Abstract
The coronavirus disease (COVID-19) pandemic is sweeping the globe. Even with a number of effective vaccines being approved and available to the public, new cases and escalating mortality are climbing every day. ACE2 (angiotensin-converting enzyme 2) is the primary receptor for the COVID-19 causative virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and its complexation with spike proteins plays a crucial role in viral entry into host cells and the subsequent infection. Blocking this binding event or reducing the accessibility of the virus to the ACE2 receptor, represents an alternative strategy to prevent COVID-19. In addition, the biological significance of ACE2 in modulating the innate immune system and tissue repair cascades and anchors its therapeutic potential for treating the infected patients. In this viewpoint article, we review the current efforts of exploiting ACE2 as a therapeutic target to address this dire medical need. We also provide a holistic view of the pros and cons of each treatment strategy. We highlight the fundamental and translational challenges in moving these research endeavors to clinical applications.
Keywords: COVID-19, ACE2, therapy
With the coronavirus disease (COVID-19) pandemic raging globally, extraordinary measures have been taken to tackle such a daunting public health crisis. The development of effective treatment strategies for the COVID-19 infection is still at the early stages, although remdesivir and dexamethasone have shown moderate efficacy in some highly defined clinical settings (1). Although steady progress has been made in the development of vaccines against COVID-19, challenges in late-stage testing, and eventual distribution remain. Moreover, a recently reported recurrence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with a different virus strain creates further concerns about the durable effectiveness of preventive vaccines or convalescent plasma (2).
ACE2 (angiotensin-converting enzyme 2) is recognized as the primary receptor for SARS-CoV-2 (3), although several other proteins are proposed coreceptors (4–6). ACE2 was initially discovered as a homolog of ACE (angiotensin-converting enzyme) but counteracted many ACE-mediated physiological and pathophysiological effects on humans and other species, ranging from blood pressure regulation, cardiovascular function, cell death, and proliferation to host responses to a variety of insults (7–13). The discovery of ACE2 as the primary receptor for a novel coronavirus, SARS-CoV, drastically changed the focus of ACE2-related research, promoting new interest in investigating the role of ACE2 in lung diseases (14). The COVID-19 pandemic has spurred even more extensive research on ACE2, particularly its interaction with the virus and other molecules involved in viral entry. These studies have unveiled novel insights into the biology of ACE2. First, several expression surveys show a gradient of ACE2 expression in the upper and lower airways and terminal airspace compartments (15–18). The highest compartmental cell-specific expression is seen in ciliated cells of the proximal airway and the alveolar type II cells of the airspace compartment (19). Second, the binding of SARS-CoV-2 to ACE2 is distinct from that of SARS-CoV and NL63 in terms of the interaction strength between ACE2 and the respective receptor-binding domain (RBD) (20–23); other coronaviruses that use this receptor but cause severe and minor lung morbidity. Third, the coreceptor requirements of the virus–ACE2 complex are cell-specific with differential use of TMPRSS2 (transmembrane serine protease 2) or cathepsin L (24, 25). TMPRSS2, lysosomal cathepsin, and furin appear essential for SARS-CoV-2 virus processing, and entry into the cells before and after the viral S protein (spike protein) is complexed with ACE2 (24, 26–28). Although reports indicate that TMPRSS2 can cleave ACE2 (29), the interactions between ACE2 and TMPRSS2, or furin and cathepsin, after the viral S protein binds ACE2 has yet to be clarified. Preclinical and whole-cell studies showing virus-triggered shedding of the ACE2 ectodomain and the downregulation of the enzyme on interaction with the virus adds even more layers of reciprocal complexity to the ACE2 and SARS-CoV-2 relationship (30–32). Interestingly, Onabajo and colleagues recently reported a unique form of primate-specific ACE2, dACE2 (δACE2), which is an ISG (IFN-stimulated gene) and lacks 356 amino acids at its N-terminus. This isoform of ACE2 has no enzymatic activity and cannot bind to the SARS-CoV-2 S protein (33). Most importantly, they found that wild type ACE2 is not an ISG; therefore, IFN-induced dACE2 expression will unlikely affect the cellular entry of SARS-CoV-2 and promote infection. Taken together, multiple unresolved issues remain, especially how the level of ACE2 translate into virus susceptibility.
What follows is a view on trenchant aspects of therapeutic ACE2 targeting, focusing on both the enzymatic and receptor functions of ACE2.
Approaches to Targeting ACE2 for COVID-19 Therapy
Targeting ACE2 as the Receptor for SARS-CoV-2 to Mitigate the Infectivity
The binding of the S protein of SARS-CoV-2 to its cognate receptor ACE2 is the initial step for virus entry, replication, and spread. Therefore, blocking this specific binding event has been logically considered a highly promising therapy to address viral infection and potentially avoid the later consequences. The fundamental principle of targeting ACE2 to mitigate SARS-CoV-2 infection is to block the virus's accessibility to the membrane-bound ACE2.
Decoy ACE2 molecules to trap the virus.
