Abstract
Schistosomiasis is a disease of great medical and veterinary importance in tropical and subtropical regions caused by different species of parasitic flatworms of the genus Schistosoma. The emergence of natural hybrids of schistosomes indicate the risk of possible infection to humans and their zoonotic potential, specifically for Schistosoma haematobium and S. bovis. Hybrid schistosomes have the potential to replace existing species, generate new resistances, pathologies and extending host ranges. Hybrids may also confuse the serological, molecular and parasitological diagnosis. Currently, LAMP technology based on detection of nucleic acids is used for detection of many agents, including schistosomes. Here, we evaluate our previously developed species-specific LAMP assays for S. haematobium, S. mansoni, S. bovis and also the genus-specific LAMP for the simultaneous detection of several Schistosoma species against both DNA from pure and, for the first time, S. haematobium x S. bovis hybrids. Proper operation was evaluated with DNA from hybrid schistosomes and with human urine samples artificially contaminated with parasites’ DNA. LAMP was performed with and without prior DNA extraction. The genus-specific LAMP properly amplified pure Schistosoma species and different S. haematobium-S. bovis hybrids with different sensitivity. The Schistosoma spp.-LAMP method is potentially adaptable for field diagnosis and disease surveillance in schistosomiasis endemic areas where human infections by schistosome hybrids are increasingly common.
Keywords: LAMP, schistosomiasis, schistosome hybrids, Schistosoma haematobium, Schistosoma bovis, molecular diagnosis, species-specific LAMP, genus-specific LAMP
1. Introduction
Environmental changes due to ecosystem decline, biodiversity loss and climate change are some issues with potential ecological risk that we are facing as human beings. These changes driven by increasing economic development, migration, agricultural and livestock practices and deforestation have consequences in emerging infectious diseases (EIDs) [1,2,3,4,5,6]. Changes in biodiversity have the potential to either increase or reduce the incidence of infectious disease in humans because they involve interactions among species. The appearance of diseases in non-endemic areas and the increase in encounters between different species, when ecological and geographic barriers are lost, lead to the emergence of new hybrid forms [4]. Hybridization of parasites is an emerging public health issue [6], mainly because hybrid forms have the potential to generate new resistances, pathologies, be more virulent as well as affect new hosts [4,6].
Schistosomiasis is one of the most important parasitic diseases of humans in terms of morbidity and mortality, ranking secondary to malaria. The World Health Organization (WHO) estimates that almost 240 million people are affected worldwide (up to 90% in Africa) with 700 million people living in tropical and subtropical endemic areas in over 78 countries [7,8,9]. The parasitic flatworms responsible of schistosomiasis are digenetic trematodes worms of the genus Schistosoma that infect both humans and animals. The three main species infecting humans are S. haematobium, S. mansoni (both in Africa and the Middle East; S. mansoni is also present in the Americas) and S. japonicum (Asia). Other schistosome species have been linked to human infections, including S. intercalatum, S. guineensis (both in West and Central Africa), S. mekongi (in Kong Island) [10,11] and S. malayensis (Malaysia) [12], or with potential to infect humans, such us S. mattheei (in Africa) [13]. Livestock schistosomiasis due to S. bovis, S. curassoni and S. mattheei in cattle, sheep and goats is a common parasitic infection in sub-Saharan Africa, and it is an important cause of animal mortality and morbidity [5,14]. The emergence of natural hybrids of S. haematobium-S. guineensis [15], S. haematobium-S. intercalatum [16,17], S. haematobium-S. mattheei [18] and mainly S. haematobium and the cattle schistosome S. bovis [2,19,20,21,22] clearly indicate the risk of hybrids that can potentially infect humans and their zoonotic potential [23]. Schistosoma bovis is one of the most significant veterinary problems in Africa [24,25] and to date is considered as a possible emerging health threat after the molecular characterization of S. haematobium-S. bovis hybrids from children in Senegal [2], in Côte d’Ivoire [26], in Benin [23], in Niger [27], in Mali [28] and in a schistosomiasis outbreak in Corsica, France [19]. S. bovis is phylogenetically a close relative of S. haematobium, and their close relationship and overlapping geographical distribution allows these to hybridize in the wild, increasing their genetic diversity and the risk of zoonotic transmission from animal reservoirs to humans [22]. Moreover, zoonotic hybrids could replace existing species and parasite strains extending intermediate and definitive host ranges, complicating transmission or presenting and increasing infectivity and virulence [6]. Furthermore, hybrid schistosomes forms may confuse the serological, molecular and, especially, parasitological diagnostic because the presence of excreted ova with atypical morphology [29,30].
However, in endemic countries schistosomiasis is definitively diagnosed by microscopic examination of excreted eggs in stool (S. mansoni, S. japonicum, S. intercalum, S. guineensis and S. mekongi) by the Kato-Katz method (KK) or in urine (S. haematobium) by filtration or sedimentation techniques. Typically, microscopy is relatively time-consuming and lacks in sensitivity, mainly in areas with low-intensity infections [7,31,32]. Numerous serological diagnostic approaches, including enzyme-linked immunosorbent assay (ELISA) and indirect hemagglutination (IHA) tests, in addition to other assays based on the antibody and antigen detection have been widely evaluated, but cross-reactivity, differences in sensitivity and a lack of standardization have been reported [33,34,35]. To try to solve these disadvantages, a large number of more sensitive and accurate PCR-based molecular methods have also been developed both for the diagnosis of human and animal schistosomiasis [36], being especially valuable in simultaneous detection and identification of Schistosoma species [37]. However, the complex PCR-based techniques are expensive and difficult to apply routinely in field conditions in endemic areas of schistosomiasis. In this sense, loop-mediated isothermal amplification (LAMP) technology [38] has been recently revealed as a versatile alternative, having great potential for molecular diagnosis in limited-resource settings in endemic areas [39,40]. To date, a number of LAMP approaches have been developed to detect specifically S. haematobium, S. mansoni and S. japonicum in urine, stool, and snails specimens, as recently summarized by Avendaño and Patarroyo [41]. In addition, a novel species-specific LAMP to detect S. bovis and a genus-specific LAMP to detect different Schistosoma species (including S. haematobium, S. intercalatum, S. mansoni and S. bovis) have been recently reported by our group [42]. Due to the schistosome hybridization rapid emergence and spread, and the consequences for disease prevalence, pathological characteristics and treatment, a LAMP test to detect several Schistosoma species (including hybrids forms) would be very useful for the diagnosis and management of schistosomiasis.
Thus, in this study we examined the utility of our recently developed genus-specific LAMP assay to detect Schistosoma species in the detection of different hybrid schistosome molecular profiles. Moreover, we evaluated the Schistosoma spp.-LAMP in simulated human urine samples spiked with serially diluted DNA from hybrid specimens using both urine with and without prior DNA extraction.
