Abstract
The recent surge in applications of deuterated pharmaceutical agents has created an urgent demand for synthetic methods that efficiently generate deuterated building blocks. Here we show that N-heterocyclic carbenes (NHC) promote a reversible hydrogen-deuterium exchange (HDE) reaction with simple aldehydes, which leads to a practical approach to synthetically valuable C-1 deuterated aldehydes. The reactivity of the well-established NHC catalysed formation of Breslow intermediates from aldehydes is reengineered to overcome the overwhelmingly kinetically favorable, irreversible benzoin condensation reaction and achieve the critical reversibility to drive the formation of desired deuterated products when an excess of D2O is employed. Notably, this operationally simple and cost-effective protocol serves as a general and truly practical approach to all types of 1-D-aldehydes including aryl, -alkyl and -alkenyl aldehydes and enables chemoselective late-stage deuterium incorporation into complex, native therapeutic agents and natural products with uniformly high levels (>95%) of deuterium incorporation for a total of 104 substrates tested.
Introduction
The recent surge in applications of deuterated pharmaceutical agents1-3, including the FDA approval of the first deuterated drug, austedo in 20174, has created an urgent demand for synthetic methods that efficiently generate deuterated building blocks5-14. As one of the most widely utilized substances in synthesis15-24, aldehydes serve as a synthetic linchpin enabling quick access to a wide array of fascinating and structurally diverse deuterated building blocks and to biologically important molecular architectures. Traditionally, deuterated aldehydes are produced from esters (Fig. 1a, reaction A)25,26 or amides (reaction B)27,28 by using reduction and/or oxidation sequences. Although these reagents can be employed to prepare both deuterated aromatic and aliphatic aldehydes, their high cost restricts their broad use. Recently, Denmark, Xie, and Sheng reported several efficient approaches to the synthesis of aromatic deuterated aldehydes from the corresponding aryl iodides (reaction C)29, carboxylic acids (reaction D)30,31, or benzyl halides (reaction E)32. High yielding hydrogen-deuterium exchange (HDE) processes catalyzed by Ir (Fig. 1b, reaction F, D2 as deuterium source)33 or Ru (reaction G, D2O as deuterium source)34 have been developed to produce deuterated aromatic aldehydes directly without the need for functional group manipulation. However, it is recognized that an intrinsic difficulty exists in controlling reactivity (reactions C29 and F33), which often cause accompanying non-selective deuteration of aromatic rings. Furthermore, the protocols result in only moderate degrees of D-incorporation (14-84%) and their use is restricted to aromatic aldehydes. Clearly, an urgent need exists for the development of a unique activation mode for HDE processes that tolerate all types of aldehydes and is capable of achieving useful levels of deuterium incorporation (>95%).
Fig. 1. Methods for the synthesis of deuterated aldehydes.
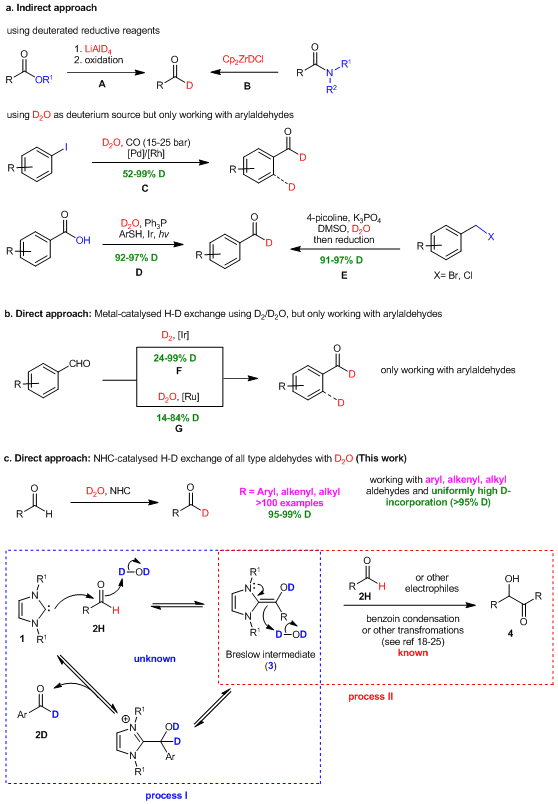
a, Indirect approaches involving transformations of different functional groups to deuterated aldehydes. b, Direct approach employing transition metal catalyzed hydrogen-deuterium exchange of aromatic formyl groups. c, The current work using organocatalytic direct hydrogen-deuterium exchange of the aromatic, aliphatic and α, β-unsaturated formyl group.
