Abstract
The kidney plays a dominant role in the pathogenesis of essential hypertension, but the initial pathogenic events in the kidney leading to hypertension are not known. Exposure to mercury has been linked to many diseases including hypertension in epidemiological and experimental studies, so we studied the distribution and prevalence of mercury in the human kidney. Paraffin sections of kidneys were available from 129 people ranging in age from 1 to 104 years who had forensic/coronial autopsies. One individual had injected himself with metallic mercury, the other 128 were from varied clinicopathological backgrounds without known exposure to mercury. Sections were stained for inorganic mercury using autometallography. Laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) was used on six samples to confirm the presence of autometallography-detected mercury and to look for other toxic metals. In the 128 people without known mercury exposure, mercury was found in: (1) proximal tubules of the cortex and Henle thin loops of the medulla, in 25% of kidneys (and also in the man who injected himself with mercury), (2) proximal tubules only in 16% of kidneys, and (3) Henle thin loops only in 23% of kidneys. The age-related proportion of people who had any mercury in their kidney was 0% at 1–20 years, 66% at 21–40 years, 77% at 41–60 years, 84% at 61–80 years, and 64% at 81–104 years. LA-ICP-MS confirmed the presence of mercury in samples staining with autometallography and showed cadmium, lead, iron, nickel, and silver in some kidneys. In conclusion, mercury is found commonly in the adult human kidney, where it appears to accumulate in proximal tubules and Henle thin loops until an advanced age. Dysfunctions of both these cortical and medullary regions have been implicated in the pathogenesis of essential hypertension, so these findings suggest that further studies of the effects of mercury on blood pressure are warranted.
Keywords: mercury, kidney, essential hypertension, environmental toxicity, heavy metal, toxic metal, risk factor, cadmium, elemental analysis, renal cell carcinoma
1. Introduction
High systolic blood pressure is the leading risk factor for global disease burden, when ranked by risk-attributable disability-adjusted life-years, and accounts for 10.4 million deaths annually [1]. The cause of most cases of hypertension, however, remains unknown [2]. The kidney has long been considered to play a central role in the pathogenesis of essential hypertension, with the most likely mechanism being impaired renal sodium excretion [3,4]. The initial pathogenetic factors leading to this are unclear, but increased sodium reabsorption in the proximal tubules [5,6], and/or oxidative damage leading to medullary ischemia [7,8], are suspected to play roles in raising blood pressure.
Epidemiological, experimental, and clinical reports suggest an association between hypertension and exposure to mercury [9,10,11,12,13,14,15,16,17,18]. A meta-analysis of 29 studies investigating the relationship between mercury biomarkers and hypertension concluded that mercury is indeed associated with hypertension and that a dose–response relationship exists between the two [9]. Furthermore, hypertension is more common in the high methylmercury exposure area of Minamata in Japan than in a nearby area of low methylmercury exposure [10], and mortality from hypertension is greater in Minamata city than in the surrounding region [11,12]. Several animal studies indicate that exposure to methylmercury gives rise to increased blood pressure [13,14,15], and accidental exposure to mercury in humans can result in increased blood pressure [16]. The mechanisms underlying mercury-induced hypertension remain unclear, but either proximal tubule dysfunction causing sodium retention or the generation of free radicals causing vasoconstriction in the medulla are possibilities [17,18].
Autometallography is a histochemical technique used to locate inorganic mercury within cells [19]. Autometallography-detected mercury has been found in the renal proximal tubules of frogs [20], fish [21], whales [22], mice [23], rats [24,25,26], dogs [27], primates [28], and two humans [29,30] who had been exposed to mercury. Mechanisms and consequences of mercury uptake and elimination in proximal tubules have been studied extensively [31,32,33,34,35,36,37]. Proximal tubules take up mercury at low mercury exposure levels, such as those resulting from the placement of even a few mercury-containing amalgam dental fillings in primates [28] or after exposure to single low doses of inorganic mercury in mice [38]. Rarely have other parts of the kidney, such as the glomeruli, Henle loops, distal tubules, or collecting ducts been shown to contain mercury after experimental exposures, and then only after administration of large doses of mercury [27].
Given the potential roles of both the kidney and of mercury in hypertension, we sought to determine the distribution and the prevalence of mercury in the human kidney. To do this, mercury was located in the kidneys of a large number of people over a wide range of ages, using two elemental bio-imaging techniques, autometallography and laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS).
2. Materials and Methods
2.1. Ethics
This study (The role of toxic metals in human diseases, X2014-029) was approved by the Human Research Committee, Sydney Local Health District (Royal Prince Alfred Hospital Zone). This institutional review board waived the need for written informed consent from relatives of individuals studied since this was a de-identified retrospective study of archived paraffin-embedded tissue.
2.2. Sample Collection
Paraffin-embedded kidney tissue blocks were obtained from the tissue archive of The New South Wales Department of Forensic Medicine. These had been taken as part of standard tissue sampling from the autopsies of 129 people (81 male, 48 female) with a mean age of 54 years, SD 28 years, median 47 years, and age range of 1–104 years. Females had a higher mean age (60 years, SD 31 years) than males (50 years, SD 25 years) (p = 0.041). Major pre-mortem medical conditions were: none known (N = 53), neurodegenerative disease (N = 41), psychosis (N = 29), epilepsy (N = 2), and one each of anorexia nervosa, Down syndrome, post-traumatic stress disorder, and self-injection with metallic mercury.
The samples were categorised into two groups: (1) Known mercury exposure. A man who injected himself intravenously with metallic mercury and later committed suicide was exposed to a consistently high level of circulating inorganic mercury for 5 months. At autopsy, mercury was found in several of his organs, including the heart and brain [30,39,40,41]. (2) Unknown mercury exposure. In 128 people without known sources of mercury exposure, causes of death were: suicide (N = 29), trauma (N = 21), cardiovascular (N = 21), drowning (N = 14), drug overdose (N = 14), infection (N = 10), undetermined (N = 6), choking (N = 4), cerebrovascular (N = 3), respiratory (N = 2), and one each of cancer, asphyxia, sudden unexpected death from epilepsy, and undernutrition.
2.3. Autometallography
Paraffin blocks were sectioned at 7 μm with a Feather S35 stainless steel disposable microtome blade, deparaffinised, and stained for inorganic mercury with silver nitrate autometallography, which represents the presence of mercury as black silver grains surrounding the mercury [42]. Autometallography is a sensitive amplification technique that can detect as few as 10 mercury sulphide/selenide molecules in a cell [43]. Sections were placed in physical developer containing 50% gum arabic, citrate buffer, hydroquinone, and silver nitrate at 26 °C for 80 min in the dark; washed in 5% sodium thiosulphate to remove unbound silver; counterstained with mercury-free hematoxylin; and viewed with bright-field microscopy. Each staining run included a control section of mouse spinal cord where motor neuron cell bodies contained mercury following an intraperitoneal injection of mercuric chloride; sections were from archived paraffin blocks of a previously published experiment approved by the Animal Ethics Committee of the University of Sydney [44]. Sections were stained with hematoxylin only to act as a control for the autometallography.
Microscopic identification of different subsets of kidney cells was based on standard histological criteria [45]. To help characterise the cell types in the kidney, autometallography-stained sections from six samples were immunostained with CD10 (Novocastra-Leica, clone 56C6), which stains proximal tubules and collecting ducts prominently [46], using a Leica Bond III staining platform (Leica Biosystems, Melbourne, Australia). Heat-mediated antigen retrieval was undertaken and a 1:25 dilution of the primary antibody was incubated at ambient temperature for 30 min. Bond Polymer Refine Red Detection (DS9390) was used so as not to obscure the black autometallography grains.
2.4. Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry (LA-ICP-MS)
To confirm which metal autometallography was demonstrating, since autometallography can also detect inorganic silver and bismuth [47,48], and to look for the presence of other toxic metals, 7 μm paraffin sections of six kidney samples were deparaffinised and subjected to LA-ICP-MS for mercury, silver, bismuth, aluminium, gold, cadmium, chromium, iron, nickel, and lead. Analyses were carried out on a New Wave Research NWR-193 laser or a Teledyne Cetac LSX-213 G2+ laser hyphenated to an Agilent Technologies 7700x ICP-MS, with argon used as the carrier gas. LA-ICP-MS conditions were optimised on NIST 612 Trace Element in Glass CRM and the sample was ablated with a 50 µm spot size and a scan speed of 100 µm/s at a frequency of 20 Hz. The data were collated into a single image file using in-house developed software and visualised using FIJI.
2.5. Statistical Analyses
Prism v8.4 software was used for chi-square analyses to compare categorical variables and aging trends, and t-tests to compare continuous variables. Significance was assessed at the 0.05 level.
3. Results
3.1. Distribution of Mercury in the Kidney
3.1.1. Known Mercury Exposure (N = 1)
In the man who injected himself with metallic mercury, autometallography of the kidney showed black mercury staining dispersed throughout the cytoplasm of proximal tubule cells in the cortex, more in straight than in convoluted tubules (Figure 1). In the medulla, autometallography showed mercury as black particulate deposits in cells of Henle thin loops (Figure 1). No significant mercury was seen in glomeruli, distal tubules, juxtaglomerular apparatus, or collecting ducts.
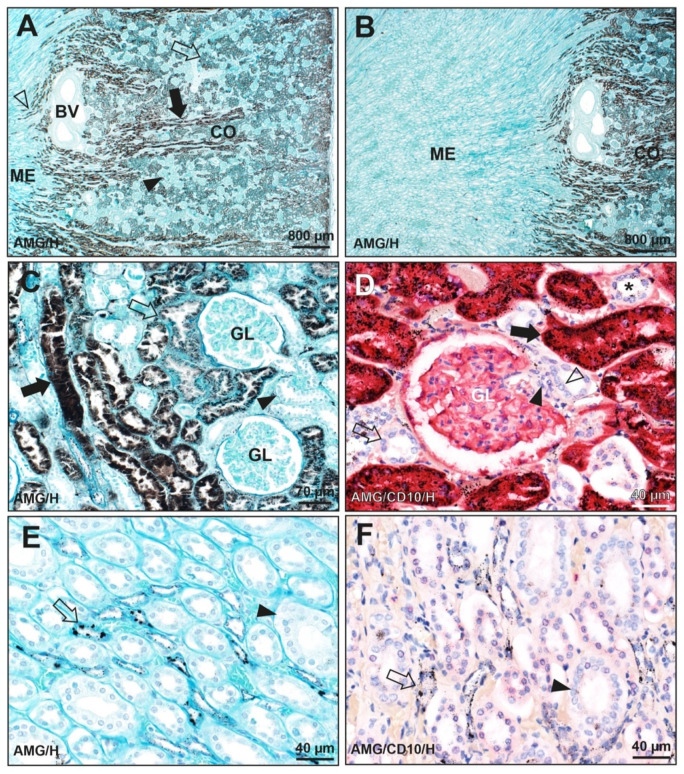
Figure 1.
