Abstract
Diseases caused by flaviviruses, including dengue fever and Japanese encephalitis, are major health problems in Vietnam. This cross-sectional study explored the feasibility of domestic dogs as sentinels to better understand risks of mosquito-borne diseases in Hanoi city. A total of 475 dogs serum samples from 221 households in six districts of Hanoi were analyzed by a competitive enzyme-linked immunosorbent assay (cELISA) for antibodies to the pr-E protein of West Nile virus and other flaviviruses due to cross-reactivity. The overall flavivirus seroprevalence in the dog population was 70.7% (95% CI = 66.4–74.8%). At the animal level, significant associations between seropositive dogs and district location, age, breed and keeping practice were determined. At the household level, the major risk factors were rural and peri-urban locations, presence of pigs, coil burning and households without mosquito-borne disease experience (p < 0.05). Mosquito control by using larvicides or electric traps could lower seropositivity, but other measures did not contribute to significant risk mitigation of flavivirus exposure in dogs. These results will support better control of mosquito-borne diseases in Hanoi, and they indicate that dogs can be used as sentinels for flavivirus exposure.
Keywords: dogs, mosquito-borne flavivirus, seroprevalence, Hanoi, Vietnam
1. Introduction
Viruses within the genus Flavivirus of the family Flaviviridae are responsible for a number of vector-borne diseases in humans, such as dengue, Japanese encephalitis (JE), Zika, yellow fever, West Nile (WN) and many others worldwide [1].
In nature, mosquito-borne flaviviruses circulate between arthropod vectors, generally Aedes spp. mosquitoes for dengue virus (DENV), Zika virus (ZIKV) and yellow fever virus (YFV), and Culex spp. mosquitoes for Japanese encephalitis virus (JEV) and West Nile virus (WNV), and vertebrate hosts [2]. Mosquitoes acquire flaviviruses mainly through horizontal transmission by taking a bloodmeal from a viremic animal, or possibly through vertical transmission from mother to offspring [3], while vertebrate hosts become infected by the probing process of blood feeding of an infected mosquito vector [4].
The rapidly urbanizing Hanoi, the capital of Vietnam, has a high density of people and different domestic animals [5]. In 2018, there were 7.9 million people, 1.8 million pigs, 136 thousand cattle, 23.5 thousand buffaloes, 11.5 thousand goats, 0.4 thousand horses, 31.5 million poultry and 450.3 thousand dogs in Hanoi [6]. People in the city are exposed to flaviviruses, mainly DENV and JEV [7].
In Hanoi, there are indigenous and exotic breeds of dogs, as well as crossbreeds. They are important as companion pets, for guarding property or as a human food source. Dogs are the closest animals to human dwellings, and they could be exposed to vector-borne pathogens to the same extent as their owners. Due to very low level of viremia, dogs do not usually show any clinical signs of flaviviral infections, nor transmit the disease to humans, but flavivirus seroprevalences in dog populations may be valuable as sentinels to evaluate risk factors for humans [8,9]. The objective of this study was to assess the association between risk factors at the household level and flavivirus exposure in the dog population in Hanoi city.
2. Materials and Methods
2.1. Study Design
A total of six districts including two more rural, where large populations of livestock are kept (with more than 1000 large ruminants, 15,000 pigs and 150,000 poultry per district), two peri-urban (less than 1000 large ruminants, 15,000 pigs and 150,000 poultry per district) and two urban districts with no livestock keeping in Hanoi were purposively selected to represent a gradient of livestock keeping.
Sample size was calculated as 475 dogs with 50% of the assumed true seroprevalence due to no previous data available for flavivirus prevalence in the dog population of Hanoi city, 5% desired precision, a 95% confidence level and a test assumed with 95% sensitivity and 95% specificity [10]. An additional 5% of the sample size was compensated in case of insufficient samples for testing.
The multi-stage cluster sampling strategy was applied in which random selection of 120 global positioning system (GPS) points in six districts was conducted, and within a radius of 2 km from each GPS point, about five households keeping a dog(s) were visited during September and October 2018. Here, the owners were interviewed by a structured questionnaire (Supplementary file, Household questionnaire) form that was pre-tested for comprehensibility, of which demographic characteristics of the respondents and their dogs, potential risk factors related to livestock keeping and mosquito prevention practices were included, and dog blood was taken for serology. A house keeping at least a ruminant or a pig or five small animals such as chickens, ducks, geese or rabbits was defined as a livestock-keeping household.
2.2. Sample Collection and Storage
Dog blood sampling was conducted by trained veterinarians of the Hanoi Sub-Department of Livestock Production and Animal Health, using venipuncture of Vena cephalica or V. saphena. If a household had several dogs, the maximum number of dogs sampled was five. The sample size was calculated from 50% of the expected seroprevalence within a flock with a 95% confidence level, and the same test sensitivity and specificity levels at 95%. The samples were stored in a cool box in the field and transferred to the National Institute for Veterinary Research (NIVR) on the sampling day. Sera were centrifuged and separated immediately and kept at −20 °C condition until tested. A total of 502 dogs from 225 households surrounding 44 GPS points in six districts of Hanoi were sampled (Table 1).