Here we wish to report a powerful and useful strategy that organocatalytic N-heterocyclic carbenes (NHC) promote hydrogen-deuterium exchange (HDE) reactions with all types of aldehydes including aryl, -alkyl and -alkenyl ones (Fig. 1c). This process enables to directly convert aldehydes to C-1 deuterated counterparts using cheap, safe and operationally convenient D2O as the deuterium source under mild reaction conditions without requiring additional functionality manipulation and with minimal byproduct production.
Results
Design plan
Activation of the inert formyl C-H bond of aldehydes by N-heterocyclic carbenes (NHCs) (1) via Breslow intermediate35 is a well-established strategy in organic synthesis (Fig. 1c). The nucleophilic nature of intermediate 3 has been used advantageously to promote reactions with various electrophiles in a number of impressive organic transformations including self-benzoin condensation (Process II)18-25. Recently, we questioned whether reactions that form Breslow intermediates could be redirected to create an unprecedented isodesmic HDE process for transforming readily accessible aldehydes into their 1-D-analogs. Specifically, we proffered that reaction of an aldehyde with an NHC carried out in D2O would reversibly produce the deuterated Breslow intermediate 3 (Process I) and that 3 could react with D2O to produce the desired deuterated aldehyde 2D with concurrent regeneration of the catalyst. Driving the reversible reaction towards the desired product 2D could be achieved by the formation of more stable C-D bond (bond energy of C-D as 341.4kJ/mol and C–H as 338kJ/mol) and by employing a large access amount of D2O. Nonetheless, we recognized that the thermodynamic process could be complicated by the existence of competitive, kinetically favored benzoin condensation reaction that is known to occur under the reaction conditions (Process II). This could be particularly problematic in HDE reactions of electron-withdrawing group (EWG) substituted aromatic aldehydes, which should be more prone to undergo condensation reactions with Breslow intermediates (e.g., 4). Furthermore, with respect to aliphatic aldehydes and enals, multiple reactive sites tend to generate more side products, which lead to lower reaction efficiency. This creates an significant challenge aimed at achieving high reaction yield while without sacrificing high level of deuterium incorporation of the HDE process.
