Key Points
Question
What are the outcomes of a dedicated hypertension program for patients with primary aldosteronism (PA)?
Findings
In this population-based cohort study in Alberta, Canada, substantial variation was noted in the investigation and treatment rates for PA. Receipt of screening, subtyping, and disease-targeted treatment was most strongly linked to certain geographic zones and clinician specialty, reflecting the resource-limited nature of adrenal vein sampling and adrenalectomy and underscoring the benefits of a dedicated hypertension program for patients with PA.
Meaning
A system-level approach to assist with investigation and treatment of PA may be highly effective in closing care gaps and improving clinical outcomes.
This population-based cohort study aims to understand the outcomes of a specialized clinic on rates of evaluation and treatment of primary aldosteronism in Alberta, Canada.
Abstract
Importance
Primary aldosteronism (PA) is one of the most common causes of secondary hypertension but remains largely unrecognized and untreated.
Objective
To understand the outcomes of a specialized clinic on rates of evaluation and treatment of PA in the context of secondary factors.
Design, Setting, and Participants
This population-based cohort study was conducted in Alberta, Canada, using linked administrative data between April 1, 2012, and July 31, 2019, on adults identified as having hypertension.
Main Outcomes and Measures
We evaluated each step of the diagnostic and care pathway for PA to determine the proportion of people with hypertension who received screening, subtyping, and targeted treatment for PA. Variations in diagnosis and treatment were examined according to individual-level, clinician-level, and system-level characteristics.
Results
Of the 1.1 million adults with hypertension, 7941 people (0.7%) were screened for PA. Among those who were screened, 1703 (21.4%) had positive test results consistent with possible PA, and 1005 (59.0%) of these were further investigated to distinguish between unilateral and bilateral forms of PA. Only 731 individuals (42.9%) with a positive screen result received disease-targeted treatment. Geographic zones and clinician specialty were the strongest determinants of screening, subtyping, and treatment of PA, with the highest rates corresponding to the location of the provincial endocrine hypertension program.
Conclusions and Relevance
In this cohort, less than 1% of patients expected to have PA were ever formally diagnosed and treated. These findings suggest that a system-level approach to assist with investigation and treatment of PA may be highly effective in closing care gaps and improving clinical outcomes.
Introduction
Primary aldosteronism (PA) accounts for at least 10% of cases of hypertension and is the most common cause of remediable high blood pressure.1,2 Unidentified and untreated PA is associated with an excess risk of cardiovascular disease and premature mortality, independent of blood pressure, along with adverse effects on quality of life.3,4,5 Although highly effective, disease-targeted treatments are available, this condition remains largely unrecognized.2,6,7
Clinical practice guidelines recommend thorough consideration and investigation of PA in patients who require 3 or more drugs to control their blood pressure, as well as those with hypokalemia or adrenal nodules.8,9 Based on these criteria, half of patients with hypertension should be investigated for PA,8,10 but it is estimated that no more than 10% of eligible patients are screened and less than 1% to 2% of affected individuals are ever diagnosed or treated.6,7,11,12,13 Accordingly, an understanding of the barriers to diagnosis and treatment is critical to advancing appropriate care and improving clinical outcomes for patients living with PA.
The current recommended care pathway for PA is lengthy and complicated. It begins with screening, then confirmatory testing in those who have positive results, subtyping in those with a diagnosis, and finally targeted treatment with an aldosterone antagonist or surgery.8 Diagnostic gaps anywhere along the way may result in missed cases and lost opportunities for intervention. Addressing this, we conducted this study to examine the yield of each step of the clinical pathway and sought to determine the factors associated with diagnosis and treatment, with a particular focus on the potential outcomes of a regional endocrine hypertension program.
Methods
This study was approved by the Conjoint Health Research Ethics Board at the University of Calgary. A waiver of consent was granted for access to personal identifiable health information consistent with the conditions of section 50 of the Health Information Act of Alberta, Canada.
Study Setting and Data Sources
We conducted a retrospective, population-based cohort study in Alberta, Canada, a province of more than 4.3 million people. We obtained data from linked administrative databases of Alberta Health, a government ministry that provides universal health care coverage to more than 99% of residents. These databases include a demographic population registry, physician claims, hospital separation data, outpatient prescription drug dispensation records, diagnostic imaging data, and laboratory services data. These data are considered to be high quality and comprehensive.14,15,16
Definitions and Measures
Hypertension
We included beneficiaries of insurance and Alberta health care who were 18 years and older, from April 1, 2012, to July 31, 2019. Using a validated case definition (based on 1 hospitalization [with the International Classification of Disease, Tenth Revision, Canadian Version (ICD-10-CA) codes I10-I13 and I15] or 2 physician claims [using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 401-405] within 2 years), we identified prevalent cases of hypertension.15
Screening, Subtyping, and Treatment
The typical diagnostic and treatment pathway for PA was examined (eFigure in the Supplement). The number of people with hypertension who were screened for PA with an aldosterone-to-renin ratio (ARR) and the proportion with abnormally high results were determined. If multiple ARRs were measured in the same individual during the study period, only the first ARR was considered for categorization. To account for differences in laboratory assays, we used cutoffs specific to the unit of measurement in alignment with each local laboratory standard (ie, >60 pmol/L per mIU/L, >100 pmol/L per ng/L, and >550 pmol/L per ng/mL/h)17,18; these cutoffs constituted an abnormally high ARR. The frequency of confirmatory testing (eg, oral sodium loading, saline infusion testing, fludrocortisone suppression, or captopril challenge) could not be determined because these encounters were not coded. Individuals with abnormally high ARRs were considered candidates for further PA workup and treatment. Next, we considered patients with positive ARR screening results who had abdominal cross-sectional imaging (with computed tomography [CT] or magnetic resonance imaging [MRI], irrespective of initial clinical indication) and/or adrenal vein sampling (AVS) to have received subtype classification.8,19 Finally, we estimated the number of people with positive screening results who received targeted treatment after a positive ARR test result with a potassium-sparing diuretic (using the Anatomical Therapeutic Chemical code C03D) and/or adrenalectomy (based on physician claims).
Covariates
Medications
For each person, we determined the number of antihypertensive drugs dispensed within 100 days following ARR testing. These were categorized according to drug classes using Anatomical Therapeutic Chemical codes: β-blockers, C07 (excluding C07AA07, C07AA12, and C07AG02); renin-angiotensin system inhibitors, C09; thiazide and thiazide-like diuretics, C03 (excluding C03BA08); calcium channel blockers, C08; and other antihypertensive drugs, C02 (excluding C02KX01).20,21 Individuals were classified according to having fewer than 3 drug classes or 3 or more drug classes.