Table 1.
Number of households visited with dogs sampled.
| Category | Number of Dogs Sampled | Number of Households |
|---|---|---|
| Not enough serum | 16 | 1 |
| cELISA doubtful | 11 | 3 |
| cELISA positive | 336 | 221 |
| cELISA negative | 139 | |
| Sum | 502 | 225 |
2.3. Laboratory Technique
Competitive Enzyme-Linked Immunosorbent Assay (cELISA)
A commercial cELISA kit for detection of IgG antibodies against WNV manufactured by IDvet company (Grabels, France) was employed. In principle, samples to be tested and controls are added to the microwells precoated with the pr-E protein of WNV. However, the protein includes epitopes that are common to all flaviviruses, causing serological cross-reactions; hence, this ELISA kit does not only detect antibodies against WNV but also cross-reactive antibodies induced by other flaviviruses [11]. Anti-pr-E antibodies, if present, form an antigen–antibody complex. An anti-pr-E antibody horseradish peroxidase (HRP) conjugate binds to the remaining free pr-E epitopes, forming an antigen–conjugate–peroxidase complex. The kit is not species-dependent [8,12,13]. The analyses were performed according to the manufacturer’s instructions. Calculation of the S/N percentage (S/N%) was equal to the value of optical density (OD) of the sample divided by the value of OD of the negative control then multiplied by 100. Samples presenting an S/N% less than or equal to 40% were considered positive; higher than 40% and less than or equal to 50% were considered doubtful; higher than 50% were considered negative.
Each dog serum was tested in duplicate on the same ELISA plate.
2.4. Statistical Analysis
A total of twenty-seven serum samples were excluded in the analysis: 16 samples had insufficient volumes and 11 samples showed doubtful results by the cELISA. Four households that had all their samples within the group of the 27 excluded sera were removed. Households were considered positive if at least one dog showed seropositivity. Data obtained from the questionnaires and the laboratory results were recorded in an Excel® spreadsheet and then transferred into STATA/SE 15.0 (StataCorp LLC, College Station, TX, USA) for analysis. Descriptive statistics for the categorical variables for dogs and households displayed by the cELISA results were used. A chi-square test was used to evaluate the association of the explanatory variables in the univariable analyses. At the household level, all independent variables were compared to assess the correlation among variables. A stepwise selection of variables based on univariable analyses with a cutoff value of 0.25 [14] and correlation measurements was applied in logistic regression models. Variables changing more than 25% from the coefficients of other variables were classified as confounding factors and they were forced into the model if the affected variables were significant. The likelihood ratio test was performed to build a parsimonious model, and the Hosmer–Lemeshow goodness-of-fit test was used for model fitness [15]. A p-value <0.05 was considered statistically significant.
2.5. Ethics
Ethical approval was obtained from the Ethical Review Board for Biomedical Research of Hanoi University of Public Health (Number 406/2018/YTCC-HD3, approved on 6 August 2018). The purpose of this study was explained to dog owners by veterinary officers of the Hanoi Sub-Department of Livestock Production and Animal Health to solicit informed consent to participate in the study.
3. Results
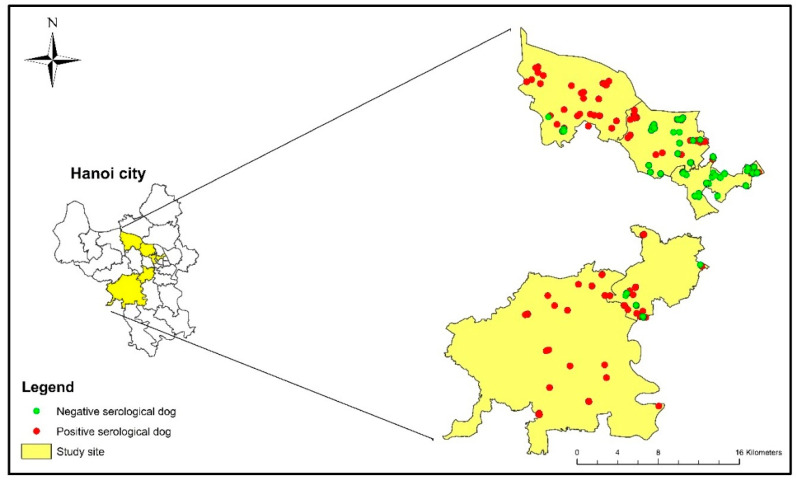
A total of 486 dogs from 224 households surrounding 44 GPS points in six districts of Hanoi were examined for the presence of antibodies against flaviviruses (Table 1). The geographical distribution of dogs tested in individual households is shown in Figure 1.
Figure 1.
Distribution of flavivirus seroprevalence in dogs rising in Hanoi by cELISA.
3.1. Seroprevalence by cELISA
The cELISA results revealed 336 positive samples to the pr-E protein of WNV, 139 negative samples and 11 doubtful samples. The doubtful samples were removed from further analyses.
