Abstract
Auditory verbal hallucinations (AVH) are a hallmark of psychotic experience. Various mechanisms including misattribution of inner speech and imbalance between bottom-up and top-down factors in auditory perception potentially due to aberrant connectivity between frontal and temporo-parietal areas have been suggested to underlie AVH. Experimental evidence for disturbed connectivity of networks sustaining auditory-verbal processing is, however, sparse. We compared functional resting-state connectivity in 49 psychotic patients with frequent AVH and 49 matched controls. The analysis was seeded from the left middle temporal gyrus (MTG), thalamus, angular gyrus (AG) and inferior frontal gyrus (IFG) as these regions are implicated in extracting meaning from impoverished speech-like sounds. Aberrant connectivity was found for all seeds. Decreased connectivity was observed between the left MTG and its right homotope, between the left AG and the surrounding inferior parietal cortex (IPC) and the left inferior temporal gyrus, between the left thalamus and the right cerebellum, as well as between the left IFG and left IPC, and dorsolateral and ventrolateral prefrontal cortex (DLPFC/VLPFC). Increased connectivity was observed between the left IFG and the supplementary motor area (SMA) and the left insula and between the left thalamus and the left fusiform gyrus/hippocampus. The predisposition to experience AVH might result from decoupling between the speech production system (IFG, insula and SMA) and the self-monitoring system (DLPFC, VLPFC, IPC) leading to misattribution of inner speech. Furthermore, decreased connectivity between nodes involved in speech processing (AG, MTG) and other regions implicated in auditory processing might reflect aberrant top-down influences in AVH.
Keywords: Functional connectivity, Psychosis, Resting state, Schizophrenia, Speech monitoring
Introduction
Auditory verbal hallucinations (AVH), i.e. perceiving speech in the absence of an external stimulation, are a common symptom of various psychiatric disorders, but in particular of schizophrenia and related psychotic syndromes (Hugdahl 2009). Several neuro-cognitive mechanisms have been suggested to contribute to this highly distressing psychopathology, including dysfunction in verbal self-monitoring resulting in misattribution of inner speech as external voices, dysfunctions in episodic memory, or imbalances between top-down and bottom-up factors contributing to auditory perception (for a review see Hugdahl 2009). According to the latter hypothesis, aberrant dominance of top-down predictions might lead to spontaneous percepts in the absence of actual sensory stimulation which are experienced as (auditory-verbal) hallucinations (Grossberg 2000). Indeed, research has demonstrated that an increased tendency to hear messages in meaningless noise is associated with a subsequent conversion to schizophrenia in individuals with prodromal symptoms (Hoffman et al. 2007) and that semantic expectations can induce misperceptions in healthy but hallucination-prone individuals (Vercammen and Aleman 2008).
While excessive weighting of top-down predictions may thus contribute to the experience of hallucinations, predictive mechanisms are an integral component of sensory processing. In particular in speech perception, where bottom-up signalling is often ambiguous, expectations acting as priors in the processing of auditory stimuli are known to support perceptual processes, e.g. by facilitating the processing of degraded speech (Sohoglu et al. 2012; Wild et al. 2012). In a previous study (Clos et al. 2012), we showed that such predictions in the form of lexical-semantic expectations led to a sudden percept of an understandable sentence from a degraded sentence rather than hearing unintelligible noise. In this large group of 29 healthy participants four brain regions were associated with this phenomenon, which might approximate the abnormal interactions between top-down and bottom-up factors presumably involved in AVH. (1) The left middle temporal gyrus (MTG) which is thought to map sound to meaning and is known to be a key region in semantic processing (Binder et al. 2009). (2) The left angular gyrus (AG) that might contribute to speech processing by providing top-down semantic constraints (Price 2010; Seghier et al. 2010). (3) The left thalamus that might filter those signals present in the auditory signal that have been predicted by the cortex (Alitto and Usrey 2003). (4) Finally, Broca’s region in the left inferior frontal gyrus (IFG) was particularly responsive when incorrect lexical-semantic expectations resulted in an unsuccessful attempt to decode the degraded speech. This response pattern suggests that Broca’s region performs a search for meaningful information in ambiguous auditory signals (Giraud et al. 2004) and potentially prevents misinterpretation in the presence of misleading expectations (Price 2010).
If disturbed integration of top-down and bottom-up auditory processing is indeed involved in the pathophysiology of AVH, we would expect that such disturbances should be reflected in aberrant interactions of these regions. We therefore investigated resting-state functional connectivity of the left MTG, AG, thalamus and IFG in patients with chronic AVH relative to healthy controls. When addressing the pathophysiology of AVH, imaging network connectivity in a task free “resting-” state may provide a particularly promising approach for several reasons. First, psychotic symptoms have been suggested to arise from dysconnectivity between distinct brain regions (e.g. Friston 1998; Stephan et al. 2009). Second, spontaneous correlations between brain regions in the absence of structured external stimulation have been linked with predictive processes reflecting anticipation and interpretation of external events to prepare appropriate responses to these events (Deco et al. 2011; Raichle 2010). Third, as a fundamental characteristic of AVH is their emergence in absence of external driving inputs, resting-state connectivity might be more sensitive to reveal differences in network connectivity underlying the predisposition towards experiencing AVH than paradigms involving sensory stimulation. Finally, it has recently been suggested that abnormally elevated levels of resting state activity (Northoff and Qin 2011) and instability of the default mode network (Jardri et al. 2012) might play a role in the generation of AVH. Furthermore, approaches investigating networks rather than concentrating on isolated regions might be particularly suited to understand pathophysiology of AVH (Allen et al. 2012).
Previous resting state connectivity studies in patients with AVH have found evidence for aberrant functional connectivity between frontal and temporoparietal (Hoffman et al. 2011; Vercammen et al. 2010) and between temporal language areas (Gavrilescu et al. 2010). However, these studies employed a network approach of functional connectivity limited to pre-defined regions that were chosen based on previously observed activation during the experience of AVH. In contrast, we computed a whole-brain connectivity analysis seeded from regions that were associated with decoding degraded speech based on prior expectations. Top-down influences from higher-order cortical regions have been suggested to influence speech perception at lower auditory processing areas such as primary auditory cortex or thalamus (Davis and Johnsrude 2007). These higher-order regions are very likely to include the left MTG and AG, since they are form-independent semantic regions (Davis and Johnsrude 2003) that contribute to speech restoration by means of prior knowledge (Heinrich et al. 2008; Shahin et al. 2009). In particular the left AG has been demonstrated to be involved in top-down activation of semantic concepts (Obleser et al. 2007; Obleser and Kotz 2010; Seghier 2012; Seghier et al. 2010). Accordingly, we hypothesised that disturbed interactions in auditory processing would be reflected in altered coupling of ‘higher’ nodes in speech processing such as the left AG and MTG with other, ‘lower’ auditory speech processing areas. However, it has been suggested that a single neurocognitive deficit by itself is unlikely sufficient to account for a multifaceted phenomenon such as AVH (Jones 2010; Seal et al. 2004). Therefore, additional neurocognitive mechanisms such as dysfunctional monitoring of self-produced speech (Allen et al. 2007a) might also be involved. Verbal self-monitoring and misattribution of speech has been linked with activations left fronto-temporal regions and anterior cingulate cortex (Allen et al. 2005, 2007b; Simons et al. 2010). Likewise, involvement of the parietal cortex in speech monitoring has also been proposed (Danckert et al. 2004). Generation of inner speech and auditory-verbal imagery on the other hand is associated with the (particularly left) IFG/insula, supplementary motor area, temporo-parietal cortex and cerebellum (Shergill et al. 2001). Therefore, we would expect that misattribution of inner speech due to dysfunctional verbal self-monitoring should be mirrored in disturbed connectivity between the left IFG and areas involved in speech articulation and associated monitoring processes.
