Summary
The human pathogen Pseudomonas aeruginosa harbors three paralogous zinc proteases annotated as AmpD, AmpDh2, and AmpDh3, which turn over the cell wall and cell-wall-derived muropeptides. AmpD is cytoplasmic and plays a role in recycling of cell-wall muropeptides, with a link to antibiotic resistance. AmpDh2 is a periplasmic soluble enzyme with the former anchored to the inner leaflet of the outer membrane. We document herein that the type VI secretion system locus II (H2-T6SS) of P. aeruginosa delivers AmpDh3 (but not AmpD or AmpDh2) to the periplasm of a prey bacterium upon contact. AmpDh3 hydrolyzes the cell-wall peptidoglycan of the prey bacterium, which leads to its killing, thereby providing a growth advantage for P. aeruginosa in bacterial competition. We also document that the periplasmic protein PA0808, heretofore of unknown function, affords self-protection from lysis by AmpDh3. Cognates of the AmpDh3-PA0808 pair are widely distributed across Gram-negative bacteria. Taken together, these findings underscore the importance of their function as an evolutionary advantage and that of the H2-T6SS as the means for manifestation of the effect.
Keywords: Type 6 secretion system, Peptidoglycan hydrolase, Cell-wall degradation, Bacterial competition
Graphical Abstract
Introduction
The Type VI secretion system (T6SS) is widely distributed in Gram-negative bacteria and functions as a “molecular syringe” that delivers toxic effectors to prokaryotic and eukaryotic target cells (Ho et al., 2014). T6SS accomplishes the feat using a complex machinery consisting of 14 core conserved proteins essential for the basic secretory functions. The additional components that exist might fine-tune the process or increase the flexibility of these transporters (Boyer et al., 2009). The core structure of T6SS resembles the tail of the T4 bacteriophage (Filloux, 2013a). TssM (IcmF), TssL and TssJ form the base plate of the T6SS by anchored to the cell envelope (Cascales and Cambillau, 2012). The hemolysin-coregulated protein (Hcp) forms a tube, which is topped by a spike comprising both the valine-glycine repeat G (VgrG) trimer and PAAR domain-containing protein tip (Coulthurst, 2019). Effectors bind to the surface of Hcp, VgrG or PAAR carriers, which secrete the substrate by rapid contractions and assembly the Hcp tube with sheath subunits (TssB/TssC) for another firing (Durand et al., 2014; Coulthurst, 2019). Significant energy is required to penetrate the physical barriers of cell or contract the tube at each point of this process (Cascales and Cambillau, 2012). ClpV, a member of the AAA+ protein family, provides energy for T6SS substrate transportation by ATP hydrolysis, which is crucial for T6SS secretion (Bingle et al., 2008; Silverman et al., 2012). It is known that some bacteria often harbor more than one T6SS-encoding locus (Cianfanelli et al., 2016). In the genome of P. aeruginosa, three such loci have been identified, designated as H1-, H2-, and H3-T6SS, for three variants of the secretion system (Mougous et al., 2006). The expression of T6SS is regulated by multiple elements at the transcriptional and posttranslational levels. For example, both H1- and H2-T6SS are repressed by the hybrid sensor RetS, which is implicated in the Gac-Rsm signaling pathway (Mougous et al., 2006; Burkinshaw et al., 2018). H2-T6SS and H3-T6SS are co-regulated by the Las and Rhl quorum-sensing systems (Lesic et al., 2009), as well as are repressed by RsmA and AmrZ (Allsopp et al., 2017).
Some toxic effectors have been biochemically characterized to be delivered to prey organisms by the T6SS of a donor bacterium (Russell et al., 2014). The known effectors are shown to target cell-wall (peptidoglycan hydrolases), nucleic acids (nucleases), and membranes (phospholipases) (Ho et al., 2014; Basler, 2015). The well-studied examples are the cell-wall-degrading effectors that include the T6S amidase (Tae) and the T6S glycoside hydrolase (Tge) families (Russell et al., 2011; Whitney et al., 2013). In P. aeruginosa, Tse1 and Tse3 belong to the Tae and Tge families, respectively. These enzymes act in the periplasm of the prey cells, thereby causing cell lysis by degrading the cell-wall peptidoglycan (Russell et al., 2011). The phospholipase Tle family represents an additional set of T6SS toxins (Russell et al., 2013). Bacteria also encode specific auto-immunity proteins, which mitigate the activity of the enzymes in the donor organism. The genes for these auto-immune proteins are usually found in bicistronic operons along with the toxic proteins, which lead to self-resistance mechanism (Russell et al., 2011; Ma et al., 2014; Tang et al., 2017). It has also been reported that the P. aeruginosa H2-T6SS-dependent phospholipase D (PldB), an effector, contributes not only to interbacterial competitive fitness, but also to bacterial internalization into human epithelial cells (Wettstadt et al., 2019). Recent studies have reported that not all T6SS substrates are cytotoxic to target cells. For example, a T6SS substrate TseM in Burkholderia thailandensis was shown to bind to the Mn2+ ion and increase its acquisition, and thus helps bacteria survive oxidative stress and have a growth advantage in competition among bacteria (Si et al., 2017). In P. aeruginosa, the TseF protein secreted to extracellular milieu by H3-T6SS, is involved in iron uptake by interacting with the outer-membrane vesicles (OMVs) and the quinolone signal (PQS) system (Lin et al., 2017).
As indicated earlier, most peptidoglycan hydrolases are secreted proteins. P. aeruginosa has three paralogous peptidoglycan/muropeptide hydrolases, designated as AmpD, AmpDh2 and AmpDh3 (Juan et al., 2006). AmpD is the cytoplasmic protease at the crossroads of antibiotic resistance and cell-wall recycling (Zhang et al., 2013). Both AmpDh2 and AmpDh3 are periplasmic enzymes with roles in turnover of the cell wall (Zhang et al., 2013). Biochemical data and structural analysis show that the reaction of these enzymes is hydrolysis of the amide bond between the lactyl moiety of muramyl or 1,6-anhydromuramyl moiety and the N-terminal l-Ala of the stem peptide (Lee et al., 2013; Martinez-Caballero et al., 2013; Zhang et al., 2013). In addition, these three AmpD enzymes are critical for P. aeruginosa virulence (Moya et al., 2008a). Herein we document that the P. aeruginosa AmpDh3 as another cell-wall-targeting effector, exhibited antibacterial activity by degrading the prey organism’s peptidoglycan via its amidase activity, which is mediated by H2-T6SS. Furthermore, this deleterious activity of the AmpDh3 is counteracted by an auto-immunity protein, PA0808. P. aeruginosa deploys the effector AmpDh3 to target bacterial competitors. Our findings reveal that the wide distribution and high conservation of the genes for AmpDh3/PA0808 pair across bacterial genomes implicate the pair as playing roles in niche competition within a polymicrobial environment.
Results
AmpDh3 secretion is under the control of H2-T6SS
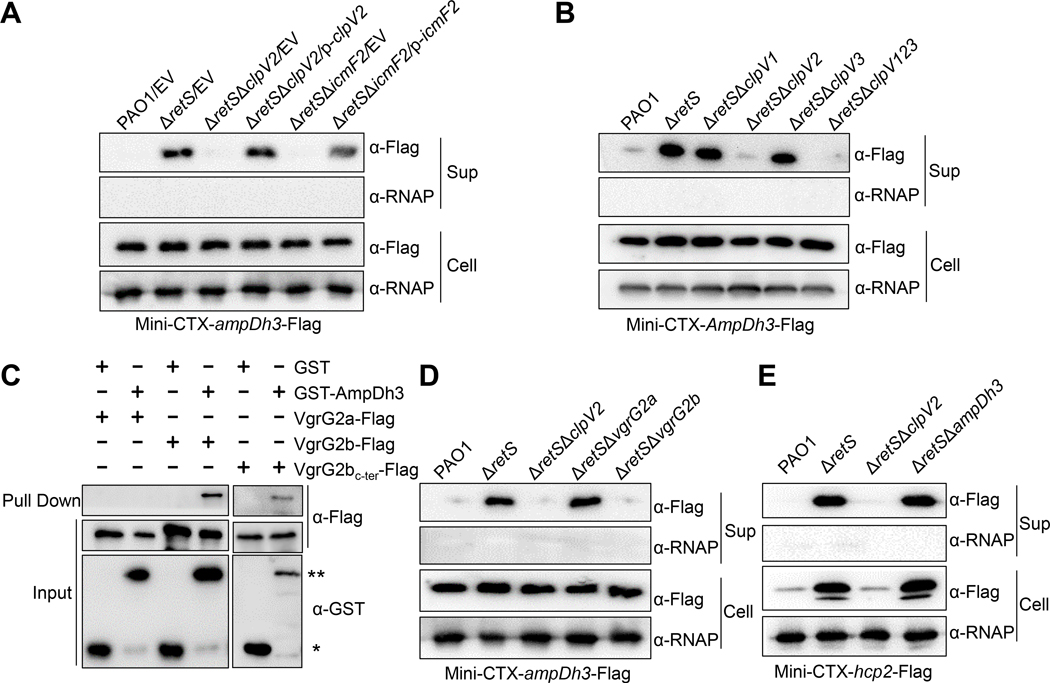
In light of the fact that H2-T6SS is repressed by the hybrid sensor RetS (Burkinshaw et al., 2018), we had previously performed a secretome analysis for the ΔretS and ΔretSΔclpV2, which lacks the core structural gene clpV2 of H2-T6SS in the ΔretS background, and we had speculated that secretion of AmpDh3 is likely dependent on H2-T6SS (Han et al., 2019). To experimentally demonstrate this observation, we cloned the ampDh3 gene fused with a Flag-tag sequence at the C-terminus in Mini-CTX-lacZ plasmid, and the ampDh3-Flag fusion was integrated into wild-type PAO1, ΔretS, and ΔretSΔclpV2 strains, respectively. Western-blot analysis showed that deletion of retS significantly improved AmpDh3 secretion compared to the wild-type parent, whereas the ΔretSΔclpV2 double mutant abrogated AmpDh3 secretion. Indeed, this effect was fully complemented by ectopic expression of clpV2 (Fig. 1A). To further determine whether the AmpDh3 secretion is dependent on H2-T6SS, we assessed the abundance of AmpDh3 in the deletion mutant of icmF2, which encodes a key T6SS structural protein (Mougous et al., 2006). Compared to ΔretS, protein levels of AmpDh3 were not detected in the ΔretSΔicmF2 mutant (Fig. 1A). These data suggest that H2-T6SS contributes to AmpDh3 secretion. In contrast, deletion of clpV1 or clpV3 from H1- or H3-T6SS clusters did not influence the levels of AmpDh3 (Fig. 1B), indicating that the secretion of AmpDh3 is dependent on H2-T6SS but not H1- and H3-T6SS.
