Abstract
Enteric hyperoxaluria is a distinct entity that can occur as a result of a diverse set of gastrointestinal disorders that promote fat malabsorption. This, in turn, leads to excess absorption of dietary oxalate and increased urinary oxalate excretion. Hyperoxaluria increases the risk of kidney stones and, in more severe cases, CKD and even kidney failure. The prevalence of enteric hyperoxaluria has increased over recent decades, largely because of the increased use of malabsorptive bariatric surgical procedures for medically complicated obesity. This systematic review of enteric hyperoxaluria was completed as part of a Kidney Health Initiative–sponsored project to describe enteric hyperoxaluria pathophysiology, causes, outcomes, and therapies. Current therapeutic options are limited to correcting the underlying gastrointestinal disorder, intensive dietary modifications, and use of calcium salts to bind oxalate in the gut. Evidence for the effect of these treatments on clinically significant outcomes, including kidney stone events or CKD, is currently lacking. Thus, further research is needed to better define the precise factors that influence risk of adverse outcomes, the long-term efficacy of available treatment strategies, and to develop new therapeutic approaches.
Keywords: chronic kidney disease, hyperoxaluria, fat malabsorption, nephrolithiasis
Introduction
Hyperoxaluria is an important pathogenic factor in the formation of calcium oxalate kidney stones (hereafter referred to simply as stones), the most common type that occur in humans. Marked hyperoxaluria can result from genetic causes of hepatic overproduction (primary hyperoxaluria), or through gastrointestinal (GI) conditions causing fat malabsorption and increased oxalate absorption in the GI tract (enteric hyperoxaluria). Both primary hyperoxaluria and enteric hyperoxaluria are associated not only with stone formation, but may progress to crystal nephropathy, CKD, and kidney failure. Novel treatments for both conditions are rapidly emerging, but, because both are relatively rare conditions, there is a paucity of clinical trial data at this time. A workgroup within the Oxalosis and Hyperoxaluria Foundation (OHF), in coordination with the Kidney Health Initiative (KHI), was formed in 2017 to define possible trial end points, with the objective of expanding treatment options for enteric hyperoxaluria. As a first step in that process, a multidisciplinary workgroup performed a systematic review of the literature on the pathophysiology and current treatment options for enteric hyperoxaluria; this document is a summary of this initial endeavor.
Pathophysiology of Enteric Hyperoxaluria
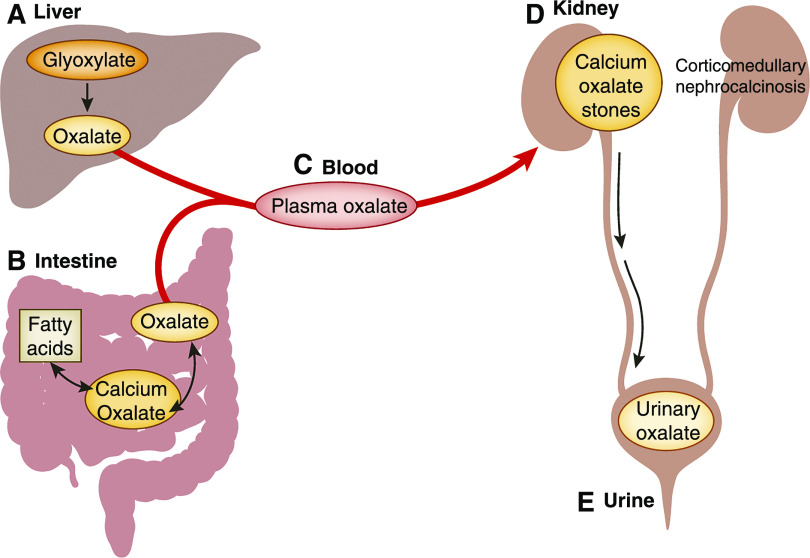
Oxalate arises from a variety of dietary and endogenous sources and is an end product of human metabolism. While a small fraction of ingested oxalate is absorbed under normal circumstances (typically 5%–10%), the majority of urine oxalate is derived from endogenous hepatic production (1,2). Any oxalate absorbed from the diet or endogenously produced is delivered to the kidney, where it is freely filtered and excreted as urinary oxalate (3). An increase in either GI oxalate absorption or hepatic oxalate production increases plasma oxalate and thus urinary oxalate, and contributes to the risk of stone formation and other adverse kidney outcomes, such as nephrocalcinosis (Figure 1) (4).
Figure 1.
Urinary oxalate is derived from a combinaiton of hepatic metabolism and gastrointestinal absorption in enteric hyperoxaluria. (A) Oxalate is an end product of glyoxalate metabolism in the liver. The remainder of systemic oxalate comes from ingestion of certain fruits and vegetables. The oxalate in these plants is in the form of relatively insoluble calcium oxalate crystals. Hence, under normal conditions, only 5%–10% of ingested oxalate is absorbed and the remainder passes into the stool. However, in patients with fat malabsorption of any cause (enteric hyperoxaluria), undigested fatty acids reach the large intestine where they can combine with calcium; (B) the net effect is to release more free oxalate into the intestinal lumen. It is thought that this free oxalate is largely absorbed paracellularly in the large intestine. Due to the fat malabsorption, among patients with enteric hyperoxaluria, the net percentage of ingested oxalate that ends up being absorbed can increase to ≥30%. Any oxalate that comes from the liver or is absorbed from the diet is delivered by (C) the blood to (D) the kidneys, where it is excreted via a combination of glomerular filtration and proximal tubular secretion. The increased delivery of oxalate to the kidneys leads to (E) increased urinary oxalate excretion, which in turn places these patients at risk of calcium oxalate kidney stones, corticomedullary nephrocalcinosis, and oxalate nephropathy.
Enteric hyperoxaluria can result from increased bioavailability of dietary oxalate or increased GI oxalate permeability. Decreased intestinal secretion of oxalate into the gut lumen has also been associated with hyperoxaluria in animal models (5,6). The bioavailability of dietary oxalate is, in part, determined by the calcium content of ingested food because calcium can form insoluble calcium oxalate salts in the intestine lumen that are eliminated in the stool. An increase in the dietary intake of free fatty acids, or their malabsorption in the small intestine, can result in increased fat delivery to the colon where it can bind dietary calcium, thus increasing free oxalate that can be absorbed into the bloodstream. Bile salts and fatty acids not only determine the bioavailability of soluble oxalate in the intestine, they can also increase the local membrane permeability in the bowel and thereby facilitate oxalate absorption (7). Thus, the net effect of fat malabsorption from any cause is increased absorption of luminal oxalate into the blood.