Materials and methods
Participants
Forty-nine patients with chronic psychosis, as diagnosed according to DSM-IV criteria by an independent psychiatrist using the “Comprehensive Assessment of Symptoms and History (CASH)” (Andreasen et al. 1992), who all experienced AVH several times a day for at least 1 year were included in the study (see Table 1 for clinical and demographic details). The medication types and medication levels as well as the overall symptomatology of the patients were unfortunately too diverse to allow matching of the patients. Psychopathology was assessed on the day of scanning with the Positive and Negative Syndrome Scale (PANSS; Kay et al. 1987). For comparison, 49 healthy (as confirmed by the CASH interview) individuals matched for age, sex and handedness were also included. All participants provided written informed consent into the study, which was approved by the Humans Ethics Committee of the University Medical Center Utrecht. Note that an analysis of these resting state data (addressing a different question) has previously been performed and published (Sommer et al. 2012). In particular, this previous analysis examined connectivity of the left superior temporal gyrus and right inferior frontal gyrus as activity in these two regions was associated with the acute experience of AVH in psychotic patients.
Table 1.
Demographic and clinical description of participants
| Group | Patients, n = 49 | Control subjects, n = 49 |
|---|---|---|
| Agea | 37.3 years ± 11.9 | 39.5 years ± 14.8 |
| Sexb | 22 males, 27 females | 19 males, 30 females |
| Handednessc | 43 right, 6 non-right | 38 right, 11 non-right |
| Mean time with AVH | 13.9 years ± 12.2 | No AVH |
| Diagnosis | 43 schizophrenia 4 schizoaffective disorder 1 schizophreniform disorder 1 psychosis NOS |
No psychiatric Diagnosis |
| Antipsychotic medication | 10 clozapine, mean dose 464 mg 3 flufenazine, mean dose 30 mg 7 risperidon, mean dose 3.6 mg 8 olanzapine, mean dose 14.2 mg 7 quetiapine, mean dose 514 mg 1 penfluridol 10 mg 5 haloperidol, mean dose 4 mg 1 aripriprazol, 15 mg 7 medication-free |
All medication-free |
| Mean PANSS scores | Positive scale 16.2 ± 3.7 | |
| Negative scale 16.3 ± 5.2 | ||
| Total 63 ± 13.3 | ||
| Years of education after primary schoold | 5.8 years ± 2.2 |
± denotes the standard deviation
T(2,96) = −0.92, p = 0.41
χ′2(1) = 0.38, p = 0.54
χ′2(1) = 1.78, p = 0.18
Information missing for 15 patients
Data acquisition and processing
Resting state scans were obtained on a Philips Achieva 3T MRI scanner using the following parameter: 40 (coronal) slices, TR/TE 21.75/32.4 ms, flip angle 10°, FOV 224 × 256 × 160, matrix 64 × 64 × 40, voxel-size 4 mm isotropic (PRESTO scans typically have shorter TR than TE times, as the whole head is scanned with each volume in stead of the slab-wise read-out of EPI scans). This scan sequence achieves full brain coverage within 609 ms (yielding 600 images in approximately 6 min) by combining a 3D-PRESTO pulse sequence with parallel imaging (SENSE) in two directions, using a commercial 8-channel SENSE headcoil (Neggers et al. 2008). Participants were instructed to lie in the scanner as still as possible with their eyes closed yet stay awake (which was confirmed by postscan debriefing). After scanning, all participants were asked whether they had experienced AVH during the scan. We did not instruct the subjects before the scan to keep track of any other aspects of their hallucinations (such as duration, content, frequency, etc.) because we did not want the participants to focus on the AVH during the resting state scan as this may have recruited additional attentional processes. Moreover, spontaneous reports by the patients did not yield specific or reliable information on, in particular, the duration or frequency of the experienced hallucinations.
The resting state scans were first corrected for head movement by affine registration using a two-pass procedure. The mean PRESTO image for each subject was then spatially normalised to the MNI single subject template (Holmes et al. 1998) using the unified segmentation approach (Ashburner and Friston 2005) and the ensuing deformation was applied to the individual PRESTO volumes. Finally, images were smoothed by a 5-mm FWHM Gaussian kernel. Regions of interest (ROIs) used as seeds for the functional connectivity analysis were based on the activation clusters obtained from Clos et al. (2012, cf introduction and Table 2). Voxel time courses were extracted for all voxels within a 5-mm radius sphere around the centre of the particular clusters.
Table 2.
Overview of seed regions
| Region | Cytoarchitectonic area (percent overlap) | x | y | z |
|---|---|---|---|---|
| L MTG | −57 | −27 | −5 | |
| L IFG (Broca’s region) | Area 44a (56 % overlap) Area 45a (44 % overlap) |
−51 | 20 | 15 |
| L Thalamus | −6 | −11 | 5 | |
| L AG | PGab (78 % overlap) PFmb (9 % overlap) PF2 (3 % overlap) |
−48 | −56 | 29 |
x, y, z coordinates refer to the centre of gravity in MNI space, L left
Resting state fMRI connectivity analyses can be confounded by physiological noise stemming from cardiac or respiratory signals but in particular also motion-related effects (Bandettini and Bullmore 2008; Fox et al. 2009). In order to reduce the potential of these effects to induce spurious correlations, variance explained by the following nuisance variables was removed from the time series (cf. Bandettini and Bullmore 2008; Eickhoff et al. 2011; Fox et al. 2009): (1) motion parameters derived from image realignment and their first derivative; (2) mean grey, white matter and CSF signal intensity per time-point which should account for the global signal changes of non-interest; (3) coherent signal drifts reflected by the first five PCA components on the entire whole-brain data (CompCor approach, cf. Behzadi et al. 2007). All nuisance variables entered the model as first and all but the PCA components also as second-order terms as previously described by Behzadi et al. (2007) and shown by Chai et al. (2012) to increase specificity and sensitivity of the analyses and detect valid correlation and anti-correlations during rest, which are not an artefact of the preprocessing method, but may reflect valid biological signals. The data were then band-pass filtered preserving frequencies between 0.01 and 0.08 Hz (Biswal et al. 1995; Greicius et al. 2003). The time course of each ROI was expressed as the first eigenvariate of the processed time series of all voxels associated with that region.
