Abstract
ApoD is a 25 to 30 kDa glycosylated protein, member of the lipocalin superfamily. As a transporter of several small hydrophobic molecules, its known biological functions are mostly associated to lipid metabolism and neuroprotection.
ApoD is a multi-ligand, multi-function protein that is involved lipid trafficking, food intake, inflammation, antioxidative response and development and in different types of cancers.
An important aspect of ApoD’s role in lipid metabolism appears to involve the transport of arachidonic acid, and the modulation of eicosanoid production and delivery in metabolic tissues. ApoD expression in metabolic tissues has been associated positively and negatively with insulin sensitivity and glucose homeostasis in a tissue dependent manner.
ApoD levels rise considerably in association with aging and neuropathologies such as Alzheimer’s disease, stroke, meningoencephalitis, moto-neuron disease, multiple sclerosis, schizophrenia and Parkinson’s disease. ApoD is also modulated in several animal models of nervous system injury/pathology.
Keywords: Apolipoprotein D, Lipocalin, Lipid transport, Lipid metabolism, Arachidonic acid, Neuroprotection
1. Introduction and characteristics of apolipoprotein D
Apolipoprotein D (ApoD) is a 29 kDa glycoprotein first detected in 1963, as a distinct component of the human plasma lipoprotein system (Ayrault-Jarrier, Levy, and Polonovski 1963), and partially characterized in 1973 (McConathy and Alaupovic, 1976). The deduced amino acid sequence from the cloned human cDNA (Drayna et al, 1986) revealed that it is an atypical lipoprotein. It harbors a high degree of homology with the lipocalins, a family of proteins that transport small hydrophobic molecules. Several studies have shown that ApoD is poorly expressed in the liver and intestines, the organs that normally produce apolipoproteins. Instead, ApoD is widely expressed in several other tissues across many species (Drayna et al., 1986, Smith et al., 1990; Provost et al., 1990; Séguin et al., 1995). A large number of potential ligands of different structure and function have been identified. Due to this apparent ligand heterogeneity and wide tissue distribution, a role as a multi-ligand, multi-function protein has been proposed for ApoD.
2. ApoD structure
2.1. The gene
The ApoD gene is located on human chromosome 3 (Drayna et al., 1987) and mouse chromosome 16 (Warden et al., 1992). The promoter of the human ApoD gene contains a large number of regulatory elements among which response elements to steroids, oestrogen, progesterone and glucocorticoids. It also contains response elements to fatty acids, liver X receptors (LXR), acute phase and serum as well as elements involved in the immune response such as Nuclear Factor kappa B (NF-κB) (Lambert et al., 1993; Do Carmo et al., 2002; Hummasti et al., 2004; Do Carmo et al., 2007). The presence of such a large number of regulatory elements contributes to the complex regulation of ApoD expression.
2.2. The protein
ApoD is highly conserved amongst mammalian species. The human protein shares 87.3% identity with the Rhesus monkey protein and is remarkably similar to the rabbit (79.3%), the mouse (73.1%) and the rat (73.1%) ApoD (source: Uniprot Database). The amino acid sequence also shares significant similarity with the lipocalin family: 25% with retinol binding protein and 30 to 40% with insect bilin binding protein (Drayna et al., 1987; Weech et al., 1991). The protein is composed of 169 residues, including a secretion peptide signal of 20 amino acids. Two glycosylation sites (asparagines 45 and 78) account for the molecular weight of the mature protein varying from 20 to 32 kDa (Weech et al., 1991; Yang et al., 1994; Schindler et al., 1995; Rassart et al., 2000). An intriguing characteristic of ApoD is that its glycosylation level varies depending on its production site. Cerebral ApoD has a lower molecular weight compared to peripheral ApoD. N-glycosylation removal revealed that this is due to differential glycosylation. Interestingly, this difference in glycosylation level could influence ApoD’s function and might be an evidence of different functional roles between brain and peripheral ApoD (Li et al., 2016).
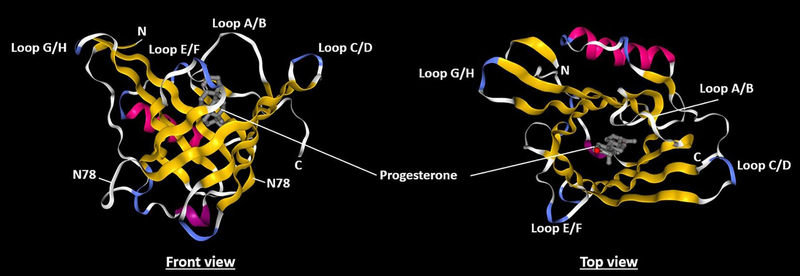
The tertiary structure of ApoD is formed from 8 antiparallel β-sheets creating a hydrophobic cavity capable of receiving different ligands (Peitsch and Boguski, 1990; Eichinger et al., 2007) (Fig. 1). The human ApoD contains 5 cysteine residues, 4 of which are involved in intramolecular disulfide bonds. The last cysteine residue can form intermolecular disulfide bridges with another ApoD protein (dimers) or with proteins associated with High density lipoproteins (HDL) namely apolipoproteins A-I and A-II (apoA-I, apoA-II) and Lecithin cholesterol acyltransferase (LCAT) (Fielding and Fielding, 1980; Weech et al., 1991; Blanco-Vaca et al., 1992; Yang et al., 1994). While ApoD has mostly been described in its monomeric form, dimers (Bhatia et al., 2013) and tetramers (Kielkopf, 2018) have been observed. Oligomerization is a common feature of lipocalins and is known to impact lipocalin ligand binding. ApoD has been shown to dimerize during oxidative stress especially in the hippocampal region of late stage Alzheimer’s disease patients. ApoD dimerization seems to occur as an antioxidative response following the interaction of methionine 93 with lipid hydroperoxides, unstable lipid species produced in response to excessive free radical production (Bhatia et al., 2012) which must be cleared from the organism to prevent membrane and tissue damage.
Fig. 1. Structure of human ApoD in complex with progesterone.
(adapted from Eichinger et al., 2007). Left: front view showing the 8 anti-parallel β-strands (yellow) structure forming the hydrophobic cavity typical of the lipocalins. Loops A/B, C/D, E/F and G/H link the β-strands at the open end of the protein. Other loops are present at the opposite end of the protein (white). There are 2 α-helix (purple) located close to the cavity, one of which is able to close the cavity. The two glycosylated residues (N45 and N78) are shown. The 2 disulfide bridges are not visible. Right: top view of the cavity showing the progesterone ligand (grey). Loops A/B, E/F and G/H are rich in hydrophobic residues and very likely enable the protein to interact with HDL and with membranes. For details, see Eichinger et al., 2007.
Also, disulfide linked ApoD homodimers have been found in urine (Blanco-Vaca and Pownall, 1993) and tears (Holzfeind et al., 1995), while tetramers have been observed in breast cyst fluids (ApoD’s main form in that fluid) (Kielkopf, 2018). Additionally, hydrophobic residues are located in close proximity to the open end of ApoD’s binding pocket and may facilitate interactions with cell membranes and lipoproteins (Eichinger et al., 2007). ApoD associates most strongly with HDL and to a lower extent to low and very low density lipoproteins (LDL and VLDL) (Perdomo et al., 2010). Biosensor experiments have shown that ApoD mediates HDL2 and 3 interactions with LDL and the cell surface (Braesch-Andersen et al., 2014). In addition to its association with plasma lipoproteins, ApoD appear to be a very specific marker of the exosome-containing fraction of the central nervous system (Pascua-Maestro et al., 2018).
2.3. ApoD ligands
2.3.1. Arachidonic acid
Arachidonic acid (AA) is the ligand that binds to ApoD with the best known affinity (Ka of 108 M−1 compared to a Ka of 106 M−1 for progesterone) (Morais Cabral et al., 1995). AA is the precursor of prostaglandin synthesis, bioactive molecules that are involved in several biological pathways such as inflammation, platelet aggregation and cellular regulation (Kuehl and Egan, 1980). Indeed, under the action of phospholipase A2, the AA present in membranes is liberated and becomes the substrate of lipoxygenases and cyclooxygenases (mostly Cox2) leading to prostaglandin and leukotriene formation in inflammatory situations (Smith et al., 2000). Thus, as an AA transporter, ApoD could play an important role in cellular protection and regulation.
2.3.2. Steroids
ApoD (initially named GCDFP-24) is the major protein constituent of human mammary cyst fluid. It binds with a relatively strong affinity to progesterone and pregnenolone and with a weaker affinity to oestrogen (Pearlman et al., 1973; Lea, 1988; Balbin et al., 1990; Vogt and Skerra, 2001). However, another study shows a lack of interaction between ApoD and β-estradiol, suggesting that oestrogen is possibly not a natural ligand of ApoD (Ruiz et al., 2013).
2.3.3. Cholesterol
Depending upon the experimental design, poor or lack of direct binding between ApoD and cholesterol has been reported (Morais Cabral et al., 1995; Patel et al., 1997; Ruiz et al., 2013; Marcoux-Legault, Brissette and Rassart, unpublished results). Cholesterol is a major membrane and lipoprotein constituent. ApoD was identified as a component of HDL. Thus, in association with LCAT, ApoA-I or CETP (Cholesteryl Ester Transfer Protein), it could be involved in the reverse transport of cholesterol to the liver (Spreyer et al., 1990; Weech et al., 1991; Blanco-Vaca et al., 1992; Yang et al., 1994). The esterification activity of LCAT is increased in the presence of ApoD (Fielding and Fielding, 1980; Steyrer and Kostner, 1988), two proteins that were shown to form heterodimers (Weech et al., 1991; Blanco-Vaca et al., 1992).
2.3.4. Other ligands
Molecular modeling studies have revealed that components derived from the heme, such as bilirubin, could be putative ApoD ligands. Accordingly, binding between ApoD and bilirubin was demonstrated (Ka of 3.107 M−1) (Peitsch and Boguski, 1990) and this property could promote the interaction of bilirubin with HDL (Goessling and Zucker, 2000). Other ligands have been identified such as E-3-methyl-2-hexenoic acid, a molecule present in axillary secretions (Zeng et al., 1996); retinoic acid, which is involved in cellular differentiation; as well as sphingomyelin and sphingolipids which are major components in HDL and in cellular membranes (Breustedt et al., 2006; Rhinn and Dolle, 2012; Vance, 2012; Ruiz et al., 2013). However, as for cholesterol, the binding of some ligands is rather weak and their biological relevance remains controversial. The fact that ApoD may bind such a large variety of ligands could be the reflection of a multi-ligand, multi-function activity.
3. ApoD expression
3.1. Tissue expression
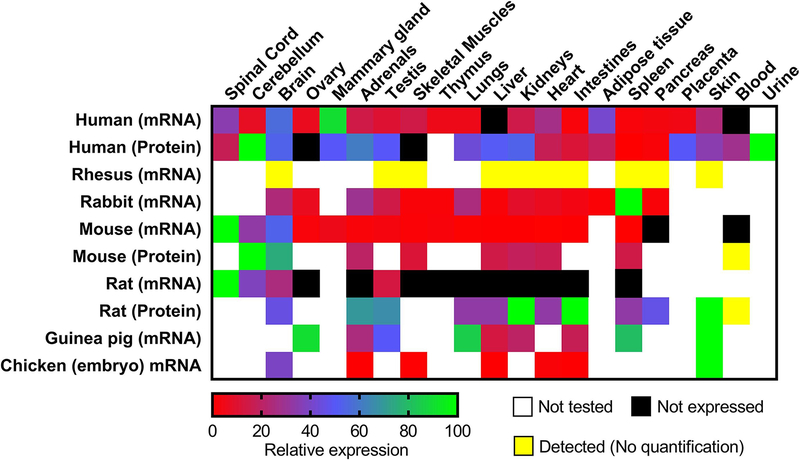
ApoD is expressed at different levels in a wide range of tissues in several mammalian species both during developmental and adult stages (Fig. 2). The wide species distribution of ApoD supports its evolutionary importance in the chordate lineage (Sanchez et al., 2003; Ganfornina et al., 2005) and its amino-acid homology is very high among mammals (Rassart et al., 2000). Since its characterization in mammalian species, it has also been detected in chicken (Ganfornina et al., 2005), Drosophila (Sanchez et al., 2000), plants (Frenette-Charron et al., 2002) and bacteria (Bishop et al., 1995). Mostly associated with plasmatic HDL, avian ApoD is present in rapidly growing oocytes, suggesting a role in the transport of lipids during embryogenesis (Vieira et al., 1995; Yao and Vieira, 2002). In humans, ApoD is poorly expressed in the liver and intestines, the major sites of apolipoprotein synthesis.
