Abstract
Social media use (SMU) is an inherent element in the daily life and neurodevelopment of adolescents, but broad concerns exist regarding the untoward effects of social media on adolescents. We conducted a prospective, cross-sectional study that sought to examine the acute effects of SMU on clinical measures and biomarkers of stress in healthy and depressed adolescents. After at least 24 hours of abstinence from social media, depressed adolescents (n=30) and healthy control adolescents (n=30) underwent baseline clinical assessment of their prior SMU, depressive symptom severity, self-esteem, and bullying. Participants provided salivary samples that were analyzed for α-amylase and cortisol levels. After 20 minutes of unsupervised SMU, saliva analyses and clinical assessments were repeated. After 20 minutes of SMU, salivary cortisol and α-amylase levels were significantly higher in adolescents with depression but not in healthy control adolescents. Furthermore, small but statistically significant changes in depressive symptom severity occurred in all participants. These changes in depressive symptoms were not clinically meaningful. SMU did not significantly change self-esteem measures among participants. Adolescents with depression appeared to have more physiological reactivity after SMU compared with healthy adolescents. Further research should characterize SMU as a clinical dimension and risk factor among adolescents with depression and other psychiatric disorders.
1. Introduction
At least 95% of adolescents own a smartphone and spend considerable time on social media platforms (Anderson and Jiang, 2018). Contemporary adolescents grow up in a digital milieu that most likely impacts their cognitive and emotional development (Rideout and Robb, 2018; Carson, Gansner, and Khang, 2018; Shafi, Romanowicz, and Croarkin, 2018). Adolescents spend an average of 6 to 9 hours per day on digital media and 2 to 4 hours daily on social media (Carson, Gansner, and Khang, 2018; Crone and Konijn, 2018). National surveys have reported that the frequency and intensity of adolescent social media use continues to increase. For example, 70% to 80% of adolescents check social media platforms more than once per day (Rideout and Robb, 2018; Carson, Gansner, and Khang, 2018). The social, clinical, and neurodevelopmental effects of these behavior patterns are poorly understood (Crone and Konijn, 2018; Katz, Peckins, and Lyon, 2019; Banyai et al., 2017).
Adolescence is a sensitive period of psychological and neurobiological development, when neural networks are likely to be more influenced by digital environments and stressors (Crone and Konijn, 2018; Katz, Peckins, and Lyon, 2019; Banyai et al., 2017; Woods and Scott, 2016). Despite the constant presence of social media, its effects on the development, physiological stress response, and psychological functioning of adolescents is poorly characterized (Vanman, Baker, and Tobin, 2018; Luby and Kertz, 2019; Andreassen et al., 2012; Shafi et al., 2020). Evidence increasingly supports associations between adolescent social media use (SMU) and self-esteem, mood, and sleep habits (Woods and Scott, 2016; Vanman, Baker, and Tobin, 2018; Luby and Kertz, 2019; Andreassen et al., 2012; Shafi et al., 2020; Nesi, Wolff, and Hunt, 2019; Tamir and Mitchell, 2012; Odgers, 2018). SMU may have a role in the recent, stark increase in adolescent suicidality. SMU is an evolving public health challenge that warrants further study (Andreassen et al., 2012; Shafi et al., 2020; Nesi, Wolff, and Hunt, 2019; Tamir and Mitchell, 2012; Odgers, 2018). Notably, recent research on adolescent SMU fails to consider physiological markers of stress or biological measures (Tamir and Mitchell, 2012).
Symptoms of problematic SMU have been characterized as being similar to those of addictive disorders, and problematic SMU is considered a particularly relevant risk factor for suboptimal interpersonal and academic outcomes (Luby and Kertz, 2019; Andreassen et al., 2012; Shafi et al., 2020). Problematic SMU has also been associated with psychiatric symptom severity and high-risk behaviors among inpatient psychiatric populations (Shafi et al., 2020; Nesi, Wolff, and Hunt, 2019). Exploration of adolescent social media habits and identification of problematic SMU may be salient additions to the psychiatric interview as potential markers of impulsivity (Carson, Gansner, and Khang, 2018; Shafi, Romanowicz, and Croarkin, 2018; Shafi et al., 2020). Notably, SMU is most likely highly complex, with variation among individuals with and without psychiatric disease (Andreassen et al., 2012; Shafi et al., 2020; Nesi, Wolff, and Hunt, 2019; Tamir and Mitchell, 2012; Odgers, 2018).
In this study, we sought to assess the impact of acute SMU on clinical symptoms and physiological markers of stress in a sample of healthy and depressed adolescents. The glucocorticoid hormone cortisol is a reliable measure of an individual’s hypothalamic pituitary axis response to psychological stress, especially in cases of social-evaluative threats (Vanman, Baker, and Tobin, 2018; Morris, Mielock, and Rao, 2016; Morris et al., 2017). α-Amylase is a surrogate biomarker of autonomic (sympathetic) nervous system activation and may increase more rapidly in response to stress than cortisol (Morris, Mielock, and Rao, 2016; Morris et al., 2017). We hypothesized that a brief exposure to social media would negatively affect self-esteem, depressive symptom severity, and physiological markers of stress. Specifically, we hypothesized that salivary cortisol and α-Amylase would increase in all participants after social media use. We hypothesized that depressed adolescents would have a greater increase in cortisol and α-Amylase levels compared to healthy controls.
2. Material and Methods
The investigation was carried out in accordance with the latest version of the Declaration of Helsinki. The study design was reviewed and approved by the local institutional review board. Informed consent of the participants (provided by parents of adolescents) was obtained after the nature of the study procedures has been fully explained. All adolescent participants provided informed assent. The reporting of this study is in compliance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement (von Elm et al., 2008).
2.1. Participant Recruitment and Eligibility
Adolescent patients with depression were recruited from a child and adolescent inpatient psychiatric unit at a large medical center. Healthy control adolescents, matched for age, sex, and race with depressed adolescents, were recruited with flyers and advertisements. Recruitment and enrollment took place from 2018 through 2019. Participants were aged 13 to 17 years, in grades 7 to 12, and had the capacity to provide informed assent. Depressed participants met criteria for major depressive disorder (MDD) based on a Mini-International Neuropsychiatric Interview for Children and Adolescents [MINI-KID; a structured, diagnostic interview (Sheehan et al., 2010)] and the Quick Inventory of Depressive Symptoms–adolescent (17-item) clinician-rated (QIDS-A17-C); score of 11 or higher (Bernstein et al., 2010). Healthy control participants had no unstable medical conditions and no psychiatric diagnoses (based on a MINI-KID interview and a QIDS-A17-C score <6). Participants requiring corticosteroid-based medication (including corticosteroid inhalers for asthma) at the time of the study were not eligible for participation (Khoury et al., 2020).