The use of global signal regression as a preprocessing step in fMRI connectivity analyses has recently evoked some criticism that is not only limited to the potential introduction of artificial anticorrelations (Murphy et al. 2009) but furthermore this correction has been discussed to potentially alter interregional correlations within a group and change differences in connectivity between groups because the true noise may vary systematically (Saad et al. 2012). Therefore, we performed a supplementary analysis in which we repeated the analysis without the global signal regression step to evaluate the impact of this correction method on observed group differences of connectivity. This supplementary analysis was thus not performed to establish secondary findings but only to ensure that the results of the main analysis were not confounded by the preprocessing. As the purpose of the supplementary analysis was thus solely to confirm that the results remain stable when changing the preprocessing parameters, there is no problem with circularity although these two analyses are not independent from each other.
Data analysis
For each subject we computed linear (Pearson) correlation coefficients between the time series of the seeds and any other grey matter voxel, which were then transformed into Fisher’s Z scores. Group analysis was then performed on these by an analysis of variance (ANOVA) across subjects using appropriate non-sphericity correction. In the ANOVA, both the main effect of functional connectivity and the group-difference between patients and controls were modelled for each seed. Inference on this random-effects analysis was then sought using linear contrasts. First, main effects of connectivity (across both groups) were calculated for each seed and a conjunction analysis over the four seeds was performed on these to reveal regions showing significant coupling common to all four seeds. Subsequently, functional connectivity maps of the left MTG, AG, IFG and thalamus were assessed for significant differences between patients and controls using t tests testing for increased or decreased connectivity in patients compared with controls. For all analyses, results were regarded as significant if they passed p < 0.05 (FWE-corrected at cluster-level for multiple comparisons; cluster-forming threshold at voxel-level: p < 0.001). Anatomical localizations in cytoarchitectonically defined areas were obtained using the SPM Anatomy Toolbox (Eickhoff et al. 2005, 2007).
We computed a follow-up analysis to test for state vs. trait effects of AVH on functional connectivity with our seed regions. We divided the patients into those who actually experienced hallucinations during the scan and those who did not. We extracted individual functional connectivity between the seed regions and 5 mm spheres centred on the peak voxel of those clusters that had shown significant aberrant connectivity in the main analysis. Subsequently, we computed a one-way ANOVA on these individual functional connectivity scores between the subgroups. It should be noted that this inference procedure is not circular, as targets were defined by a main-effect of aberrant connectivity across the entire group of patients and then evaluated for differences between two subsets (acutely hallucinating/not hallucinating) within it. In a second follow-up analysis we assessed whole-brain correlations between functional connectivity to the seeds and the PANSS positive score (reflecting psychotic psychopathology), the PANSS negative score (reflecting impairments in emotional and cognitive processes) as well as the PANSS item P3 (reflecting hallucinations). To this end, the Fisher’s Z-images of all patients were correlated with this clinical score and inference was sought on the whole-brain level. Again results were regarded as significant if they passed p < 0.05 (FWE-corrected at cluster-level; cluster-forming threshold at voxel-level: p < 0.001).
Results
All participants reported that they had stayed awake during the resting-state scan. While none of the healthy controls reported experiences of AVH during the scan, 31 of the 49 patients did. The main effects of connectivity of the four seeds computed across all participants are displayed in Fig. S1. The conjunction over these main effects of connectivity revealed no single cluster that was significantly correlated with all seeds.
Differences between patients and controls
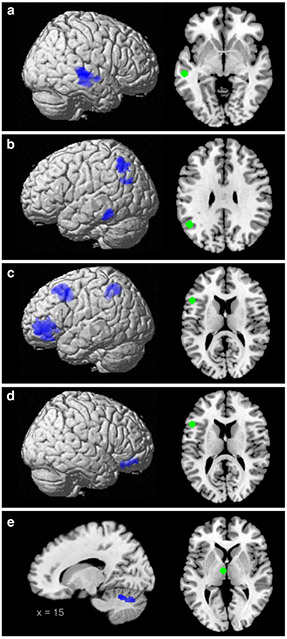
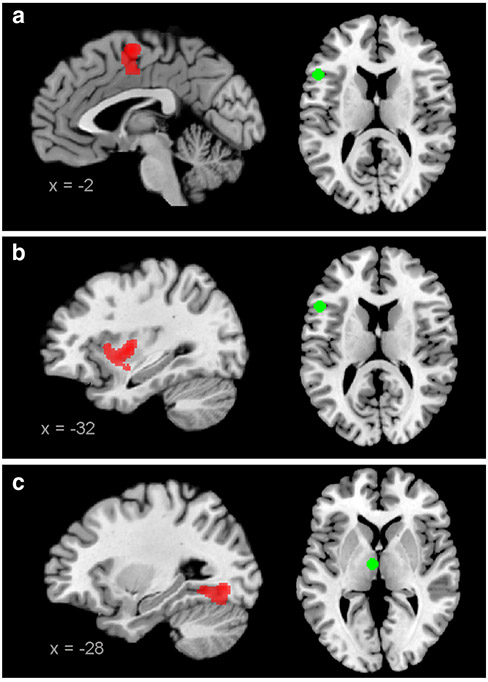
Attenuated coupling in patients compared with controls was observed for all four seeds (Fig. 1 and Table 3). First, the left MTG showed a reduced connectivity with a cluster including parts of the right superior temporal gyrus, the superior temporal sulcus and the middle temporal gyrus (STG/STS/MTG). Second, the left AG showed a reduced local connectivity with the surrounding inferior parietal cortex (IPC) overlapping with the cytoarchitectonic areas PFm/PGa/PGp (Caspers et al. 2006) and with the left inferior temporal gyrus (ITG). Third, for the left IFG, reduced coupling was observed with the bilateral ventrolateral prefrontal cortex (VLPFC; parts of the IFG anterior-ventrally to area 45), with the left dorso-lateral prefrontal cortex (DLPFC; part of the middle frontal gyrus extending to the dorsal potion of area 44/45; Amunts et al. 1999) and with the left inferior parietal cortex (IPC; area PFm/PF and hIP1/hIP2; Caspers et al. 2006; Choi et al. 2006). Finally, the thalamic seed displayed decreased connectivity with the right cerebellum. Additionally, also increased connectivity was observed between the left IFG and the supplementary motor area (area 6; Geyer 2004) and the left insula/putamen, and between the thalamic seed and the left fusiform gyrus/hippocampus (area SUB and hOC4v; Amunts et al. 2005; Rottschy et al. 2007); see Fig. 2 and Table 3.
Fig. 1.
Decreased connectivity in patients. Regions displaying decreased connectivity with the four seeds (green) in patients. a MTG, b AG, c and d IFG, e thalamus. All images are thresholded at p < 0.05 (FWE-corrected at cluster-level; cluster forming threshold at voxel level: p < 0.001)
Table 3.