Autometallography of the kidney of a man who had injected himself with metallic mercury (K24). (A) Black-staining mercury is seen in the renal cortex (CO) in cells of both the straight (filled arrow) and convoluted (open arrow) proximal tubules, with more mercury in straight tubules. The pale cortical regions (closed arrowhead) contain glomeruli and distal tubules. Some mercury-containing proximal straight tubules (open arrowhead) extend a short distance into the pale-staining medulla (ME). Large pale-staining blood vessels (BV) are present near the cortico-medullary junction. (B) A microscopic field to the left of the image in A shows the difference between the dark-staining mercury in the renal cortex (CO) and the pale medulla (ME). (C) Mercury is seen in cells of the proximal straight tubules (filled arrow), with less in the proximal convoluted tubules (open arrow). No mercury is seen in two glomeruli (GL) or in distal tubules (arrowhead). (D) Red CD10 immunostaining is seen in proximal tubule cells containing black mercury grains (filled arrow). In CD10-negative distal tubules, either no (open arrow) or minimal (asterisk) mercury staining is seen. No mercury is seen in the macula densa (open arrowhead) or Lacis cells (filled arrowhead) of the juxtaglomerular apparatus or in a glomerulus (GL) whose podocytes stain lightly with CD10. (E) Cells in Henle thin loops in the medulla contain black mercury granules of varying size (arrow). Collecting tubules (arrowhead) do not contain mercury. (F) Cells in Henle thin loops (not CD10-immunostained) in the medulla contain black mercury granules of varying size (arrow). Collecting tubules that stain with CD10 (arrowhead) do not contain mercury. Light brown/yellow staining is from red blood cells in capillaries. AMG/H autometallography/hematoxylin, AMG/CD10/H autometallography/CD10 immunostaining/hematoxylin, K identity number (see Table 1).
3.1.2. Unknown Mercury Exposure (N = 128)
Three patterns of mercury staining were found in the kidneys of the 128 people without known mercury exposure (Figure 2 and Figure 3 and Table 1). (1) Mercury was seen in cells of proximal tubules of the cortex as well as in Henle thin loops of the medulla in 32 of the 128 (25%) kidneys, with more mercury in the proximal convoluted than straight tubules. This was the same pattern seen in the man who injected himself with mercury. (2) Mercury was present in proximal tubules only in 21 of the 128 (16%) kidneys. (3) Mercury was seen in Henle thin loops only in 29 of the 128 (23%) kidneys. Mercury was not seen in glomeruli, distal tubules, juxtaglomerular apparatus, or collecting ducts.
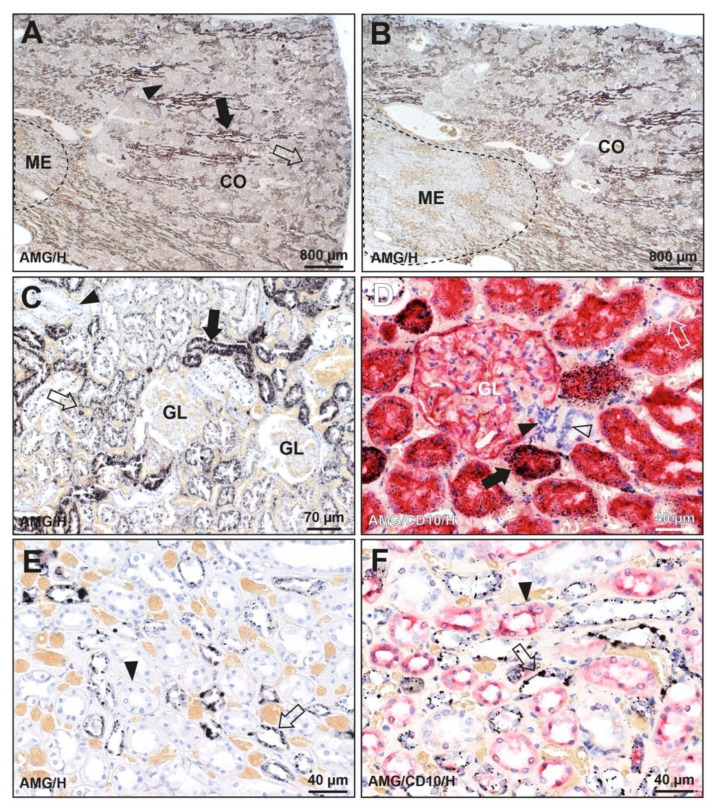
Figure 2.
Mercury in the kidney of a man with no known mercury exposure (K44). (A) Black-staining mercury is seen in the renal cortex (CO) in cells of the straight (filled arrow) and convoluted (open arrow) proximal tubules, with more mercury in straight tubules. The pale cortical regions (arrowhead) contain glomeruli and distal tubules. The medulla (ME, dashed outline) shows no mercury staining at this magnification. (B) A microscopic field to the left of the image in A shows the difference between the plentiful mercury in the renal cortex (CO) and no obvious mercury staining in the medulla (ME, dashed outline). (C) Mercury is seen in cells of the proximal straight tubules (filled arrow), with less in the proximal convoluted tubules (open arrow). No mercury is seen in two glomeruli (GL), or in distal tubules (arrowhead). (D) Red CD10 immunostaining shows proximal tubule cells containing black mercury grains (filled arrow). No mercury is seen in CD10-negative distal tubules (open arrow), in the macula densa (open arrowhead) or Lacis cells (filled arrowhead) of the juxtaglomerular apparatus or in a glomerulus (GL) whose podocytes stain lightly with CD10. (E) Cells in Henle thin loops in the medulla contain black mercury granules of varying size (arrow). Collecting tubules (arrowhead) do not contain mercury. (F) Cells in Henle thin loops (not CD10-immunostained) in the medulla contain mercury granules of varying size (arrow). Collecting tubules that stain with CD10 (arrowhead) do not contain mercury. Light brown/yellow staining is from red blood cells in capillaries. AMG/H autometallography/hematoxylin, AMG/CD10/H autometallography/CD10 immunostaining/hematoxylin. K identity number (see Table 1).
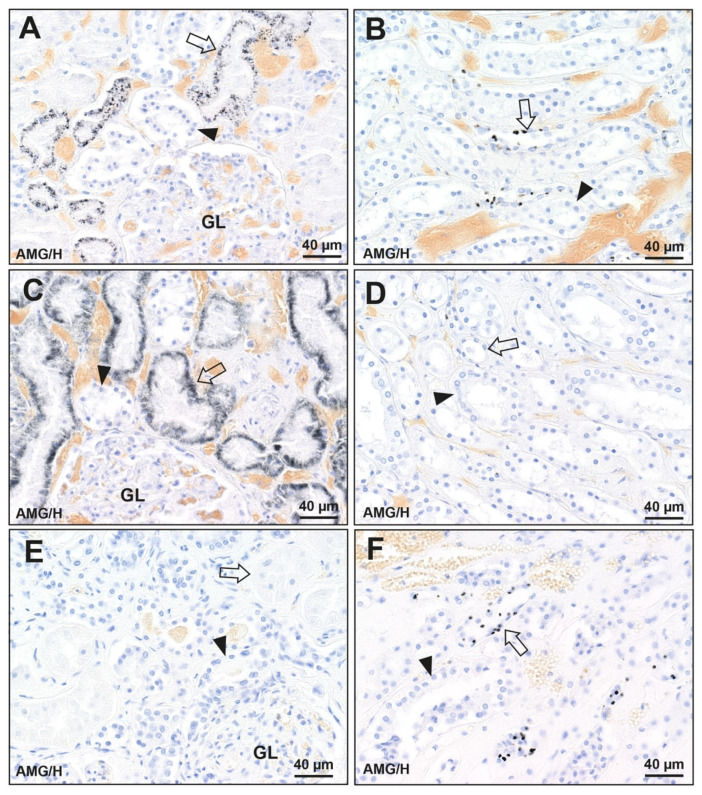
Figure 3.
Patterns of mercury distribution in three kidneys (no known mercury exposure). (A,B) Mercury in the cortex and medulla (K19). (A) In the cortex, black-staining mercury is seen in cells of proximal tubules (arrow) but not distal tubules (arrowhead) or glomeruli (GL). (B) In the medulla, discrete mercury granules are seen in cells of Henle thin loops (arrow) but not in collecting ducts (arrowhead). (C,D) Mercury in the cortex only (K79). (C) In the cortex, mercury is seen in cells of proximal tubules (arrow) but not distal tubules (arrowhead) or glomeruli (GL). (D) In the medulla, no mercury is seen in cells of Henle thin loops (arrow) or collecting ducts (arrowhead). (E,F) Mercury in the medulla only (K101). (E) In the cortex, no mercury is seen in cells of proximal tubules (arrow), distal tubules (arrowhead), or glomeruli (GL). (F) In the medulla, mercury is seen in cells of Henle thin loops (arrow) but not in collecting ducts (arrowhead). AMG/H autometallography/hematoxylin, K identify number (see Table 1).
Table 1.
