Abstract
Many meta‐analyses have been published about the efficacy of hydroxychloroquine (HCQ) in coronavirus disease 2019 (COVID‐19). Most of them included observational studies, and few have assessed HCQ as a prophylaxis or evaluated its safety profile. We searched multiple databases and preprint servers for randomized controlled trials (RCTs) that assessed HCQ for the treatment or prevention of COVID‐19. We summarized the effect of HCQ on mortality, viral clearance, and other clinical outcomes. Out of 768 papers screened, 21 RCTs with a total of 14,138 patients were included. A total of 9 inpatient and 3 outpatient RCTs assessed mortality in 8596 patients with a pooled risk difference of 0.01 (95% confidence interval [CI] 0.00–0.03, I 2 = 1%, p = 0.07). Six studies assessed viral clearance at 7 days with a pooled risk ratio (RR) of 1.11 (95% CI 0.86–1.42, I 2 = 61%, p = 0.44) and 5 studies at 14 days with a pooled RR of 0.96 (95% CI 0.89–1.04, I 2 = 0%, p = 0.34). Several trials showed no significant effect of HCQ on other clinical outcomes and. Five prevention RCTs with 5012 patients found no effect of HCQ on the risk of acquiring COVID‐19. Thirteen trials showed that HCQ was associated with increased risk of adverse events. We observed, with high level of certainty of evidence, that HCQ is not effective in reducing mortality in patients with COVID‐19. Lower certainty evidence also suggests that HCQ neither improves viral clearance and other clinical outcomes, nor prevents COVID‐19 infection in patients with high‐risk exposure. HCQ is associated with an increased rate of adverse events.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Antimalarial agents have been shown to exert in vitro anti severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) activity, with conflicting clinical results. The data of the published meta‐analyses came mainly from observational studies, hence limiting the reliability of their findings.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study explores the efficacy and safety of hydroxychloroquine (HCQ)in coronavirus disease 2019 (COVID‐19) disease, using data from a large number of randomized clinical trials.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Our study provides conclusive evidence that HCQ has no benefit in the treatment or prevention of COVID‐19 disease. HCQ therapy was also associated with higher incidence of adverse events.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Our findings will stimulate further mechanistic studies to resolve the contradictory findings of clinical and in vitro findings.
INTRODUCTION
The novel severe acute respiratory syndrome‐coronavirus 2 (SARS‐COV‐2) that emerged from Wuhan China in December 2019 has resulted in over 27 million cases and close to 900,000 deaths. In addition to the enormous death toll, the economic damage caused by this virus has led to an increase in the demand for the development of effective therapies for managing this disease. In an effort to find a quick solution, many existing drugs have been repurposed as potential treatments in patients with coronavirus disease 2019 (COVID‐19), with chloroquine (CQ) and hydroxychloroquine (HCQ) being among one of the first drugs used for this purpose.
CQ and HCQ received a significant amount of attention for the treatment of patients with COVID‐19 because of their reported in vitro antiviral activity and the results of early clinical trials. In vitro, HCQ interferes with several cellular processes, like endocytosis, exosome release, and phagolysosomal fusion. These, in turn, can affect several stages of the virus’s life cycle from cell entry and replication, to viral particle assembly and release. 1 An early commentary from China on CQ reported improvements in many clinical outcomes, such as disease progression, radiologic findings, and disease duration. 2 Later, a nonrandomized clinical trial (non‐RCT) from France, also suggested a significant effect of HCQ in reducing time to viral clearance. 3
Despite the promising early reports, many subsequent clinical trials and observational studies demonstrated disappointing results. In this meta‐analysis, we systematically review the efficacy and safety of HCQ for the treatment or prevention of COVID‐19 reported by RCTs.
METHODS
We included double‐blinded and open label RCTs that assessed the efficacy and safety of HCQ in comparison to either placebo or standard of care (SOC), for the treatment or prevention of COVID‐19. We followed Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines for study design, search protocol, screening, and reporting. 4
Literature search
The literature was searched by a medical librarian for the concepts of RCTs of CG or HCQ and COVID‐19. The search strategies were created using a combination of keywords and standardized index terms. Searches were run up to October 6, 2020, in Ovid EBM Reviews, Ovid Embase (1974+), Ovid Medline (1946+ including electronic publications ahead of print, in‐process, and other nonindexed citations), PubMed.gov (1946+), Scopus (1970+), and Web of Science Core Collection (1975+). All results were exported to Endnote where obvious duplicates were removed leaving 314 citations. Search strategies are provided in the Supplementary File. We also searched Medrxiv.org and Research Square preprint server for eligible RCTs and identified 454 citations.
Data collection
Two reviewers independently identified eligible studies (Z.K. and T.K.) and extracted the data into a prespecified data collection form. Discrepancies were resolved with a third reviewer (I.T.). Data were collected on the following prespecified outcomes:
Mortality, viral clearance, disease progression, symptom resolution and clinical recovery, need for mechanical ventilation, and requirement for hospitalization (outpatient trials) for the treatment RCTs.
Risk of acquiring COVID‐19 infection in individuals with high‐risk exposure for the prevention RCTs.
Additionally, we collected data on adverse reactions; these include arrhythmias, elevated liver enzymes, gastrointestinal adverse events (diarrhea and vomiting), neurologic adverse events (dizziness, fatigue, and irritability), headaches, visual symptoms, and rashes.
The reviewers independently assessed the risk of bias for each study using the Cochrane risk‐of‐bias tool for randomized trials 5 and resolved differences among themselves. RoB 2 is structured into 6 domains of bias: (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, (5) selection of the reported result, and (6) overall bias. Within each domain, a series of questions aim to elicit information about features of the trial that are relevant to the risk of bias. A proposed judgment about the risk of bias arising from each domain is generated by an algorithm, based on answers to the signaling questions. Judgment can be “low” or “high” risk of bias or can express “some concerns.”
Certainty of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. 6 , 7 This method evaluates the certainty of evidence by assessing the following domains: limitations, indirectness, inconsistency, imprecision, and publication bias.
