Key Points
Question
Is the receipt of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) associated with worse clinical outcomes among patients with COVID-19?
Findings
In this systematic review and meta-analysis of 52 studies that evaluated clinical outcomes among 101 949 total patients with COVID-19 who did and did not receive ACEIs or ARBs, a significantly lower risk of multivariable-adjusted mortality and severe adverse events was found among patients who received ACEIs or ARBs compared with patients who did not. A subgroup analysis of patients with hypertension indicated significant decreases in mortality and severe adverse events among patients receiving ACEIs or ARBs in both unadjusted and adjusted analyses.
Meaning
The study’s findings suggest that ACEIs and ARBs may be associated with protective benefits for patients with COVID-19 and that patients may continue receiving ACEIs and ARBs for the treatment of any condition without an increased risk of worse outcomes unless specifically advised to avoid them by treating clinicians.
Abstract
Importance
The chronic receipt of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) has been assumed to exacerbate complications associated with COVID-19 and produce worse clinical outcomes.
Objective
To conduct an updated and comprehensive systematic review and meta-analysis comparing mortality and severe adverse events (AEs) associated with receipt vs nonreceipt of ACEIs or ARBs among patients with COVID-19.
Data Sources
PubMed and Embase databases were systematically searched from December 31, 2019, until September 1, 2020.
Study Selection
The meta-analysis included any study design, with the exception of narrative reviews or opinion-based articles, in which COVID-19 was diagnosed through laboratory or radiological test results and in which clinical outcomes (unadjusted or adjusted) associated with COVID-19 were assessed among adult patients (≥18 years) receiving ACEIs or ARBs.
Data Extraction and Synthesis
Three authors independently extracted data on mortality and severe AEs associated with COVID-19. Severe AEs were defined as intensive care unit admission or the need for assisted ventilation. For each outcome, a random-effects model was used to compare the odds ratio (OR) between patients receiving ACEIs or ARBs vs those not receiving ACEIs or ARBs.
Main Outcomes and Measures
Unadjusted and adjusted ORs for mortality and severe AEs associated with COVID-19.
Results
A total of 1788 records from the PubMed and Embase databases were identified; after removal of duplicates, 1664 records were screened, and 71 articles underwent full-text evaluation. Clinical data were pooled from 52 eligible studies (40 cohort studies, 6 case series, 4 case-control studies, 1 randomized clinical trial, and 1 cross-sectional study) enrolling 101 949 total patients, of whom 26 545 (26.0%) were receiving ACEIs or ARBs. When adjusted for covariates, significant reductions in the risk of death (adjusted OR [aOR], 0.57; 95% CI, 0.43-0.76; P < .001) and severe AEs (aOR, 0.68; 95% CI, 0.53-0.88; P < .001) were found. Unadjusted and adjusted analyses of a subgroup of patients with hypertension indicated decreases in the risk of death (unadjusted OR, 0.66 [95% CI, 0.49-0.91]; P = .01; aOR, 0.51 [95% CI, 0.32-0.84]; P = .008) and severe AEs (unadjusted OR, 0.70 [95% CI, 0.54-0.91]; P = .007; aOR, 0.55 [95% CI, 0.36-0.85]; P = .007).
Conclusions and Relevance
In this systematic review and meta-analysis, receipt of ACEIs or ARBs was not associated with a higher risk of multivariable-adjusted mortality and severe AEs among patients with COVID-19 who had either hypertension or multiple comorbidities, supporting the recommendations of medical societies. On the contrary, ACEIs and ARBs may be associated with protective benefits, particularly among patients with hypertension. Future randomized clinical trials are warranted to establish causality.
This systematic review and meta-analysis examines the association between the receipt of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and clinical outcomes among adult patients with coronavirus disease 2019.
Introduction
Coronavirus disease 2019 (COVID-19), a rapidly evolving pandemic infecting more than 93 million people worldwide to date,1 is associated with worse clinical outcomes in patients with existing cardiovascular diseases, including hypertension and diabetes.2,3 Renin-angiotensin-aldosterone system (RAAS) inhibitors, specifically angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), which are frequently used for the treatment of cardiovascular conditions, are subjects of debate because angiotensin-converting enzyme 2 acts as a binding site for the virus to gain cellular entry.4 This debate has elicited several theories suggesting that the chronic receipt of RAAS inhibitors may exacerbate COVID-19 and produce worse outcomes.4
Several observational studies have since evaluated the association of ACEIs and ARBs (ACEIs/ARBs) with clinical outcomes in patients with COVID-19. Although a few studies have reported an increased risk of severe disease,5,6 most have found no association7,8 or even beneficial associations with the receipt of these drugs.9,10 A previous meta-analysis11 that examined 16 studies with 28 000 total patients reported no significant association between the receipt of ACEIs/ARBs and mortality or severe adverse events (AEs) among individuals with multiple comorbidities (OR, 0.67; 95% CI, 0.44-1.03; P = .07) and a significant association between ACEIs/ARBs and protective benefits among individuals with hypertension (OR, 0.67; 95% CI, 0.50-0.91; P = .01). Since the publication of that meta-analysis, more original studies have been published, allowing increased statistical power to further investigate specific subgroups.
Given the increasing number of COVID-19 cases and an evolving second wave of infections, it is important to summarize the data thus far to provide an updated perspective and an understanding of the association between ACEIs/ARBs and clinical COVID-19 outcomes. The inclusion of more studies and patients will allow the identification of more accurate associations with smaller CIs, producing findings that are more likely to represent true associations. In addition, the inclusion of only peer-reviewed studies will reinforce the conclusions. Findings from the present meta-analysis will be relevant for the clinical management of millions of patients receiving these drugs worldwide.12
Methods
Study Selection
The PubMed and Embase databases were systematically searched from December 31, 2019, until September 1, 2020, for studies published in English. Terms such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, coronavirus disease 2019, and SARS-COV-2 were used for a comprehensive search. Additional details about the search strategy are available in eMethods in the Supplement. The references of retrieved articles were manually screened for relevant studies to expand the search. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
All studies identified in our search were screened by 3 authors (R.B., M.D., and V.T.) using article titles and abstracts. Duplicate studies and multiple reports using the same data were removed. Any article identified as having the potential to fulfill our inclusion criteria underwent full-text evaluation. We included studies meeting the following criteria: (1) any study design, with the exception of narrative reviews and opinion-based articles; (2) adult (≥18 years) study population; (3) participants with COVID-19 diagnosed through laboratory or radiological test results; and (4) assessment of clinical or mortality outcomes (unadjusted or adjusted) among patients receiving ACEIs/ARBs. The mortality and clinical severity data of patients receiving ACEIs/ARBs were compared with those of patients not receiving ACEIs/ARBs.
Data Extraction and Quality Assessment
Three authors (R.B., V.T., and M.D.) independently extracted relevant data from included studies using a standardized extraction form. Any disagreements were resolved by discussion. The data extracted included the type of study, the number and characteristics of patients receiving ACEIs/ARBs, and mortality and severe AEs associated with COVID-19.
Severe AEs were defined as intensive care unit admission or the need for invasive or noninvasive ventilation. Studies reporting severe AEs based on information from the Chinese Center for Disease Control and Prevention13 were included. To avoid double-counting of patients in studies reporting multiple severe AE outcomes, we included the outcome with the largest number of patients in our analyses. For instances in which distinct data for ACEIs/ARBs were available, an aggregate was used given the small likelihood of combined receipt of both drugs.
The Newcastle-Ottawa Scale,14 a 9-point measure assessing the quality of cohort studies and case-control studies or case series, was used to evaluate the observational studies included. The Cochrane Risk of Bias 2 tool was used to assess the risk of bias in randomized clinical trials.15
Statistical Analysis
For each outcome, a random-effects model was used to compare the odds ratios (ORs) and 95% CIs between patients who did and did not receive ACEIs/ARBs using Review Manager software, version 5.3 (Nordic Cochrane Center), and OpenMeta[Analyst] software, version 10.12 (Center for Evidence Synthesis, Brown University).16 For studies reporting hazard ratios (HRs), those HRs were converted to ORs using methodology defined in the Cochrane Handbook for Systematic Reviews of Interventions.17 Results from studies were grouped according to a prespecified variable (patients with hypertension [hypertension subgroup] vs patients with multiple mixed comorbidities [mixed subgroup]), and a series of subgroup analyses were performed. We also conducted a sensitivity analysis, in which studies reporting HRs (which were converted to ORs) were excluded to assess the robustness of results.