Serum Potassium
We determined the frequency of hypokalemia, defined as a serum potassium level less than 3.5 mEq/L (to convert to millimoles per liter, multiply by 1.0). This was measured at any time prior to ARR testing.
Clinician Specialty
We considered the specialty of the ordering clinician who initiated the ARR screen. Family physicians, cardiologists, nephrologists, general internists, endocrinologists, and others (eg, general surgeons and urologists) were included.
Area of Residence
We determined the geographic zone of residence for each patient using their postal codes at the time of hypertension diagnosis. Specifically, health care in Alberta is organized according to 5 geographic zones (South, Calgary, Central, Edmonton, and North).22 Of these, Calgary and Edmonton are the largest, with 1.6 million and 1.3 million residents, respectively, and they provide most of the tertiary care services in the province. Uniquely in Calgary, there is an endocrine hypertension program primarily dedicated to the detection and management of PA.23
Statistical Analysis
The prevalence of hypertension was calculated for the entire province, based on the number of beneficiaries with health insurance in Alberta who were 18 years or older. We evaluated each step of the care pathway for PA and reported the proportion of individuals who received screening, subtyping, and targeted treatment, respectively, along with associated 95% CIs. Stratified analysis was performed according to individual-level, clinician-level, and system-level characteristics. Continuous variables were recoded into ordinal categories (eg, age <40, 40-59, and ≥60 years) or dichotomized (eg, presence or absence of hypokalemia). The χ2 test was used to compare groups. Finally, choropleth maps were created using QGIS version 3.8 (The Open Source Geospatial Foundation; http://www.osgeo.org) to visualize regional variations in workup and treatment. Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc).
Results
A total of 1.1 million people were identified to have hypertension, representing 29.2% of the adult population of Alberta (eTable 1 in the Supplement). The mean age (SD) was 58.9 (15.4) years; 512 805 (47.9%) were women, and 557 977 (52.1%) were men. Of these, 7941 individuals (0.7%) were screened for PA. An elevated ratio, consistent with possible PA, was detected in 1703 people (21.4%). There were 1005 people among those with a positive screen result (59.0%) who were subtyped. Slightly more than half of those with positive screen results received a CT or MRI (975 [57.2%]), and comparatively few underwent AVS (250 [14.7%]). Disease-specific treatment was provided to less than half of patients with a positive screen result (731 [42.9%]). Of these, nearly all received medical therapy (692 of 731 [94.7%]), and relatively few had an adrenalectomy (120 of 731 [16.4%]).
Screening and Diagnosis
Screening rates were highest in individuals younger than 40 years (2202 [1.8%]), patients with hypokalemia (2818 [1.0%]), and those taking 3 or more drugs for hypertension (1992 [2.4%]) (Table 1). Patients with hypokalemia and those taking 3 or more antihypertensive drugs had the greatest frequency of positive ARR test results (790 [28.0%] and 574 [28.8%], respectively).
Table 1. Screening, Subtyping, and Treatment Rates According to Patient Characteristics.
| Characteristic | Overall | Age, y | Sex | Hypokalemia | No. of antihypertensive drugsa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| <40 | 40-59 | ≥60 | Male | Female | Present | Absent | <3 | ≥3 | ||
| Patients with hypertension | ||||||||||
| No. | 1 070 783 | 120 607 | 430 129 | 520 047 | 557 981 | 512 801 | 278 704 | 792 079 | 988 564 | 82 219 |
| % (95% CI) | NA | 11.3 (11.2-11.3) | 40.2 (40.1-40.3) | 48.6 (48.5-48.7) | 52.1 (52.0-52.2) | 47.9 (47.8-48.0) | 26.0 (25.9-26.1) | 74.0 (73.9-74.1) | 92.3 (92.3-92.4) | 7.7 (7.6-7.8) |
| Screening among those with hypertension | ||||||||||
| Patients screened | ||||||||||
| No. | 7941 | 2202 | 3181 | 2558 | 3770 | 4171 | 2818 | 5123 | 5949 | 1992 |
| % (95% CI) | 0.7 (0.7-0.8) | 1.8 (1.8-1.9) | 0.7 (0.7-0.8) | 0.5 (0.5-0.5) | 0.7 (0.7-0.7) | 0.8 (0.8-0.8) | 1.0 (1.0-1.1) | 0.7 (0.6-0.7) | 0.6 (0.6-0.6) | 2.4 (2.3-2.5) |
