Abstract
Omeprazole (OM) is one of the most prescribed drugs worldwide for the treatment of hyperacidity and gastric reflux. However, concerns regarding its safety have emerged recently, and the drug is reported to enhance the risk for anxiety and cognitive deficits, particularly in elderly patients. The present study investigated these adverse effects, if any, in adult male rats. Associated changes in brain serotonin (5-hydroxytryptamine; 5-HT) and dopamine metabolism and the expression of 5-HT-1A receptors in the raphe and hippocampus were also determined. The drug was injected i.p. in doses of 10 and 20 mg/kg for 15 days. Both doses of OM decreased motor activity in an open field and impaired learning and memory in the Morris water maze test. Anxiety monitored in an elevated plus maze test was enhanced in rats treated with 20 mg/kg OM only. The levels of 5-HT and its metabolite 5-hydroxyindoleacetic acid and of homovanillic acid, a metabolite of dopamine, determined by HPLC-EC, were decreased in the brain of OM treated rats. The expression of 5-HT-1A receptor, determined by qRT-PCR, was reduced markedly in the hippocampus and moderately in the raphe. Our results provide evidence that OM use can reduce raphe hippocampal serotonin neurotransmission to lead to anxiety/depression and cognitive impairment. There is a need for increased awareness and prescription guidelines for therapeutic use of OM and possibly also other proton pump inhibitors.
Keywords: Omeprazole, Motor activity, Anxiety-like behavior, Learning and memory, Serotonin, Serotonin-1A receptors
Introduction
Omeprazole (OM) is one of the most widely prescribed proton pump inhibitors (PPIs) for treating gastric hyperacidity and reflux. It is a member of benzimidazole family and has been shown to block H+/K+-ATPase pump in the parietal cells of the stomach in the first 4 h after the administration [1]. In humans, the oral bioavailability of OM is about 40–50%, and elimination half-life from plasma is less than 1 h, and within 3–4 h it is entirely cleared from the plasma [2]. Its inhibitory action lasts up to approximately 48 to72 h [1]. It can cross the blood brain barrier rapidly to produce intracellular acidification of microglia by irreversible inhibition of microglial lysosome H+/K+-ATPase pump, similar to its effect on the gastric parietal cells [1, 3].
The safety of PPIs with respect to cognition has been questioned in clinical studies. These studies show that long-term use of PPIs impairs cognitive functions in elderly patients [4–7]. A study conducted in healthy volunteers, observed varying degree of influence on different cognitive functions by PPIs: with OM having the highest impact on cognitive impairment [8, 9]. In addition, some clinical studies also show anxiety and depression among PPIs users [10, 11].
Despite the above reported concerns about the safety of OM in clinical studies, the research in animals on these adverse effects is, however, lacking. The present study is therefore designed to examine the effects of OM on learning and memory and on anxiety/depression-like behavior in rats. Morris water maze (MWM) test is used to evaluate learning and memory. Open field (OF) activity and elevated plus maze (EPM) test are used to assess potential anxiety-like behavior. Because of a role of brain serotonin (5-hydroxytryptamine; 5-HT) and dopamine in anxiety and in learning and memory [12–14], the effect of OM on the metabolism of 5-HT and dopamine in the brain are also determined.
At least 14 different types and subtypes of 5-HT receptors have been identified in the brain [15]. The 5-HT-1A receptor, the most widely studied subtype is expressed presynaptically as autoreceptors on soma and dendrites of the 5-HT neurons in the raphe region [16]. Postsynaptically, it is expressed as heteroreceptors in brain regions where serotonergic neurons innervate [17, 18]. In view of a role of 5-HT-1A autoreceptors as well hippocampal heteroreceptors in anxiety/depression [18–20], and cognition [17, 21, 22], effects of OM on 5-HT-1A receptor expression are determined in the raphe and hippocampus of rats treated with OM.
Material and methods
Animals
Male albino Wistar rats, weighing 200–220 g (8–9 weeks of age), were taken from the institutional animal research facility. Rats were caged individually, five days before beginning of the actual experiment under standard temperature (22 ± 2 °C) with 12 h light and dark cycle for familiarization with the environment. They were free access to standard rodent diet and tap water ad libitum till the end of the experiment. All behavioral activities were carried out during the light phase (10:00–15:00 h). The study was designed and conducted in accordance with the National Institute of Health (NIH) guidelines for the Care and Use of Laboratory Animals and a protocol (ASP No.: 2018-0001) approved by the Institutional Committee for Animal Care and Use. To avoid time and order effects all experiments were carried out in balanced design.
Drug and doses
Commercially available OM (Risek®, GetzPharma) parenteral preparation, purchased locally, was used in this study. The drug was dissolved in water-for-injection (WFI) supplied along-with the parenteral preparation. It was injected i.p. in doses of 10 and 20 mg/mL/kg. Control animals were injected with WFI 1 mL/kg.
In the present study, OM was used in doses of 10 and 20 mg/kg, the doses which preclinical studies show are effective against aspirin or indomethacin-induced gastric ulcers in rats [34, 47]. The doses of OM recommended for treating gastric ulcers and reflux is 20–80 mg/kg [2]. The human equivalent dose for 20 mg/kg rats is 3.78 mg/kg [48].
Experiment 1: Dose-related effects of OM on anxiety/depression and learning/memory
Twenty-four rats were used in this experiment. The rats divided into three equal group were assigned as; WFI treated (1 mL/kg), low-dose OM treated (10 mg/kg) and high-dose OM treated (20 mg/kg) groups. The drug or WFI were administered intraperitoneally during 09:00–09:30 h daily for 15 days. Body weights and food intakes were monitored daily. Activity in OF was monitored on day 1 and day 7; 1 h after the respective day treatment. Behavior in EPM was also monitored on day 1 and day 7; 2 h after the respective day treatment. MWM training was conducted on day 10 at 10:00–12:00 h and learning acquisition was monitored on the same day at 14:00–15:00 h. Memory retention was assessed next day i.e. day 11, and also on day 14. Performance in probe test was monitored on day 15. The animals kept back in their cages were decapitated 1 h after the probe test. Whole brain were taken out and stored at − 80 °C till the determination of brain 5-HT and dopamine metabolism.