Statistical heterogeneity was assessed using the I2 statistic. Potential publication bias was assessed using funnel plots. The statistical significance threshold was P < .05.
Results
Our search identified 1788 records from the PubMed and Embase databases; after removal of duplicates, 1664 records were screened, and 71 articles underwent full-text evaluation (eFigure 1 in the Supplement). Of those, 52 studies (40 cohort studies, 6 case series, 4 case-control studies, 1 randomized clinical trial, and 1 cross-sectional study) with 101 949 total participants met inclusion criteria and were included in the meta-analysis. The cohorts and methodological characteristics of the studies are described in the Table. Ten studies were ranked as having moderate quality and 41 studies were ranked as having high quality based on the Newcastle-Ottawa Scale (eTable in the Supplement). Funnel plots indicated no substantial publication biases (eFigure 2 and eFigure 3 in the Supplement).
Table. Baseline Characteristics of Included Studies.
| Source | Study type and location | Participants receiving ACEIs or ARBs, No. | Total participants, No. | Participant age, y | Male sex, % | Participants with hypertension, % | Participant clinical characteristics, % | Outcomes measured | Adjustment methods |
|---|---|---|---|---|---|---|---|---|---|
| Amat-Santos et al,18 2020 | Ongoing open-label RCT (RASTAVI), Spain | 5 | 11 | Median (IQR), 86 (84-88) | 54.5 | 54.5 | CKD: 36.4 Diabetes: 18.2 CAD: 18.2 |
Mortality | NA |
| Conversano et al,19 2020 | Retrospective case series, Italy | 68 | 96 | NR | NR | 100 | Hypertension: 100 CHD: 28.1 Diabetes: 22.9 Heart failure: 8.3 |
Mortality; median follow-up of 28 d | NA |
| Bae et al,20 2020 | Retrospective cohort study, US | 78 | 590 | NR | 48.8 | 25.4 | Diabetes: 26.3 CAD: 5.3 Heart failure: 3.6 |
Mortality and critical severity (ICU admission) | Multivariate logistic regression with propensity matching for age and comorbidities (hypertension, dyslipidemia, diabetes or prediabetes, CAD, heart failure, CVA, chronic lung disease, and CKD or ESRD) |
| Bean et al,21 2020 | Prospective multicenter cohort study, United Kingdom | 399 | 1200 | Mean (SD), 68 (17) | 57.2 | 53.8 | Diabetes: 34.8 IHD: 13.3 Heart failure: 8.9 |
Mortality and critical severity (ICU admission); follow-up of 21 d | Multivariate logistic regression adjusted for age, sex, and comorbidities (hypertension, diabetes, IHD, heart failure, and CKD) |
| Bravi et al,7 2020 | Retrospective case-control study, Italy | 450 | 543 | Mean (SD), 58 (21) | 47.3 | 33.9 | Diabetes: 12.1 COPD: 6.0 |
Very severe or lethal (ICU admission or death) | Multivariate logistic regression adjusted for age, sex, and comorbidities (diabetes, major cardiovascular diseases, COPD, cancer, and renal diseases) |
| Cannata et al,22 2020 | Prospective cohort study, Italy | 173 | 397 | NR | NR | NR | NR | Mortality; follow-up until death or discharge | Multivariate logistic regression adjusted for age, BMI, comorbidities (diabetes, COPD, LVEF<50%, and cancer), vital parameters, and laboratory values within 24 h after admission |
| Chen et al,23 2020 | Retrospective, cohort study, China | 355 | 1182 | Median (IQR), 68 (60-75) | 49.1 | 100 | Diabetes: 22.1 CHD: 17.4 CKD: 5.3 |
Mortality and critical severity (need for IMV); follow-up of 45 d | Multivariable Cox proportional hazards regression analyses adjusted for age, sex, comorbidities (CHD and diabetes), laboratory findings (creatinine levels), and receipt of various medications |
| Chen et al,24 2020 | Retrospective cohort study, China | 81 | 312 | Median (IQR), 69 (61-77) | 55.1 | 93.9 | CAD: 25.6 | Mortality | Multivariable logistic regression models adjusted for clinically relevant parameters (age, sex, comorbidities, and laboratory findings) that differed between the 2 groups |
| Chen et al,25 2020 | Retrospective cohort study, China | 32 | 71 | NR | NR | NR | Diabetes: 100 Hypertension: 100 |
Mortality and clinical outcome of discharge or death in hospital |
NA |
| Hippisley-Cox et al,26 2020 | Prospective cohort study, United Kingdom | 4281 | 19 486 | Mean (SD), 62 (21) | 48.12 | 38.9 | CVD: 18.2 COPD: 7.3 Diabetes: 7.0 |
Critical severity (ICU admission) | Cox proportional hazards models using imputed analysis including all exposure and explanatory variables |
| Felice et al,9 2020 | Prospective cohort study, Italy | 82 | 133 | NA | 64.7 | 100 | Diabetes: 25.6 Heart failure: 18.0 COPD: 10.5 |
Mortality and critical severity (ICU admission); mean follow-up of 15.8 d | Multivariable logistic regression adjusted for age, sex, BMI, days with duration of symptoms before admission, and comorbidities (previous cardiovascular events, diabetes, and cancer) |
| Feng et al,4 2020 | Retrospective cohort study, China | 33 | 113 | NR | NR | 100 | NR | Mortality and critical severity based on CCDC report | NA |
| Fosbol et al,27 2020 | Retrospective cohort study, Denmark | 895 | 4480 | NR | 47.9 | 18.8 | COPD:14.2 Diabetes: 9.2 CVD: 9.0 Heart failure: 5.4 |
Mortality and critical severity (ICU admission); median follow-up of 34 d | Cox regression adjusted for sex, highest obtained education, income, comorbidities (myocardial infarction, heart failure, CKD, stroke, peripheral artery disease, AF, diabetes, COPD, and cancer) and various medications |
| Gao et al,28 2020 | Retrospective cohort study, China | 183 | 850 | Mean (SD), 64 (11) | 52.1 | 100 | Diabetes: 26.8 COPD: 1.3 Heart failure: 1.2 |
Mortality and critical severity based on CCDC report | Cox proportional hazards model adjusted for age, sex, medical history (diabetes and IHD), sex, RAAS inhibitor (ACEI or ARB), insulin-treated diabetes, myocardial infarction, treatment by PCI or CABG, renal failure, chronic heart failure, asthma, COPD, and stroke |
| Gormez et al,29 2020 | Retrospective cohort study, Turkey | 49 | 247 | NR | 62.3% | 31.6% | Diabetes: 39.7% COPD: 5.7% CKD: 4.0% Heart failure: 0.8% |
Critical severity (ICU admission); median follow-up of 13 d (ICU) and 7 d (other) | Bayesian logistic regression adjusted for age, sex, D-dimer level, neutrophil to lymphocyte ratio, CRP, and history of hypertension |
| Grasselli et al,30 2020 | Retrospective cohort study, Italy | NR | 1608 | NR | NR | 100% | NR | Mortality | Multivariable Cox proportional hazards regression models including baseline characteristics, comorbidities, medications, and physiological variables at admission |
| Guo et al,31 2020 | Retrospective case series, China | 19 | 187 | Mean (SD), 59 (15) | 48.7 | 32.6 | Diabetes: 15.0 CHD: 11.2 |
Mortality | NA |
| Hu et al,64 2020 | Retrospective cohort study, China | 65 | 149 | Median (IQR), 57 (50-66) | 59.1 | 100 | Diabetes: 20.1 CKD: 4.0 COPD: 1.3 |
Mortality and critical severity (ICU admission) | NA |
| Huang et al,32 2020 | Prospective cohort study, China | 20 | 50 | NR | 54.0 | 100 | Diabetes: 8.0 COPD: 2.0 |
Mortality and critical severity based on CCDC report | NA |
| Hwang et al,33 2020 | Retrospective cohort study, South Korea | 13 | 103 | Mean (SD), 68 (15) | 50.5 | 55.3 | Diabetes: 34.0 CKD: 16.5 CVD: 11.7 |
Mortality | NA |
| Iaccarino et al,34 2020 | Cross-sectional study, Italy | 655 | 1591 | Mean (SD), 67 (0.4) | 64.0 | 54.9 | Diabetes: 16.9 CAD: 13.6 Heart failure: 11.8 CKD: 5.5 |
Mortality | NA |
| Jung et al,35 2021 | Prospective study (part of COVIP), Germany | 157 | 324 | Median (IQR), 75 (70-93) | 69.1 | 65.1 | Diabetes: 29.4 Heart failure: 14.1 |
Mortality | Multivariate logistic regression with propensity matching for age, BMI, sex, sequential organ failure assessment score, comorbidities (heart failure, IHD, renal insufficiency, chronic pulmonary disease, hypertension, and diabetes) |
| Jung et al,36 2020 | Retrospective cohort study, Korea | 377 | 1954 | NR | NR | NR | NR | Mortality and critical severity (need for IMV) | Multivariate logistic regression analysis adjusted for age, sex, Charlson Comorbidity Index score, immunosuppression, and hospital type |
| Khan et al,37 2020 | Prospective cohort study, United Kingdom | 27 | 88 | Mean (SD), 72 (14) | 56.8 | 100 | NR | Mortality and critical severity (ICU admission); follow-up for 60 d or until discharge or death | NA |