2. Materials and Methods
2.1. Schistosoma Species DNA Samples
Genomic DNA (gDNA) samples from several hybrid schistosomes (miracidia) and pure adults S. haematobium, S. mansoni, and S. bovis species were used in our study. The gDNA of schistosome hybrids was obtained from parasites collected in previous studies carried out in Agboville (Côte d’Ivoire) by Angora et al. [26] and in Corsica, France, by Boissier et al. [19]. The schistosome hybrids profiles, according to the rapid diagnostic mitochondrial cox1 analysis and by sequencing of the cox1 and ITS regions, respectively, as described elsewhere [26], are specified in Table 1. Pure S. haematobium gDNA (Egyptian strain) was kindly provided by the Laboratoire Interactions Hôtes-Pathogènes-Environnements (IHPE), University Perpignan Via Domitia, Perpignan, France. The laboratory strain from Egypt is experimentally maintained in the culturing facilities at the University of Perpignan and was originally provided by the Biomedical Research Institute, Rockville, Maryland [43]. S. bovis was provided by the laboratory of Animal Parasitology of the Institute of Natural Resources and Agrobiology of Salamanca where it has been maintained in hamsters and sheep experimentally infected. S. mansoni is maintained by serial passages in mice routinely infected in the Laboratory of Parasitic and Molecular Immunology, CIETUS, University of Salamanca, Salamanca, Spain. S. mansoni gDNA (Brazilian strain) and S. bovis gDNA (Spanish strain) were obtained from frozen adult male and female worms using the NucleoSpin Tissue Kit (Macherey-Nagel, GmbH & Co., Dueren, Germany) following the manufacturers’ instructions. All DNA samples were measured using a Nanodrop ND-100 spectrophotometer (Nanodrop Technologies) and then diluted to final 20 ng/µL and 10 ng/µL concentrations. Subsequently, from 10 ng/µL concentration serial 10-fold dilutions were prepared with ultrapure water ranging from 10−1 to 10−6 and stored at −20 °C until use.
Table 1.
Hybrid schistosomes genetic profiles according to the rapid diagnostic (RD-PCR) mitochondrial cox1 analysis and by sequencing of the cox1 and ITS regions, respectively, from studies in Agboville (Côte d’Ivoire) and Corsica (c), France. The abbreviations Sb/Sb, Sh/Sb or Sh/Sh indicate that at the diagnostic sites two chromatogram peaks were visible after sequencing.
| Study Location |
RD-PCR Analysis |
Sequence Analysis |
Abbreviation | |
|---|---|---|---|---|
| cox1 | cox 1 haplotypes | ITS2 alleles | ||
| Agboville | S. haematobium | S. haematobium | S. bovis + S. bovis | Sh-Sb/Sb |
| S. bovis | S. bovis | S. haematobium + S. bovis | Sb-Sh/Sb | |
| S. bovis | S. bovis | S. haematobium+ S. haematobium | Sb-Sh/Sh | |
| S. haematobium | S. haematobium | S. haematobium + S. bovis | Sh-Sh/Sb | |
| Corsica | S. bovis | S. bovis | S. haematobium + S. haematobium | Sb-Sh/Shc |
Sb-Sh/Shc: Hybrid schistosome from Corsica, France.
2.2. Urine Samples Spiked with gDNA from Schistosoma Species
Fresh urine was collected from healthy staff donors with no history of travel to endemic areas of schistosomiasis to assess both specificity and sensitivity of the Schisto-LAMP assays. Urine was divided into aliquots of 100 μL each and then artificially spiked with 2 μL of 10-fold serially diluted gDNA from Schistosoma species ranging from 20 ng/μL to 100 fg/μL, thus resulting in a set of artificial urine samples with a final hybrid schistosomes gDNA concentration ranging from 0.8 ng/μL to 4 fg/μL. These fresh simulated urine samples were prepared when required and analyzed in Schisto-LAMP assays following two procedures. In the first procedure, we used the “Rapid-Heat LAMP method” as described elsewhere by Gandasegui et al. [44]. In brief, each aliquot of urine was heated at 95 °C for 15 min and shortly spun to pellet the debris. Subsequently, 2 μL of the supernatant was used directly as template for LAMP reactions. After analysis, the remaining volume of each aliquot was stored at −20 °C. In the second procedure, the frozen simulated urine samples were thawed and DNA was extracted using the i-genomic Urine DNA Extraction Mini Kit (Intron Biotechnology, UK) following the manufacturers’ instructions. DNA obtained from aliquots was stored at −20 °C until use in a second LAMP screening.
2.3. Schisto-LAMP Assays
LAMP assays were accomplished using the reaction mixtures and specific primer sets previously described elsewhere by our group for detection of species-specific S. mansoni based on a mitochondrial minisatellite DNA region [45], S. haematobium, based on the ribosomal intergenic spacer (IGS) [44], and S. bovis, based on the mitochondrial NADH subunit 1 [42]. A genus-specific LAMP assay designed on the internal transcribed spacer 1 (ITS-1) for the simultaneous detection of different species, including S. mansoni, S. haematobium, S. intercalatum and S. bovis, was also applied [42]. The reactions were carried out using previously described conditions, with the exception of the final reaction volume, which was reduced from 25 μL to 15 μL. Briefly, LAMP reaction mixtures (15 μL) contained 40 pmol each of FIP and BIP primers, 5 pmol each of F3 and B3 primers, 0.4 μM of each LB and LF primers (if applicable) (Table 2), 1.4 mM of each dNTP (Bioron), 1x Isothermal Amplification Buffer—20 mM Tris-HCl (pH 8.8), 50 mM KCl, 10 mM (NH4)2SO4, 2 mM MgSO4, 0.1% Tween20 (New England Biolabs Ltd., Ipswich, MA, USA)—supplementary with 6 mM MgSO4 and 8 U of Bst 2.0 WarmStart DNA polymerase (New England Biolabs Ltd., Ipswich, MA, USA) with 2 μL of template. Schisto-LAMP reactions were performed in 0.5 mL tubes that were incubated in a heat block at 65 °C for 60 min and then heated at 80 °C for 5–10 min to stop the reaction.
Table 2.
