Abstract
Background/Aims
Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, there have been concerns about the association between exposure to renin-angiotensin-aldosterone system (RAAS) inhibitors and the risk and severity of COVID-19.
Methods
We performed a case-control study that utilized up-to-date data on the South Korean population provided by the Korean National Health Insurance System. Of the 62,909 patients with hypertension or heart failure tested for COVID-19, there were 1,644 (2.6%) confirmed cases. After case-control matching, multivariable-adjusted conditional logistic regression analysis was performed.
Results
Comparison between patients exposed to RAAS inhibitors and those not exposed to RAAS inhibitors revealed that the adjusted odds ratio (OR) and 95% confidence interval (CI) for COVID-19 infection and death were 0.981 (95% CI, 0.849 to 1.135) and 0.875 (95% CI, 0.548 to 1.396), respectively. Subgroup analysis for the major confounders, age and region of diagnosis, resulted in OR of 0.912 (95% CI, 0.751 to 1.108) and 0.942 (95% CI, 0.791 to 1.121), respectively.
Conclusions
The present study demonstrated no evidence of association between RAAS inhibitor exposure and risk and severity of COVID-19.
Keywords: Renin-angiotensin system, COVID-19, Hypertension, Heart failure, Republic of Korea
INTRODUCTION
Since report of the first coronavirus disease 2019 (COVID-19) case in South Korea on January 20, 2020, various clustered outbreaks have contributed to its explosive spread during the first 2 months, which was followed by a slow reduction and plateaued state of newly confirmed cases [1]. Since the onset of the pandemic, there have been concerns regarding the association between renin-angiotensin-aldosterone system (RAAS) inhibitor exposure and risk and severity of COVID-19 infection [2]. The culprit virus, severe acute respiratory syndrome coronavirus 2 is known to infect host cells via membrane-bound angiotensin-converting enzyme (ACE) 2 [3]. The proximity of ACE2 to the ACE within the RAAS has raised concerns over the use of RAAS inhibitors, including ACE inhibitors or angiotensin receptor blockers (ARBs), which may affect risks to COVID-19 infection [3-5].
Several observational studies to date have demonstrated a lack of evidence supporting the association between RAAS inhibitor usage and the risk and severity of COVID-19 infection, thus upholding the current medical recommendation that patients should not discontinue these medications [6-10]. However, due to limitations of observational studies, exploratory, rather than definitive interpretation of these results could be performed, as there may have been confounders that were unaccounted for [11].
Due to the magnitude and persistent nature of the COVID-19 pandemic, and continuation of antihypertensive treatments, opportunities for randomized control trials or prospective cohort studies are limited. Therefore, despite their limitations, rigorous observational studies and pertinent follow-ups are the best tools we have for the present.
In July 2020, we published an article on the above subject using data provided by the Korean National Health Insurance System [12]. Since its publication, updates have been made to the dataset, with additional data provided by the Korean Centers for Disease Control and Prevention (KCDC), as well as the increased size of confirmed cases. As more accurate analysis was made possible, especially on COVID-19 death rates due to the addition of KCDC data, we performed an updated analysis to further strengthen our knowledge of the association between RAAS inhibitors and the risk and severity of COVID-19 infection.
METHODS
Data source
We analyzed the data obtained from the National Health Insurance claims of South Korea (https://hira-covid19.net/). Based on the insurance claims sent to the Health Insurance Review and Assessment Service on May 15, 2020, the current population-based dataset is comprised of all tested COVID-19 cases, including suspected and confirmed cases, as well as a history of medical services used by these individuals for the past 3 years. Data were completely anonymized and contained no identifiable information. This study was approved, and informed consent was waived by the Institutional Review Board of the Gwangju Institute of Science and Technology (20200413-EX-02-02).