2.2. Procedures and Measures
2.2.1. Saliva Sample Collection, Processing, and Analysis
Participants refrained from all Internet use, including SMU (ie, were completely “unplugged”), for at least 24 hours before the study visit. To avoid cortisol increases before the appointment, participants agreed to avoid consumption of caffeine and alcohol for 24 hours and to not exercise for 24 hours before the study visit. Participants did not eat or drink (except water) for 2 hours before the study visit. Participants abstained from nicotine or tobacco products for at least 1 hour before the study (Kirschbaum and Hellhammer, 1989a; Kirschbaum and Hellhammer, 1989b; Munsch, 2014). The research team maintained a consistent appointment start time (1:00-3:00 PM) for the 1.5-hour study visits to minimize the impact of diurnal fluctuations in cortisol (Kirschbaum and Hellhammer, 1989a; Kirschbaum and Hellhammer, 1989b; Munsch, 2014).
Saliva was collected with the SalivaBio passive drool saliva collection kit (Salimetrics). To ensure sample quality and to prevent degradation of the analytes of interest, samples were immediately centrifuged after collection, and supernatants were stored at −80°C until biological assays were performed. Samples were analyzed with the salivary cortisol enzyme-linked immunosorbent assay kit (Salimetrics), in accordance with manufacturer instructions. We quantified salivary cortisol levels before and after SMU. We used a salivary α-amylase kinetic enzyme assay kit (Salimetrics) to quantify α-amylase levels.
2.2.2. Clinical Measures
Basic demographic information, medical history, and psychiatric history were obtained. The MINI-KID was administered at baseline. For depressed participants, the MINI-KID was used to confirm the diagnosis of MDD and to identify co-occurring conditions; in control participants, it was used to confirm the absence of psychiatric diagnoses. The QIDS-A17-C was used to confirm baseline symptom severity in depressed participants and the absence of symptoms in healthy controls.
We used additional instruments to evaluate the participants’ SMU, exposure to bullying, self-esteem, and depressive symptoms. The Bergen Social Media Addiction Scale (BSMAS), a previously validated, self-report measure, was used to evaluate social media habits (Banyai et al., 2017; Andreassen et al., 2012). The BSMAS consists of 6 items that measure risk of social media addiction during the past year; total scores can range from 6 to 30, with higher scores representing greater risk of social media addiction (scores >12 indicate addictive behavior) (Banyai et al., 2017; Andreassen et al., 2012). Bullying exposure was assessed with the Illinois Bully Scale, a self-report, 18-point scale (Cronbach α score, 0.87) that assesses the presence and frequency of bullying (as the perpetrator) and of being bullied (as the victim) (Espelage and Holt, 2013). Cyberbullying was assessed with the Cyberbullying Scale, a contemporary, self-report measure that quantifies bullying in online mediums (Cronbach α score, 0.94 for American adolescents) (Stewart et al., 2014). The Rosenberg Self-Esteem Scale (Gray-Little, Williams, and Hancock, 1997), is a 10-item self-report questionnaire that uses a 4-point Likert scale to assess the global perceived sense of self-worth (positive and negative feelings about the self). Rosenberg Self-Esteem Scale scores can range from 0 to 40, with higher scores representing greater self-esteem; participants completed the scale at baseline and after 20 minutes of SMU (acute SMU). We used the Quick Inventory of Depressive Symptomatology–adolescent (17 item)–self-report (QIDS-A17-SR) to assess depressive symptom severity at baseline and after acute SMU. QIDS-A17-SR scores can range from 0 to 27, with higher scores indicating more severe depressive symptoms.
The MINI-KID interviews and all clinical rating scales described above were supervised and reviewed by 2 board-certified child and adolescent psychiatrists (M.R. and P.E.C.).
2.2.3. Social Media Exposure
Participants were asked to engage with social media on a personal device of their choosing. Participants and parents were assured that the online activity would neither be monitored nor serve as data points. The SMU occurred while the participant was in a private office with no access to means for self-harm. Participants completed SMU in an uninterrupted manner. Study staff remained outside the room. They monitored participants discretely through a small glass window and asked participants to cease SMU after 20 minutes.
2.2.4. Independent Variable, Covariates, and Outcome Variables
The primary independent variable was group membership (ie, depressed vs healthy control). Depression severity was assessed with the QIDS-A17-SR score.
Sex, age (years), pre-exposure BSMAS score, and pre-exposure cyberbullying score were selected a priori to be covariates in the models. These variables were anticipated to bolster precision when evaluating the acute effects of social media exposure on study outcomes.
Outcome variables were the self-report measures of depression symptom severity and self-esteem and the physiological measures of stress response (salivary cortisol and α-amylase).
2.3. Statistical Analysis
Demographic and clinical characteristics for the sample of 60 adolescents were described by using the sample mean (SD) for continuous variables and the frequency and percentage for categorical variables. To identify any differences between characteristics of the participants with MDD (n=30) and healthy controls (n=30), we used the 2-sample independent t test with the Satterthwaite method for unequal variances (continuous variables) and the Fisher exact test (categorical variables). A power analysis showed that a sample size of 30 participants per group would provide power greater than 0.9 for detecting changes in clinical assessments and greater than 0.8 for detecting changes in salivary cortisol.
The change over time in depression severity, self-esteem, salivary cortisol, and salivary α-amylase was compared between the MDD and healthy control groups by using a linear mixed-model analysis of repeated measures. A separate mixed model was conducted for each outcome measure. Each mixed model contained fixed-effects terms for group (MDD vs healthy control), time, and group × time interaction. Age, sex, pre-exposure BSMAS score, and pre-exposure cyberbullying score were included as covariates in each model. Restricted maximum likelihood estimation and type 3 tests of fixed effects were used, with the Kenward-Roger correction applied to the compound symmetry covariance structure (Kenward and Roger, 1997). Least squares means (LSMs; adjusted group means) were estimated as part of the mixed model to interpret the group effect (LSM difference between groups). Simple group effects at each period and within-group contrasts (change) from pre- to post-exposure were also assessed. The Cohen d (d) was calculated and interpreted as the effect size estimator.