| Elevated ARR among those screened | ||||||||||
| No. | 1703 | 339 | 761 | 603 | 745 | 958 | 790 | 913 | 1129 | 574 |
| % (95% CI) | 21.4 (20.5-22.4) | 15.4 (13.9-17.0) | 23.9 (22.5-25.5) | 23.6 (21.9-25.3) | 19.8 (18.5-21.1) | 23.0 (21.7-24.3) | 28.0 (26.4-29.7) | 17.8 (16.8-18.9) | 19.0 (18.0-20.0.) | 28.8 (26.3-30.9) |
| Subtyping among those who had positive screening results | ||||||||||
| Received a CT or MRI of the abdomen | ||||||||||
| No. | 975 | 162 | 448 | 365 | 441 | 534 | 509 | 466 | 624 | 351 |
| % (95% CI) | 57.2 (54.9-59.6) | 47.8 (42.4-53.3) | 58.9 (55.3-62.4) | 60.5 (56.5-64.5) | 59.2 (55.6-62.7) | 55.7 (52.5-58.9) | 64.4 (61.0-67.8) | 51.0 (47.7-54.3) | 55.3 (52.3-58.2) | 61.2 (57.0-65.2) |
| Received adrenal vein sampling | ||||||||||
| No. | 250 | 56 | 146 | 48 | 135 | 115 | 141 | 109 | 156 | 94 |
| % (95% CI) | 14.7 (13.0-16.4) | 16.5 (12.7-20.9) | 19.2 (16.5-22.0) | 8.0 (5.9-10.4) | 18.1 (15.4-21.1) | 12.0 (10.0-14.2) | 17.9 (15.2-20.7) | 11.9 (9.9-14.2) | 13.8 (11.9-16.0) | 16.4 (13.4-19.7) |
| Received any subtyping | ||||||||||
| No. | 1005 | 175 | 460 | 370 | 452 | 553 | 521 | 484 | 642 | 363 |
| % (95% CI) | 59.0 (56.6-61.4) | 51.6 (46.2-57.1) | 60.5 (56.9-63.9) | 61.4 (57.3-65.3) | 60.7 (57.1-64.2) | 57.7 (54.5-60.9) | 66.0 (62.5-69.3) | 53.0 (49.7-56.3) | 56.9 (53.9-59.8) | 63.2 (59.2-67.2) |
| Targeted treatment among those who had positive screening results | ||||||||||
| Received a potassium-sparing diuretic | ||||||||||
| No. | 692 | 111 | 330 | 251 | 340 | 352 | 368 | 324 | 352 | 340 |
| % (95% CI) | 40.6 (38.3-43.0) | 32.7 (27.8-38.0) | 43.4 (39.8-47.0) | 41.6 (37.7-45.7) | 45.6 (42.0-49.3) | 36.7 (33.7-39.9) | 46.6 (43.1-50.1) | 35.5 (32.4-38.7) | 31.2 (28.5-34.0) | 59.2 (55.1-63.6) |
| Received an adrenalectomy | ||||||||||
| No. | 120 | 22 | 76 | 22 | 74 | 46 | 84 | 36 | 71 | 49 |
| % (95% CI) | 7.0 (5.9-8.4) | 6.5 (4.1-9.7) | 10.0 (7.9-12.3) | 3.6 (2.3-5.5) | 9.9 (7.9-12.3) | 4.8 (3.5-6.4) | 10.6 (8.6-13.0) | 3.9 (2.8-5.4) | 6.3 (4.9-7.9) | 8.5 (6.4-11.1) |
| Received any targeted treatment | ||||||||||
| No. | 731 | 118 | 356 | 257 | 363 | 368 | 389 | 342 | 385 | 346 |
| % (95% CI) | 42.9 (40.6-45.3) | 34.8 (29.7-40.1) | 46.8 (43.2-50.4) | 42.6 (38.6-46.7) | 48.7 (45.1-52.4) | 38.4 (35.3-41.6) | 49.2 (45.7-52.8) | 37.5 (34.3-40.7) | 34.1 (31.3-36.9) | 60.3 (56.1-64.3) |
Abbreviations: ARR, aldosterone-to-renin ratio; CT, computed tomography; MRI, magnetic resonance imaging; NA, not applicable.
Based on the number of drugs dispensed within 100 days of the index date.
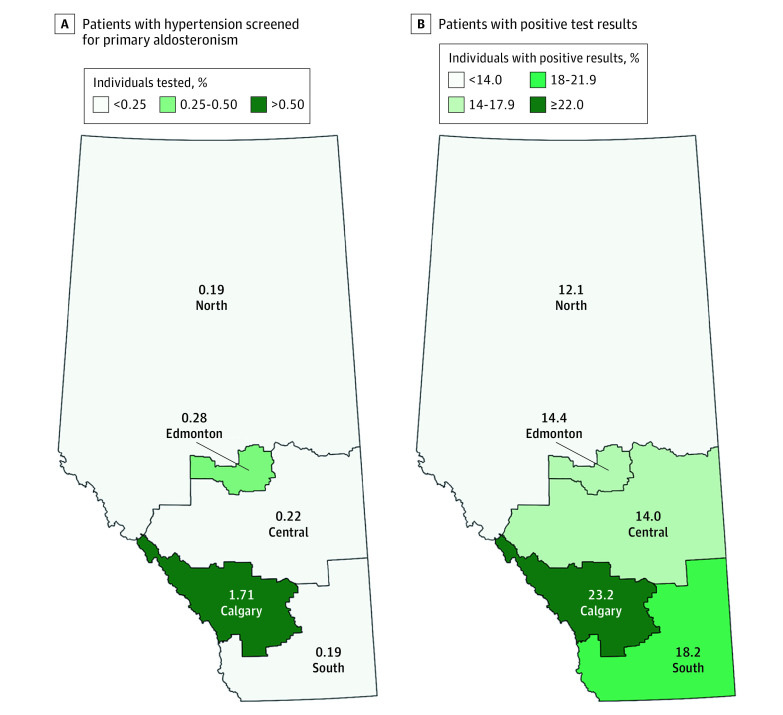
We examined for variations according to clinician and geographic factors. The proportion of patients screened for PA was approximately 6-fold to 9-fold higher in the Calgary zone than other jurisdictions (1.71% [95% CI, 1.67%-1.76%] of people were screened among adults with hypertension in Calgary, vs 0.19% [95% CI, 0.16%-0.21%] in the South and North zones to 0.28% [95% CI, 0.26%-0.30%] in the Central zone; P < .001 for general χ2 test; Figure 1A). While the proportion of people with elevated ARRs was similar irrespective of the specialty of the ordering clinician, patients living in the Calgary zone had a much higher positivity rate when screening was performed compared with the rest of the province, accounting for almost a quarter of tests performed there (23.2% [95% CI, 22.2%-24.3%] in Calgary, vs 12.1% [95% CI, 8.1%-17.1%] in the North to 18.2% [95% CI, 12.4%-25.2%] in the South; P < .001 for general χ2 test; Figure 1B; eTable 2 in the Supplement). Compared with family physicians in other regions in the province, those in the Calgary zone screened the most numerous patients and had the highest positivity rate (21.5% [95% CI, 19.6%-23.4%] in Calgary, vs 11.0% [95% CI, 5.1%-19.8%] in the North zone to 16.7% [95% CI, 6.4%-32.8%] in the South zone; P < .001; eTable 2 in the Supplement).
Figure 1. Screening and Positivity Rates.
A, Proportion of patients with hypertension screened for primary aldosteronism performed according to zone of residence. B, Percentage of patients with positive screening test results among those tested.
