Abstract
Multidisciplinary approaches to conservation and wildlife management are often effective in addressing complex, multi-factor problems. Emerging fields such as conservation physiology and conservation behaviour can provide innovative solutions and management strategies for target species and systems. Sensory ecology combines the study of ‘how animals acquire’ and process sensory stimuli from their environments, and the ecological and evolutionary significance of ‘how animals respond’ to this information. We review the benefits that sensory ecology can bring to wildlife conservation and management by discussing case studies across major taxa and sensory modalities. Conservation practices informed by a sensory ecology approach include the amelioration of sensory traps, control of invasive species, reduction of human–wildlife conflicts and relocation and establishment of new populations of endangered species. We illustrate that sensory ecology can facilitate the understanding of mechanistic ecological and physiological explanations underlying particular conservation issues and also can help develop innovative solutions to ameliorate conservation problems.
Keywords: Conservation, multidisciplinary, sensory ecology, sensory modality
Introduction
Animals possess a variety of sensory systems that perceive salient features of the environment and facilitate critical, fitness-enhancing decisions (Dusenbery, 1992). Sensory systems thus evolved to allow animals to detect a variety of potentially important signals and cues such as light, sound, chemical, mechanical (Bradbury and Vehrencamp, 2011), magnetic (Wiltschko and Wiltschko, 2005) and electric (Bullock, 1973; Himstedt et al., 1982; Scheichet al., 1986) stimuli. Presence and acuity of sensory systems vary across species (Smith, 2008), allowing them to inhabit different environments or different functional niches within the same environment (Horodysky et al., 2010; Safi and Siemers, 2010). The field of sensory ecology studies how animals acquire, process and use sensory stimuli from their environment (Dusenbery, 1992). In the past decade, sensory ecology has seen an increase in research activity and associated literature, which has enhanced mechanistic understanding of animal behaviour (Stevens, 2013; Ruxton et al., 2018).
Insights from sensory ecology have important implications for conservation and management of wildlife with connections to the emerging disciplines of conservation behaviour (Buchholz, 2007; Blumstein and Fernández-Juricic, 2010) and conservation physiology (Cooke et al., 2013) and their intersection (Cooke et al., 2014; Horodysky et al., 2016). For example, by quantifying the range of stimuli that animals perceive, we can predict potential responses to environmental change, including urbanization and other human development, enabling better management decisions and informing future infrastructure designs to minimize harm to wildlife (Lim et al., 2008, Blumstein and Fernández-Juricic, 2010). In particular, as the human footprint expands across the Earth, sound and light pollution have increased and these can negatively affect animals by masking their natural sensory cues and signals or distracting and confusing them, potentially imposing negative fitness consequences (Halfwerk and Slabbekoorn, 2015; Dominoni et al., 2020). Increasing research focus on sensory mechanisms in focal species can provide vital information on how anthropogenic light or sound pollution can impact the decision-making processes of wild animals. Understanding the perceptual worlds of different species also helps prevent and ameliorate ecological and sensory traps and reduce human–wildlife conflict (Madliger, 2012) and has been beneficial in some instances at increasing the success rates of species translocations and re-introductions (Swaisgood, 2010). We can also exploit sensory perceptions to better manage target species as demonstrated by control of destructive, invasive species (Cruz et al., 2009; Johnson et al., 2009), predator and pest control (Maguire et al. 2009., Witzgall et al., 2010) and deterring animals from dangerous sites (Elvidge et al., 2018).
Although ecological research on visual and auditory senses has been conducted for nearly a century (Bayliss et al., 1936; Clarke, 1936; Hailman, 1977; Lythgoe, 1979; Neuweiler, 1989), only in the past two decades has the literature on sensory ecology expanded to include more taxa and sensory modalities with several important syntheses on the topic (see Endler and Basolo, 1998; Dangles et al., 2009; Stevens, 2013; Cronin et al., 2014; Martin, 2017). Similarly, an increasing amount of research is being conducted in applied sensory ecology, and we are seeing more case studies successfully applying this knowledge to aid in animal conservation or management. Several recent general reviews discuss the potential implications of sensory ecology for conservation biology (Madliger, 2012; Fernández-Juricic, 2016; Madliger et al., 2016; Dominoni et al., 2020), although most syntheses to date are species-, taxa- or sensory modality-specific (for example, Laiolo, 2010; Campbell-Palmer and Rosell, 2011; Martin, 2012; Jordan et al., 2013; Sorensen and Johnson, 2016). As the volume of primary literature and case studies of successful integration of sensory ecology and conservation science continue to grow, there is need for a more comprehensive review to understand the current state of knowledge and to inform future research and application.
Here, we present a comprehensive overview of the benefits to wildlife conservation and management that emerge from an understanding of sensory ecology and use the term ‘wildlife’ broadly to include all animal taxa. We review three major sensory modalities (vision, audition and chemoreception) as well as less understood modalities (electroreception, magnetoreception) and present case studies highlighting sensory ecology approaches relevant to conservation and management. In particular, we discuss case studies where sensory ecology has been demonstrated to successfully benefit a conservation problem (see Table 1), and also sensory ecology research that has furthered our understanding of certain conservation problems with potential to aid in the development of an innovative solution (see Table 2). Our review includes both vertebrate (mammals, birds, fish, reptiles, amphibians) and invertebrate studies where supporting literature is available and identifies major gaps in knowledge and avenues for future research (Fig. 1). We also discuss the importance of considering multimodal stimuli and some of the challenges associated with using sensory ecology knowledge to inform conservation and management.
Table 1. Summary of successful applications of sensory ecology in conservation and wildlife management.
| Sensory modality | Taxa | Species | Conservation issue solved | Overview | Reference |
|---|---|---|---|---|---|
| Vision | Bird | Canada geese (Branta canadensis Linnaeus) | Reduce airstrikes | Development of artificial lights to minimize collisions with aircraft | Blackwell et al., 2012 |
| Fairy terns (Sterna nereis davisae) | Relocation | Visual decoys attracted endangered fairy terns to safe breeding areas | Jeffries and Brunton, 2001 | ||
| Griffon vultures (Gyps fulvus) | Relocation | Cliff paintings that mimicked droppings attracted vultures to nest on suitable cliffs | Sarrazin et al., 1996 | ||
| Fish | White sturgeon (Acipenser transmomtanus) | Reduce entrapment and entrainment | Behavioural guidance of age-0 white sturgeon using coloured and strobing lights | Ford et al., 2018 | |
| Invertebrate | Mayflies (Ephemeroptera), stoneflies (Trichoptera), dolichopodid dipterans, and tabanid flies (Tabanidae) | Ameliorate sensory trap | Fragmenting the solar-active area of solar panels reduced attractiveness of these panels to aquatic insects | Horváth et al., 2010 | |
| Reptile | Green sea turtle (Chelonia mydas) | Ameliorate sensory trap | New dimmer, amber lights on Florida beaches reduced misguidance of sea turtle hatchlings away from the ocean | Witherington et al., 2014 | |
| Green sea turtle (Chelonia mydas) | Reduce bycatch | Bycatch of turtles in commercial bottom gillnet fisheries reduced through use of LED and chemical light stick deterrents. | Wang et al., 2013 | ||
| Audition | Bird | Black-capped vireo (Vireo atricapilla) | Relocation | Playing recordings of conspecific song attracted birds to more suitable habitats safe from brood parasitic species. | Ward and Schlossberg,2004 |
| European starling (Sturnus vulgaris) | Reduce airstrikes | Use of sound frequencies to create a ‘sonic net’ to deter birds from airfields | Swaddle et al., 2016 | ||
| Mammal | Harbour porpoise (Phocoena phocoena) | Reduce bycatch | Acoustic alarms successfully reduced bycatch of porpoises in gillnet fisheries, without reduced catch of target species | Larsen and Eigaard, 2014 | |
| Fish | Asian carps (Hypophthalmichthys nobilis, H molitrix Valenciennes) | Invasive species control | Sound barriers effective at controlling spread of invasive Asian carps in the Great Lakes | Ruebush et al., 2012 | |
| Multiple | Relocation | Playback of healthy reef sounds increased fish abundance and species richness in degraded coral reef habitat | Gordon et al., 2019 | ||
| Chemoreception | Bird | Hooded plover (Thinornis rubricollis) | Reduce predation on threatened species | Conditioned taste aversion successful at reducing predation on threatened hooded plover eggs | Maguire et al., 2009 |
| Mammal | Common goat (Capra hircus) | Invasive species control | Female goats captured, sterilized and put in a chemically induced estrus to release pheromones to attract males for ineffective mating. Eradicated invasive goats from certain Galapagos Islands | Cruz et al., 2009 | |
| Fish | Sea lamprey (Petromyzon marinus) | Invasive species control | Pheromone-based trapping highly effective, species-specific, method of capturing invasive sea lamprey in the Great Lakes | Johnson et al., 2009 | |
| Invertebrate | Gypsy moth (Lymantria dispar L.) | Invasive species control | Invasive gypsy moths in the USA have devasting effects of forests that can be effectively controlled with pheromone traps | Tobin and Blackburn, 2007 | |
| Electroception | Fish | Hammerhead sharks (Sphyrna lewini) | Reduce bycatch | Commercial trawl fishing hooks made from electrorepulsive metals successful at reducing bycatch of sharks | Hutchinson et al., 2012 |
This table summarizes case studies where sensory ecology has been demonstrated to benefit conservation or wildlife management. We summarize relevant literature from all sensory modalities (vision, olfaction, chemoreception, electroreception and magnetoreception), across six major taxa (birds, mammals, fish, invertebrates, reptiles and amphibians) where valid case studies exist. We note that no relevant literature was found for amphibian species.
Table 2. Areas of sensory ecology research that have furthered our understanding of sensory issues, with potential to help solve conservation issues.
| Sensory modality | Taxa | Species | Overview | Reference | Conservation potential |
|---|---|---|---|---|---|
| Vision | Bird | Brown-headed cowbird (Molothrus ater) | Certain wavelengths are more likely to enhance detection and avoidance by individuals | Doppler et al., 2015; Goller et al., 2018 | Using these lights on aircraft to reduce collisions. |
| Various | Birds have more laterally projected vision, and this may contribute to increased collisions with human infrastructure | Martin, 2011 | Warning signs placed on the ground before human infrastructure may be more beneficial to helping birds avoid collisions | ||
| Mammal | Laboratory Wistar rats | Artificial light at night negatively affects sleep in rats | Stenvers et al., 2016 | Increase our understanding of the negative impacts artificial light at night can have on various animals | |
| Tammar wallabies (Macropus eugenii) | Artificial light at night can affect reproductive timing of tammar wallabies | Robert et al., 2015 | Increase our understanding of the effects of artificial light at night. Reduce artificial light at night during reproductive seasons of tammar wallabies | ||
| Invertebrate | Various | Artificial light at night causes temporal disorientation, phototaxis and visual desensitisation | Owens and Lewis, 2018; Wakefield et al., 2016 | Increase our understanding on this sensory trap. Possible solutions such as reducing the intensity or duration of artificial light at night, or using frequencies less attractive to insects | |
| Reptile | Green sea turtle (Chelonia mydas) | Sea turtles are more likely to consume plastic waste that resembles their common prey | Schuyler et al., 2014 | Increase our understanding of this sensory trap. Reduce plastic waste in ocean | |
| Audition | Bird | Western bluebird (Siatia Mexicana) | Noisy gas compressor stations increase physiological stress and reduce hatching success compared with quieter sites | Kleist et al., 2018 | Use of newer or quieter compressor stations or using improved sound insulation at compressor sites |
| Ovenbird (Seiurus aurocapilla) | Noisy compressor sites reduce mating success by dampening conspecific calls | Habib et al., 2007 | Use of newer or quieter compressor stations or using improved sound insulation at compressor sites | ||
| Various | Roadway traffic noise alone compromises avian density and overall body condition of birds in the area | Ware et al., 2015 | Increase our understanding of the impacts of noise pollution | ||
| Mammal | Pinnipeds | Acoustic deterrents effective at deterring pinnipeds from fish farms and fisheries | Götz and Janik, 2013 | This knowledge could be used in a conservation context to prevent bycatch, or vessel collisions, of pinnipeds | |
| Brazilian free-tailed bat (Tadarida brasiliensis) | Activity of bats was lower at noisy compressor sites compared with quieter stations | Bunkley et al., 2015 | Use of newer or quieter compressor stations or using improved sound insulation at compressor sites | ||
| Greater mouse-eared bats (Myotis myotis) | Smooth vertical building surfaces can act as acoustic mirrors to echolocating bats, resulting in collisions | Greif et al., 2017 | Redesign of certain buildings making them more ‘visible’ to echolocating bats | ||
| Killer whales (Orcinus orca) | Noise from shipping traffic is a chronic stressor for killer whales and can disrupt foraging behaviour, pod communication, and echolocating clicks to detect prey | Williams et al., 2019b | Re-routing ships to avoid major whale habitat, use of quieter ships, reducing speed at which ships travel near important whale habitat | ||
| Fish | Ambon damselfish (Pomacentrus amboinensis) | Ambon damselfish physiologically stressed by motorboat noise and show reduced ability to evade predators | Simpson et al., 2016 | Banning motorboats from certain areas of important damselfish habitat | |
| Invertebrate | Zooplankton | Seismic studies used to detect petroleum in the ocean can cause significant mortality of zooplankton | McCauley et al., 2017 | Further our understanding. Reduce the number of seismic studies conducted in the ocean | |
| Field crickets (Gryllus bimaculatus) | Traffic noise distracted female field crickets meaning they did not orient to male auditory calls | Schmidt et al., 2014 | Further our understanding | ||
| Hermit crab (Coenobita clypeatus) | Sound of motorboat noise distracts crabs and modifies their risk assessment allowing humans to get closer before retracting in their shells | Chan et al., 2010 | Banning motorboats from certain areas of important crab habitat | ||
| Squid (Doryteuthis pealeii) | Pile driving sounds elicited body pattern changes, inking, jetting and startle responses | Jones et al., 2020 | Further our understanding. Future consideration in placement of wind farms to avoid important squid habitat | ||
| Chemoreception | Bird | Seabirds (order: Procellariiformes) | Marine plastics release DMS, which is also a common odorant released by the prey of many seabirds. Resulting plastic ingestion can lead to mortality | Savoca et al., 2016 | Potential to synthesize plastics without chemical compounds attractive to seabirds or other marine life |
| Mammal | Harvest mice (Micromys minutus) | Captive breeding improved in mice by familiarizing females with male scents | Roberts and Gosling, 2004 | Proof of concept. This technique could be used to improve breeding success of endangered species, or species in captivity | |
| Pygmy loris (Nycticebus pygmaeus) | Urine scent has been shown to influence mate choice in threatened female pygmy loris | Fisher et al., 2003 | This knowledge can be used to promote more beneficial mate pairing in this threatened species. Proof of concept—this technique could be used to promote more beneficial mate parings in other endangered species | ||
| Fish | Sheephead swordtail fish (Xiphophorus birchmanni) | Water polluted by sewage effluent chemicals has been shown to reduce female preference for male olfactory cues | Fisher et al., 2006 | Further our understanding of the negative impacts of water pollution on fish species, which could aid in stressing the need to reduce sewage effluent in natural aquatic habitats | |
| Invertebrate | Spanish moon moth (Graellsia isabellae); Valley elderberry longhorn beetle (Desmocerus californicus dimorphus); Rare click beetle (Betarman bisbimaculatus) | Pheromone trapping effective at attracting endangered species | Millar et al., 2010; Ray et al., 2014; Konig et al., 2016 | Potential to use pheromone trapping as an effective way at monitoring populations of endangered invertebrates | |
| Magnetoreception | Bird | European robin (Erithacus rubecula) | Electromagnetic noise from human activity disrupts magnetic compass orientation | Engels et al., 2014 | Investigate use of potential electromagnetic frequencies that do not disrupt magnetic compass orientation |
| Invertebrate | Edible crab (Cancer pagurus) | Electromagnetic field emissions attracted crabs to underwater cable sites and reduced foraging behaviour | Scott et al., 2018 | Consideration of the location of underwater cable routes in areas of important crab habitat | |
| Multisensory | Fish | Sea lamprey (Petromyzoa marinus) | ‘Pull’ of attractive light frequencies and ‘push’ of odor repellents effective at controlling juvenile sea lamprey movement | Johnson et al., 2019 | Potential for more effectively controlling movement of sea lamprey to either (a) protect individuals from entrapment/entrainment, or (b) control spread of invasive lamprey in certain areas |
This table summarizes sensory ecology research that has furthered our understanding of certain conservation issues, with potential to help develop innovative solutions to these problems. Although the case studies summarized here have not been demonstrated to benefit conservation practices, we do discuss potential ways in which the knowledge could be applied for the benefit of conservation and wildlife management. We summarize relevant literature from all sensory modalities (vision, olfaction, chemoreception, electroreception and magnetoreception), across six major taxa (birds, mammals, fish, invertebrates, reptiles and amphibians) where appropriate research exists. We note that no relevant literature was found for amphibian species.
Figure 1.