Mercury (autometallography (AMG) staining) in the proximal tubules and Henle thin loops of 129 kidneys.
| ID No. | Age | Sex | Proximal Tubule AMG | Henle Loop AMG | ID No. | Age | Sex | Proximal Tubule AMG | Henle Loop AMG | ID No. | Age | Sex | Proximal Tubule AMG | Henle Loop AMG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K1 | 1 | F | − | − | K44 | 39 | M | + | + | K87 | 69 | M | − | − |
| K2 | 2 | M | − | − | K45 | 39 | M | − | + | K88 | 70 | M | − | − |
| K3 | 2 | F | − | − | K46 | 39 | M | − | − | K89 | 70 | M | + | + |
| K4 | 2 | F | − | − | K47 | 39 | M | + | + | K90 | 71 | F | − | + |
| K5 | 3 | M | − | − | K48 | 40 | F | − | + | K91 | 72 | F | + | + |
| K6 | 4 | M | − | − | K49 | 40 | F | + | + | K92 | 72 | F | + | + |
| K7 | 9 | M | − | − | K50 | 40 | M | − | + | K93 | 74 | F | − | + |
| K8 | 16 | M | − | − | K51 | 41 | M | − | − | K94 | 75 | M | − | − |
| K9 | 18 | M | − | − | K52 | 41 | F | − | − | K95 | 76 | F | + | + |
| K10 | 18 | F | − | − | K53 | 41 | M | − | + | K96 | 76 | F | + | + |
| K11 | 18 | F | − | − | K54 | 42 | M | + | + | K97 | 77 | F | + | − |
| K12 | 20 | M | − | − | K55 | 43 | M | − | − | K98 | 77 | M | + | − |
| K13 | 20 | F | − | − | K56 | 43 | M | − | − | K99 | 79 | M | − | + |
| K14 | 20 | M | − | − | K57 | 44 | M | − | + | K100 | 80 | F | + | + |
| K15 | 23 | M | − | − | K58 | 44 | M | + | − | K101 | 80 | M | − | + |
| K16 | 24 | M * | + | + | K59 | 45 | M | − | + | K102 | 81 | M | + | + |
| K17 | 24 | M | − | + | K60 | 45 | M | + | − | K103 | 83 | M | + | + |
| K18 | 25 | F | − | − | K61 | 45 | M | − | − | K104 | 85 | M | − | − |
| K19 | 25 | M | + | + | K62 | 46 | M | + | − | K105 | 86 | M | + | + |
| K20 | 26 | F | + | + | K63 | 46 | F | + | + | K106 | 86 | F | + | + |
| K21 | 28 | M | − | − | K64 | 46 | M | + | − | K107 | 87 | M | − | − |
| K22 | 29 | F | − | − | K65 | 47 | M | − | + | K108 | 87 | F | − | + |
| K23 | 29 | M | + | − | K66 | 47 | M | + | + | K109 | 89 | F | − | + |
| K24 | 30 | M | − | − | K67 | 48 | F | + | + | K110 | 95 | F | − | − |
| K25 | 30 | M | − | + | K68 | 49 | F | + | + | K111 | 95 | F | − | + |
| K26 | 30 | M | − | − | K69 | 49 | M | + | + | K112 | 95 | F | − | + |
| K27 | 31 | M | + | − | K70 | 49 | M | + | − | K113 | 95 | M | − | − |
| K28 | 32 | M | − | + | K71 | 49 | M | + | + | K114 | 95 | M | − | + |
| K29 | 32 | M | + | − | K72 | 53 | M | − | + | K115 | 95 | F | + | − |
| K30 | 33 | F | − | − | K73 | 55 | M | − | + | K116 | 95 | M | − | − |
| K31 | 33 | M | − | − | K74 | 58 | M | − | − | K117 | 96 | F | − | − |
| K32 | 34 | M | − | − | K75 | 59 | F | + | + | K118 | 96 | M | + | + |
| K33 | 35 | M | + | + | K76 | 59 | M | + | + | K119 | 96 | M | + | − |
| K34 | 35 | F | + | − | K77 | 61 | M | + | − | K120 | 96 | F | + | − |
| K35 | 35 | F | − | − | K78 | 61 | M | + | + | K121 | 96 | F | − | − |
| K36 | 36 | F | + | + | K79 | 61 | M | + | − | K122 | 97 | F | − | + |
| K37 | 36 | M | − | + | K80 | 61 | M | − | − | K123 | 97 | F | + | + |
| K38 | 37 | M | + | − | K81 | 61 | F | + | + | K124 | 98 | M | − | − |
| K39 | 38 | M | − | − | K82 | 62 | M | − | + | K125 | 98 | M | − | + |
| K40 | 38 | F | + | + | K83 | 63 | F | − | + | K126 | 99 | F | + | − |
| K41 | 38 | M | + | − | K84 | 66 | M | + | − | K127 | 100 | M | − | − |
| K42 | 38 | M | + | − | K85 | 67 | F | + | + | K128 | 104 | F | − | − |
| K43 | 38 | F | − | + | K86 | 67 | M | − | + | K129 | 104 | F | − | + |
AMG autometallography, ID no. identity number, F female, M male, * mercury self-injection (K16).
3.2. Prevalence of Mercury in the Kidney
Overall, mercury (either cortical or medullary) was detected on autometallography in the kidneys of 82 of the 128 people (64%) without known mercury exposure. The proportion of people who had mercury in their kidneys varied in different age ranges (Figure 4). People in the first two decades of life had no kidney mercury, followed by 66% of people with kidney mercury in the subsequent 21–40 years age group. The prevalence of kidney mercury increased to 77% in the 41–60 years group, reaching a maximum of 84% of people in the 61–80 years group, then falling back to 64% of people in the final 81–104 years group. The overall trend for aging to increase the proportion of mercury-positive kidneys was significant (p < 0.0001).
Figure 4.
Prevalence of mercury in the human kidney at different ages. No kidney mercury was seen in the first two decades of life. In the 41–60 years age range, mercury was found in 66% of people, rising to 77% in the 41–60 years group and 84% in the 61–80 years group, before falling to 64% in the 81–104 years age range. Numbers above bars = numbers in age groups.
The 48 females in the study had a slightly (non-significant) higher proportion of mercury-positive kidneys (69%) compared to the 80 males (61%), probably because females had a higher mean age (60 years SD 31 years) than males (50 years SD 25).
3.3. Metals Detected in the Kidney on LA-ICP-MS
LA-ICP-MS of six kidney samples (three with cortex only in the field of view) confirmed the presence of mercury in the cortex of four samples that stained positively for inorganic mercury with autometallography (Figure 5, Table 2). In two samples where autometallography did not detect mercury in the cortex, mercury was seen on LA-ICP-MS, indicating the presence of organic mercury, which is not detected by autometallography. The cortex of all six samples contained cadmium and two contained silver. Lead was seen in the cortex of three samples and in the medulla of one. Iron was widespread in the cortex and medulla, probably due to iron in intravascular red blood cells. The distribution of nickel in the cortex of two samples was similar to that of iron, suggesting this too was due to circulating metal. Despite mercury being found on autometallography in scattered Henle thin loops, no mercury was detectable in the medulla on LA-ICP-MS, probably because of the higher sensitivity of mercury detection by autometallography compared to LA-ICP-MS [49]. No chromium, aluminium, bismuth, or gold was seen in any kidneys (data not shown).
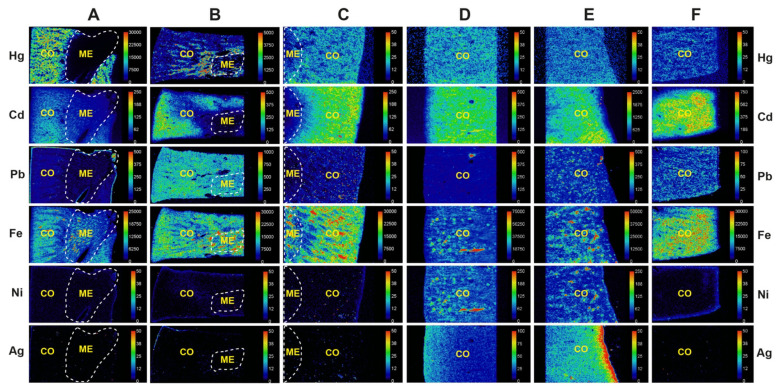
Figure 5.
LA-ICP-MS detection of metals in six kidney samples. (A) AMG+ve cortex and medulla (K16). Mercury is detected in the cortex but not the medulla. Cadmium is present in the cortex, and iron in the cortex and medulla. (B) AMG+ve cortex and medulla (K32). Mercury is seen in the cortex, but not in the medulla. Cadmium, lead, and iron are seen in the cortex, and lead and iron in the medulla. (C) AMG+ve cortex and medulla (K19). Mercury, cadmium, and iron are seen in the cortex, and iron in the medulla. (D) AMG+ cortex (K69). Mercury, cadmium, iron, nickel, and silver are present in the cortex. (E) AMG-ve cortex (K51). Mercury, cadmium, lead, iron, nickel, and silver (with edge effect) are seen in the cortex. (F) AMG-ve cortex (K39). Mercury, cadmium, lead, and iron are present in the cortex. Scale = counts per second (proportional to abundance). CO cortex, ME medulla (within dashed outlines), AMG autometallography, K identify number (see Table 1).
Table 2.
Metals detected by laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) in six human kidneys.
| ID | Site | AMG | LA-ICP-MS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hg | Cd | Pb | Fe | Ni | Ag | Cr | Al | Bi | Au | |||
| K16 | Cortex | Positive | + | + | − | + | − | − | − | − | − | − |
| Medulla | Positive | − | − | − | + | − | − | − | − | − | − | |
| K32 | Cortex | Positive | + | + | + | + | + | − | − | − | − | − |
| Medulla | Positive | + | − | + | + | + | − | − | − | − | − | |
| K19 | Cortex | Positive | + | + | − | + | − | − | − | − | − | − |
| Medulla | Positive | − | − | − | + | − | − | − | − | − | − | |
| K69 | Cortex | Positive | + | + | + | + | − | + | − | − | − | − |
| K51 | Cortex | Negative | + | + | + | + | + | + | − | − | − | − |
| K29 | Cortex | Negative | + | + | + | + | − | − | − | − | − | − |
AMG autometallography, ID identity number, + detected, − not detected, K identity number (see Table 1).
4. Discussion
Key findings of this study are that mercury was found commonly in the proximal tubules and Henle thin loops of human adult kidneys, and that the proportion of people with mercury in their kidneys increased throughout most of adult life. In addition, several other toxic metals, most commonly cadmium and lead, were found in some kidneys.
