Abstract
Purpose of Review
A growing body of evidence suggests adverse neurodevelopmental effects of early-life exposure to fluoride that may differ depending on timing of exposure and sex of the exposed. We conducted a literature search to identify the animal and human epidemiologic studies that examined sex-specific neurodevelopmental differences in response to prenatal and postnatal exposure to fluoride.
Recent Findings
Six of 138 animal studies and 15 of 106 human epidemiologic studies tested for sex-specific effects. Prenatal exposure to fluoride was associated with a male susceptibility to adverse behavioural effects in four of six animal studies and lower IQ in one of three prospective cohort studies. The body of evidence examining sex-effects associated with postnatal fluoride exposure was scarce, and many animal and cross-sectional human studies were considered to have a high risk of bias.
Summary
Compared to females, male offspring appear to be more sensitive to prenatal, but not postnatal, exposure to fluoride. We discuss several sex-specific mechanisms and emphasize the need for future research.
Keywords: fluoride, sex-differences, neurodevelopment, prenatal, exposure
Introduction
Exposure to fluoride can come from a variety of sources, including dental products, drinking water, food, pharmaceuticals, and insecticides. In some developed countries, fluoride is added to drinking water to reach the level of 0.7 mg/L, which is considered the optimal level to provide a balance of protecting against dental caries while limiting the risk of fluorosis (discolouration of tooth enamel) [1]. In endemic areas, high levels of naturally occurring fluoride (>1.2 mg/L) are found in drinking water [2].
There is a growing body of literature suggesting that early-life exposure to fluoride may increase the risk for a variety of neurodevelopmental problems, including lowered IQ scores and difficulties with attention [3]. Developmental neurotoxicity of fluoride has been documented in populations living in endemic areas, as well as optimally fluoridated areas in which urinary fluoride levels in pregnant women are roughly twice as high as compared with pregnant women from non-fluoridated areas [4]. In 2019, our group published the first prospective study of a birth cohort receiving optimally fluoridated water [5]. We measured urinary fluoride concentrations in 512 pregnant women and found a 4.49 IQ point loss in boys, but not girls, per 1 mg/L increase in maternal urinary fluoride. These findings, which reignited the debate about the safety of water fluoridation, elicited a number of critiques concerning the biological plausibility of fluoride exerting a sex-specific effect on brain development [6,7].
In recent years, the fields of psychology, toxicology, and environmental epidemiology have embraced the concept of sex-specific vulnerability associated with neurotoxic exposures [8–10]. Studies have linked in utero exposure to endocrine disrupting chemicals (EDCs) with adverse neurodevelopmental effects in one sex only, with many studies showing males at a heightened vulnerability as compared to females [11–14]. In addition, recent studies have shown that males are more vulnerable to early-life lead exposure as compared to females [15–20], suggesting that male fetuses may be more vulnerable than females [21]. Despite these findings, sex has not been adequately accounted for in neurotoxicological or epidemiological research more broadly [22–24], and has not been examined in regards to fluoride specifically.
We review animal and human epidemiologic studies that tested sex-specific neurodevelopmental effects of early-life exposure to fluoride and examine whether sex-specific effects may vary depending on timing of exposure. Finally, we discuss potential mechanisms by which early-life exposure to fluoride and other neurotoxicants may exert a differing effect on the male versus female developing brain.
Methods
Search Strategy
We used the Fluoride (2019) assessment from the open-source Health Assessment Workspace Collaborative (HAWC) web-application, https://hawcproject.org/study/assessment/405/. This comprehensive assessment lists animal studies, human studies, and in-vitro studies inclusive until August 31, 2019. Titles and abstracts were extracted from HAWC and reviewed to determine whether the following criteria were met: (1) fluoride exposure during gestation or early postnatal period (weanlings or childhood until age 18); (2) both sexes included in the sample (relevant for animal studies only); (3) outcomes related to neurodevelopment (e.g. behavioural, cognitive, sensorimotor outcome); and (4) study was written in English or an English version of the study was available. Using the studies that met our inclusion criteria, we then identified those that (a) reported exposure and outcome results stratified by sex or (b) included an interaction term in the model to test for sex differences.
Animal studies (Figure 1) were reviewed by two research assistants (RC and RP) and human studies (Figure 2) were reviewed by one research assistant (JR). A full text review was completed for those articles that met inclusion criteria based on information from the abstract or if there was ambiguity concerning the inclusion/exclusion criteria within the abstract. A full text review did not occur only if it was very clear from the abstract that the article did not meet inclusion criteria. After the research assistants assessed each citation for eligibility, the lead author (RG) independently re-reviewed each citation for eligibility. Conflicts were resolved by discussion and consensus. Quality of studies and potential for bias were assessed using the framework adopted by the National Toxicology Program (NTP) 2019 draft monograph [25], the NTP’s 2016 Research Report for the Systematic Review of the Effects of Fluoride on Learning and Memory in Animal Studies [24], and the HAWC web-application. Overall conclusions were based on a narrative analysis of patterns of responses across the different neurodevelopmental studies with quality of the study taken into consideration.
Figure 1.
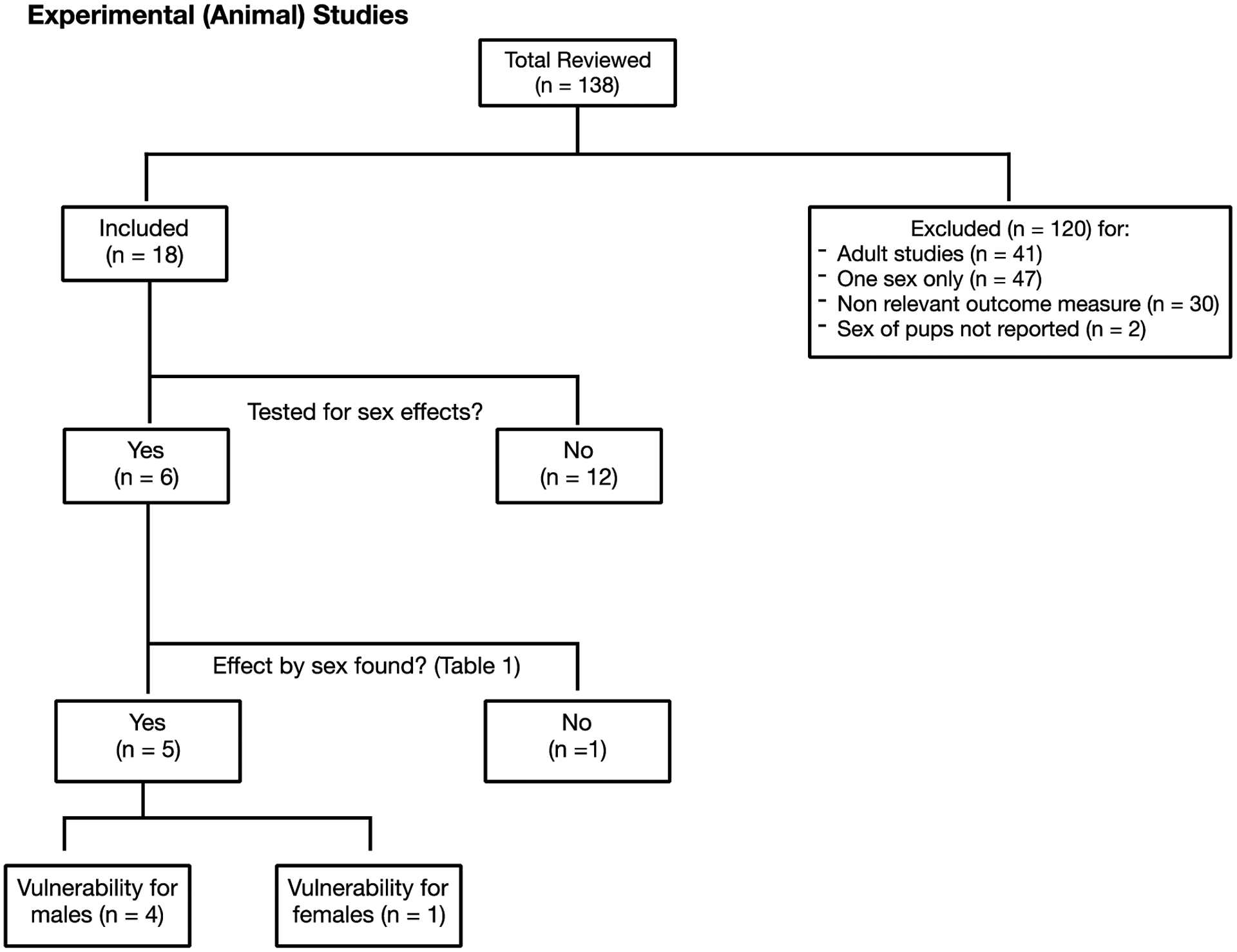
Flowchart of Inclusion Criteria for Experimental (Animal) Studies
Figure 2.
