Abstract
Deployment of RNA-guided DNA endonuclease CRISPR-Cas technology has led to radical advances in biology. As the functional diversity of CRISPR-Cas and parallel systems is further explored, RNA manipulation is emerging as a powerful mode of CRISPR-based engineering. In this Perspective, we chart progress in the RNA-targeting CRISPR-Cas (RCas) field and illustrate how continuing evolution in scientific discovery translates into applications for RNA biology and insights into mysteries, obstacles, and alternative technologies that lie ahead.
Mirroring prior efforts with DNA, biologists have leveraged nature’s molecular diversity to target RNA in living cells since the turn of the 21st century. In a breakthrough for RNA biology, studies showed that the MS2 bacteriophage viral coat protein (VCP) could be programmed along with its cognate RNA loop binding partner to image and stabilise mRNA in eukaryotic cells1,2. Three years later researchers converted a gene-expression inhibition system, RNA interference (RNAi), into one of the most widely applicable tools in the field3. For the next fifteen years, these two systems—VCP and RNAi—would come to define RNA targeting, even as other promising technologies rose from obscurity.
One such technology, CRISPR-Cas (clustered regularly inter-spaced short palindromic repeats and CRISPR-associated proteins) originates in prokaryotes, in which it acts as an adaptive immune system against phage invaders4. Canonically, Cas proteins and CRISPR RNA (crRNA) form a complex to catalyse the interference of foreign nucleic acids by recognising protospacer sequences mapping to spacer sequences present on crRNA5. CRISPR-Cas (often simply ‘CRISPR’) systems display enormous evolutionary diversity earned through postulated convergence, divergence, and horizontal gene transfer6. For instance, class 1 systems require multiple subunits for nucleic acid interference, whereas in class 2 systems an efficient single effector suffices. With an evolving classification nomenclature, unique class 1 and class 2 CRISPR systems have been found to target double-stranded DNA (dsDNA), single-stranded DNA (ssDNA) and/or single-stranded RNA (ssRNA)7.
Because of their potential for RNA programmability, built-in enzymatic interface, and remarkable ease of use, CRISPR systems have matured into an essential toolkit for genome engineering. Soon after reported uses of DNA-targeting CRISPR-Cas (which we term ‘DCas’, not to be confused with catalytically inactive Cas, ‘dCas’) in mammalian cells via Cas9 (ref.8), biology researchers applied DCas to high-throughput genomic screens and isogenic background mutant cell line generation, among other transformative applications9. Today the RCas field is seeing similar progress, driven by a bioinformatics race to discover and characterise CRISPR systems.
Discovery, diversity, and parallel systems
RCas identification.
Beyond adapting DCas systems to target RNA10, RCas identification has been accomplished through bioinformatic discovery (Fig. 1a)11. Whereas such computational analysis of metagenomic data can be generalised to encapsulate class 1 and class 2 systems12, in practice the single-effector, dual-component class 2 RCas systems hold promise as prospective transcriptomic engineering tools. For this reason, researchers have largely focused their CRISPR system searches on putative single effectors, initially proximal to a Cas1 gene essential to CRISPR adaptive immunity13, and now merely proximal to a repetitive motif sequence resembling a CRISPR array of ‘direct repeat’ sequences and intervening ‘spacer’ sequences mapping to phage genomes11,14, the two elements composing crRNA. After multiple constraint considerations, followed by grouping of putative effectors by sequence homology, and finally predictions of functional domains, a computational pipeline will ultimately yield a menu of Cas effector candidates.
Fig. 1 |. RNA-targeting CRISPR-Cas (RCas) and parallel systems.
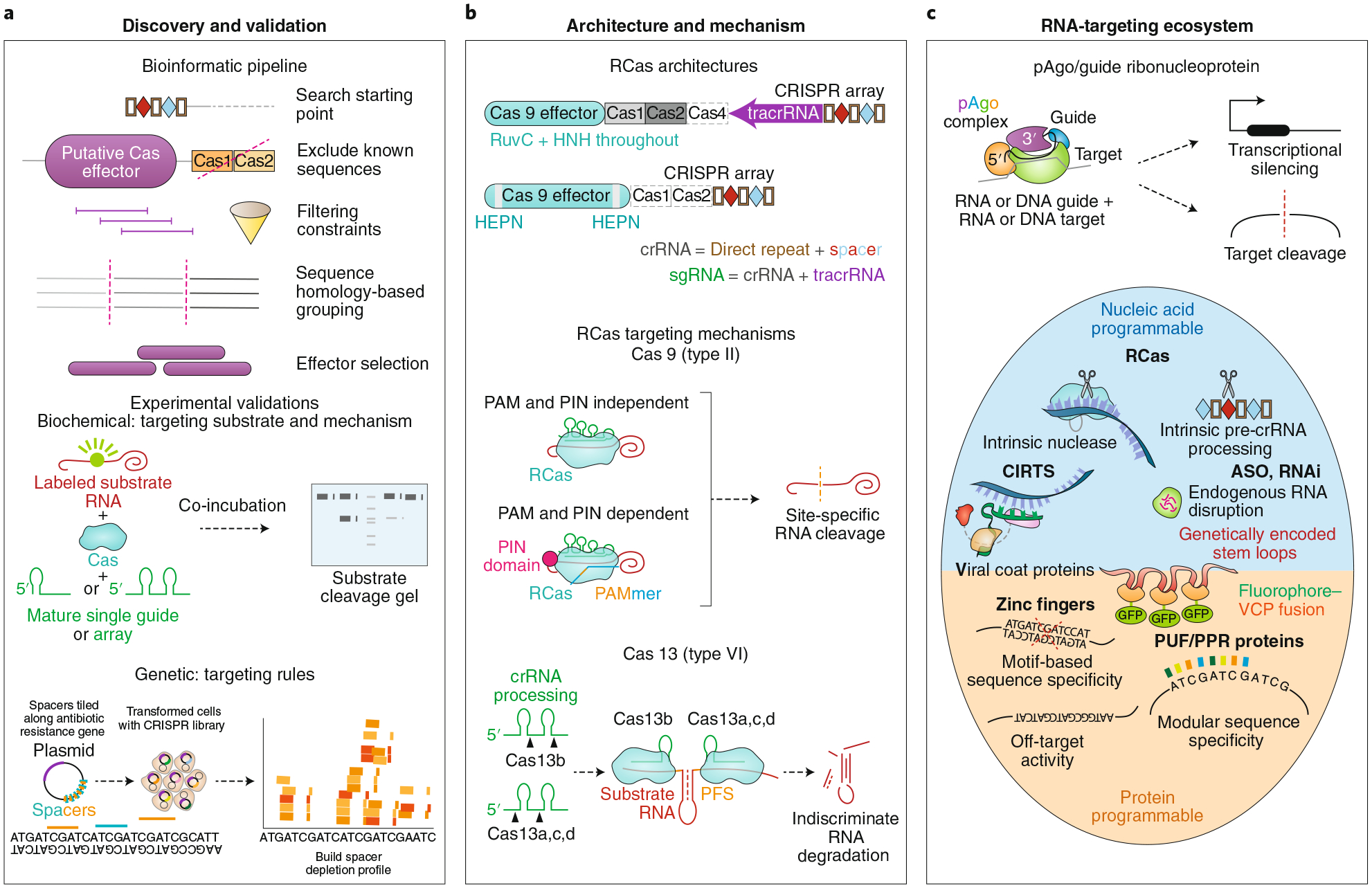
a, Cas proteins, including RCas, are discovered in metagenomic sequencing of bacteria and archaea using an established bioinformatic pipeline. Starting with a CRISPR array search seed, nearby putative Cas effectors are filtered on the basis of various criteria, including co-correlation with CRISPR arrays, size, and predicted protein domains. After grouping based on homology, distinct groups of effectors are selected for experimental validation follow-up. Researchers deduce the targeting substrate and mechanism through biochemical incubation of substrate nucleic acids with purified CRISPR ribonucleoprotein complexes and by observing resultant cleavage and binding. Genetic interrogation of targeting rules can be achieved through a spacer depletion screen assay consisting of a CRISPR library with spacers targeting an antibiotic resistance gene. Bacterial co-transformation via plasmids containing (i) the spacer library and Cas effector and (ii) the