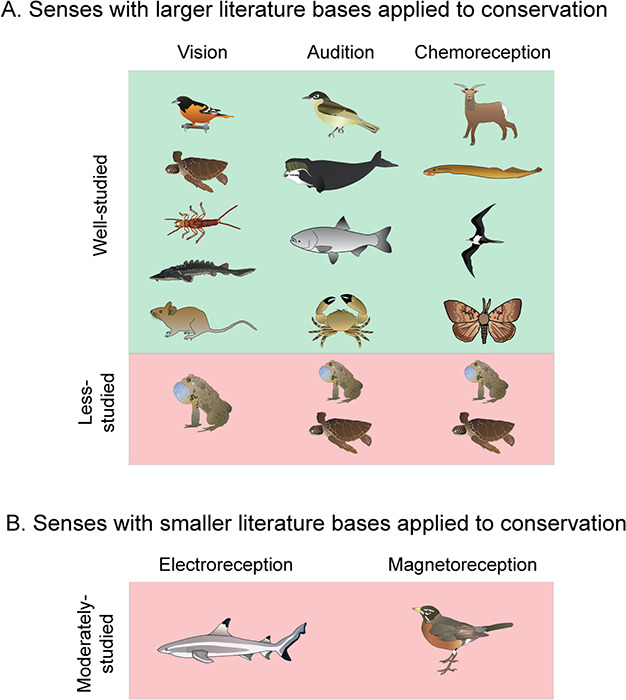
This figure provides a visual representation of taxa with known case studies demonstrating the application of sensory ecology to benefit wildlife conservation and management. Animals are categorized into six taxa: birds, mammals, invertebrates, fish, reptiles or amphibians. Each of these taxa is visually represented in the figure by a species from that taxon for which there has been a notable research demonstrating strong benefits of the integration of sensory ecology with conservation. Part (A) represents the three common senses, vision, audition and chemoreception, for which there is a larger amount of literature linking sensory ecology and conservation. Each of the six taxa specified by this paper are here categorized as either ‘well studied’ or ‘less studied’ depending on the presence or absence, respectively, of known literature linking sensory ecology to the conservation of species (or multiple species) of that taxon. Part (B) represents two sensory modalities, electroreception and magnetoreception, which have considerably smaller literature bases linking sensory ecology to conservation. However, we do note a single case study for each of these sensory modalities for which sensory ecology has benefited conservation of a species. Select Images by S. Bell, J. Hawkey, L. Fishman, K. Kraeer, and T. Saxby, courtesy of the Integration and Application Network, University of Maryland Center for Environmental Science (ian.umces.edu/symbols/).
Vision
Vision as a sensory modality can be defined not only as the ability to detect and respond to light stimuli, but also the ability to detect spatial structure and form an image (Stevens, 2013). Light is electromagnetic radiation exploited by many animals in the form of visual cues and signals (Stevens, 2013). Composed of photons travelling in waves, different types of electromagnetic radiation are grouped into functional categories by wavelength, ranging from very short and relatively high-energy (gamma and x-rays) to long and relatively low-energy (radio waves and microwaves) along the electromagnetic spectrum. Species, and even individuals, vary in the wavelength of light they are able to detect. Visible light, as defined by human detection ability (400–700 nm), is very roughly intermediate in the spectrum, yet photoreceptors in animal eyes are often sensitive enough to respond to wavelength differences between captured photons and perceive differences as colour. Beyond the light spectrum visible to humans, ultraviolet and infrared visual sensitivity is employed by numerous other species across different taxa. Photons also vary in properties beyond wavelength, notably the direction of their vibrating electric fields. All photons travel with a vibrating electric field that is perpendicular to the direction of motion or propagation, and the orientation of this electric field to the axis of propagation is referred to as the e-vector angle (Johnsen, 2012). Natural, unpolarized light consists of photons all with different e-vector angles, whereas polarized light consists of photons that all have mostly the same e-vector angle. While many species cannot perceive polarized light, others are capable of detecting it to inform spatial orientation, including some birds, fishes, reptiles, amphibians and both terrestrial and aquatic invertebrates (Cronin et al., 2003; Douglas et al., 2007).
In this section we review visual ecology research for five major taxa (birds, reptiles, fish, invertebrates and mammals) that has benefited conservation and wildlife management. Amphibian vision has been relatively understudied for the context of conservation (Fig. 1), and thus we do not cover this animal class here. Understanding what various species perceive has been important in understanding certain conservation issues and sensory traps, for example bird collisions, turtle hatchling misguidance and aquatic insects mistaking solar farms and roadways for water. Furthering our understanding of these visual traps has led to innovative solutions to these problems, as we will highlight through various case studies. Exploiting species vision has also proven to be beneficial to species relocation and translocation efforts, as well as guiding animals around, or alerting them to, particular hazards in their environment. Throughout this section we discuss case studies of successful application of visual ecology knowledge that has proven beneficial to a particular conservation problem (Table 1), as well as knowledge that has highlighted a particular conservation problem and has potential to lead to an effective solution (Table 2).
Birds
Birds are visually oriented animals whose cone photoreceptor cells also have pseudoorganelles (oil droplets) that can enhance color discrimination (Martin, 2017). Color perception in birds is then a function of the spectral sensitivity of their visual pigments as well as the absorbance properties of their oil droplets (Martin and Osorio, 2008), and it has been suggested that avian color perception may vary considerably among species (Hart and Hunt, 2007). This variation poses a challenge for using visual beacons (e.g. LED lights) to prevent different bird species from colliding with human infrastructure (including buildings, wind turbines, aircraft, etc.). It has been estimated that millions of birds collide with buildings annually (Machtans et al., 2013) and thousands are reported to collide with aircraft (Dolbeer et al., 2015). However, artificial lighting can help prevent these collisions and some strategies to standardize the development of visual deterrent beacons have been implemented (Fernández-Juricic, 2016) by (a) characterizing key properties of the visual system in species with high frequencies of collisions, (b) including information of these visual properties on avian visual models to predict which wavelengths may be most stimulating to retinal photoreceptors and (c) conducting behavioral studies to assess which of these most-stimulating light colours can lead to changes in obstacle detection and avoidance behaviors. More specifically, these strategies have been applied to the development of aircraft running lights to minimize bird–aircraft collisions (Blackwell et al., 2012; Blackwell and Fernández-Juricic, 2013).
Sensory physiology has allowed for the characterization of the brown-headed cowbird (Molothrus ater) visual system (Blackwell et al., 2009; Fernández-Juricic et al., 2013), which subsequently allowed researchers to develop potential aircraft lighting to increase detection and avoidance by this species. Researchers have developed species-specific visual models (Goller et al., 2018), which yielded four wavelengths with high chances of stimulating the retinal cells of brown-headed cowbirds (380 nm, 470 nm, 525 nm and 630 nm, roughly corresponding to ultraviolet, blue, green and orange, respectively). In turn, behavioral studies have shown that LED lights with a 470 nm (blue) peak led to quicker visual detection (Doppler et al., 2015) and avoidance behavior (Goller et al., 2018). Collisions between birds and aircraft are not only often fatal to the bird but also can cause damage to aircraft thus representing a threat to public safety (Dolbeer, 2006), and aircraft running lights optimized for bird detection and avoidance offer great potential for reducing such collisions. Similarly, furthering our knowledge on avian vision has also helped understand bird–building collisions. Birds often focus on reflections of vegetation in the glass of buildings and are also known to have more lateral vision focused towards the ground (Martin, 2011), which can lead to collisions. As a result of this knowledge, certain cities such as New York City (NYCAS, 2007), Toronto (CTGDS, 2007) and San Francisco (SFPD, 2011) have released guidelines for bird-friendly building designs to minimize collision.
Avian vision can also be exploited to aid in the successful relocation of endangered species. Visual conspecific decoys have been used to successfully attract endangered fairy terns (Sterna nereis davisae) to safe breeding areas (Jeffries and Brunton, 2001), and painting rocks to mimic faecal droppings was successful at attracting griffon vultures (Gyps fulvus) to nest on cliffs that had not been chosen as nesting sites by this species for ~60 years (Sarrazin et al., 1996). Such manipulations may be effective in certain situations for social animals that use visual cues to detect conspecifics, and the efficacy of such manipulations has been formally reviewed (Putman and Blumstein, 2019).
Mammals
Although mammals typically view the world through the ‘visible’ range of light, some species are also cabable of detecting ultraviolet wavelengths. For example the Arctic reindeer (Rangifer tarandus) that exploits ultraviolet vision to search for lichens and other food sources in dark winter periods (Hogg et al., 2011). Other mammalian species have also evolved eyes better adapted to visualizing their environments, such as marine and aquatic mammals (Mass and Supin, 2007) and nocturnal mammals, which typically have evolved larger eyes to capture more light in dark environments (Hall et al., 2012). Nocturnal mammals can be negatively affected by artificial light at night. In mammals, like most other taxa, the daily light–dark cycle is responsible for synchronizing the internal circadian clock, which is responsible for many metabolic, and ultimately behavioural, functions. Although there has been a great amount of research on mammalian vision, to our knowledge there has been very little application of mammalian visual ecology to benefit conservation effects to date. Therefore in this section we focus on research highlighting the effects of artificial light at night on noctural animals, and discuss the potential of this knowledge for conservation purposes.
Artificial light at night has been shown to increase body mass in mice (laboratory Swiss–Webster mice; Fonken et al., 2010), affect sleep in rat species (laboratory Wistar rats; Stenvers et al., 2016) and affect reproductive timing in wild tammar wallabies (Macropus eugenii: Robert et al., 2015). These behavioural changes can all negatively affect fitness and may therefore have population-level effects. More research into the effect of light at night on various species helps understand to what extent this light pollution is having across all species, which will further emphasize a need to better regulate light at night or help us develop innovative solutions to more environmentally friendly night lighting. Interestingly, there have been some beneficial effects of artificial lighting on certain mammalian species. Artificial light at night can cause phototaxis for a number of invertebrate species including moths (as will be discussed later in this section). Nocturnal feeding mammals can take advantage of this higher density of invertebrates around night lighting. One study found moth consumption of Cape serotine bats (Neoromicia capensis) under night lighting conditions to increase 6-fold compared with unlit conditions (Minnaar et al., 2015). In this instance artificial light at night is beneficial to the predator species but leaves the prey species more vulnerable to consumption.
Fish
Most fish species have well-developed eyes, evolved to allow them to effectively see in subaqueous environments (Guthrie, 1986; Wagner, 2011). Even in deep sea bathypelagic zones, (beyond 1000 m in depth) where the only light present is that which emanates from bioluminescent animals, bathypelagic fish species have well-developed eyes (Landgren et al., 2014). Beyond the visible light range, certain fish species are capable of detecting ultraviolet (Flamarique and Hawryshyn, 1998, Smith et al., 2002) and infrared radiation (Meuthen et al., 2012). Research into fish vision has shown that certain light features can act as attractants or repellants for various species of fish, and this knowledge can be successfully exploited to help protect fish by repelling or guiding them away from harmful obstacles in waterbodies. Visual guidance in fish can therefore be an effective tool in conservation and wildlife management.
It has been known for some time that white or mercury vapour light (a high intensity discharge lamp) can be an effective repellant or attractant for various fish species (Haymes et al., 1984; Patrick et al., 1985), and strobe lighting mechanisms can be effective at guiding fish around human infrastructure such as dams to prevent entrapment or injury (Brown, 2000). However, the responses to various light wavelengths are species specific, which can cause problems when using light to guide or repel fish to or from certain areas. Fish species can have different capabilities in the detection and processing of visual stimuli (Horodysky et al., 2008; Horodysky et al., 2010; Morshedian and Fain, 2015). This is important to know when attempting to use visual cues as attractants or deterrents (Elvidge et al., 2019) or to evaluate the efficacy of bycatch reduction strategies without reducing target catch. In this context, sensory physiology has been used to determine peak sensitivities of various fish species based on the absorbance properties of the visual pigments in the retina (Sillman et al., 2007). Additionally, the prediction of peak sensitivities and subsequent behavioural assays have determined fish response to these light frequencies. For example, studies have documented peak sensitivity and positive phototaxis of white sturgeon (Acipenser transmontanus) to green, red and blue light (Sillman et al., 2007; Ford et al., 2018), both positive and negative phototaxis in response to different colours in lake sturgeon (Acipenser fulvescens: Sillman et al., 2007; Elvidge et al., 2019), and negative phototaxis in response to blue light in the American eel (Anguilla rostrata: Elvidge et al., 2018). Here, an understanding of sensory physiology leads to a better understanding of behavioural responses to different portions of the light spectrum. This could be highly beneficial in many circumstances, including attracting fish towards fishways enabling them to bypass dams or other obstacles, or in deterring fish from harmful infrastructure such as hydroelectric turbines or boat propellers, however, differences in species-specific responses to various wavelengths must be well considered.
Invertebrates
There is a large amount of literature and research on insect vision, particularly for model species such as Drosophila melanogaster (Borst, 2009). Insects have anatomically and physiologically different eyes than vertebrates (Borst, 2009); however, most insects do have well-developed eyes and rely heavily on their vision. Some insect species are capable of detecting ultraviolet radiation, such as butterflies (Bicyclus anynana) for selecting mates (e.g. Lyytinen et al., 2004), and others can detect infrared radiation, such as black fire beetles (Melanophila acuminata), which use infrared to detect forest fires from distances indicating suitable low-risk places for females to lay eggs (e.g. Schmitz and Bleckmann, 1998). Certain insects also have eyes adapted for noctural vision (Warrant, 2017) and a number of species (in particular aquatic species) are capable of detecting polarized light and this has important implications for their survival. However, human development has led to several visual traps, such as artificial light at night, solar farms and roadways, which negatively impact certain insect species.
Solar farms and roadways can reflect polarized light, and for certain aquatic insects, their vision perceives these reflected light sources as a water surface on which they can lay their eggs (Schwind, 1995; Horváth et al., 2010). This knowledge has led to better solar panel designs to reduce their attractiveness to aquatic insects. One study was successful at reducing the attractiveness of solar panels by 10- to 26-fold by fragmenting their solar-active area with white partitions (Horváth et al., 2010). Artificial light at night has also been shown to have negative effects on certain insects, including spatial and temporal disorientation, attraction through positive phototaxis and visual desensitization due to high illumination (Grubisic et al., 2018; Owens and Lewis, 2018). A greater understanding of these sensory issues could lead to innovative solutions such as reducing the intensity or duration of lights at night, or using light frequencies that are less attractive to insects (Wakefield et al., 2016).
Reptiles
Vision is important for many reptile species, which can differ in their retinal physiology and morphology to adapt to diurnal, nocturnal or crepuscular activity (Katti et al., 2019). Reptiles can detect electromagnetic radiation from ultraviolet (Kawamura and Yokoyama, 1998) to infrared wavelengths (Gracheva et al., 2010). Research into the sensory ecology of reptile vision has been important in conservation, particularly of certain turtle species. There are a number of conservation issues for turtle species resulting from visual traps due to anthropogenic activity, such as plastic ingestion in marine environments, misguidance of turtle hatchlings and bycatch in commercial fisheries.
Turtle visual ecology research has offered insight and further understanding into a common sensory trap for marine wildlife: plastic ingestion. Sea turtles are known to be vulnerable to plastic waste because turtle vision may perceive certain plastics as prey (Fritts, 1982). Plastic bags or balloons can be confused with jellyfish, a common prey species for sea turtles, and research has suggested that turtles are more likely to consume waste that resembles their prey (Schuyler et al., 2014). When ingested, the plastic cannot be digested and may have consequences including physical blockage of the digestive system, often resulting in death (Wilcox et al., 2018). The amount of plastic waste in the ocean is expected to keep increasing (Jambeck et al., 2015) and it has been estimated that 52% of all sea turtles globally have ingested plastic waste of some kind (Schuyler et al., 2015). Plastic ingestion can therefore become a serious threat to the conservation of wild sea turtles and furthering our knowledge of this sensory trap can help highlight the dangers of plastic waste to wildlife and promote actions to reduce plastic waste in our oceans.
We also note two case studies demonstrating the success of turtle visual ecology in increasing conservation efforts. For green sea turtle hatchlings (Chelonia mydas), seaward migration following emergence occurs predominantly during the night and hatchlings rely on light from the horizon above the sea to guide them to water (Lohmann et al., 1997). Artificial light at night has been demonstrated to misguide sea turtle hatchlings as they emerge from nests on beaches and attempt to navigate towards the ocean (Thums et al., 2016). These artificial lights are now the brightest light source on Wobiri Beach (North West Cape, Western Australia) and mask the light from the sea horizon, thus causing hatchlings to orient away from the sea which greatly reduces survival. Because sea turtle hatchlings are particularly vulnerable to disorientation from artificial light at night, new management initiatives have been implemented on Florida beaches (Witherington et al., 2014). In these areas, typical streetlights have been replaced with dimmer amber lights that are directed downwards instead of outwards toward the nesting sites. This initiative appears to be successful as after 1 year hatchling mortality from disorientation decreased significantly and remained low in following years (Witherington et al., 2014). Visual ecology of green turtles has also been beneficial for protecting adults, as well as hatchlings. A large amount of research into sea turtle sensory biology has been focused on reducing bycatch in fishing lines and nets (Horodysky et al., 2016). One trial successfully reduced bycatch rates of green sea turtles in commercial bottom gillnets (targeting fish) by 40% and 60% through use of LED and chemical light stick visual deterrents, respectively (Wang et al., 2013). No significant effect on target species catch rate or catch value was found when using these visual deterrents, making this a viable option for use in commercial gillnet fisheries to help reduce bycatch of sea turtles.
Audition
Auditon, more commonly known as hearing, can be defined as the detection of acoustic stimuli (vibrations transmited through a medium; Pollack et al., 2016). Acoustic stimuli is typically referred to as ‘sound’ when these vibrations occur in fluid mediums (air or water), and as ‘substrate vibrations’ in a solid medium (Windmill and Jackson, 2016). In this paper we will use these definitions of sound and substrate vibrations. Vibrational waves of acoustic stimuli can vary in frequency, wavelength and intensity (Stevens, 2013). Frequency is defined as the number of wave cycles of a particular sound that occur per second, and thus is directly related to wavelength and defines the pitch of a sound. Sound intensity is energy transported by a sound wave and can be perceived as volume by a receiver (Lefebvre, 1999). Most animals have sensory organs that are capable of detecting a range of acoustic stimuli. Vertebrate hearing is often associated with the ear, a sensory organ with many features that allow it to detect certain sounds and propagate this information to sensory neurons (Ruggero et al., 1992; Ren, 2002). However, for certain species of fish and amphibians sound detection is also associated with specialized neuromast cells also capable of detecting acousti stimuli (Fay and Popper, 1998). Invertebrates also have sensory receptors capable of detecting a broad range of sound frequencies (Pollack et al., 2016). Sound stimuli can be detected by animals for a variety of purposes from prey detection and predator avoidance, mating and breeding and social interactions and communication. Although relatively understudied when compared with sound, substrate vibrations in animal communication are more exploited than once thought (Hill, 2001). Substrate vibrations have been shown to be important for a wide range of purposes such as predator–prey interactions, foraging, mate choice and breeding and materal care (Hill, 2001; Hill, 2009), and in all taxa from bees (Kirchner, 1997) to elephants (O’Connell-Rodwell et al., 2001). Some animals are also capable of producing sound frequencies for the specific purpose of detecting the echoes of this sound. This is a form of active auditon, known as echolocation, commonly used by cetaceans and bats (Jones, 2005).
