Abstract
The tumor microenvironment contributes to disease progression through multiple mechanisms, including immune suppression mediated in part by fibroblast activation protein (FAP)-expressing cells. Herein, a review of FAP biology is presented, supplemented with primary data. This includes FAP expression in prostate cancer and activation of latent reservoirs of TGF-β and VEGF to produce a positive feedback loop. This collectively suggests a normal wound repair process subverted during cancer pathophysiology. There has been immense interest in targeting FAP for diagnostic, monitoring and therapeutic purposes. Until recently, this development has outpaced an understanding of the biology; impeding optimal translation into the clinic. A summary of these applications is provided with an emphasis on eliminating tumor-infiltrating FAP-positive cells to overcome stromal barriers to immuno-oncological responses.
Keywords: : FAP, fibroblast activation protein, immunotherapy, prostate cancer, stroma, TGF-β, wound healing
Accumulating evidence suggests the tumor microenvironment (TME) is a central regulator of antitumor immune responses in multiple malignances, including prostate cancer. This includes suppression of both endogenous immune activity as well as exogenously induced responses via immunotherapy. Despite a growing recognition of their importance, the cell types and signaling pathways mitigating robust antitumor immune responses thus far have not been successfully targeted clinically. This is in large part due to a limited understanding of the complex and dynamic interactions between the many different cellular and acellular components within the TME with respect to their impact on immune surveillance. Recapitulation of this environment in standard two dimensional culture is not possible. Appropriate model systems and the careful dissection of the interplay between the TME, tumor cells and the host immune system are still under development. Consequently, this has impeded optimal and effective clinical translation of innovative strategies targeting the TME in prostate cancer and other tumor types. Such TME-targeted strategies have the potential to overcome stromal barriers to immuno-oncological responses and expand the range of tumor types that are amenable to immunotherapy such as immune checkpoint inhibitors.
Tumor-infiltrating mesenchymal stem cells (MSCs) and their progeny, carcinoma-associated fibroblasts (CAFs), have emerged as key architects of the pro tumorigenic and immunosuppressive TME that promotes disease progression and immune escape [1–3]. This tumor-supportive state is characterized by altered extracellular matrix (ECM) components and ratios, distinct metabolic profiles and products, increased angiogenesis, and the aberrant expression and activation of signaling molecules; in addition to inducing the recruitment and polarization of immune cells into suppressor phenotypes, including M2-polarized tumor-associated macrophages (TAMs), regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSCs). It should be noted that the full complexity of tumor-infiltrating mesenchymal cells has only recently begun to be appreciated as a result of high-dimensional single cell profiling techniques [4–8]; though earlier data generated using more traditional methods already supported distinct populations with functional differences [9–14]. While an incomplete understanding of this heterogeneity with respect to their pro and anti tumorigenic properties urges caution, elevated expression of fibroblast activation protein (FAP) is frequently identified as marking the pro tumorigenic ‘bad guys’ in the TME [15].
Materials & methods
Reagents
Schneider’s S2 cells were used to express recombinant human FAP (rhFAP), which was purified from the culture supernatant using Ni-NTA resin (Qiagen, CA, USA) following induction with 500 μM CuSO4 as previously described [16]. Recombinant human plasmin (#P1867) and TGF-β (T7039) were purchased from (Sigma, MO, USA).
Cell lines & primary cultures
Human WPMY-1 prostate fibroblasts, human umbilical vein endothelial cell (HUVEC) endothelial cells and prostate cancer cell lines (i.e., LNCaP, LAPC-4, CWR22Rv1, V-CaP, PC3 and DU145) were purchased from ATCC (VA, USA). All cell lines are routinely tested for mycoplasma using the MycoSensor PCR Assay (Agilent Technologies, CA, USA) and authenticated via short tandem repeat (STR) analysis in the Johns Hopkins Genetic Resources Core Facility. WPMY-1 cultured in DMEM supplemented with 10% FBS, 1% L-Glutamine and 1% Pen/Strep. HUVEC cells were grown in EGM-2 media supplemented with manufacturer supplied additives. LNCaP, CWR22Rv1, PC3 and DU145 were cultured in RPMI supplemented with 10% FBS, 1% L-Glutamine and 1% Pen/Strep. LAPC-4 were cultured in IMDM supplemented with 1 nM R1881, 10% FBS, 1% L-Glutamine and 1% Pen/Strep. V-CaP were cultured in DMEM (ATCC) supplemented with 10% FBS, and 1% L-Glutamine. All cells grown in a humidified incubator with 5% CO2 at 37°C under standard conditions. Schneider’s S2 cells (Invitrogen, CA, USA) were grown as a suspension culture at room temperature in DES medium (Invitrogen) supplemented with heat-inactivated FBS.
All primary human tissues were collected in accordance with institution review board-approved protocols as previously described [17]. Primary human prostate epithelial cells were obtained from radical prostatectomy tissue and cultured in calcium serum-free media as previously described [18]. Bone marrow-derived MSCs (BM-MSC-9c) were purchased from RoosterBio (MD, USA) and obtained from an 18–30 years old healthy male BM donor. Primary prostate cancer stromal cells (PrCSC-44c) were generated from radical prostatectomy tissue (Gleason 4 + 3). mCRPC stromal cells (mCRPC-2-1c) were generated from a bone metastasis obtained from a mCRPC patient at the time of rapid autopsy as previously described [17]. The normal prostate stromal cells used in the flow cytometry experiment were obtained from an 18–20 years old male donor and purchased from Lonza Biosciences (MD, USA). All primary human stromal cells were cultured in Rooster High Performance Media (RoosterBio) as previously described in a humidified incubator with 5% CO2 at 37°C under standard conditions [17,19].
FAP immunoblotting
FAP protein expression in human cells detected by western blot analysis according to standard methods. Briefly, cells were harvested and counted. Cell lysates were made by resuspending a known quantity of cells in RIPA buffer. SDS-PAGE gels were loaded with the 20 μl (i.e., 10,000 cells) per sample. Following transfer, blots were probed using antibodies against FAP (Santa Cruz, TX, USA, clone SS-13, 1:500) and β-actin (Sigma, clone AC-15, 1:20,000) as a loading control. Antibody specificity documented using genetically validated controls.
qRT-PCR analysis of FAP expression
FAP mRNA expression in human cells detect by quantitative reverse transcription (qRT-PCR) according to standard methods. Briefly, RNA was isolated from a known quantity of cells using the Qiagen RNeasy kit and contaminating DNA removed using the Ambion DNA-free Kit (TX, USA). cDNA was generated from 1 μg of total RNA using Oligo-dT primers and Superscript reverse transcriptase III (Invitrogen). PCR amplification of FAP cDNA performed using Platinum Pfx DNA polymerase (Invitrogen) according to manufacturer’s instructions using the following primers: (FP) 5′-CTGTGCTTGCCTTATTGGTG-3′; (RP) 5′-AATATCCTTCAGTGTGAGTGCTC-3′). Results confirmed with an independent set of primers (data not shown).
Generation of ECM from fibroblasts & endothelial cells
ECMs produced from human WPMY-1 fibroblasts or HUVEC endothelial cells were prepared according to previously published protocols [20]. Briefly, tissue culture dishes were coated with a 0.2% gelatin solution and incubated for 1 h at 37°C. Gelatin was cross-linked to the plate surface with a 1% glutaraldehyde solution followed by the addition of 1 M ethanolamine for 30 min at room temperature each with 3× phosphate-buffered saline (PBS) washes between each step. Cells were harvested from a semi-confluent dish and seeded on the gelatin-coated plates at 5 × 105 cells/ml in medium containing L-acorbic acid (50 μg/ml). Media supplemented with L-ascorbic acid was changed every 48 h for 9 days. Matrices were denuded of cells by incubation in a 0.5% Triton X-100, 20 mM NH4OH solution in PBS for 3–5 min at 37°C. Extraction buffer was diluted with PBS and aspirated. Matrices were washed 3× with PBS prior to coating with PBS supplemented with penicillin, streptomycin and fungizone. Plates were stored at 4°C until use for a maximum of 2 weeks.
Fibroblast-derived 3D matrices were assessed for quality using a cell attachment assay as previously described [20]. Cells were labeled with Hoechst 33342 (Invitrogen) for 15 min at 37°C and rinsed with PBS × 4. Cells were trypsinized and plated onto dishes coated with the fibroblast-derived 3D matrices described above or the 2D matrix control (i.e., fibronectin-coated plates [5 μg/ml]). Cells were allowed to attach for 10 min at 37°C and then washed with PBS. Cells were then fixed with a 16% paraformaldehyde, 4% sucrose solution in PBS and counted using a Nikon (NY, USA) Eclipse Ti fluorescent scope equipped with a Nikon DS-Qi1Mc camera and NIS-Elements AR3.0 imaging software. Five random images from each one of three dishes per group were analyzed at 20× magnification. Ratio of adhered cells on 3D versus 2D matrices was approximately fourfold; thus documenting deposition of a high-quality 3D matrix.
Digestion of ECM & quantification of growth factors by ELISA
Denuded matrices were incubated in the presence or absence of rhFAP at 37°C for up to 24 h in serum-free media. Supernatants were collected and clarified by centrifugation at 3000 r.p.m. for 5 min. TGF-β1 concentrations were determined by ELISA according to manufacturer’s instructions (R & D Systems, MN, USA). VEGF concentrations were also determined by ELISA according to manufacturer’s instructions (Bender Medsystems, Vienna, Austria). All samples were measured in duplicate from three independent experiments.
Synthesis of fluorescence-quenched peptides based upon LTBP sequences
Substrates synthesized as previously described [16]. Briefly, standard Fmoc solid-phase coupling on NovaTag Dnp resin (substitution level: 0.4 mmol/g, [Novabiochem, CA, USA]) followed by N-terminal capping with the fluorophore, 7-methoxycoumarin-4-acetic acid (MCa). Capping was performed overnight (2×) with MCa-Osu and HOBt in NMP. Protecting groups of capped peptides were cleaved with 95% TFA, 2.5% TIS and 2.5% water. Reversed phase HPLC and MALDI-TOF analyses were used to confirm purity and mass.
Proteolytic assays of LTBP fluorescence-quenched peptides
Protease assays performed as previously described [16]. Briefly, peptide stocks in DMSO (10 mM) were prepared and subsequently used to generate twofold dilution series ranging from 0.1 to 1.5 μM. rhFAP was added to each assay at the indicated concentration. Peptide proteolysis was monitored (ex 340/em 430 nm) using a DTX 880 multimode detector (Beckman Dickinson, NJ, USA) every 5 min for 1 h at 37°C in 100 mM Tris, 100 mM NaCl at pH 7.8 in 10% DMSO and 0.3% Brij-35. MCa (Novabiochem) standard curves were run with each assay. Calculation of kinetic constants performed according to standard methods using SigmaPlot software.
Determination of pH optimum for FAP enzymatic activity
A series of Tris buffers (100 mM) were prepared at pH values of 6.8, 7.0, 7.2, 7.4, 7.6, 7.8, 8.0, 8.2, 8.4, 8.6, 8.9, 9.1 and 9.4 as measured using a Beckman Dickinson Φ350 pH/Temp/mV meter (CA, USA) at 25°C. AP-AFC (500 μM) (Calbiochem, Darmstadt, Germany) was incubated for 1 h at 37°C in the presence or absence of 6 μM FAP in Tris buffer (100 mM) at each of the indicated pH values. All samples contained 0.3% Brij-35 and 10% DMSO (substrate vehicle). The fluorescence of the cleavage product resulting from protease activity was monitored (ex 480/em 535) every 3 min for 1 h using a DTX 880 multimode detector (Beckman Dickinson). All samples run in triplicate and the rate of hydrolysis (k) was determined during the linear phase of the reaction. All buffer solutions were confirmed to be approximately 0.3 units lower under the respective assay conditions.