Statistical analysis
The efficacy outcomes of interest in this review are mortality and viral clearance as well as hospitalization requirement (outpatient trials) in the treatment RCTs and the incidence of infection in patients with high‐risk exposure in the prevention RCTs. The safety outcomes were the occurrence of adverse events. The meta‐analysis was performed using the Mantel–Haenszel method for dichotomous data. Outcomes were reported as risk ratios (RRs) or risk differences (RDs) whenever appropriate with 95% confidence interval (CI). We reported pooled RD and 95% CI when studies had zero events and we used RD to calculate the number needed to harm (NNH) and 95% CI for each adverse event. We evaluated statistical heterogeneity using the I 2 statistic, which estimates the variability percentage in effect estimates that is due to heterogeneity rather than to chance. 8
Fixed and random‐effects models were used depending on statistical heterogeneity. We constructed funnel plots to assess for asymmetry and publication bias. All statistical analyses were performed using Review Manager version 5.4.
RESULTS
Out of 768 papers screened for eligibility, 21 RCTs 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 with a total of 14,138 patients were included (Figure 1). Sixteen RCTs assessed the efficacy and safety of HCQ in patients with confirmed COVID‐19 disease, among which, 10 studies were multicenter 9 , 29 and 6 studies were single center, 11 , 12 , 13 , 15 , 19 , 28 2 were double‐blind, 17 , 25 and 14 were open‐label. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 19 , 22 , 24 , 28 , 29 Thirteen studies 9 , 10 , 11 , 12 , 13 , 14 , 15 , 24 , 27 , 28 , 29 were conducted in the inpatient setting, whereas three studies 17 , 20 , 21 were conducted in the outpatient setting. Five RCTs studied the role of HCQ in the prevention of COVID‐19 disease. 20 , 21 , 23 , 26 , 27 The study design of all these trials is described in Table S1. The quality of the RCTs was assessed using the Cochrane ROB tool; the results of which are shown in Figure S1. Seventeen studies are at low risk of bias, 9 , 10 , 11 , 29 one study was at a moderate, 15 and three studies at high risk of bias. 12 , 18 , 19 The study with moderate risk of bias used patients who refused treatment as controls, but this had no effect on the baseline characteristics of the patients. All studies with high risk of bias had deviations from treatment protocol or baseline differences that were likely to affect the study outcomes.
Figure 1.
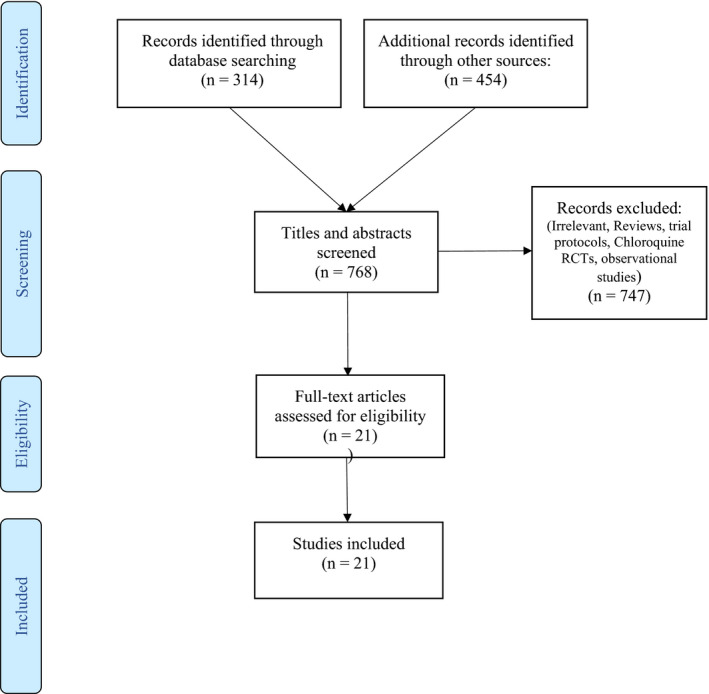
Prisma flow diagram of eligible studies. RCT, randomized control trial
OUTCOMES
Efficacy
Treatment RCTs
Sixteen RCTs studied the effect of HCQ on several outcomes that include, mortality, viral clearance, disease progression, disease severity scores, symptom resolution or clinical recovery, need for mechanical ventilation, hospital length of stay, need for hospitalization, and resolution of computerized tomography scan changes. A summary of the findings, and strength of evidence for each outcome is shown in Table 1.
Table 1.
Summary of outcomes, key findings, and strength of evidence
| Outcome | No. of studies and study setting | Findings and magnitude of effect | ARD | NNT or NNH | Strength of evidence |
|---|---|---|---|---|---|
| Mortality (treatment RCTs) | 11 inpatient and 3 outpatient trials | 13 RCTs, 10 at low and 3 high risk of bias, with consistent and precise pooled RR found no significant association between HCQ and mortality. RR 1.09 [95% CI 0.99–1.20] I 2 = 0% | 0.01 [95% CI 0.00–0.03] | NNH 100 [95% CI 100–NC] | High: We are very certain of the effect of HCQ on short‐term mortality in patients with COVID‐19 |
| Viral clearance at 7 days (treatment RCTs) | 6 inpatient trials | 6 RCTs, 4 at low risk and 2 high risk of bias, with inconsistent and imprecise RR found no association between HCQ and viral clearance. Pooled RR 1.11 [95% CI 0.86–1.42] I 2 = 61% | 0.05 [95% CI −0.06 to 0.16] | NNT 20 [95% CI 6.25 to −16.7] | Very Low: We are very uncertain of the effect of HCQ on viral clearance at 7 days in patients with COVID‐19 |
| Viral clearance at 14 days (treatment RCTs) | 5 inpatient trials | 5 RCTs, 2 at low risk, 1 at moderate, and 2 at high risk of bias, with consistent and precise RRs found a trend towards slower viral clearance at 14 days. Pooled RR 0.96 [95% CI 0.89–1.04] I 2 = 0%. | −0.03 [95% CI −0.09 to 0.03] | NNH 33.3 [95% CI −33.3 to 11.1] | Moderate: We are uncertain of the effect of HCQ on viral clearance at 14 days in patients with COVID‐19 |
| Disease progression |
8 inpatients 2 outpatient trials |
10 RCTs, 6 at low, 1 at moderate, and 3 at high risk of bias, with consistent and imprecise RRs, found no significant effect of HCQ on disease progression. RR 1.06 [95% CI 0.99–1.14] I 2 = 20.3%. | −0.02 [95% CI −0.0 to 0.04] | NNH 50 [95% CI 25–NC] | Low: We are uncertain of the effect of HCQ on disease progression in patients with COVID‐19 |
