunction through Aberrant N-Glycosylation of a Chaperone. Cell Rep. 2020;31:107840. doi: 10.1016/j.celrep.2020.107840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sabbatino F., Favoino E., Wang Y., Wang X., Villani V., Cai L., Yang L., Ferrone S., Ferrone C.R. Grp94-specific monoclonal antibody to counteract BRAF inhibitor resistance in BRAF(V600E) melanoma. J. Transl Med. 2015;13:K12. doi: 10.1186/1479-5876-13-S1-K12. [DOI] [Google Scholar]
- 161.Melendez K., Wallen E.S., Edwards B.S., Mobarak C.D., Bear D.G., Moseley P.L. Heat shock protein 70 and glycoprotein 96 are differentially expressed on the surface of malignant and nonmalignant breast cells. Cell Stress Chaperones. 2006;11:334–342. doi: 10.1379/CSC-187.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hou J., Deng M., Li X., Liu W., Chu X., Wang J., Chen F., Meng S. Chaperone gp96 mediates ER-α36 cell membrane expression. Oncotarget. 2015;6:31857–31867. doi: 10.18632/oncotarget.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Singh S., Kumar N.K., Dwiwedi P., Charan J., Kaur R., Sidhu P., Chugh V.K. Monoclonal Antibodies: A Review. Curr. Clin. Pharmacol. 2018;13:85–99. doi: 10.2174/1574884712666170809124728. [DOI] [PubMed] [Google Scholar]
- 164.Yang H.Y., Kang K.J., Chung J.E., Shim H. Construction of a large synthetic human scFv library with six diversified CDRs and high functional diversity. Mol. Cells. 2009;27:225–235. doi: 10.1007/s10059-009-0028-9. [DOI] [PubMed] [Google Scholar]
- 165.Santini D., Vincenzi B., Addeo R., Garufi C., Masi G., Scartozzi M., Mancuso A., Frezza A.M., Venditti O., Imperatori M., et al. Cetuximab rechallenge in metastatic colorectal cancer patients: How to come away from acquired resistance? Ann. Oncol. 2012;23:2313–2318. doi: 10.1093/annonc/mdr623. [DOI] [PubMed] [Google Scholar]
- 166.Todd P.A., Brogden R.N. Muromonab CD3. A review of its pharmacology and therapeutic potential. Drugs. 1989;37:871–899. doi: 10.2165/00003495-198937060-00004. [DOI] [PubMed] [Google Scholar]
- 167.Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 168.Tabasinezhad M., Talebkhan Y., Wenzel W., Rahimi H., Omidinia E., Mahboudi F. Trends in therapeutic antibody affinity maturation: From in-vitro towards next-generation sequencing approaches. Immunol. Lett. 2019;212:106–113. doi: 10.1016/j.imlet.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 169.Van Cutsem E., Köhne C.H., Hitre E., Zaluski J., Chang Chien C.R., Makhson A., D’Haens G., Pintér T., Lim R., Bodoky G., et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.