Abstract
CRF is the main activator of the hypothalamic-pituitary-adrenal (HPA) axis in response to stress. CRF neurons are found mainly in the hypothalamus, but CRF positive cells and CRF1 receptors are also found in extrahypothalamic structures, including amygdala (CeA), hippocampus, NAc and VTA. CRF release in the hypothalamus is regulated by inhibitory GABAergic interneurons and extrahypothalamic glutamatergic inputs, and disruption of this balance is found in stress-related disorders and addiction. (3α,5α)3-hydroxypregnan-20-one (3α,5α-THP), the most potent positive modulator of GABAA receptors, attenuates the stress response reducing hypothalamic CRF mRNA expression and ACTH and corticosterone serum levels. In this study, we explored 3α,5α-THP regulation of hypothalamic and extrahypothalamic CRF mRNA and peptide expression, in male and female Sprague Dawley rats, following vehicle or 3α,5α-THP administration (15 mg/kg). In the hypothalamus, we found sex differences in CRF mRNA expression (females +74%, p < 0.01) and CRF peptide levels (females − 71%, p < 0.001). 3α,5α-THP administration reduced hypothalamic CRF mRNA expression only in males (− 50%, p < 0.05) and did not alter CRF peptide expression in either sex. In hippocampus and CeA, 3α,5α-THP administration reduced CRF peptide concentrations only in the male (hippocampus − 29%, p < 0.05; CeA − 62%, p < 0.01). In contrast, 3α,5α-THP injection increased CRF peptide concentration in the VTA of both males (+32%, p < 0.01) and females (+26%, p < 0.01). The results show sex and region-specific regulation of CRF signals and the response to 3α,5α-THP administration. This data may be key to successful development of therapeutic approaches for stress-related disorders and addiction.
Keywords: 3α, 5α-THP; CRF; HPA axis; Extrahypothalamic CRF; Sex differences
1. Introduction
Corticotropin releasing factor (CRF) is a 41 amino acid polypeptide, well known as the main activator of the hypothalamic-pituitary-adrenal (HPA) axis in response to stress stimuli. CRF exerts its effects through two G protein-coupled receptors, CRF1 (brain) and CRF2 (peripheral) (Dautzenberg and Hauger, 2002). The highest concentrations of CRF-containing neurons and CRF1 are in the paraventricular nucleus (PVN) of the hypothalamus (HYP) (Rivier and Vale, 1983), but positive CRF cells have been found in extrahypothalamic regions, mainly in the central nucleus of amygdala (CeA), bed nucleus of the stria terminalis (BNST) (Peng et al., 2017), hippocampus (HP), nucleus accumbens (NAc), cortex and olfactory bulbs (Deussing and Chen, 2018). CRF1 is distributed widely in the brain, and highly concentrated in PVN, pituitary, hippocampus, ventral tegmental area (VTA), CeA, NAc, cortex and cerebellum (Deussing and Chen, 2018). Activation of pituitary CRF1 by hypothalamic CRF neurons results in activation of the HPA axis and triggers the neuroendocrine responses to stress (Koob and Heinrichs, 1999). By contrast, activation of extrahypothalamic CRF1 receptors, mainly in the amygdala and BNST, results in behavioral stress and anxiety-like responses. In the PVN, CRF release is regulated by GABAergic inputs that inhibit release, and glutamatergic inputs that increase release (Tasker et al., 1998). Moreover, the PVN receives inhibitory afferents from hippocampus and prefrontal cortex (indirectly), excitatory signals from amygdala, and both inhibitory and excitatory inputs from BNST (indirectly) (Ulrich-Lai and Herman, 2009; Ziegler and Herman, 2000). In basal conditions, the CRF neurons are under the inhibitory influence of GABAergic interneurons located in the PVN (Cullinan et al., 2008), while during stress norepinephrine and glutamate stimulate CRF release (Herman et al., 2002). Disruption in this inhibitory/excitatory balance has been associated with stress-related psychiatric disorders, like depression and anxiety (de Kloet et al., 2005; Reul and Holsboer, 2002). Moreover, HPA axis hyperactivity has been found in addiction disorders (Zorrilla et al., 2014). In fact, it has been observed that exacerbation of CRF signaling in extrahypothalamic regions, such as CeA, NAc and VTA, is related to positive reinforcement during the first stage of addiction, while increases in CRF levels in extended amygdala and dorsal striatum have been linked with negative emotional state and habitual drug-seeking in later stages of addiction (Zorrilla et al., 2014). Additionally, abnormal CRF physiology has been linked with neurogenerative disease, including Huntington’s chorea (Heuser et al., 1991) and Alzheimer’s disease (De Souza, 1995). Despite the negative correlation between aberrant CRF signaling and several neurologicaldiseases, there is evidence that CRF can also have a neuroprotective role. In rat primary cerebellar granule neurons undergoing apoptosis, CRF promotes neuronal survival through activation of CRF1 involving, partially, a cAMP-dependent mechanism (PKA) and subsequent inhibition of a proapoptotic kinase (glycogen synthase kinase-3β); furthermore, CRF protected cortical and hippocampal neurons from β-amyloid peptide injury (Facci et al., 2003). Aberrant CRF release may be restored by activation of GABAergic inputs by (3α,5α)3-hydroxypregnan-20-one (3α,5α-THP or allopregnanolone) and (3α,5α)-3,21-dihydroxypregnan-20-one (THDOC), neurosteroids that are elevated in brain and released from the adrenals into circulation with corticosteroids during the acute stress response (Purdy et al., 1991). 3α,5α-THP and THDOC are the most potent positive modulators of GABAA receptors (Morrow et al., 1987), mediating both phasic and tonic inhibition (Harrison et al., 1987; Mody, 2001). Previous data indicated that 3α,5α-THP and THDOC are able to reduce HPA activation precipitated by stress through effects on CRF expression. In fact, 3α, 5α-THP administration before stress attenuated the stress-induced adrenocorticotrophic hormone (ACTH) and corticosterone increase (Owens et al., 1992; Patchev et al., 1996) and reduced basal CRF mRNA expression in male rats (Patchev et al., 1994). Furthermore, intracerebroventricular (ICV) administration of 3α,5α-THP antibody prevented its regulation of the HPA axis and enhanced the stress response (Purdy et al., 1991). This evidence is based on studies in male subjects and has not been explored at the level of gene and protein expression in male and female rats or in the extrahypothalamic CRF system. To explore the possible sex difference in 3α,5α-THP regulation of CRF system, we studied CRF signaling across the hypothalamus and the extrahypothalamic regions (HP, CeA, VTA and NAc), measuring 3α, 5α-THP, CRF mRNA and peptide levels in both male and female Sprague Dawley rats, in basal condition and after 3α,5α-THP administration (15 mg/kg).
2. Materials and methods
2.1. Animals
Male and female (>PN60) Sprague-Dawley rats bred in-house from Envigo stock were used in all experiments. Animals were pair-housed in a temperature and humidity controlled 12 h light/dark facility with ad libitum access to food and water. A total number of 110 male and 100 female Sprague-Dawley rats were used to perform the experiments in this study. To avoid estrus-related changes in endogenous 3α,5α-THP that could affect our results, all experiments were conducted between 8 and 11.30 a.m. Previous studies have shown that the main progesterone peak in the rat estrous cycle occurs in the early evening of proestrus and returns to basal levels by the morning of estrous (Freeman, 2006; Nequin et al., 1979). All procedures were performed in accordance with guidelines approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill (IACUC approval number: 18–168).
2.2. 3α,5α-THP administration
A dose of 15 mg/kg of 3α,5α-THP has been used in the experiments. This dose is known to have an anxiolytic (Crawley et al., 1986) and anti-convulsant (Devaud et al., 1995), but no hypnotic effect (Mendelson et al., 1987). This dose was chosen based on our previous data, showing inhibition of CRF expression in male rats (Balan et al., 2019). Briefly, 3α,5α-THP was dissolved in hydroxypropyl-β-cyclodextrin (45% w/v in water) at a concentration of 7.5 mg/ml. Solutions were prepared the day before the experiment and kept stirring at 4 °C overnight. According to their weight, all rats received an IP injection of 3α,5α-THP (15 mg/kg) or an equivalent volume of vehicle (VEH). 45 min after 3α, 5α-THP administration, rats were euthanized by rapid decapitation, and brains and blood were collected and immediately frozen at −80 °C until the assay. Data from VEH groups are described as the baseline measure in experiments.