Fig. 1. AmpDh3 is secreted by H2-T6SS.
(A) Deletion of H2-T6SS clusters reduced AmpDh3 secretion. Western blot analysis of AmpDh3-Flag in the concentrated supernatant of the bacterial cultures (Sup) and the whole cell-pellet associated (Cell) protein fractions from the indicated strains are shown. An antibody against RNA polymerase (α-RNAP) was used as a loading control in this and subsequent blots and EV represents the empty vector (pUCP26). (B). The secretion of AmpDh3 is independent on H1- and H3-T6SS. The cultured condition is the same as described in A. (C) Interactions between AmpDh3 and VgrG2b were documented by individual incubation of GST or GST-AmpDh3 with the cell lysates of PAO1 expressing VgrG2a-Flag, VgrG2b-Flag or VgrG2bc-ter-Flag, and protein complexes were captured by glutathione beads. The single and double asterisks indicated GST and GST-AmpDh3, respectively. (D) Deletion of vgrG2b, but not of vgrG2a, decreased the secretion of AmpDh3. (E) AmpDh3 did not affect the secretion of Hcp2. (A-E) Data are representative of three independent replicates.
The components of the aforementioned VgrG, Hcp or PAAR have been shown to be essential for T6SS (Durand et al., 2014; Cianfanelli et al., 2016). Therefore, we explored whether secretion of AmpDh3 is dependent on VgrG proteins, including VgrG2a and VgrG2b. First, we examined the interaction between AmpDh3 and VgrGs. Our data show that the fusion product of glutathione S-transferase (GST)-AmpDh3 was able to retain VgrG2b, but GST by itself was not (Fig. 1C). In contrast, no interaction was observed between GST-AmpDh3 and VgrG2a (Fig. 1C). VgrG2a and VgrG2b are nearly 100% identical except for the C-terminal extension on VgrG2b (Stover et al., 2000). To further ascertain the site of the interaction, we constructed a pMM-vgrG2bc-ter-Flag plasmid and performed pull down assay. As expected, the interaction between VgrG2bc-ter and AmpDh3 was observed (Fig. 1C). Furthermore, we found that AmpDh3 secretion was dependent on VgrG2b, but not on VgrG2a (Fig. 1D). These data suggested that AmpDh3 is a substrate for the H2-T6SS, and its secretion is dependent on the VgrG2b carrier protein.
Given that the T6SS-dependent effectors alone are unable to reach their target cellular compartment and need additional components for its secretion (Russell et al., 2011), we reasoned that the AmpDh3 function should be linked to export by the T6SS. To investigate whether AmpDh3 affects T6SS apparatus assembly, we examined whether the enzyme is required for export of the effector Hcp2. Our data showed that the secretion of Hcp2 was not altered in the ΔampDh3 strain, indicating that AmpDh3 is not required for the assembly of T6SS apparatus (Fig. 1E). These findings suggest that AmpDh3 is an effector enzyme of the T6SS apparatus.
AmpDh3 is a toxin with catalytic activity and catalysis is required for its antibacterial activity
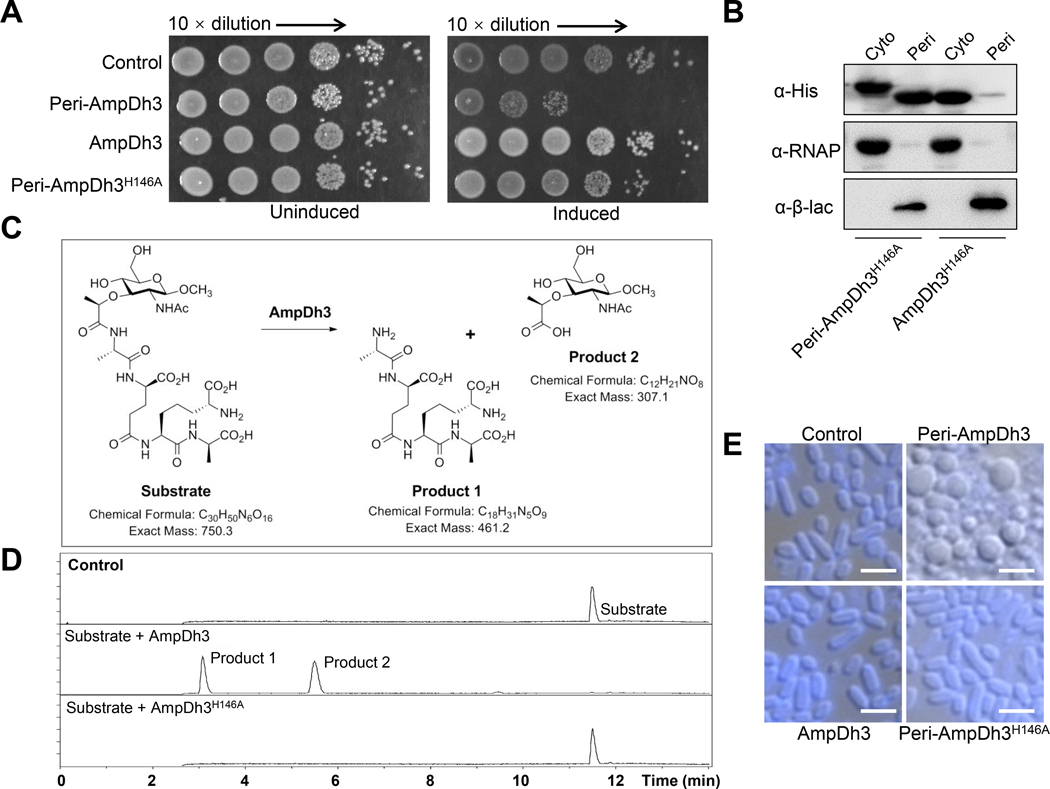
AmpDh3 was previously described as a soluble periplasmic zinc protease of P. aeruginosa, which turns over the cell-wall peptidoglycan (Lee et al., 2013). An unregulated AmpDh3 degradation of the cell-wall peptidoglycan would undoubtedly be detrimental to the bacterium. To confirm this hypothesis, we cloned the ampDh3 gene from P. aeruginosa PAO1 into pET22b (Peri-AmpDh3, which harbored a pelB signal peptide and is the form localized to the periplasm) and transformed E. coli with the plasmid. Importantly, induction of expression of ampDh3 by IPTG impaired E. coli growth (Fig. 2A). However, ectopic expression of ampDh3 in the cytoplasm of E. coli was tolerated significantly better (Fig. 2A and Fig. S1A). We next engineered a mutant that resulted in an inactive periplasmic AmpDh3 variant with alteration in a key active-site residue (H146A). The inactive protein variant, Peri-AmpDh3H146A, barely exhibited toxicity when expressed (Fig. 2A and Fig. S1A). Western-blot analysis showed that the Peri-AmpDh3H146A was able to be localized in periplasm, but not AmpDh3H146A (Fig. 2B). In accordance with our in vitro studies, Peri-AmpDh3H146A was produced in the similar expression levels as that seen for the native enzyme (Fig. S1B). To demonstrate the presence of activity in the native wild-type enzyme (AmpDh3) and abrogation of activity for the H146A variant (AmpDh3H146A), both proteins were assayed with a synthetic substrate (Fig. 2C), NAM-tetrapeptide (l-Ala-d-γ-Glu-meso-DAP-d-Ala), which was prepared in 29 synthetic steps by the methodology developed in our laboratories (Zhang et al., 2013). Wild-type AmpDh3 turned over NAM-tetrapeptide substrate to tetrapeptide (l-Ala-d-γ-Glu-meso-DAP-d-Ala) and NAM (Fig. 2C). As depicted in Fig. 2C–D and Fig. S2A by ultra-performance liquid chromatography (UPLC) and analyzed by mass spectrometry (MS), the wild-type enzyme is catalytically competent, whereas the variant is totally inactive. Furthermore, we examined cells producing Peri-AmpDh3 or Peri-AmpDh3H146A using fluorescence microscopy. AmpDh3 turns over the cell-wall peptidoglycan with its amidase activity, the cellular shape of E. coli producing Peri-AmpDh3 will be amorphous or spherical. As expect, the shapes of E. coli producing Peri-AmpDh3 displayed amorphous or spherical, whereas cells producing Peri-AmpDh3H146A or empty vector were swollen and filamentous (Fig. 2E and Fig. S2B), which were consistent with our biochemical data.