| Mechanical ventilation | 5 inpatient trials | 5 RCTs, 5 at low risk of bias, with consistent and imprecise RR, found no significant effect for HCQ on mechanical ventilation RR 1.11 [95% CI 0.94–1.31] I 2 = 0%. | 0.01 [95% CI −0.01 to 0.02] | NNH 100 [95% CI −100 to 50] | Moderate: We are moderately certain that the effect of HCQ on mechanical ventilation in patients with COVID‐19 |
| Symptom resolution | 7 inpatient and 3 outpatient trials | 10 RCTs, 8 at low and 2 at high risk of bias, with inconsistent but precise RR, found no significant effect for HCQ on symptom resolution RR 0.96 [95% CI 0.89–1.04] I 2 = 67.2%. | −0.02 [95% CI −0.07 to 0.03] | NNH 50 [95% CI −33 to 14.3] | Moderate: We are moderately certain of the effect of HCQ on symptom resolution in patients with COVID‐19 |
| Need for hospitalization | 3 outpatient and 5 prevention trials | 8 RCTs, 7 at low risk and 1 at a high risk of bias with inconsistent but imprecise effect estimates found no significant effect for HCQ on need for hospitalization RR 0.80 [95% CI 0.54–1.20] I 2 = 0%. | 0.00 [95% CI −0.01 to 0.01] | NNT NC [95% CI −100 to 100] | Low: We are uncertain of the effect of HCQ on need for hospitalization in patients with COVID‐19 |
| Risk of infection (prevention RCTs) | 5 outpatient trials | 5 RCTs with low risk of bias, consistent and imprecise results found no significant association between HCQ and the incidence of infection in patients with high‐risk exposure RR 0.85 [95% CI 0.69–1.04, I 2 = 0%, p = 0.10] | −0.01 [95% CI −0.03 to 0.00] | NNT 100 [95% CI 33–NC] | Moderate: We are moderately certain of the effect of HCQ on decreasing the risk of infection in patients with a high‐risk exposure to COVID‐19 |
Abbreviations: ARD, absolute risk difference; CI, confidence interval; COVID‐19, coronavirus disease 2019; EE, effect estimate; HCQ, hydroxychloroquine; NC, not calculable; NNH, number needed to harm; NNT, number needed to treat; RCT, randomized controlled trial; RR, risk ratio.
Effect of HCQ on mortality
A total of 9 inpatient 9 , 10 , 12 , 13 , 14 , 18 , 24 , 28 , 29 and 3 outpatient RCTs 17 , 19 , 21 assessed mortality in 8596 patients. In hospitalized patients, there was a trend toward increased mortality in the HCQ group compared with the control risk difference of 0.02 (95% CI 0.00–0.03 I 2 = 0%, p = 0.07). There was no significant difference between treatment groups in nonhospitalized patients (RD −0.00, 95% CI −0.01 to 0.01 I 2 = 0%, p = 1.0). The total pooled effect estimate of RD in combined hospitalized and nonhospitalized patients was 0.01 (95% CI 0.00–0.03, I 2 = 1%, p = 0.07; Figure 2). We reported the risk difference because many studies reported no mortality in either group. However, the mortality RR from six inpatient studies also showed a trend of increased mortality with HCQ with pooled RR of 1.09 (95% CI 0.99–1.20, I 2 = 0%, p = 0.07; Figure S2). Funnel plot analysis indicates that there was no evidence of publication bias among HCQ treatment RCTs (Figure S3). Because Cavalcanti et al. 9 had an extra arm of a combination of HCQ and azithromycin, we performed a sensitivity analysis by including the patients from this arm in the mortality analysis and found no change in the pooled effect estimates (Figure S4a). Similarly, the pooled effect estimates did not change when we excluded three studies at high risk of bias 12 , 18 , 19 (Figure S4b). Because one of the included trials 14 was very large with a calculated weight of 51.9%, a sensitivity analysis after removing this trial did not affect the overall pooled estimates.
Figure 2.
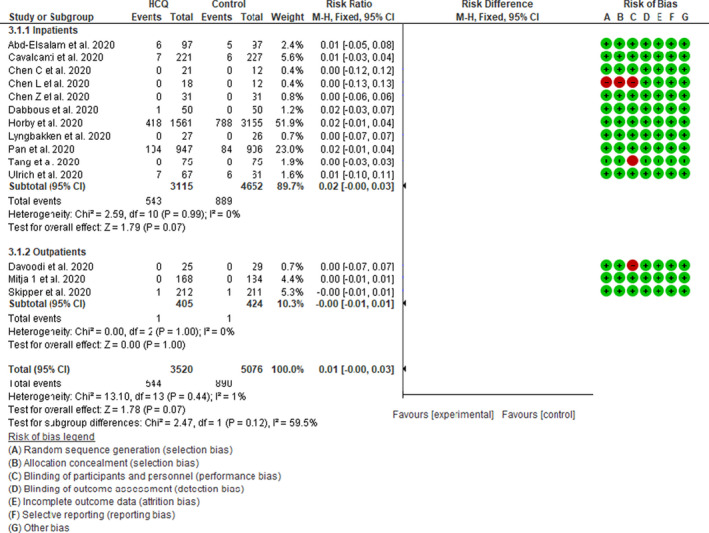
Effect of hydroxychloroquine on short‐term mortality in patients with coronavirus disease 2019 (COVID‐19): Fixed effect model forest plot. CI, confidence interval; HCQ, hydroxychloroquine; M‐H, Mantel‐Haenszel
Effect of HCQ on viral clearance
Six studies 10 , 11 , 12 , 15 , 18 , 25 assessed viral clearance and two studies assessed viral load 16 , 28 at different points in time in response to HCQ or its comparators. The pooled RR of viral clearance of 6 studies 11 , 12 , 15 , 18 , 24 , 25 at 7 days was 1.11 (95% CI 0.86–1.42, I 2 = 61%, p = 0.44) and the pooled RR of viral clearance of 5 studies 10 , 12 , 15 , 18 , 24 at 14 days was 0.96 (95% CI 0.89–1.04, I 2 = 0%, p = 0.34; Figure 3a,b). Mitja et al. 16 assessed the viral load at 3, 7, and 14 days and observed no significant effects of HCQ on viral load in comparison with the control group. Additionally, Lyngbakken et al. 28 found no difference in the rate of decline in viral load at 96 hours between the HCQ and control groups. These findings suggest that HCQ has no effect on the rate of viral clearance in patients with COVID‐19. The pooled effect estimate did not change when we excluded the two studies at high risk of bias 12 , 18 (Figure S5a,b).
