Abstract
PubMed, Scopus, and ISI Web of Knowledge databases were searched to identify studies published up to December 2020 on the involvement of urinary and male genital systems in COVID‐19. Sixteen studies involving a total of 575 patients (538 males and 37 females) were included in this systematic review. The COVID‐19 phase was available for 479 patients: 426 in the acute and 53 in the recovery phase. De novo lower urinary tract symptoms (LUTS) were observed in 43 patients and deterioration of pre‐existing LUTS in 7. Bladder hemorrhage was observed in three patients and acute urinary retention in one. Regarding the male genital system, scrotal discomfort was observed in 8 patients, swelling in 14, pain in 16, and erythema in 1; low flow priapism was observed in 2 patients. Ultrasound examination identified acute orchitis in 10 patients, acute epididymitis in 7, and acute epididymo‐orchitis in 16. A case–control study reported that patients with moderate COVID‐19 show a significant reduction in sperm concertation, the total number of sperms per ejaculate, progressive motility, and complete motility. In contrast to what is known from the first studies on the subject, this review also includes subsequent studies that give evidence of the involvement of the lower urinary tract and male genital system in COVID‐19.
Keywords: coronavirus, disease control, COVID‐19, infection, genital tract, SARS‐CoV‐2, urinary tract
1. INTRODUCTION
The COVID‐19 pandemic is one of the greatest recorded catastrophes in history, and has affected millions of human victims, severely limited the freedom of billions of people for long periods, and led to economic collapse in many countries; and everything is still going on. 1 , 2 , 3 , 4 However, doctors, researchers, politicians, and women and men of goodwill, each according to their abilities, have been able to give a concrete answer to this global disaster. In fact, about a year after its onset, basic research and clinical trials have greatly expanded knowledge on SARS‐CoV‐2 infection and the associated disease called COVID‐19, patients are treated more effectively and most patients recover, the spread of the infection has been contained in many countries, and some vaccines against this virus have become available.
SARS‐CoV‐2 belongs to the β‐coronavirus cluster. 1 , 5 Entry of SARS‐CoV‐2 into host cells depends on cellular expression of both angiotensin‐converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2). 1 , 6 , 7 , 8 , 9 , 10 Zou et al. analyzed the RNA sequencing datasets of cells of major human physiological systems to evaluate the expression of the ACE2 receptor and constructed a risk map of the different vulnerability of various organs to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 11 This physiological variability is a biological background that may explain the different involvement of individual organs or tissues in the clinical presentation of COVID‐19. Evidence has recently emerged that the lower urinary tract is a potential target for SARS‐CoV‐2 infection, due to a significant ACE2 expression in urothelial cells. 11 ACE2 has also been found in the human testis where it regulates the physiology of Leydig cells, Sertoli cell, and spermatogonia. 12 In addition, Song et al. 13 analyzed epithelial cells of the normal human prostate and found that ACE2 and TMPRSS2 are expressed in 0.32% and 18.65% of epithelial cells, respectively. Therefore, the urinary and male genital system is now regarded as at risk for SARS‐CoV‐2 infection and potentially responsible for some nonrespiratory symptoms of COVID‐19. 7 The signs and symptoms relative to the involvement of the urinary and male genital systems are sometimes vague, and in any case, little known, therefore clinicians should pay particular attention to detecting them in their patients. 14 , 15
The purpose of this systematic review is to highlight currently available literature data on the involvement of the urinary and male genital systems in SARS‐CoV‐2 infection, to offer young specialists in urology and infectious diseases an overview on this topic to better carry out their activity in this new clinical reality and, hopefully, develop the desire to perform in‐depth studies and research on the subject.
2. METHODS
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement. 16
2.1. Literature search
A comprehensive literature search was performed using a combination of keywords (MeSH terms and free text words) including “COVID‐19”/”SARS‐CoV‐2,” “Urology”/“Urogenital System,” “signs,” “symptoms,” “laboratory,” and “radiology.” Three databases (PubMed, Scopus, and ISI Web of Knowledge) were searched for articles published in English up to December 2020. Additional articles were sought from the reference lists of the included studies.