2.3. Western blotting
The brains were dissected using a brain block into nucleus accumbens (NAc), central nucleus of amygdala (CeA), hypothalamus (HYP), hippocampus (HP) and ventral tegmental area (VTA). The regions were homogenized and sonicated in ice-cold CelLytic MT lysis buffer (Sigma-Aldrich, St. Luis, MI) with 1x HALT protease and phosphatase inhibitor (Thermo Fisher Scientific, Waltham, MA), left on ice for 30 min and then centrifugated at 14,000×g for 30 min at 4 °C. Supernatant containing the total protein was transferred to new tubes and used immediately or stored at − 80 °C. The Pierce BCA assay kit (Thermo Fisher Scientific, Waltham, MA) was subsequently used to determine sample protein concentrations. The proteins (40 μg/lane) were resolved by NuPAGE™ 10% Bis-Tris Midi Protein Gel (Thermo Fisher Scientific, Waltham, MA) electrophoresis and transferred onto nitrocellulose membranes (Thermo Fisher Scientific, Waltham, MA) using the iBlot 2 Dry Blotting System (Thermo Fisher Scientific, Waltham, MA). The blots were blocked for 1 h (room temperature) in LI-COR Blocking Buffer + PBS (1:1 v/v). Primary antibodies were added to the blot in LI-COR Blocking Buffer + PBST (1:1 v/v, 0.1% Tween 20) (LI-COR Biosciences, Lincoln, NE) and incubated in CRF antibody (Immunostar, Hudson, WI) for 2 days and β-actin antibody (Novus Biologicals, Oakville, ON, Canada) overnight at 4 °C (to control for equivalent loading and transfer of proteins). The blots were washed 3 times with PBST then incubated for 1 h at room temperature with secondary antibodies conjugated to a fluorophore (goat anti-rabbit IgG Alexa Fluor Plus 680 (CRF) and goat anti-mouse IgG Alexa Fluor 790 (β-actin) (Thermo Fisher Scientific, Waltham, MA). The blots were then washed 3x with PBST, and 1x with PBS to remove the excess Tween 20. The bands were visualized using the Odyssey CLx System (LI-COR Biosciences). Optical density for CRF signals were normalized to β-actin and expressed as a ratio (CRF/β-actin).
2.4. Real-time quantitative PCR
RNA extraction.
The brains were microdissected into NAc, CeA, HYP, HP and VTA, transferred to RNase-free tubes and homogenized in ice-cold TRIzol (Thermo Fisher Scientific, Waltham, MA). Total RNA was extracted and purified using the Direct-zol RNA kit (Zymo Research, Irvine, CA) according to the manufacturer’s instructions. The RNA was quantified and quality controlled using a Nanodrop (all 260/280, and 230/260 values ≥ 1.8; Thermo Fisher Scientific, Waltham, MA). cDNA synthesis. Purified RNA (8 μg) was reversed transcribed to a cDNA library using SuperScript® III First-Strand Synthesis System for RT-PCR (Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s instructions. Briefly, samples were inserted into a thermocycler (Axygen® Maxygene Thermal Cycler II, Corning Life Sciences), with the following temperature protocol: 3 min at 25 °C for annealing, 10 min at 45 °C for reverse transcription, 5 min at 85 °C to inactivate the reaction, and 1 min at 4 °C for cool down. Next, samples were removed, diluted 1:10 with water (200 μl total), and stored at − 20 °C before RT-PCR experiments. qPCR. Each sample was run in triplicate on a 96-well plate, using 20 μl total volume per well with the following components: PowerUp™ SYBR™ Green Master Mix (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA) (containing ROX dye for passive reference), forward and reverse primers (Eton Biosciences Inc., Research Triangle Park, NC), RNase-free water and cDNA template. Real-time quantitative PCR reactions were performed at 95 °C for 10 min (initial activation), followed by 40 cycles of denaturation (95 °C for 15s), annealing (60 °C for 30s), and extension (72 °C for 45s) and a melt curve analysis using on a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA). Melt curve analysis was used to ensure proper amplicon expression rather than nonspecific amplification or primer-dimer formation. Real-time qPCR reactions included no template controls without cDNA added from each region, as well as a negative control sample where no cell material was added to the lysis solution, but underwent reverse transcription and preamplification reactions. All primer sequences are included in Table 1. Data were analyzed using the ΔΔCT method and expressed as fold control, normalized to GAPDH.
Table 1.
Primer sequences used for the qPCR assay.
| Name | 5′-Sequence-3′ |
|---|---|
| Rat CRF forward | CGCCCATCTCTCTGGATCT |
| Rat CRF reverse | ATCAGTTTCCTGTTGCTGTGAG |
| Rat GAPDH forward | AACGACCCCTTCATTGAC |
| Rat GAPDH reverse | TCCACGACATACTCAGCAC |
2.5. 3α,5α-THP determination
Plasma and brain 3α,5α-THP concentrations were analyzed in duplicate by quantitative sandwich enzyme-linked immunosorbent assay (ELISA) using the DetectX® 3α,5α-THP Immunoassay kit (Arbor Assay, Ann Arbor, MI), following the manufacturer’s instructions. Plasma was collected by spinning down the whole blood at 4 °C for 15 min at 1730×g. 500 μl of plasma was used to extract the neurosteroids by ethyl ether (Sigma-Aldrich, St. Luis, MI) at 5:1 (v/v) solvent:sample ratio. The mixture was vortexed for 2 min, allowed to separate, then the sample was frozen in dry ice/ethanol bath and the ether was collected into a clean tube; this was repeated 2 additional times, pooling the ether. The collected ether was dried down under a nitrogen stream, dissolved in 1 ml of dichloromethane (Optima, Thermo Fisher Scientific, Waltham, MA) and evaporated to dryness in a speed vacuum concentrator (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The brains were dissected using a brain block into NAc, CeA, HYP, HP and VTA, and processed adapting the protocol from (Snelling et al., 2014). Briefly, each region was transferred into a glass tube and homogenized in 1 ml Milli-Q water. Homogenates were applied to solid phase extraction columns (Strata-X 33u Polymeric RP 60mg/3 ml (8B–S100-UBJ), Phenomenex, Torrance, CA) that had been preconditioned with 3 ml dichloromethane (Optima, Thermo Fisher Scientific, Waltham, MA), 3 ml acetonitrile (Optima, Thermo Fisher Scientific, Waltham, MA) and 3 ml Milli-Q water. The column containing the sample was washed with 1 ml Milli-Q water and 1 ml 30% methanol (Optima, Thermo Fisher Scientific, Waltham, MA) in water in order to remove high polar impurities. Columns were dried under vacuum for 30 min, then washed with 1 ml n-Hexane (Optima, Thermo Fisher Scientific, Waltham, MA) to remove lipids and dried again for 10 min. Neuroactive steroids were then eluted with 4 ml acetonitrile; the extracts were evaporated in a speed vacuum concentrator (Thermo Fisher Scientific, Inc., Waltham, MA, USA), dissolved in 1 ml of methylene chloride and dried down. Extracted samples (plasma or brain) were kept frozen until the day of the assay, then dissolved in 50 μl of methanol (Thermo Fisher Scientific, Inc., Waltham, MA, USA), placed in an ultrasonic bath for 30 min, then diluted with 450 μl of assay buffer. 50 μl of each extracted sample was used to perform the ELISA analysis following the manufacturer’s instructions.
2.6. Statistical analysis
All results are presented as means ± SEM. Significant interactions were further detected by two-way ANOVA and Tukey Honest Significant Differences (HSD) test. All data were analyzed with GraphPad Prism 8 (GraphPad Software, San Diego, CA). A value of p <0.05 was considered statistically significant.
3. Results
3.1. Sex difference in serum 3α,5α-THP concentration following 3α,5α-THP administration
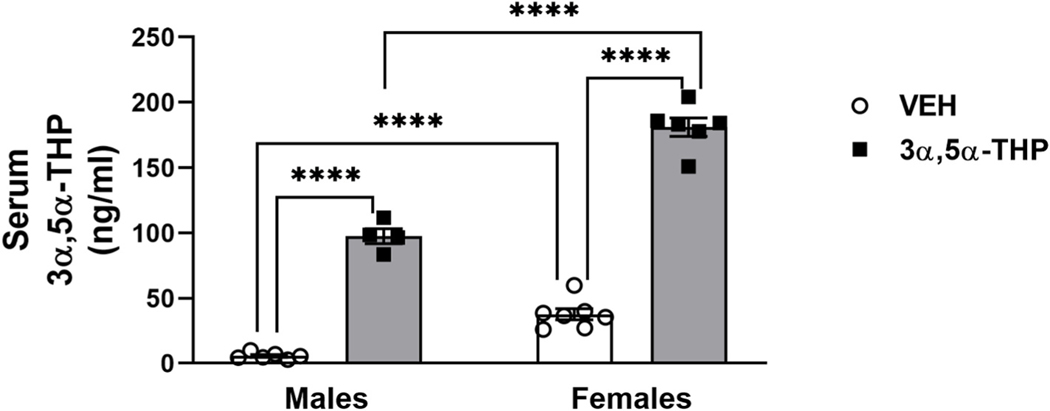
As previously demonstrated (Caruso et al., 2013; Sze and Brunton, 2020), basal serum 3α,5α-THP levels were higher in female than male rats (Fig. 1. Two-way ANOVA: F (1,19) sex = 130.92, p < 0.0001; F (1, 19) treatment = 543.2, p < 0.0001; F (1,19) interaction between factors = 26.2, p < 0.0001). The variability in female rats (11%) was similar to male rats (18%) in the VEH groups, suggesting that variability due to estrus phase was not a factor. Following 3α,5α-THP administration, serum 3α,5α-THP levels increased in both male and female rats. Moreover, we found a significant sex difference in the magnitude of the mean increase in serum 3α,5α-THP levels (t (8) = 14.77, p < 0.001), despite the identical dose of 3α,5α-THP in both groups.