An attractive strategy to target ACE2 in COVID-19 therapy is to use sACE2 (soluble ACE2) as a decoy receptor capable of trapping the virus to prevent cellular engagement (34–37). sACE2 can bind its cognate ligand, the viral S protein, but is unable to reform a membrane-bound ACE2, which consequently blocks the mechanism of virus entry into the host cells (38). As such, sACE2 ostensibly limits SARS-CoV-2 cell attachment, cell entry, and viral replication (36). Moreover, sACE2 has been proven to be an enzymatic activity in some clinical trials or disease models (39, 40). Although this sounds like a highly promising solution, there are several technical hurdles to overcome. First, because a full-length ectodomain of human ACE2 comprises 740 amino acids, a truncated form of sACE2 is needed to reduce immunogenicity. Although variable shorter sACE2 fragments have been reported to maintain the enzymatic activity (36, 37, 41), and the rationale of designing a therapeutic short form of ACE2 is proposed (42), whether the truncated ACE2 can be functional as a decoy receptor is yet to be validated. Recently, another group has reported that computational modeling–based ACE2 truncation enhances binding to the RBD of SARS-CoV-2 than binding to full-length ACE2 (42). Second, sACE2 is unstable, especially in the setting of lung infection or inflammation. As such, sACE2 may quickly degrade, attenuating its ability to trap the virus efficiently. A similar approach was explored for SARS in dealing with such drawbacks. Researchers generated a chimeric sACE2 with an engineered human IgG Fc fragment onto the sACE2 C-terminus to stabilize the protein. The results showed that the chimeric sACE2 exhibited a greatly extended plasma half-life in mice, from less than 2 hours of the original recombinant ACE2 to over a week of the chimeric (43). Similar approaches had been adopted to test the potential in COVID-19 prevention and treatment and the preliminary results are promising that significantly attenuated SARS-CoV-2 infection in a murine model is achieved by administrating an IgG1 Fc chimeric sACE2 (44). A recent report by Zoufaly and colleagues demonstrates that the soluble form of hrsACE2 (APN01 [human recombinant sACE2]) (0.4 mg/kg) treatment in a single patient with COVID-19 results in a reduced viral load in plasma, tracheal suction, and nasopharyngeal swab. In addition, leveraged proinflammatory cytokine levels are observed and accompanied by bacterial coinfection during hrsACE2 therapy by intravenous infusion (45). Moreover, because the Fc fragment might bind to its receptor CD16, the potential risk of the chimeric ACE2 becoming an alternative receptor to bind the virus and subsequently facilitating the viral transduction is potentially problematic. Such a problem is solved by mutating Fc fragment to block Fc binding to CD16 (44). Other potential approaches using sACE2 to prevent and treat COVID-19 have emerged recently as well (46). For instance, using novel LNPs (lipid nanoparticle) to package a soluble form of human ACE2, Kim and colleagues reported that instilling the sACE2 into mouse lungs resulted in a strongly inhibited (over 90%) SARS-CoV-2 pseudovirus infection, and the sACE2 can be detected even 48 hours after administration (47). Chan and colleagues, by using deep mutagenesis for understanding the specificity of the interaction between ACE2 and the S protein of SARS-CoV-2 virus, engineered high-affinity decoy ACE2 receptors that showed potent SARS-CoV-2 and SARS-CoV-1 neutralization in vitro (48). Overall, recent development in identifying a modified form of sACE2 with high-affinity to the S protein from SARS-CoV-2, prolonged stability, and low immunogenicity is exciting to effectively use sACE2 as a decoy receptor in COVID-19 prevention and therapy.
Pseudoligands to dominate the binding site of ACE2 for SARS-CoV-2.
This idea stems from a vaccine design strategy (49). The critical step is to generate a pseudoligand that has a high affinity to the receptor, such as a truncated form of viral S protein that only contains the RBD or an engineered artificial receptor-binding motif. Although advancements in structural biology make this approach feasible, the risk exists that on binding the de novo designed ligand to the receptor, other intracellular signaling pathways and subcellular responses might also be triggered, which can be beneficial or detrimental to the host. For example, it has been shown that binding of the RBD of SARS-CoV-2 S protein to ACE2 also triggers an inflammatory response, eliciting cytokine production (50). The concerns over these uncertainties warrant vigilance in the design and application of pseudoligands for COVID-19 prevention and treatment in that a cytokine storm has been frequently observed in many severe cases of COVID-19, contributing to multiple organ failures and deaths (51).
Blocking antibodies against ACE2 viral docking sites.
Developing an antibody against ACE2 on the basis of the epitopes residing on viral docking sites would seem a reasonable strategy for preventing COVID-19 infection and the early treatment of patients with COVID-19. Moreover, because the catalytic domain of ACE2 is separate from the viral binding domain, blocking the docking sites may not interfere with ACE2 enzymatic activity (13). Therefore, special attention should be given to the pulmonary ACE2 activity with all relevant substrates when a newly designed blocking antibody is tested preclinically.
ACE2 inhibitor against viral docking sites.
NAAE (N-[2-aminoethyl]-1 aziridine-ethanamine) is, thus far, the only discovered ACE2 inhibitor that inhibits SARS-CoV S protein–mediated cell fusion. In a report (52), investigators found that NAAE blocks the membrane fusion of SARS-CoV S protein–expressing cells and ACE2-expressing cells with an IC50 in a micromolar concentration range, comparable to the ability of NAAE to inhibit ACE2 enzymatic activity. The authors reasoned that the SARS-CoV S protein–binding residues of ACE2 are shifted on NAAE interaction to a sufficient degree that ACE2 binding to the S protein is inhibited. Therefore, it is conceivable that NAAE is potentially useful for mitigating SARS-CoV-2 infectivity as well. However, this inhibitor also displays high potency to inhibit ACE2 catalytic activity, an alarming feature that needs to be considered when choosing this inhibitor in COVID-19 therapy (52).
Agents to enhance ACE2 shedding.
ACE2 can be released from the cell’s plasma membrane to the extracellular milieu as sACE2 (38, 53). Increased sACE2 leads to reduced membrane-bound ACE2 since so far, no evidence exists that suggests the shedding process induces ACE2 expression. Therefore, promoting ACE2 shedding will likely reduce viral binding to ACE2 on the cell surface, while potentially preserving local ACE2 in the soluble form. Interestingly, the S protein from SARS-CoV binds to ACE2 and induces ACE2 shedding (54), a typical host-pathogen interaction phenomenon. However, whether this can be harnessed in the setting of active infection to mitigate tissue damage is unclear.
ACE2 shedding depends on the activity of ADAM17, or TACE, a metalloprotease (53). Moreover, reports indicated that ADAM10 might also be involved in the shedding under certain circumstances (38). Notably, the shedding is inducible by various biomolecular stimuli, including agonist-PMA (phorbol 12-myristate 13-acetate) for ADAM17, ionomycin for ADAM10 (55), bacterial endotoxin, cytokines, and chemokines. Because ADAM17 is the major protease responsible for ACE2 shedding, enhancing ADAM17 activity represents a tractable strategy to increase ACE2 shedding and reduce the SARS-CoV-2 infectivity. However, given that ADAM17 also involves many other biological processes, such as TNF-α processing, the alteration of ADAM17 activity may have detrimental effects, such as significantly elevated TNF-α or other cytokines that exacerbate inflammatory responses.
Agents to promote or inhibit ACE2 internalization.
Like many cell surface proteins, ACE2 undergoes regulated internalization in a clathrin-dependent fashion (56). Therefore, designing or identifying small molecules that can bind with ACE2 and subsequently trigger internalization could be considered an effective way to lower the ACE2 cell surface density for preventing binding, thus reducing viral entry. Moreover, it has been reported that RBD from SARS-CoV S protein binds to ACE2 and induces ACE2 internalization (57). Many agonists or antagonists of transmembrane proteins, once engaged with the target protein, trigger internalization (58–60). As such, inhibitors for clathrin could be used in blocking the endocytosis of the virus-ACE2 complex. Several clinical trials of such types of inhibitors are underway (56, 61). When considering such a strategy, a caveat is that promoting ACE2 internalization will reduce enzymatically active ACE2 on the cell surface, potentially resulting in the heightened inflammatory response and lung damage (details are shown below).
A summary of design strategies that leverage ACE2 as the therapeutic target is listed in Table 1.