Fig. 2. AmpDh3 is toxic to E. coli when sequestered in the periplasm.
(A) Growth of E. coli strain BL21 (DE3) pLysS harboring a vector expressing the Peri-ampDh3, ampDh3 or Peri-ampDh3H146A on LB-LS agar with or without 0.03 mM IPTG at 37 °C. An empty vector was included as control. Overnight cultures were spotted on LB-LS medium plates by successive ten-fold dilution. (B) Cytoplasmic (Cyto) and periplasmic (Peri) fractions of E. coli expressing Peri-AmpDh3H146A or AmpDh3H146A were examined by Western blot. The tagged proteins were detected using a His-tag antibody. RNA polymerase (RNAP) and β-lactamase (β-lac) were used as cytoplasmic or periplasmic fraction controls, respectively. (A-B) Data are representative of three independent replicates. (C) The amidase reaction of AmpDh3 with substrate is shown. (D) The LC/MS traces reveal that the AmpDh3H146A protein failed to degrade the substrate, whereas the conversion to the two products was clean for the wild-type AmpDh3. Mass spectra of Substrate, Product 1, and Product 2 are shown in Fig. S2A. (E) Representative micrographs of strains shown in panel A acquired before complete lysis. The cellular membranes were stained by the lipophilic dye TMA-DPH and the full microscopic fields were shown in Fig. S2B. All images were gained at the same magnification. Scale bar: 5 μm.
In contrast to other bacteria, analyses of the genomic sequences of P. aeruginosa reveal that it possesses three paralogous genes, designated as ampD, ampDh2, and ampDh3 (Juan et al., 2006). We next tested whether AmpD or AmpDh2 have any deleterious effects (antibacterial activity) in the periplasm of a recipient host. To test this possibility, we cloned the ampD and ampDh2 genes from P. aeruginosa PAO1 into pET22b (Peri-AmpD and Peri-AmpDh2, both with the sequence for the pelB signal peptide for targeting the respective proteins to the periplasm) and transformed these plasmids into E. coli, respectively. Similar to the case of AmpDh3, both AmpD and AmpDh2 displayed antibacterial activity (Fig. S3A–B). This is consistent for the periplasmic AmpDh2, as it turns over the cell wall (Martinez-Caballero et al., 2013). It is less obvious for AmpD, as this enzyme is regulated by dramatic conformational changes induced by its muropeptide substrate, once it is internalized to the cytoplasm (Carrasco-Lopez et al., 2011). Meanwhile, we determined whether these proteins are secreted by T6SS using western-blot assays. The results showed that no secreted AmpD protein was detected in the concentrated supernatant (Sup) of the bacterial cultures from the indicated strains (Fig. S3C), confirming that indeed AmpD is not transported, consistent with its cytoplasmic location (Zhang et al., 2013). In addition, the protein abundance of AmpDh2 in the ΔretS mutant is comparable to that of the ΔretSΔclpV1, ΔretSΔclpV2, ΔretSΔclpV3 and ΔretSΔclpV123 mutants, indicating its secretion is T6SS independent (Fig. S3D).
PA0808 is the auto-immunity protein for AmpDh3 and mitigates its self-deleterious effect
Previous studies have revealed that individual P. aeruginosa can target themselves with toxic effectors by direct injection via the T6SS (Ho et al., 2014; Jiang et al., 2014). We reasoned that uncontrolled AmpDh3 activity would be deleterious in the periplasm (the antibacterial effect), so P. aeruginosa should harbor an auto-immunity protein within the periplasm. Typically, T6SS auto-immunity proteins are genetically linked to the respective toxin that they regulate (Russell et al., 2011; Jiang et al., 2014). In P. aeruginosa genome, PA0808, encoding a hypothetical protein of unknown function, is located downstream of the ampDh3 gene (Stover et al., 2000). We wondered whether it might be an auto-immunity protein for AmpDh3.
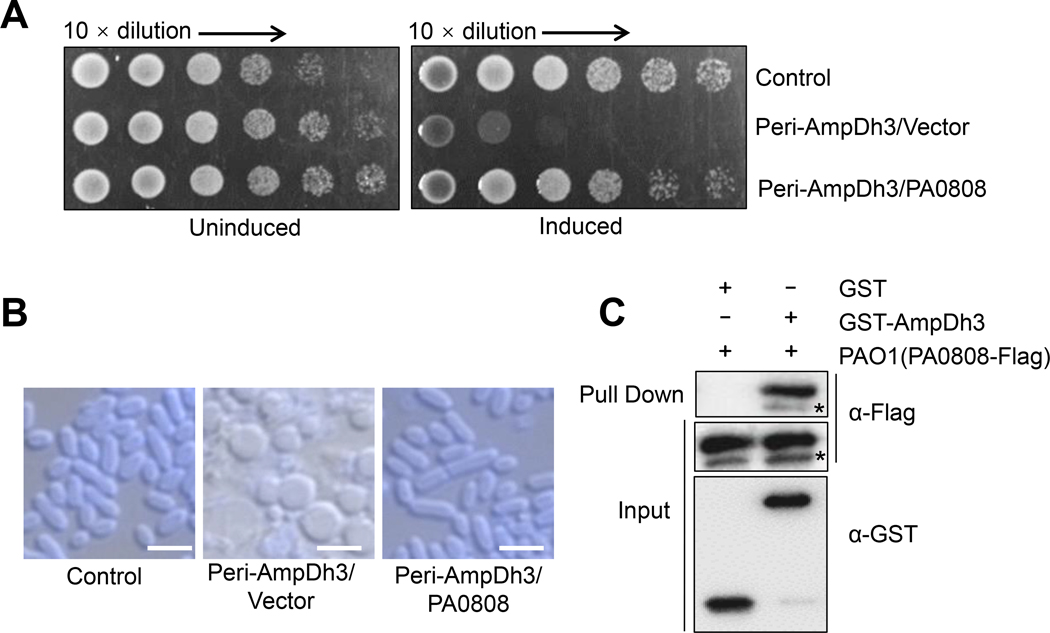
To test this hypothesis, we engineered expression plasmids that harbored ampDh3 (PA0807) alone and ampDh3 with the putative auto-immunity gene (PA0808) for heterologous expression in E. coli BL21 (DE3) pLysS strain. In this case, we fused the ampDh3 gene to a signal peptide for its export to the periplasm. The results showed that growth of E. coli was inhibited by periplasmic expression of ampDh3 alone, but the effects of the enzyme were mitigated when ampDh3 was co-expressed with PA0808 (Fig. 3A and Fig. S4A). Western blot showed that the protein levels for AmpDh3 were comparable to PA0808, when they co-expressed in E. coli (Fig. S4B). Indeed, E. coli cells producing Peri-AmpDh3 and PA0808 exhibited a swollen and filamentous phenotype (Fig. 3B), which is distinct from the amorphous or spherical cells producing Peri-AmpDh3 alone. This confirms that the activity of periplasmic AmpDh3 is inhibited by PA0808.
Fig. 3. Auto-immunity effect of PA0808 on the activity of AmpDh3.
(A) Growth of E. coli strain BL21 (DE3) pLysS expressing either Peri-AmpDh3 or Peri-AmpDh3 and PA0808 on LB-LS agar or LB-LS agar with 0.03 mM IPTG and 0.1 mM L-Arabinose at 37 °C. Overnight cultures were spotted on LB-LS medium plates by ten-fold dilution. (B) Micrographs of the indicated strains shown in panel A acquired before complete lysis with membranes stained with TMA-DPH. The full microscopic images were supplemented in Fig. S4C. Scale bar: 5 μm. (C) The purified GST-AmpDh3 and the cell lysates of P. aeruginosa expressing PA0808-Flag were incubated with MagneGST™ glutathione beads, and bound proteins were eluted and detected by western-blot assays; note that the asterisk indicates small isoform of the PA0808 that is consistent with cleavage of the predicted signal peptides. (A, C) Data are representative of three independent replicates.