Study population
Fig. 1 represents the study population of this case-control study. The study was performed on 234,427 subjects, all of whom were tested for COVID-19 with diagnosis codes of B342, B972, Z208, Z290, U18, U181, Z038, Z115, U071, and U072. The main study population was established based on the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes for hypertension (I10, I11) [13,14] or heart failure (I11.0, I13.0, I13.2, I50.0, I50.1, I50.9) [15] with at least one annual claim for prescription of an antihypertensive drug. A total of 62,909 subjects aged ≥ 19 years were analyzed. The laboratory diagnosis of COVID-19 in South Korea was based on guidelines provided by the KCDC and World Health Organization, which recommended polymerase chain reaction amplification of the viral E gene as a screening test, and amplification of the RdRp region of the orf1b gene as a confirmatory test [16]. The current dataset, especially concerning the confirmed and death cases, was updated by the COVID-19 patient dataset provided by the KCDC.
Figure 1.
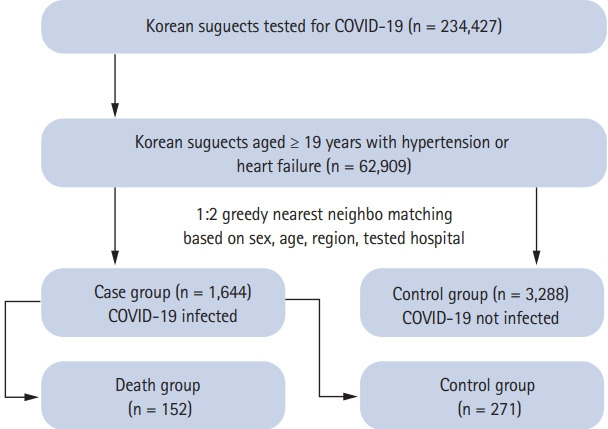
Flow of study subjects. COVID-19, coronavirus disease 2019.
As of May 26, the claims data of 7,590 out of 11,018 confirmed patients were matched and analyzed. Among the study group, there were 1,644 (2.6%) confirmed COVID-19 cases, which were designated as the case group; 61,265 (97.4%) uninfected cases were designated as the control group. Cases and controls were matched in 1:2 ratio based on covariates such as sex, age, region, and tested hospital. Subjects were classified into either Daegu and Gyeongbuk regions or other regions, as a binary variable. The hospitals where subjects had been tested were classified into tertiary hospitals and others. The matching was exact in sex, region, and tested hospital, but greedy nearest neighbor matching was performed on age, with a caliper of 0.1 in propensity scores. The final number of subjects was 1,644 and 3,288, for the case and control groups, respectively.
We analyzed the number of deaths in the case group, which was 165 (10.0%). We also matched cases versus controls in a 1:2 ratio within the infection group in the same manner as explained above. The final number of subjects was 152 and 271 for the case and control groups, respectively.
Classification of exposure to RAAS inhibitors
Exposure to RAAS inhibitors was defined by the type of drugs administered within 1 year. RAAS inhibitors include ACE inhibitors and ARBs. The classifications of the study cohort were composed of the following groups: non-exposure to RAAS inhibitors, exposure to RAAS inhibitors, exposure to ACE inhibitors, and exposure to ARBs. Two additional analyses were performed to verify the robustness of our study. For those with at least one claim within 6 months and 3 months for prescription of an antihypertensive drug, further classification was performed based on exposure to RAAS inhibitors, and additional analyses were performed.
Definition of covariates
Covariates were designated based on diagnosis codes of ICD-10. The covariates considered in this study were diabetes, dyslipidemia [13], myocardial infarction (MI), stroke [17], heart failure [15], liver disease, cancer, chronic obstructive pulmonary disease (COPD) [18], asthma [19], end-stage renal disease (ESRD) with dialysis [14], and immunocompromised status including autoimmune diseases and HIV infections [20]. Definitions of each comorbidity are presented in Supplementary Table 1. The most widely used comorbidity index, the Charlson comorbidity index (CCI) [21], was also applied as a covariate, and was classified as 0, 1, or ≥ 2.
Statistical analysis
Baseline characteristics of each group were presented as mean with standard deviation for continuous variables, and number with percentage (%) for categorical variables. Comparisons between case and control groups were performed using Student’s t tests for continuous variables, and chi-square or Fisher’s exact tests for categorical variables. Following case-control matching, the odds ratio (OR) and 95% confidence interval (CI) were calculated using conditional logistic regression analysis. Multivariable-adjusted conditional logistic regression analysis for infection outcomes and death was performed with adjustments for presence of diabetes, dyslipidemia, MI, stroke, heart failure, liver disease, cancer, COPD, asthma, ESRD with dialysis, immunocompromised status, and CCI. A subgroup analysis of COVID-19 infection according to age and region was also conducted to evaluate risk stratification. Statistical analyses were performed using the SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA). A p < 0.05 was considered to be statistically significant.
