To the Editor: The mRNA-1273 vaccine against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) elicited high neutralizing-antibody titers in phase 1 trial participants1,2 and has been shown to be highly efficacious in preventing symptomatic Covid-19 disease and severe disease.3 The emergence of SARS-CoV-2 variants in the United Kingdom (the B.1.1.7 lineage), South Africa (the B.1.351 lineage), Brazil (the P.1 lineage), and California (the B.1.427/B.1.429 lineage) has led to concerns about increased transmission and the potential of these variants to circumvent immunity elicited by natural infection or vaccination. The recent identification in the United Kingdom of a B.1.1.7 variant that includes the E484K mutation (B.1.1.7+E484K) furthers these concerns.
We assayed the neutralizing activity against recombinant vesicular stomatitis virus (rVSV)–based SARS-CoV-2 (a pseudovirus-based model) in serum samples obtained from eight participants in the phase 1 trial. The samples were obtained 1 week after the participants had received the second dose of mRNA-1273 vaccine. We tested pseudoviruses bearing the spike proteins from the original Wuhan-Hu-1 isolate, the D614G variant, and the B.1.1.7, B.1.351, P.1, B.1.427/B.1.429, B.1.1.7+E484K, and other variants (20E [EU1], 20A.EU2, N439K-D614G, and the mink cluster 5 variant that was first identified in Denmark).
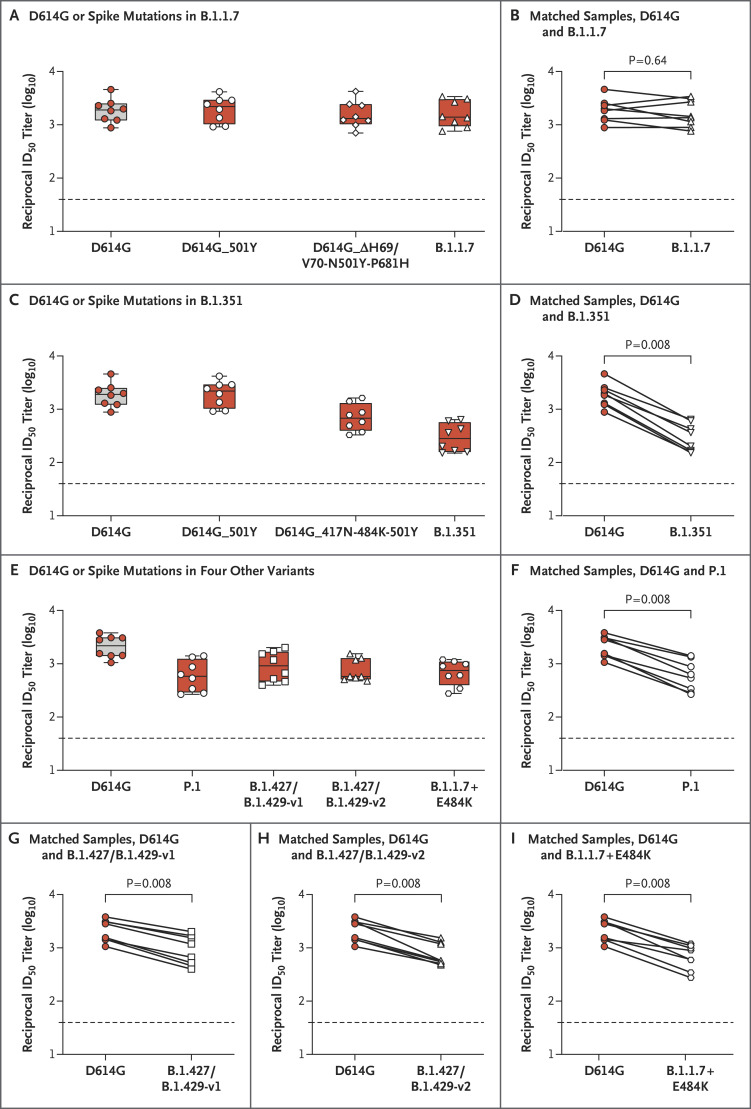
Both the full panel of mutations in S and a subset of mutations affecting the receptor-binding domain (RBD) region of the B.1.1.7 variant had no significant effect on neutralization by serum obtained from participants who had received the mRNA-1273 vaccine in the phase 1 trial (Figure 1A and 1B). In contrast, we observed a decrease in titers of neutralizing antibodies against the P.1 variant, the B.1.427/B.1.429 variant (versions 1 and 2), the B.1.1.7+E484K variant, and the B.1.351 variant as well as a subset of its mutations in the RBD. We detected reductions by a factor of between 2.3 and 6.4 in titers of neutralizing antibodies against this panel of variants (Figure 1C through 1I). The largest effect on neutralization, reduction by a factor of 6.4, was measured against the B.1.351 variant (Figure 1C and 1D). However, the geometric mean neutralizing titer against B.1.351 was 1:290, and all the serum samples fully neutralized the rVSV pseudovirus, albeit at relatively low dilutions (Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). The effect of the E484K mutation was observed by comparing neutralizing activity against the B.1.1.7 variant with neutralizing activity against the B.1.1.7+E484K variant. We found a significant reduction in neutralizing titers when the E484K mutation was present (Figure 1B and 1I). Using both rVSV and lentiviral neutralization assays, we observed a similar trend in serum samples obtained from macaque monkeys (Figs. S2 and S3).