| Lam et al,38 2020 | Retrospective cohort study, US | 335 | 614 | NR | 55.0 | 100 | Diabetes: 40.7 CKD: 15.4 COPD: 13.4 Heart failure: 13.4 |
Mortality and critical severity (ICU admission) | NA |
| Li et al,39 2020 | Retrospective case series, China | 115 | 362 | Median (IQR), 66 (59-73) | 53.2 | 100 | Diabetes: 35.2% CVD: 18.8% CHD: 17.1% Heart failure: 2.8% |
Mortality and critical severity based on CCDC report | NA |
| Liabeuf et al,40 2020 | Retrospective cohort study, France | 96 | 268 | Median (IQR), 73 (61-84) | 58.2 | 56.7 | Diabetes: 20.5 COPD: 9.7 CKD: 7.1 |
Mortality and critical severity (ICU admission) | Multivariate logistic regression model adjusted for age, sex, BMI, and CHD |
| Liu et al,41 2021 | Retrospective single-center case series, China | 74 | 157 | NR | 46.5 | 100 | Diabetes: 27.3 CAD: 10.2 |
Critical severity based on CCDC criteria | NA |
| Lopez-Otero et al,42 2020 | Retrospective single-center cohort study, Spain | 210 | 965 | Mean (SD), 60 (20) | 43.9 | 30.9 | Diabetes: 12.8 CAD: 4.4 |
Mortality and critical severity (ICU admission) | Multivariate logistic regression models adjusted for variables with P < .05 in the univariate analysis (fever, oxygen saturation <95%, age, sex, obesity, health personnel, and dependency status) |
| Mancia et al,43 2020 | Case–control study, Italy | 2896 | 6272 | Mean (SD), 68 (13) | 63.3 | NR | CHD: 7.5 Heart failure: 5.1 |
Critical severity (assisted ventilation) and fatal infection | NA |
| Matsuzawa et al,44 2020 | Retrospective cohort study, Japan | 21 | 39 | Mean (SD), 71 (12) | 69.2 | 100 | Diabetes: 35.9 CKD: 7.7 COPD: 2.6 Heart failure: 2.6 |
Mortality and critical severity (ICU admission) | Multivariate logistic regression model adjusted for age, sex, and presence of diabetes |
| Mehta et al,6 2020 | Retrospective cohort study, US | 212 | 1735 | NR | NR | NR | NR | Mortality and critical severity (ICU admission) | Propensity score estimated using multivariable logistic regression model adjusted for age, sex, and comorbidities (hypertension, diabetes, heart failure, COPD, and CAD) |
| Meng et al,45 2020 | Retrospective cohort study, China | 17 | 42 | Median (IQR), 65 (56-69) | 57.1 | 100 | CHD: 19.0 Diabetes: 14.2 |
Mortality | NA |
| Mostaza et al,46 2020 | Retrospective cohort study, Spain | 192 | 404 | Mean (SD), 85 (5) | 54.7 | 73.8 | Diabetes: 28.0 Heart failure: 18.8 CKD: 15.6 |
Mortality; follow-up until death or discharge | NA |
| Oussalah et al,47 2020 | Retrospective longitudinal cohort study, France | 44 | 149 | Median (IQR), 65 (54-77) | 61.0 | 49.6 | Diabetes: 28.6 COPD: 11.3 CKD: 6.0 |
Mortality and critical severity (ICU admission and IMV) | NA |
| Pan et al,48 2020 | Retrospective cohort study, China | 41 | 282 | Median (IQR), 69 (62-76) | 50.7 | 100 | Diabetes: 27.2 CVD: 7.8 COPD: 2.8 |
Mortality and critical severity (ICU admission) | NA |
| Reynolds et al,49 2020 | Retrospective cohort study, US | 1293 | 2141 | NR | NR | 100 | NR | Severity (ICU admission, need for assisted ventilation, or death) | Multivariable logistic regression adjusted for demographic characteristics and comorbidities |
| Richardson et al,50 2020 | Case series, US | 456 | 1366 | NR | NR | 100 | NR | Mortality and critical severity (ICU admission); median follow-up of 4 d | NA |
| Rossi et al,51 2020 | Prospective cohort study, Italy | 818 | 2653 | NR | 50.1 | 18.1 | Diabetes: 12.0 Heart failure: 5.8 COPD: 5.4 CKD: 2.5 |
Mortality | Multivariate proportional hazards models adjusted for age, sex, and Charlson Comorbidity Index score |
| Sardu et al,52 2020 | Prospective cohort study, Italy | 45 | 62 | Mean (SD), 58 (18) | 66.1 | 100 | Diabetes: 25.8 COPD: 16.1 CVD: 11.3 |
Mortality and critical severity (ICU admission) | NA |
| Selcuk et al,5 2020 | Retrospective cohort study, Turkey | 74 | 113 | NR | 52.2 | 100 | Diabetes: 42.5 CAD: 24.8 Heart failure: 8.0 |
Mortality and critical severity (ICU admission) | Multivariate logistic regression analysis adjusted for age, CAD, receipt of ACEIs or ARBs, and laboratory findings (WBC count and D-dimer, creatinine, plasma glucose, and lactate dehydrogenase levels) |
| Senkal et al,53 2020 | Retrospective cohort study, Turkey | 165 | 248 | NR | NR | 100 | NR | Mortality and critical severity (ICU admission) | Cases matched to controls according to age, sex, number of days ill before hospital admission, comorbidities (diabetes, COPD or asthma, CAD, chronic heart failure, and CKD), current smoking status, and various medications |
| Shah et al,54 2020 | Retrospective cohort study, US | 207 | 531 | NR | 41.1 | 80.0 | Diabetes: 42.9 Chronic heart failure: 14.9 COPD: 7.5 CAD: 7.0 |
Mortality and critical severity (ICU admission) | Multivariable logistic regression analysis adjusted for age, sex, BMI, baseline comorbidities, and presenting illness severity |
| Tan et al,55 2020 | Retrospective cohort study, China | 31 | 100 | NR | 51.0 | 100 | Diabetes: 28.0 CHD: 18.0 |
Mortality and critical severity (ICU admission) | NA |
| Tedeschi et al,56 2020 | Prospective cohort study, Italy | 175 | 311 | Median (IQR), 76 (67-83) | 72.3 | 100 | CVD: 42.1 Diabetes: 23.8 COPD: 15.8 |
Mortality | Multivariate Cox regression analysis adjusted for age, sex, presence of comorbidities, and COPD |
| Trifiro et al,57 2020 | Retrospective cohort study, Italy | 9522 | 42 926 | Median (IQR), 69 (57-79) | 62.6 | 13.1 | Diabetes: 18.0 COPD: 3.5 Heart failure: 6.5 |
Mortality and critical severity (ICU admission) | Mixed-effects Cox proportional hazards model adjusted for center, age, sex, Charlson Comorbidity Index score, drug receipt, and comorbidities (pneumonia and influenza, IHD, AF, heart failure, hypertension, CVD, diabetes, liver disease, dementia, renal failure, COPD, cancer, and rheumatic diseases) |
| Xu et al,58 2020 | Retrospective cohort study, China | 40 | 101 | Median (IQR), 65 (58-73) | 52.5 | 100 | Diabetes: 18.8 COPD: 2.0 Heart failure: 1.0 |
Mortality and critical severity (ICU admission) | Multivariable analysis with logistic model adjusted for age and sex |
| Yang et al,59 2020 | Retrospective case-control study, China | 43 | 126 | Median (IQR), 66 (61-73) | 49.2 | 100 | Diabetes: 30.2 | Death and severity based on CCDC report | NA |
| Yuan et al,60 2020 | Retrospective cohort study, China | 196 | 733 | NR | NR | 100 | NR | Mortality | Propensity score estimated using multivariable logistic regression adjusted for age, sex, history of hypertension, chronic heart disease, diabetes, tumor, COPD, chronic liver disease, CKD, and baseline vital signs |
| Zhang et al,61 2020 | Retrospective cohort study, China | 188 | 1128 | Median (IQR), 64 (56-69) | 53.5 | 100 | Diabetes: 21.3 CHD: 11.6 CVD: 3.6 COPD: 0.5 |
Mortality and critical severity (ICU admission); follow-up of 28 d | Cox proportional hazards model adjusted for age, sex, comorbidities (CHD, CKD, CVD, and diabetes), and in-hospital medications |
| Zhou et al,10 2020 | Retrospective cohort study, China | 989 | 3572 | NR | NR | NR | NR | Mortality; follow-up of 28 d | Propensity score matching for age, sex, disease severity, comorbidities, and receipt of calcium channel blocker medication |
| Zhou et al,8 2020 | Retrospective case series, China | 15 | 36 | Mean (SD), 65 (10) | 52.8 | 100 | Diabetes: 25.0 CVD: 19.4 |
Mortality and transfer to high-level hospital | Multivariate logistic regression adjusted for age, sex, hospitalization time, and time from onset to hospital admission |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass graft; CAD, coronary artery disease; CCDC, Chinese Center for Disease Control and Prevention; CHD, coronary heart disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVIP, Corona Virus Disease in Very Elderly Intensive Care Patients study; CRP, C-reactive protein; CVA, cardiovascular accident; CVD, cerebrovascular disease; ESRD, end-stage renal disease; ICU, intensive care unit; IHD, ischemic heart disease; IMV, invasive mechanical ventilation; IQR, interquartile range; LVEF, left ventricular ejection fraction; NA, not applicable; NR, not reported; PCI, percutaneous coronary intervention; RAAS, renin-angiotensin-aldosterone system; RASTAVI, Remodeling After Transcatheter Aortic Valve Implantation clinical trial; RCT, randomized clinical trial; WBC, white blood cell.
