Abstract
Background:
Certain nutrients have shown protective effects against frailty, but less is known about the influence of individual food groups. Thus, this study aimed to investigate the relationship between the intake of different food groups and physical frailty in a cohort of community-dwelling older adults in Cork, Ireland.
Methods:
One hundred and forty-two (n = 81 females, n = 61 males, age 74.1 ± 6.80 years) Irish community-dwelling volunteers aged ⩾65 years participated in this cross-sectional study. Dietary intake was assessed using a validated food frequency questionnaire (FFQ). Frailty was identified as having 3 or more of the following criteria: weight loss, exhaustion, weakness, slow walking speed and low physical activity. Relationships between intakes of food groups and frailty score were determined using Spearman’s rank (and partial rank) correlations and ordinal logistic regression analysis.
Results:
Negative Spearman’s rank correlations were observed between frailty score and fish and fish products, fruit and vegetables and nuts and seeds, while positive correlations were found between frailty score and potatoes, fats and oils and sugars, preserves and snacks (P < .05). After adjustment for confounders, partial rank correlations remained statistically significant (P < .05) for all of the above dietary variables, with the exception of nuts and seeds (P > .05). Following ordinal logistic regression, the odds ratios (ORs) (95%CI) for frailty incidence for those in the lowest tertile of food group intake compared to the highest were; 3.04 (1.09-8.85) for fish and fish products, 4.34 (1.54-13.13) for fruit and vegetables, 1.52 (0.58-4.15) for nuts and seeds, 0.54 (0.19-1.51) for potatoes, 0.58 (0.17-1.95) for fats and oils and 0.49 (0.16-1.47) for sugars, preserves and snacks.
Conclusion:
This study suggests that intakes of selected food groups are independently associated with frailty. These findings may hold significant relevance for the development of future frailty prevention strategies.
Keywords: Frailty, dietary intake, food groups, elderly
Introduction
Frailty is defined as a reduced ability to cope with everyday stressors due to ageing-associated functional decline in multiple physiological systems,1 and has been described as one of the most problematic expressions of population ageing.2 Frailty is closely related to the incidence of disability in older adults and is linked to increased falls, fractures, hospitalisation and death.3 Studies suggest the prevalence of frailty in community-dwelling elderly ranges between 5% and 27% worldwide, with significant variation between countries.4,5 In Ireland, the prevalence of frailty is estimated to be 5% to 24%, depending on the classification used.6 The weight of this condition has substantial economic relevance, with frailty increasing healthcare expenditure by up to 101% and the average 3-month healthcare cost estimated at €3659 per frail person in Europe.7,8 This extensive burden highlights the need for effective frailty prevention strategies, demanding clear understanding of contributing factors and modifiable areas to be addressed.
Evidence suggests that a relationship exists between nutrition and frailty.9-13 Food intake often decreases with older age14 and malnutrition can be more prevalent in older adults due to factors such as reduced appetite, disease, disability and social factors such as isolation and poverty.15 However, diet is modifiable, and nutrition is fast becoming an active target in health promoting efforts for this age group due to emerging evidence on the relationship between diet and several health outcomes in older adults.16 Research to date on the link between diet and frailty is primarily based on investigation of overall dietary quality9,10 and protein intake,11-13 due to the well-established contribution of dietary protein to muscle health in this age group.17 Yet, there has been inadequate investigation on the relationship with individual food group intake. As food group recommendations, rather than nutrient goals, are used as a national guide for healthy eating,18 identifying which food groups might protect against frailty could arguably be of greater value than identifying associations with nutrients. Additionally, promoting intake of particular food groups in education interventions, rather than highlighting nutrient intake recommendations, may be a more comprehendible approach for the older adult, as cognitive decline and literacy issues are prevalent in this cohort.19
The aim of this study was to analyse the relationship between intake of specific food groups and frailty in a cohort of Irish older adults, identifying dietary areas to target for future frailty prevention strategies.
Methods
Study population
A total of 204 participants aged ⩾65 years were recruited on a voluntary basis to participate in this cross-sectional study. Advertisement for study recruits took place in health clinics, community centres, sports centres, churches, parish newsletters and through communication with local radio stations and elderly groups in the city and county of Cork, Republic of Ireland, from February to June 2019 inclusive. Study participants were invited to attend screening sessions in small groups at local community centres and health clinics, where their dietary intake and physical function were assessed by trained researchers. Exclusion criteria included those that received a mini-cog score20 of <3 (n = 9), were unable to walk 15 ft (n = 1), had invalid dietary intake data (n = 33) and/or had incomplete baseline frailty data (n = 19), leaving 142 (n = 61 male, n = 81 female) participants with complete sets of data for analysis. Data collection took place between March and July 2019 inclusive. All participants were informed about the research methods and gave written consent before completing the physical tests and questionnaires. The methods of the study were approved by the Cork Institute of Technology Research Ethics Committee (Cork, Ireland) in December 2018.
