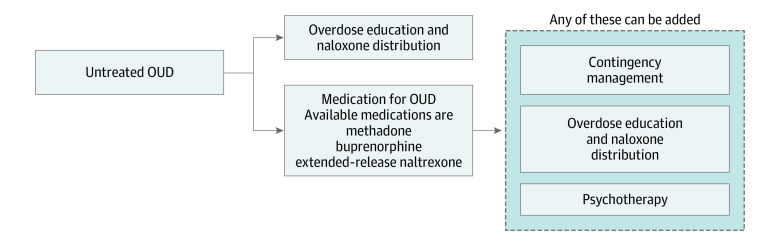
This study evaluates the cost-effectiveness of medication-assisted treatment and treatment add-ons (eg, contingency management) for opioid use disorder.
Key Points
Question
What is the cost-effectiveness of medication-assisted treatment (MAT) and treatment add-ons (eg, contingency management) for opioid use disorder in the United States?
Findings
In this cost-effectiveness study, MAT combined with contingency management and overdose education and naloxone distribution to treat opioid use disorder was associated with significant health benefits and cost savings compared with no treatment.
Meaning
A significant fraction of individuals with opioid use disorder in the United States do not receive any form of MAT; expanding access to MAT, overdose education and naloxone distribution, and contingency management may generate significant societal cost savings and, more importantly, save numerous lives.
Abstract
Importance
Opioid use disorder (OUD) is a significant cause of morbidity and mortality in the US, yet many individuals with OUD do not receive treatment.
Objective
To assess the cost-effectiveness of OUD treatments and association of these treatments with outcomes in the US.
Design and Setting
This model-based cost-effectiveness analysis included a US population with OUD.
Interventions
Medication-assisted treatment (MAT) with buprenorphine, methadone, or injectable extended-release naltrexone; psychotherapy (beyond standard counseling); overdose education and naloxone distribution (OEND); and contingency management (CM).
Main Outcomes and Measures
Fatal and nonfatal overdoses and deaths throughout 5 years, discounted lifetime quality-adjusted life-years (QALYs), and costs.
Results
In the base case, in the absence of treatment, 42 717 overdoses (4132 fatal, 38 585 nonfatal) and 12 660 deaths were estimated to occur in a cohort of 100 000 patients over 5 years, and 11.58 discounted lifetime QALYs were estimated to be experienced per person. An estimated reduction in overdoses was associated with MAT with methadone (10.7%), MAT with buprenorphine or naltrexone (22.0%), and when combined with CM and psychotherapy (range, 21.0%-31.4%). Estimated deceased deaths were associated with MAT with methadone (6%), MAT with buprenorphine or naltrexone (13.9%), and when combined with CM, OEND, and psychotherapy (16.9%). MAT yielded discounted gains of 1.02 to 1.07 QALYs per person. Including only health care sector costs, methadone cost $16 000/QALY gained compared with no treatment, followed by methadone with OEND ($22 000/QALY gained), then by buprenorphine with OEND and CM ($42 000/QALY gained), and then by buprenorphine with OEND, CM, and psychotherapy ($250 000/QALY gained). MAT with naltrexone was dominated by other treatment alternatives. When criminal justice costs were included, all forms of MAT (with buprenorphine, methadone, and naltrexone) were associated with cost savings compared with no treatment, yielding savings of $25 000 to $105 000 in lifetime costs per person. The largest cost savings were associated with methadone plus CM. Results were qualitatively unchanged over a wide range of sensitivity analyses. An analysis using demographic and cost data for Veterans Health Administration patients yielded similar findings.
Conclusions and Relevance
In this cost-effectiveness analysis, expanded access to MAT, combined with OEND and CM, was associated with cost-saving reductions in morbidity and mortality from OUD. Lack of widespread MAT availability limits access to a cost-saving medical intervention that reduces morbidity and mortality from OUD. Opioid overdoses in the US likely reached a record high in 2020 because of COVID-19 increasing substance use, exacerbating stress and social isolation, and interfering with opioid treatment. It is essential to understand the cost-effectiveness of alternative forms of MAT to treat OUD.