Food intake and body weight
Pre-weighed food was given to each animal in its respective cage hopper. Cumulative food intake and change in body weight during the treatment were monitored. Percentage change in body weight was calculated as: {(Body weight on day 15/starting day body weight) × 100}.
Open field (OF) activity
A large square area of 76 × 76 cm having 42 cm surrounding walls was used as OF. The floor of the OF was divided by black lines into 25 squares of equal size (15 cm). Activity in the OF was monitored as described earlier [23, 24]. A rat was gently placed in the center of the OF and the total number of squares crossed with all four paws were counted from 0 to 5 min, starting immediately after the placement.
Activity in an elevated plus maze (EPM)
The EPM, as described before [23, 24], was made up of white plastic. It had four arms arising from a central square giving shape of plus to the maze. Each arm was 50 cm long and 10 cm wide. Two arms were open and had no side walls. Other two arms were closed and had 15 cm high side walls. The maze was elevated 60 cm from the ground. To monitor anxiety-like behavior in the maze, a rat was placed in the central square of the maze with its face facing the junction of open and closed arm and permitted to explore the entire maze for a duration of 5 min. Time spent and number of entries in open arm were noted from 0 to 5 min.
Morris water maze (MWM) test
The apparatus and protocol were adopted as mentioned previously [25, 26]. The maze, made up of white plastic, was a circular pool having 90 cm diameter and 60 cm height. It was filled up to the height of 30 cm with tap-water, made opaque with milk. The maze was divided virtually into four equal quadrants (north, south, east and west). A square platform (10 × 10 cm) and 28 cm height was placed in the center of the north quadrant. The platform 2 cm below the surface of water was not visible. The procedure consisted of two phases: the training phase and the test phase.
The training phase consisted of three trials was conducted on day 10, 30 min post injection. In every trial, a rat placed in one of the quadrants except the north quadrant (having hidden platform), was given 2 min to search the hidden platform. Rats succeeded to find the platform, were left on it for 10 s. Rats not able to find the platform were directed to find it with the help of a rod. In the test phase, learning acquisition was monitored after 2 h of training and memory retention was assessed on day 11 (next day after training) and day 14 (four day after training) also. To monitor these, rat was placed in the water pool from the quadrant, opposite to the platform quadrant, and time taken to locate the submerged platform was monitored.
Probe test
This test was conducted on day 15 (5 days after training), 45 min post injection. During the probe test, escape platform was removed. The rat was placed in the water pool from quadrant, opposite to the platform quadrant, and permitted to swim for 60 s as reported previously [24]. The memory in the probe test was assessed as; latency time to enter the quadrant which initially had a platform, total time passed and number of entries in that quadrant. An increase in latency time and a decrease in the number of entries and time passed in the quadrant that initially had a platform was considered as memory impairment.
Neurochemical analysis
Frozen brain samples were homogenized in an extraction medium which was composed of; sodium meta-bisulfite (0.1%), cysteine (0.01%), EDTA (0.01%) and perchloric acid (0.4 M). The homogenate was centrifuged at 6000 RPM for 15 min. The supernatant was collected and processed for further analysis as reported before [24]. A commercially available separation column (Waters® Spherisorb® S5 5um ODS2 4.6 × 150 mm) was used as the stationary phase. The Mobile phase was 0.1 M sodium phosphate buffer (pH 2.9), containing methanol (14%), sodium octyl sulfate (0.023%) and EDTA (0.005%). Sample aliquots (20 μL) were injected by auto-sampler/injector. Separation was achieved at an operating potential of 0.8 V and a pressure of 2000–3000 psi with a flow rate of 1 mL/min. Serotonin, dopamine and their metabolites were detected using an electrochemical detector (Waters®, e2465 ECD) while tryptophan was determined by measuring absorbance at 273 nm wavelength on UV detector (Waters®, e2489 UV/Vis) via alliance Waters e2695 separation module (Waters®, Massachusetts, USA).
Experiment 2: Serotonin—1A receptor expression
Twelve rats were used in this experiment. The rats divided into two equal group were assigned as; controls (WFI, 1 mL/kg) and high-dose OM treated (20 mg/kg). Control and test animals were injected accordingly, daily for 15 days at 9:00–9:30 h. On day 15, 2 h post treatment, animals were decapitated to collect the raphe (dorsal and median) region and hippocampus. The samples were frozen immediately in liquid nitrogen and stored at − 80 °C for determining the 5-HT-1A receptor expression by qRT-PCR.
RNA extraction and reverse transcription
The samples were homogenized in TRIzol® reagent (Invitrogen, Thermo Fisher Scientific) and total RNA were extracted according to the described protocol by the manufacturer. The total RNA concentration was measured as reported before [26] using a NanoDrop (2000/2000c, Thermo Scientific). All RNA samples were stored at − 80 °C freezer without freeze-thawing until the subsequent reverse transcription was performed. The cDNA was generated as reported before [26], from 1 µg of total RNA of each sample with random primers in Master cycler proS (Eppendorf) via Revert Aid First Strand cDNA synthesis kit (ThermoFisher Scientific) following manufacturer’s instructions. The cDNA was then stored at − 20 °C for qRT-PCR.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Specific primers of 5-HT-1A and beta-actin were used as reported previously [26]. These were purchased locally from Penicon Pharmaceuticals (Table 1). Real-time PCR assay was performed in AriaMx G8830A (Agilent Technologies, USA) with Maxima SYBR Green/ROX qPCR Master Mix 2X (Thermo Scientific) according to the manufacturer’s guidelines. The thermocycling program was used as described earlier [26] with denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s. Data were acquired and analyzed using an AriaMx Agilent HRM software. 5-HT-1A receptor mRNA was co-amplified with the beta-actin mRNA which was used as an internal loading control. The 5-HT-1A receptor mRNA levels were normalized with beta-actin mRNA.
Table 1.