Out of the remaining 475 dogs in 221 households, the flavivirus seropositivity was 70.7% (95% CI = 66.4–74.8%). The seroprevalences of males and females were 73.2% (95% CI = 66.6–79.0%) and 75.7% (95% CI = 67.9–82.1%), respectively.
Seroprevalences of crossbreed (91.3%; 95% CI = 84.1–95.5%) and local breed (71.6%; 95% CI = 65.6–77.7%) were significantly higher than exotic breed (33.3%; 95% CI = 17.3–54.4%). Significantly higher seropositivity was found in dogs under 12 months old (77.5%; 95% CI = 72.1–82.1%) compared to dogs above 12 months old (56.1%; 95% CI = 46.5–65.2%). The seroprevalence was significantly higher in rural districts (93.3%; 95% CI = 88.1–96.4%) as compared to peri-urban districts (74.5%; 95% CI = 68.5–79.7%) and urban districts (22.5%; 95% CI = 14.9–32.4%). Dogs kept outside the house showed a significantly higher seroprevalence (79.5%; 95% CI = 70.9–86.0%) than indoor dogs (58.2%; 95% CI = 47.8–68.0%).
Univariable analyses at the animal level (Table 2) identified significant associations between seropositive dogs and district location, age of dog, breed of dog and keeping practice of dog in the households.
Table 2.
Results from univariable analysis showing the association between seropositivity of dogs and exposure variables.
| Exposure Variable | Label | Total Test | Positive | Seroprevalence (95% CI) |
OR (95% CI) |
p-Value |
|---|---|---|---|---|---|---|
| Sex | Male | 198 | 145 | 73.2 (66.6–79.0) |
0.88 (0.52–1.48) |
0.607 |
| Female | 140 | 106 | 75.7 (67.9–82.1) |
1 | ||
| Breed | Local | 215 | 153 | 71.6 (65.6–77.7) |
4.94 (1.87–13.9) |
<0.001 |
| Crossbreed | 104 | 95 | 91.3 (84.1–95.5) |
21.11 (6.28–72.6) |
||
| Exotic | 24 | 8 | 33.3 (17.3–54.4) |
1 | ||
| Age group | ≤12 months | 271 | 210 | 77.5 (72.1–82.1) |
2.70 (1.62–4.46) |
<0.001 |
| >12 months | 107 | 60 | 56.1 (46.5–65.2) |
1 | ||
| District | Rural | 151 | 141 | 93.3 (88.1–96.4) |
48.6 (20.4–121) |
<0.001 |
| Peri-urban | 235 | 175 | 74.5 (68.5–79.7) |
10.06 (5.47–18.9) |
||
| Urban | 89 | 20 | 22.5 (14.9–32.4) |
1 | ||
| Dog keeping at house | Outside | 112 | 89 | 79.5 (70.9–86.0) |
2.77 (1.43–5.42) |
0.001 |
| Inside | 91 | 53 | 58.2 (47.8–68.0) |
1 |
Abbreviations: OR, odds ratio; CI, confidence interval.
3.2. Univariable Analysis Results at Household Level
The results obtained by univariable analyses at the household level (Table 3) revealed that households with seropositivity against flavivirus were significantly associated with district location, presence of livestock such as pigs or chickens, mosquito-borne disease history in the family and mosquito coil burning measures.
Table 3.
Results from univariable analysis showing the association between seropositivity of households and exposure variables.
| Exposure Variable | Label | Total HH Tested | HH Positive | OR (95%CI) |
p-Value |
|---|---|---|---|---|---|
| District | Rural | 71 | 67 | 40.6 (12.9–127) |
<0.001 |
| Peri-urban | 85 | 75 | 18.2 (7.77–42.5) |
<0.001 | |
| Urban | 65 | 19 | 1 | - | |
| Household keeping livestock in general | Yes | 81 | 75 | 8.13 (3.31–20.0) |
<0.001 |
| No | 137 | 83 | 1 | ||
| Household keeping pig | Yes | 70 | 64 | 6.13 (2.49–15.1) |
<0.001 |
| No | 148 | 94 | 1 | ||
| Household keeping chicken | Yes | 38 | 35 | 5.41 (1.60–18.3) |
0.007 |
| No | 180 | 123 | 1 | ||
| Household that has cat | Yes | 32 | 27 | 2.27 (0.83–6.19) |
0.110 |
| No | 186 | 131 | 1 | ||
| Family member no experience with mosquito disease | Yes | 199 | 149 | 5.96 (1.94–18.3) |
0.002 |
| No | 15 | 5 | 1 | ||
| Mosquito Prevention Practice by Using: | |||||
| Window/door screen | Yes | 23 | 15 | 0.71 (0.28–1.76) |
0.457 |
| No | 190 | 138 | 1 | ||
| Repellent | Yes | 39 | 31 | 1.65 (0.71–3.83) |
0.243 |
| No | 174 | 122 | 1 | ||
| Mosquito net | Yes | 189 | 137 | 1.32 (0.53–3.26) |
0.551 |
| No | 24 | 16 | 1 | ||
| Electric racket/portable electric trap | Yes | 126 | 86 | 0.64 (0.34–1.20) |
0.164 |
| No | 87 | 67 | 1 | ||
| Mosquito coil/incense stick | Yes | 40 | 35 | 3.26 (1.21–8.78) |
0.019 |
| No | 173 | 118 | 1 | ||
| Lid covered on water tank | Yes | 77 | 50 | 0.59 (0.32–1.09) |
0.094 |
| No | 136 | 103 | 1 | ||
| Chemical/larvicide in water container | Yes | 12 | 7 | 0.527 (0.16–1.73) |
0.291 |
| No | 201 | 146 | 1 | ||
| Insecticides spraying | Yes | 102 | 74 | 1.07 (0.59–1.95) |
0.823 |
| No | 111 | 79 | 1 | ||
| Breeding site elimination | Yes | 51 | 40 | 1.58 (0.75–3.33) |
0.232 |
| No | 162 | 113 | 1 | ||
| Fish in water container | Yes | 69 | 54 | 1.64 (0.84–3.20) |
0.151 |
| No | 144 | 99 | 1 |
Abbreviations: HH, household; OR, odds ratio; CI, confidence interval.
