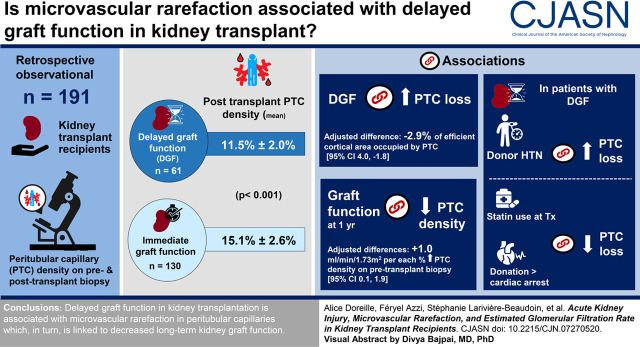
Visual Abstract
Keywords: acute renal failure, chronic kidney failure, delayed graft function, pathophysiology of renal disease and progression, microvascular rarefaction, kidney transplantation, acute kidney injury
Abstract
Background and objectives
Animal studies suggest that microvascular rarefaction is a key factor in the acute kidney disease to CKD transition. Hence, delayed graft function appears as a unique human model of AKI to further explore the role of microvascular rarefaction in kidney transplant recipients. Here, we assessed whether delayed graft function is associated with peritubular capillary loss and evaluated the association between this loss and long-term kidney graft function.
Design, setting, participants, & measurements
This observational, retrospective cohort study included 61 participants who experienced delayed graft function and 130 who had immediate graft function. We used linear regression models to evaluate associations between delayed graft function and peritubular capillary density expressed as the percentage of efficient cortical area occupied by peritubular capillaries in pre- and post-transplant graft biopsies. eGFRs 1 and 3 years post-transplant were secondary outcomes.
Results
Post-transplant biopsies were performed at a median of 113 days (interquartile range, 101–128) after transplantation. Peritubular capillary density went from 15.4% to 11.5% in patients with delayed graft function (median change, −3.7%; interquartile range, −6.6% to −0.8%) and from 19.7% to 15.1% in those with immediate graft function (median change, −4.5%; interquartile range, −8.0% to −0.8%). Although the unadjusted change in peritubular capillary density was similar between patients with and without delayed graft function, delayed graft function was associated with more peritubular capillary loss in the multivariable analysis (adjusted difference in change, −2.9%; 95% confidence interval, −4.0 to −1.8). Pretransplant peritubular capillary density and change in peritubular capillary density were associated with eGFR 1 and 3 years post-transplantation.
Conclusions
Perioperative AKI is associated with lower density in peritubular capillaries before transplantation and with loss of peritubular capillaries following transplantation. Lower peritubular capillary density is linked to lower long-term eGFR.
Introduction
AKI is a well-established risk factor for progressive CKD in the general population (1). Microvascular injury is emerging as a pivotal element to explain this AKI to CKD transition (2,3). Because the regenerative capacity of endothelial cells in peritubular capillaries appears limited (4), microvascular damage occurring during an episode of AKI can lead to permanent peritubular capillary rarefaction (4–6). Through exacerbation of hypoxia, decreased peritubular capillary density is associated with interstitial fibrosis and progressive kidney dysfunction in animal models (3,7–14) and patients with native CKDs (15–18). We showed recently, using an animal model of AKI where early microvascular and tubular injuries were differentially regulated, that peritubular capillary injury predominates over early tubular injury as a predictor of kidney fibrosis (13). To our knowledge, changes in kidney microvascular density have never been studied in human AKI (19).
AKI in the immediate post-transplant period manifests as delayed graft function (DGF) and occurs in the context of ischemia-reperfusion injury. DGF is associated with reduced long-term kidney graft survival in most studies (20). Yet, patients with DGF constitute a heterogeneous group with variable prognosis. Indeed, some factors, such as donor age and donor type, highly modulate the effect of DGF on allograft survival (21,22). The precise mechanisms that explain the variable negative effect of DGF on allograft kidney function remain incompletely understood. DGF represents a unique setting of predictable AKI, where the exact time of ischemia-reperfusion is known and kidney biopsy samples are often available. Although early peritubular capillary loss has been described in kidney transplant recipients (23,24), the association between DGF and microvascular density in peritubular capillaries is currently unclear.
As microvascular rarefaction following ischemia-reperfusion injury has been reported in animal studies, we hypothesized that kidney transplant recipients who experienced DGF, a marker of severe ischemia-reperfusion injury, would show peritubular capillary loss compared with those with immediate graft function. Hence, our primary aim was to assess whether DGF is associated with peritubular capillary loss compared with immediate graft function. Then, we set out to determine the extent to which peritubular capillary loss is linked with subsequent eGFR. Last, we asked whether some clinical factors were associated with peritubular capillary loss specifically in patients with and without DGF.
Materials and Methods
Participants, Setting, and Study Design
We performed a single-center, observational, retrospective cohort study among kidney transplant recipients who participated in the University of Montreal Kidney Transplant Biobank (Centre Hospitalier de l’Université de Montréal site). We included all patients transplanted between June 2008 and 2017 for whom a graft biopsy was available pretransplantation and post-transplantation, whether this post-transplant biopsy was performed for surveillance purposes or to investigate graft dysfunction. Recipients of nonkidney solid organ transplants were excluded. Patients were followed for 3 years after transplantation. The study was approved by the local ethics committee (reference no. 16.204), and all patients provided informed written consent. A detailed version of this section can be found in Supplemental Appendix.
Main Outcomes
The change in peritubular capillary density on the preimplantation versus the postimplantation biopsy was the primary outcome. Peritubular capillary density was measured by immunohistochemistry on pre- and post-transplant biopsies with CD34 mAbs (Figure 1A) (24,25). To score peritubular capillary density, CD34-positive staining was quantified using VIS, an image analysis software (Figure 1, B–F). Peritubular capillary density was defined as the percentage of efficient cortical area occupied by peritubular capillaries. eGFRs 1 and 3 years post-transplant were secondary outcomes and were estimated with the Modification of Diet in Renal Disease four-variable equation (26). Patients who lost their graft were imputed with an eGFR of 0 ml/min per 1.73 m2.
Figure 1.
