Abstract
Introduction
The goal of this study was to assess the changes in arterial spin labeling (ASL) cerebral blood flow (CBF) and arterial transit time (ATT), and in apparent diffusion coefficient (ADC), before and after an acetazolamide challenge in moyamoya patients, as function of arterial stenosis severity.
Methods
Pre-operative patients diagnosed with moyamoya disease who could undergo MRI at 3.0T were recruited for this study. A multi-delay pseudo-continuous ASL and a diffusion-weighted sequence were acquired before and 15 min after acetazolamide injection. The severity of anterior, middle, and posterior cerebral artery pathology was graded on time-of-flight MR angiographic images. CBF, ATT, and ADC were measured on standardized regions of interest as function of the vessel stenosis severity.
Results
Thirty patients were included. Fifty-four percent of all vessels were normal, 28% mildly/moderately stenosed, and 18% severely stenosed/occluded. Post-acetazolamide, a significantly larger CBF (ml/100 g/min) increase was observed in territories of normal (+19.6 ± 14.9) compared to mildly/moderately stenosed (+14.2 ± 27.2, p = 0.007), and severely stenosed/occluded arteries (+9.9 ± 24.2, p < 0.0001). ATT was longer in territories of vessel anomalies compared with normal regions at baseline. ATT decreases were observed in all territories post-acetazolamide. ADC did not decrease after acetazolamide in any regions, and no correlation was found between ADC changes and baseline ATT, change in ATT, or CVR.
Conclusion
The hemodynamic response in moyamoya disease, as measured with ASL CBF, is impaired mostly in territories with severe arterial stenosis/occlusion, while ATT was prolonged in all non-normal regions. No significant changes in ADC were observed after acetazolamide.
Keywords: Arterial spin labeling, Moyamoya disease, Acetazolamide challenge, Cerebral blood flow, Apparent diffusion coefficient
Introduction
Moyamoya disease is characterized by progressive stenosis of the distal intracranial internal carotid arteries and the proximal parts of the anterior and middle cerebral arteries [1]. It is associated with the development of a characteristic compensatory network of collateral small vessels. This condition predisposes those patients to stroke and hemorrhage.
Cerebrovascular reserve (CVR) is defined as the change in cerebral blood flow (CBF) after reducing vascular resistance [2]. It has been proposed that CVR could help stratify the risk of ischemic events in moyamoya patients [3], using acetazolamide, a cerebral vasodilatative agent. CVR impairment has indeed been shown to be strongly associated with increased risk of ischemic events in carotid stenosis or occlusion [4] and other chronic steno-occlusive diseases, by demonstrating the inability of regions of the brain to augment CBF, because autoregulatory vasodilation is already maximized. For example, moyamoya patients with poor CVR are at higher risk of clinical complications post-extracranial-intracranial bypass operation [5]. Despite this value, and although there is a long history of the use of vasodilatory agents in patients with advanced cerebrovascular disease, there exist concerns about the safety of acetazolamide in moyamoya patients due to the potential for CBF reduction (“cerebrovascular steal”) [6].
Given concerns of chronic gadolinium deposition in the brain after repeated intravenous injection of gadolinium-based contrast agents [7, 8], arterial spin labeling (ASL) is a promising non-contrast alternative [9] to the routine gadolinium-based dynamic susceptibility contrast (DSC) perfusion imaging, in particular to test CVR, because it permits repeated measurement without issues such as residual contrast from previous injection or contrast leakage. ASL is also of interest in moyamoya patients, because those patients are often young and frequently require multiple MR examinations over their lifetimes.
However, the ASL signal is strongly dependent on the time the blood takes to reach the tissue of interest from the labeling plane. Therefore, ASL using a single post-label delay might not properly reflect the true CBF, while an ASL acquisition with multiple post-label delay times might mitigate this effect [10]. Multi-delay ASL has been shown to correlate with CT perfusion in moyamoya [11], and moyamoya disease staging has been shown to be feasible with ASL-4D MR-angiography [12].
The purpose of this study was to assess the changes in CBF and arterial transit time (ATT), using a multi-delay pseudo-continuous ASL sequence, and to characterize the correlation between CVR and baseline ATT. In addition, we evaluate the safety of acetazolamide challenge for measuring CVR in patients with moyamoya disease by looking for diffusion changes post-acetazolamide.