RESULTS
Baseline characteristics
Before matching, the case and control groups consisted of 1,644 and 61,265 subjects, respectively. Baseline characteristics of each group before matching are shown in Supplementary Table 2. After matching, a total of 4,932 subjects were identified and analyzed. The mean age was 65.5 years, and 2,142 (43.4%) subjects were men. The baseline characteristics of the case and control groups are presented in Table 1. The proportions of dyslipidemia, MI, stroke, heart failure, liver disease, cancer, COPD, asthma, ESRD with dialysis, and higher CCI scores were significantly higher in the control group as compared to the case group. The mortality rate was 2.7% in the control group and 10.0% in the case group (p < 0.0001). The proportion of RAAS inhibitor exposure was 74.9% in the control group and 74.0% in the case group (p = 0.5172). There were no significant differences in the exposure to RAAS inhibitors between the case and control groups.
Table 1.
Baseline characteristics of subjects according to coronavirus disease 2019 infection (n = 4,932)
| Characteristic | Control (n = 3,288) | Case (n = 1,644) | p value |
|---|---|---|---|
| Male sex | 1,428 (43.4) | 714 (43.4) | 1.0000 |
| Age, yr | 65.5 ± 13.7 | 65.5 ± 13.7 | 1.0000 |
| Age over 65 yr | 1,700 (51.7) | 850 (51.7) | 1.0000 |
| Region of diagnosis | |||
| Daegu & Gyeongbuk | 2,196 (66.8) | 1,098 (66.8) | 1.0000 |
| Tested hospital | |||
| 3rd | 646 (19.7) | 323 (19.7) | 1.0000 |
| Comorbidities | |||
| Diabetes | 1,114 (33.9) | 512 (31.1) | 0.0539 |
| Dyslipidemia | 2,013 (61.2) | 917 (55.8) | 0.0002 |
| MI & Stroke | 1,212 (36.9) | 456 (27.7) | < 0.0001 |
| Heart failure | 971 (29.5) | 339 (20.6) | < 0.0001 |
| Liver disease | 2,173 (66.1) | 962 (58.5) | < 0.0001 |
| Cancer | 601 (18.3) | 172 (10.5) | < 0.0001 |
| COPD | 1,276 (38.8) | 459 (27.9) | < 0.0001 |
| Asthma | 1,195 (36.3) | 466 (28.4) | < 0.0001 |
| ESRD with dialysis | 191 (5.8) | 18 (1.1) | < 0.0001 |
| Immunocompromised status | 422 (12.8) | 186 (11.3) | 0.1257 |
| Charlson comorbidity index | |||
| 0 | 185 (5.6) | 196 (11.9) | < 0.0001 |
| 1 | 407 (12.4) | 278 (16.9) | |
| ≥ 2 | 2,696 (82.0) | 1,170 (71.2) | |
| Exposure to RAAS inhibitors | |||
| RAAS inhibitors | 2,462 (74.9) | 1,217 (74.0) | 0.5172 |
| ACE inhibitors | 192 (5.8) | 85 (5.2) | 0.3360 |
| ARBs | 2,364 (71.9) | 1,172 (71.3) | 0.6549 |
| Death | 90 (2.7) | 165 (10.0) | < 0.0001 |
Values are presented as number (%) or mean ± SD.
MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; RAAS, renin-angiotensin-aldosterone system; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
In hypertensive or heart failure subjects with confirmed COVID-19 infection, baseline characteristics of the case and control groups according to death were analyzed in Table 2. As shown in Table 2, the prevalence of diabetes, heart failure, cancer, COPD, and CCI scores were higher in the case group when compared to the control group.
Table 2.