Primer sets used in this work for Schisto-LAMP assays. For S. mansoni, S. haematobium, S. bovis and Schistosoma spp.: F3, forward outer primer; B3, backward outer primer; FIP, forward inner primer (comprising F1c and F2 sequences); BIP, backward inner primer (comprising B1c and B2 sequences); LF, loop forward primer; LB = loop backward primer. bp, base pairs.
| Schisto-LAMP | Primer Sets | Sequence 5′→3′ | Length (bp) | Ref. |
|---|---|---|---|---|
| S. mansoni | F3 | TTATCGTCTATAGTACGGTAGG | 22 | [45] |
| B3 | ATACTTTAACCCCCACCAA | 19 | ||
| FIP | GCCAAGTAGAGACACAAACATCTT-TGGGTAAGGTAGAAAATGTTGT | 47 | ||
| BIP | AGAAGTGTTTAACTTGATGAAGGGG-AAACAAAACCGAAACCACTA | 45 | ||
| S. haematobium | F3 | CTTTCTAAGCCCGCGATA | 18 | [44] |
| B3 | GCGCATTACACTTGGTCT | 18 | ||
| FIP | TACCCCTAACTTCGTGGTCTCC-CCCCCTTATTTTAGGGTGC | 41 | ||
| BIP | CTCCCTATATAACATGGCGAGTAAG-ACTATGAAATCAGTGTTTTTCGG | 48 | ||
| S. bovis | F3 | TTCATTGTTAGGTTGCGT | 18 | [42] |
| B3 | TCTATATTCTACTCTAATCCCTCT | 24 | ||
| FIP | TCAGTATCATCTCAAACATCACACT-AGTAGTATGTTCTGTCTTAAGTT | 48 | ||
| BIP | TTTGTAGTACCTCTGGTTTACATCA-TTCACTCTCAGACTCTACAT | 45 | ||
| LF | ACTTAGACCATGAACATCAACCTAT | 25 | ||
| LB | TACTAAGTGAGAGTAATCGAACACC | 25 | ||
| Schistosoma spp. | F3 | TTGACCGGGGTACCTAGC | 18 | [42] |
| B3 | CGTGAATGGCAAGCCAAAC | 19 | ||
| FIP | ATCGCCCTTGGCAGATCAGG-CTGTCGTATGCCCTGATGG | 39 | ||
| BIP | ATATGCATGCAAATCCGCCCCG-CGGATCGCTTCAACAGTGTA | 43 | ||
| LF | CAGATCAGGCAACCCGAAAG | 22 |
2.4. Specificity and Sensibility of Schisto-LAMP Assays in Detecting Schistosome Hybrids
The specificity of Schisto-LAMP assays to amplify both pure Schistosoma species (S. haematobium, S. mansoni and S. bovis) and hybrids was tested against parasite DNA samples used as controls, as mentioned above. To determine the lower detection limit of the Schisto-LAMP assays, gDNA from hybrid schistosomes 10-fold serially diluted was used as template for amplification. Moreover, the sensitivity was also assayed with the simulated urine samples artificially spiked with hybrid schistosomes gDNA both without prior DNA extraction and after DNA extraction by using the commercial kit.
2.5. Detection of LAMP Products
LAMP results were visually detected by the naked eye by adding 2 μL (1:10, 10,000x) SYBR Green I fluorescent dye (Invitrogen, Carlsbad, California, USA) to each reaction tube post-amplification. Green fluorescence was observed in LAMP-positive reactions and original orange in LAMP-negative reactions. In addition, the LAMP products (3–5 μL) were visualized by Midori Green Advance DNA (Nippon Genetics Europe GmbH, Dueren, Germany) staining in 1.5% agarose gels to corroborate the colorimetric results. The LAMP amplifications showed a characteristic ladder-like band pattern.
3. Results
3.1. Schisto-LAMP Assays Performance
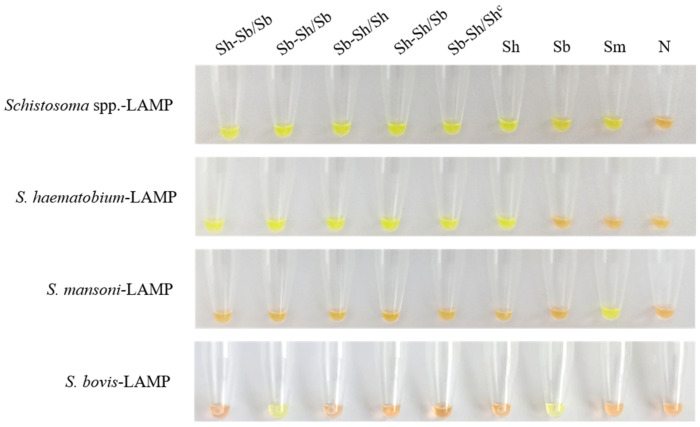
The results obtained in testing the different Schisto-LAMP assays against both the hybrid schistosomes gDNA and pure Schistosoma species used as controls are shown in Figure 1. All DNA samples tested positive by the genus-specific LAMP. The species-specific LAMP for detecting S. haematobium amplified all DNA samples with the exception of S. bovis and S. mansoni DNA. As expected, the species-specific LAMP for S. mansoni only amplified DNA from this parasite. Finally, the species-specific LAMP for S. bovis amplified both pure S. bovis DNA and S. bovis-S. haematobium x S. bovis hybrid parasite DNA.
Figure 1.
Schisto-LAMP assays performance in testing DNA samples from both pure Schistosoma species and hybrids. Schistosoma spp.-LAMP, the genus-specific LAMP for detecting several schistosome species; S. haematobium-LAMP, S. mansoni-LAMP and S. bovis-LAMP, the species-specific LAMP assays for detecting S. haematobium, S. mansoni and S. bovis, respectively. Sh-Sb/Sb, Sb-Sh/Sb, Sb-Sh/Sh, Sh-Sh/Sb, DNA from schistosomes hybrids from Agboville. Sb-Sh/Shc, corsican hybrid schistosome. Sh, Sb, Sm, DNA from pure S. haematobium, S. bovis and S. mansoni, respectively. N, negative control (no DNA template).