Methods
Patient demographics
We declare that this human study has been approved by the ethic committee at Stanford University and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study. Inclusion criteria were patients with catheter angiography proven moyamoya disease, scanned in the context of pre-operative assessment at our institution from June 2013 to January 2015. Exclusion criteria were the usual contraindications to MRI.
Acquisition parameters
Images were acquired at 3.0 Tesla (MR750 and MR750w, GE Healthcare, Waukesha, WI, USA). The protocol consisted of anatomical T1-weighted, T2-weighted, fluid attenuated inversion recovery (FLAIR), time-of-flight intracranial MR angiogram (TOF MRA), ASL, and diffusion-weighted imaging (DWI). Fifteen minutes after intravenous injection of 1 g acetazolamide, the ASL and DWI sequences were repeated. The ASL sequence consisted of a three-dimensional, multi-delay pseudo-continuous ASL, with a fast spin echo stack-of-spiral readout with eight interleaves. Other parameters were as follows: TR = 4844 ms, TE = 11 ms, time of labeling = 2 s, post-label delays = 0.7, 1.3,1.9, 2.5, and 3.0 s, field of view = 240 × 240 mm2, number of slices = 36, voxel size = 6 × 6 × 5 mm3, and NEX = 1, scan time = 3:36 min (which includes all five post-label delay times and a proton density images required to calculate CBF). The diffusion-weighted sequence was acquired with a b value of 1000 s/mm2, from which, using the b = 0 images, the ADC was calculated. Imaging parameters were TR = 5000 s, TE = 87 ms, bandwidth = 1953 Hz/s, and voxel size = 1.9 × 1.9 × 5 mm3. The parameters for the time-of-flight intracranial MR angiogram were as follows: TR = 21 ms, TE = 2.3 ms, flip angle = 15°, bandwidth = 195 Hz/s, and voxel size = 0.5 × 0.5 × 1.2 mm3.
Image post-processing
CBF and ATT measurements were performed using the method ofDai et al. [10]: Briefly, the ATTwas determined using an ASL signal-weighted delay method. Then, using this ATT, CBF was determined as follows:
where T1a is the longitudinal relaxation time of blood and was assumed to be 1.6 s, ΔM is the ASL difference signal intensity, M0 is the longitudinal relaxed brain tissue signal, α is the combined efficiency of labeling and background suppression (assumed to be 0.63), ω is the post-label delay, and τ is the labeling duration. A CBF value was calculated for each of the five post-label delay ASL images, and an SNR-weighted (correcting for the dependence of SNR to post-labeling delay and labeling duration (Eq. [2] in Dai et al. [10])) average was used to determine the final CBF.
Severity of disease
The severity of disease of the anterior, middle, and posterior cerebral arteries was graded as normal, mild/moderate, or severe/occluded in consensus by two experienced neuroradiologists, based on the MRA images (Fig. 1a). Supraclinoid internal carotid artery stenosis was handled as both anterior and middle cerebral artery disease, unless the anterior cerebral artery was supplied by a dominant contralateral artery through the anterior communicating artery. The presence of a fetal origin of the posterior cerebral artery was noted. The severity of previous infarction was categorized as normal, with small infarct, and with large infarct.
Fig. 1.

a Severity score of vessel stenosis on maximum intensity projection (anterior and superior views) of TOF MRA. Examples of normal (no visible stenosis, left), mildly/moderately stenosed (middle), and severely stenosed/occluded (right), right middle cerebral artery (MCA). b Definition of the 20 regions of interest per patient on the MNI template, corresponding to the anterior, middle, and posterior cerebral artery territories
Regions of interest and data analysis
All images were co-registered to the MNI brain template using MINC [13]. Twenty standardized regions of interest of 2 cm thickness, corresponding to ASPECTS levels [14] were defined on the brain template [15] in two axial planes, the first plane being at the level of the basal ganglia and the second just above the superior aspect of the lateral ventricle (Fig. 1b). The regions were divided to correspond to the vascular territory of the major cerebral arteries. The values of the CBF and ATT calculated from the ASL acquisition, as well as the ADC from the DWI sequence, were measured in each region of interest and analyzed as function of vessel stenosis severity. The Pearson’s correlation coefficient between CVR (with CVR = CBFpost − acetazolamide − CBFpre − acetazolamide) and baseline ATT was calculated, as well as between changes in ADC (ΔADC = ADCpost − acetazolamide − ADCpre − acetazolamide) and respectively CVR, baseline ATT, and ATT change (ΔATT = ATTpost − acetazolamide − ATTpre − acetazolamide).