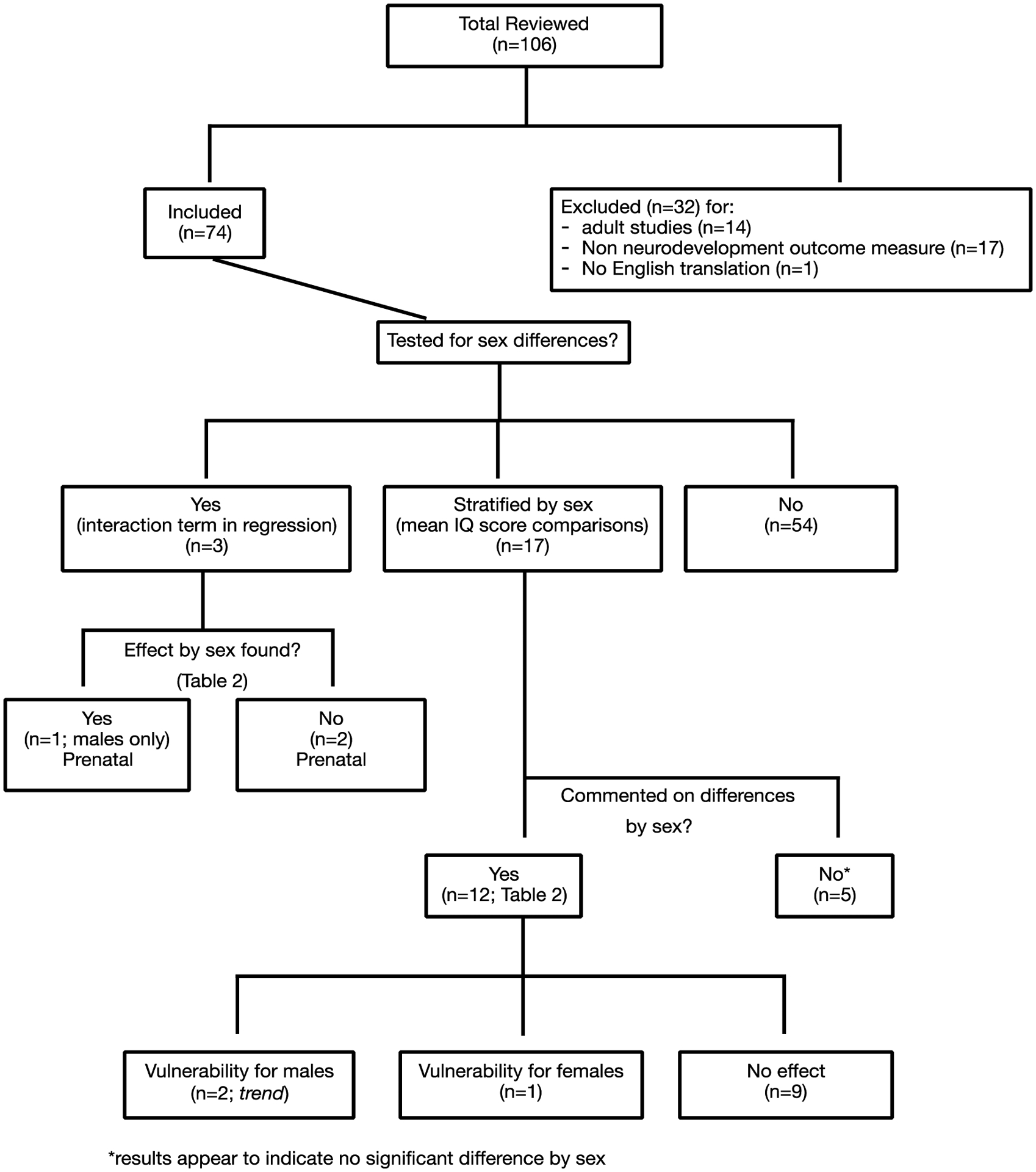
Flowchart of Inclusion Criteria for Epidemiological (Human) Studies
Results
Data Extraction and Management: Animal Studies
Author name, year of publication, species, sample size, exposure period, dose, and key findings related to sex-specific effects were extracted from the eligible animal studies. Of 138 animal studies that were reviewed, 120 were excluded for the following reasons: dosed adult species (n = 41), included one sex only (n = 47), did not include a neurodevelopmental outcome measure (n = 30), and the sex of the subjects was not reported (n = 2) (Figure 1). From the 18 eligible studies, six reported testing for sex differences (Table 1); the other 12 studies included both sexes, but did not test for sex differences nor presented data by sex and exposure group.
Table 1.