Introduction
Opioid use disorder (OUD) has become a public health crisis and is a significant cause of morbidity, mortality, lost productivity, and criminal justice system cost in the US.1,2 In 2018, at least 2 million people in the US had a substance use disorder related to prescription opioid pain medication.3 Veterans Health Administration (VA) patients have been particularly affected, with 7-times higher prevalence of OUD care receipt than patients in commercial health plans.4 Opioid overdose deaths reached a record high of 50 042 in 20195 and likely increased further in 2020 because of COVID-19 increasing substance use, exacerbating stress and social isolation, and interfering with opioid treatment.6,7
Multiple health care interventions are available for patients with OUD, including pharmacotherapies such as methadone (a full opioid agonist offered only in federally licensed opioid treatment programs), buprenorphine (a partial opioid agonist offered in opioid treatment programs and in office-based models, eg, primary care practices), and extended-release naltrexone (an opioid antagonist offered in a range of specialty addiction programs as well as in office-based settings). An overdose rescue medication, naloxone, coupled with health education, is offered in a range of clinical settings as well as in community outreach programs (eg, needle exchange programs, mobile methadone vans). Psychosocial services are offered independently and in conjunction with the preceding medications and include psychotherapy as well as a behavior change strategy known as contingency management (CM). Although some studies have assessed the effectiveness and cost-effectiveness of various interventions to treat OUD,8,9,10,11,12,13,14,15,16,17 no study has examined the effect and cost-effectiveness of specific forms of medication-assisted treatment (MAT) (with methadone, buprenorphine, or extended-release naltrexone) with combinations of potential add-on treatments (eg, CM), to our knowledge. Moreover, many prior studies evaluated treatment from a relatively narrow health care perspective without accounting for societal savings such as those that accrue from the criminal justice system.18 Determining the most effective and cost-effective interventions to treat OUD is important because treatment resources are limited, and unmet treatment need is substantial.3,19
We developed a mathematical model to assess the cost-effectiveness of interventions to treat OUD and the association of these treatments with outcomes. We performed an analysis for the US general population and an analysis specifically for the markedly different VA patient population because VA is the nation’s largest provider of substance use disorder treatment. We evaluated costs and quality-adjusted life-years (QALYs) for different treatments. We conducted cost-effectiveness analyses from a strictly health care sector perspective and from a broader perspective that also included criminal justice costs.
Methods
Overview