In this section we review case studies of how auditory ecology has benefited wildlife conservation and management across four major taxa (birds, mammals, fish and invertebrates). Reptile and amphibian auditory ecology has been relatively understudied in the context of conservation, and thus we do not discuss these taxa in this section (Fig. 1). Anthropogenic development and activity ultimately creates unnatural sounds and vibrations in the environment, which can have many negative effects of various animal species (Slabbekoorn et al., 2018). From a conservation perspective, it is important to identify and understand the seriousness of these effects on animals in order to act to mitigate these problems. Auditory stimuli have also been shown to be effective at both attracting animals to certain habitats (e.g. through conspecific cues and signals to aid in species relocation), and also at deterring animals from habitats (e.g. certain sound frequencies have been effective at deterring animals from dangerous environments), which can both be beneficial to conservation and wildlife management.
Birds
Avian species often rely heavily on hearing for a number of behaviours including hunting/foraging, predator avoidance, territorial defense and conspecific attraction for reproductive purposes (Winkler, 2001). Avian hearing is typically restricted to lower frequencies (below 10 kHz) and they cannot detect ultrasonic frequencies (above 20 kHz; Köppl, 2015). However, some bird species, such as pigeons, chickens and guinea fowl, are capable of infrasonic hearing (frequencies below 20 Hz) for purposes such as long-range detection of auditory cues from landmarks or weather events (Hagstrum, 2000; Zeyl et al., 2020). Some bird species, such as oilbirds and swiftlets, are even capable of echolocation to detect food such as fruits and insects, respectively (Brinkløv et al., 2013).
Anthropogenic development and activity can result in loud, unnatural sounds (‘sound pollution’), and this can have a number of negative consequences for bird species in many contexts (Ortega, 2012). For example, noisy natural gas compressor stations in New Mexico, USA, caused significantly increased levels of the stress hormone corticosterone in a community of nesting western bluebirds (Sialia mexicana) and a significant reduction in hatching success in noisy sites compared with control, quiet sites (Kleist et al., 2018). Sound pollution from compressor sites has also been shown to reduce mating success by dampening conspecific calls of ovenbirds (Seiurus aurocapilla: Habib et al., 2007). In one study, the sound of roadway traffic alone was enough to compromise both avian density and condition. Ware et al. (2015) created a ‘phantom road’ by amplifying traffic noise in a rural habitat with no road. The results of this study showed that the sound of traffic alone caused 31% of individuals of various species to avoid the area, and those that remained despite the noise had reduced overall body condition (a size-adjusted metric of body mass that signifies energy stores birds need for migration). Further examples show sound pollution can alter bird song (Gentry et al., 2018) and alter bird song timing (Nordt and Klenke, 2013). For example, shifting European blackbird (Turdus merula) song to earlier hours of the morning to avoid rush hour traffic causing sleep deprivation for the bird (Nordt and Klenke, 2013).
Conservation practitioners can also exploit bird auditory ecology to influence habitat selection behaviour to guide birds to settle in more appropriate areas. In a study by Ward and Schlossberg (2004), the black-capped vireo (Vireo atricapilla), a territorial songbird, was attracted to suitable habitat sites that were uninhabited by the species by playing recordings of the bird’s song. The researchers were successful at attracting birds to the experimental sites where the bird song was played, compared with control sites where no black-capped vireos were attracted over the same time period. In this example, the researchers attracted birds to sites where the brood-parasitic brown-headed cowbird (Molothrus ater) was controlled, and thus higher nesting success was seen compared with nearby black-capped vireo populations in uncontrolled habitats (Ward and Schlossberg, 2004). Similar studies with other songbirds have also proven successful, with 12/14 species in which playback of bird song were tested successfully attracting birds to settle in the area (Ahlering et al., 2006). Here we see how avian auditory ecology can be exploited to attract bird species to more suitable, safe habitats. However, we can also exploit avian auditory ecology to deter bird species from dangerous, unfavourable habitats. Airfields are an area with increased numbers of airstrikes between birds and aircraft. One study demonstrated that spatially controlled sound frequencies emitted around an airfield (a ‘sonic net’) were able to successfully deter birds from the area (Swaddle et al., 2016). These sound frequencies were chosen to overlap with the frequency range of the European starling (Sturnus vulgaris), and results showed an 82% reduction in the number of starlings at airfields with sonic nets compared with control areas. Previously we discussed how avian vision can be exploited to make aircraft more visible to birds to reduce airstrikes, and perhaps using a combination of approaches, and targeting more than one sense (multisensory approaches), may be more effective at either repelling species from unsuitable dangerous habitats, or attracting species to more suitable habitats.
Mammals
Mammalian species are capable of producing and detecting a wide range of sound frequencies, from high-frequency bat calls (Manley, 2012) to low frequency whale songs (Darling, 2015). Mammals use auditory cues and signals for mating, hunting, predator avoidance, foraging and social communications (Suthers, 1978). Several mammals, most notably species of bats and toothed cetaceans, rely on echolocation to hunt prey, as well as to navigate in low light conditions (Thomas et al., 2004). Sound pollution affects echolocation in pallid bats (Myotis myotis), potentially interfering with signal reception and processing. Natural gas compressor stations reduce pallid bat activity levels by as much as 40% at louder stations compared to quieter ones (Bunkley et al., 2015). Similarly, frog-eating fringe-lipped bats (Trachops cirrhosus) can shift to active echolocation from passively listening for frog vocalizations when anthropogenic noise is present (Gomes et al., 2016). Noise pollution can also interfere with acoustic communication if it masks acoustic signals, reducing the ability of animals to coordinate socially for mating, territoriality, and other behaviours. In the case of the endangered Stephens’ kangaroo rat (Dipodomys stephensi), traffic noise not only masked foot-drumming signals but also served as an acoustic model that kangaroo rats appeared to mimic (Shier et al., 2012). This behavior may have important biological and fitness consequences compromising conservation. For example, noise pollution might cause distraction from true conspecific signals (lost mating opportunities), attraction to dangerous roads (mortality risk) or increased stress (may lead to decreased body condition and all the other problems with stress).
Sound can also travel through water, and sound pollution from boats and aquatic infrastructure has many negative impacts on marine mammals (Popper and Hawkins, 2012). Noise from shipping traffic is a chronic habitat-level stressor for many species of whales, including killer whales (Orcinus orca: Williams et al., 2019b). For a population of southern resident killer whales, shipping noise disturbance is thought to be one of the three main stressors responsible for declining population numbers, along with lower numbers of prey (salmonid fishes), and ocean contaminant levels (DFO, 2008; NMFS, 2008). Shipping and boating noise disrupts foraging behaviour (Williams et al., 2006), pod communication (Williams et al., 2014) and echolocation clicks used to hunt prey (Holt, 2008). However, reducing the speed of ships, relocating major shipping routes and removing noisier ships and replacing them with newer, quieter ships would reduce the intensity of sound pollution (Williams et al., 2019b). Although anthropogenic sound pollution is a threat to many cetaceans, this sense can also be exploited to help reduce bycatch of these species in commercial fisheries, or to reduce pinniped predation on fish farms and fisheries. Indeed, acoustic alarms effectively reduce bycatch of harbour porpoises (Phocoena phocoena) without reducing target Atlantic cod (Gadus morhua) catches in Danish gillnet fisheries (Larsen and Eigaard, 2014). Finally, Götz and Janik (2013) have shown how acoustic deterrent devices, and specifically those that capitalize on the startle reflex system, may be effective pinniped deterrents from fish farms and fisheries, thus reducing human–wildlife conflicts in this example.
Fish
Fish are capable of detecting a range of sound frequencies through the inner ear (Popper and Fay, 2011), as well as a range of low-frequency vibrations and mechanical disturbance (hydrodynamic stimulation) using specialized receptors in their lateral line (Bleckmann and Zelick, 2009; Higgs and Radford, 2016). This allows fish to detect distant motion and vibrations through specific mechanoreceptors. Hearing is important for many fish species for school cohesion, mate choice and spawning, finding suitable habitats (e.g. detection of ‘reef sounds’) and territory defense (Putland et al. 2019).
Sound pollution from boats, windfarms and hydroelectric facilities can cause a number of problems for certain fish species (Slabbekoorn et al., 2010). Both marine and freshwater fishes are affected by sound from boats, ships, offshore windfarms and hydroelectric facilities (Mickle and Higgs, 2018; Popper and Hawkins, 2012, 2019). Compared to marine fishes, the effects of sound pollution on fish in the freshwater environment have been less well studied, but these species still face important threats from hydroelectric facilities and are affected by sound pollution from boats (Graham and Cooke, 2008; Mickle and Higgs, 2018; Rountree et al., 2020). Sound has been shown to have negative effects on the behaviours of various fish species, often causing a decrease in foraging behaviour (Codarin et al., 2009; Purser and Radford, 2011; McLaughlin and Kunc, 2015; Sabet et al., 2015). Exposure to sound pollution also causes physiological stress. For example, intermittent noise elicited a stress response in the giant kelpfish (Heterostichus rostratus), although the stress response was not seen when kelpfish were exposed to a constant source of sound indicating that the variability in sound pollution may be a more important factor than its presence alone (Nichols et al., 2015). Ambon damselfish (Pomacentrus amboinensis) exposed to motorboat noise also show signs of physiological stress and, as a result, have a reduced ability to evade predators (Simpson et al., 2016).
Sound can be strategically used to deter fish from certain areas. As previously mentioned, turbines pose a significant threat to fish who could be severely injured or killed by the fast-moving turbine blades or screws. Barriers of sound, light and bubbles can be effective at deterring fish movement through dangerous dam structures (Nestler et al., 1992; Ross et al., 1995; Noatch and Suski, 2012). Sound barriers can also be effective at controlling the spread of invasive Asian carps (Hypophthalmichthys nobilis, H. molitrix) in North America, including into the Laurentian Great Lakes (Ruebush et al., 2012). These invasive species are exerting generally negative effects on native communities, and thus control of their distribution could be very beneficial to ecosystem functioning. Sound barriers also blocked movement of native fish species present in the area, which nay negatively impact population processes within resident communities. Sound is being investigated as a deterrent for invasive sea lamprey in the Great Lakes basin and to date, low-frequency sounds have induced the strongest behavioral responses (Mickleet al., 2019).
Invertebrates
For insects, acoustic stimuli are very important for intraspecific communication, predator avoidance and prey detection, and as a result hearing has evolved multiple times in parallel across seven insect orders (Hedwig, 2014). Insects have developed tympanal ears, characterized by a membrane (tympanum) that vibrates in response to sound (Hoy and Robert, 1996), and these ears can be found on various body parts in different insect species (Hoy and Robert, 1996; Göpfert and Hennig, 2016). Other invertebrates, such as arachnids and crustaceans, do not have tympanal ears, although some species may still be able to detect certain sound frequencies but relatively little is known (Barth, 2000; Stumpner and von Helverson, 2001; Edmonds et al., 2016). However, for many arthropods, substrate vibrations are an important stimulus (Hill, 2008). For example, spiders exploit substrate vibrations for multiple purposes including mating behaviour whereby spiders send out vibrations through leaf litter (Uetz and Roberts, 2002), and detecting vibrations in their webs (Barth et al., 1988; Landolfa and Barth, 1996). Although we found no case studies demonstrating the successful application of invertebrate auditory ecology for conservation purposes, we do highlight important research on invertebrate hearing and problems resulting from anthropogenic activity and resulting sound pollution.
Invertebrates, both aquatic and terrestrial, are affected in numerous ways by sound pollution. Noisy compressor stations at oil and natural gas facilities have been shown to have negative consequences on invertebrate populations. One study found the abundance of a number of different arthropod species to be negatively associated with noisy compressor sites, and this might have significant knock-on impacts for the surrounding ecosystem (Bunkley et al., 2017). In the marine environment, zooplankton are integral to the productivity of the ocean as the primary food source for a vast array of marine species, including many species of fish and cetaceans. One study found that seismic surveys, an acoustic imaging technique used to search for petroleum in the ocean, can cause significant mortality for zooplankton (McCauley et al., 2017). In particular, the abundance of zooplankton decreased by 64% following an acoustic impulse signal, affecting zooplankton up to 1.2 km away from the signal source. Based on these findings, seismic surveys might be having significant negative impacts on ocean ecology that is not widely acknowledged.
As well as effects on abundance, sound pollution can affect the behaviour of invertebrates. For example, bow-winged grasshoppers (Chorthippus biguttulus) found near loud roadways (Lampe et al., 2012) and the cicada (Cryptotympana takasagona) found in louder environments (Shieh et al., 2012) both emit higher frequency calls than their conspecifics in quieter environments. This is an adaptation to prevent masking of their auditory calls to potential mates, highlighting that sound pollution can have negative implications for mating and reproductive success. Indeed, another study found that traffic noise resulted in failure of female field crickets (Gryllus bimaculatus) to orient to male auditory calls (Schmidt et al., 2014). However, the failure of females to orient may not have been the result of male auditory signal masking, but instead because females were distracted by traffic noise.
Distraction, in addition to masking and stress, is also a potential consequence of acoustic noise (Chan and Blumstein, 2011). Studies on terrestrial Caribbean hermit crabs (Coenobita clypeatus: Chan et al., 2010) led to the development of the distracted prey hypothesis (Chan and Blumstein, 2011). The hypothesis notes that any stimulus that can be detected has the potential to re-direct the limited attention that a species has, and this can have negative consequences for risk assessment. For hermit crabs the sounds of boat motor noise modified risk assessment by permitting humans to get closer to individual crabs before they retreated into their shells.
Chemoreception
Both biotic and abiotic components within an environment release molecules and chemical compounds that can provide information to individuals. Animals are often able to detect chemical stimuli through olfaction, gustation and chemesthesis. Olfaction is the ability to detect (chemical) odours without physical contact with the source (Eisthen, 2002) and is often a vital component of reproductive and social behaviours, individual or group recognition, as well as predator–prey interactions. Chemesthesis is the detection of chemical stimuli via receptors and neurons found in the integument of animals (Slack, 2016). Gustation (or taste) also involves the detection of chemicals or molecules but uses different families of chemoreceptors and different signalling pathways to the brain (Wyatt, 2014). Through gustation animals perceive chemical stimuli as tastes or flavours, whereas through olfaction they are perceived as smells. Chemical stimuli can be broadly categorized as environmental odors (chemical stimuli from abiotic sources such as water, fire, soil types or habitats) and semiochemicals (produced by other animals for the purpose of inter- or intraspecific interactions). Semiochemicals can be further categorized as pheromones, signature mixes and allelochemicals. Pheromones are involved in intraspecific communication and elicit an innate response for a specific purpose, such as mating, alarm cues or mother–offspring interactions (Wyatt, 2010). Signature mixes, on the other hand, are variable chemical mixtures that are learned, and typically allow an animal to identify an individual or social group (Wyatt, 2010). Finally, allelochemicals are important for interspecific interactions and may function in various ways that benefit the emitter, the receiver or both (Wyatt, 2014).
In this section, we will discuss how chemosensory ecology, in particular olfaction and gustation, can be exploited in conservation and wildlife management. Research has led to the development of highly effective animal control techniques through creating species-specific olfactory traps. Examples include controlling pest, invasive or overabundant species, and mitigating human–wildlife conflicts. The majority of the literature on pest control through exploiting olfactory systems focuses on insects as various species are common pests in agriculture and vectors for diseases, and thus there is a strong need to control their populations in some instances (Witzgall et al., 2010). However, invasive species of other major taxa have also been successfully managed through exploiting their olfactory sense. Olfactory traps can also be exploited by wildlife managers to help control overabundant, or invasive populations that might have negative effects on an ecosystem, or overabundant predator populations, which cause further threat to endangered prey populations (Baker, 2009; Cruz et al., 2009; Johnson et al., 2009). Unfortunately, human activity is also causing unwanted, inadvertent olfactory traps in environments that have negative effects on certain species. There are case studies of this in marine environments causing harmful effects on fish and birds (Savoca et al., 2016; Williams et al., 2019a).
Birds
It was once thought that birds were anosmic or have very limited olfactory capabilities. However, furthering research into avian olfaction has revealed a great importance for this sense across many bird species (Balthazart and Taziaux, 2009; Prada and Furton, 2018). Birds rely on olfaction for a number of purposes, including searching for food (Wenzel, 1968; Graves, 1992; Nevitt, 2000; Nevitt, 2008), homing behaviour and navigation (Wallraff, 2004; Gagliardo, 2013) and for nest localization (Bonadonna et al., 2003; Bonadonna and Nevitt, 2004). In particular, certain species of seabirds rely heavily on olfaction (Nevitt, 2008). Plastic waste in the ocean is often ingested by seabirds, but the reasons why have been unclear. However, a recent sensory physiology study has begun to explain this common ecological trap. Savoca et al. (2016) showed that microplastics that have been in the marine environment for extended periods of time produce dimethyl sulfide (DMS). DMS is also a common odorant that is released by the prey of many seabirds, thus causing seabirds to ingest plastic mistaking it as a viable food source. Plastic is also ingested by a number of other marine animals, including species of sea turtles and whales, and further research is necessary to determine whether DMS released from these plastics also acts as an olfactory trap for these species (Savoca et al., 2016).