Frailty measurement
Frailty was assessed using the validated methodology and phenotypic definition described by Fried et al,21 which is composed of the following 5 criteria; self-reported weight loss, exhaustion, low physical activity, weakness and slowness. A minor modification was made to physical activity measurement, which was assessed using the previously validated Physical Activity Scale for the Elderly (PASE); a 5-minute self-reported questionnaire based on leisure time, household and occupational activity.22 Certain activity examples provided in the questionnaire were revised to ensure suitability for an Irish population. For example, moderate intensity activities such as ballroom dancing, ice-skating and softball were replaced with brisk walking, cycling with light effort and dancing for leisure.
Population-specific cut off points were adapted based on the study sample for low physical activity, weakness and slowness (Table 1). Participants were considered pre-frail and frail if they received a positive score for 1 to 2 and ⩾3 of the 5 criteria, respectively.21 Those with a score of 0 were considered non-frail.
Table 1.
Definitions used for frailty phenotype.
| Characteristic | Definition | Inclusion criteria |
|---|---|---|
| Weight loss | Self-reported weight loss. | Those who lost more than 10l bs (or 4.5 kg) in the last year. |
| Exhaustion | ‘How often in the last week did you feel that (a) everything you did was effort or (b) you could not get going?’ | Those who reported 3 or more days in the last week for either or both. |
| Low Physical Activity | Measured using the PASE Questionnaire.22 | Those in the lowest quintile of physical activity stratified by sex: |
| Male PASE score ⩽96.32 | ||
| Female PASE score ⩽75.00 | ||
| Weakness | Measurement of handgrip strength using a Jamar Plus digital hand-held dynamometer. The average of 3 readings from the hand with the highest handgrip value was recorded. | Those in the lowest quintile of grip strength stratified by sex and BMI: |
| Male | ||
| ⩽22.14 kg for BMI ⩽25.7 kg/m2 | ||
| ⩽29.40 kg for BMI 25.8-28.2 kg/m2 | ||
| ⩽23.54 kg for BMI 28.3-30.2 kg/m2 | ||
| ⩽22.0 kg for BMI >30.2 kg/m2 | ||
| Female | ||
| ⩽16.60 kg for BMI ⩽25.3 kg/m2 | ||
| ⩽15.38 kg for BMI 25.4-28.6 kg/m2 | ||
| ⩽16.08 kg for BMI 28.7-31.6 kg/m2 | ||
| ⩽13.14 kg for BMI of >31.6 kg/m2 | ||
| Slowness | Measurement of time to walk 15 ft. Average time from 2 trials with 1.5 and 1 m allowed for acceleration and deceleration, respectively, was used. | Those in the slowest quintile of walking speed stratified by sex and height: |
| Male | ||
| Walking time ⩾4.57 s for height ⩽173.0 cm | ||
| Walking time ⩾3.90 s for height >173.0 cm | ||
| Female | ||
| Walking time ⩾5.02 s for height ⩽158.0 cm | ||
| Walking time ⩾3.94 s for height >158.0 cm | ||
| Classification: | ||
| 0 positive criteria = non-frail | ||
| 1-2 positive criteria = pre-frail | ||
| ⩾3 positive criteria = frail | ||
Abbreviations: BMI: body mass index; PASE: physical activity scale for the elderly.
Dietary intake assessment
Dietary intake was assessed using the validated Food Frequency Questionnaire (FFQ) used in the European Prospective Investigation into Cancer and Nutrition (EPIC) – Norfolk Study.23 The semi-quantitative FFQ estimates the average consumption of foods during the last 12 months with participants asked to tick responses ranging from never or less than once a month to 6+ per day. Additional questions are given on types of fat used and dietary supplement use. The FFQs were self-completed by participants, with the guidance of trained researchers. An introduction was given to participants prior to completion, where they were given detailed instructions on how to complete the FFQ. Those FFQs that were missing 10 or more responses were excluded from the analysis to improve reliability and prevent under-reporting.24 Dietary intake data were analysed and transformed into food group intake data using the specific FETA software (version 2.53) created for the EPIC study.24 Intake data on the following food and beverage groups were attained; meat and meat products, fish and fish products, milk and milk products, fruit and vegetables, cereals, potatoes, soups and sauces, nuts and seeds, fats and oils, sugars, preserves and snacks and alcoholic and non-alcoholic beverages.
Other measures
Body mass index (BMI) was calculated as weight (kg)/height (m)2. A calibrated Tanita Scales (model DC-360s, Tanita, Tokyo, Japan) was used for weight (kg) measurement. Participants were asked to remove any outdoor clothing and shoes. As previously recommended,25 1.2 and 0.8 kg were subtracted for clothes weight for males and females, respectively. A SECA portable stadiometer (model 213, SECA North America, Hanover, MD) was used for height (cm) measurement.