All humans are exposed to mercury emitted into the environment from both anthropogenic and natural sources (Figure 6) [50]. Common human exposures to mercury are from consuming mercury-contaminated fish, occupations such as gold mining, and from mercury-containing dental amalgam fillings [51]. Methylmercury crosses cell membranes readily, mostly by the formation of methylmercury-cysteine complexes that enter cells on neutral amino acid carriers [52] and is slowly converted in cells into more toxic inorganic mercury (Hg2+) [53]. Mercury vapor also passes through the cell membrane freely and is oxidised to Hg2+ within the cell or is oxidised in circulating red blood cells to Hg2+, which crosses some cell membranes (such as those of renal tubules) via transporters [52,54]. Once inside the cell, mercury attaches preferentially to intracellular membranous structures such as lysosomes, mitochondria, and the nuclear envelope [55].
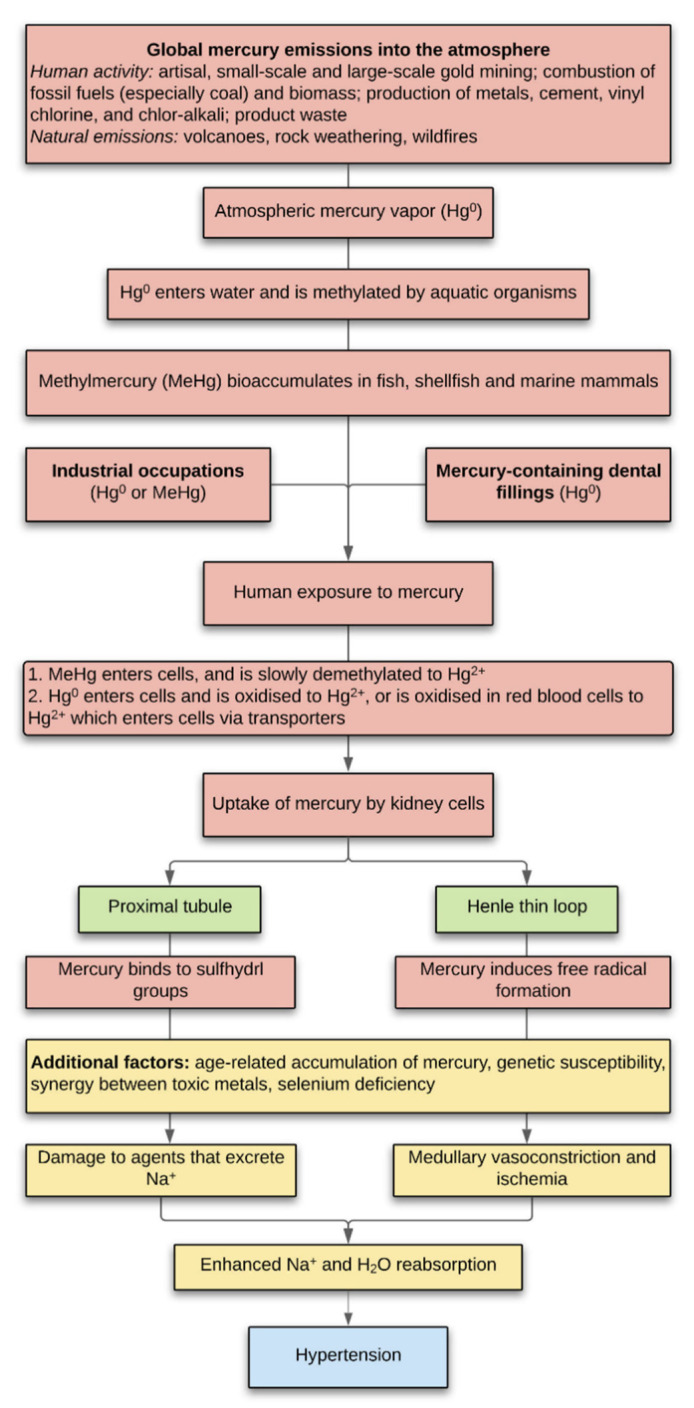
Figure 6.
Potential mechanisms of kidney mercury-induced hypertension. Exposure to mercury results in organic and inorganic mercury being taken up by the cells of proximal tubules and/or Henle thin loops. In proximal tubules of the cortex, mercury-initiated damage to agents that excrete sodium in response to elevated blood pressure (for example by selective binding of mercury to sulfhydryl-rich proteins) would enhance sodium reabsorption. In the medulla, free radicals induced by mercury in Henle thin loops could result in medullary ischemia, also with enhancement of sodium reabsorption. Both or either of these cortical and medullary mechanisms would increase sodium and concomitant water reabsorption, with resultant hypertension. Mercury toxicity would be accentuated by bioaccumulation of mercury over time, genetic susceptibilities to mercury toxicity, the presence of other heavy metals, or deficiencies in mercury-protective mechanisms such as selenium.
Chemical analyses of mercury in human kidneys report more mercury in people who had mercury amalgam dental fillings [56,57,58]. Autometallography of kidney tissue sections from single individuals found mercury in renal tubules both 5 months [30] and 17 years [29] after mercury exposure. The renal cortex appeared to contain more mercury on atomic absorption in eight people who committed suicide than in 10 others [59], though case numbers were small and the authors could not rule out the role of chance. In our study, there was no significant difference in the proportion of people with mercury in their kidneys who committed suicide (N = 19 of 29, 66%) compared to others (N = 64 of 100, 64%).
The location of mercury in renal proximal tubules and Henle thin loops suggests two pathways by which mercury in the kidney could contribute to essential hypertension (Figure 6): (1) Several studies have stressed the importance of the role proximal tubules play in the pathogenesis of hypertension [5,6,60,61]. Mercury could preferentially damage humoral or hormonal agents that decrease ion transport in the proximal tubule [62], with resultant increased reabsorption of sodium and water. One mechanism could be that mercury, which has an affinity for sulfhydryl groups (found mostly in cysteine) can selectively inactivate proteins with a high sulfhydryl content [63]. A comparison of the sulfhydryl content of the humoral and hormonal agents that either decrease or increase ion transport in the proximal tubule [62] could provide evidence to support this hypothesis. (2) Experimental evidence in rats indicates that oxidative stress in the renal medulla results in vasoconstriction and medullary ischemia, which leads to enhanced sodium and water reabsorption and subsequent hypertension [8,64]. In these rats, it is suggested that reactive oxygen species are released by the Henle thick ascending limbs into surrounding capillaries. In our human samples, mercury was located in the Henle thin loops, and since mercury is known to promote oxidative stress [37,65,66], a similar mechanism of medullary ischemia could lead to human hypertension. Both these damaging effects of mercury within the kidney would be augmented by bioaccumulation of the metal with aging [67], genetic susceptibilities to mercury toxicity [68], the presence of other heavy metals [69,70], and a lack of mercury-protective selenium [71].
The finding that mercury is found commonly in adult human kidneys could help explain several epidemiological findings in hypertension [72,73,74,75,76,77,78,79,80,81]. (1) The incidence of hypertension rises with age [72,82], so it was of interest that in our study, the proportion of people with mercury in their kidneys also increased with age, at roughly the same rate. In our final age group of people over the age of 80 years, the proportion with kidney mercury fell back, which suggests a “survivor” effect, possibly because people who have been exposed to less mercury during their lives would tend to live longer [83]. (2) Younger men have higher blood pressure than younger women on average, and in some animals, males are predisposed to higher blood pressures than females [73,74]. One factor that could contribute to this gender difference is that the kidney of the male mouse takes up more of a given dose of mercury than does the female kidney [84]. Unfortunately, we did not have quantitative data from our project that could assess whether more mercury was present in male than female kidneys. (3) Renal cell carcinoma arises from proximal tubule cells, and appears to be associated with hypertension [75,76]. It may therefore be relevant to the pathogenesis of renal cell carcinoma that our study showed that human proximal tubules commonly contain mercury, which is genotoxic [85,86]. In addition to causing somatic mutations in adult kidney cells, mercury in proximal tubule progenitor cells in the foetus could be genotoxic since mercury in non-toxic doses passes through the placenta and enters foetal renal tubules [87]. It would be of interest to assess how often proximal tubules adjacent to human renal cell carcinomas contain mercury, in the same way as has been done in breast and pancreatic cancers [49,88]. However, because mercury is so commonly found in adult human proximal tubules, large numbers of tumour and non-tumour samples would be needed to assess whether kidney mercury is in fact associated with renal cell carcinoma. (4) Firefighters who worked for 10 years or more with wildfires have greater odds of being diagnosed with hypertension than those working fewer than 10 years with wildfires [77]. So it is worth noting that wildfires have long been recognised as a source of mercury emissions [89,90], especially if they affect regions where the soil has previously been polluted with mercury from activities such as gold mining [91]. (5) People who live in the vicinity of volcanoes tend to have higher blood pressures [78,79], and volcanic eruptions are sources of mercury [92]. (6) Exposure to severe particulate atmospheric pollution has been linked to higher blood pressure [80,81], and atmospheric pollution often contains mercury [93].
Estimates of mean blood pressure by world region indicate that the prevalence of high blood pressure has decreased over time in regions such as North America and Western Europe, but has increased over time in others such as China, India, and Southeast Asia [94]. Several factors could underlie this regional heterogeneity in hypertension prevalence, such as variations in sodium uptake [94]. Of note, however, geographic regions of increases and decreases in hypertension prevalence over time [94] overlap with regions where increases and decreases of anthropogenic emissions of mercury have been reported [50], as well as with regions with increases and decreases in discharges of mercury into rivers [95]. For example, the United States has had reductions in mercury atmospheric emissions and discharges of mercury into rivers, and a decreased prevalence of hypertension; on the other hand, China and India have had increases in mercury atmospheric emissions and discharges of mercury into rivers, and an increased prevalence of hypertension [50,94,95]. Mercury exposure, therefore, needs to be considered when assessing possible reasons for the variation in the prevalence of hypertension between different world regions.