2.2. Selection criteria
All articles identified from the literature search were screened by two independent reviewers (M. C. and N. L.) with any discrepancies resolved by a third author (C. S.). To assess the eligibility for the systematic review, PICOS (participants, intervention, comparisons, outcomes, study type) criteria were used. PICOS criteria were set as follows: (P)articipants—the subjects infected with SARS‐CoV‐2; (I)ntervention—the evaluation of signs, symptoms, laboratory or radiological findings relative to the urinary and/or male genital tract; (O)utcome—the evidence of signs and/or symptoms and/or laboratory findings and/or radiological evaluations indicative of male genital and/or urinary tract involvement; (S)tudy types—prospective and retrospective studies, review articles, meta‐analysis, case series, case reports, letters to editors; studies related to nephrology, obstetrics, and gynecology were not included.
2.3. Data collection
The following data were extracted from eligible studies: authors, year of publication, study period, study design, sample size, site of the study, patients' age and gender, disease state (acute vs. recovery), results of rhino‐pharyngeal swab, SARS‐CoV‐2 antibody status, COVID‐19 severity, signs and/or symptoms regarding the lower urinary tract or the male genital tract, symptom scores, findings from radiological and/or laboratory investigations regarding the lower urinary tract or the male genital tract, strategies adopted to manage these conditions.
3. RESULTS
The search strategy revealed a total of 339 results. Screening of the titles and abstracts revealed 25 papers eligible for inclusion. Further assessment of eligibility, based on full‐text papers, led to the exclusion of nine papers. Finally, 16 studies involving a total of 575 patients infected with SARS‐CoV‐2 who had been evaluated for urinary and/or male genital involvement between January and June 2020 were included in the final analysis 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 (Figure 1). The characteristics of selected studies are summarized in Table 1.
Figure 1.
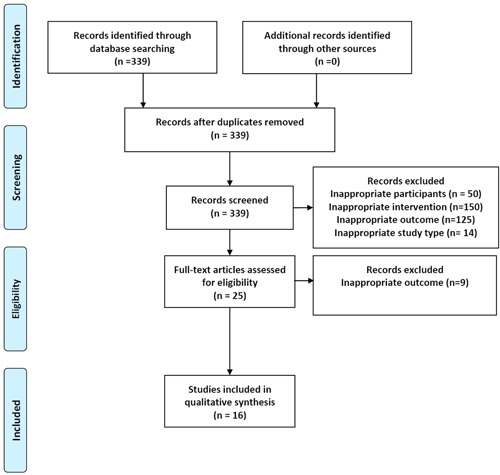
Flow diagram of the systematic review
Table 1.
Study characteristics, demographic, and clinical characteristics of patients included
| Studies [ref.] | Study design | Time frame | City and country | No. of COVID‐19 patients | Age (years), mean (SD) | Sex (M:F) |
Disease phase, n |
Positive rinopharyngel swab, n |
Positive antibody status, n |
COVID‐19 severity, n |
|---|---|---|---|---|---|---|---|---|---|---|
| Holtmann et al. 17 | CC | April–May 2020 | Duesseldorf, | 14 | 42.7 (10.4) | 14:0 | Recovery (18) | 17 | IgA: 17 | Mild (14) |
| Holtmann et al. 17 | Germany | 4 | 40.8 (8.7) | 4:0 | IgG: 16 | Moderate (4) | ||||
| Pan et al. 18 | CS | January –March 2020 | Wuhan, China | 34 | 37 (31–49)a | 34:0 | Recovery (34) | 34 | n/a | n/a |
| Guo et al. 19 | CS | February –April 2020 | Shandong, China | 23 | 41.04 (11.56) | 23:0 | Acute (23) | 23 |
IgM: 9 IgG: 22 |
Mild (18) Moderate (5) |
| Chen et al. 20 | R | February –March 2020 | Wuhan, China | 83 | 54.2 (38.0–69.0)a | 83:0 | Acute (83) | 83 | n/a | Mild and moderate (83) |
