Abstract
Endothelial dysfunction is a hallmark of type 2 diabetes that can have severe consequences on vascular function, including hypertension and changes in blood flow, as well as exercise performance. Because endothelium is also the barrier for insulin movement into tissues, it acts as a gatekeeper for transport and glucose uptake. For this reason, endothelial dysfunction is a tempting area for pharmacological and/or exercise intervention with insulin-based therapies. In this review, we describe the current state of drugs that can be used to treat endothelial dysfunction in type 2 diabetes and diabetes-related diseases (e.g., obesity) at the molecular levels, and also discuss their role in exercise.
Keywords: Endothelial dysfunction, Exercise, Vascular function, Insulin
1. INTRODUCTION
The endothelium is an important modulator of microvascular function. In resistance arteries, the heterocellular communication between the endothelium and smooth muscle cells determines regional blood flow and contributes to systemic blood pressure regulation. Endothelial dysfunction refers to a condition in which the endothelial-derived vasodilation is impaired, shifting toward a vasoconstrictive, pro-thrombotic and pro-inflammatory state [1]. As such, identifying therapeutic interventions to maintain a healthy endothelium or reverse endothelial dysfunction is a worthy research objective.
One of the most important markers of endothelial dysfunction is represented by the reduction of Nitric Oxide (NO) bioactivity, due to its actions as a potent vasodilator. [1] Bioavailability of NO reflects a balance between production via endothelial Nitric Oxide Synthase (eNOS) and the conversion to nitrates (NO−3) and nitrites (NO−2). Decreasing NO activity can be due to diminished eNOS expression. Reactive oxygen species (ROS) can rapidly degrade NO to form peroxynitrite, reducing the amount of bioavailable NO resulting in further endothelial cell dysfunction [2].
Endothelium-dependent vasodilation impairments, which are a hallmark of endothelial dysfunction, can be assessed by measuring responses to endothelium-dependent vasodilators such as acetylcholine (ACh) [3]. Clinically this is relevant since endothelial dysfunction is considered an early marker of vascular complications of type 2 diabetes and heart disease risks [3]. Functional impairment of endothelial activity precedes the development of morphological alterations leading to the progression of type 2 diabetes (T2D) [1]. Therapies aimed at the reduction of hyperglycemia, dyslipidemia, and insulin resistance may effectively improve endothelial function and delay or prevent the onset of vascular complications [1].
2. ENDOTHELIAL DYSFUNCTION AND DIABETES
Vascular dysfunction limits the delivery of oxygen, hormones and substrates to metabolically active tissues, thereby affecting nutrient availability throughout the arterial tree for energy metabolism [4–6]. Interestingly, work by our group and others demonstrate that people with insulin resistance may have normal fasting vascular function, but impaired conduit or microvascular insulin action [7–10]. This highlights the role which regulatory processes play in disease as well as makes the critical distinction between the fed vs. fasted state in regards to vascular function. These data support the hypothesis that endothelial cells in people at risk for diabetes become insulin resistant as best demonstrated in a study by Baron and colleagues in which leg blood flow was diminished to insulin across sub- to supra-physiologic doses in parallel to leg glucose uptake in people with T2D when compared to lean or obese individuals [11]. More recently, most but not all studies support a role for insulin action on increasing limb blood flow in glucose and insulin delivery to the muscle via a NO-mediated mechanism [11–15]. In fact, some have even suggested that vascular insulin resistance precedes that of metabolic responses to insulin, thereby placing endothelial cell function at the center of glycemic regulation [16]. How diabetes promotes attenuated endothelial cell function remains an area of intense investigation, but hyperglycemia has been suggested to promote oxidative stress-mediated inflammation in the endothelium thereby blunting eNOS-VEGF pathways related to angiogenesis. [17–22]. Collectively, endothelial dysfunction has clinical and public health significance for promoting varying degrees of CVD risk including heart failure, strokes, and death compared with healthy normoglycemic individuals [23].
3. INSULIN
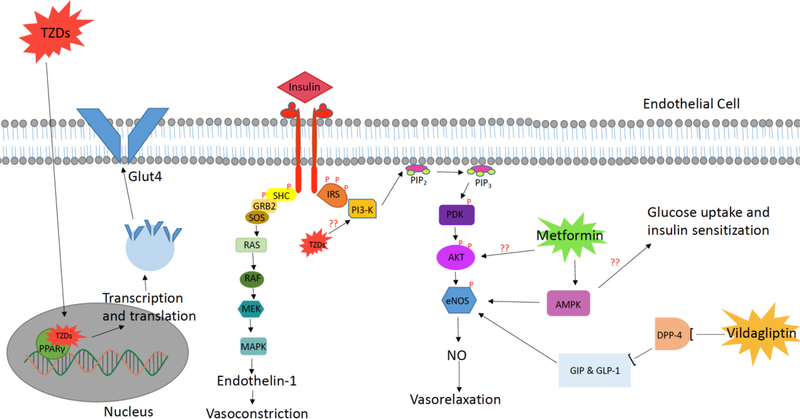
Aside from insulin’s essential effects on whole-body glucose and lipid metabolism, insulin also affects vascular biology. Insulin signaling mediates vascular function by stimulating signal transduction and mediating endothelial cell function [24]. Specifically, insulin signaling increases amino acid transport, glycogen synthesis, DNA synthesis, and gene expression in vascular cells. [25] As seen in Fig. 1, the physiological effects of insulin within the vasculature are mediated by insulin binding to its receptor on the surface of endothelial cells, which triggers the insulin receptor kinase (IR) phosphorylation of the insulin receptor substrate (IRS-1) [26]. Phosphorylation of IRS-1 leads to the subsequent recruitment and activation of phosphatidylinositol 3-kinase (PI3K) which then phosphorylates AKT and activates it to directly phosphorylate eNOS on Ser1177 [27]. Phosphorylation of Ser1177 has been shownto be both necessary and sufficient for eNOS to produce NO [27]. The end result of this signaling pathway is insulin’s induction of vasorelaxation via NO production and the stimulation of NO-dependent basal blood flow. Insulin also stimulates renal reabsorption of sodium, sympathetic nervous system activity, and induces the production of vasoactive factors including endothelin 1 and NO [28]. An imbalance between the release of endothelin-1 and NO in an insulin-resistance state may be involved in the pathophysiology of hypertension and atherosclerosis in conjunction with endothelial dysfunction [28]. Vascular smooth muscle cells (VSMCs) also contain insulin receptors; however, a study using bovine aortic samples noted that aortic endothelial cells contain a 10-fold higher concentration of insulin receptors compared to aortic VSMCs. This study also measured insulin binding affinity in retinal capillaries to test microvascular insulin receptor number and affinity. The results were congruent with the data seen from aortic segments in that the insulin receptor number was increased in endothelial cells when compared to smooth muscle cells or vascular supporting cells [29]. These data show the importance of insulin signaling specifically in vascular endothelial cells. Furthermore, systemic insulin resistance, a condition linked to T2D and cardiovascular disease (CVD), has been connected with impaired vascular insulin signaling [25].