3.2. Sensitivity of Genus-Specific-LAMP Assay in Detection of Hybrid Schistosomes
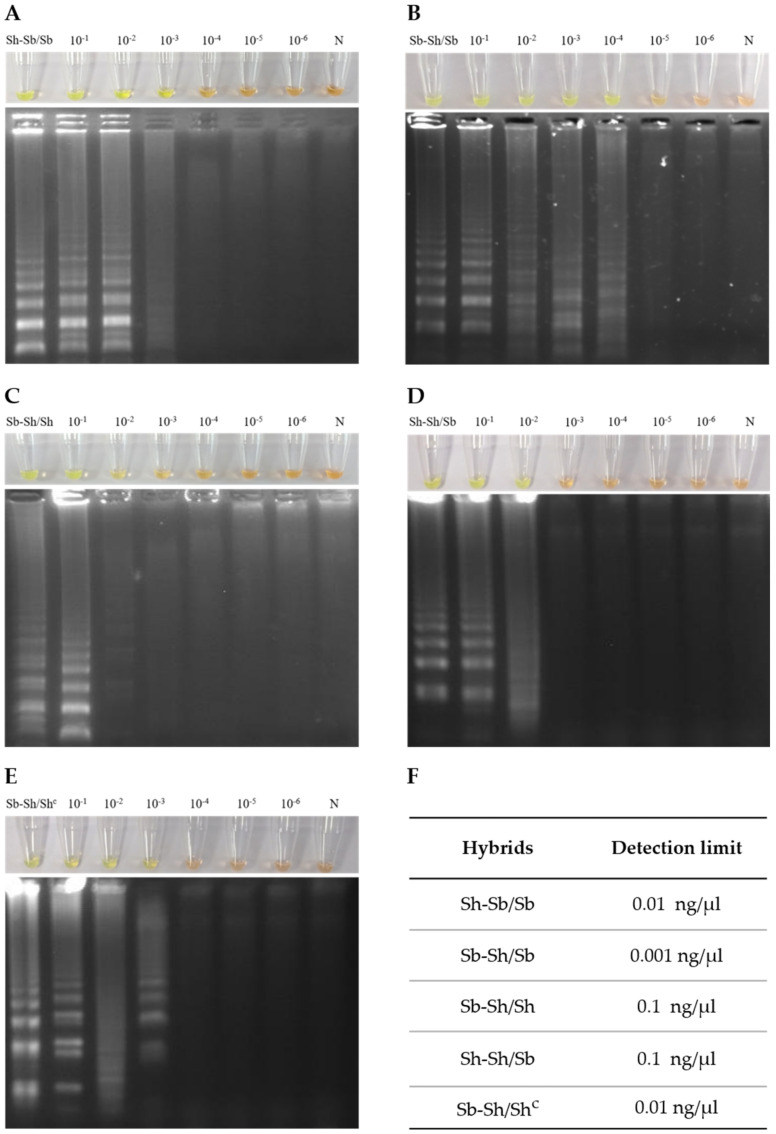
The genus-specific LAMP assay detection limit was different for the different hybrid schistosomes tested (Figure 2). The hybrids Sb-Sh/Sh and Sh-Sh/Sb DNA amplification detection limit was 0.1 ng/µL, whereas the hybrids Sh-Sb/Sb and Sb-Sh/Sh (Corsican hybrid) detection limit was 0.01 ng/µL. The lowest limit of detection was 0.001 ng/µL for hybrid Sb-Sh/Sb.
Figure 2.
Assessment of genus-specific LAMP analytical sensitivity for hybrid schistosomes using gDNA serial dilutions. The figure shows the genus-specific LAMP results by color change (top) and in agarose electrophoresis (bottom) for each hybrid schistosome tested: (A) Sh-Sb/Sb; (B) Sb-Sh/Sb; (C) Sb-Sh/Sh; (D) Sh-Sh/Sb and (E) Sb-Sh/Shc). (F) Summary table indicating the genus-specific LAMP detection limit for detecting each hybrid. Lanes 10−1–10−6: 10-fold serial dilutions. N, negative control (no DNA template).
3.3. Detection Limit of Genus-Specific LAMP Assay in Simulated Human Urine Samples
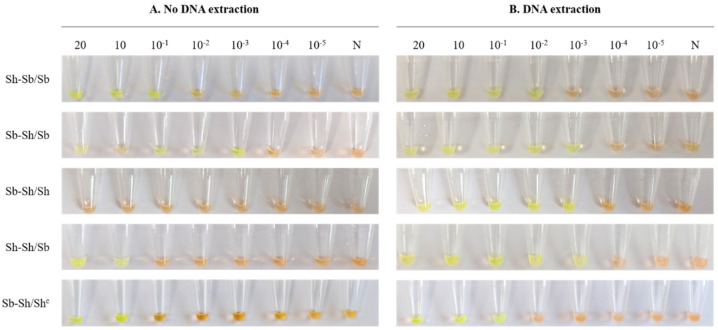
The detection limit of genus-specific LAMP for hybrid schistosomes in simulated urine samples spiked with serial dilutions of gDNA is shown in Figure 3. As observed by colorimetric change, the sensitivity was generally lower when using the urine samples without prior extraction of DNA (for Sh-Sb/Sb: 0.1 ng/µL; Sh-Sh/Sb and Sb-Sh/Shc: 1 ng/µL) than when using a commercial kit for extraction (for Sh-Sb/Sb: 0.01 ng/µL; Sh-Sh/Sb: 0.001 ng/µL and Sb-Sh/Shc: 0.1 ng/µL). It should be noted that we obtained the same detection limit of genus-specific LAMP assay for hybrid Sb-Sh/Sb using both procedures for analysis (0.001 ng/µL). Unexpectedly, hybrid Sb-Sh/Sh did not amplify using the simple heat method but did when a prior DNA extraction was carried out, reaching a limit of detection of 0.001 ng/µL.
Figure 3.
Sensitivity of the genus-specific LAMP assay in simulated human urine samples artificially spiked with gDNA from different hybrid schistosomes. (A) Sensitivity assessment of LAMP when performing a simple heating method from serial dilutions of hybrid schistosomes gDNA. (B) Sensitivity assessment of LAMP when performing the DNA extraction with the i-genomic Urine DNA Extraction Mini Kit (Intron Biotechnology, UK) from serial dilutions of hybrid schistosomes gDNA. Lanes 20, 10 and 10−1–10−5: 20 ng, 10 ng and 10-fold serial dilutions, respectively; Sh-Sb/Sb, Sb-Sh/Sb, Sb-Sh/Sh, Sh-Sh/Sb, and Sb-Sh/Shc: gDNA from hybrid schistosomes; N: negative controls (no DNA template).
4. Discussion
Schistosoma species hybridization in nature is an emergent issue for public health [4,6]. Molecular data from hybridizations between schistosome collections have identified new species distributions [46], interspecies both human-specific and animal-specific hybridization [22,47,48,49], and surprising host associations and multi-host transmission [23,50,51]. Molecular detection of the hybrid schistosomes adds a new perspective to the diagnosis, epidemiology and control of schistosomiasis.
In this work, we tested our previously developed species-specific LAMP assays for S. haematobium [44], S. mansoni [45], S. bovis and also the genus-specific LAMP for the simultaneous detection of several Schistosoma species [42] against both gDNA from pure and, for the first time, hybrid schistosomes. These hybrids were obtained in studies conducted in Côte d’Ivoire [26] and Corsica, France [19], and subsequently well characterized by amplification and sequencing of a partial fragment of the mitochondrial cytochrome c oxidase subunit 1 (cox1) and the complete nuclear ribosomal DNA internal transcribed spacer (ITS).