Statistical analysis
Statistical analyses were performed using RStudio 1.2.1335 for Windows. Descriptive statistics were used to describe the characteristics of the study group and Shapiro-Wilk test was used to test for normality of the data. Differences in characteristics between sexes were analysed using Pearson’s Chi-squared test for categorical variables and analysis of variance (ANOVA) for numerical data with normal distribution. As food group intake data was non-normal, the Kruskal-Wallis test was used for between-group comparisons. Unadjusted Spearman’s rank correlation coefficients were calculated to initially establish correlations between food intake and frailty score, followed by partial rank correlation coefficients adjusted for the following covariates; energy intake (kcal/day), age (years), gender (male/female) and BMI (kg/m2). To visualise the relationship, scatterplots (with regression lines and standard errors) were created for the partial correlation of each food group against frailty score, adjusted for the covariates mentioned above. Dietary data was normalised prior to multiple regression analyses by organising each food group into gender-specific tertiles of intake. The cut-off values for each tertile were determined by using the PERCENTILE function in Microsoft Excel, and participants were then categorised into their corresponding tertile for intake of each food group (lower, middle, upper). Ordinal logistic regression was applied to model the relationship between food group intakes and frailty, with frailty category entered as the dependant variable. A cut-point of P < .1 for correlation with univariate analyses (Table 3) was used for inclusion of dietary variables in the model.26 Variance inflation factors (VIFs) were calculated to assess for multi-collinearity (a VIF of <4 was used as a cut-point for inclusion)27 and the Brant test was performed to test for the assumption of proportional odds. The model was adjusted for energy intake (kcal/day), age (years) gender (male/female), BMI (kg/m2) and use of dietary supplements (yes/no). Odds ratios (ORs) complemented by 95% confidence intervals (95%CIs) were calculated for each dietary variable in the model, with the upper tertile of intake used as reference. A significance level of 5% was used for interpretation of all statistical analyses.
Table 3.
Spearman’s rank (rho) and partial rank† (partial r) correlation coefficients of dietary variables with frailty score.
| Variable | Rho | Partial r |
|---|---|---|
| Meat and meat products | 0.147 | 0.164 |
| Fish and fish products | −0.257** | −0.297*** |
| Milk and milk products | 0.077 | 0.056 |
| Fruit and vegetables | −0.363*** | −0.346*** |
| Cereals and cereal products | −0.007 | −0.070 |
| Potatoes | 0.199* | 0.179* |
| Soups and sauces | −0.103 | −0.124 |
| Nuts and seeds | −0.242** | −0.148 |
| Fats and oils | 0.179* | 0.199* |
| Sugars, preserves and snacks | 0.217** | 0.299** |
| Alcoholic beverages | 0.041 | 0.120 |
| Non-alcoholic beverages | 0.019 | −0.015 |
Adjusted for gender, age, energy intake and body mass index.
P < .05; **P < .01; ***P < .001; values in bold indicate statistical significance (P < .05)
Results
The main characteristics of the study sample by frailty status are presented in Table 2. A total of 81 (57.0%) participants were female, 93 (65.5%) participants lived in an urban area and 111 (78.2%) participants were overweight. Of the 142 participants, 17 (12.0%) participants were classified as frail, 49 (34.5%) participants were pre-frail and 76 (53.5%) participants were non-frail. The prevalence of pre-frailty and frailty increased with increasing age (P < .001). No significant differences in gender, BMI, area of residence or weight between frailty classes were observed. Compared to those who were non-frail, frail participants had a diet that was higher in meat, milk products, potatoes, fats, sugars, snacks and alcohol, and lower in fish, fruit and vegetables, cereal products and nuts and seeds. However, the Kruskal-Wallis test found these between-group differences to be statistically insignificant (P > .05).
Table 2.
Characteristics of study sample by frailty status (n = 142).
| Characteristics | Total (n = 142) | Non-frail (n = 76) | Pre-frail (n = 49) | Frail (n = 17) |
|---|---|---|---|---|
| Female sex, n (%) | 81 (57.0%) | 40 (52.6%) | 32 (65.3%) | 9 (52.9%) |
| Age (y, mean ± SD)*** | 74.1 ± 6.80 | 71.7 ± 5.23 | 75.2 ±7.04 | 82.1 ± 5.71 |
| Living in urban area n (%) | 93 (65.5%) | 51 (67.1%) | 32 (65.3%) | 10 (58.8%) |
| Weight (kg, mean ± SD) | 76.9 ± 14.23 | 77.3 ± 11.8 | 75.1 ± 16.09 | 80.2 ± 18.29 |
| Body Mass Index (kg/m2) | 28.8 ± 5.01 | 28.4 ± 3.71 | 28.6 ± 6.25 | 30.7 ± 5.90 |
| Energy intake, kcal/day, median (IQR) | 1758.0 (1386.73-2094.31) | 1677.8 (1386.73- 2190.86) | 1846.0 (1441.42-2097.10) | 1767.0 (1585.67-2215.73) |
| Food group intakes, g/day, median (IQR) | ||||
| Meat and meat products | 92.5 (68.67-124.83) | 88.0 (60.42-121.65) | 105.4 (73.43-124.79) | 112.8 (91.78-150.05) |
| Fish and fish products | 32.1 (16.10-53.73) | 32.1 (24.06-66.39) | 32.1 (16.10-49.02) | 19.3 (12.34-27.23) |
| Milk and milk products | 307.0 (181.24-448.81) | 296.6 (168.40-440.68) | 347.2 (225.02-449.56) | 288.4 (180.62-490.66) |
| Fruit and vegetables | 462.1 (334.81-665.57) | 521.3 (411.68-775.60) | 407.2 (284.17-543.70) | 349.1 (212.45-443.35) |
| Cereals and cereal products | 262.3 (189.53-332.03) | 253.2 (177.45-317.09) | 275.6 (202.55-349.65) | 251.3 (194.03-299.46) |
| Potatoes | 116.4 (71.39-136.97) | 106.3 (64.93-127.23) | 116.4 (71.39-142.64) | 133.8 (125.00-144.35) |
| Soups and sauces | 44.1 (22.48-102.33) | 52.3 (23.63-106.13) | 39.2 (20.30-103.43) | 40.9 (20.30-66.40) |
| Nuts and seeds | 0 (0-4.20) | 0 (0-8.70) | 0 (0-2.10) | 0 (0-0) |
| Fats and oils | 24.0 (13.51-34.60) | 20.3 (12.96- 31.80) | 25.9 (12.52-36.76) | 32.0 (24.22-39.19) |
| Sugars, preserves and snacks | 29.53 (17.78-62.47) | 24.7 (14.11-58.99) | 29.8 (18.00-63.75) | 70.9 (31.00-83.52) |
| Alcoholic beverages | 8.75 (0-56.96) | 8.8 (0-57.39) | 9.9 (0-56.07) | 8.8 (0-23.00) |
| Non-alcoholic beverages | 679.42 (505.67-946.90) | 671.0 (514.66-902.38) | 693.0 (491.80-965.00) | 705.0 (556.70-855.00) |
Abbreviations: IQR: inter quartile range; SD: standard deviation.