Oxalate homeostasis could potentially be affected by the gut microbiota and genetic anomalies. Research over the past 20 years has established the role of the gut microbiome in oxalate homeostasis (8,9). Certain bacterial strains, including Oxalobacter spp., Bifidobacterium spp., and Lactobacillus spp., can degrade oxalate and may be capable of modulating intestinal oxalate secretion (6,10). Additionally, animal studies suggest that inherited defects in the SLC6 family of anion exchangers could predispose to enteric hyperoxaluria (11,12). However, the role of either in human disease remains to be defined.
Causes of Enteric Hyperoxaluria
Enteric hyperoxaluria is frequently observed in conjunction with digestive diseases that affect fat absorption such as Crohn's disease, cystic fibrosis, chronic biliary disease, pancreatic pathologies, and short bowel syndrome (13). Chronic diarrhea associated with fat malabsorption may result in a low urine volume, hypocitraturia, and hypomagnesuria, which further increases the risk for stone formation.
Surgical procedures used for weight loss have been linked with enteric hyperoxaluria and higher stone risk due to altered fat absorption. The jejunoileal bypass procedure was almost universally associated with enteric hyperoxaluria but, due to this and other complications, was abandoned by the early 1980s (14). In the intervening years, that procedure was largely replaced by the Roux-en-Y gastric bypass (RYGB) and biliopancreatic diversion procedures, which can also result in enteric hyperoxaluria, calcium oxalate kidney stones, and oxalate nephropathy. Nondiverting procedures for bariatric surgery (i.e., restrictive), such as sleeve gastrectomy, appear to only rarely cause enteric hyperoxaluria and are not associated with a markedly higher risk of kidney stones (15). Over the last 10 years, use of sleeve gastrectomy has increased and RYGB has decreased; consequently, the incidence of enteric hyperoxaluria associated with bariatric surgery may fall over time. Nevertheless, tens of thousands of RYGB surgeries are still performed annually, and the numbers of at-risk patients previously treated with RYGB and other malabsorptive procedures will be substantial for decades to come.
A recent study demonstrated a higher risk for nephrolithiasis associated with use of antibiotics (16) that can alter the gut microbiome (17). Because good observational evidence backs up the potential role of Oxalobacter, Bifidobacterium, Lactobacillus, and other gut microbiota in maintaining oxalate homeostasis, this correlation has validity, but more studies are needed to establish the cause and effect of this relationship and the exact mechanism(s) involved.
Prevalence of Enteric Hyperoxaluria in the United States
The prevalence of enteric hyperoxaluria and its major complications (kidney stones and CKD) remains poorly defined. The total prevalence of patients in the United States in 2019 with enteric conditions associated with kidney failure and/or recurrent kidney stones is estimated at 250,000. The most common causes were RYGB surgery for obesity (approximately 60%), inflammatory bowel disease (approximately 20%), celiac disease (8%), and chronic pancreatitis (4%).
Nephrolithiasis in Enteric Hyperoxaluria
Kidney stones are a well-recognized complication of enteric hyperoxaluria (18), and urinary oxalate excretion appears to correlate with stone risk. In a study of 762 patients after RYGB, urinary oxalate increased over time and was highest in patients who developed stones (63 mg/d in stone formers compared with 42 mg/d in non-stone formers, P<0.001) (15). In another series, stone formation risk was also increased after RYGB (odds ratio, 1.4; 95% confidence interval [95% CI], 1.1 to 1.8; P=0.006), and those with stones had a greater increase in urinary oxalate excretion (19). Finally, an analysis of seven studies including 277 patients before and after RYGB identified a 50% increase in urinary oxalate as one of the key lithogenic risk factors after RYGB (20).
The reported stone incidence 10 months to 6 years after RYGB ranges from 0.02% to 18% (Table 1). In studies where preoperative and nonoperative control data were reported, stone incidence was up to 7% higher postoperatively compared with preoperatively, and 3%–7% higher compared with subjects who were obese and who did not undergo surgery. A recent meta-analysis reported the pooled relative risk of stone formation from RYGB to be 1.79 (95% CI, 1.54 to 2.10) (21). After procedures that cause more pronounced malabsorption (i.e., very long limb RYGB or biliopancreatic diversion), patients have a five-fold greater risk of stones compared with nonoperative controls who are obese (15). In a detailed literature review of 24 studies that, in aggregate, included 6777 patients who had undergone bariatric surgery and had a stone history and 7089 non–stone forming controls, the incidence of stones was two-fold higher in the non-stone formers (9%) and four-fold higher in those with a previous history of stones (17%) (20).
Table 1.
Published studies reporting kidney stone rates in patients with enteric hyperoxaluria due to bariatric surgery
| Article | Population | Stone Incidence | ||
|---|---|---|---|---|
| Bariatric Surgery | Comparison Group | P Value | ||
| Prospective | ||||
| Valezi et al. (19) | 151 obese patients who underwent RYGB | 1 yr postop, 18% | Preop, 11% | 0.001 |
| Retrospective | ||||
| Lieske et al. (15) | 762 patients who underwent bariatric surgery and matched control individuals, who were equally obese, without surgery | 6 yr postop, 11% | No surgery, 4% | <0.001 |
| Matlaga et al. (83) | 4639 patients who underwent RYGB surgery, and a control group of 4639 patients who were obese and who did not have surgery, in a national private insurance claims database in a 5-year period (2002–2006) | 4.6 yr postop, 8% | No surgery, 5% | <0.001 |
| Costa-Matos et al. (84) | 58 Brazilian patients who underwent RYGB between 2000 and 2005 | 10 mo to 6 yr postop, 0.02% | Preop, 0.02% | N/A |
| Durrani et al. (85) | 972 patients who underwent RYGB | 2.4 yr postop, 6% overall, 3% de novo, and 31% of patients with prior stones | Preop, 9% | N/A |
RYGB, Roux-en-Y gastric bypass; postop, postoperative; preop, preoperative; N/A, not applicable.
Malabsorptive conditions other than bariatric surgery are also associated with kidney stone risk, with the reported stone incidence ranging from 2% to 38% across studies (Table 2). Stone incidence is 35% in patients with inflammatory bowel disease and hyperoxaluria, compared with 23% in patients with inflammatory bowel disease who have normal urinary oxalate excretion (22). Among patients who undergo bowel resection, the incidence of stones correlated with the length of resection (23,24). However, the segment of bowel removed is important, because loss of small intestine favors fat malabsorption, whereas the pathologic oxalate absorption associated with enteric hyperoxaluria occurs largely in the colon. Thus, patients who have resection of the colon, for example for ulcerative colitis, are at low risk for enteric hyperoxaluria.
Table 2.