Overview of regions showing altered functional connectivity in patients
| Region | Overlap with cytoarchitectonic area | x | y | z | Z score | Cluster size |
|---|---|---|---|---|---|---|
| Decreased connectivity with left MTG | ||||||
| R STG/STS/MTG | 66 | −24 | −2 | 5.64 | 1,307 | |
| Decreased connectivity with left AG | ||||||
| L IPC | PFma (14 % overlap) PGaa (13 % overlap) PGpa (11 % overlap) |
−50 | −59 | 57 | 4.81 | 659 |
| L ITG | −71 | −41 | −18 | 5.02 | 422 | |
| Decreased connectivity with Broca’region | ||||||
| L VLPFC | Area 45b (13 % overlap) | −56 | 42 | −5 | 6.27 | 1,913 |
| R VLPFC | 44 | 39 | −15 | 4.84 | 452 | |
| L DLPFC | Area 44b (6 % overlap) | −35 | 20 | 51 | 5.65 | 1,612 |
| L IPC | PFma (24 % overlap) PFa (13 % overlap) hIP1c (9 % overlap) hIP2c (21 % overlap) |
−45 | −45 | 45 | 4.33 | 658 |
| Increased connectivity with Broca’sregion | ||||||
| SMA | Area 6d (83 % overlap) | 0 | −5 | 59 | 5.27 | 667 |
| L insula/putamen | −32 | −5 | 2 | 4.14 | 918 | |
| Decreased connectivity with left thalamus | ||||||
| R cerebellum | 5 | −78 | −27 | 4.96 | 632 | |
| Increased connectivity with left thalamus | ||||||
| L hippocampus/fusiform gyrus | SUBe (16 % overlap) hOC4vf (8 % overlap) |
−12 | −35 | −11 | 5.61 | 1,656 |
All activations p < 0.05 (cluster-level FWE corrected)
x, y, z coordinates refer to the peak voxel in MNI space, R right, L left
Fig. 2.
Increased connectivity in patients. Regions displaying increased connectivity with the seeds (green) in patients. a and b IFG, c thalamus. All images are thresholded at p < 0.05 (FWE-corrected at cluster-level; cluster forming threshold at voxel level: p < 0.001)
In order to assure that global signal regression did not introduce any systematic confounds we repeated the analysis on the same dataset but pre-processed without the removal of global signals. The results were well comparable to the group differences reported above. In particular, even though additional regions showed aberrant connectivity, we could replicate the previously observed differences in functional connectivity of all seeds (see Fig. S2 and S3). Accordingly, these group differences are very unlikely to be an artefact of the global signal correction.
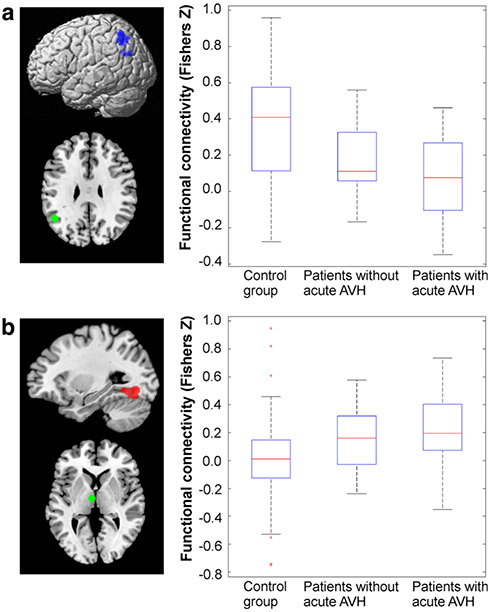
In order to assess state-effects of acute AVH on functional connectivity with our seed regions, we performed follow-up analyses for those regions that showed the disturbed connectivity in the overall group of trait carriers as reported above. This analysis revealed that the reduction in connectivity between the AG and the surrounding left inferior parietal cortex and the increase in connectivity between the thalamus and the left fusiform gyrus/hippocampus were more pronounced in those patients who hallucinated during scanning compared with those that did not (Fig. 3). That is, the connectivity linking these regions provided evidence for both trait and state-effects. For the remaining regions, no systematic differences were found between acutely hallucinating patients and those showing merely the trait to experience AVH. As it might be conceivable that the two subgroups (acutely vs. non-acutely hallucinating patients) showed a difference with respect to their medication status, we directly tested for these. We did not find a significant difference between groups with respect to the current medication (χ′2 = 6.0973, p = 0.53). Therefore, the subgroups and the associated connectivity differences are unlikely to arise from medication confounds.
Fig. 3.
State-trait effects. a The reduction in connectivity between the left AG and the left IPC in is more pronounced in patients with acute AVH. b The increase in connectivity between the left thalamus and the left fusiform gyrus is more pronounced in patients with acute AVH. Red crosses represent data points outside 1.5 × IQR, i.e. outliers
Correlations with PANSS-scores
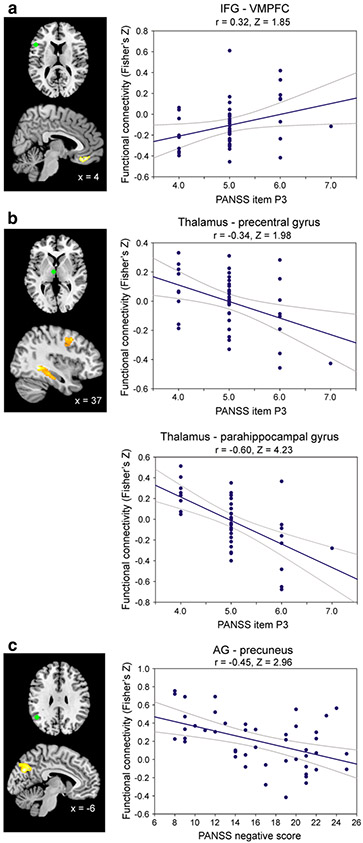
No significant correlations between PANSS-positive score and the connectivity of any of the seed regions to other brain areas were found. However, when correlating only the score on the P3 item of the PANSS (i.e. hallucinatory behaviour), a significant positive relationship was observed with the connectivity between the left IFG and the ventromedial prefrontal cortex (VMPFC; Fig. 4a), indicating that higher connectivity between these two areas is associated with more severe hallucinations. Furthermore, connectivity between the thalamic seed and the right parahippocampal gyrus (area CA/SUB; Amunts et al. 2005) and between the thalamus and the right precentral gyrus was negatively correlated to the severity of hallucinations (Fig. 4b). Thus, reduced thalamic coupling was associated with more severe hallucinations. Finally, the PANSS summary score of negative symptoms showed a negative correlation with the connectivity between the seed in the left AG and the left superior parietal lobe/precuneus (area 7 M/7P; Scheperjans et al. 2008) See Fig. 4c and Table 4 for details.
Fig. 4.
Correlations with PANSS-scores. Connectivity between the seeds (green) and brain regions showing a significant relationship with hallucination severity (a, b) and with negative symptoms (c) as measured by the PANSS. All images are thresholded at p < 0.05 (FWE-corrected at cluster-level; cluster forming threshold at voxel level: p < 0.001)
Table 4.