Fig. 1. Serum 3α,5α-THP levels are higher in female vs male rats following vehicle and 3α,5α-THP administration.
As expected, female rats showed higher levels of circulating 3α,5α-THP (males = 5.703 ± 1.02 ng/ml vs females = 37.59 ± 4.2 ng/ml, p = 0.0006). Following 3α,5α-THP (15 mg/kg) IP administration, plasma 3α,5α-THP levels were increased in both male and female rats (males VEH = 5.703 ± 1.02 ng/ml vs 3α,5α-THP = 97.46 ± 5.8 ng/ml, p < 0.0001; females VEH = 37.59 ± 4.2 ng/ml vs 3α,5α-THP = 181 ± 7 ng/ml, p < 0.0001); this increase was significantly different between male and female rats (males = 91.76 ± 5.8 ng/ml vs females = 143.41 ± 7 ng/ml; t (8) = 14.77, p < 0.001) (n = 4–7 per group). Significant effect was found using Two-way ANOVA, followed by Tukey HSD test, ****p < 0.0001. Data are represented as mean ± SEM. VEH = rats treated with vehicle; 3α,5α-THP = rats treated with 3α,5α-THP.
3.2. Sex differences in hypothalamic CRF mRNA and peptide expression following 3α,5α-THP administration
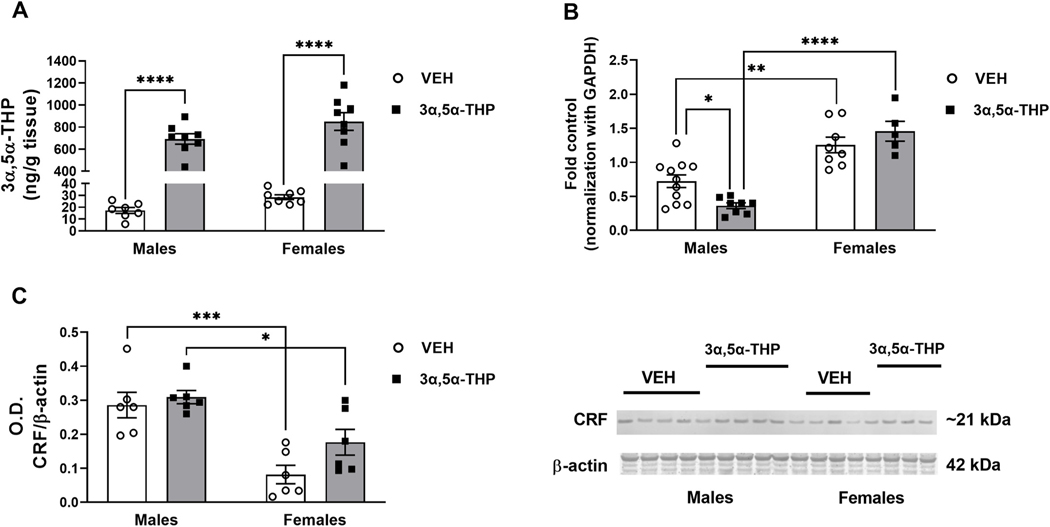
To investigate CRF system activity and the effect of 3α,5α-THP administration (15 mg/kg) in male and female rats, we measured the amount of 3α,5α-THP, CRF mRNA and CRF peptide in hypothalamus and extra-hypothalamic regions of Sprague Dawley rats. No sex difference in hypothalamic 3α,5α-THP content was found between the VEH groups and females showed less variability than males (15% males vs 7% females). 3α,5α-THP injection increased the hypothalamic 3α,5α-THP content in both male and female rats (Fig. 2A. Two-way ANOVA: F (1,27) treatment = 239.4, p < 0.0001), confirming that 3α,5α-THP crossed the blood brain barrier following IP administration and reached the hypothalamus. No sex difference in this response was observed. Female rats exhibited elevated baseline levels of CRF mRNA expression vs. male rats (Fig. 2B. Two-way ANOVA: F (1,28) sex = 28.66, p = 0.0108; F (1,28) interaction between factors = 62.48, p < 0.0001). As shown previously (Patchev et al., 1994), and consistent with the increase in hypothalamic 3α,5α-THP levels following the IP administration, CRF mRNA expression was reduced by 50% in male rats treated with 3α,5α-THP. In contrast, female rats did not show any change in CRF mRNA levels after 3α,5α-THP administration. As expected, CRF mRNA levels in female rats were significantly higher than male rats injected with 3α,5α-THP.Control CRF peptide expression exhibited the opposite sex difference compared to CRF mRNA. Control CRF peptide in female rats was lower than male rats (Fig. 2C. Two-way ANOVA: F (1,20) sex =7.458, p < 0.0001). Moreover, 3α,5α-THP administration did not significantly alter control CRF protein levels in male or female rats. Finally, CRF peptide levels were higher in male rats treated with 3α, 5α-THP than female rats.
Fig. 2. Sex differences in CRF signals after 3α,5α-THP administration in hypothalamus.
A. 3α,5α-THP (15 mg/kg) IP administration increased 3α,5α-THP levels in hypothalamus of male and female rats (males VEH = 17.29 ng/g tissue vs 3α,5α-THP = 692.2 ± 47 ng/g tissue, p < 0.0001; females VEH = 28.5 ± 2 ng/g tissue vs 3α,5α-THP = 850.8 ± 80.6 ng/g tissue, p < 0.0001) (n = 7–8 rats per group). B. The results of the qPCR analysis showed higher hypothalamic baseline CRF mRNA in female rats (males = 0.7224 ± 0.09 vs females = 1.255 ± 0.11, p = 0.0018. 3α,5α-THP injection decreased CRF mRNA only in male rats (males VEH = 0.7224 ± 0.09 vs 3α,5α-THP = 0.3624 ± 0.04, p = 0.0461) (n = 5–11 rats per group); after the treatment, females mRNA expression was higher than males (males = 0.3624 ± 0.04 vs females = 1.457 ± 0.15, p < 0.0001). C. In contrast to the mRNA results, in basal condition western blotting showed in female rats have lower hypothalamic CRF peptide levels than males (males = 0.2857 ± 0.04 vs females = 0.0816 ± 0.03, p = 0.0009); 3α,5α-THP injection did not affect the basal levels of CRF peptide, but male rats treated with 3α,5α-THP resulted in higher CRF levels than the same group of female rats (males = 0.3091 ± 0.02 vs females = 0.1766 ± 0.04, p = 0.0343) (n = 6 per group). Significant effect was found using Two-way ANOVA, followed by Tukey HSD test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are represented as mean ± SEM. VEH = rats treated with vehicle; 3α,5α-THP = rats treated with 3α,5α-THP.