P < .001 (for between-group difference); values in bold indicate statistical significance (P < .05).
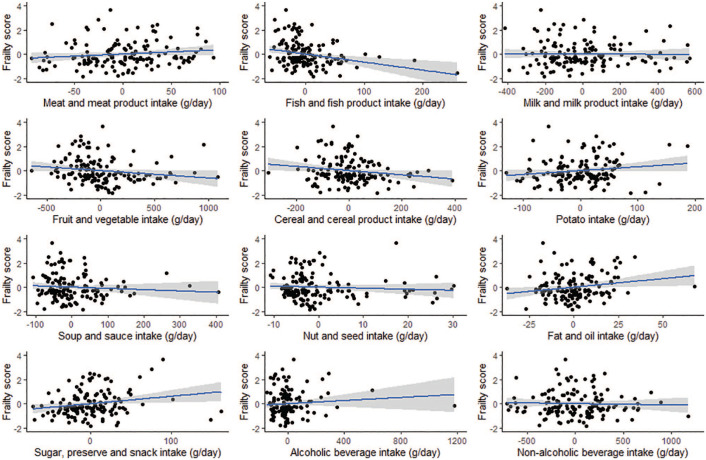
Spearman’s rank correlation and partial rank correlation coefficients for all dietary variables are presented in Table 3, with scatterplots for partial correlations of food groups with frailty score shown in Figure 1. There were a number of outliers in the dataset (Figure 1). Biological data is heterogeneous and often contains unusual, but not impossible values.28 These outliers were not excluded from the analysis, as on assessment of the FFQ responses, they were not found to be unrealistic. For example for alcoholic beverages, 1 participant’s intake appears to be outlying at 1296 g/day. However, the corresponding FFQ showed a plausible response (5-6 half pints of beer per day). All energy intakes were also examined to verify plausibility and a reasonable range of 893.6 to 3423.2 kcal/day was noted.29
Figure 1.
Partial regression plots (± standard error) for intakes of food groups with frailty score in Irish older adults (n = 142) adjusted for gender, age, energy intake and body mass index.
Prior to adjustment for confounders (using Spearman’s rho) intakes of the following food groups showed significant negative correlations with frailty score; fish and fish products, fruit and vegetables and nuts and seeds; while intakes of potatoes, sugars, preserves and snacks and fats and oils were positively correlated with frailty score (P < .05). Following adjustment for gender, age, energy intake and BMI (using partial correlation coefficients), all of the above correlations remained statistically significant (P < .05), with the exception of nuts and seeds (P > .05).
No multi-collinearity was detected by the VIFs of each dietary variable (all <4),27 therefore all variables with a P-value of <.1 for univariate analyses26 were included in the regression model (Table 4). The Brant test confirmed that the assumption of proportional odds was met. The ORs and 95%CIs for incident frailty by tertiles of food group intake are presented in Table 4. In adjusted analyses, the ORs (95%CI) for those in the lowest tertile of food group intake compared to the highest were; 3.04 (1.09-8.85) for fish and fish products, 4.34 (1.54-13.13) for fruit and vegetables, 1.52 (0.58-4.15) for nuts and seeds, 0.54 (0.19-1.51) for potatoes, 0.58 (0.17-1.95) for fats and oils and 0.49 (0.16-1.47) for sugars, preserves and snacks.