Fig. (1). Effects of Insulin and pharmacological agents on endothelial insulin signaling.
Shown are the effects of Insulin, TZDs, Metformin, and Vildagliptin on endothelial insulin signaling and downstream vasorelaxation, vasoconstriction, and glucose uptake. Question marks represent places in which mechanisms are unknown throughout the available literature. Illustrated here is the PI3K signaling pathway downstream of insulin receptor activation resulting in NO production and vasodilation. Shown in tandem is the metabolic and mitogenic pathway of insulin receptor activation which controls the secretion of endothelin-1 and leads to vasoconstriction. Also displayed are the molecular targets of metformin acting as an activator of AKT downstream of PI3K as well as stimulating AMPK. TZDs are shown here also stimulating both PI3K as well as PPARγ which in conjunction increase glucose uptake and NO production. Vildagliptin is presented as a DPP-4 inhibitor with downstream effects on eNOS, subsequently increasing NO levels which result in vasorelaxation.
The beneficial effects of insulin on blood flow and blood pressure are reduced in patients with T2D or insulin resistance [30]. It has been shown that exposure to high glucose levels compromised endothelial-dependent vasorelaxation in diabetic rabbit aortas and prolonged exposure to elevated serum glucose also increases the production of proteinoids which act as vasoconstrictors [30]. This result is likely seen due to constantly elevated levels of glucose triggering insulin resistance in the aortic endothelial cells that ultimately lead to decreased levels of NO and eNOS from loss of insulin signaling. Diminished endothelium-dependent vasodilation is associated with insulin resistance and can be seen in the vasculature as dysfunctions in insulin- stimulated endothelial function [31]. Early introduction of insulin therapy to patients with diabetes may improve endothelial function leading to improved microvascular endothelial-dependent vasodilation [31]. After 2 months of insulin therapy (0.05 mU/kg/min for 20 minutes) patients had a better response to ACh stimulation than prior to starting insulin treatment [31]. This shows that a reduction in hyperglycemia via insulin treatment results in better endothelial cell function (patients saw between a 58 and 120% increase in blood flow) due to the decreased damaging effect of hyperglycemia and the increased NO production from insulin signaling.
3.1. Insulin and Exercise
Despite the improvements in glycemic control, insulin therapy can predispose patients to weight gain, inflammation, dyslipidemia and atherosclerosis [32]. However, exercise may be used adjunctively to improve body composition, inflammation and cardiovascular risk associated with insulin therapy. Balducci et al. (2012) observed that twelve months of exercise (2×/wk; 75 min aerobic and resistance) reduced waist circumference, blood pressure, LDL-C and overall coronary heart disease risk score compared to insulin-treatment only in insulin-dependent adults [33]. Similarly, 5 months of low-intensity aerobic and resistance exercise attenuated the increased need in exogenous insulin requirements in adults with T2D [34]. These data highlight that exercise can increase insulin sensitivity and the body’s ability to produce insulin, resulting in less exogenous insulin usage. In addition to metabolic-mediated improvements of exercise, further benefits are also observed for endothelial function, like 4 months of cycling (3d/wk; 60–70% HRmax; 60min) led to improvements in flow-mediated vasodilation in adults with type 1 diabetes [35]. It was speculated that contracting skeletal muscle may have activated AKT and eNOS in the vascular endothelium as well as triggered the release of myokines that increased blood flow pathway stimulation. In either case, it seems clear that exercise adds to the beneficial effects of insulin therapy specifically in the vasculature [36].
4. THIAZOLIDINESDIONES (TZDs)
Given the crucial role insulin plays on vasculature function, drugs that work to sensitize tissues to insulin should show a beneficial effect on disease states, like T2D and CVD, which are associated with vascular dysfunction [25]. Sensitizing endothelial cells to insulin would allow for the increased production of eNOS as well as NO subsequently causing vascular relaxation.
Thiazolidinediones (TZDs) are a group of insulin-sensitizing therapeutic drugs that activate peroxisome proliferator-activated receptor γ (PPAR-γ) with effects illustrated in Fig. 1. This nuclear receptor, which is highly expressed on vascular smooth muscle cells as well as the endothelium, enhances insulin-mediated glucose uptake. PPAR-γ expression increases sensitivity to serum insulin levels by altering the transcription of several genes involved in glucose and lipid metabolism, lipoprotein lipase, fatty acid transporters, glucokinase, and the GLUT4 glucose transporter [37, 38]. TZDs provide sustained glycemic control mediated primarily by reductions in insulin resistance [37]. It is proposed that TZDs anti-inflammatory properties and activation of both AMP Kinase (AMPK) and PI3K signaling contribute to the reversal of insulin resistance and increased NO production through increasing endothelial cell function. This result was seen in T2D patients with both impaired glucose tolerance and endothelial cell dysfunction who were administered 600mg/d of various TZDs [37]. Patients with T2D also had an increased VO2max (i.e. maximal oxygen consumption or aerobic fitness), insulin sensitivity, and endothelial function when administered rosiglitazone (4mg/d) compared with a placebo [39]. Through the promotion of an insulin-sensitive state, TZDs can reduce microvascular complications associated with endothelial dysfunction by increasing eNOS and NO production.