Experimental (Animal) Studies Reporting Sex-Specific Neurodevelopmental Effects of Gestational or Postnatal Exposure to Fluoride
| Reference (risk of bias) | Species | Sample size | Timing and dose of fluoride | Outcome and assessment timing | Main findings |
|---|---|---|---|---|---|
| Baran-Poesina et al., 2013[26] (high risk of bias; excluded from NTP meta-analysis) | NMRI mice | 3 groups with 16 per group | Gestation (GD8 to PND0) Groups: 5 mg/kg-d 10 mg/kg-d Control |
Clearing time in classic maze in 21-day intervals (3 evaluations) (higher clearing times indicate worse performance) | Overall, greatest effect of ↑ clearing time by 385% observed in males as compared to non-exposed controls as opposed to females. |
| Bartos et al., 2015[31] (low risk of bias) | Wistar rats | 10 males and 10 females per condition | Gestation to postnatal (GD0 to PND21) Groups: 5 mg/L 10 mg/L Control |
Sensorimotor development (righting reflex, cliff aversion, negative geotaxis, eye/ear opening) starting on PND3 and locomotor activities, including maze test, on PND45 and PND90. | No differences by sex on most activities. 45-day-old females showed ↓ anxiety (i.e., elevated time spent in the open arms and high number of entries in same arms) in the plus maze test as compared to controls while no difference observed in males. |
| Bera et al., 2007[27] (high risk of bias; excluded from NTP meta-analysis) | Wistar rats | 4 groups with 10 per group with 6–12 per test | Gestation to postnatal (GD 1 to PND 9) Groups: 1 mg/kg/mL 2.5 mg/kg/mL 5 mg/kg/mL Control |
Locomotor activity test on PND21, 40, and 60; learning (novel object exploration test), rotarod test, and active avoidance conditioning test on PND40. | Females – no differences Males – ↓ learning and memory associated with 1 and 2.5 mg/kg/mL exposure in perinatal period. |
| Flace et al., 2010[28] (probable low risk of bias) | Wistar rats | 40 males and 40 females divided into 3 groups | Gestation to postnatal (GD0 to PND9) Groups: 2.5 mg/kg/mL 5 mg/kg/mL Control |
Emotionality and sensory-motor gating: ultrasonic vocalization at PND 10; acoustic startle reflex (ASR) at PND 90; pre-pulse inhibition (PPI) at PND 90 | Females – no differences Males – ↓ peak intensity values of the ASR in 90-day-old treated rats with both doses as compared to controls; ↓ ultrasonic calls by 10-day-old male rats in the 5mg group as compared to controls; ↓ latency to peak intensity of the ASR in 90-day-old male rats in the 5mg group as compared to controls; PPI peak values increased in 90-day-old male rats in the 5mg group |
| Jiang et al., 2014[30] (probable low risk of bias) | Sprague-Dawley rats | 12 males and 12 females per group | Gestation to postnatal (GD0 to PND60) Groups: 25 mg/L 50 mg/L 100 mg/L |
Morris Water Maze at 2- months-old | No significant differences by sex. |
| Mullenix et al., 1995[29] (probable high risk of bias; excluded from NTP meta- analysis)1 | Sprague-Dawley rats | 1. 13 in control group; 7–9 in experimental group | 1. Late gestation (GD 14–18 or 17–19). Dams injected with 0.13 mg/kg NaF or control. | Measures of spontaneous behaviours at 9, 14, and 19 weeks. |
1. Late gestation : Females - no effect Males treated on GD 17–19 were more active (i.e. ↑ instances of grooming and head turning) than controls. |
| 2. 19–27 per sex per group | 2. Weanlings (PND19) received water containing 0, 75, 100, or 125 ppm F for 6 or 16 weeks. |
2. Weanlings: Females – reduced behaviours (i.e. ↓ head turns, ↓ grooming bouts) relative to controls in the 100 ppm × 6 weeks and 125 ppm × 16 weeks group Males – reduced behaviours (i.e. ↓ head turns, ↓ grooming bouts) relative to controls, but only in the high exposure group (125 ppm × 16 weeks) Overall, gestational exposure was associated with ↓ spontaneous behaviours in males whereas exposure in weanlings was associated with effects in both sexes |
Abbreviations: NMRI = Naval Medical Research Institute; GD = gestational day; F = fluoride; PND = post-natal day; ppm = part per million
Data not reported for a third exposure group (12-week old rats) because this group did not meet our inclusion criteria of exposure during early-life.
All six animal studies examined the gestational to early postnatal (i.e., pre-weaning) period to mimic the human gestational period (Table 1). Of the six studies, four reported a male-specific effect of fluoride, including slower performance time [26], worse learning/memory [27], and reduced sensory-motor function [28] and spontaneous behaviours [29]. One study did not report a sex-specific effect [30] and one demonstrated lower anxiety behaviour in female rats (measured by more time spent in the maze) as compared to controls whereas treated males did not differ from controls [31]. Fluoride exposure concentrations varied considerably across the animal studies reflecting differences in between-species pharmacokinetics, diets, and age at dosing [32].
Data Extraction and Management: Epidemiologic (Human) Studies
Of 106 epidemiological studies that were reviewed, 74 were considered eligible and were included in the critical review (Figure 2). The remaining 32 articles were excluded for having a non-neurodevelopment outcome measure (n = 17), exposure to fluoride in adulthood (n = 14), and not translated into English (n = 1). Of the 74 studies included, 54 did not test for sex differences or report outcomes stratified by sex and exposure, leaving 20 studies that reported outcome data separately by sex.
Three of the 20 were birth cohort studies with prenatal fluoride exposure [5,33,34] (see Table 2) that were deemed to have low risk of bias. Among these three studies, one study reported a lower IQ score in males, but not females, with increasing fetal exposure to fluoride [5]. The remaining 17 studies were cross-sectional (postnatal/lifetime exposure) and presented sex-stratified results for exposure and outcome data, though only 12 of them specifically reported on whether there were significant sex-specific IQ outcomes across fluoride exposure grouping (Table 2).
Table 2.
Epidemiological (Human) Studies Reporting Sex-Specific Neurodevelopmental Effects of Prenatal or Postnatal Exposure to Fluoride
| Reference (risk of bias) | Country | Timing of exposure; sample size | Exposure measure and level | Assessment measure and timing | Sex differences |
|---|---|---|---|---|---|
| Birth cohort studies | |||||
| Bashash et al., 2018 (probably low risk)[34] | Mexico | Prenatal; n = 210 |
Maternal urine: 0.85 mg/L (IQR: 0.46) |
Children (CRS-R, CPT-II: ages 6–12 years) | No significant interaction between sex and maternal urinary fluoride. |
| Green et al., 2019 (probably low risk)[5] | Canada | Prenatal; n = 512 |
Maternal urine: 0.51 mg/L (range: 0.06–2.44) |
Children (WPPSI-III: age 3 years) | Significant ↓ in FSIQ in boys (adjusted β = −4.49, 95% CI: −8.38, − 0.60), but not girls, per 1 mg F/L increase in maternal urine. |
| Valdez Jimenez et al., 2017 (probably low risk)[33] | Mexico | Prenatal; n = 65 |
Maternal urine: 1.9–2.7 mg/L across trimesters |
Infants (BSDI-II: ages 3–15 months) | No differences between sex. |
| Cross-sectional studiesa | |||||
| Aravind et al., 2016 (probably high risk)[43] | India | Postnatal; n = 288 |
Drinking water: Low: <1.2ppm Medium: 1.2–2ppm High: >3ppm |
Children (RSPM: ages 10–12 years) | No significant differences between mean IQ scores by sex across the 3 villages. |
| Chen et al., 2008 (probably high risk)[44] | China | Postnatal; n = 640 |
Residency: Endemic fluoride area (dental fluorosis = 85%) Control area (dental fluorosis = 15%) |
Children (Chinese Raven’s test: ages 7–14- years) | No significant differences in the average IQ scores by sex. |