Subtyping
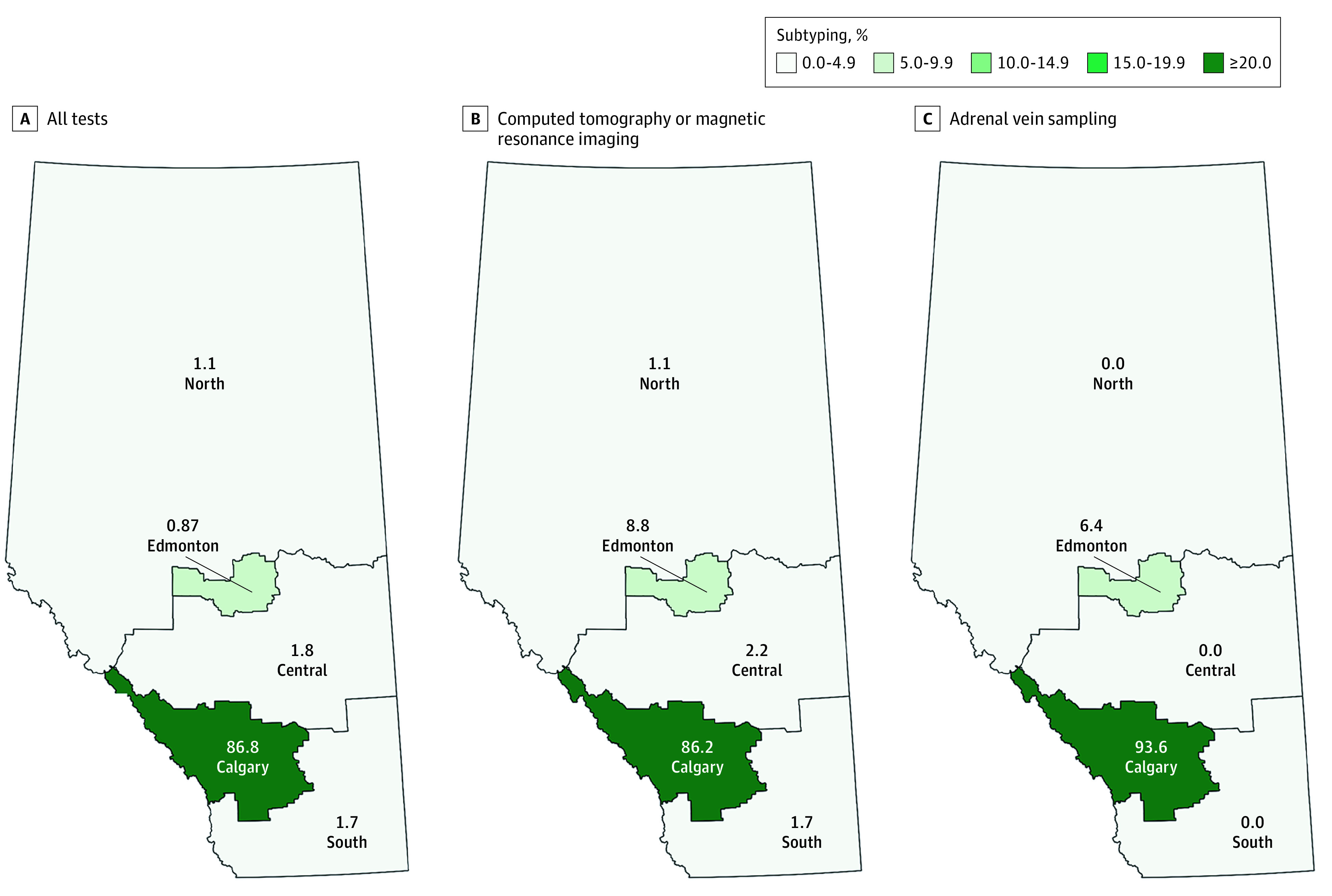
There was little difference in the proportion of people who were subtyped when examined across age strata, sex, occurrence of hypokalemia, or number of antihypertensive drugs taken (Table 1). Overall rates were also similar across the province and between clinician specialties (Table 2). Computed tomography and MRI scans were the most common modalities used for subtyping, with little regional variation and few differences between clinician specialties. However, when an endocrinologist ordered the initial screening test, patients had a 2-fold to 3-fold higher rate of receiving AVS, as compared with cases for whom a clinician from another specialty was involved (26.7% [95% CI, 21.5%-32.4%] for endocrinology, vs 8.8% [95% CI, 5.5%-13.1%] to 13.6% [95% CI, 11.2%-16.2%] for other specialties; P < .001 for general χ2 tests), despite there being a similar proportion of positive ARR screening test results for all types of physicians.
Table 2. Screening, Subtyping, and Treatment Rates According to Clinician Characteristics and Health Zone.
| Characteristic | Overall | Specialty ordering ARR screening | Zone of residencea | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Family medicine | Cardiology, nephrology, and general internal medicine | Endocrinology | Other | South | Calgary | Central | Edmonton | North | ||
| Screening among those with hypertension | ||||||||||
| Patients screened | ||||||||||
| No. | 7941 | 2245 | 3381 | 1166 | 1149 | 154 | 6317 | 286 | 925 | 223 |
| % (95% CI) | NA | 28.3 (27.3-29.3) | 42.6 (41.5-43.7) | 14.7 (13.9-15.5) | 14.5 (13.7-15.3) | 1.9 (1.6-2.3) | 79.9 (79.0-80.8) | 3.6 (3.2-4.1) | 11.7 (11.0-12.4) | 2.8 (2.5-3.2) |
| Elevated ARR among those screened | ||||||||||
| No. | 1703 | 445 | 752 | 266 | 240 | 28 | 1468 | 40 | 133 | 27 |
| % (95% CI) | 21.4 (20.5-22.4) | 19.8 (18.2-21.5) | 22.2 (20.9-23.7) | 22.8 (20.4-25.3) | 20.9 (18.6-23.4) | 18.2 (12.4-25.2) | 23.2 (22.2-24.3) | 14.0 (10.2-18.6) | 14.4 (12.2-16.8) | 12.1 (8.1-17.1) |
| Subtyping among those who had positive screening results | ||||||||||
| Received a CT or MRI of the abdomen | ||||||||||
| No. | 975 | 222 | 432 | 174 | 147 | 18 | 828 | 25 | 83 | 19 |
| % (95% CI) | 57.2 (54.9-59.6) | 49.9 (45.1-54.6) | 57.5 (53.8-61.0) | 65.4 (59.4-71.1) | 61.3 (54.8-67.5) | 64.3 (44.1-81.4) | 56.4 (53.8-59.0) | 62.5 (45.8-77.3) | 62.4 (53.6-70.6) | 70.4 (49.8-86.3) |
| Received adrenal vein sampling | ||||||||||
| No. | 250 | 56 | 102 | 71 | 21 | 6 | 218 | 6 | 17 | 3 |
| % (95% CI) | 14.7 (13.0-16.4) | 12.6 (9.7-16.0) | 13.6 (11.2-16.2) | 26.7 (21.5-32.4) | 8.8 (5.5-13.1) | 21.4 (8.3-40.9) | 14.8 (13.1-16.8) | 15.0 (5.7-29.8) | 12.8 (7.6-19.7) | 11.1 (2.4-29.2) |
| Received any subtyping | ||||||||||
| No. | 1005 | 227 | 444 | 183 | 151 | 19 | 854 | 25 | 86 | 19 |
| % (95% CI) | 59.0 (56.6-61.4) | 51.0 (46.3-55.7) | 59.0 (55.4-62.6) | 68.8 (62.8-74.3) | 62.9 (56.5-69.0) | 67.9 (47.6-84.1) | 58.2 (55.6-60.7) | 62.5 (45.8-77.3) | 64.7 (55.9-72.7) | 70.4 (49.8-86.3) |
| Targeted treatment among those who had positive screening results | ||||||||||
| Received a potassium-sparing diuretic | ||||||||||
| No. | 692 | 168 | 323 | 129 | 72 | 16 | 592 | 14 | 58 | 12 |
| % (95% CI) | 40.6 (38.3-43.0) | 37.7 (33.2-42.4) | 42.9 (39.4-46.6) | 48.5 (42.3-54.7) | 30.0 (24.3-36.2) | 57.1 (37.2-75.5) | 40.3 (37.8-42.9) | 35.0 (20.6-51.7) | 43.6 (35.0-52.5) | 44.4 (25.5-64.7) |
| Received an adrenalectomy | ||||||||||