Overview of regions connected to seeds showing significant correlations with the PANSS
| Region | Overlap with cytoarchitectonic area | x | y | z | Z score | Cluster size |
|---|---|---|---|---|---|---|
| Connections with Broca’s region positively correlated with item P3 (hallucination severity) | ||||||
| vmPFC | 3 | 38 | −20 | 4.25 | 377 | |
| Connections with the thalamus negatively correlated with item P3 (hallucination severity) | ||||||
| R parahippocampal gyrus | CAa (13 % overlap) SUBa (7 % overlap) |
41 | −38 | −12 | 5.31 | 467 |
| R precentral gyrus | Area 6b (3 % overlap) | 42 | −3 | 41 | 4.34 | 485 |
| Connections with the AG negatively correlated with the negative scale | ||||||
| L precuneus/SPL | 7 Mc (16 % overlap) 7Pc (12 % overlap) |
−6 | −75 | 29 | 4.99 | 693 |
All activations p < 0.05 (cluster-level FWE corrected)
x, y, z coordinates refer to the peak voxel in MNI space, R right, L left
Discussion
Here we examined whether patients with auditory verbal hallucinations show aberrant whole-brain connectivity of regions that previously have been associated with decoding degraded speech based on prior expectations (Clos et al. 2012). To this end, we assessed resting-state connectivity seeded from the left MTG, AG, IFG and thalamus in 49 psychotic patients with frequent AVH and 49 matched healthy controls to reveal disturbances in networks implicated in top-down/bottom-up interactions in speech processing. Predictions should be particularly important for speech perception because of the transient and often ambiguous nature of auditory signals; however, excessive influence of these expectations might lead to experiences of AVH (Grossberg 2000). The crucial role of predictive processes in speech perception could explain why perception in the auditory-verbal domain is particularly vulnerable to hallucinations, as AVH are the most frequent variant of hallucinations in schizophrenia (Hugdahl 2009).
The results indicated several neurocognitive mechanisms that might underlie AVH. In particular, our analysis revealed (1) aberrant coupling in the speech processing system (connections of the left MTG, AG and the thalamus with regions involved in auditory-verbal processing), (2) increased coupling in the speech production system (connections between the left IFG and regions involved in speech production/articulation) and (3) attenuated coupling in the auditory monitoring system (connections between the left IFG and regions implicated in attentional control). Since these potential deficits are not mutually exclusive and most likely all contribute to the pathophysiology of AVH, these findings are in accordance with a multidimensional framework of AVH (Seal et al. 2004). Furthermore, connectivity with the left IFG and with the left thalamus showed a relationship with hallucination severity as assessed by the P3 item of the PANSS. We subsequently interpret these results in the light of previous findings and proposed models of AVH. Note, however, that the exact mechanisms and causal interactions underlying AVH can of course not be derived from correlational methods such as resting-state connectivity. Therefore, these interpretations represent merely a reasoning on the most likely underlying processes, given our current findings and previous results on AVH as found in the literature.
Speech processing
Decreased connectivity was observed between the left MTG and the right MTG/STG. While the left MTG is known to be especially important for semantic processing of speech (Binder et al. 2009), its right-sided homotope is particularly tuned to process emotional prosody in speech (Mitchell et al. 2003). Furthermore, the right temporal cortex has been reported to be more recruited with increasing semantic complexity of language (Jung-Beeman 2005; Pugh et al. 1996). In particular, the right hemisphere might be involved in integrating context information (Kircher et al. 2001) and be more sensitive to distant semantic relations (Sass et al. 2009) required for understanding figurative language and metaphors (Bottini et al. 1994; Nichelli et al. 1995; Mashal et al. 2007). Their attenuated coupling in patients with AVH suggests that disturbed balance of the interhemispheric connectivity linking left and right MTG/STG contributes to AVH, potentially through disturbed semantic processing. Indeed, interhemispheric projections to homotopic regions are known to be particularly dominant in the auditory system (Cipolloni and Pandya 1989) and reduced interhemispheric functional connectivity between auditory areas has previously been noted in schizophrenic patients with AVH (Gavrilescu et al. 2010). Furthermore, observations of right MTG activation prior to the onset of AVH (Shergill et al. 2004) and more bilateral language dominance in patients with AVH when listening to speech rather than the usual left-lateralized pattern in healthy controls (Sommer et al. 2001) suggest that this interhemispheric decoupling could lead to hyperactivity of the right node that ultimately plays a role in experiences of AVH. However, it has been suggested that decreased lateralization might actually be linked more with psychosis than with the experience AVH per se (Diederen et al. 2010a). Since we do not have a psychotic control group without AVH, we cannot infer from the present data whether this decreased interplay between left and right MTG is indeed contributing to AVH rather than reflecting more general aspects of psychosis.
The left AG also showed a decreased connectivity with regions involved in speech processing, namely the surrounding left IPC and the left ITG. The left AG is known to be a hierarchically high node in the speech processing system with access to higher-order concepts and long-term memory that has been suggested to provide top-down semantic constraints in language processing (Price 2010; Seghier et al. 2010). Since both the left IPC and the left ITG are involved in semantic analysis and speech processing (Binder et al. 2009; Price 2010) the decreased connectivity with the left AG might potentially reflect disturbances between bottom-up and top-down aspects in the speech processing system.
Furthermore, decreased connectivity between the thalamic seed and the cerebellum and increased connectivity between the thalamic seed and the left fusiform gyrus/hippocampus in patients with AVH was observed. The left fusiform and the adjacent parahippocampal gyrus are implicated in semantic processing of language (Binder et al. 2009). The additional role of hippocampal structures in verbal memory recollection has previously been linked with AVH (Diederen et al. 2010b). The aberrant thalamic connectivity with these regions could hence reflect disturbed feedback of semantic-verbal memory recollection which might influence the filtering and amplification processes of auditory signals in the thalamus.
Together, these results point toward an association between AVH and disturbed top-down and bottom-up interactions in auditory-verbal processing. Moreover, the pattern of reduced connectivity between the left AG and the surrounding IPC and the pattern of increased connectivity between the left thalamus and the left fusiform gyrus/ hippocampus differentiated between state and trait. This observation suggests that aberrant coupling between these areas is particularly important for the acute experience of AVH. However, the state vs. trait analysis may have actually been underpowered because the acutely hallucinating patients (most likely) were not experiencing AVH during the entire scanning period. Unfortunately, however, reports on the nature and extent of hallucinations were not precise and reliable enough to quantitatively assess these aspects and, e.g. correct for the duration spent hallucinating. Therefore, the lack of state vs. trait connectivity differences for the other disturbed connections might potentially be due to the low power.
Inner speech production
In addition to these aberrant connectivity patterns in the speech processing system, Broca’s region in the left IFG showed increased coupling with the SMA and with the left insula in the patient group. Activation of these three areas is associated with generation of (inner) speech (Price 2010) and auditory imagery of alien speech (Shergill et al. 2001). Therefore, increased connectivity of this network might potentially reflect increased recruitment of these pathways in AVH. This interpretation would be in accordance with accounts hypothesising that AVH are caused by dysfunctional monitoring of self-produced speech (Frith 1995). Previously, observations of reduced SMA response in AVH compared with deliberate imagining alien speech (Shergill et al. 2000) and in verbal imagery in schizophrenic patients with AVH compared with healthy controls and schizophrenic patients without AVH (McGuire et al. 1996) have been reported. These reduced SMA responses could be reconciled with the increased connectivity observed in our study if this connection with Broca’s region results in higher tonic SMA recruitment and, accordingly, attenuated phasic responses. Moreover, a recent study by Linden et al. (2011) revealed that auditory verbal imagery was associated with SMA activity, clearly preceding activity in Broca’s region and the STS. In contrast, in non-clinical persons with AVH these areas became active nearly coincidentally. This coincidence of activations is in line with the increased connectivity we observed between Broca’s region and the SMA. In sum, these findings suggest that the increased coupling between Broca’s region with the SMA and with the insula might underlie an increased generation of inner speech/inner dialogue of alien voices in psychotic patients.