Most studies included in the meta-analysis were retrospective20,21,33,44 or observational4,6,26,37,61,62,63 and were conducted in China,4,8,10,23,24,25,28,31,32,35,39,41,45,48,55,58,59,60,62,64,65,66 Europe,5,7,9,18,19,22,30,34,40,43,46,47,51,52,53,56,57 or North America.6,20,49,50,54 Two studies22,38 included a subgroup of patients receiving ACEIs/ARBs that were explicitly discontinued during hospital admission. The results from this subgroup of patients were included in the group of patients receiving ACEIs/ARBs and compared with those not receiving ACEIs/ARBs. In studies in which both multivariate and propensity-matched scores were reported,28,42,61 data from the multivariate analyses were used. A total of 26 545 of 101 949 patients (26.0%) overall and 4813 of 11 696 patients (41.2%) in the hypertension subgroup were receiving ACEIs/ARBs (Table).
Mortality
A total of 41 studies (69 577 total participants) that compared mortality rates of patients receiving vs not receiving ACEIs/ARBs were included in the meta-analysis. Overall, the results of the pooled unadjusted meta-analysis indicated no increases in the risk of death among those who received ACEIs/ARBs (unadjusted OR, 1.05; 95% CI, 0.86-1.29; P = .61; I2 = 85.0%) compared with those who did not (Figure 1). The subgroup analysis revealed significant reductions in mortality among patients in the hypertension subgroup who were receiving ACEIs/ARBs (unadjusted OR, 0.66; 95% CI, 0.49-0.91; P = .01). In contrast, the mixed subgroup comprising patients with multiple comorbidities indicated significant increases in mortality among those receiving ACEIs/ARBs (unadjusted OR, 1.46; 95% CI, 1.15-1.85; P = .002)
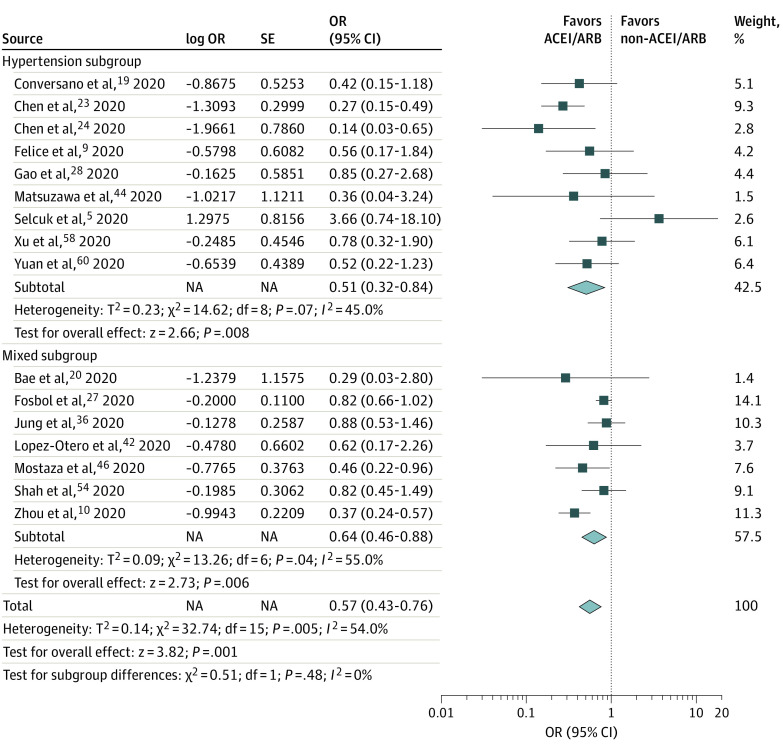
Figure 1. Subgroup Analysis of Unadjusted Mortality Among Patients Who Did and Did Not Receive ACEIs or ARBs.
Subgroup analysis of mortality in 41 studies of patients who did and did not receive ACEIs or ARBs. A total of 19 studies included a mixed subgroup (a sample population with multiple mixed comorbidities), and 22 studies included a hypertension subgroup (a sample population with hypertension). Diamonds represent 95% CIs for subtotal and total ORs. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; and OR, odds ratio.
However, a pooled analysis of 17 studies (17 392 total participants) using an adjusted analysis of mortality found reductions in the risk of death among patients receiving vs not receiving ACEIs/ARBs (adjusted OR [aOR], 0.57; 95% CI, 0.43-0.76; P < .001; I2 = 54.0%) (Figure 2). A significant decrease in the risk of death was observed in both subgroups (for the hypertension subgroup, aOR, 0.51 [95% CI, 0.32-0.84]; P = .008; for the mixed subgroup, aOR, 0.64 [95% CI, 0.46-0.88]; P = .006).
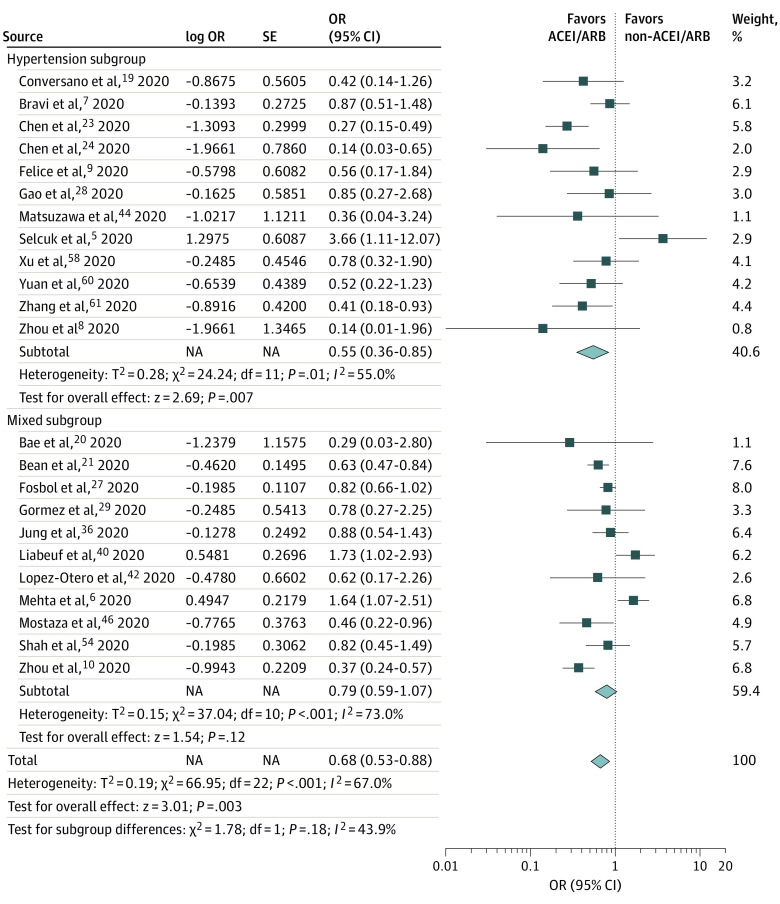
Figure 2. Subgroup Analysis of Adjusted Mortality Among Patients Who Did and Did Not Receive ACEIs or ARBs.