Published studies reporting kidney stone rates in patients with enteric hyperoxaluria associated with other malabsorptive conditions
| Article | Population | Stone Incidence |
|---|---|---|
| Prospective | ||
| Caudarella et al. (86) | 66 patients with Crohn's disease or ulcerative colitis. Patients with Crohn's disease had ileal resection and right hemicolectomy with end-end ileotransversostomy. Patients with ulcerative colitis had proctocolectomy with ileal pouch anal anastomosis | Preop, 8% |
| Postop, 12% | ||
| Anderson et al. (23) | 107 patients with Crohn's disease receiving various bowel resections | Preop, 2% |
| Postop, 10% | ||
| Hylander et al. (22) | 87 patients with chronic IBD, stratified by kidney oxalate excretion. 26 patients had increased urinary oxalate, while 61 patients had normal urinary oxalate | Normal urinary oxalate, 23% |
| Hyperoxaluric, 35% | ||
| Retrospective | ||
| McAuliffe et al. (87) | 33,386 patients with IBD, stratified by severity of disease, followed for at least 6 mo | Incidence per 1000 person-years (95% CI): |
| Mild IBD, 13.5 (12.5 to 14.6) | ||
| Moderate-severe IBD, 10.8 (8.80 to 13.2) | ||
| Cury et al. (88) | 168 Brazilian patients with IBD (93 with Crohn's disease, 75 with ulcerative colitis) | Crohn's disease, 38% |
| Ulcerative colitis, 38% | ||
| Siener et al. (64) | 51 patients with fat malabsorption due to intestinal diseases such as Crohn's disease, exocrine pancreatic insufficiency, short bowel syndrome, and primary biliary cholangitis | Overall, 20% |
| Pancreatic insufficiency, 29% | ||
| Crohn's disease, 13% | ||
| Hueppelshaeuser et al. (89) | 27 children and 19 adults with Crohn's disease | 28% |
| Mukewar et al. (90) | 218 patients with ileal pouch anal anastomosis | 37% |
| Repiso et al. (91) | 157 patients diagnosed with Crohn's disease in Spain | 8% |
| Ben-Ami et al. (92) | 312 patients with Crohn's disease in Israel, followed for 7.2 yr | 4% |
| Christodoulou et al. (93) | 256 patients with IBD in Greece, followed for 8.1 yr | Crohn's disease, 5% |
| Ulcerative colitis, 6% | ||
| Nightingale et al. (94) | 86 patients with <200 cm of residual small bowel after resection, followed for 22 mo | With colon, 24% |
| Without colon, 0% | ||
| Trnka et al. (95) | 113 patients with IBD after resections, followed for 19.8 yr | 19% |
| Bambach et al. (24) | 426 patients after bowel surgery, stratified by procedure, followed for 8 yr | Overall, 9% |
| Resection with intact colon, 7% | ||
| Ileostomy only, 9% | ||
| Ileostomy with resection, 15% | ||
| Knudsen et al. (96) | 228 patients with chronic IBD | 15% |
| Maratka et al. (97) | 512 patients with ulcerative colitis in Czechoslovakia | Overall, 2% |
| After resection, 13% | ||
95% confidence interval, 95% CI; preop, preoperative; postop, postoperative; IBD, inflammatory bowel disease.
Compared with age-matched controls without cystic fibrosis (1%–2%), 3%–6% of patients with cystic fibrosis have nephrolithiasis. Suggested mechanisms include hyperoxaluria due to fat malabsorption, hypocitraturia, antibiotic exposure, and lack of Oxalobacter formigenes colonization (25). Most recently, in a mouse model of cystic fibrosis, SLC26A6 expression and SLC26A6-mediated intestinal oxalate secretion was defective and was associated with a 2.5-fold increase in urine oxalate excretion and plasma oxalate concentration (26).
Other Kidney Complications and Natural History
It is increasingly apparent that urinary oxalate is a risk factor for other kidney pathologies, including AKI and CKD (27–30). AKI occurs after patients consume ethylene glycol, which is hepatically metabolized to oxalate, or with heavy consumption of fruits such as rhubarb and star fruit (both very oxalate rich) (31).
Calcium oxalate crystals can precipitate within kidney tubules, bind to epithelial cells, and cause obstruction. Attached crystals can be phagocytosed and transcytosed into the kidney interstitium (32). The resulting release of inflammatory mediators can contribute to oxalate nephropathy and the potential for progressive loss of kidney function (33). Evidence in animal studies suggests that the inflammation induced by calcium oxalate crystals could be mediated by the NLRP3 inflammasome, the same pathway of crystal-induced inflammation observed in gout (uric acid) and arteriosclerosis (cholesterol) (34). Stimulation of TGF-β release by NLRP3 is associated with kidney fibrosis and progressive loss of kidney function in animal models of hyperoxaluria (35). Similarly, kidney biopsy specimens of patients after RYGB show diffuse tubular degenerative changes, abundant tubular calcium oxalate deposits, and various degrees of tubulointerstitial scarring (28).
Oxalate nephropathy in enteric hyperoxaluria can lead to progressive kidney deterioration and, eventually, kidney failure requiring dialysis (27–29,36,37). Patients with obesity syndromes necessitating bariatric surgery often suffer from underlying CKD (27,38). Thus, postoperative hyperoxaluria and kidney stone formation place this population at further long-term risk for kidney failure. Patients with pancreatic insufficiency, as can occur in cystic fibrosis, can also develop kidney failure due to oxalate nephropathy, particularly if they are nonadherent with pancreatic enzyme replacement therapy (39).
When GFR falls below 30–40 ml/min per 1.73 m2, plasma oxalate levels increase. This, in turn, can promote calcium oxalate crystallization and deposition in extrarenal tissues, a process termed systemic oxalosis (40). Rarely, patients with CKD and severe secondary hyperoxaluria can present with systemic crystal deposition in the retina, joints, and skin, particularly when consuming high oral doses of vitamin C, which is metabolized to oxalate (41,42).
After patients receive a kidney transplant for kidney failure due to oxalate nephropathy, they remain at risk for recurrent oxalate nephropathy in the allograft (43). In a small report of three patients with enteric hyperoxaluria who developed kidney failure requiring transplantation, two developed recurrent oxalate nephropathy and one required a second transplant (37).
Finally, in addition to the attendant kidney risks, there is increasing evidence that hyperoxaluria may be a factor in the pathogenesis of cardiovascular complications. Elevated plasma oxalate has been associated with reactive cardiac fibrosis, increased vascular calcification, and hemodynamic effects such as reduced pulse wave velocity and central aortic BPs (44–47). Thus, the effect of enteric hyperoxaluria likely extends far beyond stones.