3.3. Region specific sex-dependent regulation of CRF peptide expression following 3α,5α-THP administration in hippocampus and central nucleus of amygdala
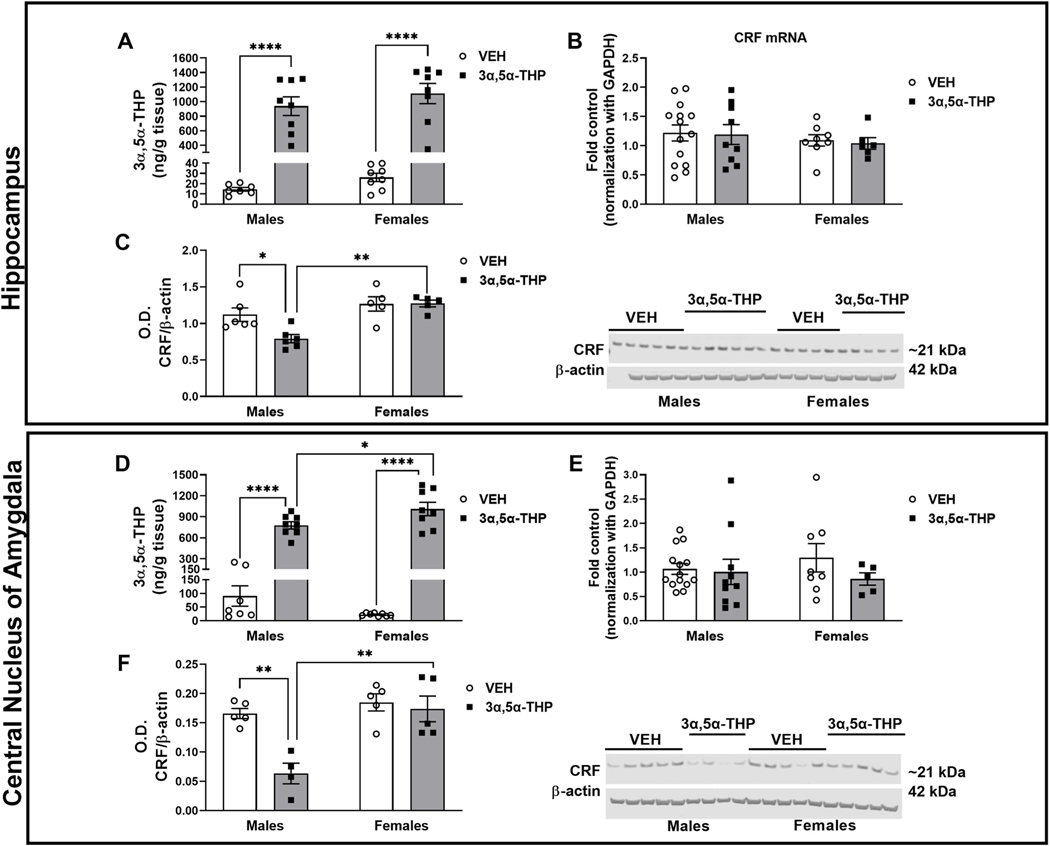
To explore the effect of 3α,5α-THP on CRF signals in the extra-hypothalamic CRF circuits, we analyzed the 3α,5α-THP and CRF content of hippocampus, central nucleus of amygdala, ventral tegmental area and nucleus accumbens of male and female rats, under basal conditions and after 3α,5α-THP injection. Hippocampus. As expected, 3α,5α-THP administration enhanced endogenous 3α,5α-THP levels in hippocampus, in both male and female rats (Fig. 3A. Two-way ANOVA: F (1,27) treatment = 105.6, p < 0.0001). No sex differences were detected both in VEH groups or following 3α,5α-THP injection. We did not find a sex difference in baseline CRF mRNA or peptide expression in hippocampus. Moreover, 3α,5α-THP administration did not alter CRF mRNA levels in male or female rats (Fig. 3B). In contrast, 3α,5α-THP injection reduced CRF peptide levels in hippocampus of male rats (Fig. 3C. Two-way ANOVA: F (1,18) sex = 15.52, p < 0.0007; F (1,18) interaction between factors = 4.726, p = 0.0433), but there was no effect of 3α,5α-THP administration on CRF protein levels in female rats. Therefore, hippocampal CRF peptide levels in male rats were 48% lower than female rats treated with 3α,5α-THP.
Fig. 3. 3α,5α-THP administration decreased CRF peptide in hippocampus and central nucleus of amygdala of male rats.
Hippocampus: A. 3α,5α-THP (15 mg/kg) IP administration increased 3α,5α-THP levels in hippocampus of male and female rats (males VEH = 14.52 ± 1.8 ng/g tissue vs 3α,5α-THP = 938.3 ± 128.2 ng/g tissue, p < 0.0001; females VEH = 26.08 ± 4 ng/g tissue vs 3α,5α-THP = 1112 ± 138.3 ng/g tissue, p < 0.0001) (n = 6–8 rats per group). B. qPCR analysis did not show any sex or treatment differences in CRF mRNA expression (n = 6–14 rats per group). C. Western blotting analysis did not show any sex differences in baseline CRF peptide levels; however, 3α,5α-THP injection reduced CRF protein expression in male (VEH CRF ratio = 1.12 vs 3α,5α-THP CRF ratio = 0.7906, p = 0.0254), but not in female rats. Male rats treated with 3α,5α-THP exhibited lower CRF levels than the same group of female rats (male = 0.7906 ± 0.06 vs. female = 1.273 ± 0.05, p = 0.0017) (n = 5–6 per group). Central nucleus of amygdala: D. 3α,5α-THP (15 mg/kg) IP administration increased 3α,5α-THP levels in male and female rats, but the increase in female rats is higher than male rats (males VEH = 90.48 ± 37.24 ng/g tissue vs 3α,5α-THP = 778.1 ± 51.39 ng/g tissue, p < 0.0001; females VEH = 22.4 ± 1.96 ng/g tissue vs 3α,5α-THP = 1011 ± 95.73 ng/g tissue, p < 0.0001; males 3α,5α-THP = 778.1 ± 51.39 ng/g tissue vs females 3α,5α-THP = 1011 ± 95.73 ng/g tissue, p = 0.0386) (n = 6–8 rats per group). E. qPCR analysis did not show any sex or treatment differences in CRF mRNA expression (n = 6–14 rats per group). F. The results of western blotting analysis did not show any sex differences in CRF peptide levels under basal condition. However, 3α,5α-THP injection significantly reduced CRF protein expression in male (males VEH = 0.1658 ± 0.01 vs 3α,5α-THP = 0.0632 ± 0.02, p = 0.0033), but not in female rats. Male rats treated with 3α,5α-THP exhibited lower CRF levels than female rats (males = 0.0632 ± 0.02 vs females = 17.36 ± 0.02, p = 0.0017) (n = 5–6 per group). Significant effect was found using Two-Way ANOVA, followed by Tukey HSD test, *p < 0.05, **p < 0.01, ****p < 0.0001. Data are represented as mean ± SEM. VEH = rats treated with vehicle; 3α,5α-THP = rats treated with 3α,5α-THP.
Central nucleus of amygdala.
As expected, both male and female rats injected with 3α,5α-THP displayed an increase in 3α,5α-THP levels, but this increase was 3653% higher in female compared to male rats (Fig. 3D. Two-way ANOVA: F (1,27) treatment = 203.6, p < 0.0001). No sex difference in control 3α,5α-THP content was observed and females exhibited less variability than males in control 3α,5α-THP levels (41% for males and 0.09% for females). As in the hippocampus, CRF mRNA expression in CeA did not exhibit baseline sex differences or any effect of 3α,5α-THP administration (Fig. 3E) in male or female rats. There was no sex difference in baseline CRF peptide levels in the central nucleus of amygdala. 3α,5α-THP administration reduced CRF peptide concentration in male rat amygdala (Fig. 3F. Two-way ANOVA: F (1,15) sex = 15.44, p < 0.0013; F (1,15) treatment = 11.91, p = 0.036; F (1,15) interaction between factors = 7.672, p = 0.0143), but no effect of 3α,5α-THP was observed in female rats. CRF peptide levels in male rats treated with 3α,5α-THP were lower than the female rats, likely the result of the differential regulation of CRF peptide in male rats. All data obtained with the qPCR were normalized using GAPDH as housekeeping control.
To be sure the GAPDH was not influence by the 3α,5α-THP treatment, we analyzed the raw CT values and we did not find any difference between groups in the regions we studied (HYP Two-way ANOVA: F (1,30) sex = 1.457, p = 0.2368; F (1, 30) treatment = 0.3998, p = 0.5320; F (1, 30) interaction between factors = 0.08115, p = 0.7777. HP Two-way ANOVA: F (1, 26) sex = 1.856, p = 0.1847; F (1, 26) treatment = 1.978, p = 0.1714; F (1, 26) interaction between factors = 0.03684, p = 0.8493. CeA Two-way ANOVA: F (1, 25) sex = 3.693, p = 0.0661; F (1, 25) treatment = 0.2140, p = 0.6477; F (1, 25) interaction between factors = 6.557e-005, p = 0.9936. VTA Two-way ANOVA: F (1, 18) sex = 0.7535, p = 0.3968; F (1, 18) treatment = 0.03400, p = 0.8558; F (1, 18) interaction between factors = 0.4886, p = 0.4935. NAc Two-way ANOVA: F (1, 22) sex = 0.009891, p = 0.9217; F (1, 22) treatment = 0.003593, p = 0.9527; F (1, 22) interaction between factors = 4.142, p = 0.0540) (data not showed).