Figure 3.
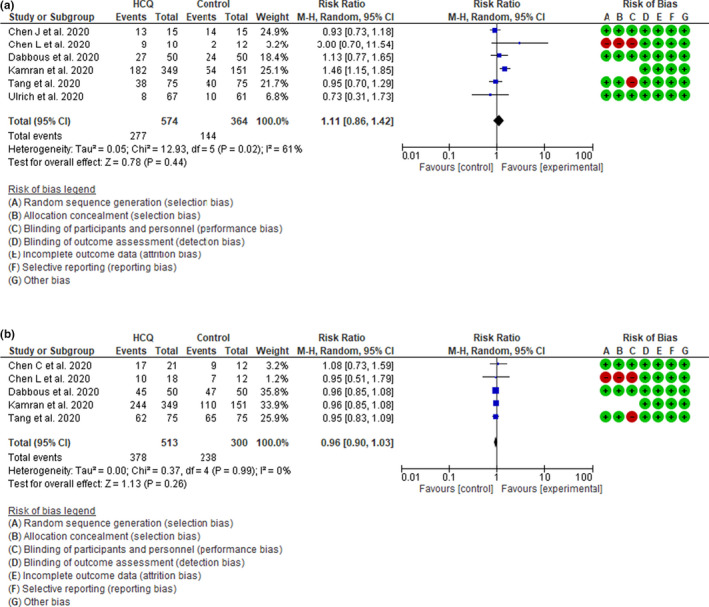
(a) Effect of hydroxychloroquine (HCQ) on viral clearance at 7 days in patients with coronavirus disease 2019 (COVID‐19): Random effect model forest plot. (b) Effect of HCQ on viral clearance at 14 days in patients with COVID‐19: Random effect model forest plot. CI, confidence interval; M‐H, Mantel‐Haenszel
Effect of HCQ on disease progression
Eight inpatient 9 , 10 , 11 , 12 , 13 , 14 , 15 , 18 and two outpatient 17 , 19 RCTs with a total of 6410 patients assessed the effect of HCQ on COVID‐19 disease progression. HCQ demonstrated a trend toward a higher risk of disease progression among the inpatient studies with an RR of 1.07 (95% CI 0.99–1.15, I 2 = 0%, p = 0.07). The RR for the outpatient trials was 0.85 (95% CI 0.58–1.26, I 2 = 0%, p = 0.42) with the pooled RR for the 10 trials of 1.06 (95% CI 0.99–1.14, I 2 = 0%, p = 0.12; Figure 4a). These findings indicate that HCQ might be associated with a trend of worse disease progression in COVID‐19 disease.
Figure 4.
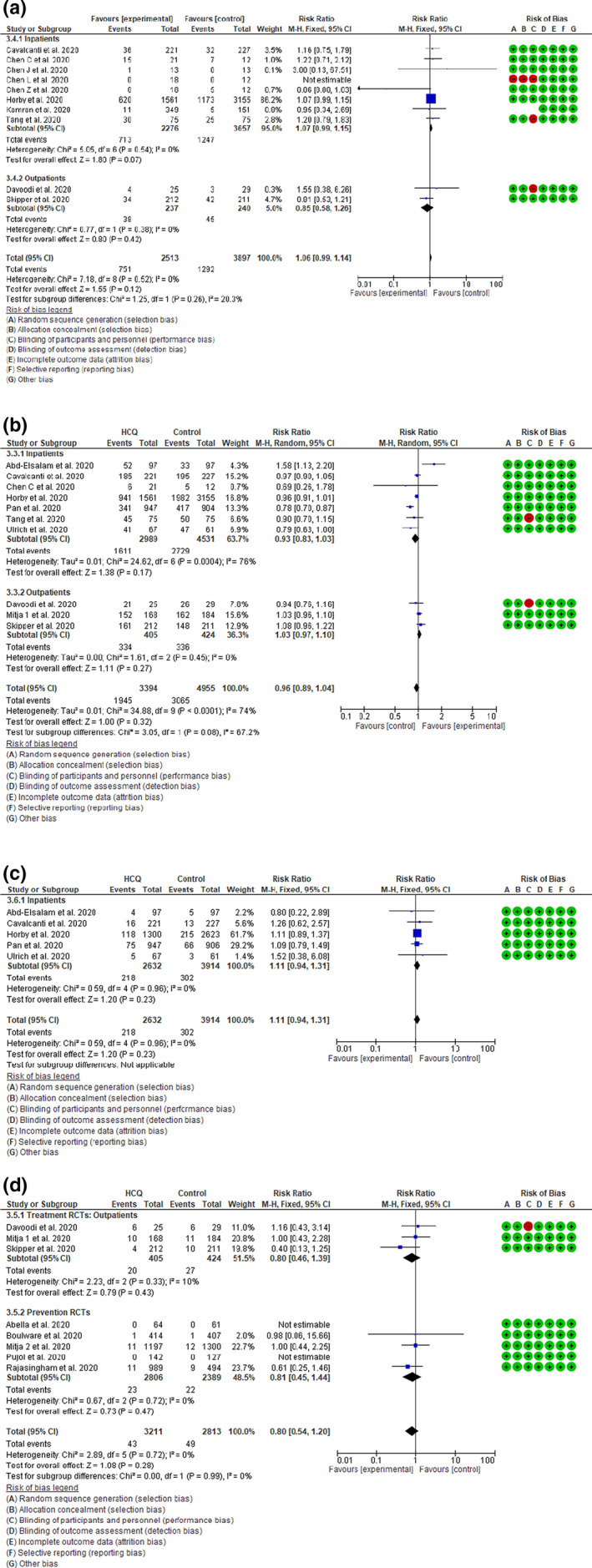
(a) Effect of hydroxychloroquine (HCQ) on disease progression in patients with coronavirus disease 2019 (COVID‐19): Random effect model forest plot. (b) Effect of HCQ on symptom resolution or clinical recovery in patients with COVID‐19: Random effect model forest plot. (c) Effect of HCQ on requirement for mechanical ventilation in patients with COVID‐19: Fixed effect model forest plot. (d) Effect of HCQ on need for hospitalization in patients with COVID‐19: Fixed effect model forest plot. M‐H, Mantel‐Haenszel; RCT, randomized controlled trial
Effect of HCQ on symptom resolution or clinical recovery
Seven inpatient 9 , 10 , 14 , 18 , 22 , 25 , 29 and 3 outpatient 16 , 17 , 19 RCTs with a total of 8349 patients assessed the effect of HCQ on symptom resolution or clinical recovery among patients with COVID‐19. The pooled RR of the 7 inpatient studies was 0.93 (95% CI 0.83–1.03, I 2 = 76%, p = 0.17) and for the outpatient trials was 1.03 (95% CI 0.97–1.10, I 2 = 0%, p = 0.27). The pooled effect estimates of all 10 studies was 0.96 (95% CI 0.89–1.04, I 2 = 74%, p = 0.32; Figure 4b). Therefore, HCQ therapy did not result into better symptom resolution or clinical recovery among patients with COVID‐19. We performed a sensitivity analysis by excluding the two studies at high risk of bias 18 , 19 and did not find any change in the pooled effect estimates (Figure S6).