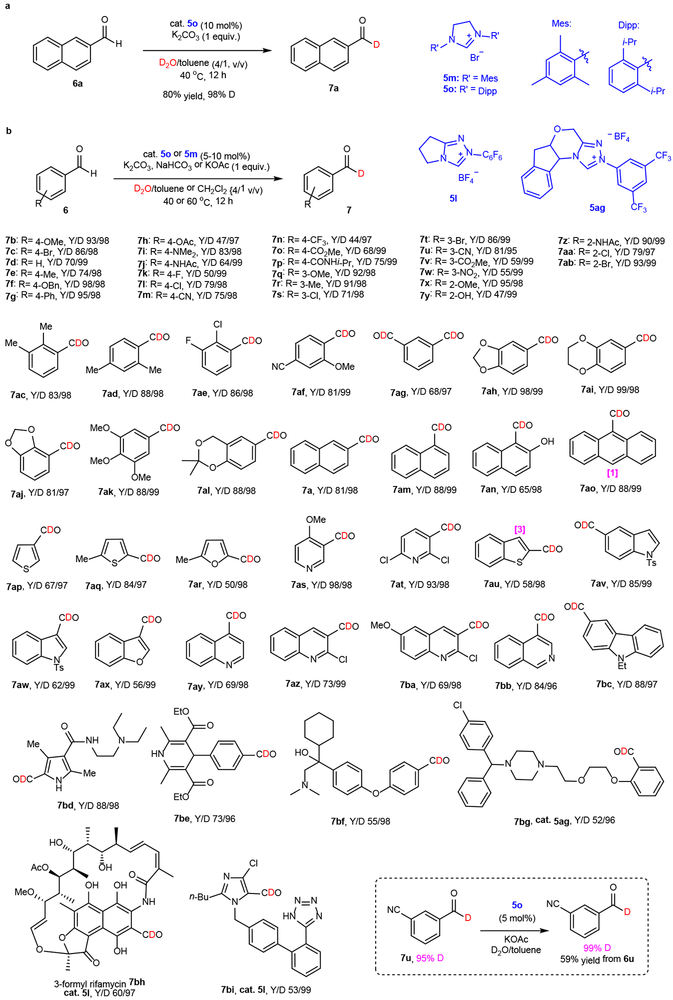
Exploration and reaction optimization for aromatic aldehydes
Guided by the reasoning that reactions of bulky NHC catalysts would form Breslow intermediates that are more reluctant to undergo undesired benzoin condensation and the electronic effect of substrates on reactivity, we explored the studies aimed at elucidating a general and optimal protocol for the HDE process by using electron-neutral 2-naphthaldehyde (6a) as a reactant and varied the NHC catalysts (see structures in Supplementary Figure 1), catalyst loading, base, solvent, time and temperature, and amount of D2O (see Supplementary Table 1). This effort led to establishment of the reagents, time and temperatures that promote most efficient HED reactions. Specifically, for effective reactions, it is important to utilize bulky NHC catalysts such as the N,N-dimesityl- or 2,6-diisopropy-imidazolium bromides 5m or 5o, 5-10 mol% K2CO3, NaHCO3 or KOAc as base (1.0 equiv), toluene or CH2Cl2 containing 1/4 v/v D2O as solvent, temperatures in the 40-60 °C range and a 12 h reaction time (Fig. 2a).
Fig. 2. Exploration and the scope of NHC catalysed HDE with aromatic aldehydes (61 examples).
a, Exploration of NHC catalysed HDE with model aromatic aldehyde. b, Scope of NHC catalysed HDE with aromatic aldehydes. The reaction details are given in supplemental materials (see section 4 in SI). Catalyst 5o was used, except for the following products: 7i, 7j, 7z, 7ad, 7ao, 7av-7ax, 7bd, and 7bf (cat. 5m); 7bg, and 7i (cat 5ag); and 7bh-7bi (cat. 5l). Y refers to isolated yields of deuterated products. It is noted that protium aldehyde starting materials are completely converted in these reactions and the major side products arise from benzoin condensation, and D refers to D-incorporation percentages based on the calculation described in Supplementary Equation 1. Some sites in compounds 7h, 7j, 7z, 7ao, and 7au (shown in their structures and the deuteration percentage indicated in bracket) are slightly deuterated observed by 2H NMR.