Fig. 2. Relative ApoD mRNA and protein expression in tissues of different species.
ApoD expression in the figure is an average value from the literature. ApoD expression in a tissue is expressed as a percentage of the strongest ApoD tissue expression for that species. Tissue expression between different species should not be compared as they are only relative to their own species. Tissues in which ApoD was detected but not quantified are noted in yellow. Expression data were obtained from multiple sources: Human (Uhlen et al. 2015) data available at https://www.proteinatlas.org/ENSG00000189058-APOD/tissue, (Fishilevich et al. 2016; Ben-Ari Fuchs et al. 2016) data available at https://www.genecards.org/cgi-bin/carddisp.pl?gene=APOD, (Séguin et al.,1995; Drayna et al. 1986); Rhesus (Smith et alo., 1990); Rabbit (Provost et al. 1990); Mouse (Séguin et al., 1995; Do Carmo et al. 2009a; Li et al. 2016; Cofer and Ross 1996); Rat (Séguin, Desforges, and Rassart 1995; Boyles et al., 1990b); Guinea pig (Provost et al. 1995); Chicken (Ganfornina et al. 2005).
In contrast, ApoD mRNA expression is strongest in the mammary glands and central nervous system (Fig. 2). However, as it is a secreted protein, ApoD protein levels vary significantly from mRNA expression. While ApoD doesn’t appear to be produced in the liver, the protein is present in high amounts. Similarly, mRNA expression is weak in the adrenals, testis, lungs, kidneys and placenta, but strong at the protein level. ApoD has been detected in multiple tissues in the monkey (Smith et al., 1990), but the absence of quantified data limits the comparison to human expression. Rats and mice both have an ApoD mRNA expression almost exclusively limited to the CNS (Boyles et al., 1990b; Séguin et al 1995). In mice, most of the protein is also found in the CNS (Li et al. 2016). In rats, however, moderate to high protein levels are found in peripheral tissues (Boyles et al., 1990b).
3.2. Cellular expression
ApoD mRNA is mostly expressed by fibroblasts, particularly in fibroblasts located in the proximity of blood vessels (Smith et al., 1990; Provost et al., 1991b). In the nervous system, expression is strong in white matter glial cells, in particular astrocytes and oligodendrocytes. However, in the peripheral nervous system, expression is detected in endoneural fibroblasts (Boyles et al., 1989; Boyles et al., 1990b; Provost et al., 1991a).
3.3. Modulation of ApoD in physiological conditions
In normal individuals, serum ApoD concentration varies between 5 and 23 mg/100 ml (Camato et al, 1989) but its concentration has been shown to be affected in several studies.
ApoD expression is modulated in pathologies that present metabolic defects such as familial HDL deficiency and Tangier diseases (Alaupovic et al., 1981), familial LCAT deficiency (Albers et al., 1985) and type 2 diabetes (Baker et al., 1994; Hansen et al., 2004). The common link between these diseases could be the activation of pro-inflammatory and/or pro-angiogenic pathways related to the metabolism of AA, the best ApoD ligand (Hummasti et al., 2004; Labrie et al., 2015). A neuroprotective and anti-inflammatory effect of ApoD could result from its capacity to stabilize AA at the cellular membrane or to sequester it, thus preventing its transformation into pro-inflammatory molecules (Thomas et al., 2003a, 2003b; Desmarais et al., 2019).
Tangier disease is caused by a mutation in the ATP-binding Cassette A1 transporter (ABCA1) gene, which control cholesterol efflux (Bodzioch et al., 1999). ABCA1 loss of function causes cholesterol accumulation in peripheral organs and an increased risk of atherosclerosis (Bodzioch et al., 1999). Considering that ApoD can bind cholesterol, it could facilitate removal of cholesterol excess throughreverse transport via HDL and therefore protect against atherosclerosis (Pfrieger, 2003; Tall, 2008). Moreover, the protein profile of HDL isolated from atherosclerotic patients shows an ApoD increase, which could be a compensatory mechanism for cholesterol accumulation (Vaisar et al., 2007). In addition to its accumulation in HDL particles, ApoD is present in atheroma, lipid plaques in arteries, and is associated with excessive cholesterol accumulation in atherosclerotic patients and mouse models of atherosclerosis (Sarjeant et al., 2003; Perdomo and Dong, 2009).
3.4. Growth and differentiation
Studies on primary cultures of human fibroblasts have shown that a forced arrest in cell growth can induce ApoD expression (Provost et al 1991a). This induction has also been noted in non-proliferating quiescent and senescent fibroblast cultures and in several other cell types (Do Carmo et al. 2002).
In normal cell culture conditions, ApoD is secreted and reinternalized (Sarjeant et al 2003, Leung et al 2004, Do Carmo et al 2007). ApoD is able to selectively modulate the proliferative response of vascular smooth muscle cells to growth factors by inhibiting nuclear translocation of the active form of ERK1/2 (extracellular signal-regulated kinase 1/2) (Sarjeant et al., 2003).
ApoD expression can be induced in some stress conditions, such as oxidative and inflammatory stresses or UV treatment (Do Carmo et al., 2007). This induction is observed specifically at peroxide concentrations (oxidative stress) and UV doses that induce growth arrest but not apoptosis. Nuclear factors PARP-1 (Poly ADP-ribose polymerase-1) and APEX (Apurinic/Apyrimidinic Endonuclease-I), factors that are upregulated in growth-arrested cells, can both bind the ApoD promoter and induce ApoD expression (Levros et al., 2010). Both APEX and PARP-1 are known to be induced in pathologic situations such as oxidative stress and neurodegenerative conditions (Fritz et al., 2003; Duan et al., 2007). Moreover, in several stress situations, ApoD is localized to the perinuclear level (Do Carmo et al 2007).
Several studies have shown an inverse correlation between ApoD expression and cell growth. In parallel, studies on breast cancer cell lines have shown that ApoD expression is inhibited by oestrogens (cell growth inducer) and stimulated by androgens (cell growth inhibitor) (Simard et al., 1990; Simard et al., 1991). Moreover, ApoD expression concomitant with inhibition of cell growth was observed in some breast cancer cell lines treated with Interleukin-1α and retinoic acid (Blais et al., 1994; Lopez-Boado et al., 1994). So far, the only known exception is Interleukin-6 as it inhibits both cell proliferation and ApoD expression (Blais et al., 1995).
Beside cell growth, ApoD expression is also linked to cell differentiation. Two mediators of cell differentiation, retinoic acid and 1,25-dihydroxyvitamin D3 both induce ApoD expression in mammary cancer cells (Lopez-Boado et al., 1994; Lopez-Boado et al., 1997). This induction appears to be mediated by retinoic acid nuclear receptors (RAR) that reduce cell growth. This suggests that ApoD could be a marker of RAR-mediated differentiation and growth arrest in mammary cancer cells (Lopez-Boado et al., 1996). A similar correlation was also observed in human prostate cancer tissue (Aspinall et al., 1995), where ApoD is mostly present in glandular prostatic epithelium and could be associated with a non-proliferative or differentiated state. In summary, ApoD expression is stimulated in growth arrest, cell senescence and during oxidative and inflammatory stresses. In contrast, ApoD expression is reduced during cell growth.
3.5. Influence of metabolic pathways
There is a positive correlation between adipose mass, circulating leptin levels and hypothalamic ApoD expression (Liu et al., 2001). However, this correlation disappears in ob/ob obese mice (leptin deficient) and db/db mice (harboring a mutation in the leptin receptor). These mice present a reduced level of hypothalamic ApoD compared to wild type mice. The same study shows a specific and direct interaction of ApoD and the cytoplasmic portion of the long form of the leptin receptor (Liu et al., 2001). This suggests that ApoD in the hypothalamus is involved in the signaling pathway that controls appetite and probably fat accumulation. Plasma ApoD levels decrease significantly during normal uncomplicated pregnancy. ApoD is further decreased in women with excessive gestational weight gain and their newborns. In these women, ApoD concentration was tightly associated with lipid parameters (Do Carmo et al., 2009b). In the adipose tissues of morbidly obese women (BMI over 40), ApoD protein expression is positively correlated with parameters of metabolic health. Women with high levels of ApoD in their round ligament, an adipose depot that form on the liver of obese individuals, have lower insulin resistance, lower insulin blood levels as well as lower circulating Tumor Necrosis Factor-α (TNF-α) and Plasminogen Activator Inhibitor-1 levels (Desmarais et al., 2018). This could indicate that ApoD’s higher expression is beneficial in obesogenic conditions. Since TNF-α is a potent disruptor of insulin signaling (Akash et al., 2017), it is possible that ApoD’s association with increased insulin sensitivity is linked to an anti-inflammatory effect of ApoD in the round ligament. This association has yet to be evaluated in men.
Interestingly, ApoD has been identified as a LXR-regulated gene that is upregulated following adipocyte differentiation. It has been hypothesised that ApoD could transport ligands for LXR or PPAR (peroxisome proliferator-activated receptor) in differentiated adipocytes (Hummasti et al., 2004). Treatment of 3T3-L1 adipocytes with AA, ApoD’s preferential ligand, increases the level of glucose transporters GLUT1 and GLUT4 incorporation into the plasma membranes, and consequently increases glucose uptake. This phenomenon is dependent upon an intact PPARγ and lipoxygenase activities but not on cyclooxygenase activity (Nugent et al., 2000). Part of ApoD’s effect on glucose homeostasis in adipose tissues could therefore be due to a modulation of eicosanoid production or delivery.
A role for ApoD in hepatic lipid metabolism was highlighted by a study on transgenic mice overexpressing human ApoD in their central nervous system (CNS) (Do Carmo et al., 2009a). These mice also showed ApoD transgenic protein accumulation in the liver and slowly developed a hepatic and muscular steatosis accompanied with insulin resistance (Do Carmo et al., 2009a). However, these mice were not obese or diabetic and they did not develop inflammation. The development of this pathology was triggered at least in part by precise signaling events involving prostanoids derived from AA and PPARγ activation (Labrie et al., 2015; Desmarais et al., 2019). ApoD-induced lipid accumulation was not due to de novo lipogenesis but rather from increased lipid uptake. An important characteristic of ApoD-induced hepatic steatosis was that omega-3 and omega-6 fatty acids (including AA) were preferentially accumulated over saturated and mono-unsaturated fatty acids. Additionally, omega-3 fatty acids (mainly DHA) appeared to be accumulated at an even greater pace than omega-6 fatty acids, resulting in a more favorable omega-3/omega-6 balance in transgenic mice compared to wild type controls (Desmarais et al., 2019). This intriguing phenomenon suggests that DHA and/or other omega-3 fatty acids might also be ApoD ligands. Together, the favorable omega-3/omega-6 balance, the production of anti-inflammatory eicosanoids and the activation of PPARγ appear to prevent chronic inflammation despite extensive lipid accumulation (Desmarais et al., 2019). As for the adipose tissues, the full extent of ApoD’s involvement in AA metabolism is unknown. Interestingly, ApoD overexpression in HepG2 cells increases PPARγ transcriptional activity, a phenomenon that is amplified by the addition of AA (Labrie et al., 2015). Earlier articles show that ApoD can limit inflammation by sequestering AA in cell membranes (Thomas et al., 2003b; Do Carmo et al., 2008) but ApoD might also influence the fate of AA upon release.
An additional effect of ApoD might occur through its capacity to associate with osteopontin (OPN) (Jin et al., 2006). OPN is a negatively charged extracellular protein found in bones and produced by various cell types including kidney, placenta, arterial smooth muscle and immune cells. OPN promotes cell migration, adhesion, and activation of T lymphocytes and macrophages (Gravallese, 2003; Jürets et al., 2016). OPN is overexpressed in atherosclerosis and in obesity-induced adipose tissue inflammation where it mediates macrophage infiltration (Giachelli et al., 1995; Nomiyama et al., 2007). Interestingly, ApoD reduces cell invasiveness by blocking OPN-mediated cell adhesion and proliferation (Jin et al., 2006). Remarkably, OPN-deficient obese mice display improved insulin sensitivity and decreased adipose tissue macrophage infiltration as well as decreased inflammation (Nomiyama et al., 2007). Therefore, blockage of OPN function by ApoD could further explain its anti-inflammatory and insulin sensitizing effects. OPN is also an important factor in liver inflammation, fibrosis and carcinogenesis (Bruha et al., 2020). The interaction between ApoD and OPN could constitute a previously overlooked mechanism by which ApoD can limit the deleterious effects occurring during the development of hepatic steatosis in transgenic mice (Do Carmo et al., 2009a).