Generally, auto-immunity proteins abrogate the activity of their corresponding toxin proteins by direct protein-protein interaction (Russell et al., 2011; Jiang et al., 2014). To determine if PA0808 protein could interact with AmpDh3, we performed protein pull-down assay. The P. aeruginosa expressing PA0808-Flag was sonicated, and the cell lysates were incubated with GST-AmpDh3 or GST proteins. The mixtures were further purified by GST beads. Western-blot analysis showed that GST-AmpDh3 was able to retain PA0808, but GST alone was not (Fig. 3C), indicating that physical interaction between AmpDh3 and PA0808 exists. These findings suggest that PA0808 can act as an auto-immunity protein against AmpDh3 toxicity.
AmpDh3 is a VgrG2b-dependent T6SS substrate
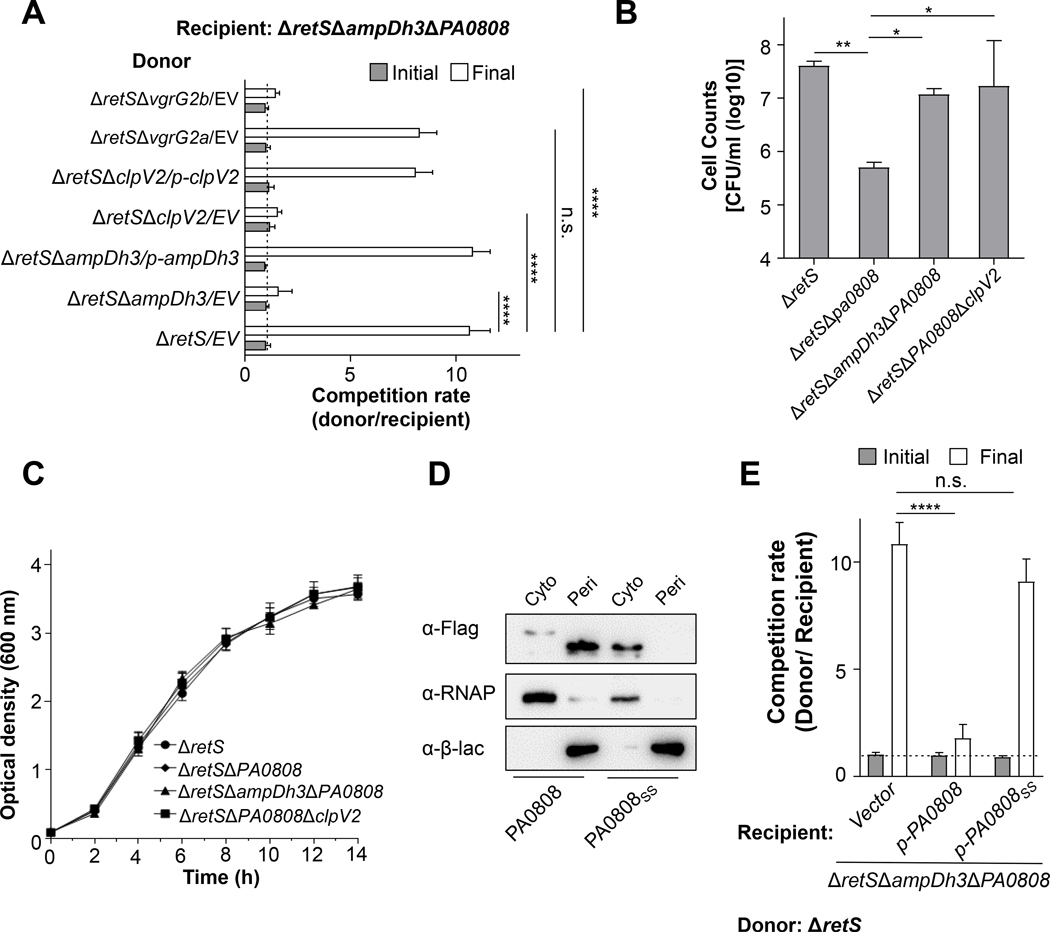
As mentioned above, AmpDh3 is delivered by H2-T6SS via direct interaction with VgrG2b. The transfer kills the recipient bacteria. In addition, we found that PA0808 is the auto-immunity protein of AmpDh3. To assess whether AmpDh3-mediated antibacterial activity is dependent on VgrG2b, we performed bacterial growth competition assays using a series of T6SS-defective mutants (donors) with recipient cells (ΔretSΔampDh3ΔPA0808). Our data showed that the vgrG2a mutant has equal competition advantages to ΔretS parental strain, whereas the ΔampDh3 and ΔvgrG2b mutants showed loss of advantage, similar to the H2-T6SS ΔclpV2 mutant (Fig. 4A). These results clearly suggest that VgrG2b is an effective carrier for AmpDh3 delivery.
Fig. 4. PA0808 access to the periplasm provides a growth advantage to donor cells.
(A) Growth-competition assays between the indicated donor and recipient strains under T6SS-inducing conditions. The initial c.f.u of the donor and recipient bacteria was equal and is denoted by the dashed line. Error bars represent the mean ± s.d. of three biological replicates, and significance was calculated using one-way ANOVA Dunnett’s multiple comparison test, ****P<0.0001; n.s. indicates no significance. (B) Quantification of intercellular self-intoxication of P. aeruginosa. Indicated strains were spotted on a nitrocellulose membrane placed on LB-LS 3% agar, and the final CFU/mL was determined after incubated for 24 h at 37°C. Error bars indicate mean ± s.d. of three biological replicates, and statistical significance was calculated using one-way ANOVA Dunnett’s comparison test, *P<0.05, **P<0.01. (C) Growth rates of the indicated strains in liquid medium is depicted. Error bars indicate the mean ± s.d. of three biological replicates. (D) Western-blot assays of cytoplasmic (Cyto) and periplasm (Peri) fractions of P. aeruginosa producing PA0808 or PA0808SS. Equal samples of the cyto and peri from the P. aeruginosa strain were collected and loaded in each panel. RNA polymerase (RNAP) and β-lactamase (β-lac) were used as cytoplasmic or periplasmic fraction controls respectively. Data are representative of three independent replicates. (E) Growth-competition assays indicate that PA0808 serves as the auto-immunity protein for AmpDh3 under T6SS-inducing conditions. The recipient P. aeruginosa strains are the mutant of the whole ampDh3-PA0808 gene locus in addition to retS gene with different vectors. The donor strain is ΔretS mutant strain. PA0808SS is a truncated PA0808 lacking the N-terminal signal-sequence. Experiments were initiated with equal c.f.u of donor and recipient bacteria as denoted by the dashed line. Error bars indicate the mean ± s.d. of three biological replicates, and significance was calculated using one-way ANOVA Dunnett’s multiple comparison test, ****P<0.0001; n.s. indicates no significance.
The auto-immunity protein PA0808 mitigates the deleterious activity of AmpDh3 in the periplasm
Most genes encoding auto-immunity determinants are in the vicinity of that of their respective toxins In addition, it has been shown that a strain grown on a solid substrate is considered conductive to T6SS-based effector delivery (Russell et al., 2011). Similar to the case of Tsi1 and Tsi3 (Russell et al., 2011), we could also engineer the deletion mutant of PA0808 in P. aeruginosa strain in the presence of AmpDh3. Deletion of PA0808 resulted in a high proportion of growth attenuation when it is grown on a solid substrate for 24h (Fig. 4B). In contrast, this growth suppression was not observed in liquid media (Fig. 4C), indicating that T6SS-dependent effector AmpDh3 is denied access to donor cell periplasm, but targets the periplasm of the recipient cell under a contact-dependent condition (solid growth support).
Because AmpDh3 is active in the periplasm, we reasoned that its auto-immunity protein should encode an N-terminal signal peptide. Bioinformatic analyses indicated that PA0808 indeed has a predicted signal peptide (Fig. S5A) for periplasmic export. Separation of the periplasmic and cytoplasmic contents showed PA0808 protein was dominant in the periplasmic compartment (Fig. 4D). As expected, PA0808 lacking the signal-sequence (PA0808SS) was located exclusively in the cytoplasm. Indeed, PA0808SS still could interact with AmpDh3 in vitro (Fig. S6A). To further determine the effect of PA0808 and PA0808SS location on AmpDh3 toxicity to the prey cells, growth competition assays were performed between ΔretSΔampDh3ΔPA0808 expressing the PA0808 or PA0808SS gene or an empty vector (recipient) and ΔretS donor to see whether the auto-immunity protein was able to rescue a fitness deficiency in the recipient strain. As shown in Fig. 4E, the ΔretSΔampDh3ΔPA0808 recipient P. aeruginosa, which are incapable of self-intoxication, display a growth advantage against donor strain. In the ΔretSΔampDh3ΔPA0808 recipient strain, expression of wild-type PA0808 rescues the fitness; however, expression of PA0808SS fails to restore the growth phenotype (Fig. 4E), supporting that PA0808 functions in the periplasm. Collectively, these results demonstrate that the auto-immunity protein PA0808 is periplasmic and that it mitigates the AmpDh3 antibacterial activity.