Scope of NHC catalysed HDE with aromatic aldehydes
Having established optimal conditions, we next explored the aldehyde scope of the NHC catalyzed HDE process. A study using aromatic aldehydes (Fig. 2b) showed that the conditions needed to bring about maximum yields and high levels of D-incorporation are substrate dependent (61 examples, see section 4 in SI for detailed reaction conditions including catalyst and catalyst loading, reaction temperature and solvent). Under appropriate conditions, HDE reactions of structurally diverse benzaldehydes and heteroaromatic aldehydes take place with uniformly high to excellent levels of D-incorporation (95-99%). Electron-poor aromatic aldehydes are competent substrates to deliver corresponding deuterated aldehydes in moderate to good yields (44-86%) (7c, 7k-7p, 7s-7w, 7aa-ab, and 7ae). Of particular note is the observation that the strong electron-withdrawing 3-nitro group substituted benzaldehyde 7w undergoes smooth HDE when 5o (5 mol%) is utilized as the catalyst, KOAc is employed as the base and 80 °C is the temperature. In contrast, reactions of electron-rich aryl aldehydes (7b, 7e, 7f, 7q, 7r, 7x-7z, 7ac, 7ad, 7ah-al) take place in higher yields as a consequence of the attenuation of undesired benzoin condensations. Notably, free phenolic hydroxyl groups have limited effects on the exchange reactions, as is demonstrated by the observations that 7y and 7an produce the deuterio-analogs with 98% D-incorporation. Moreover, the methodology can be employed to introduce deuterium into a substrate (6ag) containing two aldehyde groups. In this case, efficient double HDE occurs to produce the bis-deuterated counterpart 7ag in high yield and with an excellent level of D-incorporation. In addition to benzaldehyde derivatives, various electron-rich (7ap-7ar, 7au-7ax, 7bc, and 7bd) and poor (7as, 7at, 7ay-7bb) heteroaromatics, and polycyclic aromatics (7am-7ao) are viable substrates for the HDE reaction. Finally, advantages associated with the mild nature and selectivity of the metal free methodology are illustrated by the tolerance of transition metal sensitive halogen substituted substrates (7c, 7l, 7s, 7t, 7aa, 7ab, 7at, 7az and 7ba). Of further significance is the observation that the protocol enables late-stage HDE exchange reactions of structurally complex aldehydes (7be-7bi) in impressive yields (52-73%) and levels of D-incorporation (96-98%). Importantly, the level of D-incorporation can be improved by simply carrying out a second HDE reaction with the product of an initial HDE reaction (eg. 7u from 95% to 99%). Inversely, the C-1 deuterated aldehydes can be converted back to the corresponding protium products under identical conditions in the presence of excess H2O, as demonstrated experimentally with 7a and 7c.
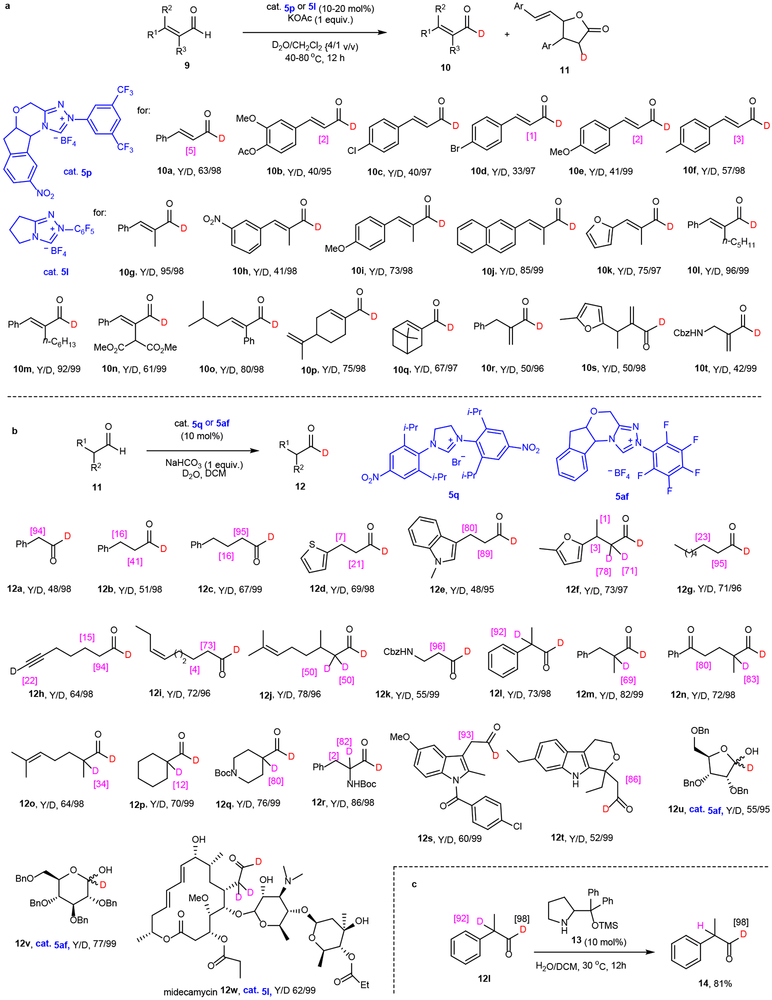
Scope of NHC catalyzed HDE with enals and aliphatic aldehydes