Safety evaluation
The safety of the acetazolamide challenge in patients with moyamoya disease was assessed as follows: (1) The medical records were searched for any documentation of neurological deficits within the first 24 h after acetazolamide challenge. (2) New focal diffusion restrictions after acetazolamide challenge were searched by an experienced neuroradiologist by reviewing the images pre- and post-acetazolamide challenge. (3) Sub-clinical, “pre-stroke”, apparent diffusion coefficient (ADC) changes post-acetazolamide challenge were studied as function of baseline ATT, change in ATT, or CVR.
Statistical analysis
Statistical analysis was performed using Matlab version 2014a (MathWorks, Natick, Massachusetts). Statistical significance was assessed using unpaired and paired two-tailed Student’s t test. Significance level was set to α < 0.05 and corrected for false discovery rate using the Benjamini–Hochberg procedure.
Results
Thirty patients (mean age ± standard deviation: 40 ± 14 years; 18 (60%) women; equally split between bilateral and unilateral disease [15 in each group]) were included in this study; no patients were excluded, such that this represents a consecutive series of patients. Seven of 60 posterior cerebral arteries were of fetal origin. Fifty-four percent of cerebral arteries were normal, 28% mildly/moderately stenosed, and 18% severely stenosed or occluded (Table 1). No infarct was found in 98% of territories where the arteries were found normal, and 2% showed small infarcts. In territories where the artery was mildly/moderately stenosed, no infarct was found in 84%, small infarcts in 14%, and large infarcts in 2%. In territories where the artery was severely stenosed or occluded, no infarct was found in 76%, small infarcts in 21%, and large infarcts in 3%. Representative examples of pre- and post-acetazolamide imaging of CBF, ATT, and ADC in several patients are shown in Fig. 2.
Table 1.
Severity of the stenosis of intracranial arteries as assessed on TOF MRA. Data are number of vessels. The denominator of the percentage value is always the number of patients (30), except in the total column, where it is the total number of vessel segments (180)