On the one hand, the risk of households being seropositive in rural districts (OR = 40.6, p < 0.001) and peri-urban districts (OR = 12.8, p < 0.001) was significantly higher than in urban districts. Likewise, the risk of seropositivity was higher (p < 0.01) in households keeping livestock, pigs and/or chickens than houses without livestock. Families without a reported previous human case of a mosquito-borne disease had a higher risk of having seropositive dogs (OR = 5.96, p = 0.002).
There was no significant difference in seroprevalence depending on the presence of cats in the houses.
On the other hand, burning coils to control mosquitoes was significantly associated with an increased proportion of positive households (OR = 3.263, p = 0.019). Other practices at households including door/window screening, use of repellent, mosquito net, mosquito electric trap or racket, lid covered on water tanks, larvicides, insecticides, eliminating the breeding site of mosquitoes and keeping fish in water tanks did not show a significant risk associated with flavivirus exposure.
3.3. Multivariable Analysis Results
The paired variables between district location and livestock keeping (r = −0.75), between district location and pig (r = −0.71) and between livestock keeping and pig production (r = 0.89) were strongly correlated. Of the 81 households keeping livestock, 86% (n = 70) of the households kept pigs and the final models of pig keeping and livestock keeping variables were not different. Therefore, the variable for livestock keeping was taken out from the modeling. There were two multivariable logistic regression models built (Table 4).
Table 4.
Final multivariable analysis of risk factors for dog-keeping households.
| Exposure Variable | Categories | Coef. | ORs | 95% CI | p-Value |
|---|---|---|---|---|---|
| Model 1. Without the variables for livestock keeping and pig keeping | |||||
| District | Rural | 3.70 | 40.6 | 12.3–134 | <0.001 |
| Peri-urban | 2.81 | 16.7 | 6.96–40.2 | <0.001 | |
| Urban | Ref | Ref | |||
| No larvicides in water containers | Yes | 1.68 | 5.39 | 1.06–27.3 | 0.042 |
| No | Ref | Ref | |||
| Coil burning | Yes | 0.78 | 2.18 | 0.58–8.11 | 0.247 |
| No | Ref | Ref | |||
| Model 2. Without the variables for district location and livestock keeping | |||||
| Pig keeping | Yes | 1.75 | 5.76 | 2.27–14.6 | <0.001 |
| No | Ref | Ref | |||
| No use of mosquito electric racket/trap | Yes | 0.73 | 2.08 | 1.05–4.14 | 0.036 |
| No | Ref | Ref | |||
| Coil burning | Yes | 1.13 | 3.09 | 1.04–9.17 | 0.042 |
| No | Ref | Ref | |||
| No experience with mosquito disease in family | Yes | 1.60 | 4.94 | 1.50–16.3 | 0.009 |
| No | Ref | Ref | |||
Abbreviations: Coef., coefficients; Ref, reference; OR, odds ratio; CI, confidence interval.
In model 1 that excluded the variables of livestock keeping and pig keeping, coil burning had an effect of more than 33% on the coefficient of larvicides use and the change in this exposure variable became insignificant; therefore, this confounding factor was added back to the model. The final model determined district location and use of larvicides in water tanks were significantly associated with the positivity of houses (p < 0.05).
Model 2 without the variables of district location and livestock keeping identified significant risks of the positivity of households as pig keeping, mosquito electric trap use, coil burning and mosquito-borne disease history of family (p < 0.05).
Both models showed a good fit (Hosmer–Lemeshow statistic test, p = 0.114 and 0.541, respectively).