antibiotic resistance gene, followed by computational analysis of depleted spacers in the sequenced surviving population, will yield any targeting-dependent sequence requirements vis-à-vis the spacer. b, Type II and VI systems, encapsulating Cas9 and Cas13, respectively, represent the most commonly used RCas systems. Cas9 binds to a tracrRNA and crRNA (often combined into a single sgRNA) to target RNA. Depending on the Cas9 variant, a PAMmer oligonucleotide comprising a PAM sequence may be required for RNA binding10 and an additional domain (such as PIN domain endonuclease) may be required for RNA cleavage47. Cas13 binds to a crRNA for effective RNA targeting and subsequent indiscriminate cleavage. c, Prokaryotic Argonaute (pAgo) systems resemble CRISPR-Cas systems in their ability to target nucleic acids with a programmable guide. They exist as part of a broader RNA-targeting ecosystem that includes antisense oligonucleotides (ASOs), RNA interference (RNAi), CRISPR-Cas-inspired RNA targeting systems (CIRTS), viral coat proteins (VCPs), zinc fingers, Pumilio and FBF homology proteins (PUFs), and pentatricopeptide repeat proteins (PPRs), among others.
Each considered candidate must be experimentally validated to determine CRISPR functionality. If crRNA processing via the putative Cas effector is confirmed experimentally, researchers can readily identify the spacer length and direct repeat orientation. With this knowledge in hand, the Cas effector and its crRNA partner (and potentially transactivating crRNA or ‘tracrRNA’) can be co-expressed to interrogate the targeting substrate (DNA versus RNA), targeting mechanism (protein domains responsible), and targeting rules (substrate sequence preferences).
Researchers test for the targeting substrate by incubating various nucleic acids with biochemically reconstituted CRISPR systems and observing any resulting nucleic acid cleavage or binding. Once established, the targeting mechanism can be teased out by mutating amino acid residues in conserved protein domains that abolish activity. As for discerning targeting rules, in our view the most elegant assay to date (an evolution over previously described randomised PAM depletion screens and bacterial essential gene tiling screens14,15) involves the depletion of a CRISPR array library of spacers tiled against an antibiotic resistance gene plasmid16. In this logical integration of molecular biology, biochemistry, and genetics, researchers have systematically discovered and characterised RCas systems.
Diverse RCas platforms.
Class 1 RNA-targeting CRISPR systems, namely the Cmr complex (type III-B and type III-C)17 and Csm complex (type III-A and type III-D)18,19, have been well characterised. Due to their relative simplicity, however, the class 2 type II and VI loci embodied by Cas9 and Cas13, respectively, define the RCas transcriptomic engineering space (Fig. 1b).
Native Cas9 complexed with its crRNA and tracrRNA (both RNAs often combined into a single-guide RNA or ‘sgRNA’) necessitates a protospacer adjacent motif (PAM) to recognise and cleave its dsDNA target effectively20. Given this mechanism, researchers devised an RCas system by co-incubating the oligonucleotide PAMmer containing such a PAM with the remainder of the CRISPR system, thereby catalysing a single cleavage event in a target RNA10. Subsequently, a number of PAM-independent RNA-cleaving Cas9 systems have been characterised that exhibit a naturally ambiguous DCas and RCas functionality21, which may also be exploited for RNA targeting.
The type VI Cas13, unlike Cas9, has thus far been shown to target ssRNA exclusively7. Additionally, whereas the Cas9-crRNA-tracrRNA ribonucleoprotein utilises RuvC (endonuclease domain named for a DNA repair protein in Escherichia coli) and HNH (endonuclease domain with characteristic histidine and asparagine residues) catalytic domains to induce a single dsDNA break in a substrate, upon Cas13-crRNA complex recognition of target ssRNA containing a protospacer flanking sequence (PFS) the dual higher eukaryotes and prokaryotes nucleotide-binding domain (HEPN) domains of Cas13 initiate indiscriminate RNA cleavage in a substrate22. Furthermore, whereas Cas9 relies on endogenously expressed RNase III for crRNA processing, the Cas13 effector processes its own array23. Thus far several Cas13 variants have been discovered and characterised, with the Cas13b crRNA comprising a 5′ spacer and 3′ direct repeat like Cas9 (ref.14) and the Cas13a, Cas13c, and Cas13d crRNAs comprising a 3′ spacer and 5′ direct repeat16,22–24. Structures of Cas13 reveal further architectural distinctions among the characterised variants and offer blueprints for rationally designed molecular engineering25–27.
Non-Cas RNA-targeting systems.
RCas may be poised to dominate the RNA-targeting field, yet it remains one technology in an ever-expanding ecosystem divided roughly between nucleic acid-programmable and protein-programmable systems (Fig. 1c).