Table 1.
Design Strategies That Leverage ACE2 as the Therapeutic Target
| Design Strategy | Therapeutic Agent | Regimen | Preclinical/Clinical Significance | Refs. |
|---|---|---|---|---|
| Decoy receptor | rhACE2 | Single dose of 100–800 μg/kg or repeated dose of 400 μg/kg, i.v. for 30 min | Decreased AngII and higher Ang1–7 and Ang1–5 | 39 |
| GSK2586881 (rhACE2) | Escalating doses (0.1 mg/kg–0.8 mg/kg) or twice-daily dose (0.4 mg/kg), i.v. | ARDS patients demonstrated reduced Ang-II level and increased Ang1–7 and Ang1–5; elevated SF-D level and trends of decreased IL-6 | 40 | |
| APN01 (hrsACE2) | 0.4 mg/kg, i.v., two times a day for 7 d | Reduced viral load in plasma and airway, accompanied by decreased proinflammatory cytokine levels in plasma and bacterial lung infection | 45 | |
| hACE2-IgG1 fusion and mutations on the enzymatic domain | Single dose of 4 mg/kg, i.v. | Prolonged half-life (∼145 h), attenuated SARS-CoV-2 infection in mouse model | 44 | |
| Dimeric sACE2 mutant | 20–200 nM | Higher affinity of SARS-CoV-1 and SARS-CoV-2 S-protein binding; enhanced neutralization of the viruses in vitro | 48 | |
| Lipid nanoparticle-packaged-hACE2-ectodomain-encoded mRNA | Not reported | Prolonged half-life of hsACE2 in circulation and mouse lung; inhibition of SARS-CoV-2 pseudovirus infection | 47 | |
| Engineered sACE2 | Not reported | Enhanced affinity to SARS-CoV-2 RBD, abolished enzymatic activity, and improved neutralization potency in vitro | 46 | |
| ACE2 inhibitor | NAAE | 0.5–2 mM | Blocking SARS-CoV S protein–mediated cell fusion and inhibiting ACE2 enzymatic activity in vitro | 52 |
| ACE2 shedding promoter | ADAM17 | PMA (10 ng/ml) | Enhancing ACE2 shedding, but facilitating proinflammatory cytokine release and functioning | 38, 53 |
| ADAM10 | Ionomycin (2.5 μM) | Enhancing ACE2 shedding, but activating HER2 signaling as well | 38, 55 | |
| ACE2 internalization affecter | Clathrin inhibitor Chlorpromazine | 5–20 μM | Facilitating ACE2 endocytosis, reducing virus-receptor binding capacity in vitro | 56 |
| Arbidol (umifenovir, favipiravir) | Not reported | Decreased viral endocytosis | 61 |
Definition of abbreviations: ACE2 = angiotensin-converting enzyme 2; AngII = angiotensin II; ARDS = acute respiratory distress syndrome; HER2 = human epidermal growth factor receptor 2; hrsACE2 = human recombinant soluble ACE2; NAAE = N-(2-aminoethyl)-1 aziridine-ethanamine; PMA = phorbol 12-myristate 13-acetate; RBD = receptor binding domain; Refs. = references; rhACE2 = recombinant human ACE2; sACE2 = soluble ACE2; SARS-CoV-1 = severe acute respiratory syndrome coronavirus 1; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; S protein = spike protein; SF-D = serum and/or synovial fluid.
Targeting ACE2 as a Carboxypeptidase to Modulate Inflammatory Responses
Conventionally, ACE2 is viewed as a component of the renin–angiotensin system to regulate cell functions in the cardiovascular and renal systems. Accumulating evidence indicates that ACE2 is also a potent regulator of the inflammatory response (62). As of today, the antiinflammatory function of ACE2 is achieved through its enzymatic activity and no evidence suggests that ACE2 itself has effects on the inflammatory process. Our recent study revealed that the initial reduction of pulmonary ACE2, while the host encounters a bacterial lung infection, is crucial for recruiting the inflammatory neutrophils into the lungs (63). The subsequent recovery of pulmonary ACE2 is equally critical to prevent exuberant neutrophil accumulation and safeguard the lung inflammation progression, suggesting a highly promising new strategy in inflammatory lung disease therapy (63). Clinical observations and public reports suggest that the most vulnerable populations to SARS-CoV-2 are the elderly and those with preexisting conditions, such as diabetes and hypertension (64), which are the same group of people who have impaired immunity. Not unexpectedly, this is the group of people that display the most severe disease progression and high mortality, mainly because of the exaggerated inflammatory response (51). This suggests that a dysregulated inflammatory response plays a critical role in the initiation, progression, and prognosis of COVID-19. Therefore, the logistics for targeting ACE2 enzymatic activity in COVID-19 therapy is rational.
Agents to enhance ACE2 enzymatic activities.
There are several ways to enhance ACE2 activity. Although inducing ACE2 expression can increase ACE2 activity, more membrane-bound ACE2 would further facilitate the viral entry. Two commercially available reagents, DIZE and XNT (65), are reported to increase ACE2 activity (66), enabling their potential use in patients with COVID-19 to alleviate inflammatory injury. However, the off-target potential, the ACE2-independent effects (67), and the relatively high toxicity challenge their clinical utility. A clinical trial was conducted using sACE2 as a therapy to treat patients with acute respiratory distress syndrome (40). The preliminary results only demonstrated minimal beneficial effects, such as an elevated surfactant-D and a trend of decreased IL-6. Therefore, novel ACE2 activators with high efficacy, specificity, and low toxicity are urgently needed. The accumulating evidence indicates that ACEi (angiotensin-converting enzyme inhibitor) and ARBs can increase ACE2 activity, although the underlying mechanisms remain elusive (68).
Agents to simulate ACE2 activity.
ACE2 is a carboxypeptidase that can hydrolyze AngII (angiotensin II), apelin 13, dynorphin-A, and des-Arg9–bradykinin (69). The primary product peptide is Ang1–7, which is generated from AngII with high catalytic efficiency (70). The antiinflammatory effects of ACE2 primarily reside in Ang1–7 (71, 72). Accordingly, a reasonable therapeutic approach is the delivery of Ang1–7 to attenuate viral morbidity. Although Ang1–7 has been used in clinical trials for pulmonary hypertension, its short half-life constrains therapeutic delivery options (73). Alternative approaches include stabilized forms of Ang1–7, which have demonstrated efficacy in preclinical settings (74). AVE0991, an analog of Ang1–7, might also be used as a substitute for ACE2 enzymatic activity (75).
Agents to reduce ACE2 degradation.