Behaviour of certain species can be manipulated through taste conditioning. Conditioned taste aversion (CTA) is one such method used to manipulate the behaviour of an individual. Through this method, individuals are taught to associate certain food items with a negative taste experience. True CTA has been successfully used to reduce predation of endangered bird eggs. In one study that aimed to reduce red fox (Vulpes vulpes) predation on threatened hooded plover (Thinornis rubricollis) eggs, model eggs that mimicked those of the hooded plover were produced and treated with a CTA-inducing chemical (Maguire et al., 2009). Control eggs were also produced without the CTA chemical treatment and placed in the experimental setting. This study found CTA to be successful in reducing predation on both treated and control eggs, showing promise for CTA as an effective way to deter predators and protect endangered species. CTA has also been successfully implemented in this way to reduce predation by grey wolves (Canis lupus; Gustavson, 1982), coyotes (C. latrans; Ellins and Catalano, 1980) and brushtail possum (Trichosurus vulpecula; Clapperton et al., 1996), and to reduce crop damage by African elephants (Loxodonta spp.; Osborn, 2002).
Mammals
For mammals, the main olfactory organ is the nose, within which there are many olfactory subsystems. In particular, the main olfactory epithelium and vomeronasal organ are the most widely studied and contain different classes of receptors (Trotier, 2011; Wackermannová et al., 2016). Mammals rely heavily on olfaction during mating, locating food, avoiding predators and for individual recognition and social behaviour. Pheromones are widely used for communication in mammalian species, for example in mating, territorial defense, alarm signals and mother–offspring interactions (Brennan, 2010). During mating, some mammalian females release pheromones to attract male conspecifics, and this knowledge can be exploited for conservation purposes. Due to the strong, innate response mating pheromones often elicit, the use of pheromones can be highly effective at controlling invasive, destructive mammalian populations, as we will discuss here. Mammals are also capable of detecting chemical stimuli through gustation (or ‘taste’). There are five taste modalities that chemical stimuli can be categorized through gustation: sweet, bitter, sour, salty and umami (Yarmolinsky et al., 2009). Bitter and sour tastes are typically ‘bad’ tastes and alert the animal to harmful foods, for example toxins, noxious plants and spoiled food. Although we will not discuss gustation in the context of mammalian conservation in this section, mammalian gustation can be exploited for the conservation of other taxa (e.g. birds) as was previously discussed.
Common goat (Capra hircus) females release sexpheromones that attract males for reproduction purposes. These goats were introduced by humans to the Galapagos archipelago where they quickly established a fast-growing population that had destructive effects on native biota and eventually demanded eradication of the population (Robertson et al., 2017). Initially, large numbers of the goats were removed by ground and aerial hunting. However, when the goat population reached low density, they became increasingly difficult to hunt. The remaining goats were then eradicated through a very effective sensory trap. Female goats were captured, sterilized and put in a chemically induced estrus, which caused them to produce pheromones that were detected by males as an attractant for mating. Males mated with these sterilized ‘Judas’ goats, resulting in no offspring. This technique eventually resulted in complete eradication of the invasive goat population on certain Galapagos Islands (Cruz et al., 2009), which greatly helped restoration of native flora. In this case study, male goat olfaction was exploited by creating a sensory trap to eradicate an invasive population. Other eradication techniques had been attempted before implementing this sensory trap, but they were not successful at complete eradication of the goat population, thus highlighting the advantages of the sensory ecology approach to invasive species management. In Australia, feral goats (Capra hircus) were controlled through predator olfactory deterrents. Cox et al. (2012) demonstrated that feral goats avoided areas with dingo (Canis lupus dingo), lion (Panthera leo) and tiger (P. tigris) odors, which could have implications to shift goat grazing areas to other areas where they may not be causing as much damage or competition with endangered native species. Olfactory predator deterrents have also been shown to be successful with a number of other species including certain marsupials (Parsons and Blumstein, 2010) and vervet monkeys (Cercopithecus aethiops; Willems and Hill, 2009).
Olfaction can also be exploited to support at-risk species. For example, urine scent has been shown to influence mate choice in female pygmy loris (Nycticebus pygmaeus), a threatened primate species (Fisher et al., 2003). This knowledge can be used to promote successful mate pairings that maximize genetic compatibility and diversity to aid in supporting healthy populations. In another example, improved conservation breeding in captivity or translocation programs could be achieved by familiarizing females with male scents to reduce aggression and increase breeding success, as demonstrated with harvest mice (Micromys minutus: Roberts and Gosling, 2004).
Fish
Fish detect chemical stimuli in the aquatic environment and rely on these cues for reproduction, feeding, alarm (Smith, 1992) and, in some species, vast migrations (Yamamoto et al., 2010; Bett et al., 2016; Sorensen and Johnson, 2016). Fish are capable of detecting chemical stimuli through olfaction, gustation and solitary chemosensory cells (SCCs; Hansen and Reutter, 2004). SCCs occur on the skin, gills and oropharynx of fish and have been suggested to function to locate food, predators or conspecifics (Sbarbati and Osculati, 2003; Hansen and Reutter, 2004). For fish, olfaction is a ‘distance’ sense, allowing fish to detect chemical stimuli from conspecifics, food, predators and even habitats at sometimes great distances. For example, olfaction is important in long distance spawning migrations, as demonstrated by salmonid fishes as reproductive adults navigate from marine or lacustrine foraging environments to their natal tributary streams following very dilute stream-specific chemical odours over great distances (Yamamoto et al., 2010; Bett et al., 2016). It is important that salmonid populations return to their specific natal streams because their eggs are genetically programmed with specific incubation times and growth rates adapted to the specific environmental conditions of that stream (Dittman and Quinn, 1996). Straying of salmonids to spawn in non-natal streams will often result in death of the offspring due to mismatches between environmental conditions and localized adaptations (Bett et al., 2017). Elevated carbon dioxide concentrations in seawater can disrupt the olfactory senses that salmon rely so heavily upon to navigate to natal streams (Williams et al., 2019a). Knowledge of the sensory implications of increasing ocean acidification, an unfortunate result of global climate change, offers more insight into yet another threat faced by this species.
Sex pheromones in species of invasive fish have been successfully exploited to control populations of some species. Sea lamprey (Petromyzon marinus) are invasive species to the Laurentian Great Lakes and are destructive parasites of valued sport fishes. Researchers found that male sea lamprey release a mixture of sex pheromones and identified several components (Buchinger et al., 2015). The major component has been synthesized and attracts ovulated females into baited traps from over hundreds of meters (Johnson et al., 2009). These studies of pheromone- or sensory-based pest control could provide a highly effective, species-specific way of controlling invasive or harmful species. This example of control of sea lamprey with sex pheromones represents the first of its type demonstrated in a vertebrate species. Further research in this field could lead to more sensory-based pest control techniques in a range of other species and taxa.
Invertebrates
Most invertebrates rely heavily on olfaction and as a result have evolved very sensitive olfactory systems (Hildebrand and Shepherd, 1997). This is a very broad, diverse topic across all invertebrate species; however, we will only focus specifically on insect olfaction as this field of research has shown most relevance to conservation and management. Many invertebrate species use pheromones for communication and mating, and these pheromones will often elicit a strong innate response. Farmers and conservationists can therefore exploit this innate response to lure insects to sensory pheromone traps, and there are now numerous examples of how insect species can be controlled and monitored in this way (El-Sayed et al., 2006; Baker, 2009). This so-called ‘mass trapping’ uses specific chemical lures, usually sex aggregation pheromones or food odors, to attract certain insects to lethal traps (El-Sayed et al., 2006). Many scientific studies describe the use of mass trapping for control of pest insect species, for example in agricultural practices; however, these pheromone traps are also beneficial in conservation science for monitoring endangered species. Numerous examples illustrate the use of pheromones to attract endangered insects (e.g. Millaret al., 2010; Ray et al., 2014; Konig et al., 2016). Pheromone-based trapping is highly beneficial for monitoring populations and sampling of threatened species because it is highly species specific and effective at even low population densities (Larsson, 2016). Chemical ecology has also been exploited for the control of destructive, invasive insect species that devastate native flora and fauna (Smith, 1998; El-Sayedet al., 2006; Larsson, 2016). The gypsy moth (Lymantria dispar) is an invasive species in the United States that feeds on many woody plants and has devastating effects on forests (Davidson et al., 1999) and there is therefore a need to control numbers of this species. Pheromone trapping of insects can be a highly effective conservation tool, allowing us to both monitor endangered species, and control invasive species, to conserve native flora and fauna (Tobin and Blackburn, 2007).
Other Sensory Modalities
Electroreception
Electroreception is defined as the detection of electric information within the environment (Stevens, 2013). Water, unlike air, is a good conductor of electricity and thus species that are capable of electroreception typically live in aquatic or moist environments. This sensory modality is particularly widespread in fishes and amphibians (Crampton, 2019) but is also important in other taxa. Studies have demonstrated the importance of electroreception in mammalian species such as the platypus (Ornithorhynchus anatinu; Scheich et al., 1986), star-nosed mole (Condylura cristata; Gould et al., 1993) and Guiana dolphin (Sotalia guianensis; Czech-Damal et al., 2012), and also aquatic invertebrates such as the crayfish (Cherax destructor; Patullo and Macmillan, 2007; Patullo and Macmillan, 2010) and fish species including sea lamprey (Chung-Davidson et al., 2004; Johnson et al., 2016).
Electroreception, like audition, can be passive or active. Passive electroreception is the ability to detect weak direct current fields or low-frequency sinusoidal fields (Crampton, 2019). These electric stimuli are detected by highly specialized cells (electroreceptors) located in pores of the animal exposed to water (Peters et al., 2007). Passive electroreception is important for detection of prey in several aquatic species, such as the spotted dogfish (Scyliorhinus canicular) that can detect its prey, the flatfish (Pleuronectes platessa), using only electrical stimuli (Kalmijn, 1971). Active electroreception is less common than passive electroreception. It can be defined as the ability of certain animals, in particular teleost fishes, to produce electric fields and measure distortion of these fields by surrounding objects in the environment (Alvez-Gomes, 2001). These species are defined as electrogenic, and they create electric fields through specialized muscle fibres that consist of electrocytes (Stoddard and Markham, 2008). These electrocytes are capable of polarizing the skin of the fish, which creates a surrounding electric field. Any objects close to the fish will then cause distortions in this electric field, which, in turn, will be detected by electroreceptors on the fish allowing it to localize surrounding objects and individuals (Von der Emde, 1999). Active electroception has been shown to be important for communication (Dunlap et al., 2010), mating (Curtis and Stoddard, 2003), recognition (Nagel et al., 2018) and hunting (Hanika and Kramer, 2000).
Understanding electroreception may have applied benefits as seen in successful case studies that effectively reduced shark bycatch by exploiting their electrorepulsive behaviour (Brill et al., 2009; Hutchinson et al., 2012). A major threat to many aquatic species is bycatch. Commercial fisheries often deploy large nets or trawl fishing hooks to catch large numbers of target fish species. However, non-target species can also be caught by these commercial fishing practices and bycatch of sharks in this way has contributed to mortality and declining numbers of certain species (Ferretti et al., 2008; Molina and Cooke, 2012; Gallagher et al., 2014). Sharks are capable of detecting electric fields, whereas target fish species often cannot, leading to the use of electropositive metals on fishing hooks (O'Connell et al., 2014) and reduced shark catch rates (Hutchinson et al., 2012). This result appeared to be species specific, however, as the electropositive hooks reduced bycatch of hammerhead sharks (Sphyrna lewini) but not sandbar sharks (Carcharhinus plumbeus: Hutchinsonet al., 2012).
Magnetoreception
Magnetoreception is the ability to sense the earth’s magnetic field, but is not as well understood as other sensory modalities (Wiltschko and Wiltschko, 2005; Lohmann et al., 2007; Gould, 2010; Wiltschko and Wiltschko, 2012; Mouritsen, 2018). Magnetoreception has been shown across most taxa and is exploited for orientation and navigation. Because magnetic fields pass through animal tissues, identifying receptors and understanding the physiological basis of detection have been difficult (Walker et al., 1997; Johnsen and Lohmann, 2005). However, there are now several proposed mechanisms of magnetic field detection in various animals including highly specialized electroreceptors, magnetic-particle-based magnetoreception and radical-pair-based magnetoreception (Mouritsen, 2018).
Magnetoreception is important for migratory birds (Wiltschko and Wiltschko, 2005) and insects such as the monarch butterfly (Danaus plexippus; Guerra et al., 2014). Magnetoreception is also important for shorter-distance movements in birds, for example in homing behaviour of pigeons (Columba livia f. domestica: Wiltschko et al. 2010), and in home range navigation of domestic chickens (Gallus gallus: Wiltschko et al., 2007). Animals such as rodents and migratory salmonids do not use an inclination-based compass, but instead use a ‘polarity compass’, whereby they are able to detect the polarity of the field lines allowing them to perceive north and south (Wiltschko and Wiltschko, 2005). This compass is important in long-distance migrations as smolts heading to the ocean (Quinn and Brannon, 1982), and again as adults returning to natal streams (Putman et al., 2013; Lohmann and Lohmann, 2019). A third and final mechanism of using magnetic fields for navigation is known as ‘magnetic maps’, where an individual can determine its position relative to a target location for migration (Lohmann et al., 2007). This type of navigation by magnetoreception is used by spiny lobsters (Panulirus argus; Boles and Lohmann, 2003), and various species of sea turtles (Lohmann et al., 2007).
Magnetoreception has been clearly demonstrated to be a vital sense for orientation and navigation in a growing number of species. However, compared to other sensory modalities, it has been relatively under-studied and there are still many questions concerning the sensory ecology and physiology of magnetoreception. However, in recent years, the impacts of human electromagnetic noise on animal magnetic compasses have been investigated, and researchers have documented interfering effects of such noise on bird magnetic orientation. Electromagnetic noise from human activity has been shown to disrupt magnetic compass orientation in European robins (Erithacus rubecula) in both natural and labortary settings (Engels et al., 2014; Schwarze et al., 2016). These findings may suggest that growing anthropogenic development may be causing widespread negative effects on bird migration and may represent a more serious conservation issue. Other studies have since raised concern that electromagnetic noise may be having similar disruptive effects on the magnetic compass of other animals, such as the monarch butterfly (Guerra et al., 2014; Reppert et al., 2016); however, there is no evidence to support this yet. Anthropogenic activity may also be affecting animal magnetoreception in ways that we are not yet aware of, and furthering research into this sensory modality may reveal potential sensory traps, or ways conservation practioners can exploit magnetoreception for applied purposes.
Multimodal stimuli
Animals often receive and process multimodal stimuli (Partan and Marler, 1999; Munoz and Blumstein, 2012; Munoz and Blumstein, 2020). Multimodal cues and signals target multiple sensory systems in the receiver. The receiver must then integrate this multisensory information to make decisions and modify behaviour appropriately (Munoz and Blumstein, 2012). Multimodal signalling is thought to have evolved to either increase the information content in a signal (i.e. non-redundant signalling whereby different sensory components of a signal provide different information to the receiver) or to increase the robustness of the signal reaching the receiver (redundant signalling whereby each component of the signal provides the receiver with the same information; Hebets and Papaj, 2005; Partan and Marler, 2005). For example, non-redundant multimodal begging signals from reed warbler (Acrocephalus scirpaceus) offspring provide their parents with increased information (Kilner et al., 1999). The area of the visual gape displayed by the brood provides information on the age and size of the brood, whereas the vocal calls provide information on the hunger levels of the brood, and both signals allow parents to adjust their feeding rates accordingly. We see an example of redundant multimodal signalling during wolf spider (Schizocosa stridulans) courtship behaviour that includes both visual and seismic signals (Hebets, 2008). Visual signals, although not necessary for mating success, are used as they can travel farther than seismic signals and thus increase the probability of being detected. However, when mates come closer, seismic signals become a dominant and necessary signal for mating success. In this example the multimodal components of the signal are redundant, both providing the same information to the receiver but increasing the probability the signal will be received (see Partan and Marler, 1999; Partan and Marler, 2005; Munoz and Blumstein, 2012; Higham and Hebets, 2013; Stevens, 2013).
Studying multisensory integration is complex and requires a number of considerations. Animals may not use all available stimuli, sometimes only responding to one sensory cue or signal. The sensory information that an animal uses may also change depending on various factors such as reproductive state (Kasurak et al., 2012), environmental conditions (Munoz and Blumstein, 2020) and seasonality (Gall et al., 2013). For example, one study found that female round gobies (Neogobius melanostomus) only integrated vibrational and olfactory sexual stimuli from males when they are reproductive, which aids in finding the specific location of reproductive males and nest sites (Kasurak et al., 2012). Interspecific multisensory integration adds another level of complexity where we must now consider the different sensory systems, sensory thresholds and cognitive abilities of various species (Munoz and Blumstein, 2020). Interspecific multimodal signaling is important in aposematism (Rowe and Guilford, 1999), for example, the warning signals of seven-spot ladybirds (Coccinella septempunctata) are multimodal signals comprising visual and chemical warning signals (Marples et al., 1994). In another example, plant–pollinator interactions benefit from interspecific multimodal signaling, as we see multimodal signals enhance decision making in common eastern bumble bees (Bombus impatiens), allowing individuals to learn and detect more rewarding flowers faster (Kulahci et al., 2008). Even intraspecific multisensory integration is relevant in mate choice contexts as shown by female brown-headed cowbirds: females with better auditory temporal resolution prefer shorter and high frequency male songs, whereas females with better temporal visual resolution prefer less intense male visual displays (Ronald et al., 2018). Understanding and predicting animal integration of multisensory cues and signals often require complex models and frameworks. Not only do we need to predict what sensory cues and signals an animal will respond to at a particular time, but also how that animal will respond in its given condition and environment. There are now several models and frameworks that can be applied to multimodal signalling in animals and have helped further our understanding of this complex signalling (see Munoz and Blumstein, 2012; Wilkins et al., 2015; Hebets et al., 2016; Munoz and Blumstein, 2020).