To demonstrate the wide substrate scope of the NHC catalysed HDE process, we explored its use in forming 1-D-substituted enals (Fig. 3a), potentially more problematic substrates owing to their higher tendency to form γ-butyrolactones 11 under NHC catalysis conditions36,37. Furthermore, unexpectedly the conditions and NHC catalysts used to promote HDE reactions of aromatic aldehydes do not lead to deuteration of enals like cinnamaldehyde (Supplementary Table 3 and 4). An intense investigation of this process showed that HDE reaction of cinnamaldehyde promoted by the triazolium salt 5p generates the corresponding 1-D-analog 10a in 63% yield with 97% D-incorporation. As demonstrated by the examples displayed in Figure 3a, the process using 5p represents a general approach to the preparation of deuterated cinnamaldehyde derivatives 10b-f (95-99% D-incorporation in albeit moderate yields). In HDE reactions of 2,3-disubstituted enals 9g-q, use of triazolium salt 5l rather than 5p enables formation of the corresponding 1-D-aldehydes with uniformly high levels of D-incorporation (97-99%). Finally, under the mild reaction conditions, HDE reactions of sensitive β-nonsubstituted enals 9r-t take place smoothly to produce the corresponding deuterated aldehydes 10r-t.
Fig. 3. Scope of NHC catalyzed HDE with enals (20 examples) and aliphatic aldehydes (23 examples).
a, Scope of NHC catalysed HDE reactions of enals. The reaction details are given in section 4 in SI. b, Scope of NHC catalysed HDE with aliphatic aldehydes. c, Amine catalyzed selective α-deuteration of bis-deuterated aldehydes. Some sites in 10a, 10b, 10d, 10e, 10f and 12f (shown in their structures and the deuteration percentage indicated in bracket) are slightly deuterated observed by 2H NMR.
The roles played by aliphatic aldehydes (alkanals) in organic synthesis are equal to those of aromatic aldehydes and enals. However, the preparation of 1-D analogs of alkanals remains an unsolved problem. Furthermore, as compared with aromatic and α,β-unsaturated counterparts, alkanals have been used less often in NHC catalysed processes18-25. These issues are associated with the presence in alkanals of multiple reactive sites, particularly enolizable α-positions, at which side reactions can occur. An extensive exploratory study aimed at assessing the applicability of the NHC promoted HDE reactions to alkanals demonstrated that optimal conditions are substrate dependent (Supplementary Table 4). An extensive evaluation of catalysts revealed the nitro-group containing imidazolinium salt 5q is an ideal choice. In reactions using 5q as catalyst, NaHCO3 as base and CH2Cl2 as solvent, a large number of structurally diverse alkanals, such as linear 11a-k (R2 = H), α-branched 11l-q, amine α-functionalized 11r and complex 11s-w derivatives are efficiently transformed to their respective 1-D-analogs (Fig. 3b). Notably, benzyl protected D-ribofuranose and D-glucose bearing hemiacetal can effectively participate in the process with 5af as promoter to give only α-deuterated products 12u-v. This protocol is complementary to a Ru-catalyzed HDE process, in which deuterium substitutes hydrogen occurring at the hydroxyl tethered carbon38. Furthermore, the protocol enables highly chemoselective deuteration of the aldehyde moiety in the densely functionalized macrolide antibiotic midecamycin (11w). However, a limitation of this reaction was also uncovered. For example, deuteration also takes place at α- and/or β-positions of alkanals. However, this problem is easily solved by subjecting the bis-deuterio product to a base promoted exchange reaction in H2O. For example, treatment of 12l with pyrrolidine derivative 13 in H2O/CH2Cl2 forms the 1-D-aldehyde 14 in 81% yield (Fig. 3c).