The exact mechanism behind improved endothelial cell function is not completely understood but a proposed mechanism of action is that PPAR-γ activation results in downstream anti-inflammatory effects. TZDs, such as pioglitazone and rosiglitazone, have a known anti-inflammatory effect through the suppression of TNF-a, leptin, and lipolysis which together decrease plasma free fatty acid (FFA) concentrations and increase adiponectin levels [40]. It has been experimentally shown that increasing FFA levels impairs endothelium-dependent vasodilation. This was performed by infusing, over 2 hours, the femoral artery of healthy lean patients with exogenous lipids to increase serum FFA. Vasodilation was quantified by measuring the response to methacholine chloride (Mch) an endothelium-dependent vasodilator. Vasodilation was reduced by 20% in the group that was infused with lipids [41]. The anti-inflammatory properties of PPAR-γ lead to decreased circulating adipokine levels (TNF-α and leptin) causing adiponectin levels to rise, which reduces vascular expression of adhesion molecules (VCAM). Decreased VCAM leads to reduced white blood cell adhesion to the endothelial cell surface, thereby attenuating the monocyte response to inflammation and impairing the inflammatory activity of macrophages [42].
Another function of TZDs is to reduce systemic and vascular oxidative stress. It has long been known that oxidative stress contributes to vascular dysfunction in diabetes as documented in a 1991 study showing the increased accumulation of oxidized sugars in diabetic rats [43]. Coronary arterioles from T2D male mice were isolated and analyzed to look at changes in blood flow and vasodilation. It was seen that diabetic mice had reductions in vasodilation as well as blood flow. The cause of the diminished vascular capacity was due to increased superoxide production, which was measured via dihydroethidium staining and lucigenin enhanced chemiluminescence [38]. Rosiglitazone causes a reduction of nicotinamide adenine dinucleotide (NAD(P)H) oxidase activity, which diminishes oxidative stress within the cell. This subsequently enhances NO activity specifically in the vasculature because of the reduction in vascular NAD(P)H oxidase derived superoxide production [38].
4.1. TZDs and Exercise
Lifestyle modifications including caloric restriction with exercise may be a supplemental way to combat the weight gain seen with TZDs and decrease the variability of beneficial responses. In fact, 20 weeks of moderate-intensity exercise (45min; 60–75% heart rate reserve; 4d/wk) and caloric restriction (500kcal/d) led to an average weight loss of 11.8kg, compared to pioglitazone (30mg/d), which increased weight by approximately 2.7kg in sedentary adults [44]. Another study found that 4 weeks of submaximal cycling (30min, 4d/wk) decreased bodyweight, increased exercise capacity (watts) and improved flow-mediated dilation (FMD) compared to 8mg/d of rosiglitazone in patients with coronary artery disease and prediabetes [45]. Moreover, Kadoglou et al. observed that 8 months of rosiglitazone (8mg/d) and aerobic exercise (60min; 50–80% VO2max; 4d/wk) elicited more pronounced decreases in fasting insulin, HOMA-IR, resistin, IL-6 and TNF-α than rosiglitazone alone in adults with T2D. The combined therapy also saw a 12% greater improvement in VO2 max than exercise alone. These studies together highlight that the best metabolic benefit regarding health outcomes may be observed when lifestyle and TZD therapy are combined [46].
While aerobic and resistance exercise, as well as TZDs, have been shown to improve endothelial function in adults with T2D; to date, there has been no study examining the specific effects of TZDs plus exercise on arterial physiology [47–49]. TZDs are thought to be a potential therapy for vascular disease via the increase in adiponectin expression through activation of PPARγ. Adiponectin activates the AMPK/Akt/eNOS pathway and ultimately increases the amount of NO produced [50]. However, a meta-analysis found that TZD therapy is most likely to lead to increased endothelial function following at least 12 weeks of treatment and if the patient is not older than 65 years of age [50]. On the other hand, exercise has been shown to increase adiponectin expression and also has a direct mechanical effect on the vasculature via increases in blood flow and shear stress [36]. Furthermore, as little as 2 weeks of aerobic exercise improves endothelial function and adiponectin levels in adults with obesity [7, 51]. As previously discussed, a combination of exercise and rosiglitazone was found to increase adiponectin levels more than individual therapies [46]. Based on these findings, it could be hypothesized that exercise and TZDs may work synergistically to improve endothelial function as they are not directly competing to utilize the same mechanistic pathway. Nonetheless, clinical trials are needed to confirm and determine the appropriate exercise and TZD dosage for maximum health benefits.
4.2. TZDs and Mitochondrial Dynamics
TZDs have a known function of exerting anti-diabetic effects. In isolated rat soleus muscle, it has been shown that TZDs reduce the activity of respiratory Complex I in the mitochondria [52]. Since TZDs have been shown to change mitochondrial dynamics in skeletal muscle, other groups like Artwohl et al. proposed a similar mechanism for TZDs in endothelial cells. It is hypothesized that TZDs have an antiproliferative action on endothelial cells which can explain their vascular protective effect as individuals with T2D often have a proatherogenic vascular environment that is often characterized by accelerated proliferation of both endothelial and smooth muscle cells. This study concluded that the effects seen from TZD treatment in vitro are independent of PPARγ activation and correlate with lactate release. The correlation with lactate release is then extrapolated to be linked with possible inhibition of mitochondrial complex I function [53].