Inhibitors for TMPRSS2 have been proposed in treating patients with COVID-19 (27, 76). TMPRSS2 activates the virus’s S protein and hydrolyzes ACE2 into nonfunctional fragments (27, 29, 76, 77). Thus, the TMPRSS2 inhibitors that preserve ACE2 functions might serve as an ACE2 activator in this context, in addition to their viral fusion blocking effect (76, 78).
A list of therapeutic agents that leverage ACE2 as an inflammation modulator is included in Table 2.
Table 2.
Therapeutic Agents That Leverage ACE2 as an Inflammation Modulator
| Design Strategy | Therapeutic Agent | Important Observations | Refs. |
|---|---|---|---|
| Enhancing ACE2 enzymatic activity | rhACE2 | Elevated circulating: tissue ACE2 activity reduced; inflammatory responses induced by a bacterial lung infection | 63 |
| DIZE | Enhanced ACE2 activity, but with off-target effects; reduced infarct area; attenuated LV remodeling | 66, 67 | |
| XNT | Prevention of glycemia and improved cardiac function; off-target effects were noticed | 65, 66 | |
| Simulating ACE2 enzymatic activity | Ang1–7 or AVE0991 | Ang1–7 represses the increased NADPH oxidase; rescues the dilated cardiomyopathy in ACE2ko mice | 75 |
| Reducing ACE2 degradation | TMPRSS2 inhibitors (camostat, nafamostat) | TMPRSS2 hydrolyzes ACE2 and thus degrades ACE2 | 29, 76–78 |
Definition of abbreviations: ACE2ko = ACE2 knockout; DIZE = diminazene aceturate; LV = left ventricle; TMPRSS2 = transmembrane serine protease 2; XNT = 9H-Xanthen-9-one.
Collective Considerations for Targeting ACE2 in COVID-19 Therapy
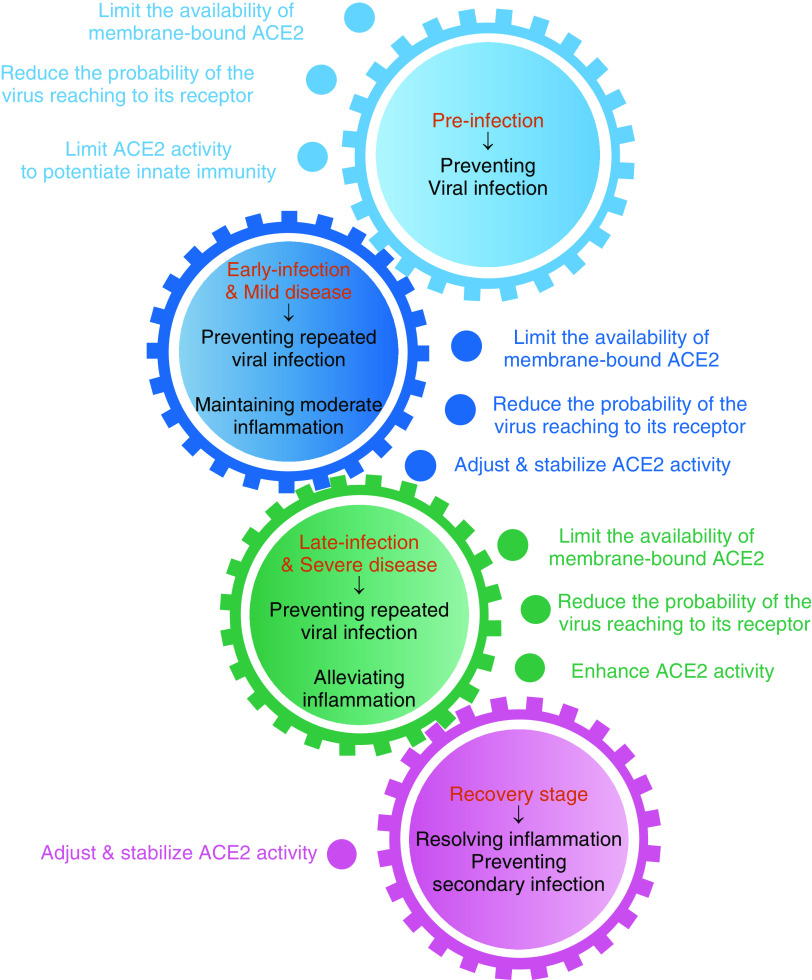
ACE2 is a multifunctional protein; consequently, leveraging ACE2 as a therapeutic target for COVID-19 requires collective considerations to balance the pros and cons at the stage of disease progression for each patient. Some basics about ACE2 have to be clear: 1) The enzymatic activity and viral receptor property of ACE2 are independent, as most known ACE2 inhibitors reduce ACE2 enzymatic activity. However, only NAAE is reported to dampen the binding of the SARS-CoV S protein to ACE2 (52), suggesting segregation of the enzymatic activity and S protein–binding of ACE2. Moreover, studies indicated that the binding of a virus to ACE2 does not necessarily interfere with the enzymatic activity of the latter (37, 79); and 2) soluble ACE2 and membrane-bound ACE2 share the same enzymatic and viral binding functionalities (37, 38, 80). However, only the membrane-bound ACE2 can facilitate viral entry and subsequent infectivity (37, 38) (Figure 1). Having these essential prerequisites in mind, it seems feasible to target ACE2 as an alternative option for COVID-19 therapy. However, when planning a therapeutic ACE2-targeting regimen, clinicians will need to determine the stage of disease and comorbidities that could prove consequential. During the initial infection stage, approaches to reduce viral infectivity would be prioritized at the expense of allowing an appropriate immune response to contain the virus. At more advanced stages of COVID-19 with exuberant inflammation, not requiring an active viral driver, strategies to enhance ACE2 activity should be entertained. Foundational studies showing lung and systemic ACE2 expression and activity during different disease stages are crucial for the optimal use of these interventions. Regarding comorbidities, when using decoy ACE2 for prevention purposes, the recipient’s age and preexisting conditions must be considered. An elderly or a potentially immunocompromised recipient might require the coadministration of an ACE2 inhibitor to reduce the possibility that decoy ACE2 (with enzymatic activity) may attenuate the innate immune response that will lead to unexpected, enhanced infectivity.
Figure 1.