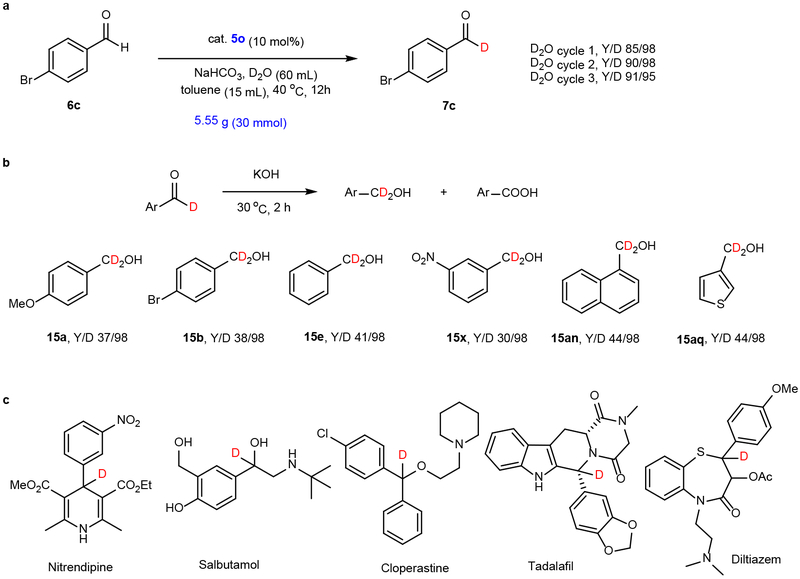
Synthetic application and D2O recycling
A gram-scale HDE reaction was conducted using aldehyde 6c and the same reaction conditions employed in the small-scale process (Fig 2b). The gram-scale counterpart formed 7c in a similar yield and with a comparable level of D-incorporation (Fig. 4a). Furthermore, the recovered D2O containing solvent can be used in a second and third reaction without causing a decrease in yield and D-incorporation level.
Fig. 4. Gram scale synthesis, recycling and reuse of D2O and a synthetic application.
a, In gram scale reactions, carried out using the same conditions employed for small scale, similar yields and levels of deuteration incorporation level are obtained. Moreover, D2O can be recycled and reused for subsequent HDE reactions without loss of the efficiency. b, Cannizzaro reaction for the synthesis of α, α-dideuterated benzylic alcohols. c, Standard protocols can be used to prepare deuterated therapeutics from the corresponding deuterated aromatic aldehydes. The benzylic positions in the therapeutics are easily metabolized sites (See Supplementary methods for detail).
As stated above, aldehydes are versatile starting materials and intermediates in organic synthesis. In the last phase of this investigation, we demonstrated that 1-D-aldehydes formed in the HDE process can be utilized in transformations that produce the deuterated analogs of important substances including aryl methanols and pharmaceuticals (Fig. 4b). For example, Cannizzaro reactions39 of 1-D-aldehydes conducted in concentrated KOH were found to produce the corresponding dideuterated benzyl alcohols in high yields. Furthermore, several deuterated analogs of clinically important drugs were synthesized using appropriate deuterated aldehydes as starting materials (Fig. 4c).
Conclusion
In summary, we have developed an NHC promoted reversible hydrogen-deuterium exchange (HDE) reaction for the efficient synthesis of synthetically valued C-1 deuterated aldehydes. A reactivity of NHC catalysis is successfully implemented to overcome the overwhelmingly kinetically favorable, irreversible benzoin condensation reaction and achieve the desired thermodynamic reversibility to drive the formation of desired deuterated products. The unique capacities of the NHC catalysed reversible processes have been demonstrated by directly converting aldehydes to C-1 deuterated counterparts using D2O as the deuterium source under mild reaction conditions without requiring additional functionality manipulation and thus with minimal byproduct formation. Furthermore, this operationally simple and cost-effective protocol serves as a general and truly practical approach to all types of 1-D-aldehydes including aryl, -alkyl and -alkenyl aldehydes, which are difficult to access by existing methods, and enables chemoselective late-stage deuterium incorporation into complex, native therapeutic agents and natural products (total 104 examples) with uniformly high levels (>95%) of deuterium incorporation. We anticipate that the approach will enable facile access to a wide range of synthetically versatile compounds and that it will accelerate the construction of new deuterated compounds for drug discovery.