Statistical analyses
All statistics were performed using GraphPad Prism 8.3.0. p-values were calculated using a Student’s t-test and values <0.05 were considered statistically significant. All statistical analyses were paired and two-sided. All error bars represent ± standard error.
Results
FAP & expression patterns
FAP is a 97 kDa serine protease in the S9 family that is 760 amino acids in length consisting of a β-propeller and an α/β-hydrolase domain [21,22]. It is enzymatically active as a homodimer and can heterodimerize with dipeptidyl peptidase (DPP)-IV when co-expressed by the same cell [22–24]. Members of the S9 family are relatively unique in their ability to hydrolyze substrates with a proline in the P1 position of the protease cleavage site [16,21]. Among the family, FAP is the only member with both dipeptidase and true endopeptidase activity [25]. The other S9 proteases are either dipeptide exopeptidases that require a free amino terminus for hydrolysis, or those that have limited oligopeptidase activity [1]. For example, prolyl oligopeptidase (POP [also known as PREP]) can only cleave after proline residues ≤30 amino acids from the amino terminus [26]; whereas, FAP can cleave accessible substrates anywhere within a protein as exemplified by its ability to digest collagen I-derived gelatin [16,25]. Though not expressed at high levels in normal tissue, FAP can be detected in mesenchymal tissues during embryonic development and at low levels in benign adult tissues [15], often associated with inflammation and tissue remodeling during wound healing. This expression is likely associated with infiltrating or tissue-resident MSCs and their reactive fibroblast progeny [17,27], which are important regulators of the overall tissue repair and homeostatic surveillance mechanisms [28,29]. A subset of pancreatic alpha cells in Langerhans islets also express FAP, potentially playing a role in glucose and lipid homeostasis [30,31].
A soluble form of FAP missing the short cytoplasmic tail (6 amino acids) and transmembrane domain has also been identified in plasma [32]. The mechanism for this localization and its physiological relevance is unclear, though it is thought to play a role in fibrinolysis. Additionally, the percentage of circulating FAP that is enzymatically active has not been sufficiently characterized across different donor ages and disease types, particularly different cancers; however, at least some fraction of it in the serum appears to be functional [33–35]. One study determined circulating FAP ranged from 49 to 159 ng/ml with approximately 20% being enzymatically active [36]. There are potentially confounding variables in earlier analyses of this type due to the overlapping substrate specificities of FAP and related family members, especially POP, and the poor discrimination between these activities using commonly available reagents. Fortunately, there have been a series of small molecule inhibitors and activity-based probes recently developed with high specificity for FAP and selectivity over POP as well as other family members that should be able to more definitively answer this question [37–40].
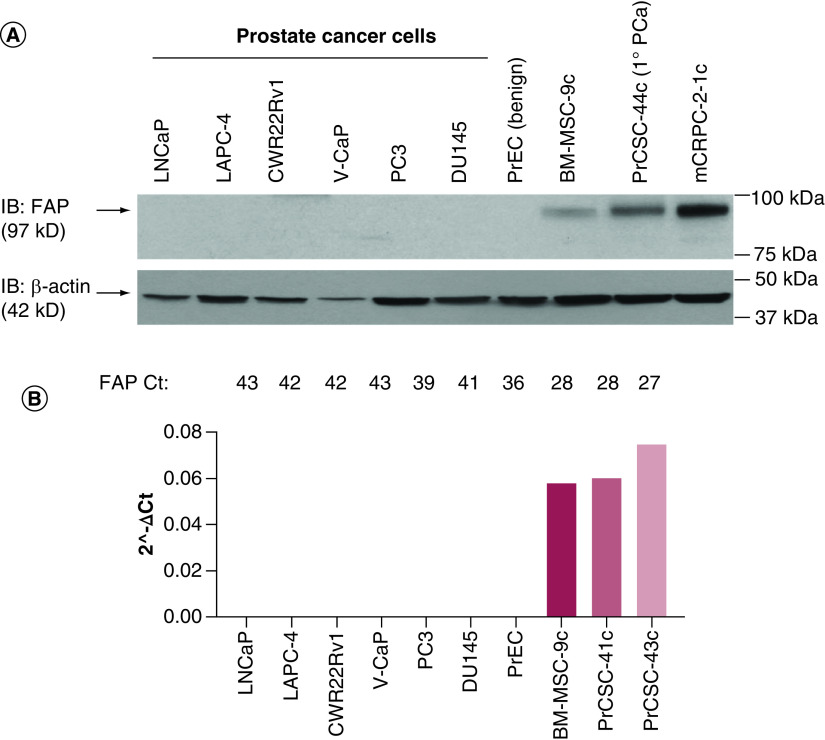
In contrast to the typically low levels of expression in healthy adult tissues, FAP is highly upregulated in the TME in the vast majority (>90%) of solid tumors [41]. Several studies have documented that increased FAP expression is associated with worse overall survival across many tumor types, including breast, colorectal and pancreatic cancers [42]. While this has not been conclusively documented in prostate cancer, a small study indicated elevated FAP expression is a principal factor of the prostate cancer reactive stroma [43], which Rowley, Ayala and colleagues have shown is a significant predictor of disease-free survival [44]. Our own work has confirmed that FAP is not expressed by benign or malignant prostate epithelial cells at the protein (Figure 1A) or mRNA level (Figure 1B). However, FAP is upregulated on prostate cancer-associated stromal cells (MSCs + CAFs) and appears to increase with disease progression, in addition to being elevated relative to BM-MSCs (Figure 1), which may provide a therapeutic index. More recently, a fibroblast-rich stroma defined as vimentin-positive and alpha-smooth muscle actin (αSMA)-negative was shown to be associated with aggressive prostate cancer and predict disease-specific outcome in a multivariate analysis [45]. In addition to MSCs and CAFs, FAP expression has been detected on a subset of M2-polarized TAMs in murine lung cancer and human breast cancer [46,47]. FAP was independently discovered as ‘seprase’, a 170 kDa membrane-associated heterodimer of FAP and DPPIV localized to invadopodia on melanocytes [23,48,49]. There is evidence suggesting FAP may also be expressed by some malignant epithelial cells in certain tumor types; however, in at least some instances these data should be interpreted with caution due to widespread availability of poorly validated reagents, particularly antibodies lacking specificity [15,50]. This is a problem that has plagued the study of FAP for many years, but recently reagents that have been validated with appropriate controls are entering the marketplace and investigators are increasingly cognizant of the importance of this issue [51].
Figure 1. . Fibroblast activation protein expression in epithelial and stromal cells derived from benign and malignant prostate tissue.
(A) FAP immunoblot documenting no FAP expression in benign or malignant prostate epithelial cells. However, FAP is detected on primary BM-MSCs and prostate cancer stromal cells derived from primary (1° PCa) and metastatic castration-resistant prostate cancer lesions. (B) qRT-PCR documenting high levels of FAP mRNA detected in BM-MSCs and prostate cancer stromal cells, but not in prostate cancer epithelial cells. Results confirmed with an independent set of primers.
FAP: Fibroblast activation protein; BM-MSC: Bone marrow-derived mesenchymal stem cell.
FAP substrates
Despite significant clinical interest since its initial discovery more than 30 years ago [52], there is still little known about the physiological role of FAP. The best characterized substrate is collagen I (Col-I), which requires initial cleavage by collagenases (e.g., matrix metalloproteinase [MMP]-1) before FAP can further digest it [16,53]. Similar data demonstrating sequential cleavage of perlecan by MMP-7 prior to FAP has also been reported in prostate cancer models [54]. Recent proteomics data using endogenous substrates from cellular sources has identified Col-III, Col-V, fibrillin-2, ECM protein-I (ECM-I) and lysyl oxidase homolog-I (LOX-L1) as additional ECM-associated substrates [55]. Collectively, digestion of these basement membrane and ECM components are thought to promote tumor invasion and metastasis. However, it should be noted that FAP does not cleave Col-IV or fibronectin, so it is not a general basement membrane and ECM degrader [16,25]. Other substrates identified through biochemical assays include FGF21, α2-antiplasmin, neuropeptide Y (NPY), complement C1q tumor necrosis factor-related protein-6 (C1qT6), C-X-C motif chemokine ligand 5 (CXCL5), chemokine ligand 2/monocyte chemoattractant protein-1 (CCL2/MCP-1) and colony-stimulating factor-1 (CSF-1) with putative roles in metabolism, fibrinolysis, neural regulation and immune signaling [32,34,55–57]. For the other substrates, there is in vitro evidence of direct proteolysis by FAP; however, elevated levels of soluble CSF-1 in the presence of enzymatically active FAP are likely mediated via an indirect mechanism as FAP does not appear to be directly shed it from the cell surface [55]. TGF-β and VEGF have also been identified as substrates in a proteomics screen and will be discussed in greater detail below.
While this growing list of FAP substrates represent important steps forward, there remains much work to be done to determine the physiologic and pathophysiologic relevance of these and other putative substrates. For example, FAP cleaves a dipeptide (Ala-Pro) from the N-terminus of secreted CXCL5, but this proteolysis does not appear to impact is biological function based on a neutrophil chemotaxis activity [55]. Similarly, FAP-mediated cleavage of eight amino acids from the N-terminus of secreted CCL2 also did not appear to regulate monocyte chemotaxis [55]. This is surprising because earlier work using CCL2 truncation mutants demonstrated that removal of these eight amino acids produced a dominant negative receptor antagonist [58–60]. This discrepancy is currently unresolved, but potentially related to species-specific differences or other experimental conditions that varied between different studies. Additional evidence indicates there is crosstalk between FAP and a STAT3-CCL2 axis that is functionally relevant, leading to elevated levels of CCL2 and increased recruitment of MDSCs [61,62].