ApoD-null mice display non-fasting hypertriglyceridemia and a 30% to 50% increase in food intake, yet do not show an increase in body weight, and have normal glucose tolerance and islet morphology. In these mice, adipose tissue lipoprotein lipase (LPL) activity is reduced by 35% and triglyceride hepatic content is low (Jimenez-Palomares et al., 2011). Inversely, hepatic transitory overexpression of ApoD in CD-1 mice increases plasmatic LPL activity and postprandial triglyceride clearance. In vitro, ApoD dose-dependently increases free fatty acid release from VLDL-triglycerides (Perdomo et al., 2010).
ApoD-null female mice display progressive bone loss with aging with a 50% reduction in trabecular bone volume (Martineau et al, 2016). Histochemistry indicates significantly higher osteoblast surface and number of osteoclasts in femora. Also, ApoD-null females and males present a 24% reduction in cortical bone volume. These results indicate that ApoD influences bone metabolism in mice in a genderspecific manner (Martineau et al., 2016). The receptor activator of nuclear factor-κB ligand (RANKL), the key final effector of osteoclastogenesis and bone resorption, is overexpressed in bone marrow mesenchymal cells isolated from ApoD-null mice. This is reversed in vitro by treatment with human ApoD. RANKL expression and secretion by osteoblasts is stimulated by inflammatory cytokines such as IL-1, TNF-α, and M-CSF (Weitzmann, 2013). In this study, ApoD was suggested to act as an anti-inflammatory and antioxidant agent. An additional mechanism for ApoD’s role in bone metabolism could involve ApoD’s blockage of OPN function (Jin et al., 2006). OPN is implicated in the activation of the bone resorption process; it serves as an attachment protein for osteoclast to adhere to the bone matrix (Rittling et al., 1998; Weber and Cantor, 1996).
In summary, ApoD appears to be a beneficial actor in both lipid and bone metabolism as it is associated with inflammation resorption as well as bone turnover regulation.
3.6. Role in development processes
A modulation of ApoD expression is observed during pregnancy and embryonic development. Low levels of ApoD transcripts are observed in guinea pig embryos (Provost et al., 1991a). In mice, embryonic ApoD expression begins between days 8 and 9 and is selectively modulated until birth (Sanchez et al., 2002). In rats, the induction of ApoD during embryo development coincides with active myelination and synaptogenesis (Ong et al., 1999). In chickens, ApoD is present in the rapidly growing oocyte and is associated with mobilization and transport of lipids (Vieira et al., 1995).
In humans, ApoD is strongly expressed in the endometrium during implantation and in the placenta (Drayna et al., 1986; Kao et al., 2002). ApoD plasmatic levels decrease during pregnancy and are even lower in women that gained excessive weight (Do Carmo et al., 2009b).
Androgen insensitivity syndrome is the most common cause of sexual development disorder and is usually due to a defective androgen receptor. The ApoD transcript is one of the few transcripts significantly upregulated by dihydrotestosterone in human male genital fibroblasts and is under consideration as a marker of androgen sensitivity (Appari et al., 2009). The function of ApoD in male genital formation is still unclear. Tanase-Nakao et al. proposed that ApoD could be involved in genital masculinization by regulating angiogenesis. They also recognized that dynamic cell-to-cell interactions are necessary for genital masculinization (Tanase-Nakao et al., 2019). ApoD is implicated in astrocyte to neuron communication through extracellular vesicle exchange (Pascua-Maestro et al., 2018). The authors show that ApoD, mostly expressed by astrocytes, is exclusively transported to neurons by extracellular vesicle and internalized to promote integrity and survival. It is plausible that ApoD could be similarly implicated in the cell-to-cell interactions taking place during genital masculinization.
During angiogenesis, endothelial and mural cells need to communicate to produce functional blood vessels. A multitude of genes, including ApoD, are modulated during this process. One study showed that ApoD is downregulated by NOTCH3 in mural cell during angiogenesis. That same study showed that ApoD negatively regulates cell adhesion by reducing focal contacts and that ApoD downregulation by endothelial cells is necessary for vessel maturation (Pajaniappan et al., 2011). ApoD overexpression in response to LXR activation was also shown to suppress the tube formation of human umbilical vein endothelial cells. ApoD, interacting with SR-B1, appears to suppress phosphorylation of AKT, which in turn inhibits NF-κB and eNOS. The resulting effect is the reduced expression of Snail and matrix metalloproteinases and thus the suppression of the angiogenesis process (Lai et al., 2017). Other studies however, showed that ApoD and platelet-derived growth factor (PDGF)-BB have synergetic action in promoting vascular smooth muscle migration (Leung et al 2004) and that ApoD is strongly expressed in both developing and mature perivascular fibroblasts (Smith et al., 1990; Provost et al 1991b; Sanchez et al., 2002) suggesting that ApoD expression might only be suppressed at specific steps during the angiogenesis process.
3.7. Role in cancer
ApoD is expressed and modulated in several cancers. It is overexpressed in numerous types of tissue carcinomas, such as breast, ovaries, prostate, skin and CNS. In prostate, pancreas and skin, a high level of ApoD expression is related to cancer invasiveness (Aspinall et al., 1995; Zhang et al., 1998; Ashida et al., 2004; Hall et al., 2004). Additionally, higher ApoD expression was also associated with shorter survival and recurrence-free survival in non-small cell lung cancer (Cury et al., 2019). However, early research on breast and CNS cancers strongly associated increased ApoD expression to a highly differentiated, non-invasive and non-metastatic state (Serra Diaz et al., 1999; Hunter et al., 2002; Porter et al., 2003). ApoD overexpression is often linked to a decrease of cancer relapse and thus better survival for patients with these cancer types (Diez-Itza et al., 1994). Some studies suggest that growth arrest due to differentiation in these cancers explains the high ApoD expression. This increase of ApoD expression could be an unfavorable factor for cancer progression (Ryu et al., 2001; Iacobuzio-Donahue et al., 2002; Miranda et al., 2003; West et al., 2004). It remains to be determined whether ApoD expression is a cause or a consequence of cell transformation. Interestingly, ApoD reduces OPN-mediated adherence, invasiveness, and proliferation of Rama37 breast cancer cells in vitro (Jin et al., 2006). OPN expression is increased in transformed cells and is inversely correlated with breast cancer patient survival (Rudland et al., 2002). OPN is thought to facilitate invasion by upregulating the expression of matrix metalloproteinase 9 through NF-κB activation (Zhang et al., 2005).
A recent study performed on a cohort of patients diagnosed with invasive ductal breast carcinoma shows that ApoD could be an independent predictor of metastasis free survival and overall survival in invasive breast cancer. Increased ApoD expression was associated with poor patient outcome (Jankovic-Karasoulos et al., 2020), a result shared with another research team studying breast cancer outcomes in patients over the age of 70 (Soiland et al., 2009). It is important to note that patients in this last study were under tamoxifen therapy, a drug used to treat estrogen receptor-positive tumors (Harbeck et al., 2019). ApoD can bind tamoxifen and is likely able to interfere with this treatment approach. Accordingly, ApoD negative patients on tamoxifen had better outcomes compared with ApoD positive patients under the same tamoxifen therapy (Soiland et al., 2009). Future studies on ApoD’s role in cancer progression should take caution to separate ApoD’s biological effects from its potential interference with treatment agents. Although both effects are of considerable importance for breast cancer research and the development of therapies.
What differentiates ApoD’s effect on cancer outcomes in different cancer types is largely unknown. Zhou and Luo (Zhou and Luo, 2020) argue that ApoD’s effect on cancer depends on which cell types are transformed. According to them, ApoD is beneficial in parenchymal tumors because it helps to limit cell cycle progression and induces cell senescence. ApoD interferes with arachidonic acid metabolism by sequestering it in cell membranes. Therefore, ApoD reduces the production of prostaglandins and leukotrienes, which can promote cell proliferation and neo-angiogenesis (Romano and Claria, 2003; Matsuyama et al., 2005). ApoD would hence indirectly improve prognosis via several mechanisms. However, ApoD may be deleterious when it affects stromal cells because the cell senescence it induces stimulates the production of inflammatory cytokines (TNF-α and IL-1β) and matrix metalloproteinases. These two factors lead to the degradation of the extracellular matrix and promote tumor malignancy (Kleiner and Stetler-Stevenson, 1999). These opposing effects could explain some of the contradictory reports concerning ApoD’s association with cancer progression and outcomes. More mechanistic studies are needed to better understand ApoD’s role in cancer.
3.8. ApoD and the nervous system
ApoD is expressed by Schwann cells of the peripheral nervous system (Garcia-Mateo et al., 2014). ApoD expression is low in the peripheral nervous fibers of the rat. However, ApoD and apolipoprotein E (ApoE) expression increases drastically after a nerve crush injury (Boyles et al., 1990b). ApoD expression is higher on the site of a crush injury than on the site of a sectioned nerve (Boyles et al., 1990a; Boyles et al., 1990b; Spreyer et al., 1990). This was also observed in the rabbit and marmoset monkey (Boyles et al., 1990a; Boyles et al., 1990b). After a peripheral nerve lesion, myelin degradation releases a large quantity of lipids including cholesterol, which are stocked and reutilized during regeneration. ApoD could be involved in this regeneration process as a lipid transporter. This longstanding hypothesis was confirmed by a study on nerve regeneration in ApoD-null mice and in transgenic mice overexpressing the human ApoD protein (Ganfornina et al., 2010). This study showed that axonal regeneration and remyelination are delayed in the absence of ApoD and stimulated in the transgenic animals. ApoD appears to be essential for proper myelin extracellular leaflet compaction. The repulsive force generated by densely layered, negatively charged glycocalyx proteins can prevent, if not cleared, the extensive bilayer-bilayer membrane interactions that need to occur during myelination. ApoD deficiency in mice is associated with improper glycocalyx clearance and incomplete myelin compaction (Garcia-Mateo et al., 2018).
In human, ApoD is concentrated in the cytosol of white matter glial cells (Navarro et al., 1998; Hu et al., 2001). In the grey matter, ApoD is mostly located in protoplasmic astrocytes and in a few neurons (Navarro et al., 1998; Navarro et al., 2004). ApoD is also detected in the cytosol of perivascular cells and in the pericyte lysosomes of neocortex blood vessel walls (Hu et al., 2001). This suggests a potential role for ApoD in the transport of small hydrophobic molecules in glial cells and in the blood vessels of the CNS. Interestingly, increased ApoD expression is observed in the cerebral cortex of aged patients, possibly due to the increased number of reactive astrocytes (Kalman et al., 2000; del Valle et al., 2003). Increased ApoD expression is also observed in several neurodegenerative diseases.
4. ApoD and neurodegenerative diseases
4.1. Alzheimer’s disease
ApoD protein levels are strongly increased in the cerebrospinal fluid, hippocampus and cerebral cortex of Alzheimer’s patients (Terrisse et al., 1998; Kalman et al., 2000; Desai et al., 2005). These elevated levels are correlated with the number of neurofibrillary tangles (NFT) but not with senile plaques (Belloir et al., 2001; Glockner and Ohm, 2003). ApoD transcription is altered before these NFT appear and the ApoD protein is rarely observed within these structures (Belloir et al., 2001). The close relationship between Alzheimer’s disease and ApoE has been clearly established and the E4 isoform represents an important risk factor in the development of the disease (Henderson and Finch, 1989). In the hippocampus and cerebrospinal fluid, ApoD levels correlate with the Braak degeneration stage (Glockner and Ohm, 2003) and with the ApoE4 genotype (Glockner and Ohm, 2003). Surprisingly though, it is independent of ApoE protein concentration (Terrisse et al., 1998). In ApoE deficient mice, brain cholesterol levels remain unaltered, possibly because of a compensatory process involving increased ApoD and ABCA1 protein levels (Jansen et al., 2009). In contrast, knock-in mice expressing the ApoE3 and E4 isoforms present a significant increase in cholesterol and its precursors in the brain. Interestingly, ApoD is poorly expressed in these mice’s brains (Jansen et al., 2009). In contrast to the ApoE2 isoform, both E3 and E4 isoforms are able to bind the ApoD promoter and repress its activity in U87 human glioblastomas (Levros et al., 2013). Thus, the poor increase of ApoD expression in mice expressing ApoE3 and E4 could be due to a direct action on the ApoD promoter accompanied with the activator effect of cholesterol accumulation.