5. METFORMIN
Metformin is a drug used by over 120 million patients with T2D worldwide to improve glycemic control and insulin sensitivity. The beneficial effects seen in patients taking metformin have been attributed to the stimulation of AMPK activity as shown in Figure 1 [55]. Increased AMPK action decreases insulin resistance via promoting glucose uptake in muscle cells while also inhibiting hepatic glucose release. [54] Insulin resistance has been seen to mediate endothelial dysfunction, therefore metformin’s insulin-sensitizing effect improves endothelial-dependent vasodilation [55]. To test the beneficial effects of metformin on insulin resistance, endothelial cells were cultured in high glucose media and were also given insulin. These cells function as a model for insulin resistance as they are unable to take up the amount of glucose as endothelial cells cultured in regular media. The NO, eNOS, and endothelin levels were measured in metformin-treated (10−3mmol/L) control cells. Metformin treatment significantly increased NO levels and reduced endothelin-1 concentration in insulin-resistant cells compared with the non-treated controls. Metformin also increased eNOS protein expression in the insulin-resistant cells [56].
Metformin has been seen to increase AKT and eNOS phosphorylation in mouse microvascular endothelial cells that have been cultured in high glucose media. Exposing the aorta from hyperglycemic db/db mice to a 3hr treatment of 50uM metformin significantly increased vasodilation in response to stimulation with ACh. These data lead to the conclusion that metformin can both treat and reverse hyperglycemia-induced endothelial cell dysfunction. Metformin is thought to reverse the negative effects of high glucose on eNOS and AKT phosphorylation leading to improved vascular function, and reduction in both insulin resistance and hyperglycemia [55].
5.1. Metformin and Exercise
Coupling metformin treatment with exercise to reduce the incidence of T2D and vascular dysfunction results in irresolute clinical trial data. The landmark U.S. diabetes prevention program reported that 150min/wk of physical activity and weight loss of 7% reduced T2D incidence by 58% compared to the 31% decrease with metformin (1700 mg/d) in adults with impaired glucose [57]. While these findings suggest lifestyle was better than metformin alone, another study observed that both regular physical activity (recommended >30min/d) and metformin (500mg/d) reduced the progression of impaired glucose tolerance to T2D in native Asian Indians [58]. However, there were no synergistic improvements when the two therapies were combined. Interestingly, in a randomized controlled trial, the combined effect of metformin and exercise was tested compared to either treatment in adults with prediabetes. The results showed that while 12 weeks of metformin (2000mg/d) plus cycling (3d/wk; aerobic: 45min at 70% HRpeak) and resistance exercise (2d/wk 2×12 at 70% of 1RM) increased insulin sensitivity, individuals randomized to exercise-only saw a 25–30% higher increase than the combined or metformin-only group [59]. Likewise in another study, 12 weeks of treadmill exercise (3d/wk; 45 min at 85& HRmax) with metformin (2000mg/d) saw no improvements in whole-body insulin sensitivity and VO2max in an aged population of overweight to obese insulin-resistant adults. Further, cellular experiments in these older individuals indicated that metformin blunted skeletal muscle mitochondria respiration adaptations [60]. Interestingly, this blunting effect of metformin on metabolic adaptation was also observed with regard to weightlifting alone. Indeed, a recent study showed that 14 weeks of progressive resistance exercise training only increased total lean mass and thigh muscle mass in healthy older adults, whereas the combined treatment of resistance exercise with metformin appeared to blunt these changes through competing AMPK/mTOR pathways [61]. The effect of metformin on insulin sensitivity, aerobic fitness and muscle also seems to be of clinical relevance as some have reported increased glucagon levels that coincided with the glucose-lowering effect of exercise in patients with T2D [62]. Moreover, metformin seems to interfere with the ability of exercise to lower blood pressure in people with prediabetes [63].
Taken together, these data suggest that metabolically, metformin does not enhance the benefits of physical activity, and likely blunts some adaptation. While no study has looked specifically at outcomes of endothelial cells in exercise and metformin models, competition via the activation of the AMPK pathway would likely result in a downstream reduction of NO production and subsequent endothelial function. Whether metformin alters the endothelial function and/or arterial reactivity following exercise in humans awaits further investigation.
5.2. Metformin and Mitochondrial Dynamics
Mitochondria are exceptionally dynamic organelles that play a critical role in energy metabolism, stress responses, cell death, and ROS production. Increased mitochondrial fission is associated with increased amounts of ROS released from the organelle which subsequently has been shown to impair endothelial nitric oxide synthase production of NO [64].
Metformin has also shown promising results in altering mitochondrial fission and fusion dynamics that exert cardiovascular protective effects. A study conducted by Wang et al. reported a novel mechanism by which metformin reduces mitochondrial fragmentation decreasing mitochondrial-derived superoxide release and therefore improving endothelial-dependent vasodilation. In this study, metformin was also seen to reduce vascular inflammation and suppression of atherosclerotic lesions in streptozotocin-induced diabetic ApoE null mice. This group suggests that metformin exerts its cardiovascular protective effects by inhibiting dynamin-related protein (Drp1), a key protein involved in the fragmentation of mitochondria. Also, in ApoE and AMPK-α-2 null mice, the reduction in Drp1 expression was lost. Taken together, these data suggest that metformin exerts beneficial effects on mitochondrial dynamics through AMPK activation and decreases in ROS. This diminishes Drp1 expression and subsequently reduces mitochondrial fragmentation as well as promotes proper endothelial cell-dependent vasodilation [65].
6. VILDAGLIPTIN
Hormonal treatments, such as incretins which include glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), have been used as a treatment for T2D [66]. Dipeptidyl peptidase-4 (DPP-4) rapidly degrades these incretin hormones making them inactive metabolites and diminishing their benefit for patients with diabetes [67]. DPP-4 inhibitors have been shown to reduce the breakdown of GLP-1 and increase beta-cell function, which increases the insulin section [68]. Vildagliptin is an effective DPP-4 inhibitor that can be used to treat T2D [68]. Figure 1 shows that GLP-1 and GIP have protective effects on the vasculature, specifically on endothelial function via the upregulation of protein expression and action of eNOS [69, 70]. Individuals with T2D often have reduced postprandial secretion of GLP-1 and GIP [71]. Increasing GLP-1 with vildagliptin induces vasodilation in an endothelial cell-dependent manner in both humans and animal brachial arteries. It has also been shown that 4 weeks of injections of vildagliptin into the arms of patients with T2D improved endothelial-dependent vasodilation [66]. Greater vasodilation was seen in the patient’s vascular bed when administered with vildagliptin and ACh compared to patients treated with an endothelium-independent vasodilator sodium nitroprusside [3].