Statistical analyses were conducted with SAS software, version 9.4 (SAS Institute Inc). The level of significance was set at α=0.05 (2-tailed), and we implemented the false-discovery rate procedure to control false-positives over the multiple tests (Benjamini and Hochberg, 1995).
3. Results
3.1. Participant Characteristics
Of the 60 youth (30 with MDD and 30 healthy controls), 63.33% were females, 80% were white (non-Hispanic), and the mean (SD) age was 15.05 (1.18) years (age range, 13-17 years). Mean (SD) age at first use of social media was 11.38 (1.53) years. The BSMAS and cyberbullying scores at baseline (mean [SD], 14.45 [5.86] and 5.68 [7.10], respectively) suggested social media addiction and minimal cyber victimization (Banyai et al., 2017; Andreassen et al., 2012; Stewart et al., 2014). Demographic and clinical characteristics of the 60 participants are shown in Table 1. Two participants with MDD were taking medications for Attention Deficit Hyperactivity Disorder (methylphenidate, clonidine). Sixteen of the participants with MDD were taking either a selective serotonin reuptake inhibitor or a serotonin-norepinephrine reuptake inhibitor. One participant with MDD was taking topiramate and one healthy control participant was taking levetiracetam. There were no differences in BMI or smoking status amongst healthy subjects and those with MDD. Self-reported descriptions of general social media application use, perceived changes in mood with social media use, and social media application use during the study are summarized in Table 2.
Table 1.
Demographic and clinical characteristics of the overall sample and by group
| Characteristic | Overall Sample (N=60) |
MDD (n=30) |
Healthy Control (n=30) |
p-value (FDR) |
|---|---|---|---|---|
| Patient Demographics | ||||
| Age, years, M (SD) | 15.05 (1.18) | 15.26 (1.22) | 14.83 (1.11) | 0.15 (0.23) |
| Grade in School, Median (IQR) | 10.00 (02) | 10.00 (02) | 9.00 (02) | 0.14 (0.23) |
| Female Sex, % (n) | 63.33% (38) | 63.33% (19) | 63.33% (19) | 1.00 (1.00) |
| White, Non-Hispanic, % (n) | 80.00% (48) | 80.00% (24) | 80.00% (24) | 1.00 (1.00) |
| Patient Factors | ||||
| BMI, kg/m2, M (SD) | 24.55 (6.48) | 25.65 (6.85) | 23.34 (5.93) | 0.18 (0.25) |
| Age at First use of Social Media, M (SD) | 11.38 (1.53) | 11.03 (1.37) | 11.73 (1.61) | 0.07 (0.14) |
| Baseline QIDS-C Total, M (SD) | 10.08 (7.42) | 16.86 (3.79) | 3.30 (1.58) | <0.0001 (0.0004) |
| Baseline Social Media Addiction Score, M (SD) | 14.45 (5.86) | 17.56 (5.47) | 11.33 (4.46) | <0.0001 (0.0004) |
| Baseline Cyberbullying Score, M (SD) | 5.60 (7.10) | 8.43 (8.50) | 2.76 (3.72) | 0.001 (0.002) |
| Baseline Bullying Perpetrator Score, M (SD) | 1.78 (2.40) | 2.00 (1.85) | 1.56 (2.86) | 0.49 (0.57) |
| Baseline Bullying Victim Score, M (SD) | 2.38 (3.63) | 3.80 (4.41) | 0.96 (1.79) | 0.002 (0.004) |
| Psychotropic Medication, % (n) | 30.00% (18) | 56.67% (17) | 33.33% (01) | <0.0001 (0.0004) |
| History of Depression, % (n) | 45.00% (27) | 90.00% (27) | 0.00% (00) | <0.0001 (0.0004) |
| Current Smoker, % (n) | 3.33% (02) | 6.67% (02) | 0.00% (00) | 0.49 (0.57) |
Note. M=Sample Mean; SD=Standard Deviation. Two-independent sample t-test with the Satterthwaite method for unequal variances (continuous variables), Median one-way ANOVA for median values, and Fisher’s exact test (categorical variables) were used to identify any differences between characteristics of the two groups. P-value (two-tailed) associated with the test of group differences (MDD vs. Healthy Control) on each characteristic. QIDS-C Total = Clinician-rated. FDR = False Discovery Rate. IQR = Interquartile Range.
Table 2.
Summary of self-reported social media use
| Self-Reported Descriptions of Typical Social Media Use | MDD (n=30) | Healthy Controls (n=30) |
|---|---|---|
| Commonly use Facebook | 23% (n=7) | 17% (n=5) |
| Commonly use Snapchat | 80% (n=24) | 60% (n=18) |
| Commonly use Instagram | 86% (n=26) | 53% (n=16) |
| Commonly Use Twitter | 13% (n=4) | 17% (n=5) |
| Commonly use 1 application | 23% (n=7) | 37% (n=11) |
| Commonly use 3 or more applications | 33% (n=10) | 20% (n=6) |
| Negative mood change after social media use | 27% (n=8) | 10% (n=3) |
| Self-Reported Descriptions of Application Used During 20 Minute Research Study Period | ||
| 23% (n=7) | 23% (n=7) | |
| SnapChat | 83% (n=25) | 73% (n=22) |
| 83% (n=25) | 63% (n=19) | |
| 10% (n=3) | 10% (n=3) |
3.2. Depression Severity and Self-Esteem
For depression severity, the mixed-model repeated-measures analysis showed a significant main effect of the group (F=46.73, degrees of freedom [df]=1,54; raw P<.0001; adjusted P=.0002), a significant time effect (F=12.03, df=1,58; raw P=.001; adjusted P=.004), but no significant group × time interaction effect (F=2.33, df=1,58; raw P=.1325; adjusted P=.1767). The least squares group means for adjusted QIDS-A17-SR scores were significantly different between the 2 groups at pre-exposure, with MDD=13.81 (SE, 0.88) and healthy control=4.41 (SE, 0.71) (P<.0001; adjusted P=.0002; d=1.889), and at post-exposure, with MDD=12.61 (SE, 1.06) and healthy control=3.95 (SE, 0.68) (P<.0001; adjusted P=.0002; d=1.599) (Table 3, Supplemental Figure 1). Also, the pattern of the adjusted LSMs showed a significant albeit small improvement (decrease) in severity of depression symptoms (adjusted QIDS-A17-SR scores) from pre-exposure to post-exposure for the MDD group (13.81 [SE, 0.88] at pre-exposure vs 12.61 [SE, 1.06] at post-exposure; LSM decrease=−1.20 [SE, 0.43]; mean change, 8.69% decrease; raw P=.0081; adjusted P=.0216; d=0.194) and for the healthy control group (4.41 [SE, 0.71] at pre-exposure vs 3.95 [SE, 0.68] at post-exposure; LSM decrease=−0.46 [SE, 0.19]; mean change, 10.43% decrease; raw P=.0228; adjusted P=.0456; d=0.256).