4.2. Schizophrenia
Schizophrenia is a severe and chronic psychotic disorder caused by neurotransmitter activity malfunction that affects serotoninergic, glutamatergic and dopaminergic systems (Jones and Pilowsky, 2002; Konradi and Heckers, 2003). This neuropsychiatric pathology is characterized by an alteration in phospholipid metabolism as well as a defect in AA incorporation in platelets and erythrocytes that could contribute to the impairment of neurotransmitter activity (Laugharne et al., 1996; Yao et al., 1996; Horrobin, 1998). AA deficiency has been observed in schizophrenic patients (Thomas et al., 2003b; Yao et al., 2005). As previously mentioned, AA is the best-known ligand of ApoD (Morais Cabral et al., 1995). The plasma and post-mortem brains of schizophrenic patients also show an alteration of ApoD expression (Thomas et al., 2001; Mahadik et al., 2002; Thomas et al., 2003a). Increased ApoD protein levels are found in the brains of post-mortem patients as well as in rats treated with clozapine, an antipsychotic atypical drug effective against schizophrenia (Thomas et al., 2001; Mahadik et al., 2002; Ciapparelli et al., 2003). Levels of AA increased at the erythrocyte membrane in clozapine-treated schizophrenic patients (Vaddadi, 1992; Walker et al., 1999). Thus, an increased expression of ApoD, induced by antipsychotic drugs like clozapine, could facilitate AA membrane incorporation. This close relationship between ApoD expression and AA membrane levels in the presence of clozapine has been demonstrated in mice (Thomas and Yao, 2007).
4.3. Niemann-Pick type C disease
Niemann-Pick type C (NPC) disease is a genetic disorder affecting cellular cholesterol transport, which results in accumulation of non-esterified cholesterol in late endosomes and lysosomes (Kolodny, 2000). An important increase in ApoD expression was observed in the plasma and the brain of a murine model of NPC disease. High levels of ApoD were also observed in the heart, adipose tissue and thymus (Yoshida et al., 1996; Suresh et al., 1998). NPC disease is characterized by a chronic progressive neurodegeneration suggesting a higher vulnerability of neuronal cells following the disruption of intracellular cholesterol trafficking (Suresh et al., 1998; Sevin et al., 2007). In comparison with other tissues, the brain in strongly enriched in cholesterol as it is found in high concentrations in the myelin sheath surrounding axons. Thus, an increased expression of ApoD which binds cholesterol could constitute an attempt at remediating the intracellular cholesterol disruption experienced by NPC mice.
4.4. Other neuropathologies and neurodegenerative lesions
In a rat model of entorhinal cortex lesion, ApoD increases during CNS re-innervation and repair (Terrisse et al, 1999) and its expression is detected in astrocytes and neurons. ApoD mRNA and protein levels increase in the ipsilateral region of the hippocampus as early as 2 days post-lesion, remain high for 10 days and return to normal after 14 days, a period considered necessary for complete re-innervation. Similar results were obtained after kainic acid injection in the hippocampus (Ong et al., 1997; Montpied et al., 1999) or after an experimentally induced stroke (Rickhag et al., 2006; Rickhag et al., 2008). In an experimental model of maternal hyper and hypothyroidism associated with oxidative stress changes in the brain, the hippocampal region of rat pups showed a proportional increase of ApoD expression (Salami et al 2019).
In humans, ApoD expression is strongly increased in glial cells of the substantia nigra of patients with Parkinson’s disease (Ordonez et al., 2006). Increased ApoD expression is also observed in the cerebrospinal fluid of patients having experienced a stroke (Terrisse et al, 1998) and in patients with multiple sclerosis (Reindl et al., 2001). There is a significant correlation of ApoD concentration with age in patients older than 65 years (Waldner et al., 2018). Also, a significant increase of ApoD was detected in the plasma of patients with Parkinson’s disease compared with healthy patients suggesting that it could be a marker for disease progression. Altogether, it appears that ApoD plays an important role in neuron preservation and protection following neurologic impairment.
4.5. Neuroprotective role of ApoD
Several studies confirm the neuroprotective role of ApoD in the presence of different neurodegenerative inducers such as oxidative stress, inflammatory stress and excitotoxicity (Do Carmo et al., 2008; Ganfornina et al., 2008; Najyb et al., 2016).
Transgenic mice overexpressing the human ApoD protein are an appropriate model to study its neuroprotective role. Human ApoD overexpression in the mouse brain increases survival in neurodegenerative conditions. The mice are less sensitive to oxidative stress induced by paraquat, a generator of free oxygen radicals and display a reduced level of lipid peroxidation (Ganfornina et al., 2008). In contrast, mice lacking ApoD present an increased sensitivity to oxidative stress, increased brain lipid peroxidation and impairment of locomotor and learning abilities (Ganfornina et al., 2008). Similarly, the loss of Lazarillo (the Drosophila homolog of ApoD) increases lipid peroxidation and sensitivity to oxidative stress (Sanchez et al., 2000; Sanchez et al., 2006). These Drosophila mutants show reduced longevity. In contrast, Lazarillo overexpression increases longevity by 26% and allows for a strong resistance against oxidative stress (Walker et al., 2006; Hull-Thompson et al., 2009; Ruiz et al., 2012). Overexpression of human ApoD in Drosophila shows similar effects suggesting that the ApoD role is conserved (Muffat et al., 2008). Moreover, the antioxidative role of ApoD is due to the residue methionine 93 which prevents lipid peroxidation (Bhatia et al., 2012; Oakley et al., 2012).
A neuroprotective role for ApoD has also been observed following an inflammatory stress. Intracerebral injection of the human coronavirus OC43 in mice causes encephalitis and an inflammatory demyelination of the CNS very similar to multiple sclerosis (Jacomy and Talbot, 2003) accompanied by cellular death. However, transgenic mice overexpressing human ApoD treated by the OC43 coronavirus presented a better survival rate and a reduced level of inflammation (Do Carmo et al., 2008). This protection appeared to be correlated with a restricted activity of the phospholipase A2 pathway. Interestingly, ApoD needs to be contained in extracellular vesicles to exert its full neuroprotective effects (Pascua-Maestro et al., 2018).
ApoD-null mice and transgenic mice overexpressing human ApoD were crossed with APP-PS1 amyloidogenic mice, a mouse model of Alzheimer’s disease (Li et al 2015). The absence of ApoD caused a 2-fold increase in hippocampal amyloid plaque load. In contrast, neuronal expression of the ApoD transgene reduced hippocampal plaque load by about 35% and was associated with a 60% decrease in amyloid β 1–40 peptide levels, as well as a 34% decrease in insoluble amyloid β 1–42 peptide. This study provides clear evidence that ApoD regulates amyloid plaque pathology, at least in the mouse.
Kainic acid (KA), an analog of glutamate, can induce the over-activation of glutamate receptors and is widely used as a model to explore excitotoxic processes in neurodegenerative injury (Zheng et al., 2011). Because of the high density of kainate receptors, the hippocampus is more sensible to KA-induced neurotoxicity (Darstein et al., 2003). ApoD levels are increased in the hippocampus of KA-treated rats (Ong et al., 1997; Montpied et al., 1999). Also, glutamatergic pathways seem to be particularly affected in ApoD-null mice with a 20% decrease in the density of kainate receptors in the CA 2–3 subfields of the hippocampus, a global decrease in AMPA receptors and a global increase in muscarinic M2/M4 receptors (Boer et al., 2010). These changes may contribute to impairments in learning and memory, motor tasks and orientation-based tasks observed in these animals (Ganfornina et al., 2008), all of which involve glutamatergic neurotransmission.
Transgenic mice overexpressing human ApoD show an increased resistance to KA-induced seizures, a significant reduction of inflammatory responses and a stronger protection against KA-induced cell apoptosis in the hippocampus. This ApoD-mediated protection against KA-induced toxicity was imputed in part to increased plasma membrane Ca2 + ATPase type 2 expression (1.7 fold), decreased NMDA receptor subunit NR2B levels (30%) and a modified lipid metabolism. Indeed, ApoD can diminish intracellular cholesterol content in primary hippocampal neurons and in the brain of transgenic mice. In addition, ApoD can be internalized by neurons, a process that is accentuated in aging and injury (Najyb et al. 2016). ApoD protection appears to be conferred not only through its anti-inflammatory properties but also through the regulation of cholesterol distribution in neurons, and by affecting the levels of proteins limiting excitotoxic effects. Though ApoD’s mode of action has yet to be fully understood, the studies presented above provide clues on the mechanisms involved in ApoD-mediated protection from neurodegenerative conditions. One thing is clear however, ApoD is an important factor in brain homeostasis, repair, and resistance to neurodegenerative stress.
4.6. The ApoD receptor
Several studies have shown that ApoD is secreted and reinternalized (Sarjeant et al 2003, Leung et al 2004, Do Carmo et al 2007; Najyb et al., 2016). This suggests that reinternalization could be receptormediated. The transmembrane glycoprotein basigin (BSG; CD147) was identified as a receptor for ApoD (Najyb et al, 2015). BSG is a membrane glycoprotein receptor, member of the immunoglobulin family, involved in several pathologies such as cancer and Alzheimer disease (Iacono et al., 2007). Internalized ApoD localizes with BSG into vesicular compartments. Downregulation of BSG disrupts the cellular internalization of ApoD. In contrast, overexpression of BSG in SH-5YSY cells, which poorly express BSG, restores the uptake of ApoD. Cyclophilin A, a known ligand of BSG, competitively reduces ApoD internalization confirming that BSG is a key player in the ApoD internalization process.
In summary, BSG is a likely ApoD receptor. This provides additional clues on the mechanisms involved in ApoD-mediated functions, including neuroprotection. Interestingly, BSG is a key player in tumor progression toward metastasis. Its activation results in increased proliferation, invasiveness, angiogenesis and drug resistance, and in limiting apoptosis (Xin et al., 2016). Considering the link between ApoD and cancer progression, it would be reasonable to assume that ApoD could be involved in these BSG-dependent mechanisms.
5. Conclusion
Despite numerous studies on ApoD, little is understood about its physiological function(s). In the nervous system, its role is very likely one of neuroprotection and repair, most likely due to its antioxidant and anti-inflammatory properties. However, ApoD appears to be involved in several other situations and pathologies such as development, cell growth and differentiation, cancer and neurodegenerative diseases. Because of its capacity to bind a wide variety of ligands from bilirubin to its best-known ligand arachidonic acid, it seems quite possible that ApoD acts through different pathways in each tissue or organ where it is found. An interesting prospect for future research is ApoD’s interaction with OPN. Blockage of OPN function could have multiple health benefits including the limitation of adipose tissue inflammation, bone loss and better outcomes for some cancer types. ApoD affects longevity, is involved in bone and lipid metabolism, acts as an antioxidant and limits inflammation. These are all critical systems relevant to the pathophysiology of aging in which ApoD could serve as a therapeutic target. However, much needs to be learned before this eventuality can be realistically considered.
Acknowledgements
Part of this work was supported by the Canadian Institutes of Health Research grant MOP-15677 (ER).
This review and the corresponding Gene Wiki article are written as part of the Gene Wiki Review series–a series resulting from a collaboration between the journal GENE and the Gene Wiki Initiative. The Gene Wiki Initiative is supported by National Institutes of Health (GM089820). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE.