As expected, in our trials, species-specific LAMP for S. mansoni only amplified DNA from pure parasite but not from other schistosomes nor hybrids S. bovis-S. haematobium or S. haematobium-S. bovis, thus corroborating again its high specificity in detection of S. mansoni. The S. mansoni-LAMP was originally designing on a 620 bp sequence corresponding to a specific mitochondrial S. mansoni minisatellite DNA region [52] and has been already specifically tested by our group in stool samples from experimentally infected mice [45], in both human stool and snail samples in field conditions [53], and also in human urine samples [54]. Notwithstanding, it should be very interesting to assess this S. mansoni-LAMP in S. mansoni-S. haematobium hybrid parasites detection since these hybrid forms have been already described in a study carried out in schoolchildren from northern Senegal [49] and, more recently, in a migrant boy from Côte d’Ivoire entering France [55].
The species-specific LAMP for S. bovis amplified gDNA from pure S. bovis but not from S. haematobium nor S. mansoni, showing its high specificity in detecting only that species. The two hybrids with a S. haematobium mitochondrial (cox1) profile (Sh-Sb/Sb and Sh-Sh/Sb) did not amplify. Unexpectedly, among those hybrids with a S. bovis mitochondrial (cox1) profile (Sb-Sh/Sb, Sb-Sh/Sh, and Sb-Sh/Shc), the only one amplified was S. bovis-S. haematobium/S. bovis but not those with a S. haematobium double-banded ITS rDNA profile. Our S. bovis-LAMP is based on a 678 bp sequence derived from mitochondrial NADH subunit 1 (NADH-1) first reported by Xiao et al. (2010). Sequences generated from the mitochondrial (mt) DNA, including cox1 and NADH 1, are the most commonly used mitochondrial markers for studies on flatworms helping to establish the population and genetic relationship among Schistosoma species [56]. mtDNA is usually maternally inherited in almost all metazoans and is considered to be clonal and rarely or never undergoes recombination. Nevertheless, mtDNA rapidly accumulate mutations over time and shows a higher level of divergence among species relative to intra-specific variation [47,57]. This could be a possible explanation for lack of amplification in both hybrid Sb-Sh/Sh (from Côte d’Ivoire) and Sb-Sh/Shc (from Corsica) profiles. The fact that only the hybrid Sb-Sh/Sb was amplified by S. bovis-specific LAMP may be interpreted because the hybrid line is the result of an initial cross between a male S. haematobium and a female S. bovis, leading to introgression of S. bovis mtDNA into S. haematobium [2], most likely favoring the amplification of the S. bovis profile.
As expected, the genus-specific LAMP achieved DNA amplification of pure Schistosoma species (S. mansoni, S. haematobium and S. bovis) as verified previously by our group [42], but also, and very interestingly, all hybrid schistosomes tested. The Schistosoma spp.-LAMP design is based on a 457 bp ITS-1 sequence type from S. haematobium [58]. ITS-1 and ITS-2 are sequences of non-functional RNA situated between structural ribosomal RNAs on a common precursor transcript. ITS-2 has been widely used in trematode identification because it is usually conserved within species but more variable among species [59,60]. The schistosome ITS-2 is particularly powerful marker to detect introgression. This region can retain both parental copies for several generations before they are homogenized by concerted evolution, the nuclear DNA profiles resulting in double chromatogram peaks at the species-specific mutation sites [6]. On the other hand, the schistosome ITS-1 contains an original main repeating sequence unit from the 3’ end of the 18S rRNA gene which, in turn, contains a sub-repeat that varies slightly in size and composition [61]. There is a high degree of sequence conservation between the repeats, but variation in sequence patterns and their number occur both within and between species. For example, the ITS-1 region of S. haematobium contains two tandemly repeated elements, whereas S. japonicum group of species contains as many as seven repeats [62]. In general, multiple repeats and intra-individual variation in numbers and abundance of these is a feature of the Asian schistosomes (S. japonicum and S. indicum groups), but not generally of African schistosomes (S. mansoni and S. haematobium groups), in which an absence of intra-individual variation in the ITS-1 was reported [63]. In this regard, since the hybrid schistosomes tested has been molecularly characterized by analysis of mitochondrial cox1 as S. haematobium/S. bovis species designation (hence, African schistosomes), our genus-specific LAMP could detect the hybrids probably because of that lack of intra-individual variation in the ITS-1 type targeted sequence. Additionally, processes such as hybridization could cause the sharing of different ITS types among Schistosoma species [57], which would likely affect the sensitivity in detecting gDNA from the different hybrid specimens. In this sense, using Schistosoma spp-LAMP, we previously reported a limit of detection for pure S. haematobium and S. bovis species of 0.1 pg and 10 pg, respectively [42]; however, a lower sensitivity ranging from 100 pg to 1 pg was now obtained when testing the different crossing of S. haematobium and S. bovis species. Interestingly, for hybrid Sb-Sh/Sb a limit of detection 10 times higher than for pure S. bovis was obtained (1 pg vs. 10 pg). Despite the variation in sensitivity of the Schistosoma spp.-LAMP between the detection of pure Schistosoma species and hybrid forms, it is very important to highlight the possibility of amplifying both pure and hybrid schistosomes in order to use LAMP as a single molecular tool for the diagnosis and surveillance of schistosomiasis, mainly in endemic areas of the disease. As mentioned above for S. mansoni-LAMP, it would also be very interesting to test if the Schistosoma spp.-LAMP assay (that detects pure S. mansoni) could amplify the more surprising hybridization between S. mansoni and S. haematobium that infect humans [6,55].
Similarly remarkably, the species-specific LAMP for S. haematobium amplified all gDNA from hybrid forms in addition to the pure S. haematobium gDNA. Our S. haematobium-LAMP is based on a 2522 bp sequence of S. haematobium ribosomal intergenic spacer (IGS) DNA (GenBank: AJ223838) [64] that amplifies a highly specific sub-sequence target of 199 bp of that species [44]. The IGS of Schistosoma species contains many repeats, and recombination is a relatively frequent event, although sequences of S. haematobium are well conserved within the IGS [64]. Since the molecular characterization of the hybrids showed a S. haematobium signature (either by cox1 or ITS-2 genetic profiles), amplification of the S. haematobium IGS sequence could be possible in all hybrid schistosomes tested.
Regarding urine samples analysis, we are aware that our Schistosoma spp.-LAMP has not been tested with clinical specimens, but results obtained in simulated human urine samples indicate that, although with some differences, the LAMP test is sensitive enough to detect hybrid schistosomes at a low level in urine. Better results were obtained when applying a commercial kit for DNA extraction than heated urine, because of the well-known effectiveness of this procedure to isolate genomic DNA from urine samples suitable for further molecular analyses [65]. However, amplification (with the unexplainable exception in hybrid Sb-Sh/Sh) was also obtained just with heated whole urine without prior DNA extraction at an acceptable level. This inexpensive and simple rapid-heating procedure could be potentially very useful under certain circumstances when a large number of samples must be tested, mainly in low-resource settings in endemic areas.