Broad distribution of the effector/auto-immunity gene pair across genera
The X-ray structure of AmpDh3 has been solved to high resolution (Lee et al., 2013). The protein is tetrameric, both in solution and in the crystals. Each monomer is comprised of an N-terminal coiled-coil loop (residues 1–19), a catalytic domain (residues 20–173) and a globular C-terminal domain (residues 174–255). According to the syntenic analysis of ampDh3 and PA0808, orthologues of these two adjacent genes are transcribed either in consistent or opposite directions in various bacteria (Fig. 5A). The established phylogenetic trees revealed that the AmpDh3 and PA0808 orthologues are widely distributed in α-proteobacteria, γ-proteobacteria and flavobacteria (Fig. 5B). Next, we sought to determine whether the bacteriolytic activity of AmpDh3 could be repressed by PA0808 orthologues proteins. To this end, EW82_RS06645 from Chryseobacterium sp. YR561 or B5X62_RS13770 from Soonwooa buanensis was co-expressed with AmpDh3 in E. coli. As shown in Fig. 5C, the bacteriolytic activity of AmpDh3 was inhibited by EW82_RS06645 or B5X62_RS13770, suggesting the broad distributed effector/auto-immunity gene pair can provide an across-protection for the effector (Fig. 5C and Fig. S6B–C). The fact that AmpDh3 and PA0808 orthologues exist across genera indicates that these genes likely descended from a common ancestor and they are expected to played similar functions.
Fig. 5. Synteny analyses and phylogenetic tree established the existence of AmpDh3 and PA0808 orthologues.
(A) Genetic organization of the AmpDh3-PA0808 in P. aeruginosa and 4 additional bacteria. The counterparts of orthologues are shown in the same color, and the direction of the arrow represents the direction of transcription. (B) Phylogenetic tree of AmpDh3 (PA0807) and PA0808 for 15 genera is shown. The relations were established using the neighbor-joining method, the bootstrap values are shown next to the branches, and evolutionary distances were computed using the Poisson correction method. Analyses were performed with MEGA 7 software. (C) The bacteriolytic activity of AmpDh3 was inhibited by EW82_RS06645 or B5X62_RS13770. Growth of E. coli strain BL21 (DE3) pLysS harboring the vector expressing either Peri-AmpDh3 alone or Peri-AmpDh3 with PA0808, EW82_RS06645 or B5X62_RS13770 on LB-LS agar or LB-LS agar with 0.03 mM IPTG and 0.1 mM L-Arabinose at 37°C. Overnight cultures were spotted on LB-LS medium plates by ten-fold dilution. Data are representative of three independent replicates.
AmpDh3 contributes to interspecies competition
We determined whether AmpDh3 is toxic to other Gram-negative grey bacteria. To this end, we performed growth competition assays between P. aeruginosa and E. coli or Yersinia pseudotuberculosis as prey. Our results revealed that the recovery was impaired in competition experiments of the P. aeruginosa ΔampDh3 and ΔclpV2 strains over E. coli or Y. pseudotuberculosis (Fig. 6A–B). In addition, this growth advantage provided by AmpDh3 was not observed in liquid medium (Fig. 6A–B). These data indicate that H2-T6SS can deliver AmpDh3 to other bacterial species and this protein is important for P. aeruginosa for interspecies bacterial interactions.
Fig. 6. P. aeruginosa AmpDh3 targets interspecies bacteria.
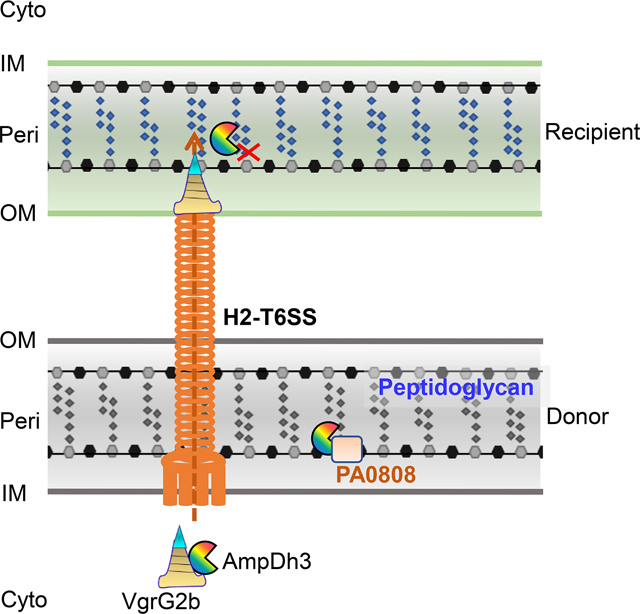
(A, B) Quantification of prey strains (E. coli or Y. pseudotuberculosis) after co-incubation with the indicated P. aeruginosa strains is depicted in growth-competition assays on solid (dark bars) or in liquid medium (blank bars). EV is the empty vector. Error bars represent the mean ± s.d. of three biological replicates, and significance was calculated using one-way ANOVA Dunnett’s multiple comparison test, ***P<0.001; ****P<0.0001. (C) The proposed model depicts P. aeruginosa H2-T6SS delivery of AmpDh3 to the periplasm of the recipient cell. AmpDh3 and auto-immunity protein PA0808 are shown as “packman” and rectangle, respectively. Data from this study indicate that AmpDh3 interacts with VgrG2b, an event necessary for secretion. The sites of reactions for AmpDh3 are indicated by red X; the outer membrane (OM), periplasm (Peri), inner membrane (IM), and cytoplasm (Cyto) of both bacteria are shown.
Discussion
Previous studies show that the three AmpD homologues (AmpD, AmpDh2 and AmpDh3) are responsible for a stepwise ampC upregulation and thus leads to constitutive hyperexpression of the chromosomal cephalosporinase conferring high-level β-lactam resistance (Juan et al., 2006; Moya et al., 2008b). The crystal structure demonstrates that AmpDh3 is tetrameric and functions in peptidoglycan/muropeptide hydrolases, which is involved in cell-wall remodeling (Lee et al., 2013). In this study, we reveal that the P. aeruginosa H2-T6SS delivers a cell-wall amidase effector, AmpDh3, to the periplasm of the recipient cells, which secure a fitness advantage in competition with other bacteria. Additionally, we demonstrate that PA0808, a heretofore protein of unknown function, serves in mitigating the deleterious auto-toxicity of AmpDh3 (Fig. 6C). Although there are numerous other similar T6SS effectors that have already been identified in P. aeruginosa, we here demonstrated that AmpDh3, a cell-wall remodeling enzyme, as a specific effector for being secreted by H2-T6SS via its association with VgrG2b, which extends understanding the function of AmpDh3 and T6SS.
Effectors can be translocated by the T6SS either fused by covalent bonds to structural components or by noncovalent interaction with one of the core components (Whitney et al., 2014; Cianfanelli et al., 2016). Both Hcp and VgrG proteins have been shown to play a role in the export of T6SS effectors (Cianfanelli et al., 2016). Various examples of effector domains fused to PAAR domains for T6SS delivery have been described (Ma et al., 2014; Salomon et al., 2014; Whitney et al., 2014). In these examples, effectors together with the expelled Hcp-VgrG-PAAR are simultaneously delivered into a target cell in one lethal shot (Shneider et al., 2013). Another obvious characteristic of many effectors is thought to be located in the same operon together with their cognate VgrG, Hcp or PAAR proteins (Silverman et al., 2013; Ma et al., 2014). Bioinformatics revealed that the gene ampDh3 is distant from the operons for VgrG or Hcp (Stover et al., 2000). However, we documented that it can directly interact with VgrG2b (Fig. 1C). This observation was further confirmed by the western-blot assays that documented that the secretion of AmpDh3 was dependent on VgrG2b (Fig. 1D). In addition, intraspecies growth competition assays showed that inactivation of vgrG2b impaired AmpDh3-mediated competition (Fig. 4A). Similarly, several effectors that are distant from T6SS operon were also found in other bacteria. For example, the T6SS-effector Ssp6 of Serratia marcescens inhibits bacterial growth by causing depolarisation of the inner membrane in intoxicated cells (Mariano et al., 2019), and KatN, a novel T6SS effector in Enterohemorrhagic E. coli, is a Mn-containing catalase (Wan et al., 2017).These findings indicate that these kinds of effectors maybe universal among bacteria.