Subgroup analysis of adjusted mortality in 16 studies of patients who did and did not receive ACEIs or ARBs. A total of 7 studies included a mixed subgroup (a sample population with multiple mixed comorbidities), and 9 studies included a hypertension subgroup (a sample population with hypertension). Diamonds represent 95% CIs for subtotal and total ORs. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; and OR, odds ratio.
Severe Adverse Events
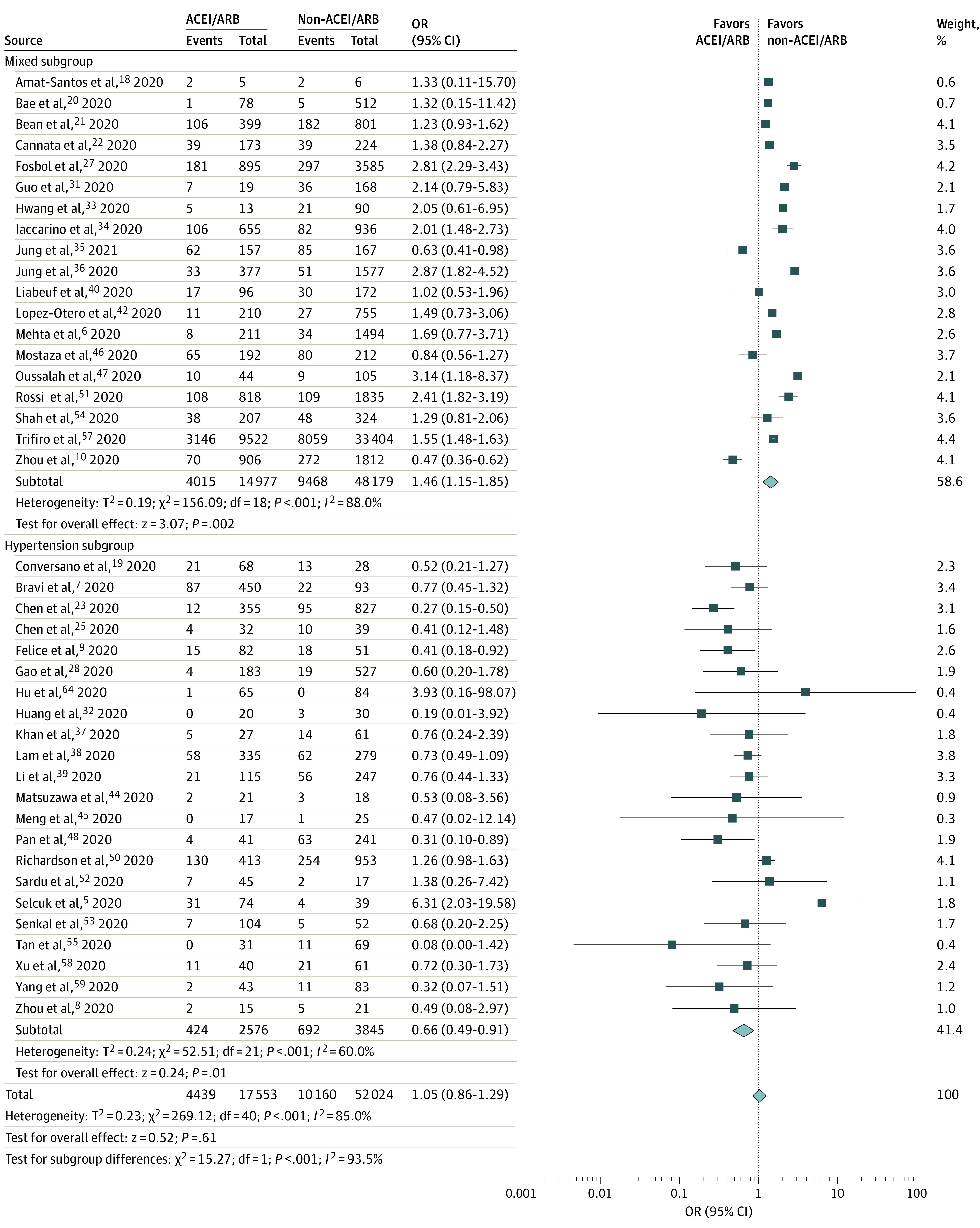
Unadjusted values for severe AEs were reported in 48 studies that included a total of 98 985 participants. A pooled analysis found comparable results among patients who did and did not receive ACEIs/ARBs (unadjusted OR, 1.11; 95% CI, 0.95-1.31; P = .20; I2 = 86.0%) (Figure 3). Notably, the 26 studies including a hypertension subgroup (unadjusted OR, 0.70; 95% CI, 0.54-0.91; P = .007) and the 33 studies including a mixed subgroup (unadjusted OR, 1.50; 95% CI, 1.25-1.81; P < .001) reported statistically significant results.
Figure 3. Subgroup Analysis of Unadjusted Mortality and Severe Adverse Events Among Patients Who Did and Did Not Receive ACEIs or ARBs.
Subgroup analysis of mortality and severe adverse events in 48 studies of patients who did and did not receive ACEIs or ARBs. A total of 22 studies included a mixed subgroup (a sample population with multiple mixed comorbidities), and 26 studies included a hypertension subgroup (a sample population with hypertension). Diamonds represent 95% CIs for subtotal and total ORs. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; and OR, odds ratio.