5. Conclusions
In conclusion, the results of this preliminary study demonstrated that the genus-specific LAMP assay could be a potential molecular tool to be use for detection, not only for different pure schistosome species, but also for hybrids S. haematobium-S. bovis in urine samples. Although further research for evaluation of the assay for the application in clinical samples is required, the method is potentially adaptable for field diagnosis and disease surveillance in schistosomiasis endemic areas where human infections by schistosome hybrids are increasingly common.
Author Contributions
Conceptualization, B.C.-V., P.F.-S. and A.M.; Methodology, B.C.-V., P.F.-S., B.F.-S. and J.G.-B.D.; Investigation, B.C.-V., P.F.-S., B.F.-S. and J.G.-B.D.; Project administration, P.F.-S.; Resources, J.B., E.K.A. and A.O.P; writing—original draft preparation, B.C.-V., P.F.-S. and A.M.A.; writing—review and editing, B.C.-V., P.F.-S., A.M., J.B., E.K.A. and A.O.; Funding acquisition, P.F.-S. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Institute of Health Carlos III, ISCIII, Spain (www.isciii.es), grants: RICET RD16/0027/0018 (A.M.), PI19/01727 (P.F.-S.), European Union cofinancing by FEDER (Fondo Europeo de Desarrollo Regional) ‘Una manera de hacer Europa’. We also acknowledge support by the Predoctoral Fellowship Program of University of Salamanca and cofinancing by Santander Bank, and Predoctoral Fellowship Program of Junta de Castilla y León cofinancing by Fondo Social Europeo.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huyse T., Webster B.L., Geldof S., Stothard J.R., Diaw O.T., Polman K., Rollinson D. Bidirectional introgressive hybridization between a cattle and human schistosome species. PLoS Pathog. 2009;5:e1000571. doi: 10.1371/journal.ppat.1000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keesing F., Belden L.K., Daszak P., Dobson A., Harvell C.D., Holt R.D., Hudson P., Jolles A., Jones K.E., Mitchell C.E., et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King K.C., Stelkens R.B., Webster J.P., Smith D.F., Brockhurst M.A. Hybridization in parasites: Consequences for adaptive evolution, pathogenesis, and public health in a changing world. PLoS Pathog. 2015;11:1–12. doi: 10.1371/journal.ppat.1005098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webster J.P., Gower C.M., Knowles S.C.L., Molyneux D.H., Fenton A. One health—An ecological and evolutionary framework for tackling Neglected Zoonotic Diseases. Evol. Appl. 2016;9:313–333. doi: 10.1111/eva.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leger E., Webster J.P. Hybridizations within the genus Schistosoma: Implications for evolution, epidemiology and control. Parasitology. 2017;144:65–80. doi: 10.1017/S0031182016001190. [DOI] [PubMed] [Google Scholar]
- 7.Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. [(accessed on 18 November 2020)];2018 Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis.
- 10.Ohmae H., Sinuon M., Kirinoki M., Matsumoto J., Chigusa Y., Socheat D., Matsuda H. Schistosomiasis mekongi: From discovery to control. Parasitol. Int. 2004;53:135–142. doi: 10.1016/j.parint.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Webster B.L., Southgate V.R., Timothy D., Littlewood J. A revision of the interrelationships of Schistosoma including the recently described Schistosoma guineensis. Int. J. Parasitol. 2006;36:947–955. doi: 10.1016/j.ijpara.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Latif B., Heo C.C., Razuin R., Shamalaa D.V., Tappe D. Autochthonous human schistosomiasis, Malaysia. Emerg. Infect. Dis. 2013;19:1340–1341. doi: 10.3201/eid1908.121710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weyher A.H., Phillips-Conroy J.E., Fischer K., Weil G.J., Chansa W., Fischer P.U. Molecular identification of Schistosoma mattheei from feces of kinda (Papio cynocephalus kindae) and grayfoot baboons (Papio ursinus griseipes) in Zambia. J. Parasitol. 2010;96:184–190. doi: 10.1645/GE-2186.1. [DOI] [PubMed] [Google Scholar]
- 14.De Bont J., Vercruysse J. The epidemiology and control of cattle schistosomiasis. Parasitol. Today. 1997;13:255–262. doi: 10.1016/S0169-4758(97)01057-0. [DOI] [PubMed] [Google Scholar]
- 15.Webster B.L., Tchuem Tchuenté L.A., Jourdane J., Southgate V.R. The interaction of Schistosoma haematobium and S. guineensis in Cameroon. J. Helminthol. 2005;79:193–197. doi: 10.1079/JOH2005306. [DOI] [PubMed] [Google Scholar]
- 16.Southgate V.R., van Wijk H.B., Wright C.A. Schistosomiasis at Loum, Cameroun; Schistosoma haematobium, Schistosoma haematobium, S. intercalatum and their natural hybrid. Z. Parasitenkd. 1976;49:145–159. doi: 10.1007/BF00382422. [DOI] [PubMed] [Google Scholar]
- 17.Webster B.L., Southgate V.R., Tchuem Tchuenté L.-A. Isoenzyme analysis of Schistosoma haematobium, S. intercalatum and their hybrids and occurrences of natural hybridization in Cameroon. J. Helminthol. 2003;77:269–274. doi: 10.1079/JOH2003166. [DOI] [PubMed] [Google Scholar]
- 18.Webster B.L., Alharbi M.H., Kayuni S., Makaula P., Halstead F., Christiansen R., Juziwelo L., Stanton M.C., LaCourse E.J., Rollinson D., et al. Schistosome interactions within the Schistosoma haematobium group, Malawi. Emerg. Infect. Dis. 2019;25:1245–1247. doi: 10.3201/eid2506.190020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boissier J., Grech-Angelini S., Webster B.L., Allienne J.F., Huyse T., Mas-Coma S., Toulza E., Barré-Cardi H., Rollinson D., Kincaid-Smith J., et al. Outbreak of urogenital schistosomiasis in Corsica (France): An epidemiological case study. Lancet Infect. Dis. 2016;16:971–979. doi: 10.1016/S1473-3099(16)00175-4. [DOI] [PubMed] [Google Scholar]