Baseline characteristics of subjects with confirmed coronavirus disease 2019 infection according to death (n = 423)
| Characteristic | Control (n = 271) | Case (n = 152) | p value |
|---|---|---|---|
| Male sex | 126 (46.5) | 73 (48.0) | 0.7620 |
| Age, yr | 77.6 ± 8.9 | 78.4 ± 9.0 | 0.3785 |
| Age over 65 | 243 (89.7) | 138 (90.8) | 0.7113 |
| Region of diagnosis | |||
| Daegu & Gyeongbuk | 224 (82.7) | 125 (82.2) | 0.9131 |
| Tested hospital | |||
| 3rd | 76 (28.0) | 47 (30.9) | 0.5319 |
| Comorbidities | |||
| Diabetes | 86 (31.7) | 89 (58.6) | < 0.0001 |
| Dyslipidemia | 152 (56.1) | 81 (53.3) | 0.5787 |
| MI & Stroke | 108 (39.9) | 64 (42.1) | 0.6508 |
| Heart failure | 73 (26.9) | 70 (46.1) | < 0.0001 |
| Liver disease | 156 (57.6) | 91 (59.9) | 0.6446 |
| Cancer | 25 (9.2) | 28 (18.4) | 0.0061 |
| COPD | 95 (35.1) | 75 (49.3) | 0.0040 |
| Asthma | 89 (32.8) | 61 (40.1) | 0.1326 |
| ESRD with dialysis | 2 (0.7) | 4 (2.6) | 0.1940 |
| Immunocompromised status | 35 (12.9) | 17 (11.2) | 0.6029 |
| Charlson comorbidity index | |||
| 0 | 16 (5.9) | 2 (1.3) | 0.0018 |
| 1 | 36 (13.3) | 8 (5.3) | |
| ≥ 2 | 219 (80.8) | 142 (93.4) | |
| Exposure to RAAS inhibitors | |||
| RAAS inhibitors | 185 (68.3) | 104 (68.4) | 0.9737 |
| ACE inhibitors | 22 (8.1) | 11 (7.2) | 0.7457 |
| ARBs | 172 (63.5) | 101 (66.5) | 0.5389 |
Values are presented as number (%) or mean ± SD.
MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; RAAS, renin-angiotensin-aldosterone system; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Association between exposure to RAAS inhibitors and risk and severity of COVID-19 infection
Table 3 shows the results of the logistic regression analysis for COVID-19 infection and death based on exposure to RAAS inhibitors. The adjusted OR for COVID-19 infection between those exposed to RAAS inhibitors and those not exposed to RAAS inhibitors within 1 year was 0.981 (95% CI, 0.849 to 1.135). When comparing exposure to RAAS inhibitors and non-exposure to RAAS inhibitors based on death, the adjusted OR were 0.875 (95% CI, 0.548 to 1.396). Additional analyses on at least one instance of RAAS inhibitor within 6 months and 3 months did not yield any significant different between case and control groups (p values > 0.05).
Table 3.
OR and 95% CI for outcome of coronavirus disease 2019 according to exposure to RAAS inhibitors
| Variable | Control group | Case group | Crude OR (95% CI) | p value | Adjusted ORa (95% CI) | p value | ||
|---|---|---|---|---|---|---|---|---|
| Within 1 year | ||||||||
| Infection | 3,288 (100) | 1,644 (100) | ||||||
| Without exposure to RAAS inhibitors | 826 (25.1) | 427 (26.0) | 1.000 | 1.000 | ||||
| Exposure to RAAS inhibitors | 2,462 (74.9) | 1,217 (74.0) | 0.955 (0.833–1.095) | 0.5135 | 0.981 (0.849–1.135) | 0.7984 | ||
| Exposure to ACE inhibitors | 192 (5.8) | 85 (5.2) | 0.879 (0.676–1.143) | 0.3368 | 1.074 (0.813–1.419) | 0.6171 | ||