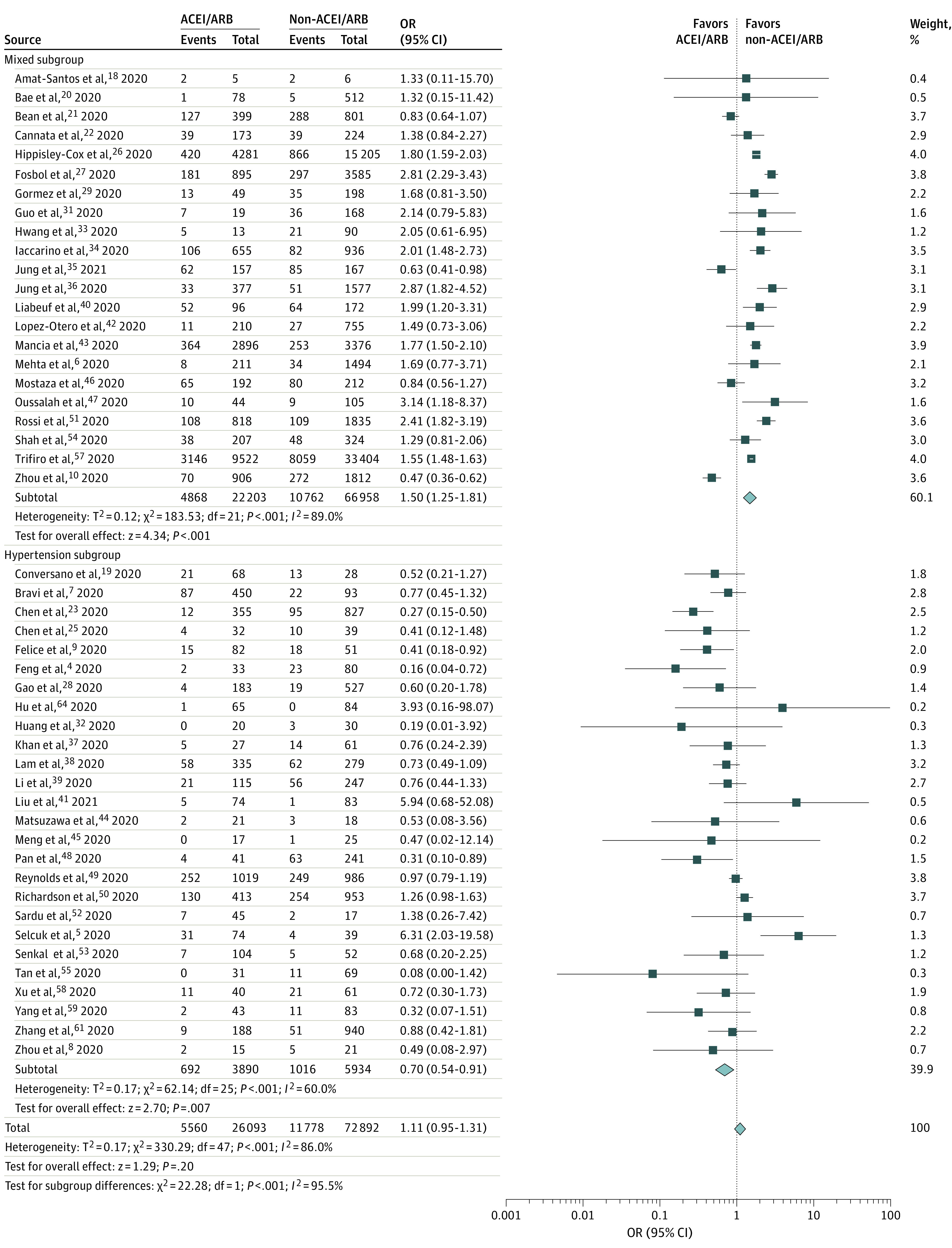
A total of 23 studies (23 129 total participants) reported an adjusted risk of severe AEs associated with the receipt of ACEIs/ARBs in a COVID-19 cohort. The adjusted covariates for each study are listed in the Table. A significant decrease in severe AEs was found in patients who received ACEIs/ARBs compared with those who did not (aOR, 0.68; 95% CI, 0.53-0.88; P = .003; I2 = 67.0%) (Figure 4). This reduced risk remained significant among the hypertension subgroup in 12 studies (aOR, 0.55; 95% CI, 0.36-0.85; P = .007). However, in the mixed subgroup, the decreased risk was not statistically significant (OR, 0.79; 95% CI, 0.59-1.07; P = .12). A sensitivity analysis that excluded studies reporting HRs indicated statistically significant results for mortality but nonsignificant results for severe AEs (eFigure 4 and eFigure 5 in the Supplement). Subgroup analyses of studies of moderate quality (OR, 0.36; 95% CI, 0.25-0.51; P < .001) and high quality (OR, 0.78; 95% CI, 0.60-1.00; P = .05) indicated reduced risk of adjusted severe AEs among both hypertension and mixed subgroups (eFigure 6 in the Supplement).
Figure 4. Subgroup Analysis of Adjusted Mortality and Severe Adverse Events Among Patients Who Did and Did Not Receive ACEIs or ARBs.
Subgroup analysis of adjusted mortality and severe adverse events in 23 studies of patients who did and did not receive ACEIs or ARBs. A total of 11 studies included a mixed subgroup (sample population with multiple mixed comorbidities), and 12 studies included a hypertension subgroup (defined as a sample population with hypertension). Diamonds represent 95% CIs for subtotal and total ORs. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; and OR, odds ratio.
Discussion
The results of this systematic review and meta-analysis of 52 studies with 101 949 total patients indicated a significant association between the receipt of ACEIs/ARBs and reductions in mortality and severe AEs among patients in the hypertension subgroup. In the mixed subgroup of patients with multiple comorbidities, this association was observed only when the analysis was adjusted for significant covariates.
Our results are consistent with those of another meta-analysis67 comprising 18 studies and 17 311 patients with hypertension. This previous meta-analysis reported a lower risk (risk ratio, 0.84; 95% CI, 0.73-0.95; P = .007) of the composite outcome (death, intensive care unit admission, mechanical ventilation, and progression to severe or critical pneumonia) among patients receiving ACEIs/ARBs. The present meta-analysis extends this finding to patients with multiple mixed comorbidities, suggesting that ACEIs/ARBs may have a substantial protective role in COVID-19 outcomes across all patient groups.
Notably, the protective implications of ACEIs/ARBs in the mixed subgroup were observed only after adjustments to potential and important confounders, such as age and comorbidities. This finding suggests that comorbidities may have an important role in COVID-19 clinical outcomes and that ACEIs/ARBs might be associated with further improvements in potential outcomes. In a large retrospective cohort study by Fosbol et al27 that included 4480 patients with COVID-19, an unadjusted analysis indicated worse outcomes among those who received ACEIs/ARBs. However, after multivariate adjustments, this finding was no longer statistically significant. Similar results were observed in multiple small retrospective cohort studies.29,36,42,54 It is worth noting that most studies included in the meta-analyses were retrospective and observational; with these study designs, unmeasured confounding factors and potential biases are inevitable. In addition, patients receiving ACEIs/ARBs are more likely to have heart failure, cardiovascular disease, hypertension, and comorbidities, which are associated with an increased risk of death among patients with COVID-19.3 Therefore, it is necessary to adjust for these confounders when evaluating the protective benefits of ACEIs/ARBs for mortality and severe AEs.
The potential mechanisms underlying the beneficial consequences of ACEIs/ARBs remain unknown. Our results suggest that these benefits are not solely associated with better blood pressure control, as patients receiving antihypertensive medications that were not ACEIs/ARBs had comparably inferior clinical outcomes in the adjusted subgroup analysis. Concerns about upregulation of angiotensin-converting enzyme 2 receptors with the receipt of RAAS inhibitors, which are derived from inconsistent results in studies with small samples,68,69 have been challenged by reports of deactivation of RAAS61 with chronic receipt of ACEIs/ARBs. Such downregulation may limit the inflammatory process, reducing acute lung injury among patients with COVID-19.49
Nevertheless, the findings of the present meta-analysis are consistent with those of national and international scientific experts,70,71,72 who recommend continuation of ACEIs/ARBs unless they are clinically contraindicated. This meta-analysis also indicated that, after adjustment for case mix, patients with hypertension and COVID-19 who received ACEIs/ARBs were 0.55 times as likely to experience a severe AE than those who did not receive ACEIs/ARBs, with a similar extent of benefit observed in the combined hypertension and mixed comorbidities subgroups. Although our study clarifies the association between RAAS inhibitors and mortality among patients with COVID-19, future randomized clinical trials are warranted to establish causality.
Limitations
This study has limitations. First, the study was limited by the insufficient data and varying study designs available, which did not allow for comparison of these analyses with a control group. The meta-analysis was primarily composed of observational studies because studies with higher levels of evidence, such as randomized clinical trials, were lacking. Second, the meta-analysis indicated substantial unadjusted and moderate adjusted levels of heterogeneity, which is typical in observational studies that include patients with diverse characteristics across large geographic regions. Nevertheless, measures were taken to maintain a homogeneous study population. A standard definition for severe AEs was used, and patients with unconfirmed COVID-19 were excluded. Third, we did not define the criteria for chronic receipt of ACEIs/ARBs. Insufficient description was available to distinguish between study participants, which is likely a factor associated with the increased heterogeneity observed in the study. With these limitations in mind, there were no data indicating that the receipt of ACEIs/ARBs was associated with harm if patients subsequently contracted COVID-19; on the contrary, ACEIs/ARBs may be associated with substantial protective benefits.