FAP & TGF-β bioavailability
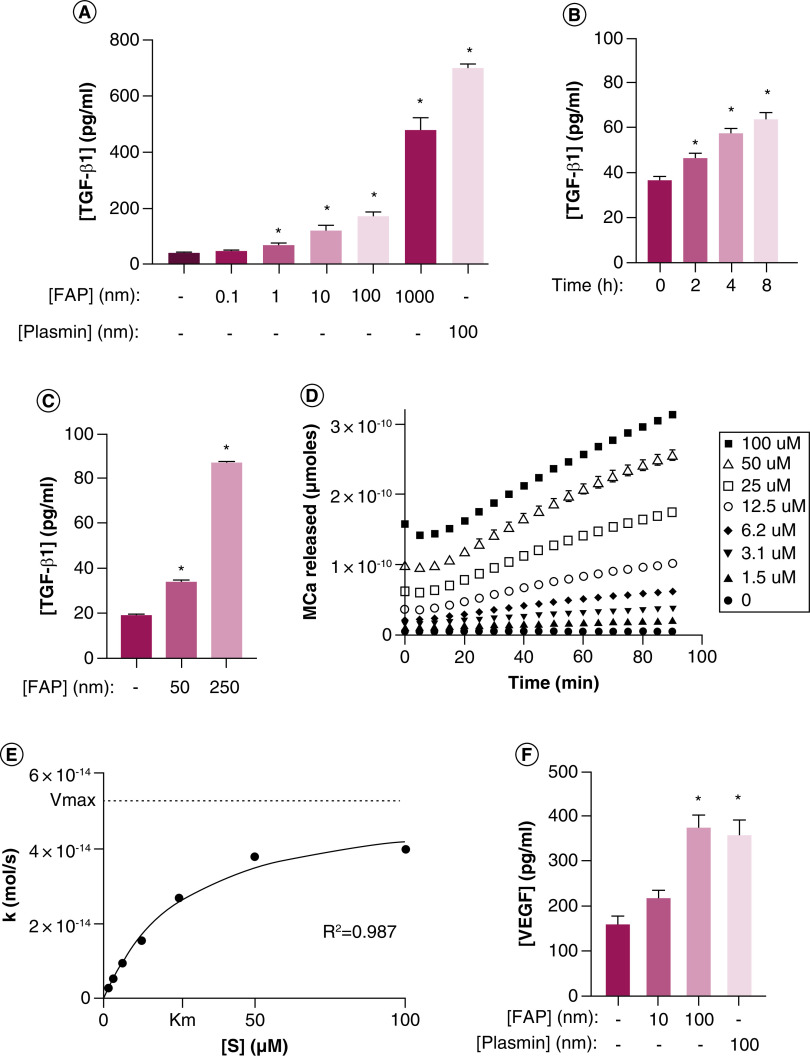
TGF-β has well known roles in multiple physiologic and pathophysiologic processes, including development, wound healing, the immune system, tissue homeostasis and cancer [63,64]. In addition to the study mentioned above, an earlier proteomics study also identified CCL2 and various ECM-associated proteins as substrates, in addition to implicating FAP in VEGF and TGF-β signaling [65]. This is interesting, because we demonstrated in independent studies that treating HUVEC with increasing concentrations of exogenous rhFAP produces a dose-dependent increase in TGF-β1 in the supernatant (Figure 2A) [66]. Endothelial cells deposit a basement membrane that is rich in Col-IV, fibronectin, and a variety of growth factors, including latent reservoirs of TGF-β, on their basal surface [67–69]. Therefore, to determine whether this increase in TGF-β1 was cell-dependent, HUVECs were allowed to deposit basement membrane on tissue culture dishes over 9 days in the presence of L-ascorbic acid (50 μg/ml) and then denuded of cells using mild detergents according to previously published protocols [70]. De-cellularized matrices were treated with rhFAP (50 nM) to assess a time-dependent increase in TGF-β1 in the conditioned media (Figure 2B) [66]. Similar de-cellularized matrices were produced from immortalized human prostate fibroblasts (WPMY-1 [71]) and treated with increasing concentrations of rhFAP to document a similar dose-dependent increase in TGF-β1 (Figure 2C) [66]. These data suggest that FAP can activate latent reservoirs of TGF-β in the basement membrane and ECM in a cell-independent manner.
Figure 2. . Fibroblast activation protein activates latent reservoirs of TGF-β sequestered in the basement membrane and extracellular matrix.
(A) HUVEC treated with increasing concentrations of exogenous recombinant human FAP (rhFAP) for 2.5 h at 37°C demonstrates a dose-dependent increase in TGF-β1 in the supernatant compared with vehicle-treated controls as measured by ELISA from two independent experiments performed in duplicate. Plasmin used as a positive control. (B) rhFAP (50 nM) releases TGF-β1 from HUVEC-derived basement membrane denuded of cells in a time-dependent manner as determined by ELISA of conditioned media at the indicated time points. Experiment performed in duplicate. (C) FAP releases TGF-β1 from human fibroblast (WPMY-1) derived ECM denuded of cells in a concentration-dependent manner over 24 h at 37°C. Three independent experiments performed in duplicate. (D) FAP-dependent cleavage of a fluorescence-quenched peptide substrate (Mca-EIPESGPSSG-Dnp) designed from the hinge region of LTBP-4 in the presence of FAP (100 nM) at 37°C. No Mca released in the absence of FAP. All experiments performed in triplicate with a representative plot shown here. (E) Representative plot of hydrolysis rate (k) versus substrate concentration ([S]) for the peptide presented in panel D. (F) Increasing concentrations of VEGF detected in conditioned media from human prostate cancer (PC3) cells treated with increasing concentrations of exogenous rhFAP release as measured by ELISA and measured in triplicate. Plasmin used as a positive control. All error bars represent standard error (SE). p-values < 0.05 (*) relative to the untreated controls.
ECM: Extracellular matrix; FAP: Fibroblast activation protein.
TGF-β is synthesized as a proprotein that is cleaved intracellularly by a furin-like protease following homodimerization. The propeptide portion, called the latency-associated protein, has a high affinity for the TGF-β dimer and forms a latent complex with the growth factor through non covalent electrostatic interactions that is known as the small latent complex (SLC) [72]. This SLC is further linked via disulfide bonds to one of the latent TGF-β binding proteins (LTBP), all three of which together constitute the large latent complex (LLC). The vast majority of TGF-β secreted from cells is in this LLC form, which associates with ECM proteins such as fibrillin, fibronectin and Col-IV [72,73]. This ECM-bound form constitutes a latent reservoir of TGF-β awaiting activation in response to tissue injury or other physiologic cues. There is also a pool of latent TGF-β associated with the cell surface of specific cell types including Tregs, endothelium and macrophages via interactions with transmembrane Glycoprotein A Repetitions Predominant Protein (GARP/LRRC32) or Leucine-Rich Repeat-Containing Protein 33 (LRRC33) [64,74].
There are four members of the LTBP family, which is critically involved in the regulation of TGF-β bioavailability [75,76]. Each of the LTBPs is expressed in a tissue-specific, but partially overlapping pattern in the body and genetic evidence suggests they have non redundant isoform-specific functions despite their similarities [72,77]. Latent TGF-β can be released from the ECM-associated LLC by pH changes, reactive oxygen species, heat, thrombospondin-1 (TSP-1), integrin-mediated conformational changes or cleavage by proteases, including plasmin, elastase, PSA and various MMPs [72,76,78,79]. Protease-induced activation of the LLC occurs via cleavage of LTBP in the proline-rich, protease-sensitive hinge region and results in release of the SLC. Once released, this SLC binds to integrins via RGD motifs or cell surface receptors such as mannose-6-phosphate (M6P) receptors, which leads to release of the active TGF-β dimer and presentation to the cognate receptor complex to stimulate signaling [80,81]. Following activation, soluble TGF-β has a short half-life and is cleared very quickly from the extracellular space if not bound to its cognate receptor [72,82]. This complex system of activation allows for dynamic local regulation of TGF-β bioactivity at multiple levels in a tightly controlled temporal and spatial fashion.
Based on this information, we hypothesized that FAP-mediated release of TGF-β from the ECM occurs via proteolysis of the proline-rich, protease-sensitive hinge region of LTBP and subsequent release of the SLC into the extracellular fluid or conditioned media. We synthesized a series of fluorescence-quenched peptide substrates representing potential FAP cleavage sites located within the hinge region and flanking sequences from each member of the LTBP family (LTBP1-4) based on the known substrate preference (P2Gly-P1Pro). These peptide substrates were ten amino acids in length and flanking the predicted cleavage site from the P7-P3′ positions (Table 1). We were unsuccessful in synthesizing a few of the peptides for unknown technical reasons. Hydrolysis rates for each of the successfully synthesized peptides were determined with representative kinetic plots for one of the substrates from LTBP-4 shown in Figure 2D & E. Enzyme kinetic parameters for these substrates are presented in Table 1. Of the peptides that were successfully synthesized, FAP only cleaves a single peptide located in the hinge region of LTBP-1, -2 and -4 with LTBP-4 the most efficiently cleaved of these substrates. These data demonstrate that FAP can release latent TGF-β from fibroblast-derived ECM and endothelial cell-derived basement membrane. This release is the result of a specific proteolytic event in the LTBP hinge region, but FAP-mediated digestion of fibrillin also likely contributes to increased TGF-β bioavailability in this context. Based on the homology with the LTBP family, we predicted fibrillins may be FAP substrates as well [66]; an assertion verified in a recent proteomics study [55]. Interestingly, all four LTBPs were also identified in these proteomics screens, however, they did not reach statistical significance and thus only included in the supplemental data [55,65].
Table 1. . Enzyme kinetics for fibroblast activation protein-mediated hydrolysis of fluorescence-quenched peptides designed from hinge regions and flanking sequences of the LTBP1-4 isoforms.
| Position | Sequence | Km (μM) | kcat (s-1) | kcat/Km (M-1s-1) | |
|---|---|---|---|---|---|
| LTBP1 | 672–681 | ISEEKGPCYR | NC | ||
| 706–715 | VGKAWGPHCE | NC | |||
| 748–757† | HHVGKGPVFV | NC | |||
| 786–795† | FSREHGPGVA | 10.5 ± 0.5 | 2.1E-0.3 ± 2.4E-0.5 | 208 ± 25 | |
| 797–806† | PEVATAPPEK | NC | |||
| 849–858 | VPVEVAPEAS | NC | |||
| 862–871 | ASQVIAPTQV | NC | |||
| LTBP2 | 674–683 | CYRSLGPGTC | NC | ||
| 761–770† | SGALPGPAER | 6.3 ± 0.4 | 8.8E-0.4 ± 6.2E-0.4 | 147 ± 108 | |
| 852–861 | ATNVCGPGTC | NC | |||
| LTBP3 | 490–499† | FLHPDGPPKP | NC | ||
| 504–513† | ESPSQAPPPE | NC | |||
| 572–581† | SAVEIAPTQV | US | |||
| 597–606 | GECVPGPPDY | NC | |||
| LTBP4 | 365–374 | ISEAKGPCFR | NC | ||
| 420–429 | EICPAGPGYH | US | |||
| 481–490† | PDPRPGPELP | NC | |||
| 494–503† | IPAWTGPEIP | US | |||
| 501–510† | EIPESGPSSG | 24.2 ± 1.5 | 1.4E-0.2 ± 2.9E-0.3 | 565 ± 81 | |
| 515–524 | NPQVCGPGRC | US |
Located in the hinge region.
NC: Not cleaved under the conditions tested; US: Unable to synthesize.
Whether preferential cleavage of one family member over another by FAP is of biological significance in this context is not known. Relative to LTBP-1 and -3, LTBP-4 is a poor binder of latent TGF-β1, which is the only TGF-β isoform it binds [64,77]. Homozygous mutations in LTBP-4 are associated with phenotypes in multiple tissues; however, these defects are thought to be related to its role in elastic fiber formation in the ECM rather than TGF-β binding [64]. In contrast to the other family members, LTBP-2 lacks the latency-associated protein binding domain and therefore does not sequester TGF-β in the ECM despite its name [64]. Similar to LTBP-4, it is thought to primarily play a role in fibrillar elastogenesis [64]. Increased migration of melanoma cells via adhesion to LTBP-2 has been demonstrated via interactions with integrin α3β1 [83]. There is also evidence that FAP clusters with integrin α3β1 on invadopodia of invasive melanoma cells in a collagen-dependent manner [84,85]. Therefore, α3β1-dependent clustering of FAP to invadopodia at the leading edge of melanoma or other cancer cells may mediate increased invasion via proteolysis of LTBP-2 and other ECM-associated proteins, including Col-I and fibrillin.