Table 3.
The effect of MDD vs. Normal Control on depression severity, self-esteem, and stress response
| Pre-Exposure | Post-Exposure | Change from Pre to Post | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome and Group | LSM (SE ) | 95% CI | F Statistic | p-value | LSM (SE ) | 95% CI | F Statistic | p-value | LSM (SE ) | 95% CI | F Statistic | p-value |
| QIDS-A17-SRa | ||||||||||||
| MDD | 13.81 (0.88) | 12.04 to 15.59 | 12.61 (1.06) | 10.49 to 14.73 | −1.20 (0.43) | −2.07 to −0.32 | F(1,58)=7.53 | 0.0081 | ||||
| Normal Control | 4.41 (0.71) | 2.98 to 5.85 | 3.95 (0.68) | 2.57 to 5.32 | −0.46 (0.19) | −0.86 to −0.06 | F(1,58)=5.47 | 0.0228 | ||||
| LSM Group Difference | 9.39 (1.28) | 6.82 to 11.96 | F(1,58)=53.53 | 0.0001 | 8.66 (1.39) | 5.86 to 11.46 | F(1,58)=38.38 | 0.0001 | ||||
| Self-Esteemb | ||||||||||||
| MDD | 22.73 (1.07) | 20.58 to 24.88 | 23.80 (1.14) | 21.52 to 26.08 | 1.06 (0.58) | −0.10 to 2.24 | F(1,58)=3.30 | 0.0744 | ||||
| Normal Control | 33.69 (0.89) | 31.91 to 35.47 | 33.89 (0.79) | 32.31 to 35.48 | 0.20 (0.44) | −0.68 to 1.08 | F(1,58)=0.21 | 0.6511 | ||||
| LSM Group Difference | −10.96 (1.55) | −14.06 to −7.85 | F(1,58)=49.95 | 0.0001 | −10.09 (1.53) | −13.16 to −7.02 | F(1,58)=43.40 | 0.0001 | ||||
| Cortisolc | ||||||||||||
| MDD | 0.16 (0.01) | 0.13 to 0.19 | 0.22 (0.02) | 0.17 to 0.27 | 0.06 (0.02) | 0.02 to 0.10 | F(1,56)=8.49 | 0.0051 | ||||
| Normal Control | 0.18 (0.02) | 0.13 to 0.22 | 0.17 (0.02) | 0.12 to 0.21 | −0.01 (0.30) | −0.03 to 0.01 | F(1,56)=0.46 | 0.5013 | ||||
| LSM Group Difference | −0.02 (0.02) | −0.07 to 0.04 | F(1,56)=0.32 | 0.5739 | 0.05 (0.03) | −0.01 to 0.12 | F(1,56)=2.31 | 0.1343 | ||||
| Amylased | ||||||||||||
| MDD | 154.02 (24.07) | 105.80 to 202.24 | 192.60 (27.79) | 136.93 to 248.27 | 38.58 (13.92) | 10.68 to 66.47 | F(1,56)=7.68 | 0.0076 | ||||
| Normal Control | 203.49 (23.77) | 155.86 to 251.11 | 211.32 (22.41) | 166.41 to 256.23 | 7.83 (8.85) | −9.89 to 25.56 | F(1,56)=0.78 | 0.3798 | ||||
| LSM Group Difference | −49.46 (35.75) | −121.08 to 22.14 | F(1,56)=1.91 | 0.1719 | −18.72 (33.24) | −85.31 to 47.87 | F(1,56)=0.32 | 0.5756 | ||||
Note. LSM = Least Squares Mean Estimate adjusted for age, sex, pre-exposure social media addiction score, and pre-exposure cyberbullying score; SE = Standard Error; LSM Group Difference = Difference of LSM estimates (MDD vs. normal control); 95% CI = 95% Confidence Interval for the group LSM estimate; p-value = associated with the test (F-statistic) of the LSM group difference at pre-exposure and at post-exposure as well as the change from pre to post within each group; p-values were adjusted using the FDR and are reported in the results section.
Higher score equals greater depression severity;
Higher score equals greater self-esteem;
Higher cortisol levels (μg/dL) equals greater stress response;
Higher amylase levels (U/mL) equals greater stress response.
For self-esteem, the mixed-model repeated-measures analysis showed a significant main effect of group (F=49.43, df=1,54; raw P<.0001; adjusted P=.0002) but no significant time effect (F=2.98, df=1,58; raw P=.0896; adjusted P=.0896) or group × time interaction effect (F=1.40, df=1,58; raw P=.2423; adjusted P=.2423). The LS group means (adjusted self-esteem scores) were significantly different between the 2 groups at pre-exposure (MDD=22.73 [SE, 1.07] vs healthy control=33.69 [SE, 0.89]; P<.0001; adjusted P=.0002; d=1.825) (Table 3, Supplemental Figure 2) and at post-exposure (MDD=23.80 [SE, 1.14] vs healthy control=33.89 [SE, 0.79]; P<.0001; adjusted P=.0002; d=1.701).