Primer sequences with annealing temperatures and amplicon size
| Gene | Primers | Annealing temperature (°C) | Amplicon size (bp) |
|---|---|---|---|
| Beta-actin |
F: 5′-ACCCACACTGTGCCCATCTA R: 5′-CGGAACCGCTCATTGCC |
58.5 57.1 |
285 |
| 5-HT-1A |
F: 5′-CCCCCCAAGAAGAGCCTGAA R: 5′-GGCAGCCAGCAGAGGATGAA |
59.4 60.1 |
335 |
Statistical analysis
Results are presented as means ± SD. Statistical analysis was done by IBM SPSS Statistics version 21. One-way ANOVA was used to analyse the data on food intake, change in body weight, and also on tryptophan, serotonin and dopamine and their metabolites. Two-way ANOVA, repeated measures design (RMD) was used to analyse the data on OF and EPM activities and also on learning/memory in the MWM test. Data on probe test were analyzed by one-way ANOVA. The post-hoc comparisons were done by Tukey’s test. Data on 5-HT-1A receptor expression were analyzed by Mann–Whitney U test. p values < 0.05 were considered as statistically significant.
Results
OM effects on body weight and food intake
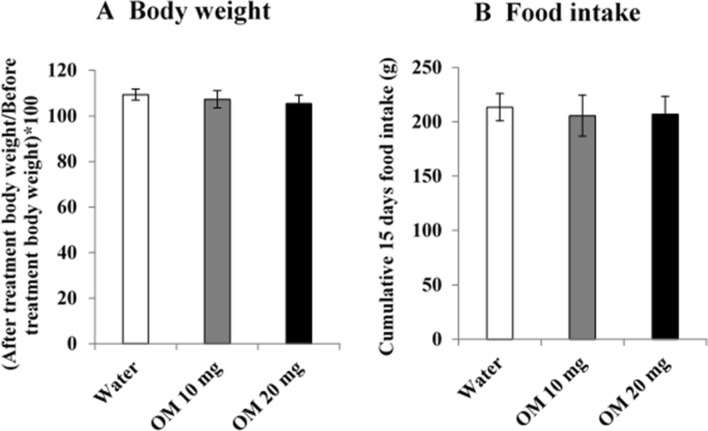
Figure 1 shows the effects of daily OM treatment for 15 days in doses of 10 and 20 mg/kg on cumulative food intake and body weight change. Data analyzed by one-way ANOVA revealed the drug effects were not significant for body weight F(2,21) = 2.813, p > 0.05 and food intake F(2,21) = 0.543, p > 0.05.
Fig. 1.
Dose-related effects of OM on a percentage change in body weight and b cumulative food intake. Values are means ± SD (n = 8). Data analyzed by one-way ANOVA showed no significant difference between the groups
OM effects on open field activity
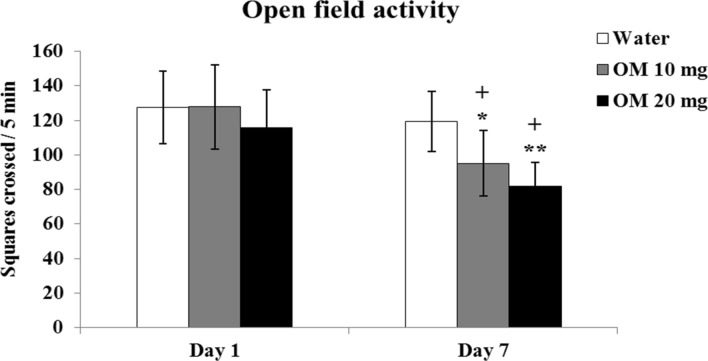
Figure 2 shows the results of daily OM administration in doses of 10 and 20 mg/kg on motor activity in OF monitored on day 1 and day 7. Data on OF activity was analyzed by 2-way ANOVA (RMD) showed significant effect of the drug F(2,21) = 17.746, p < 0.01, repeated measures (days) F(2,42) = 113.69, p < 0.01 and a significant interaction F(4,42) = 3.191, p < 0.05 between the drug and days. Post-hoc tests revealed that the OM administration on day 1 produced no effect on motor activity. Following repeated administration, activity decreased on day 7. The decrease was greater in high dose (20 mg/kg) compared to low dose (10 mg/kg) OM treated rats. Taken together, the results show that OM decreases motor/exploratory activity on repeated administration in a dose-dependent manner.
Fig. 2.
Dose-related effects of OM on motor/exploratory activity in OF. Values are means ± SD (n = 8). Significant differences using Tukey’s test: *p < 0.05, **p < 0.01 from respective day values of water treated group, +p < 0.01 from day 1 values of similar treated group, following 2-way ANOVA (RMD)
OM effects on elevated plus maze activity
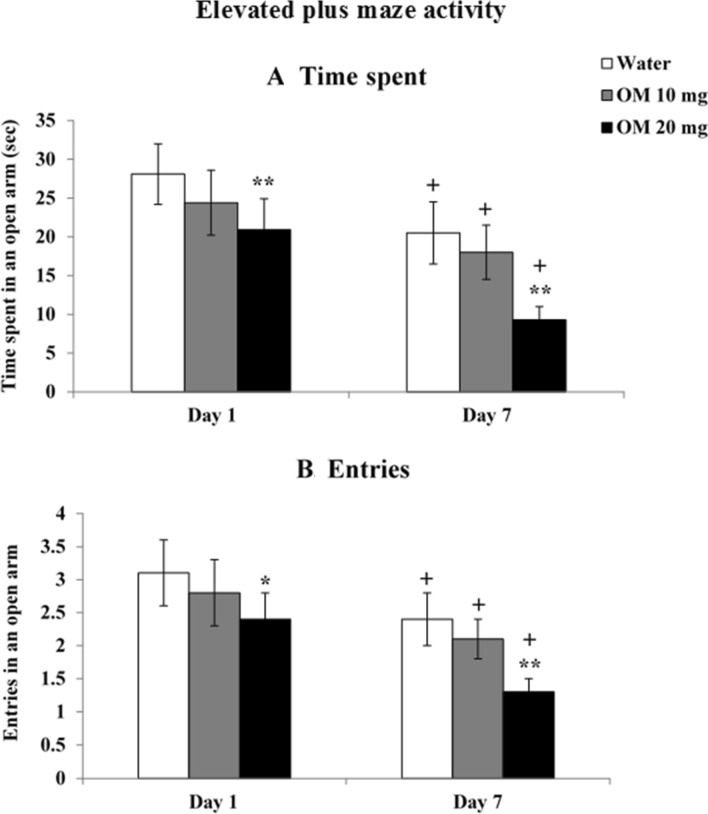
Figure 3 shows the results of daily OM administration in doses of 10 and 20 mg/kg on EPM activity monitored as time spent and number of entries in open arm on day 1 and day 7.