FAP & VEGF bioavailability
Similar to TGF-β, latent repositories of VEGF isoforms bound to heparin sulfate proteoglycans can be sequestered in the ECM [86,87]. Increased concentrations of VEGF could be detected in the conditioned media of human prostate cancer (PC3) cells treated with FAP in a dose-dependent manner (Figure 2F). Interestingly, there is evidence suggesting ECM-bound VEGF promotes endothelial cell adhesion, migration and survival through interactions with integrin α3β1 [88]. There is also evidence integrin α3β1 regulates endothelial VEGF production [89]. We have previously shown that primary cultures of human prostate stromal cells, which are heterogeneous cultures of FAP-positive MSCs and CAFs, are essential for angiogenesis using an in vitro 3D fibrin matrix assay that accurately recapitulates each of the major physiologic changes necessary for new vessel formation [90]. Since this experiment was not performed in a cell-free system, we cannot discount the involvement of integrins or the possibility of direct effects of FAP or secondary mediators, such as TGF-β, on the cells themselves. There is ample evidence of crosstalk between the two pathways and TGF-β is known to upregulate VEGF expression in endothelial cells [91,92]. However, the possibility that FAP proteolysis releases VEGF from the ECM in a manner analogous to that described for TGF-β as part of a broader integrin-dependent growth factor activation complex localized to invadopodia is provocative. This is supported by increased levels of pro-angiogenic factors (VEGF-C and angiopoietin) in conditioned media and decreased levels of the anti-angiogenic factor PEDF in a FAP-dependent manner [65]. Further evidence that FAP plays an important role in angiogenesis is provided from transgenic models in which FAP deletion decreases blood vessel density in syngeneic CT26 colon tumors [93].
FAP & immunosurveillance
The immunosuppressive properties of TGF-β are well-documented with significant effects on multiple innate and adaptive immune cell types [64,74,94,95]. Beyond its ability to activate TGF-β, the strongest evidence that FAP plays an important role in immune surveillance is derived from transgenic models. Eliminating FAP-expressing cells via an elegant transgenic model in which the diphtheria toxin receptor is driven off the Fap promoter was shown to induce adaptive immune-mediated control of murine LL2 Lewis lung carcinoma and KPC pancreatic ductal adenocarcinoma models [96]. Targeting FAP+ cells via diphtheria toxin was also shown to synergize with immune checkpoint blockade in these KPC tumors [97]. Because FAP has been identified on multiple cell types including CAFs, MSCs and TAMs; all of which have well-described immunosuppressive properties [1,17,27,46,47], it is important to define which of these FAP+ populations is important in mediating this immunosuppression. Using BM chimeric mice, it was demonstrated that both the CD45- (i.e., mesenchymal) and CD45+ F4/80+ (i.e., TAM) FAP+ populations contribute to this protection from antitumor immunity [46].
The above diphtheria-based approach clearly documents that FAP+ cells are essential in immuno surveillance. There is also evidence that the enzymatic activity of FAP plays a role in this process. Crossing the same KPC pancreatic cancer model onto a Fap-null background had no impact on tumor initiation, but significantly slowed tumor progression leading to an increase in overall survival [98]. Using Fap-deficient hosts, the same group documented reduced tumor burden and increased survival in the K-rasG12D endogenous lung carcinoma and transplanted CT26 colon cancer models [93]. These results were interpreted to be due to effects on stromagenesis (i.e., angiogenesis, ECM composition and organization, etc.), which was convincingly demonstrated; however, an immunological component likely also played a role in this antitumor response, but was not investigated in the study. The role of FAP enzymatic activity in immunosurveillance is further supported by data demonstrating that FAP+ cells promote the recruitment of MDSCs into the TME of Hepa1-6 liver cancer xenografts via STAT3-CCL2 signaling, and this phenomenon could be recapitulated by stably expressing FAP in normal fibroblasts [61]. Similar recruitment was observed in a model of intrahepatic cholangiocarcinoma and could be reversed by FAP knockdown in CAFs [62]. However, the physiologic substrates driving these antitumor responses are currently unclear and an active area of important ongoing studies with significant potential for therapeutic impact.
FAP, tissue repair & cancer as a wound that does not heal
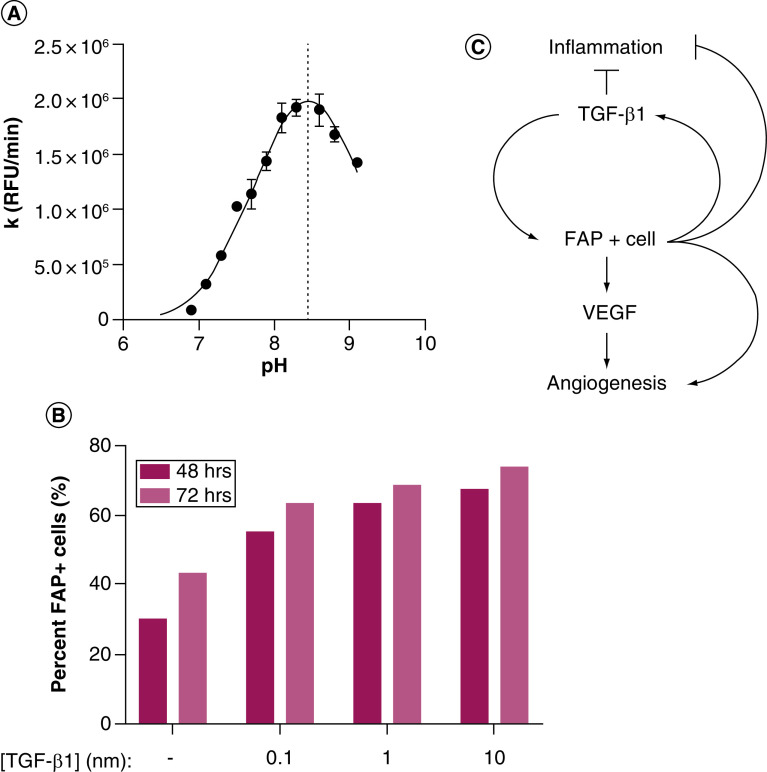
Taken together, these observations suggest a model in which FAP activates latent reservoirs of TGF-β and VEGF sequestered in the basement membrane and ECM in response to tissue damage. Following injury, tissue-resident fibroblasts are converted to a FAP-expressing activated phenotype, possibly via TGF-β released from platelets [99]. Additionally, BM-derived FAP+ MSCs may infiltrate sites of tissue damage along with immune cells in response to inflammatory stimuli, such as CCL2, CCL5, CXCL12 and TGF-β [29]. Notably, all of these chemokines are highly upregulated in prostate cancer and many other tumor types [100–103]. A third possibility is that the extracellular, soluble form of FAP in circulation leaks into areas of tissue damage following vessel damage to activate TGF-β, other growth factors, and bioactive molecules to aid in the repair process. Early work characterizing the enzymology of FAP documented a pH optimum of 8.5 for enzymatic activity [104]; an observation we have independently confirmed (Figure 3A). Interestingly, areas of tissue damage, particularly in the context of chronic inflammation, often lead to an alkaline microenvironment [105,106]. This suggests that soluble FAP at neutral pH in the circulation may be in a minimally active state that becomes maximally active under alkaline conditions in areas of tissue damage and chronic inflammation; a supposition that holds equally true for the activity of membrane-bound FAP as well. This is not without precedent, as other proteases involved in ECM remodeling and tissue repair, such as MMPs, also have a more basic pH optimum [106,107]. Maximal proliferation of keratinocytes and fibroblasts has also been reported under alkaline conditions [108].
Figure 3. . Fibroblast activation protein pH profile and positive feedback loop with TGF-β.
(A) FAP is optimally active under alkaline conditions. The rate of substrate (AP-AFC) hydrolysis (k) was measured in the presence or absence of FAP in a series of Tris buffers at the indicated pH values at 37°C. All samples run in triplicate. (B) TGF-β1 induces FAP expression on human prostate fibroblasts (WPMY-1) in a concentration-dependent manner as determined by flow cytometry. (C) Model of positive feedback loop between FAP+ cells and TGF-β1 regulating angiogenesis and inflammation during wound healing and chronic inflammation with implications for cancer initiation and progression. All error bars represent standard error.
FAP: Fibroblast activation protein.
Once FAP is present at the site of tissue damage, a positive feedback loop may be established that reinforces the wound healing response through activation of latent reservoirs of TGF-β and VEGF. TGF-β is a well-known inducer of the activated CAF phenotype and stimulates further expression of FAP [43,109]. A phenomenon we have independently confirmed using immortalized human prostate fibroblasts (Figure 3B). These data provide further evidence of crosstalk between FAP and TGF-β that is amplified via a positive feedback loop in tissue repair and chronic inflammation (Figure 3C). Following successful resolution of tissue damage, this feedback loop must be interrupted by an as yet poorly defined mechanism to maintain tissue homeostasis under non pathological conditions. This could be the loss of FAP activity due to pH normalization, downregulation of FAP expression, increased expression of an inhibitor, egress or death of FAP-positive cells, depletion of latent TGF-β reservoirs or some combination of these factors. A failure to engage such a negative regulator of this process would lead to a chronic cycle of injury response, including persistent inflammation, excessive ECM remodeling and fibrosis, in addition to stimulation of angiogenesis. All of which play a role in tumor initiation and progression; drawing obvious parallels to the analogy of cancer as a ‘wound that does not heal’ [110].
Discussion
Therapeutic strategies targeting FAP
A multitude of strategies have been developed since FAP's initial discovery in an attempt to exploit its restricted expression pattern and unique substrate specificity for clinical benefit. These approaches range from diagnostic and prognostic applications as discussed above to imaging and therapeutic targeting using a diverse series of modalities, several which have been evaluated clinically with mixed success. The therapeutic approaches generally fall into one of two categories – those designed to kill FAP+ cells (e.g., prodrugs, vaccines or immunotherapies) or those relying on inhibition of FAP enzymatic activity (e.g., small molecules). The first of these to be tested clinically was sibrotuzumab, a humanized monoclonal anti-FAP antibody based on the F19 antibody that was initially used to discover and characterize FAP expression [30,41,111,112]. Though sibrotuzumab was ineffective against metastatic lung and colorectal cancers in Phase I and II studies, it was well-tolerated and demonstrated tumor targeting [111,112]. The Phase I trial utilized Iodine-131 (131I)-radiolabeled sibrotuzumab, whereas the Phase II study evaluated unlabeled antibody for antitumor efficacy. As sibrotuzumab does not inhibit FAP enzymatic activity and was not optimized for antibody-dependent cellular cytotoxicity (ADCC), the lack of efficacy is not entirely unanticipated. Revisiting this space with an optimized antibody, naked or conjugated, may be warranted, particularly if eliminating FAP+ cells is documented to be both safe and required for efficacy.