Table 4.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between food group intake and frailty incidence (n = 142).
| Food group | OR (95%CI) | |
|---|---|---|
| Tertile 2† | Tertile 1 (lower)† | |
| Fish and fish products | 2.31 (0.84-6.67) | 3.04 (1.09-8.85)* |
| Fruit and vegetables | 1.30 (0.44-3.85) | 4.34 (1.54-13.13)** |
| Nuts and seeds | 1.41 (0.32-6.04) | 1.52 (0.58-4.15) |
| Potatoes | 0.34 (0.12-0.90) | 0.54 (0.19-1.51) |
| Fats and oils | 0.50 (0.19-1.34) | 0.58 (0.17-1.95) |
| Sugars, preserves and snacks | 0.82 (0.30-2.23) | 0.49 (0.16-1.47) |
Model adjusted for gender, age, energy intake, body mass index and use of dietary supplements.
P < .05; **P < .01; †compared to tertile 3 (upper); values in bold indicate statistical significance (P < .05).
Discussion
The current study investigated the association between intake of individual food groups and frailty in a cohort of community-dwelling adults aged ⩾65 years in Ireland. The prevalence of pre-frailty and frailty in the study sample was 34.5% and 12.0%, respectively. These findings are similar to those reported on a national level by The Irish Longitudinal Study of Ageing (TILDA)8 when the same method of classification was used, and are in line with the overall estimated prevalence of frailty among European community-dwelling elderly.30
This study revealed a significant association between frailty and dietary intake; specifically with intakes of fish, fruit and vegetables, potatoes, fats and oils and sugars and snacks. One of the strongest relationships observed was between fish and fish products and frailty, with those in the lowest tertile of intake being 3.04 times more likely to be frail, compared to those with the highest intakes (P = .016). This relationship may be explained by the rich content of high quality protein and branch chain amino acids (BCAAs) found in fish.31 Dietary protein plays a well-recognised role in muscle health and strength in older adults17 by stimulating muscle protein synthesis through activation of the target of rapamycin (TOR) in skeletal muscle.32 This is an important consideration for older adults, as the muscle protein synthesis response to protein ingestion becomes blunted with ageing.33 Fish may thus provide a valuable protein source to those at risk of functional decline and promoting fish intake is worthy of investigation for frailty prevention. Interestingly, however, no association was observed between frailty and meat intake, the primary protein source in the Irish diet.34 This may be explained by the types of meat habitually consumed by the Irish population. Ham and bacon are the top sources of meat in Ireland, consumed by 73% of the population.34 These products can be highly processed and high in salt and fat, excessive intakes of which have several health implications,35 possibly counteracting any benefits of the rich protein content on frailty status.
The current study indicates that fruit and vegetable consumption is also associated with frailty status, with a low intake translating to a 4.34 higher odds of being frail (P = .002). This finding is consistent with prior research in European older adults.36 Fruit and vegetables are rich in fibre and micronutrients, each with a specific role in preventing the onset of several diseases and health conditions,37 possibly translating to a lower frailty risk. Additionally, fruit and vegetables are a rich source of phytochemicals, a number of which function as antioxidants37 and some bearing strong anti-inflammatory properties.38 Oxidative stress and inflammatory markers are both elevated in frail persons.39,40 Higher oxidative stress can contribute to muscle atrophy,41 while similarly, inflammation is linked to measures of muscle mass, strength and function in older subjects.42 It is likely, therefore, that these properties contribute to the relationship observed. Despite their potential, however, increasing fruit and vegetable intake is yet to feature in frailty interventions, and clinical trials are warranted to explore this prospect.
This study revealed positive, yet weaker, associations between frailty and intake of potatoes, sugars, preserves and snacks and fats and oils. To the best of our knowledge, this relationship has not been reported elsewhere. Research has, however, drawn a recurrent link between reduced frailty risk and adherence to a Mediterranean style diet, high in fruit and vegetables, whole grains, nuts and seeds and fish and low in sugars, saturated fats and meat products.43,44 The findings of this research support this hypothesis. There are a number of factors which may explain the correlations observed. Those with a higher intake of sugars, potatoes, snacks and fats may be inclined to have a higher level of adiposity and fat mass, recently found to be characteristics of frail persons due to factors such as inflammation, oxidative stress, insulin resistance and increased risk of disease.45 It is also possible that those who consume sugars, snacks and fats more often have a poorer overall diet quality, lacking in essential nutrients for overall health maintenance,46 and, thus, increasing frailty risk. Excessive intake of sugars has also been linked to a number of adverse health outcomes in older adults, which may, in turn, increase the risk for frailty. Such outcomes include weakened bones, osteoporosis47 and cognitive decline,48 each shown to be linked to the incidence of frailty.49,50 Nonetheless, more detailed investigation with larger study samples is essential to explicate this relationship.
The estimated dietary intake of this cohort was similar to that reported by older adults in the most recent National Adult Nutrition Survey (NANS) for most foods.34 The difference in average energy intake between the 2 reports was trivial (11 kcal). The average intake of meat and meat products was, however, slightly higher in this study (92.5 g vs 67.0 g), while intake of fish and fish products was lower (32.1 g vs 50.0 g). There was a notably higher intake of fruit and vegetables in this study (462.0 g vs 285.0 g), while a smaller survey in Cork elderly estimated a range in between these 2 figures (328-378 g).51 It should be noted that the NANS report was completed 9 years prior to the current study and dietary trends may have somewhat diverged in this period. Additionally, participants of the current study were living in a local region of Ireland, only, and not nationwide. Thus, parallel estimates were not anticipated. Another interesting finding was that those who were frail had a slightly, although non-significantly higher BMI than those who were pre-frail and non-frail. Those who were frailer also tended to be older and since ageing is generally accompanied by decline in physical stature,52 this may have resulted in the higher BMI scores in this group.
