Abstract
The physiological actions of estrogens are primarily mediated by the nuclear hormone receptors estrogen receptor alpha (ERα) and beta (ERβ). Activities of these nuclear steroid hormone receptors in etiology and progression of many hormone-responsive cancers are well-established, yet the specific role of each receptor, and their various expressed isoforms, in estrogen-responsive cancers remains unclear. Recent advances in nuclear receptor profiling, characterization of expressed splice variants, and the availability of new experimental cancer models, has extended the understanding of the complex interplay between the differentially expressed nuclear estrogen receptors. In this review, we discuss proposed roles of ERβ in several subtypes of cancers that lack significant ERα expression and define current understanding of how different ERs collaborate to regulate cellular processes.
Introduction
The steroid hormone 17β-estradiol (E2) is the most potent physiological estrogen and is responsible for a myriad of functions, including cell growth and differentiation. Perturbations to estrogen signaling not only disrupt normal function and development, but are also involved in the initiation, progression, and severity of several types of estrogen responsive cancers [1–3]. The physiological actions of estrogens are largely mediated though the activities of nuclear hormone receptors NR3A1 (ERα) and NR3A2 (ERβ), which act in cell, tissue, and temporally specific fashions to regulate complex and dynamic gene-expression networks. ERα and ERβ share 96% homology in the DNA-binding region and 59% in the ligand-binding region (Figure 1) [4]. Although both full length receptors have comparable binding affinities for endogenous ligands (e.g. E2), they differ significantly in their affinity for various natural and synthetic ligands including phytoestrogens and pharmaceuticals (Table 1) [5]. Alternative mRNA splicing differentially regulates expression of ERα and ERβ isoforms that produce receptors with distinctive ligand-binding properties, subcellular localization, response to post-translational modification, and ligand-dependent and independent activities (Figure 1). Cell-specific expression patterns of both canonical receptor and alternatively spliced receptor isoforms play a role in mediating the diverse responsiveness to ligand binding [3].
Figure 1.
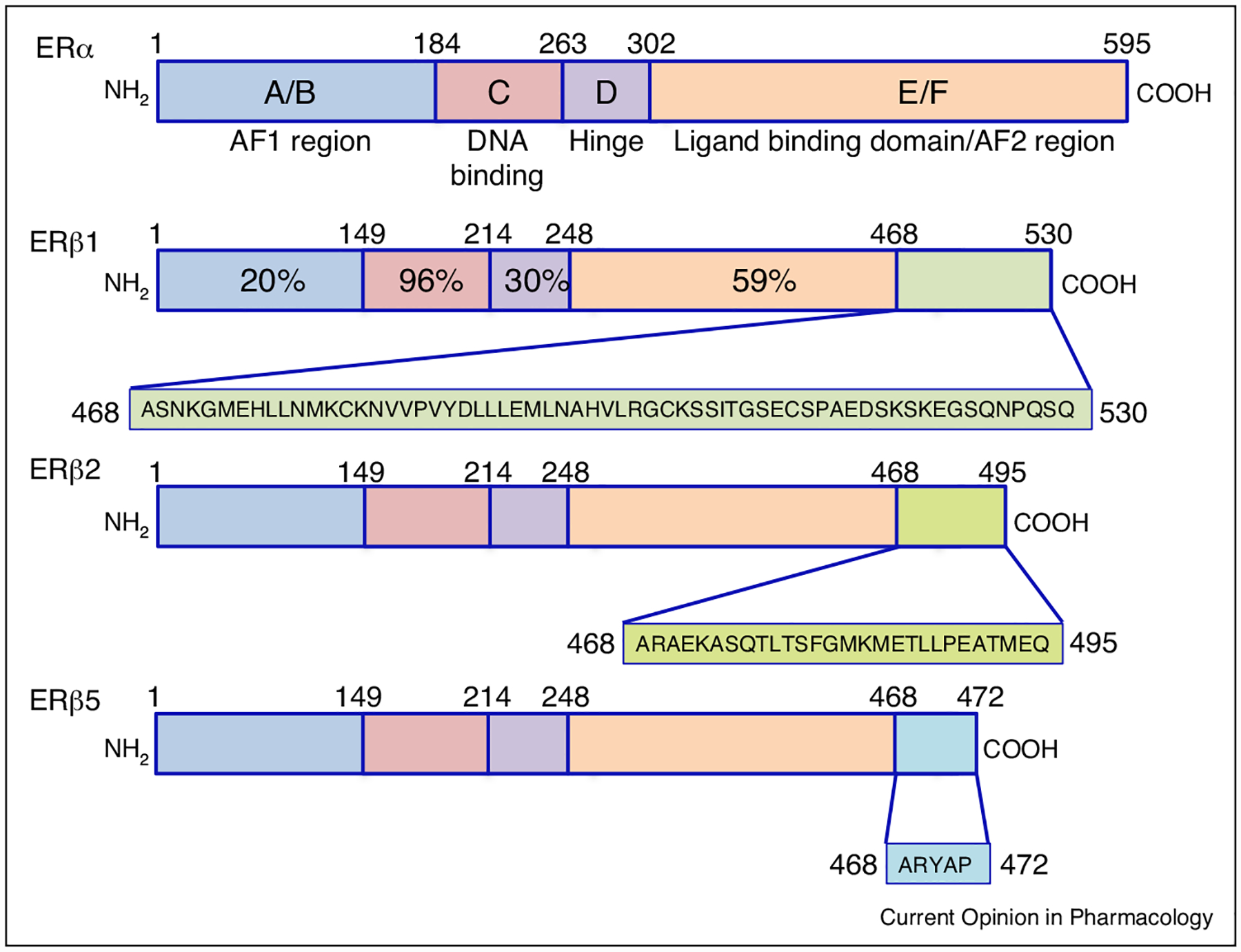
Comparative representation of ERβ isoforms. Each receptor is represented by a colored bar with structural domains conserved across the steroid/thyroid super-family of nuclear receptors indicated. Shading in the carboxyl terminus of each ERβ isoform is representative of the predicted differential amino acid sequences in each receptor isoform as a result of alternative splicing. Percentage on ERβ1 highlights the homology shared between each domain of ERα and ERβ1. The amino-terminal A/B regions have a transactivation domain with ligand-independent function and recruits co-activators and co-repressors. The C region contains the DNA-binding domain (DBD), which is needed for binding to specific estrogen response elements (EREs) in estrogen responsive genes. The D region contains several functional domains, including the hinge domain to connect C and E/F. The carboxy-terminal regions E and F contain the ligand-binding domain, which is needed for ligand binding, receptor dimerization, and nuclear translocation. ERβ variants are identical in the first 468 amino acid (AA) sequences, and divergence of AAs are shown after 468 in different colors and representative boxes underneath the variant protein structure.