Fig. 3.
Dose-related effects of OM on a time spent and b number of entries in open arm. Values are means ± SD (n = 8). Significant differences using Tukey’s test: *p < 0.05, **p < 0.01 from respective day values of water treated group and +p < 0.01 from day 1 values of similar treated group, following 2-way ANOVA (RMD)
Time spent in open arm
Figure 3a shows data on time spent in open arm were analyzed by 2-way ANOVA (RMD) showed a significant effect of the drug F(2,21) = 34.768, p < 0.01, repeated measures (days) F(2,42) = 280.807, p < 0.01 and a significant interaction F(4,42) = 3.895, p < 0.01 between the drug and days. Post-hoc tests revealed that both single and repeated OM administration in high (20 mg/kg) dose, decreased time spent in open arm. Decreases were not significant for low dose at single or repeated administration. Habituation effect was also observed in water as well OM treated rats on repeated administration
Entries in open arm
Figure 3b shows data-analyzed by 2-way ANOVA (RMD) on the number of entries in open arm showed a significant effect of the drug F(2,21) = 46.798, p < 0.01, repeated measures (days) F(2,42) = 176.451, p < 0.01 and a significant interaction (F = 4.908 df = 4,42 p < 0.01) between the drug and days. Post-hoc tests revealed that both single and repeated OM administration in high (20 mg/kg) dose decreased number of entries in open arm. Decreases were not significant for low dose at single or repeated administration. Habituation effect was also observed in water as well OM treated rats on repeated administration
Taken together, it shows that OM produces anxiogenic effects at higher dose (20 mg/kg) which are greater on repeated administration.
OM effects on learning and memory in the MWM test
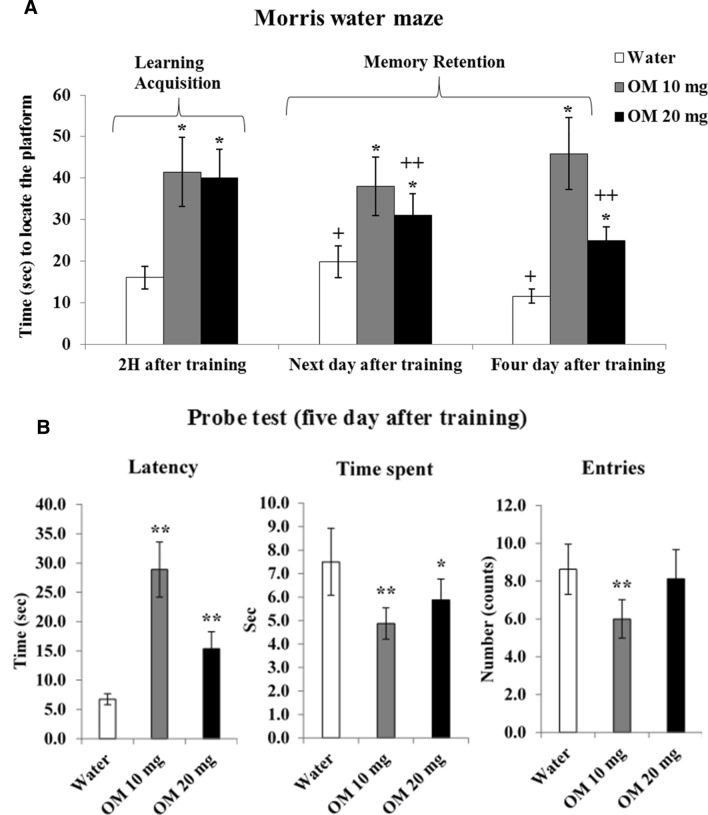
Figure 4a shows the results of daily OM administration in doses of 10 and 20 mg/kg on learning and memory in the MWM test, assessed on day 10 (2 h after training), day 11 (next day after training) and day 14 (four day after training). Data analyzed by 2-way ANOVA (RMD) showed a significant effect of drug F(2,21) = 75.233, p < 0.01, repeated measures F(2,42) = 6.874, p < 0.01 and a significant interaction F(4,42) = 12.332, p < 0.05 between the drug and repeated measures. The post-hoc test revealed that both low (10 mg/kg) and high (20 mg/kg) dose of OM impaired learning acquisition (examined after 2 h of training) in treating rats comparably. Impairment in memory retention was greater in low-dose than high-dose OM treated rats, observed on the next day and four days after training. Low dose (10 mg/kg) OM treated rats took more time to find the hidden platform than high dose (20 mg/kg) OM treated rats, showing greater impairment occurred with low dose (10 mg/kg) of OM. Taken together, it shows that OM impairs learning and memory on repeated administration.
Fig. 4.
a Dose-related effects of OM on learning and memory, assessed in the MWM test. Values are means ± SD (n = 8). Significant differences using Tukey’s test: *p < 0.01 from respective water treated group and +p < 0.05, ++p < 0.01 from learning acquisition (2 h after training) of similar treated group, following 2-way ANOVA (RMD). b Dose-related effects of OM on spatial memory assessed in the probe test as; latency time to reach the quadrant which initially had a platform, total time passed and number of entries in that quadrant. Values are means ± SD (n = 8). Significant differences using Tukey’s test: *p < 0.05, **p < 0.01 from respective water treated group, followed by one-way ANOVA
OM effects on spatial memory in the probe test
Figure 4b shows the results of daily OM administration in doses of 10 and 20 mg/kg on spatial memory assessed in the probe test on day 15 (five day after training). The performance was evaluated as, latency time to reach the quadrant which initially had a platform, total time passed in that quadrant and number of entries in that quadrant. Data analyzed by one-way ANOVA, showed a significant drug effect on latency time F(2,21) = 91.284, p < 0.01, time spent F(2,21) = 12.907, p < 0.01 and number of entries F(2,21) = 9.053, p < 0.01. Post-hoc comparison revealed that low dose (10 mg/kg) of OM increased latency time with decreased time spent and number of entries. High dose (20 mg/kg) OM treated rats also showed increased latency time with decreased time passed in the platform quadrant. Data on number of entries were not significant for high dose (20 mg/kg) of OM. Taken together, it shows that OM impairs spatial memory on repeated administration.