6.1. Vildagliptin and Exercise
In addition to vildagliptin, aerobic exercise increases postprandial incretin concentrations in adults with obesity [72,73]. This effect may be, in part, mediated through lower circulating plasma DPP-4 [74]. Despite the beneficial effects of exercise in relation to incretin hormones, there are limited studies looking at the combined effects of DPP-4 inhibitors and exercise. One of the few human studies looking at this drug in combination with exercise found that 12 weeks of sitagliptin (100mg/d) and exercise impacted C-peptide area under the curve and beta-cell function equivalently to sitagliptin only. However, the combined therapy did result in increased circulating HDL-C and slightly lower glucose concentrations in adults with long-standing type 1 diabetes [75]. Benefits of this combination therapy are also supported in rodent models. Data in diabetic KK/Ta mice showed that a combination of exercise and alogliptin was effective against high-fat diet-induced lipid accumulation in the liver [76]. Likewise, MK-0626, another DPP-4 inhibitor, was found to increase mitochondrial biogenesis and exercise capacity in mice induced with heart failure [77]. However, an important consideration is that no data in rodents or humans appear to specifically test whether the combination of DPP-4 inhibitors plus exercise impacts cardiometabolic health to a greater extent than exercise or DPP-4 inhibitor alone.
While these drugs are all a part of the same class, it is necessary to recognize that there may be a difference in overall efficacy amongst DPP-4 inhibitors. This is important as each type of DPP-4 inhibitor may differentially impact outcomes regarding combination therapy with exercise. Interestingly, vildagliptin was found to induce lower mean amplitudes of glycemic excursions than sitagliptin as well as induce greater decreases in HbA1c than alogliptin in patients with T2D [78,79]. However, in another study conducted in patients undergoing either an exercise/diet program or a combination therapy of lifestyle with metformin, vildagliptin and sitagliptin induced similar alterations in incretin hormones, glucose concentration and insulin secretion [80]. These data collectively highlight the importance of not only needing more exercise trials with DPP-4 inhibitors, but also how results and interpretation of outcomes may differ depending on the type of DPP-4 inhibitor studied. Further, no study has addressed the implications of exercise when added to vildagliptin in regards to endothelial cells. This has public health relevance for understanding both T2D and CVD risk reduction.
6.2. Vildagliptin and Mitochondrial Dynamics
Vildagliptin has also been shown to alter mitochondrial dynamics in endothelial cells in vitro. Human umbilical vein endothelial cells were cultured in hyperglycemic media and then analyzed for ROS, mtDNA damage, and ATP synthesis changes when treated with vildagliptin or vehicle. In vildagliptin treated cells mitochondrial ROS production and mtDNA damage were significantly reduced while ATP synthesis was enhanced. Vildagliptin treatment also reduced the expression of Drp1 and fission-1 (Fis1). Drp1 translocation to the mitochondria was blocked resulting in reduced mitochondrial fragmentation that is usually seen in hyperglycemic endothelial cells. Blocking mitochondrial fission will have an endothelial protective effect by reducing mitochondrial damage that is induced by hyperglycemia. [81]
CONCLUSION
In conclusion, the implications of current pharmacological regimens for insulin-based therapies with and without exercise have been described in relation to molecular and functional outcomes of endothelial dysfunction. Exercise aids in weight management, increases insulin sensitivity, and improves blood flow in T2D; however, the effects when combined with pharmacological agents are less understood and warrant further mechanistic study. There are also several deficiencies with these agents, especially when the focus is endothelial dysfunction. More recent novel work in the area of endothelial dysfunction has revealed targets that may be able to be exploited at the pharmacological level. This includes the fat mass obesity (Fto) protein in endothelium and how it regulates prostaglandin D2 to protect against insulin resistance and promotes glucose uptake as well as vascular function [82]. Another example is the role of peroxynitrite regulating TRPV4 in endothelium to restore vasodilation in obese mice [83]. It is tantalizing that, in both examples, endothelial dysfunction was reversed in isolated human tissue, which highlights a possible novel translational pipeline.
FUNDING
This work was supported by NIH HL088554 (BEI).