Table 1.
Binding affinities of estrogenic ligands.
| Compounds | Ki ERα | EC50 ERα | IC50 ERα | Ki ERβ | EC50 ERβ | IC50 ERβ |
|---|---|---|---|---|---|---|
| Endogenous | ||||||
| 17-β-Estradiol | 0.04 | 0.017 | 0.12 | 0.11 | 0.068 | 0.18 |
| Estriol | 0.35 | 0.16 | – | 0.63 | 0.41 | – |
| Estrone | 1.01 | 0.66 | – | 3.1 | 1.6 | – |
| Phytoestrogens | ||||||
| Genistein | 126 | 38 | – | 12.8 | 5.8 | – |
| Coumestrol | 80 | 16 | – | 27 | 6.9 | – |
| Daidzein | 262 | 150 | – | 85.3 | 57 | – |
| Pharmaceuticals | ||||||
| PPT | 0.4 | 0.085 | – | 92.8 | Not detected | – |
| DPN | 32.4 | 27 | – | 1.7 | 2.3 | – |
| Fulvestrant (ICI 182 780) | 0.42 | – | 2.2 | 1.3 | – | 1.3 |
| 4-OH-tamoxifen | 2.3 | – | 2.2 | 4.8 | – | 1.1 |
Endogenous ligands, phytoestrogens, and pharmaceuticals inhibitor constant (Ki), half maximal effective concentration (EC50), and half maximal inhibitory concentration (IC50) for ERα and ERβ [78]. All concentration values are in nM.
Receptor-specific activities in cancer
ERα influences both tumor development and progression and is largely associated with poor prognosis and malignancy in breast, prostate, ovarian, and endometrial cancer [6]. In ER-positive (ER+) breast cancer (BC), ERα mediates estrogen actions and promotes tumor cell proliferation and metastasis [6]. Functional studies in ERα knockout mice have shown that ERα is required for the onset of mammary tumor development and prostate cancer progression [7–9]. In advanced stages of prostate cancer (PCa), ERα is up-regulated and can stimulate osteoblastic tumor growth in human PCa cell lines [10–12]. In these ER+ cancers, ERβ expression appears to oppose the growth promoting activities of estrogen, suggesting a dichotomous model in which ERα stimulates and ERβ suppresses estrogen-responsive tumors [13]. This view of ERβ as purely suppressive in cancer is inadequate, given the growing body of evidence demonstrating ERβ activation in ERα-negative cancers is proliferative [14,15].
Activities of ERβ in breast cancer
Increased endogenous and exogenous estrogens are a risk factor for the initiation and progression of BC [16–18], but tumor responsiveness to estrogens depends on nuclear receptor expression. Based on expression of ERα, progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), there are three major classes of BC tumors: Triple negative phenotypic, luminal (A and B), and human epidermal growth factor receptor 2 (HER2)-enriched [19]. ERβ is tumor suppressive in ERα positive (ER+) BC [20,21]. Several studies demonstrate that ERβ activation reduces proliferation [22] and angiogenesis in ER+ BC cell lines and tumor formation in mice [23].
Two subtypes of BC tumors are ERα negative (ER−): (1) HER2-enriched and (2) triple negative breast cancer (TNBC). Both subtypes are also progesterone receptor negative but differ in HER2 expression status [19]. Though TNBC lacks ERα, nuclear and extranuclear localized ERβ is expressed in TNBC tumor specimens [24,25], and both in vitro and in vivo studies have shown that ERβ is proliferative in the absence of ERα [26,27]. Disregarding the potential proliferative activities of ERβ in TNBC, a recent prospective, open label clinical trial evaluated efficacy of oral E2 (Estrace, 10 mg) in TNBC (www.clinicaltrial.gov Identifier: NCT01083641). As anticipated, the trial was unsuccessful and terminated with 65% of patients exhibiting disease progression after the first 28-day cycle of drug treatment [28]. Although death occurred in 88% of patients, and 77% were removed from the study treatment for disease progression, the authors argued that additional ERβ agonist treatment is recommended in TNBC. That interpretation is inconsistent with the study findings and understanding of current literature on the actions of ERβ in TNBC. Out-lined below, several studies highlight evidence for a proliferative role of ERβ in TNBC, and support contra-indication of ER agonists for TNBC pharmacotherapy.
High ERβ1 expression correlates with increased expression of the proliferative marker, Ki-67, which was also associated with poor prognosis in TNBC [29,30]. ERβ activation in ER− breast cancer stem cells (BSCs) increased mammosphere growth, and ERβ knockdown with shRNA in TNBC xenografts reduced tumor volume by 50%. Further, treatment of TNBC-derived BSCs with an ERβ selective antagonist (PHTPP) reduced mammosphere formation by 45% [31••] and suppressed TNBC growth in vitro [32•]. Additionally, treatment with the non-selective anti-estrogen Fulvestrant decreased tumor volume and cell proliferation in TNBC explants in two different ER− cell lines [MDA-MB-231 (ER−, high ERβ), MDA-MD-468 (ER−, low ERβ)] [33]. These seminal findings indicate that ERβ has proliferative activities in ER− BSCs and TNBC.