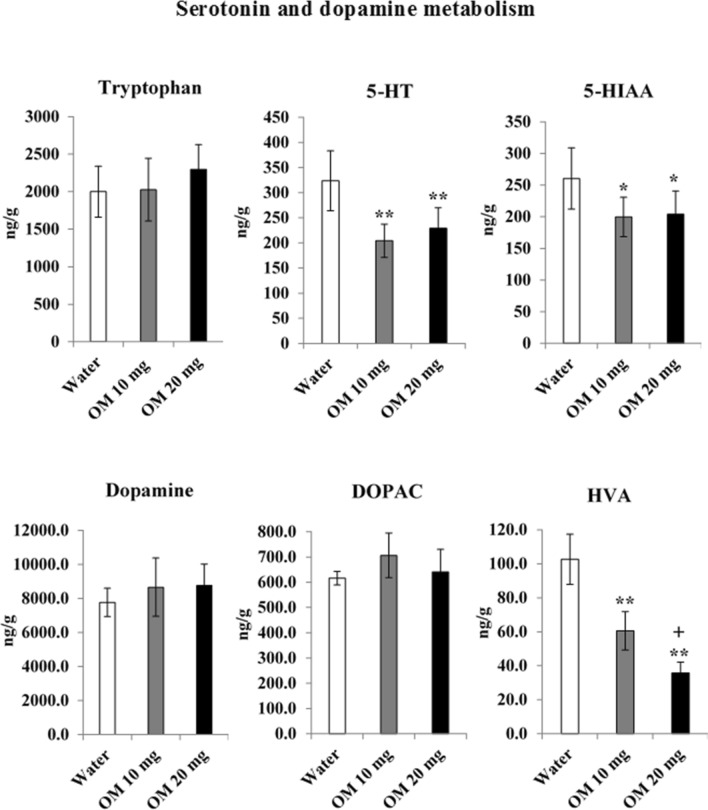
OM effects on brain serotonin and dopamine metabolism
Figure 5 shows the effects of OM treatment for 15 days in doses of 10 and 20 mg/kg on tryptophan, 5-HT, 5-HIAA, dopamine, DOPAC (3, 4-Dihydroxyphenylacetic acid) and HVA in the whole brain. Data-analyzed by one-way ANOVA showed significant effect of the drug (df = 2.21) on, 5-HT (F = 15.127, p < 0.01), 5-HIAA (F = 5.917, p < 0.01) and HVA (F = 70.729, p < 0.01) levels. However, no significant effect was found on tryptophan (F = 1.623, p > 0.05), dopamine (F = 3.125, p > 0.05) and DOPAC (F = 1.402, p > 0.05) levels. Post-hoc tests revealed that both low (10 mg/kg) and high (20 mg/kg) doses of OM reduced 5-HT and 5-HIAA levels in treating rats comparably. Both doses of OM decreased HVA level too, and this decrease was greater with high dose (20 mg/kg) of OM. There was no significant effect on brain tryptophan, dopamine and DOPAC concentrations. In general, OM decreased dopamine and serotonin metabolism and greater effect was produced on serotonin metabolism as both levels (5-HT and 5-HIAA) were reduced. However, a large decrease in HVA level also occurred.
Fig. 5.
Dose-related effects of OM on brain tryptophan, 5-HT, 5-HIAA, dopamine, DOPAC and HVA levels. Values are means ± SD (n = 8). Significant differences using Tukey’s test: *p < 0.05, **p < 0.01 from water treated group, and +p < 0.01 from low dose (10 mg/kg) of OM treated group, following one-way ANOVA
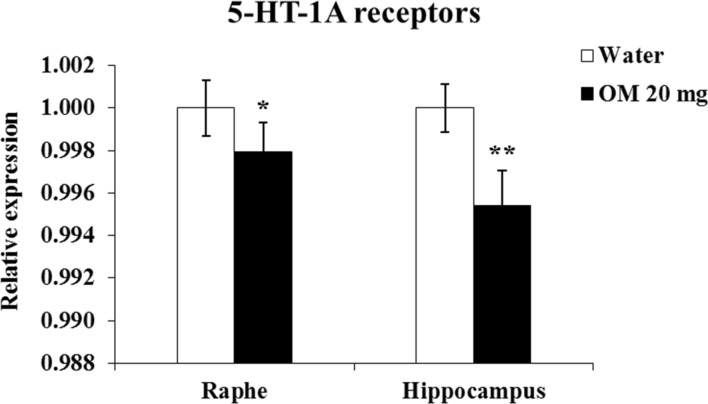
OM effects on 5-HT-1A receptor expression in the raphe and hippocampus
Figure 6 shows the effects of daily OM administration at a dose of 20 mg/kg for 15 days on 5-HT-1A receptor expression in the raphe region and hippocampus. Mann–Whitney U test showed a significant decrease (p < 0.01) in 5-HT-1A receptor expression in the hippocampus of OM-treated animals. Similar decreases of 5-HT-1A receptor expression in the raphe also reached significance (p = 0.05).
Fig. 6.
Effects of 20 mg/kg OM on 5-HT-1A receptor expression in the raphe and hippocampus by qRT-PCR. Values are means ± SD (n = 6). Significant differences using Mann–Whitney U test: *p = 0.05, **p < 0.01 from water-treated group
Discussion
The aim of this study was three fold. Firstly, we were interested in knowing whether repeated administration of OM produces anxiety and impairs memory. Secondarily, we wanted to know the associated changes in brain serotonin and dopamine metabolism. Thirdly, to determine the 5-HT-1A receptor expression in the raphe and hippocampus after repeated treatment with OM. Our results support clinical investigations [11, 27] and suggest a greater risk of anxiety and dementia in PPIs users. In addition, our results show that long-term use of OM can decrease brain serotonin and dopamine metabolism to produce these effects. A decrease in the expression of 5-HT-1A receptors in the raphe and hippocampus, supports the notion that a decrease in 5-HT1A heteroreceptor mediated serotonin neurotransmission via hippocampus is involved in these effects of OM.