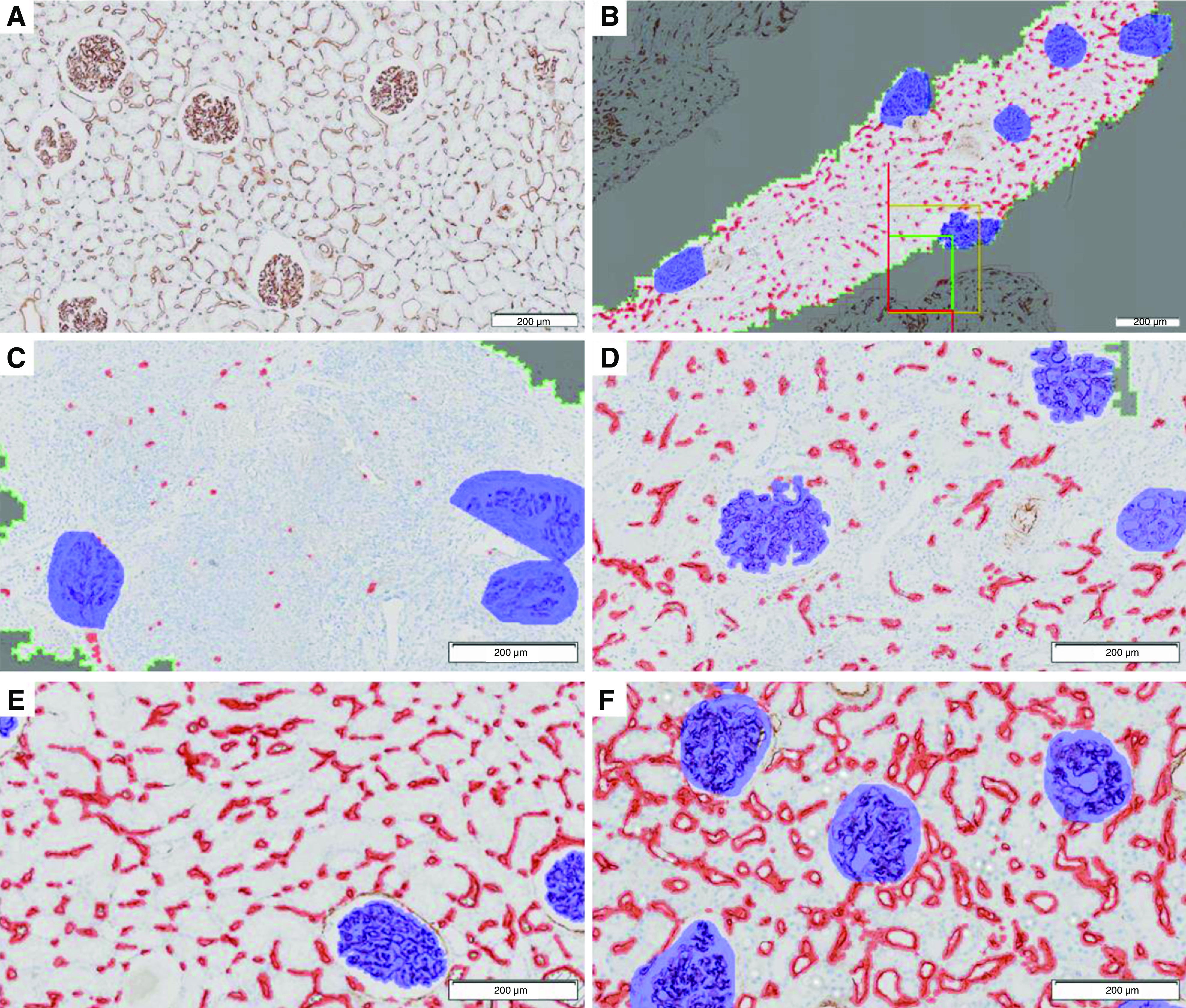
Immunohistochemical quantification of peritubular capillary density. Biopsies from different patients are provided as examples for illustrative purposes. (A) Endothelial marking with CD34 immunohistochemistry. (B) In the delimited cortical area (within the green line), marking of peritubular capillary (red) and glomeruli (blue). (C–F) Four examples of different peritubular capillary densities expressed as the percentage of the efficient cortical area occupied by peritubular capillary. (C) Peritubular capillary density is 1.2%. (D) Peritubular capillary density is 7.8%. (E) Peritubular capillary density is 15.8%. (F) Peritubular capillary density is 30.2%.
Independent Variables
DGF was the main independent variable of interest, and it was defined as the need for dialysis in the first week post-transplant, failure of serum creatinine to decrease by >10% on 3 consecutive days post-transplant, or serum creatinine 2.8 mg/dl on day 5 post-transplant in the presence of scintigraphic evidence of acute tubular necrosis (27–29). We also collected data on recipient-, donor-, and procedure-related characteristics and rejection (30) to adjust for potential confounding in multivariable models.
Statistical Analyses
First, we fit a multivariable linear regression model to determine whether DGF was associated with the extent of change in peritubular capillary density between the preimplantation and the post-transplant biopsies. We subtracted the preimplantation from the post-transplant peritubular capillary density, and hence, a negative estimate for the difference signifies that an independent variable is associated with more peritubular capillary loss compared with the reference category (or loss per unit change for a continuous independent variable). In the model, all variables were included that were associated with (1) DGF (Table 1) and (2) change in peritubular capillary density adjusted for pretransplant peritubular capillary density with a P=0.15 to control for potential confounders. Pretransplant peritubular capillary density was strongly associated with peritubular capillary density change and confounded the association between multiple independent variables and peritubular capillary change. Hence, we decided to assess the univariable associations between independent variables and the outcome while keeping the pretransplant peritubular capillary density constant when selecting the variables to include in the multivariable model.
Table 1.
Characteristics of patients who underwent a kidney transplant at Centre Hospitalier de l’Université de Montréal between 2008 and 2017 for whom a graft biopsy was available pretransplantation and post-transplantation
| Characteristics | Delayed Graft Function, n=61 | Immediate Graft Function, n=130 |
|---|---|---|
| Recipient characteristics | ||
| Age, yr, mean (SD) | 48 (12) | 49 (13) |
| Sex, women, n (%) | 17 (28) | 51 (39) |
| Smoker or ex-smoker, n (%) | 34 (56) | 63 (48) |
| Weight, kg, mean (SD) | 77 (18) | 71 (15) |
| Height, m, mean (SD) | 1.70 (0.10) | 1.69 (0.09) |
| Race, n (%) | ||
| White | 46 (75) | 96 (74) |
| Black | 6 (10) | 10 (8) |
| Cause of CKD, n (%) | ||
| Glomerular diseases | 22 (36) | 51 (39) |
| Diabetes | 9 (15) | 13 (10) |
| Hypertension/vascular | 5 (8) | 6 (4) |
| Polycystic kidney disease | 10 (16) | 30 (23) |
| Autoimmune disease | 3 (5) | 7 (5) |
| Urologic | 9 (15) | 14 (11) |
| Unknown | 11 (15) | 15 (10) |
| Diabetes, n (%) | 14 (23) | 17 (13) |
| First transplantation, n (%) | 62 (83) | 131 (90) |
| Preemptive transplantation, n (%) | 5 (8) | 20 (15) |
| Median time on dialysis before transplantation, mo (IQR) | 45 (18–44) | 31 (9–54) |
| Pretransplant panel-reactive antibodies >0%, n (%) | 10 (16) | 17 (13) |
| Statin use at transplantation, n (%) | 24 (39) | 69 (53) |
| ACE inhibitor use at transplantation, n (%) | 16 (26) | 67 (52) |
| Donor characteristics | ||
| Donor type, n (%) | ||
| Living donor | 4 (7) | 56 (43) |
| Neurologic determination of death | 43 (70) | 69 (53) |
| Donation after cardiac death | 14 (23) | 5 (4) |
| Age, yr, mean (SD) | 47 (15) | 47 (13) |
| Sex, women, n (%) | 34 (56) | 65 (50) |
| Hypertension, n (%) | 14 (23) | 16 (12) |
| Diabetes, n (%) | 5 (8) | 4 (3) |
| Smoker or ex-smoker, n (%) | 29 (48) | 46 (35) |
| Mean donor creatinine, mg/dl (SD) | 0.77 (0.38) | 0.76 (0.25) |
| Median number of HLA mismatches (IQR) | 4 (3–4) | 4 (3–5) |
| Use of hypothermic perfusion machine, n (%) | 22 (36) | 71 (55) |
| Preimplantation biopsy findings | ||
| Median % global glomerulosclerosis (IQR) | 4 (0–7) | 3 (1–6) |
| Interstitial fibrosis (ci) score, n (%) | ||
| 0 | 36 (59) | 95 (73) |
| 1 | 22 (36) | 31 (24) |
| 2 | 3 (5) | 4 (3) |
| 3 | 0 (0) | 0 (0) |
| Vascular fibrous intimal thickening (cv) score, n (%) | ||
| 0 | 36 (59) | 62 (48) |
| 1 | 12 (20) | 29 (22) |
| 2 | 11 (18) | 35 (27) |
| 3 | 2 (3) | 4 (3) |
| Peritransplantation events | ||
| Cold ischemic time, h, mean (SD) | 10 (5) | 8 (5) |
| Warm ischemic time, min, mean (SD) | 39 (11) | 38 (10) |
| Induction, n (%) thymoglobulin | 28 (47) | 27 (21) |
| Post-transplant biopsy | ||
| Median time to biopsy, d (IQR) | 106 (21–117) | 117 (104–129) |
| Surveillance biopsy, n (%) | 54 (89) | 121 (93) |
| Rejection before/on surveillance biopsy, n (%) | 18 (30) | 23 (18) |
| T cell mediated, borderline | 13 (72) | 13 (56) |
| T cell mediated, Banff grade 1A | 3 (17) | 7 (30) |
| T cell mediated, Banff grade 2A | 2 (11) | 0 (0) |
| Antibody mediated | 0 (0) | 2 (9) |
| Chronic T cell–mediated rejection | 0 (0) | 1 (4) |
IQR, interquartile range; ACE, angiotensin-converting enzyme; ci, Banff score for interstitial fibrosis; cv, Banff score for vascular fibrous intimal thickening.