3.4. Regulation of 3α,5α-THP levels, CRF mRNA or peptide expression in ventral tegmental area and nucleus accumbens following 3α,5α-THP administration
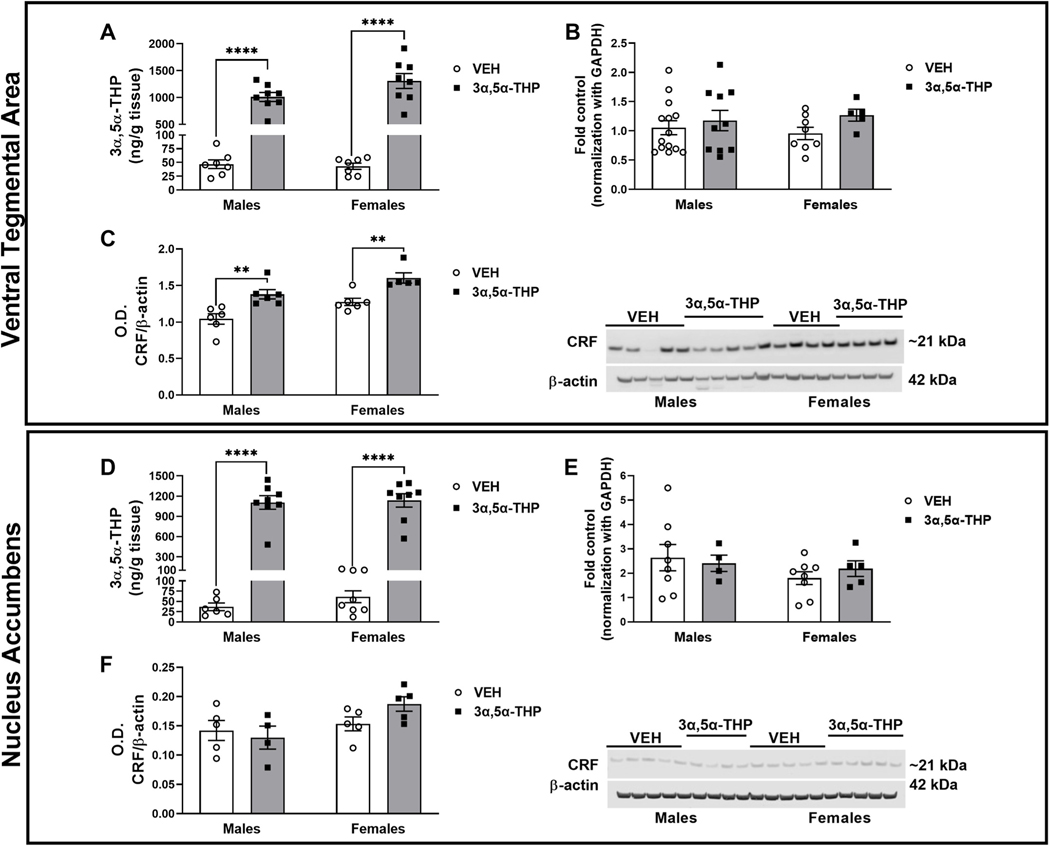
Ventral tegmental area. No sex difference in baseline 3α,5α-THP content was observed and variability was similar in male and female subjects (17% for males and 13% for females). As expected, both male and female rats injected with 3α,5α-THP displayed an increase in VTA 3α,5α-THP levels with no apparent sex difference (Fig. 4A. Two-way ANOVA: F (1,26) treatment = 163.9, p < 0.0001). CRF mRNA expression was not influenced by sex and 3α,5α-THP injection did not alter the baseline levels (Fig. 4B). In both male and female rats, 3α,5α-THP injection increased CRF peptide levels in VTA (Fig. 4C. Two-way ANOVA: F (1,19) sex = 12.52, p < 0.0022; F (1,19) treatment = 26.6, p < 0.0001).
Fig. 4. 3α,5α-THP administration increased CRF peptide levels in ventral tegmental area (VTA), while did not affect CRF signaling in nucleus accumbens (NAc).
Ventral Tegmental Area: A. 3α,5α-THP (15 mg/kg) IP administration increased 3α,5α-THP levels in VTA of male and female rats (males VEH = 46.83 ± 7.94 ng/g tissue vs 3α,5α-THP = 1011 ± 83.39 ng/g tissue, p < 0.0001; females VEH = 43.11 ± 5.7 ng/g tissue vs 3α,5α-THP = 1308 ± 138.7 ng/g tissue, p < 0.0001) (n = 6–8 rats per group). B. qPCR analysis did not show any sex or treatment difference in CRF mRNA expression (n = 5–12 rats per group). C. Western blotting analysis showed an increase in both male and female rats following 3α,5α-THP injection (V males VEH = 1.043 ± 0.07 vs 3α,5α-THP = 1.380 ± 0.06, p = 0.0062; females VEH = 1.276 ± 0.05 vs females 3α,5α-THP = 1.602 ± 0.07, p = 0.0115) (n = 5–6 per group). Nucleus accumbens: D. 3α,5α-THP (15 mg/kg) IP administration increased 3α,5α-THP levels in male and female rats (males VEH = 36.78 ± 9.3 ng/g tissue vs 3α,5α-THP = 1105 ± 100.6 ng/g tissue, p < 0.0001; females VEH = 61.46 ± 14.51 ng/g tissue vs 3α,5α-THP = 1136 ± 100.7 ng/g tissue, p < 0.0001) (n = 6–8 rats per group). E. qPCR analysis did not show any sex or treatment differences in CRF mRNA expression (n = 4–8 rats per group). F. Western blotting analysis did not detect any change in CRF protein levels due by sex or treatment with 3α,5α-THP (n = 5–6 per group). Significant effect was found using Two-Way ANOVA, followed by Tukey HSD test, **p < 0.01, ****p < 0.0001. Data are represented as mean ± SEM. VEH = rats treated with vehicle; 3α,5α-THP = rats treated with 3α,5α-THP.
Nucleus accumbens.
Baseline 3α,5α-THP levels did not show any sex difference in NAc and the variability was similar between male and female rats (25% for males and 24% for females). Following 3α,5α-THP administration, 3α,5α-THP levels in the NAc increased of both male and female rats (Fig. 4D. Two-way ANOVA: F (1,26) treatment = 192.0, p < 0.0001). No sex difference was observed. There was no significant difference in CRF mRNA expression between male and female rats in the vehicle condition or following 3α,5α-THP injection (Fig. 4E). Finally, we did not detect any sex or 3α,5α-THP treatment difference in CRF protein levels (Fig. 4F).
4. Discussion
The aim of this work was to investigate the impact of sex as biological variable in 3α,5α-THP regulation of CRF gene and protein expression, in the hypothalamus and in extrahypothalamic regions, such as hippocampus, central nucleus of amygdala, nucleus accumbens and ventral tegmental area. Our results show a sex and region-specific regulation of CRF that differs at the level of gene and protein expression. Furthermore, control 3α,5α-THP levels and CRF expression were differentially affected by sex compared to the effects of 3α,5α-THP administration. The results portray a very complex relationship between 3α,5α-THP and CRF signaling in the hypothalamus and extrahypothalamic CRF circuits that was not previously elucidated in the literature.
4.1. Circulating and brain 3α,5α-THP
It is already established that females exhibit enhanced HPA axis activity, in terms of greater serum levels of corticosterone (Handa et al., 1994) and 3α,5α-THP (Caruso et al., 2013). Consistent with this, in our study, female Sprague Dawley rats displayed higher serum 3α,5α-THP concentration compared to male rats. Despite the sex difference found in circulating levels, our results showed 3α,5α-THP content was no different between males and females across the brain regions examined. This evidence demonstrates a region-specific 3α,5α-THP modulation and endorses the theory of the de novo synthesis of neurosteroids in the brain (Corpechot et al., 1993), independent of circulating levels. The sex and region-specific variations in steroidogenesis could be linked to differences in precursors (such as progesterone) (Caruso et al., 2010) and/or in expression and activity of the enzymes involving in the conversion of 3α,5α-THP. Previous data showed higher levels of 11β-hydroxylase and 5α-reductase in female brains (Li et al., 1997; Mellon and Deschepper, 1993). Moreover, 3α,5α-THP administration (15 mg/kg, IP) dramatically increased plasma and brain allopregnanolone content, with a significant sex difference only in serum levels. Despite the identical dose of 3α,5α-THP in both groups, female rats showed a greater absolute increase in serum 3α,5α-THP than male rats following 3α,5α-THP administration. This disparity could be explained as a higher uptake of 3α,5α-THP in the female rats. Furthermore, this difference could be due by a greater activation of pregnane xenobiotic receptor (PXR), a ubiquitous and promiscuous nuclear receptor, localized mostly in liver and excretory organs, but also in the brain, to metabolize xenobiotics (Frye et al., 2014b). PXR mRNA and protein were found higher in female rats in proestrous vs those in diestrous or male rats (Frye et al., 2011), suggesting an ovarian steroids-dependent regulation of PXR (Frye et al., 2014a). PXR is an upstream factor of TSPO (a crucial element for the translocation of cholesterol into the mitochondria) that has been shown to be necessary for neurosteroidogenesis of 3α,5α-THP in the VTA of Long-Evans female rats (Frye et al., 2014b). Activation of PXR increased 3α,5α-THP levels in VTA of female rats (Frye et al., 2014b); targeting PXR, 3α,5α-THP is able to regulate its own synthesis (Frye et al., 2014a). New studies are needed to address the possibility of a greater activation of PXR in female rats to explain the greater increase in serum 3α,5α-THP concentration than male rats, following 3α,5α-THP administration.