To assess anxiety-like behavior [28–31], we monitored exploratory activity in an OF and time passed for open arm exploration in an EPM test. A marked decrease in OF exploration, observed in the present study in OM treated rats, supports the notion that the drug is anxiogenic. The EPM test also affirms our findings from the OF test as total time passed and the number of entries in open arm decreased in OM treated rats.
Clinical studies link anxiety producing effect of PPIs with increased level of gastrin in the blood [10, 32] which can stimulate cholecystokinin type B receptors (CCKB), in the brain to lead to anxiety and hypoactivity [10, 33]. The present study shows that the OM-induced decreases of 5-HT, dopamine, and decreases of 5-HT1-A receptor expression are involved in these behavioral deficits.
Several studies show an association between PPIs use and cognitive impairment or dementia [4–8]. In the present study, we conducted MWM test to assess potential cognitive impairment in OM treated rats. It is a widely used model for evaluating learning and memory in laboratory animals [26, 35]. We monitored learning and memory in terms of time, the trained animal took to find the hidden platform in the MWM task, and found that OM treated rats took more time to locate the hidden platform compared to water treated animals. The finding suggested impaired learning acquisition and memory retention. The results of the probe test also showed memory impairment as OM treated animals took more time to reach the platform quadrant and also passed less time in that quadrant. Moreover, entries in that compartment were also reduced. Overall, the present study shows impaired learning and memory in rats treated with OM.
A role of tryptophan and serotonin in learning/memory is well documented [12, 36]. Animal studies show that tryptophan administration increases brain tryptophan and serotonin and improves cognitive performance [37, 38]. Conversely, decreases of brain 5-HT metabolism are linked with memory deficits [39]. In this study, the 5-HT depleting effects of OM together with reduced 5-HT-1A receptor expression in the hippocampus implies that reduced serotonin neurotransmission via hippocampus plays an important role in memory impairing effects of OM. In addition, a large decrease in the HVA level, support a role of dopamine in the memory impairing effects of OM. A clinical study shows an association between low plasma HVA levels and greater errors in cognitive tasks [14], but similar studies on 5-HT and its metabolite 5-HIAA are lacking.
Because of the large decrease in brain serotonin in OM treated animals, the concentration of tryptophan, an essential amino acid and the sole precursor of 5-HT, was also determined. As tryptophan levels were not altered, the findings tend to suggest that the 5-HT depleting effect of OM is potentially due to decrease in the activity of the rate limiting enzyme, tryptophan hydroxylase [40–42].
Previous studies show that long-term use of PPIs produces vitamin B12 deficiency [43, 44], which may be linked with the decreases of serotonin and dopamine metabolism observed in the present study. A deficiency of B12 can impair the metabolism of biogenic amines [45]. In the current study, a decrease in the brain levels of 5-HT, 5-HIAA, and HVA may be due to the ability of OM to affect the vitamin B12 status. In future studies, it will be interesting to determine the potential use of vitamin B12 supplementation for restoring 5-HT levels and associated anxiety or depression.
To further understand the role of 5-HT in OM-induced anxiety and memory deficits, effects of OM on 5-HT-1A receptor expression were determined. Antidepressant drugs downregulate 5-HT-1A autoreceptors function to enhance the availability of 5-HT in terminal regions [46]. While stress-induced behavioral depression is associated with an upregulation of 5-HT-1A autoreceptors function [16, 18]. Here, in the present study, we were expecting an increase in the expression of 5-HT-1A autoreceptors. Surprisingly, we found a moderate but significant decrease in the expression of these receptors, suggesting that the decreases of 5-HT and 5-HIAA in the brain of OM treated animals are not due to greater activity of 5-HT-1A autoreceptors mediated feedback control. We suggest that the decreases occur as an adaptive mechanism to overcome OM-induced decreases of 5-HT metabolism. A decrease in 5-HT-1A receptor expression in the hippocampus is relevant that the decrease in 5-HT-1A receptor dependent neurotransmission [18] via hippocampus is involved in anxiety-like behavior and memory deficits of OM treated rats. The present study shows an important role of 5-HT-1A receptors in OM-induced anxiety/depression. Studies on the role of other 5-HT receptors, other than 5-HT1A [49, 50], serotonin transporter [51, 52], and signaling molecules [53–55] can improve our understanding of OM-induced behavioral deficits.
Overall, the present study shows that long-term use of OM can produce anxiety, reduce motor activity and impair cognition. Although factors, such as excessive secretion of gastrin and low vitamin B12 status may contribute to these behavioral deficits, the present findings tend to suggest that the decrease of 5-HT neurotransmission via 5-HT-1A receptors in the hippocampus is involved in these behavioral effects of OM. It suggests a need for increased awareness and prescription guidelines for therapeutic use of OM and possibly also other PPIs. Co-use of drugs or supplements for increasing serotonin neurotransmission can potentially improve the therapeutic use of these commonly used drugs.
Acknowledgements
The authors would like to thank Dr. Panjwani Center for Molecular Medicine and Drug Research (PCMD), University of Karachi for providing faculty research Grants.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Sadia Basharat Ali, Email: sadia.basharat@iccs.edu.
Khalid Mahmood, Email: qureshi.qau@gmail.com.
Raheel Saeed, Email: rahisaeed@hotmail.com.
Tabinda Salman, Email: tabinda.salman89@gmail.com.
Muhammad Iqbal Choudhary, Email: iqbalhej@yahoo.com.
Darakhshan Jabeen Haleem, Email: djhaleem@uok.edu.pk.