The differing roles of ERβ in BC are also dependent on ERβ splice variant expression; ERβ2 and ERβ5 do not possess a ligand-binding region but alter estrogen signaling through heterodimerization with ERβ1 and ERα. Recent meta-analysis evaluating ERβ1, ERβ2, and ERβ5 expression in BC patients (ER+/−) showed that ERβ1 was positively associated with increased 5-year overall (survival rate from study inclusion until patient death) and disease free (time of survival after primary treatment) survival [34•]. However, when the analysis was stratified by ERα status (+ or −), the positive association of ERβ1 with 5-year overall survival was lost, indicating that overall survival is dependent on the coexpression of ERα. In ER+ BC, increased cytoplasmic ERβ2 was associated with decreased overall survival [35]. Histologic and experimental data support a proliferative role for ERβ2 in TNBC cells [36•]; however, few studies have analyzed the association between ERβ variants in ER− BC.
One established mediator of ERβ actions in TNBC is increased insulin-like growth factor (IGF 1/2) expression and signaling [37]. IGF1/2 modulates expression of ERβ in TNBC, and inhibitors for both IGF1R (BMS-754807) and HER2 (neratinib) decreased cell proliferation in ER− cell lines, providing two potential therapeutic targets for ER− BC [38••].
ERβ actions in prostate cancer
Androgens and the androgen receptor (AR) are crucial to the development and progression of prostate cancer (PCa). The role of estrogens in suppressing androgenic actions (through the hypothalamic–pituitary–gonadal axis and negative luteinizing hormone feedback) in PCa has been noted for over 70 years [39]. The foundational studies of Huggins and Hodges showed that injection of E2 suppressed levels of testosterone in PCa patients and reduced the influence of androgens on PCa advancement [40,41]. Further epidemiological evidence indicates that the ratio of circulating testosterone to E2 is significant to the progression of PCa; low testosterone and/or high E2 was associated with higher risk of PCa. Whereas the importance of AR in PCa is well established, roles for other steroid receptors (ERβ) on AR and PCa progression remain controversial, as several studies present contradictory findings.
ERβ is expressed throughout normal prostate, while ERα expression is differentially localized to stromal cells and the androgen-independent basal cell layer [42]. ERβ expression is typically reduced in advanced stages of PCa [43,44]. ERβ activation in the prostate is typically tumor suppressive, with ERβ agonists displaying anti-proliferative activity in cell and mouse models of prostate cancer [45,46]. ERβ activation upregulated tumor repressor genes (PTEN, T-cadherin13, Smad7) and loss of ERβ in older mice (18 months) increased AR expression in the ventral prostate [47•]. This suggests that ERβ is an important potent regulator of AR signaling which acts to suppress tumor formation. Epidemiological evidence also suggests that diets high in phyotestrogens (ERβ selective partial agonists) may lower the risk of PCa [48,49].
However, a recent large cohort study positively associated expression of ERβ with reduced biochemical-failure-free survival supporting an adverse role for ERβ in PCa [50•]. Treatment with E2 in a xenograft model that only expressed ERβ (DU-145) elicited divergent effects wherein low doses of E2 increased tumor growth, and high E2 exposure decreased tumor volume [51•]. These seemingly contradictory results indicate differential responses of ERβ based on concentration of E2.
As with BC, possible explanations for the contradictory actions of ERβ in PCa are opposing actions of the ERβ2 isoform. ERβ2 expression was increased in advanced PCa and metastatic cancer and was associated with reduced overall survival in PCa patients [52]. In vitro models have also shown that ERβ2 can upregulate several proteins associated with osteolytic metastasis [53]. These findings suggest that ERβ2 can promote PCa tumor progression, although further work is necessary to clarify the differential roles of ERβ and its isoforms in PCa.
ERβ action in medulloblastoma
Estrogens play a critical role throughout cerebellar development by regulating gene expression and modulating growth factor related signal transduction pathways. Patterns of E2 binding and expression of both ERα and ERβ vary temporally and by cell type during cerebellar development in rodent models [54,55]. Wherein ERβ expression is highest during periods of cellular differentiation, ERβ is present in the rodent cerebellum from birth and persists in Purkinje cells, granule cells and other interneurons through adulthood. In the first postnatal week of development, expression of ERβ is localized primarily in post-mitotic granule cell precursors, with ERβ expression induced in Purkinje cells when migrating granule cells are nearby [55]. Similar patterns of ERβ expression are observed in the infant human cerebellum [56,57].
Medulloblastoma (MB) are a heterogeneous group of brain tumors that are associated with the posterior fossa or cerebellum and comprise nearly 10% of all childhood tumors [58]. Cerebellar MB arise from ERβ+ granule cell precursors (GCPs) which normally differentiate in the mitotically active external germinal layer of the cerebellum, and then migrate to form the internal granular layer [59]. GCPs are estrogen-responsive and express ERβ, but not ERα, and increased estrogen activation of ERβ signaling increases GCP mitogenesis, migration and upregulates neuroprotective mechanisms in mature granule cells [60].