Monitoring and misattribution of inner speech
However, an increased frequency of inner dialogues alone is not sufficient for AVH because the voices in AVH are perceived as externally generated and intrusive unlike inner speech. Furthermore, psychotic patients can distinguish between their self-produced inner speech and the voices in AVH (Hoffman et al. 2008). It has been suggested that self-produced inner speech is actually misattributed to an external source in psychotic patients due to impairments in verbal self-monitoring. As a result, the self-produced verbal thoughts are erroneously experienced as coming from another person’s voice (Allen et al. 2007a). This hypothesised impairment in verbal self-monitoring might be represented in the reduced coupling between Broca’s region and the DLPFC, VLPFC and IPC since accurate self-monitoring has been previously associated with a frontoparietal network (Danckert et al. 2004). Importantly, schizophrenic patients were shown to have a specific impairment in self-monitoring but not dysfunctional attentional control processes in general (Turken et al. 2003). This finding suggests that the network underlying self-monitoring might not be identical to the frontoparietal network associated with attentional control (Danckert et al. 2004). Alterations of right IPC function have been associated with delusions of control, i.e. the impression that one’s movements are unintended and externally caused due to impairments of the action selfmonitoring system (Blakemore 2003). Furthermore, VLPFC activation is linked to the executive control network by being the place of interaction between short-term memory and executive processing (Owen et al. 1996). In particular, the left side of this network is not only associated with language production and processing (Price 2010) but also with auditory-verbal imagery (Shergill et al. 2001) and might therefore preferentially monitor language-related processes. Therefore, aberrant connectivity in this network might cause misattributions of inner speech and auditory verbal imagery equivalent to delusions in action control resulting from dysfunction of the right-sided action-monitoring system.
AVH would thus, at least in part, result from imbalance in connectivity with Broca’s region. First, increased coupling with the SMA and the left insula would lead to aberrant recruitment of pathways subserving inner speech. Second, the resulting dialogues are experienced as alien because of relative decoupling of the verbal monitoring system (VLPFC, DLPF and IPC) from Broca’s region.
Correlations with the PANSS
Finally, we observed correlations between altered connectivity and hallucination severity. First, higher connectivity between Broca’s region and the VMPFC was associated with increased hallucination severity. The VMPFC is implicated in anticipation of upcoming stimuli (Stephens et al. 2010), perceptual set (Summerfield et al. 2006) and importantly, was found in our previous study (Clos et al. 2012) to be especially responsive when prior information matched with the following stimuli. In contrast, Broca’s region was particularly responsive to mismatches of stimuli when the presence of meaning was hard to determine which might reflect search for meaning and the prevention of misinterpretation (Novick et al. 2005; Price 2010). The observation that increased connectivity between these two regions is associated with higher hallucination behaviour might point to a mechanism where expectations in the VMPFC override bottom-up sensory evidence and/or that the mismatch detection usually performed by Broca’s region is dysfunctional. This could, in consequence, lead to detection of perceptual matches when these are not supported by sensory information.
Furthermore, attenuated coupling between the thalamus and both the right parahippocampal gyrus as well as the right precentral gyrus was associated with higher hallucination severity. This again suggests the extent of hallucinations is linked with effects of memory retrieval in verbal imagery (Diederen et al. 2010b) and auditory attention (Westernhausen et al. 2010) on auditory processing in the thalamus.
Finally, we observed a negative relationship between the connectivty between the left AG and the left precuneus/superior parietal lobe with negative symptoms. This finding is interesting given the involvement of both areas in the ‘default mode’ network (e.g. Binder et al. 2009) and abnormalities in this network observed in schizophrenia (Skudlarski et al. 2010; Whitfield-Gabrieli et al. 2009).
Limitations and technical considerations
By using regions as seeds that are important for top-down guided speech decoding, we employed a model-based approach for assessing differences in connectivity between patients and controls. While such an approach has limited sensitivity to discover aberrations that might equally contribute to AVH but were not connected to our seed regions, it provides higher specificity with regard to the functional interpretation of the implicated network. Furthermore, one must acknowledge that the observed differences between patients and controls might also be due to other symptoms than AVH as patients and controls also differed in variables such as the presence of other psychotic or cognitive symptoms. Since we did not use a psychotic patient group without AVH as an additional control group, we cannot be entirely certain that the observed differences are indeed specifically due to AVH. A similar argument holds true for a potential medication confound as the types and levels of antipsychotic medication used by the patients were too heterogeneous to test for effects of medication on overall connectivity patterns. However, the a-priori selection of regions specifically involved in decoding of degraded speech sounds should reduce the influence of variables other than AVH on the observed connectivity pattern. Moreover, these factors should only vary unsystematically between acutely and non-acutely hallucinating patients. Still, it should be noted that this last state vs. trait analysis might possibly have been underpowered because the acute hallucinations were not present continuously during scanning.
Conclusions
The observed differences in connectivity point towards two main, complementary mechanisms that might underlie AVH rather than to a single unidimensional neurocognitive deficit. First, decoupling between Broca’s area and the verbal monitoring system might lead to misattribution of auditory verbal imagery produced by the increased interaction of Broca’s area with the SMA and the left insula. Furthermore, abnormal connectivity between nodes involved in speech processing (AG, MTG) and other regions implicated in auditory-verbal processing might reflect aberrant interactions that can elicit percepts in the absence of stimulation. In particular, the aberrant coupling with the left AG and the left thalamus might represent a more specific contribution to the acute state of AVH. Finally, these disturbed connections might be possible targets for future repetitive transcranial magnetic stimulation (rTMS) or transcranial direct current stimulation (tDCS) intervention studies. We would hypothesise that inhibitory stimulation of the speech production network and excitatory stimulation of the verbal monitoring system might be particularly promising given that stimulation of the temporo-parietal speech perception system has received more attention but only provided rather mixed evidence for reduction of hallucination severity (Slotema et al. 2012).
Supplementary Material
Acknowledgments
This work was supported by the Human Brain Project (R01-MH074457-01A1 to SBE), the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model SBE, MC), and the DFG (IRTG 1328 to SBE).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s00429-013-0519-5) contains supplementary material, which is available to authorized users.
Contributor Information
Mareike Clos, Institute of Neuroscience and Medicine (INM-1, INM-2), Research Centre Jülich, 52425 Jülich, Germany.
Kelly M. J. Diederen, Neuroscience Division, University Medical Center Utrecht and Rudolf Magnus Institute for Neuroscience, Utrecht, Netherlands
Anne Lotte Meijering, Neuroscience Division, University Medical Center Utrecht and Rudolf Magnus Institute for Neuroscience, Utrecht, Netherlands.