The corresponding Gene Wiki entry for this review can be found here: https://en.wikipedia.org/wiki/Apolipoprotein_D
Abbreviations:
- AA
Arachidonic Acid
- ABCA1
ATP-binding Cassette A1 transporter
- AMPA-R
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- APEX
Apurinic/Apyrimidinic Endonuclease-I
- ApoA-I
Apolipoprotein A-I
- ApoA-II
Apolipoprotein A-II
- ApoD
Apolipoprotein D
- ApoE
Apolipoprotein E
- BSG
Basigin
- BMI
Body Mass index
- CETP
Cholesteryl Ester Transfer Protein
- CNS
Central Nervous System
- eNOS
Endothelial Nitric Oxide Synthase
- ERK1/2
Extracellular signal-Regulated Kinase 1/2
- GLUT
Glucose Transporter
- HDL
High Density Lipoprotein
- KA
Kainic Acid
- LCAT
Lecithin Cholesterol Acyltransferase
- LDL
Low Density Lipoprotein
- LPL
Lipoprotein Lipase
- LXR
Liver X Receptor
- NF-κB
Nuclear Factor kappa B
- NFT
Neurofibrillary Tangles
- NMDA R
N-methyl-D-Aspartate Receptor
- NPC
Niemann-Pick type C
- PARP
Poly ADP-Ribose Polymerase-1
- PDGF
Platelet-Derived Growth Factor
- PPAR
Peroxisome proliferator-activated receptor
- RAR
Retinoic Acid Nuclear Receptor
- TNF-α
Tumor Necrosis Factor-α
- VLDL
Very Low Density Lipoprotein
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Akash MSH, Rehman K, Liaqat A, 2017. Tumor Necrosis Factor-Alpha: Role in development of insulin resistance and pathogenesis of Type 2 Diabetes Mellitus. J Cell Biochem. 119, 105–110. [DOI] [PubMed] [Google Scholar]
- Alaupovic P, Schaefer EJ, McConathy WJ, Fesmire JD, Brewer HB Jr., 1981. Plasma apolipoprotein concentrations in familial apolipoprotein A-I and A-II deficiency (Tangier disease). Metabolism 30, 805–809. [DOI] [PubMed] [Google Scholar]
- Albers JJ, Adolphson J, Chen CH, Murayama N, Honma S, Akanuma Y, 1985. Defective enzyme causes lecithin-cholesterol acyltransferase deficiency in a Japanese kindred. Biochim Biophys Acta 835, 253–257. [DOI] [PubMed] [Google Scholar]
- Appari M, Werner R, Wünsch L, Cario G, Demeter J, Hiort O, Riepe F, Brooks JD, Holterhus PM, 2009. Apolipoprotein D (APOD) is a putative biomarker of androgen receptor function in androgen insensitivity syndrome. J Mol Med (Berl) 87, 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida S, Nakagawa H, Katagiri T, Furihata M, Iiizumi M, Anazawa Y, Tsunoda T, Takata R, Kasahara K, Miki T, Fujioka T, Shuin T, Nakamura Y, 2004. Molecular features of the transition from prostatic intraepithelial neoplasia (PIN) to prostate cancer: genome-wide gene-expression profiles of prostate cancers and PINs. Cancer Res 64, 5963–5972. [DOI] [PubMed] [Google Scholar]
- Aspinall JO, Bentel JM, Horsfall DJ, Haagensen DE, Marshall VR, Tilley WD, 1995. Differential expression of apolipoprotein-D and prostate specific antigen in benign and malignant prostate tissues. J Urol 154, 622–628. [DOI] [PubMed] [Google Scholar]
- Ayrault-Jarrier M, Levy G, Polonovski J, 1963. Study of human serum alpha-lipoproteins by immunoelectrophoresis. Bull Soc. Chim. Biol 45, 703–716. [PubMed] [Google Scholar]
- Baker WA, Hitman GA, Hawrami K, McCarthy MI, Riikonen A, Tuomilehto-Wolf E, Nissinen A, Tuomilehto J, Mohan V, Viswanathan M, et al. (1994) Apolipoprotein D gene polymorphism: a new genetic marker for type 2 diabetic subjects in Nauru and south India. Diabetic medicine: a journal of the British Diabetic Association 11:947–952. [DOI] [PubMed] [Google Scholar]
- Balbin M, Freije JM, Fueyo A, Sanchez LM, Lopez-Otin C, 1990. Apolipoprotein D is the major protein component in cyst fluid from women with human breast gross cystic disease. Biochem J 271, 803–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloir B, Kovari E, Surini-Demiri M, Savioz A, 2001. Altered apolipoprotein D expression in the brain of patients with Alzheimer disease. J Neurosci Res 64, 61–69. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Fuchs S, Lieder I, Stelzer G, Mazor Y, Buzhor E, Kaplan S, Bogoch Y, Plaschkes I, Shitrit A, Rappaport N, Kohn A, Edgar R, Shenhav L, Safran M, Lancet D, Guan-Golan Y, Warshawsky D, and Shtrichman R. 2016. ‘GeneAnalytics: An Integrative Gene Set Analysis Tool for Next Generation Sequencing, RNAseq and Microarray Data’, OMICS, 20:139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop RE, Penfold SS, Frost LS, Holtje JV, Weiner JH, 1995. Stationary phase expression of a novel Escherichia coli outer membrane lipoprotein and its relationship with mammalian apolipoprotein D. Implications for the origin of lipocalins. J Biol Chem 270, 23097–23103. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Jenner AM, Li H, Ruberu K, Spiro AS, Shepherd CE, Kril JJ, Kain N, Don A, Garner B, 2013. Increased apolipoprotein D dimer formation in Alzheimer’s disease hippocampus is associated with lipid conjugated diene levels. J Alzheimers Dis. 35, 475–486. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Knoch B, Wong J, Kim WS, Else PL, Oakley AJ, Garner B, 2012. Selective reduction of hydroperoxyeicosatetraenoic acids to their hydroxy derivatives by apolipoprotein D: implications for lipid antioxidant activity and Alzheimer’s disease. Biochem J. 442, 713–721. [DOI] [PubMed] [Google Scholar]
- Blanco-Vaca F, Via DP, Yang CY, Massey JB, Pownall HJ, 1992. Characterization of disulfide-linked heterodimers containing apolipoprotein D in human plasma lipoproteins. J. Lipid Res 33, 1785–1796. [PubMed] [Google Scholar]
- Blanco-Vaca F, Pownall HJ, 1993. Disulfide linked dimers of apolipoprotein D in urine. Electrophoresis 14, 1086–1087. [DOI] [PubMed] [Google Scholar]
- Blais Y, Sugimoto K, Carriere MC, Haagensen DE, Labrie F, Simard J, 1994. Potent stimulatory effect of interleukin-1 alpha on apolipoprotein D and gross cystic disease fluid protein-15 expression in human breast-cancer cells. Int. J. Cancer 59, 400–407. [DOI] [PubMed] [Google Scholar]
- Blais Y, Sugimoto K, Carriere MC, Haagensen DE, Labrie F, Simard J, 1995. Interleukin-6 inhibits the potent stimulatory action of androgens, glucocorticoids and interleukin-1 alpha on apolipoprotein D and GCDFP-15 expression in human breast cancer cells. Int. J. Cancer 62, 732–737. [DOI] [PubMed] [Google Scholar]
- Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G, 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22, 347–351. [DOI] [PubMed] [Google Scholar]
- Boer S, Sanchez D, Reinieren I, van den Boom T, Udawela M, Scarr E, Ganfornina MD, Dean B, 2010. Decreased kainate receptors in the hippocampus of apolipoprotein D knockout mice. Prog Neuropsychopharmacol Biol Psychiatry 34, 271–278. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Notterpek LM, Anderson LJ, 1990a. Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve. Identification of apolipoprotein D, apolipoprotein A-IV, apolipoprotein E, and apolipoprotein A-I. J Biol Chem 265, 17805–17815. [PubMed] [Google Scholar]
- Boyles JK, Notterpek LM, Wardell MR, Rall SC Jr., 1990b. Identification, characterization, and tissue distribution of apolipoprotein D in the rat. J Lipid Res 31, 2243–2256. [PubMed] [Google Scholar]
- Boyles JK, Zoellner CD, Anderson LJ, Kosik LM, Pitas RE, Weisgraber KH, Hui DY, Mahley RW, Gebicke-Haerter PJ, Ignatius MJ, et al. , 1989. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest 83, 1015–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braesch-Andersen S, Beckman L, Paulie S, Kumagai-Braesch M, 2014. ApoD mediates binding of HDL to LDL and to growing T24 carcinoma. PLoS ONE 9, e115180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breustedt DA, Schonfeld DL, Skerra A, 2006. Comparative ligand-binding analysis of ten human lipocalins. Biochim Biophys Acta 1764, 161–173. [DOI] [PubMed] [Google Scholar]
- Bruha R, Vitek L, Smid V, 2020. Osteopontin - A potential biomarker of advanced liver disease. Ann Hepatol 10.1016/j.aohep.2020.01.001. [DOI] [PubMed] [Google Scholar]
- Camato R, Marcel YL, Milne RW, Lussier-Cacan S, Weech PK, 1989. Protein polymorphism of a human plasma apolipoprotein D antigenic epitope. J Lipid Res 30, 865–875. [PubMed] [Google Scholar]
- Ciapparelli A, Dell’Osso L, Bandettini di Poggio A, Cecconi D, Fenzi M, Chiavacci MC, Bottai M, Ramacciotti CE, Cassano GB, 2003. Clozapine in treatment-resistant patients with schizophrenia, schizoaffective disorders or psychotic bipolar disorders : a naturalistic 48-month follow-up study. J Clin Psychiatry 64, 451–458. [DOI] [PubMed] [Google Scholar]
- Cofer Shelagh, Ross Susan R., 1996. The murine gene encoding apolipoprotein D exhibits a unique expression pattern as compared to other species. Gene 171, 261–263. [DOI] [PubMed] [Google Scholar]
- Cury SS, de Moraes D, Freire PP, de Oliveira G, Marques DVP, Fernandez GJ, Dal-Pai-Silva M, Hasimoto ÉN, Dos Reis PP, Rogatto SR, Carvalho RF, 2019. Tumor Transcriptome Reveals High Expression of IL-8 in Non-Small Cell Lung Cancer Patients with Low Pectoralis Muscle Area and Reduced Survival. Cancers (Basel) 11, E1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF, 2003. Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J Neurosc 23, 8013–8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Valle E, Navarro A, Astudillo A, Tolivia J, 2003. Apolipoprotein D expression in human brain reactive astrocytes. J Histochem and Cytochem 51, 1285–1290. [DOI] [PubMed] [Google Scholar]
- Desai PP, Ikonomovic MD, Abrahamson EE, Hamilton RL, Isanski BA, Hope CE, Klunk WE, DeKosky ST, Kamboh MI, 2005. Apolipoprotein D is a component of compact but not diffuse amyloid-beta plaques in Alzheimer’s disease temporal cortex. Neurobiol Dis 20, 574–582. [DOI] [PubMed] [Google Scholar]
- Desmarais F, Bergeron KF, Lacaille M, Lemieux I, Bergeron J, Biron S, Rassart E, Joanisse DR, Mauriege P, Mounier C, 2018. High ApoD protein level in the round ligament fat depot of severely obese women is associated with an improved inflammatory profile. Endocrine 61, 248–257. [DOI] [PubMed] [Google Scholar]
- Desmarais F, Bergeron KF, Rassart E, Mounier C, 2019. Apolipoprotein D overexpression alters hepatic prostaglandin and omega fatty acid metabolism during the development of a non-inflammatory hepatic steatosis. Biochim Biophys Acta Mol Cell Biol Lipids 1864, 522–531. [DOI] [PubMed] [Google Scholar]
- Diez-Itza I, Vizoso F, Merino AM, Sanchez LM, Tolivia J, Fernandez J, Ruibal A, Lopez-Otin C, 1994. Expression and prognostic significance of apolipoprotein D in breast cancer. Am J Pathol 144, 310–320. [PMC free article] [PubMed] [Google Scholar]
- Do Carmo S, Forest JC, Giguere Y, Masse A, Lafond J, Rassart E, 2009a. Modulation of Apolipoprotein D levels in human pregnancy and association with gestational weight gain. Reprod Biol Endocrinol 7, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Carmo S, Fournier D, Mounier C, Rassart E, 2009b. Human apolipoprotein D overexpression in transgenic mice induces insulin resistance and alters lipid metabolism. Am J Physiol Endocrinol Metab 296, E802–E811. [DOI] [PubMed] [Google Scholar]
- Do Carmo S, Jacomy H, Talbot PJ, Rassart E, 2008. Neuroprotective effect of apolipoprotein D against human coronavirus OC43-induced encephalitis in mice. J Neurosc 28, 10330–10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Carmo S, Levros LC Jr., Rassart E, 2007. Modulation of apolipoprotein D expression and translocation under specific stress conditions. Biochim Biophys Acta 1773, 954–969. [DOI] [PubMed] [Google Scholar]
- Do Carmo S, Seguin D, Milne R, Rassart E, 2002. Modulation of apolipoprotein D and apolipoprotein E mRNA expression by growth arrest and identification of key elements in the promoter. J Biol Chem 277, 5514–5523. [DOI] [PubMed] [Google Scholar]