Most of effectors secreted by the T6SS have been described as weapons for bacterial warfare (Filloux, 2013b; Russell et al., 2014) whereas only a few T6SS effectors are shown to target eukaryotic cells (Jiang et al., 2014). Tse1 and Tse3, both secreted by the H1-T6SS (Russell et al., 2011). Tse1, a peptidase, targets the peptide cross-links of the cell-wall peptidoglycan, whereas the Tse3 as a glycoside hydrolase degrades the glycan backbone of the cell wall (Whitney et al., 2013). Similar to Tse1, AmpDh3 appears to target cell-wall. However, the two proteins are unrelated to each other and lack significant primary sequence homology (Fig. S5B). In addition, Tse1 and AmpDh3 are secreted by the H1-T6SS and H2-T6SS, respectively, which suggest a highly selected trait. Similar to AmpDh3, the other two paralogs, AmpD and AmpDh2, exhibit antibacterial activity. However, AmpD was located in cytoplasm and the secretion of AmpDh2 is independent on T6SS (Fig. S3C–D). Taken together, our findings highlight that P. aeruginosa can take a fitness advantage by exploiting different T6SS effectors to compete with other bacteria in the complex environments.
Polymicrobial communities are prevalent in cystic fibrosis (CF) lung disease (Peters et al., 2012). P. aeruginosa has been reported as the dominant organism, displacing the resident microbial community (Cox et al., 2010). It can secret a set of exoproducts, such as pyocyanin, elastase, and rhamnolipids to kill Staphylococcus aureus, a pathogen that commonly inhabit the CF lung as well (O’Brien and Fothergill, 2017). On the other hand, secretions can shape interactions between P. aeruginosa and other commonly encountered members of the lung microbiome by producing an array of phenazines and other signal molecules (O’Brien and Fothergill, 2017). Although interactions between P. aeruginosa and some bacterial species have been investigated, those of P. aeruginosa with other common inhabitants of the CF lung (e.g., Gram-negative bacteria) remain unknown. In polymicrobial infections, P. aeruginosa often compete with other Gram-negative bacteria for access to nutrient-rich host tissue (Gjodsbol et al., 2006; Russell et al., 2011). Factors such as T6SS, that may increase the competitiveness of P. aeruginosa with the resident CF lung microflora, are thus likely to have important implications for disease progression and therapeutic outcomes.
Here, we describe the function of a PAO1 H2-T6SS effector, highlighting an additional strategy used by this organism to adapt to environment changing. As type VI amidase effectors are widely distributed in bacteria, they may represent potent targets for antimicrobial drug development to combat this chronic infection disease that is the killer in CF patients.
Experimental Procedures
Bacterial strains and growth conditions
Bacterial strains and plasmids used in this study are listed in table S1. P. aeruginosa strains were grown in Luria-Bertani (LB), LB low salt (LB-LS, 1% tryptone, 0.5% yeast extract) or Pseudomonas isolate agar (PIA) at 37°C with appropriate antibiotics, when needed. Antibiotics were used at the following concentrations, where appropriate: for E. coli, carbenicillin at 100 μg/mL, kanamycin at 50 μg/mL, tetracycline at 10 μg/mL and gentamicin at 25 μg/mL; for P. aeruginosa carbenicillin at 300 μg/mL, and tetracycline at 100 μg/mL; for Y. pseudotuberculosis gentamicin at 25 μg/mL.
Plasmid construction
Primers used in this study are listed in table S2. Plasmid Mini-CTX-ampDh3-Flag was constructed for protein secretion assay. Briefly, primer CTX-ampDh3-Flag-S/A was used to amplify the ampDh3 gene from genomic DNA. The PCR product was digested and inserted into mini-CTX to generate mini-CTX-ampDh3-Flag. Plasmid pMM-ampD-Flag was constructed for protein secretion assay. Briefly, primer pMM-ampD-Flag-S/A was used to amplify the ampD gene from genomic DNA. The PCR product was digested and inserted into pMMB67H to generate pMM-ampD-Flag. The plasmids pMM-ampD-Flag, pMM-vgrG2bc-ter-Flag, pMM-PA0808-Flag and pMM-PA0808ss-Flag were constructed in similar manners. Plasmid pAK-ampDh3 was constructed for complement mutant; primer pAK-ampDh3-S/A was used to amplify the ampDh3 gene fragment from genomic DNA. The PCR product was digested and inserted into similarly digested pAK1900 to produce pAK-ampDh3.
Plasmid Peri-ampDh3 was constructed by PCR amplification using the primers Peri-ampDh3-S/A. The PCR product was digested and inserted into similarly digested pET22b to produce Peri-ampDh3. The plasmids Peri-ampD, Peri-ampDh2 were constructed in similar manners. To constructed the N-terminal pelB signal sequence lack plasmid pET22b-ampDh3, DNA fragment was constructed by PCR amplification using the primers ampDh3-S/Peri-ampDh3-A. The PCR product was digested and inserted into similarly digested pET22b to produce pET22b-ampDh3. The plasmids pET22b-ampD, pET22b-ampDh2 were constructed in similar manners.
Site-directed mutagenesis was carried out by overlap PCR to substitute the histidine residue at position 146 of AmpDh3 into an alanine residue (AmpDh3H146A) as described previously (Wang et al., 2015). Briefly, DNA of mutant ampDh3H146A was amplified by two rounds of PCR. Primer pairs Peri-ampDh3-S/ampDh3H146A-A and ampDh3H146A-S/Peri-ampDh3-A were used to amplify segments 1 and 2 respectively. The second round of PCR was carried out by using Peri-ampDh3-S/A as primer pair while fragment 1 and fragment 2 as templates to obtain the ampDh3H146A fragment. The DNA fragment was digested and cloned into similar digested pET22b to produce Peri-ampDh3H146A.
To constructed the N-terminal pelB signal sequence lack plasmid pET22b-ampDh3H146A, DNA fragment was constructed by PCR amplification using the primers pET22b-ampDh3-S/Peri-ampDh3-A form Peri-ampDhH146A The DNA fragment was digested and cloned into similar digested pET22b to produce pET22b-ampDh3H146A. To construct the N-terminal pelB signal sequence fused plasmid pET28a-pelB-ampDh3, primers pET28a-pelB-ampDh3-S/Peri-ampDh3-A were used to amplify the pelB-ampDh3 fragment from Peri-ampDh3 plasmid DNA and was subcloned into similarly digested pET28a. Plasmids pBAD22a-PA0808 was constructed by PCR amplification using the primers pBAD-PA0808-S/pBAD-PA0808-A. The PCR product was digested and inserted into similarly digested pBAD22a to produce pBAD22a-PA0808. The fragments of EW82 RS06645 and B5X62 RS13770 were synthesized according to the NCBI genome sequence and cloned into pBAD22a plasmid, respectively.
Construction of P. aeruginosa deletion mutants
The plasmid pEX-ampDh3 was used to construct the ampDh3 in-frame deletion mutant of P. aeruginosa described previously (Kong et al., 2015). upstream and downstream fragments flanking ampDh3 were amplified with primer pair pEX-ampDh3-Up-S/A and pEX-ampDh3-Down-S/A, respectively. The two PCR products were digested with appropriate restriction enzymes and directionally cloned into pEX18Ap (Hoang et al., 1998) to produce pEX-ampDh3. The pEX-ampDh3 was electroporated into P. aeruginosa with selection for carbenicillin. Colonies showing both carbenicillin susceptibility and sucrose (10%) resistance were selected on LB agar plates, which indicates a double-crossover event and gene replacement occurring. Deletion events were verified by PCR and DNA sequencing. The ΔampDh3, ΔretSΔampDh3, ΔretSΔPA0808, ΔretSΔampDh3ΔPA0808 and ΔretSΔclpV2ΔPA0808 mutants were constructed by similar manners.
Protein expression and purification
The experiments were performed as described previously (Liang et al., 2014). To express His6-tagged AmpDh3 and AmpDh3H146A, plasmids pET28a-ampDh3 was constructed by PCR amplification using the primers pET28a-ampDh3-S/A. The PCR product was digested and inserted into similarly digested pET28a to produce pET28a-ampDh3. To construct the site-directed mutagenesis plasmid pET28a-ampDh3H146A, primer pair pET28a-ampDh3-S/A was used to amplify the ampDh3H146A fragment from pET22b-ampDh3H146A plasmid DNA and was subcloned into similarly digested pET28a. To express GST-tagged AmpDh3, plasmid pGEX-6p-1-ampDh3 was constructed by PCR amplification using the primers pGEX-ampDh3-S/A. The PCR product was digested and inserted into similarly digested pGEX-6p-1 to produce pGEX-6p-1-ampDh3. Then the plasmids were transformed into E. coli BL21(DE3) respectively. For protein production, bacteria were grown at 37 °C in LB medium to an OD600 of 0.6, shifted to 16 °C and cultivated for an additional 16 h with 0.5 mM IPTG. Harvested cells were disrupted by sonification and purified with the Ni-NTA column or GSTrap HP column (GE Healthcare) according to manufacturer’s instructions. The samples were concentrated and stored at −80°C until use.