MB tumors express ERβ, and dysregulation of ER signaling during cerebellar development drives MB progression [60]. Estrogen increased the growth of MB tumors in gonadectomized male and female mice with xenografts from a human cell line established from a 6-year old male’s tumor, D283Med [61], and in a mouse model of MB. Knockout of the ERβ gene (Esr2) inhibited MB growth in both sexes (Figure 2) [62••]. ERβ-mediated tumor development may be driven through a DNA repair mechanism, since ERβ-mediated nuclear interactions between proteins in the insulin-like growth factor (IGF) pathway inhibited homologous recombination-directed DNA repair in MB [63]. IGF signaling has also been implicated in tumorigenesis, and is key in the progression of MB [64]. The IGF pathway is upregulated in human MB [65], and Igf2 amplification can increase incidence and is required for progression of MB in mice [66]. IGF1R is a receptor tyrosine kinase that is considered a key target in high-risk metastatic MB [67], and tyrosine kinase inhibitors (TKIs) are an emerging treatment in MB [68•]. There are several ongoing clinical trials using erlotinib, a receptor TKI, to treat central nervous system tumors including MB (www.clinicaltrials.gov Identifiers: NCT00360854, NCT00077454, NCT02689336).
Figure 2.
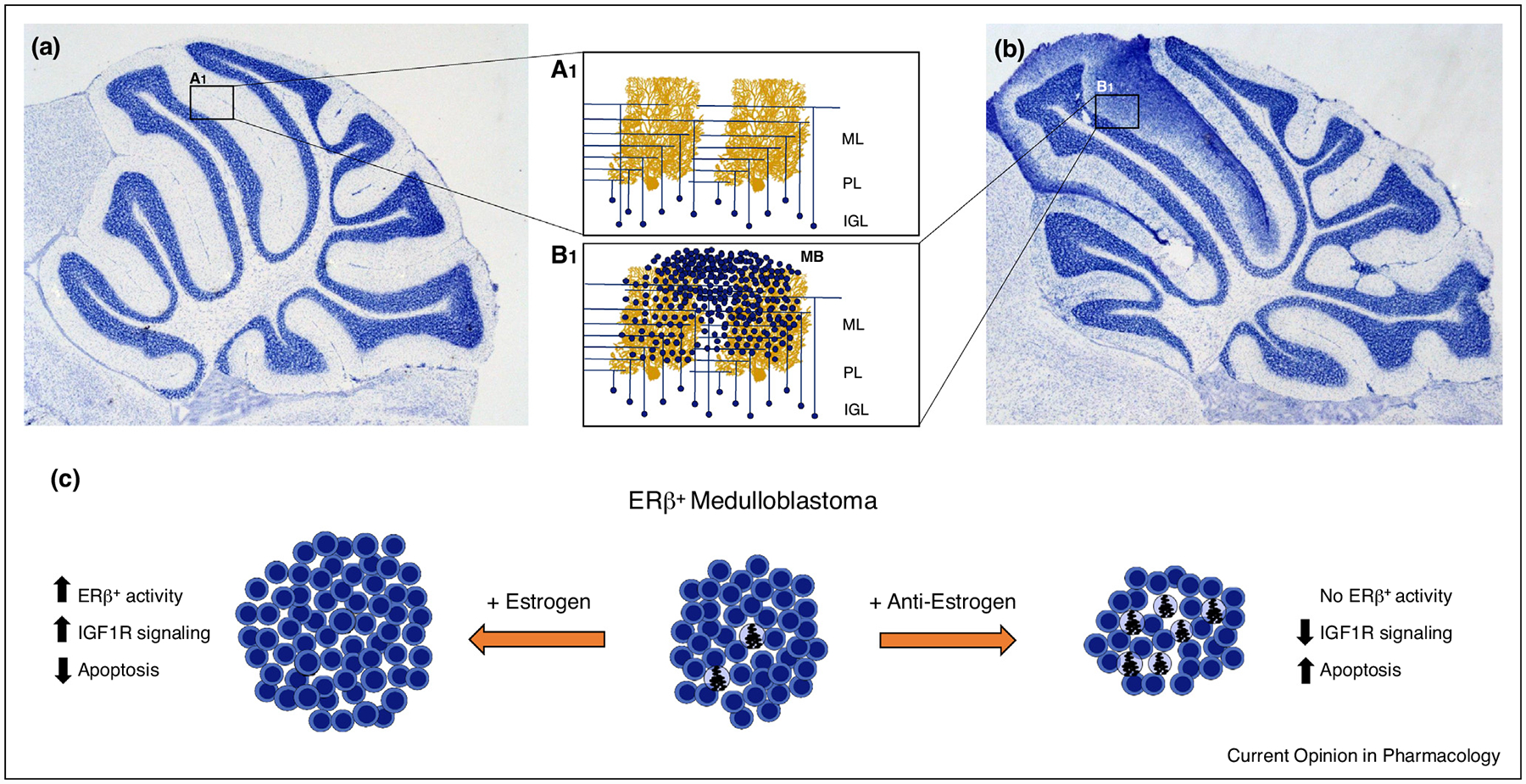
ERβ activation drives progression of medulloblastoma through cytoprotective and anti-apoptotic mechanisms. Granule cells in the IGL of the healthy mouse cerebellum (a) only weakly express ERβ, leading to a highly structured network of mossy fiber and parallel fiber synapses on Purkinje cell dendrites [A1]. Dysregulation of granule cell mitogenesis results in cerebellar overgrowth, excessive granule cell numbers in the IGL and tumor formation associated with the ML (b). In MB cells (c), ERβ is highly expressed and estrogen drives tumor formation and progression through cytoprotective mechanisms that decrease apoptosis. Addition of anti-estrogens (+ anti-estrogen) block the protective ERβ actions increases apoptosis resulting in decreased tumor size. Abbreviations: ML, molecular layer; PL, Purkinje cell layer; IGL, internal granular layer. Purkinje cell adapted from Cell Image Library CCDB_3687.