However, the pattern of adjusted LSMs showed no significant improvement (increase) in self-esteem (adjusted self-esteem scores) from pre-exposure to post-exposure for the MDD group (22.73 [SE, 1.07] at pre-exposure vs 23.80 [SE, 1.14] at post-exposure; LSM increase=1.07 [SE, 0.58]; mean change, 4.70% increase; raw P=.0744; adjusted P=.1190; d=0.215) or the healthy control group (33.69 [SE, 0.89] at pre-exposure vs 33.89 [SE, 0.79] at post-exposure; LSM increase=0.20 [SE, 0.44]; mean change, 0.59% increase; raw P=.6511; adjusted P=.6511; d=0.045).
3.3. Cortisol and α-Amylase
For cortisol and α-amylase, the mixed model showed a significant group × time interaction effect (F=8.23 for cortisol, F=3.47 for α-amylase, df=1,56; raw cortisol P=.0058 [adjusted P=.0232], raw amylase P=.0677 [adjusted P=.1354]) and a significant time effect (F=4.87 for cortisol, F=7.92 for α-amylase, df=1,56; raw ps<0.0315, adjusted ps=0.0420) but no significant main effect for group (F=0.40 for cortisol, F=1.03 for α-amylase, df=1,53; raw ps<0.5281; adjusted ps<0.5281). The least squares simple group means (adjusted cortisol/α-amylase levels) were not significantly different between the 2 groups at pre-exposure for cortisol (MDD=0.16 [SE, 0.01] vs healthy control=0.18 [SE, 0.02]; P=.5739; adjusted P=.5739; d=0.146) (Table 3), at pre-exposure for amylase (MDD=154.02 [SE, 24.07] vs healthy control=203.49 [SE, 23.77]; P=.1719; adjusted P=.2292; d=0.356) (Table 3,), at post-exposure for cortisol (MDD=0.22 [SE, 0.02] vs healthy control=0.17 [SE, 0.02]; P=.1343; adjusted P=.1791; d=0.392) or at post-exposure for amylase (MDD=192.60 [SE, 27.79] vs healthy control=211.32 [SE, 22.41]; P=.5756; adjusted P=.5756; d=0.146)
However, the pattern of the adjusted LSM (Figure 1A) showed a significant increase in stress response (adjusted cortisol levels) from pre-exposure to post-exposure for the MDD group (0.16 [SE, 0.01] at pre-exposure vs 0.22 [SE, 0.02] at post-exposure; LSM increase=0.06 [SE, 0.02]; mean change, 37.50% increase; raw P=.0051; adjusted P=.0216; d=0.545) but not for the healthy control group (0.18 [SE, 0.02] at pre-exposure vs 0.17 [SE, 0.02] at post-exposure; LSM decrease=−0.01 [SE, 0.01]; mean change, 5.55% decrease; raw P=.5013; adjusted P=.5729; d=0.066). For α-amylase (Figure 1B), we noted a significant increase in stress response (adjusted amylase levels) from pre-exposure to post-exposure for the MDD group (154.02 [SE, 24.07] at pre-exposure vs 192.60 [SE, 27.79] at post-exposure; LSM increase=38.58 [SE, 13.92]; mean change, 25.05% increase; raw P=.0076; adjusted P=.0216; d=0.257) but not for the healthy control group (203.49 [SE, 23.77] at pre-exposure vs 211.32 [SE, 22.41] at post-exposure; LSM increase=7.83 [SE, 8.85]; mean change, 3.85% increase; raw P=.3798; adjusted P=.5064; d=0.077). Medication status, BMI, and smoking status did not impact the basic findings related to cortisol an α-amylase.
Figure 1.
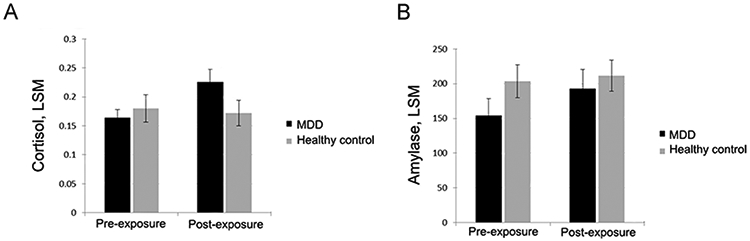
A. Adjusted LSM for Cortisol. In this linear mixed model, LSMs were adjusted for age, sex, pre-exposure social media addiction score, and pre-exposure cyberbullying score. Error bars indicate standard error. No significant group differences were observed at pre-exposure (adjusted P=.5739) or post-exposure (adjusted P=.1791). A significant increase in cortisol levels occurred from pre-exposure to post-exposure for the MDD group (LSM increase, 0.06 [SE, 0.02]; adjusted P=.0216) but not for the healthy control group (LSM decrease, −0.01 [SE, 0.01]; adjusted P=.5729). Greater cortisol levels represent a greater stress response. LSM indicates least squares mean; MDD, major depressive disorder. Figure 1B. Adjusted LSM for Amylase. In this linear mixed model, LSMs were adjusted for age, sex, pre-exposure social media addiction score, and pre-exposure cyberbullying score. Error bars indicate standard error. No significant group differences were observed at pre-exposure (adjusted P=.2292) or post-exposure (adjusted P=.5756). A significant increase in amylase levels occurred from pre-exposure to post-exposure for the MDD group (LSM increase, 38.58 [SE, 13.92]; adjusted P=.0216) but not for the healthy control group (LSM increase, 7.83 [SE, 8.85]; adjusted P=.5064). Greater amylase levels (U/mL) represent greater stress response. LSM indicates least squares mean; MDD, major depressive disorder.
4. Discussion
This is the first study to use physiological biomarkers and clinical measures to assess the potential impact of a brief (20-minute) period of SMU after at least 24 hours of abstinence. Depressed participants had an elevated physiological stress response after acute SMU, as indicated by significant increases in salivary α-amylase and cortisol (Figure 2). This finding is consistent with previous work showing an increase in cortisol reactivity among depressed adolescents after being subjected to psychosocial stressors (Hellman et al., 2015). Thus, for adolescents with MDD, SMU could be characterized as a pervasive psychosocial stressor, especially for those with problematic use. The elevated α-amylase and cortisol levels among these adolescents with MDD suggest that SMU may contribute to biological vulnerabilities for further morbidity and co-occurring diagnoses such as substance use (Rao, Hammen, and Poland, 2009; Rao, Hammen, and Poland, 2010).