Then, to understand whether peritubular capillary loss was associated with subsequent eGFR 1 and 3 years post-transplant, we fit multivariable linear regression models with eGFR 1 and 3 years post-transplantation as the dependent variables. Last, we fit a multivariable linear regression model where peritubular capillary loss was the dependent variable specifically in the subgroups of patients with and without DGF. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
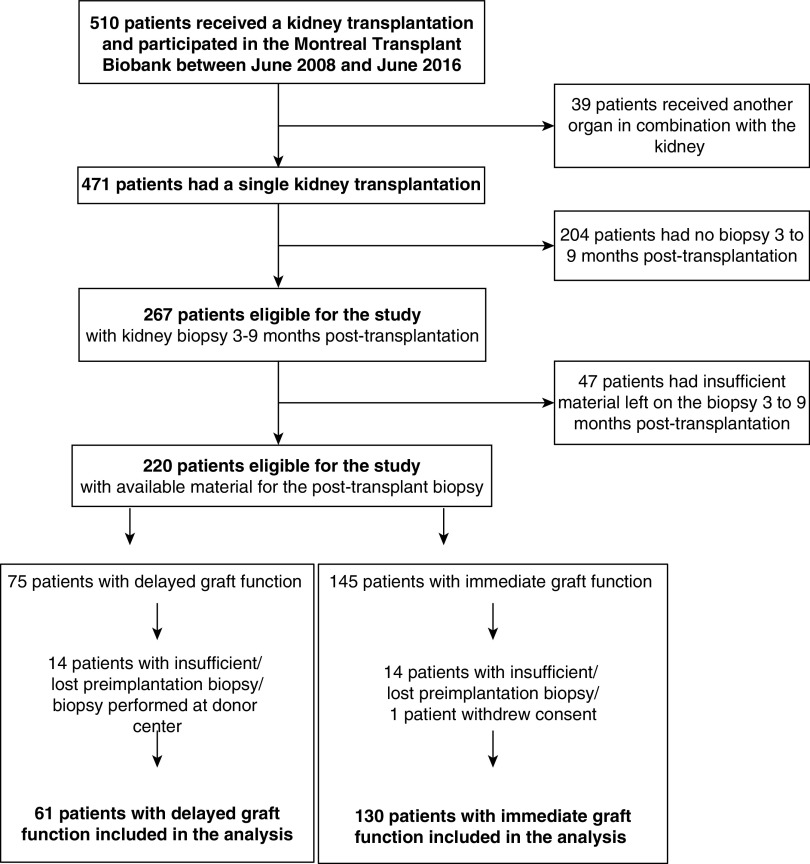
Among the 191 patients eligible for the study, 61 patients (32%) experienced DGF, and 130 patients (68%) had immediate graft function (Figure 2). Supplemental Table 1 provides baseline characteristics for patients with available post-transplant biopsies and those who did not. All characteristics were similar in both groups.
Figure 2.
Patient flow chart.
Among patients with DGF, 16 required hemodialysis, and in two, dialysis was needed for a week or more. Post-transplant biopsies were performed for surveillance purposes (92%) or to investigate allograft dysfunction (8%). All patients were alive 3 years after transplantation. Two patients had experienced graft loss.
When compared with those with immediate graft function (Table 1), recipients who experienced DGF were heavier, more likely to have diabetes, and more likely to have received a kidney from a deceased donor or from a donor with a history of hypertension. Recipients with DGF were also more likely to have longer cold ischemic time and to receive thymoglobulin as induction therapy, but they were less likely to use statins or renin-angiotensin system blockers at transplantation. Patients who experienced DGF were also more likely to have their post-transplant biopsy performed early and to experience an acute rejection episode either before or on the 3- to 9-month post-transplant biopsy.
Peritubular capillary density on pre- and post-transplant biopsies was lower in patients with DGF than in those with immediate graft function (P<0.001), whereas the unadjusted change in peritubular capillary density was similar in both groups (Table 2). When we stratified patients with DGF in subgroups according to the criteria used to define DGF (need for dialysis in the first week post-transplant versus serum creatinine–based definitions of DGF), we observed similar pretransplant peritubular capillary density and eGFR 1 and 3 years post-transplant in all subgroups (Supplemental Table 2).
Table 2.
Peritubular capillary density in pre- and post-transplant biopsies in all study participants, patients with delayed graft function, and patients with immediate graft function
| Peritubular Capillary Densities | All Participants | Delayed Graft Function | Immediate Graft Function |
|---|---|---|---|
| Mean peritubular capillary density on the pretransplant biopsy, % (SD)a | 18.3 (5.0) | 15.4 (4.0) | 19.7 (4.8) |
| Mean peritubular capillary density on the post-transplant biopsy, % (SD)a | 13.9 (3.0) | 11.5 (2.0) | 15.1 (2.6) |
| Median change in peritubular capillary density (post-transplant density minus pretransplant density), % (IQR) | −4.0 (−7.5 to −0.8) | −3.7 (−6.6 to −0.8) | −4.5 (−8.0 to −0.8) |
IQR, interquartile range.
P<0.001 for the difference in peritubular capillary density between patients with delayed graft function and patients with immediate graft function.
Peritubular capillary density on the post-transplant biopsy and eGFR 3 years post-transplant were directly correlated (Rho=0.30; P<0.001) (Figure 3B), and both were lower in patients with DGF than in patients with immediate graft function (Figure 3).
Figure 3.