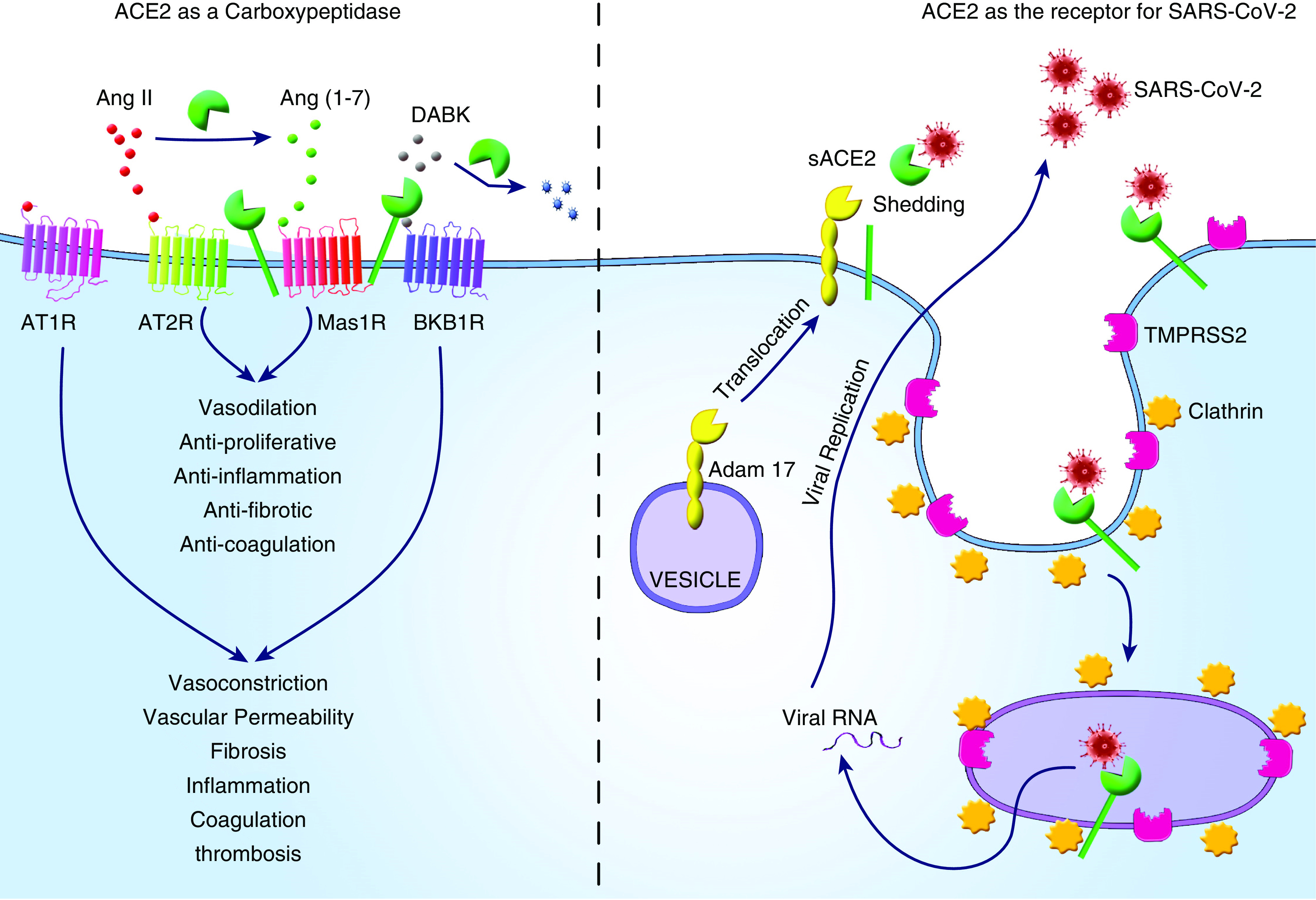
Schematic illustration of ACE2 (angiotensin-converting enzyme 2) in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and coronavirus disease (COVID-19) pathogenesis. The role of ACE2 in SARS-CoV-2 infection and the pathogenesis of COVID-19 encompasses its function as the viral receptor to facilitate viral entry and also as carboxypeptidase to regulate the activities of the renin–angiotensin system and kinin–kallikrein system, which in turn modulates cellular responses related to inflammation, fibrosis, coagulation, and thrombosis. The vertical dashed line highlights two distinct functions of ACE2 in the pathogenesis of COVID-19. Adapted from Reference 86. Ang II = angiotensin II; AT1R = angiotensin II receptor type 1; AT2R = angiotensin II receptor type 2; BKB1R = bradykinin receptor B1; DABK = des-Arg9-bradykinin; Mas1R = Mas1 receptor; sACE2 = soluble ACE2; TMPRSS2 = transmembrane serine protease.
Drug delivery issues.
Our proposed strategies for targeting ACE2 in COVID-19 therapy are illustrated in Figure 2. Given the many important biological roles that ACE2 plays in regulating cardiovascular functions and innate immune systems, caution must be taken in leveraging ACE2 as a therapeutic target. Once a therapeutic compound is identified and optimized, efforts should be devoted to developing formulations that enable its specific delivery to the target sites to maximize the intended preventive or therapeutic efficacy and reduce harmful side effects.
Figure 2.
Proposed COVID-19 therapeutic strategy by targeting ACE2. The strategy is based on the status of SARS-CoV-2 infection and the progression of COVID-19; ACE2-targeted intervention strategies need to adjust accordingly to prioritize the therapeutic goal for each patient.
For ACE2-based therapies, the primary delivery targets are lung epithelial cells in the airway and airspace compartments. Because blocking the virus's accessibility to ACE2 is the key to preventing viral infection, inhalable delivery would seem to be a logical option to selectively and locally deposit pseudoligands, docking antibodies, and/or ACE2 inhibitors to the lungs. Similarly, therapeutic compounds to promote ACE2 internalization or shedding also need to be delivered to the lung epithelial cells. There are three critical considerations in the development of their inhalable formulations. The first is the safety requirements in administering an aerosolized drug to patients who have a high risk of viral dissemination to clinical personnel. Advancements in delivery hardware and lessons learned from treating current patients with COVID-19 will likely address this challenge. The second is the nebulization stability of these therapeutic compounds. Although small molecule therapeutics might not encounter any stability issues, protein or peptide drugs may suffer from potential denaturing because of the mechanical forces or interface enrichment during the nebulization process (81). The use of nanoscale carriers may help stabilize their molecular and structural integrity (82) but their stability, such as size and morphology, also needs to be carefully considered and assessed. A third hurdle is the need to penetrate the airway mucous layer. To promote mucous penetration, spherical nanoparticles with optimized size and surface chemistry can improve delivery efficiency (83). When using the soluble form of ACE2 or decoy ACE2 to block viral entry, it is preferred that they stick on the top of the mucous layers without any significant contact with lung cells. In this case, charged nanoparticles of a high aspect ratio might better serve the purpose (84). Timing and dosage control are incredibly essential to modulate inflammatory responses by altering the enzymatic activities of ACE2. Depending on the severity of symptoms and patient conditions, the administration of ACE2 inhibitors and activators should be assessed case by case, with careful control over the dose, dosing time, and frequency.