There are a few studies that have focused on the impacts of anthropogenic activity using a multisensory/multimodal perspective. Rabin et al. (2006) studied the effects of auditory noise from wind turbines on California ground squirrels (Spermophilus beecheyi), finding that squirrels were less responsive to auditory predator signals, and instead increased their alertness to obtain more visual cues. Partan et al. (2010) found a similar multimodal shift from reliance on audio to visual cues in the eastern grey squirrel (Sciurus carolinensis). Assessing how human development, and planned development, affects multisensory perception in animals will allow a more comprehensive measurement of the impacts of such development on affected animals (Munoz and Blumstein, 2012; Partan, 2017). In a recent study, researchers found multisensory stimuli may have beneficial implications for controlling sea lamprey movement. Johnson et al. (2019) studied the impacts of visual and olfactory stimuli applied together on juvenile sea lamprey movement. Results showed that light attractant stimuli, and conspecific odorant repellent stimuli, can be used together in a ‘push and pull’ configuration to better control movement of these fish. Such findings of exploiting multisensory attractants and repellents together could be useful to more effectively reduce turbine entrainment of native species, or instead to increase trapping of invasive species. Understanding multisensory perception may also be beneficial to improving translocation and reintroduction programs designed to help recover species by returning them to suitable available habitat. Translocation, reintroduction and captive release of species are often unsuccessful, and failure of animals to detect and recognize various stimuli in their new environment is frequently attributed to these low success rates (Stamps and Swaisgood, 2007). For example, introduced species may fail to recognize the chemical scent or auditory calls of suitable prey or fail to identify and evade predators, thus resulting in unsuccessful introduction. Understanding the multisensory perceptions of an animal in its habitat may help increase the success rates of introducing these animals to novel habitats (Munoz and Blumstein, 2012). Finally, many unimodal repellents fail (Lecker et al., 2015). Often this is because animals habituate to unreinforced unimodal threat stimuli. There have been suggestions that by designing multisensory repellents, habituation can be delayed (Lecker et al., 2015).
Benefits, challenges and future directions for applied sensory ecology
Benefits
Sensory-based approaches can be effective in conservation interventions, and throughout this paper we see recurrent applications of sensory ecology for certain conservation challenges. Through an extensive review of the literature, we have highlighted many successful applications of sensory ecology to conservation and wildlife management (Table 1), as well as a large amount of research furthering the field of sensory ecology with potential to benefit conservation science (Table 2). In particular, sensory approaches can be beneficial, and successful, for ameliorating sensory traps, reducing harm to wildlife (i.e. from bycatch or airstrikes), and wildlife relocation. Through emphasizing these success stories, and discussing the benefits, challenges and future potential of sensory ecology, we hope to encourage wildlife managers and conservationists to consider sensory-based approaches to conservation issues. We also highlight gaps in the literature to encourage future research and development in the fields of applied sensory ecology and sensory physiology.
Many conservation issues are a consequence of human activity, such as rapid environmental change, habitat loss or climate change (Sutherland et al., 2019; Sutherland et al., 2020). As these issues continue, it is important to predict how species will respond to such environmental changes, which species will be most susceptible, and ultimately how to protect and restore those at risk. Sensory approaches are needed to more accurately predict how, why and when human activities may threaten wildlife populations. Such approaches avoid biases of human perception and can predict which types of environmental changes are actually perceived as stressful by wildlife, and how various species might respond to these changes. Further, research identifying potential sensory traps can help guide development and construction of human structures (e.g. building facades, wind turbines) and equipment (e.g. fishing gear) towards more wildlife-friendly options. For example, new beach developments in important turtle habitat should use specific lighting to prevent misguiding hatchlings (Witherington et al., 2014), while future solar farm constructions should consider new designs that are less attractive to aquatic insects (Horváth et al., 2010). As the human population continues to grow, and development expands across the globe, identifying and developing solutions to prevent sensory traps will be highly beneficial in protecting species into the future.
Challenges
Many challenges remain for integrating sensory ecology with wildlife conservation and management. Environmental heterogeneity, both natural and anthropogenic, makes it difficult to understand or predict with high degrees of accuracy what an animal may perceive in nature (Dangles et al., 2009). For example, what an animal can see can be influenced by air or water clarity when detectability and acuity of visual cues are reduced under conditions of fog or turbidity (air and water, respectively). Similarly, wind and water velocity (current) limits the ability to detect and identify cues, as well as locate cue sources. Individual variability in sensory sensitivity adds another layer of complexity (Dangles et al., 2009): just as some humans have better eyesight, hearing or smell than others, the same is true within and between other species (e.g. Ronald et al., 2017). Sensory physiology studies can be logistically challenging, and some questions are best addressed under laboratory conditions where background noise can be minimized (Dangles et al., 2009). Researchers with the understanding and ability to conduct sensory physiological assays in laboratory environments may not have the expertise to do so in ecological field environments and vice versa, creating a communication barrier between lab and field researchers. However, sensory physiology interfaces with many disciplines and can connect physiologists, ecologists and practitioners to maximize research efficiency and benefit research and management efforts (Caro and Sherman, 2013; Lennox and Cooke, 2014).
Future Directions
The growing number of success stories emerging from the integration of sensory ecology with conservation and wildlife management are promising signs that highlight the benefits of studying the sensory perceptions of species of interest. Increasing exposure and emphasis on these success stories is hoped to encourage conservationists and wildlife managers to consider sensory approaches to solve conservation issues in the future, and perhaps even encourage funding opportunities for such interdisciplinary research. Greater exposure of sensory-based conservation science can be achieved through review articles, conference presentations (Madliger et al., 2017) and integration of this cross-disciplinary field at graduate and undergraduate levels.
Throughout this paper we highlighted a large amount of research furthering our understanding of species sensory ecology with potential to benefit conservation sciences (Table 2). Furthering our understanding of what organisms perceive, and which types of sensory stimuli are causing harmful or negative effects on organisms (i.e. artificial light at night, noise pollution, etc.), will be necessary for managers to know where future regulation or management changes are needed to protect wildlife. For example, there are a number of studies documenting the negative effects that noise pollution can have for many species across taxa (Table 2). There is great potential for managers and decision-makers to make regulation changes that benefit wildlife based on these findings, for example rerouting of major shipping routes to avoid important whale habitat (Williams et al., 2019b), or development of quieter compressor stations to reduce stress to wildlife (Habib et al., 2007; Bunkley et al., 2015; Kleist et al., 2018). Table 2 provides recommendations for future conservation and management decisions, based on a sensory understanding, that could benefit wildlife. We also recommend that furthering research into sensory ecology of species of interest will help to highlight other conservation problems that can begin to be addressed.
Despite a relatively small amount of research in the field of applied sensory ecology, we do observe certain taxonomic biases (Fig. 1). Specifically, we note research biases towards avian and mammalian species, a common observation in the field of conservation (Donaldson et al., 2016; Troudet et al., 2017). Amphibian sensory ecology is largely understudied when compared with other vertebrate groups and we identified no research in this field currently being applied to conservation. Reptiles are also relatively understudied in sensory ecology, and we identified endangered green sea turtles (Chelonia mydas) as the only species for which sensory ecology has been studied with applications for conservation. Donaldson et al. (2016) also reported green sea turtles as an outlier in reptile biodiversity conservation papers, again highlighting bias towards this reptile species. Despite invertebrates also being largely understudied compared with vertebrate groups in conservation fields (Donaldson et al., 2016; Troudet et al., 2017), we found a number of invertebrate sensory ecology studies with potential benefits to conservation (Table 2), and one demonstrated application of aquatic insect visual ecology ameliorating a sensory trap (Horváth et al., 2010). However, we recognize that compared with all vertebrate taxonomic groups discussed in this paper, invertebrates were again relatively understudied. Furthering sensory research for understudied taxa could provide additional benefits to the conservation and protection of these species and reduce taxonomic biases in this field.
To improve communication and collaboration across sensory ecology, physiology and conservation science fields there is a need for increased research efforts demonstrating sensory-based conservation strategies, and a clear understanding of conservation needs (see Greggor et al., 2016 for and in-depth discussion into resolving this interdisciplinary communication issue). Relating to sensory ecology, further research into multimodal stimuli (e.g. Partan et al., 2010; Johnson et al., 2019), multi-species approaches (e.g. Spoelstra et al., 2015) and linking sensory physiology to behaviour (e.g. Sillman et al., 2007; Fernandez-Juricic et al., 2013; Elvidge et al., 2019) would benefit conservation.
Conclusions
Every organism lives in its own sensory world, each perceiving the environment through different sensory organs. Knowledge of how and what different organisms perceive has helped us understand seemingly counter-intuitive, maladaptive behaviours including birds colliding with moving vehicles (DeVault et al., 2015), aquatic insects laying eggs on solar panels (Horváth et al., 2010) and marine animals ingesting plastics (Savoca et al., 2016). Furthering our understanding of these sensory problems can lead to solutions on how to resolve and prevent them in the future. As we have demonstrated through numerous case studies, sensory ecology has proven to be a valuable and effective tool in wildlife conservation and management, and we have generated suggestions for where and when mechanistic studies of perceptual mechanisms may provide informative insights. Promotion and exposure of the benefits sensory ecology can provide for conservation sciences is needed, and conservationists and wildlife managers are encouraged to consider sensory-based approaches to conservation issues.
Acknowledgements
Any use of trade, product or firm name is for descriptive purposes only and does not imply endorsement by the US Government. We would like to thank Jayne E. Yack for her contributions to this paper.
Funding
N.S.J. is supported by the Great Lakes Fishery Commission. D.T.B. is supported by the Natural Sciences Foundation. S.J.C. is supported by the Natural Sciences and Engineering Research Council of Canada. A.Z.H. was supported by National Science Foundation Awards # 1600691 and 1846004.
References
- Ahlering MA, Johnson DH, Faaborg J (2006) Conspecific attraction in a grassland bird, the Baird’s sparrow. J Field Ornithol 77: 365–371. [Google Scholar]
- Alvez-Gomes JA (2001) The evolution of electroreception and bioelectrogenesis in teleost fish: a phylogenetic perspective. J Fish Biol 58: 1489–1511. [Google Scholar]
- Baker T (2009) Use of pheromones in IPM. In E Radcliffe, W Hutchison, R Cancelado, eds, Integrated Pest Management: Concepts, Tactics, Strategies and Case Studies. Cambridge University Press, New York, pp. 273–285. [Google Scholar]
- Balthazart J, Taziaux M (2009) The underestimated role of olfaction in avian reproduction? Behav Brain Res 200: 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth FG (2000) How to catch the wind: spider hairs specialized for sensing the movement of air. Naturwissenschaften 87: 51–58. [DOI] [PubMed] [Google Scholar]
- Barth FG, Bleckmann H, Bohnenberger J, Seyfarth EA (1988) Spiders of the genus Cupiennius Simon 1891 (Araneae, Ctenidae) : II. On the vibratory environment of a wandering spider. Oecologia 77: 194–201. [DOI] [PubMed] [Google Scholar]
- Bayliss LE, Lythgoe RJ, Tansley K (1936) Some new forms of visual purple found in sea fishes with a note on the visual cells of origin. Proc Royal Soc Ser B Biol Sci 120: 95–113. [Google Scholar]
- Bett NN, Hinch SG, Burnett NJ, Donaldson MR, Naman SM (2017) Causes and consequences of straying into small populations of Pacific salmon. Fisheries 42: 220–230. [Google Scholar]
- Bett NN, Hinch SG, Dittman AH, Yun SS (2016) Evidence of olfactory imprinting at an early life stage in pink salmon (Oncorhynchus gorbuscha). Sci Rep 6: 36393. doi: 10.1038/srep36393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell BF, DeVault TL, Seamans TW, Lima SL, Baumhardt P, Fernández-Juricic E (2012) Exploiting avian vision with aircraft lighting to reduce bird strikes. J Appl Ecol 49: 758–766. [Google Scholar]
- Blackwell BF, Fernández-Juricic E, Seamans TW, Dolan T (2009) Avian visual system configuration and behavioural response to object approach. Anim Behav 77: 673–684. [Google Scholar]
- Blackwell B, Fernández-Juricic E (2013) Behavior and physiology in the development and application of visual deterrents at airports. In T DeVault, B Blackwell, J Belant, eds, Wildlife Management in Airport Environments. The Johns Hopkins University Press, Baltimore, pp. 11–22. [Google Scholar]
- Bleckmann H, Zelick R (2009) Lateral line system of fish. Integr Zool 4: 13–25. [DOI] [PubMed] [Google Scholar]
- Blumstein D, Fernández-Juricic E (2010) A Primer of Conservation Behavior. Sinauer, Sunderland. [Google Scholar]
- Boles LC, Lohmann KJ (2003) True navigation and magnetic maps in spiny lobsters. Nature 421: 60–63. [DOI] [PubMed] [Google Scholar]
- Bonadonna F, Cunningham GB, Jouventin P, Hesters F, Nevitt GA (2003) Evidence for nest-odour recognition in two species of diving petrel. J Exp Biol 206: 3719–3722. [DOI] [PubMed] [Google Scholar]
- Bonadonna F, Nevitt GA (2004) Partner-specific odor recognition in an Antarctic seabird. Science 306: 835. [DOI] [PubMed] [Google Scholar]
- Borst A (2009) Drosophila’s view on insect vision. Curr Biol 19: R36–R47. [DOI] [PubMed] [Google Scholar]
- Bradbury J, Vehrencamp S (2011) Principles of Animal Communication, EdEd 2. Oxford University Press, New York. [Google Scholar]
- Brennan P (2010) Pheromones in mammalian behavior. In A Menini, ed, The Neurobiology of Olfaction. CRC Press, Cambridge, pp. 157–181. [Google Scholar]
- Brill R, Bushnell P, Smith L, Speaks C, Sundaram R, Stroud E, Wang J (2009) The repulsive and feeding-deterrent effects of electropositive metals on juvenile sandbar sharks (Carcharhinus plumbeus). Fish Bull 107: 298–307. [Google Scholar]
- Brinkløv S, Fenton MB, Ratcliffe JM (2013) Echolocation in oilbirds and swiftlets. Front Physiol 4: 123. doi: 10.3389/fphys.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R (2000) The potential of strobe lighting as a cost-effective means for reducing impingement and entrainment. Environ Sci Policy 3: 405–416. [Google Scholar]
- Buchholz R (2007) Behavioural biology: an effective and relevant conservation tool. Trends Ecol Evol 22: 401–407. [DOI] [PubMed] [Google Scholar]
- Buchinger TJ, Siefkes MJ, Zielinski BS, Brant CO, Li W (2015) Chemical cues and pheromones in the sea lamprey (Petromyzon marinus). Front Zool 12: 32. doi: 10.1186/s12983-015-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TH (1973) Seeing the world through a new sense: electroception in fish. Am Sci 61: 316–325. [PubMed] [Google Scholar]