| Exposure to ARBs | 2,364 (71.9) | 1,172 (71.3) | 0.969 (0.848–1.108) | 0.6488 | 0.964 (0.837–1.112) | 0.6172 | ||
| Death | 271 (100) | 152 (100) | ||||||
| Without exposure to RAAS inhibitors | 86 (31.7) | 48 (31.6) | 1.000 | 1.000 | ||||
| Exposure to RAAS inhibitors | 185 (68.3) | 104 (68.4) | 1.007 (0.657–1.544) | 0.9737 | 0.875 (0.548–1.396) | 0.5742 | ||
| Exposure to ACE inhibitors | 22 (8.1) | 11 (7.2) | 0.883 (0.416–1.874) | 0.7459 | 0.706 (0.316–1.576) | 0.3951 | ||
| Exposure to ARBs | 172 (63.5) | 101 (66.5) | 1.140 (0.751–1.731) | 0.5390 | 1.021 (0.646–1.615) | 0.9281 | ||
| Within 6 months | ||||||||
| Infection | 3,205 (100) | 1,603 (100) | ||||||
| Without exposure to RAAS inhibitors | 830 (25.9) | 432 (27.0) | 1.000 | 1.000 | ||||
| Exposure to RAAS inhibitors | 2,375 (74.1) | 1,171 (73.0) | 0.947 (0.826–1.086) | 0.4351 | 0.971 (0.843–1.120) | 0.6899 | ||
| Exposure to ACE inhibitors | 167 (5.2) | 72 (4.5) | 0.853 (0.641–1.135) | 0.2755 | 1.045 (0.774–1.412) | 0.7730 | ||
| Exposure to ARBs | 2,278 (71.1) | 1,125 (70.2) | 0.957 (0.837–1.093) | 0.5170 | 0.955 (0.832–1.098) | 0.5186 | ||
| Death | 263 (100) | 145 (100) | ||||||
| Without exposure to RAAS inhibitors | 87 (33.1) | 48 (33.1) | 1.000 | 1.000 | ||||
| Exposure to RAAS inhibitors | 176 (66.9) | 97 (66.9) | 0.999 (0.649–1.537) | 0.9961 | 0.862 (0.540–1.375) | 0.5324 | ||
| Exposure to ACE inhibitors | 18 (6.8) | 9 (6.2) | 0.901 (0.394–2.060) | 0.8043 | 0.654 (0.272–1.574) | 0.3433 | ||
| Exposure to ARBs | 163 (62.0) | 93 (64.1) | 1.097 (0.720–1.671) | 0.6657 | 1.026 (0.649–1.620) | 0.9132 | ||
| Within 3 months | ||||||||
| Infection | 3,101 (100) | 1,551 (100) | ||||||
| Without exposure to RAAS in- hibitors | 823 (26.5) | 427 (27.5) | 1.000 | 1.000 | ||||
| Exposure to RAAS inhibitors | 2,278 (73.5) | 1,124 (72.5) | 0.951 (0.829–1.091) | 0.4735 | 0.939 (0.813–1.085) | 0.3954 | ||
| Exposure to ACE inhibitors | 161 (5.2) | 64 (4.1) | 0.784 (0.581–1.056) | 0.1090 | 0.914 (0.668–1.250) | 0.5727 | ||
| Exposure to ARBs | 2,173 (70.1) | 1,080 (69.6) | 0.979 (0.857–1.119) | 0.7583 | 0.949 (0.825–1.092) | 0.4639 | ||
| Death | 256 (100) | 140 (100) | ||||||
| Without exposure to RAAS inhibitors | 87 (34.0) | 46 (32.9) | 1.000 | 1.000 | ||||
| Exposure to RAAS inhibitors | 169 (66.0) | 94 (67.1) | 1.052 (0.679–1.629) | 0.8208 | 0.946 (0.590–1.517) | 0.8173 | ||
| Exposure to ACE inhibitors | 18 (7.0) | 8 (5.7) | 0.801 (0.339–1.893) | 0.6136 | 0.587 (0.236–1.457) | 0.2505 | ||
| Exposure to ARBs | 156 (60.9) | 89 (63.6) | 1.119 (0.731–1.713) | 0.6060 | 1.102 (0.692–1.755) | 0.6824 | ||
Values are presented as number (%).
OR, odds ratio; CI, confidence interval; RAAS, renin-angiotensin-aldosterone system; ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blocker.
Adjusted for diabetes, dyslipidemia, myocardial infarction, stroke, heart failure, liver disease, cancer, chronic obstructive pulmonary disease, asthma, end-stage renal disease with dialysis, immunocompromised status, and Charlson comorbidity index.