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Potenza MA, Gagliardi S, Nacci C, Carratu’ MR, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem 2009; 16(1): 94–112. 10.2174/092986709787002853 [DOI] [PubMed] [Google Scholar]
- [2].Landmesser U, Dikalov S, PriceS R, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 2003; 111(8): 1201–9. 10.1172/JCI200314172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].VanPoppel PCM, Netea MG, Smits P, Tack CJ. Vildagliptin Improves Endothelium-Dependent Vasodilatation in Type 2 Diabetes 2011. 10.2337/dc10-2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Eggleston EM, Jahn LA, Barrett EJ. Early microvascular recruitment modulates subsequent insulin-mediated skeletal muscle glucose metabolism during lipid infusion. Diabetes Care 2013; 36(1): 104–10. 10.2337/dc11-2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab 2009; 94(9): 3543–9. 10.1210/jc.2009-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vincent MA, Clerk LH, Lindner JR, et al. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 2006; 290(6): E1191–7. 10.1152/ajpendo.00497.2005 [DOI] [PubMed] [Google Scholar]
- [7].Malin SK, Gilbertson NM, Eichner NZM, Heiston E, Miller S, Weltman A. Impact of Short-Term Continuous and Interval Exercise Training on Endothelial Function and Glucose Metabolism in Prediabetes. J Diabetes Res 2019; 20194912174 10.1155/2019/4912174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gilbertson NM, Miller SL, Eichner NZM, Malin SK. Endothelial function following interval exercise plus low-calorie diet treatment in obese females. Physiol Rep 2019; 7(18): e14239. 10.14814/phy2.14239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rattigan S, Wheatley C, Richards SM, Barrett EJ, Clark MG. Exercise and insulin-mediated capillary recruitment in muscle. Exerc Sport Sci Rev 2005; 33(1): 43–8. [PubMed] [Google Scholar]
- [10].Jahn LA, Hartline L, Rao N, et al. Insulin enhances endothelial function throughout the arterial tree in healthy but not metabolic syndrome subjects. J Clin Endocrinol Metab 2016; 101(3): 1198–206. 10.1210/jc.2015-3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baron AD, Brechtel-Hook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol 1996; 271(6 Pt 1): E1067–72. [DOI] [PubMed] [Google Scholar]
- [12].Raitakari M, Knuuti MJ, Ruotsalainen U, et al. Insulin increases blood volume in human skeletal muscle: studies using [15O]CO and positron emission tomography. Am J Physiol 1995; 269(6 Pt 1): E1000–5. [DOI] [PubMed] [Google Scholar]
- [13].Vollenweider P, Tappy L, Randin D, et al. Differential effects of hyperinsulinemia and carbohydrate metabolism on sympathetic nerve activity and muscle blood flow in humans. J Clin Invest 1993; 92(1): 147–54. 10.1172/JCI116542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Taddei S, Virdis A, Mattei P, Natali A, Ferrannini E, Salvetti A. Effect of insulin on acetylcholine-induced vasodilation in normotensive subjects and patients with essential hypertension. Circulation 1995; 92(10): 2911–8. 10.1161/01.CIR.92.10.2911 [DOI] [PubMed] [Google Scholar]
- [15].Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 1994; 94(3): 1172–9. 10.1172/JCI117433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vincent MA, Clerk LH, Lindner JR, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004; 53(6): 1418–23. 10.2337/diabetes.53.6.1418 [DOI] [PubMed] [Google Scholar]
- [17].Lira VA, Brown DL, Lira AK, et al. Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J Physiol 2010; 588(Pt 18): 3551–66. 10.1113/jphysiol.2010.194035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Srinivasan S, Hatley ME, Bolick DT, et al. Hyperglycaemiainduced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia 2004; 47(10): 1727–34. 10.1007/s00125-004-1525-1 [DOI] [PubMed] [Google Scholar]
- [19].Chen Z, Peng IC, Sun W, et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res 2009; 104(4): 496–505. 10.1161/CIRCRESAHA.108.187567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee-Young RS, Ayala JE, Hunley CF, et al. Endothelial nitric oxide synthase is central to skeletal muscle metabolic regulation and enzymatic signaling during exercise in vivo. Am J Physiol Regul Integr Comp Physiol 2010; 298(5): R1399–408. 10.1152/ajpregu.00004.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cook S, Hugli O, Egli M, et al. Partial gene deletion of endothelial nitric oxide synthase predisposes to exaggerated high-fat diet-induced insulin resistance and arterial hypertension. Diabetes 2004; 53(8): 2067–72. 10.2337/diabetes.53.8.2067 [DOI] [PubMed] [Google Scholar]
- [22].Cosentino F, Hishikawa K, Katusic ZS, Lüscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 1997; 96(1): 25–8. 10.1161/01.CIR.96.1.25 [DOI] [PubMed] [Google Scholar]
- [23].Færch K, Vistisen D, Johansen NB, Jørgensen ME. Cardiovascular risk stratification and management in pre-diabetes. Curr Diab Rep 2014; 14(6): 493. 10.1007/s11892-014-0493-1 [DOI] [PubMed] [Google Scholar]
- [24].Rask-Madsen C, Li Q, Freund B, et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab 2010; 11(5): 379–89. 10.1016/j.cmet.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jiang ZY, Lin YW, Clemont A, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest 1999; 104(4): 447–57. 10.1172/JCI5971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zeng G, Nystrom FH, Ravichandran LV, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 2000; 101(13): 1539–45. 10.1161/01.CIR.101.13.1539 [DOI] [PubMed] [Google Scholar]
- [27].Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999; 399(6736): 601–5. 10.1038/21224 [DOI] [PubMed] [Google Scholar]
- [28].Cardillo C, Nambi SS, Kilcoyne CM, et al. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation 1999; 100(8): 820–5. 10.1161/01.CIR.100.8.820 [DOI] [PubMed] [Google Scholar]
- [29].King GL, Buzney SM, Kahn CR, et al. Differential responsiveness to insulin of endothelial and support cells from micro- and macrovessels. J Clin Invest 1983; 71(4): 974–9. 10.1172/JCI110852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tesfamariam B. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. The American journal of physiology 1992; 08263: H321–H326. [DOI] [PubMed] [Google Scholar]