The second FAP-targeted therapy to be evaluated in the clinic was PT-100 (Talabostat), a dipeptide boronic acid (Val-boroPro). Again, it was well-tolerated, but ultimately failed in multiple Phase II clinical trials both as a monotherapy for metastatic colorectal cancer [113] and in combination with docetaxel for advanced non-small-cell lung cancer [114] or cisplatin for late-stage melanoma [115]. Emblematic of the problems associated with many of these early FAP-targeted approaches (i.e., lack of specificity) is that Val-boroPro was initially designed as a DPPIV inhibitor (IC50: <4 nM), later developed as FAP inhibitor (FAPI) (IC50: 560 nM), and recently undergone a rebirth as a DPP8/9 inhibitor (IC50: 4 and 100 nM, respectively) able to induce monocyte and macrophage pyroptosis [116–119]. The latter property is the basis for its current clinical development by Bioxcel Therapeutics (rebranded as BXCL701) as a treatment for neuroendocrine prostate cancer in combination with anti-PD1 (pembrolizumab) (Phase Ib/II: NCT03910660) or as a monotherapy in a phase 0 ‘window of opportunity’ trial of pancreatic cancer (NCT04123574). The broad activity of PT-100/BXCL701 against members of the DPP family is not necessarily bad, though it may limit the therapeutic index given the lack of tumor-specific or -selective expression for many of these enzymes. Despite these concerns, the results of these new combination trials with immune checkpoint blockade will be eagerly anticipated.
The repertoire of FAP-targeted strategies designed to kill FAP+ cells that have been explored preclinically is quite large, including antibody–drug conjugates, bispecific antibodies, vaccines, FAP-activated prodrugs and FAP-directed chimeric antigen receptors (CARs) [15,31]. The underlying rationale is that the restricted expression pattern and/or unique substrate specificity of FAP can be used to localize or selectively activate a cytotoxic molecule or immune response to the FAP+ TME. CARs are among the most advanced of these approaches thus far with several having been developed. Adoptive transfer of these anti-FAP CARs demonstrated efficacy against multiple preclinical tumor models including mesothelioma, melanoma, lung, colon, kidney and breast cancers [120–124]. One of these, again based on the F19 antibody, has already made it into early clinical testing with a first-in-man protocol for patients with malignant pleural mesothelioma (NCT01722149). This recently completed small study of four patients injected with re-directed FAP-specific T cells into the pleural effusion has only reported data from one patient reported thus far [125]. While no significant adverse events were reported, the trial was stopped prior to the estimated enrollment of six patients. There is preclinical evidence suggesting potential for significant toxicity with this approach as both a FAP-targeted CAR developed at the NIH by Rosenberg and colleagues in addition to conditional deletion of FAP+ cells using the diphtheria toxin-based approach described above produced cachexia and hematopoietic defects in recipient animals [122,126]. Other causes for potential concern regarding toxicity associated with FAP-targeted agents are related to FAP expression in pancreatic islet cells and fibroblastic reticular cells in lymph nodes [31,127]. It should be noted, however, that none of the other FAP-specific CARs or other FAP-targeted therapies described above produce similar toxicity, including when administered to some of the same models and genetic backgrounds. These discrepant results may be due to differences in the binding affinities and epitope specificities of antibodies used to generate the single chain variable fragments (scFv) that form the targeting moieties in these CAR constructs [123]. These differences may allow discrimination between FAP-high cells in the TME and FAP-low cells in normal tissues such as the BM and pancreas to spare toxicity in contrast to approaches that eliminate all or nearly all FAP+ cells in the body. Another possibility is that the total body irradiation preconditioning regimen used by Rosenberg et al. and not the other studies induced activation and proliferation of FAP+ MSCs in the BM, which support the hematopoietic stem cell (HSC) niche and aid in its recovery following irradiation [128]. Toxicity induced by eliminating rare HSC-supporting BM stromal cells was recently demonstrated using ROR1-targeted CARs following lymphodepletion with high dose irradiation or cyclophosphamide preconditioning regimens [129]. Additional considerations include species-specific differences in FAP biochemistry and cell biology between humans and mice. Most notably, the level of circulating FAP in mice is 20-fold that of humans [37]. The efficacy and safety profiles of other FAP-targeted CARs that are actively moving toward the clinic will be informative and guide future development of promising cell-based therapies directed against stromal targets.
In the case of FAP-activated prodrugs, the unique post prolyl endopeptidase activity of FAP is used to selectively activate a cytotoxic molecule within the TME following systemic administration in an inactive prodrug form to spare toxicity to normal host tissues [130,131]. This logic has been used to engineer a variety of FAP-targeted prodrugs based on different cytotoxic ‘warheads’, including melittin, doxorubicin, bufadienolide, desacetylvinblastine monohydrazide, emetine and thapsigargin [132–139]. Because FAP is predominantly expressed by stromal cells with a low proliferative index in vivo in mice and humans, molecules with a proliferation-independent mechanism of action are particularly important for stromal-targeted therapies [134]. These different prodrugs have demonstrated significant antitumor efficacy against multiple tumor types, including breast, hepatocellular, lung and prostate cancers [132–139]. Overall, these different prodrug platforms were well-tolerated and showed reduced toxicity compared the parent compounds despite some activation in peripheral tissues [132–139]. The contribution of FAP-specific versus non targeted activation in these non tumor tissues is unclear. Many of these prodrugs are based on short peptide (2–7 amino acid) sequences that are potentially proteolyzed by related family members based on overlapping substrate specificities as discussed above. This is particularly of concern with POP as the N-termini for most of these substrates were ‘capped’ in such a way as to prevent recognition by strict exopeptidases, such as DDPIV. Again, the expression profile and enzymatic activity of FAP, POP and other S9 family members in plasma and tissues across different age groups and disease states has not been adequately characterized. These data along with more rigorous evaluation of these strategies using FAP-knockout hosts will likely be very informative for determining the FAP-specific efficacy at disease sites versus normal organ toxicities to safely move these approaches forward in the clinic. Additional work characterizing the immunologic impact of targeting FAP-positive cells via prodrugs or other strategies, timing of dosing regimens to maximize therapeutic benefit and combination treatments that exploit unique vulnerabilities with these FAP-depleted tumors are also needed. Current studies performed using pharmacologic-, immunotoxin- or cell-based approaches in syngeneic models support a re-polarization of the tumor-infiltrating immune profile toward an antitumor phenotype, including increasing the CD8:CD4 ratio and the expression of cytotoxic effector molecules in addition to suppressing macrophage and MDSC infiltration [96,123,140,141]. These antitumor immune responses can potentially be further augmented in the context of an antitumor vaccine [141] or using FAP-targeted 4-1BB agonist bispecific antibodies [142,143].
FAP-directed radioconjugates for imaging & therapy
As noted above, the first translated anti-FAP agent, sibrotuzumab, was radiolabeled with beta particle and single photon-emitting Iodine-131 (131I). This enabled planar gamma camera imaging and single photon emission computed tomography (CT) imaging to delineate biodistribution of 131I-sibrotuzumab in patients with advanced FAP-positive malignancies. The radioconjugate demonstrated high specific uptake in sites of tumor >1.5 cm, usually by 1–2 days post injection. However, the radio conjugate was eliminated slowly from normal organs and blood pool, resulting in high background signal and limited imaging quality [111], which discouraged further development.
There is evidence suggesting that radiolabeled anti-FAP antibodies have antitumor potential in the preclinical setting. Two engineered anti-FAP antibodies, ESC11 and ESC14, labeled with the beta-emitting radionuclide Lutetium-177 (177Lu) have successfully extended survival of FAP-positive melanoma xenografts in nude mice [144]. Both antibodies induced rapid internalization of cell surface FAP, providing therapeutic impact by altering the TME. Emitted beta particles damaged targeted FAP-expressing cells and adjacent neighboring cells, which contributed to tumor suppression [144]. Beyond cancer, radiolabeled anti-FAP antibodies have been evaluated preclinically in other disease contexts as well, including inflammatory rheumatoid arthritis (RA), indicating the predictive value of FAP-targeted imaging [145–147]. Given the abundant FAP expression on fibroblast-like synoviocytes in RA, imaging studies using the anti-FAP antibody 28H1 labeled with Indium-111 (111In), Technetium-99 (99 mTc), or Zirconium-89 (89Zr) demonstrated selective accumulation in inflamed joints in mouse models. Radioactivity uptake was correlated with arthritis severity and can be quantified to monitor RA treatment efficacy [145–147].
Small molecule conjugates derived from the potent FAP-inhibitor (4-quinolinoyl)-gly-2-cyanopyrrolidine scaffold [38] have recently been modified with a variety of radioisotopes for imaging and therapy. This class of compounds are termed FAPI, and many have been rapidly evaluated in preclinical and clinical contexts already. From the diagnostic imaging perspective, Gallium-68 (68Ga)-FAPI positron emission tomography/CT (PET/CT) provides high-contrast images with quality comparable to that of the current clinical standard Fluorine-18 (18F)-FDG imaging. Proof-of-concept was provided by imaging metastatic breast and pancreatic cancer patients with 68Ga-FAPI-02, which demonstrated rapid accumulation in metastases [148]. 68Ga-FAPI-02 can be targeted to FAP-expressing cells with high affinity and then internalized rapidly. Its low uptake in normal tissues and fast clearance from circulation also contributed to high-contrast imaging [148]. To improve the uptake profile and increase tracer retention in tumors, a series of compounds based on FAPI-02 were developed – among them FAPI-04 has been most extensively investigated, which is based on a di-fluoronated proline analog of a highly specific small molecule FAPI [38]. Compared with 68Ga-FAPI-02, 68Ga-FAPI-04 showed higher accumulation and longer retention time in tumors without a significant increase in background activity [149]. Subsequently, 68Ga-FAPI-04 was evaluated in 80 patients with 28 different tumor types, including 13 prostate cancer patients, achieving remarkably high image contrast and excellent tumor delineation with PET/CT [150]. Further progress has been made with the recent development of FAPI-46, which has a longer tumor retention time than FAPI-04 [151]. Dosimetry and biodistribution studies of 68Ga-FAPI-46 PET imaging in cancer patients has generated favorable profiles with clinical diagnostic potential [151]. FAPI PET imaging identified tumors not found with conventional CT and MRI in patients with glioblastomas and head and neck cancers, providing additional information for subsequent treatment planning [152,153]. Traditional 18F-FDG PET imaging, which identifies cells with high glucose consumption, is limited by poor performance in some cancers including liver, pancreatic, brain and prostate cancers. As an orthogonal approach and due to its low background uptake in brain and liver [154], 68Ga-FAPI PET imaging may enable high contrast imaging of tumor types not sensitive to 18F-FDG. A pilot study in patients with suspected hepatic carcinoma demonstrated the diagnostic value of 68Ga-FAPI-04 PET imaging in this disease setting [155]. An additional advantage of this approach is that 68Ga-FAPI PET imaging is independent of blood sugar level, sparing patients from dietary preparation for 18F-FDG imaging. FAPI PET imaging can be performed 10 min to 1 h after injection, potentially reducing patient waiting time as well [154]. In addition to the quinoline-based FAPI derivatives, other FAPIs are emerging as promising imaging radiotracers, including UAMC1110-based compounds containing a squaramide linker, which have achieved comparable imaging quality relative to 68Ga-FAPI-04 in animal models [156].