| No. | 120 | 29 | 44 | 31 | 16 | 2 | 99 | 2 | 14 | 3 |
| % (95% CI) | 7.0 (5.9-8.4) | 6.5 (4.4-9.2) | 5.8 (4.3-7.8) | 11.7 (8.1-16.1) | 6.7 (3.9-10.6) | 7.1 (0.1-23.5) | 6.7 (5.5-8.2) | 5.0 (0.1-16.9) | 10.5 (5.9-17.0) | 11.1 (2.4-29.2) |
| Received any targeted treatment | ||||||||||
| No. | 731 | 176 | 331 | 145 | 79 | 16 | 625 | 14 | 62 | 14 |
| % (95% CI) | 42.9 (40.6-45.3) | 39.6 (35.0-44.3) | 44.0 (40.4-47.7) | 54.5 (48.3-60.6) | 32.9 (27.0-39.3) | 57.1 (37.2-75.5) | 42.6 (40.0-45.2) | 35.0 (20.6-51.7) | 46.6 (37.9-55.5) | 51.8 (31.9-71.3) |
Abbreviations: ARR, aldosterone-to-renin ratio; CT, computed tomography; MRI, magnetic resonance imaging; NA, not applicable.
Thirty-six patients who were screened and 7 patients with positive screening results had no zone listed.
In relative terms, patients with positive ARR screening results from all parts of the province had similar rates of CT and MRI scans (ranging from 56.4% [95% CI, 53.8%-59.0%] to 70.4% [95% CI, 49.8%-86.3%]) as well as AVS (ranging from 11.1% [95% CI, 2.4%-29.2%] to 21.4% [95% CI, 8.3%-40.9%]). However, considering absolute numbers, most patients investigated for unilateral vs bilateral disease were residents of the Calgary zone (828 of 973 [85.1%] for a CT or MRI; 218 of 250 [87.2%] for AVS; 854 of 1003 [85.1%] for all tests), and this was concordant with the large number of people who were screened for PA in Calgary (Table 2). Correspondingly, when considering the location where the service was provided, subtyping was most intensely delivered in the Calgary zone (86.2% [95% CI, 83.8%-88.3%] for a CT or MRI; 93.6% [95% CI, 89.8%-96.3%] for AVS; and 86.8% [95% CI, 84.5%-88.8%] for all tests) (Figure 2).
Figure 2. Subtyping Among Patients With Positive Screening Test Results, According to Zones.
Treatment
Among the subset of people who received further subtyping, targeted treatment rates were approximately 5% to 10% higher for both medical therapy and surgery (receiving a potassium-sparing diuretic: among those with a positive screen result, 40.6% [95% CI, 38.3%-43.0%] vs those with further subtyping, 49.7% [95% CI, 46.6%-52.9%]; adrenalectomy, 7.0% [95% CI, 5.9%-8.4%] vs 11.9% [95% CI, 10.0%-14.1%]; any targeted treatment, 42.9% [95% CI, 40.6%-45.3%] vs 53.6% [95% CI, 50.5%-56.7%]), although overall disease-specific treatment was received in less than half of potentially eligible patients (eTable 1 in the Supplement). Clinical characteristics associated with higher rates of disease-specific treatment included male sex (receiving a potassium-sparing diuretic: men, 45.6% [95% CI, 42.0%-49.3%] vs women, 36.7% [95% CI, 33.7%-39.9%]; adrenalectomy: 9.9% [95% CI, 7.9%-12.3%] vs 4.8% [95% CI, 3.5%-6.4%]; any targeted treatment, 48.7% [95% CI, 45.1%-52.4%] vs 38.4% [95% CI, 35.3%-41.6%]; P < .001), occurrence of hypokalemia (receiving a potassium-sparing diuretic: those with hypokalemia, 46.6% [95% CI, 43.1%-50.1%] vs those without hypokalemia, 35.5% [95% CI, 32.4%-38.7%]; adrenalectomy: 10.6% [95% CI, 8.6%-13.0%] vs 3.9% [95% CI, 2.8%-5.4%]; any targeted treatment, 49.2% [95% CI, 45.7%-52.8%] vs 37.5% [95% CI, 34.3%-40.7%]; P < .001), and the use of 3 antihypertensive drugs or more (receiving a potassium-sparing diuretic: those with <3 drugs, 31.2% [95% CI, 28.5%-30.4%] vs those with ≥3 drugs, 59.2% [95% CI, 55.1%-63.6%]; adrenalectomy: 6.3% [95% CI, 4.9%-7.9%] vs 8.5% [95% CI, 6.4%-11.1%]; any targeted treatment, 34.1% [95% CI, 31.3%-36.9%] vs 60.3% [95% CI, 56.1%-64.3%]; P < .001) (Table 1).