Increased retention of sodium and water due to renal dysfunction is not the sole mechanism suspected to underlie essential hypertension, since noradrenaline excess causing increased sympathetic output is a frequent finding in people with raised blood pressure [96,97,98]. This is reflected in medication regimens used to treat hypertension, which often include diuretics to promote natriuresis combined with beta-adrenoreceptor blockers that reduce sympathetic overactivity [99]. Recent work has shown that mercury is found commonly in the adult human adrenal medulla and could lead to increased noradrenaline output [100]. This raises the possibility that two hits of mercury, one in the kidney and one in the adrenal medulla, could underlie the combined renal and sympathetic dysfunction found in many people with essential hypertension.
Toxic metals other than mercury that were found in our kidney samples were cadmium, lead, and silver. Several epidemiological studies have examined possible links between serum or urine cadmium levels and hypertension, but with inconsistent results, leading to calls for future longitudinal studies [18,101]. Increased levels of lead in blood [102,103,104] and bone [105] have been associated with hypertension. Acute exposure to silver nitrate causes a decrease in blood pressure, and silver is not currently thought to be toxic to the cardiovascular system [106]. Synergistic toxic effects have been described for a range of heavy metals, especially mercury and cadmium [70,107]. It is, therefore, of interest that some of our kidney samples had mercury, cadmium, and lead together in the cortex. The effects of these metal interactions on the kidney are complex, however, at least in the rat, where paradoxical decreases of hypertensive effects have been described for mercury/lead combinations [69]. We were unable to determine the physiological role of mercury and these other metals on blood pressure in this autopsy study, and further experiments will be required to measure the effects on blood pressure of long-term exposure to single and combinations of toxic metals.
It has long been known that selenium interacts with mercury and appears to decrease mercury toxicity [108,109,110,111,112,113]. More recently, it has been proposed that one deleterious effect of mercury could be its binding to selenium, thus reducing the ability of selenium to participate in selenoenzyme activity [114,115,116]. The kidneys of most people appear to have enough selenium to detoxify mercury [117,118,119], though the trapping of the freely available renal selenium by mercury may have adverse effects [119]. These kidney studies have relied on chemical analyses, so the mercury and selenium levels in individual cells could not be measured. One way of assessing the mercury–selenium status of individual cells is by synchrotron X-ray fluorescence microscopy, which has detected the equivalent of 1:1 mercury–selenium molar ratios within individual neurons [120]. However, this technique requires frozen sections and allows only a small window of tissue sampling. We were unable to reliably measure selenium in the current project, since trace selenium analysis is often refractory to LA-ICP-MS imaging using standard single-quadrupole MS technology, due to polyatomic interferences from the large volumes of argon gas used in creating the plasma and as the carrier gas. Future studies using triple quadrupole-ICP-MS would be needed for the accurate determination of renal selenium levels [121]. A complicating factor in unravelling the relationship between selenium and mercury toxicity is that genetic polymorphism may affect selenium status and responses to selenium therapy [122], so future studies in this field may need to take these genetic variants into account. Studies of the relationship between human selenium levels and hypertension have given mixed results. A recent study of Inuit in Canada suggested that high selenium exposure decreased the risk of hypertension [123], but high serum selenium levels have been associated with an increased prevalence of hypertension [124,125], and most workers are of the opinion that further studies of the effects of selenium on hypertension are needed [126].
This study has several limitations. (1) This was a retrospective forensic/coronial autopsy study, so we did not have access to detailed clinical medical information to allow us to determine whether individuals had been diagnosed with hypertension during life or if they had been taking antihypertensive medication. Large prospective autopsy studies of people with known blood pressure recordings would be needed to ascertain a link between kidney mercury and hypertension. In such a study, further information gathered could include renal function tests, blood, urine, hair and toenail levels of toxic metals, selenium levels, and whole-genome analyses to look for genetic susceptibility variants. (2) We did not have access to occupational data, places lived, dental records, or dietary habits to assess whether individuals had any known sources of mercury exposure. However, we do know from a previous study that over 90% of Australians over the age of 40 years eat seafood regularly and that over 80% have mercury-containing dental fillings [127], both common sources of human exposure to mercury [51]. (3) We had only modest numbers of individuals in the 0–20 years group. Hypertension is unusual at this age [82], and large numbers of samples would be needed to give an accurate estimation of the proportion of people in this early age group who have kidney mercury. (4) Forensic/coronial autopsy populations, aimed largely at investigating unnatural deaths, cannot exactly replicate conditions in general populations. We tried to minimise the differences by studying people with a range of disorders, as well as those without known medical conditions who died suddenly and unexpectedly. (5) We were unable to quantify the amount of mercury in the kidney using these techniques so the results are qualitative in nature.
5. Conclusions
In conclusion, mercury is found commonly in the proximal tubules and Henle thin loops of adult human kidneys and increases in aging kidneys until an advanced age. Dysfunctions of both these kidney regions have been implicated in the pathogenesis of essential hypertension. Our study was on human autopsy tissue, so the functional implications of our findings will require confirmation with future experimental studies of the effects of renal toxic metals on blood pressure. Precautionary measures to lessen the possibility of mercury-induced hypertension would include making efforts to reduce the burning of fossil fuels such as coal, reduce artisanal gold mining, limit the consumption of fish, such as shark and swordfish, that contain more mercury than selenium [128], consider alternatives to mercury-containing amalgam dental fillings and ensure an adequate intake of selenium-containing foods [129].
Acknowledgments
Ki Lam performed the CD10 immunostaining in the Department of Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital, Sydney, Australia.
Author Contributions
Conceptualization, R.P.; methodology, R.P. and D.P.B.; software, R.P. and D.P.B.; formal analysis, R.P., D.P.B., and P.A.D.; investigation, R.P. and D.P.B.; resources, R.P. and P.A.D.; data curation, R.P. and D.P.B.; writing—original draft preparation, R.P.; writing—review and editing, D.P.B. and P.A.D.; visualization, R.P. and D.P.B.; supervision, R.P. and D.P.B.; project administration, R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. R.P. is supported by the Aimee Stacy Memorial and Ignacy Burnett bequests. P.A.D. is supported by the Australian Research Council Discovery Project Grant DP190102361. D.P.B. is supported by an Australian Research Council Discovery Early Career Researcher Award DE180100194. P.A.D. and D.P.B. are both supported by the USA National Institute of Health R21 Exploratory/Development Grant 1R21AR072950.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Sydney Local Health District (Royal Prince Alfred Hospital Zone) (protocol code X2014-029, date of approval 26 May 2014).
Informed Consent Statement
The Institutional Ethics Committee waived the need for written informed consent from relatives of individuals studied since this was a de-identified retrospective study of archived paraffin-embedded tissue.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poulter N.R., Prabhakaran D., Caulfield M. Hypertension. Lancet. 2015;386:801–812. doi: 10.1016/S0140-6736(14)61468-9. [DOI] [PubMed] [Google Scholar]
- 3.Guyton A.C. Dominant role of the kidneys and accessory role of whole-body autoregulation in the pathogenesis of hypertension. Am. J. Hypertens. 1989;2:575–585. doi: 10.1093/ajh/2.7.575. [DOI] [PubMed] [Google Scholar]
- 4.Crowley S.D., Coffman T.M. The inextricable role of the kidney in hypertension. J. Clin. Investig. 2014;124:2341–2347. doi: 10.1172/JCI72274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X.C., Zhuo J.L. Recent Updates on the Proximal Tubule Renin-Angiotensin System in Angiotensin II-Dependent Hypertension. Curr. Hypertens. Rep. 2016;18:63. doi: 10.1007/s11906-016-0668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horita S., Nakamura M., Suzuki M., Satoh N., Suzuki A., Homma Y., Nangaku M. The role of renal proximal tubule transport in the regulation of blood pressure. Kidney Res. Clin. Pract. 2017;36:12–21. doi: 10.23876/j.krcp.2017.36.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung J., Basile D.P., Pratt J.H. Sodium reabsorption in the thick ascending limb in relation to blood pressure: A clinical perspective. Hypertension. 2011;57:873–879. doi: 10.1161/HYPERTENSIONAHA.108.120246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowley A.W., Jr., Abe M., Mori T., O’Connor P.M., Ohsaki Y., Zheleznova N.N. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am. J. Physiol. Renal Physiol. 2015;308:F179–F197. doi: 10.1152/ajprenal.00455.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu X.F., Singh K., Chan H.M. Mercury Exposure, Blood Pressure, and Hypertension: A Systematic Review and Dose-response Meta-analysis. Environ. Health Perspect. 2018;126:076002. doi: 10.1289/EHP2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yorifuji T., Tsuda T., Kashima S., Takao S., Harada M. Long-term exposure to methylmercury and its effects on hypertension in Minamata. Environ. Res. 2010;110:40–46. doi: 10.1016/j.envres.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Inoue S., Yorifuji T., Tsuda T., Doi H. Short-term effect of severe exposure to methylmercury on atherosclerotic heart disease and hypertension mortality in Minamata. Sci. Total Environ. 2012;417–418:291–293. doi: 10.1016/j.scitotenv.2011.11.076. [DOI] [PubMed] [Google Scholar]