| Khan et al., 2015 (probably high risk)[45] | India | Postnatal; n = 429 | Village: Low fluoride area (0.19 ppm) High fluoride area (2.41 ppm) |
Children (RCPM: ages 6–11 years) | No significant differences between mean IQ scores by sex. |
| Li et al., 2008 (probably high risk)[46] | China | Postnatal; n = 956 | Residency: Endemic fluoride area (dental fluorosis = 58%) Control area (dental fluorosis = 12%) |
Children (Standardized Raven’s: Rural version: ages 6–13 years) | No significant differences between mean IQ scores by sex across residencies. |
| Li et al., 2010 (probably high risk)[47] | China | Postnatal; n = 676 | Residency in area with high fluoride in drinking water (2.47 ppm) | Combined Raven’s Test: Rural Area Version: ages 7–10 years | No significant differences between mean IQ scores by sex. |
| Poureslami et al., 2011 (probably high risk)[48] | Iran | Postnatal; n = 120 | Residency: Low fluoride area (0.41 ppm) High fluoride area (2.38 ppm) |
Children (RCPM: ages 7–9 years) | No significant differences between mean IQ scores by sex across residences. |
| Razdan et al., 2017 (probably high risk)[49] | India | Postnatal; n = 219 | Residency: Low: 0.60 ppm Medium: 1.70 ppm High: 4.49 ppm |
Children (IQ: RSPM: ages 12–14-years) | No significant differences between mean IQ scores by sex across residencies. |
| Seraj et al., 2012 (probably low risk)[37] | Iran | Postnatal; n = 293 | Drinking water: Low: 0.80 ppm Medium: 3.1 ppm High: 5.2 ppm |
Children (RCPM: ages 6–11 years) | Trend level association (p=.07) showing ↓ IQ scores in males (89.9 ± 15.9) compared with females (93.4 ± 17.4) collapsed across residencies. |
| Sudhir et al., 2009 (probably high risk)[50] | India | Postnatal; n = 1000 | Residency: Low: <0.6 ppm Medium: 0.7–1.2 ppm High: 1.3–3.1 ppm Very high: >4.1 ppm |
Children (RSPM: ages 13–15 years) | No significant differences between mean IQ scores by sex across residencies. |
| Trivedi et al., 2007 (probably high risk)[36] | India | Postnatal; n = 190 | Drinking water: Medium: 2.01 ppm High: 5.55 ppm Children’s urine: Medium fluoride area: 2.30 ppm High fluoride area: 6.13 ppm |
Children (IQ score estimated via a questionnaire: ages 12–13 years) | Trend level association (p=.09) showing ↓ IQ scores in males in the high fluoride area (90.24 ± 1.58) relative to females (94.15 ± 1.35). No difference in IQ by sex in the low fluoride area. |
| Xiang et al., 2003 (probably low risk)[35] | China | Postnatal; n = 512 | Drinking water: Low: 0.36 ppm High: 2.47 ppm Children’s urine Low fluoride area: 1.11 ppm High fluoride area: 3.47 ppm |
Children (Combined Raven’s Test: Rural Area Version: ages 8–13 years) | IQ scores significantly ↓ in girls (88.7 ± 12.2) relative to boys (94.7 ± 13.1) in the high fluoride region. No difference in IQ by sex in the low fluoride area. |
| Zhao et al., 1996 (probably high risk)[51] | China | Postnatal; n = 320 | Residency: Low: 0.91 ppm High: 4.12 ppm |
Rui wen IQ test rural edition | No significant differences between mean IQ scores by sex across residencies. |
Outcomes reported comparing mean IQ scores for boys and girls.
Abbreviations: IQR = Interquartile Range; BSID-II = Bayley Scales of Infant Development-II; CRS-R = Conners’ Rating Scales-Revised; CPT-II = Conners’ Continuous Performance Test; WPPSI-III = Wechsler Preschool and Primary Scale of Intelligence-III; MSCA = McCarthy Scales of Children’s Abilities; RCPM = Raven’s Coloured Progressive Matrices; RSPM: Raven’s Standard Progressive Matrices)
Of the 12 cross-sectional studies that reported on sex-specific differences, one reported significantly lower IQ scores for females relative to males [35], two reported trend level effects for males [36,37], and the remaining nine studies reported no significant differences in IQ scores by sex (Table 2). Of the five that did not report sex-specific IQ outcomes across exposure, our post-hoc independent analysis (student’s t-test) did not suggest differences in IQ scores by sex [38–42] (not included in Table 2).
It is important to note that the majority of the cross-sectional studies (10 out of 12) were deemed to have a high risk of bias. Because of the methodological limitations of these studies (limited exposure assessment, no adjustment for potential confounders, ecological), the results should be interpreted cautiously. The two “lower risk” studies both showed sex-specific effects (though in opposite directions and one was a trend).
Conclusions
In this review, we summarize and discuss the animal and human epidemiologic literature that tested for sex-specific neurotoxic effects of early-life exposure to fluoride. Until recently, most animal studies (i.e., 120 out of 138; 87%) either excluded females or pooled males and females in the analyses, consistent with the conclusion made by the NTP in 2016 that our knowledge on sex-specific differences is sparse. We identified six studies that evaluated sex differences in animals treated with fluoride during the gestational period. The majority of these studies (four out of six [26–29]) suggested a male vulnerability to prenatal exposure to fluoride whereas one suggested a female vulnerability [31] and one suggested no differences by sex [30]. Further research is needed to confirm whether there is a vulnerability by sex as three of the studies showing a male-specific finding were considered to pose an overall high risk of bias, primarily due to no randomization, lack of information on exposure, lack of blinding for outcome assessment [26,27] and one lacked adequate exposure information and control for litter effects [29].
Sex-specific effects of fluoride were also not directly evaluated in the majority (54 out of 74; 73%) of the human studies. Instead, males and females were pooled for analyses, or sex was included as a covariate. Of those that did compare IQ scores across exposure and sex, most did not find significant differences in IQ scores by sex (nine out of 12). Two found trend levels of lower IQ scores in boys in the fluoridated region or collapsed across regions [36,37], as compared to girls in the same region, and one found significantly lower IQ scores of girls in the fluoridated region as compared to boys in the fluoridated region [35].
In one of the three birth cohort studies in which sex was taken into account [5], a significant interaction was observed between sex and IQ outcomes. This Canadian study observed a 4.49 point decrement in IQ score in boys (95% CI: −8.38, −0.60), but not girls (B = 2.40; 95% CI: −2.53, 7.33), with increasing levels of fetal exposure estimated via urinary fluoride concentration averaged over three trimesters in pregnancy. Although only one out of the three studies found a prenatal effect on males, it could be that the other two studies did not have enough power to test for sex effects due to smaller sample sizes and uneven distribution by sex [33]. Another reason for the discrepant findings could be that neurotoxic responses to fluoride vary by sex[52]; as such, sex-specific differences may not be observed across the different neurodevelopmental outcomes (i.e. child IQ versus infant cognition versus parent-rated attention scores). Our review did not include a 2017 prospective cohort study [53] conducted in Mexico City that found an association between prenatal fluoride exposure and child IQ because results by sex and exposure levels were not reported. However, a newer study by our group comparing this Mexican cohort with our Canadian cohort found that prenatal fluoride exposure was associated with lower performance IQ in boys, but not girls, across both cohorts [54]. Still, sex-specific effects of prenatal exposure to fluoride are limited by the few birth cohort studies conducted to date.