Like CRISPR, prokaryotic Argonaute (pAgo) systems are thought to have evolved to protect prokaryotes from phage invaders28. Programmable with either a DNA or RNA guide depending on the variant, pAgo has been demonstrated as an RNA-targeting tool29. Mechanistically similar to pAgo, eukaryotic Argonaute systems are limited to RNAi in engineering applications because of their endogenous origin and therefore inability to orthogonalise30. Another nucleic-acid-programmable technology, antisense oligonucleotides (ASOs), have been an effective means to target RNA as a consequence of their modified bases and lack of a critical protein component31. Finally, recent efforts to reprogram human RNA-programmable elements have led to the development of the CRISPR-Cas-inspired RNA-targeting systems (CIRTS) and RNA scaffolds that recruit endogenous factors32,33, though their specificity and sensitivity remain uncharacterised.
On the opposite end of the spectrum, researchers are considering single-component, protein-programmable methods for targeting RNA. Akin to DNA-targeting zinc finger nucleases, RNA motif-recognising zinc fingers have been successfully concatemerised to bind ssRNA34, although widespread use has thus far been hindered by limited identifiable RNA-targeting motifs. Composed of individual nucleotide-recognising subunits, tandem repeats of the more modular Pumilio and FBF homology proteins (PUFs) present a more feasible option35, as evidenced by contemporaneous research to a eukaryotic RCas study36. Akin to PUFs, pentatricopeptide repeat proteins (PPRs) could also be engineered37.
Currently, direct comparisons of RCas to non-Cas RNA-targeting systems are limited in scope, though two studies suggest higher RNA knockdown efficiency and specificity of Cas13 than short hairpin RNA (shRNA) expression on selected transcripts24,38. Each RNA-targeting system possesses a distinct set of modalities, and all possess attractive features independent of these modalities. For example, whereas ASOs must be delivered chemically or physically, RCas and the other systems can be vectorised for AAV delivery39. However, ASOs (and shRNAs) would escape protein-mediated immunogenicity issues. Although all of these systems will undoubtedly play roles in the burgeoning RNA-targeting field, in our opinion the genetically encoded, nucleic-acid-programmable, eukaryotic-orthogonal, dual-component RCas has the largest breadth in terms of applications.
Applications in basic research and industry
Biological.
RCas can be deployed to biological purposes ranging from detection (Fig. 2a) and modulation (Fig. 2b) to programming (Fig. 2c). In mammalian cells RCas9 was exploited to knock down mRNA and to track mRNA trafficking to stress granules40. Subsequent studies uncovered similar capabilities for Cas13a, Cas13b, and Cas13d24,38,41, with additional modalities explored. For example, similarly to ASOs, catalytically inactive Cas13 has been shown to disrupt the recognition of 5′ splice sites, 3′ splice sites, and branch points, leading to efficient exon exclusion in cultured cells24. With the fusion of an adenosine deaminase acting on RNA (ADAR) enzyme to Cas13, researchers have demonstrated programmable direct adenosine-to-inosine conversion41, although the efficiency and specificity of this site-directed RNA editing over other ADAR-fusion approaches have been strongly disputed42,43. In fact, site-directed ASOs or even genetically expressed single-component guide RNAs stand likely to outcompete RCas in this domain33,44,45.
Fig. 2 |. Applications of RCas in basic biology.
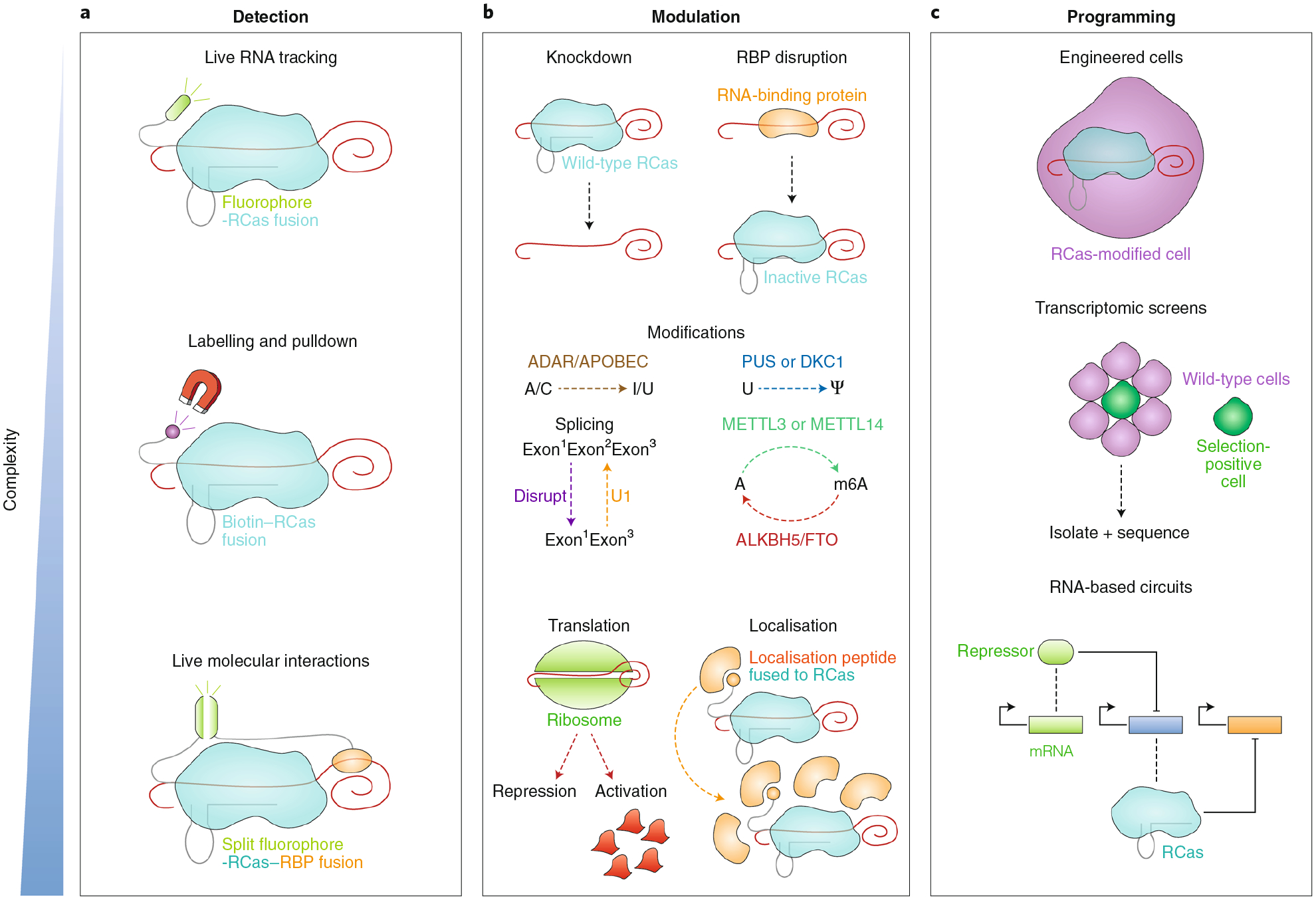
a, RCas can detect RNA in cells. Fusing a fluorophore to catalytically inactive RCas allows live-cell RNA tracking. Labelling the same RCas, for example, with biotin, followed by crosslinking enables pulldown of proximal proteins or RNA. Live protein-RNA interactions can be studied by fusing complementary split fluorophores to RCas and a protein. b, RCas can modulate RNA transcripts in cells. Expression of wild-type RCas results in RNA knockdown, whereas catalytically inactive RCas disrupts the binding of RBPs to RNA. Fusing an RNA-modifying effector to RCas enables the sequence modification of transcripts, such as A-to-I editing facilitated by the ADAR deaminase domain, U-to-pseudouridine editing by PUS (pseudouridine synthase) or DKC1 (dyskerin) enzymes, or m6A (methylation of adenine at the 6 position) by METTL3 or METTL14 enzymes. Other more challenging RNA modulations, such as translational activation/repression and localisation (by fusing a signal peptide to catalytically inactive RCas), could be achieved. c, RCas can be used to program cells. Researchers may engineer cells through any of the modulations previously described. RCas technologies can also be used to empower transcriptomic screens, facilitating selection of phenotypically positive cells among a pool of unperturbed wild-type cells. Finally, RCas can serve as a programmable element in RNA-based circuits, for example serving as a repressor for a transcript that in turn codes for an activating protein.