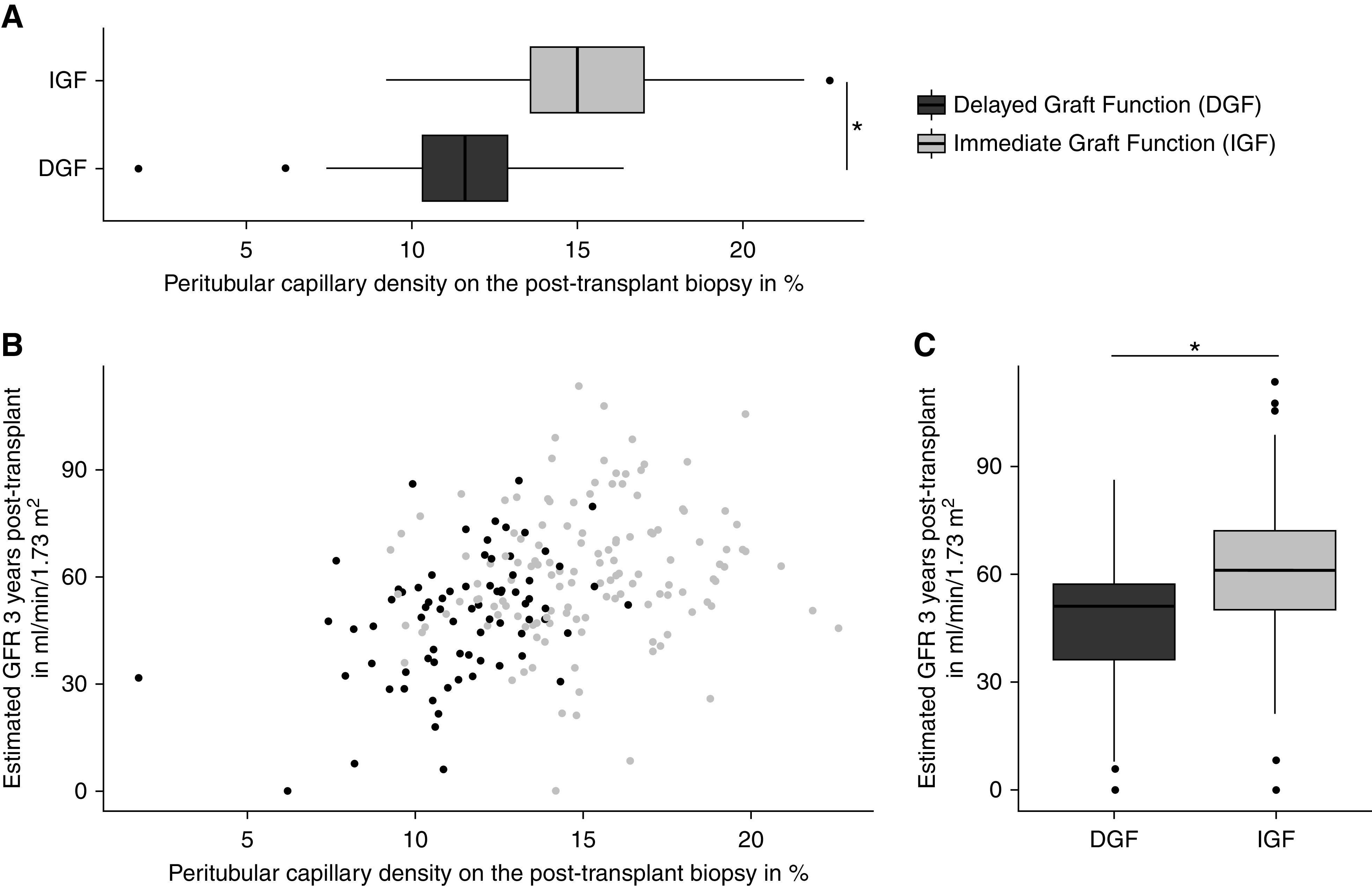
Peritubular capillary density post-transplantation is correlated with eGFR 3 years post-transplantation. (A) Peritubular capillary density on the post-transplantation biopsy is lower in patients with delayed graft function (DGF; mean: 11.5%±2.0% of efficient cortical area occupied by peritubular capillaries) than in patients with immediate graft function (IGF; mean: 15.1%±2.6% of efficient cortical area occupied by peritubular capillaries; P<0.001). (B) Peritubular capillary density post-transplantation correlates with eGFR 3 years after transplantation in patients with DGF and IGF (Rho=0.30; P<0.001). (C) eGFR 3 years post-transplantation is lower in patients with DGF (mean: 51±18 ml/min per 1.73 m2) compared with patients with IGF (mean: 61±19 ml/min per 1.73 m2; P<0.001). *P<0.001.
Delayed Graft Function Is Associated with Peritubular Capillary Loss Independent of Pretransplant Peritubular Capillary Density
In the multivariable analysis, peritubular capillary density on the preimplantation biopsy (adjusted difference in change, −0.9% per percentage higher; 95% confidence interval [95% CI], −1.0 to −0.9) was strongly associated with the change in peritubular capillary density: the higher the pretransplant peritubular capillary density, the more loss was observed. We found that DGF was independently associated with more peritubular capillary loss when adjusted for pretransplant peritubular capillary density (adjusted difference in change, −2.9%; 95% CI, −4.0 to −1.8) (Table 3), as were donor hypertension (adjusted difference, −1.2%; 95% CI, −2.2 to −0.2) and surveillance rather than indication biopsies (adjusted difference, 1.5%; 95% CI, 0.2 to 2.7). Rejection, whether C4d positive or negative prior to or on the post-transplant biopsy, was not associated with peritubular capillary loss. Results of univariable analyses for peritubular capillary loss adjusted for pretransplant peritubular capillary density are presented in Supplemental Table 3. We performed two sensitivity analyses. In the first one, we ran the same multivariable model using readings by the independent blinded pathologist. The association between DGF and peritubular capillary loss was similar to those obtained by the first reader (adjusted difference in change, −2.8%; 95% CI, −3.8 to −1.9; P<0.001) (Supplemental Table 4). Then, we excluded recipients who received donors with a history of hypertension or diabetes, leaving 159 patients for this subgroup analysis. Again, we observed a similar association between DGF and peritubular capillary loss (adjusted difference in change: β, −3.2%; 95% CI, −4.6 to −1.9; P<0.001).
Table 3.
Associations of delayed graft function and other clinical characteristics with change in peritubular capillary density in the fully adjusted multivariable model (n=191)
| Clinical Characteristics | Mean Change, % (SD) | Unadjusted Difference in Change, % (95% Confidence Interval) | P Value | Difference in Change Adjusted only for Pretransplant Peritubular Capillary Density, % (95% Confidence Interval) | P Value | Fully Adjusted Difference in Change, % (95% Confidence Interval)a | P Value |
|---|---|---|---|---|---|---|---|
| Postoperative graft function | 0.60 | <0.001 | <0.001 | ||||
| Immediate graft function | −4.6 (5.2) | 0 | 0 | 0 | |||
| Delayed graft function | −3.9 (4.1) | 0.5 (−1.4 to 2.5) | −3.4 (−4.4 to −2.4) | −2.9 (−4.0 to −1.8) | |||
| Peritubular capillary density on the preimplantation biopsy, per 1% higher | −0.8 (−0.9 to −0.8) | <0.001 | −0.8 (−0.9 to −0.8) | <0.001 | −0.9 (−1.0 to −0.9) | <0.001 | |
| Donor type | |||||||
| Neurologically deceased | −3.4 (4.9) | 0 | 0 | 0 | |||
| Living | −6.3 (4.5) | −2.8 (−4.3 to −1.3) | <0.001 | 0.7 (−0.2 to 1.6) | 0.13 | −0.0 (−1.0 to 0.9) | 0.97 |
| Cardiocirculatory arrest | −4.3 (4.2) | −1.0 (−3.2 to 1.3) | 0.41 | −0.6 (−1.8 to 0.7) | 0.39 | 0.3 (−1.0 to 1.5) | 0.67 |
| Donor age per 10 yr higher | −0.2 (−0.7 to 0.2) | 0.29 | −0.2 (−1.6 to 0.2) | 0.11 | −0.1 (−0.4 to 0.1) | 0.33 | |
| Rejection | 0.25 | 0.12 | 0.74 | ||||
| Absence of rejection | −4.2 (5.1) | 0 | 0 | 0 | |||
| Rejection | −5.2 (4.0) | −1.0 (−2.7 to 0.7) | −0.7 (−1.3 to 1.5) | −0.2 (−1.0 to 0.7) | |||
| Donor BP | 0.47 | 0.003 | 0.02 | ||||
| Normal BP | −4.5 (4.9) | 0 | 0 | 0 | |||
| Donor hypertension | −3.8 (4.6) | 0.7 (−1.2 to 2.6) | −1.6 (−2.6 to −0.5) | −1.2 (−2.2 to −0.2) | |||
| Recipient statin use | 0.90 | 0.15 | 0.19 | ||||
| No statin use at transplant | −4.3 (4.9) | 0 | 0 | 0 | |||
| Statin at time of transplant | −4.4 (4.8) | −0.1 (−1.5 to 1.3) | 0.6 (−0.2 to 1.3) | 0.5 (−0.2 to 1.1) | |||
| Biopsy type | 2.2 (−0.3 to 4.7) | 0.07 | 2.3 (1.0 to 3.6) | <0.001 | 1.5 (0.2 to 2.7) | 0.02 | |
| For cause | −6.8 (3.6) | ||||||
| Surveillance | −4.2 (4.9) | ||||||
| KRT before transplantation | 0.77 | 0.05 | 0.19 | ||||
| Dialysis pretransplant | −4.3 (4.8) | 0 | 0 | 0 | |||
| Preemptive transplantation | −4.9 (5.5) | −0.3 (−2.4 to 1.7) | 1.1 (−0.0 to 1.2) | 0.7 (−0.4 to 1.7) | |||
| Modality of graft transportation | 0.03 | 0.02 | 0.14 | ||||
| Cold storage | −5.1 (4.7) | 0 | 0 | 0 | |||
| Hypothermic perfusion | −3.7 (4.9) | 1.6 (0.2 to 2.9) | 0.9 (0.1 to 1.6) | 0.5 (−0.2 to 1.3) | |||
| cv score on the pretransplant biopsy, per 1 point higher | 0.2 (−0.6 to 1.0) | 0.62 | 0.7 (−0.1 to 1.4) | 0.09 | 0.6 (−0.2 to 1.3) | 0.12 | |
| Time to post-transplant biopsy, per 1 mo higher | 0.2 (−0.3 to 0.7) | 0.42 | 0.3 (0.1 to 0.6) | 0.01 | 0.0 (−0.2 to 0.3) | 0.85 |
cv, Banff score for vascular fibrous intimal thickening.