| Chen et al. 20 | 59 | 64.0 (47.0–78.0) a | 59:0 | Acute (59) | 59 | Severe and critical (59) | ||||
| Bridwell et al. 21 | CR | n/a | USA | 1 | 37 (–) | 1:0 | Acute (1) | 1 | n/a | n/a |
| Gagliardi et al. 22 | CR | n/a | Versilia, Italy | 1 | 14 (–) | 1:0 | Acute (1) | 1 | n/a | n/a |
| Lamamri et al. 23 | CR | n/a | Le Chesnay, France | 1 | 62 (–) | 1:0 | Acute (1) | 1 | n/a | Critical (1) |
| La Marca et al. 24 | CR | April 2020 | Modena, Italy | 1 | 43 (–) | 1:0 | Acute (1) | 1 | n/a | Critical (1) |
| Kim et al. 25 | CR | n/a | USA | 1 | 42 (–) | 1:0 | Acute (1) | n/a | n/a | n/a |
| Lamb et al. 26 | CC | n/a | MI, USA | 4 | 68.2 (–) | 1:3 | n/a | 4 | n/a | n/a |
| Mumm et al. 15 | CS | March–April 2020 | Munich, Germany | 7 | 62 (59–78)a | 7:0 | Acute (1) | 7 | n/a | n/a |
| Luciani et al. 27 | CS | February –March 2020 | Trento, Italy | 3 | 74 (9.1) | 3:0 | Acute (1) | 3 | n/a | n/a |
| Dhar et al. 28 | CS | May–June 2020 | MI, USA | 39 | 63.5 (n/a) | 32:7 | Recovery (1) | 39 | n/a | n/a |
| Kaya et al. 29 | CS | May–June 2020 | Turkey | 19 | 38.9 (13) | 19:0 | n/a | n/a | n/a | n/a |
| Kaya et al. 23 | 27 | 32.3 (8.9) | 0:27 | |||||||
| Lam et al. 30 | CR | March 2020 | Pembrokeshire, UK | 1 | 67 (–) | 1:0 | Acute (1) | n/a | n/a | Severe (1) |
| Alkhatatbeh et al. 31 | R | March– May 2020 | Amman, Jordan | 253 | 43 (n/a) | 253:0 | Acute (253) | 253 | n/a |
Asymptomatic (53) Mild (152) Severe (36) Critical (12) |
Abbreviations: CC, case control; CR, case report; CS, case series; n/a, not available; R, retrospective; SD, standard deviation.
Median (range).
3.1. Patients' demographics and COVD‐19 characteristics
Patients' demographics are reported in Table 1. Overall, 199 patients were from China, 78 from Europe, 45 from the United States, and 253 from Jordan. The COVID‐19 phase was available for 479 patients: 426 in the acute and 53 in the recovery phase. COVID‐19 severity was available for 439 patients, but it was classified differently by the authors. The patients classified as asymptomatic or with mild, moderate, severe, and critical disease were 53, 166, 9, 37, and 14, respectively; Chen et al., however, merged patients with mild/moderate disease (83 cases) and those with severe and critical disease (59 cases). 20
3.2. Urinary tract involvement in COVID‐19 patients
Urinary tract involvement was reported in five studies (Table 2). 26 , 27 , 28 , 29 In detail, de novo lower urinary tract symptoms (LUTS) and deterioration of pre‐existing LUTS occurred in 43 and 7 patients, respectively. Validated questionnaires to score LUTS were adopted in two studies. 28 , 29 Dhar et al. 28 scored symptoms according to the Overactive Bladder Symptom Score and found a median overactive bladder symptom score of 18 in both men and women. Kaya et al. 29 adopted the International Prostate Symptom Score (IPSS) and the Urinary Symptom Profile (USP) for men and women, respectively and found, both in men and women, a statistically significant worsening in terms of both mean IPSS and mean UPS stress urinary incontinence/overactive bladder subscores.
Table 2.