4. Discussion
This study indicated that the overall seroprevalence against flaviviruses in dogs in Hanoi was as high as 70.7%. By the same cELISA as used here, previously reported prevalences of flavivirus seropositivity in dogs have been highly varied, e.g., 5.7% in China [8], 62% in Morocco [16] and 42.1% in Romania [17]. Notwithstanding the circulation in Cambodia, Myanmar, Thailand, Indonesia, Malaysia, the Philippines and China, WNV has never been reported in Vietnam [18]. However, several other flaviviruses have been long present in Vietnam. Specifically, JEV was isolated already in 1951 and subsequent virus isolations have been performed from humans, pigs and birds [19,20]. In 1958, DENV was first reported in the northern region of Vietnam [21]; seropositivity for Zika was first confirmed in 1954 and since then, the first cases of ZIKV were reported in 2015 [22]. Flaviviruses are well known to have serological cross-reactions, and therefore the results of the ELISA are not sufficiently virus-specific. The plaque reduction neutralization test (PRNT), the most specific serological test for flaviviruses [23], was not conducted against all flaviviruses endemically circulating in Vietnam as well as WNV; therefore, we acknowledge this limitation in our study. Serum neutralization assays for multiple flaviviruses are suggested for confirmation in future studies.
Dog puppies under 12 months of age showed more than two times higher odds of having flavivirus antibodies as compared to adult dogs. Naturally, old animals have had more chances of being exposed to infectious agents. However, maternal antibodies from infected mother dogs to their puppies through colostrum could be maintained for a period, which could explain this higher rate. However, maternal immunity to flaviviruses in dogs is still unknown. Generally, immunity relies on flavivirus antibody persistence in vertebrate hosts, but the mechanism(s) for persistence is still poorly understood [24,25]. Further studies on flavivirus immunology post-infection in dogs are suggested.
Dogs with greater outdoor exposure obtained a higher level of flavivirus seroprevalence, which was also consistent with earlier findings [8,26]. Generally, exotic breeds of dogs imported to Vietnam have a very high economical value and they are closer to their owner. In contrast, crossbreed and indigenous dogs that have lower value may be kept outdoors more; therefore, they have a greater possibility of being infected with flaviviruses through mosquito feeding. Significantly higher seroprevalences to flavivirus of local and crossbreed dogs due to more frequent outdoor keeping than exotic ones were also found in this study.
Location of households was significantly associated with seropositivity among the dogs, while sex of dogs was not a risk factor, which is similar to a previous study [8]. Rural dogs and peri-urban dogs showed forty times and eighteen times higher risk of exposure as compared to urban dogs, respectively. This is likely related to the greater livestock presence in rural than in urban Hanoi [5], since more mosquitoes have been found in livestock shelters than in non-livestock-keeping households [27]. In fact, vector distribution varies depending on mosquito species. For instance, Culex spp. mosquitoes, the major vector of JEV, prefer to breed in polluted aquatic habitats such as rice production areas, wetlands and ponds [28]. A previous study found high abundance of Culex tritaeniorhynchus, Cx. vishnui, Cx. gelidus and Cx. fuscocephala in cultivating rice fields of a Hanoi rural area [29]. In contrast, Aedes spp. mosquitoes, the main vector for dengue, have been more adapted to human environments and have their breeding sites in clean and undisturbed water; therefore, they are close to aquatic items surrounding human dwellings [30]. An entomological survey in Hanoi revealed that concrete water tanks, clay jars and drums were abundant in Aedes aegypti and Ae. albopictus larvae [31].
Households keeping livestock, especially pigs or/and chickens, were at higher risk of flavivirus exposure in dogs than houses without a livestock presence. In Vietnam, the seroprevalence of JEV in pigs, another domestic species, has been reported at above 70% [9,32,33]. Pigs are known amplifying hosts for JEV and known to attract mosquitoes; thus, pig keeping increases the risk of both viral circulation and the number of mosquitoes that act as vectors [34].
The risk of flavivirus exposure in dogs was about six times higher in families without historical infection of mosquito-borne diseases compared to households that reported human cases of DENV or JEV. The reduced risk of flavivirus seropositivity in dogs in households with experience of mosquito-borne disease could also be correlated to the risk mitigation behavior of these families.
A limitation of our study is that mosquito control practices of households were not directly observed, or the frequency of implementation was not recorded; therefore, the evaluation of mosquito prevention could not be performed.
However, some mosquito prevention measures applied in households were reported in this study. In particular, window screening can block entry points for mosquitoes in a house [35], while repellents can influence mosquito olfaction [36]. Mosquito bed nets are the most commonly used measure by people in Hanoi, followed by anti-mosquito products such as insecticides, elimination of breeding sites and electric rackets, in order to prevent mosquitoes [37]. Water containers are the most likely breeding sites of some mosquito species such as Ae. albopictus, Ae. niveus gp. and Cx. quinquefasciatus [38]. Previous studies in Vietnam concluded that the use of an appropriate cover on water storage containers effectively reduces pre-adult mosquito infestation levels [39,40]. Keeping fish as predators of mosquito larvae, a biological control for mosquito-borne diseases, has also been studied [41,42].
However, some methods that protect humans and pets from mosquito bites in this study such as using a window screen, repellents, mosquito nets and insecticides spraying did not show any efficiency of lowering seropositivity against flavivirus for the dogs.