Figure 1. Neutralization of SARS-CoV-2 Pseudoviruses in Serum Samples.
Serum samples obtained from participants who received the mRNA-1273 vaccine in a phase 1 trial were collected on day 36 (7 days after the participants received the second dose of the vaccine). Neutralization was measured with the use of a recombinant vesicular stomatitis virus (rVSV)–based pseudovirus neutralization assay that incorporated D614G or the indicated spike mutations present in the B.1.1.7 variant (Panels A and B), the B.1.351 variant (Panels C and D), or the P.1 variant, the B.1.427/B.1.429 (versions 1 and 2) variants, and the B.1.1.7+E484K variant (Panels E through I). The red dots indicate the results from serum samples of the individual participants; the white dots, white diamonds, and white triangles the same samples tested against the variants shown on the x axis; and the horizontal dashed lines the lower limit of quantification. The reciprocal neutralizing titers on the pseudovirus neutralization assay at a 50% inhibitory dilution (ID50) are shown. In Panels A, C, and E, boxes and horizontal bars denote the interquartile range (IQR) and the median neutralizing titer, respectively. Whisker end points are equal to the maximum and minimum values below or above the median at 1.5 times the IQR. In Panels B, D, F, G, H, and I, the lines connect the D614G and variant neutralization titers in matched samples. We detected reductions by a factor of 1.2 in titers of neutralizing antibodies against the B.1.1.7 variant (Panel B), a factor of 6.4 against the B.1.351 variant (Panel D), a factor of 3.5 against the P.1 variant (Panel F), a factor of 2.3 against the B.1.427/B.1.429-v1 variant (Panel G), a factor of 2.8 against the B.1.427/B.1.429-v2 variant (Panel H), and a factor of 3.1 against the B.1.1.7+E484K variant (Panel I). Statistical analysis of matched pairs was performed with the use of the Wilcoxon signed-rank test.
The rVSV-based pseudovirus neutralization assay was also used to assess the neutralizing activity of serum obtained from participants who had received the mRNA-1273 vaccine in the phase 1 trial against the full-length spike protein of the dominant strain in 2020 (D614G), as well as against 20E (EU1), 20A.EU2, N439K-D614G, and mink cluster 5 variants (Table S1). We observed levels of neutralization against these variants that were similar to those against the Wuhan-Hu-1 (D614) isolate (Fig. S4).
Protection conferred by the mRNA-1273 vaccine against the P.1, B.1.427/B.1.429, and B.1.351 variants remains to be determined. Our findings underscore the importance of continued viral surveillance and evaluation of vaccine efficacy against new variants and may help to facilitate the establishment of correlates of protection in both nonhuman primates and humans.
Supplementary Appendix
Disclosure Forms
Preliminary Version
A preliminary version of this letter was published on February 17, 2021, at NEJM.org. This letter was published on March 17, 2021, at NEJM.org.
Footnotes
Supported by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH); the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, Department of Health and Human Services (contract 75A50120C00034); and a research fellowship (to Dr. Corbett) that was partially funded by the Undergraduate Scholarship Program, Office of Intramural Training and Education, Office of the Director, NIH.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med 2020;383:1920-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020;383:2427-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.