Blockade of ER signaling can inhibite both the growth and migration of MB through ERβ-mediated IGF-like signaling mechanisms [61]. Treatment of human MB cell lines with high doses of genistein that block TK activity and cisplatin show synergistic growth inhibition and cytotoxicity [69]. E2 and lower physiological levels of genistein and other dietary soy estrogens could decrease sensitivity of MB to cytotoxic cisplatin treatment. The cytoprotective effects of these estrogens was dependent on ERβ activity [70••]. Those findings support the therapeutic potential of anti-estrogen drugs and indicate that it may be prudent for patients to avoid environmental estrogen exposure during treatment. An ongoing clinical trial is using Tamoxifen, a selective estrogen receptor modulator, in combination with classical chemotherapy to treat patients with solid tumors including MB (www.clinicaltrials.gov Identifier NCT00002608); this study could provide further evidence that anti-estrogen activity improves patient outcomes.
Overall, MB occurs more often in males; however, the gender bias of incidence differs by population and tumor subtypes [71]. Contradicting the proliferative role of ERβ activation on MB development, tumors from females showed significantly lower growth and fewer proliferative markers in a mouse with D283Med human tumor xenografts [72]. In an ionizing radiation-induced mouse model, an ERβ-selective agonist inhibited MB development via anti-proliferative and pro-apoptotic pathways in ovariectomized females, while an ERα agonist had no effect [73]. The authors conclude that ERβ serves a tumor suppressive role, but an alternative explanation is that ERβ activation was cytoprotective against the ionizing radiation induction of GCPs in this model.
The physiological actions of ERβ in the cerebellum and on MB are modified by sex, ER splice variation, and neurotrophic and growth factors. Purkinje cells express aromatase and estrogens are synthesized de novo in the cerebellum, and this activity could be involved in reported sex differences. Tumors from both sexes expressed similar amounts of the ERβ isoforms ERβ2 and ERβ5, whereas ERβ1 was absent in tumors from males but persisted in females [72]. ERα and ERβ have several splice variants that present differentially across MB tumor tissue [72], but the clinical evaluation of specific ER isoforms in MB deserves further exploration. Immunohistochemical analysis of human cerebella revealed ERs in normal tissue and in primary MB tumor samples. In primary tumor samples, ERβ was detected in each sample, whereas ERα was essentially absent [61]. Additionally, primary tumors with the lowest levels of ERβ were associated with better clinical prognosis compared to tumors associated with the highest level of ERβ expression [74]. An alternative isoform in mouse reduced the ligand-mediated activity of the primary ERβ isoform, demonstrating that certain isoforms could modulate activity of other isoforms [75].
Secreted neurotrophic factors including brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) are highly expressed in granule and Purkinje cells and may be involved in mediating the expression of ERβ during cerebellar development [55]. BDNF acts on early GCPs in the EGL to promote commitment, followed by NT-3 to promote maturation into granule cells [76]. Growth factors also modify actions of ERβ activation on MB. As previously mentioned, the IGF pathway is up-regulated in MB, and activation of ERβ both promoted tumor proliferation and inhibited apoptosis of MB cells via up-regulation of IGF1 receptor expression and signaling activity [62••].
Conclusions
The general claim that ERβ is tumor-suppressive and ERα is tumor-promoting in cancer is incomplete, as a multifaceted environment of other coregulators, presence of other steroid receptors, transcriptional regulators, and endogenous and exogenous ligands influence ER actions. The role of ERβ in carcinogenesis or tumor suppression is highly dependent on the co-expression of ERα. In addition to expression of ERα, ERβ actions depend on the specific cell types (stem cells, stromal, and so on) and other estrogen modulators (IGF signaling).
Highlighting the importance of cell-type specific activities, in a comparison between BC stem cells (ERα−) and PCa stem cells (ERα+), treatment with an ERβ agonist elicited contrasting results. In the ERα-negative model, treatment induced cell proliferation and tumor development in mouse xenografts. Alternatively, in PCa stem cells, treatment with an ERβ agonist prompted apoptosis. These results highlight that presence or absence of ERα contributes to the duality of ERβ activities in stem cells.
IGF signaling is also an important modulator of estrogen in the etiology of ERβ cancers. In TNBC cells, IGF-2 increased expression of ERβ, and in a MB murine model, ERβ activation upregulated IGF-1 expression and activity. The extent to which NR/IGF crosstalk mechanisms influence hormone responsive cancers is uncertain and warrants further investigation.
Understanding the role of ERβ signaling in the development and progression of BC, PCa, and MB is critical to the development of therapeutic interventions. Blockade of ERβ has demonstrated anti-tumorigenic properties in cell and animal models; however, as demonstrated in this review and others, ERβ’s influence depends on multiple factors. Compensatory mechanisms of steroid receptors might require treatment of multiple levels of the steroid hormone pathway and other growth factors to reduce the proliferative role of estrogen in cancer.