- [31].Rask-Madsen C, Ihlemann N, Krarup T, et al. Insulin therapy improves insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart disease. Diabetes 2001; 50(11): 2611–8. 10.2337/diabetes.50.11.2611 [DOI] [PubMed] [Google Scholar]
- [32].Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3): 422–34. 10.1016/j.pcad.2017.09.001 [DOI] [PubMed] [Google Scholar]
- [33].Balducci S, Zanuso S, Cardelli P, et al. Italian Diabetes Exercise Study (IDES) Investigators. Supervised exercise training counterbalances the adverse effects of insulin therapy in overweight/obese subjects with type 2 diabetes. Diabetes Care 2012; 35(1): 39–41. 10.2337/dc11-1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].DeFeyter HM, Praet SF, van den Broek NM, et al. Exercise training improves glycemic control in long-standing insulin-treated type 2 diabetic patients. Diabetes Care 2007; 30(10): 2511–3. 10.2337/dc07-0183 [DOI] [PubMed] [Google Scholar]
- [35].Fuchsjäger-Mayrl G, Pleiner J, Wiesinger GF, et al. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care 2002; 25(10): 1795–801. 10.2337/diacare.25.10.1795 [DOI] [PubMed] [Google Scholar]
- [36].Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 2017; 97(2): 495–528. 10.1152/physrev.00014.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hauner H. The mode of action of thiazolidinediones. Diabetes Metab Res Rev 2002; 18(S2)(Suppl. 2): S10–5. 10.1002/dmrr.249 [DOI] [PubMed] [Google Scholar]
- [38].Bagi Z, Koller A, Kaley G. PPARgamma activation, by reducing oxidative stress, increases NO bioavailability in coronary arterioles of mice with Type 2 diabetes. Am J Physiol Heart Circ Physiol 2004; 286(2): H742–8. 10.1152/ajpheart.00718.2003 [DOI] [PubMed] [Google Scholar]
- [39].Regensteiner JG, Bauer TA, Reusch JE. Rosiglitazone improves exercise capacity in individuals with type 2 diabetes. Diabetes Care 2005; 28(12): 2877–83. 10.2337/diacare.28.12.2877 [DOI] [PubMed] [Google Scholar]
- [40].Wakino S, Law RE, Hsueh WA. Vascular protective effects by activation of nuclear receptor PPARgamma. J Diabetes Complications 2002; 16(1): 46–9. 10.1016/S1056-8727(01)00197-0 [DOI] [PubMed] [Google Scholar]
- [41].Steinberg HO, Tarshoby M, Monestel R, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 1997; 100(5): 1230–9. 10.1172/JCI119636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tummala PE, Chen XL, Sundell CL, et al. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: A potential link between the renin-angiotensin system and atherosclerosis. Circulation 1999; 100(11): 1223–9. 10.1161/01.CIR.100.11.1223 [DOI] [PubMed] [Google Scholar]
- [43].Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes (New York, NY) 1991; 0440: 405–412. [DOI] [PubMed] [Google Scholar]
- [44].Shadid S, Jensen MD. Effects of pioglitazone versus diet and exercise on metabolic health and fat distribution in upper body obesity. Diabetes Care 2003; 26(11): 3148–52. 10.2337/diacare.26.11.3148 [DOI] [PubMed] [Google Scholar]
- [45].Sixt S, Rastan A, Desch S, et al. Exercise training but not rosiglitazone improves endothelial function in prediabetic patients with coronary disease. Eur J Cardiovasc Prev Rehabil 2008; 15(4): 473–8. 10.1097/HJR.0b013e3283002733 [DOI] [PubMed] [Google Scholar]
- [46].Kadoglou NP, Iliadis F, Liapis CD, Perrea D, Angelopoulou N, Alevizos M. Beneficial effects of combined treatment with rosiglitazone and exercise on cardiovascular risk factors in patients with type 2 diabetes. Diabetes Care 2007; 30(9): 2242–4. 10.2337/dc07-0341 [DOI] [PubMed] [Google Scholar]
- [47].Okada S, Hiuge A, Makino H, et al. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J Atheroscler Thromb 2010; 17(8): 828–33. 10.5551/jat.3798 [DOI] [PubMed] [Google Scholar]
- [48].Cohen ND, Dunstan DW, Robinson C, Vulikh E, Zimmet PZ, Shaw JE. Improved endothelial function following a 14-month resistance exercise training program in adults with type 2 diabetes. Diabetes Res Clin Pract 2008; 79(3): 405–11. 10.1016/j.diabres.2007.09.020 [DOI] [PubMed] [Google Scholar]
- [49].Naka KK, Kalantaridou SN, Kravariti M, et al. Effect of the insulin sensitizers metformin and pioglitazone on endothelial function in young women with polycystic ovary syndrome: a prospective randomized study. Fertil Steril 2011; 95(1): 203–9. 10.1016/j.fertnstert.2010.06.058 [DOI] [PubMed] [Google Scholar]
- [50].Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci 2017; 18(6): 1321. 10.3390/ijms18061321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kelly KR, Blaszczak A, Haus JM,et al. A7- dexercise program increases high-molecular weight adiponectin in obese adults. Med Sci Sports Exerc 2012; 44(1): 69–74. 10.1249/MSS.0b013e318228bf85 [DOI] [PubMed] [Google Scholar]
- [52].Brunmair B, Staniek K, Gras F, et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes 2004; 53(4): 1052–9. 10.2337/diabetes.53.4.1052 [DOI] [PubMed] [Google Scholar]
- [53].Artwohl M, Fürnsinn C, Waldhäusl W,et al. Thiazolidinediones inhibit proliferation of microvascular and macrovascular cells by a PPARgamma-independent mechanism. Diabetologia 2005; 48(3): 586–94. 10.1007/s00125-005-1672-z [DOI] [PubMed] [Google Scholar]
- [54].Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108(8): 1167–74. 10.1172/JCI13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ghosh S, Lakshmanan AP, Hwang MJ, et al. Metformin improves endothelial function in aortic tissue and microvascular endothelial cells subjected to diabetic hyperglycaemic conditions. Biochem Pharmacol 2015; 98(3): 412–21. 10.1016/j.bcp.2015.10.008 [DOI] [PubMed] [Google Scholar]
- [56].Chen H, Li J, Yang O, Kong J, Lin G. Effect of metformin on insulin-resistant endothelial cell function. Oncol Lett 2015; 9(3): 1149–53. 10.3892/ol.2015.2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Knowler WC, Barrett-Connor E, Fowler SE,et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346(6): 393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006; 49(2): 289–97. 10.1007/s00125-005-0097-z [DOI] [PubMed] [Google Scholar]