Clinical considerations.
COVID-19 is a disorder punctuated by clinical transitions, including exposure to infection, fever or upper respiratory symptomatology, hypoxia, hypoxia-induced respiratory failure, respiratory failure results in acute respiratory distress syndrome, or multiorgan system failure. Each of these transitions reflects specific physiologic, immune, and homeostatic perturbations that reasonably require specific interventions. Thus, a strategy anchored on a single perturbation is not likely to be effective in all stages of COVID-19. Targeting ACE2 as a therapy for COVID-19 has to be considered holistically integrating the infection stage, the degree of inflammation, coagulation status, and disease progression for each patient. During the initial infection stage, approaches to reduce viral infectivity would be prioritized at the expense of allowing an appropriate immune response to contain the virus. At more advanced stages of COVID-19 with exuberant inflammation not requiring an active viral driver, strategies to enhance ACE2 activity would be appealing. Besides, although the enzymatic activity and viral receptor capacity of ACE2 are not coupled, in clinical practice, manipulating one function of ACE2 might affect the other function of ACE2, resulting in severe clinical consequences. By this view, reducing membrane-bound ACE2 and increasing sACE2 (harboring decoy and enzymatic functions) may be ideal for this disorder. However, if soluble and membrane-bound ACE2 can both be induced with a single agent (i.e., ARBs or ACEis), the soluble agent's dual effects might override the possibility of feeding viral uptake, a singular caution. The form and site of ACE2 modulation and the disease evolution stage will dictate the best precision-based use of this therapy. Foundational studies showing lung and systemic ACE2 expression and activity during different disease stages will guide these optimal use interventions. In addition, preclinical and clinical studies will be essential to assess competing effects.
Other considerations.
Given ACE2 as the essential receptor for SARS-CoV-2, it is conceivable that targeting ACE2 provides an effective means for COVID-19 prevention and treatment. However, this candidate approach requires careful consideration of the pros and cons of available reagents and the clinical contexts in which they are likely to be effective. More importantly, because ACE2 is a multifunctional protein, all functions of ACE2, either separately or collectively, need to be considered in the context of COVID-19 progression. For instance, an underexplored nonenzymatic ACE2 function is its role as a cotransporter for neutral amino acid transporter B0AT1, or SLC6A19 (85), underscoring the multifaceted character of ACE2. Although SLC6A19 does not express in airway epithelial cells, it is abundantly expressed in enterocytes and some renal cells. Although there is no evidence that SLAC6A19 directly binds to or indirectly interacts with the S protein of SARS-CoV-2, a supercomplex formed by a dimer of ACE2–SLC6A19, in which ACE2 binds the viral protein and SLC6A19 stabilizes the heterodimer, could be possible. Altogether, the structural data suggest that even if SLAC6A19 does not directly interact with the virus, it may play a role in the virus's internalization, stabilizing, or participating in the receptor’s conformational changes (20).
Conclusions
Until effective anti–SARS-CoV-2 vaccines are ready for the public, alternative therapeutic options are urgently needed. Because ACE2 is the receptor for the virus and a modulator of inflammatory responses, targeting ACE2 is a logical option for COVID-19 treatment. Although only a select few ACE2-related reagents are tested in humans (e.g., sACE2, DIZE, Ang1–7), many have already been evaluated extensively in animal models. In conclusion, ACE2 targeting is an underexplored approach to COVID-19 therapeutics and may avail novel primary or adjunctive pipelines for this pandemic.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Zongyuan Wang for artistically optimizing the figures.
Footnotes
Supported by the U.S. National Institutes of Health grants AI149321 and AI148446 (H.J.).
Author Contributions: H.J., E.N., and H.C. contributed to this article equally.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0322PS on December 9, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bai C, Chotirmall SH, Rello J, Alba GA, Ginns LC, Krishnan JA, et al. Updated guidance on the management of COVID-19: from an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020) Eur Respir Rev. 2020;29:200287. doi: 10.1183/16000617.0287-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.To KK, Hung IF, Ip JD, Chu AW, Chan WM, Tam AR, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. doi: 10.1093/cid/ciaa1275. [online ahead of print] 25 Aug 2020; DOI: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, Painter CD, et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183:1043–1057.e15. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, Du P, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Therapy. 2020;5:283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amraie R, Napoleon MA, Yin W, Berrigan J, Suder E, Zhao G, et al. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2 and are differentially expressed in lung and kidney epithelial and endothelial cells [preprint]. bioRxiv. 2020 [accessed 2020 Nov 20]. Available from: https://www.biorxiv.org/content/10.1101/2020.06.22.165803v1.
- 7.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, et al. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–728, 18, 728. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- 8.Patel VB, Bodiga S, Fan D, Das SK, Wang Z, Wang W, et al. Cardioprotective effects mediated by angiotensin II type 1 receptor blockade and enhancing angiotensin 1-7 in experimental heart failure in angiotensin-converting enzyme 2-null mice. Hypertension. 2012;59:1195–1203. doi: 10.1161/HYPERTENSIONAHA.112.191650. [DOI] [PubMed] [Google Scholar]
- 9.Kassiri Z, Zhong J, Guo D, Basu R, Wang X, Liu PP, et al. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail. 2009;2:446–455. doi: 10.1161/CIRCHEARTFAILURE.108.840124. [DOI] [PubMed] [Google Scholar]
- 10.Lovren F, Pan Y, Quan A, Teoh H, Wang G, Shukla PC, et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol. 2008;295:H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 11.Nunes-Souza V, Alenina N, Qadri F, Penninger JM, Santos RA, Bader M, et al. CD36/sirtuin 1 axis impairment contributes to hepatic steatosis in ACE2-deficient mice. Oxid Med Cell Longev. 2016;2016:6487509. doi: 10.1155/2016/6487509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Huang XR, Chen HY, Fung E, Liu J, Lan HY. Deletion of angiotensin-converting enzyme-2 promotes hypertensive nephropathy by targeting Smad7 for ubiquitin degradation. Hypertension. 2017;70:822–830. doi: 10.1161/HYPERTENSIONAHA.117.09600. [DOI] [PubMed] [Google Scholar]
- 13.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Rostami MR, Leopold PL, Mezey JG, O’Beirne SL, Strulovici-Barel Y, et al. Expression of the SARS-CoV-2 ACE2 receptor in the human airway epithelium. Am J Respir Crit Care Med. 2020;202:219–229. doi: 10.1164/rccm.202003-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16:e9610. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radzikowska U, Ding M, Tan G, Zhakparov D, Peng Y, Wawrzyniak P, et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020;75:2829–2845. doi: 10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, III, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446, e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brielle ES, Schneidman-Duhovny D, Linial M. The SARS-CoV-2 exerts a distinctive strategy for interacting with the ACE2 human receptor. Viruses. 2020;12:497. doi: 10.3390/v12050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim H, Baek A, Kim J, Kim MS, Liu J, Nam KY, et al. Hot spot profiles of SARS-CoV-2 and human ACE2 receptor protein protein interaction obtained by density functional tight binding fragment molecular orbital method. Sci Rep. 2020;10:16862. doi: 10.1038/s41598-020-73820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang K, Li S, Pintilie G, Chmielewski D, Schmid MF, Simmons G, et al. A 3.4Å cryo-EM structure of the human coronavirus spike trimer computationally derived from vitrified NL63 virus particles [preprint]. bioRxiv. 2020 doi: 10.1017/qrd.2020.16. [accessed 2020 Nov 20]. Available from: https://www.biorxiv.org/content/10.1101/2020.08.11.245696v1. [DOI] [PMC free article] [PubMed]
- 24.Bestle D, Heindl MR, Limburg H, Van Lam van T, Pilgram O, Moulton H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020;3:e202000786. doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muus C, Luecken MD, Eraslan G, Waghray A, Heimberg G, Sikkema L, et al. The NHLBI LungMAP Consortium, and The Human Cell Atlas Lung Biological Network. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells [preprint]. bioRxiv. 2020 [accessed 2020 Aug 10]. Available from: https://www.biorxiv.org/content/10.1101/2020.04.19.049254v1.