Conclusions
This comprehensive systematic review and meta-analysis of 52 studies indicated no higher risks of multivariable-adjusted mortality or severe AEs associated with the receipt of ACEIs/ARBs, which is consistent with recommendations for the continuation of these medications among patients for whom they are prescribed for the treatment of any condition. On the contrary, ACEIs and ARBs may be associated with protective benefits, particularly among patients with hypertension. Future randomized clinical trials are warranted to confirm the beneficial implications of these medications.
eMethods. Search Strategy in PubMed
eTable. Quality Assessment of Included Clinical Trials: Newcastle Ottawa Scale for Nonrandomized Clinical Trials and Cochrane Collaboration Tool for Assessing Risk of Bias in Randomized Clinical Trials
eFigure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram of the Clinical Trial Selection Process
eFigure 2. Funnel Plot for Unadjusted Critical or Fatal Outcomes for Patients With COVID-19 Receiving ACEIs or ARBs
eFigure 3. Funnel Plot for Unadjusted Death for Patients With COVID-19 Receiving ACEIs or ARBs
eFigure 4. Sensitivity Analysis Excluding Studies Reporting Hazard Ratios for Critical or Fatal Outcomes
eFigure 5. Sensitivity Analysis Excluding Studies Reporting Hazard Ratios for Mortality Outcomes
eFigure 6. Subgroup Analysis of Studies With Moderate or High Quality for Adjusted Critical or Fatal Outcomes
References
- 1.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Updated February 13, 2021. Accessed January 18, 2021. https://covid19.who.int/
- 2.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811-818. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531-538. doi: 10.1007/s00392-020-01626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y, Ling Y, Bai T, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380-1388 doi: 10.1164/rccm.202002-0445OC . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selcuk M, Cınar T, Keskin M, et al. Is the use of ACE inb/ARBs associated with higher in-hospital mortality in Covid-19 pneumonia patients? Clin Exp Hypertens. 2020;42(8):738-742. doi: 10.1080/10641963.2020.1783549 [DOI] [PubMed] [Google Scholar]
- 6.Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(9):1020-1026. doi: 10.1001/jamacardio.2020.1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bravi F, Flacco ME, Carradori T, et al. Predictors of severe or lethal COVID-19, including angiotensin converting enzyme inhibitors and angiotensin II receptor blockers, in a sample of infected Italian citizens. PLoS One. 2020;15(6):e0235248. doi: 10.1371/journal.pone.0235248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, Zhu J, Xu T. Clinical characteristics of coronavirus disease 2019 (COVID-19) patients with hypertension on renin-angiotensin system inhibitors. Clin Exp Hypertens. 2020;42(7):656-660. doi: 10.1080/10641963.2020.1764018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felice C, Nardin C, Di Tanna GL, et al. Use of RAAS inhibitors and risk of clinical deterioration in COVID-19: results from an Italian cohort of 133 hypertensives. Am J Hypertens. 2020;33(10):944-948. doi: 10.1093/ajh/hpaa096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Liu YM, Xie J, et al. Comparative impacts of ACE (angiotensin-converting enzyme) inhibitors versus angiotensin II receptor blockers on the risk of COVID-19 mortality. Hypertension. 2020;76(2):e15-e17. doi: 10.1161/HYPERTENSIONAHA.120.15622 [DOI] [PubMed] [Google Scholar]
- 11.Baral R, White M, Vassiliou VS. Effect of renin-angiotensin-aldosterone system inhibitors in patients with COVID-19: a systematic review and meta-analysis of 28,872 patients. Curr Atheroscler Rep. 2020;22(10):61. doi: 10.1007/s11883-020-00880-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. COVID-19 and the use of angiotensin-converting enzyme inhibitors and receptor blockers: scientific brief. May 7, 2020. Accessed October 20, 2020. https://www.who.int/news-room/commentaries/detail/covid-19-and-the-use-of-angiotensin-converting-enzyme-inhibitors-and-receptor-blockers
- 13.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute; 2019. Accessed October 20, 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 15.Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 16.Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49(5):1-15. doi: 10.18637/jss.v049.i05 [DOI] [Google Scholar]
- 17.Higgins JPT, Li T, Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.1. Cochrane; 2020. Accessed October 20, 2020. https://training.cochrane.org/handbook/current/chapter-06
- 18.Amat-Santos IJ, Santos-Martinez S, Lopez-Otero D, et al. Ramipril in high-risk patients with COVID-19. J Am Coll Cardiol. 2020;76(3):268-276. doi: 10.1016/j.jacc.2020.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conversano A, Melillo F, Napolano A, et al. Renin-angiotensin-aldosterone system inhibitors and outcome in patients with SARS-CoV-2 pneumonia: a case series study. Hypertension. 2020;76(2):e10-e12. doi: 10.1161/HYPERTENSIONAHA [DOI] [PubMed] [Google Scholar]
- 20.Bae DJ, Tehrani DM, Rabadia SV, et al. Angiotensin converting enzyme inhibitor and angiotensin II receptor blocker use among outpatients diagnosed with COVID-19. Am J Cardiol. 2020;132:150-157. doi: 10.1016/j.amjcard.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bean DM, Kraljevic Z, Searle T, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur J Heart Fail. 2020;22(6):967-974. doi: 10.1002/ejhf.1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannata F, Chiarito M, Reimers B, et al. Continuation versus discontinuation of ACE inhibitors or angiotensin II receptor blockers in COVID-19: effects on blood pressure control and mortality. Eur Heart J Cardiovasc Pharmacother. 2020:6(6):412-414. doi: 10.1093/ehjcvp/pvaa056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Wang F, Chen P, et al. Mortality and pre-hospitalization use of renin-angiotensin system inhibitors in hypertensive COVID-19 patients. J Am Heart Assoc. 2020;9(21):e017736. doi: 10.1161/JAHA.120.017736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen FF, Zhong M, Liu Y, et al. The characteristics and outcomes of 681 severe cases with COVID-19 in China. J Crit Care. 2020;60:32-37. doi: 10.1016/j.jcrc.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Yang D, Cheng B, et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43(7):1399-1407. doi: 10.2337/dc20-0660 [DOI] [PubMed] [Google Scholar]
- 26.Hippisley-Cox J, Young D, Coupland C, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106(19):1503-1511. doi: 10.1136/heartjnl-2020-317393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fosbol EL, Butt JH, Ostergaard L, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020;324(2):168-177. doi: 10.1001/jama.2020.11301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao C, Cai Y, Zhang K, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058-2066. doi: 10.1093/eurheartj/ehaa433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gormez S, Ekicibasi E, Degirmencioglu A, et al. Association between renin-angiotensin-aldosterone system inhibitor treatment, neutrophil-lymphocyte ratio, D-dimer and clinical severity of COVID-19 in hospitalized patients: a multicenter, observational study. J Hum Hypertens. 2020:1-10. doi: 10.1038/s41371-020-00405-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345-1355. doi: 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo X, Zhu Y, Hong Y. Decreased mortality of COVID-19 with renin-angiotensin-aldosterone system inhibitors therapy in patients with hypertension: a meta-analysis. Hypertension. 2020;76(2):e13-e14. doi: 10.1161/HYPERTENSIONAHA.120.15572 [DOI] [PubMed] [Google Scholar]
- 32.Huang Z, Cao J, Yao Y, et al. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann Transl Med. 2020;8(7):430. doi: 10.21037/atm.2020.03.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang JM, Kim JH, Park JS, Chang MC, Park D. Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurol Sci. 2020;41(9):2317-2324. doi: 10.1007/s10072-020-04541-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M; SARS-RAS Investigators. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the Italian Society of Hypertension. Hypertension. 2020;76(2):366-372. doi: 10.1161/HYPERTENSIONAHA.120.15324 [DOI] [PubMed] [Google Scholar]