Current Therapies
There are no specific therapies for enteric hyperoxaluria. Current management includes treating the underlying disease with the goal of reducing fat malabsorption. Because that is not always possible or sufficient, measures to reduce GI absorption of oxalate (and hence its excretion through kidneys) and/or measures to reduce calcium oxalate crystallization in the urine and tubular fluid are needed. Examples include reducing dietary consumption of fat or oxalate, using calcium supplements to bind oxalate in the gut, and increasing daily fluid intake to reduce calcium oxalate concentration. Finally, other treatments such as bile acids or bile acid sequestrants, probiotics, oxalate-degrading enzymes, and calcium supplements have also been studied.
Treatment of the Underlying Disease
Whenever possible, treatment of the underlying GI disease is critical for reducing fat malabsorption and resultant enteric hyperoxaluria. There are a large number of patients with morbid obesity who underwent malabsorptive bariatric surgery over the last 3 decades and are at risk for developing profound hyperoxaluria and kidney failure (29,48). If oxalate nephropathy develops, reversal of the bypass may result in improved kidney function and reduction in oxalate excretion, although reversal can carry significant risks and is rarely done (49,50).
In patients with celiac disease, treatment with a gluten-free diet lowers stone incidence comparably to control levels (51). Among patients with pancreatic insufficiency, such as secondary to cystic fibrosis, sustained use of pancreatic enzyme therapy reduces steatorrhea and thus may prevent stone formation (52). In inflammatory bowel disease, enteric hyperoxaluria can be prevented both by medical therapy to reduce disease activity, and avoiding, if possible, surgical resection of the small intestine (53).
Dietary Measures
Increased fluid intake, a general stone-prevention tool, is a standard component of enteric hyperoxaluria treatment. However, patients with enteric hyperoxaluria may have short gut or diarrhea, which limits the amount of fluid intake they may tolerate. Low-oxalate diets are an obvious treatment strategy (50,54). Although studies that used a low-oxalate diet (<50 mg, or 0.57 mmol, per day) are small, many (55,56), but not all (54,57), suggest this strategy reduces urinary oxalate excretion. Most studies also incorporated other diet interventions, such as lower protein or sodium intake, which limits the ability to combine them for analysis. Although a low-fat, low-oxalate, moderate-protein, and normal-calcium diet has been demonstrated to reduce calcium oxalate supersaturation in enteric hyperoxaluria, this diet can be difficult for patients to maintain and may have a minimal effect on urinary oxalate excretion (50,54). One current recommendation is to lower daily oxalate intake to 50 mg with maintenance of normal dietary calcium, although this may be difficult to chronically maintain and accurate information about food oxalate content is not readily available (58).
Urinary oxalate excretion in enteric hyperoxaluria also correlates directly with the fat content of stool, and limiting fat intake has been shown to reduce urinary oxalate (59,60). A low-fat diet also improves steatorrhea, leading to less fecal fluid loss, which further diminishes the risk of stone formation.
Calcium supplements have been used to bind oxalate in the gut and thus promote excretion in the stool. Multiple small studies in the 1970s demonstrated this treatment reduced urinary oxalate in patients with diverse causes of enteric hyperoxaluria, without a significant increase in urinary calcium (61,62). Although this has theoretic benefit to reduce risk for oxalate nephropathy, the effectiveness of calcium supplementation to reduce stone formation in enteric hyperoxaluria remains to be demonstrated. Treatment algorithms currently suggest administration of elemental calcium, primarily when consuming oxalate-rich foods, and monitoring 24-hour urine oxalate and calcium excretion. Calcium citrate has superior bioavailability and a potential advantage for bone health compared with calcium carbonate (63), and patients with enteric hyperoxaluria often manifest hypocitraturia and may benefit from oral citrate therapy (59,64). However, it is unclear which calcium salt is a more effective oxalate binder (59,60).
Other Approaches
The use of natural, conjugated bile acids may benefit those with significant terminal ileum resection who are known to be deficient in bile acids, although definitive evidence is lacking for this approach (65). Older studies have demonstrated variable efficacy for the use of bile acid sequestrants (e.g., cholestyramine) to reduce urinary oxalate excretion in enteric hyperoxaluria (66–69).
Enzymatic degradation of oxalate in the GI tract using an oral preparation of oxalate decarboxylase is a newer potential treatment strategy (70). A phase 2, open-label trial in 16 participants with idiopathic and enteric hyperoxaluria found that oral oxalate decarboxylase taken for 4 days decreased urinary oxalate excretion by 14 mg/d, with a greater reduction observed in patients with enteric (−22.0 mg/d) versus idiopathic (−10.2 mg/d) hyperoxaluria (71). A phase 3 randomized controlled trial in patients with enteric hyperoxaluria treated for 28 days was recently completed, and a follow-up phase 3 randomized controlled trial will evaluate the effect of oral oxalate decarboxylase on stone recurrence in patients with enteric hyperoxaluria (NCT03847090). Future research may identify methods to manipulate oxalate transport in the colon and small intestine, but much work remains to better understand these pathways (72–74).
Future Directions and Knowledge Gaps
Although much has been learned about oxalate absorption from the GI tract over the last 50 years, many gaps remain. Overall, the literature is limited, and the majority of clinical studies have been small, single center, and nonrandomized. Further, there is no definitive evidence that reducing urinary oxalate decreases stone formation or preserves kidney function, despite the known pathophysiologic mechanisms that would suggest this should be beneficial. Enteric hyperoxaluria also has many different underlying etiologies, and controlled trials are limited. Hence, there is a significant gap in data on existing treatments for enteric hyperoxaluria, and future studies should aim to include larger populations in randomized controlled trials.
Mechanisms of Intestinal Oxalate Transport
Over the last 20 years, major progress has been made regarding the molecular identification of oxalate transporters. Several investigators have confirmed the SLC26A6 protein can actively secrete oxalate at the apical membrane of enterocytes, and loss of SLC26A6 results in hyperoxaluria and oxalate nephrolithiasis in animal models (11,12). Similar to SLC26A6, functional expression in Xenopus oocytes revealed that SLC26A1 can mediate oxalate transport (75,76). Moreover, one line of SLC26A1-knockout mice developed hyperoxalemia, hyperoxaluria, and calcium oxalate nephrolithiasis analogous to SLC26A6-knockout mice (77), whereas another strain did not (78). It has also been proposed that active, transcellular oxalate absorption occurs at the apical membrane via SLC26A3 (79). However, heterologous expression studies have demonstrated poor oxalate affinity of human SLC26A3 (80), and the lower urinary oxalate excretion of SLC23A3-knockout mice may be due to the diarrhea these mice manifest, rather than reduced transcellular oxalate absorption. Overall, to date, the exact molecular mechanisms of oxalate transport in the human intestine remain incompletely understood. Further studies are needed to identify the molecular identity and physiologic function of oxalate transporters and their importance in humans with enteric hyperoxaluria.