| Left | Right | Total | |||||
|---|---|---|---|---|---|---|---|
| TOF MRA score | ACA | MCA | PCA | ACA | MCA | PCA | |
| Normal | 16 (53%) | 9 (30%) | 25 (83%) | 16 (53%) | 6 (20%) | 24 (80%) | 96 (54%) |
| Mildly/moderately stenosed | 6 (20%) | 12 (40%) | 5 (17%) | 6 (20%) | 16 (53%) | 6 (20%) | 51 (28%) |
| Severely stenosed/occluded | 8 (27%) | 9 (30%) | 0 (0%) | 8 (27%) | 8 (27%) | 0 (0%) | 33 (18%) |
Fig. 2.
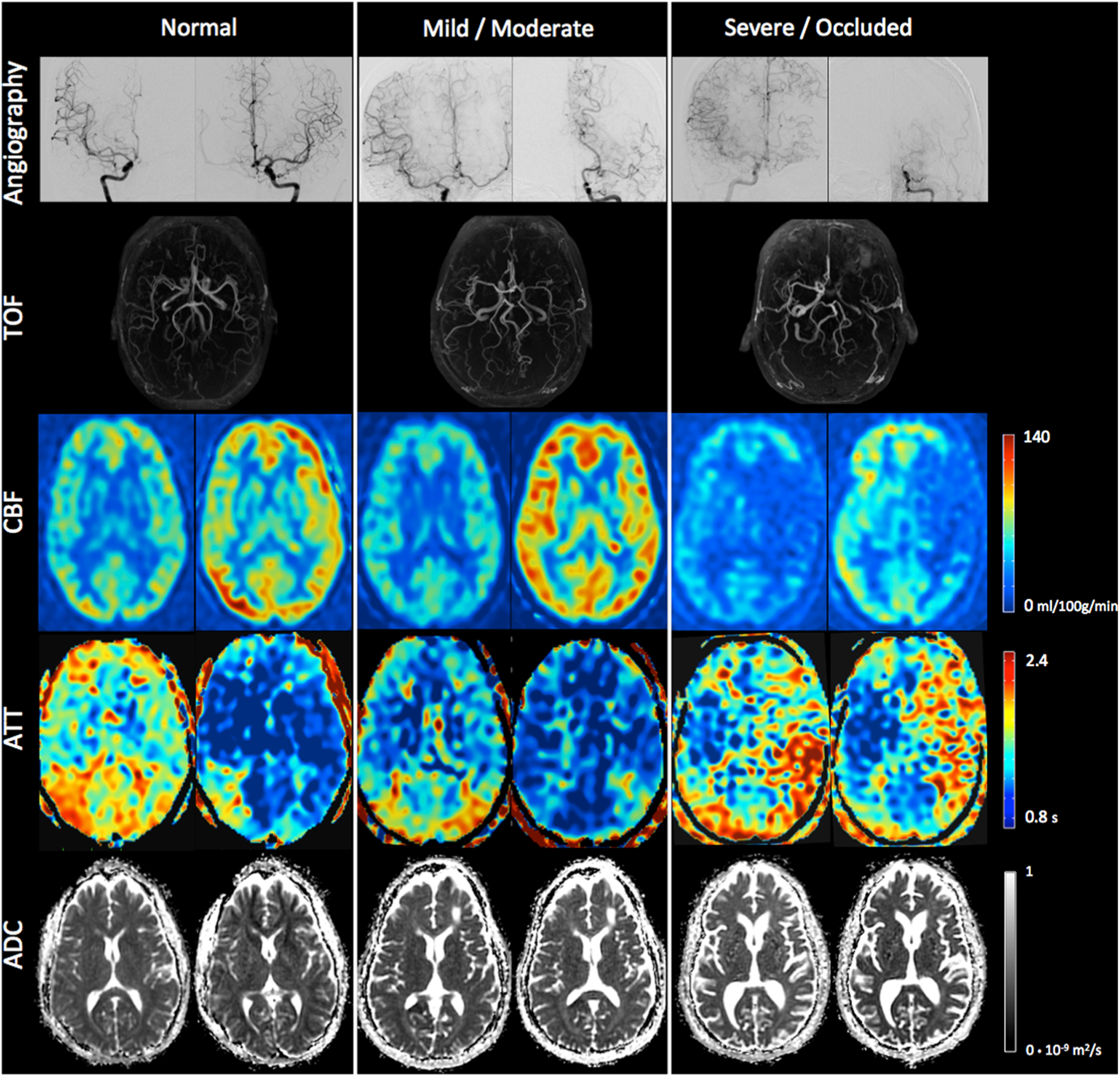
(Top) Catheter angiography (right and left internal carotid injection) and TOF. (Bottom) CBF, ATT, and ADC at baseline (left) and after acetazolamide challenge (right) in patients with normal, mild/moderate, and occluded left MCA, respectively. All of the other territories were without arterial stenosis in those three patients. A significant CBF increase is visible in all territories after acetazolamide, except in the severely stenosed/occluded left MCA. A decrease in ATT is visible in all territories after acetazolamide. Interestingly, slightly prolonged ATT is visible in the posterior part of the right MCA in the patient with normal vessels on the MR angiogram, suggesting the ASL ATT measurement is very sensitive, and can depict even mild delays
Interestingly, a higher baseline CBF was measured in regions corresponding to severely stenosed or occluded arteries (54.7 ± 16.1 ml/100 g/min) and mild/moderately stenosed vessels (52.3 ± 18.3 ml/100 g/min, p < 0.0001) compared to normal (48.3 ± 14.4 ml/100 g/min, p = 0.008) (Tables 2 and 3). After acetazolamide challenge, a significant CBF increase was observed in all territories, and this increase was significantly larger in territories corresponding to the arteries with no stenosis (19.6 ± 14.9 ml/100 g/min) compared to in the territories of mild/moderate stenosis (14.2 ± 27.2 ml/100 g/min, p = 0.007), and compared to in the territories of severely stenosed/occluded arteries (9.9 ± 24.2 ml/100 g/min, p < 0.0001) (Table 2).
Table 2.