This finding reported by Green et al [5] that males might be more susceptible to fluoride toxicity than females in the prenatal period is consistent with the majority of the animal studies that tested sex-specific effects of prenatal fluoride. In contrast, sex-specific effects were not observed by Green et al. when water fluoride concentration was used. Rather, IQ decrements were found in boys and girls combined (B = −5.29; 95% CI: −10.39, −0.19), consistent with the majority of other epidemiologic studies that reported an inverse association between IQ and fluoride in drinking water. It is probable that fluoride level in drinking water is capturing exposure that occurs beyond the prenatal period, including fluoride consumed in infant formula and water drunk in early childhood as children presumably drink the same type of water as their mother. Indeed, in the same Canadian birth cohort (note: paper was published after our review cut-off), an increase of 0.5 mg/L in the water fluoride concentration (approximately equal to the difference between fluoridated and non-fluoridated regions) was associated with a 7.9-point lower IQ score (95% CI: −12.84, −3.01) in formula-fed infants and 6.3-point lower IQ score (95% CI: −10.45, −1.94) in breastfed children in both boys and girls [55]. These results were found after controlling for fetal fluoride exposure, suggesting that postnatal exposure to fluoride may affect both sexes. Yet, firm conclusions are limited by the small number of epidemiologic studies that have tested for sex-specific effects, including the low quality of evidence among the studies that did, as well as the fact that these studies did not address windows of vulnerability.
Mechanisms of Fluoride’s Neurotoxicity and Possible Mechanisms of Sex-Related Differences
Studies from endemic fluorosis areas and animal studies have demonstrated that fluoride can affect the synthesis and excretion of certain neurotransmitters, thus impacting the various stages of brain development and altering brain structure and/or function [56]. There is also evidence that fluoride may reduce the concentration of cholinesterase activity in the brain [57]. Animal studies and human fetal brain studies have also shown alterations in dopamine, serotonin, norepinephrine, and epinephrine in the hippocampus and neocortex regions [56,58].
At lower levels of fluoride, mechanisms of developmental neurotoxicity are not well understood but it is possible that fluoride affects thyroid function [32], especially among individuals who are iodine deficient [59]. Until mid-gestation when the fetal thyroid gland becomes functional, the fetus is exclusively dependent on maternal thyroid hormones for regulating crucial brain development processes [60–62]. Even relatively subtle changes in circulating levels of thyroid hormone in pregnancy (i.e. subclinical hypothyroidism) can have adverse neurodevelopmental outcomes, including lowered IQ [62–67], increased risk for attention disorders [68,69], and altered brain structure [60,61]. Since males and females may respond differently to thyroid hormone insufficiencies [70,71], it might be the case that fluoride exposure contributes to sex-specific effects that are mediated by disruption to thyroid hormones.
Sex hormones exert organizational effects during critical periods of brain development [72], including the development of the hypothalamus [73], which is involved in the release of thyrotropin-releasing hormone and is a region associated with elevated fluoride following high level exposure to fluoride in weanling rats [29]. Estradiol can exert neuroprotective effects [74] [75], including enhancing cell proliferation and synaptic density, and protecting neurons from oxidative stress [76], which may be induced by fluoride intoxication [77]. Females may have better mechanisms for mitigating against oxidative stress in the prenatal and perinatal period due to increased antioxidant status in the mitochondria as compared to males [78,79]. In addition, increasing levels of fluoride exposure may also impact levels of sex hormones in adolescents. Indeed, a recent study found an inverse relationship between plasma fluoride and sex steroid hormones (testosterone, sex-hormone binding globulin, estradiol) in male adolescents [80]. Thus, interactions between fluoride exposure and sex hormones may contribute to sex-specific neurotoxic effects.
In addition to biological differences, there may be differences in critical windows of exposure between male and females reflecting differences in neurological development [81]. For example, timing of remethylation of imprinted genes during gametogenesis differs by sex [82]. Gray matter and total brain volume may also differ between the sexes with males peaking at around eleven years of age and females at nine [83][84]. In terms of fluoride’s neurotoxicity specifically, one study showed that fluoride levels in key brain regions in rats differed by sex with hippocampal fluoride levels increased in females but not males [29]. It could be that postnatally, since males may experience more prolonged growth spurts than females, more of their plasma fluoride is directed towards developing bones as opposed to the brain. Fluoride exposure may also differ between the sexes due to contextual factors, such as differences in beverage consumption or inadvertent ingestion of fluoridated toothpaste among young children [52]. Indeed, patterns of ingestion, metabolism and toxicokinetic have been shown to vary by sex [9,85]. Finally, the blood-brain barrier’s permeability has also been shown to differ across sex depending on substances [86,87].
Future Directions
This review highlights that both sexes are vulnerable to fluoride neurotoxicity, but effects may vary depending on timing of exposure. Sex-specific differences in clinical expressions of adverse neurodevelopmental trajectories are in line with males having a higher prevalence of many neurodevelopmental disorders (attention-deficit hyperactivity disorder, learning disabilities, Autism Spectrum Disorder, and intellectual disabilities) [88]. Given the biological plausibility for sex-specific vulnerabilities related to early-life exposure to fluoride, we disagree with comments made by epidemiologist John Ioannidis and others who stated that a drop in IQ for boys, but not girls, “makes no sense”[89]. Considering the pattern of the existing evidence and the clinical significance of the male preponderance in the neurodevelopmental literature, future high-quality investigations into possible sex-related mechanisms underlying fluoride neurotoxicity is necessary.