For most RCas applications, Cas9 may be indistinguishable from Cas13, with two notable exceptions. First, Cas13 interacts exclusively with RNA in its native context, whereas Cas9 may, depending on PAM (or PAMmer) recognition requirements and fusion of extra protein domains, competitively bind to both DNA and RNA21. Second, the self-processing CRISPR array capability of Cas13 enables multiplexing, as long as there remains sufficient RCas and individual guide expression.
Degradation or destabilisation of a target RNA is likely the most common application for researchers, and either the Cas13 (b or d) or Cas9-endonuclease platforms may be suited for this purpose. Although Cas13-mediated RNA knockdown has shown greater than 95% efficiency for multiple targets in human cells24,41, there remain concerns that catalytically active Cas13-induced prokaryotic cellular dormancy may have implications for eukaryotic cells46. Cas9-PIN (PilT N terminus) domain endonuclease fusions have likewise reduced certain repetitive RNA elements in human cells by greater than 95%47, but a comprehensive comparison of these systems on an identical set of transcripts and conditions has yet to emerge. With respect to other RCas modalities, researchers have achieved up to 30% endogenous RNA A-to-I editing with Cas13-ADAR fusions41. Given these results, RNA biology researchers would be well positioned to use RCas rather than ASOs or RNAi for targeting their RNA transcript of interest. CRISPR interference (CRISPRi), an alternative DCas-based methodology that represses gene activity on the transcriptional level, has shown more varied levels of efficacy that largely depend on guide RNA position48.
When considering an application involving RNA biology, researchers should assess whether RCas is in fact more advantageous than DCas. Despite the difficulty of predicting and identifying DNA off-target effects49, desired functions that demand a more sustained phenotype, such as systemic in vivo splicing modulation50, may benefit from the DCas platform instead. If, however, one is intent on using a reversible, graded-dosage, or an RNA substrate-specific (such as noncoding RNA) biological response, RCas offers many unique opportunities.
Among cellular RNA species, over 100 chemical modifications have been identified and are increasingly being implicated in a host of biological regulations51. It stands to reason that the majority of these modifications may be programmable via fusing their responsible enzymes to RCas. A group has recently developed a programmable m6A methylation-and-demethylation platform via a Cas9-METTL3 (methyltransferase like 3)/METTL14 (methyltransferase like 14) and Cas9-ALKBH5 (AlkB homolog 5, RNA demethylase)/FTO (fat mass and obesity-associated protein) fusion, sgRNA, and corresponding PAMmer52. Modulation of more intricate cellular processes, such as translational regulation and localisation, might require more complex engineering machinery to attain phenotypically significant changes. For example, researchers have programmed inducible recruitment of genomic DNA to subcellular compartments via catalytically inactive Cas9 (ref.53), and an analogous approach may be taken to study RNA cellular localisation.
As the scientific community expands its census of RNA-binding proteins (RBPs), RCas will become indispensable for dissecting RBP functionalities in various cell types. Of the more than 1,500 human RBPs curated54, hundreds have already been characterised via enhanced crosslinking and immunoprecipitation (eCLIP)55. Live-cell RBP–RNA tracking and labelling and pulldown similar to those in reported efforts with DCas and DNA-binding proteins56 will complement our understanding of RBP-RNA interactions.
Some of the most tantalising RCas applications involve programming RNA to compute functional outputs within cells. Assuming that sufficient RCas expression can be achieved, researchers may implement phenotypic RCas screens (e.g., transcriptome-wide RNA modification), similarly to previously reported DCas screens57, to uncover RNA-mediated pathways. In parallel to DCas gene circuits acting on DNA58, RCas gene circuits could control dynamic cellular processes while circumventing direct, irreversible genomic manipulation, a chief concern in human therapeutics and other sensitive biotechnological applications59.
Biotechnological.
Among the potential commercial applications for RCas (Fig. 3), nucleic acid detection may be the most feasible. As exploited in a proof of concept for Cas13a23, RCas diagnostic assays rely on the catalytic ability of type VI Cas effectors to degrade both target and collateral RNA with single-nucleotide sensitivity60. Combined with orthogonal sequence-specific cleavage preferences of various type VI orthologs61, this principle has given rise to fluorescence and colorimetric readouts—based on lateral flow detection—of multiple nucleic acid inputs in parallel with attomolar range sensitivity62 (Fig. 3a). Diagnostic technologies based on both type VI and type V Cas effectors that function analogously with respect to collateral ssDNA cleavage have efficiently identified different ZIKA, Dengue63, and human papilloma64 viral strains isolated from clinical samples.
Fig. 3 |. Opportunities for RCas in biotechnology.