Data Availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgements
Financial support was provided by the NIH (5R01GM125920-03), the National Natural Science Foundation of China (21702058 and 21202112), East China University of Science and Technology, the ‘111’ Project, and the Priority Academic Program Development of the Jiangsu Higher Education Institutes (PAPD).
Footnotes
Competing interests The authors declare no competing interests.
Additional Information Supplementary information and chemical compound information accompany this paper at www.nature.com/naturec.
References
- 1.Pirali T, Serafini M, Cargnin S & Genazzani AA Applications of deuterium in medicinal chemistry, J. Med. Chem 62, 5276–5297 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Mullard A Deuterated drugs draw heavier backing. Nat. Rev. Drug Discovery 15, 219–221 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Atzrodt J, Derdau V, Kerr WJ & Reid M Deuterium- and tritium-labelled compounds: Applications in the life sciences. Angew. Chem. Int. Ed 57, 1758–1784 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Schmidt C First deuterated drug approved. Nat. Biotechnol 35, 493–494 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Atzrodt J, Derdau V, Kerr WJ & Reid M C-H Functionalization for hydrogen isotope exchange. Angew. Chem. Int. Ed 57, 3022–3047 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Sattler A Hydrogen/deuterium (H/D) exchange catalysis in alkanes. ACS Catal. 8, 2296–2312 (2018). [Google Scholar]
- 7.Yu RP, Hesk D, Rivera N, Pelczer I & Chirik PJ Iron-catalysed tritiation of pharmaceuticals. Nature 529, 195–199 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Loh YY et al. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science 358, 1182–1187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koniarczyk J, Hesk D, Overgard A, Davies IW & McNally A A general strategy for site-selective incorporation of deuterium and tritium into pyridines, diazines and pharmaceuticals. J. Am. Chem. Soc 140, 1990–1993 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Hale LVA & Szymczak NK Stereoretentive deuteration of α-chiral amines with D2O. J. Am. Chem. Soc 138, 13489–13492 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valero M, Weck R, Ggssregen S, Atzrodt J & Derdau V Highly selective directed iridium-catalyzed hydrogen isotope exchange reactions of aliphatic amides. Angew. Chem. Int. Ed 57, 8159–8163 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X et al. General and practical potassium methoxide/disilane-mediated dehalogenative deuteration of (hetero)arylhalides. J. Am. Chem. Soc 140, 10970–10974 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Puleo TR, Strong AJ & Bandar JS Catalytic α-selective deuteration of styrene derivatives. J. Am. Chem. Soc 141, 1467–1472 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Liang X & Duttwyler S Efficient Brønsted-acid-catalyzed deuteration of arenes and their transformation to functionalized deuterated products. Asian J. Org. Chem 6, 1064–1071 (2017). [Google Scholar]
- 15.Taddei M & Mann A Hydroformylation for organic synthesis in Topics in Current Chemistry; Springer: Berlin, 2013; Vol. 342. [Google Scholar]
- 16.Erkkilä A, Majander I & M. Pihko PM, Iminium catalysis. Chem. Rev 107, 5416–5470 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee S, Yang JW, Hoffmann S & List B Asymmetric enamine catalysis. Chem. Rev 107, 5471–5569 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Enders D, Niemeier O & Henseler A Organocatalysis by N-heterocyclic carbenes. Chem. Rev 107, 5606–5655 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Flanigan DM, Romanov-Michailidis F, White NA & Rovis T Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev 115, 9307–9387 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bugaut X & Glorius F Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev 2012, 41, 3511–3522. [DOI] [PubMed] [Google Scholar]