HCQ treatment and requirement of mechanical ventilation
Five inpatient 9 , 14 , 22 , 25 , 29 RCTs with a total of 6546 patients assessed the effect of HCQ on the need for mechanical ventilation in COVID‐19 disease. No patients enrolled in the outpatient studies required mechanical ventilation. The pooled RR of all 5 studies was 1.11 (95% CI 0.94–1.31, I 2 = 0%, p = 0.23) indicating that HCQ therapy does not reduce the risk of requiring mechanical ventilation (Figure 4c).
HCQ treatment and need for hospitalization
Eight RCTs, 3 treating outpatients with mild COVID‐19 disease 16 , 17 , 19 and 5 prevention trials using HCQ as a prophylaxis 20 , 21 , 23 , 26 , 27 assessed the effect of HCQ on the need for hospitalization in these patient populations. The pooled RR was 0.80 (95% CI 0.54–1.20, I 2 = 0%, p = 0.28; Figure 4d), which indicates that HCQ failed to reduce the need for hospitalization among nonhospitalized patients with COVID‐19 with mild to moderate disease and among individuals with high‐risk exposure.
Prevention RCTs
Five studies 20 , 21 , 23 , 26 , 27 with 5012 patients assessed the efficacy of HCQ as a prophylactic treatment for patients with a high‐risk exposure to COVID‐19. There was no significant difference in the incidence of infection between the HCQ and control groups, RR 0.85 (95% CI 0.69–1.04, I 2 = 0%, p = 0.10; Figure 5).
Figure 5.
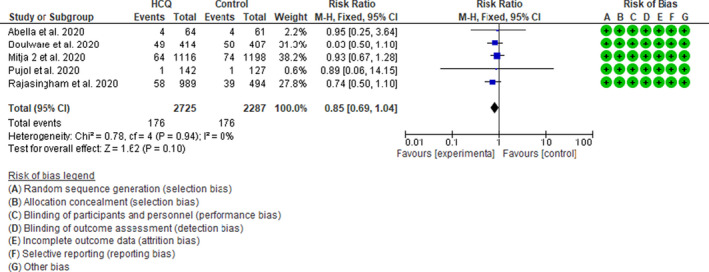
Effect of hydroxychloroquine (HCQ) on incidence of infection in prevention trials in patients with coronavirus disease 2019 (COVID‐19): fixed effect model forest plot. CI, confidence interval; M‐H, Mantel‐Haenszel
Safety
Although the occurrence of life‐threatening adverse events was very minimal among the patients of the included studies, the use of HCQ was associated with a significantly increased risk of any adverse effects.
Thirteen studies 9 , 23 , 25 , 26 , 27 reported on the incidence of any adverse event in both the treatment and control groups with a total of 6494 patients. HCQ was associated with significant increased risk of any adverse event with the pooled risk difference of 0.21 (95% CI 0.07–0.34, I 2 = 97%, p = 0.003) and RR of 2.29 (95% CI 1.37–3.82, I 2 = 96%, p = 0.002; Table 2, Figure S7a,b). The NNH was 4.76 (95% CI 2.94–14.3). We observed similar increased risk of any adverse event with HCQ therapy when we pooled the inpatient, outpatient, and postexposure prevention studies separately (Figure S7a,b).
Table 2.
Summary of adverse events
| Adverse event | No. of RCTs | No. of patients | RD | NNH |
|---|---|---|---|---|
| Any adverse events | 13 | 6494 | 0.21 [95% CI 0.07–0.34] | 4.76 [95% CI 2.94–14.3] |
| Arrhythmias | 8 | 9767 | 0.00 [95% CI 0.00–0.01] | NC [95% CI 100–NC] |
| LFT elevation | 5 | 789 | 0.03 [95% CI 0.00–0.07] | 33.3 [95% CI 14.3–NC] |
| GI symptoms | 11 | 4936 | 0.15 [95% CI 0.04–0.26] | 6.66 [95% CI 3.84–25] |
| Neurologic symptoms | 4 | 2648 | 0.02 [95% CI 0.00–0.03] | 50 [95% CI 33.3–NC] |
| Headache | 9 | 5454 | 0.04 [95% CI −0.03 to 0.11] | 25 [95% CI 9.09 to −33.3] |
| Visual symptoms | 7 | 3323 | 0.01 [95% CI 0.00–0.01] | 100 [95% CI 100–NC] |
| Skin rash | 6 | 2977 | 0.01 [95% CI 0.00–0.02] | 100 [95% CI 50–NC] |
Abbreviations: CI, confidence interval; GI, gastrointestinal; LFT, liver function tests; NC, not calculable; NNH, number needed to harm; NNT, number needed to treat; RCT, randomized controlled trial; RD, risk difference.