- 26.Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39:e105114. doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280, e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung MK, Karnik S, Saef J, Bergmann C, Barnard J, Lederman MM, et al. SARS-CoV-2 and ACE2: the biology and clinical data settling the ARB and ACEI controversy. EBioMedicine. 2020;58:102907. doi: 10.1016/j.ebiom.2020.102907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onabajo OO, Banday AR, Stanifer ML, Yan W, Obajemu A, Santer DM, et al. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nat Genet. 2020;52:1283–1293. doi: 10.1038/s41588-020-00731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei C, Fu W, Qian K, Li T, Zhang S, Ding M, et al. Potent neutralization of 2019 novel coronavirus by recombinant ACE2-Ig [preprint]. bioRxiv. 2020 [accessed 2020 Sep 23]. Available from: https://www.biorxiv.org/content/10.1101/2020.02.01.929976v2.
- 35.Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batlle D, Wysocki J, Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond) 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 37.Davidson AM, Wysocki J, Batlle D. Interaction of SARS-CoV-2 and other coronavirus with ACE (Angiotensin-Converting Enzyme)-2 as their main receptor: therapeutic implications. Hypertension. 2020;76:1339–1349. doi: 10.1161/HYPERTENSIONAHA.120.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia HP, Look DC, Tan P, Shi L, Hickey M, Gakhar L, et al. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009;297:L84–L96. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haschke M, Schuster M, Poglitsch M, Loibner H, Salzberg M, Bruggisser M, et al. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin Pharmacokinet. 2013;52:783–792. doi: 10.1007/s40262-013-0072-7. [DOI] [PubMed] [Google Scholar]
- 40.Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wysocki J, Schulze A, Batlle D. Novel variants of angiotensin converting enzyme-2 of shorter molecular size to target the kidney renin angiotensin system. Biomolecules. 2019;9:886. doi: 10.3390/biom9120886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basit A, Ali T, Rehman SU. Truncated human angiotensin converting enzyme 2; a potential inhibitor of SARS-CoV-2 spike glycoprotein and potent COVID-19 therapeutic agent. J Biomol Struct Dyn. doi: 10.1080/07391102.2020.1768150. [online ahead of print] 20 May 2020; DOI: 10.1080/07391102.2020.1768150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu P, Wysocki J, Souma T, Ye M, Ramirez V, Zhou B, et al. Novel ACE2-Fc chimeric fusion provides long-lasting hypertension control and organ protection in mouse models of systemic renin angiotensin system activation. Kidney Int. 2018;94:114–125. doi: 10.1016/j.kint.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 44.Iwanaga N, Cooper L, Rong L, Beddingfield B, Crabtree J, Tripp RA, et al. Novel ACE2-IgG1 fusions with improved activity against SARS-CoV2 [preprint]. bioRxiv. 2020 doi: 10.1016/j.isci.2021.103670. [accessed 2020 Oct 15]. Available from: https://www.biorxiv.org/content/10.1101/2020.06.15.152157v1. [DOI] [PMC free article] [PubMed]
- 45.Zoufaly A, Poglitsch MP, Aberle JH, Hoepler W, Seitz T, Traugott M, et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020;8:1154–1158. doi: 10.1016/S2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glasgow A, Glasgow J, Limonta D, Solomon P, Lui I, Zhang Y, et al. Engineered ACE2 receptor traps potently neutralize SARS-CoV-2. Proc Natl Acad Sci USA. 2020;117:28046–28055. doi: 10.1073/pnas.2016093117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, Mukherjee A, Nelson D, Jozic A, Sahay G. Rapid generation of circulating and mucosal decoy ACE2 using mRNA nanotherapeutics for the potential treatment of SARS-CoV-2 [preprint]. bioRxiv. 2020 doi: 10.1002/advs.202202556. [accessed 2020 Nov 5]. Available from: https://www.biorxiv.org/content/10.1101/2020.07.24.205583v1. [DOI] [PMC free article] [PubMed]
- 48.Chan KK, Dorosky D, Sharma P, Abbasi SA, Dye JM, Kranz DM, et al. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science. 2020;369:1261–1265. doi: 10.1126/science.abc0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujii H, Inobe M, Hayakawa Y, Kimura F, Murakami M, Onishi Y, et al. Vaccination with B7-1+ tumor and anti-adhesion therapy with RGD pseudo-peptide (FC-336) efficiently induce anti-metastatic effect. Clin Exp Metastasis. 1998;16:141–148. doi: 10.1023/a:1021985002088. [DOI] [PubMed] [Google Scholar]
- 50.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huentelman MJ, Zubcevic J, Hernández Prada JA, Xiao X, Dimitrov DS, Raizada MK, et al. Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 2004;44:903–906. doi: 10.1161/01.HYP.0000146120.29648.36. [DOI] [PubMed] [Google Scholar]
- 53.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, et al. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J Biol Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haga S, Yamamoto N, Nakai-Murakami C, Osawa Y, Tokunaga K, Sata T, et al. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci USA. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu PCLX, Liu X, Li Y, Covington M, Wynn R, Huber R, et al. Identification of ADAM10 as a major source of HER2 ectodomain sheddase activity in HER2 overexpressing breast cancer cells. Cancer Biol Ther. 2006;5:657–664. doi: 10.4161/cbt.5.6.2708. [DOI] [PubMed] [Google Scholar]