References
- 1.Özay R, Türkoğlu ME, Gürer B, Dolgun H, Evirgen O, Ergüder Bİ, Şekerci Z, et al. The protective effect of omeprazole against traumatic brain injury: an experimental study. World Neurosurg. 2017;104:634–643. doi: 10.1016/j.wneu.2017.04.136. [DOI] [PubMed] [Google Scholar]
- 2.Bendas ER, Abdelbary AA. Instantaneous enteric nano-encapsulation of omeprazole: pharmaceutical and pharmacological evaluation. Int J Pharm. 2014;468:97–104. doi: 10.1016/j.ijpharm.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Cheng FC, Ho YF, Hung LC, Chen CF, Tsai TH. Determination and pharmacokinetic profile of omeprazole in rat blood, brain and bile by microdialysis and high-performance liquid chromatography. J Chromatogr A. 2002;949:35–42. doi: 10.1016/S0021-9673(01)01225-0. [DOI] [PubMed] [Google Scholar]
- 4.Haenisch B, von Holt K, Wiese B, Prokein J, Lange C, Ernst A, Luppa M, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265:419–428. doi: 10.1007/s00406-014-0554-0. [DOI] [PubMed] [Google Scholar]
- 5.Herghelegiu AM, Prada GI, Nacu R. Prolonged use of proton pomp inhibitors and cognitive function in older adults. Farmacia. 2016;64:262–267. [Google Scholar]
- 6.Gomm W, von Holt K, Thomé F, Broich K, Maier W, Fink A, Haenisch B, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410–416. doi: 10.1001/jamaneurol.2015.4791. [DOI] [PubMed] [Google Scholar]
- 7.Nevado-Holgado AJ, Kim CH, Winchester L, Gallacher J, Lovestone S. Commonly prescribed drugs associate with cognitive function: a cross-sectional study in UK Biobank. BMJ Open. 2016;6:e012177. doi: 10.1136/bmjopen-2016-012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akter S, Hassan MR, Shahriar M, Akter N, Abbas MG, Bhuiyan MA. Cognitive impact after short-term exposure to different proton pump inhibitors: assessment using CANTAB software. Alzheimer's Res Therapy. 2015;7:79. doi: 10.1186/s13195-015-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maravelias C, Stefanidou M, Dona A, Athanaselis S, Spiliopoulou C. Drug-facilitated sexual assault provoked by the victim's religious beliefs: a case report. Am J Forensic Med Pathol. 2009;30:384–385. doi: 10.1097/PAF.0b013e3181c03e2e. [DOI] [PubMed] [Google Scholar]
- 10.Laudisio A, Incalzi RA, Gemma A, Giovannini S, Monaco MRL, Vetrano DL, Zuccalà G, et al. Use of proton-pump inhibitors is associated with depression: a population-based study. Int Psychogeriatr. 2018;30:153–159. doi: 10.1017/S1041610217001715. [DOI] [PubMed] [Google Scholar]
- 11.Masumoto S, Sato M, Maeno T, Ichinohe Y, Maeno T. Association between potentially inappropriate medications and anxiety in Japanese older patients. Geriatr Gerontol Int. 2017;17:2520–2526. doi: 10.1111/ggi.13128. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins T, Nguyen J, Polglaze K, Bertrand P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8:56. doi: 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Zhao J, Guo W. Emotional roles of mono-aminergic neurotransmitters in major depressive disorder and anxiety disorders. Front Psychol. 2018;9:2201. doi: 10.3389/fpsyg.2018.02201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagy O, Kelemen O, Benedek G, Myers CE, Shohamy D, Gluck MA, Kéri S. Dopaminergic contribution to cognitive sequence learning. J Neural Transm. 2007;114:607–612. doi: 10.1007/s00702-007-0654-3. [DOI] [PubMed] [Google Scholar]
- 15.Yohn CN, Gergues MM, Samuels BA. The role of 5-HT receptors in depression. Mol Brain. 2017;10:28. doi: 10.1186/s13041-017-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vahid-Ansari F, Zhang M, Zahrai A, Albert PR. Overcoming resistance to selective serotonin reuptake inhibitors: targeting serotonin, serotonin-1A receptors and adult neuroplasticity. Front Neurosci. 2019;13:404. doi: 10.3389/fnins.2019.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altieri SC, Garcia-Garcia AL, Leonardo ED, Andrews AM. Rethinking 5-HT1A receptors: emerging modes of inhibitory feedback of relevance to emotion-related behavior. ACS Chem Neurosci. 2012;4:72–83. doi: 10.1021/cn3002174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haleem DJ. Behavioral deficits and exaggerated feedback control over raphe-hippocampal serotonin neurotransmission in restrained rats. Pharmacol Rep. 2011;63:888–897. doi: 10.1016/S1734-1140(11)70604-1. [DOI] [PubMed] [Google Scholar]
- 19.Albert PR, Le François B, Vahid-Ansari F. Genetic, epigenetic and posttranscriptional mechanisms for treatment of major depression: the 5-HT1A receptor gene as a paradigm. J Psychiatry Neurosci JPN. 2019;44:164. doi: 10.1503/jpn.180209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. P5-HT 1A receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology. 2014;231:623–636. doi: 10.1007/s00213-013-3425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Tóth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin1A receptors. Proc Natl Acad Sci. 2000;97:14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff M, Costet P, Gross C, Hen R, Segu L, Buhot MC. Age-dependent effects of serotonin-1A receptor gene deletion in spatial learning abilities in mice. Mol Brain Res. 2004;130:39–48. doi: 10.1016/j.molbrainres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Cheema MAR, Nawaz S, Gul S, Salman T, Naqvi S, Dar A, Haleem DJ. Neurochemical and behavioral effects of Nigella sativa and Olea europaea oil in rats. Nutr Neurosci. 2018;21:185–194. doi: 10.1080/1028415X.2016.1257417. [DOI] [PubMed] [Google Scholar]
- 24.Haleem DJ, Mahmood K. Brain serotonin in high-fat diet-induced weight gain, anxiety and spatial memory in rats. Nutr Neurosci. 2019 doi: 10.1080/1028415X.2019.1619983. [DOI] [PubMed] [Google Scholar]
- 25.Haleem DJ, Nawaz S, Salman T. Dose related effects of buspirone on pain, learning/memory and food intake. Regul Toxicol Pharmacol. 2018;99:182–190. doi: 10.1016/j.yrtph.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Salman T, Nawaz S, Waraich RS, Haleem DJ. Repeated administration of methylphenidate produces reinforcement and downregulates 5-HT-1A receptor expression in the nucleus accumbens. Life Sci. 2019;218:139–146. doi: 10.1016/j.lfs.2018.12.046. [DOI] [PubMed] [Google Scholar]