Studies describing urinary tract involvement
| Studies [Ref.] |
Pre‐existing urological conditions type (no./totala) |
Time from exposure/diagnosis to onset of symptoms (days) mean (range) |
Signs/symptoms type (no./totala) |
Symptom score | Laboratory findings | Management | |
|---|---|---|---|---|---|---|---|
|
Urinalysis pathologic finding type no./totalb |
Urine culture findings type no./totalb |
||||||
| Lamb et al. 26 | n/a | n/a |
De novo urgency (4/4) De novo urge incontinence (4/4) De novo frequency (4/4) De novo nocturia (4/4) |
n/a | n/a | n/a | n/a |
| Mumm et al. 15 |
BPH (1/7) |
n/a | Increased urinary frequency (7/7) | n/a |
Microhematuria (3/7) Leukocyturia (2/4) |
Negative (6/6) | n/a |
| Luciani et al. 27 |
Radiation cystitis (1/3) BPH (2/3) |
6.3 (5‐8) |
Hematuria (3/3) Urinary retention (1/3) |
n/a | n/a | n/a |
Endoscopy (1/3) Embolization (1/3) Conservative (1/3) |
| Dhar et al. 28 | n/a | n/a |
De novo urgency (39/39) De novo urge incontinence (39/39) De novo frequency (39/39) De novo nicturia (39/39) |
n/a | Negative (39/39) | n/a | |
| Kaya et al. 29 | n/a | n/a | n/a |
IPSS total Pre‐COVID‐19: 6.1 (7.3)e During hospitalization: 6.2 (7.5)e Post hospitalization: 5.7 (7.2)e (p : .148) IPSS storage Pre‐COVID‐19: 3.2 (4.1)e During hospitalization: 3.2 (4.3)e Post hospitalization: 2.8 (4)e (p : .054) IPSS voiding Pre‐COVID‐19: 2.9 (3.5)e During hospitalization: 3.1 (3.5)e Post hospitalization: 2.9 (3.4)e (p : .933) |
n/a | n/a | n/a |
| Kaya et al. 29 | n/a | n/a | n/a |
USP Scale (stress urinary incontinence) Pre‐COVID‐19: 0.5 (1.9)e During hospitalization: 0.7 (1.9)e Post hospitalization: 0.5 (1.9)e (p : .05) USP Scale (overactive bladder) Pre‐COVID‐19: 2.2 (2.9)e During hospitalization: 2.3 (3)e Post hospitalization: 1.9 (2.6)e (p : .051) USP Scale (slow current) Pre‐COVID‐19: 0.1 (0.4)e During hospitalization: 0 (0)e Post hospitalization: 0 (0)e (p : .368) |
n/a | n/a | n/a |
Abbreviations: BPH, benign prostate hyperplasia; IPSS, International Prostate Symptom Score; USP, urinary symptom profile.
Total patients with signs and/or symptoms and/or laboratory findings of urinary tract involvement.
Total patients for whom the laboratory evaluation was available.
Overactive Bladder symptom score.
Median (range).
Mean (standard deviation).
Other signs of lower urinary tract involvement were bladder hemorrhage in three patients and acute urinary retention in one. 27 Microhematuria and leukocyturia were reported in three and two patients, respectively. Urine culture was negative in all 45 patients investigated. Radiologic data were not available in these five studies.
3.3. Male genital tract involvement in COVID‐19 patients
Male genital tract involvement was investigated in 11 studies 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 30 , 31 (Table 3). Scrotal discomfort was described in 8 patients, swelling in 14, pain in 16, and erythema in 1. Low flow priapism was reported in 2 patients. 23 , 30 Scrotal ultrasound was performed in 4 studies 20 , 21 , 22 , 24 : acute orchitis was found in 10 patients, acute epididymitis in 7, and acute epididymo‐orchitis in 16 20 , 22 , 24 ; one case of bilateral nonspecific intratesticular increased blood flow was also described. 21
Table 3.
Studies describing male genital tract involvement
| Study [Ref.] | Prior andrological conditions, (n) | Signs or symptoms, (n) | Ultrasound data, (n) | Laboratory data | Management |
|---|---|---|---|---|---|
| Holtmann et al. 17 | 0 | ‐ | ‐ | Sperm concentration, mean (SD): 95.9 (50.5) 106/ml* | n/a |
| Total no. progressive motility (SD) (×106): 125.3 (96.4)* | |||||
| Total no. complete motility (SD) (×106): 157.1 (120.8)* | |||||
| Total no. immotile (SD) (×106): 86.6 (66.5)* | |||||
| Holtmann et al. 17 | 0 | Scrotal discomfort (1) | ‐ | Sperm concentration, mean (SD): 16.2 (22.4) 106/ml** | n/a |
| Progressive motility (×106): 2.4 (2.7)** | |||||
| Total no. complete motility (SD) (×106): 4.7 (5.5)** | |||||
| Total no. immotile (SD) (×106):7.2 (9.4)** | |||||
| Pan et al. 18 | 0 | Scrotal discomfort (6) | ‐ | n/a | n/a |
| Guo et al. 19 | n/a | n/a | n/a | Semen volume (ml): 2.3 (1.35–3.0) | n/a |
| Sperm concentration: 95 (56–155.5) 106/ml | |||||
| Motility (PR, %): 50 (37.5) | |||||
| Motility (PR + NP, %): 65(57.5–76) | |||||
| Normal forms (%):16 (12–22) | |||||
| Chen et al. 20 | n/a | Scrotal swelling/pain (3) | Acute orchitis (3) | n/a | n/a |
| Acute epididymitis (3) | |||||
| Epididymo‐orchitis (5) | |||||
| Chen et al. 20 | n/a | Scrotal swelling/pain (10) | Acute orchitis (7) | n/a | n/a |
| Acute epididymitis (4) | |||||
| Epididymo‐orchitis (10) | |||||
| Bridwell et al. 14 | n/a | Scrotal erythema (1) | Bilateral nonspecific increased blood flow (1) | Urinalysis: unremarkable | Acetaminophen + cefepime + azithromycin |
| Scrotal discomfort (1) | |||||