From the therapeutic perspective, FAPI work as enzymatic inhibitors in the TME. Additionally, FAPI derivatives can be chelated with beta- or alpha-emitting radioisotopes to irradiate tumor cells. Beta particle-emitting Yttrium-90 (90Y)-FAPI-04 was administered to one patient with metastatic breast cancer and associated with pain reduction [149]. In mouse models bearing FAP-positive human pancreatic xenografts, alpha particle-emitting Actinium-225 (225Ac)-FAPI-04 treatment targeted the tumor stroma leading to significant tumor growth suppression [157]. To amplify therapeutic benefit, future work is expected to focus on increasing tumor uptake and prolongation of tumor retention time. Preclinical studies have raised some concerns with respect to potential toxicity related to FAP-targeted approaches. Specifically, the short residency time in tumors may require higher injected quantities, which may consequently incur radiotoxic effects on clearance organs such as the kidney and bladder. This does not necessarily preclude the administration of radiotracer quantities for imaging purposes. It should be noted that all clinical studies reported thus far have reported a favorable safety profile; however, rigorous, phased trials are required for definitive conclusions.
Conclusion & future perspective
The previous decade has witnessed an explosion of molecular information that we possess about cancer cells. While an appreciation for the role of the TME in tumor progression is well-documented, characterization of these cells which are crucial to cancer’s progression and lethality, have lagged behind. Emerging tools and techniques are finally enabling the interrogation of these heterogeneous cellular and acellular components with ever-increasing resolution. As these technologies and our ability to integrate the resulting data matures, deeper mechanistic insights into precisely defined pathways driving the pro and anti tumorigenic effects of these heterogeneous populations will continue to emerge. For example, are these distinct populations derived from tissue-resident sources with tissue-specific functions, or are they recruited from systemic sources such as the BM and subsequently polarized in response to local factors produced in the tissue or TME? Is there a hierarchical relationship among different mesenchymal cell lineages with stem, progenitor and terminally differentiated pools akin to the hematopoietic system? Are these more differentiated phenotypes stable, or do they exist along a dynamic continuum that are polarized and potentially re-polarized to perform niche- and damage-specific functions? Is FAP the best target to overcome stromal barriers to immuno-oncological responses, or are there others that are either more suitable or complementary? Evidence in support of each of these overlapping and non exclusive viewpoints has emerged over the last few years [17,29,158–161], but we as a community are just beginning to scratch the surface of these complex relationships. A greater understanding of the underlying biology at the mesenchymal-immune interface in both normal tissue repair and pathophysiological processes such as cancer will reveal novel targets for intervention that can be either disrupted or restored for therapeutic benefit.
Executive summary.
The tumor microenvironment (TME) is a central regulator of antitumor immune responses.
Fibroblast activation protein (FAP)-positive cells are key architects of the immunosuppressive TME.
Tumor-infiltrating FAP-expressing cells include mesenchymal stem cells, carcinoma-associated fibroblasts and tumor-associated macrophages.
FAP is a serine protease with unique substrate specificity and restricted expression pattern that can be exploited to reverse the immunosuppressive TME and promote effect antitumor immune responses.
Beyond FAP+ cells, the enzymatic activity of FAP is implicated in immunosuppression via a multiple mechanisms, potentially including activating latent reservoirs of TGF-β and VEGF sequestered in the extracellular matrix.
Multiple FAP-targeted strategies designed to kill FAP+ cells and/or inhibit its enzymatic activity have been developed, some of which have advanced into clinical evaluation.
Though well-tolerated and demonstrating tumor-targeting, these approaches have not been successful therapeutically thus far.
This may be due to a poor understanding of the biology underlying FAP and FAP+ cells that has impeded optimal development and implementation of these approaches.
The tumor-targeting potential of FAP-specific antibodies and small molecules in particular are rapidly being developed for imaging and monitoring purposes.
Additional stromal targets mediating the immunosuppressive TME are likely to be identified that can be evaluated to determine if an improved therapeutic index is available.
This will be enhanced through a greater understanding of the biology that exists at the intersection of an antitumor immune responses and the heterogeneous mesenchymal cells comprising the TME.
Acknowledgments
The authors would like to acknowledge the SKCCC Flow Cytometry and Tissue Services Cores supported by the SKCCC Cancer Center Support Grant [CCSG, (P30 CA006973)].
Footnotes
Author contributions
WN Brennen, DLJ Thorek, W Jiang, TE Krueger, SR Denmeade and JT Isaacs conceived of the idea and wrote the manuscript. WN Brennen, TE Krueger and L Antony performed the experiments. All authors reviewed and approved the manuscript.
Financial & competing interests disclosure
The work was supported by Abbvie (C109738FE, [WN Brennen]), Allegheny Health Network-Johns Hopkins University Cancer Research Fund (WN Brennen, DLJ Thorek), Emerson Collective Cancer Research Fund (643396, [WN Brennen, DLJ Thorek]), the Department of Defense (W81XWH-17-1-0528, [WN Brennen]), W81XWH-16-1-0410 (JT Isaacs, SR Denmeade), and the NIH-Prostate SPORE Grant (P50 CA058236, [SR Denmeade, JT Isaacs]). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol. Cancer Ther. 11(2), 257–266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplan AI, Sorrell JM. The MSC curtain that stops the immune system. Immunol. Lett. 2015) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 3.Krueger TE, Thorek DLJ, Meeker AK, Isaacs JT, Brennen WN. Tumor-infiltrating mesenchymal stem cells: drivers of the immunosuppressive tumor microenvironment in prostate cancer? Prostate 79(3), 320–330 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa A, Kieffer Y, Scholer-Dahirel A et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33(3), 463–479 e410 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Lambrechts D, Wauters E, Boeckx B et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 24(8), 1277–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Bartoschek M, Oskolkov N, Bocci M et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 9(1), 5150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elyada E, Bolisetty M, Laise P et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9(8), 1102–1123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon OJ, Zhang Y, Li Y et al. Functional heterogeneity of mouse prostate stromal cells revealed by single-cell RNA-Seq. iScience 13, 328–338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther. 5(12), 1640–1646 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Franco OE, Jiang M, Strand DW et al. Altered TGF-beta signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 71(4), 1272–1281 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohlund D, Handly-Santana A, Biffi G et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214(3), 579–596 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avery D, Govindaraju P, Jacob M, Todd L, Monslow J, Pure E. Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts. Matrix Biol. 67, 90–106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cremasco V, Astarita JL, Grauel AL et al. FAP delineates heterogeneous and functionally divergent stromal cells in immune-excluded breast tumors. Cancer Immunol. Res. 6(12), 1472–1485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei X, Zhang L, Zhou Z et al. Spatially restricted stromal Wnt signaling restrains prostate epithelial progenitor growth through direct and indirect mechanisms. Cell Stem Cell 24(5), 753–768 e756 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pure E, Blomberg R. Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics. Oncogene 37(32), 4343–4357 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal S, Brennen WN, Kole TP et al. Fibroblast activation protein peptide substrates identified from human collagen I derived gelatin cleavage sites. Biochemistry 47(3), 1076–1086 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennen WN, Zhang B, Kulac I et al. Mesenchymal stem cell infiltration during neoplastic transformation of the human prostate. Oncotarget 8(29), 46710–46727 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litvinov IV, Vander Griend DJ, Xu Y, Antony L, Dalrymple SL, Isaacs JT. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Res. 66(17), 8598–8607 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennen WN, Kisteman LN, Isaacs JT. Rapid selection of mesenchymal stem and progenitor cells in primary prostate stromal cultures. Prostate 76(6), 552–564 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beacham DA, Amatangelo MD, Cukierman E. Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr. Protoc. Cell Biol. 33, 10.19.11–10.19.21 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Aertgeerts K, Levin I, Shi L et al. Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein alpha. J. Biol. Chem. 280(20), 19441–19444 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Scanlan MJ, Raj BK, Calvo B et al. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc. Natl Acad. Sci. USA 91(12), 5657–5661 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghersi G, Dong H, Goldstein LA et al. Regulation of fibroblast migration on collagenous matrix by a cell surface peptidase complex. J. Biol. Chem. 277(32), 29231–29241 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Wonganu B, Berger BW. A specific, transmembrane interface regulates fibroblast activation protein (FAP) homodimerization, trafficking and exopeptidase activity. Biochim. Biophys. Acta 1858(8), 1876–1882 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J. Biol. Chem. 274(51), 36505–36512 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Horsman JA, Mannisto PT, Venalainen JI. On the role of prolyl oligopeptidase in health and disease. Neuropeptides 41(1), 1–24 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Bae S, Park CW, Son HK et al. Fibroblast activation protein alpha identifies mesenchymal stromal cells from human bone marrow. Br. J. Haematol. 142(5), 827–830 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell 9(1), 11–15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennen WN, Denmeade SR, Isaacs JT. Mesenchymal stem cells as a vector for the inflammatory prostate microenvironment. Endocr. Relat. Cancer 20(5), R269–290 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc. Natl Acad. Sci. USA 85(9), 3110–3114 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busek P, Hrabal P, Fric P, Sedo A. Co-expression of the homologous proteases fibroblast activation protein and dipeptidyl peptidase-IV in the adult human Langerhans islets. Histochem. Cell Biol. 143(5), 497–504 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Lee KN, Jackson KW, Christiansen VJ, Lee CS, Chun JG, Mckee PA. Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein. Blood 107(4), 1397–1404 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Zhen EY, Jin Z, Ackermann BL, Thomas MK, Gutierrez JA. Circulating FGF21 proteolytic processing mediated by fibroblast activation protein. Biochem. J. 473(5), 605–614 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunshee DR, Bainbridge TW, Kljavin NM et al. Fibroblast activation protein cleaves and inactivates fibroblast growth factor 21. J. Biol. Chem. 291(11), 5986–5996 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bracke A, Van Elzen R, Van Der Veken P, Augustyns K, De Meester I, Lambeir AM. The development and validation of a combined kinetic fluorometric activity assay for fibroblast activation protein alpha and prolyl oligopeptidase in plasma. Clin. Chim. Acta 495, 154–160 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Uitte De Willige S, Keane FM, Bowen DG et al. Circulating fibroblast activation protein activity and antigen levels correlate strongly when measured in liver disease and coronary heart disease. PLoS ONE 12(6), e0178987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keane FM, Yao TW, Seelk S et al. Quantitation of fibroblast activation protein (FAP)-specific protease activity in mouse, baboon and human fluids and organs. FEBS Open Bio. 4, 43–54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jansen K, Heirbaut L, Verkerk R et al. Extended structure-activity relationship and pharmacokinetic investigation of (4-quinolinoyl)glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J. Med. Chem. 57(7), 3053–3074 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Bainbridge TW, Dunshee DR, Kljavin NM, Skelton NJ, Sonoda J, Ernst JA. Selective homogeneous assay for circulating endopeptidase fibroblast activation protein (FAP). Sci. Rep. 7(1), 12524 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Decker A, Vliegen G, Van Rompaey D et al. Novel small molecule-derived, highly selective substrates for fibroblast activation protein (FAP). ACS Med. Chem. Lett. 10, 1173–1179 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes the development of highly specific activity-based probes for fibroblast activation protein (FAP) based on a previously developed small-molecule dipeptide inhibitor scaffold.