It is important to consider the limitations of this study when interpreting the results. Firstly, as dietary intake was self-reported, it is possible that some social desirability and recall bias may have impacted the results. Consequentially, the diet-disease relationship may have been attenuated and some weaker associations may not have been detected by this study. Much consideration was given, however, when choosing an appropriate assessment tool that has been well-validated and is suitable for an older population. The EPIC FFQ has been shown to be capable of producing results similar to the more labour intensive 7-day food diary in older adults53 and has been frequently used as a dietary assessment tool in older populations.51,54,55 A mini-cog score20 of 3 or more was also included as inclusion criteria to minimise dietary recall error, and a researcher was available at all times to provide guidance to participants where necessary when completing the FFQs to avoid any reporting errors and optimise accuracy. The generalisability of the results of this study may also be somewhat limited by the small sample size of 142. Due to the short time period allocated for the study, a large study group was not attainable in this instance. Further research with an increased sample size is desirable to confirm these findings. Finally, the cross-sectional design of this study has certain limitations. Dietary intake may be affected by the presence of frailty as well as vice versa, and causal relationships are difficult to establish from cross-sectional analysis. A prospective cohort design would be more advantageous in clarifying the exact contribution of diet to frailty incidence. However, cross-sectional observation was appropriate for the objectives of this study, which will assist in providing a basis for future longitudinal research.
Conclusion
A significant relationship was observed between frailty and food group intake in this cohort. Higher consumption of fish and fish products, fruit and vegetables and nuts and seeds were linked to a lower frailty risk. Contrastingly, higher intake of sugars, preserves and snacks, potatoes and fats and oils were positively associated with frailty risk, a finding which has not been reported elsewhere. Further research with larger study samples and longitudinal design is needed to fully elucidate this relationship. Additionally, intervention studies are warranted to establish if replacing sugars, fats, snacks and potatoes with more fish, fruit and vegetables and nuts and seeds can reduce and/or prevent the prevalence of the condition.
Acknowledgments
The authors would like to thank Cork Institute of Technology for providing the funding for this research, and the participants of the study for their time.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Cork Institute of Technology funded this research through the Risam Scholarship. We declare that the funding body has no role in the design of the study.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AM and TC formed the study idea. MOC and TA carried out the data collection under the supervision of AM and TC. Data analysis and interpretation were performed by MOC, SL, TC, JW and AM. All authors contributed to the writing of and approved the final manuscript.
Ethical Approval/Patient Consent: All participants were informed about the research methods and gave written consent before participating. The methods of the study were approved by the Cork Institute of Technology Research Ethics Committee (Cork, Ireland) in December 2018.
ORCID iDs: Maeve Lorraine O’Connell  https://orcid.org/0000-0002-2411-2782
https://orcid.org/0000-0002-2411-2782
Seán Lacey  https://orcid.org/0000-0003-3005-6294
https://orcid.org/0000-0003-3005-6294
References
- 1. Xue Q. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clegg A, Young J, Iliffe S, Rikkert M, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752-762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li G, Ioannidis G, Pickard L, et al. Frailty index of deficit accumulation and falls: data from the Global Longitudinal Study of Osteoporosis in Women (GLOW) Hamilton cohort. BMC Musculoskelet Disord. 2014;15:185. doi: 10.1186/1471-2474-15-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biritwum R, Minicuci N, Yawson A, et al. Prevalence of and factors associated with frailty and disability in older adults from China, Ghana, India, Mexico, Russia and South Africa. Maturitas. 2016;91:8-18. doi: 10.1016/j.maturitas.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 5. Choi J, Ahn A, Kim S, Won C. Global prevalence of physical frailty by Fried’s criteria in community-dwelling elderly with national population-based surveys. JAMDA. 2015;16:548-550. doi: 10.1016/j.jamda.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 6. Roe L, Normand C, Wren M, Browne J, O’Halloran A. The impact of frailty on healthcare utilisation in Ireland: evidence from the Irish longitudinal study on ageing. BMC Geriatr. 2017;17:1-12. doi: 10.1186/s12877-017-0579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hajek A, Bock J, Saum K, et al. Frailty and healthcare costs—longitudinal results of a prospective cohort study. Age Ageing. 2017;47:233-241. doi: 10.1093/ageing/afx157. [DOI] [PubMed] [Google Scholar]