CBF, ATT, and ADC measured in vascular territories based on the level of arterial stenosis. Data are mean ± standard deviation
| TOF MRA score | Normal | Mildly/moderately stenosed | Severely stenosed/occluded | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-acetazolamide | Difference | % change | p value | Baseline | Post-acetazolamide | Difference | % change | p value | Baseline | Post-acetazolamide | Difference | % change | p value | |
| CBF (ml/100 g/min) | 48.3 ± 14.4 | 67.9 ± 19.9 | 19.6 ± 14.9 | 45 ± 45 | <0.0001 | 52.3 ± 18.3 | 66.5 ± 24.2 | 14.2 ± 27.2 | 36 ± 52 | <0.0001 | 54.7 ± 16.1 | 64.6 ± 25.8 | 9.9 ± 24.2 | 24 ± 52 | <0.0001 |
| ATT (ms) | 1359 ± 418 | 1192 ±466 | −167 ± 271 | −12 ± 24 | <0.0001 | 1466 ± 408 | 1313 ± 414 | −153 ± 276 | −10 ± 20 | <0.0001 | 1478 ± 480 | 1263 ± 409 | −216 ± 328 | −12 ± 21 | <0.0001 |
| ADC (10−6 mm2/s) | 988 ± 169 | 974 ± 164 | −13 ± 104 | −1 ± 11 | 0.04 | 1062 ± 225 | 1050 ± 237 | −12 ± 117 | −1 ± 10 | 0.14 | 1076 ± 252 | 1055 ± 283 | −22 ± 218 | −1 ± 22 | 0.25 |
Table 3.
p value for comparison between groups of vessel occlusion severity for CBF, ATT, and ADC, at baseline and post-acetazolamide challenge
| Baseline | Post-acetazolamide | |||||
|---|---|---|---|---|---|---|
| Normal vs mild/moderate | Normal vs severe/occluded | Mild/moderate vs severe/occluded | Normal vs mild/moderate | Normal vs severe/occluded | Mild/moderate vs severe/occluded | |
| CBF | 0.008 | <0.0001 | 0.21 | 0.51 | 0.17 | 0.49 |
| ATT | 0.006 | 0.01 | 0.79 | 0.004 | 0.14 | 0.27 |
| ADC | <0.0001 | <0.0001 | 0.58 | <0.0001 | 0.0005 | 0.87 |
At baseline, ATT was significantly higher in regions of territories of vessel anomalies pooled together (1470 ± 436 ms) compared with normal regions (1359 ± 418 ms, p = 0.002). After correcting for multiple comparisons, ATT was significantly higher in regions with severe/occlusive (1478 ± 480 ms, p = 0.01) and mild/moderate (1466 ± 408 ms, p = 0.006) vessel disease when compared with normal regions (Tables 2 and 3).
After acetazolamide challenge, ATT was significantly higher in regions of territories of vessel anomalies pooled together (1293 ± 412 ms) compared with normal regions (1192 ± 466 ms, p = 0.005), as well as after correcting for multiple comparisons in regions with mild/moderate (1313 ± 414 ms, p = 0.004) vessel disease when compared with normal regions, but not in regions with severe/occlusive (1263 ± 409 ms, p = 0.14) vessel disease when compared with normal regions (Tables 2 and 3). The acetazolamide challenge induced a significant ATT decreases in all regions, but those decreases did not differ by severity of vessel involvement (Table 2).
Finally, we found a significant negative correlation between CVR and baseline ATT that was visible in all three groups, but which was statistically significant only for the regions of mild/moderate stenosis (normal: r = −0.39, p < 0.0001; mild/moderate stenosis: r = −0.53, p < 0.0001; severe stenosis/occlusion: r = −0.47, p < 0.006; Fig. 3).
Fig. 3.