Subgroup analysis of COVID-19 infection based on exposure to RAAS inhibitors
Table 4 shows the results of subgroup analyses based on age and region. The adjusted OR for COVID-19 infection when comparing exposure to RAAS inhibitors and non-exposure to RAAS inhibitors in those over 65 years was 0.912 (95% CI, 0.751 to 1.108); in those under 65 years, the adjusted OR was 1.073 (95% CI, 0.858 to 1.340). Furthermore, regional analysis of Daegu and Gyeongbuk versus other regions did not yield any significant association between COVID-19 infection and RAAS inhibitor exposure (p values > 0.05).
Table 4.
Subgroup analysis for coronavirus disease 2019 infection according to exposure to RAAS inhibitors
| Subgroup | Control group | Case group | Crude OR (95% CI) | p value | Adjusted ORa (95% CI) | p value | |
|---|---|---|---|---|---|---|---|
| Age over 65 | 1,700 (100) | 850 (100) | |||||
| Without exposure to RAAS inhibitors | 468 (27.5) | 268 (31.5) | 1.000 | 1.000 | |||
| Exposure to RAAS inhibitors | 1,232 (72.5) | 582 (68.5) | 0.820 (0.682–0.984) | 0.0331 | 0.912 (0.751–1.108) | 0.3531 | |
| Exposure to ACE inhibitors | 129 (7.6) | 50 (5.9) | 0.765 (0.548–1.069) | 0.1168 | 0.878 (0.615–1.254) | 0.4746 | |
| Exposure to ARBs | 1,166 (68.6) | 552 (64.9) | 0.842 (0.704–1.007) | 0.0590 | 0.901 (0.745–1.089) | 0.2793 | |
| Age under 65 | 1,588 (100) | 794 (100) | |||||
| Without exposure to RAAS inhibitors | 358 (22.5) | 159 (20.0) | 1.000 | 1.000 | |||
| Exposure to RAAS inhibitors | 1,230 (77.5) | 635 (80.0) | 1.160 (0.942–1.430) | 0.1628 | 1.073 (0.858–1.340) | 0.5385 | |
| Exposure to ACE inhibitors | 63 (4.0) | 35 (4.4) | 1.119 (0.730–1.714) | 0.6060 | 1.529 (0.964–2.424) | 0.0710 | |
| Exposure to ARBs | 1,198 (75.4) | 620 (78.1) | 1.162 (0.947–1.425) | 0.1505 | 1.044 (0.838–1.299) | 0.7020 | |
| Daegu & Gyeongbuk | 2,196 (100) | 1,098 (100) | |||||
| Without exposure to RAAS inhibitors | 557 (25.4) | 299 (27.2) | 1.000 | 1.000 | |||
| Exposure to RAAS inhibitors | 1,639 (74.6) | 799 (72.8) | 0.907 (0.768–1.070) | 0.2456 | 0.942 (0.791–1.121) | 0.4999 | |
| Exposure to ACE inhibitors | 144 (6.6) | 63 (5.7) | 0.868 (0.640–1.177) | 0.3624 | 1.053 (0.765–1.448) | 0.7532 | |
| Exposure to ARBs | 1,567 (71.4) | 765 (69.7) | 0.919 (0.782–1.081) | 0.3075 | 0.923 (0.778–1.094) | 0.3541 | |
| Etc. | 1,092 (100) | 546 (100) | |||||
| Without exposure to RAAS inhibitors | 269 (24.6) | 128 (23.4) | 1.000 | 1.000 | |||
| Exposure to RAAS inhibitors | 823 (75.4) | 418 (76.6) | 1.069 (0.838–1.363) | 0.5926 | 1.036 (0.792–1.354) | 0.7981 | |
| Exposure to ACE inhibitors | 48 (4.4) | 22 (4.0) | 0.913 (0.545–1.529) | 0.7298 | 1.079 (0.600–1.939) | 0.7998 | |
| Exposure to ARBs | 797 (73.0) | 407 (74.5) | 1.087 (0.856–1.380) | 0.4928 | 1.035 (0.796–1.345) | 0.7986 | |
Values are presented as number (%).