Potential Role of the Intestinal Microbiome in Oxalate Metabolism and Transport
Work over the last 2 decades has identified O. formigenes as an obligate anaerobe that degrades oxalate and stimulates oxalate absorption in the gut (5,81). However, use of O. formigenes as a therapeutic agent in humans has not reduced urine oxalate excretion or plasma oxalate concentration in clinical trials of patients with primary hyperoxaluria (5), and has not been extensively studied in patients with enteric hyperoxaluria. Humans are variably colonized, and epidemiologic evidence suggests associations of colonization with kidney stone risk in various patient populations. Emerging evidence suggests other elements of the intestinal microbiome might also participate in oxalate degradation, and maintaining biodiversity of the microbiome is important to maintain adequate oxalate metabolism (81,82).
Conclusion
Multiple GI disorders associated with fat malabsorption can cause oxalate overabsorption and result in enteric hyperoxaluria. This disorder is most commonly recognized in short bowel syndrome and after malabsorptive bariatric procedures (most often RYGB), but can also occur in inflammatory bowel disease and with pancreatic insufficiency of any cause. Enteric hyperoxaluria is associated with a higher risk of kidney stones and, less often, CKD. However, there is limited published literature to suggest uniformly effective measures to mitigate the consequences of enteric hyperoxaluria. Clearly more research and novel therapeutic approaches are needed, as well as careful analysis of appropriate end points for clinical trials.
Disclosures
M. Allain reports employment at the American Society of Nephrology. D. Assimos has been on editorial boards for Journal of Endourology, Journal of Urology, and Urolithiasis. M.A. Baum is on the scientific advisory boards for Dicerna, Dent Disease Foundation, Oxalosis/Hyperoxaluria Foundation, and Retrophin, and is a consultant for Retrophin. A. Kausz is employed by and has ownership interest in Allena Pharmaceuticals, Inc, which is developing a product for the treatment of enteric hyperoxalura. She is also on the KHI Board of Directors. F. Knauf is a consultant for Allena Pharmaceuticals, Alnylam Pharmaceuticals, and OxThera Pharmaceuticals, and receives funding from Dicerna Pharmaceuticals and Fresenius Medical Care. C.B. Langman is a consultant for Alexion Pharmaceuticals, Allena Pharmaceuticals, and Dicerna Pharmaceuticals, and receives researching funding from Lurie Children’s Hospital, Achillion and Alexicon Pharmaceuticals; Lurie Children’s Hospital of Chicago, Alexion; and Lurie Children’s Hospital, Ultragenyx. J.C. Lieske reports receiving grants and other from Allena, and reports serving on the advisory boards of Novobiom and Synlogic, during the conduct of the study. He is a consultant for Allena, Alnylam, American Board of Internal Medicine, Dicerna, Orfan, OxThera, Retrophin, and Siemens; receives funding from Allena, Alnylam, Dicerna, OxThera, Retrophin, and Siemens; and serves on the advisory board for Orfan. D. Milliner reports serving as consultant (contracts paid directly to Mayo Clinic) for Allena Pharmaceuticals, consultant (contracts paid directly to Mayo Clinic) and on the advisory committee for Alnylam Pharmaceuticals, consultant and chair of Data Safety and Monitoring Committtee (contracts paid directly to Mayo Clinic) for Dicerna Pharmaceuticals, consultant and chair of the Data Safety and Monitoring Board (contracts paid directly to Mayo Clinic) for OxThera Pharmaceuticals, member of Synologic Medical Advisory Panel (contracts paid directly to Mayo Clinic) for Synlogic, and on the editorial board for Urolithiasis. D. Milliner also reports receiving National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant U54-DK083908, outside the submitted work. G. Tasian is a consultant for Allena Pharmaceuticals; reports receiving grants from the Patient-Centered Outcomes Research Institute and NIDDK; and reports receiving personal fees from Allena Pharmaceuticals, Dicerna, and Novome, outside the submitted work. M. West reports employment at the American Society of Nephrology, serving as the Director of the KHI. KHI is a public-private partnership with the US Food and Drug Administration. M. West received no direct financial compensation. E. Worcester is a consultant for Allena, Alnylam, Dicerna, and OxThera. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
We would like to acknowledge Kim Hollander and Julie Bertarelli from the OHF (www.ohf.org) for their initiation of this project and continued support, along with the patients with enteric hyperoxaluria and their families who motivated this work.
This work was supported by the KHI, a public-private partnership between the American Society of Nephrology, the US Food and Drug Administration, and >100 member organizations and companies to enhance patient safety and foster innovation in kidney disease.
KHI funds were used to defray costs incurred during the conduct of the project, including project management support, which was expertly provided by American Society of Nephrology staff members Meaghan Allain and Melissa West. There was no honorarium or other financial support provided to KHI workgroup members. The authors of this paper had final review authority and are fully responsible for its content. KHI makes every effort to avoid actual, potential, or perceived conflicts of interest that may arise as a result of industry relationships or personal interests among the members of the workgroup. More information on KHI, the workgroup, or the conflict of interest policy can be found at www.kidneyhealthinitiative.org.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Holmes RP, Goodman HO, Assimos DG: Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int 59: 270–276, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Hesse A, Schneeberger W, Engfeld S, von Unruh GD, Sauerbruch T: Intestinal hyperabsorption of oxalate in calcium oxalate stone formers: Application of a new test with [13C2]oxalate. J Am Soc Nephrol 10: S329–S333, 1999 [PubMed] [Google Scholar]
- 3.Knauf F, Velazquez H, Pfann V, Jiang Z, Aronson PS: Characterization of renal NaCl and oxalate transport in Slc26a6-/- mice. Am J Physiol Renal Physiol 316: F128–F133, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curhan GC, Taylor EN: 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73: 489–496, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Arvans D, Jung YC, Antonopoulos D, Koval J, Granja I, Bashir M, Karrar E, Roy-Chowdhury J, Musch M, Asplin J, Chang E, Hassan H: Oxalobacter formigenes-derived bioactive factors stimulate oxalate transport by intestinal epithelial cells. J Am Soc Nephrol 28: 876–887, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatch M, Freel RW: A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis 41: 379–384, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobbins JW, Binder HJ: Effect of bile salts and fatty acids on the colonic absorption of oxalate. Gastroenterology 70: 1096–1100, 1976. [PubMed] [Google Scholar]