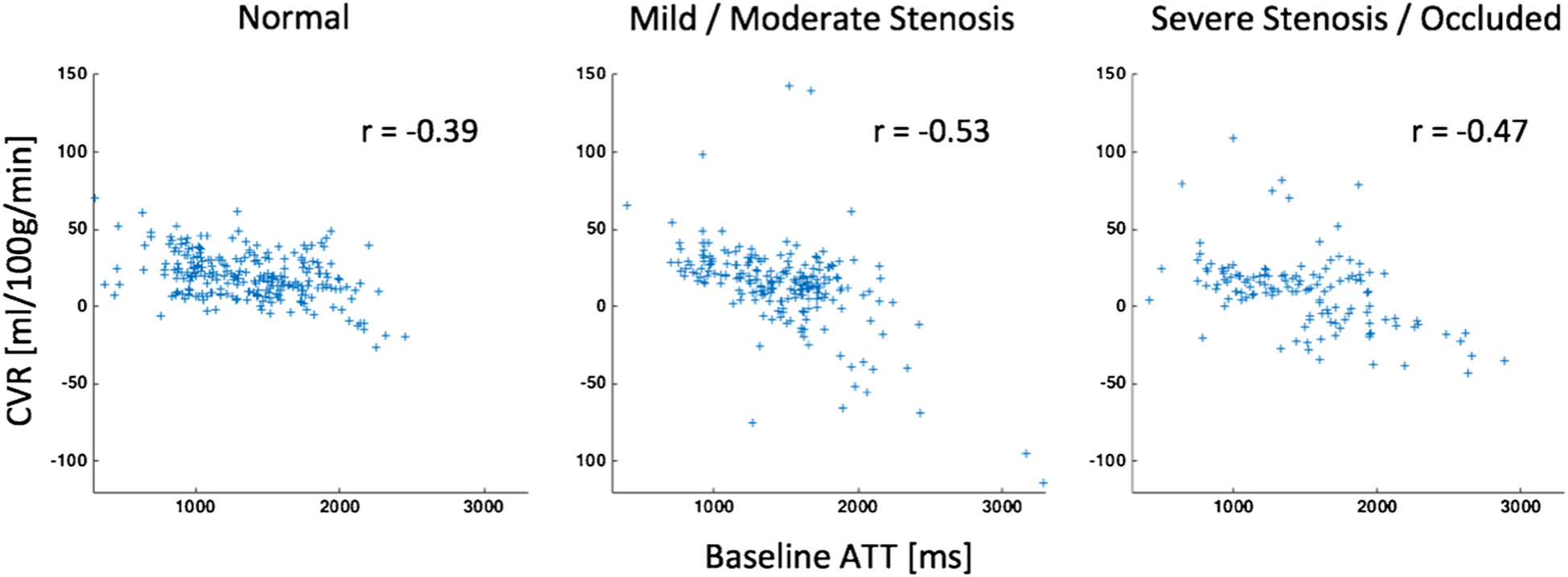
Scatterplots between the CVR (difference in CBF between after acetazolamide and baseline) and baseline ATT. A significant decrease in CVR with increasing baseline ATT is visible in all three groups
Concerning the safety of the acetazolamide challenge, while at baseline and after acetazolamide challenge, ADC was higher in territories with vessel disease compared with the normal vessels (all p < 0.0006), there was no evidence for ADC decrease in any of the regions after acetazolamide (Table 2). We also found no correlation between change in ADC and respectively CVR (r = 0.07, p = 0.10), baseline ATT (r = 0.0725, p = 0.08), and change in ATT (r = 0.05, p = 0.24) (Fig. 4). The detailed review of the DWI images before and after the acetazolamide challenge showed no appearance of new DWI restriction foci, in any of the 30 patients. After the acetazolamide challenge, 1 patient presented with migraine, and 1 patient presented with right face numbness; both of which resolved without medication. Many of these patients had subsequent bypass surgery thereafter, making it difficult to assess for new neurological deficits.
Fig. 4.

Scatterplots between the difference in ADC (ΔADC), and respectively CVR, baseline ATT, and change in ATT (ΔATT). The absence of significant correlation (r) in all three graphs speaks for the safety of the acetazolamide challenge to test CVR in moyamoya patients
Discussion
This study demonstrates in a large, consecutive cohort of pre-operative moyamoya patients, that their CVR, as measured with multi-delay ASL CBF, is gradually impaired as function of vessel impairment. We also found, paradoxically, that baseline CBF was gradually higher in the territories as function of vessel impairment. The increased heterogeneity in the most affected territories might be explained by the presence of retained spins in proximal feeding vessels even at the longest post-label delay time (3.0 s) that are destined to perfuse more distal slices. Longer labeling time or post-label delays could be considered to mitigate this effect in multi-delay ASL imaging but would require longer scans and additional signal averaging, given the T1 decay of the label that occurs. Another explanation for this paradoxical higher apparent CBF in the affected territories may be due to the labeled spins spending more time in the arterial blood compartment (with its longer T1) compared with the brain tissue itself, where the label will decay faster. We also observed a negative correlation between CVR and baseline ATT, which suggests that ATT might be of a certain value to evaluate the CVR if an acetazolamide challenge cannot be obtained.
ATT, a measurement only possible with multi-delay ASL, was longer in territories of vessel stenosis compared to normal, both at baseline and after acetazolamide. A significant ATT decrease of about 150–200 ms after acetazolamide administration was seen in all territories, but without any differences based on feeding vessel status. This suggests that there is an increase in flow velocity between labeling plane and imaging plane, but it is not possible to figure out whether this increase arises predominantly in smaller or larger arteries. This ATT decrease may be important for studies that use single-delay ASL to measure CBF, since it is possible that changes in ATT may cause inaccurate measurement of CBF changes following acetazolamide. This effect was explicitly accounted for in the model used in this work.