- 8. Bock J, König H, Brenner H, et al. Associations of frailty with health care costs – results of the ESTHER cohort study. BMC Health Serv Res. 2016;16:1-11. doi: 10.1186/s12913-016-1360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parsons T, Papachristou E, Atkins J, et al. Physical frailty in older men: prospective associations with diet quality and patterns. Age Ageing. 2019;48:355-360. doi: 10.1093/ageing/afy216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shikany J, Barrett-Connor E, Ensrud K, et al. Macronutrients, diet quality, and frailty in older men. J Gerontol A Biol Sci. 2013;69:695-701. doi: 10.1093/gerona/glt196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Isanejad M, Sirola J, Rikkonen T, et al. Higher protein intake is associated with a lower likelihood of frailty among older women, Kuopio OSTPRE-Fracture Prevention Study. Eur J Nutr. 2019;59:1181-1189. doi: 10.1007/s00394-019-01978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coelho-Júnior H, Rodrigues B, Uchida M, Marzetti E. Low protein intake is associated with frailty in older adults: a systematic review and meta-analysis of observational studies. Nutrients. 2018;10:1334. doi: 10.3390/nu10091334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beasley J, LaCroix A, Neuhouser M, et al. Protein intake and incident frailty in the Women’s health initiative observational study. J Am Geriatr Soc. 2010;58:1063-1071. doi: 10.1111/j.1532-5415.2010.02866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giezenaar C, Chapman I, Luscombe-Marsh N, Feinle-Bisset C, Horowitz M, Soenen S. Ageing is associated with decreases in appetite and energy intake - a meta-analysis in healthy adults. Nutrients. 2016;8:28. doi: 10.3390/nu8010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hickson M. Malnutrition and ageing. Postgrad Med J. 2006;82:2-8. doi: 10.1136/pgmj.2005.037564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nowson C, Service C, Appleton J, Grieger J. The impact of dietary factors on indices of chronic disease in older people: A systematic review. J Nutr Health Aging. 2017;22:282-296. doi: 10.1007/s12603-017-0920-5. [DOI] [PubMed] [Google Scholar]
- 17. Paddon-Jones D, Leidy H. Dietary protein and muscle in older persons. Curr Opin Clin Nutr Metab Care. 2014;17:5–11. doi: 10.1097/MCO.0000000000000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Department of Health. The food pyramid. Published 2016. Accessed May 10, 2020. https://www.safefood.eu/SafeFood/media/SafeFoodLibrary/Documents/Healthy%20Eating/M9617-DEPARTMENT-OF-HEALTH_Food-Pyramid-Poster_Simple-Version-NEW.pdf.
- 19. Federman A, Sano M, Wolf M, Siu A, Halm E. Health literacy and cognitive performance in older adults. J Am Geriatr Soc. 2009;57:1475-1480. doi: 10.1111/j.1532-5415.2009.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steenland N, Auman C, Patel P, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. J Alzheimers Dis. 2008;15:419-427. doi: 10.3233/jad-2008-15308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fried L, Tangen C, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci. 2001;56:M146-M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 22. Washburn R, Smith K, Jette A, Janney C. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153-162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 23. Loh Y, Jakszyn P, Luben R, Mulligan A, Mitrou P, Khaw K. N-nitroso compounds and cancer incidence: the European prospective investigation into cancer and nutrition (EPIC)–Norfolk study. Am J Clin Nutr. 2011;93:1053-1061. doi: 10.3945/ajcn.111.012377. [DOI] [PubMed] [Google Scholar]
- 24. Mulligan A, Luben R, Bhaniani Parry-Smith D, et al. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open. 2014;4:1-11. doi: 10.1136/bmjopen-2013-004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whigham L, Schoeller D, Johnson L, Atkinson R. Effect of clothing weight on body weight. Int J Obes. 2012;37:160-161. doi: 10.1038/ijo.2012.20. [DOI] [PubMed] [Google Scholar]
- 26. Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: logistic regression. Perspect Clin Res. 2017;8:148-151. doi: 10.4103/picr.PICR_87_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hair J, Black W, Babin B, Anderson R. Multivariate Data Analysis. Prentice Hall; 2009. [Google Scholar]
- 28. Woolley C, Handel I, Bronsvoort M, Schoenebeck J, Clements D. Is it time to stop sweeping data cleaning under the carpet? A novel algorithm for outlier management in growth data. Plos One. 2020;15:e0228154. doi: 10.1371/journal.pone.0228154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. ter Borg S, Verlaan S, Mijnarends DM, Schols J, de Groot L, Luiking YC: Macronutrient intake and inadequacies of community-dwelling older adults, a systematic review. Ann Nutr Metab. 2015;66:242-255. doi: 10.1159/000435862 [DOI] [PubMed] [Google Scholar]
- 30. O’Caoimh R, Galluzzo L, Rodríguez-Laso A, et al. Prevalence of frailty at population level in European ADVANTAGE joint action member states: a systematic review and meta-analysis. Ann 1st Super Sanita. 2018;54:226-238. doi: 10.4415/ANN_18_03_10 [DOI] [PubMed] [Google Scholar]
- 31. Khalili Tilami S, Sampels S. Nutritional value of fish: lipids, proteins, vitamins, and minerals. Rev Fish Sci Aquac. 2017;26:243-253. doi: 10.1080/23308249.2017.1399104 [DOI] [Google Scholar]
- 32. Drummond M, Rasmussen B. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr. 2008;11:222-226. doi: 10.1097/MCO.0b013e3282fa17fb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wall B, Gorissen S, Pennings B, et al. aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. Plos One. 2015;10(11):e0140903. doi: 10.1371/journal.pone.0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Irish Universities Nutrition Alliance. National adult nutrition survey summary report March 2011. Accessed November 4, 2019. https://irp-cdn.multiscreensite.com/46a7ad27/files/uploaded/The%20National%20Adult%20Nutrition%20Survey%20Summary%20Report%20March%202011.pdf Published 2011.