Furthermore, we also analyzed several types of HCQ‐induced adverse events individually. We found that HCQ therapy was associated with a higher rate of gastrointestinal symptoms (defined as vomiting or diarrhea) RR 3.32 (95% CI 1.66–6.67). HCQ was associated with a trend toward higher incidence of nonlethal cardiovascular arrhythmias 1.37 (95% CI 1.00–1.88) liver enzyme elevation RR 1.66 (95% CI 0.98–2.79), and neurologic symptoms (defined as fatigue, dizziness, or irritability) 1.38 (95% CI 0.99–1.93). The results of these analyses are shown in Table 2 and Figures S8–S14. There was no statistically significant difference in the incidence of headaches (RR 1.74, 95% CI 0.64–4.73), rashes (RR 1.50, 95% CI 0.91–2.50), or visual symptoms (RR 1.91, 95% CI 0.92–3.95). It is noteworthy to mention that in the Horby et al. 14 study reported one case of Torsades de Pointes (TdP) in the HCQ group. In addition, Dabbous et al 24 reported one case of lethal myocarditis with HCQ and WHO Solidarity Trial Consortium et al 29 reported death due to any cardiac cause in four patients from the HCQ group and two from the control group.
DISCUSSION
Main findings
Our systematic review and meta‐analysis included 21 RCTs with a total of 14,138 patients. We found with high certainty that HCQ is not effective in reducing short‐term mortality of patients with COVID‐19 with different disease severities. Additionally, lower quality evidence suggests that HCQ had no impact on viral clearance rate at 7 and 14 days and did not improve other important clinical outcomes, such as disease progression, symptom relief or clinical recovery, need for mechanical ventilation, or need for hospitalization. Further, in postexposure prophylaxis RCTs, we found, with moderate certainty, that HCQ failed in preventing COVID‐19 infection.
There was a higher rate of adverse events among patients taking HCQ compared with controls. In terms of clinical recovery or symptom resolution, only one small trial by Abd‐Esalam et al. showed benefit of HCQ in terms of improving clinical recovery. 22 In contrary, the large SOLIDARITY trial showed that SOC was better than HCQ. 29 This is likely due to differences in the studied patient populations and variation of the standard therapies in these two studies, sample size, and the time of ascertainment of the outcome. The other five studies showed no benefit of HCQ on clinical recovery or symptom resolution with their effect estimates crossing the unity line.
Although several meta‐analyses addressing the role of HCQ in COVID‐19 disease have been published 30 , 31 , 32 , 33 , 34 , 35 , 36 among others, in all of these meta‐analyses, the bulk of their data were derived from observational studies, with the exception of 3 studies. Many of these observational studies suffer from serious methodological weaknesses, including treatment selection bias, immortal time bias, competing risk bias, and residual confounding. Moreover, some meta‐analyses pooled unadjusted effect estimates of the included studies, which result in a very biased results. 31 Only three authors 35 , 36 , 37 included only RCTs in their meta‐analyses similar to us; however, in contrast to ours, these meta‐analyses were small (included 4–7 RCTs). One meta‐analysis by Hussain et al. 36 had a total of 381 patient with only 2 trials (111 patients) had data on mortality. The second meta‐analysis by Pathak et al. 35 pooled different clinical outcomes together as a composite end point. Recently, Lewis et al. published a meta‐analysis that included only 4 trials that addressed only the efficacy and safety of HCQ for COVID‐19 prophylaxis. 37 Chowdhury et al. 38 performed a systematic review of 7 RCTs without meta‐analysis and concluded that there was not sufficient data to support the routine use of HCQ in the treatment of COVID‐19.
These small systematic reviews / metanalyses of the HCQ RCTs had conflicting results ranging from being efficacious 38 to not effective 35 or harmful. 35 To date, our meta‐analysis is the largest meta‐analysis of RCTs (21 trials) that addressed the efficacy and safety of HCQ in the prevention and treatment of COVID‐19 disease, which gives it the power to better answer these questions.
Mechanisms
Despite the promising results from in vitro studies and early in vivo studies, the majority of subsequent studies failed to demonstrate any significant benefits for HCQ in COVID‐19. There are many possible reasons for this observation. The most important are: first, many in vitro studies introduced the virus after pretreating the cells with HCQ or CQ. For instance, Vincent et al. 39 demonstrated that cells treated 5 hours after viral adsorption required up to 5 times the dose given to cells pretreated with CQ to achieve a similar level of viral inhibition. However, the five trials included in this review failed to demonstrate any effectiveness of HCQ as a prophylaxis in patients with high‐risk exposure. Additionally, a study conducted on 14,250 patients found that chronic use of HCQ was not associated with decreased SARS‐CoV‐2 infection. 40
Second, a wide range of 50% effective concentration (EC50) values for HCQ were reported ranging from 0.72 to 17.31 µM. The lowest reported EC50 was from a study by Yao et al. 41 Based on their result, the lowest possible EC50 would be ~ 0.48 mg/L considering the HCQ plasma protein binding of 50%. Only 2 studies reported on the pharmacokinetics of HCQ treatment in patients with COVID‐19. In a study by Gautret et al., 3 the proportion of patients who achieved the minimum suggested effective plasma concentration of 0.48 mg/L was 53.6%. In another study by Perinel et al., 42 only 61% of their patients achieved what they considered a therapeutic level of 1 mg/L. In a prevention trial, Rajasingham et al. 26 measured HCQ concentrations in the dried whole blood samples from 180 patients receiving prophylaxis doses of HCQ of either 400 mg per week or 400 mg twice a week. The blood levels of HCQ were ranging from 0.098 mg/L in the once/week to 0.2 mg/L in the twice a week regimens. 