Important publications:
2. Grandjean (2019) – recent meta-analysis on fluoride’s developmental neurotoxicity
4. Green et al (2019) – our study showing a prenatal effect of fluoride exposure on males, and one of the reasons we wished to pursue this critical review on sex differences to fluoride exposure
6. Gochfield (2017) - report emphasizing the importance of sex differences in research studies
13. Desrochers et al (2018) – study on the same cohort as ours showing prenatal lead exposure affecting males’ intelligence only
22. NTP draft monograph (2019) – the document we used to obtain references and estimates of biases
Funding:
Vanier Canadian Graduate Scholarship, NIEHS R01 #ES030365; R21 #ES27044
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.U.S. Department of Health and Human Services Federal Panel on Community Water Fluoridation. U.S. public health service recommendation for fluoride concentration in drinking water for the prevention of dental caries. Public Health Rep [Internet] 2015;130(1):1–14. Available from: http://www.publichealthreports.org/fluorideguidelines.cfm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fawell J Fluoride in Drinking-water. 2013. [Google Scholar]
- 3.Grandjean P Developmental fluoride neurotoxicity: An updated review. Environ Heal A Glob Access Sci Source 2019;18(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Till C, Green R, Grundy J, Hornung R, Neufeld R, Martinez-Mier EA, et al. Community Water Fluoridation and Urinary Fluoride Concentrations in a National Sample of Pregnant Women in Canada. Enviromental Heal Perspect 2018;126(10):107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green R; Lanphear B; Hornung R; Flora D; Martinez-Mier EA; Neufeld R; Ayotte P; Muckle G; Till C Fluoride Exposure during Fetal Development and Intellectual Abilities in a Canadian Birth Cohort. JAMA Pediatr 2019;173(10):940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Science Media Centre. expert reaction to study looking at maternal exposure to fluoride and IQ in children [Internet]. Sci. Media Cent 2019;Available from: https://www.sciencemediacentre.org/expert-reaction-to-study-looking-at-maternal-exposure-to-fluoride-and-iq-in-children/ [Google Scholar]
- 7.American Dental Association. Responses to fluoride study flood in from all over the globe. ADA News 2019; [Google Scholar]
- 8.Mergler D Neurotoxic exposures and effects: Gender and sex matter! Hänninen Lecture 2011. Neurotoxicology [Internet] 2012;33(4):644–51. Available from: http://www.sciencedirect.com/science/article/pii/S0161813X12001246 [DOI] [PubMed] [Google Scholar]
- 9.Gochfeld M Sex Differences in Human and Animal Toxicology. Toxicol Pathol 2017;45(1):172–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbuckle TE. Are there sex and gender differences in acute exposure to chemicals in the same setting? Environ Res 2006;101(2):195–204. [DOI] [PubMed] [Google Scholar]
- 11.Braun J, Yolton K, Stacy SL, Erar B, Papandonatos GD, Bellinger DC, et al. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology 2017;62:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans SF, Kobrosly RW, Barrett ES, Thurston SW, Calafat AM, Weiss B, et al. Prenatal Bisphenol A Exposure and maternally reported behavior in boys and girls. Neurotoxicology [Internet] 2014;45:91–9. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4362616/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harley KG, Schall RA, Chevrier J, Tyler K, Aguirre H, Bradman A, et al. Research | Children ‘ s Health Prenatal and Postnatal Bisphenol A Exposure and Body Mass Index in Childhood in the CHAMACOS Cohort. 2013;514(4):514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roen EL, Wang Y, Calafat AM, Wang S, Margolis A, Herbstman J, et al. Bisphenol A Exposure and Behavioral Problems among Inner City Children at 7–9 Years of Age. Environ Res [Internet] 2015;142:739–45. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4545741/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brubaker CJ, Dietrich KN, Lanphear BP, Cecil KM. The influence of age of lead exposure on adult gray matter volume. Neurotoxicology 2010;31(3):259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desrochers-Couture M, Oulhote Y, Arbuckle TE, Fraser WD, Séguin JR, Ouellet E, et al. Prenatal, concurrent, and sex-specific associations between blood lead concentrations and IQ in preschool Canadian children. Environ Int [Internet] 2018;121:1235–42. Available from: http://www.sciencedirect.com/science/article/pii/S0160412018312510 [DOI] [PubMed] [Google Scholar]
- 17.Jedrychowski W, Perera FP, Jankowski J, Mrozek-Budzyn D, Mroz E, Flak E, et al. Very low prenatal exposure to lead and mental development of children in infancy and early childhood: Krakow prospective cohort study. Neuroepidemiology [Internet] 2009;32(4):270–8. Available from: https://www.ncbi.nlm.nih.gov/pubmed/19223686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ris MD, Dietrich KIMN, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and neuropsychological outcome in adolescence. J Int Neuropsychol Soc [Internet] 2004;10(2):261–70. Available from: https://www.cambridge.org/core/article/early-exposure-to-lead-and-neuropsychological-outcome-in-adolescence/CE1C294E68F705297C1FD964C39B82AB [DOI] [PubMed] [Google Scholar]
- 19.Cecil KM, Brubaker CJ, Adler CM, Dietrich KN, Altaye M, Egelhoff JC, et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med 2008;5(5):0741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh G, Singh V, Sobolewski M, Cory-Slechta DA, Schneider JS. Sex-Dependent Effects of Developmental Lead Exposure on the Brain. Front Genet [Internet] 2018;9:89. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29662502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dipietro J, Voegtline K. The gestational foundation of sex differences in development and vulnerability. Neuroscience 2017;342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gochfeld M Sex-Gender Research Sensitivity and Healthcare Disparities. J Women’s Heal [Internet] 2010;19(2):189–94. Available from: 10.1089/jwh.2009.1632 [DOI] [PubMed] [Google Scholar]
- 23.Weiss B Same sex, no sex, and unaware sex in neurotoxicology. Neurotoxicology [Internet] 2011;32(5):509–17. Available from: http://www.sciencedirect.com/science/article/pii/S0161813X10001841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Toxicology Program (NTP). Systematic literature review on the effects of fluoride on learning and memory in animal studies. Research Triangle Park, N.C., United States: 2016. [PubMed] [Google Scholar]
- 25.NTP. Draft Ntp Monograph on the Systematic Review of Fluoride Exposure and Neurodevelopmental and Cognitive Health Effects. 2019;129. [PubMed] [Google Scholar]
- 26.Bǎran-Poesina V, Negreș S, Dobrescu D, Dimcevici-Poesina N, Dimcevici-Poesina A, Feghiu A, et al. Experimental pharmacological researches regarding the influence of sodium fluoride in allopathic and homeopathic doses on central nervous system’s performances. A correlation between behavioral response in classic maze test and morphological aspects of ce. Farmacia 2013;61:781–99. [Google Scholar]
- 27.Bera I, Sabatini R, Auteri P, Flace P, Sisto G, Montagnani M, et al. Neurofunctional effects of developmental sodium fluoride exposure in rats. Eur Rev Med Pharmacol Sci 2007;11(4):211–24. [PubMed] [Google Scholar]
- 28.Flace P, Benagiano V, Vermesan D, Sabatini R, Inchingolo AM, Auteri P, et al. Effects of developmental fluoride exposure on rat ultrasonic vocalization, acoustic startle reflex and pre-pulse inhibition (European Review for Medical and Pharmacological Sciences (2010) 14, 6 (507–512)). Eur Rev Med Pharmacol Sci 2010;14(12):1074. [PubMed] [Google Scholar]