Bacterial competition assay
Bacterial competition assays were performed as described previously (Russell et al., 2011), with minor modifications. Briefly, overnight cultures of relevant P. aeruginosa strains and the competitor strain containing pBBR1-MCS5 vector (conferring gentamicin resistance) were mixed in a 5:1 or 1:1 ratio, the mixture was serially diluted, spread on PIA or LB plates containing gentamicin and the CFU of the strains were measured by plate counts. For solid plate assay, 5 μL of this mixture was spotted on a nitrocellulose membrane placed on LB-LS 3% agar, for liquid assay the mixture was diluted into fresh LB at 1%. The assays were incubated for 24 h at 37°C, after which the cells were re-suspended in 1 mL LB broth and serially diluted, spread on PIA or LB plates containing gentamicin, and the final CFU was determined. The CFU rate or CFU/ml were calculated with appropriate dilution method and normalized with P. aeruginosa strains. For interspecies competition, the rate of P. aeruginosa: Prey is 5:1; for intraspecies competition, the rate of Donor: Recipient is 1:1.
Protein secretion assay
Secretion assays were performed according to described methods (Mougous et al., 2006). Briefly, overnight bacterial cultures were subcultured in LB and incubated until OD600 reached 0.9 at 37°C. A 0.5-mL culture was centrifuged and the cell pellet was resuspended in SDS-loading buffer; this whole-cell lysate sample was defined as cell. 2 mL of the culture was centrifuged, and the supernatant was filtered through a 0.22 μm PES filter (Millipore). For each 1 mL of culture supernatant, 180 μL of 100% trichloroacetic acid was added, and the fractions were incubated on ice for 4 h before centrifugation at 15,000 g and 4°C for 15 min. The resulting protein pellet was washed with ice-cold acetone three times. The supernatant protein pellets were re-suspended in SDS-loading buffer. All samples were normalized to the OD600 of the culture and volume used in preparation.
Western blot analysis
For western blots, samples were loaded and separated by SDS-PAGE. were transferred onto PVDF membranes (Millipore), and blocking with 5% milk for 1 hour at room temperature. The proteins were hybridized probed with primary antibody at room temperature for 2 hours, and further incubated with HRP-conjugated secondary antibodies in TBST buffer for 1 hour. The signal was detected by the use of an ECL Plus kit (GE Healthcare) following the manufacturer’s protocol.
GST Pull-down assay
For GST Pull-down assays, 100 μg purified GST or equal amount GST-AmpDh3 was mixed with 25 μL of pre-washed MagneGST™ glutathione beads slurry (Promega) for 2 h at 4 °C. Unbinding proteins were washed, and 1 mL of cell lysis, which expressed the indicated Flag-tag protein, was added and induced for 4 h at 4°C. The beads were sufficient washed, and retained proteins (Pull Down) and the input (Input) samples were separated by SDS-PAGE, and the Western bolt was carried out using a standard protocol.
Amidase activity assay
Amidase activity assays were performed according to described methods (Lee et al., 2013). The AmpDh3 or AmpDh3H146A were incubated with the NAM-tetrapeptide substrate in 20 mM phosphate buffer (pH 8.0), 150 mM NaCl, 100 μM ZnCl2 for 30 min at RT. The reaction was stopped by addition of 0.2% trifluoroacetic acid and was flash frozen until LC/MS analysis. The reaction mixture was separated and analyses with a C18 reversed-phase UPLC column (Acquity UPLC® HSS T3, 1.8 μm, 2.1×150 mm; Waters) on a Dionex Ultimate 3000 Rapid Separation UPLC system. Four-step-LC gradient consists of 100%A/0%B for 5 min, a 5-min linear gradient to 92%A/8%B, an 1-min linear gradient to 100%A/0%B, and then 100%A/0%B for 3 min with a flow rate of 0.4 mL/min (A = 0.1% formic acid in water; B = 0.1% formic acid in acetonitrile). MS instrumentation and conditions were reported previously (Lee et al., 2013).
E. coli toxicity assay
E. coli BL21(DE3) pLysS cells harbouring the expression plasmids were grown overnight in liquid LB shaking at 37°C. The optical density of the cells was determined and serially diluted in LB medium at ten-fold. A total of 5 μL of this bacterial dilution was spotted on LB-LS agar with the following concentrations of inducer. 0.03 mM IPTG for AmpD; 0.1 mM IPTG for AmpDh2; 0.03 mM IPTG for AmpDh3, 0.03 mM IPTG and 0.1 mM L-Arabinose for AmpDh3 and PA0808. Pictures were taken after 16 hours growth at 37°C.
For growth curves, E. coli BL21(DE3) pLysS cells harbouring the pET22b plasmids were grown overnight in liquid LB shaking at 37°C. Then the cultures were subcultured to a starting optical density at 600nm (OD600) at 0.1 in LB-LS. Cultures were grown to OD600 0.4 and induced with 0.03 mM IPTG. And the optical density was taken in 6 hours. E. coli BL21(DE3) pLysS cells harbouring the pET22b and pBAD22a plasmids were grown overnight in liquid LB shaking at 37°C. Then the cultures were subcultured to a starting optical density at 600nm (OD600) at 0.1 in LB-LS with 0.1 mM l-Arabinose. Cultures were grown to OD600 0.4 and induced with 0.03 mM IPTG. Growth was measured with optical density at the indicated time points. Samples for western blot analysis were taken 1 hour or the indicated time point after induction.
Subcellular fractionation
Subcellular fractionation assays were performed according to described methods (Imperi et al., 2009; Russell et al., 2011). E. coli BL21(DE3) harbouring expression vectors Peri-ampDh3H146A or pET22b-ampDh3H146A were grown overnight and sub-inoculated into LB. Cells were grown to an OD600 of 0.4 and induced with 0.03 mM IPTG for 1 hour. P. aeruginosa ΔPA0808 harbouring expression vectors pMMB67H-PA0808-Flag or pMMB67H-PA0808ss-Flag were grown overnight and sub-inoculated into LB. Cells were grown to an OD600 of 1.5 then 10 mL of cell culture were harvested, and resuspended in 500 μL of 20 mM PBS, pH 7.3, 20% sucrose, 2.5 mM EDTA. After 20 min incubation at room temperature, 500 μL ice-cold ddH2O was added and gently shaken for 5 min. The suspension was centrifuged (7,000 g for 20 min at 4°C) to collect the supernatant which containing periplasmic proteins. The pellet was resuspended in SDS-loading buffer; this sample was defined as cytoplasmic (Cyto). For each of supernatant, trichloroacetic acid was added and incubated on ice for 1 h before centrifugation. The resulting protein pellet was washed with ice-cold acetone. The supernatant protein pellets were re-suspended in SDS-loading buffer, this sample was defined as periplasmic (Peri).
Fluorescence microscopy
The bacterial were cultured as the growth curve experiments in E. coli toxicity assay. After 1 h induction, cells were harvested and re-suspended in PBS with 0.2 mM TMA-DPH (1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene p-toluenesulfonate) (Thermo Fisher Scientific) and incubated for 30 min. The stained cells were placed on 1% agarose pads and examined with a spinning-disk confocal system equipped with a CSU-W1 spinning-disk head (Yokogawa) and an iXon Ultra 888 EMCCD (Andor) on a DMi8 microscope body (Leica) with a HCX PL Apo 1.44 N.A. 100× oil immersion objective. Confocal images were processed with the Fiji ImageJ.
Self-intoxication assays
Self-intoxication assays. P. aeruginosa ΔretS and the mutant strains were grown overnight in LB. The optical density of the cells was determined and serially diluted in LB medium to OD600 at 0.001, the cells were spotted on a nitrocellulose membrane placed on LB-LS 3% agar. After incubated for 24 h at 37 °C, the cells were re-suspended in 1 mL LB broth and serially diluted, spread on LB plates, and the final CFU was determined. For P. aeruginosa growth curves, cells were grown overnight in LB. The optical density of the cells was determined and sub-inoculated 1:1,000 into LB-LS. Growth was measured with optical density at the indicated time points.
Supplementary Material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31622003, 31670080, and 31870060 to HL, and 31800127 to TW), China Postdoctoral Science Foundation (2018M641008 to TW) and Postdoctoral Science Research Plan in ShaanXi Province of China (2018BSHEDZZ244 to TW). The work in the USA was supported by grants from the National Institute of Health (GM61629 and GM131685 to SM). We thank other members of the Liang lab for providing reagents, general support and critical reading of the manuscript.