26 Yao et al. proposed a dosing regimen for HCQ consisting of 400 mg twice daily for one day followed by 200 mg twice daily for 4 days based on their physiologically‐based pharmacokinetic models and simulation. 41 It is noteworthy to mention that this proposed regimen was based on the estimated free lung trough concentration to in vitro EC50 ratio because HCQ achieves high tissue concentrations. However, HCQ is known to accumulate in the acidic compartments of the cells like lysosomes and gets sequestered by these organelles reaching up to 80 μM when the extracellular concentrations are in the 0.5 μM range. 43 Based on these properties, Fan et al. suggested that translation of the in vitro to in vivo antiviral activity of HCQ and estimation of the appropriate dosing regimen should be based on the free HCQ plasma concentrations, which are similar to the extracellular concentrations rather than the lung tissue concentrations. 44 They concluded from their repeated calculations that were based on Yao et al.’s EC50 measurements that current dosing regimens of HCQ may not have adequate in vivo antiviral activity against SARS‐Cov‐2. 44 Similarly, Garcia‐Cremades et al. used a complex model that integrated in vivo and in vitro data and population pharmacokinetic model of HCQ and found out that the extrapolated patient EC50 is 4.7 μM (1.58 mg/L). They predicted that HCQ dose of 400 mg or higher twice daily for 5 days will be necessary to achieve adequate antiviral concentration but with a higher risk QT prolongation. 45
The narrow therapeutic window for HCQ, the increased rate of adverse events associated with its use, and the difficulty of achieving adequate therapeutic concentration make HCQ not a good option for the treatment of COVID‐19. 46
Adverse events
The occurrence of life‐threatening adverse events was very uncommon among the included studies. Horby et al. 14 reported ventricular arrhythmias (6 in HCQ vs. 9 in SOC) and one case of TdP in the HCQ group. However, Cavalcanti et al. 9 reported significant increase in corrected QT (QTc) prolongation of greater than 480 ms among patients on HCQ or HCQ, azithromycin combination in comparison to SOC patients (14.3%, 16.5%, and 1.7%, respectively). Similarly, Ulrich et al. 25 reported significant increase in the mean QTc duration among HCQ patients (16 ms +/‐ 30.0 vs. 2.1 ms +/‐ 25.3, p = 0.029) with three patients in the HCQ developing QTc greater than 500 ms compared with one patient in the control arm. In the SOLIDARITY trial, 29 death due to any cardiac cause occurred in four patients compared to two in the control arm. Other reported cardiac adverse events in association with HCQ treatment include one case of syncope associated with new supraventricular tachycardia 26 and one fatal myocarditis 24 but a cause‐and‐effect relationship between HCQ and these events cannot be made. Nonetheless, these observations indicate that HCQ use among patients with COVID‐19 is potentially associated with nontrivial serious cardiac adverse events despite the short duration of the therapy and the exclusion of patients with underlying QT prolongation, history of arrhythmias and cardiac risk factors, and the inclusion of trials that enrolled nonhospitalized and asymptomatic patients.
On the other hand, the incidence of noncardiac side effects was very common among HCQ‐treated patients with COVID‐19 with overall incidence of 45.1% in comparison to 15.0% for placebo or SOC. Several types of adverse events have reported among patients with COVID‐19 treated with HCQ with gastrointestinal side effects being the most significant.
Strengths and limitations
This review has several strengths. We included published and unpublished studies thereby limiting publication bias. Our review is, to our knowledge, the first large meta‐analysis that analyzed data only from RCTs, thereby minimizing the risk of treatment selection bias, immortal time bias, competing risk bias, and residual confounding that would otherwise undermine the results of observational studies. Our meta‐analysis has also certain limitations. First, the results of our meta‐analysis are affected by inherent limitations of the individual trials included in this study, such as the significant variations in the use of other medical therapies, which might affect patient outcomes, variations in HCQ dosing regimens, and reported patient outcomes. Second, we could not analyze viral clearance at different time points due to the discrepancy in outcomes reported in individual RCTs. Finally, we could not quantitatively analyze other outcomes of interest, such as hospital length of stay and radiological improvement of COVID‐19 pneumonia, because RCTs did not report on these outcomes consistently.
CONCLUSIONS
In this systematic review, we observed, with high level of certainty of evidence, that HCQ is not effective in reducing mortality in patients with COVID‐19. Lower certainty evidence also suggests that HCQ neither improve other clinical outcomes in COVID‐19, nor prevent COVID‐19 infection in patients with high‐risk exposure. HCQ is associated with an increased rate of adverse events.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
Z.K., I.T., and T.K. wrote the manuscript. I.T. and D.G. designed the research. I.T. and Z.K. performed the research. Z.K., I.T., and T.K. analyzed the data.
Supporting information
Supplementary Material
Supplementary Material
Funding information
No funding was received for this work.
REFERENCES
- 1. Tripathy S, Dassarma B, Roy S, Chabalala H, Matsabisa MG. A review on possible modes of action of chloroquine/hydroxychloroquine: repurposing against SAR‐CoV‐2 (COVID‐19) pandemic. Int J Antimicrob Agents. 2020;56:106028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72‐73. [DOI] [PubMed] [Google Scholar]
- 3. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 6. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murad MH. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc. 2017;92:423‐433. [DOI] [PubMed] [Google Scholar]
- 8. Deeks J, Higgins J, Altman D, et al. Cochrane Handbook for Systematic Reviews of Interventions Chapter 10: analysing data and undertaking meta‐analyses. 2020.