- 29.Mullenix PJ, Denbesten PK, Schunior A, Kernan WJ. Neurotoxicity of sodium fluoride in rats. Neurotoxicol Teratol [Internet] 1995. [cited 2018 Apr 13];17(2):169–77. Available from: https://www.sciencedirect.com/science/article/pii/089203629400070T [DOI] [PubMed] [Google Scholar]
- 30.Jiang C, Zhang S, Liu H, Guan Z, Zeng Q, Zhang C, et al. Low Glucose Utilization and Neurodegenerative Changes Caused by Sodium Fluoride Exposure in Rat’s Developmental Brain. NeuroMolecular Med [Internet] 2014;16(1):94–105. Available from: 10.1007/s12017-013-8260-z [DOI] [PubMed] [Google Scholar]
- 31.Bartos M, Gumilar F, Bras C, Gallegos CE, Giannuzzi L, Cancela LM, et al. Neurobehavioural effects of exposure to fluoride in the earliest stages of rat development. Physiol Behav [Internet] 2015;147:205–12. Available from: http://www.sciencedirect.com/science/article/pii/S0031938415002516 [DOI] [PubMed] [Google Scholar]
- 32.National Research Council (NRC). Fluoride in Drinking Water: A Scientific Review of EPA’s Standards. Washington, DC: National Academies Press: 2006. [Google Scholar]
- 33.Valdez Jiménez L, López Guzmán OD, Cervantes Flores M, Costilla-Salazar R, Calderón Hernández J, Alcaraz Contreras Y, et al. In utero exposure to fluoride and cognitive development delay in infants. Neurotoxicology [Internet] 2017;59:65–70. Available from: http://www.sciencedirect.com/science/article/pii/S0161813X16302571 [DOI] [PubMed] [Google Scholar]
- 34.Bashash M, Marchand M, Hu H, Till C, Martinez-Mier EA, Sanchez BN, et al. Prenatal fluoride exposure and attention deficit hyperactivity disorder (ADHD) symptoms in children at 6–12 years of age in Mexico City. Environ Int [Internet] 2018;121:658–66. Available from: http://www.sciencedirect.com/science/article/pii/S0160412018311814 [DOI] [PubMed] [Google Scholar]
- 35.Xiang Q, Liang Y, Chen L, Wang C, Chen B, Chen X, et al. Effect of fluoride in drinking water on children’s intelligence. Fluoride 2003;36(2):84–94. [Google Scholar]
- 36.Trivedi MH, Verma RJ, Chinoy NJ, Patel RS, Sathawara NG. Effect of high fluoride concentration in drinking water on children’s intelligence. J Dent Med 2006;19(2):80–6. [Google Scholar]
- 37.Seraj B, Shahrabi M, Shadfar M, Ahmadi R, Fallahzadeh M, Eslamlu HF, et al. Effect of high water fluoride concentration on the intellectual development of children in makoo/iran. J Dent (Tehran) [Internet] 2012;9(3):221–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23119131%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3484826 [PMC free article] [PubMed] [Google Scholar]
- 38.Cui Y, Zhang B, Ma J, Wang Y, Zhao L, Hou C, et al. Dopamine receptor D2 gene polymorphism, urine fluoride, and intelligence impairment of children in China: A school-based cross-sectional study. Ecotoxicol Environ Saf [Internet] 2018;165(August):270–7. Available from: 10.1016/j.ecoenv.2018.09.018 [DOI] [PubMed] [Google Scholar]
- 39.Li XS, Zhi JL, Gao RO. Effect of fluoride exposure on intelligence in children. Fluoride - Q Reports 1995;28(4):189–92. [Google Scholar]
- 40.Kundu H, Basavaraj P, Singla A, Gupta R, Singh K, Jain S. Effect of fluoride in drinking water on children′s intelligence in high and low fluoride areas of Delhi. J Indian Assoc Public Heal Dent 2015;13(2):116. [Google Scholar]
- 41.Shivaprakash PK, Ohri K, Noorani H. Relation between dental fluorosis and intelligence quotient in school children of Bagalkot district. J Indian Soc Pedod Prev Dent 2011;29(2):117–20. [DOI] [PubMed] [Google Scholar]
- 42.Trivedi M, Sangai NP, Patel RS, Payak M, Vyas SJ. Assessment of groundwater quality with special reference to fluoride and its impact on iq of schoolchildren in six villages of the mundra region, kachchh, gujarat, India. 2012. [Google Scholar]
- 43.Aravind A, Dhanya RS, Narayan A, Sam G, Adarsh VJ, Kiran M. Effect of fluoridated water on intelligence in 10–12-year-old school children. J Int Soc Prev Community Dent [Internet] 2016;6(Suppl 3):S237–42. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28217543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Han F, Zhou Z, Zhang H, Jiao X, Zhang S, et al. Research on the intellectual development of children in high fluoride areas. Fluoride 2008;41(2):120–4. [Google Scholar]
- 45.Khan SA, Singh RK, Navit S, Chadha D, Johri N, Navit P, et al. Relationship Between Dental Fluorosis and Intelligence Quotient of School Going Children In and Around Lucknow District: A Cross-Sectional Study. J Clin Diagnostic Res [Internet] 2015;9(11):ZC10–5. Available from: file:///back_issues.asp?issn=0973-709x&year=2015&month=November&volume=9&issue=11&page=ZC10-ZC15&id=6726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Jing X, Chen D, Lin L, Wang Z. Effects of endemic fluoride poisoning on the intellectual development of children in baotou. Fluoride 2008;41(2):161–4. [Google Scholar]
- 47.Li, Hou, Yu. Inves & ga & on and Analysis of Children ‘ s IQ and Dental Fluorosis in a High Fluoride Area. 2010;26(3):230–1. [Google Scholar]
- 48.Poureslami HR, Horri A, Garrusi B. A comparative study of the IQ of children age 7–9 in a high and a low fluoride water city in Iran. Fluoride 2011;44(3):163–7. [Google Scholar]
- 49.Razdan P, Patthi B, Kumar JK, Agnihotri N, Chaudhari P, Prasad M. Effect of fluoride concentration in drinking water on intelligence quotient of 12–14-year-old children in Mathura District: A cross-sectional study. J Int Soc Prev Community Dent 2017;7(5):252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sudhir KM, Chandu GN, Prashant GM, Reddy VVS. Effect of fluoride exposure on Intelligence Quotient (IQ) among 13–15 year old school children of known endemic area of fluorosis , Nalgonda District , Andhra Pradesh. J Indian Assoc Public Heal Dent [Internet] 2009;2009(13):88–94. Available from: http://fluoridealert.org/wp-content/uploads/sudhir-2009.pdf [Google Scholar]
- 51.Zhao LB, Liang GH, Zhang DN, Wu XR. EFFECT OF A HIGH FLUORIDE WATER SUPPLY ON CHILDREN ‘ S INTELLIGENCE. Fluoride 1996;29(4):190–2. [Google Scholar]
- 52.Bellinger DC. Effect modification in epidemiologic studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicol Teratol [Internet] 2000;22(1):133–40. Available from: http://www.sciencedirect.com/science/article/pii/S0892036299000537 [DOI] [PubMed] [Google Scholar]
- 53.Bashash M, Thomas D, Hu H, Martinez-mier EA, Sanchez BN, Basu N, et al. Prenatal Fluoride Exposure and Cognitive Outcomes in Children at 4 and 6 – 12 Years of Age in Mexico. Enviromental Heal Perspect [Internet] 2017;1:1–12. Available from: https://sakai.duke.edu/access/content/group/2949fc9f-7dc1-4ec1-aaf7-63b50fcc322e/GGuidryLecture4172018/EHP655.alt_.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green R, Bashash M, Lanphear B, Peterson K, Flora D, Schnaas L, et al. Prenatal fluoride exposure and verbal versus non-verbal IQ: Results from the ELEMENT and MIREC pregnancy cohort studies. J Int Neuropsychol Soc 2020;INS confer. [Google Scholar]
- 55.Till C, Green R, Flora D, Hornung R, Martinez-mier EA, Blazer M, et al. Fluoride exposure from infant formula and child IQ in a Canadian birth cohort. Environ Int [Internet] 2020;134(November 2019):105315. Available from: 10.1016/j.envint.2019.105315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu Y, Yang W, Dong Z, Wan C, Zhang J, Liu J, et al. Neurotransmitter and receptor changes in the brains of fetuses from areas of endemic fluorosis. Fluoride 2008;41(2):134–8. [Google Scholar]
- 57.Gao Q, Liu Y-J, Wu C-X, Long Y-G, Guan Z-Z. Effects of fluoride on learning and memory and cholinesterase activity in rat brains. Chinese J Endem 2008;27:128–30. [Google Scholar]