Burning a mosquito coil indoors generates smoke that can control mosquitoes [43,44,45]. In this study, a significant increase in the seropositivity of dogs in the houses burning coils as compared to dogs from the houses without coils was identified, but dogs’ behavior in reaction to coil smoke is unclear. If dogs are sensitive to coil smoke, they may avoid it the same way as mosquito vectors and thus still be at risk of infection. A previous study suggested that mosquito coils do not significantly affect the risk of a mosquito-borne disease in humans if the coils are burnt just once per week [46].
Multivariable logistic regression models identified more rural location, pig grazing, no application of mosquito control measures such as electric rackets or larvicides in home water tanks, burning coils and family without mosquito-borne disease experience as the main risk factors for flavivirus exposure of dog-keeping households.
5. Conclusions
This study indicated a high flavivirus seroprevalence in the dog population of Hanoi. The main risk factors for households were rural area, presence of pigs, coil burning, no use of either larvicides or mosquito electric racquet/trap at home and no experience of mosquito-borne infections.
Some common mosquito control measures of local people in Hanoi did not significantly mitigate the risk of flavivirus infection in the households.
Understanding the risk factors associated with flavivirus prevalence in dogs could facilitate better mosquito-borne disease control in Hanoi.
Acknowledgments
We thank the Sub-Department of Livestock Production and Animal Health of Hanoi city for their great contribution to the field investigation. We are grateful to the National Institute for Veterinary Research (Ministry of Agriculture and Rural Development) and the National Institute for Hygiene and Epidemiology (Ministry of Health) for their kind supports to laboratory testing.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/13/3/507/s1, Household questionnaire.
Author Contributions
All authors contributed to the conception and design of this study. Conceptualization, L.P.-T., J.L.; field investigation, L.P.-T., T.N.-T., M.C.-X.; laboratory testing, V.B.-N., A.B.-N., D.L.-T., T.N.-T.T., H.V.-T.B.; data analysis and mapping, L.P.-T., H.S.L.; supervision, J.L., U.M., Å.L., H.N.-V.; L.P.-T. wrote the first draft. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas, grant number 2016-00364). The project was also supported by the CGIAR Research Program on Agriculture for Nutrition and Health.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Hanoi University of Public Health (protocol code 406/2018/YTCC-HD3 on 6 August 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets supporting our findings are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Holbrook M.R. Historical Perspectives on Flavivirus Research. Viruses. 2017;9:97. doi: 10.3390/v9050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y.-J.S., Higgs S., Horne K.M., VanLandingham D.L. Flavivirus-Mosquito Interactions. Viruses. 2014;6:4703–4730. doi: 10.3390/v6114703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brackney D.E. Implications of autophagy on arbovirus infection of mosquitoes. Curr. Opin. Insect Sci. 2017;22:1–6. doi: 10.1016/j.cois.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colpitts T.M., Conway M.J., Montgomery R.R., Fikrig E. West Nile Virus: Biology, Transmission, and Human Infection. Clin. Microbiol. Rev. 2012;25:635–648. doi: 10.1128/CMR.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pham-Thanh L., Magnusson U., Can-Xuan M., Nguyen-Viet H., Lundkvist Å., Lindahl J. Livestock Development in Hanoi City, Vietnam—Challenges and Policies. Front. Vet. Sci. 2020;7:566. doi: 10.3389/fvets.2020.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanoi Statistics Office . Hanoi Statistical Yearbook 2018. Hanoi Statistics Office; Hanoi, Vietnam: 2019. [Google Scholar]
- 7.Nguyen-Tien T., Lundkvist Å., Lindahl J. Urban transmission of mosquito-borne flaviviruses—A review of the risk for humans in Vietnam. Infect. Ecol. Epidemiol. 2019;9:1660129. doi: 10.1080/20008686.2019.1660129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan D., Ji W., Yu D., Chu J., Wang C., Yang Z., Hua X. Serological evidence of West Nile virus in dogs and cats in China. Arch. Virol. 2011;156:893–895. doi: 10.1007/s00705-010-0913-8. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson S. Ph.D. Thesis. Sveriges lantbruksuniversitet; Uppsala, Sweden: 2013. Seroprevalence of Japanese Encephalitis Virus in Pigs and Dogs in the Mekong Delta. [Google Scholar]