- 56.Inoue Y, Tanaka N, Tanaka Y, Inoue S, Morita K, Zhuang M, et al. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang S, Guo F, Liu K, Wang H, Rao S, Yang P, et al. Endocytosis of the receptor-binding domain of SARS-CoV spike protein together with virus receptor ACE2. Virus Res. 2008;136:8–15. doi: 10.1016/j.virusres.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stepanovska B, Huwiler A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol Res. 2020;154:104170. doi: 10.1016/j.phrs.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Yang H, Liu H, Zeng Q, Imperato GH, Addorisio ME, Li J, et al. Inhibition of HMGB1/RAGE-mediated endocytosis by HMGB1 antagonist box A, anti-HMGB1 antibodies, and cholinergic agonists suppresses inflammation. Mol Med. 2019;25:13. doi: 10.1186/s10020-019-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mishra RK, Shum AK, Platanias LC, Miller RJ, Schiltz GE. Discovery and characterization of novel small-molecule CXCR4 receptor agonists and antagonists. Sci Rep. 2016;6:30155. doi: 10.1038/srep30155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Touret F, Gilles M, Barral K, Nougairède A, van Helden J, Decroly E, et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci Rep. 2020;10:13093. doi: 10.1038/s41598-020-70143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jia H. Pulmonary Angiotensin-Converting Enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46:239–248. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 63.Sodhi CP, Nguyen J, Yamaguchi Y, Werts AD, Lu P, Ladd MR, et al. A dynamic variation of pulmonary ACE2 is required to modulate neutrophilic inflammation in response to Pseudomonas aeruginosa lung infection in mice. J Immunol. 2019;203:3000–3012. doi: 10.4049/jimmunol.1900579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murça TM, Moraes PL, Capuruço CA, Santos SH, Melo MB, Santos RA, et al. Oral administration of an angiotensin-converting enzyme 2 activator ameliorates diabetes-induced cardiac dysfunction. Regul Pept. 2012;177:107–115. doi: 10.1016/j.regpep.2012.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qi Y, Zhang J, Cole-Jeffrey CT, Shenoy V, Espejo A, Hanna M, et al. Diminazene aceturate enhances angiotensin-converting enzyme 2 activity and attenuates ischemia-induced cardiac pathophysiology. Hypertension. 2013;62:746–752. doi: 10.1161/HYPERTENSIONAHA.113.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haber PK, Ye M, Wysocki J, Maier C, Haque SK, Batlle D. Angiotensin-converting enzyme 2-independent action of presumed angiotensin-converting enzyme 2 activators: studies in vivo, ex vivo, and in vitro. Hypertension. 2014;63:774–782. doi: 10.1161/HYPERTENSIONAHA.113.02856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burchill LJ, Velkoska E, Dean RG, Griggs K, Patel SK, Burrell LM. Combination renin-angiotensin system blockade and angiotensin-converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin Sci (Lond) 2012;123:649–658. doi: 10.1042/CS20120162. [DOI] [PubMed] [Google Scholar]
- 69.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 70.Clarke NE, Turner AJ. Angiotensin-converting enzyme 2: the first decade. Int J Hypertens. 2012;2012:307315. doi: 10.1155/2012/307315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khajah MA, Fateel MM, Ananthalakshmi KV, Luqmani YA. Anti-inflammatory action of angiotensin 1-7 in experimental colitis. PLoS One. 2016;11:e0150861. doi: 10.1371/journal.pone.0150861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bossi F, Bernardi S, De Nardo D, Bramante A, Candido R, Carretta R, et al. Angiotensin 1-7 significantly reduces diabetes-induced leukocyte recruitment both in vivo and in vitro. Atherosclerosis. 2016;244:121–130. doi: 10.1016/j.atherosclerosis.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 73.Breitling S, Krauszman A, Parihar R, Walther T, Friedberg MK, Kuebler WM. Dose-dependent, therapeutic potential of angiotensin-(1-7) for the treatment of pulmonary arterial hypertension. Pulm Circ. 2015;5:649–657. doi: 10.1086/683696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cassis P, Locatelli M, Corna D, Villa S, Rottoli D, Cerullo D, et al. Addition of cyclic angiotensin-(1-7) to angiotensin-converting enzyme inhibitor therapy has a positive add-on effect in experimental diabetic nephropathy. Kidney Int. 2019;96:906–917. doi: 10.1016/j.kint.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 75.Bodiga S, Zhong JC, Wang W, Basu R, Lo J, Liu GC, et al. Enhanced susceptibility to biomechanical stress in ACE2 null mice is prevented by loss of the p47(phox) NADPH oxidase subunit. Cardiovasc Res. 2011;91:151–161. doi: 10.1093/cvr/cvr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoffmann M, Hofmann-Winkler H, Smith JC, Kruger N, Sorensen LK, Sogaard OS, et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity [preprint]. bioRxiv. 2020 doi: 10.1016/j.ebiom.2021.103255. [accessed 2020 Nov 5]. Available from: https://www.biorxiv.org/content/10.1101/2020.08.05.237651v1. [DOI] [PMC free article] [PubMed]
- 77.Shulla A, Heald-Sargent T, Subramanya G, Zhao J, Perlman S, Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamamoto M, Kiso M, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Imai M, Takeda M, et al. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12:629. doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li W, Zhang C, Sui J, Kuhn JH, Moore MJ, Luo S, et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.C SK, Kumar SA, Wei H. Comparative docking studies to understand the binding affinity of nicotine with soluble ACE2 (sACE2)-SARS-CoV-2 complex over sACE2. Toxicol Rep. 2020;7:1366–1372. doi: 10.1016/j.toxrep.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson CF, Chakroun RW, Su H, Mitrut RE, Cui H. Interface-enrichment-induced instability and drug-loading-enhanced stability in inhalable delivery of supramolecular filaments. ACS Nano. 2019;13:12957–12968. doi: 10.1021/acsnano.9b05556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 83.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lock LL, Reyes CD, Zhang P, Cui H.Tuning cellular uptake of molecular probes by rational design of their assembly into supramolecular nanoprobes J Am Chem Soc 20161383533–3540.: [DOI] [PubMed] [Google Scholar]
- 85.Camargo SM, Singer D, Makrides V, Huggel K, Pos KM, Wagner CA, et al. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136:872–882. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jia H, Yue X, Lazartigues E. ACE2 mouse models: a toolbox for cardiovascular and pulmonary research. Nat Communications. 2020;22:5165. doi: 10.1038/s41467-020-18880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.