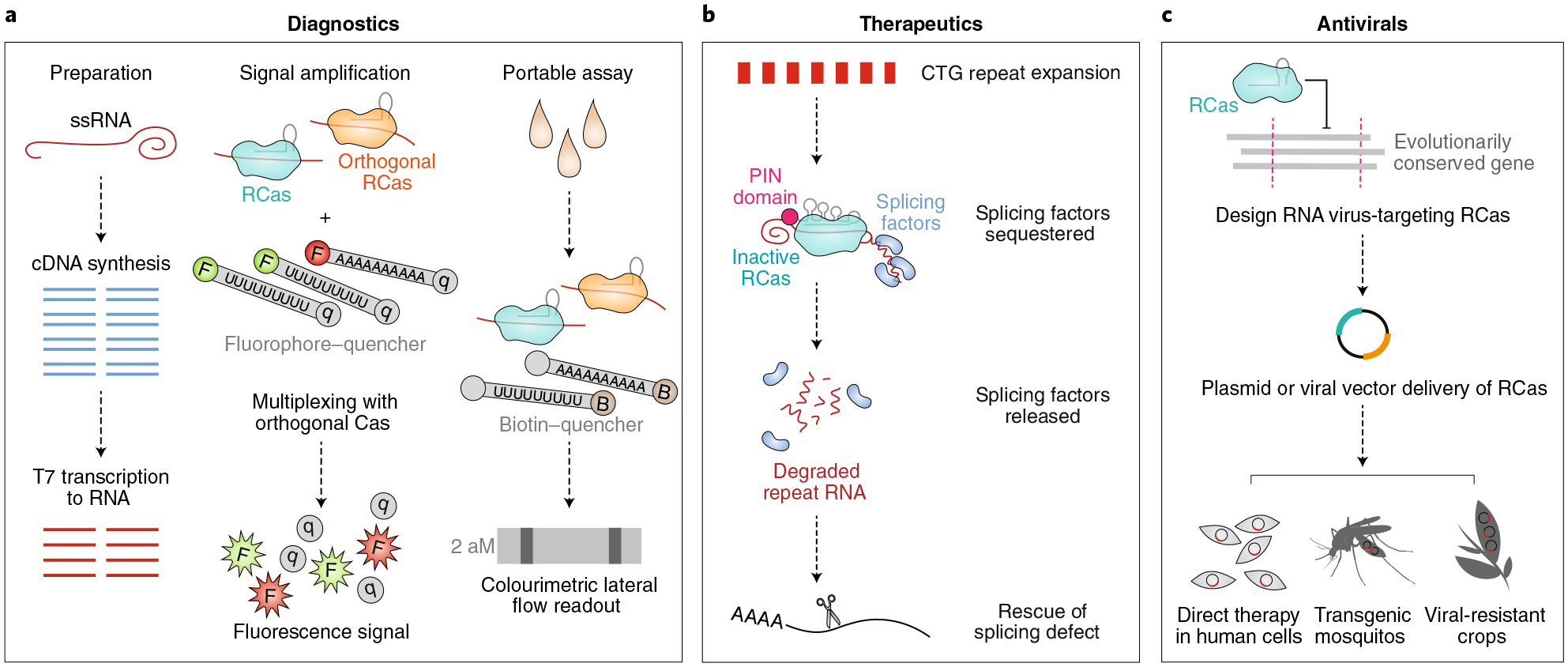
a, By leveraging the indiscriminate ssRNA cleavage of Cas13 upon target RNA recognition, researchers can quantitate the amount of any given nucleic acid species in a complex mixture. Briefly, RNA is converted into cDNA and then reverse transcribed back into RNA (or, alternatively, DNA is reverse transcribed into RNA). This amplified RNA is intermixed with short RNA fragments containing a fluorophore–quencher or biotin–quencher pair, as shown in the ‘signal amplification’ and ‘portable assay’ panels. Upon incubation of the mixture with Cas13 and a target-specific crRNA, fluorescence or colourimetric lateral flow readout corresponding exponentially to the concentration of a target RNA species can be quantitated for diagnostic purposes. b, RCas directed to CTG repeat expansions on RNA releases sequestered splicing factors by degrading the toxic repeat expansions, thus reversing associated RNA-splicing defects. Likewise, RCas can be applied to correct other RNA repeat expansions and RNA-related disorders in humans. c, By leveraging RCas to target evolutionarily conserved genomic regions of ssRNA viruses, researchers may invent antiviral human cell therapies, transgenic mosquitos with diminished capacity to spread infectious disease, and disease-resistant crops.
The versatility afforded by the RCas platform in diagnostic assays may be translated to developments in human therapy, particularly for diseases related to RNA mis-splicing and RBP-RNA aggregation, including muscular dystrophy and amyotrophic lateral sclerosis65. Current therapies against genetic neuropathological disorders utilise ASOs66, which require continual drug administration67. Gene correction by DCas provides an alternative approach, but may induce off-target effects resulting in permanent unintended signatures in the DNA of the recipient cell or tissue49. RCas treatments (with current clinical limitations discussed in the next section) could theoretically circumvent issues inherent both to ASOs and DCas systems. With the AAV packaging of a more compact RCas9, researchers efficiently eliminated toxic repetitive RNA foci, dysregulated splicing events, and toxic polyglutamine protein aggregation—three molecular hallmarks of neurodegenerative diseases—in patient cells47 and in a preclinical in vivo model of myotonic dystrophy68 (Fig. 3b). Furthermore, a recent study packaged Cas13d into AAV to correct Tau mis-splicing in a patient-derived human-induced pluripotent stem-cell-differentiated neuron model of frontotemporal dementia24.
Beyond its conceivable therapeutic use to halt or reverse RNA-mediated diseases, RCas could also benefit the development of antiviral drugs (Fig. 3c). Cas13b targeted to conserved regions of three distinct ssRNA viruses infecting human cells was shown to reduce viral infectivity by up to 300-fold, depending on the virus and the guide selected69. Similar antiviral methods could be extended to the agricultural industry, wherein RNA viruses habitually threaten commercial plants with loss of crop yield and quality. Promisingly, Cas13a has been shown to incorporate stably into the plant genome and substantially hinder ssRNA viruses such as the tobacco mosaic virus and turnip mosaic virus70. Finally, in an antiviral application spanning both human therapeutics and agriculture, mosquitos and other RNA virus-harbouring pests could be engineered with RCas-containing gene drives to eliminate infectious diseases in a wild population, as has been demonstrated under laboratory settings with DCas71. RCas holds great potential to transform fundamental biology and biotechnology, yet hurdles await scientists and engineers at each step of development.
Considerations, concerns, and challenges
Molecular scale.