- 9. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild‐to‐moderate COVID‐19. N Engl J Med. 2020;383(21):2041‐2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen CP, Lin YC, Chen TC, et al. A multicenter, randomized, open‐label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate coronavirus disease 2019 (COVID‐19). PLoS One. 2020;15(12):e0242763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen J, Liu D, Liu L, et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID‐19. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:215‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen L, Zhang Z, Fu J, et al. Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID‐19: a prospective open‐label randomized controlled study. medRxiv. 10.1101/2020.06.19.20136093 [DOI] [Google Scholar]
- 13. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial. medRxiv. 10.1101/2020.03.22.20040758 [DOI] [Google Scholar]
- 14. Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with COVID‐19: preliminary results from a multi‐centre, randomized, controlled trial. medRxiv. 10.1101/2020.07.15.20151852 [DOI] [Google Scholar]
- 15. Kamran SM, Mirza Z, Naseem A, Azam R. Clearing the fog: is HCQ effective in reducing COVID‐19 progression: a randomized controlled trial. medRxiv. https://doi.org/2020.07.30.20165365. [Google Scholar]
- 16. Mitjà O, Corbacho‐Monné M, Ubals M, et al. Hydroxychloroquine for early treatment of adults with mild covid‐19: a randomized‐controlled trial [published online ahead of print July 16, 2020]. Clin Infect Dis. 10.1093/cud.cuaa1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID‐19. Ann Intern Med. 2020;173(8):623‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients mainly with mild to moderate COVID‐19: an open‐label, randomized, controlled trial. BMJ. 2020;369:m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davoodi L, Abedi SM, Salehifar E, et al. Febuxostat therapy in outpatients with suspected COVID‐19: A clinical trial. Int J Clin Pract. 2020;74(11):e13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID‐19. N Engl J Med. 2020;383:517‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mitjà O, Corbacho‐Monné M, Ubals M, et al. A cluster‐randomized trial of hydroxychloroquine as prevention of COVID‐19 transmission and disease. N Engl J Med. 2020;384(5)417‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abd‐Elsalam S, Esmail ES, Khalaf M, et al. Hydroxychloroquine in the treatment of COVID‐19: a multicenter randomized controlled study. Am J Trop Med Hygiene. 2020;103:1635‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Abella BS, Jolkovsky EL, Biney BT, et al. Efficacy and safety of hydroxychloroquine vs placebo for pre‐exposure SARS‐CoV‐2 prophylaxis among health care workers. JAMA Intern Med. 2021;181(2):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dabbous H, El‐Sayed M, El Assal G, et al. A randomized controlled study of favipiravir vs hydroxychloroquine in COVID‐19 management: what have we learned so far?. Research Square. 2020. 10.21203/rs.3.rs-83677/v1 [DOI] [Google Scholar]
- 25. Ulrich RJ, Troxel AB, Carmody E, et al. Treating COVID‐19 with hydroxychloroquine (TEACH): a multicenter, double‐blind, randomized controlled trial in hospitalized patients. Open Forum Infect Dis. 2020;7(10):ofaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajasingham R, Bangdiwala AS, Nicol MR, et al. Hydroxychloroquine as pre‐exposure prophylaxis for COVID‐19 in healthcare workers: a randomized trial [published online ahead of print October 17, 2020]. Clin Infect Dis. 10.1093/cid/ciaa1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grau‐Pujol B, Camprubí D, Marti‐Soler H, et al. Pre‐exposure prophylaxis with hydroxychloroquine for COVID‐19: initial results of a double‐blind, placebo‐controlled randomized clinical trial. Research Square. 2020. 10.21203/rs.3.rs-72132/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lyngbakken MN, Berdal J‐E, Eskesen A, et al. A pragmatic randomized controlled trial reports the efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics. Nat Commun. 2020;11(1):5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. WHO Solidarity Trial Consortium , Pan H, Peto R, et al. Repurposed antiviral drugs for COVID‐19 –interim WHO SOLIDARITY trial results. N Eng J Med. 2020;384(6):497‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fiolet T, Guihur A, Rebeaud ME, Mulot M, Peiffer‐Smadja N, Mahamat‐Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID‐19) patients: a systematic review and meta‐analysis. Clin Microbiol Infect. 2021;27(1):19‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ullah W, Zahid S, Nadeem N, et al. Meta‐analysis comparing culprit‐only versus complete multivessel percutaneous coronary intervention in patients with ST‐elevation myocardial infarction. Am J Cardiol. 2021;139:34‐39. [DOI] [PubMed] [Google Scholar]
- 32. Kashour Z, Riaz M, Garbati MA, et al. Efficacy of chloroquine or hydroxychloroquine in COVID‐19 patients: a systematic review and meta‐analysis. J Antimicrob Chemother. 2021;76(1):30‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elavarasi A, Prasad M, Seth T, et al. Chloroquine and hydroxychloroquine for the treatment of COVID‐19: a systematic review and meta‐analysis. J Gen Intern Med. 2020;35(11):3308‐3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Putman M, Chock YPE, Tam H, et al. Antirheumatic disease therapies for the treatment of COVID‐19: a systematic review and meta‐analysis. Arthritis Rheumatol. 2021;73(1):36‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pathak DSK, Salunke AA, Thivari P, et al. No benefit of hydroxychloroquine in COVID‐19: results of systematic review and meta‐analysis of randomized controlled trials". Diabetes Metab Syndr. 2020;14:1673‐1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hussain N, Chung E, Heyl J, et al. A meta‐analysis on the effects of hydroxychloroquine on COVID‐19. Cureus. 2020;12:e10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lewis K, Chaudhuri D, Alshamsi F, et al. The efficacy and safety of hydroxychloroquine for COVID‐19 prophylaxis: a systematic review and meta‐analysis of randomized trials. PLoS One. 2021;16:e0244778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chowdhury MS, Rathod J, Gernsheimer J. A rapid systematic review of clinical trials utilizing chloroquine and hydroxychloroquine as a treatment for COVID‐19. Acad Emerg Med. 2020;27:493‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gendelman O, Amital H, Bragazzi NL, Watad A, Chodick G. Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS‐CoV‐2: Insights from a large healthcare database analysis. Autoimmun Rev. 2020;19:102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020;71(15):732‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perinel S, Launay M, Botelho‐Nevers É, et al. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID‐19 patients. Clin Infect Dis. 2020;71(16):2227‐2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cutler DJ. Possible mechanisms of action of antimalarials in rheumatic disease. Agents Actions Suppl. 1993;44:139‐143. [PubMed] [Google Scholar]
- 44. Fan J, Zhang X, Liu J, et al. Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating patients with coronavirus disease 2019. Clin Infect Dis. 2020;71(12):3232‐3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garcia‐Cremades M, Solans BP, Hughes E, et al. Optimizing hydroxychloroquine dosing for patients with COVID‐19: an integrative modeling approach for effective drug repurposing. Clin Pharmacol Ther. 2020;108:253‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kashour T, Tleyjeh IM. It is time to drop hydroxychloroquine from our COVID‐19 armamentarium. Med Hypotheses. 2020;144:110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material