- 35.Jung C, Bruno RR, Wernly B, et al. Inhibitors of the renin–angiotensin–aldosterone system and COVID-19 in critically ill elderly patients. Eur Heart J Cardiovasc Pharmacother. 2021;7(1):76-77. doi: 10.1093/ehjcvp/pvaa083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung SY, Choi JC, You SH, Kim WY. Association of renin-angiotensin-aldosterone system inhibitors with coronavirus disease 2019 (COVID-19)–related outcomes in Korea: a nationwide population-based cohort study. Clin Infect Dis. 2020;71(16):2121-2128. doi: 10.1093/cid/ciaa624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan KS, Reed-Embleton H, Lewis J, Bain P, Mahmud S. Angiotensin converting enzyme inhibitors do not increase the risk of poor outcomes in COVID-19 disease: a multi-centre observational study. Scott Med J. 2020;65(4):149-153. doi: 10.1177/0036933020951926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam KW, Chow KW, Vo J, et al. Continued in-hospital angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use in hypertensive COVID-19 patients is associated with positive clinical outcome. J Infect Dis. 2020;222(8):1256-1264. doi: 10.1093/infdis/jiaa447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825-830. doi: 10.1001/jamacardio.2020.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liabeuf S, Moragny J, Bennis Y, et al. Association between renin–angiotensin system inhibitors and COVID-19 complications. Eur Heart J Cardiovasc Pharmacother. Published online June 12, 2020. doi: 10.1093/ehjcvp/pvaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Liu Y, Chen K, et al. Efficacy of ACEIs/ARBs vs CCBs on the progression of COVID-19 patients with hypertension in Wuhan: a hospital-based retrospective cohort study. J Med Virol. 2021;93(2):854-862. doi: 10.1002/jmv.26315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Otero D, Lopez-Pais J, Cacho-Antonio CE, et al. Impact of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on COVID-19 in a Western population. Rev Espanola Cardiol. 2020;74(2):175-182. doi: 10.1016/j.rec.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382(25):2431-2440. doi: 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuzawa Y, Ogawa H, Kimura K, et al. Renin-angiotensin system inhibitors and the severity of coronavirus disease 2019 in Kanagawa, Japan: a retrospective cohort study. Hypertens Res. 2020;43(11):1257-1266. doi: 10.1038/s41440-020-00535-8 [DOI] [PubMed] [Google Scholar]
- 45.Meng J, Xiao G, Zhang J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757-760. doi: 10.1080/22221751.2020.1746200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mostaza JM, Garcia-Iglesias F, Gonzalez-Alegre T, et al. ; Carlos III COVID Working Group . Clinical course and prognostic factors of COVID-19 infection in an elderly hospitalized population. Arch Gerontol Geriatr. 2020;91:104204. doi: 10.1016/j.archger.2020.104204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oussalah A, Gleye S, Urmes IC, et al. Long-term ACE inhibitor/ARB use is associated with severe renal dysfunction and acute kidney injury in patients with severe COVID-19: results from a referral center cohort in the northeast of France. Clin Infect Dis. 2020;71(9):2447-2456. doi: 10.1093/cid/ciaa677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan W, Zhang J, Wang M, et al. Clinical features of COVID-19 in patients with essential hypertension and the impacts of renin-angiotensin-aldosterone system inhibitors on the prognosis of COVID-19 patients. Hypertension. 2020;76(3):732-741. doi: 10.1161/HYPERTENSIONAHA.120.15289 [DOI] [PubMed] [Google Scholar]
- 49.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–angiotensin–aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020;382(25):2441-2448. doi: 10.1056/NEJMoa2008975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi PG, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R; Reggio Emilia COVID-19 Working Group. Characteristics and outcomes of a cohort of COVID-19 patients in the province of Reggio Emilia, Italy. PLoS One. 2020;15(8):e0238281. doi: 10.1371/journal.pone.0238281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sardu C, Maggi P, Messina V, et al. Could anti-hypertensive drug therapy affect the clinical prognosis of hypertensive patients with COVID-19 infection? data from centers of southern Italy. J Am Heart Assoc. 2020;9(17):e016948. doi: 10.1161/JAHA.120.016948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Senkal N, Meral R, Medetalibeyoqlu A, Konyaoqlu H, Kose M, Tukek T. Association between chronic ACE inhibitor exposure and decreased odds of severe disease in patients with COVID-19. Anatol J Cardiol. 2020;24(1):21-29. doi: 10.14744/AnatolJCardiol.2020.57431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah P, Owens J, Franklin J, Jani Y, Kumar A, Doshi R. Baseline use of angiotensin-converting enzyme inhibitor/AT1 blocker and outcomes in hospitalized coronavirus disease 2019 African-American patients. J Hypertens. 2020;38(12):2537-2541. doi: 10.1097/HJH.0000000000002584 [DOI] [PubMed] [Google Scholar]
- 55.Tan ND, Qiu Y, Xing XB, Ghosh S, Chen MH, Mao R. Associations between angiotensin-converting enzyme inhibitors and angiotensin II receptor blocker use, gastrointestinal symptoms, and mortality among patients with COVID-19. Gastroenterology. 2020;159(3):1170-1172. doi: 10.1053/j.gastro.2020.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tedeschi S, Giannella M, Bartoletti M, et al. Clinical impact of renin-angiotensin system inhibitors on in-hospital mortality of patients with hypertension hospitalized for coronavirus disease 2019. Clin Infect Dis. 2020;71(15):899-901. doi: 10.1093/cid/ciaa492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trifiro G, Massari M, Da Cas R, et al. ; ITA-COVID-19: RAAS Inhibitor Group . Renin-angiotensin-aldosterone system inhibitors and risk of death in patients hospitalised with COVID-19: a retrospective Italian cohort study of 43,000 patients. Drug Saf. 2020;43(12):1297-1308. doi: 10.1007/s40264-020-00994-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J, Huang C, Fan G, et al. Use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in context of COVID-19 outbreak: a retrospective analysis. Front Med. 2020;14(5):601-612. doi: 10.1007/s11684-020-0800-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang G, Tan Z, Zhou L, et al. Effects of angiotensin II receptor blockers and ACE (angiotensin-converting enzyme) inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID-19 and hypertension: a single-center retrospective study. Hypertension. 2020;76(1):51-58. doi: 10.1161/HYPERTENSIONAHA.120.15143 [DOI] [PubMed] [Google Scholar]
- 60.Yuan Y, Liu D, Zeng S, et al. In-hospital use of ACEI/ARB is associated with lower risk of mortality and critic illness in COVID-19 patients with hypertension. J Infect. 2020:81(5):816-846. doi: 10.1016/j.jinf.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671-1681. doi: 10.1161/CIRCRESAHA.120.317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng YD, Meng K, Guan HQ, et al. [Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(6):450-455. doi: 10.3760/cma.j.cn112148-20200220-00105 [DOI] [PubMed] [Google Scholar]
- 63.de Abajo FJ, Rodriguez-Martin S, Lerma V, et al. ; MED-ACE2-COVID19 Study Group . Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395(10238):1705-1714. doi: 10.1016/S0140-6736(20)31030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu J, Zhang X, Zhang X, et al. COVID-19 is more severe in patients with hypertension; ACEI/ARB treatment does not influence clinical severity and outcome. J Infect. 2020;81(6):979-997. doi: 10.1016/j.jinf.2020.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan H, Valdes AM, Vijay A, et al. Role of drugs affecting the renin-angiotensin-aldosterone system on susceptibility and severity of COVID-19: a large case-control study from Zheijang Province, China. medRxiv. Preprint posted online April 29, 2020. doi: 10.1101/2020.04.24.20077875 [DOI] [Google Scholar]
- 66.Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41(19):1798-1800. doi: 10.1093/eurheartj/ehaa231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barochiner J, Martinez R. Use of inhibitors of the renin-angiotensin system in hypertensive patients and COVID-19 severity: a systematic review and meta-analysis. J Clin Pharm Ther. 2020;45(6):1244-1252. doi: 10.1111/jcpt.13246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kreutz R, Algharably EAE, Azizi M, et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res. 2020;116(10):1688-1699. doi: 10.1093/cvr/cvaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furuhashi M, Moniwa N, Mita T, et al. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28(1):15-21. doi: 10.1093/ajh/hpu086 [DOI] [PubMed] [Google Scholar]
- 70.de Simone G. Position statement of the ESC Council on Hypertension on ACE-inhibitors and angiotensin receptor blockers. European Society of Cardiology; 2020. Accessed May 25, 2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang
- 71.Bozkurt B, Kovacs R, Harrington B. HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. American College of Cardiology; 2020. Accessed May 3, 2020. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19 [DOI] [PMC free article] [PubMed]
- 72.Kreutz R, Januszewicz A. Letter on the COVID-19 pandemic from the president of the European Society of Hypertension. April 7, 2020. Accessed May 25, 2020. https://www.eshonline.org/esh-content/uploads/2020/06/ESH-LETTER-COVID-19-by-ESH-President-and-Secretary-April-7-2020.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search Strategy in PubMed
eTable. Quality Assessment of Included Clinical Trials: Newcastle Ottawa Scale for Nonrandomized Clinical Trials and Cochrane Collaboration Tool for Assessing Risk of Bias in Randomized Clinical Trials
eFigure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram of the Clinical Trial Selection Process
eFigure 2. Funnel Plot for Unadjusted Critical or Fatal Outcomes for Patients With COVID-19 Receiving ACEIs or ARBs
eFigure 3. Funnel Plot for Unadjusted Death for Patients With COVID-19 Receiving ACEIs or ARBs
eFigure 4. Sensitivity Analysis Excluding Studies Reporting Hazard Ratios for Critical or Fatal Outcomes
eFigure 5. Sensitivity Analysis Excluding Studies Reporting Hazard Ratios for Mortality Outcomes
eFigure 6. Subgroup Analysis of Studies With Moderate or High Quality for Adjusted Critical or Fatal Outcomes