The design of RCas encompasses many decisions, including which RCas variant to select, which sequence of RNA to target, whether to use a modified guide RNA, and whether to fuse localisation (for example, nuclear import or export) or effector modules72. Dauntingly, even the effector orientation (N or C terminus) and linker peptide between RCas and effector can significantly impact solubility and bioactivity73. Given the complexity of selecting these parameters and the more dynamic nature of RCas, DCas designs will generally be simpler to validate experimentally.
Researchers should also determine the most appropriate mode of delivery. For DCas, researchers have traditionally selected genetic (DNA or RNA) or ribonucleoprotein (RNP) constructs delivered through viral, chemical, or physical means39. RNP delivery often entails the chemical modification of constituent RNA for greater cellular stability74. Although RNP delivery has successfully been implemented in the mouse brain75, the necessity of sustained RCas expression for most applications will limit in vivo delivery to transduction via high-copy viral vectors such as AAV. For in vitro work in cell lines with an expedited (i.e., 24–72 h) temporal readout, chemical (e.g., lipofection) or physical (e.g., nucleofection) delivery should suffice.
Cellular level.
Critical to any targeted drug, proper dosage—in this case stoichiometry between RCas and substrate RNA—can be manipulated by pulsing delivery, by chemically, radiatively, or enzymatically inducing RCas activity76–78, or by abolishing activity through small molecules such as proteolytic protacs79 or inhibitory anti-CRISPR peptides80 if one does not desire constitutive activity. (In fact, the small protein Csx27 may play such an inhibitory role for Cas13b14.) Nevertheless, dosage will depend on many factors beyond experimental control, particularly the rate of RNA turnover81. RNA accessibility in target design14,38 and RBP site competition55 will also contribute to dosage constraints. For long-term RCas durability, researchers should be cautious of potential transcriptional and translational repression caused in part by endogenous cellular machinery82.
Of equal or greater concern to researchers is RCas specificity, namely the differential between on-target and off-target RNA activity. Specificity can be decoupled into RNA binding and cleavage83, and, in the case of fusions to effector modules, RNA modulation as well. (Incidentally, the recent discovery of promiscuous RNA-editing activity by Cas9-APOBEC (apolipoprotein B mRNA editing catalytic polypeptide-like) fusions highlights the need for improved off-target measurements and for a revisit of specificity issues seemingly inherent to effector module fusions to Cas proteins84.) Although certain low-level off-target DNA-editing sites are challenging to detect, RNA sequencing to adequate read depth generally captures RNA modulation comprehensively. Researchers have a number of tools to understand and improve RCas specificity. FRET and chip-based assays developed to assess DCas specificity85,86 and directed-evolution approaches to generate more specific DCas variants87 could readily be translated to comparable problems in RCas specificity. In addition, machine-learning techniques trained on RNA binding and sequencing data could predict more efficient and specific guides for target RNA88. These concerns aside, RCas reversibility and RNA abundance lessen the impact of RNA-targeting specificity relative to DCas editing.
System wide.
An RCas construct may harbour optimal molecular and cellular characteristics yet still suffer from system-wide challenges. Leakage, either tropism- or dosage-mediated, can result in RCas expression in undesired cell types. AAV serotypes possess natural tropisms to particular tissue types89,90, which can be manipulated through in vivo selection methods91. Likewise, surface chemistry largely governs nanoparticle tropism92, a variable shown to exhibit high tunability in modulating recipient cell targeting specificity93. In addition to tropism-mediated concerns, at certain dosages leakage may result either from stochastic delivery to unintended cells or through cellular expulsion via extracellular vesicles94. Regardless of the mechanism, RCas leakage would prove less challenging than DCas leakage, in which a single misplaced DCas molecule can hypothetically wreak havoc on an entire system (e.g., when mutating an oncogene or tumour-suppressor gene).
Finally, in our view, the chief challenge for RCas therapeutics will be immunogenicity. Unlike DCas therapeutics, in which Cas expression is generally transient, most RCas therapeutics will require sustained expression for a desired phenotypic change. The presence of foreign protein and RNA may initially stimulate a non-specific innate immune response95. Persistence in the system may additionally spur an adaptive immune response, as demonstrated by the presence of pre-existing antibodies or reactive T cells to Cas9 in human populations96,97. This can result in cytotoxicity, inflammation, and potentially fatality. Given the challenge that Cas-mediated immunogenicity presents for RNA-targeting therapeutics, researchers have proposed numerous workarounds, including immunosuppression98, immunosilencing of human T cell epitopes present on Cas proteins99, and immune circumvention with orthogonal orthologues of Cas proteins and AAV100. Another aforementioned approach, CIRTS, involves constructing CRISPR-like systems from elements of the human proteome such as histone RNA hairpin-binding domain and TATA-binding protein32, though it runs the risk of interfering with the native RNA transcripts to which these proteins bind. The efficacy of these immunotolerance solutions among a diverse human population remains to be seen.
Outlook and future directions
Despite the concerns discussed above, RNA-targeting CRISPR-Cas systems have exhibited effectiveness in biotechnology and biomedicine. Still, the RCas field is in its infancy. In the coming years researchers will likely employ established bioinformatic discovery tools to uncover additional RCas systems in metagenomic sequencing. Accordingly, we may identify more compact class 2 systems, along with associated functional neighborhood Cas genes101 such as the previously described Csx27, Csx28, and WYL domain genes14,16, perhaps including uncharacterised anti-CRISPR genes80. As the full RCas diversity is explored, enzymes such as the ssDNA- and ssRNA-cleaving Cas12g102 will continue to blur the line between DNA and RNA targeting. Analogous to DNA- and RNA-binding proteins, we may discover that these simultaneously DCas and RCas systems play physiologically consequential roles in their host systems103,104.
More innovations also await in RCas engineering. Similar to a recently published donor template-free search-and-replace genome editing system built from DCas105, researchers will undoubtedly augment characterised systems by rational design and directed evolution to mitigate any perceived shortcomings of the existing RNA-targeting toolkit. At the same time—just as DNA-targeting restriction enzymes have gradually been displaced by DCas for in vivo applications—alternative RNA-targeting technologies, including de novo designed proteins106, will challenge RCas for impending hegemony in transcriptomic engineering. Yet regardless of its ultimate scientific or industrial purposes, RCas will continue to illuminate RNA biology.
Acknowledgements
The authors wish to acknowledge J. Schwartz and J. Schmok for their helpful comments in preparing this manuscript. G.W.Y. is supported by grants from the NIH (NS103172, MH107367, EY029166, HG009889, HG004659), from TargetALS, the ALS Association and a Chan-Zuckerberg Initiative Neurodegeneration Challenge Network grant.