- Drayna D, Fielding C, McLean J, Baer B, Castro G, Chen E, Comstock L, Henzel W, Kohr W, Rhee L, et al. , 1986. Cloning and expression of human apolipoprotein D cDNA. J Biol Chem 261, 16535–16539. [PubMed] [Google Scholar]
- Drayna DT, McLean JW, Wion KL, Trent JM, Drabkin HA, Lawn RM, 1987. Human apolipoprotein D gene: gene sequence, chromosome localization, and homology to the alpha 2u-globulin superfamily. DNA 6, 199–204. [DOI] [PubMed] [Google Scholar]
- Duan Y, Gross RA, Sheu SS, 2007. Ca2+-dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(ADP-ribose) polymerase-1 activation during glutamate excitotoxicity. J Physiol 585, 741–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger A, Nasreen A, Kim HJ, Skerra A, 2007. Structural insight into the dual ligand specificity and mode of high density lipoprotein association of apolipoprotein D. J Biol Chem 282, 31068–31075. [DOI] [PubMed] [Google Scholar]
- Fielding PE, Fielding CJ, 1980. A cholesteryl ester transfer complex in human plasma. Proc Natl Acad Sc USA 77, 3327–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishilevich S, Zimmerman S, Kohn A, Iny Stein T, Olender T, Kolker E, Safran M, and Lancet D. 2016. ‘Genic insights from integrated human proteomics in GeneCards’, Database (Oxford), 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette-Charron JB, Breton G, Badawi M, Sarhan F, 2002. Molecular and structural analyses of a novel temperature stress-induced lipocalin from wheat and Arabidopsis. FEBS Lett 517, 129–132. [DOI] [PubMed] [Google Scholar]
- Fritz G, Grosch S, Tomicic M, Kaina B, 2003. APE/Ref-1 and the mammalian response to genotoxic stress. Toxicology 193, 67–78. [DOI] [PubMed] [Google Scholar]
- Ganfornina MD, Do Carmo S, Lora JM, Torres-Schumann S, Vogel M, Allhorn M, Gonzalez C, Bastiani MJ, Rassart E, Sanchez D, 2008. Apolipoprotein D is involved in the mechanisms regulating protection from oxidative stress. Aging Cell 7, 506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina MD, Do Carmo S, Martinez E, Tolivia J, Navarro A, Rassart E, Sanchez D, 2010. ApoD, a glia-derived apolipoprotein, is required for peripheral nerve functional integrity and a timely response to injury. Glia 58, 1320–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina MD, Sanchez D, Pagano A, Tonachini L, Descalzi-Cancedda F, Martinez S, 2005. Molecular characterization and developmental expression pattern of the chicken apolipoprotein D gene: implications for the evolution of vertebrate lipocalins. Dev Dyn 232, 191–199. [DOI] [PubMed] [Google Scholar]
- Garcia-Mateo N, Ganfornina MD., Montero O, Gijón MA, Murphy RC, and Sanchez D 2014. Schwann Cell-Derived Apolipoprotein D Controls the Dynamics of PostInjury Myelin Recognition and Degradation. Front Cell Neurosc. 8: article 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mateo N, Pascua-Maestro R, Pérez-Castellanos A, Lillo C, Sanchez D, Ganfornina MD, 2018. Myelin extracellular leaflet compaction requires apolipoprotein D membrane management to optimize lysosomal-dependent recycling and glycocalyx removal. Glia 66, 670–687. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Liaw L, Murry CE, Schwartz SM, Almeida M, 1995. Osteopontin expression in cardiovascular diseases. Ann N Y Acad Sci 760, 109–126. [DOI] [PubMed] [Google Scholar]
- Glockner F, Ohm TG, 2003. Hippocampal apolipoprotein D level depends on Braak stage and APOE genotype. Neuroscience 122, 103–110. [DOI] [PubMed] [Google Scholar]
- Goessling W, Zucker SD, 2000. Role of apolipoprotein D in the transport of bilirubin in plasma. Am J Physiol Gastrointest liver Physiol 279, 356–365. [DOI] [PubMed] [Google Scholar]
- Gravallese EM, 2003. Osteopontin: a bridge between bone and the immune system. Journal of Clinical Investigation 112, 147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RE, Horsfall DJ, Stahl J, Vivekanandan S, Ricciardelli C, Stapleton AM, Scardino PT, Neufing P, Tilley WD, 2004. Apolipoprotein-D: a novel cellular marker for HGPIN and prostate cancer. Prostate 58, 103–108. [DOI] [PubMed] [Google Scholar]
- Hansen L, Gaster M, Oakeley EJ, Brusgaard K, Damsgaard Nielsen EM, Beck-Nielsen H, Pedersen O, Hemmings BA, 2004. Expression profiling of insulin action in human myotubes: induction of inflammatory and pro-angiogenic pathways in relationship with glycogen synthesis and type 2 diabetes. Biochem Biophys Res Commun 323, 685–695. [DOI] [PubMed] [Google Scholar]
- Harbeck N, Penault-Liorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F, 2019. Breast Cancer. Nat. Rev. 5 (1), 66. 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Finch CE, 1989. The neurobiology of Alzheimer’s disease. J Neurosurgery 70, 335–353. [DOI] [PubMed] [Google Scholar]
- Holzfeind P, Merschak P, Dieplinger H, Redl B, 1995. The human lacrimal gland synthesizes apolipoprotein D mRNA in addition to tear prealbumin mRNA, both species encoding members of the lipocalin superfamily. Exp. Eye Res. 61, 495–500. [DOI] [PubMed] [Google Scholar]
- Horrobin DF, 1998. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophrenia Res 30, 193–208. [DOI] [PubMed] [Google Scholar]
- Hu CY, Ong WY, Sundaram RK, Chan C, Patel SC, 2001. Immunocytochemical localization of apolipoprotein D in oligodendrocyte precursor-like cells, perivascular cells, and pericytes in the human cerebral cortex. J Neurocytology 30, 209–218. [DOI] [PubMed] [Google Scholar]
- Hull-Thompson J, Muffat J, Sanchez D, Walker DW, Benzer S, Ganfornina MD, Jasper H, 2009. Control of metabolic homeostasis by stress signaling is mediated by the lipocalin NLaz. PLoS Genet. 5, e1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummasti S, Laffitte BA, Watson MA, Galardi C, Chao LC, Ramamurthy L, Moore JT, Tontonoz P, 2004. Liver X receptors are regulators of adipocyte gene expression but not differentiation: identification of ApoD as a direct target. J Lipid Res 45, 616–625. [DOI] [PubMed] [Google Scholar]
- Hunter S, Young A, Olson J, Brat DJ, Bowers G, Wilcox JN, Jaye D, Mendrinos S, Neish A, 2002. Differential expression between pilocytic and anaplastic astrocytomas: identification of Neuropathol. Exp Neurol 61, 275–281. [DOI] [PubMed] [Google Scholar]
- Iacobuzio-Donahue CA, Ryu B, Hruban RH, Kern SE, 2002. Exploring the host desmoplastic response to pancreatic carcinoma: gene expression of stromal and neoplastic cells at the site of primary invasion. Am J Pathol 160, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono KT, Brown AL, Greene MI, Saouaf SJ, 2007. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp. Mol. Pathol. 83, 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacomy H, Talbot PJ, 2003. Vacuolating encephalitis in mice infected by human coronavirus OC43. Virology 315, 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic-Karasoulos T, Bianco-Miotto T, Butler MS, Butler LM, McNeil CM, O’Toole SA, Millar EKA, Sakko AJ, Ruiz AI, Birrell SN, Sutherland RL, Hickey TE, Tilley WD, Ricciardelli C. (2020). Elevated levels of tumor apolipoprotein-D independently predict poor outcome in breast cancer patients. Histopathology, January 28. doi: 10.1111/his.14081. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Jansen PJ, Lutjohann D, Thelen KM, von Bergmann K, van Leuven F, Ramaekers FC, Monique M, 2009. Absence of ApoE upregulates murine brain ApoD and ABCA1 levels, but does not affect brain sterol levels, while human ApoE3 and human ApoE4 upregulate brain cholesterol precursor levels. J Alzheimers Dis 18, 319–329. [DOI] [PubMed] [Google Scholar]
- Jimenez-Palomares M, Cózar-Castellano I, Ganfornina MD, Sánchez D, Perdomo G, 2011. Genetic deficiency of apolipoprotein D in the mouse is associated with non-fasting hypertriglyceridemia and hyperinsulinemia. Metabolism 60, 1767–1774. [DOI] [PubMed] [Google Scholar]
- Jin D, El-Tanani M, Campbell FC, 2006. Identification of apolipoprotein D as a novel inhibitor of osteopontin-induced neoplastic transformation. Int. J. Oncol. 29, 1591–1599. [PubMed] [Google Scholar]
- Jones HM, Pilowsky LS, 2002. Dopamine and antipsychotic drug action revisited. Br J Psychiatry 181, 271–275. [DOI] [PubMed] [Google Scholar]
- Jürets A, Le Bras M, Staffler G, Stein G, Leitner L, Neuhofer A, Tardelli M, Turkof E, Zeyda M, Stulnig TM, 2016. Inhibition of Cellular Adhesion by Immunological Targeting of Osteopontin Neoepitopes Generated through Matrix Metalloproteinase and Thrombin Cleavage. PLoS ONE 11, e0148333. 10.1371/journal.pone.0148333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman J, McConathy W, Araoz C, Kasa P, Lacko AG, 2000. Apolipoprotein D in the aging brain and in Alzheimer’s dementia. Neurol Res 22, 330–336. [DOI] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC, 2002. Global gene profiling in human endometrium during the window of implantation. Endocrinology 143, 2119–2138. [DOI] [PubMed] [Google Scholar]
- Kielkopf CS, 2018. Low JKK, Mok YF, Bhatia S, Palasovski T, Oakley AJ, Whitten AE, Garner B, Brown SHJ Identification of a novel tetrameric structure for human apolipoprotein-D. J Struct Biol 203, 205–218. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Stetler-Stevenson WG, 1999. Matrix metalloproteinases and metastasis. Cancer Chemother. Pharmacol. 43, S42–S51. [DOI] [PubMed] [Google Scholar]
- Kolodny EH (2000) Niemann-Pick disease. Current opinion in hematology 7:48–52. [DOI] [PubMed] [Google Scholar]
- Konradi C, Heckers S, 2003. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol. Ther. 97, 153–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl FA Jr., Egan RW, 1980. Prostaglandins, arachidonic acid, and inflammation. Science 210, 978–984. [DOI] [PubMed] [Google Scholar]
- Labrie M, Lalonde S, Najyb O, Thiery M, Daneault C, Des Rosiers C, Rassart E, Mounier C, 2015. Apolipoprotein D Transgenic Mice Develop Hepatic Steatosis through Activation of PPARgamma and Fatty Acid Uptake. PLoS ONE 10, e0130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CJ, Cheng HC, Lin CY, Huang SH, Chen TH, Chung CJ, Chang CH, Wang HD, Chuu CP, 2017. Activation of liver X receptor suppresses angiogenesis via induction of ApoD. FASEB J 31, 5568–5576. [DOI] [PubMed] [Google Scholar]
- Lambert J, Provost PR, Marcel YL, Rassart E, 1993. Structure of the human apolipoprotein D gene promoter region. Biochim Biophys Acta 1172, 190–192. [DOI] [PubMed] [Google Scholar]
- Laugharne JD, Mellor JE, Peet M, 1996. Fatty acids and schizophrenia. Lipids 31 (Suppl), S163–S165. [DOI] [PubMed] [Google Scholar]
- Lea OA, 1988. Binding properties of progesterone-binding Cyst protein, PBCP. Steroids 52, 337–338. [DOI] [PubMed] [Google Scholar]
- Leung WC, Lawrie A, Demaries S, Massaeli H, Burry A, Yablonsky S, Sarjeant JM, Fera E, Rassart E, Pickering JG, Rabinovitch M, 2004. Apolipoprotein D and platelet-derived growth factor-BB synergism mediates vascular smooth muscle cell migration. Circ. Res. 95, 179–186. [DOI] [PubMed] [Google Scholar]