- 20.De la Torre-Escudero E., Pérez-Sánchez R., Manzano-Román R., Oleaga A. Schistosoma bovis-host interplay: Proteomics for knowing and acting. Mol. Biochem. Parasitol. 2017;215:30–39. doi: 10.1016/j.molbiopara.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Kincaid-Smith J., Rey O., Toulza E., Berry A., Boissier J. Emerging Schistosomiasis in Europe: A need to quantify the risks. Trends Parasitol. 2017;33:600–609. doi: 10.1016/j.pt.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Oey H., Zakrzewski M., Gravermann K., Young N.D., Korhonen P.K., Gobert G.N., Nawaratna S., Hasan S., Martínez D.M., You H., et al. Whole-genome sequence of the bovine blood fluke Schistosoma bovis supports interspecific hybridization with S. haematobium. PLoS Pathog. 2019;15:1–16. doi: 10.1371/journal.ppat.1007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savassi B.A.E.S., Mouahid G., Lasica C., Mahaman S.D.K., Garcia A., Courtin D., Allienne J.F., Ibikounlé M., Moné H. Cattle as natural host for Schistosoma haematobium (Bilharz, 1852) Weinland, 1858 x Schistosoma bovis Sonsino, 1876 interactions, with new cercarial emergence and genetic patterns. Parasitol. Res. 2020;119:2189–2205. doi: 10.1007/s00436-020-06709-0. [DOI] [PubMed] [Google Scholar]
- 24.De Bont J., Vercruysse J., Southgate V.R., Rollinson D., Kaukas A. Cattle Schistosomiasis in Zambia. J. Helminthol. 1994;68:295–299. doi: 10.1017/S0022149X00001516. [DOI] [PubMed] [Google Scholar]
- 25.Charlier J., van der Voort M., Kenyon F., Skuce P., Vercruysse J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014;30:361–367. doi: 10.1016/j.pt.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Angora E.K., Allienne J.F., Rey O., Menan H., Touré A.O., Coulibaly J.T., Raso G., Yavo W., N’Goran E.K., Utzinger J., et al. High prevalence of Schistosoma haematobium x Schistosoma bovis hybrids in schoolchildren in Côte d’Ivoire. Parasitology. 2020;147:287–294. doi: 10.1017/S0031182019001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pennance T., Allan F., Emery A., Rabone M., Cable J., Garba A.D., Hamidou A.A., Webster J.P., Rollinson D., Webster B.L. Interactions between Schistosoma haematobium group species and their Bulinus spp. intermediate hosts along the Niger River Valley. Parasites Vectors. 2020;13:1–15. doi: 10.1186/s13071-020-04136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soentjens P., Cnops L., Huyse T., Yansouni C., De Vos D., Bottieau E., Clerinx J., Van Esbroeck M. Diagnosis and clinical management of Schistosoma haematobium-Schistosoma bovis hybrid infection in a cluster of travelers returning from Mali. Clin. Infect. Dis. 2016;63:1626–1629. doi: 10.1093/cid/ciw493. [DOI] [PubMed] [Google Scholar]
- 29.Holtfreter M.C., Moné H., Müller-Stöver I., Mouahid G., Richter J. Schistosoma haematobium infections acquired in Corsica, France, August 2013. Eurosurveillance. 2014;19:2013–2015. doi: 10.2807/1560-7917.ES2014.19.22.20821. [DOI] [PubMed] [Google Scholar]
- 30.Moné H., Holtfreter M.C., Mouahid G., Richter J. Difficulties in Schistosomiasis assessment, Corsica, France. Emerg. Infect. Dis. 2016;22:762–763. doi: 10.3201/eid2204.160110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bärenbold O., Raso G., Coulibaly J.T., N’Goran E.K., Utzinger J., Vounatsou P. Estimating sensitivity of the Kato-Katz technique for the diagnosis of Schistosoma mansoni and hookworm in relation to infection intensity. PLoS Negl. Trop. Dis. 2017;11:1–14. doi: 10.1371/journal.pntd.0005953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chuah C., Gobert G.N., Latif B., Heo C.C., Leow C.Y. Schistosomiasis in Malaysia: A review. Acta Trop. 2019;190:137–143. doi: 10.1016/j.actatropica.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Weerakoon K.G.A.D., Gobert G.N., Cai P., McManus D.P. Advances in the diagnosis of human schistosomiasis. Clin. Microbiol. Rev. 2015;28:939–967. doi: 10.1128/CMR.00137-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinz R., Schwarz N.G., Hahn A., Frickmann H. Serological approaches for the diagnosis of schistosomiasis—A review. Mol. Cell. Probes. 2017;31:2–21. doi: 10.1016/j.mcp.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 35.McManus D.P., Dunne D.W., Sacko M., Utzinger J., Vennervald B.J., Zhou X.N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018;4:1–19. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 36.Weerakoon K.G., Gordon C.A., McManus D.P. DNA diagnostics for schistosomiasis control. Trop. Med. Infect. Dis. 2018;3:81. doi: 10.3390/tropicalmed3030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schols R., Carolus H., Hammoud C., Mulero S., Mudavanhu A., Huyse T. A rapid diagnostic multiplex PCR approach for xenomonitoring of human and animal schistosomiasis in a “One Health” context. Trans. R. Soc. Trop. Med. Hyg. 2019;113:722–729. doi: 10.1093/trstmh/trz067. [DOI] [PubMed] [Google Scholar]
- 38.Notomi T., Okayama H., Masubuchi H., Yonekawa T., Watanabe K., Amino N., Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:e63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong Y.P., Othman S., Lau Y.L., Radu S., Chee H.Y. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018;124:626–643. doi: 10.1111/jam.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mori Y., Notomi T. Loop-mediated isothermal amplification (LAMP): Expansion of its practical application as a tool to achieve universal health coverage. J. Infect. Chemother. 2020;26:13–17. doi: 10.1016/j.jiac.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Avendaño C., Patarroyo M.A. Loop-mediated isothermal amplification as point-of-care diagnosis for neglected parasitic infections. Int. J. Mol. Sci. 2020;21:7981. doi: 10.3390/ijms21217981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-Soto P., Avendaño C., Sala-Vizcaíno A., Crego-Vicente B., Febrer-Sendra B., García-Bernalt Diego J., Oleaga A., López-Abán J., Vicente B., Patarroyo M.A., et al. Molecular markers for detecting Schistosoma species by Loop-Mediated Isothermal Amplification. Dis. Markers. 2020;2020:1–11. doi: 10.1155/2020/8042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis F.A., Liang Y.S., Raghavan N., Knight M. The NIH-NIAID schistosomiasis resource center. PLoS Negl. Trop. Dis. 2008;2:1–4. doi: 10.1371/journal.pntd.0000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gandasegui J., Fernández-Soto P., Carranza-Rodríguez C., Pérez-Arellano J.L., Vicente B., López-Abán J., Muro A. The rapid-heat LAMPellet method: A potential diagnostic method for human urogenital schistosomiasis. PLoS Negl. Trop. Dis. 2015;9:1–23. doi: 10.1371/journal.pntd.0003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández-Soto P., Gandasegui Arahuetes J., Sánchez Hernández A., López Abán J., Vicente Santiago B., Muro A. A Loop-Mediated Isothermal Amplification (LAMP) assay for early detection of Schistosoma mansoni in stool samples: A diagnostic approach in a murine model. PLoS Negl. Trop. Dis. 2014;8:e3126. doi: 10.1371/journal.pntd.0003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pennance T., Ame S.M., Amour A.K., Suleiman K.R., Allan F., Rollinson D., Webster B.L. Occurrence of Schistosoma bovis on Pemba Island, Zanzibar: Implications for urogenital schistosomiasis transmission monitoring. Parasitology. 2018;145:1732. doi: 10.1017/S0031182018001622. [DOI] [PubMed] [Google Scholar]