Footnotes
Competing interests
A.A.S. declares inventorship on the following published patents, applied for by the Broad Institute of MIT and Harvard and the Massachusetts Institute of Technology: WO2018035250A1 on methods for bioinformatic discovery of class 2 CRISPR-Cas systems; WO2017070605 on systems, methods, and compositions for targeting nucleic acids with type VI-B CRISPR-Cas systems. G.W.Y is co-founder, member of the Board of Directors, on the SAB, equity holder, and paid consultant for Locana and Eclipse BioInnovations. G.W.Y. is a Distinguished Visiting Professor at the National University of Singapore. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
References
- 1.Bertrand E et al. Localization of ASH1 mRNA particles in living yeast. Mol. Cell 2, 437–445 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Coller JM, Gray NK & Wickens MP mRNA stabilization by poly(A) binding protein is independent of poly(A) and requires translation. Genes Dev. 12, 3226–3235 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elbashir SM et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Barrangou R et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Marraffini LA CRISPR-Cas immunity in prokaryotes. Nature 526, 55–61 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Koonin EV, Makarova KS & Zhang F Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol 37, 67–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makarova KS, Wolf YI & Koonin EV Classification and nomenclature of CRISPR-Cas systems: where from here? CRISPR J. 1, 325–336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mali P et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu PD, Lander ES & Zhang F Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connell MR et al. Programmable RNA recognition and cleavage by CRISPR/Cas9. Nature 516, 263–266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shmakov S et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Microbiol 15, 169–182 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawley AB, Henriksen JR & Barrangou R CRISPRdisco: an automated pipeline for the discovery and analysis of CRISPR-Cas systems. CRISPR J. 1, 171–181 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shmakov S et al. Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol. Cell 60, 385–397 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smargon AA et al. Cas13b is a type VI-B CRISPR-associated RNA-guided RNase differentially regulated by accessory proteins Csx27 and Csx28. Mol. Cell 65, 618–630.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang W, Bikard D, Cox D, Zhang F & Marraffini LA RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol 31, 233–239 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan WX et al. Cas13d is a compact RNA-targeting type VI CRISPR effector positively modulated by a WYL-domain-containing accessory protein. Mol. Cell 70, 327–339.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hale CR et al. RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell 139, 945–956 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staals RH et al. RNA targeting by the type III-A CRISPR-Cas Csm complex of Thermus thermophilus. Mol. Cell 56, 518–530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamulaitis G et al. Programmable RNA shredding by the type III-A CRISPR-Cas system of Streptococcus thermophilus. Mol. Cell 56, 506–517 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Jinek M et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strutt SC, Torrez RM, Kaya E, Negrete OA & Doudna JA RNA-dependent RNA targeting by CRISPR-Cas9. eLife 7, e32724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abudayyeh OO et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 353, aaf5573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.East-Seletsky A et al. Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature 538, 270–273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konermann S et al. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell 173, 665–676.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L et al. The molecular architecture for RNA-guided RNA cleavage by Cas13a. Cell 170, 714–726.e10 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Zhang B et al. Structural insights into Cas13b-guided CRISPR RNA maturation and recognition. Cell Res. 28, 1198–1201 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C et al. Structural basis for the RNA-guided ribonuclease activity of CRISPR-Cas13d. Cell 175, 212–223.e17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makarova KS, Wolf YI, van der Oost J & Koonin EV Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements. Biol. Direct 4, 29 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lapinaite A, Doudna JA & Cate JHD Programmable RNA recognition using a CRISPR-associated Argonaute. Proc. Natl Acad. Sci. USA 115, 3368–3373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters L & Meister G Argonaute proteins: mediators of RNA silencing. Mol. Cell 26, 611–623 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Dias N & Stein CA Antisense oligonucleotides: basic concepts and mechanisms. Mol. Cancer Ther 1, 347–355 (2002). [PubMed] [Google Scholar]
- 32.Rauch S et al. Programmable RNA-guided RNA effector proteins built from human parts. Cell 178, 122–134.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katrekar D et al. In vivo RNA editing of point mutations via RNA-guided adenosine deaminases. Nat. Methods 16, 239–242 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandevenne M et al. Engineering specificity changes on a RanBP2 zinc finger that binds single-stranded RNA. Angew. Chem. Int. Edn Engl 53, 7848–7852 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Filipovska A, Razif MF, Nygård KK & Rackham O A universal code for RNA recognition by PUF proteins. Nat. Chem. Biol 7, 425–427 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Adamala KP, Martin-Alarcon DA & Boyden ES Programmable RNA-binding protein composed of repeats of a single modular unit. Proc. Natl Acad. Sci. USA 113, E2579–E2588 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barkan A & Small I Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant Biol 65, 415–442 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Abudayyeh OO et al. RNA targeting with CRISPR-Cas13. Nature 550, 280–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glass Z, Lee M, Li Y & Xu Q Engineering the delivery system for CRISPR-based genome editing. Trends Biotechnol. 36, 173–185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelles DA et al. Programmable RNA tracking in live cells with CRISPR/Cas9. Cell 165, 488–496 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox DBT et al. RNA editing with CRISPR-Cas13. Science 358, 1019–1027 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel P et al. Efficient and precise editing of endogenous transcripts with SNAP-tagged ADARs. Nat. Methods 15, 535–538 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogel P & Stafforst T Critical review on engineering deaminases for site-directed RNA editing. Curr. Opin. Biotechnol 55, 74–80 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Merkle T et al. Precise RNA editing by recruiting endogenous ADARs with antisense oligonucleotides. Nat. Biotechnol 37, 133–138 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Qu L et al. Programmable RNA editing by recruiting endogenous ADAR using engineered RNAs. Nat. Biotechnol 37, 1059–1069 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Meeske AJ, Nakandakari-Higa S & Marraffini LA Cas13-induced cellular dormancy prevents the rise of CRISPR-resistant bacteriophage. Nature 570, 241–245 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Batra R et al. Elimination of toxic microsatellite repeat expansion RNA by RNA-targeting Cas9. Cell 170, 899–912.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi LS et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang XH, Tee LY, Wang XG, Huang QS & Yang SH Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther. Nucleic Acids 4, e264 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson CE et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roundtree IA, Evans ME, Pan T & He C Dynamic RNA modifications in gene expression regulation. Cell 169, 1187–1200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu XM, Zhou J, Mao Y, Ji Q & Qian SB Programmable RNA N6-methyladenosine editing by CRISPR-Cas9 conjugates. Nat. Chem. Biol 15, 865–871 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H et al. CRISPR-mediated programmable 3D genome positioning and nuclear organization. Cell 175, 1405–1417.