In addition to the variables included in the table, estimates are adjusted for biopsy size to correct for technical variations in measurement.
Peritubular Capillary Density Is Associated with Lower Estimated Glomerular Filtration Rate 1 and 3 Years Post-Transplant
To investigate the association between microvascular rarefaction and subsequent eGFR, we then asked whether peritubular capillary loss was independently linked with poorer graft function 1 year post-transplant. We found that pretransplant peritubular capillary density (adjusted difference, +1.0 ml/min per 1.73 m2 per each percentage higher; 95% CI, 0.1 to 1.9) and glomerulosclerosis on the pretransplant biopsy (adjusted difference, −0.6 ml/min per 1.73 m2 per each percentage higher; 95% CI, −1.1 to −0.1) were associated with eGFR 1 year post-transplant. Glomerular disease as a cause of CKD prior to transplantation, donor sex, and having a surveillance rather than an indication biopsy were also associated with 1-year eGFR (Table 4). Change in peritubular capillary density was significantly associated with 1-year eGFR when DGF was not included in the multivariable model (adjusted difference, +1.0 ml/min per 1.73 m2 per each percentage higher; 95% CI, 0.3 to 1.8), but this was not the case when DGF was included (adjusted difference, +0.8 ml/min per 1.73 m2 per each percentage higher; 95% CI, −0.1 to 1.6). The results of univariable analyses can be found in Supplemental Table 5.
Table 4.
Factors associated with eGFR 1 year post-transplant
| Clinical Characteristics | Mean Change, ml/min per 1.73 m2 (SD) | Unadjusted Difference, ml/min per 1.73 m2 (95% Confidence Interval) | P Value | Adjusted Difference without Delayed Graft Function, ml/min per 1.73 m2 (95% Confidence Interval)a | P Value | Adjusted Difference with Delayed Graft Function, ml/min per 1.73 m2 (95% Confidence Interval)a | P Value |
|---|---|---|---|---|---|---|---|
| Pretransplant peritubular capillary density per 1% higher | 0.6 (0.1 to 1.0) | 0.01 | 1.3 (0.6 to 2.0) | <0.001 | 1.0 (0.1 to 1.9) | 0.03 | |
| Peritubular capillary change per 1% higher | 0.0 (−0.4 to 0.5) | 0.92 | 1.0 (0.3 to 1.8) | 0.007 | 0.8 (−0.1 to 1.6) | 0.07 | |
| Postoperative graft function | <0.001 | not included | 0.23 | ||||
| Immediate graft function | 60.7 (15.6) | 0 | 0 | ||||
| Delayed graft function | 51.1 (13.1) | −9.2 (−13.7 to −4.6) | −3.1 (−8.3 to 2.0) | ||||
| Biopsy type | <0.001 | 0.005 | 0.004 | ||||
| For cause | 43.9 (15.7) | 0 | 0 | 0 | |||
| Surveillance | 59.0 (14.8) | 15.7 (8.6 to 22.8) | 10.5 (3.3 to 17.7) | 10.7 (3.5 to 17.0) | |||
| Donor sex | 0.001 | 0.005 | 0.004 | ||||
| Man donor | 61.7 (15.7) | 0 | 0 | 0 | |||
| Woman donor | 54.1 (14.4) | −7.5 (−11.8 to −3.3) | −5.8 (−9.8 to −1.8) | −5.9 (−9.9 to −1.9) | |||
| Donor BP | 0.01 | 0.19 | 0.17 | ||||
| Normal BP | 58.9 (15.8) | 0 | 0 | 0 | |||
| Donor hypertension | 51.7 (11.4) | −7.6 (−13.6 to −1.6) | −3.7 (−9.3 to 1.9) | −3.9 (−9.5 to 1.7) | |||
| Donor age per 10 yr higher | −2.6 (−4.2 to −1.0) | <0.001 | −1.0 (−2.6 to −0.7) | 0.24 | −1.0 (−2.7 to 0.1) | 0.21 | |
| Glomerulosclerosis on the pretransplant biopsy per 1% higher | −0.7 (−1.1 to −0.3) | 0.001 | −0.6 (−1.1 to −0.1) | 0.01 | −0.6 (−1.1 to −0.1) | 0.02 | |
| ci score on the pretransplant biopsy per 1 point higher | −3.0 (−7.0 to 1.0) | 0.14 | 1.9 (−2.6 to 6.4) | 0.41 | 2.1 (−2.4 to 6.6) | 0.36 | |
| No. of previous transplants | 0.13 | 0.44 | 0.51 | ||||
| First transplants | 58.4 (15.7) | 0 | 0 | 0 | |||
| Repeated transplants | 53.3 (12.5) | −5.1 (−11.7 to 1.5) | −2.3 (−8.2 to 3.6) | −2.0 (−7.9 to 3.9) | |||
| Cause of CKD | 0.03 | 0.03 | 0.03 | ||||
| Other | 55.9 (15.4) | 0 | 0 | 0 | |||
| Glomerular diseases | 60.8 (15.0) | 4.9 (0.4 to 9.4) | 4.6 (0.5 to 8.7) | 4.7 (0.6 to 8.8) |
ci, Banff score for interstitial fibrosis.
All of the variables included in the multivariable model are listed in the table.
We then examined whether these results held true in the long term and evaluated the association between peritubular capillary density and graft function 3 years post-transplant. Results of univariable analyses are presented in Supplemental Table 6. We found that higher peritubular capillary density on the preimplantation biopsy (adjusted difference, +1.7 ml/min per 1.73 m2 per percentage higher; 95% CI, 0.7 to 2.6) and peritubular capillary change (adjusted difference, 1.4 ml/min per 1.73 m2 per each percentage higher; 95% CI, 0.4 to 2.3) were associated with 3-year eGFR, but this was significant only when DGF was not included in the multivariable model (Supplemental Table 7). Peritubular capillary density was the only histologic finding associated with eGFR 3 years post-transplant. Donor age was also associated with eGFR 3 years post-transplant.