Acknowledgements
This work was supported in part by North Carolina State University College of Sciences and by the National Institutes of Health NIEHS grant P30ES025128. This work was partially funded through an NIEHS-funded training grant: T32-ES07046.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Colditz GA: Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J Natl Cancer Inst 1998, 90:814–823. [DOI] [PubMed] [Google Scholar]
- 2.Hankinson SE, Colditz GA, Willett WC: The lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res 2004, 6:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas C, Gustafsson JÅ: The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer 2011, 11:597–608. [DOI] [PubMed] [Google Scholar]
- 4.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Treuter E, Warner M, Hartman J, Tujague M, Stro A: Estrogen receptors: how do they signal and what are their targets. Physiol Rev 2007, 87:905–931. [DOI] [PubMed] [Google Scholar]
- 5.Kuiper GGJM: Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor. Endocrinology 1998, 139:4252–4263. [DOI] [PubMed] [Google Scholar]
- 6.Pearce ST, Jordan VC: The biological role of estrogen receptors α and β in cancer. Crit Rev Oncol Hematol 2004, 50:3–22. [DOI] [PubMed] [Google Scholar]
- 7.Ali S, Coombes RC: Estrogen receptor alpha in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia 2000, 5:271–281. [DOI] [PubMed] [Google Scholar]
- 8.Bocchinfuso WP, Hively WP, Couse JF, Varmus HE, Korach KS: A mouse mammary tumor virus Wnt-1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor-alpha. Cancer Res 1999, 59:1869–1876. [PubMed] [Google Scholar]
- 9.Bocchinfuso WP, Korach KS: Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia 1997, 2:323–334. [DOI] [PubMed] [Google Scholar]
- 10.Bonkhoff H, Fixemer T, Hunsicker I, Remberger K: Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Am J Pathol 1999, 155:641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, Singh H, Ho SM: Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol 2001, 159:79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Royuela M, de Miguel MP, Bethencourt FR, Sanchez-Chapado M, Fraile B, Arenas MI, Paniagua R: Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J Endocrinol 2001, 168:447–454. [DOI] [PubMed] [Google Scholar]
- 13.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC: Estrogen receptor β inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res 2004, 64:423–428. [DOI] [PubMed] [Google Scholar]
- 14.Leygue E, Murphy LC: A bi-faceted role of estrogen receptor β in breast cancer. Endocr Relat Cancer 2013:20. [DOI] [PubMed] [Google Scholar]
- 15.Fox EM, Davis RJ, Shupnik MA: ERβ in breast cancer — onlooker, passive player, or active protector? Steroids 2008, 73:1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leygue E, Dotzlaw H, Watson PH, Leigh MC: Altered estrogen receptor α and β messenger RNA expression during human breast tumorigenesis. Cancer Res 1998, 58:3197. [PubMed] [Google Scholar]
- 17.Khan SA, Rogers MA, Khurana KK, Meguid MM, Numann PJ: Estrogen receptor expression in benign breast epithelium and breast cancer risk. J Natl Cancer Inst 1998, 90:37–42. [DOI] [PubMed] [Google Scholar]
- 18.Travis RC, Key TJ: Oestrogen exposure and breast cancer risk. Breast Cancer Res 2003, 5:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, Shi B: Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res 2015, 5:2929–2943. [PMC free article] [PubMed] [Google Scholar]
- 20.Omoto Y, Iwase H: Clinical significance of estrogen receptor? In breast and prostate cancer from biological aspects. Cancer Sci 2015, 106:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haldosén LA, Zhao C, Dahlman-Wright K: Estrogen receptor beta in breast cancer. Mol Cell Endocrinol 2014, 382:665–672. [DOI] [PubMed] [Google Scholar]
- 22.Ström A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson J-Å: Estrogen receptor β inhibits 17β-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci U S A 2004, 101:1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartman J, Lindberg K, Morani A, Inzunza J, Strom A, Gustafsson J-A: Estrogen receptor beta inhibits angiogenesis and growth of T47D breast cancer xenografts. Cancer Res 2006, 66:11207–11213. [DOI] [PubMed] [Google Scholar]
- 24.Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC: Expression of oestrogen receptor-β in oestrogen receptor-α negative human breast tumours. Br J Cancer 2006, 95:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanle EK, Onitilo AA, Huang W, Kim K, Zang C, Engel JM, Xu W, Wisinski KB: Prognostic significance of full-length estrogen receptor beta expression in stage I-III triple negative breast cancer. Am J Transl Res 2015, 7:1246. [PMC free article] [PubMed] [Google Scholar]
- 26.Piperigkou Z, Bouris P, Onisto M, Franchi M, Kletsas D, Theocharis AD, Karamanos NK: Estrogen receptor beta modulates breast cancer cells functional properties, signaling and expression of matrix molecules. Matrix Biol 2016, 56:4–23. [DOI] [PubMed] [Google Scholar]
- 27.Tonetti DA, Rubenstein R, DeLeon M, Zhao H, Pappas SG, Bentrem DJ, Chen B, Constantinou A, Jordan VC: Stable transfection of an estrogen receptor beta cDNA isoform into MDA-MB-231 breast cancer cells. J Steroid Biochem Mol Biol 2003, 87:47–55. [DOI] [PubMed] [Google Scholar]
- 28.Wisinski KB, Xu W, Tevaarwerk AJ, Saha S, Kim K, Traynor A, Dietrich L, Hegeman R, Patel D, Blank J: Targeting estrogen receptor beta in a phase 2 study of high-dose estradiol in metastatic triple-negative breast cancer: a Wisconsin Oncology Network Study. Clin Breast Cancer 2016, 16:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oueslati M, Bittaieb I, Sassi N, Jemaa AB, Gamoudi A, Rahal K, Oueslati R: ERα and ERβ co-expression: an indicator of aggressive tumors and hormonal sensitivity. Oncol Lett 2017, 14:1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keam B, Im S-A, Lee K-H, Han S-W, Oh D-Y, Kim JH, Lee S-H, Han W, Kim D-W, Kim T-Y et al. : Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res 2011, 13:R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. ••.Ma R, Karthik G-M, Lövrot J, Haglund F, Rosin G, Katchy A, Zhang X, Viberg L, Frisell J, Williams C et al. : Estrogen receptor β as a therapeutic target in breast cancer stem cells. J Natl Cancer Inst 2017, 109:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates the proliferative role of ER β in BSCs and shows that antagonist of ER β reduce tumor growth and mammosphere formation.
- 32. •.Hamilton N, Márquez-Garbán D, Mah V, Fernando G, Elshimali Y, Garbán H, Elashoff D, Vadgama J, Goodglick L, Pietras R: Biologic roles of estrogen receptor-β and insulin-like growth factor-2 in triple-negative breast cancer. Biomed Res Int 2015:2015. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work highlights the interplay between IGF-2 and ER β in vitro, specifically that IGF-2 upregulates expression of ER β in a triple negative breast cancer cell line.