4.2. Hypothalamic CRF
Our results showed sex differences in both CRF mRNA and peptide expression. CRF mRNA expression was higher in female rats, consistent with other studies in PVN (Duncko et al., 2001; Viau et al., 2005). In contrast, female rats showed lower levels (− 71.5 ± 16.3%, p < 0.001) of CRF peptide compared to male rats. This discrepancy between mRNA expression and protein concentrations is already well known (de Sousa Abreu et al., 2009), and typically attributed to other levels of regulation between transcript and protein product (Maier et al., 2009). It is possible that, in the hypothalamus of female rats, the regulation of CRF transcript messenger is independent of 3α,5α-THP concentrations. This theory is consistent with the lack of effect of 3α,5α-THP treatment on the transcript levels. Using two-way ANOVA, we did not detect any sex difference in basal 3α,5α-THP content, however, we found higher basal 3α, 5α-THP level in hypothalamus of female rats (males = 17.29 ng/g tissue vs females = 28.5 ng/g tissue; t (13) = 3.489, p = 0.004). This higher amount of 3α,5α-THP may be related to the lower CRF peptide levels in female rats, but further study is needed to establish this relationship and possible mechanism. As previously demonstrated (Patchev et al., 1994), 3α,5α-THP administration reduced CRF mRNA expression in males. This result shows a clear sexual dimorphism in 3α,5α-THP regulation of hypothalamic CRF signals. In fact, for 25 years, our knowledge was based on experiments on male subjects showing that 3α,5α-THP inhibited CRF and HPA axis activity in the hypothalamus, but here we show this effect is not present in female rats. Moreover, 3 α,5α-THP administration (15 mg/kg) did not impact hypothalamic CRF peptide levels in either male or female rats. On the contrary, other studies, demonstrated an activation of HPA axis following intracerebroventricular infusion of 3α, 5α-THP (Naert et al., 2007), increasing CRF synthesis and release in hypothalamus. The differences in 3α,5α-THP effects on CRF mRNA vs. peptide concentration could indicate that 3α,5α-THP acts through multiple mechanisms to influence the HPA axis.
4.3. Extrahypothalamic CRF
The data obtained from the extrahypothalamic regions elucidates the regional specificity of the effects of 3α,5α-THP on CRF signals. Despite the sex difference in 3α,5α-THP content in hippocampus (comparing VEH groups: Males = 14.52 ng/g tissue vs females = 26.08 ng/g tissue, t (13) = 2.485, p = 0.0273), there was no sex difference in CRF mRNA or peptide expression, suggesting that difference in 3α,5α-THP does not influence either CRF mRNA or peptide levels in this region. Furthermore, we did not find any sex difference in CRF mRNA or peptide expression in other extrahypothalamic areas examined (CeA, VTA and NAc). 3α,5α-THP administration did not affect CRF messenger levels in the extrahypothalamic regions examined. This result supports the observation of region specificity, where 3α,5α-THP inhibition of CRF mRNA levels is only found in hypothalamus (Patchev et al., 1996), but not in extrahypothalamic regions where CRF plays a major role in brain reward circuits and emotional behavior (Zorrilla et al., 2014). The second important discovery of this study is that 3 α,5α-THP administration (15 mg/kg) decreased CRF peptide concentrations in hippocampus and CeA only in male rats. In contrast, CRF peptide concentration increased following 3α,5α-THP administration in VTA of both male and female rats. These results show an interaction between regional heterogeneity and sex in the response to 3α,5α-THP and further suggest complex regulation of CRF by 3α,5α-THP that may involve multiple different pathways or mechanisms, for example through the innate immune system. Previous studies showed that Toll-like receptor 4 (TLR4) activation led to an increase in CRF release (June et al., 2015; Balan et al., 2018) and that 3α,5α-THP was able to regulate the TLR4 pathway and CRF levels in VTA (Balan et al., 2019).
The data obtained clearly showed the absence of linkage between the messenger transcript and the protein concentration, at baseline and following 3α,5α-THP administration. Genome-wide correlation between expression levels of mRNA and protein from individual genes showed that in 50% of the studies this correlation is discordant (Koussounadis et al., 2015). This discrepancy may be due by a post-transcriptional regulation (Maier et al., 2009; Schwanhausser et al., 2011). Moreover, CRF gene expression is regulated by estrogen (Lalmansingh and Uht, 2008; Vamvakopoulos and Chrousos, 1993), suggesting that the sex differences observed in CRF transcription and expression (in both hypothalamic and extra-hypothalamic areas) may be hormone regulated. The females used in the present study were not monitored for estrous phase in order to avoid the stress of monitoring procedures, but were unlikely to be sampled during the estrus phase (when 3α,5α-THP levels are elevated) due to its short duration across the 4 day cycle. The variability in 3α,5α-THP in female rats was similar to male rats in the vehicle groups and our experiments were performed in the morning, to avoid potential circadian fluctuations in neurosteroids. Moreover, oestrous cycle phase does not affect basal corticosterone concentrations or the corticosterone response to swim stress (Ogle and Kitay, 1977; Sze and Brunton, 2020). However, we cannot exclude the possibility that the estrous cycle could influence the results of this study. Future studies need to address to clarify the potential role of the estrous cycle.
Finally, GABAergic inhibition has been considered crucial in the regulation of CRF neurons. Neurosteroids control the activity of CRF neurons through a tonic current mediated by GABAAR δ subunits and can alter the Cl− gradient such that GABA has an excitatory action on CRF neurons (Sarkar et al., 2011). This phenomenon may explain the differences found between the hypothalamus and the extrahypothalamic regions in 3α,5α-THP regulation on CRF transcription and expression.
4.4. Possible mechanisms of 3α,5α-THP regulation of CRF signaling
The lack of correlation between 3α,5α-THP levels and CRF transcription and expression (baseline and following 3α,5α-THP administration) suggested that 3α,5α-THP is acting through other mechanism to regulate HPA axis activity.
One possibility is that 3α,5α-THP exerts its effects through the neuroimmune system, mainly through toll-like receptors (TLRs), located in neurons and glial cells (Takeda et al., 2003). TLRs identify all types of microbes that enter in the human body as well as endogenous inputs like tissue damage, cellular and psychological stress (Aguirre et al., 2013; Akhter et al., 2019; Zhang et al., 2008). TLR activation contributes to various inflammatory, neurological and psychiatric conditions (Bhattacharya et al., 2016; Crews et al., 2017a, 2017b; Dantzer et al., 2008). TLR4 activity in brain enhances CRF production (Balan et al., 2018; June et al., 2015) and TLR4 activity is enhanced by CRF in both macrophages (Tsatsanis et al., 2006) and brain (Whitman et al., 2013). We previously demonstrated that both 3α,5α-THP and pregnenolone inhibit TLR signaling in the innate immune system in both RAW246.7 macrophage cells and the brain of alcohol preferring P rats that exhibit innate TLR4 activation (Balan et al., 2019). In particular, 3α,5α-THP was able to inhibit TLR4 pathways that lead to the production of pro-inflammatory cytokines and chemokines (Boero et al., 2020). Thus, 3α,5α-THP may regulate the HPA axis through its effects on neuroimmune TLR signals.