- 12.Yorifuji T., Tsuda T. Epidemiological studies of neurological signs and symptoms and blood pressure in populations near the industrial methylmercury contamination at Minamata, Japan. Arch. Environ. Occup. Health. 2016;71:231–236. doi: 10.1080/19338244.2015.1084261. [DOI] [PubMed] [Google Scholar]
- 13.Wakita Y. Hypertension induced by methyl mercury in rats. Toxicol. Appl. Pharmacol. 1987;89:144–147. doi: 10.1016/0041-008X(87)90185-2. [DOI] [PubMed] [Google Scholar]
- 14.Grotto D., de Castro M.M., Barcelos G.R., Garcia S.C., Barbosa F., Jr. Low level and sub-chronic exposure to methylmercury induces hypertension in rats: Nitric oxide depletion and oxidative damage as possible mechanisms. Arch. Toxicol. 2009;83:653–662. doi: 10.1007/s00204-009-0437-8. [DOI] [PubMed] [Google Scholar]
- 15.Wildemann T.M., Mirhosseini N., Siciliano S.D., Weber L.P. Cardiovascular responses to lead are biphasic, while methylmercury, but not inorganic mercury, monotonically increases blood pressure in rats. Toxicology. 2015;328:1–11. doi: 10.1016/j.tox.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Torres A.D., Rai A.N., Hardiek M.L. Mercury intoxication and arterial hypertension: Report of two patients and review of the literature. Pediatrics. 2000;105:E34. doi: 10.1542/peds.105.3.e34. [DOI] [PubMed] [Google Scholar]
- 17.Houston M.C. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J. Clin, Hypertens. 2011;13:621–627. doi: 10.1111/j.1751-7176.2011.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Da Cunha Martins A., Jr., Carneiro M.F.H., Grotto D., Adeyemi J.A., Barbosa F., Jr. Arsenic, cadmium, and mercury-induced hypertension: Mechanisms and epidemiological findings. J. Toxicol. Environ. Health B Crit. Rev. 2018;21:61–82. doi: 10.1080/10937404.2018.1432025. [DOI] [PubMed] [Google Scholar]
- 19.Danscher G. Applications of autometallography to heavy metal toxicology. Pharmacol. Toxicol. 1991;68:414–423. doi: 10.1111/j.1600-0773.1991.tb01264.x. [DOI] [PubMed] [Google Scholar]
- 20.Loumbourdis N.S., Danscher G. Autometallographic tracing of Hg-S quantum dots in frogs exposed to inorganic mercury. Biometals. 2008;21:311–319. doi: 10.1007/s10534-007-9120-9. [DOI] [PubMed] [Google Scholar]
- 21.Baatrup E., Danscher G. Cytochemical demonstration of mercury deposits in trout liver and kidney following methyl mercury intoxication: Differentiation of two mercury pools by selenium. Ecotoxicol. Environ. Saf. 1987;14:129–141. doi: 10.1016/0147-6513(87)90055-8. [DOI] [PubMed] [Google Scholar]
- 22.Woshner V.M., O’Hara T.M., Eurell J.A., Wallig M.A., Bratton G.R., Suydam R.S., Beasley V.R. Distribution of inorganic mercury in liver and kidney of beluga and bowhead whales through autometallographic development of light microscopic tissue sections. Toxicol. Pathol. 2002;30:209–215. doi: 10.1080/019262302753559542. [DOI] [PubMed] [Google Scholar]
- 23.Pamphlett R., Kum Jew S., Cherepanoff S. Mercury in the retina and optic nerve following prenatal exposure to mercury vapor. PLoS ONE. 2019;14:e0220859. doi: 10.1371/journal.pone.0220859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danscher G., Zimmer J. An improved Timm sulphide silver method for light and electron microscopic localization of heavy metals in biological tissues. Histochemistry. 1978;55:27–40. doi: 10.1007/BF00496691. [DOI] [PubMed] [Google Scholar]
- 25.Danscher G. Autometallography. A new technique for light and electron microscopic visualization of metals in biological tissues (gold, silver, metal sulphides and metal selenides) Histochemistry. 1984;81:331–335. doi: 10.1007/BF00514327. [DOI] [PubMed] [Google Scholar]
- 26.Norgaard J.O., Moller-Madsen B., Hertel N., Danscher G. Silver enhancement of tissue mercury: Demonstration of mercury in autometallographic silver grains from rat kidneys. J. Histochem. Cytochem. 1989;37:1545–1547. doi: 10.1177/37.10.2778309. [DOI] [PubMed] [Google Scholar]
- 27.Hansen J.C., Reske-Nielsen E., Thorlacius-Ussing O., Rungby J., Danscher G. Distribution of dietary mercury in a dog. Quantitation and localization of total mercury in organs and central nervous system. Sci. Total Environ. 1989;78:23–43. doi: 10.1016/0048-9697(89)90020-X. [DOI] [PubMed] [Google Scholar]
- 28.Danscher G., Horsted-Bindslev P., Rungby J. Traces of mercury in organs from primates with amalgam fillings. Exp. Mol. Pathol. 1990;52:291–299. doi: 10.1016/0014-4800(90)90070-T. [DOI] [PubMed] [Google Scholar]
- 29.Opitz H., Schweinsberg F., Grossmann T., Wendt-Gallitelli M.F., Meyermann R. Demonstration of mercury in the human brain and other organs 17 years after metallic mercury exposure. Clin. Neuropathol. 1996;15:139–144. [PubMed] [Google Scholar]
- 30.Pamphlett R., Kum Jew S. Uptake of inorganic mercury by human locus ceruleus and corticomotor neurons: Implications for amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2013;1:13. doi: 10.1186/2051-5960-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zalups R.K., Cherian M.G., Barfuss D.W. Mercury-metallothionein and the renal accumulation and handling of mercury. Toxicology. 1993;83:61–78. doi: 10.1016/0300-483X(93)90092-7. [DOI] [PubMed] [Google Scholar]
- 32.Zalups R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000;52:113–143. [PubMed] [Google Scholar]
- 33.Zalups R.K., Aslamkhan A.G., Ahmad S. Human organic anion transporter 1 mediates cellular uptake of cysteine-S conjugates of inorganic mercury. Kidney Int. 2004;66:251–261. doi: 10.1111/j.1523-1755.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 34.Zalups R.K., Joshee L., Bridges C.C. Novel Hg2+-induced nephropathy in rats and mice lacking Mrp2: Evidence of axial heterogeneity in the handling of Hg2+ along the proximal tubule. Toxicol. Sci. 2014;142:250–260. doi: 10.1093/toxsci/kfu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bridges C.C., Zalups R.K., Joshee L. Toxicological significance of renal Bcrp: Another potential transporter in the elimination of mercuric ions from proximal tubular cells. Toxicol. Appl. Pharmacol. 2015;285:110–117. doi: 10.1016/j.taap.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bridges C.C., Zalups R.K. The aging kidney and the nephrotoxic effects of mercury. J. Toxicol. Environ. Health B Crit. Rev. 2017;20:55–80. doi: 10.1080/10937404.2016.1243501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orr S.E., Barnes M.C., Joshee L., Uchakina O., McKallip R.J., Bridges C.C. Potential mechanisms of cellular injury following exposure to a physiologically relevant species of inorganic mercury. Toxicol. Lett. 2019;304:13–20. doi: 10.1016/j.toxlet.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Pamphlett R., Waley P. Motor neuron uptake of low dose inorganic mercury. J. Neurol. Sci. 1996;135:63–67. doi: 10.1016/0022-510X(95)00258-4. [DOI] [PubMed] [Google Scholar]
- 39.Kedziora A., Duflou J. Attempted suicide by intravenous injection of mercury: A rare cause of cardiac granulomas. A case report. Am. J. Forensic Med. Pathol. 1995;16:172–176. doi: 10.1097/00000433-199506000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Pamphlett R., Waley P. Uptake of inorganic mercury by the human brain. Acta Neuropathol. 1996;92:525–527. doi: 10.1007/s004010050556. [DOI] [PubMed] [Google Scholar]
- 41.Pamphlett R., Kum Jew S. Inorganic mercury in human astrocytes, oligodendrocytes, corticomotoneurons and the locus ceruleus: Implications for multiple sclerosis, neurodegenerative disorders and gliomas. Biometals. 2018;31:807–819. doi: 10.1007/s10534-018-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danscher G., Moller-Madsen B. Silver amplification of mercury sulfide and selenide: A histochemical method for light and electron microscopic localization of mercury in tissue. J. Histochem. Cytochem. 1985;33:219–228. doi: 10.1177/33.3.2579122. [DOI] [PubMed] [Google Scholar]
- 43.Danscher G., Rungby J. Differentiation of histochemically visualized mercury and silver. Histochem. J. 1986;18:109–114. doi: 10.1007/BF01675364. [DOI] [PubMed] [Google Scholar]
- 44.Pamphlett R., Png F.Y. Shrinkage of motor axons following systemic exposure to inorganic mercury. J. Neuropathol. Exp. Neurol. 1998;57:360–366. doi: 10.1097/00005072-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Clapp W.L., Croker B.P. Kidney. In: Mills S.E., editor. Histology for Pathologists. 4th ed. Wolters Klower; Philadelphia, PA, USA: 2012. pp. 891–970. [Google Scholar]
- 46.Faa G., Gerosa C., Fanni D., Nemolato S., Marinelli V., Locci A., Senes G., Mais V., Van Eyken P., Iacovidou N., et al. CD10 in the developing human kidney: Immunoreactivity and possible role in renal embryogenesis. J. Matern. Fetal Neonatal Med. 2012;25:904–911. doi: 10.3109/14767058.2011.599457. [DOI] [PubMed] [Google Scholar]
- 47.Danscher G., Stoltenberg M., Juhl S. How to detect gold, silver and mercury in human brain and other tissues by autometallographic silver amplification. Neuropathol. Appl. Neurobiol. 1994;20:454–467. doi: 10.1111/j.1365-2990.1994.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 48.Danscher G., Stoltenberg M., Kemp K., Pamphlett R. Bismuth autometallography: Protocol, specificity, and differentiation. J. Histochem. Cytochem. 2000;48:1503–1510. doi: 10.1177/002215540004801107. [DOI] [PubMed] [Google Scholar]