At baseline and after acetazolamide, we observed a stepwise ADC increase with increasing vessel stenosis severity, which is explained by an increasing number of chronic ischemic lesions in territories with higher stenosis. Importantly, no significant ADC changes were observed following acetazolamide, regardless of vessel status, and no correlation were found between changes in ADC and CVR, baseline ATT, and changes in ATT. Those findings support the safety of acetazolamide challenge for measuring CVR in patients with significant cerebrovascular disease, in agreement with prior literature as well [16]. An interesting follow-up experiment would be to perform a more sophisticated DWI experiment incorporating intravoxel incoherent motion [17] and observe the changes in perfusion fraction after acetazolamide challenge in the various territories.
Given concerns of chronic gadolinium deposition in the brain of patients (which has also been observed in patients with normal renal function) after repeated intravenous injection of gadolinium-based contrast agents [7, 8, 18, 19], ASL offers a robust non-contrast method for measuring CBF and ATT to evaluate cerebrovascular reserve. Single-delay ASL has previously been shown to be useful to monitor hemodynamic changes in moyamoya patients [20–22]. Noguchi et al. [3] found ASL equivalent to SPECT for mapping CVR in moyamoya patients, using an ASL method that was synchronized to the pulse detected on a finger. Yun et al. [20] found a significant increase in CVR with ASL and single photon emission computed tomography (SPECT), in moyamoya patients 6 months after bypass surgery. Perfusion measurement with multi-delay ASL may add value compared to single-delay ASL [23], adding ATT information and potentially improving in the accuracy of CBF measurement.
There are limitations to this study. It is a single center study, in a pre-operative set of patients. While both stable xenon computed tomography (CT) and 15O–H2O positron emission tomography (PET) are considered reference standard measurements for CBF [24], they were not performed as part of this study. Therefore, we cannot prove the CBF obtained by the presented method is similar to a gold standard measurement, but two recent studies showed a significant correlation between multiple post-label delay ASL CBF and PET CBF in healthy volunteers [25] and in patients with chronic occlusive cerebrovascular disease [26]. Furthermore, there exists no reference standard for ATT measurements, so the accuracy of the values measured in this study cannot be evaluated. The analysis of vascular territories was based on standard territories in healthy normal subjects and may not be entirely accurate in a moyamoya cohort in whom extensive collateralization may cause vascular territory boundaries to shift. Infarcted territories were not specifically excluded from the analysis, as the aim of this work was to study changes in perfusion and ADC. The timing of the MRI to assess for irreversible ischemia after acetazolamide might not be optimal, as only early sign of ischemia would be visible, and a later DWI, such as at 24–72 h, might reveal lesions not visible immediately postvascular challenge.
In conclusion, the current study demonstrates that multi-delay ASL can be used as a non-invasive imaging tool to monitor CBF and ATT changes following acetazolamide challenge. Furthermore, acetazolamide does not appear to adversely affect ADC measurements, even in regions with severe arterial stenoses or occlusions.
Acknowledgements
CF was supported by the Swiss National Science Foundation.
Footnotes
Compliance with ethical standards We declare that all human and animal studies have been approved by the Stanford University Ethics Committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest SC consults for iSchemaView and GZ receives research support from GE Healthcare.