| Gagliardi et al. 15 | n/a | Scrotal pain and swelling (1) | Epididymo‐orchitis (1) | Urinalysis: unremarkable | Broad‐spectrum antibiotics |
| Urine culture: sterile | |||||
| Lamamri et al. 16 | n/a | Low flow priapism (1) | n/a | n/a | Cavernosal blood aspiration + intracavernosal injection of ethylephrine + thromboprophylaxis |
| Lam et al. 31 | Paraphimosis (1) | Low flow priapism (1) | n/a | n/a | Conservative management |
| La Marca et al. 24 | n/a | Scrotal pain (1) | Epididymo‐orchitis (1) | n/a | Amoxicillin/clavulanic acid + azithromycin |
| Kim et al. 25 | n/a | Scrotal pain (1) | n/a | n/a | Cefpodoxime + azithromycin |
| Alkhatatbeh et al. 32 | 0 | 0 | 0 | n/a | n/a |
P < .05: mild vs. moderate.
P < .05: moderate vs. control.
Alkhatatbeh et al. 22 observed 253 COVID‐19 male patients and failed to observe symptoms or signs of orchitis across all age groups and different disease status. Two studies investigated spermatogenesis in patients with COVID‐19 infection. 17 , 19 Holtmann et al. investigated semen parameters in patients with mild or moderate COVID‐19 infection after a mean period of 43.5 and 47.0 days from diagnosis, respectively. As compared with healthy controls and with patients with mild disease, those with a moderate COVID‐19 infection showed a statistically significant reduction in sperm concertation, the total number of sperms per ejaculate, progressive motility, and complete motility, whereas no statistically significant difference was found between controls and patients with mild disease. 17 Accordingly, Guo et al. 19 found total sperm count, total motile sperm count, and sperm morphology within normal ranges in a population mainly composed of patients with mild COVID‐19, tested after a median interval of 32 days from diagnosis.
4. DISCUSSION
The different expression in ACE2 and TMPRSS2 in human tissues is one of the main reasons for their different involvement in COVID‐19. Although not considered among the systems most frequently and/or intensely affected by SARS‐CoV‐2 infection, the lower urinary tract and the male genital systems have nevertheless been identified by recent studies as part of COVID‐19 pathology. It follows that urologists and infectious disease specialists should become aware of the recent evidence on this topic, to fully experience their clinical and research activity in this sector as well. Chan et al. 32 have systematically reviewed the data available in literature until April 2020 and, not having found the presence of patients with urogenital symptoms among those included in their analysis, concluded that involvement of the urogenital system in COVID‐19 was unlikely. Subsequently, the persistence of the SARS‐CoV‐2 pandemic has stimulated further research on the topic, and this has led us to carry out an updated systematic review ensuring a timely systematic review of the available evidence. The results of this systematic review demonstrate that the male genital system and the lower urinary tract are both involved in COVID‐19.
De novo LUTS or worsening of pre‐existing LUTS represent the most common involvement of the lower urinary tract in COVID‐19, with storage symptoms being the most remarkable complaints. Although considered multifactorial by some authors, 33 , 34 , 35 the pathogenesis of LUTS has not yet been elucidated. 34 , 35 Theoretically, the bladder could become infected with SARS‐CoV‐2 by the hematogenous route or by the propagation from urethral cells, where the presence of ACE2 receptors has been demonstrated. 15 However, if it is not fully understood which cells, luminal or basal urothelial, effectively express ACE2 receptor, 15 SARS‐CoV‐2 has been infrequently detected in the urine of infected individuals. A systematic review of the literature considering urine samples from 533 patients from 14 studies found evidence of SARS‐CoV‐2 in only 24 patients (4.5%). 35 The infrequent presence of SARS‐CoV‐2 in urine samples from infected subjects has been interpreted as a sign in favor of the spread of the virus from the urethral endothelium to the bladder. 15 Some authors have recently hypothesized that, in patients with COVID‐19 and de novo severe urinary symptoms, an increase in inflammatory cytokines released into the urine and/or active in the bladder may be responsible for COVID‐19 Associated Cystitis (CAC) and of the associated bladder dysfunctions. 26
Recent literature data have reported that patients with CAC, both men and women, frequently refer to de novo lower urinary tract symptoms such as an increase in urinary frequency and nocturia, stressing the need that de novo urinary symptoms should be considered among the complex symptomatology of COVID‐19. Therefore, physicians caring for COVID‐19 patients, in ambulatory care, clinical wards, or emergency rooms, should be aware of CAC.