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerstberger S, Hafner M & Tuschl T A census of human RNA-binding proteins. Nat. Rev. Genet 15, 829–845 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Nostrand EL et al. A large-scale binding and functional map of human RNA binding proteins. Preprint at bioRxiv 10.1101/179648 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myers SA et al. Discovery of proteins associated with a predefined genomic locus via dCas9-APEX-mediated proximity labeling. Nat. Methods 15, 437–439 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shalem O et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jusiak B, Cleto S, Perez-Piñera P & Lu TK Engineering synthetic gene circuits in living cells with CRISPR technology. Trends Biotechnol. 34, 535–547 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Wroblewska L et al. Mammalian synthetic circuits with RNA binding proteins for RNA-only delivery. Nat. Biotechnol 33, 839–841 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gootenberg JS et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 356, 438–442 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.East-Seletsky A, O’Connell MR, Burstein D, Knott GJ & Doudna JA RNA targeting by functionally orthogonal type VI-A CRISPR-Cas enzymes. Mol. Cell 66, 373–383.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gootenberg JS et al. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 360, 439–444 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Myhrvold C et al. Field-deployable viral diagnostics using CRISPR-Cas13. Science 360, 444–448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen JS et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360, 436–439 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jain A & Vale RD RNA phase transitions in repeat expansion disorders. Nature 546, 243–247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lagier-Tourenne C et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc. Natl Acad. Sci. USA 110, E4530–E4539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geary RS, Norris D, Yu R & Bennett CF Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev 87, 46–51 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Batra R et al. Reversal of molecular pathology by RNA-targeting Cas9 in a myotonic dystrophy mouse model. Preprint at bioRxiv 10.1101/184408 (2017). [DOI] [Google Scholar]
- 69.Freije CA et al. Programmable inhibition and detection of RNA viruses using Cas13. Mol. Cell 76, 826–837.e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aman R et al. RNA virus interference via CRISPR/Cas13a system in plants. Genome Biol. 19, 1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gantz VM et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl Acad. Sci. USA 112, E6736–E6743 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Konermann S et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517, 583–588 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen X, Zaro JL & Shen WC Fusion protein linkers: property, design and functionality. Adv. Drug Deliv. Rev 65, 1357–1369 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khvorova A & Watts JK The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol 35, 238–248 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Staahl BT et al. Efficient genome editing in the mouse brain by local delivery of engineered Cas9 ribonucleoprotein complexes. Nat. Biotechnol 35, 431–434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu JH, Davis KM & Liu DR Chemical biology approaches to genome editing: understanding, controlling, and delivering programmable nucleases. Cell Chem. Biol 23, 57–73 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Oakes BL et al. CRISPR-Cas9 circular permutants as programmable scaffolds for genome modification. Cell 176, 254–267.e16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richter F et al. Switchable Cas9. Curr. Opin. Biotechnol 48, 119–126 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Sakamoto KM et al. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl Acad. Sci. USA 98, 8554–8559 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pawluk A, Davidson AR & Maxwell KL Anti-CRISPR: discovery, mechanism and function. Nat. Rev. Microbiol 16, 12–17 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Larsson E, Sander C & Marks D mRNA turnover rate limits siRNA and microRNA efficacy. Mol. Syst. Biol 6, 433 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iwakawa HO & Tomari Y The functions of MicroRNAs: mRNA decay and translational repression. Trends Cell Biol. 25, 651–665 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Tambe A, East-Seletsky A, Knott GJ, Doudna JA & O’Connell MR RNA Binding and HEPN-nuclease activation are decoupled in CRISPR-Cas13a. Cell Rep. 24, 1025–1036 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grünewald J et al. Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature 569, 433–437 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen JS et al. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature 550, 407–410 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jung C et al. Massively parallel biophysical analysis of CRISPR-Cas complexes on next generation sequencing chips. Cell 170, 35–47.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu JH et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 556, 57–63 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim HK et al. Deep learning improves prediction of CRISPR-Cpf1 guide RNA activity. Nat. Biotechnol 36, 239–241 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Srivastava A In vivo tissue-tropism of adeno-associated viral vectors. Curr. Opin. Virol 21, 75–80 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zincarelli C, Soltys S, Rengo G & Rabinowitz JE Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther 16, 1073–1080 (2008). [DOI] [PubMed] [Google Scholar]
- 91.Choudhury SR et al. In vivo selection yields AAV-B1 capsid for central nervous system and muscle gene therapy. Mol. Ther 24, 1247–1257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song E et al. Surface chemistry governs cellular tropism of nanoparticles in the brain. Nat. Commun 8, 15322 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhai J et al. Epidermal growth factor receptor-targeted lipid nanoparticles retain self-assembled nanostructures and provide high specificity. Nanoscale 7, 2905–2913 (2015). [DOI] [PubMed] [Google Scholar]
- 94.Shah R, Patel T & Freedman JE Circulating extracellular vesicles in human disease. N. Engl. J. Med 379, 958–966 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Kim S et al. CRISPR RNAs trigger innate immune responses in human cells. Genome Res. 28, 367–373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Charlesworth CT et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med 25, 249–254 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wagner DL et al. High prevalence of Streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat. Med 25, 242–248 (2019). [DOI] [PubMed] [Google Scholar]
- 98.Chew WL Immunity to CRISPR Cas9 and Cas12a therapeutics. Wiley Interdiscip. Rev. Syst. Biol. Med 10, e1408 (2018). [DOI] [PubMed] [Google Scholar]
- 99.Ferdosi SR et al. Multifunctional CRISPR-Cas9 with engineered immunosilenced human T cell epitopes. Nat. Commun 10, 1842 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moreno AM et al. Immune-orthogonal orthologues of AAV capsids and of Cas9 circumvent the immune response to the administration of gene therapy. Nat. Biomed. Eng 3, 806–816 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shmakov SA, Makarova KS, Wolf YI, Severinov KV & Koonin EV Systematic prediction of genes functionally linked to CRISPR-Cas systems by gene neighborhood analysis. Proc. Natl Acad. Sci. USA 115, E5307–E5316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yan WX et al. Functionally diverse type V CRISPR-Cas systems. Science 363, 88–91 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dugar G et al. CRISPR RNA-dependent binding and cleavage of endogenous RNAs by the Campylobacter jejuni Cas9. Mol. Cell 69, 893–905.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hudson WH & Ortlund EA The structure, function and evolution of proteins that bind DNA and RNA. Nat. Rev. Mol. Cell Biol 15, 749–760 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anzalone AV et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang PS, Boyken SE & Baker D The coming of age of de novo protein design. Nature 537, 320–327 (2016). [DOI] [PubMed] [Google Scholar]