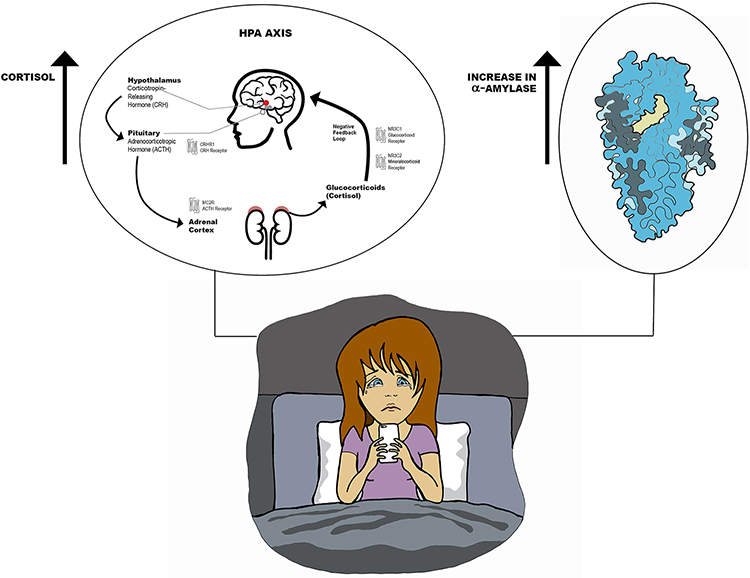
Figure 2.
Effect of Social Media Use on Salivary Cortisol and α-Amylase Levels (©2020 Anosha Zanjani; used with permission). A brief period of social media use increased salivary markers of stress in depressed adolescents. Salivary cortisol is an index of hypothalamo-pituitary-adrenal (HPA) activation and noradrenergic activity. Salivary α-amylase is an indirect measure of sympathetic-adreno-medullary activity and central norepinephrine activity.
Depressed participants had a significantly higher score on the BSMAS compared with healthy control participants, indicating more problematic SMU. A 2-year longitudinal study showed that problematic SMU is associated with sleep disruptions and increased depressive symptoms (Raudsepp, 2019). Previous studies have also indicated an increasing concern about the impact of problematic SMU on depressed adolescents because it has been associated with more severe psychiatric symptoms, self-harm, and suicidality, and it could potentially be a marker of impulsivity (Luby and Kertz, 2019; Shafi et al., 2020; Nesi, Wolff, and Hunt, 2019; Odgers, 2018).
Of note, we observed no significant difference in the age of first SMU. Both groups reported starting at the preadolescent age of 11 years, which is younger than the stipulated minimum age requirement (13 years) by the main social media applications (SnapChat, Instagram, Facebook). This places adolescents at risk of having their data collected without parental consent. In addition, it subjects them to online environments at a young age, when they are more neurobiologically susceptible to the effects of peer rejection and when engagement can influence their cognitive development (Crone and Konijn, 2018; Firth et al., 2019).
SMU involves complex and poorly understood brain network activity. Gindrat and colleagues (Gindrat et al., 2015) reported a motor level interaction with the interface of smartphones and altered cortical regions associated with sensory and motor networks of the thumb. Firth and colleagues (Firth et al., 2019) highlighted how online activities can affect attentional ability by promoting multitasking over sustained attention and focus. SMU also likely affects reward processing in adolescents. Recent, related work underscores the ontogenetic complexity of reward processing in adolescents with psychiatric symptoms and the likely bidirectional relationships with SMU (Bradley et al., 2017).
The baseline BSMAS score was higher for the MDD group and indicated social media addiction, whereas the control group score was under the threshold addiction score of 12 (Banyai et al., 2017; Andreassen et al., 2012). SMU appears to be different for depressed and healthy adolescent participants, with depressed adolescents more commonly having problematic SMU, having significantly higher BSMAS scores, and being more physiologically reactive to SMU compared with controls. Prior work suggested that heightened cortisol reactivity, as seen in depression, can increase an individual’s risk of a substance use disorder, and stressful experiences can heighten this vulnerability (Rao, Hammen, and Poland, 2009; Rao, Hammen, and Poland, 2010).
There is a pressing and broad need to update clinical practice parameters about the increasing importance and clinical relevance of adolescent SMU, which should be regarded as an emerging public health issue (Rideout and Robb ,2018; Carson, Gansner, and Khang ,2018; Shafi, Romanowicz, and Croarkin ,2018). However, older psychiatrists may underestimate or discount the potential pernicious effects of SMU in adolescents. At least 44% of practicing psychiatrists are older than 55 years, making them less likely to have personal SMU experience that is comparable to their younger patients (Ilango et al., 2020).
This study was the first to assess the acute impact of SMU on physiological stress responses among psychiatrically hospitalized adolescents. Because of the novel nature of this work, we note several study limitations. The sample size was small and limited with respect to demographic characteristics (eg, adolescents with medical illnesses). Additionally, the study session had a short duration and the impact of SMU could be more reliably ascertained with longitudinal studies and digital monitoring applications (Carson, Gansner, and Khang, 2018). It also cannot be ruled out that the difference between depressed and healthy control adolescents in terms of stress reactivity may have been related to exposure to a research setting as opposed to SMU. The research setting may have been more stressful for depressed adolescents as compared to healthy control adolescents. Prior work also suggests that depressed adolescents have increased neurobiological reactivity in general (Dhami et al., 2020).
The artificial study setting and the participants’ awareness about being observed during SMU may have changed their behavior. However, although studies in this area are very limited, personal media devices have become ubiquitous in daily life and are often used in public places (eg, buses, hospitals, restaurants) where being observed is likely (Nesi, Wolff, and Hunt ,2019). Efforts at maintaining a sense of privacy and simulating a more natural setting were made by providing a private area, having participants use a personal device, discretely observing participants, and not collecting or directly monitoring the adolescent’s SMU. Thus, we relied on the adolescents’ account of their use.
For control participants, we ascertained whether the adolescent could agree to abstain from SMU for 24 hours, and we relied on parents to assist with restricting access (eg, securing the phone). Participants and parents were asked about their time of last SMU to confirm adherence, but the study ultimately relied on participant self-reports. Conversely, enforcing social media abstinence among hospitalized adolescents was possible because the inpatient unit policy does not permit personal electronic devices or SMU (Nesi, Wolff, and Hung ,2019; Stanton, Drake af Hagelsrum, and Stasiak, 2015).