Another possible mechanism is 3α,5α-THP action on nuclear receptors (NR). NR are a family of ligand-activated transcription factors that are activated by steroid hormones (Sever and Glass, 2013). While 3α,5α-THP has no effects on progesterone or glucocorticoid NRs, evidence indicates that NR nurr1 and nur77 regulate CRF expression in PVN (Murphy and Conneely, 1997) and other data suggested a reciprocal regulation between NR factors and CRF, dependent on pituitary adenylate cyclase activating polypeptide (PACAP) (McEwen, 1991; Stroth et al., 2011). This hypothesis needs to be verified. The PXR is an important component of the NR family. PXR is activated by a large number of endogenous and exogenous compounds, including neurosteroids (Kliewer et al., 2002). PXR controls cholesterol metabolism: for 3α,5α-THP synthesis, cholesterol is translocated into the mitochondria, where interacting with other enzymes (such as cytochrome P450Scc, 5α-reductase and 3α-hydroxysteroid dehydrogenase) will lead to the production of pregnenolone, progesterone, dihydroprogesterone and, ultimately, 3α,5α-THP (Frye et al., 2014a; 2014b). PXR is an essential upstream factor for 3α,5α-THP neurosteroidogenesis in hippocampus and VTA of Long-Evans female rats. Blocking PXR in VTA produced a reduction in 3α,5α-THP content in VTA and a reduction in mating behavior in female rats (Frye et al., 2014b). Moreover, activation of PXR increased the release of corticosterone into systemic circulation, without increasing the secretion of ACTH (Zhai et al., 2007). Acting through PXR, 3α,5α-THP may regulate its own synthesis as well as circulating levels of corticosterone. This possibility should be explored.
The evidence that CRF has a neuroprotective role may explain the differential regulation of CRF signaling following 3α,5α-THP administration. In apoptotic cerebellar granular neurons, CRF neuroprotective action was mediated by CRF1 activation and increased cAMP that inhibited proapoptotic kinase (Facci et al., 2003). CRF prevented glutamate-induced neurotoxicity in hippocampal slice cultures (Elliott-Hunt et al., 2002). Furthermore, CRF was capable of rescuing cortical and hippocampal neurons from β-amyloids-induced death, acting via PKA and MAP kinase pathways (Facci et al., 2003). Those data may explain the failure of CRF1 antagonist molecules as a therapy for addiction disorders: blocking CRF1 led to a decrease in the CRF-mediated neuroprotection. We found sex and region-dependent regulation in extrahypothalamic CRF signaling following 3α,5α-THP administration. In VTA, 3α,5α-THP administration led to an increase in CRF peptide concentration in both male and female rats: this increase may have a protective effect in this specific region, that has a role in the positive reinforcement during the early phases of addiction.
5. Conclusion
The data presented in this study demonstrated robust sex and region-specific differences in the 3α,5α-THP regulation of CRF transcription and expression. For the first time we have shown that 3α,5α-THP administration reduced hypothalamic CRF mRNA expression and CRF peptide levels in hippocampus and CeA, but only in male rats. Those new data add a new component to our previous hypothesis (Boero et al., 2020; Morrow et al., 2020), elucidating a sex-dependent regulation of 3α, 5α-THP on CRF synthesis and release. Multiple mechanisms at different levels are involved in the regulation of CRF signaling across brain. These results provide a better understanding of the incredible complexity of CRF regulation, the role of 3α,5α-THP in its regulation and the role of sex as a biological variable influencing CRF system and the stress response. Future studies will address the role of stress in this regulation. Our data represents an important finding in order to develop sex-specific therapeutic approaches for diseases involving aberrant CRF signaling, such as addiction and stress-related disorders.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institutes of Health [NIAAA-R01-AA024095 (ALM, JB)] and the Bowles Center for Alcohol Studies at the University of North Carolina at Chapel Hill.
Footnotes
Declaration of competing interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2021.108463.
References
- Aguirre A, Maturana CJ, Harcha PA, Saez JC, 2013. Possible involvement of TLRs and hemichannels in stress-induced CNS dysfunction via mastocytes, and glia activation. Mediat. Inflamm 2013, 893521 10.1155/2013/893521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter N, Madhoun A, Arefanian H, Wilson A, Kochumon S, Thomas R, Shenouda S, Al-Mulla F, Ahmad R, Sindhu S, 2019. Oxidative stress induces expression of the toll-like receptors (TLRs) 2 and 4 in the human peripheral blood mononuclear cells: implications for metabolic inflammation. Cell. Physiol. Biochem 53 (1), 1–18. 10.33594/000000117. [DOI] [PubMed] [Google Scholar]
- Balan I, Beattie MC, O’Buckley TK, Aurelian L, Morrow AL, 2019. Endogenous neurosteroid (3α,5α)3-hydroxypregnan-20-one inhibits toll-like-4 receptor activation and pro-inflammatory signaling in macrophages and brain. Sci. Rep 9 (1), 1220. 10.1038/s41598-018-37409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan I, Warnock KT, Puche A, Gondre-Lewis MC, Aurelian L, 2018. Innately activated TLR4 signal in the nucleus accumbens is sustained by CRF amplification loop and regulates impulsivity. Brain Behav. Immun 69, 139–153. 10.1016/j.bbi.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Derecki NC, Lovenberg TW, Drevets WC, 2016. Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology (Berl) 233 (9), 1623–1636. 10.1007/s00213-016-4214-0. [DOI] [PubMed] [Google Scholar]
- Boero G, Porcu P, Morrow AL, 2020. Pleiotropic actions of allopregnanolone underlie therapeutic benefits in stress-related disease. Neurobiol Stress 12, 100203. 10.1016/j.ynstr.2019.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso D, Pesaresi M, Abbiati F, Calabrese D, Giatti S, Garcia-Segura LM, Melcangi RC, 2013. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology 38 (10), 2278–2290. 10.1016/j.psyneuen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Caruso D, Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Melcangi RC, 2010. Effect of short-and long-term gonadectomy on neuroactive steroid levels in the central and peripheral nervous system of male and female rats. J. Neuroendocrinol 22 (11), 1137–1147. 10.1111/j.1365-2826.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, Mouren M, Prasad VVK, Banner C, Sjövall J, Baulieu EE, Robel P, 1993. Neurosteroids: 3α-hydroxy-5α-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology 133, 1003–1009. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Glowa JR, Majewska MD, Paul SM, 1986. Anxiolytic activity of an endogenous adrenal steroid. Brain Res. 398, 382–385. 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, Coleman LG Jr., 2017a. The role of neuroimmune signaling in alcoholism. Neuropharmacology 122, 56–73. 10.1016/j.neuropharm.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Walter TJ, Coleman LG Jr., Vetreno RP, 2017b. Toll-like receptor signaling and stages of addiction. Psychopharmacology (Berl) 234 (9–10), 1483–1498. 10.1007/s00213-017-4560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Ziegler DR, Herman JP, 2008. Functional role of local GABAergic influences on the HPA axis. Brain Struct. Funct 213 (1–2), 63–72. 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9 (1), 46–56. 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL, 2002. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol. Sci 23 (2), 71–77. 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F, 2005. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci 6 (6), 463–475. 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C, 2009. Global signatures of protein and mRNA expression levels. Mol. Biosyst 5 (12), 1512–1526. 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EB, 1995. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology 20 (8), 789–819. 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Deussing JM, Chen A, 2018. The corticotropin-releasing factor family: physiology of the stress response. Physiol. Rev 98 (4), 2225–2286. 10.1152/physrev.00042.2017. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Morrow AL, 1995. The neurosteroid, 3α-hydroxy-5α-pregnan-20-one, protects against bicuculline-induced seizures during ethanol withdrawal in rats. Alcohol Clin. Exp. Res 19, 350–355. 10.1111/j.1530-0277.1995.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Duncko R, Kiss A, Skultetyova I, Rusnak M, Jezova D, 2001. Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology 26 (1), 77–89. 10.1016/s0306-4530(00)00040-8. [DOI] [PubMed] [Google Scholar]
- Elliott-Hunt CR, Kazlauskaite J, Wilde GJ, Grammatopoulos DK, Hillhouse EW, 2002. Potential signalling pathways underlying corticotrophin-releasing hormone-mediated neuroprotection from excitotoxicity in rat hippocampus. J. Neurochem 80 (3), 416–425. 10.1046/j.0022-3042.2001.00712.x. [DOI] [PubMed] [Google Scholar]
- Facci L, Stevens DA, Pangallo M, Franceschini D, Skaper SD, Strijbos PJ, 2003. Corticotropin-releasing factor (CRF) and related peptides confer neuroprotection via type 1 CRF receptors. Neuropharmacology 45 (5), 623–636. 10.1016/s0028-3908(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Freeman M, 2006. The neuroendocrine control of the ovarian cycle of the rat. In: Neill P,T, Pfaff J, Challis D, de Kretser J, Richards D, Wassarman J,P (Eds.), Knobil and Neill’s Physiology of Reproduction, vol. 3. Academic Press, Cambridge, MA, pp. 2327–2388. [Google Scholar]