- 10.Humphry R.W., Cameron A., Gunn G.J. A practical approach to calculate sample size for herd prevalence surveys. Prev. Vet. Med. 2004;65:173–188. doi: 10.1016/j.prevetmed.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Beck C., Lowenski S., Durand B., Bahuon C., Zientara S., Lecollinet S. Improved reliability of serological tools for the diagnosis of West Nile fever in horses within Europe. PLoS Neglected Trop. Dis. 2017;11:e0005936. doi: 10.1371/journal.pntd.0005936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azmi K., Tirosh-Levy S., Manasrah M., Mizrahi R., Nasereddin A., Al-Jawabreh A., Ereqat S., Abdeen Z., Lustig Y., Gelman B., et al. West Nile Virus: Seroprevalence in Animals in Palestine and Israel. Vector Borne Zoonotic Dis. 2017;17:558–566. doi: 10.1089/vbz.2016.2090. [DOI] [PubMed] [Google Scholar]
- 13.Yildirim Y., Yilmaz V., Yazici K., Ozic C., Ozkul A. Molecular and serological investigation of West Nile virus (WNV) infection in donkeys, horses and native geese in Turkey. Revue Méd. Vét. 2018;169:87–92. [Google Scholar]
- 14.Zhang Z. Model building strategy for logistic regression: Purposeful selection. Ann. Transl. Med. 2016;4:111. doi: 10.21037/atm.2016.02.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagerland M.W., Hosmer D.W. A Generalized Hosmer–Lemeshow Goodness-of-Fit Test for Multinomial Logistic Regression Models. Stata J. Promot. Commun. Stat. Stata. 2012;12:447–453. doi: 10.1177/1536867X1201200307. [DOI] [Google Scholar]
- 16.Durand B., Haskouri H., Lowenski S., Vachiéry N., Beck C., Lecollinet S. Seroprevalence of West Nile and Usutu viruses in military working horses and dogs, Morocco, 2012: Dog as an alternative WNV sentinel species? Epidemiol. Infect. 2016;144:1857–1864. doi: 10.1017/S095026881600011X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexandra L.O., Crivei L., Ratoi I., Raileanu C., Porea D., Anita D., Savuta G. Endo-crine and Behavioural Response of Dog in Stress Conditions Natalia. Bull. UASVM Vet. Med. 2018;73:197–198. [Google Scholar]
- 18.Chancey C., Grinev A., Volkova E., Rios M. The Global Ecology and Epidemiology of West Nile Virus. Bio. Med. Res. Int. 2015;2015:1–20. doi: 10.1155/2015/376230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yen N.T., Duffy M.R., Hills S.L., Fischer M., Hien N.T., Hong N.M. Surveillance for Japanese Encephalitis in Vietnam, 1998–2007. Am. J. Trop. Med. Hyg. 2010;83:816–819. doi: 10.4269/ajtmh.2010.10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Do L.P., Bui T.M., Hasebe F., Morita K., Phan N.T. Molecular epidemiology of Japanese encephalitis in northern Vietnam, 1964–2011: Genotype replacement. Virol. J. 2015;12:51. doi: 10.1186/s12985-015-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thi K.L.P. Ph.D. Thesis. Université Montpellier; Montpellier, France: 2015. Epidemiology and Dynamic of Dengue and Chikungunya in Several Provinces in Vietnam. [Google Scholar]
- 22.Duong V., Dussart P., Buchy P. Zika virus in Asia. Int. J. Infect. Dis. 2017;54:121–128. doi: 10.1016/j.ijid.2016.11.420. [DOI] [PubMed] [Google Scholar]
- 23.WHO . Guidelines for PRNT of Human Antibodies to Dengue Viruses. WHO; Geneva, Switzerland: 2007. [Google Scholar]
- 24.Mlera L., Melik W., Bloom M.E. The role of viral persistence in flavivirus biology. Pathog. Dis. 2014;71:137–163. doi: 10.1111/2049-632X.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuno G., MacKenzie J.S., Junglen S., Hubálek Z., Gubler D.J., Plyusnin A. Vertebrate Reservoirs of Arboviruses: Myth, Synonym of Amplifier, or Reality? Viruses. 2017;9:185. doi: 10.3390/v9070185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kile J.C., Panella N.A., Komar N., Chow C.C., MacNeil A., Robbins B., Bunning M.L. Serologic survey of cats and dogs during an epidemic of West Nile virus infection in humans. J. Am. Veter. Med. Assoc. 2005;226:1349–1353. doi: 10.2460/javma.2005.226.1349. [DOI] [PubMed] [Google Scholar]
- 27.Jakobsen F., Nguyen-Tien T., Pham-Thanh L., Bui V.N., Nguyen-Viet H., Tran-Hai S., Lundkvist Å., Bui-Ngoc A., Lindahl J.F. Urban livestock-keeping and dengue in urban and peri-urban Hanoi, Vietnam. PLoS Negl. Trop. Dis. 2019;13:e0007774. doi: 10.1371/journal.pntd.0007774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa M., Nam V.S., Tuno N., Takagi M., Yen N.T. Influence of the Distribution of Host Species on Adult Abundance of Japanese Encephalitis Vectors—Culex vishnui Subgroup and Culex gelidus—In a Rice-Cultivating Village in Northern Vietnam. Am. J. Trop. Med. Hyg. 2008;78:159–168. doi: 10.4269/ajtmh.2008.78.159. [DOI] [PubMed] [Google Scholar]