- 8.Kaufman DW, Kelly JP, Curhan GC, Anderson TE, Dretler SP, Preminger GM, Cave DR: Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol 19: 1197–1203, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denburg MR, Koepsell K, Lee JJ, Gerber J, Bittinger K, Tasian GE: Perturbations of the gut microbiome and metabolome in children with calcium oxalate kidney stone disease. J Am Soc Nephrol 31: 1358–1369, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight J, Deora R, Assimos DG, Holmes RP: The genetic composition of Oxalobacter formigenes and its relationship to colonization and calcium oxalate stone disease. Urolithiasis 41: 187–196, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freel RW, Hatch M, Green M, Soleimani M: Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719–G728, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS: Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Hylander E, Jarnum S, Jensen HJ, Thale M: Enteric hyperoxaluria: Dependence on small intestinal resection, colectomy, and steatorrhoea in chronic inflammatory bowel disease. Scand J Gastroenterol 13: 577–588, 1978. [DOI] [PubMed] [Google Scholar]
- 14.Requarth JA, Burchard KW, Colacchio TA, Stukel TA, Mott LA, Greenberg ER, Weismann RE: Long-term morbidity following jejunoileal bypass. The continuing potential need for surgical reversal. Arch Surg 130: 318–325, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Lieske JC, Mehta RA, Milliner DS, Rule AD, Bergstralh EJ, Sarr MG: Kidney stones are common after bariatric surgery. Kidney Int 87: 839–845, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tasian GE, Jemielita T, Goldfarb DS, Copelovitch L, Gerber JS, Wu Q, Denburg MR: Oral antibiotic exposure and kidney stone disease. J Am Soc Nephrol 29: 1731–1740, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi S, Goldfarb DS: The use of antibiotics and risk of kidney stones. Curr Opin Nephrol Hypertens 28: 311–315, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canales BK, Hatch M: Kidney stone incidence and metabolic urinary changes after modern bariatric surgery: Review of clinical studies, experimental models, and prevention strategies. Surg Obes Relat Dis 10: 734–742, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valezi AC, Fuganti PE, Junior JM, Delfino VD: Urinary evaluation after RYGBP: A lithogenic profile with early postoperative increase in the incidence of urolithiasis. Obes Surg 23: 1575–1580, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Canales BK, Gonzalez RD: Kidney stone risk following Roux-en-Y gastric bypass surgery. Transl Androl Urol 3: 242–249, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Upala S, Jaruvongvanich V, Sanguankeo A: Risk of nephrolithiasis, hyperoxaluria, and calcium oxalate supersaturation increased after Roux-en-Y gastric bypass surgery: A systematic review and meta-analysis. Surg Obes Relat Dis 12: 1513–1521, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Hylander E, Jarnum S, Frandsen I: Urolithiasis and hyperoxaluria in chronic inflammatory bowel disease. Scand J Gastroenterol 14: 475–479, 1979. [PubMed] [Google Scholar]
- 23.Andersson H, Bosaeus I, Fasth S, Hellberg R, Hultén L: Cholelithiasis and urolithiasis in Crohn’s disease. Scand J Gastroenterol 22: 253–256, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Bambach CP, Robertson WG, Peacock M, Hill GL: Effect of intestinal surgery on the risk of urinary stone formation. Gut 22: 257–263, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibney EM, Goldfarb DS: The association of nephrolithiasis with cystic fibrosis. Am J Kidney Dis 42: 1–11, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Knauf F, Thomson RB, Heneghan JF, Jiang Z, Adebamiro A, Thomson CL, Barone C, Asplin JR, Egan ME, Alper SL, Aronson PS: Loss of cystic fibrosis transmembrane regulator impairs intestinal oxalate secretion. J Am Soc Nephrol 28: 242–249, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troxell ML, Houghton DC, Hawkey M, Batiuk TD, Bennett WM: Enteric oxalate nephropathy in the renal allograft: An underrecognized complication of bariatric surgery. Am J Transplant 13: 501–509, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Nasr SH, D’Agati VD, Said SM, Stokes MB, Largoza MV, Radhakrishnan J, Markowitz GS: Oxalate nephropathy complicating Roux-en-Y gastric bypass: An underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol 3: 1676–1683, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson WK, Houghton SG, Milliner DS, Lieske JC, Sarr MG: Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: Potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis 1: 481–485, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Cartery C, Faguer S, Karras A, Cointault O, Buscail L, Modesto A, Ribes D, Rostaing L, Chauveau D, Giraud P: Oxalate nephropathy associated with chronic pancreatitis. Clin J Am Soc Nephrol 6: 1895–1902, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glew RH, Sun Y, Horowitz BL, Konstantinov KN, Barry M, Fair JR, Massie L, Tzamaloukas AH: Nephropathy in dietary hyperoxaluria: A potentially preventable acute or chronic kidney disease. World J Nephrol 3: 122–142, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan SR, Kok DJ: Modulators of urinary stone formation. Front Biosci 9: 1450–1482, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Knauf F, Asplin JR, Granja I, Schmidt IM, Moeckel GW, David RJ, Flavell RA, Aronson PS: NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int 84: 895–901, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurts C: A crystal-clear mechanism of chronic kidney disease. Kidney Int 84: 859–861, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Anders HJ, Suarez-Alvarez B, Grigorescu M, Foresto-Neto O, Steiger S, Desai J, Marschner JA, Honarpisheh M, Shi C, Jordan J, Müller L, Burzlaff N, Bäuerle T, Mulay SR: The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int 93: 656–669, 2018. [DOI] [PubMed] [Google Scholar]