- Frye CA, Koonce CJ, Walf AA, 2014a. Novel receptor targets for production and action of allopregnanolone in the central nervous system: a focus on pregnane xenobiotic receptor. Front. Cell. Neurosci 8, 106. 10.3389/fncel.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Walf AA, 2014b. Role of pregnane xenobiotic receptor in the midbrain ventral tegmental area for estradiol- and 3alpha,5alpha-THP-facilitated lordosis of female rats. Psychopharmacology (Berl) 231 (17), 3365–3374. 10.1007/s00213-013-3406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Walf AA, Rusconi JC, 2011. Effects and mechanisms of 3alpha,5alpha,-THP on emotion, motivation, and reward functions involving pregnane xenobiotic receptor. Front. Neurosci 5, 136. 10.3389/fnins.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA, 1994. Gonadal steroid hormone receptors and sex differences in the hypothalamic-pituitary-adrenal axis. Horm. Behav 28, 464–476. 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL, 1987. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acid-A receptor complex. J. Pharmacol. Exp. Therapeut 241, 346–353. [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE, 2002. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol. Biochem. Behav 71 (3), 457–468. 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Heuser IJ, Chase TN, Mouradian MM, 1991. The limbic-hypothalamic-pituitary-adrenal axis in Huntington’s disease. Biol. Psychiatr 30 (9), 943–952. 10.1016/0006-3223(91)90007-9. [DOI] [PubMed] [Google Scholar]
- June HL, Liu J, Warnock KT, Bell KA, Balan I, Bollino D, Puche A, Aurelian L, 2015. CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology 40 (6), 1549–1559. 10.1038/npp.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, Willson TM, 2002. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr. Rev 23 (5), 687–702. 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, 1999. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 848 (1–2), 141–152. 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Koussounadis A, Langdon SP, Um IH, Harrison DJ, Smith VA, 2015. Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci. Rep 5, 10775. 10.1038/srep10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalmansingh AS, Uht RM, 2008. Estradiol regulates corticotropin-releasing hormone gene (crh) expression in a rapid and phasic manner that parallels estrogen receptor-alpha and -beta recruitment to a 3’,5’-cyclic adenosine 5’-monophosphate regulatory region of the proximal crh promoter. Endocrinology 149 (1), 346–357. 10.1210/en.2007-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Bertics PJ, Karavolas HJ, 1997. Regional distribution of cytosolic and particulate 5alpha-dihydroprogesterone 3alpha-hydroxysteroid oxidoreductases in female rat brain. J. Steroid Biochem. Mol. Biol 60 (5–6), 311–318. [DOI] [PubMed] [Google Scholar]
- Maier T, Guell M, Serrano L, 2009. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 583 (24), 3966–3973. 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 1991. Non-genomic and genomic effects of steroids on neural activity. Trends Pharmacol. Sci 12 (4), 141–147. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Deschepper CF, 1993. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 629, 283–292. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Martin JV, Perlis M, Wagner R, Majewska MD, Paul SM, 1987. Sleep induction by an adrenal steroid in the rat. Psychopharmacology 93, 226–229. 10.1007/bf00179939. [DOI] [PubMed] [Google Scholar]
- Mody I, 2001. Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem. Res 26 (8–9), 907–913. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Boero G, Porcu P, 2020. A rationale for allopregnanolone treatment of alcohol use disorders: basic and clinical studies. Alcohol Clin. Exp. Res 44 (2), 320–339. 10.1111/acer.14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM, 1987. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur. J. Pharmacol 142, 483–485. 10.1016/0014-2999(87)90094-X. [DOI] [PubMed] [Google Scholar]
- Murphy EP, Conneely OM, 1997. Neuroendocrine regulation of the hypothalamic pituitary adrenal axis by the nurr1/nur77 subfamily of nuclear receptors. Mol. Endocrinol 11 (1), 39–47. 10.1210/mend.11.1.9874. [DOI] [PubMed] [Google Scholar]
- Naert G, Maurice T, Tapia-Arancibia L, Givalois L, 2007. Neuroactive steroids modulate HPA axis activity and cerebral brain-derived neurotrophic factor (BDNF) protein levels in adult male rats. Psychoneuroendocrinology 32 (8–10), 1062–1078. 10.1016/j.psyneuen.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Nequin LG, Alvarez J, Schwartz NB, 1979. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol. Reprod 20 (3), 659–670. 10.1095/biolreprod20.3.659. [DOI] [PubMed] [Google Scholar]
- Ogle TF, Kitay JI, 1977. Ovarian and adrenal steroids during pregnancy and the oestrous cycle in the rat. J. Endocrinol 74 (1), 89–98. 10.1677/joe.0.0740089. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Ritchie JC, Nemeroff CB, 1992. 5α-Pregnane-3α,21-diol-20-one (THDOC) attenuates mild stress-induced increases in plasma corticosterone via a non-glucocorticoid mechanism: comparison with alprazolam. Brain Res. 573, 353–355. 10.1016/0006-8993(92)90788-B. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hassan AHS, Holsboer F, Almeida OFX, 1996. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 15, 533–540. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OFX, 1994. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 62, 265–271. 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Peng J, Long B, Yuan J, Peng X, Ni H, Li X, Gong H, Luo Q, Li A, 2017. A quantitative analysis of the distribution of CRH neurons in whole mouse brain. Front. Neuroanat 11, 63. 10.3389/fnana.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH Jr., Paul SM, 1991. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. U.S.A 88, 4553–4557. 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, Holsboer F, 2002. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr. Opin. Pharmacol 2 (1), 23–33. 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W, 1983. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature 305. 10.1038/305325a0, 5932, 325–7. [DOI] [PubMed] [Google Scholar]
- Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J, 2011. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J. Neurosci 31 (50), 18198–18210. 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M, 2011. Global quantification of mammalian gene expression control. Nature 473 (7347), 337–342. 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- Sever R, Glass CK, 2013. Signaling by nuclear receptors. Cold Spring Harb Perspect Biol 5 (3), a016709. 10.1101/cshperspect.a016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelling C, Tanchuck-Nipper MA, Ford MM, Jensen JP, Cozzoli DK, Ramaker MJ, Helms M, Crabbe JC, Rossi DJ, Finn DA, 2014. Quantification of ten neuroactive steroids in plasma in Withdrawal Seizure-Prone and -Resistant mice during chronic ethanol withdrawal. Psychopharmacology (Berl) 231 (17), 3401–3414. 10.1007/s00213-014-3618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Liu Y, Aguilera G, Eiden LE, 2011. Pituitary adenylate cyclase-activating polypeptide controls stimulus-transcription coupling in the hypothalamic-pituitary-adrenal axis to mediate sustained hormone secretion during stress. J. Neuroendocrinol 23 (10), 944–955. 10.1111/j.1365-2826.2011.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze Y, Brunton PJ, 2020. Sex, stress and steroids. Eur. J. Neurosci 52 (1), 2487–2515. 10.1111/ejn.14615. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S, 2003. Toll-like receptors. Annu. Rev. Immunol 21, 335–376. 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Boudaba C, Schrader LA, 1998. Local glutamatergic and gabaergic synaptic circuits and metabotropic glutamate receptors in the hypothalamic paraventricular and supraoptic nuclei. Adv. Exp. Med. Biol 449, 117–121. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Androulidaki A, Alissafi T, Charalampopoulos I, Dermitzaki E, Roger T, Gravanis A, Margioris AN, 2006. Corticotropin-releasing factor and the urocortins induce the expression of TLR4 in macrophages via activation of the transcription factors PU.1 and AP-1. J. Immunol 176 (3), 1869–1877. 10.4049/jimmunol.176.3.1869. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP, 2009. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci 10 (6), 397–409. 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP, 1993. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J. Clin. Invest 92 (4), 1896–1902. 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M, 2005. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology 146 (1), 137–146. 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Whitman BA, Knapp DJ, Werner DF, Crews FT, Breese GR, 2013. The cytokine mRNA increase induced by withdrawal from chronic ethanol in the sterile environment of brain is mediated by CRF and HMGB1 release. Alcohol Clin. Exp. Res 37 (12), 2086–2097. 10.1111/acer.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Pai HV, Zhou J, Amico JA, Vollmer RR, Xie W, 2007. Activation of pregnane X receptor disrupts glucocorticoid and mineralocorticoid homeostasis. Mol. Endocrinol 21 (1), 138–147. 10.1210/me.2006-0291. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Woodruff M, Zhang Y, Miao J, Hanley G, Stuart C, Zeng X, Prabhakar S, Moorman J, Zhao B, Yin D, 2008. Toll-like receptor 4 mediates chronic restraint stress-induced immune suppression. J. Neuroimmunol 194 (1–2), 115–122. 10.1016/j.jneuroim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Herman JP, 2000. Local integration of glutamate signaling in the hypothalamic paraventricular region: regulation of glucocorticoid stress responses. Endocrinology 141 (12), 4801–4804. 10.1210/endo.141.12.7949. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF, 2014. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front. Neuroendocrinol 35 (2), 234–244. 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.