Noteworthily, it has been also underlined that an increase in urinary frequency, in addition to fever, should be considered an important symptom of overlap with urosepsis in the context of the differential diagnosis of COVID‐19. 15
Scrotal discomfort or pain with radiological evidence of inflammatory changes of the testis and/or epididymis is the most frequently described male genital complaints in patients with SARS‐CoV‐2 infection. The pathogenesis of orchitis relies on the hematogenous spreading of SARS‐CoV2 to testis tissue, where ACE2 is expressed in the Leydig cells, Sertoli cells, and spermatogonia. 20 Postmortem studies of patients who had died of severe acute respiratory syndrome (SARS) showed the presence of orchitis with histological evidence of immune‐mediated inflammatory damage. 36 Alkhatatbeh et al., 31 however, failed to observe any symptoms or signs of orchitis in their series, but the relatively small sample size might have failed to capture a rare complication; in addition, most patients in this study were asymptomatic or had mild‐to‐moderate symptoms and the authors hypothesized that viral threshold required to cross the blood–testis barrier could be not achieved. 31 The involvement of the male genital system in patients with SARS‐CoV‐2 infection may theoretically impair fertility. Available evidence demonstrates that although a mild COVID‐19 infection is not likely to affect spermatogenesis, semen can be impaired after a moderate infection. 17 However, data about semen analysis performed before the outbreak of the pandemic were not available for patients involved in these studies thus limiting the diagnosis of pre‐existing male infertility. 17 Moreover, the long‐term effects of SARS‐CoV‐2 on male reproductive function are lacking. 17
Low flow priapism was observed in two patients with severe COVID‐19. 23 , 30 Once recognized by health‐care professionals, low flow priapism should be treated promptly to prevent immediate and chronic functional complications. 23 , 30 The pathogenesis of priapism in patients with COVID‐19 has been attributed to thrombotic complications. The reporting of further cases would be of interest to strengthen this evidence. 23 , 30
We recognize the limitations of the data published so far on the involvement of the urogenital system by SARS‐CoV‐2, coming from a few studies, often carried out with low methodological quality, and including few patients, often heterogeneous and with short follow‐up. Furthermore, demographics, epidemiological data, and symptoms were recorded in precoded questionnaires only in a few studies. As a result, available data on the involvement of urologic and male genital systems in COVID‐19 are sparse and patchy. However, unlike what emerged during the first wave of the pandemic, we now know that this involvement occurs in 3%–5% of cases with SARS‐CoV‐2 infection and that in some cases the clinical manifestations are relevant.
5. CONCLUSIONS
The data highlighted in this systematic review demonstrate that patients with COVID‐19 may have signs, symptoms, and radiological and laboratory features indicative of involvement of the lower urinary tract and of the male genital system. De novo or worsening LUTS, and testis and/or epididymal discomfort or pain are the most common clinical findings. Moreover, spermatogenesis can be impaired in patients with moderate infection. Current knowledge is therefore sufficient to alert all health‐care professionals involved in the management of patients with SARS‐CoV‐19 infection to focus their attention also on the lower urinary tract and male genital system.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization: Massimiliano Creta, Caterina Sagnelli, Giuseppe Celentano, and Luigi Napolitano. Methodology: Massimiliano Creta, Caterina Sagnelli, Giuseppe Celentano, and Luigi Napolitano. Validation: Massimiliano Creta, Caterina Sagnelli, Giuseppe Celentano, Luigi Napolitano, Roberto La Rocca, Marco Capece, Gianluigi Califano, Armando Calogero, Antonello Sica, Francesco Mangiapia, Massimo Ciccozzi, Ferdinando Fusco, Vincenzo Mirone, Evangelista Sagnelli, and Nicola Longo. Data curation: Massimiliano Creta, Caterina SagGiuseppe Celentanonelli, Giuseppe Celentano, Luigi Napolitano, Roberto La Rocca, Marco Capece, Gianluigi Califano, Armando Calogero, Antonello Sica, Francesco Mangiapia, Massimo Ciccozzi, Ferdinando Fusco, Vincenzo Mirone, Evangelista Sagnelli, and Nicola Longo. Writing—original draft preparation: Massimiliano Creta, Caterina Sagnelli, Giuseppe Celentano, and Luigi Napolitano. Writing—review and editing: Massimiliano Creta, Caterina Sagnelli, Giuseppe Celentano, and Luigi Napolitano, and Evangelista Sagnelli. Supervision: Massimiliano Creta, Caterina Sagnelli, Giuseppe Celentano, and Luigi Napolitano. All authors have read and agreed to the published version of the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.26883.