- 41.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc. Natl Acad. Sci. USA 87(18), 7235–7239 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F, Qi L, Liu B et al. Fibroblast activation protein overexpression and clinical implications in solid tumors: a meta-analysis. PLoS ONE 10(3), e0116683 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res. 8(9), 2912–2923 (2002). [PubMed] [Google Scholar]
- 44.Ayala G, Tuxhorn JA, Wheeler TM et al. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin. Cancer Res. 9(13), 4792–4801 (2003). [PubMed] [Google Scholar]
- 45.Blom S, Erickson A, Ostman A et al. Fibroblast as a critical stromal cell type determining prognosis in prostate cancer. Prostate 79(13), 1505–1513 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arnold JN, Magiera L, Kraman M, Fearon DT. Tumoral immune suppression by macrophages expressing fibroblast activation protein-alpha and heme oxygenase-1. Cancer Immunol. Res. 2(2), 121–126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tchou J, Zhang PJ, Bi Y et al. Fibroblast activation protein expression by stromal cells and tumor-associated macrophages in human breast cancer. Hum. Pathol. 44(11), 2549–2557 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aoyama A, Chen WT. A 170-kDa membrane-bound protease is associated with the expression of invasiveness by human malignant melanoma cells. Proc. Natl Acad. Sci. USA 87(21), 8296–8300 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldstein LA, Ghersi G, Pineiro-Sanchez ML et al. Molecular cloning of seprase: a serine integral membrane protease from human melanoma. Biochim. Biophys. Acta 1361(1), 11–19 (1997). [DOI] [PubMed] [Google Scholar]
- 50.Jacob M, Chang L, Pure E. Fibroblast activation protein in remodeling tissues. Curr. Mol. Med. 12(10), 1220–1243 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Sfanos KS, Yegnasubramanian S, Nelson WG et al. If this is true, what does it imply? How end-user antibody validation facilitates insights into biology and disease. Asian J. Urol. 6(1), 10–25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rettig WJ, Chesa PG, Beresford HR et al. Differential expression of cell surface antigens and glial fibrillary acidic protein in human astrocytoma subsets. Cancer Res. 46(12 Pt 1), 6406–6412 (1986). [PubMed] [Google Scholar]
- 53.Christiansen VJ, Jackson KW, Lee KN, Mckee PA. Effect of fibroblast activation protein and alpha2-antiplasmin cleaving enzyme on collagen types I, III, and IV. Arch. Biochem. Biophys. 457(2), 177–186 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grindel BJ, Martinez JR, Pennington CL et al. Matrilysin/matrix metalloproteinase-7(MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. Matrix Biol. 36, 64–76 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang HE, Hamson EJ, Koczorowska MM et al. Identification of novel natural substrates of fibroblast activation protein-alpha by differential degradomics and proteomics. Mol. Cell Proteomics 18(1), 65–85 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coppage AL, Heard KR, Dimare MT et al. Human FGF-21 is a substrate of fibroblast activation protein. PLoS ONE 11(3), e0151269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong PF, Gall MG, Bachovchin WW, Mccaughan GW, Keane FM, Gorrell MD. Neuropeptide Y is a physiological substrate of fibroblast activation protein: enzyme kinetics in blood plasma and expression of Y2R and Y5R in human liver cirrhosis and hepatocellular carcinoma. Peptides 75, 80–95 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 29(6), 313–326 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gong JH, Clark-Lewis I. Antagonists of monocyte chemoattractant protein 1 identified by modification of functionally critical NH2-terminal residues. J. Exp. Med. 181(2), 631–640 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ni W, Egashira K, Kitamoto S et al. New anti-monocyte chemoattractant protein-1 gene therapy attenuates atherosclerosis in apolipoprotein E-knockout mice. Circulation 103(16), 2096–2101 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Yang X, Lin Y, Shi Y et al. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res. 76(14), 4124–4135 (2016). [DOI] [PubMed] [Google Scholar]; • A FAP-STAT3-CCL2 signaling axis in carcinoma-associated fibroblasts drives an inflammatory program that promotes tumor growth in part via increased recruitment of myeloid-derived suppressor cells.
- 62.Lin Y, Li B, Yang X et al. Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment. Neoplasia 21(12), 1133–1142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.David CJ, Massague J. Contextual determinants of TGFbeta action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 19(7), 419–435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lodyga M, Hinz B. TGF-beta1 - a truly transforming growth factor in fibrosis and immunity. Semin. Cell Dev. Biol. 2019) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 65.Koczorowska MM, Tholen S, Bucher F et al. Fibroblast activation protein-alpha, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations. Mol. Oncol. 10(1), 40–58 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brennen WN, Denmeade SR. Fibroblast activation protein (FAP) as a mediator of TGF-beta activation (Abstract #1125). Presented at: 100th AACR Annual Meeting. Denver, CO, USA: (April 18-22, 2009). [Google Scholar]
- 67.Vlodavsky I, Eldor A, Hyam E, Atzom R, Fuks Z. Platelet interaction with the extracellular matrix produced by cultured endothelial cells: a model to study the thrombogenicity of isolated subendothelial basal lamina. Thromb. Res. 28(2), 179–191 (1982). [DOI] [PubMed] [Google Scholar]
- 68.Benezra M, Vlodavsky I, Ishai-Michaeli R, Neufeld G, Bar-Shavit R. Thrombin-induced release of active basic fibroblast growth factor-heparan sulfate complexes from subendothelial extracellular matrix. Blood 81(12), 3324–3331 (1993). [PubMed] [Google Scholar]
- 69.Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J. Biol. Chem. 270(9), 4689–4696 (1995). [DOI] [PubMed] [Google Scholar]
- 70.Beacham DA, Amatangelo MD, Cukierman E. Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr. Protoc. Cell Biol. 10.9 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Webber MM, Trakul N, Thraves PS et al. A human prostatic stromal myofibroblast cell line WPMY-1: a model for stromal-epithelial interactions in prostatic neoplasia. Carcinogenesis 20(7), 1185–1192 (1999). [DOI] [PubMed] [Google Scholar]
- 72.Hyytiainen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit. Rev. Clin. Lab. Sci. 41(3), 233–264 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Taipale J, Saharinen J, Hedman K, Keski-Oja J. Latent transforming growth factor-beta 1 and its binding protein are components of extracellular matrix microfibrils. J. Histochem. Cytochem. 44(8), 875–889 (1996). [DOI] [PubMed] [Google Scholar]
- 74.Batlle E, Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity 50(4), 924–940 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neptune ER, Frischmeyer PA, Arking DE et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 33(3), 407–411 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J. Biol. Chem. 280(9), 7409–7412 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB. Latent TGF-beta-binding proteins. Matrix Biol. 47, 44–53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J. Cell Sci. 116(Pt 2), 217–224 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Williams SA, Singh P, Isaacs JT, Denmeade SR. Does PSA play a role as a promoting agent during the initiation and/or progression of prostate cancer? Prostate 67(3), 312–329 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc. Natl Acad. Sci. USA 88(2), 580–584 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koli K, Saharinen J, Hyytiainen M, Penttinen C, Keski-Oja J. Latency, activation, and binding proteins of TGF-beta. Microsc. Res. Tech. 52(4), 354–362 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Wakefield LM, Winokur TS, Hollands RS, Christopherson K, Levinson AD, Sporn MB. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J. Clin. Invest. 86(6), 1976–1984 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vehvilainen P, Hyytiainen M, Keski-Oja J. Latent transforming growth factor-beta-binding protein 2 is an adhesion protein for melanoma cells. J. Biol. Chem. 278(27), 24705–24713 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Mueller SC, Ghersi G, Akiyama SK et al. A novel protease-docking function of integrin at invadopodia. J. Biol. Chem. 274(35), 24947–24952 (1999). [DOI] [PubMed] [Google Scholar]
- 85.Kennedy A, Dong H, Chen D, Chen WT. Elevation of seprase expression and promotion of an invasive phenotype by collagenous matrices in ovarian tumor cells. Int. J. Cancer 124(1), 27–35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 267(36), 26031–26037 (1992). [PubMed] [Google Scholar]
- 87.Ferrara N, Gerber HP, Lecouter J. The biology of VEGF and its receptors. Nat. Med. 9(6), 669–676 (2003). [DOI] [PubMed] [Google Scholar]
- 88.Genersch E, Ferletta M, Virtanen I, Haller H, Ekblom P. Integrin alphavbeta3 binding to human alpha5-laminins facilitates FGF-2- and VEGF-induced proliferation of human ECV304 carcinoma cells. Eur. J. Cell Biol. 82(3), 105–117 (2003). [DOI] [PubMed] [Google Scholar]
- 89.Da Silva RG, Tavora B, Robinson SD et al. Endothelial alpha3beta1-integrin represses pathological angiogenesis and sustains endothelial-VEGF. Am. J. Pathol. 177(3), 1534–1548 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brennen WN, Nguyen H, Dalrymple SL et al. Assessing angiogenic responses induced by primary human prostate stromal cells in a three-dimensional fibrin matrix assay. Oncotarget 7(44), 71298–71308 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J. Biol. Chem. 270(21), 12607–12613 (1995). [DOI] [PubMed] [Google Scholar]
- 92.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 16(5), 585–601 (2008). [DOI] [PubMed] [Google Scholar]
- 93.Santos AM, Jung J, Aziz N, Kissil JL, Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J. Clin. Invest. 119(12), 3613–3625 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• FAP-null mice were used to provide proof-of-principle data documenting that targeting the tumor microenvironment can provide therapeutic benefit in epithelial-derived solid tumors via disrupting stromagenesis.
- 94.Chakravarthy A, Khan L, Bensler NP, Bose P, De Carvalho DD. TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 9(1), 4692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiao S, Subudhi SK, Aparicio A et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 179(5), 1177–1190 e1113 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Kraman M, Bambrough PJ, Arnold JN et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 330(6005), 827–830 (2010). [DOI] [PubMed] [Google Scholar]; •• Depletion of FAP-expressing cells via a transgenic diphtheria toxin-based approach promoted immunologic control of tumor growth in an IFN-γ- and TNF-α-dependent manner.
- 97.Feig C, Jones JO, Kraman M et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110(50), 20212–20217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• CXCL12 derived from carcinoma-associated fibroblasts inhibits T-cell trafficking to the tumor and pharmacologic inhibition of CXCR4 enhanced immune checkpoint blockade efficacy.