- Bunkley JP, McClure CJW, Kawahara AY, Francis CD, Barber JR (2017) Anthropogenic noise changes arthropod abundances. Ecol Evol 7: 2977–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunkley JP, McClure CJW, Kleist NJ, Francis CD, Barber JR (2015) Anthropogenic noise alters bat activity levels and echolocation calls. Global Ecol Conserv 3: 62–71. [Google Scholar]
- Campbell-Palmer R, Rosell F (2011) The importance of chemical communication studies to mammalian conservation biology: a review. Biol Conserv 144: 1919–1930. [Google Scholar]
- Caro T, Sherman PW (2013) Eighteen reasons animal behaviourists avoid involvement in conservation. Anim Behav 85: 305–312. [Google Scholar]
- Chan A, Blumstein DT (2011) Attention, noise, and implications for wildlife conservation and management. Appl Anim Behav Sci 131: 1–7. [Google Scholar]
- Chan A, Giraldo-Perez P, Smith S, Blumstein DT (2010) Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis. Biol Lett 6: 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung-Davidson YW, Yun SS, Teeter J, Li W (2004) Brain pathways and behavioral responses to weak electric fields in parasitic sea lampreys (Petromyzon marinus). Behav Neurosci 118: 611–619. [DOI] [PubMed] [Google Scholar]
- Clapperton BK, Matthews LR, Fawkes MS, Pearson AJ (1996) Lithium and cyanide-induced conditioned food aversions in brushtail possums.J Wildlife Manag 60: 195–201. [Google Scholar]
- Clarke GL (1936) On the depth at which fish can see. Ecology 17: 452–456. [Google Scholar]
- Codarin A, Wysocki LE, Ladich F, Picciulin M (2009) Effects of ambient and boat noise on hearing and communication in three fish species living in a marine protected area (Miramare, Italy). Mar Pollut Bull 58: 1880–1887. [DOI] [PubMed] [Google Scholar]
- Cooke SJ et al. (2014) Physiology, behavior, and conservation. Physiol Biochem Zool 87: 1–14. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1, cot001.doi: 10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TE, Murray PJ, Hall GP, Li XH (2012) Manipulating resource use by goats with predator fecal odors. Wildl Soc Bull 36: 802–806. [Google Scholar]
- Crampton WGR (2019) Electroreception, electrogenesis and electric signal evolution. J Fish Biol 95: 92–134. [DOI] [PubMed] [Google Scholar]
- Cronin TW, Johnsen S, Marshall NJ, Warrant EJ (2014) Visual Ecology. Princeton University Press, Princeton and Oxford. [Google Scholar]
- Cronin TW, Shashar N, Caldwell RL, Marshall J, Cheroske AG, Chiou TH (2003) Polarization vision and its role in biological signaling. Integr Comp Biol 43: 549–558. [DOI] [PubMed] [Google Scholar]
- Cruz F, Carrion V, Campbell KJ, Lavoie C, Donlan CJ (2009) Bio-economics of large-scale eradication of feral goats from Santiago Island, Galapagos. J Wildlife Manag 73: 191–200. [Google Scholar]
- CTGDS (2007) Bird-Friendly Development Guideline. City of Toronto Green Develoment Standard. ON, Canada, Toronto. [Google Scholar]
- Curtis CC, Stoddard PK (2003) Mate preference in female electric fish, Brachyhypopomus pinnicaudatus. Anim Behav 66: 329–336. [Google Scholar]
- Czech-Damal NU, Liebschner A, Miersch L, Klauer G, Hanke FD, Marshall C, Dehnhardt G, Hanke W (2012) Electroreception in the Guiana dolphin (Sotalia guianensis). Proc Royal Soc B Biol Sci 279: 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangles O, Irschick D, Chittka L, Casas J (2009) Variability in sensory ecology: expanding the bridge between physiology and evolutionary biology. Q Rev Biol 84: 51–74. [DOI] [PubMed] [Google Scholar]
- Darling JD (2015) Low frequency, ca. 40 Hz, pulse trains recorded in the humpback whale assembly in Hawaii. J Acoust Soc Am 138: EL452–EL458. [DOI] [PubMed] [Google Scholar]
- Davidson CB, Gottschalk KW, Johnson JE (1999) Tree mortality following defoliation by the European gypsy moth (Lymantria dispar L.) in the United States: a review. Forest Sci 45: 74–84. [Google Scholar]
- DeVault TL, Blackwell BF, Seamans TW, Lima SL, Fernández-Juricic E (2015) Speed kills: ineffective avian escape responses to oncoming vehicles. Proc R Soc B 282: 20142188. doi: 10.1098/rspb.2014.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DFO (2008) Recovery Strategy for the Northern and Southern Resident Killer Whales (Orcinus orca) in Canada. Department of Fisheries and Oceans, Ottawa, ON, CA. [Google Scholar]
- Dittman AH, Quinn TP (1996) Homing in Pacific salmon: mechanisms and ecological basis. J Exp Biol 199: 83–91. [DOI] [PubMed] [Google Scholar]
- Dolbeer RA (2006) Height distribution of birds recorded by collisions with civil aircraft. J Wildlife Manag 70: 1345–1350. [Google Scholar]
- Dolbeer RA, Weller JR, Anderson AL, Begier MJ (2015) Wildlife Strikes to Civil Aircraft in the United States 1990–2015. In Federal Aviation Administration National Wildlife Strike Database. USA, Washington DC. [Google Scholar]
- Dominoni DM et al. (2020) Why and how conservation biology can benefit from sensory ecology. Nat Ecol Evol 4: 502–511. [DOI] [PubMed] [Google Scholar]
- Donaldson MR, Burnett NJ, Braun DC, Suski CD, Hinch SG, Cooke SJ, Kerr JT, Hutchings J (2016) Taxonomic bias and international biodiversity conservation research. FACETS 1: 105–113. [Google Scholar]
- Doppler M, Blackwell BF, DeVault TL, Fernández-Juricic E (2015) Cowbird responses to aircraft with lights tuned to their eyes: implications for bird-aircraft collisions. The Condor 117: 165–177. [Google Scholar]
- Douglas JM, Cronin TW, Chiou TH, Dominy NJ (2007) Light habits and the role of polarized iridescence in the sensory ecology of neotropical nymphalid butterflies (Lepidoptera: Nymphalidae). J Exp Biol 210: 788–799. [DOI] [PubMed] [Google Scholar]
- Dunlap KD, DiBenedictis BT, Banever SR (2010) Chirping response of weakly electric knife fish (Apteronotus leptorhynchus) to low-frequency electric signals and to heterospecific electric fish. J Exp Biol 213: 2243–2242. [DOI] [PubMed] [Google Scholar]
- Dusenbery D (1992) Sensory Ecology: How Organisms Acquire and Respond to Information. W H Freeman, New York. [Google Scholar]
- Edmonds NS, Firmin CJ, Goldsmith D, Faulkner RC, Wood DT (2016) A review of crustacean sensitivity to high amplitude underwatwer noise: data needs for effective risk assessment in relation to UK commercial species. Mar Pollut Bull 108: 5–11. [DOI] [PubMed] [Google Scholar]
- Eisthen HL (2002) Why are olfactory systems of different animals so similar? Brain Behav Evol 59: 273–293. [DOI] [PubMed] [Google Scholar]
- El-Sayed AM, Suckling DM, Wearing CH, Byers JA (2006) Potential of mass trapping for long-term pest management and eradication of invasive species. J Econ Entomol 99: 1550–1564. [DOI] [PubMed] [Google Scholar]
- Ellins SR, Catalano SM (1980) Field application of the conditioned taste aversion paradigm to the control of coyote predation on sheep and turkeys. Behav Neural Biol 29: 532–536. [DOI] [PubMed] [Google Scholar]
- Elvidge CK, Ford MI, Pratt TC, Smokorowski KE, Sills M, Patrick PH, Cooke SJ (2018) Behavioural guidance of yellow-stage American eel Anguilla rostrata with a light-emitting diode device. Endanger Species Res 35: 159–168. [Google Scholar]
- Elvidge CK, Reid CH, Ford MI, Sills M, Patrick PH, Gibson D, Backhouse S, Cooke SJ (2019) Ontogeny of light avoidance in juvenile lake sturgeon. J Appl Ichthyol 35: 202–209. [Google Scholar]
- Endler JA, Basolo AL (1998) Sensory ecology, receiver biases and sexual selection. Trends Ecol Evol 13: 415–420. [DOI] [PubMed] [Google Scholar]
- Engels S, Schneider N-L, Lefeldt N, Hein CM, Zapka M, Michalik A, Elbers D, Kittel A, Hore PJ, Mouritsen H (2014) Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory bird. Nature 509: 353–356. [DOI] [PubMed] [Google Scholar]
- Fay RR, Popper AN (1998) Comparative Hearing: Fish and Amphibians. Springer, New York. [Google Scholar]
- Fernández-Juricic E (2016) The role of animal sensory perception in behavior-based management. In D Saltz, O Berger-Tal, eds, Conservation Behaviour: Applying Behavioural Ecology to Wildlife Conservation and Management. Cambridge University Press, Cambridge, pp. 149–175. [Google Scholar]
- Fernández-Juricic E, Ojeda A, Deisher M, Burry B, Baumhardt P, Stark A, Elmore AG, Ensminger AL (2013) Do male and female cowbirds see their world differently? Implications for sex differences in the sensory system of an avian brood parasite. PLoS One 8: e58985.doi: 10.1371/journal.pone.0058985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti F, Myers RA, Serena F, Lotze HK (2008) Loss of large predatory sharks from the Mediterranean Sea. Conserv Biol 22: 952–964. [DOI] [PubMed] [Google Scholar]
- Fisher HS, Swaisgood RR, Fitch-Snyder H (2003) Odor familiarity and female preferences for males in a threatened primate, the pygmy Loris Nycticebus pygmaeus: applications for genetic management of small populations. Naturwissenschaften 90: 509–512. [DOI] [PubMed] [Google Scholar]
- Fisher HS, Wong BBM, Rosenthal GG (2006) Alteration of the chemical environment disrupts communication in a freshwater fish. Proc Royal Soc B Biol Sci 273: 1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamarique IN, Hawryshyn CW (1998) The common white sucker (Catostomus commersoni): a fish with ultraviolet sensitivity that lacks polarization sensitivity. J Comp Physiol A 182: 331–341. [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ (2010) Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A 107: 18664–18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MI, Elvidge CK, Baker D, Pratt TC, Smokorowski KE, Sills M, Patrick P, Cooke SJ (2018) Preferences of age-0 white sturgeon for different colours and strobe rates of LED lights may inform behavioural guidance strategies. Environ Biol Fishes 101: 667–674. [Google Scholar]
- Fritts TH (1982) Plastic bags in the intestinal tracts of leatherback marine turtles. Herpetol Rev 13: 72–73. [Google Scholar]
- Gagliardo A (2013) Forty years of olfactory navigation in birds. J Exp Biol 216: 2165–2171. [DOI] [PubMed] [Google Scholar]
- Gall MD, Salameh TS, Lucas JR (2013) Songbird frequency selectivity and temporal resolution vary with sex and season. Proc R Soc B 280: 20122296. doi: 10.1098/rspb.2012.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher AJ, Orbesen ES, Hammerschlag N, Serafy JE (2014) Vulnerability of oceanic sharks as pelagic longline bycatch. Glob Ecol Conserv 1: 50–59. [Google Scholar]
- Gentry KE, McKenna MF, Luther DA (2018) Evidence of suboscine song plasticity in response to traffic noise fluctuations and temporary road closures. Bioacoustics Int J Anim Sound Record 27: 165–181. [Google Scholar]
- Goller B, Blackwell BF, DeVault TL, Baumhardt P, Fernández-Juricic E (2018) Assessing bird avoidance of high-contrast lights using a choice test approach: implications for reducing human-induced avian mortality. PeerJ 6, e5404. doi: 10.7717/peerj.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes DGE, Page RA, Geipel I, Taylor RC, Ryan MJ, Halfwerk W (2016) Bats perceptually weight prey cues across sensory systems when hunting in noise. Science 353: 1277–1280. [DOI] [PubMed] [Google Scholar]
- Göpfert MC, Hennig RM (2016) Hearing in insects. Annu Rev Entomol 61: 257–276. [DOI] [PubMed] [Google Scholar]
- Gordon TAC, Radford AN, Davidson IK, Barnes K, McCloskey K, Nedelec SL, Meekan MG, McCormick MI, Simpson SD (2019) Acoustic enrichment can enhance fish community development on degraded coral reef habitat. Nat Commun 10: 5414. doi: 10.1038/s41467-019-13186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz T, Janik VM (2013) Acoustic deterrent devices to prevent pinniped depredation: efficiency, conservation concerns and possible solutions. Mar Ecol Prog Ser 492: 285–302. [Google Scholar]
- Gould E, McShea W, Grand T (1993) Funtion of the star in the star-nosed mole, Condylura cristata. J Mammal 74: 108–116. [Google Scholar]
- Gould JL (2010) Magnetoreception. Curr Biol 20: 431–435. [DOI] [PubMed] [Google Scholar]
- Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sanchez EE, Perez JC, Weissman JS, Julius D (2010) Molecular basis of infrared detection by snakes. Nature 464: 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Cooke SJ (2008) The effects of noise disturbance from various recreational boating activities common to inland waters on the cardiac physiology of a freshwater fish, the largemouth bass (Micropterus salmoides). Aquat Conserv Mar Freshw Ecosyst 18: 1315–1324. [Google Scholar]
- Graves GR (1992) Greater yellow-headed vulture (Cathartes melambrotus) locates food by olfaction. J Raptor Res 26: 38–39. [Google Scholar]
- Greggor AL et al. (2016) Research priorities from animal behaviour for maximizing conservation progress. Trends Ecol Evol 31: 953–964. [DOI] [PubMed] [Google Scholar]
- Greif S, Zsebok S, Schmieder D, Siemers BM (2017) Acoustic mirrors as sensory traps for bats. Science 357: 1045–1047. [DOI] [PubMed] [Google Scholar]
- Grubisic M, van Grunsven RHA, Kyba CCM, Manfrin A, Hölker F (2018) Insect declines and agroecosystems: does light pollution matter? Ann Appl Biol 173: 180–189. [Google Scholar]
- Guerra PA, Gegear RJ, Reppert SM (2014) A magnetic compass aids in monarch butterfly migration. Nat Commun 5: 4164. doi: 10.1038/ncomms5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson CR (1982) An evaluation of taste-aversion control of wolf (Canis lupus) predation in nothern Minesota. Appl Anim Ethol 9: 63–71. [Google Scholar]
- Guthrie D (1986) Role of vision in fish behaviour. In T Pitcher, ed, The Behaviour of Teleost Fishes. Springer, Boston, pp. 75–113. [Google Scholar]
- Habib L, Bayne EM, Boutin S (2007) Chronic industrial noise affects pairing success and age structure of ovenbirds Seiurus aurocapilla. J Appl Ecol 44: 176–184. [Google Scholar]
- Hagstrum JT (2000) Infrasound and the avian navigation map. J Exp Biol 203: 1103–1111. [DOI] [PubMed] [Google Scholar]
- Hailman J (1977) Optical Signals: Animal Communication and Light. Indianna University Press, Bloomington. [Google Scholar]
- Halfwerk W, Slabbekoorn H (2015) Pollution going multimodal: the complex impact of the human-altered sensory environment on animal perception and performance. Biol Lett 11: 20141051.doi: 10.1098/rsbl.2014.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MI, Kamilar JM, Kirk EC (2012) Eye shape and the nocturnal bottleneck of mammals. Proc Royal Soc B 279: 4962–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanika S, Kramer B (2000) Electrosensory prey detection in the African sharptooth catfish, Clarias gariepinus (Clariidae), of a weakly electric mormyrid fish, the bulldog (Marcusenius macrolepidotus). Behav Ecol Sociobiol 48: 218–228. [Google Scholar]
- Hansen A, Reutter K (2004) Chemosensory Systems in Fish: Structural, Functional and Ecological Aspects. In G von der Emde, J Mogdans, B Kapoor, eds, The Senses of Fish. Springer, Dordrecht, pp. 55–89. [Google Scholar]
- Hart NS, Hunt DM (2007) Avian visual pigments: characteristics, spectral tuning, and evolution. Am Nat 169: S7–S26. [DOI] [PubMed] [Google Scholar]
- Haymes GT, Patrick PH, Onisto LJ (1984) Attraction of fish to mercury vapor light and its application in a generating station forebay. Int Rev Gesamten Hydrobiol 69: 867–876. [Google Scholar]
- Hebets EA (2008) Seismic signal dominance in the multimodal courtship display of the wolf spider Schizocosa stridulans Stratton 1991. Behav Ecol 19: 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebets EA, Barron AB, Balakrishnan CN, Hauber ME, Mason PH, Hoke KL (2016) A systems approach to animal communication. Proc Royal Soc B 283: 20152889. doi: 10.1098/rspb.2015.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebets EA, Papaj DR (2005) Complex signal function: developing a testable framework for testable hypotheses. Behav Ecol Sociobiol 57: 197–214. [Google Scholar]
- Hedwig B (2014) Insect Hearing and Acoustic Communication. Springer Science and Business Media, New York. [Google Scholar]
- Higgs D, Radford C (2016) The potential overlapping roles of the ear and lateral line in driving “acoustic” responses. In J Sisneros, ed, Fish Hearing and Bioacoustics: An Anothology in Honor of Arthur N. Popper and Richard R. Fay. Springer International Publishing, Cham, pp. 39–52. [DOI] [PubMed] [Google Scholar]
- Higham J, Hebets EA (2013) An introduction to multimodal communication. Behav Ecol Sociobiol 67: 1381–1388. [Google Scholar]
- Hildebrand JG, Shepherd GM (1997) Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 20: 595–631. [DOI] [PubMed] [Google Scholar]
- Hill PSM (2001) Vibration and animal communication: a review. Am Zool 41: 1135–1142. [Google Scholar]
- Hill PSM (2008) Vibration Communication in Animals. Harvard University Press, Cambridge. [Google Scholar]
- Hill PSM (2009) How do animals use substrate-borne vibrations as an information source? Naturwissenschaften 96: 1355–1371. [DOI] [PubMed] [Google Scholar]
- Himstedt W, Kopp J, Schmidt W (1982) Electroreception guides feeding behaviour in amphibians. Naturwissenschaften 69: 552–553. [Google Scholar]
- Hogg C, Neveu M, Stokkan KA, Folkow L, Cottrill P, Douglas R, Hunt DM, Jeffery G (2011) Arctic reindeer extend their visual range into the ultraviolet. J Exp Biol 214: 2014–2019. [DOI] [PubMed] [Google Scholar]
- Holt MM (2008) Sound exposure and southern resident killer whales (Orcinus orca): a review of current knowledge and data gaps. U.S. Dept. Commer., NOAA Tech. Memo NMFS-NWFSC-89. [Google Scholar]