RAAS, renin-angiotensin-aldosterone system; OR, odds ratio; CI, confidence interval; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Adjusted for diabetes, dyslipidemia, myocardial infarction, stroke, heart failure, liver disease, cancer, chronic obstructive pulmonary disease, asthma, end-stage renal disease with dialysis, immunocompromised status, and Charlson comorbidity index.
DISCUSSION
In our case-control study, we matched 1,644 patients with hypertension or heart failure who were tested positive for COVID-19 with 3,288 patients who were tested negative, by sex, age, region of diagnosis, and tested hospital. Multivariable logistic regression analysis showed no association between exposure to RAAS inhibitors and COVID-19 infections or death. Subgroup analyses of age and region showed no significant difference between the two groups when adjusted for covariates. Overall, this study shows no evidence of any association between exposure to RAAS inhibitors and the risk and severity of COVID-19 infection.
Compared to our previous study, the current analysis consisting of updated data contains some improvements and clarifications [12]. Most importantly, compared with the similar mortality (3.9% vs. 4.0%) between the control and case groups in the previous analysis, the current analysis showed significant differences between the two groups by 2.7% and 10.0%, which corresponds better with overall mortality of COVID-19 patients as well as mortality of patients with hypertension, as reported in other observational studies [22,23]. This clarification may be due to mitigation of insurance claim data limitation by incorporating data from the KCDC, which oversees the COVID-19 pandemic in South Korea. We only included death and COVID-19 infection as our target of analysis in order to control the quality of our data, as the KCDC only provided data for these two variables. Moreover, since RAAS inhibitors are not only used in hypertension but also in the majority of cases of heart failure, we included those with heart failure to the study group and analyzed accordingly.
Additional analysis was performed within the subject group; baseline characteristics of the case and control groups according to death are presented in Table 2. According to this analysis, the prevalence of diabetes, heart failure, cancer, COPD, and CCI scores were higher in the case group. Although no positive relationships could be drawn regarding the association between RAAS inhibitor exposure and the risk and severity of COVID-19 infection, there may be a positive relationship between diabetes, heart failure, cancer, COPD, and CCI scores and COVID-19 mortality. A recent cross-sectional, observational, multicenter, nationwide study performed in Italy also discussed the factors that may contribute to the mortality of COVID-19 patients. After correction by multivariate analysis, factors such as age, diabetes mellitus, COPD, and chronic kidney disease but not hypertension predicted mortality, which partially agrees with the results of our study [22].
Since discussions regarding the topic of this study began in April 2020, many investigators have synchronously shown that there is no association between exposure to RAAS inhibitors and the risk and severity of COVID-19 infections, with detailed differences in study setting [6,24]. A recent study by Jung et al. [23], using the same dataset as our previous study, have shown no significant differences between RAAS inhibitor users and nonusers in terms of adverse outcomes among confirmed cases of COVID-19 infection. Compared with this study, our analyses have used an updated dataset with the inclusion of KCDC mortality data, enabling us to minimize the limitation of a claims data based study. To conclude, regardless of study design and the accumulation of data, current case-control observational study indicates no evidence of association between RAAS inhibitor usage and risk and severity of COVID-19 infection.
This study has a few limitations. First, although we incorporated data from the KCDC, the possibility of discrepancy between the actual therapeutic practice and insurance claim remains. To obtain validation, we used widely accepted definitions of clinical outcomes and covariates from previously performed studies [13- 15,17-20]. Also, considering the rigorous control of National Health Insurance system of South Korea in the diagnosis of COVID-19, hypertension, heart failure, and prescription of drugs, results are not likely to be confounded. Second, due to the innate limitation of this being an observational study, not every confounding factor could be considered and controlled. Third, South Korean medical practices show a preference for ARBs over ACE inhibitors, with the use of ACE inhibitors in monotherapy only accounting for 1.9%, thereby contributing to the possible weakness of this study [25]. Lastly, due to the retrospective observational design, causal inferences could not be made regarding the relationship between exposure to RAAS inhibitors and the risk and severity of COVID-19 infection, which calls for further investigations and possibly, randomized control trials.
KEY MESSAGE
1. Analysis on updated data show no association between exposure to renin-angiotensin-aldosterone system (RAAS) inhibitors and the risk and severity of coronavirus disease 2019 (COVID-19) infection in South Korea.