- 47.Webster B.L., Diaw O.T., Seye M.M., Webster J.P., Rollinson D. Introgressive hybridization of Schistosoma haematobium group species in Senegal: Species barrier break down between ruminant and human schistosomes. PLoS Negl. Trop. Dis. 2013;7:e2110. doi: 10.1371/journal.pntd.0002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Léger E., Garba A., Hamidou A.A., Webster B.L., Pennance T., Rollinson D., Webster J.P. Introgressed animal schistosomes Schistosoma curassoni and S. bovis naturally infecting humans. Emerg. Infect. Dis. 2016;22:2212–2214. doi: 10.3201/eid2212.160644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huyse T., Van Den Broeck F., Hellemans B., Volckaert F.A.M., Polman K. Hybridisation between the two major African schistosome species of humans. Int. J. Parasitol. 2013;43:687–689. doi: 10.1016/j.ijpara.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Catalano S., Sène M., Diouf N.D., Fall C.B., Borlase A., Léger E., Bâ K., Webster J.P. Rodents as natural hosts of zoonotic Schistosoma species and hybrids: An epidemiological and evolutionary perspective from West Africa. J. Infect. Dis. 2018;218:429–433. doi: 10.1093/infdis/jiy029. [DOI] [PubMed] [Google Scholar]
- 51.Catalano S., Léger E., Fall C.B., Borlase A., Diop S.D., Berger D., Webster B.L., Faye B., Diouf N.D., Rollinson D., et al. Multihost transmission of Schistosoma mansoni. Emerg. Infect. Dis. 2020;26:1234–1242. doi: 10.3201/eid2606.200107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pena H.B., De Souza C.P., Simpson A.J.G., Pena S.D.J. Intracellular promiscuity in Schistosoma mansoni: Nuclear transcribed DNA sequences are part of a mitochondrial minisatellite region. Proc. Natl. Acad. Sci. USA. 1995;92:915–919. doi: 10.1073/pnas.92.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandasegui J., Fernández-Soto P., Muro A., Simões Barbosa C., Lopes de Melo F., Loyo R., de Souza Gomes E.C. A field survey using LAMP assay for detection of Schistosoma mansoni in a low-transmission area of schistosomiasis in Umbuzeiro, Brazil: Assessment in human and snail samples. PLoS Negl. Trop. Dis. 2018;12:1–16. doi: 10.1371/journal.pntd.0006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernández-Soto P., Gandasegui J., Rodríguez C.C., Pérez-Arellano J.L., Crego-Vicente B., García-Bernalt Diego J., López-Abán J., Vicente B., Muro A. Detection of Schistosoma mansoni-derived DNA in human urine samples by loop-mediated isothermal amplification (LAMP) PLoS ONE. 2019;14:e0214125. doi: 10.1371/journal.pone.0214125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le Govic Y., Kincaid-Smith J., Allienne J.F., Rey O., de Gentile L., Boissier J. Schistosoma haematobium- Schistosoma mansoni hybrid parasite in migrant boy, France, 2017. Emerg. Infect. Dis. 2019;25:365–367. doi: 10.3201/eid2502.172028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao G.H., Mo X.H., Zou F.C., Li J., Weng Y.B., Lin R.Q., Xia C.M., Zhu X.Q. Genetic variability among Schistosoma japonicum isolates from different endemic regions in China revealed by sequences of three mitochondrial DNA genes. Vet. Parasitol. 2009;162:67–74. doi: 10.1016/j.vetpar.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 57.Vilas R., Criscione C.D., Blouin M.S. A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology. 2005;131:839–846. doi: 10.1017/S0031182005008437. [DOI] [PubMed] [Google Scholar]
- 58.Webster B.L., Culverwell C.L., Khamis I.S., Mohammed K.A., Rollinson D., Stothard J.R. DNA barcoding of Schistosoma haematobium on Zanzibar reveals substantial genetic diversity and two major phylogenetic groups. Acta Trop. 2013;128:206–217. doi: 10.1016/j.actatropica.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Nolan M.J., Cribb T.H. The use and implications of ribosomal DNA sequencing for the discrimination of digenean species. Adv. Parasitol. 2005;60:101–163. doi: 10.1016/S0065-308X(05)60002-4. [DOI] [PubMed] [Google Scholar]
- 60.Cutmore S.C., Bennett M.B., Cribb T.H. Staphylorchis cymatodes (Gorgoderidae: Anaporrhutinae) from carcharhiniform, orectolobiform and myliobatiform elasmobranchs of Australasia: Low host specificity, wide distribution and morphological plasticity. Parasitol. Int. 2010;59:579–586. doi: 10.1016/j.parint.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Kane R.A., Rollinson D. Repetitive sequences in the ribosomal DNA internal transcribed spacer of Schistosoma haematobium, Schistosoma intercalatum and Schistosoma mattheei. Mol. Biochem. Parasitol. 1994;63:153–156. doi: 10.1016/0166-6851(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 62.Dvořák J., Vaňáčová Š., Hampl V., Flegr J., Horák P. Comparison of European Trichobilharzia species based on ITS1 and ITS2 sequences. Parasitology. 2002;124:307–313. doi: 10.1017/S0031182001001238. [DOI] [PubMed] [Google Scholar]
- 63.Van Herwerden L., Blair D., Agatsuma T. Intra- and inter-specific variation in nuclear ribosomal internal transcribed spacer 1 of the Schistosoma japonicum species complex. Parasitology. 1998;116:311–317. doi: 10.1017/S003118209800242X. [DOI] [PubMed] [Google Scholar]
- 64.Kane R.A., Rollinson D. Comparison of the intergenic spacers and 3’ end regions of the large subunit (28S) ribosomal RNA gene from three species of Schistosoma. Parasitology. 1998;117:235–242. doi: 10.1017/S0031182098003059. [DOI] [PubMed] [Google Scholar]
- 65.El Bali L., Diman A., Bernard A., Roosens N.H.C., Dekeersmaecker S.C.J. Comparative study of seven commercial kits for human DNA extraction from urine samples suitable for DNA biomarker-based public health studies. J. Biomol. Tech. 2014;25:96–110. doi: 10.7171/jbt.14-2504-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.