- 98.Lo A, Li CP, Buza EL et al. Fibroblast activation protein augments progression and metastasis of pancreatic ductal adenocarcinoma. JCI Insight 2(19), e92232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Metelli A, Wu BX, Riesenberg B et al. Thrombin contributes to cancer immune evasion via proteolysis of platelet-bound GARP to activate LTGF-beta. Sci. Transl. Med. 12(525), eaay4860 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 15(10), 730–738 (2008). [DOI] [PubMed] [Google Scholar]
- 101.Wan M, Li C, Zhen G et al. Injury-activated transforming growth factor beta controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cells 30(11), 2498–2511 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y, Yu X, Lin S, Li X, Zhang S, Song YH. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 356(3), 780–784 (2007). [DOI] [PubMed] [Google Scholar]
- 103.Ponte AL, Marais E, Gallay N et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25(7), 1737–1745 (2007). [DOI] [PubMed] [Google Scholar]
- 104.Sun S, Albright CF, Fish BH et al. Expression, purification, and kinetic characterization of full-length human fibroblast activation protein. Protein Expr. Purif. 24(2), 274–281 (2002). [DOI] [PubMed] [Google Scholar]
- 105.Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch. Dermatol. Res. 298(9), 413–420 (2007). [DOI] [PubMed] [Google Scholar]
- 106.Jones EM, Cochrane CA, Percival SL. The effect of pH on the extracellular matrix and biofilms. Adv. Wound Care (New Rochelle) 4(7), 431–439 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fasciglione GF, Marini S, D'alessio S, Politi V, Coletta M. pH- and temperature-dependence of functional modulation in metalloproteinases. A comparison between neutrophil collagenase and gelatinases A and B. Biophys. J. 79(4), 2138–2149 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharpe JR, Harris KL, Jubin K, Bainbridge NJ, Jordan NR. The effect of pH in modulating skin cell behaviour. Br. J. Dermatol. 161(3), 671–673 (2009). [DOI] [PubMed] [Google Scholar]
- 109.Rettig WJ, Su SL, Fortunato SR et al. Fibroblast activation protein: purification, epitope mapping and induction by growth factors. Int. J. Cancer 58(3), 385–392 (1994). [DOI] [PubMed] [Google Scholar]
- 110.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315(26), 1650–1659 (1986). [DOI] [PubMed] [Google Scholar]
- 111.Scott AM, Wiseman G, Welt S et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res. 9(5), 1639–1647 (2003). [PubMed] [Google Scholar]
- 112.Hofheinz RD, Al-Batran SE, Hartmann F et al. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie 26(1), 44–48 (2003). [DOI] [PubMed] [Google Scholar]
- 113.Narra K, Mullins SR, Lee HO et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting Fibroblast Activation Protein in patients with metastatic colorectal cancer. Cancer Biol. Ther. 6(11), 1691–1699 (2007). [DOI] [PubMed] [Google Scholar]
- 114.Eager RM, Cunningham CC, Senzer N et al. Phase II trial of talabostat and docetaxel in advanced non-small cell lung cancer. Clin. Oncol. (R. Coll. Radiol.) 21(6), 464–472 (2009). [DOI] [PubMed] [Google Scholar]
- 115.Eager RM, Cunningham CC, Senzer NN et al. Phase II assessment of talabostat and cisplatin in second-line stage IV melanoma. BMC Cancer 9, 263 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coutts SJ, Kelly TA, Snow RJ et al. Structure-activity relationships of boronic acid inhibitors of dipeptidyl peptidase IV. 1. Variation of the P2 position of Xaa-boroPro dipeptides. J. Med. Chem. 39(10), 2087–2094 (1996). [DOI] [PubMed] [Google Scholar]
- 117.Adams S, Miller GT, Jesson MI, Watanabe T, Jones B, Wallner BP. PT-100, a small molecule dipeptidyl peptidase inhibitor, has potent antitumor effects and augments antibody-mediated cytotoxicity via a novel immune mechanism. Cancer Res. 64(15), 5471–5480 (2004). [DOI] [PubMed] [Google Scholar]
- 118.Lankas GR, Leiting B, Roy RS et al. Dipeptidyl peptidase IV inhibition for the treatment of type 2 diabetes: potential importance of selectivity over dipeptidyl peptidases 8 and 9. Diabetes 54(10), 2988–2994 (2005). [DOI] [PubMed] [Google Scholar]
- 119.Okondo MC, Johnson DC, Sridharan R et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat. Chem. Biol. 13(1), 46–53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kakarla S, Chow KK, Mata M et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol. Ther. 21(8), 1611–1620 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schuberth PC, Hagedorn C, Jensen SM et al. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J. Transl. Med. 11, 187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tran E, Chinnasamy D, Yu Z et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med. 210(6), 1125–1135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Adoptive transfer FAP-specific CAR T-cells induced cachexia and lethal bone marrow toxicity via elimination of bone marrow stromal cells, suggesting caution with FAP-targeted therapy.
- 123.Wang LC, Lo A, Scholler J et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2(2), 154–166 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Adoptive cell therapy with an independent FAP-targeted CAR based on the 73.3 monoclonal antibody produced significant antitumor effects with no evidence of toxicity, suggesting FAP-directed therapies are safe and effective but potentially context-dependent.
- 124.Lo A, Wang LS, Scholler J et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 75(14), 2800–2810 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gulati P, Ruhl J, Kannan A et al. Aberrant Lck signal via CD28 costimulation augments antigen-specific functionality and tumor control by redirected T cells with PD-1 blockade in humanized mice. Clin. Cancer Res. 24(16), 3981–3993 (2018). [DOI] [PubMed] [Google Scholar]
- 126.Roberts EW, Deonarine A, Jones JO et al. Depletion of stromal cells expressing fibroblast activation protein-alpha from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 210(6), 1137–1151 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Denton AE, Roberts EW, Linterman MA, Fearon DT. Fibroblastic reticular cells of the lymph node are required for retention of resting but not activated CD8+ T cells. Proc. Natl Acad. Sci. USA 111(33), 12139–12144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 16(3), 239–253 (2015). [DOI] [PubMed] [Google Scholar]
- 129.Srivastava S, Salter AI, Liggitt D et al. Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated toxicity to normal tissues and enables selective tumor targeting. Cancer Cell 35(3), 489–503 e488 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Denmeade SR, Isaacs JT. Engineering enzymatically activated “molecular grenades” for cancer. Oncotarget 3(7), 666–667 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Akinboye ES, Brennen WN, Denmeade SR, Isaacs JT. Albumin-linked prostate-specific antigen-activated thapsigargin- and niclosamide-based molecular grenades targeting the microenvironment in metastatic castration-resistant prostate cancer. Asian J. Urol. 6(1), 99–108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lebeau AM, Brennen WN, Aggarwal S, Denmeade SR. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol. Cancer Ther. 8(5), 1378–1386 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang S, Fang R, Xu J et al. Evaluation of the tumor targeting of a FAPalpha-based doxorubicin prodrug. J. Drug Target. 19(7), 487–496 (2011). [DOI] [PubMed] [Google Scholar]
- 134.Brennen WN, Rosen DM, Wang H, Isaacs JT, Denmeade SR. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J. Natl Cancer Inst. 104(17), 1320–1334 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brennen WN, Rosen DM, Chaux A, Netto GJ, Isaacs JT, Denmeade SR. Pharmacokinetics and toxicology of a fibroblast activation protein (FAP)-activated prodrug in murine xenograft models of human cancer. Prostate 74(13), 1308–1319 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Akinboye ES, Brennen WN, Rosen DM, Bakare O, Denmeade SR. Iterative design of emetine-based prodrug targeting fibroblast activation protein (FAP) and dipeptidyl peptidase IV DPPIV using a tandem enzymatic activation strategy. Prostate 76(8), 703–714 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deng LJ, Wang LH, Peng CK et al. Fibroblast activation protein alpha activated tripeptide bufadienolide antitumor prodrug with reduced cardiotoxicity. J. Med. Chem. 60(13), 5320–5333 (2017). [DOI] [PubMed] [Google Scholar]
- 138.Chen M, Lei X, Shi C et al. Pericyte-targeting prodrug overcomes tumor resistance to vascular disrupting agents. J. Clin. Invest. 127(10), 3689–3701 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang S, Zhang Y, Zhong J, Pan Y, Cai S, Xu J. Toxicological profile and safety pharmacology of a single dose of fibroblast activation protein-alpha-based doxorubicin prodrug: in-vitro and in-vivo evaluation. Anticancer Drugs 29(3), 253–261 (2018). [DOI] [PubMed] [Google Scholar]
- 140.Fang J, Xiao L, Joo KI et al. A potent immunotoxin targeting fibroblast activation protein for treatment of breast cancer in mice. Int. J. Cancer 138(4), 1013–1023 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fang J, Hu B, Li S, Zhang C, Liu Y, Wang P. A multi-antigen vaccine in combination with an immunotoxin targeting tumor-associated fibroblast for treating murine melanoma. Mol. Ther. Oncolytics 3, 16007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Muller D, Frey K, Kontermann RE. A novel antibody-4-1BBL fusion protein for targeted costimulation in cancer immunotherapy. J. Immunother. 31(8), 714–722 (2008). [DOI] [PubMed] [Google Scholar]
- 143.Claus C, Ferrara C, Xu W et al. Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci. Transl. Med. 11(496), eaav5989 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fischer E, Chaitanya K, Wuest T et al. Radioimmunotherapy of fibroblast activation protein positive tumors by rapidly internalizing antibodies. Clin. Cancer Res. 18(22), 6208–6218 (2012). [DOI] [PubMed] [Google Scholar]
- 145.Laverman P, Van Der Geest T, Terry SY et al. Immuno-PET and immuno-SPECT of rheumatoid arthritis with radiolabeled anti-fibroblast activation protein antibody correlates with severity of arthritis. J. Nucl. Med. 56(5), 778–783 (2015). [DOI] [PubMed] [Google Scholar]
- 146.Van Der Geest T, Laverman P, Gerrits D et al. Liposomal treatment of experimental arthritis can be monitored noninvasively with a radiolabeled anti-fibroblast activation protein antibody. J. Nucl. Med. 58(1), 151–155 (2017). [DOI] [PubMed] [Google Scholar]
- 147.Van Der Geest T, Roeleveld DM, Walgreen B et al. Imaging fibroblast activation protein to monitor therapeutic effects of neutralizing interleukin-22 in collagen-induced arthritis. Rheumatology (Oxford) 57(4), 737–747 (2018). [DOI] [PubMed] [Google Scholar]
- 148.Loktev A, Lindner T, Mier W et al. A tumor-imaging method targeting cancer-associated fibroblasts. J. Nucl. Med. 59(9), 1423–1429 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lindner T, Loktev A, Altmann A et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J. Nucl. Med. 59(9), 1415–1422 (2018). [DOI] [PubMed] [Google Scholar]
- 150.Kratochwil C, Flechsig P, Lindner T et al. (68)Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J. Nucl. Med. 60(6), 801–805 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Small molecule radio conjugate derived from FAP inhibitor achieves selective tumor uptake and high image contrast with positron emission tomography/computed tomography in 80 patients with 28 different tumor types, demonstrating theranostics potential.
- 151.Meyer C, Dahlbom M, Lindner T et al. Radiation dosimetry and biodistribution of (68)Ga-FAPI-46 PET imaging in cancer patients. J. Nucl. Med. 2019) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Syed M, Flechsig P, Liermann J et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. Mol. Imaging 2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Windisch P, Rohrich M, Regnery S et al. Fibroblast activation protein (FAP) specific PET for advanced target volume delineation in glioblastoma. Radiother. Oncol. 150, 159–163 (2020). [DOI] [PubMed] [Google Scholar]
- 154.Giesel FL, Kratochwil C, Lindner T et al. (68)Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J. Nucl. Med. 60(3), 386–392 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shi X, Xing H, Yang X et al. Fibroblast imaging of hepatic carcinoma with (68)Ga-FAPI-04 PET/CT: a pilot study in patients with suspected hepatic nodules. Eur. J. Nucl. Med. Mol. Imaging 2020) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 156.Moon ES, Elvas F, Vliegen G et al. Targeting fibroblast activation protein (FAP): next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA(5m) chelators. EJNMMI Radiopharm. Chem. 5(1), 19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Watabe T, Liu Y, Kaneda-Nakashima K et al. Theranostics targeting fibroblast activation protein in the tumor stroma: (64)Cu and (225)Ac labelled FAPI-04 in pancreatic cancer xenograft mouse models. J. Nucl. Med. 2019) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE 5(4), e10088 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348(6230), 74–80 (2015). [DOI] [PubMed] [Google Scholar]
- 160.Yu VW, Scadden DT. Heterogeneity of the bone marrow niche. Curr. Opin. Hematol. 23(4), 331–338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chan CKF, Gulati GS, Sinha R et al. Identification of the human skeletal stem cell. Cell 175(1), 43–56 e21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]