2. When the subjects on RAAS inhibitors with confirmed COVID-19 infection were analyzed according to death, the prevalence of diabetes, heart failure, cancer, chronic obstructive pulmonary disease, and Charlson comorbidity index scores were higher in the case group, requiring consideration for further investigation.
Acknowledgments
The authors appreciate the Ministry of Health and Welfare and the Health Insurance Review and Assessment Service of Korea for providing invaluable National Health Insurance claims data in a prompt manner.
Footnotes
No potential conflict of interest relevant to this article was reported.
Supplementary Material
Definition of covariates
Baseline characteristics of subjects according to coronavirus disease 2019 infection before matching (n = 62,909)
REFERENCES
- 1.Kim M, Park S, Kim Y, et al. Weekly report on the COVID-19 situation in the Republic of Korea (As of June 20, 2020) Public Health Wkly Rep. 2020;13:1859–1865. [Google Scholar]
- 2.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N Engl J. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5:1–6. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Ruiz I. RAAS inhibitors do not increase the risk of COVID-19. Nat Rev Cardiol. 2020;17:383. doi: 10.1038/s41569-020-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Son M, Seo J, Yang S. Association between renin-angiotensin-aldosterone system inhibitors and COVID-19 infection in South Korea. Hypertension. 2020;76:742–749. doi: 10.1161/HYPERTENSIONAHA.120.15464. [DOI] [PubMed] [Google Scholar]
- 13.Kim JA, Lee JS, Chung HS, et al. Impact of visit-to-visit fasting plasma glucose variability on the development of type 2 diabetes: a nationwide population-based cohort study. Diabetes Care. 2018;41:2610–2616. doi: 10.2337/dc18-0802. [DOI] [PubMed] [Google Scholar]
- 14.Kim MK, Han K, Kim HS, et al. Effects of variability in blood pressure, glucose, and cholesterol concentrations, and body mass index on end-stage renal disease in the general population of Korea. J Clin Med. 2019;8:755. doi: 10.3390/jcm8050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JH, Lim NK, Cho MC, Park HY. Epidemiology of heart failure in Korea: present and future. Korean Circ J. 2016;46:658–664. doi: 10.4070/kcj.2016.46.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong KH, Lee SW, Kim TS, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020;40:351–360. doi: 10.3343/alm.2020.40.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee G, Choi S, Kim K, et al. Association of hemoglobin concentration and its change with cardiovascular and allcause mortality. J Am Heart Assoc. 2018;7:e007723. doi: 10.1161/JAHA.117.007723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SR, Choi EK, Kwon S, et al. Effectiveness and safety of contemporary oral anticoagulants among Asians with nonvalvular atrial fibrillation. Stroke. 2019;50:2245–2249. doi: 10.1161/STROKEAHA.119.025536. [DOI] [PubMed] [Google Scholar]
- 19.Kim AM, Kang S, Park JH, Kim Y. Regional variation of hospitalization rates for asthma in Korea: association with ambient carbon monoxide and health care supply. Int J Environ Res Public Health. 2020;17:1244. doi: 10.3390/ijerph17041244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YJ, Lee CN, Lee MS, et al. Recurrence rate of herpes zoster and its risk factors: a population-based cohort study. J Korean Med Sci. 2018;34:e1. doi: 10.3346/jkms.2019.34.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. A critical review of available methods. J Clin Epidemiol. 2003;56:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 22.Iaccarino G, Grassi G, Borghi C, et al. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the Italian Society of Hypertension. Hypertension. 2020;76:366–372. doi: 10.1161/HYPERTENSIONAHA.120.15324. [DOI] [PubMed] [Google Scholar]
- 23.Jung SY, Choi JC, You SH, Kim WY. Association of renin-angiotensin-aldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study. Clin Infect Dis. 2020:ciaa624. doi: 10.1093/cid/ciaa624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korean Society Hypertension (KSH) Hypertension Epidemiology Research Working Group. Kim HC, Cho MC. Korea hypertension fact sheet 2018. Clin Hypertens. 2018;24:13. doi: 10.1186/s40885-018-0098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definition of covariates
Baseline characteristics of subjects according to coronavirus disease 2019 infection before matching (n = 62,909)