- Levros LC Jr., Do Carmo S, Edouard E, Legault P, Charfi C, Rassart E, 2010. Characterization of nuclear factors modulating the apolipoprotein D promoter during growth arrest: implication of PARP-1, APEX-1 and ERK1/2 catalytic activities. Biochim Biophys Acta 1803, 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levros LC Jr., Labrie M, Charfi C, Rassart E, 2013. Binding and repressive activities of apolipoprotein E3 and E4 isoforms on the human ApoD promoter. Mol Neurobiol 48, 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ruberu K, Karl T, Garner B, 2016. Cerebral Apolipoprotein-D Is Hypoglycosylated Compared to Peripheral Tissues and Is Variably Expressed in Mouse and Human Brain Regions’. PLoS ONE 11, e0148238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ruberu K, Munoz SS, Jenner AM, Spiro A, Zhao H, Rassart E, Sanchez D, Ganfornina M, Karl T, Garner B, 2015. Apolipoprotein D modulates amyloid pathology in APP/PS1 Alzheimer’s disease mice. Neurobiol Aging 36, 1820–1833. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chang GQ, Leibowitz SF, 2001. Apolipoprotein D interacts with the long-form leptin receptor: a hypothalamic function in the control of energy homeostasis. FASEB J 15, 1329–1331. [DOI] [PubMed] [Google Scholar]
- Lopez-Boado YS, Klaus M, Dawson MI, Lopez-Otin C, 1996. Retinoic acid-induced expression of apolipoprotein D and concomitant growth arrest in human breast cancer cells are mediated through a retinoic acid receptor RARalpha-dependent signaling pathway. J Biol Chem 271, 32105–32111. [DOI] [PubMed] [Google Scholar]
- Lopez-Boado YS, Puente XS, Alvarez S, Tolivia J, Binderup L, Lopez-Otin C, 1997. Growth inhibition of human breast cancer cells by 1,25-dihydroxyvitamin D3 is accompanied by induction of apolipoprotein D expression. Cancer Res 57, 4091–4097. [PubMed] [Google Scholar]
- Lopez-Boado YS, Tolivia J, Lopez-Otin C, 1994. Apolipoprotein D gene induction by retinoic acid is concomitant with growth arrest and cell differentiation in human breast cancer cells. J Biol Chem 269, 26871–26878. [PubMed] [Google Scholar]
- Mahadik SP, Khan MM, Evans DR, Parikh VV, 2002. Elevated plasma level of apolipoprotein D in schizophrenia and its treatment and outcome. Schizophr Res 58, 55–62. [DOI] [PubMed] [Google Scholar]
- Matsuyama M, Yoshimura R, Mitsuhashi M, Tsuchida K, Takemoto Y, Kawahito Y, et al. , 2005. 5-Lipoxygenase inhibitors attenuate growth of human renal cell carcinoma and induce apoptosis through arachidonic acid pathway. Oncol Rep 14, 73–79. [PubMed] [Google Scholar]
- Martineau C, Najyb O, Signor C, Rassart E, Moreau R, 2016. Apolipoprotein D deficiency is associated to high bone turnover, low bone mass and impaired osteoblastic function in aged female mice. Metabolism 65, 1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConathy WJ, Alaupovic P, 1976. Studies on the isolation and partial characterization of apolipoprotein D and lipoprotein D of human plasma. Biochemistry 15, 515–520. [DOI] [PubMed] [Google Scholar]
- Miranda E, Vizoso F, Martin A, Quintela I, Corte MD, Segui ME, Ordiz I, Merino AM, 2003. Apolipoprotein D expression in cutaneous malignant melanoma. J Surg Oncol 83, 99–105. [DOI] [PubMed] [Google Scholar]
- Montpied P, de Bock F, Lerner-Natoli M, Bockaert J, Rondouin G, 1999. Hippocampal alterations of apolipoprotein E and D mRNA levels in vivo and in vitro following kainate excitotoxicity. Epilepsy Res 35, 135–146. [DOI] [PubMed] [Google Scholar]
- Morais Cabral JH, Atkins GL, Sanchez LM, Lopez-Boado YS, Lopez-Otin C, Sawyer L, 1995. Arachidonic acid binds to apolipoprotein D: implications for the protein’s function. FEBS Lett 366, 53–56. [DOI] [PubMed] [Google Scholar]
- Muffat J, Walker DW, Benzer S, 2008. Human ApoD, an apolipoprotein up-regulated in neurodegenerative diseases, extends lifespan and increases stress resistance in DrosophilaDrosophila. Proc. Nat. Acad. Sc. USA 105, 7088–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najyb O, Brissette L, Rassart E, 2015. Apolipoprotein D internalization is a Basigindependant mechanism. J. of Biol. Chem. 290, 16077–16087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najyb O, Do Carmo S, Alikashani A, Rassart E, 2016. Apolipoprotein D overexpression protects against kainate-induced neurotoxicity in mice. Mol Neurobiol 54, 3948–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Del Valle E, Tolivia J, 2004. Differential expression of apolipoprotein d in human astroglial and oligodendroglial cells. J Histochem Cytochem 52, 1031–1036. [DOI] [PubMed] [Google Scholar]
- Navarro A, Tolivia J, Astudillo A, del Valle E, 1998. Pattern of apolipoprotein D immunoreactivity in human brain. Neurosci Lett 254, 17–20. [DOI] [PubMed] [Google Scholar]
- Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, et al. , 2007. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J. Clin. Investig. 117, 2877–2888. 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent C, Prins JB, Whitehead JP, Wentworth JM, Chatterjee VKK, O’Rahilly S, 2000. Arachidonic acid stimulates glucose uptake in 3T3-L1 adipocytes by increasing GLUT1 and GLUT4 levels at the plasma membrane: Evidence for involvement of lipoxygenase metabolites and peroxisome proliferator-activated receptor γ. J Biol Chem. 276, 9149–9157. [DOI] [PubMed] [Google Scholar]
- Oakley AJ, Bhatia S, Ecroyd H, Garner B, 2012. Molecular dynamics analysis of apolipoprotein-D-lipid hydroperoxide interactions: mechanism for selective oxidation of Met-93. PLoS ONE 7, e34057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WY, He Y, Suresh S, Patel SC, 1997. Differential expression of apolipoprotein D and apolipoprotein E in the kainic acid-lesioned rat hippocampus. Neuroscience 79, 359–367. [DOI] [PubMed] [Google Scholar]
- Ong WY, Lau CP, Leong SK, Kumar U, Suresh S, Patel SC, 1999. Apolipoprotein D gene expression in the rat brain and light and electron microscopic immunocytochemistry of apolipoprotein D expression in the cerebellum of neonatal, immature and adult rats. Neuroscience 90, 913–922. [DOI] [PubMed] [Google Scholar]
- Ordonez C, Navarro A, Perez C, Astudillo A, Martinez E, Tolivia J, 2006. Apolipoprotein D expression in substantia nigra of Parkinson disease. Histol Histopathol 21, 361–366. [DOI] [PubMed] [Google Scholar]
- Pajaniappan M, Glober NK, Kennard S, Liu H, Zhao N, Lilly B, 2011. Endothelial cells downregulate apolipoprotein D expression in mural cells through paracrine secretion and Notch signaling. Am J Physiol Heart Circ Physiol 301, 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascua-Maestro R, González E, Lillo C, Ganfornina MD, Falcón-Pérez JM, Sanchez D, 2018. Extracellular Vesicles Secreted by Astroglial Cells Transport Apolipoprotein D to Neurons and Mediate Neuronal Survival Upon Oxidative Stress. Front Cell Neurosci 2018 (12), 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RC, Lange D, McConathy WJ, Patel YC, Patel SC, 1997. Probing the structure of the ligand binding cavity of lipocalins by fluorescence spectroscopy. Protein Eng. 10, 621–625. [DOI] [PubMed] [Google Scholar]
- Pearlman WH, Gueriguian JL, Sawyer ME, 1973. A specific progesterone-binding component of human breast cyst fluid. J Biol Chem 248, 5736–5741. [PubMed] [Google Scholar]
- Peitsch MC, Boguski MS, 1990. Is apolipoprotein D a mammalian bilin-binding protein? New Biol 2, 197–206. [PubMed] [Google Scholar]
- Perdomo G, Kim DH, Zhang T, Qu S, Thomas EA, Toledo FG, Slusher S, Fan Y, Kelley DE, Dong HH, 2010. A role of apolipoprotein D in triglyceride metabolism. J Lipid Res 51, 1298–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdomo G, Dong H, 2009. Apolipoprotein D in lipid metabolism and its functional implication in atherosclerosis and aging. Aging 1, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW, 2003. Cholesterol homeostasis and function in neurons of the central nervous system. Cellular and molecular life sciences : CMLS 60, 1158–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D, Lahti-Domenici J, Keshaviah A, Bae YK, Argani P, Marks J, Richardson A, Cooper A, Strausberg R, Riggins GJ, Schnitt S, Gabrielson E, Gelman R, Polyak K, 2003. Molecular markers in ductal carcinoma in situ of the breast. Mol Cancer Res 1, 362–375. [PubMed] [Google Scholar]
- Provost PR, Marcel YL, Milne RW, Weech PK, Rassart E, 1991a. Apolipoprotein D transcription occurs specifically in nonproliferating quiescent and senescent fibroblast cultures. FEBS Lett 290, 139–141. [DOI] [PubMed] [Google Scholar]
- Provost PR, Tremblay Y, el-Amine M, Belanger A, 1995. Guinea pig apolipoprotein D RNA diversity, and developmental and gestational modulation of mRNA levels. Mol Cell Endocrinol 109, 225–236. [DOI] [PubMed] [Google Scholar]
- Provost PR, Villeneuve L, Weech PK, Milne RW, Marcel YL, Rassart E, 1991b. Localization of the major sites of rabbit apolipoprotein D gene transcription by in situ hybridization. J Lipid Res 32, 1959–1970. [PubMed] [Google Scholar]
- Provost PR, Weech PK, Tremblay NM, Marcel YL, Rassart E, 1990. Molecular characterization and differential mRNA tissue distribution of rabbit apolipoprotein D. J Lipid Res 31, 2057–2065. [PubMed] [Google Scholar]
- Rassart E, Bedirian A, Do Carmo S, Guinard O, Sirois J, Terrisse L, Milne R, 2000. Apolipoprotein D. Biochim Biophys Acta 1482, 185–198. [DOI] [PubMed] [Google Scholar]
- Reindl M, Knipping G, Wicher I, Dilitz E, Egg R, Deisenhammer F, Berger T, 2001. Increased intrathecal production of apolipoprotein D in multiple sclerosis. J. Neuroimmunol. 119, 327–332. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Dolle P, 2012. Retinoic acid signalling during development. Development 139, 843–858. [DOI] [PubMed] [Google Scholar]
- Rickhag M, Deierborg T, Patel S, Ruscher K, Wieloch T, 2008. Apolipoprotein D is elevated in oligodendrocytes in the peri-infarct region after experimental stroke: influence of enriched environment. J Cereb Blood Flow Metab 28, 551–562. [DOI] [PubMed] [Google Scholar]
- Rickhag M, Wieloch T, Gido G, Elmer E, Krogh M, Murray J, Lohr S, Bitter H, Chin DJ, von Schack D, Shamloo M, Nikolich K, 2006. Comprehensive regional and temporal gene expression profiling of the rat brain during the first 24 h after experimental stroke identifies dynamic ischemia-induced gene expression patterns, and reveals a biphasic activation of genes in surviving tissue. J Neurochem 96, 14–29. [DOI] [PubMed] [Google Scholar]
- Rittling SR, Matsumoto HN, McKee MD, et al. , 1998. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J Bone Miner Res. 13, 1101–1111. [DOI] [PubMed] [Google Scholar]
- Romano M, Claria J, 2003. Cyclooxygenase-2 and 5-lipoxygenase converging functions on cell proliferation and tumor angiogenesis: implications for cancer therapy. FASEB J 17, 1986–1995. [DOI] [PubMed] [Google Scholar]
- Ryu B, Jones J, Hollingsworth MA, Hruban RH, Kern SE, 2001. Invasion-specific genes in malignancy: serial analysis of gene expression comparisons of primary and passaged cancers. Cancer Res 61, 1833–1838. [PubMed] [Google Scholar]
- Rudland PS, Platt-Higgins A, El-Tanani M, et al. , 2002. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res 62 (12), 3417–3427. [PubMed] [Google Scholar]
- Ruiz M, Sanchez D, Correnti C, Strong RK, Ganfornina MD, 2013. Lipid-binding properties of human ApoD and Lazarillo-related lipocalins: functional implications for cell differentiation. FEBS J 280, 3928–3943. [DOI] [PubMed] [Google Scholar]
- Ruiz M, Wicker-Thomas C, Sanchez D, Ganfornina MD, 2012. Grasshopper Lazarillo, a GPI-anchored Lipocalin, increases DrosophilaDrosophila longevity and stress resistance, and functionally replaces its secreted homolog NLaz. Insect Biochem Mol Biol 42, 776–789. [DOI] [PubMed] [Google Scholar]