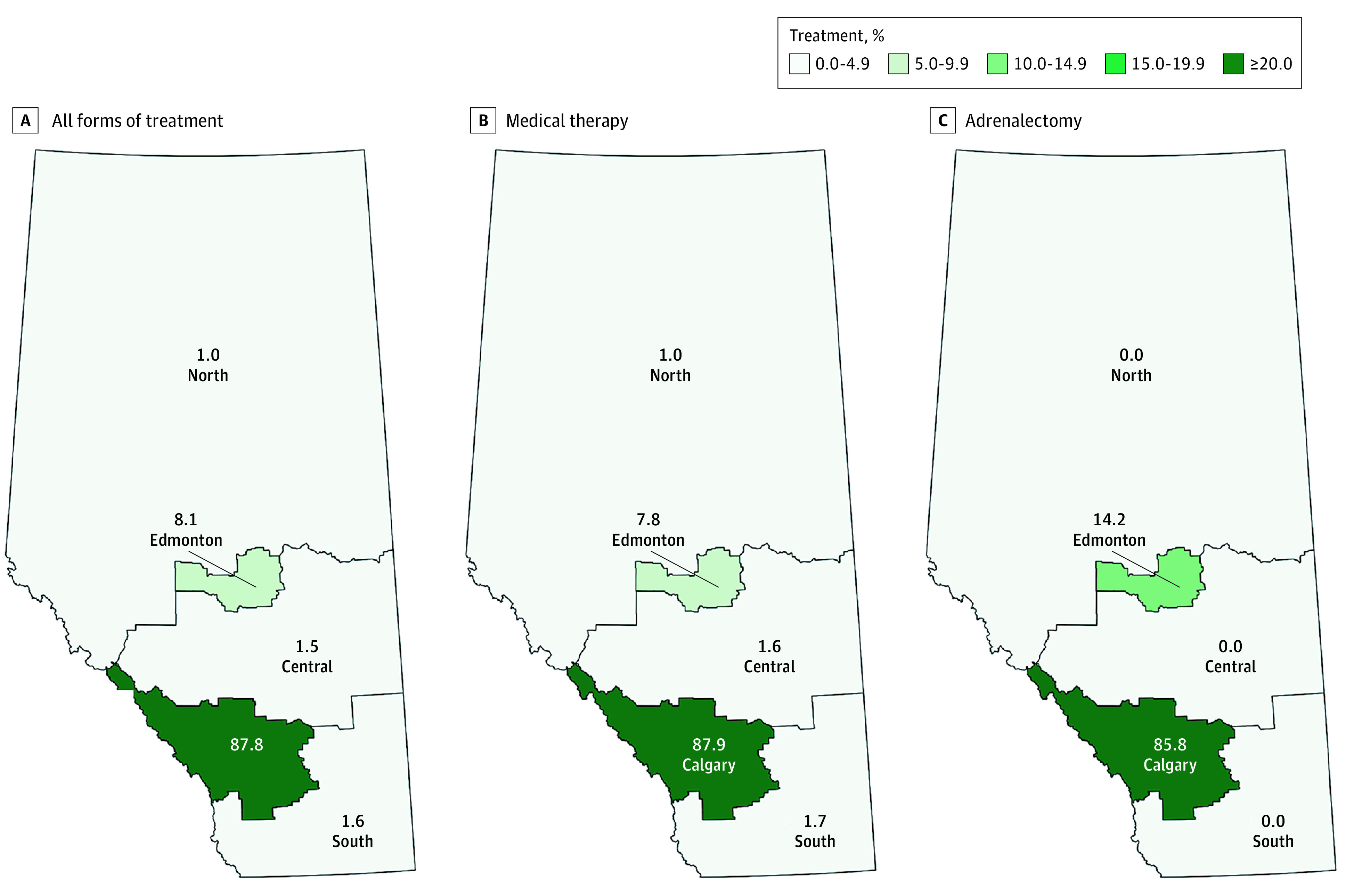
Overall disease-targeted treatment rates were the highest among endocrinologists compared with other clinicians (endocrinology, 54.5% [95% CI, 48.3%-60.6%]; family medicine, 39.6% [95% CI, 35.0%-44.3%]; cardiology, nephrology, and general internal medicine, 44.0% [95% CI, 40.4%-47.7%]; other specialties, 32.9% [95% CI, 27.0%-39.3%]; P < .001) (Table 2). Furthermore, as was consistent with the frequency of referrals for AVS, patients were twice more likely to receive adrenalectomy when the responsible physician for the workup was an endocrinologist (endocrinology, 11.7% [95% CI, 8.1%-16.1%]; family medicine, 6.5% [95% CI, 4.4%-9.2%]; cardiology, nephrology, and general internal medicine, 5.8% [95% CI, 4.3%-7.8%]; other specialties, 6.7% [95% CI, 3.9%-10.6%]; P = .001). There were large geographic variations in the proportion of patients who received disease-specific treatment (ranging from 35.0% [95% CI, 20.6%-51.7%] in the Central zone to 57.1% [95% CI, 37.2%-75.5%] in the South zone). Similar to the pattern observed with subtype investigations, most of the patients who were treated had their clinical care concentrated in Calgary (ie, the location of a dedicated endocrine hypertension program), especially for those receiving adrenalectomies (ie, 99 of 120 [82.5%]; Figure 3).
Figure 3. Disease-Targeted Treatment Among Patients With Positive Screening Test Results, According to Zone.
Discussion
We found that PA is detected and treated in Alberta at rates far lower than the expected population disease prevalence and with wide geographic disparity. In a population conservatively estimated to have at least 100 000 patients with PA (among the 1.1 million adults with hypertension), only 0.5% were potentially diagnosed and treated. Low screening rates appeared to be the greatest barrier to care, with less than 1% of people with hypertension even considered to have PA. Among patients who were screened with an ARR, an abnormally high result was closely associated with high-probability clinical features (ie, hypokalemia and the use of ≥3 antihypertensive drugs). Receipt of screening, subtyping, and disease-targeted treatment was most strongly linked to certain geographic zones and clinician specialty, perhaps reflecting the resource-limited nature of AVS and adrenalectomy and underscoring the benefits of a dedicated endocrine hypertension program for patients with PA. Potentially causally associated, the positivity rate of screening results was highest for patients living near the regional endocrine hypertension clinic, thus reflecting a greater awareness of PA in the area, such that clinicians were more likely to select patients with high-probability features of hyperaldosteronism for testing.