- 49.Pamphlett R., Satgunaseelan L., Kum Jew S., Doble P.A., Bishop D.P. Elemental bioimaging shows mercury and other toxic metals in normal breast tissue and in breast cancers. PLoS ONE. 2020;15:e0228226. doi: 10.1371/journal.pone.0228226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.UN Environment Programme . Global Mercury Assessment 2018. UN Environment Programme; Geneva, Switzerland: 2019. [Google Scholar]
- 51.Clarkson T.W., Magos L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 52.Clarkson T.W., Vyas J.B., Ballatori N. Mechanisms of mercury disposition in the body. Am. J. Ind. Med. 2007;50:757–764. doi: 10.1002/ajim.20476. [DOI] [PubMed] [Google Scholar]
- 53.Clarkson T.W. The toxicology of mercury. Crit. Rev. Clin. Lab. Sci. 1997;34:369–403. doi: 10.3109/10408369708998098. [DOI] [PubMed] [Google Scholar]
- 54.Bridges C.C., Zalups R.K. Mechanisms involved in the transport of mercuric ions in target tissues. Arch. Toxicol. 2017;91:63–81. doi: 10.1007/s00204-016-1803-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang L.W., Hartmann H.A. Electron microscopic histochemical study on the localization and distribution of mercury in the nervous system after mercury intoxication. Exp. Neurol. 1972;35:122–137. doi: 10.1016/0014-4886(72)90064-7. [DOI] [PubMed] [Google Scholar]
- 56.Nylander M., Friberg L., Lind B. Mercury concentrations in the human brain and kidneys in relation to exposure from dental amalgam fillings. Swed. Dent. J. 1987;11:179–187. [PubMed] [Google Scholar]
- 57.Barregard L., Svalander C., Schutz A., Westberg G., Sallsten G., Blohme I., Molne J., Attman P.O., Haglind P. Cadmium, mercury, and lead in kidney cortex of the general Swedish population: A study of biopsies from living kidney donors. Environ. Health Perspect. 1999;107:867–871. doi: 10.1289/ehp.107-1566723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barregard L., Fabricius-Lagging E., Lundh T., Molne J., Wallin M., Olausson M., Modigh C., Sallsten G. Cadmium, mercury, and lead in kidney cortex of living kidney donors: Impact of different exposure sources. Environ. Res. 2010;110:47–54. doi: 10.1016/j.envres.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 59.Guzzi G., Grandi M., Cattaneo C., Calza S., Minoia C., Ronchi A., Gatti A., Severi G. Dental amalgam and mercury levels in autopsy tissues: Food for thought. Am. J. Forensic Med. Pathol. 2006;27:42–45. doi: 10.1097/01.paf.0000201177.62921.c8. [DOI] [PubMed] [Google Scholar]
- 60.Doris P.A. Renal proximal tubule sodium transport and genetic mechanisms of essential hypertension. J. Hypertens. 2000;18:509–519. doi: 10.1097/00004872-200018050-00002. [DOI] [PubMed] [Google Scholar]
- 61.Quigley R., Chakravarty S., Zhao X., Imig J.D., Capdevila J.H. Increased renal proximal convoluted tubule transport contributes to hypertension in Cyp4a14 knockout mice. Nephron Physiol. 2009;113:p23–p28. doi: 10.1159/000235774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X., Armando I., Upadhyay K., Pascua A., Jose P.A. The regulation of proximal tubular salt transport in hypertension: An update. Curr. Opin. Nephrol. Hypertens. 2009;18:412–420. doi: 10.1097/MNH.0b013e32832f5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ajsuvakova O.P., Tinkov A.A., Aschner M., Rocha J.B.T., Michalke B., Skalnaya M.G., Skalny A.V., Butnariu M., Dadar M., Sarac I., et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020;417:213343. doi: 10.1016/j.ccr.2020.213343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cowley A.W., Jr. Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension. 2008;52:777–786. doi: 10.1161/HYPERTENSIONAHA.107.092858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lemos N.B., Angeli J.K., Faria Tde O., Ribeiro R.F., Jr., Vassallo D.V., Padilha A.S., Stefanon I. Low mercury concentration produces vasoconstriction, decreases nitric oxide bioavailability and increases oxidative stress in rat conductance artery. PLoS ONE. 2012;7:e49005. doi: 10.1371/journal.pone.0049005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karimi R., Vacchi-Suzzi C., Meliker J.R. Mercury exposure and a shift toward oxidative stress in avid seafood consumers. Environ. Res. 2016;146:100–107. doi: 10.1016/j.envres.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rice K.M., Walker E.M., Jr., Wu M., Gillette C., Blough E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health. 2014;47:74–83. doi: 10.3961/jpmph.2014.47.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andreoli V., Sprovieri F. Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview. Int. J. Environ. Res. Public Health. 2017;14:93. doi: 10.3390/ijerph14010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wildemann T.M., Siciliano S.D., Weber L.P. The mechanisms associated with the development of hypertension after exposure to lead, mercury species or their mixtures differs with the metal and the mixture ratio. Toxicology. 2016;339:1–8. doi: 10.1016/j.tox.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Andrade V.M., Aschner M., Marreilha Dos Santos A.P. Neurotoxicity of Metal Mixtures. In: Aschner M., Costa L.G., editors. Neurotoxicity of Metals. 2017/09/11 ed. Volume 18. Springer Nature; Cham, Switzerland: 2017. pp. 227–265. [DOI] [PubMed] [Google Scholar]
- 71.Orr S.E., George H.S., Barnes M.C., Mathis T.N., Joshee L., Barkin J., Kiefer A.M., Seney C.S., Bridges C.C. Co-administration of Selenium with Inorganic Mercury Alters the Disposition of Mercuric Ions in Rats. Biol. Trace Elem. Res. 2020;195:187–195. doi: 10.1007/s12011-019-01835-y. [DOI] [PubMed] [Google Scholar]
- 72.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart Disease and Stroke Statistics—2019 Update: A Report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 73.Sandberg K., Ji H. Sex differences in primary hypertension. Biol. Sex Differ. 2012;3:7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doumas M., Papademetriou V., Faselis C., Kokkinos P. Gender differences in hypertension: Myths and reality. Curr. Hypertens. Rep. 2013;15:321–330. doi: 10.1007/s11906-013-0359-y. [DOI] [PubMed] [Google Scholar]
- 75.Hidayat K., Du X., Zou S.Y., Shi B.M. Blood pressure and kidney cancer risk: Meta-analysis of prospective studies. J. Hypertens. 2017;35:1333–1344. doi: 10.1097/HJH.0000000000001286. [DOI] [PubMed] [Google Scholar]
- 76.Kim C.S., Han K.D., Choi H.S., Bae E.H., Ma S.K., Kim S.W. Association of Hypertension and Blood Pressure with Kidney Cancer Risk: A Nationwide Population-Based Cohort Study. Hypertension. 2020;75:1439–1446. doi: 10.1161/HYPERTENSIONAHA.120.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Semmens E.O., Domitrovich J., Conway K., Noonan C.W. A cross-sectional survey of occupational history as a wildland firefighter and health. Am. J. Ind. Med. 2016;59:330–335. doi: 10.1002/ajim.22566. [DOI] [PubMed] [Google Scholar]
- 78.Longo B.M. Adverse Health Effects Associated with Increased Activity at Kilauea Volcano: A Repeated Population-Based Survey. ISNR Public Health. 2013;2013:475962. doi: 10.1155/2013/475962. [DOI] [Google Scholar]
- 79.Brook R.D., Brook J.R., Tam E.K. Volcanic smog and cardiometabolic health: Hawaiian hypertension? J. Clin. Hypertens. 2019;21:533–535. doi: 10.1111/jch.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dong G.H., Qian Z.M., Xaverius P.K., Trevathan E., Maalouf S., Parker J., Yang L., Liu M.M., Wang D., Ren W.H., et al. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension. 2013;61:578–584. doi: 10.1161/HYPERTENSIONAHA.111.00003. [DOI] [PubMed] [Google Scholar]
- 81.Brook R.D., Sun Z., Brook J.R., Zhao X., Ruan Y., Yan J., Mukherjee B., Rao X., Duan F., Sun L., et al. Extreme Air Pollution Conditions Adversely Affect Blood Pressure and Insulin Resistance: The Air Pollution and Cardiometabolic Disease Study. Hypertension. 2016;67:77–85. doi: 10.1161/HYPERTENSIONAHA.115.06237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kit B.K., Kuklina E., Carroll M.D., Ostchega Y., Freedman D.S., Ogden C.L. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr. 2015;169:272–279. doi: 10.1001/jamapediatrics.2014.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pamphlett R., Kum Jew S., Doble P.A., Bishop D.P. Elemental Analysis of Aging Human Pituitary Glands Implicates Mercury as a Contributor to the Somatopause. Front. Endocrinol. 2019;10:419. doi: 10.3389/fendo.2019.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pamphlett R., Ewan K.B., McQuilty R., Waley P. Gender differences in the uptake of inorganic mercury by motor neurons. Neurotoxicol. Teratol. 1997;19:287–293. doi: 10.1016/S0892-0362(97)00021-4. [DOI] [PubMed] [Google Scholar]
- 85.Crespo-Lopez M.E., Macedo G.L., Pereira S.I., Arrifano G.P., Picanco-Diniz D.L., do Nascimento J.L., Herculano A.M. Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacol. Res. 2009;60:212–220. doi: 10.1016/j.phrs.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 86.Nersesyan A., Kundi M., Waldherr M., Setayesh T., Misik M., Wultsch G., Filipic M., Mazzaron Barcelos G.R., Knasmueller S. Results of micronucleus assays with individuals who are occupationally and environmentally exposed to mercury, lead and cadmium. Mutat. Res. 2016;770:119–139. doi: 10.1016/j.mrrev.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 87.Pamphlett R., Kum Jew S. Mercury Is Taken Up Selectively by Cells Involved in Joint, Bone, and Connective Tissue Disorders. Front. Med. 2019;6:168. doi: 10.3389/fmed.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pamphlett R., Colebatch A.J., Doble P.A., Bishop D.P. Mercury in Pancreatic Cells of People with and without Pancreatic Cancer. Int. J. Environ. Res. Public Health. 2020;17:8990. doi: 10.3390/ijerph17238990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Finley B.D., Swartzendruber P.C., Jaffe D.A. Particulate mercury emissions in regional wildfire plumes observed at the Mount Bachelor Observatory. Atmos. Environ. 2009;43:6074–6083. doi: 10.1016/j.atmosenv.2009.08.046. [DOI] [Google Scholar]