Statin Use, Donor Hypertension, and Donation after Cardiac Arrest Are Associated with Peritubular Capillary Loss in Patients with Delayed Graft Function
When adjusted for peritubular capillary density on the preimplantation biopsy and cold ischemia time, recipient statin use prior to/at the time of transplantation (adjusted difference in change, 1.1%; 95% CI, 0.3 to 2.0) and donation after circulatory death (adjusted difference in change, 2.0% relative to neurologic death; 95% CI, 0.5 to 3.5) were associated with preservation of peritubular capillary density, whereas donor hypertension was associated with peritubular capillary loss (adjusted difference in change, −1.1%; 95% CI, −2.1 to −0.1) (Table 5). Results from univariable analyses and the initial multivariable model can be found in Supplemental Tables 8 and 9. We then examined whether the same factors were associated with peritubular capillary loss in patients with immediate graft function. We found similar associations between change in peritubular capillary density and donor hypertension, surveillance versus for-cause biopsies, and pretransplant peritubular capillary density. However, we did not observe associations between changes in peritubular capillary density, cold ischemic time, pretransplant recipient statin use, and donation after cardiocirculatory death (Supplemental Table 10).
Table 5.
Factors associated with change in peritubular capillary density among patients with delayed graft function (n=61)
| Clinical Characteristics | Mean Change, % (SD) | Unadjusted Difference in Change, % (95% Confidence Interval) | P Value | Difference in Change Adjusted for Pretransplant Peritubular Capillary Density, % (95% Confidence Interval) | P Value | Fully Adjusted Difference in Change, % (95% Confidence Interval)a | P Value |
|---|---|---|---|---|---|---|---|
| Peritubular capillary density on the preimplantation biopsy per 1% higher | −0.9 (−1.0 to −0.8) | <0.001 | −0.9 (−1.0 to −0.8) | <0.001 | −0.8 (−0.9 to −0.7) | <0.001 | |
| Donor type | |||||||
| Neurologically deceased | −3.8 (4.4) | 0 | 0 | 0 | |||
| Living | −6.4 (2.7) | −2.5 (−6.7 to 1.8) | 0.25 | 0.6 (−2.6 to 1.4) | 0.55 | 1.0 (−1.1 to 3.1) | 0.32 |
| Cardiocirculatory arrest | −3.4 (3.3) | 0.2 (−2.3 to 2.7) | 0.85 | 0.9 (−0.3 to 2.1) | 0.12 | 2.0 (0.5 to 3.5) | 0.01 |
| Donor BP | |||||||
| Normal BP | −3.6 (4.3) | 0 | 0 | 0 | |||
| Donor hypertension | −4.9 (3.6) | −1.7 (−4.1 to 0.8) | 0.18 | −1.2 (−2.3 to −0.0) | 0.05 | −1.1 (−2.1 to −0.1) | 0.04 |
| Recipient statin use | |||||||
| No statin use at transplant | −4.3 (3.7) | 0 | 0 | 0 | |||
| Statin at time of transplant | −3.3 (4.7) | 0.9 (−1.2 to 3.1) | 0.38 | 0.9 (−0.1 to 1.8) | 0.09 | 1.1 (0.3 to 2.0) | 0.02 |
| Biopsy type | |||||||
| For cause | −7.6 (4.5) | 0 | |||||
| Surveillance | −3.4 (3.9) | 3.6 (0.2 to 7.0) | 0.04 | 2.3 (1.0 to 3.6) | <0.001 | 1.4 (−0.1 to 2.9) | 0.06 |
| Modality of graft transportation | |||||||
| Cold storage | −4.6 (3.7) | 0 | 0 | 0 | |||
| Hypothermic perfusion | −2.6 (4.6) | 1.9 (−0.2 to 4.0) | 0.08 | 1.3 (0.4 to 2.3) | 0.005 | 0.9 (−0.1 to 1.8) | 0.06 |
| Cold ischemia time per 1 h higher | 0.2 (0.0 to 0.4) | 0.02 | 0.2 (0.1 to 0.4) | 0.02 | 0.2 (0.0 to 0.3) | 0.02 |
In addition to the variables included in the table, estimates are adjusted for biopsy size to correct for technical variations in measurement.
Discussion
Here, we show that DGF or post-transplant AKI secondary to significant ischemia-reperfusion injury is associated with microvascular rarefaction compared with immediate graft function. Peritubular capillary density and glomerulosclerosis on the pretransplant biopsy, as well as peritubular capillary density loss, were associated with lower 1-year eGFR, supporting a key role of preexisting and early post-transplant microvascular damage in predicting subsequent graft function. Although DGF is associated with poorer graft outcomes in most studies (29,30), this is not always the case (31,32). We show that statin use by the recipient at the time of transplantation and donation after circulatory death were associated with less peritubular capillary loss, whereas donor hypertension was associated with more peritubular capillary loss in kidney transplant recipients with DGF.
Our results are in line with previous animal studies (3,6–13) that demonstrated a pivotal role for peritubular capillary rarefaction in the AKI to CKD transition. We found that patients who experience DGF have low pre- and post-transplant peritubular capillary density. Pretransplant peritubular capillary density was strongly associated with change in peritubular capillary density: the higher the pretransplant density, the more peritubular capillary loss. Hence, to study the effect of other independent variables on peritubular capillary change, we adjusted for the baseline value in density. The peritubular capillary loss we observed after an episode of DGF suggests that ischemia-reperfusion–induced AKI leads to endothelial damage, which promotes subsequent graft dysfunction.
Peritubular capillary rarefaction, pretransplant and developing post-transplant, is associated with 1- and 3-year graft function, but these associations were not independent of DGF. Furthermore, when DGF was added to a model that contained pretransplant and change in peritubular capillary density, its association with 1- and 3-year eGFR was importantly attenuated and no longer significant. These findings suggest that microvascular rarefaction mediates part of the association between DGF and 1- and 3-year eGFR. In humans with native kidney disease, the importance of microvascular rarefaction on CKD progression has been shown in glomerular diseases (17,18) and diabetic nephropathy (15) but not in AKI. In a cross-sectional study of allograft biopsies in kidney transplant patients with chronic allograft nephropathy, microvascular injury in peritubular capillary showed a strong correlation with interstitial fibrosis, graft dysfunction, and proteinuria (23). More recently, Steegh et al. (24) reported that early peritubular capillary loss occurring in the first 3 months after kidney transplantation correlated with kidney function 1 year post-transplantation. However, the effect of DGF on peritubular capillary rarefaction was not evaluated in this study. In contrast, we were specifically interested in the association between DGF, as a marker of ischemia-reperfusion severity in the perioperative period, and microvascular rarefaction in the AKI to CKD transition in humans. Our larger sample size allowed us to present results that were also adjusted for donor type. We did not find any association between rejection and peritubular capillary loss in our study, which we believe is due to the low numbers of C4d positive or antibody-mediated rejection (33,34) we observed. Nevertheless, we observed better preservation of peritubular capillary density in patients who underwent a surveillance rather than an indication biopsy, which indicates that clinical deterioration in graft function and associated pathologies can promote peritubular capillary loss after transplantation.
We identified factors that influence peritubular capillary loss in patients with DGF. Statin therapy at the time of transplantation was associated with preservation of peritubular capillary density in patients with DGF but not in patients with immediate graft function. Statins are known to modify endothelial function and inflammatory responses (35–40). Although this remains to be evaluated in large prospective studies, recipient pretransplant statin use may protect endothelial function during ischemia-reperfusion in the peritransplant period and preserve the kidney microvasculature.