Creta M, Sagnelli C, Celentano G, et al. SARS‐CoV‐2 infection affects the lower urinary tract and male genital system: A systematic review. J Med Virol. 2021;93:3133–3142. 10.1002/jmv.26883
Massimiliano Creta, Caterina Sagnelli, and Giuseppe Celentano equally contributed to this work.
REFERENCES
- 1. Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin‐converting enzyme 2 (ACE2), SARS‐CoV‐2 and the pathophysiology of coronavirus disease 2019 (COVID‐19). J Pathol. 2020;251(3):228‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sica A, Casale D, Rossi G, et al. The impact of the SARS‐CoV‐2 infection, with special reference to the hematological setting. J Med Virol. 2020;93:223‐233. 10.1002/jmv.26197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sagnelli C, Gentile V, Tirri R, et al. Vanvitelli COVID‐19 group. Chronic conventional disease‐modifying anti‐rheumatic drugs masking severe SARS‐CoV‐2 manifestations in an elderly rheumatic patient. J Infect. 2020;81(6):979‐997. 10.1016/j.jinf.2020.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macera M, De Angelis G, Sagnelli C, Coppola N. Vanvitelli Covid‐group. Clinical presentation of COVID‐19: case series and review of the literature. Int J Environ Res Public Health. 2020;17(14):5062. 10.3390/ijerph17145062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ciotti M, Angeletti S, Minieri M, et al. COVID‐19 outbreak: an overview. Chemotherapy. 2019;64(5‐6):215‐223. 10.1159/000507423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peluso G, Campanile S, Scotti A, et al. COVID‐19 and living donor kidney transplantation in Naples during the pandemic. BioMed Res Int. 2020;2020:5703963‐5703964. 10.1155/2020/5703963 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7. Sagnelli C, Celia B, Monari C, et al. Management of SARS‐CoV‐2 pneumonia. J Med Virol. 2020;93:1276‐1287. 10.1002/jmv.26470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mussini C, Falcone M, Nozza S, et al. Therapeutic strategies for severe COVID‐19: a position paper from the Italian Society of Infectious and Tropical Diseases (SIMIT). Clin Microbiol Infect. 2020:S1198‐743X(20)30770‐9. 10.1016/j.cmi.2020.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020;14(2):185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verma S, Saksena S, Sadri‐Ardekani H. ACE2 receptor expression in testes: implications in coronavirus disease 2019 pathogenesis. Biol Reprod. 2020;103(3):449‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song H, Seddighzadeh B, Cooperberg MR, Huang FW. Expression of ACE2, the SARS‐CoV‐2 receptor, and TMPRSS2 in prostate epithelial cells. Eur Urol. 2020;78(2):296‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sighinolfi MC, Rocco B, Mussini C. COVID‐19: importance of the awareness of the clinical syndrome by urologists. Eur Urol. 2020;78(1):e40‐e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mumm JN, Osterman A, Ruzicka M, et al. Urinary frequency as a possibly overlooked symptom in COVID‐19 patients: does SARS‐CoV‐2 cause viral cystitis? Eur Urol. 2020;78(4):624‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holtmann N, Edimiris P, Andree M, et al. Assessment of SARS‐CoV‐2 in human semen‐a cohort study. Fertil Steril. 2020;114(2):233‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan F, Xiao X, Guo J, et al. No evidence of severe acute respiratory syndrome‐coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113(6):1135‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo L, Zhao S, Li W, et al. Absence of SARS‐CoV‐2 in semen of a COVID‐19 patient cohort. Andrology. 2020;9:42‐47. 10.1111/andr.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen L, Huang X, Yi Z, et al. Ultrasound imaging findings of acute testicular infection in patients with coronavirus disease 2019: a single‐center‐based study in Wuhan China. J Ultrasound Med. 2020:jum.15558. [DOI] [PubMed] [Google Scholar]