Iris E. Sommer, Neuroscience Division, University Medical Center Utrecht and Rudolf Magnus Institute for Neuroscience, Utrecht, Netherlands
Simon B. Eickhoff, Institute of Neuroscience and Medicine (INM-1, INM-2), Research Centre Jülich, 52425 Jülich, Germany; Institute of Clinical Neuroscience and Medical Psychology, Heinrich Heine University, Düsseldorf, Germany
References
- Alitto HJ, Usrey WM (2003) Corticothalamic feedback and sensory processing. Curr Opin Neurobiol 13:440–445 [DOI] [PubMed] [Google Scholar]
- Allen PP, Amaro E, Fu CH, Williams SC, Brammer M, Johns LC, McGuire PK (2005) Neural correlates of the misattribution of self-generated speech. Hum Brain Mapp 26:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P, Aleman A, McGuire PK (2007a) Inner speech models of auditory verbal hallucinations: evidence from behavioural and neuroimaging studies. Int Rev Psychiatry 19:407–415 [DOI] [PubMed] [Google Scholar]
- Allen P, Amaro E, Fu CH, Williams SC, Brammer MJ, Johns LC, McGuire PK (2007b) Neural correlates of the misattribution of speech in schizophrenia. Br J Psychiatry 190:162–169 [DOI] [PubMed] [Google Scholar]
- Allen P, Modinos G, Hubl D, Shields G, Cachia A, Jardri R, Thomas P, Woodward T, Shotbolt P, Plaze M, Hoffman R (2012) Neuroimaging auditory hallucinations in schizophrenia: from neuroanatomy to neurochemistry and beyond. Schizophr Bull 38:695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K (1999) Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412:319–341 [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K (2005) Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 210:343–352 [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Arndt S (1992) The comprehensive assessment of symptoms and history (CASH): an instrument for assessing psychopathology and diagnosis. Arch Gen Psychiatry 49:615–623 [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26:839–851 [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Bullmore E (2008) Endogenous oscillations and networks in functional magnetic resonance imaging. Hum Brain Mapp 29:737–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT (2007) A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL (2009) Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 19:2767–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Blakemore SJ (2003) Deluding the motor system. Conscious Cogn 12:647–655 [DOI] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RS, Frith CD (1994) The role of the right hemisphere in the interpretation of figurative aspects of language. A positron emission tomography activation study. Brain 117:1241–1253 [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K (2006) The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. NeuroImage 33:430–448 [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castañón AN, Öngür D, Whitfield-Gabrieli S (2012) Anticorrelations in resting-state networks without global signal regression. Neuroimage 59:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K (2006) Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol 495:53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolloni PB, Pandya DN (1989) Connectional analysis of the ipsilateral and contralateral afferent neurons of the superior temporal region in the rhesus monkey. J Comp Neurol 281:567–585 [DOI] [PubMed] [Google Scholar]
- Clos M, Langner R, Meyer M, Oechslin MS, Zilles K, Eickhoff SB (2012) Effects of prior information on decoding degraded speech: an fMRI study. Hum Brain Mapp (Advance online publication). doi: 10.1002/hbm.22151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danckert J, Saoud M, Maruff P (2004) Attention, motor control and motor imagery in schizophrenia: implications for the role of the parietal cortex. Schizophr Res 70:241–261 [DOI] [PubMed] [Google Scholar]
- Davis MH, Johnsrude IS (2003) Hierarchical processing in spoken language comprehension. J Neurosci 23:3423–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH, Johnsrude IS (2007) Hearing speech sounds: top-down influences on the interface between audition and speech perception. Hear Res 229:132–147 [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR (2011) Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci 12:43–56 [DOI] [PubMed] [Google Scholar]
- Diederen KM, De Weijer AD, Daalman K, Blom JD, Neggers SF, Kahn RS, Sommer IE (2010a) Decreased language lateralization is characteristic of psychosis, not auditory hallucinations. Brain 133:3734–3744 [DOI] [PubMed] [Google Scholar]
- Diederen KM, Neggers SF, Daalman K, Blom JD, Goekoop R, Kahn RS, Sommer IE (2010b) Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am J Psychiatry 167:427–435 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbas MH, Evans AC, Zilles K, Amunts K (2007) Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36:511–521 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT (2011) Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57:938–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME (2009) The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101:3270–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ (1998) The disconnection hypothesis. Schizophr Res 30:115–125 [DOI] [PubMed] [Google Scholar]
- Frith CD (1995) The cognitive abnormalities underlying the symptomatology and the disability of patients with schizophrenia. Int Clin Psychopharmacol 10(Suppl. 3):87–98 [PubMed] [Google Scholar]
- Gavrilescu M, Rossel S, Stuart GW, Shea TL, Innes-Brwon H, Henshall K, McKay C, Sergejew AA, Copolov D, Egan GF (2010) Reduced connectivity of the auditory cortex in patients with auditory hallucinations: a resting state functional magnetic resonance imaging study. Psychol Med 40:1149–1158 [DOI] [PubMed] [Google Scholar]
- Geyer S (2004) The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol 174:1–92 [DOI] [PubMed] [Google Scholar]
- Giraud AL, Kell C, Thierfelder C, Sterzer P, Russ MO, Preibisch C, Kleinschmidt A (2004) Contributions of sensory input, auditory search and verbal comprehension to cortical activity during speech processing. Cereb Cortex 14:247–255 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg S (2000) How hallucinations may arise from brain mechanisms of learning, attention, and volition. J Int Neuropsychol Soc 6:583–592 [DOI] [PubMed] [Google Scholar]
- Heinrich A, Carlyon RP, Davis MH, Johnsrude IS (2008) Illusory vowels resulting from perceptual continuity: a functional magnetic resonance imaging study. J Cogn Neurosci 20:1737–1752 [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Woods SW, Hawkins KA, Pittman B, Tohen M, Preda A, Breier A, Glist J, Addington J, Perkins DO, McGlashan TH (2007) Extracting spurious messages from noise and risk of schizophrenia-spectrum disorders in a prodromal population. Br J Psychiatry 191:355–356 [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Varanko M, Gilmore J, Mishara AL (2008) Experiential features used by patients with schizophrenia to differentiate ‘voices’ from ordinary verbal thought. Psychol Med 38:1167–1176 [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Fernandez T, Pittman B, Hampson M (2011) Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry 69:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC (1998) Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22:324–333 [DOI] [PubMed] [Google Scholar]
- Hugdahl K (2009) “Hearing voices”: auditory hallucinations as failure of top-down control of bottom-up perceptual processes. Scand J Psychol 50:553–560 [DOI] [PubMed] [Google Scholar]
- Jardri R, Thomas P, Delmaire C, Delion P, Pins D (2012) The neurodynamic organization of modality-dependent hallucinations. Cereb Cortex (Advance online publication). doi: 10.1093/cercor/bhs082 [DOI] [PubMed] [Google Scholar]