Our study is consistent with and extends the findings of previous reports. Although approximately half of patients with hypertension should be screened for PA as a potential cause of high blood pressure,8,10 actual global screening rates are in fact much lower.1,7,11,13 In a survey of 500 general practitioners from Italy and Germany, only 7% to 8% of patients treated for hypertension were reportedly screened for PA.11 Although notably higher than those observed in this study, the screening rates in the earlier study11 may have potentially been overstated, because these were based on clinician recall and not verified with objective laboratory results. Surprisingly, screening rates also appear generally low for patients with resistant hypertension and those with hypokalemia, despite a high prevalence of PA in these highly selected populations.1,24,25 In a cross-sectional study13 in an academic US center, only 2% of patients with resistant hypertension were screened for PA. In another US cohort,26 less than 3% of patients with hypertension and hypokalemia were considered to have PA. Indeed, studies7,11,12,25,26 have consistently reported poor awareness of PA among physicians caring for patients with hypertension. While large screening gaps have been well documented around the world,27,28 to our knowledge, no previous studies have systematically evaluated rates of PA subtyping and treatment, although these are also commonly believed to be low as well, owing to the specialized nature of these interventions.12,29
The lack of a simple, accessible, and streamlined process for diagnosis has been raised as the major barrier to care,27,30 a point also acknowledged by PA guideline developers.31,32 The markedly low detection and treatment rate may reflect a knowledge deficit, a general reluctance of physicians to proceed with the complicated and time-consuming workup for PA, or a simple preference to attempt to control blood pressure by conventional means instead.3,33,34 Educational interventions alone may be insufficient for the task, since mere awareness of guideline recommendations does not substantially increase detection.11 Rather, a system-level approach is likely needed to improve the efficiency of diagnosis and provide expedient care for patients. Notably, in the Calgary zone (where detection and treatment rates were the highest), a simplified care pathway for PA was implemented over a decade ago with excellent clinical outcomes.23 Referrals to the endocrine hypertension program are frequently made for patients with treatment-resistant hypertension, as well as those with hypokalemia, a known adrenal mass, or abnormal screening results. Here, a multidisciplinary team (of endocrinologists, endocrine surgeons, and interventional radiologists) facilitates the workup for PA, assesses eligibility for disease-targeted treatment, and provides standardized follow-up to measure treatment response. The program appeals to many primary care physicians because a single referral streamlines the process from diagnosis to treatment, removing a major barrier to initial screening (as reflected by the large number of tests ordered by family physicians in the Calgary region in our study).
Limitations
Our study had many strengths (ie, a large cohort with longitudinal follow-up drawn from a well-defined geographic locale; complete capture of all physician encounters and laboratory tests performed; and the presence of a universal insurance system, such that access to health services was not limited by direct costs to patients), but there were some limitations. First, we considered an elevated ARR to be sufficient for PA diagnosis but were unable to account for potential inaccuracies in the screening test (ie, because of confounding medications),35 or determine the results of confirmatory testing (ie, to exclude false-positive screen results). Although medication factors may result in some differences between individual ARR measurements, these are less likely to cause significant bias in our population estimates.18 Moreover, ARRs measured without medication adjustment are increasingly accepted and used in clinical care, because discontinuation of medications is not always safe or possible.28,36,37 Admittedly, however, the lack of confirmatory testing data may have resulted in some degree of misclassification among those who were screened. As such, it is possible that the rates of downstream subtyping and targeted treatment among those with PA were slightly higher than estimated. Second, we were unable to account for the possibility that some people were not surgical candidates (eg, cases in which a contraindication to surgery existed and/or surgery was declined by the patient). However, this would likely represent a very small percentage of patients, since AVS would not have generally been offered to individuals who were not at least considering surgery. Furthermore, this limitation would not have accounted for the very low rates of medical treatment. Finally, while detection, subtyping, and treatment rates for PA were consistently higher in the Calgary zone (ie, coinciding with the provincial endocrine hypertension program) compared with other parts of the province, a causal association could not be established. However, apart from the endocrine hypertension program, there were few differences between the zones to account for the large differences seen.22 Although it is impossible to conduct a randomized clinical trial to prove the efficacy of an endocrine hypertension program on improving health outcomes, the consistency of association across multiple measures in our study are highly suggestive that our conclusions about the outcomes of the dedicated program for improving the care of patients with PA are real.
Conclusions
In summary, PA is an increasingly recognized public health problem,10 not only because of the excess risk of cardiovascular disease,3 but also because of its high prevalence.2 But only a small fraction of affected patients are ever screened or treated. Improvements may be best realized by increasing awareness of this condition, promoting screening efforts, and providing access to specialized programs that can assist with workup when appropriate. The implication of our findings is that a system-level approach to simple workup and treatment may be effective in closing care gaps and improving clinical outcomes for patients with PA. With the declining rates of blood pressure control in the US and Canada, proper workup and treatment of secondary causes of hypertension, especially PA, has become more relevant than ever.21,38
eFigure. The typical diagnostic and treatment pathway for primary aldosteronism
eTable 1. People with hypertension who were investigated and treated for primary aldosteronism in Alberta
eTable 2. Demographic and clinical factors affecting the detection rate stratified according to zone of residence
References
- 1.Käyser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826-2835. doi: 10.1210/jc.2016-1472 [DOI] [PubMed] [Google Scholar]
- 2.Brown JM, Siddiqui M, Calhoun DA, et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173(1):10-20. doi: 10.7326/M20-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monticone S, D’Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6(1):41-50. doi: 10.1016/S2213-8587(17)30319-4 [DOI] [PubMed] [Google Scholar]
- 4.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243-1248. doi: 10.1016/j.jacc.2005.01.015 [DOI] [PubMed] [Google Scholar]