- 35. Safe Food. The food pyramid – Foods and drinks high in fat, sugar and salt. Accessed November 6, 2019. https://www.safefood.eu/Healthy-Eating/The-Food-Pyramid-and-The-Eatwell-Guide/The-Food-Pyramid/Sugar-and-confectionery.aspx. Published 2019.
- 36. García-Esquinas E, Rahi B, Peres K, et al. Consumption of fruit and vegetables and risk of frailty: a dose-response analysis of 3 prospective cohorts of community-dwelling older adults. Am J Clin Nutr. 2016;104:132-142. doi: 10.3945/ajcn.115.125781. [DOI] [PubMed] [Google Scholar]
- 37. Van Duyn M, Pivonka E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional. J Am Diet Assoc. 2000;100:1511-1521. doi: 10.1016/S0002-8223(00)00420-X [DOI] [PubMed] [Google Scholar]
- 38. Zhu F, Du B, Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: a review. Crit Rev Food Sci Nutr. 2017;58:1260-1270. doi: 10.1080/10408398.2016.1251390. [DOI] [PubMed] [Google Scholar]
- 39. Soysal P, Isik A, Carvalho A, et al. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas. 2017;99:66-72. doi: 10.1016/j.maturitas.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 40. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1-8. doi: 10.1016/j.arr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 41. Powers S, Smuder A, Judge A. Oxidative stress and disuse muscle atrophy. Curr Opin Clin Nutr. 2012;15:240-245. doi: 10.1097/MCO.0b013e328352b4c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Westbury L, Fuggle N, Syddall H, et al. Relationships between markers of inflammation and muscle mass, strength and function: findings from the Hertfordshire Cohort Study. Calcified Tissue Int. 2017;102:287-295. doi: 10.1007/s00223-017-0354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ntanasi E, Yannakoulia M, Kosmidis M, et al. Adherence to Mediterranean diet and frailty. JAMDA. 2018;19:315-322. doi: 10.1016/j.jamda.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 44. León-Muñoz L, Guallar-Castillón P, López-García E, Rodríguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. JAMDA. 2014;15:899-903. doi: 10.1016/j.jamda.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 45. Xu L, Zhang J, Shen S, et al. Association between body composition and frailty in elder inpatients. Clin Interv Aging. 2020;15:313-320. doi: 10.2147/CIA.S243211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Louie J, Tapsell L. Association between intake of total vs added sugar on diet quality: a systematic review. Nutr Rev. 2015;73:837-857. doi: 10.1093/nutrit/nuv044 [DOI] [PubMed] [Google Scholar]
- 47. DiNicolantonio J, Mehta V, Zaman SB, O’Keefe JH. Not salt but sugar as aetiological in osteoporosis: a review. Mo Med. 2018;115:247-252. [PMC free article] [PubMed] [Google Scholar]
- 48. Beilharz J, Maniam J, Morris M. Diet-induced cognitive deficits: the role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients. 2015;7:6719-6738. doi: 10.3390/nu7085307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bartosch P, McGuigan F, Akesson K. Progression of frailty and prevalence of osteoporosis in a community cohort of older women—a 10-year longitudinal study. Osteoporosis Int. 2018;29:2191-2199. doi: 10.1007/s00198-018-4593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raji M, Al Snih S, Ostir G, Markides K, Ottenbacher K. Cognitive status and future risk of frailty in Older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2010;65A:1228-1234. doi: 10.1093/gerona/glq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Power SE, Jeffery IB, Ross RP, et al. Food and nutrient intake of Irish community-dwelling elderly subjects: who is at nutritional risk? J Nutr Health Aging. 2014;18:561-72. doi: 10.1007/s12603-014-0449-9. PMID: 24950145. [DOI] [PubMed] [Google Scholar]
- 52. Yamaguchi M, Yamada Y, Nanri H, et al. Association between the frequency of protein-rich food intakes and kihon-checklist frailty indices in older Japanese adults: the Kyoto-Kameoka Study. Nutrients. 2018;10:84. doi: 10.3390/nu10010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bartali B, Turrini A, Salvini S, et al. Dietary intake estimated using different methods in two Italian older populations. Arch Gerontol Geriatr. 2004;38:51-60. doi: 10.1016/s0167-4943(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 54. Bartali B, Frongillo EA, Bandinelli S, et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006;61A:589-593. doi: 10.1093/gerona/61.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cesari M, Pahor M, Bartali B, et al. Antioxidants and physical performance in elderly persons: the Invecchiare in Chianti (inCHIANTI) study. Am J Clin Nutr. 2004;79:289-294. doi: 10.1093/ajcn/79.2.289. [DOI] [PubMed] [Google Scholar]