- Salami M, Bandegi AR, Sameni HR, Vafaei AA, Pakdel A, 2019. Hippocampal UpRegulation of Apolipoprotein D in a Rat Model of Maternal Hypo- and Hyperthyroidism: Implication of Oxidative Stress. Neurochem Res 44, 2190–2201. [DOI] [PubMed] [Google Scholar]
- Sanchez D, Ganfornina MD, Gutierrez G, Marin A, 2003. Exon-intron structure and evolution of the Lipocalin gene family. Mol Biol Evol. 20, 775–783. [DOI] [PubMed] [Google Scholar]
- Sanchez D, Ganfornina MD, Martinez S, 2002. Expression pattern of the lipocalin apolipoprotein D during mouse embryogenesis. Mech Dev 110, 225–229. [DOI] [PubMed] [Google Scholar]
- Sanchez D, Ganfornina MD, Torres-Schumann S, Speese SD, Lora JM, Bastiani MJ, 2000. Characterization of two novel lipocalins expressed in the DrosophilaDrosophila embryonic nervous system. Int J Dev Biol 44, 349–359. [PubMed] [Google Scholar]
- Sanchez D, Lopez-Arias B, Torroja L, Canal I, Wang X, Bastiani MJ, Ganfornina MD, 2006. Loss of glial lazarillo, a homolog of apolipoprotein D, reduces lifespan and stress resistance in DrosophilaDrosophila. Curr Biol 16, 680–686. [DOI] [PubMed] [Google Scholar]
- Sarjeant JM, Lawrie A, Kinnear C, Yablonsky S, Leung W, Massaeli H, Prichett W, Veinot JP, Rassart E, Rabinovitch M, 2003. Apolipoprotein D inhibits platelet-derived growth factor-BB-induced vascular smooth muscle cell proliferated by preventing translocation of phosphorylated extracellular signal regulated kinase 1/2 to the nucleus. Arterioscler Thromb Vasc Biol 23, 2172–2177. [DOI] [PubMed] [Google Scholar]
- Schindler PA, Settineri CA, Collet X, Fielding CJ, Burlingame AL, 1995. Sitespecific detection and structural characterization of the glycosylation of human plasma proteins lecithin:cholesterol acyltransferase and apolipoprotein D using HPLC/electrospray mass spectrometry and sequential glycosidase digestion. Protein Sc 4, 791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguin D, Desforges M, Rassart E, 1995. Molecular characterization and differential mRNA tissue distribution of mouse apolipoprotein D. Mol Brain Res 30, 242–250. [DOI] [PubMed] [Google Scholar]
- Serra Diaz C, Vizoso F, Lamelas ML, Rodriguez JC, Gonzalez LO, Baltasar A, Medrano J, 1999. Expression and clinical significance of apolipoprotein D in male breast cancer and gynaecomastia. Br J Surg 86, 1190–1197. [DOI] [PubMed] [Google Scholar]
- Sevin M, Lesca G, Baumann N, Millat G, Lyon-Caen O, Vanier MT, Sedel F, 2007. The adult form of Niemann-Pick disease type C. Brain 130, 120–133. [DOI] [PubMed] [Google Scholar]
- Simard J, Dauvois S, Haagensen DE, Levesque C, Merand Y, Labrie F, 1990. Regulation of progesterone-binding breast cyst protein GCDFP-24 secretion by estrogens and androgens in human breast cancer cells: a new marker of steroid action in breast cancer. Endocrinol 126, 3223–3231. [DOI] [PubMed] [Google Scholar]
- Simard J, Veilleux R, de Launoit Y, Haagensen DE, Labrie F, 1991. Stimulation of apolipoprotein D secretion by steroids coincides with inhibition of cell proliferation in human LNCaP prostate cancer cells. Cancer Res 51, 4336–4341. [PubMed] [Google Scholar]
- Smith KM, Lawn RM, Wilcox JN, 1990. Cellular localization of apolipoprotein D and lecithin:cholesterol acyltransferase mRNA in rhesus monkey tissues by in situ hybridization. J Lipid Res 31, 995–1004. [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM, 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu rev Biochem 69, 145–182. [DOI] [PubMed] [Google Scholar]
- Soiland H, Skaland I, Varhaug JE, Kørner H, Janssen EA, Gudlaugsson E, Baak JP, Søreide JA, 2009. Co-expression of estrogen receptor alpha and Apolipoprotein D in node positive operable breast cancer–possible relevance for survival and effects of adjuvant tamoxifen in postmenopausal patients. Acta Oncol 48, 514–521. [DOI] [PubMed] [Google Scholar]
- Spreyer P, Schaal H, Kuhn G, Rothe T, Unterbeck A, Olek K, Muller HW, 1990. Regeneration-associated high level expression of apolipoprotein D mRNA in endoneurial fibroblasts of peripheral nerve. EMBO J 9, 2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyrer E, Kostner GM, 1988. Activation of lecithin-cholesterol acyltransferase by apolipoprotein D: comparison of proteoliposomes containing apolipoprotein D, A-I or C-I. Biochim Biophys Acta 958, 484–491. [DOI] [PubMed] [Google Scholar]
- Suresh S, Yan Z, Patel RC, Patel YC, Patel SC, 1998. Cellular cholesterol storage in the Niemann-Pick disease type C mouse is associated with increased expression and defective processing of apolipoprotein D. J Neurochem 70, 242–251. [DOI] [PubMed] [Google Scholar]
- Tall AR, 2008. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Inter Med 263, 256–273. [DOI] [PubMed] [Google Scholar]
- Tanase-Nakao K, Mizuno K, Hayashi Y, Kojima Y, Hara M, Matsumoto K, Matsubara Y, Igarashi M, Miyado M, Fukami M, 2019. Dihydrotestosterone induces minor transcriptional alterations in genital skin fibroblasts of children with and without androgen insensitivity. Endocr J 66, 387–393. [DOI] [PubMed] [Google Scholar]
- Terrisse L, Poirier J, Bertrand P, Merched A, Visvikis S, Siest G, Milne R, Rassart E, 1998. Increased levels of apolipoprotein D in cerebrospinal fluid and hippocampus of Alzheimer’s patients. J Neurochem 71, 1643–1650. [DOI] [PubMed] [Google Scholar]
- Terrisse L, Seguin D, Bertrand P, Poirier J, Milne R, Rassart E, 1999. Modulation of apolipoprotein D and apolipoprotein E expression in rat hippocampus after entorhinal cortex lesion. Mol Brain Res 70, 26–35. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Copolov DL, Sutcliffe JG, 2003a. From pharmacotherapy to pathophysiology: emerging mechanisms of apolipoprotein D in psychiatric disorders. Curr Mol Med 3, 408–418. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Dean B, Pavey G, Sutcliffe JG, 2001. Increased CNS levels of apolipoprotein D in schizophrenic and bipolar subjects: implications for the pathophysiology of psychiatric disorders. Proc Natl Acad Sci USA 98, 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EA, George RC, Sutcliffe JG, 2003b. Apolipoprotein D modulates arachidonic acid signaling in cultured cells: implications for psychiatric disorders. Prostaglandins leukot Essent Fatty Acids 69, 421–427. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Yao JK, 2007. Clozapine specifically alters the arachidonic acid pathway in mice lacking apolipoprotein D. Schizophrenia Res 89, 147–153. [DOI] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F, 2015. Proteomics Tissuebased map of the human proteome. Science 347 20151260419. [DOI] [PubMed] [Google Scholar]
- Vaddadi KS, 1992. Use of gamma-linolenic acid in the treatment of schizophrenia and tardive dyskinesia. Prostaglandins leukot Essent Fatty Acids 46, 67–70. [DOI] [PubMed] [Google Scholar]
- Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW, 2007. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 117, 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE, 2012. Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis Model Mech 5, 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira AV, Lindstedt K, Schneider WJ, Vieira PM, 1995. Identification of a circulatory and oocytic avian apolipoprotein D. Mol Reprod Dev 42, 443–446. [DOI] [PubMed] [Google Scholar]
- Vogt M, Skerra A, 2001. Bacterially produced apolipoprotein D binds progesterone and arachidonic acid, but not bilirubin or E-3M2H. J Mol Recognit 14, 79–86. [DOI] [PubMed] [Google Scholar]
- Waldner A, Dassati S, Redl B, Smania N, Gandolfi M, 2018. Apolipoprotein D Concentration in Human Plasma during Aging and in Parkinson’s Disease: A CrossSectional Study. Parkinsons Dis 2018, 3751516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DW, Muffat J, Rundel C, Benzer S, 2006. Overexpression of a DrosophilaDrosophila homolog of apolipoprotein D leads to increased stress resistance and extended lifespan. Curr Biol 16, 674–679. [DOI] [PubMed] [Google Scholar]
- Walker NP, Fox HC, Whalley LJ, 1999. Lipids and schizophrenia. Br J Psychiatry 174, 101–104. [DOI] [PubMed] [Google Scholar]
- Warden CH, Diep A, Taylor BA, Lusis AJ, 1992. Localization of the gene for apolipoprotein D on mouse chromosome 16. Genomics 12, 851–852. [DOI] [PubMed] [Google Scholar]
- Weber GF, Cantor H, 1996. The immunology of Eta-1/osteopontin. Cytokine Growth Factor Rev 7, 241–248. [DOI] [PubMed] [Google Scholar]
- Weitzmann MN, 2013. The Role of Inflammatory Cytokines, the RANKL-OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Scientifica (Cairo). 10.1155/2013/125705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RB, Harvell J, Linn SC, Liu CL, Prapong W, Hernandez-Boussard T, Montgomery K, Nielsen TO, Rubin BP, Patel R, Goldblum JR, Brown PO, van de Rijn M, 2004. ApoD in soft tissue tumors: a novel marker for dermatofibrosarcoma protuberans. Am J Surg Pathol 28, 1063–1069. [DOI] [PubMed] [Google Scholar]
- Weech PK, Provost P, Tremblay NM, Camato RN, Milne RW, Marcel YL, Rassart E, 1991. Apolipoprotein D: an atypical apolipoprotein. Prog Lipid Res 30, 259–266. [DOI] [PubMed] [Google Scholar]
- Xin X, Zeng X, Gu H, Li M, Tan H, Jin Z, Hua T, Shi R, Wang H, 2016. CD147/ EMMPRIN overexpression and prognosis in cancer: A systematic review and metaanalysis. Sci Rep 6, 32804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Gu ZW, Blanco-Vaca F, Gaskell SJ, Yang M, Massey JB, Gotto AM Jr., Pownall HJ, 1994. Structure of human apolipoprotein D: locations of the intermolecular and intramolecular disulfide links. Biochemistry 33, 12451–12455. [DOI] [PubMed] [Google Scholar]
- Yao JK, van Kammen DP, Gurklis JA, 1996. Abnormal incorporation of arachidonic acid into platelets of drug-free patients with schizophrenia. Psychiatry Res 60, 11–21. [DOI] [PubMed] [Google Scholar]
- Yao JK, Thomas EA, Reddy RD, Keshavan MS, 2005. Association of plasma apolipoproteins D with RBC membrane arachidonic acid levels in schizophrenia. Schizophr Res 72, 259–266. [DOI] [PubMed] [Google Scholar]
- Yao Y, Vieira A, 2002. Comparative 17beta-estradiol response and lipoprotein interactions of an avian apolipoprotein. Gen Comp Endocrinol 127, 89–93. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Cleaveland ES, Nagle JW, French S, Yaswen L, Ohshima T, Brady RO, Pentchev PG, Kulkarni AB, 1996. Molecular cloning of the mouse apolipoprotein D gene and its upregulated expression in Niemann-Pick disease type C mouse model. DNA Cell Biol 15, 873–882. [DOI] [PubMed] [Google Scholar]
- Zeng C, Spielman AI, Vowels BR, Leyden JJ, Biemann K, Preti G, 1996. A human axillary odorant is carried by apolipoprotein D. Proc Natl Acad Sci USA 93, 6626–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Bentel JM, Ricciardelli C, Horsfall DJ, Haagensen DE, Marshall VR, Tilley WD, 1998. Immunolocalization of apolipoprotein D, androgen receptor and prostate specific antigen in early stage prostate cancers. J Urol 159, 548–554. [DOI] [PubMed] [Google Scholar]
- Zhang DT, Yuan J, Yang L, et al. , 2005. Osteopontin Expression and Its Relation to Invasion and Metastases in Gastric Cancer. Zhonghua Zhong Liu Za Zhi 27 (3), 167–169. [PubMed] [Google Scholar]
- Zheng XY, Zhang HL, Luo Q, Zhu J, 2011. Kainic acid-induced neurodegenerative model: potentials and limitations. J Biomed Biotechnol 2011, 457079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y and Luo G (2020) Apolipoproteins, as the carrier proteins for lipids, are involved in the development of breast cancer. Clinical and Translational Oncology; doi: 10.1007/s12094-020-02354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]