- Horodysky AZ, Brill RW, Warrant EJ, Musick JA, Latour RJ (2008) Comparative visual function in five sciaenid fishes inhabiting Chesapeake Bay. J Exp Biol 211: 3601–3612. [DOI] [PubMed] [Google Scholar]
- Horodysky AZ, Brill RW, Warrant EJ, Musick JA, Latour RJ (2010) Comparative visual function in four piscivorous fishes inhabiting Chesapeake Bay. J Exp Biol 213: 1751–1761. [DOI] [PubMed] [Google Scholar]
- Horodysky AZ, Cooke SJ, Graves JE, Brill RW (2016) Fisheries conservation on the high seas: linking conservation physiology and fisheries ecology for the management of large pelagic fishes. Conserv Physiol 4, cov059. doi: 10.1093/conphys/cov059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth G, Blaho M, Egri A, Kriska G, Seres I, Robertson B (2010) Reducing the maladaptive attractiveness of solar panels to polarotactic insects. Conserv Biol 24: 1644–1653. [DOI] [PubMed] [Google Scholar]
- Hoy RR, Robert D (1996) Tympanal hearing in insects. Annu Rev Entomol 41: 433–450. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Wang JH, Swimmer Y, Holland K, Kohin S, Dewar H, Wraith J, Vetter R, Heberer C, Martinez J (2012) The effects of a lanthanide metal alloy on shark catch rates. Fish Res 131: 45–51. [Google Scholar]
- Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL (2015) Plastic waste inputs from land into the ocean. Science 347: 768–771. [DOI] [PubMed] [Google Scholar]
- Jeffries DS, Brunton DH (2001) Attracting endangered species to 'safe' habitats: responses of fairy terns to decoys. Anim Conserv 4: 301–305. [Google Scholar]
- Johnsen S (2012) The Optics of Life: A Biologist's Guide to Light in Nature. Princeton University Press, Princeton. [Google Scholar]
- Johnsen S, Lohmann KJ (2005) The physics and neurobiology of magnetoreception. Nat Rev Neurosci 6: 703–712. [DOI] [PubMed] [Google Scholar]
- Johnson NS, Miehls SM, Haro AJ, Wagner MC (2019) Push and pull of downstream moving juvenile sea lamprey (Petromyzon marinus) exposed to chemosensory and light cues. Conserv Physiol 7, coz080. doi: 10.1093/conphys/coz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NS, Miehls S, O’Connor LM, Bravener G, Barber J, Thompson H, Tix JA, Bruning T (2016) A portable trap with electric lead catches up to 75% of an invasive fish species. Sci Rep 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NS, Yun SS, Thompson HT, Brant CO, Li WM (2009) A synthesized pheromone induces upstream movement in female sea lamprey and summons them into traps. Proc Natl Acad Sci U S A 106:1021–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G (2005) Echolocation. Curr Biol 15: R484–R488. [DOI] [PubMed] [Google Scholar]
- Jones IT, Stanley JA, Mooney TA (2020) Impulsive pile driving noise elicits alarm responses in squid (Doryteuthis pealeii). Mar Pollut Bull 150: 110792. doi: 10.1016/j.marpolbul.2019.110792. [DOI] [PubMed] [Google Scholar]
- Jordan LK, Mandelman JW, McComb DM, Fordham SV, Carlson JK, Werner TB (2013) Linking sensory biology and fisheries bycatch reduction in elasmobranch fishes: a review with new directions for research. Conserv Physiol 1, cot002. doi: 10.1093/conphys/cot002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmijn AJ (1971) The electric sense of sharks and rays. J Exp Biol 55: 371–383. [DOI] [PubMed] [Google Scholar]
- Kasurak AV, Zielinski B, Higgs DM (2012) Reproductive status influences multisensory integration responses in female round gobies, Neogobius melanostomus. Anim Behav 83: 1179–1185. [Google Scholar]
- Katti C, Stacey-Solis M, Coronel-Rojas NA, Davies WIL (2019) The diversity and adaptive evolution of visual photopigments in reptiles. Front Ecol Evol 7: 352. doi: 10.3389/fevo.2019.00352. [DOI] [Google Scholar]
- Kawamura S, Yokoyama S (1998) Functional characterization of visual and nonvisual pigments of American chameleon (Anolis carolinensis). Vision Res 38: 37–44. [DOI] [PubMed] [Google Scholar]
- Kilner RM, Noble DG, Davies NB (1999) Signals of need in parent–offspring communication and their exploitation by the common cuckoo. Nature 397: 667–672. [Google Scholar]
- Kirchner W (1997) Acoustical communication in social insects. In M Lehrer, ed, Orientation and communication in arthropods. Birkhäuser Verlag, Basel, pp. 273–300. [Google Scholar]
- Kleist NJ, Guralnick RP, Cruz A, Lowry CA, Francis CD (2018) Chronic anthropogenic noise disrupts glucocorticoid signaling and has multiple effects on fitness in an avian community. Proc Natl Acad SciU S A 115: E648–E657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig C, Szallies A, Steidle JLM, Tolasch T (2016) Sex pheromone of the rare click beetle Betarmon bisbimaculatus. J Chem Ecol 42: 55–59. [DOI] [PubMed] [Google Scholar]
- Köppl C (2015) Avian hearing. In C Scanes, ed, Sturkie’s Avian Physiology, EdEd 6. Academic Press, New York, pp. 71–87. [Google Scholar]
- Kulahci IG, Dornhaus A, Papaj DR (2008) Multimodal signals enhance decision making in foraging bumble-bees. Proc Royal Soc B 275: 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiolo P (2010) The emerging significance of bioacoustics in animal species conservation. Biol Conserv 143: 1635–1645. [Google Scholar]
- Lampe U, Schmoll T, Franzke A, Reinhold K (2012) Staying tuned: grasshoppers from noisy roadside habitats produce courtship signals with elevated frequency components. Funct Ecol 26:1348–1354. [Google Scholar]
- Landgren E, Fritsches K, Brill R, Warrant E (2014) The visual ecology of a deep-sea fish, the escolar Lepidocybium flavobrunneum (Smith, 1843). Philos Trans R Soc B 369: 20130039. doi: 10.1098/rstb.2013.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfa MA, Barth FG (1996) Vibrations in the orb web of the spider Nephila clavipes: cues for discrimination and orientation. J Comp Physiol A 179: 493–508. [Google Scholar]
- Larsen F, Eigaard OR (2014) Acoustic alarms reduce bycatch of harbour porpoises in Danish North Sea gillnet fisheries. Fish Res 153: 108–112. [Google Scholar]
- Larsson MC (2016) Pheromones and other semiochemicals for monitoring rare and endangered species. J Chem Ecol 42: 853–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker CA, Parsons MH, Lecker DR, Sarno RJ, Parsons FE (2015) The temporal multimodal influence of optical and auditory cues on the repellent behaviour of ring-billed gulls (Larus delewarensis). Wildl Res 42: 232–240. [Google Scholar]
- Lefebvre J (1999) Physical basis of acoustics. In P Filippi, D Habault, J Lefebvre, A Bergassoli, eds, Acoustics. Academic Press, London, pp. 1–39. [Google Scholar]
- Lennox R, Cooke SJ (2014) State of the interface between conservation and physiology: a bibliometric analysis. Conserv Physiol 2, cou003. doi: 10.1093/conphys/cou003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MLM, Sodhi NS, Endler JA (2008) Conservation with sense. Science 319: 281. [DOI] [PubMed] [Google Scholar]
- Lohmann KJ, Lohmann CMF (2019) There and back again: natal homing by magnetic navigation in sea turtles and salmon. J Exp Biol 222, jeb184077. doi: 10.1242/jeb.184077. [DOI] [PubMed] [Google Scholar]
- Lohmann KJ, Lohmann CMF, Putman NF (2007) Magnetic maps in animals: nature's GPS. J Exp Biol 210: 3697–3705. [DOI] [PubMed] [Google Scholar]
- Lohmann K, Witherington B, Lohmann C, Salmon M (1997) Orientation, navigation, and natal beach homing in sea turtles. In P Lutz, J Musick, eds, Biology of Sea Turtles. Plenum Press, New York, pp. 107–135. [Google Scholar]
- Lythgoe J (1979) The Ecology of Vision. Oxford University Press, New York. [Google Scholar]
- Lyytinen A, Lindstrom L, Mappes J (2004) Ultraviolet reflection and predation risk in diurnal and nocturnal Lepidoptera. Behav Ecol 15: 982–987. [Google Scholar]
- Machtans CS, Wedeles CHR, Bayne EM (2013) A first estimate for Canada of the number of birds killed by colliding with building windows. Avian Conserv Ecol 8: 6. doi: 10.5751/ACE-00568-080206. [DOI] [Google Scholar]
- Madliger CL (2012) Toward improved conservation management: a consideration of sensory ecology. Biodivers Conserv 21: 3277–3286. [Google Scholar]
- Madliger CL et al. (2016) Success stories and emerging themes in conservation physiology. Conserv Physiol 4, cov057. doi: 10.1093/conphys/cov057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madliger CL, Cooke SJ, Love OP (2017) A call for more physiology at conservation conferences. Biodivers Conserv 26: 2507–2515. [Google Scholar]
- Maguire GS, Stojanovic D, Weston MA (2009) Conditioned taste aversion reduces fox depredation on model eggs on beaches. Wildl Res 36: 702–708. [Google Scholar]
- Manley GA (2012) Evolutionary paths to mammalian cochleae. J Assoc Res Otolaryngol 13: 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marples NM, van Veelen W, Brakefield PM (1994) The relative importance of color, taste and smell in the protection of an aposematic insect Coccinella septempunctata. Anim Behav 48: 967–974. [Google Scholar]
- Martin GR (2011) Understanding bird collisions with man-made objects: a sensory ecology approach. Ibis 153: 239–254. [Google Scholar]
- Martin GR (2012) Through birds' eyes: insights into avian sensory ecology. J Ornithol 153: S23–S48. [Google Scholar]
- Martin G (2017) The Sensory Ecology of Birds. Oxford University Press, Oxford. [Google Scholar]
- Martin G, Osorio D (2008) Vision in birds. In R Masland, T Albright, eds, The Senses Vol vol. 1. Academic Press, London, pp. 25–52. [Google Scholar]
- Mass AM, Supin AY (2007) Adaptive features of aquatic mammals' eye. Anat Rec 290: 701–715. [DOI] [PubMed] [Google Scholar]
- McCauley RD, Day RD, Swadling KM, Fitzgibbon QP, Watson RA, Semmens JM (2017) Widely used marine seismic survey air gun operations negatively impact zooplankton. Nat Ecol Evol 1: 0195.doi: 10.1038/s41559-017-0195. [DOI] [PubMed] [Google Scholar]
- McLaughlin KE, Kunc HP (2015) Changes in the acoustic environment alter the foraging and sheltering behaviour of the cichlid Amititlania nigrofasciata. Behav Processes 116: 75–79. [DOI] [PubMed] [Google Scholar]
- Meuthen D, Rick IP, Thünken T (2012) Visual prey detection by near-infrared cues in a fish. Naturwissenschaften 99: 1063–1066. [DOI] [PubMed] [Google Scholar]
- Mickle MF, Higgs DM (2018) Integrating techniques: a review ofthe effects of anthropogenic noise on freshwater fish. Can J Fish Aquat Sci 75: 1535–1541. [Google Scholar]
- Mickle MF, Miehls SM, Johnson NS, Higgs DM (2019) Hearing capabilities and behavioural response of sea lamprey (Petromyzon marinus) to low-frequency sounds. Can J Fish Aquat Sci 76: 1541–1548. [Google Scholar]
- Millar JG, McElfresh JS, Romero C, Vila M, Mari-Mena N, Lopez-Vaamonde C (2010) Identification of the sex pheromone of a protected species, the Spanish moon moth Graellsia isabellae. J Chem Ecol 36:923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnaar C, Boyles JG, Minnaar IA, Sole CL, McKechnie AE (2015) Stacking the odds: light pollution may shift the balance in an ancient predator-prey arms race. J Appl Ecol 52: 522–531. [Google Scholar]
- Molina JM, Cooke SJ (2012) Trends in shark bycatch research: current status and research needs. Rev Fish Biol Fish 22: 719–737. [Google Scholar]
- Morshedian A, Fain GL (2015) Single-photon sensitivity of lamprey rods with cone-like outer segments. Curr Biol 25: 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H (2018) Long-distance navigation and magnetoreception in migratory animals. Nature 558: 50–59. [DOI] [PubMed] [Google Scholar]
- Munoz NE, Blumstein DT (2012) Multisensory perception in uncertain environments. Behav Ecol 23: 457–462. [Google Scholar]
- Munoz NE, Blumstein DT (2020) Optimal multisensory integration. Behav Ecol 31: 184–193. [Google Scholar]
- Nagel R, Kirschbaum F, Engelmann J, Hofmann V, Pawelzik F, Tiedemann R (2018) Male-mediated species recognition among African weakly electric fishes. R Soc Open Sci 5: 170443. doi: 10.1098/rsos.170443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler JM, Polskey GR, Pickens J, Menezes J, Schilt C (1992) Responses of blueback hearring to high-frequency sound and implications for reducing entrainment at hydropower dams. North Am J Fish Manag 12: 667–683. [Google Scholar]
- Neuweiler G (1989) Foraging ecology and audition in echolocating bats. Trends Ecol Evol 4: 160–166. [DOI] [PubMed] [Google Scholar]
- Nevitt GA (2000) Olfactory foraging by Antarctic procellariiform seabirds: life at high Reynolds numbers. Biol Bull 198: 245–253. [DOI] [PubMed] [Google Scholar]
- Nevitt GA (2008) Sensory ecology on the high seas: the odor world of the procellariiform seabirds. J Exp Biol 211: 1706–1713. [DOI] [PubMed] [Google Scholar]
- Nichols TA, Anderson TW, Sirovic A (2015) Intermittent noise induces physiological stress in a coastal marine fish. PLoS One 10: e0139157. doi: 10.1371/journal.pone.0139157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NMFS (2008) Recovery Plan for Southern Resident Killer Whales (Orcinus orca). National Marine Fisheries Service, Northwest Region, Seattle, Washington. [Google Scholar]
- Noatch MR, Suski CD (2012) Non-physical barriers to deter fish movements. Environ Rev 20: 71–82. [Google Scholar]
- Nordt A, Klenke R (2013) Sleepless in town - drivers of the temporal shift in dawn song in urban European blackbirds. PLoS One 8, e0071476. doi: 10.1371/journal.pone.0071476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYCAS (2007) Bird-Safe Building Guidelines. New York City Audubon Society, Inc., New York City, NY, USA. [Google Scholar]
- O'Connell CP, Stroud EM, He PG (2014) The emerging field of electrosensory and semiochemical shark repellents: mechanisms of detection, overview of past studies, and future directions. Ocean Coast Manag 97: 2–11. [Google Scholar]
- O’Connell-Rodwell CE, Hart LA, Arnason BT (2001) Exploring the potential use of seismic waves as a communication channel by elephants and other large mammals. Am Zool 41: 1157–1170. [Google Scholar]
- Ortega C (2012) Effects of noise pollution on birds: a brief review of our knowledge. In C Francis, J Blickley, eds, Ornithological Monographs. American Ornithologists' Union, Chicago, pp. 6–22. [Google Scholar]
- Osborn FV (2002) Capsicum oleoresin as an elephant repellent: field trials in the communal lands of Zimbabwe. J Wildlife Manag 66: 674–677. [Google Scholar]
- Owens ACS, Lewis SM (2018) The impact of artificial light at night on nocturnal insects: a review and synthesis. Ecol Evol 8: 11337–11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MH, Blumstein DT (2010) Familiarity breeds contempt: kangaroos persistently avoid areas with experimentally deployed dingo scents. PLoS One 5: e10403. doi: 10.1371/journal.pone.0010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partan SR (2017) Multimodal shifts in noise: switching channels to communicate through rapid environmental change. Anim Behav 124: 325–337. [Google Scholar]
- Partan SR, Fulmer A, Gounard MAM, Redmond JE (2010) Multimodal alarm behavior in urban and rural gray squirrels studied by means of observation and mechanical robot. Curr Zool 56: 313–326. [Google Scholar]
- Partan SR, Marler P (1999) Communication goes mulitmodal. Science 83: 1272–1273. [DOI] [PubMed] [Google Scholar]
- Partan SR, Marler P (2005) Issues in the classification of multisensory communication signals. Am Nat 166: 231–245. [DOI] [PubMed] [Google Scholar]
- Patrick PH, Christie AE, Sager D, Hocutt C, Stauffer J (1985) Responses of fish to a strobe light air-bubble barrier. Fish Res 3: 157–172. [Google Scholar]
- Patullo BW, Macmillan DL (2007) Crayfish respond to electrical fields. Curr Biol 17: R83–R84. [DOI] [PubMed] [Google Scholar]
- Patullo BW, Macmillan DL (2010) Making sense of electrical sense in crayfish. J Exp Biol 213: 651–657. [DOI] [PubMed] [Google Scholar]
- Peters RC, Eeuwes LBM, Bretschneider F (2007) On the electrodetection threshold of aquatic vertebrates with ampullary or mucous gland electroreceptor organs. Biol Rev 82: 361–373. [DOI] [PubMed] [Google Scholar]
- Pollack G, Mason A, Popper A, Fay R (2016) Insect Hearing. Springer, Berlin. [Google Scholar]
- Popper AN, Fay RR (2011) Rethinking sound detection in fishes. Hear Res 273: 25–36. [DOI] [PubMed] [Google Scholar]
- Popper AN, Hawkins AD (2012) The Effects of Noise on Aquatic Life. Springer, New York. [Google Scholar]
- Popper AN, Hawkins AD (2019) An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes. J Fish Biol 94: 692–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada PA, Furton KG (2018) Birds and dogs: toward a comparative perspective on odor use and detection. Front Vet Sci 5: 188.doi: 10.3389/fvets.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser J, Radford AN (2011) Acoustic noise induces attention shifts and reduces foraging performance in three-spined sticklebacks (Gasterosteus aculeatus). PLoS One 6: e17478. doi: 10.1371/journal.pone.0017478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putland RL, Montgomery JC, Radford CA (2019) Ecology of fish hearing. J Fish Biol 95: 39–52. [DOI] [PubMed] [Google Scholar]
- Putman BJ, Blumstein DT (2019) What is the effectiveness of using conspecific or heterospecific acoustic playbacks for the attraction of animals for wildlife management? A systematic review protocol. Environ Evidence 8: 6. doi: 10.1186/s13750-019-0149-3. [DOI] [Google Scholar]
- Putman NF, Lohmann KJ, Putman EM, Quinn TP, Klimley AP, Noakes DLG (2013) Evidence for geomagnetic imprinting as a homing mechanism in Pacific salmon. Curr Biol 23: 312–316. [DOI] [PubMed] [Google Scholar]