The relative preservation of peritubular capillary density observed in recipients of donors after circulatory death compared with recipients of neurologically deceased donors may explain why DGF, although more common in recipients of donors after circulatory death, has less effect on long-term graft survival in this population (41). This may be due to the cytokine release that is associated with neurologic death and that activates the donor’s endothelium even before the organ is collected, making it more susceptible to ischemia-reperfusion injury (42). We hypothesize that the counterintuitive finding of preserved peritubular capillary density with longer cold ischemic time is due to the very strong association between shorter cold ischemic time and donor after circulatory arrest (5 versus 12 hours in those who received neurologically deceased donors) in patients who experienced DGF. Because of its association with impaired endothelial function (43,44), donor hypertension might also predispose the microvascular network to ischemia-reperfusion injury, explaining the association we observed between the latter and microvascular rarefaction.
Our study is limited by its retrospective nature, thus remaining hypothesis generating. It is a single-center study, with relatively low rates of graft failure in study participants and in the full cohort of patients, which may affect the generalizability. The sample size precluded the inclusion of more independent variables to better control for residual confounding, especially in the subgroup analysis of patients with DGF. The lack of precision in the assessment of interstitial fibrosis through semiquantitative Banff scores may explain why we could not detect independent associations between interstitial fibrosis and 1- or 3-year eGFR. Hence, given the observational nature of the study, we cannot exclude residual confounding nor a potential selection bias because a substantial number of eligible patients had no post- or pretransplant biopsies. Nevertheless, baseline characteristics and 1- and 3-year graft function were similar in patients with and without post-transplant biopsies. The reasons for unavailable pretransplant material (material lost/unavailable/biopsy performed at another center) were unlikely to be associated with differential peritubular capillary density changes.
In conclusion, lower donor peritubular capillary density at the time of transplantation is associated with DGF, which in turn, is associated with further loss of peritubular capillaries. Microvascular rarefaction and glomerulosclerosis at the time of transplantation, as well as peritubular capillary loss after transplantation, are associated with reduced subsequent graft function. Collectively, these results suggest that peritubular capillary rarefaction plays a role in the AKI to CKD transition. Biomarkers of microvascular injury or noninvasive assessment of the microcirculation will be needed to better diagnose and test interventions aimed at preserving kidney function in transplant patients and more generally, after AKI.
Disclosures
F. Azzi reports employment with the Centre de recherche du Centre hospitalier de l'Université de Montréal (CRCHUM) and Institut du cancer de Montréal. H. Cardinal reports that Astellas provides funding of $50,000/yr for the maintenance of the kidney transplant biobank at the Centre Hospitalier de l’Université de Montreal (CHUM); this biobank is one of the Canadian Donation and Transplantation Research Program (CDTRP)–affiliated biobanks. H. Cardinal, M. Dieudé, and M.-J. Hébert are CDTRP investigators. H. Cardinal, M.-J. Hébert, and D. Trudel are research scholars of the Fonds de Recherche du Québec santé. M. Dieudé reports employment with Université de Montréal and the CDTRP, where she serves as executive director. M.-J. Hébert reports employment with Université de Montréal and serving as a scientific advisor or member of the Institute of Research in Vegetal Biology; Montreal Institute for Learning Algorithms, the Quebec Artificial Intelligence Institute; and Ste-Justine Hospital. M.-J. Hébert is the Shire Chair in Nephrology, Transplantation and Renal Regeneration, Université de Montréal. A. Karakeussian-Rimbaud reports employment with CRCHUM. S. Larivière-Beaudoin reports employment with CRCHUM and the CDTRP. D. Trudel reports employment with CHUM. All remaining authors have nothing to disclose.
Funding
This work was supported by Kidney Foundation of Canada research grant KFOC 160003 (to H. Cardinal) and Canadian Institutes of Health Research grant PJT-148884 (to H. Cardinal).
Data Sharing Statement
Denominalized clinical data may be shared if the study investigators, the local ethics review board, and the legal department of Centre de Recherche du Centre hospitalier de l'Université de Montréal agree.
Supplementary Material
Acknowledgments
The authors thank Mrs. Véronique Barrès, Mrs. Gabriela Fragoso, and Mrs. Liliane Meunier of the molecular pathology core facility of the Centre de Recherche du Centre hospitalier de l'Université de Montréal for performing the sections, immunohistochemistry, and slide scanning and the facility core for image analysis with the Visiopharm image software. They thank Mrs. Aurélie Cleret-Buhot for her help with the image analysis software. The authors also thank Dr. Kejia Zhang from the Department of Plastic and Cosmetic Surgery in Changchun, China, who helped with the schematic drawing. The authors thank the J.-L. Lévesque Foundation for renewed support.
Access to Dr. Féryel Azzi’s expertise is possible thanks to the TransMedTech Institute and its primary funding partner, the Canada First Research Excellence Fund. Dr. Alice Doreille's expertise was made possible through a research fellowship from Agence Régionale de Santé Ile de France.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07270520/-/DCSupplemental.
Supplemental Appendix. Detailed methods.
Supplemental Table 1. Characteristics of patients with and without post-transplant biopsies.
Supplemental Table 2. Pretransplant peritubular capillary density and clinical outcome 1 and 3 years post-transplant according to the various definitions of delayed graft function
Supplemental Table 3. Associations between recipient, donor, and procedure characteristics on peritubular capillary density change in univariable analyses.
Supplemental Table 4. Associations of delayed graft function and other clinical characteristics with change in peritubular capillary density: independent blinded validation using readings from a trained pathologist (F.A.; n=191).
Supplemental Table 5. Associations between recipient, donor, and procedure characteristics on eGFR 1 year post-transplant in univariable analyses.
Supplemental Table 6. Associations between recipient, donor, and procedure characteristics and eGFR 3 years post-transplant in univariable analyses.
Supplemental Table 7. Factors associated with eGFR 3 years post-transplant.
Supplemental Table 8. Associations between recipient, donor, and procedure characteristics and peritubular capillary density change in patients with DGF change in univariable analyses (n=61).
Supplemental Table 9. Initial multivariable model for factors associated with change in peritubular capillary density among patients with delayed graft function (n=61).
Supplemental Table 10. Factors associated with change in peritubular capillary density among patients with immediate graft function (n=130).