Footnotes
Data availability statement
The data that supports the findings of this study are available in the supplementary material of this article.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Allsopp LP, Wood TE, Howard SA, Maggiorelli F, Nolan LM, Wettstadt S, and Filloux A. (2017) RsmA and AmrZ orchestrate the assembly of all three type VI secretion systems in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 114: 7707–7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler M. (2015) Type VI secretion system: secretion by a contractile nanomachine. Philos Trans R Soc Lond B Biol Sci 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle LE, Bailey CM, and Pallen MJ (2008) Type VI secretion: a beginner’s guide. Curr Opin Microbiol 11: 3–8. [DOI] [PubMed] [Google Scholar]
- Boyer F, Fichant G, Berthod J, Vandenbrouck Y, and Attree I. (2009) Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? Bmc Genomics 10: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkinshaw BJ, Liang X, Wong M, Le ANH, Lam L, and Dong TG (2018) A type VI secretion system effector delivery mechanism dependent on PAAR and a chaperone-co-chaperone complex. Nat Microbiol 3: 632–640. [DOI] [PubMed] [Google Scholar]
- Carrasco-Lopez C, Rojas-Altuve A, Zhang W, Hesek D, Lee M, Barbe S. et al. (2011) Crystal structures of bacterial peptidoglycan amidase AmpD and an unprecedented activation mechanism. J Biol Chem 286: 31714–31722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales E, and Cambillau C. (2012) Structural biology of type VI secretion systems. Philos Trans R Soc Lond B Biol Sci 367: 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfanelli FR, Monlezun L, and Coulthurst SJ (2016) Aim, Load, Fire: The Type VI Secretion System, a Bacterial Nanoweapon. Trends Microbiol 24: 51–62. [DOI] [PubMed] [Google Scholar]
- Coulthurst S. (2019) The Type VI secretion system: a versatile bacterial weapon. Microbiology 165(5): 503–515. [DOI] [PubMed] [Google Scholar]
- Cox MJ, Allgaier M, Taylor B, Baek MS, Huang YJ, Daly RA et al. (2010) Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS One 5: e11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand E, Cambillau C, Cascales E, and Journet L. (2014) VgrG, Tae, Tle, and beyond: the versatile arsenal of Type VI secretion effectors. Trends Microbiol 22: 498–507. [DOI] [PubMed] [Google Scholar]
- Filloux A. (2013a) The rise of the Type VI secretion system. F1000Prime Rep 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filloux A. (2013b) Microbiology: a weapon for bacterial warfare. Nature 500: 284–285. [DOI] [PubMed] [Google Scholar]
- Gjodsbol K, Christensen JJ, Karlsmark T, Jorgensen B, Klein BM, and Krogfelt KA (2006) Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 3: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wang T, Chen G, Pu Q, Liu Q, Zhang Y. et al. (2019) A Pseudomonas aeruginosa type VI secretion system regulated by CueR facilitates copper acquisition. PLoS Pathog 15: e1008198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BT, Dong TG, and Mekalanos JJ (2014) A view to a kill: the bacterial type VI secretion system. Cell Host Microbe 15: 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, and Schweizer HP (1998) A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212: 77–86. [DOI] [PubMed] [Google Scholar]
- Imperi F, Ciccosanti F, Perdomo AB, Tiburzi F, Mancone C, Alonzi T. et al. (2009) Analysis of the periplasmic proteome of Pseudomonas aeruginosa, a metabolically versatile opportunistic pathogen. Proteomics 9: 1901–1915. [DOI] [PubMed] [Google Scholar]
- Jiang F, Waterfield NR, Yang J, Yang G, and Jin Q. (2014) A Pseudomonas aeruginosa type VI secretion phospholipase D effector targets both prokaryotic and eukaryotic cells. Cell Host Microbe 15: 600–610. [DOI] [PubMed] [Google Scholar]
- Juan C, Moya B, Perez JL, and Oliver A. (2006) Stepwise upregulation of the Pseudomonas aeruginosa chromosomal cephalosporinase conferring high-level beta-lactam resistance involves three AmpD homologues. Antimicrob Agents Chemother 50: 1780–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Zhao J, Kang H, Zhu M, Zhou T, Deng X, and Liang H. (2015) ChIP-seq reveals the global regulator AlgR mediating cyclic di-GMP synthesis in Pseudomonas aeruginosa. Nucleic Acids Res 43: 8268–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Artola-Recolons C, Carrasco-Lopez C, Martinez-Caballero S, Hesek D, Spink E. et al. (2013) Cell-wall remodeling by the zinc-protease AmpDh3 from Pseudomonas aeruginosa. J Am Chem Soc 135: 12604–12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesic B, Starkey M, He J, Hazan R, and Rahme LG (2009) Quorum sensing differentially regulates Pseudomonas aeruginosa type VI secretion locus I and homologous loci II and III, which are required for pathogenesis. Microbiology 155: 2845–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Deng X, Li X, Ye Y, and Wu M. (2014) Molecular mechanisms of master regulator VqsM mediating quorum-sensing and antibiotic resistance in Pseudomonas aeruginosa. Nucleic Acids Res 42: 10307–10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Zhang W, Cheng J, Yang X, Zhu K, Wang Y. et al. (2017) A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat Commun 8: 14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LS, Hachani A, Lin JS, Filloux A, and Lai EM (2014) Agrobacterium tumefaciens deploys a superfamily of type VI secretion DNase effectors as weapons for interbacterial competition in planta. Cell Host Microbe 16: 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano G, Trunk K, Williams DJ, Monlezun L, Strahl H, Pitt SJ, and Coulthurst SJ (2019) A family of Type VI secretion system effector proteins that form ion-selective pores. Nat Commun 10: 5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Caballero S, Lee M, Artola-Recolons C, Carrasco-Lopez C, Hesek D, Spink E. et al. (2013) Reaction products and the X-ray structure of AmpDh2, a virulence determinant of Pseudomonas aeruginosa. J Am Chem Soc 135: 10318–10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA et al. (2006) A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312: 1526–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya B, Juan C, Alberti S, Perez JL, and Oliver A. (2008a) Benefit of having multiple ampD genes for acquiring beta-lactam resistance without losing fitness and virulence in Pseudomonas aeruginosa. Antimicrob Agents Chemother 52: 3694–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya B, Juan C, Alberti S, Perez JL, and Oliver A. (2008b) Benefit of having multiple ampD genes for acquiring beta-lactam resistance without losing fitness and virulence in Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy 52: 3694–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien S, and Fothergill JL (2017) The role of multispecies social interactions in shaping Pseudomonas aeruginosa pathogenicity in the cystic fibrosis lung. FEMS Microbiol Lett 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BM, Jabra-Rizk MA, O’May GA, Costerton JW, and Shirtliff ME (2012) Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev 25: 193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AB, Peterson SB, and Mougous JD (2014) Type VI secretion system effectors: poisons with a purpose. Nat Rev Microbiol 12: 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AB, Hood RD, Bui NK, LeRoux M, Vollmer W, and Mougous JD (2011) Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475: 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AB, LeRoux M, Hathazi K, Agnello DM, Ishikawa T, Wiggins PA et al. (2013) Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature 496: 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D, Kinch LN, Trudgian DC, Guo X, Klimko JA, Grishin NV et al. (2014) Marker for type VI secretion system effectors. Proc Natl Acad Sci U S A 111: 9271–9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneider MM, Buth SA, Ho BT, Basler M, Mekalanos JJ, and Leiman PG (2013) PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature 500: 350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si M, Zhao C, Burkinshaw B, Zhang B, Wei D, Wang Y. et al. (2017) Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc Natl Acad Sci U S A 114: E2233-E2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JM, Brunet YR, Cascales E, and Mougous JD (2012) Structure and regulation of the type VI secretion system. Annu Rev Microbiol 66: 453–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JM, Agnello DM, Zheng H, Andrews BT, Li M, Catalano CE et al. (2013) Haemolysin coregulated protein is an exported receptor and chaperone of type VI secretion substrates. Mol Cell 51: 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ et al. (2000) Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406: 959–964. [DOI] [PubMed] [Google Scholar]
- Tang JY, Bullen NP, Ahmad S, and Whitney JC (2017) Diverse NADase effector families mediate interbacterial antagonism via the type VI secretion system. J Biol Chem 293: 1504–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan B, Zhang Q, Ni J, Li S, Wen D, Li J. et al. (2017) Type VI secretion system contributes to Enterohemorrhagic Escherichia coli virulence by secreting catalase against host reactive oxygen species (ROS). PLoS Pathog 13: e1006246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Si M, Song Y, Zhu W, Gao F, Wang Y. et al. (2015) Type VI Secretion System Transports Zn2+ to Combat Multiple Stresses and Host Immunity. PLoS Pathog 11: e1005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstadt S, Wood TE, Fecht S, and Filloux A. (2019) Delivery of the Pseudomonas aeruginosa Phospholipase Effectors PldA and PldB in a VgrG- and H2-T6SS-Dependent Manner. Front Microbiol 10: 1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JC, Chou S, Russell AB, Biboy J, Gardiner TE, Ferrin MA et al. (2013) Identification, structure, and function of a novel type VI secretion peptidoglycan glycoside hydrolase effector-immunity pair. J Biol Chem 288: 26616–26624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JC, Beck CM, Goo YA, Russell AB, Harding BN, De Leon JA et al. (2014) Genetically distinct pathways guide effector export through the type VI secretion system. Mol Microbiol 92: 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Lee M, Hesek D, Lastochkin E, Boggess B, and Mobashery S. (2013) Reactions of the three AmpD enzymes of Pseudomonas aeruginosa. J Am Chem Soc 135: 4950–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.