- 36.Bobrowski AE, Langman CB: Hyperoxaluria and systemic oxalosis: Current therapy and future directions. Expert Opin Pharmacother 7: 1887–1896, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Nazzal L, Puri S, Goldfarb DS: Enteric hyperoxaluria: An important cause of end-stage kidney disease. Nephrol Dial Transplant 31: 375–382, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turgeon NA, Perez S, Mondestin M, Davis SS, Lin E, Tata S, Kirk AD, Larsen CP, Pearson TC, Sweeney JF: The impact of renal function on outcomes of bariatric surgery. J Am Soc Nephrol 23: 885–894, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Rankin AC, Walsh SB, Summers SA, Owen MP, Mansell MA: Acute oxalate nephropathy causing late renal transplant dysfunction due to enteric hyperoxaluria. Am J Transplant 8: 1755–1758, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Bhasin B, Ürekli HM, Atta MG: Primary and secondary hyperoxaluria: Understanding the enigma. World J Nephrol 4: 235–244, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nankivell BJ, Murali KM: Images in clinical medicine. Renal failure from vitamin C after transplantation. N Engl J Med 358: e4, 2008. [DOI] [PubMed] [Google Scholar]
- 42.D’Costa MR, Winkler NS, Milliner DS, Norby SM, Hickson LJ, Lieske JC: Oxalosis associated with high-dose vitamin C ingestion in a peritoneal dialysis patient. Am J Kidney Dis 74: 417–420, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karaolanis G, Lionaki S, Moris D, Palla VV, Vernadakis S: Secondary hyperoxaluria: A risk factor for kidney stone formation and renal failure in native kidneys and renal grafts. Transplant Rev (Orlando) 28: 182–187, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Gulhan B, Turkmen K, Aydin M, Gunay M, Cıkman A, Kara M: The relationship between serum oxalic acid, central hemodynamic parameters and colonization by Oxalobacter formigenes in hemodialysis patients. Cardiorenal Med 5: 164–174, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulay SR, Anders HJ: Crystal nephropathies: Mechanisms of crystal-induced kidney injury. Nat Rev Nephrol 13: 226–240, 2017. [DOI] [PubMed] [Google Scholar]
- 46.Salyer WR, Keren D: Oxalosis as a complication of chronic renal failure. Kidney Int 4: 61–66, 1973. [DOI] [PubMed] [Google Scholar]
- 47.Tomson CR, Channon SM, Parkinson IS, Morley AR, Lennard TW, Parrott NR, Laker MF: Plasma oxalate concentration and secondary oxalosis in patients with chronic renal failure. J Clin Pathol 41: 1107–1113, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel BN, Passman CM, Fernandez A, Asplin JR, Coe FL, Kim SC, Lingeman JE, Assimos DG: Prevalence of hyperoxaluria after bariatric surgery. J Urol 181: 161–166, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agrawal V, Wilfong JB, Rich CE, Gibson PC: Reversal of gastric bypass resolves hyperoxaluria and improves oxalate nephropathy secondary to Roux-en-Y gastric bypass. Case Rep Nephrol Dial 6: 114–119, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Penniston KL, Nakada SY: Effect of dietary changes on urinary oxalate excretion and calcium oxalate supersaturation in patients with hyperoxaluric stone formation. Urology 73: 484–489, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Ciacci C, Spagnuolo G, Tortora R, Bucci C, Franzese D, Zingone F, Cirillo M: Urinary stone disease in adults with celiac disease: Prevalence, incidence and urinary determinants. J Urol 180: 974–979, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Chidekel AS, Dolan TF Jr.: Cystic fibrosis and calcium oxalate nephrolithiasis. Yale J Biol Med 69: 317–321, 1996. [PMC free article] [PubMed] [Google Scholar]
- 53.Fagagnini S, Heinrich H, Rossel JB, Biedermann L, Frei P, Zeitz J, Spalinger M, Battegay E, Zimmerli L, Vavricka SR, Rogler G, Scharl M, Misselwitz B: Risk factors for gallstones and kidney stones in a cohort of patients with inflammatory bowel diseases. PLoS One 12: e0185193, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pang R, Linnes MP, O’Connor HM, Li X, Bergstralh E, Lieske JC: Controlled metabolic diet reduces calcium oxalate supersaturation but not oxalate excretion after bariatric surgery. Urology 80: 250–254, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chadwick VS, Modha K, Dowling RH: Mechanism for hyperoxaluria in patients with ileal dysfunction. N Engl J Med 289: 172–176, 1973. [DOI] [PubMed] [Google Scholar]
- 56.Earnest DL, Williams HE, Admirand WH: A physicochemical basis for treatment of enteric hyperoxaluria. Trans Assoc Am Physicians 88: 224–234, 1975. [PubMed] [Google Scholar]
- 57.Gregory JG, Park KY, Schoenberg HW: Oxalate stone disease after intestinal resection. J Urol 117: 631–634, 1977. [DOI] [PubMed] [Google Scholar]
- 58.Asplin JR: The management of patients with enteric hyperoxaluria. Urolithiasis 44: 33–43, 2016. [DOI] [PubMed] [Google Scholar]
- 59.Maalouf NM, Tondapu P, Guth ES, Livingston EH, Sakhaee K: Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J Urol 183: 1026–1030, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Canales BK, Ellen J, Khan SR, Hatch M: Steatorrhea and hyperoxaluria occur after gastric bypass surgery in obese rats regardless of dietary fat or oxalate. J Urol 190: 1102–1109, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hylander E, Jarnum S, Nielsen K: Calcium treatment of enteric hyperoxaluria after jejunoileal bypass for morbid obesity. Scand J Gastroenterol 15: 349–352, 1980. [DOI] [PubMed] [Google Scholar]
- 62.Lindsjö M, Fellström B, Ljunghall S, Wikström B, Danielson BG: Treatment of enteric hyperoxaluria with calcium-containing organic marine hydrocolloid. Lancet 2: 701–704, 1989. [DOI] [PubMed] [Google Scholar]
- 63.Tondapu P, Provost D, Adams-Huet B, Sims T, Chang C, Sakhaee K: Comparison of the absorption of calcium carbonate and calcium citrate after Roux-en-Y gastric bypass. Obes Surg 19: 1256–1261, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siener R, Petzold J, Bitterlich N, Alteheld B, Metzner C: Determinants of urolithiasis in patients with intestinal fat malabsorption. Urology 81: 17–24, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Emmett M, Guirl MJ, Santa Ana CA, Porter JL, Neimark S, Hofmann AF, Fordtran JS: Conjugated bile acid replacement therapy reduces urinary oxalate excretion in short bowel syndrome. Am J Kidney Dis 41: 230–237, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Caspary WF, Tönissen J, Lankisch PG: ‘Enteral’ hyperoxaluria. Effect of cholestyramine, calcium, neomycin, and bile acids on intestinal oxalate absorption in man. Acta Hepatogastroenterol (Stuttg) 24: 193–200, 1977. [PubMed] [Google Scholar]
- 67.Nordenvall B, Backman L, Larsson L, Tiselius HG: Effects of calcium, aluminium, magnesium and cholestyramine on hyperoxaluria in patients with jejunoileal bypass. Acta Chir Scand 149: 93–98, 1983. [PubMed] [Google Scholar]