References
- 1.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basile DP, Yoder MC: Renal endothelial dysfunction in acute kidney ischemia reperfusion injury. Cardiovasc Hematol Disord Drug Targets 14: 3–14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bábíčková J, Klinkhammer BM, Buhl EM, Djudjaj S, Hoss M, Heymann F, Tacke F, Floege J, Becker JU, Boor P: Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int 91: 70–85, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA: Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol 300: F721–F733, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon O, Hong S-M, Sutton TA, Temm CJ: Preservation of peritubular capillary endothelial integrity and increasing pericytes may be critical to recovery from postischemic acute kidney injury. Am J Physiol Renal Physiol 295: F351–F359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basile DP, Donohoe D, Roethe K, Osborn JL: Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Basile DP: The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int 72: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Ehling J, Bábíčková J, Gremse F, Klinkhammer BM, Baetke S, Knuechel R, Kiessling F, Floege J, Lammers T, Boor P: Quantitative micro-computed tomography imaging of vascular dysfunction in progressive kidney diseases. J Am Soc Nephrol 27: 520–532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang D-H, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ: Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto M, Tanaka T, Yamamoto T, Noiri E, Miyata T, Inagi R, Fujita T, Nangaku M: Hypoperfusion of peritubular capillaries induces chronic hypoxia before progression of tubulointerstitial injury in a progressive model of rat glomerulonephritis. J Am Soc Nephrol 15: 1574–1581, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Ohashi R, Kitamura H, Yamanaka N: Peritubular capillary injury during the progression of experimental glomerulonephritis in rats. J Am Soc Nephrol 11: 47–56, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Ohashi R, Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, Yamanaka N: Peritubular capillary regression during the progression of experimental obstructive nephropathy. J Am Soc Nephrol 13: 1795–1805, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Yang B, Lan S, Dieudé M, Sabo-Vatasescu JP, Karakeussian-Rimbaud A, Turgeon J, Qi S, Gunaratnam L, Patey N, Hébert MJ: Caspase-3 is a pivotal regulator of microvascular rarefaction and renal fibrosis after ischemia-reperfusion injury. J Am Soc Nephrol 29: 1900–1916, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menshikh A, Scarfe L, Delgado R, Finney C, Zhu Y, Yang H, de Caestecker MP: Capillary rarefaction is more closely associated with CKD progression after cisplatin, rhabdomyolysis, and ischemia-reperfusion-induced AKI than renal fibrosis. Am J Physiol Renal Physiol 317: F1383–F1397, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindenmeyer MT, Kretzler M, Boucherot A, Berra S, Yasuda Y, Henger A, Eichinger F, Gaiser S, Schmid H, Rastaldi MP, Schrier RW, Schlöndorff D, Cohen CD: Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol 18: 1765–1776, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Bohle A, Mackensen-Haen S, Wehrmann M: Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res 19: 191–195, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Kaukinen A, Lautenschlager I, Helin H, Karikoski R, Jalanko H: Peritubular capillaries are rarefied in congenital nephrotic syndrome of the Finnish type. Kidney Int 75: 1099–1108, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Namikoshi T, Satoh M, Horike H, Fujimoto S, Arakawa S, Sasaki T, Kashihara N: Implication of peritubular capillary loss and altered expression of vascular endothelial growth factor in IgA nephropathy. Nephron, Physiol 102: 9–16, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Basile DP: The case for capillary rarefaction in the AKI to CKD progression: Insights from multiple injury models. Am J Physiol Renal Physiol 317: F1253–F1254, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Yarlagadda SG, Coca SG, Formica RN Jr., Poggio ED, Parikh CR: Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol Dial Transplant 24: 1039–1047, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Cavaillé-Coll M, Bala S, Velidedeoglu E, Hernandez A, Archdeacon P, Gonzalez G, Neuland C, Meyer J, Albrecht R: Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant 13: 1134–1148, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Lapointe I, Lachance J-G, Noël R, Côté I, Caumartin Y, Agharazii M, Houde I, Rousseau-Gagnon M, Kim SJ, De Serres SA: Impact of donor age on long-term outcomes after delayed graft function: 10-year follow-up. Transpl Int 26: 162–169, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Ishii Y, Sawada T, Kubota K, Fuchinoue S, Teraoka S, Shimizu A: Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int 67: 321–332, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Steegh FMEG, Gelens MACJ, Nieman FHM, van Hooff JP, Cleutjens JP, van Suylen RJ, Daemen MJ, van Heurn EL, Christiaans MH, Peutz-Kootstra CJ: Early loss of peritubular capillaries after kidney transplantation. J Am Soc Nephrol 22: 1024–1029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuart LN: CD Markers. CD34, 2002. Available at: http://www.pathologyoutlines.com/topic/cdmarkerscd34.html. Accessed June 19, 2018
- 26.Shaffi K, Uhlig K, Perrone RD, Ruthazer R, Rule A, Lieske JC, Navis G, Poggio ED, Inker LA, Levey AS: Performance of creatinine-based GFR estimating equations in solid-organ transplant recipients. Am J Kidney Dis 63: 1007–1018, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humar A, Ramcharan T, Kandaswamy R, Gillingham K, Payne WD, Matas AJ: Risk factors for slow graft function after kidney transplants: A multivariate analysis. Clin Transplant 16: 425–429, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Yang B, Dieudé M, Hamelin K, Hénault-Rondeau M, Patey N, Turgeon J, Lan S, Pomerleau L, Quesnel M, Peng J, Tremblay J, Shi Y, Chan JS, Hébert MJ, Cardinal H: Anti-LG3 antibodies aggravate renal ischemia-reperfusion injury and long-term renal allograft dysfunction. Am J Transplant 16: 3416–3429, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, Parikh CR: Marked variation in the definition and diagnosis of delayed graft function: A systematic review. Nephrol Dial Transplant 23: 2995–3003, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loupy A, Haas M, Solez K, Racusen L, Glotz D, Seron D, Nankivell BJ, Colvin RB, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell L, Drachenberg C, Dragun D, de Kort H, Gibson IW, Kraus ES, Lefaucheur C, Legendre C, Liapis H, Muthukumar T, Nickeleit V, Orandi B, Park W, Rabant M, Randhawa P, Reed EF, Roufosse C, Seshan SV, Sis B, Singh HK, Schinstock C, Tambur A, Zeevi A, Mengel M: The Banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant 17: 28–41, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Legendre C, Canaud G, Martinez F: Factors influencing long-term outcome after kidney transplantation. Transpl Int 27: 19–27, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Boom H, Mallat MJ, de Fijter JW, Zwinderman AH, Paul LC: Delayed graft function influences renal function, but not survival. Kidney Int 58: 859–866, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Lipták P, Kemény E, Morvay Z, Szederkényi E, Szenohradszky P, Marofka F, Toldi J, Exner M, Iványi B: Peritubular capillary damage in acute humoral rejection: An ultrastructural study on human renal allografts. Am J Transplant 5: 2870–2876, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Regele H, Böhmig GA, Habicht A, Gollowitzer D, Schillinger M, Rockenschaub S, Watschinger B, Kerjaschki D, Exner M: Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: A contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol 13: 2371–2380, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Rosenson RS, Tangney CC: Antiatherothrombotic properties of statins: Implications for cardiovascular event reduction. JAMA 279: 1643–1650, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Wolfrum S, Jensen KS, Liao JK: Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol 23: 729–736, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Lewicki M, Ng I, Schneider AG: HMG CoA reductase inhibitors (statins) for preventing acute kidney injury after surgical procedures requiring cardiac bypass. Cochrane Database Syst Rev 3: CD010480, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Layton JB, Hansen MK, Jakobsen C-J, Kshirsagar AV, Andreasen JJ, Hjortdal VE, Rasmussen BS, Simpson RJ, Brookhart MA, Christiansen CF: Statin initiation and acute kidney injury following elective cardiovascular surgery: A population cohort study in Denmark. Eur J Cardiothorac Surg 49: 995–1000, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Tuuminen R, Nykänen AI, Saharinen P, Gautam P, Keränen MA, Arnaudova R, Rouvinen E, Helin H, Tammi R, Rilla K, Krebs R, Lemström KB: Donor simvastatin treatment prevents ischemia-reperfusion and acute kidney injury by preserving microvascular barrier function. Am J Transplant 13: 2019–2034, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Reiling J, Johnson DW, Kruger PS, Pillans P, Wall DR: Association of pre-transplant statin use with delayed graft function in kidney transplant recipients. BMC Nephrol 13: 111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh RP, Farney AC, Rogers J, Zuckerman J, Reeves-Daniel A, Hartmann E, Iskandar S, Adams P, Stratta RJ: Kidney transplantation from donation after cardiac death donors: Lack of impact of delayed graft function on post-transplant outcomes. Clin Transplant 25: 255–264, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Bouma HR, Ploeg RJ, Schuurs TA: Signal transduction pathways involved in brain death-induced renal injury. Am J Transplant 9: 989–997, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Brandes RP: Endothelial dysfunction and hypertension. Hypertension 64: 924–928, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Dinh QN, Drummond GR, Sobey CG, Chrissobolis S: Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. BioMed Res Int 2014: 406960, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.