- 5.Velema M, Dekkers T, Hermus A, et al. ; SPARTACUS investigators . Quality of life in primary aldosteronism: a comparative effectiveness study of adrenalectomy and medical treatment. J Clin Endocrinol Metab. 2018;103(1):16-24. doi: 10.1210/jc.2017-01442 [DOI] [PubMed] [Google Scholar]
- 6.Buffolo F, Monticone S, Burrello J, et al. Is primary aldosteronism still largely unrecognized? Horm Metab Res. 2017;49(12):908-914. doi: 10.1055/s-0043-119755 [DOI] [PubMed] [Google Scholar]
- 7.Rossi E, Perazzoli F, Negro A, Magnani A. Diagnostic rate of primary aldosteronism in Emilia-Romagna, northern Italy, during 16 years (2000-2015). J Hypertens. 2017;35(8):1691-1697. doi: 10.1097/HJH.0000000000001384 [DOI] [PubMed] [Google Scholar]
- 8.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916. doi: 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 9.Leung AA, Daskalopoulou SS, Dasgupta K, et al. ; Hypertension Canada . Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557-576. doi: 10.1016/j.cjca.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 10.Funder JW. Primary aldosteronism as a public health issue. Lancet Diabetes Endocrinol. 2016;4(12):972-973. doi: 10.1016/S2213-8587(16)30272-8 [DOI] [PubMed] [Google Scholar]
- 11.Mulatero P, Monticone S, Burrello J, Veglio F, Williams TA, Funder J. Guidelines for primary aldosteronism: uptake by primary care physicians in Europe. J Hypertens. 2016;34(11):2253-2257. doi: 10.1097/HJH.0000000000001088 [DOI] [PubMed] [Google Scholar]
- 12.Funder JW. Primary aldosteronism: clinical lateralization and costs. J Clin Endocrinol Metab. 2012;97(10):3450-3452. doi: 10.1210/jc.2012-3046 [DOI] [PubMed] [Google Scholar]
- 13.Jaffe G, Gray Z, Krishnan G, et al. Screening rates for primary aldosteronism in resistant hypertension: a cohort study. Hypertension. 2020;75(3):650-659. doi: 10.1161/HYPERTENSIONAHA.119.14359 [DOI] [PubMed] [Google Scholar]
- 14.Weaver CG, Clement FM, Campbell NR, et al. ; Alberta Kidney Disease Network and the Interdisciplinary Chronic Disease Collaboration . Healthcare costs attributable to hypertension: Canadian population-based cohort study. Hypertension. 2015;66(3):502-508. doi: 10.1161/HYPERTENSIONAHA.115.05702 [DOI] [PubMed] [Google Scholar]
- 15.Quan H, Khan N, Hemmelgarn BR, et al. ; Hypertension Outcome and Surveillance Team of the Canadian Hypertension Education Programs . Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54(6):1423-1428. doi: 10.1161/HYPERTENSIONAHA.109.139279 [DOI] [PubMed] [Google Scholar]
- 16.Quan H, Chen G, Walker RL, et al. ; Hypertension Outcome and Surveillance Team . Incidence, cardiovascular complications and mortality of hypertension by sex and ethnicity. Heart. 2013;99(10):715-721. doi: 10.1136/heartjnl-2012-303152 [DOI] [PubMed] [Google Scholar]
- 17.Kline GA, Prebtani APH, Leung AA, Schiffrin EL. Primary aldosteronism: a common cause of resistant hypertension. CMAJ. 2017;189(22):E773-E778. doi: 10.1503/cmaj.161486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung AA, Orton DJ, Chin A, Sadrzadeh H, Kline GA. Novel approach to establishing an aldosterone: renin ratio cutoff for primary aldosteronism. Hypertension. 2017;69(3):450-456. doi: 10.1161/HYPERTENSIONAHA.116.08407 [DOI] [PubMed] [Google Scholar]
- 19.Nwariaku FE, Miller BS, Auchus R, et al. Primary hyperaldosteronism: effect of adrenal vein sampling on surgical outcome. Arch Surg. 2006;141(5):497-502. doi: 10.1001/archsurg.141.5.497 [DOI] [PubMed] [Google Scholar]
- 20.Leung AA, Bushnik T, Hennessy D, McAlister FA, Manuel DG. Risk factors for hypertension in Canada. Health Rep. 2019;30(2):3-13. [PubMed] [Google Scholar]
- 21.Leung AA, Williams JVA, McAlister FA, Campbell NRC, Padwal RS; Hypertension Canada’s Research and Evaluation Committee . Worsening hypertension awareness, treatment, and control rates in Canadian women between 2007 and 2017. Can J Cardiol. 2020;36(5):732-739. doi: 10.1016/j.cjca.2020.02.092 [DOI] [PubMed] [Google Scholar]
- 22.Alberta Health Services . AHS map and zone overview. Published 2017. Accessed December 15, 2020. https://www.albertahealthservices.ca/assets/about/publications/ahs-ar-2017/zones.html
- 23.Kline GA, Pasieka JL, Harvey A, So B, Dias VC. High-probability features of primary aldosteronism may obviate the need for confirmatory testing without increasing false-positive diagnoses. J Clin Hypertens (Greenwich). 2014;16(7):488-496. doi: 10.1111/jch.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calhoun DA. Hyperaldosteronism as a common cause of resistant hypertension. Annu Rev Med. 2013;64:233-247. doi: 10.1146/annurev-med-042711-135929 [DOI] [PubMed] [Google Scholar]
- 25.Burrello J, Monticone S, Losano I, et al. Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension. 2020;75(4):1025-1033. doi: 10.1161/HYPERTENSIONAHA.119.14063 [DOI] [PubMed] [Google Scholar]
- 26.Ruhle BC, White MG, Alsafran S, Kaplan EL, Angelos P, Grogan RH. Keeping primary aldosteronism in mind: deficiencies in screening at-risk hypertensives. Surgery. 2019;165(1):221-227. doi: 10.1016/j.surg.2018.05.085 [DOI] [PubMed] [Google Scholar]
- 27.Williams B, Brown MJ. Investigation of primary aldosteronism in patients with resistant hypertension—authors’ reply. Lancet Diabetes Endocrinol. 2018;6(8):600-601. doi: 10.1016/S2213-8587(18)30174-8 [DOI] [PubMed] [Google Scholar]
- 28.Auchus RJ. Primary aldosteronism and a Texas two-step. Rev Endocr Metab Disord. 2011;12(1):37-42. doi: 10.1007/s11154-011-9157-5 [DOI] [PubMed] [Google Scholar]
- 29.Funder JW. Ultimately we are in furious agreement. J Hypertens. 2012;30(10):1903-1905. doi: 10.1097/HJH.0b013e328356c5be [DOI] [PubMed] [Google Scholar]
- 30.Kline GA. Primary aldosteronism: unnecessary complexity in definition and diagnosis as a barrier to wider clinical care. Clin Endocrinol (Oxf). 2015;82(6):779-784. doi: 10.1111/cen.12798 [DOI] [PubMed] [Google Scholar]
- 31.Funder JW. Primary aldosteronism: at the tipping point. Ann Intern Med. 2020;173(1):65-66. doi: 10.7326/M20-1758 [DOI] [PubMed] [Google Scholar]
- 32.Funder JW. Primary aldosteronism: present and future. Vitam Horm. 2019;109:285-302. doi: 10.1016/bs.vh.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 33.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3(8):768-774. doi: 10.1001/jamacardio.2018.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51-59. doi: 10.1016/S2213-8587(17)30367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rehan M, Raizman JE, Cavalier E, Don-Wauchope AC, Holmes DT. Laboratory challenges in primary aldosteronism screening and diagnosis. Clin Biochem. 2015;48(6):377-387. doi: 10.1016/j.clinbiochem.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 36.Rye P, Chin A, Pasieka J, So B, Harvey A, Kline G. Unadjusted plasma renin activity as a “first-look” test to decide upon further investigations for primary aldosteronism. J Clin Hypertens (Greenwich). 2015;17(7):541-546. doi: 10.1111/jch.12523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams B, MacDonald TM, Morant S, et al. ; British Hypertension Society’s PATHWAY Studies Group . Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386(10008):2059-2068. doi: 10.1016/S0140-6736(15)00257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among us adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324(12):1190-1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. The typical diagnostic and treatment pathway for primary aldosteronism
eTable 1. People with hypertension who were investigated and treated for primary aldosteronism in Alberta
eTable 2. Demographic and clinical factors affecting the detection rate stratified according to zone of residence