- 27.Clouston SA, Shapira O, Kotov R, Lei L, Waszczuk M, Bromet EJ, Luft BJ. Proton pump inhibitors and the risk of severe cognitive impairment: the role of posttraumatic stress disorder. Alzheimer's Dement Transl Res Clin Interv. 2017;3:579–583. doi: 10.1016/j.trci.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Liu Y, Zhao M, Tang W, Wang X, Dong Z, Yu S. Depression and anxiety behaviour in a rat model of chronic migraine. J Headache Pain. 2017;18:27. doi: 10.1186/s10194-017-0736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mällo T, Alttoa A, Kõiv K, Tõnissaar M, Eller M, Harro J. Rats with persistently low or high exploratory activity: behaviour in tests of anxiety and depression, and extracellular levels of dopamine. Behav Brain Res. 2007;177:269–281. doi: 10.1016/j.bbr.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 31.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- 32.Polimeni G, Cutroneo P, Gallo A, Gallo S, Spina E, Caputi AP. Rabeprazole and psychiatric symptoms. Ann Pharmacother. 2007;41:1315–1317. doi: 10.1345/aph.1K134. [DOI] [PubMed] [Google Scholar]
- 33.Roesler R, Henriques JAP, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target for psychiatric and neurological disorders. CNS Neurol Disord Drug Targets. 2006;5:197–204. doi: 10.2174/187152706776359673. [DOI] [PubMed] [Google Scholar]
- 34.El-Shinnawy NA, Abd-Elmageid SA, Alshailabi EM. Evaluation of antiulcer activity of indole-3-carbinol and/or omeprazole on aspirin-induced gastric ulcer in rats. Toxicol Ind Health. 2014;30:357–375. doi: 10.1177/0748233712457448. [DOI] [PubMed] [Google Scholar]
- 35.Deng-Bryant Y, Leung LY, Caudle K, Tortella F, Shear D. Injury models of the central nervous system. New York: Humana Press; 2016. Cognitive evaluation using Morris water maze in neurotrauma; pp. 539–551. [DOI] [PubMed] [Google Scholar]
- 36.Strasser B, Gostner JM, Fuchs D. Mood, food, and cognition: role of tryptophan and serotonin. Curr Opin Clin Nutr Metab Care. 2016;19:55–61. doi: 10.1097/MCO.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 37.Haider S, Khaliq S, Haleem DJ. Enhanced serotonergic neurotransmission in the hippocampus following tryptophan administration improves learning acquisition and memory consolidation in rats. Pharmacol Rep. 2007;59:53. [PubMed] [Google Scholar]
- 38.Khaliq SAIMA, Haider SAIDA, Ahmed SP, Perveen TAHIRA, Haleem DJ. Relationship of brain tryptophan and serotonin in improving cognitive performance in rats. Pak J Pharm Sci. 2006;19:11–15. [PubMed] [Google Scholar]
- 39.Haider SAIDA, Shameem SAIMA, Ahmed SP, Perveen TAHIRA, Haleem DJ. Repeated administration of lead decreases brain 5-HT metabolism and produces memory deficits in rats. Cell Mol Biol Lett. 2005;10:669. [PubMed] [Google Scholar]
- 40.Carneiro IBC, Toscano AE, Lacerda DC, da Cunha MDSB, De Castro RM, de Jesus Deiró TCB, Medeiros JMB. l-Tryptophan administration and increase in cerebral serotonin levels: systematic review. Eur J Pharmacol. 2018;836:129–135. doi: 10.1016/j.ejphar.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Haleem DJ. Improving therapeutics in anorexia nervosa with tryptophan. Life Sci. 2017;178:87–93. doi: 10.1016/j.lfs.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Lim LW, Shrestha S, Or YZ, Tan SZK, Chung HH, Sun Y, Lin VCL, et al. Tetratricopeptide repeat domain 9A modulates anxiety-like behavior in female mice. Sci Rep. 2016;6:37568. doi: 10.1038/srep37568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310:2435–2442. doi: 10.1001/jama.2013.280490. [DOI] [PubMed] [Google Scholar]
- 44.O'Leary F, Allman-Farinelli M, Samman S. Vitamin B 12 status, cognitive decline and dementia: a systematic review of prospective cohort studies. Br J Nutr. 2012;108:1948–1961. doi: 10.1017/S0007114512004175. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5:949–960. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- 46.Metts AV, Rubin-Falcone H, Ogden RT, Lin X, Wilner DE, Burke AK, Mann JJ, et al. Antidepressant medication exposure and 5-HT1A autoreceptor binding in major depressive disorder. Synapse. 2019;73:e22089. doi: 10.1002/syn.22089. [DOI] [PubMed] [Google Scholar]
- 47.Morjan S, Al Laham S, Atieh R. Gastroprotective efficacy of folic acid and omeprazole in indomethacin-induced gastropathy in rats. Int J Pharmacogn Phytochem Res. 2013;5:113–119. [Google Scholar]
- 48.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rojas PS, Fiedler JL. What do we really know about 5-HT1A receptor signaling in neuronal cells? Front Cell Neurosci. 2016;10:272. doi: 10.3389/fncel.2016.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masson J, Emerit MB, Hamon M, Darmon M. Serotonergic signaling: multiple effectors and pleiotropic effects. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:685–713. doi: 10.1002/wmts.50. [DOI] [Google Scholar]
- 51.Fox MA, Jensen CL, French HT, Stein AR, Huang SJ, Tolliver TJ, Murphy DL. Neurochemical, behavioral, and physiological effects of pharmacologically enhanced serotonin levels in serotonin transporter (SERT)-deficient mice. Psychopharmacology. 2008;201:203–218. doi: 10.1007/s00213-008-1268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olivier JDA, Van Der Hart MGC, Van Swelm RPL, Dederen PJ, Homberg JR, Cremers T, Ellenbroek BA, et al. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 53.Welcome MO. Cellular mechanisms and molecular signaling pathways in stress-induced anxiety, depression, and blood–brain barrier inflammation and leakage. Inflammopharmacology. 2020;28:643–665. doi: 10.1007/s10787-020-00712-8. [DOI] [PubMed] [Google Scholar]
- 54.Huang YJ, Lane HY, Lin CH. New treatment strategies of depression: based on mechanisms related to neuroplasticity. Neural Plast. 2017;2017:4605971. doi: 10.1155/2017/4605971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Réus GZ, Generoso JS, Rodrigues ALS, Quevedo J. Neurobiology of depression. New York: Academic Press; 2019. Intracellular signaling pathways implicated in the pathophysiology of depression; pp. 97–109. [Google Scholar]