References
- 1.Takeuchi K, Shimizu K (1957) Hypoplasia of the bilateral internal carotid arteries. Brain Nerve 9:37–43 [Google Scholar]
- 2.Vagal AS, Leach JL, Fernandez-Ulloa M, Zuccarello M (2009) The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol 30: 876–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noguchi T, Kawashima M, Nishihara M, Egashira Y, Azama S, Irie H (2015) Noninvasive method for mapping CVR in moyamoya disease using ASL-MRI. Eur J Radiol 84:1137–1143 [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Chazen JL, Hartman M et al. (2012) Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke 43:2884–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonucci MU, Burns TC, Pulling TM et al. (2015) Acute preoperative infarcts and poor cerebrovascular reserve are independent risk factors for severe ischemic complications following direct extracranial-intracranial bypass for moyamoya disease. AJNR Am J Neuroradiol. doi: 10.3174/ajnr.A4535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim TJ, Lee JS, Hong JM, Lim YC (2012) Intracerebral steal phenomenon: a potential mechanism for contralateral stroke after carotid artery stenting. Neurologist 18:128–129 [DOI] [PubMed] [Google Scholar]
- 7.McDonald RJ, McDonald JS, Kallmes DF et al. (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782 [DOI] [PubMed] [Google Scholar]
- 8.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841 [DOI] [PubMed] [Google Scholar]
- 9.Alsop DC, Detre JA, Golay X et al. (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73: 102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai W, Robson PM, Shankaranarayanan A, Alsop DC (2012) Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med 67: 1252–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang R, Yu S, Alger JR et al. (2014) Multi-delay arterial spin labeling perfusion MRI in moyamoya disease—comparison with CT perfusion imaging. Eur Radiol 24:1135–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchino H, Ito M, Fujima N et al. (2015) A novel application of fourdimensional magnetic resonance angiography using an arterial spin labeling technique for noninvasive diagnosis of moyamoya disease. Clin Neurol Neurosurg 137:105–111 [DOI] [PubMed] [Google Scholar]
- 13. [04/15/2015]; http://www.bic.mni.mcgill.ca/ServicesSoftware/MINC. Accessed.
- 14.Kim JJ, Fischbein NJ, Lu Y, Pham D, Dillon WP (2004) Regional angiographic grading system for collateral flow: correlation with cerebral infarction in patients with middle cerebral artery occlusion. Stroke 35:1340–1344 [DOI] [PubMed] [Google Scholar]
- 15.Fonov V, Evans AC, Botteron K et al. (2011) Unbiased average ageappropriate atlases for pediatric studies. NeuroImage 54:313–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piepgras A, Schmiedek P, Leinsinger G, Haberl RL, Kirsch CM, Einhaupl KM (1990) A simple test to assess cerebrovascular reserve capacity using transcranial Doppler sonography and acetazolamide. Stroke 21:1306–1311 [DOI] [PubMed] [Google Scholar]
- 17.Federau C, O’Brien K, Meuli R, Hagmann P, Maeder P (2014) Measuring brain perfusion with intravoxel incoherent motion (IVIM): initial clinical experience. J Magn Reson Imaging 39: 624–632 [DOI] [PubMed] [Google Scholar]
- 18.Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig Radiol 49:685–690 [DOI] [PubMed] [Google Scholar]
- 19.Robert P, Lehericy S, Grand S et al. (2015) T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Investig Radiol 50:473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yun TJ, Paeng JC, Sohn CH et al. (2015) Monitoring cerebrovascular reactivity through the use of arterial spin labeling in patients with moyamoya disease. Radiology. doi: 10.1148/radiol.2015141865:141865 [DOI] [PubMed] [Google Scholar]
- 21.Noguchi T, Kawashima M, Nishihara M, Hirai T, Matsushima T, Irie H (2013) Arterial spin-labeling MR imaging in moyamoya disease compared with clinical assessments and other MR imaging findings. Eur J Radiol 82:e840–e847 [DOI] [PubMed] [Google Scholar]
- 22.Sugino T, Mikami T, Miyata K, Suzuki K, Houkin K, Mikuni N (2013) Arterial spin-labeling magnetic resonance imaging after revascularization of moyamoya disease. J Stroke Cerebrovasc Dis 22: 811–816 [DOI] [PubMed] [Google Scholar]
- 23.Zaharchuk G, Do HM, Marks MP, Rosenberg J, Moseley ME, Steinberg GK (2011) Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke 42:2485–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wintermark M, Sesay M, Barbier E et al. (2005) Comparative overview of brain perfusion imaging techniques. Stroke 36:e83–e99 [DOI] [PubMed] [Google Scholar]
- 25.Heijtel DF, Petersen ET, Mutsaerts HJ et al. (2016) Quantitative agreement between [(15) O]H2 O PET and model free QUASAR MRI-derived cerebral blood flow and arterial blood volume. NMR Biomed 29:519–526 [DOI] [PubMed] [Google Scholar]
- 26.Tsujikawa T, Kimura H, Matsuda T et al. (2016) Arterial transit time mapping obtained by pulsed continuous 3D ASL imaging with multiple post-label delay acquisitions: comparative study with PET-CBF in patients with chronic occlusive cerebrovascular disease. PLoS One 11:e0156005. [DOI] [PMC free article] [PubMed] [Google Scholar]