We worked with the inpatient psychiatrist and providers to identify potentially eligible patients while also bearing in mind their acute level of risk of harm to themselves or others. For example, 1 patient was ineligible for safety reasons related to interactions with others on social media.
This study is the first of its kind among psychiatrically hospitalized, depressed adolescent patients. Consequently, we did not identify any publications describing the risk of deterioration associated with study participation. In the current study, one patient had an exacerbation of depressive symptoms after participation. However, the inpatient psychiatrist noted that the patient’s participation and subsequent reaction assisted with formulating management and treatment needs.
Saliva collection occurred before and after SMU. Studies evaluating cortisol response to social stressor scenarios have shown rapid increases in short periods (Morris, Mielock, and Raos 2016; Morris et al., 2017; Kirschbaum and Hellhammer, 1989b; Kirschbaum and Hellhammer,1994). Further sampling during the 20-minute SMU period may have helped determine at what points the salivary cortisol and α-amylase increase. Conversely, prior work suggests that cortisol peaks can be found 20 minutes after socially-evaluative stressors (Poppelaars et al., 2019). Further cortisol sampling 20 minutes after the SMU use might have demonstrated a greater effect with respect to cortisol. Future studies should include more delayed monitoring of salivary cortisol.
In conclusion, the present findings suggest that depressed adolescents were more physiologically reactive to acute SMU than controls. Depressed adolescents may be more physiologically reactive in general, which may cause them to be more susceptible to elevated stress biomarkers (Morris et al., 2017; Dhami et al., 2020). Future studies should include additional comparison groups, such as a sample of depressed adolescents reading print material or speaking with a friend or family member. Despite the limitations of the current study, the findings present a framework for future studies and provide evidence that even brief SMU increases the stress response in adolescents with depression. Future research findings will help inform related translational neuroscience efforts, treatment teams, parents, and adolescents with respect to healthy SMU and inpatient treatment planning for this integral aspect of adolescent life (Meshi, Tamir, and Heekeren, 2015).
Supplementary Material
Highlights.
Social media use is an inherent element of life and neurodevelopment in adolescents
The effects of social media use are not well characterized among adolescents
This study examined clinical ratings and stress biomarkers after social media use
After social media use biomarkers of stress increased in depressed adolescents
Changes in self-reported depressive symptoms before and after social media use were not considered clinically significant
Acknowledgements:
The authors thank Ms Anosha Zanjani for preparation of Figure 2.
Funding: This publication was made possible by the Mayo Clinic Clinical Translational Science Award (CTSA) through grant number UL1TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Research reported in this publication was also supported by the NIH under award R01 MH113700 and MH124655. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- BSMAS
Bergen Social Media Addiction Scale
- d
Cohen d
- df
degrees of freedom
- LSM
least squares mean
- MDD
major depressive disorder
- MINI-KID
Mini-International Neuropsychiatric Interview for Children and Adolescents
- QIDS-A17-C
Quick Inventory of Depressive Symptoms–adolescent (17-item)–clinician-rated
- QIDS-A17-SR
Quick Inventory of Depressive Symptoms–adolescent (17 item)–self-report
- SMU
Social Media Use
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the annual meeting of the American College of Neuropsychopharmacology, Orlando, Florida, December 10, 2019.
Declaration of interest: Dr Croarkin has received research grant support from Pfizer, Inc; equipment support from Neuronetics, Inc; and supplies and genotyping services from Assurex Health, Inc for investigator-initiated studies. He is the primary investigator for a multicenter study funded by Neuronetics, Inc and a site primary investigator for a study funded by NeoSync, Inc. Dr Croarkin is a consultant for Procter & Gamble Company and Myriad Neuroscience. The other authors report no financial relationships with commercial interests.
Contributor Information
Reem M. A. Shafi, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota.
Paul A. Nakonezny, Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, Texas; Department of Population and Data Sciences, University of Texas Southwestern Medical Center, Dallas, Texas..
Keith A. Miller, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota.
Jinal Desai, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota.
Ammar G. Almorsy, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota.
Anna N. Ligezka, Department of Clinical Genomics, Mayo Clinic, Rochester, Minnesota.
Brooke A. Morath, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota
Magdalena Romanowicz, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota.
Paul E. Croarkin, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota.
References
- Anderson M and Jiang J 2018. Teens, Social Media and Technology 2018 Pew Research Center. https://www.pewresearch.org/internet/2018/05/31/teens-social-media-technology [Google Scholar]
- Andreassen CS, Torsheim T, Brunborg GS, Pallesen S 2012. Development of a Facebook Addiction Scale. Psychol Rep, 110: 501–17. [DOI] [PubMed] [Google Scholar]
- Banyai F, Zsila A, Kiraly O, Maraz A, Elekes Z, Griffiths MD, Andreassen CS, Demetrovics Z 2017. Problematic Social Media Use: Results from a Large-Scale Nationally Representative Adolescent Sample. PloS One, 12: e0169839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y and Hochberg Y 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological), 57: 289–300. [Google Scholar]
- Bernstein IH, Rush AJ, Trivedi MH, Hughes CW, Macleod L, Witte BP, Jain S, Mayes TL Emslie GJ 2010. Psychometric properties of the Quick Inventory of Depressive Symptomatology in adolescents. Int J Methods Psychiatr Res, 19: 185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KAL, Case JAC, Freed RD, Stern ER, Gabbay V 2017. Neural correlates of RDoC reward constructs in adolescents with diverse psychiatric symptoms: A Reward Flanker Task pilot study. J Affect Disord, 216: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson NJ, Gansner M, Khang J 2018. Assessment of Digital Media Use in the Adolescent Psychiatric Evaluation. Child Adolesc Psychiatr Clin N Am, 27: 133–43. [DOI] [PubMed] [Google Scholar]