- 68.Smith LH, Fromm H, Hofmann AF: Acquired hyperoxaluria, nephrolithiasis, and intestinal disease. Description of a syndrome. N Engl J Med 286: 1371–1375, 1972. [DOI] [PubMed] [Google Scholar]
- 69.Stauffer JQ, Humphreys MH, Weir GJ: Acquired hyperoxaluria with regional enteritis after ileal resection. Role of dietary oxalate. Ann Intern Med 79: 383–391, 1973. [DOI] [PubMed] [Google Scholar]
- 70.Langman CB, Grujic D, Pease RM, Easter L, Nezzer J, Margolin A, Brettman L: A double-blind, placebo controlled, randomized phase 1 cross-over study with ALLN-177, an orally administered oxalate degrading enzyme. Am J Nephrol 44: 150–158, 2016. [DOI] [PubMed] [Google Scholar]
- 71.Lingeman JE, Pareek G, Easter L, Pease R, Grujic D, Brettman L, Langman CB: ALLN-177, oral enzyme therapy for hyperoxaluria. Int Urol Nephrol 51: 601–608, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lieske JC, Regnier C, Dillon JJ: Use of sevelamer hydrochloride as an oxalate binder. J Urol 179: 1407–1410, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monico CG, Rossetti S, Olson JB, Milliner DS: Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int 67: 1704–1709, 2005. [DOI] [PubMed] [Google Scholar]
- 74.Jaeger P, Portmann L, Jacquet AF, Burckhardt P: [Pyridoxine can normalize oxaluria in idiopathic renal lithiasis]. Schweiz Med Wochenschr 116: 1783–1786, 1986. [PubMed] [Google Scholar]
- 75.Xie Q, Welch R, Mercado A, Romero MF, Mount DB: Molecular characterization of the murine Slc26a6 anion exchanger: Functional comparison with Slc26a1. Am J Physiol Renal Physiol 283: F826–F838, 2002. [DOI] [PubMed] [Google Scholar]
- 76.Heneghan JF, Akhavein A, Salas MJ, Shmukler BE, Karniski LP, Vandorpe DH, Alper SL: Regulated transport of sulfate and oxalate by SLC26A2/DTDST. Am J Physiol Cell Physiol 298: C1363–C1375, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dawson PA, Russell CS, Lee S, McLeay SC, van Dongen JM, Cowley DM, Clarke LA, Markovich D: Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. J Clin Invest 120: 706–712, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whittamore JM, Stephens CE, Hatch M: Absence of the sulfate transporter SAT-1 has no impact on oxalate handling by mouse intestine and does not cause hyperoxaluria or hyperoxalemia. Am J Physiol Gastrointest Liver Physiol 316: G82–G94, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Freel RW, Whittamore JM, Hatch M: Transcellular oxalate and Cl- absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. Am J Physiol Gastrointest Liver Physiol 305: G520–G527, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H, Alper SL: SLC26 anion exchangers of guinea pig pancreatic duct: Molecular cloning and functional characterization. Am J Physiol Cell Physiol 301: C289–C303, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ticinesi A, Nouvenne A, Chiussi G, Castaldo G, Guerra A, Meschi T: Calcium oxalate nephrolithiasis and gut microbiota: Not just a gut-kidney axis. A nutritional perspective. Nutrients 12: 548, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zampini A, Nguyen AH, Rose E, Monga M, Miller AW: Defining dysbiosis in patients with urolithiasis. Sci Rep 9: 5425, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA: Effect of gastric bypass surgery on kidney stone disease. J Urol 181: 2573–2577, 2009. [DOI] [PubMed] [Google Scholar]
- 84.Costa-Matos A, Guidoni LR, Carvalho KA, Fernandes RC, Perez MD: Is there an association between urolithiasis and Roux-en-y gastric bypass surgery? Int Braz J Urol 35: 432–435, 2009. [DOI] [PubMed] [Google Scholar]
- 85.Durrani O, Morrisroe S, Jackman S, Averch T: Analysis of stone disease in morbidly obese patients undergoing gastric bypass surgery. J Endourol 20: 749–752, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Caudarella R, Rizzoli E, Pironi L, Malavolta N, Martelli G, Poggioli G, Gozzetti G, Miglioli M: Renal stone formation in patients with inflammatory bowel disease. Scanning Microsc 7: 371–379; discussion 379–380, 1993 [PubMed] [Google Scholar]
- 87.McAuliffe ME, Lanes S, Leach T, Parikh A, Faich G, Porter J, Holick C, Esposito D, Zhao Y, Fox I: Occurrence of adverse events among patients with inflammatory bowel disease in the HealthCore Integrated Research Database. Curr Med Res Opin 31: 1655–1664, 2015. [DOI] [PubMed] [Google Scholar]
- 88.Cury DB, Moss AC, Schor N: Nephrolithiasis in patients with inflammatory bowel disease in the community. Int J Nephrol Renovasc Dis 6: 139–142, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hueppelshaeuser R, von Unruh GE, Habbig S, Beck BB, Buderus S, Hesse A, Hoppe B: Enteric hyperoxaluria, recurrent urolithiasis, and systemic oxalosis in patients with Crohn’s disease. Pediatr Nephrol 27: 1103–1109, 2012. [DOI] [PubMed] [Google Scholar]
- 90.Mukewar S, Hall P, Lashner BA, Lopez R, Kiran RP, Shen B: Risk factors for nephrolithiasis in patients with ileal pouches. J Crohn’s Colitis 7: 70–78, 2013. [DOI] [PubMed] [Google Scholar]
- 91.Repiso A, Alcántara M, Muñoz-Rosas C, Rodríguez-Merlo R, Pérez-Grueso MJ, Carrobles JM, Martínez-Potenciano JL: Extraintestinal manifestations of Crohn’s disease: Prevalence and related factors. Rev Esp Enferm Dig 98: 510–517, 2006. [DOI] [PubMed] [Google Scholar]
- 92.Ben-Ami H, Ginesin Y, Behar DM, Fischer D, Edoute Y, Lavy A: Diagnosis and treatment of urinary tract complications in Crohn’s disease: An experience over 15 years. Can J Gastroenterol 16: 225–229, 2002. [DOI] [PubMed] [Google Scholar]
- 93.Christodoulou DK, Katsanos KH, Kitsanou M, Stergiopoulou C, Hatzis J, Tsianos EV: Frequency of extraintestinal manifestations in patients with inflammatory bowel disease in Northwest Greece and review of the literature. Dig Liver Dis 34: 781–786, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Nightengale ML, Sarr MG, Kelly KA, Jensen MD, Zinsmeister AR, Palumbo PJ: Prospective evaluation of vertical banded gastroplasty as the primary operation for morbid obesity. Mayo Clin Proc 66: 773–782, 1991. [DOI] [PubMed] [Google Scholar]
- 95.Trnka YM, Glotzer DJ, Kasdon EJ, Goldman H, Steer ML, Goldman LD: The long-term outcome of restorative operation in Crohn’s disease: Influence of location, prognostic factors and surgical guidelines. Ann Surg 196: 345–355, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knudsen L, Marcussen H, Fleckenstein P, Pedersen EB, Jarnum S: Urolithiasis in chronic inflammatory bowel disease. Scand J Gastroenterol 13: 433–436, 1978. [DOI] [PubMed] [Google Scholar]
- 97.Maratka Z, Nedbal J: Urolithiasis as a complication of the surgical treatment of ulcerative colitis. Gut 5: 214–217, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]