- 90.Kristensen L.J., Taylor M.P. Fields and forests in flames: Lead and mercury emissions from wildfire pyrogenic activity. Environ. Health Perspect. 2012;120:a56–a57. doi: 10.1289/ehp.1104672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abraham J., Dowling K., Florentine S. Effects of prescribed fire and post-fire rainfall on mercury mobilization and subsequent contamination assessment in a legacy mine site in Victoria, Australia. Chemosphere. 2018;190:144–153. doi: 10.1016/j.chemosphere.2017.09.117. [DOI] [PubMed] [Google Scholar]
- 92.Varekamp J.C., Buseck P.R. Global mercury flux from volcanic and geothermal sources. J. Appl. Geochem. 1986;1:65–73. doi: 10.1016/0883-2927(86)90038-7. [DOI] [Google Scholar]
- 93.Streets D.G., Devane M.K., Lu Z., Bond T.C., Sunderland E.M., Jacob D.J. All-time releases of mercury to the atmosphere from human activities. Environ. Sci. Technol. 2011;45:10485–10491. doi: 10.1021/es202765m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mills K.T., Stefanescu A., He J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amos H.M., Jacob D.J., Kocman D., Horowitz H.M., Zhang Y., Dutkiewicz S., Horvat M., Corbitt E.S., Krabbenhoft D.P., Sunderland E.M. Global biogeochemical implications of mercury discharges from rivers and sediment burial. Environ. Sci. Technol. 2014;48:9514–9522. doi: 10.1021/es502134t. [DOI] [PubMed] [Google Scholar]
- 96.Louis W.J., Doyle A.E., Anavekar S. Plasma norepinephrine levels in essential hypertension. N. Engl. J. Med. 1973;288:599–601. doi: 10.1056/NEJM197303222881203. [DOI] [PubMed] [Google Scholar]
- 97.Goldstein D.S. Plasma catecholamines and essential hypertension. An analytical review. Hypertension. 1983;5:86–99. doi: 10.1161/01.HYP.5.1.86. [DOI] [PubMed] [Google Scholar]
- 98.Guyenet P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 99.Oparil S., Acelajado M.C., Bakris G.L., Berlowitz D.R., Cifkova R., Dominiczak A.F., Grassi G., Jordan J., Poulter N.R., Rodgers A., et al. Hypertension. Nat. Rev. Dis. Primers. 2018;4:18014. doi: 10.1038/nrdp.2018.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pamphlett R., Kum Jew S., Doble P.A., Bishop D.P. Mercury in the human adrenal medulla could contribute to increased plasma noradrenaline in aging. Sci. Rep. 2021;11:2961. doi: 10.1038/s41598-021-82483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martins A.C., Almeida Lopes A.C.B., Urbano M.R., Carvalho M.F.H., Silva A.M.R., Tinkov A.A., Aschner M., Mesas A.E., Silbergeld E.K., Paoliello M.M.B. An updated systematic review on the association between Cd exposure, blood pressure and hypertension. Ecotoxicol. Environ. Saf. 2021;208:111636. doi: 10.1016/j.ecoenv.2020.111636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gambelunghe A., Sallsten G., Borne Y., Forsgard N., Hedblad B., Nilsson P., Fagerberg B., Engstrom G., Barregard L. Low-level exposure to lead, blood pressure, and hypertension in a population-based cohort. Environ. Res. 2016;149:157–163. doi: 10.1016/j.envres.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 103.Almeida Lopes A.C.B., Silbergeld E.K., Navas-Acien A., Zamoiski R., Martins A.D.C., Jr., Camargo A.E.I., Urbano M.R., Mesas A.E., Paoliello M.M.B. Association between blood lead and blood pressure: A population-based study in Brazilian adults. Environ. Health. 2017;16:27. doi: 10.1186/s12940-017-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han L., Wang X., Han R., Xu M., Zhao Y., Gao Q., Shen H., Zhang H. Association between blood lead level and blood pressure: An occupational population-based study in Jiangsu province, China. PLoS ONE. 2018;13:e0200289. doi: 10.1371/journal.pone.0200289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zheutlin A.R., Hu H., Weisskopf M.G., Sparrow D., Vokonas P.S., Park S.K. Low-Level Cumulative Lead and Resistant Hypertension: A Prospective Study of Men Participating in the Veterans Affairs Normative Aging Study. J. Am. Heart Assoc. 2018;7:e010014. doi: 10.1161/JAHA.118.010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Drake P.L., Hazelwood K.J. Exposure-related health effects of silver and silver compounds: A review. Ann. Occup. Hyg. 2005;49:575–585. doi: 10.1093/annhyg/mei019. [DOI] [PubMed] [Google Scholar]
- 107.Cobbina S.J., Chen Y., Zhou Z., Wu X., Feng W., Wang W., Mao G., Xu H., Zhang Z., Wu X., et al. Low concentration toxic metal mixture interactions: Effects on essential and non-essential metals in brain, liver, and kidneys of mice on sub-chronic exposure. Chemosphere. 2015;132:79–86. doi: 10.1016/j.chemosphere.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 108.Parizek J., Ostadalova I. The protective effect of small amounts of selenite in sublimate intoxication. Experientia. 1967;23:142–143. doi: 10.1007/BF02135970. [DOI] [PubMed] [Google Scholar]
- 109.Ganther H.E., Goudie C., Sunde M.L., Kopecky M.J., Wagner P. Selenium: Relation to decreased toxicity of methylmercury added to diets containing tuna. Science. 1972;175:1122–1124. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- 110.Kosta L., Byrne A.R., Zelenko V. Correlation between selenium and mercury in man following exposure to inorganic mercury. Nature. 1975;254:238–239. doi: 10.1038/254238a0. [DOI] [PubMed] [Google Scholar]
- 111.Sumino K., Yamamoto R., Kitamura S. A role of selenium against methylmercury toxicity. Nature. 1977;268:73–74. doi: 10.1038/268073a0. [DOI] [PubMed] [Google Scholar]
- 112.Berlin M. Interaction between selenium and inorganic mercury. Environ. Health Perspect. 1978;25:67–69. doi: 10.1289/ehp.782567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ganther H.E. Modification of methylmercury toxicity and metabolism by selenium and vitamin E: Possible mechanisms. Environ. Health Perspect. 1978;25:71–76. doi: 10.1289/ehp.782571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Drasch G., Mailänder S., Schlosser C., Roider G. Content of non-mercury-associated selenium in human tissues. Biol. Trace Elem. Res. 2000;77:219–230. doi: 10.1385/BTER:77:3:219. [DOI] [PubMed] [Google Scholar]
- 115.Ralston N.V., Blackwell J.L., 3rd, Raymond L.J. Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biol. Trace Elem. Res. 2007;119:255–268. doi: 10.1007/s12011-007-8005-7. [DOI] [PubMed] [Google Scholar]
- 116.Ralston N.V.C., Raymond L.J. Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry. Biochim. Biophys. Acta Gen. Subj. 2018:2405–2416. doi: 10.1016/j.bbagen.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 117.Nylander M., Weiner J. Mercury and selenium concentrations and their interrelations in organs from dental staff and the general population. Br. J. Ind. Med. 1991;48:729–734. doi: 10.1136/oem.48.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bjorkman L., Palm B., Nylander M., Nordberg M. Mercury and selenium distribution in human kidney cortex. Biol. Trace Elem. Res. 1994;40:255–265. doi: 10.1007/BF02950798. [DOI] [PubMed] [Google Scholar]
- 119.Drasch G., Wanghofer E., Roider G., Strobach S. Correlation of mercury and selenium in the human kidney. J. Trace Elem. Med. Biol. 1996;10:251–254. doi: 10.1016/S0946-672X(96)80043-5. [DOI] [PubMed] [Google Scholar]
- 120.Pamphlett R., Mak R., Lee J., Buckland M.E., Harding A.J., Kum Jew S., Paterson D.J., Jones M.W.M., Lay P.A. Concentrations of toxic metals and essential trace elements vary among individual neurons in the human locus ceruleus. PLoS ONE. 2020;15:e0233300. doi: 10.1371/journal.pone.0233300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bishop D.P., Clases D., Fryer F., Williams E., Wilkins S., Hare D.J., Cole N., Karst U., Doble P.A. Elemental bio-imaging using laser ablation-triple quadrupole-ICP-MS. J. Anal. At. Spectrom. 2016;31:197–202. doi: 10.1039/C5JA00293A. [DOI] [Google Scholar]
- 122.Mao J., Vanderlelie J.J., Perkins A.V., Redman C.W., Ahmadi K.R., Rayman M.P. Genetic polymorphisms that affect selenium status and response to selenium supplementation in United Kingdom pregnant women. Am. J. Clin. Nutr. 2016;103:100–106. doi: 10.3945/ajcn.115.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu X.F., Sharin T., Chan H.M. Dietary and blood selenium are inversely associated with the prevalence of stroke among Inuit in Canada. J. Trace Elem. Med. Biol. 2017;44:322–330. doi: 10.1016/j.jtemb.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 124.Laclaustra M., Navas-Acien A., Stranges S., Ordovas J.M., Guallar E. Serum selenium concentrations and hypertension in the US Population. Circ. Cardiovasc. Qual. Outcomes. 2009;2:369–376. doi: 10.1161/CIRCOUTCOMES.108.831552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Su L., Jin Y., Unverzagt F.W., Liang C., Cheng Y., Hake A.M., Kuruppu D., Ma F., Liu J., Chen C., et al. Longitudinal Association between Selenium Levels and Hypertension in a Rural Elderly Chinese Cohort. J. Nutr. Health Aging. 2016;20:983–988. doi: 10.1007/s12603-016-0700-7. [DOI] [PubMed] [Google Scholar]
- 126.Kuruppu D., Hendrie H.C., Yang L., Gao S. Selenium levels and hypertension: A systematic review of the literature. Public Health Nutr. 2014;17:1342–1352. doi: 10.1017/S1368980013000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parkin Kullmann J.A., Pamphlett R. A Comparison of Mercury Exposure from Seafood Consumption and Dental Amalgam Fillings in People with and without Amyotrophic Lateral Sclerosis (ALS): An International Online Case-Control Study. Int. J. Environ. Res. Public Health. 2018;15:2874. doi: 10.3390/ijerph15122874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ralston N.V.C., Kaneko J.J., Raymond L.J. Selenium health benefit values provide a reliable index of seafood benefits vs. risks. J. Trace Elem. Med. Biol. 2019;55:50–57. doi: 10.1016/j.jtemb.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 129.Ralston N.V., Raymond L.J. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology. 2010;278:112–123. doi: 10.1016/j.tox.2010.06.004. [DOI] [PubMed] [Google Scholar]