- 58.Dong Z, Wan C, Zhang X, Liu J. Determination of the Contents of Amino Acid and Monoamine Neurotransmitters in Fetal Brains from a Fluorosis Endemic Area. J Guiyang Med Coll 1997;18(4):241–5. [Google Scholar]
- 59.Malin AJ, Riddell J, McCague H, Till C. Fluoride exposure and thyroid function among adults living in Canada: Effect modification by iodine status. Environ Int [Internet] 2018;121:667–74. Available from: http://www.sciencedirect.com/science/article/pii/S016041201830833X [DOI] [PubMed] [Google Scholar]
- 60.Rovet JF. The Role of Thyroid Hormones for Brain Development and Cognitive Function. Paediatr Thyroidol 2014;26:26–43. [DOI] [PubMed] [Google Scholar]
- 61.Samadi A, Skocic J, Rovet JF. Children Born to Women Treated for Hypothyroidism During Pregnancy Show Abnormal Corpus Callosum Development. Thyroid 2015;25(5):494–502. [DOI] [PubMed] [Google Scholar]
- 62.Stagnaro-Green A, Rovet J. Pregnancy: Maternal thyroid function in pregnancy-a tale of two tails. Nat. Rev. Endocrinol 2016;12(1):10–1. [DOI] [PubMed] [Google Scholar]
- 63.Murphy NC, Diviney MM, Donnelly JC, Cooley SM, Kirkham CH, Foran AM, et al. The effect of maternal subclinical hypothyroidism on IQ in 7- to 8-year-old children: A case-control review. Aust New Zeal J Obstet Gynaecol 2015;55(5):459–63. [DOI] [PubMed] [Google Scholar]
- 64.Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 2015;342(2015):68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y, Wang X, Guo X, Hu P. The effects of high levels of fluoride and iodine on child intellectual ability and the metabolism of fluoride and iodine. Fluoride 2008;41(4):336–9. [Google Scholar]
- 66.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal Thyroid Deficiency during Pregnancy and Subsequent Neuropsychological Development of the Child. N Engl J Med 1999;341(8):549–55. [DOI] [PubMed] [Google Scholar]
- 67.Thompson W, Russell G, Baragwanath G, Matthews J, Vaidya B, Thompson-Coon J. Maternal thyroid hormone insufficiency during pregnancy and risk of neurodevelopmental disorders in offspring: A systematic review and meta-analysis. Clin Endocrinol (Oxf) 2018;88(4):575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Päkkilä F, Männistö T, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, et al. The impact of gestational thyroid hormone concentrations on ADHD symptoms of the child. J Clin Endocrinol Metab 2014;99(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Modesto T, Tiemeier H, Peeters RP, Jaddoe VV., Hofman A, Verhulst FC, et al. Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children. JAMA Pediatr 2015;169(9):838–45. [DOI] [PubMed] [Google Scholar]
- 70.Batista G, Hensch TK. Critical Period Regulation by Thyroid Hormones: Potential Mechanisms and Sex-Specific Aspects [Internet]. Front. Mol. Neurosci 2019;12:77. Available from: https://www.frontiersin.org/article/10.3389/fnmol.2019.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andersen SL, Andersen S, Liew Z, Vestergaard P, Olsen J. Maternal Thyroid Function in Early Pregnancy and Neuropsychological Performance of the Child at 5 Years of Age. J Clin Endocrinol Metab [Internet] 2017;103(2):660–70. Available from: 10.1210/jc.2017-02171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, de Vries GJ. Sex differences in the brain: The not so inconvenient truth. J Neurosci 2012;32(7):2241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parent A-S, Naveau E, Gerard A, Bourguignon J-P, Westbrook GL. Early developmental actions of endocrine disruptors on the hypothalamus, hippocampus, and cerebral cortex. J Toxicol Environ Health B Crit Rev [Internet] 2011;14(5–7):328–45. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21790315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci [Internet] 2012;126(1):4–16. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22289042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Torres-Rojas C, Jones BC. Sex differences in neurotoxicogenetics. Front Genet 2018;9(JUN):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chetty CS, Vemuri MC, Reddy GR, Suresh C. Protective effect of 17-β-estradiol in human neurocellular models of lead exposure. Neurotoxicology [Internet] 2007;28(2):396–401. Available from: http://www.sciencedirect.com/science/article/pii/S0161813X06000817 [DOI] [PubMed] [Google Scholar]
- 77.Zhao Q, Niu Q, Chen J, Xia T, Zhou G, Li P, et al. Roles of mitochondrial fission inhibition in developmental fluoride neurotoxicity: mechanisms of action in vitro and associations with cognition in rats and children. Arch Toxicol [Internet] 2019;93(3):709–26. Available from: 10.1007/s00204-019-02390-0 [DOI] [PubMed] [Google Scholar]
- 78.Díaz-Castro B, Pardal R, García-Flores P, Sobrino V, Durán R, Piruat JI, et al. Resistance of glia-like central and peripheral neural stem cells to genetically induced mitochondrial dysfunction--differential effects on neurogenesis. EMBO Rep [Internet] 2015;16(11):1511–9. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26392570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sastre J, Pallardó FV, García de la Asunción J, Viña J Mitochondria, oxidative stress and aging. Free Radic Res [Internet] 2000;32(3):189–98. Available from: 10.1080/10715760000300201 [DOI] [PubMed] [Google Scholar]
- 80.Bai R, Huang Y, Wang F, Guo J. Associations of fluoride exposure with sex steroid hormones among U.S. children and adolescents, NHANES 2013–2016. Environ Pollut [Internet] 2020;260:114003. Available from: http://www.sciencedirect.com/science/article/pii/S0269749119357963 [DOI] [PubMed] [Google Scholar]
- 81.M Goldstein J, Seidman L, Horton N, Makris N, Kennedy D, Caviness V, et al. Normal Sexual Dimorphism of the Adult Human Brain Assessed by In Vivo Magnetic Resonance Imaging. 2001. [DOI] [PubMed] [Google Scholar]
- 82.Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol [Internet] 2011;31(3):363–73. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3171169/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. Sexual Dimorphism of Brain Developmental Trajectories during Childhood and Adolescence. Neuroimage [Internet] 2007;36(4):1065–73. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2040300/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev [Internet] 2010;20(4):327–48. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21042938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex Differences in Pharmacokinetics and Pharmacodynamics. Annu Rev Pharmacol Toxicol [Internet] 2004;44(1):499–523. Available from: 10.1146/annurev.pharmtox.44.101802.121453 [DOI] [PubMed] [Google Scholar]
- 86.Minami T, Sakita Y, Ichida S, Dohi Y. Gender difference regarding selenium penetration into the mouse brain. Biol Trace Elem Res [Internet] 2002;89(1):85–93. Available from: 10.1385/BTER:89:1:85 [DOI] [PubMed] [Google Scholar]
- 87.Pakulski C, Drobnik L, Millo B. Age and sex as factors modifying the function of the blood-cerebrospinal fluid barrier. Med Sci Monit 2000;6(2):314–8. [PubMed] [Google Scholar]
- 88.Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. Trends in the Prevalence of Developmental Disabilities in US Children, 1997–2008. Pediatrics [Internet] 2011;Available from: http://pediatrics.aappublications.org/content/early/2011/05/19/peds.2010-2989.abstract [DOI] [PubMed] [Google Scholar]
- 89.Guarino B Study raises questions about fluoride and children’s IQ [Internet]. Washington Post 2019; Available from: https://www.washingtonpost.com/science/2019/08/19/study-raises-questions-about-fluoride-childrens-iq/?noredirect=on [Google Scholar]