- Quinn TP, Brannon EL (1982) The use of celestial and magnetic cues by orienting sockeye salmon smolts. J Comp Physiol 147:547–552. [Google Scholar]
- Rabin LA, Coss RG, Owings DH (2006) The effects of wind turbines on antipredator behavior in California ground squirrels (Spermophilus beecheyi). Biol Conserv 131: 410–420. [Google Scholar]
- Ray AM, Arnold RA, Swift I, Schapker PA, McCann S, Marshall CJ, McElfresh JS, Millar JG (2014) (R)-Desmolactone is a sex pheromone or sex attractant for the endangered valley elderberry longhorn beetle Desmocerus californicus dimorphus and several congeners (Cerambycidae: Lepturinae). PLoS One 9: e115498. doi: 10.1371/journal.pone.0115498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren TY (2002) Longitudinal pattern of basilar membrane vibration in the sensitive cochlea. Proc Natl Acad Sci U S A 99: 17101–17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Guerra PA, Merlin C (2016) Neurobiology of monarch butterfly migration. Annu Rev Entomol 61: 25–42. [DOI] [PubMed] [Google Scholar]
- Robert KA, Lesku JA, Partecke J, Chambers B (2015) Artificial light at night desynchronizes strictly seasonal reproduction in a wild mammal. Proc Royal Soc B Biol Sci 282: 20151745. doi: 10.1098/rspb.2015.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SC, Gosling LM (2004) Manipulation of olfactory signaling and mate choice for conservation breeding: a case study of harvest mice. Conserv Biol 18: 548–556. [Google Scholar]
- Robertson BA, Ostfeld RS, Keesing F (2017) Trojan females and judas goats: evolutionary traps as tools in wildlife management. Bioscience 67: 982–993. [Google Scholar]
- Ronald KL, Fernández-Juricic E, Lucas JR (2018) Mate choice in the eye and ear of the beholder? Female multimodal sensory configuration influences her preferences. Proc R Soc B 285: 20180713.doi: 10.1098/rspb.2018.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald KL, Zeng R, Stewart R, White D, Fernández-Juricic E, Lucas J (2017) What makes a multimodal signal attractive? A preference function approach. Behav Ecol 28: 677–687. [Google Scholar]
- Ross QU, Dunning DJ, Menezes JK, Kenna MJ, Tiller G (1995) Reducing impingement of alewives with high-freuency sound at a power plant on Lake Ontario. North Am J Fish Manag 15: 378–388. [Google Scholar]
- Rountree RA, Juanes F, Bolgan M (2020) Temperate freshwater soundscapes: a cacophony of undescribed biological sounds now threatened by anthropogenic noise. PLoS One 15: e0221842. doi: 10.1371/journal.pone.0221842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe C, Guilford T (1999) The evolution of multimodal warning displays. Evol Ecol 13: 655–671. [Google Scholar]
- Ruebush BC, Sass GG, Chick JH, Stafford JD (2012) In-situ tests of sound-bubble-strobe light barrier technologies to prevent range expansions of Asian carp. Aquat Invasions 7: 37–48. [Google Scholar]
- Ruggero MA, Robles L, Rich NC (1992) 2-tonne suppression in the basilar membrane of the cochlea: mechanical basis of auditory nerve rate suppression. J Neurophysiol 6: 1087–1099. [DOI] [PubMed] [Google Scholar]
- Ruxton GD, Allen WL, Sherratt TN, Speed MP (2018) Avoiding attack: the evolutionary ecology of crypsis. In Aposematism, and Mimicry, EdEd 2. Oxford University Press, Oxford. [Google Scholar]
- Sabet SS, Neo YY, Slabbekoorn H (2015) The effect of temporal variation in sound exposure on swimming and foraging behaviour of captive zebrafish. Anim Behav 107: 49–60. [Google Scholar]
- Safi K, Siemers BM (2010) Implications of sensory ecology for species coexistence: biased perception links predator diversity to prey size distribution. Evol Ecol 24: 703–713. [Google Scholar]
- Sarrazin F, Bagnolini C, Pinna JL, Danchin E (1996) Breeding biology during establishment of a reintroduced griffon vulture Gyps fulvus population. Ibis 138: 315–325. [Google Scholar]
- Savoca MS, Wohlfeil ME, Ebeler SE, Nevitt GA (2016) Marine plastic debris emits a keystone infochemical for olfactory foraging seabirds. Sci Adv 2: e1600395. doi: 10.1126/sciadv.1600395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarbati A, Osculati F (2003) Solitary chemosensory cells in mammals? Cells Tissues Organs 175: 51–55. [DOI] [PubMed] [Google Scholar]
- Scheich H, Langner G, Tidemann C, Coles RB, Guppy A (1986) Electroreception and electrolocation in platypus. Nature 319: 401–402. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Morrison A, Kunc HP (2014) Sexy voices - no choices: male song in noise fails to attract females. Anim Behav 94: 55–59. [Google Scholar]
- Schmitz H, Bleckmann H (1998) The photomechanic infrared receptor for the detection of forest fires in the beetle Melanophila acuminata (Coleoptera: Buprestidae). J Comp Physiol A 182: 647–657. [Google Scholar]
- Schuyler QA, Wilcox C, Townsend KA, Hardesty BD, Marshall NJ (2014) Mistaken identity? Visual similarities of marine debris to natural prey items of sea turtles. BMC Ecol 14: 14. doi: 10.1186/1472-6785-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler QA, Wilcox C, Townsend KA, Wedemeyer-Strombel KR, Balazs G, van Sebille E, Hardesty BD (2015) Risk analysis reveals global hotspots for marine debris ingestion by sea turtles. Glob Chang Biol 22: 567–576. [DOI] [PubMed] [Google Scholar]
- Schwarze S, Schneider N-L, Reichl T, Dreyer D, Lefeldt N, Engels S, Baker N, Hore PJ, Mouriitsen H (2016) Weak broadband electromagnetic fields are more disruptive to magnetic compass orientation in a night-migratory songbird (Erithacus rubecula) than strong narrow-band fields. Front Behav Neurosci 10: 55. doi: 10.3389/fnbeh.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwind R (1995) Spectral regions in which aquatic insects see reflected polarized light. J Comp Physiol A 177: 439–448. [Google Scholar]
- Scott K, Harsanyi P, Lyndon AR (2018) Understanding the effects of electromagnetic field emissions from marine renewable energy devices (MREDs) on the commercially important edible crab, Cancer pagurus (L.). Mar Pollut Bull 131: 580–588. [DOI] [PubMed] [Google Scholar]
- SFPD (2011) Standards for Bird-Safe Buildings. San Francisco Planning Department, San Francisco, CA, USA. [Google Scholar]
- Shieh BS, Liang SH, Chen CC, Loa HH, Liao CY (2012) Acoustic adaptations to anthropogenic noise in the cicada Cryptotympana takasagona Kato (Hemiptera: Cicadidae). Acta Ethol 15: 33–38. [Google Scholar]
- Shier DM, Lea AJ, Owen MA (2012) Beyond masking: endangered Stephen’s kangaroo rats respond to traffic noise with footdrumming. Biol Conserv 150: 53–58. [Google Scholar]
- Sillman AJ, Ong EK, Loew ER (2007) Spectral absorbance, structure, and population density of photoreceptors in the retina of the lake sturgeon (Acipenser fulvescens). Can J Zool 85: 584–587. [Google Scholar]
- Simpson SD, Radford AN, Nedelec SL, Ferrari MCO, Chivers DP, McCormick MI, Meekan MG (2016) Anthropogenic noise increases fish mortality by predation. Nat Commun 7: 10544. doi: 10.1038/ncomms10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN (2010) A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol Evol 25: 419–427. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Dooling R, Popper A, Fay R (2018) The Effects of Anthropogenic Noise on Animals. Springer, New York. [Google Scholar]
- Slack J (2016) Molecular pharmacology of chemesthesis. In F Zufall, S Munger, eds, Chemosensory Transduction: The Detection of Odors, Tastes, and Other Chemostimuli. Academic Press, London, pp. 375–391. [Google Scholar]
- Smith C (2008) Biology of Sensory Systems, EdEd 2. Wiley-Blackwell, Oxford. [Google Scholar]
- Smith EJ, Partridge JC, Parsons KN, White EM, Cuthill IC, Bennett ATD, Church SC (2002) Ultraviolet vision and mate choice in the guppy (Poecilia reticulata). Behav Ecol 13: 11–19. [Google Scholar]
- Smith JF (1992) Alarm signals in fishes. Rev Fish Biol Fish 2: 33–63. [Google Scholar]
- Smith JW (1998) Boll weevil eradication: area-wide pest management. Ann Entomol Soc Am 91: 239–247. [Google Scholar]
- Sorensen PW, Johnson NS (2016) Theory and application of semiochemicals in nuisance fish control. J Chem Ecol 42: 698–715. [DOI] [PubMed] [Google Scholar]
- Spoelstra K, van Grunsven RHA, Donners M, Gienapp P, Huigens ME, Slaterus R, Berendse F, Visser ME, Veenendaal E (2015) Experimental illumination of natural habitat - an experimental set-up to assess the direct and indirect ecological consequences of artificial light of different spectral composition. Philos Trans R Soc B 370: 20140129. doi: 10.1098/rstb.2014.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamps JA, Swaisgood RR (2007) Someplace like home: experience, habitat selection and conservation biology. Appl Anim Behav Sci 102: 392–409. [Google Scholar]
- Stenvers DJ, van Dorp R, Foppen E, Mendoza J, Opperhuizen AL, Fliers E, Bisschop PH, Meijer JH, Kalsbeek A, Deboer T (2016) Dim light at night disturbs the daily sleep-wake cycle in the rat. Sci Rep 6: 35662. doi: 10.1038/srep35662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M (2013) Sensory Ecology, Behaviour, and Evolution. Oxford University Press, Oxford. [Google Scholar]
- Stoddard PF, Markham MR (2008) Signal cloaking by electric fish. Bioscience 58: 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpner A, von Helverson D (2001) Evolution and function of auditory systems in insects. Naturwissenschaften 88: 159–170. [DOI] [PubMed] [Google Scholar]
- Sutherland WJ et al. (2020) A horizon scan of emerging global biological conservation issues for 2020. Trends Ecol Evol 35: 81–90. [DOI] [PubMed] [Google Scholar]
- Sutherland WJ, Fleishman E, Clout M, Gibbons DW, Lickorish F, Peck LS, Pretty J, Spalding M, Ockendon N (2019) Ten years on: a review of the first global conservation horizon scan. Trends Ecol Evol 34: 139–153. [DOI] [PubMed] [Google Scholar]
- Suthers RA (1978) Sensory ecology of mammals. In M Ali, ed, Sensory Ecology. Springer, Boston, MA, pp. 253–287. [Google Scholar]
- Swaddle JP, Moseley DL, Hinders MK, Smith EP (2016) A sonic net excludes birds from an airfield: implications for reducing bird strike and crop losses. Ecol Appl 26: 339–345. [DOI] [PubMed] [Google Scholar]
- Swaisgood RR (2010) The conservation-welfare nexus in reintroduction programmes: a role for sensory ecology. Anim Welf 19: 125–137. [Google Scholar]
- Thomas JA, Moss CF, Vater M (2004) Echolocation in Bats and Dolphins. The University of Chicago Press, Chicago. [Google Scholar]
- Thums M, Whiting SD, Reisser J, Pendoley KL, Pattiaratchi CB, Proietti M, Hetzel Y, Fisher R, Meekan MG (2016) Artificial light on water attracts turtle hatchlings during their near shore transit. R Soc Open Sci 3: 160142. doi: 10.1098/rsos.160142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin PC, Blackburn LM (2007) Slow the spread: a national program to manage the gypsy moth. Gen. Tech. Rep. NRS-6. Newtown Square, PA. U.S. Department of Agriculture, Forest Service, Northern Research Station.
- Trotier D (2011) Vomeronasal organ and human pheromones. Eur Ann Otorhinolaryngol Head Neck Dis 128: 184–190. [DOI] [PubMed] [Google Scholar]
- Troudet J, Grandcolas P, Blin A, Vignes-Lebbe R, Legendre F (2017) Taxonomic bias in biodiversity data and societal preferences. Sci Rep 7: 9132. doi: 10.1038/s41598-017-09084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz GW, Roberts JA (2002) Multisensory cues and multimodal communication in spiders: insights from video/audio playback studies. Brain Behav Evol 59: 222–230. [DOI] [PubMed] [Google Scholar]
- Von dermde G (1999) Active electrolocation of objects in weakly electric fish. J Exp Biol 202: 1205–1215. [DOI] [PubMed] [Google Scholar]
- Wackermannová M, Pinc L, Jebavý L (2016) Olfactory sensitivity in mammalian species. Physiol Res 65: 369–390. [DOI] [PubMed] [Google Scholar]
- Wagner H (2011) Vision in fishes: an introduction. In A Farrell, ed, Encyclopedia of Fish Physiology. Academic Press, London, pp. 98–101. [Google Scholar]
- Wakefield A, Broyles M, Stone EL, Jones G, Harris S (2016) Experimentally comparing the attractiveness of domestic lights to insects: do LEDs attract fewer insects than conventional light types? Ecol Evol 6: 8028–8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MM, Diebel CE, Haugh CV, Pankhurst PM, Montgomery JC, Green CR (1997) Structure and function of the vertebrate magnetic sense. Nature 390: 371–376. [DOI] [PubMed] [Google Scholar]
- Wallraff HG (2004) Avian olfactory navigation: its empirical foundation and conceptual state. Anim Behav 67: 189–204. [Google Scholar]
- Wang J, Barkan J, Fisler S, Godinez-Reyes C, Swimmer Y (2013) Developing ultraviolet illumination of gillnets as a method to reduce sea turtle bycatch. Biol Lett 9: 20130383. doi: 10.1098/rsbl.2013.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MP, Schlossberg S (2004) Conspecific attraction and the conservation of territorial songbirds. Conserv Biol 18:519–525. [Google Scholar]
- Ware HE, McClure CJW, Carlisle JD, Barber JR (2015) A phantom road experiment reveals traffic noise is an invisible source of habitat degradation. Proc Natl Acad Sci U S A 112:12105–12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrant EJ (2017) The remarkable visual capacities of nocturnal insects: vision at the limits with small eyes and tiny brains. Philos Trans R Soc B 372: 20160063. doi: 10.1098/rstb.2016.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel BM (1968) Olfactory prowess of the kiwi. Nature 220: 1133–1134. [DOI] [PubMed] [Google Scholar]
- Wilcox C, Puckridge M, Schuyler QA, Townsend K, Hardesty BD (2018) A quantitative analysis linking sea turtle mortality and plastic debris ingestion. Sci Rep 8: 12536. doi: 10.1038/s41598-018-30038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MR, Shizuka D, Joseph MB, Hubbard JK, Safran RJ (2015) Multimodal signalling in the North American barn swallow: a phenotype network approach. Proc Royal Soc B 282: 20151574.doi: 10.1098/rspb.2015.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems EP, Hill RA (2009) Predator-specific landscapes of fear and resource distribution: effects on spatial range use. Ecology 90: 546–555. [DOI] [PubMed] [Google Scholar]
- Williams CR, Dittman AH, McElhany P, Busch DS, Maher MT, Bammler TK, MacDonald JW, Gallagher EP (2019a) Elevated CO2 impairs olfactory-mediated neural and behavioral responses and gene expression in ocean-phase coho salmon (Oncorhynchus kisutch). Glob Chang Biol 25: 963–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Clark CW, Ponirakis D, Ashe E (2014) Acoustic quality of critical habitats for three threatened whale populations. Anim Conserv 17: 174–185. [Google Scholar]
- Williams R, Lusseau D, Hammond PS (2006) Estimating relative energetic costs of human disturbance to killer whales (Orcinus orca). Biol Conserv 133: 301–311. [Google Scholar]
- Williams R, Veirs S, Veirs V, Ashe E, Mastick N (2019b) Approaches to reduce noise from ships operating in important killer whale habitats. Mar Pollut Bull 139: 459–469. [DOI] [PubMed] [Google Scholar]
- Wiltschko R, Schiffner I, Fuhrmann P, Wiltschko W (2010) The role of the magnetite-based receptors in the beak in pigeon homing. Curr Biol 20: 1534–1538. [DOI] [PubMed] [Google Scholar]
- Wiltschko R, Wiltschko W (2012) Magnetoreception. Sens Nat 739: 126–141. [DOI] [PubMed] [Google Scholar]
- Wiltschko W, Freire R, Munro U, Ritz T, Rogers L, Thalau P, Wiltschko R (2007) The magnetic compass of domestic chickens, Gallus gallus.J Exp Biol 210: 2300–2310. [DOI] [PubMed] [Google Scholar]
- Wiltschko W, Wiltschko R (2005) Magnetic orientation and magnetoreception in birds and other animals. J Comp Physiol A 191:675–693. [DOI] [PubMed] [Google Scholar]
- Windmill J, Jackson J (2016) Mechanical specializations of insect ears. In G Pollack, A Mason, A Popper, R Fay, eds, Insect Hearing. Springer, Berlin, pp. 125–157. [Google Scholar]
- Winkler H (2001) The ecology of avian acoustical signals. In F Barth, A Schmid, eds, Ecology of Sensing. Springer, Berlin, pp. 79–104 [Google Scholar]
- Witherington BE, Martin RE, Trindell RN (2014) Understanding, assessing, and resolving light pollution problems on sea turtle nesting beaches, 3rd ed, revised. Florida Marine Research Institute TechnicalReport TR-2. [Google Scholar]
- Witzgall P, Kirsch P, Cork A (2010) Sex pheromones and their impact on pest management. J Chem Ecol 36: 80–100. [DOI] [PubMed] [Google Scholar]
- Wyatt TD (2010) Pheromones and signature mixtures: defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J Comp Physiol A 196: 685–700. [DOI] [PubMed] [Google Scholar]
- Wyatt TD (2014) Pheromones and Animal Behavior: Chemical Signals and Signatures, EdEd 2v. Cambridge University Press, Cambridge. [Google Scholar]
- Yamamoto Y, Hino H, Ueda H (2010) Olfactory imprinting of amino acids in lacustrine sockeye salmon. PLoS One 5: e8633. doi: 10.1371/journal.pone.0008633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker1 CS, Ryba NJP (2009) Common sense about taste: from mammals to insects. Cell 139: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyl JN, den Ouden O, Köppl C, Assink J, Christensen-Dalsgaard J, Patrick SC, Clusella-Trullas S (2020) Infrasonic hearing in birds: a review of audiometry and hypothesized structure– function relationships. Biol Rev 0:000–000. doi: 10.1111/brv.12596. [DOI] [PubMed] [Google Scholar]