- 29.Ohba S.-Y., Van Soai N., Van Anh D.T., Nguyen Y.T., Takagi M. Study of mosquito fauna in rice ecosystems around Hanoi, Northern Vietnam. Acta Trop. 2015;142:89–95. doi: 10.1016/j.actatropica.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Getachew D., Tekie H., Gebre-Michael T., Balkew M., Mesfin A. Breeding Sites ofAedes aegypti: Potential Dengue Vectors in Dire Dawa, East Ethiopia. Interdiscip. Perspect. Infect. Dis. 2015;2015:1–8. doi: 10.1155/2015/706276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phong T.V., Nam V.S. Key breeding Sites of Dengue Vectors in Hanoi, Vietnam, 1994–1997. Dengue Bull. 1999;23:67. [Google Scholar]
- 32.Lee H.S., Thanh T.L., Ly N.K., Nguyen-Viet H., Thakur K.K., Grace D. Seroprevalence of zoonotic diseases (leptospirosis and Japanese encephalitis) in swine in ten provinces of Vietnam: A Bayesian approach to estimate prevalence. BioRxiv. 2019;110:528. doi: 10.1371/journal.pone.0214701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindahl J.F., Ståhl K., Chirico J., Boqvist S., Thu H.T.V., Magnusson U. Circulation of Japanese Encephalitis Virus in Pigs and Mosquito Vectors within Can Tho City, Vietnam. PLoS Neglected Trop. Dis. 2013;7:e2153. doi: 10.1371/journal.pntd.0002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindahl J., Chirico J., Boqvist S., Thu H.T.V., Magnusson U. Occurrence of Japanese Encephalitis Virus Mosquito Vectors in Relation to Urban Pig Holdings. Am. J. Trop. Med. Hyg. 2012;87:1076–1082. doi: 10.4269/ajtmh.2012.12-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogoma S.B., Kannady K., Sikulu M., Chaki P.P., Govella N.J., Mukabana W.R., Killeen G.F. Window screening, ceilings and closed eaves as sustainable ways to control malaria in Dar es Salaam, Tanzania. Malar. J. 2009;8:221. doi: 10.1186/1475-2875-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paluch G., Bartholomay L., Coats J. Mosquito repellents: A review of chemical structure diversity and olfaction. Pest Manag. Sci. 2010;66:925–935. doi: 10.1002/ps.1974. [DOI] [PubMed] [Google Scholar]
- 37.Chapot L., Nguyen-Tien T., Pham-Thanh L., Nguyen-Viet H., Craven L., Lindahl J.F. A Mixed-Methods Approach to Understanding Knowledge of Mosquito-Borne Infections and Barriers for Protection in Hanoi, Vietnam. Trop. Med. Infect. Dis. 2020;5:66. doi: 10.3390/tropicalmed5020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C.D., Lee H.L., Stella-Wong S.P., Lau K.W., Sofian-Azirun M. Container survey of mosquito breeding sites in a university campus in Kuala Lumpur, Malaysia. Dengue Bull. 2009;33:187–193. [Google Scholar]
- 39.Tran H.P., Kutcher S., Kay B.H., Nguyen Y.T., Ryan P.A., Huynh T.T.T., Marquart L., O’Rourke P. Low Entomological Impact of New Water Supply Infrastructure in Southern Vietnam, with Reference to Dengue Vectors. Am. J. Trop. Med. Hyg. 2012;87:631–639. doi: 10.4269/ajtmh.2012.12-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuzuki A., Huynh T., Luu L., Tsunoda T., Takagi M., Kawada H. Effect of existing practices on reducing Aedes aegypti pre-adults in key breeding containers in Ho Chi Minh City, Vietnam. Am. J. Trop. Med. Hyg. 2009;80:752–757. doi: 10.4269/ajtmh.2009.80.752. [DOI] [PubMed] [Google Scholar]
- 41.Louca V., Lucas M.C., Green C., Majambere S., Fillinger U., Lindsay S.W. Role of fish as predators of mosquito larvae on the floodplain of the Gambia River. J. Med. Entomol. 2009;46:546–556. doi: 10.1603/033.046.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Killeen G.F., Fillinger U., Knols B.G.J. Advantages of larval control for African malaria vectors: Low mobility and behavioural responsiveness of immature mosquito stages allow high effective coverage. Malar. J. 2002;1:8. doi: 10.1186/1475-2875-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jantan I., Zaki Z., Ahmad A., Ahmad R. Evaluation of smoke from mosquito coils containing Malaysian plants against Aedes aegypti. Fitoterapia. 1999;70:237–243. doi: 10.1016/S0367-326X(99)00026-X. [DOI] [Google Scholar]
- 44.Tawatsin A., Thavara U., Chompoosri J. Field evaluation of mosquito coils derived from plants against night-biting mosquitoes in Thailand; Proceedings of the International Conference on Biopesticides 3; Kuala Lumpur, Malaysia. 21–26 April 2002; pp. 214–220. [Google Scholar]
- 45.Deshpande S.G. Renofluthrin: Novel pyrethroid insecticide mosquito coil for mosquito control. Int. J. Mosq. Res. 2016;3:1–3. [Google Scholar]
- 46.Yamamoto S.S., Louis V.R., Sié A., Sauerborn R. The effects of zooprophylaxis and other mosquito control measures against malaria in Nouna, Burkina Faso. Malar. J. 2009;8:283. doi: 10.1186/1475-2875-8-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets supporting our findings are available from the corresponding author on reasonable request.