- 21. Bridwell RE, Merrill DR, Griffith SA, Wray J, Oliver JJ. A coronavirus disease 2019 (COVID‐19) patient with bilateral orchitis: a case report. Am J Emerg Med. 2020:S0735‐6757(20)30761‐0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gagliardi L, Bertacca C, Centenari C, et al. Orchiepididymitis in a boy with COVID‐19. Pediatr Infect Dis J. 2020;39(8):e200‐e202. [DOI] [PubMed] [Google Scholar]
- 23. Lamamri M, Chebbi A, Mamane J, et al. Priapism in a patient with coronavirus disease 2019 (COVID‐19): a case report. Am J Emerg Med. 2020;39:251.e5‐251.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. La Marca A, Busani S, Donno V, Guaraldi G, Ligabue G, Girardis M. Testicular pain as an unusual presentation of COVID‐19: a brief review of SARS‐CoV‐2 and the testis. Reprod Biomed Online. 2020;41(5):903‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim J, Thomsen T, Sell N, Goldsmith AJ. Abdominal and testicular pain: an atypical presentation of COVID‐19. Am J Emerg Med. 2020;38(7):1542.e1‐1542.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lamb LE, Dhar N, Timar R, Wills M, Dhar S, Chancellor MB. COVID‐19 inflammation results in urine cytokine elevation and causes COVID‐19 associated cystitis (CAC). Med Hypotheses. 2020;145:110375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luciani LG, Gallo F, Malossini G. Re: Jan‐Niclas Mumm, Andreas Osterman, Michael Ruzicka, et al. Urinary frequency as a possible overlooked symptom in COVID‐19 patients: does SARS‐CoV‐2 cause viral cystitis? Eur Urol. 2020;78(3):e129‐e130. 10.1016/j.eururo.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dhar N, Dhar S, Timar R, Lucas S, Lamb LE, Chancellor MB. De novo urinary symptoms associated with COVID‐19: COVID‐19‐associated cystitis. J Clin Med Res. 2020;12(10):681‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaya Y, Kaya C, Kartal T, Tahta T, Tokgöz VY. Could LUTS be early symptoms of COVID‐19. Int J Clin Pract. 2020:e13850. 10.1111/ijcp.13850 [DOI] [PubMed] [Google Scholar]
- 30. Lam G, McCarthy R, Haider R. A Peculiar case of priapism: the hypercoagulable state in patients with severe COVID‐19 infection. Eur J Case Rep Intern Med. 2020;7(8):001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alkhatatbeh H, Alzaghari D, Alkhashman A, Azab M, Edwan GMA, Abufaraj M. Does severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) cause orchitis in patients with coronavirus disease 2019 (COVID‐19)? Arab J Urol. 2020;18(3):129‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan VW, Chiu PK, Yee CH, Yuan Y, Ng CF, Teoh JY. A systematic review on COVID‐19: urological manifestations, viral RNA detection and special considerations in urological conditions. World J Urol. 2020:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Creta M, Bottone F, Sannino S, et al. Effects of alpha1‐blockers on urodynamic parameters of bladder outlet obstruction in patients with lower urinary tract symptoms suggestive of benign prostatic enlargement: a review. Minerva Urol Nefrol. 2016;68(2):209‐221. [PubMed] [Google Scholar]
- 34. Fusco F, Creta M, Imperatore V, et al. Benign prostatic obstruction relief in patients with lower urinary tract symptoms suggestive of benign prostatic enlargement undergoing endoscopic surgical procedures or therapy with alpha‐blockers: a review of urodynamic studies. Adv Ther. 2017;34(4):773‐783. [DOI] [PubMed] [Google Scholar]
- 35. Kashi AH, De la Rosette J, Amini E, Abdi H, Fallah‐Karkan M, Vaezjalali M. Urinary viral shedding of COVID‐19 and its clinical associations: a systematic review and meta‐analysis of observational studies. Urol J. 2020;17(5):433‐441. [DOI] [PubMed] [Google Scholar]
- 36. Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74(2):410‐416. 10.1095/biolreprod.105.044776 [DOI] [PMC free article] [PubMed] [Google Scholar]