- Jones SR (2010) Do we need multiple models of auditory verbal hallucinations? Examining the phenomenological fit of cognitive and neurological models. Schizophr Bull 36:566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Beeman M (2005) Bilateral brain processes for comprehending natural language. Trends Cogn Sci 9:512–518 [DOI] [PubMed] [Google Scholar]
- Kay S, Fiszbein A, Opler L (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276 [DOI] [PubMed] [Google Scholar]
- Kircher TT, Brammer M, Tous Andreu N, Williams SC, McGuire PK (2001) Engagement of right temporal cortex during processing of linguistic context. Neuropsychologia 39:798–809 [DOI] [PubMed] [Google Scholar]
- Linden DE, Thornton K, Kuswanto CN, Johnston SJ, van de Ven V, Jackson MC (2011) The brain’s voices: comparing nonclinical auditory hallucinations and imagery. Cereb Cortex 21:330–337 [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung-Beeman M (2007) An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain Lang 100:115–126 [DOI] [PubMed] [Google Scholar]
- McGuire PK, Silbersweig DA, Wright I, Murray RM, Frackowiak RS, Frith CD (1996) The neural correlates of inner speech and auditory verbal imagery in schizophrenia: relationship to auditory verbal hallucinations. Br J Psychiatry 169:148–159 [DOI] [PubMed] [Google Scholar]
- Mitchell RL, Elliott R, Barry M, Cruttenden A, Woodruff PW (2003) The neural response to emotional prosody, as revealed by functional magnetic resonance imaging. Neuropsychologia 41:1410–1421 [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009) The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44:893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neggers SF, Hermans EJ, Ramsey NF (2008) Enhanced sensitivity with fast three-dimensional blood-oxygen-level-dependent functional MRI: comparison of SENSE-PRESTO and 2D-EPI at 3 T. NMR Biomed 21:663–676 [DOI] [PubMed] [Google Scholar]
- Nichelli P, Grafman J, Pietrini P, Clark K, Lee KY, Miletich R (1995) Where the brain appreciates the moral of a story. NeuroReport 6:2309–2313 [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P (2011) How can the brain’s resting state activity generate hallucinations? A ‘resting state hypothesis’ of auditory verbal hallucinations. Schizophr Res 127:202–214 [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL (2005) Cognitive control and parsing: reexamining the role of Broca’s area in sentence comprehension. Cogn Affect Behav Neurosci 5:263–281 [DOI] [PubMed] [Google Scholar]
- Obleser J, Kotz SA (2010) Expectancy constraints in degraded speech modulate the language comprehension network. Cereb Cortex 20:633–640 [DOI] [PubMed] [Google Scholar]
- Obleser J, Wise RJ, Alex Dresner M, Scott SK et al. (2007) Functional integration across brain regions improves speech perception under adverse listening conditions. J Neurosci 27:2283–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M (1996) Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex 6:31–38 [DOI] [PubMed] [Google Scholar]
- Price CJ (2010) The anatomy of language: a review of 100 fMRI studies published in 2009. Ann NY Acad Sci 1191:62–88 [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC (1996) Cerebral organization of component processes in reading. Brain 119:1221–1238 [DOI] [PubMed] [Google Scholar]
- Raichle ME (2010) Two views of brain function. Trends Cogn Sci 14:180–190 [DOI] [PubMed] [Google Scholar]
- Rottschy C, Eickhoff SB, Schleicher A, Mohlberg H, Kujovic M, Zilles K, Amunts K (2007) Ventral visual cortex in humans: cytoarchitectonic mapping of two extrastriate areas. Hum Brain Mapp 28:1045–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW (2012) Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass K, Krach S, Sachs O, Kircher T (2009) Lion-tiger-stripes: neural correlates of indirect semantic priming across processing modalities. Neuroimage 45:224–236 [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K (2008) Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex 18:846–867 [DOI] [PubMed] [Google Scholar]
- Seal ML, Aleman A, McGuire PK (2004) Compelling imagery, unanticipated speech and deceptive memory: neurocognitive models of auditory verbal hallucinations in schizophrenia. Cogn Neuropsychiatry 9:43–72 [DOI] [PubMed] [Google Scholar]
- Seghier ML (2012) The Angular Gyrus: multiple Functions and Multiple Subdivisions. Neuroscientist (Advance online publication). doi: 10.1177/1073858412440596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Fagan E, Price CJ (2010) Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. J Neurosci 30:16809–16817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin AJ, Bishop CW, Miller LM (2009) Neural mechanisms for illusory filling-in of degraded speech. Neuroimage 44:1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK (2000) Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 57:1033–1038 [DOI] [PubMed] [Google Scholar]
- Shergill SS, Bullmore ET, Brammer MJ, Williams SC, Murray RM, McGuire PK (2001) A functional study of auditory verbal imagery. Psychol Med 31:241–253 [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Amaro E, Williams SC, Murray RM, McGuire PK (2004) Temporal course of auditory hallucinations. Br J Psychiatry 185:516–517 [DOI] [PubMed] [Google Scholar]
- Simons CJ, Tracy DK, Sanghera KK, O’Daly O, Gilleen J, Dominguez MD, Krabbendam L, Shergill SS (2010) Functional magnetic resonance imaging of inner speech in schizophrenia. Biol Psychiatry 67:232–237 [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G (2010) Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry 68:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotema CW, Aleman A, Daskalakis ZJ, Sommer IE (2012) Meta-analysis of repetitive transcranial magnetic stimulation in the treatment of auditory verbal hallucinations: update and effects after one month. Schizophr Res 142:40–45 [DOI] [PubMed] [Google Scholar]
- Sohoglu E, Peelle JE, Carlyon RP, Davis MH (2012) Predictive top-down integration of prior knowledge during speech perception. J Neurosci 32:8443–8453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Kahn RS (2001) Language lateralization in schizophrenia, an fMRI study. Schizophr Res 52:57–67 [DOI] [PubMed] [Google Scholar]
- Sommer IE, Clos M, Meijering AL, Diederen KM, Eickhoff SB (2012) Resting state functional connectivity in patients with chronic hallucinations. PLoS ONE 7:e43516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD (2009) Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self monitoring. Schizophr Bull 35:509–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens GJ, Silbert LJ, Hasson U (2010) Speaker-listener neural coupling underlies successful communication. Proc Natl Acad Sci USA 107:14425–14430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, Hirsch J (2006) Predictive codes for forthcoming perception in the frontal cortex. Science 314:1311–1314 [DOI] [PubMed] [Google Scholar]
- Turken AU, Vuilleumier P, Mathalon DH, Swick D, Ford JM (2003) Are impairments of action monitoring and executive control true dissociative dysfunctions in patients with schizophrenia? Am J Psychiatry 160:1881–1883 [DOI] [PubMed] [Google Scholar]
- Vercammen A, Aleman A (2008) Semantic expectations can induce false perceptions in hallucination-prone individuals. Schizophr Bull 36:151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen A, Knegtering H, den Boer JA, Liemburg EJ, Aleman A (2010) Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol Psychiatry 67:912–918 [DOI] [PubMed] [Google Scholar]
- Westernhausen R, Moosmann M, Alho K, Belsby SO, Hämäläinen H, Medvedev S, Hugdahl K (2010) Identification of attention and cognitive control networks in a parametric auditory fMRI study. Neuropsychologia 48:2075–2081 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ (2009) Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA 106:1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CJ, Davis MH, Johnsrude IS (2012) Human auditory cortex is sensitive to the perceived clarity of speech. Neuroimage 60:1490–1502 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.