- 33.Mishra AK, Abrahamsson A, Dabrosin C: Fulvestrant inhibits growth of triple negative breast cancer and synergizes with tamoxifen in ERα positive breast cancer by up-regulation of ERβ. Oncotarget 2016, 7:56876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. •.Liu J, Guo H, Mao K, Zhang K, Deng H, Liu Q: Impact of estrogen receptor-β expression on breast cancer prognosis: a meta-analysis. Breast Cancer Res Treat 2016, 156:149–162. [DOI] [PubMed] [Google Scholar]; This meta-analysis shows that ER β1 expression associates with improved overall and disease-free survival, but that overall survival association is dependent on the positive or negative status of ERα.
- 35.Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, Harkins L, Ellis IO, Robertson JF, Paish EC, Saunders PTK et al. : Nuclear and cytoplasmic expression of ERβ1, ERβ2, and ERβ5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res 2008, 14:5228–5235. [DOI] [PubMed] [Google Scholar]
- 36. •.Bialesova L, Xu L, Gustafsson J, Haldosen L-A, Zhao C, Dahlman-Wright K: Estrogen receptor ß2 induces proliferation and invasiveness of triple negative breast cancer cells; association with regulation of PHD3 and HIF-1α. Oncotarget 2017, 8:76622–76633. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demostrates that endogenous ERß2 is proliferative in triple negative breast cancer cells and that knockdown reduces cell invasion and proliferation.
- 37.Hamilton N, Mah V, Elshimali Y, Elashoff D, Garon E, Vadgama J, Pietras R, Angeles L, Comprehensive J, Angeles L et al. : Estrogen receptor-β and the insulin-like growth factor axis as potential targets for triple-negative breast cancer.. HHS Public Access 2017, 20:373–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. ••.Chakraborty A, Hatzis C, DiGiovanna MP: Co-targeting the HER and IGF/insulin receptor axis in breast cancer, with triple targeting with endocrine therapy for hormone-sensitive disease. Breast Cancer Res Treat 2017, 163:37–50. [DOI] [PubMed] [Google Scholar]; This paper provides evidence that inhibitors of HER and IGF work to reduce cell proliferation in ER− breast cancer cell lines, and that targeting these together show potential therapeutic targets for ER− breast cancer.
- 39.Nelson WG: Commentary on huggins and hodges: “studies on prostatic cancer.”. Cancer Res 2016, 76:186–187. [DOI] [PubMed] [Google Scholar]
- 40.Huggins C, Hodges CV: Studies of prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatase in metastatic carcinoma of the prostate. Arch Surg 1941, 43:209. [DOI] [PubMed] [Google Scholar]
- 41.Huggins C: Endocrine control of prostatic cancer. Science 1943, 97:541–544. [DOI] [PubMed] [Google Scholar]
- 42.Marker PC, Donjacour AA, Dahiya R, Cunha GR: Hormonal, cellular, and molecular control of prostatic development. Dev Biol 2003, 253:165–174. [DOI] [PubMed] [Google Scholar]
- 43.Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O’Neill G et al. : Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res 2001, 61:5331–5335. [PubMed] [Google Scholar]
- 44.Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P: Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer 2004, 11:537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dey P, Strom A, Gustafsson J-A: Estrogen receptor beta upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene 2014, 33:4213–4225. [DOI] [PubMed] [Google Scholar]
- 46.Hussain S, Lawrence MG, Taylor RA, Lo CY-W, Frydenberg M, Ellem SJ, Furic L, Risbridger GP: Estrogen receptor beta activation impairs prostatic regeneration by inducing apoptosis in murine and human stem/progenitor enriched cell populations. PLoS One 2012, 7:e40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. •.Wu W, Maneix L, Insunza J, Nalvarte I, Antonson P, Kere J, Yu NY-L, Tohonen V, Katayama S, Einarsdottir E et al. : Estrogen receptor β, a regulator of androgen receptor signaling in the mouse ventral prostate. Proc Natl Acad Sci 2017, 114: E3816–E3822. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes that ER β represses prostate cancer progression through upregulating tumor supressor genes, and that loss of ER β resulted in increased expression of genes associated with poor prognosis in prostate cancer.
- 48.Hori S, Butler E, McLoughlin J: Prostate cancer and diet: food for thought? BJU Int 2011, 107:1348–1359. [DOI] [PubMed] [Google Scholar]
- 49.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA: Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139:4252–4263. [DOI] [PubMed] [Google Scholar]
- 50. •.Grindstad T, Skjefstad K, Andersen S, Ness N, Nordby Y, Al-Saad S, Fismen S, Donnem T, Khanehkenari MR, Busund LT et al. : Estrogen receptors α and β and aromatase as independent predictors for prostate cancer outcome. Sci Rep 2016, 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that ERβ expression is associated with reduced time to biochemical failure in prostate tumor samples, and proposes a negative role for ERβ in PCa progression.
- 51. •.Nakajima Y, Osakabe A, Waku T, Suzuki T, Akaogi K, Fujimura T, Homma Y, Inoue S, Yanagisawa J: Estrogen exhibits a biphasic effect on prostate tumor growth through the ERβ-KLF5 pathway. Mol Cell Biol 2015, 36 MCB. 00625–15. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that different amounts of E2 elicit biphasic effects on prostate tumor growth controlled through actions of ERβ.