- Crone EA, and Konijn EA 2018. Media use and brain development during adolescence. Nat Commun, 9: 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhami P, Atluri S, Lee JC, Knyahnytska Y, Croarkin PE, Blumberger DM, Daskalakis ZJ, Farzan F 2020. Prefrontal Cortical Reactivity and Connectivity Markers Distinguish Youth Depression from Healthy Youth. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelage DL, and Holt MK, 2013. Suicidal ideation and school bullying experiences after controlling for depression and delinquency. J Adolesc Health, 53: S27–31. [DOI] [PubMed] [Google Scholar]
- Firth J, Torous J, Stubbs B, Firth JA, Steiner GZ, Smith L Alvarez-Jimenez M, Gleeson J, Vancampfort D, Armitage CJ, Sarris J 2019. The “online brain”: how the Internet may be changing our cognition. World Psychiatry, 18: 119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindrat AD, Chytiris M, Balerna M, Rouiller EM, Ghosh A 2015. Use-dependent cortical processing from fingertips in touchscreen phone users. Curr Biol, 25: 109–16. [DOI] [PubMed] [Google Scholar]
- Gray-Little B, Williams VSL, Hancock TD 1997. An Item Response Theory Analysis of the Rosenberg Self-Esteem Scale. Personality and Social Psychology Bulletin, 23: 443–51. [Google Scholar]
- Hellman N, Morris MC, Rao U, Garber J 2015. Depression history as a moderator of relations between cortisol and shame responses to social-evaluative threat in young adults. Biol Psychol, 109: 159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango S, Schmidt A, McManus M, Kim WJ 2020. Call for Improvements to Federal Child and Adolescent Psychiatry Workforce Projection Methods. J Am Acad Child Adolesc Psychiatry, 59: 501–03. [DOI] [PubMed] [Google Scholar]
- Katz DA, Peckins MK, Lyon CC 2019. Adolescent stress reactivity: Examining physiological, psychological and peer relationship measures with a group stress protocol in a school setting. J Adolesc, 74: 45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenward MG, Roger JH 1997. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics, 53: 983–97. [PubMed] [Google Scholar]
- Khoury JE, Jamieson B, Gonzalez A, Atkinson L 2020. Child depressive symptoms: Associations with salivary cortisol and alpha amylase in two distinct challenges. Biol Psychol, 149: 107808. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH, 1989a. Response variability of salivary cortisol under psychological stimulation. J Clin Chem Clin Biochem, 27: 237. [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH 1989b. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology, 22: 150–69. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. 1994. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology, 19: 313–33. [DOI] [PubMed] [Google Scholar]
- Luby J, Kertz S 2019. Increasing Suicide Rates in Early Adolescent Girls in the United States and the Equalization of Sex Disparity in Suicide: The Need to Investigate the Role of Social Media. JAMA Netw Open, 2: e193916. [DOI] [PubMed] [Google Scholar]
- Meshi D, Tamir DI, Heekeren HR 2015. The Emerging Neuroscience of Social Media., Trends Cogn Sci, 19: 771–82. [DOI] [PubMed] [Google Scholar]
- Morris MC, Kouros CD, Mielock AS, Rao U 2017. Depressive symptom composites associated with cortisol stress reactivity in adolescents. J Affect Disord, 210: 181–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Mielock AS, Rao U 2016. Salivary stress biomarkers of recent nicotine use and dependence. Am J Drug Alcohol Abuse, 42: 640–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsch S 2014. Study protocol: psychological and physiological consequences of exposure to mass media in young women - an experimental cross-sectional and longitudinal study and the role of moderators. BMC Psychol, 2: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesi J, Wolff JC, Hunt J 2019. Patterns of Social Media Use Among Adolescents Who Are Psychiatrically Hospitalized. J Am Acad Child Adolesc Psychiatry, 58: 635–39 e1. [DOI] [PubMed] [Google Scholar]
- Odgers C 2018. Smartphones are bad for some teens, not all. Nature, 554: 432–34. [DOI] [PubMed] [Google Scholar]
- Poppelaars ES, Klackl J, Pletzer B, Wilhelm FH, Jonas E 2019. Social-evaluative threat: Stress response stages and influences of biological sex and neurotocism. Psychoneuroendocrinology, 109:104378. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen CL, Poland RE 2009. Mechanisms underlying the comorbidity between depressive and addictive disorders in adolescents: interactions between stress and HPA activity. Am J Psychiatry, 166: 361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Hammen CL, Poland RE 2010. Longitudinal course of adolescent depression: neuroendocrine and psychosocial predictors, J Am Acad Child Adolesc Psychiatry, 49: 141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudsepp L 2019. Brief report: Problematic social media use and sleep disturbances are longitudinally associated with depressive symptoms in adolescents. J Adolesc, 76: 197–201. [DOI] [PubMed] [Google Scholar]
- Rideout V, Robb MB 2018. Social media, social life: Teens reveal their experiences . In. San Francisco, CA: Common Sense Media. [Google Scholar]
- Shafi RMA, Nakonezny PA, Romanowicz M, Nandakumar AL, Suarez L, Croarkin PE 2020. Suicidality and self-injurious behavior among adolescent social media users at psychiatric hospitalization. CNS Spectr: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi RMA, Romanowicz M, Croarkin PE 2018. #SwitchedOn: a call for assessing social media use of adolescents. Lancet Psychiatry, 5: e27. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, Milo KM, Stock SL, Wilkinson B 2010. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J Clin Psychiatry, 71: 313–26. [DOI] [PubMed] [Google Scholar]
- Stanton J, Drake af Hagelsrum E, Stasiak K 2015. Access to the internet in an acute child and adolescent mental health inpatient unit. Aust N Z J Psychiatry, 49: 487–8. [DOI] [PubMed] [Google Scholar]
- Stewart RW, Drescher CF, Maack DJ, Ebesutani C, Young J 2014. The Development and Psychometric Investigation of the Cyberbullying Scale. J Interpers Violence, 29: 2218–38. [DOI] [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP 2012. Disclosing information about the self is intrinsically rewarding. Proc Natl Acad Sci U S A, 109: 8038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanman EJ, Baker R, Tobin SJ 2018. The burden of online friends: the effects of giving up Facebook on stress and well-being. J Soc Psychol, 158: 496–507. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP and Strobe Initiative. 2008. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Journal of Clinical Epidemiology, 61: 344–9. [DOI] [PubMed] [Google Scholar]
- Woods HC, Scott H 2016. #Sleepyteens: Social media use in adolescence is associated with poor sleep quality, anxiety, depression and low self-esteem. J Adolesc, 51: 41–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.