- 21.Phillips EM, Chan A & Scheidt KA Discovering new reactions with N-heterocyclic carbene catalysis. Aldrichimica Acta 42, 55–66 (2009). [PMC free article] [PubMed] [Google Scholar]
- 22.Mahatthananchai J & Bode JW, J. W. On the mechanism of N-heterocyclic carbene-catalyzed reactions involving acyl azoliums. Acc. Chem. Res 47, 696–707 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Namitharan K et al. Metal and carbene organocatalytic relay activation of alkynes for stereoselective reactions. Nat. Commun 5, 3982 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Ren Q, Li M, Yuana L & Wang J Recent advances in N-heterocyclic carbene catalyzed achiral synthesis. Org. Biomol. Chem 15, 4731–4749 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Adcock HV, Chatzopoulou E & Davies PW Divergent C–H insertion–cyclization cascades of N–allyl ynamides. Angew. Chem. Int. Ed 54, 15525–15529 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen EPK, Singh T, Harris P, Andersson PG & Madsen R Experimental and theoretical mechanistic investigation of the iridium-catalyzed dehydrogenative decarbonylation of primary alcohols. J. Am. Chem. Soc 137, 834–842 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Spletstoser JT, White JM & Georg GI One-step facile synthesis of deuterium labeled aldehydes from tertiary amides using Cp2Zr(D)Cl. Tetrahedron Lett. 45, 2787–2789 (2004). [Google Scholar]
- 28.Spletstoser JT, White JM, Tunoori AR & Georg GI Mild and selective hydrozirconation of amides to aldehydes using Cp2Zr(H)Cl: scope and mechanistic insight. J. Am. Chem. Soc 129, 3408–3419 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim MYS & Denmark SE Palladium/rhodium cooperative catalysis for the production of aryl aldehydes and their deuterated analogues using the water–gas shift reaction. Angew. Chem. Int. Ed 57, 10362–10367 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Yuan X, Zhu C & Xie J Deoxygenative deuteration of carboxylic acids with D2O. Angew. Chem. Int. Ed 58, 312–316 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Bergin E Deoxygenative deuteration. Nat. Catal 1, 898–898 (2018) [Google Scholar]
- 32.Li X et al. One-pot synthesis of deuterated aldehydes from arylmethyl halides. Org. Lett 20, 1712–1715 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Kerr WJ, Reid M & Tuttle T Iridium–catalyzed formyl-selective deuteration of aldehydes. Angew. Chem. Int. Ed 56, 7808–7812 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Isbrandt ES, Vandavasi JK, Zhang W, Jamshidi MP & Newman SG Catalytic deuteration of aldehydes with D2O. Synlett 28, 2851–2854 (2017). [Google Scholar]
- 35.Breslow R On the mechanism of thiamine action. IV. evidence from studies on model systems. J. Am. Chem. Soc 80, 3719–3726 (1958). [Google Scholar]
- 36.Sohn SS, Rosen EL, & Bode JW N-Heterocyclic carbene-catalyzed generation of homoenolates: γ-butyrolactones by direct annulations of enals and aldehydes. J. Am. Chem. Soc 126, 14370–14371 (2004).. [DOI] [PubMed] [Google Scholar]
- 37.Burstein C & Glorius F Organocatalyzed conjugate umpolung of α, β-unsaturated aldehydes for the synthesis of γ-butyrolactones. Angew. Chem. Int. Ed 43, 6205–6208 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Fujiwara Y, Iwata H, Sawama Y, Monguchi Y & Sajiki H Method for regio-, chemo- and stereoselective deuterium labeling of sugars based on ruthenium-catalysed C–H bond activation. Chem. Commun 46, 4977–4979 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Mojtahedi MM, Akbarzadeh E, Sharifi R & Abaee MS Lithium bromide as a flexible, mild, and recyclable reagent for solvent-free Cannizzaro, Tishchenko, and Meerwein-Ponndorf-Verley reactions. Org. Lett, 9, 2791–2793 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.