- [59].Malin SK, Gerber R, Chipkin SR, Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care 2012; 35(1): 131–6. 10.2337/dc11-0925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Konopka AR, Laurin JL, Schoenberg HM, et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell 2019; 18(1)e12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Walton RG, Dungan CM, Long DE, et al. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: A randomized, double-blind, placebo-controlled, multicenter trial: The MASTERS trial. Aging Cell 2019; 18(6)e13039 10.1111/acel.13039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Boulé NG, Robert C, Bell GJ, et al. Metformin and exercise in type 2 diabetes: examining treatment modality interactions. Diabetes Care 2011; 34(7): 1469–74. 10.2337/dc10-2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Malin SK, Braun B. Impact of metformin on exercise-induced metabolic adaptations to lower type 2 diabetes risk. Exerc Sport Sci Rev 2016; 44(1): 4–11. 10.1249/JES.0000000000000070 [DOI] [PubMed] [Google Scholar]
- [64].Shenouda SM, Widlansky ME, Chen K, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011; 124(4): 444–53. 10.1161/CIRCULATIONAHA.110.014506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang Q, Zhang M, Torres G, et al. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of Drp1-mediated mitochondrial fission. Diabetes 2017; 66(1): 193–205. 10.2337/db16-0915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Nyström T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004; 287(6): E1209–15. 10.1152/ajpendo.00237.2004 [DOI] [PubMed] [Google Scholar]
- [67].Deacon CF, Danielsen P, Klarskov L, Olesen M, Holst JJ. Dipeptidyl peptidase IV inhibition reduces the degradation and clearance of GIP and potentiates its insulinotropic and antihyperglycemic effects in anesthetized pigs. Diabetes 2001; 50(7): 1588–97. 10.2337/diabetes.50.7.1588 [DOI] [PubMed] [Google Scholar]
- [68].Ahrén B. Emerging dipeptidyl peptidase-4 inhibitors for the treatment of diabetes. Expert Opin Emerg Drugs 2008; 13(4): 593–607. 10.1517/14728210802584126 [DOI] [PubMed] [Google Scholar]
- [69].Ceriello A, Esposito K, Testa R,Bonfigli AR, Marra M, Giugliano D. The possible protective role of glucagon-like peptide 1 on endothelium during the meal and evidence for an “endothelial resistance” to glucagon-like peptide 1 in diabetes. Diabetes Care 2011; 34(3): 697–702. 10.2337/dc10-1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ding L, Zhang J. Glucagon-like peptide-1 ac tivates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol Sin 2012; 33(1): 75–81. 10.1038/aps.2011.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 2001; 50(3): 609–13. 10.2337/diabetes.50.3.609 [DOI] [PubMed] [Google Scholar]
- [72].Martins C, Kulseng B, King NA, Holst JJ, Blundell JE. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J Clin Endocrinol Metab 2010; 95(4): 1609–16. 10.1210/jc.2009-2082 [DOI] [PubMed] [Google Scholar]
- [73].Malin SK, Francois ME, Eichner NZM, et al. Impact of short-term exercise training intensity on ß-cell function in older obese adults with prediabetes. J Appl Physiol 2018; 125(6): 1979–86. 10.1152/japplphysiol.00680.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Malin SK, Huang H, Mulya A, Kashyap SR, Kirwan JP. Lower dipeptidyl peptidase-4 following exercise training plus weight loss is related to increased insulin sensitivity in adults with metabolic syndrome. Peptides 2013; 47: 142–7. 10.1016/j.peptides.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Seelig E, Trinh B, Hanssen H, et al. Exercise and the dipeptidyl-peptidase IV inhibitor sitagliptin do not improve beta-cell function and glucose homeostasis in long-lasting type 1 diabetes— A randomised open-label study. Endocrinology, diabetes & metabolism 2019; 2(3): e00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tanimura Y, Aoi W, Mizushima K, Higashimura Y, Naito Y. Combined treatment of dipeptidyl peptidase-4 inhibitor and exercise training improves lipid profile in KK/Ta mice. Exp Physiol 2019; 104(7): 1051–60. 10.1113/EP087449 [DOI] [PubMed] [Google Scholar]
- [77].Takada S, Masaki Y, Kinugawa S,et al. Dipeptidyl peptidase-4 inhibitor improved exercise capacity and mitochondrial biogenesis in mice with heart failure via activation of glucagon-like peptide-1 receptor signalling. Cardiovasc Res 2016; 111(4): 338–47. 10.1093/cvr/cvw182 [DOI] [PubMed] [Google Scholar]
- [78].Sakamoto M, Nishimura R, Irako T, Tsujino D, Ando K, Utsunomiya K. Comparison of vildagliptin twice daily vs. sitagliptin once daily using continuous glucose monitoring (CGM): crossover pilot study (J-VICTORIA study). Cardiovasc Diabetol 2012; 11(1): 92. 10.1186/1475-2840-11-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shigematsu E, Yamakawa T, Oba MS, et al. Arandomized controlled trial of vildagliptin versus alogliptin: effective switch from sitagliptin in patients with type 2 diabetes. J Clin Med Res 2017; 9(7): 567–72. 10.14740/jocmr3012w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Baranov O, Kahle M, Deacon CF, Holst JJ, Nauck MA. Feedback suppression of meal-induced glucagon-like peptide-1 (GLP-1) secretion mediated through elevations in intact GLP-1 caused by dipeptidyl peptidase-4 inhibition: a randomized, prospective comparison of sitagliptin and vildagliptin treatment. Diabetes Obes Metab 2016; 18(11): 1100–9. 10.1111/dom.12706 [DOI] [PubMed] [Google Scholar]
- [81].Liu H, Xiang H, Zhao S, et al. Vildagliptin improves high glucose-induced endothelial mitochondrial dysfunction via inhibiting mitochondrial fission. J Cell Mol Med 2019; 23(2): 798–810. 10.1111/jcmm.13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Krüger N, Biwer LA, Good ME, et al. Loss of endothelial FTO antagonizes obesity-induced metabolic and vascular dysfunction. Circ Res 2020; 126(2): 232–42. 10.1161/CIRCRESAHA.119.315531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ottolini M, Hong K, Cope EL, et al. Local Peroxynitrite Impairs Endothelial TRPV4 Channels and Elevates Blood Pressure in Obesity. Circulation 2020. 10.1161/CIRCULATIONAHA.119.043385 [DOI] [PMC free article] [PubMed] [Google Scholar]