- 52.Leung Y-K, Lam H-M, Wu S, Song D, Levin L, Cheng L, Wu C-L, Ho S-M: Estrogen receptor 2 and 5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr Relat Cancer 2010, 17:675–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dey P, Jonsson P, Hartman J, Williams C, Ström A, Gustafsson J-Å: Estrogen receptors β1 and β2 have opposing roles in regulating proliferation and bone metastasis genes in the prostate cancer cell line PC3. Mol Endocrinol 2012, 26:1991–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ikeda Y, Nagai A: Differential expression of the estrogen receptors alpha and beta during postnatal development of the rat cerebellum. Brain Res 2006, 1083:39–49. [DOI] [PubMed] [Google Scholar]
- 55.Jakab RL, Wong JK, Belcher SM: Estrogen receptor beta immunoreactivity in differentiating cells of the developing rat cerebellum. J Comp Neurol 2001, 430:396–409. [DOI] [PubMed] [Google Scholar]
- 56.Belcher SM: Regulated expression of estrogen receptor alpha and beta mRNA in granule cells during development of the rat cerebellum. Brain Res Dev Brain Res 1999, 115:57–69. [DOI] [PubMed] [Google Scholar]
- 57.Belcher SM: Blockade of estrogen receptor signaling to improve outlook for medulloblastoma sufferers. Fut Oncol 2009, 5:751–754. [DOI] [PubMed] [Google Scholar]
- 58.Millard NE, De Braganca KC: Medulloblastoma. J Child Neurol 2016, 31:1341–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Altman J, Bayer SA: Development of the cerebellar system. [date unknown].
- 60.Belcher SM: Rapid signaling mechanisms of estrogens in the developing cerebellum. Brain Res Rev 2008, 57:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belcher SM, Ma X, Le HH: Blockade of estrogen receptor signaling inhibits growth and migration of medulloblastoma. Endocrinology 2009, 150:1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. ••.Cookman CJ, Belcher SM: Estrogen receptor-beta upregulates IGF1R expression and activity to inhibit apoptosis and increase growth of medulloblastoma. Endocrinology 2015, 156:2395–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper details how estrogen acts specifically through estrogen receptor beta to increase the growth and inhibit the apoptosis of tumor cells in medulloblastoma through growth factor signaling.
- 63.Urbanska K, Pannizzo P, Grabacka M, Croul S, Del Valle L, Khalili K, Reiss K: Activation of PPARalpha inhibits IGF-I-mediated growth and survival responses in medulloblastoma cell lines. Int J Cancer 2008, 123:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hartmann W, Koch A, Brune H, Waha A, Schuller U, Dani I, Denkhaus D, Langmann W, Bode U, Wiestler OD et al. : Insulin-like growth factor II is involved in the proliferation control of medulloblastoma and its cerebellar precursor cells. Am J Pathol 2005, 166:1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ajeawung NF, Wang HY, Gould P, Kamnasaran D: Advances in molecular targets for the treatment of medulloblastomas. Clin Invest Med 2012, 35:E246. [DOI] [PubMed] [Google Scholar]
- 66.Corcoran RB, Bachar Raveh T, Barakat MT, Lee EY, Scott MP: Insulin-like growth factor 2 is required for progression to advanced medulloblastoma in patched1 heterozygous mice. Cancer Res 2008, 68:8788–8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Svalina MN, Kikuchi K, Abraham J, Lal S, Davare MA, Settelmeyer TP, Young MC, Peckham JL, Cho Y-J, Michalek JE et al. : IGF1R as a key target in high risk, metastatic medulloblastoma. Sci Rep 2016, 6:27012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. •.Kumar V, Kumar V, McGuire T, Coulter DW, Sharp JG, Mahato RI: Challenges and recent advances in medulloblastoma therapy. Trends Pharmacol Sci 2017, 38:1061–1084. [DOI] [PubMed] [Google Scholar]; This review discusses medulloblastoma subtypes, difficulties in designing therapeutics, and recent advances in therapeutic option that show promise to improve outcomes with less toxic treatment.
- 69.Khoshyomn S, Manske GC, Lew SM, Wald SL, Penar PL: Synergistic action of genistein and cisplatin on growth inhibition and cytotoxicity of human medulloblastoma cells. Pediatr Neurosurg 2000, 33:123–131. [DOI] [PubMed] [Google Scholar]
- 70. ••.Belcher SM, Burton CC, Cookman CJ, Kirby M, Miranda GL, Saeed FO, Wray KE: Estrogen and soy isoflavonoids decrease sensitivity of medulloblastoma and central nervous system primitive neuroectodermal tumor cells to chemotherapeutic cytotoxicity. BMC Pharmacol Toxicol 2017, 18:63. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper highlights that dietary estrogens may inhibit the effectiveness of medulloblastoma therapeutics and should be considered when designing studies or treating patients.
- 71.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho Y-J, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A et al. : Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 2012, 123:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ciucci A, Meco D, De Stefano I, Travaglia D, Zannoni GF, Scambia G, Riccardi R, Saran A, Mancuso M, Gallo D: Gender effect in experimental models of human medulloblastoma: does the estrogen receptor B signaling play a role? PLoS ONE 2014:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mancuso M, Leonardi S, Giardullo P, Pasquali E, Borra F, De Stefano I, Prisco MG, Tanori M, Scambia G, Di Majo V et al. : The estrogen receptor beta agonist diarylpropionitrile (DPN) inhibits medulloblastoma development via anti-proliferative and pro-apototic pathways. Cancer Lett 2011, 308:197–202. [DOI] [PubMed] [Google Scholar]
- 74.Eberhart CG, Kepner JL, Goldthwaite PT, Kun LE, Duffner PK, Friedman HS, Strother DR, Burger PC: Histopathologic grading of medulloblastomas. Cancer 2002, 94:552–560. [DOI] [PubMed] [Google Scholar]
- 75.Donoghue LJ, Neufeld TI, Li Y, Arao Y, Coons LA, Korach KS: Differential activation of a mouse estrogen receptor β isoform (mER β 2) with endocrine-disrupting chemicals (EDCs). Environ Health Perspect 2017, 125:634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Segal RA, Takahashi H, McKay RD: Changes in neurotrophin responsiveness during the development of cerebellar granule neurons. Neuron 1992, 9:1041–1052. [DOI] [PubMed] [Google Scholar]