Use of VA data was approved by the VA and the Stanford University institutional review board. No human subjects were involved. We considered possible combinations of MAT (with buprenorphine, methadone, or injectable extended-release naltrexone) with add-on treatments (psychotherapy, overdose education and naloxone distribution [OEND], and CM), as well as OEND alone, resulting in 26 treatment options including the possibility of no treatment (Figure 1). We use the term MAT consistent with National Institute on Drug Abuse terminology for treatment combining medications with behavioral counseling.20 We assumed that MAT included a baseline level of drug counseling and medication management services21 and also considered the addition of more intensive psychotherapy. To estimate lifetime costs and QALYs associated with different treatment options, we developed a continuous-time dynamic compartmental model in which individuals with OUD can transition between different health states including out of treatment, receiving treatment, abstinent (no illicit opioids) and not receiving treatment, and dead (Figure 2). We distinguish individuals who inject drugs from those who do not.
Figure 1. Treatment Options Considered.
OUD indicates opioid use disorder.
Figure 2. Schematic of Dynamic Compartmental Model.
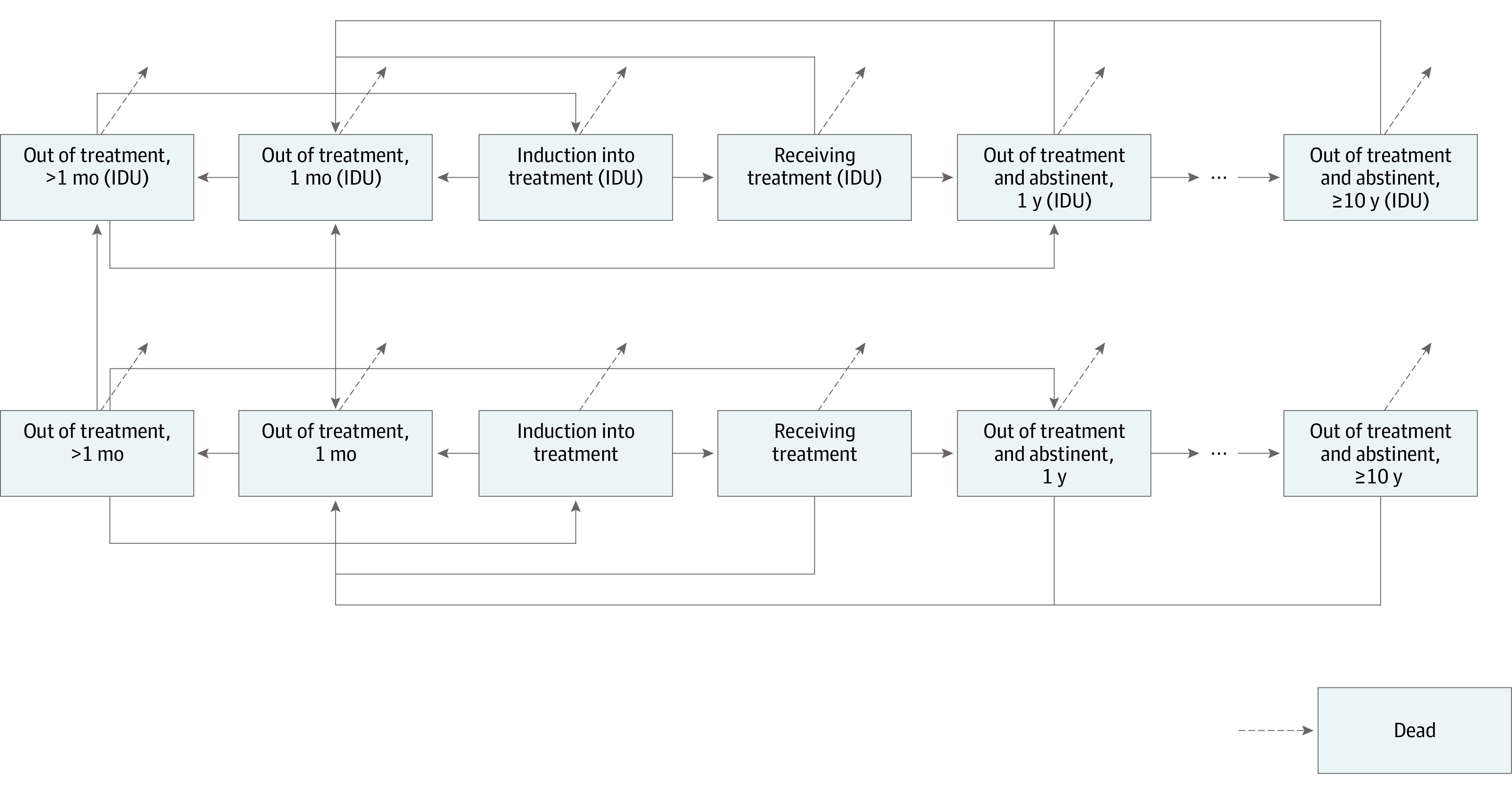
Individuals with and without injection drug use are included in this figure. IDU indicates injection drug use.
We modeled a representative cohort of individuals with OUD in the US population. We considered different combinations of sex and age (18 to 100 years) and ran the model for each individual’s lifetime for each possible treatment option. Background mortality, baseline health care cost, and criminal justice cost varied by sex and age. We weighted outcomes according to the age and sex distribution of individuals with OUD in the US (eFigure 1A in the Supplement) to assess mean per-person outcomes for each form of treatment. Model parameter values were obtained from the literature, expert opinion, and primary VA data (Table 122,24,30,48,49,50,51,54; eTable 1 the Supplement). The eMethods, eFigure 2, and eTables 2 and 3 in the Supplement provide full details of our methods.
Table 1. Base Case Parameter Values and Sources.
| Parameter | Mean (range) | Source |
|---|---|---|
| Male, % | 51.1 | Substance Abuse and Mental Health Data Archive3;US Census Bureau22 |
| Female, % | 48.9 | Substance Abuse and Mental Health Data Archive3;US Census Bureau22 |
| Age, mean, y | ||
| Male | 43.7 | Substance Abuse and Mental Health Data Archive3;US Census Bureau22 |
| Female | 45.3 | Substance Abuse and Mental Health Data Archive3;US Census Bureau22 |
| Initial fraction with OUD who inject drugs | 0.253 (0.214-0.294) | Substance Abuse and Mental Health Data Archive23 |
| Transition | ||
| Death and overdose, annual rates per person | ||
| Background mortality | CDC life tables | Arias24 |
| Nonoverdose excess mortality due to OUD, out of treatment | ||
| Out of treatment | 0.00978 (0.00744-0.01249) | Ma et al25 |
| In treatment | 0.00318 (0.00238-0.00406) | Ma et al25 |
| Multiplier for increased all-cause mortality when inducted onto methadone | 14.0 (1.08-62.16) | Ma et al25 |
| Overdose | ||
| Out of treatment | 0.103 (0.0472-0.2206) | Kelty et al26 |
| In treatment | 0.0438 (0.0205-0.0932) | Kelty et al26 |
| Overdose survival probability, per overdosea | ||
| Death after overdose, out of treatment | ||
| Without naloxone | 0.899 (0.799-0.954) | Coffin and Sullivan15 |
| With naloxone | 0.909 (0.826-0.967) | Coffin and Sullivan15 |
| Treatment discontinuation, annual rates/person | ||
| Discontinuation | ||
| From methadone | 1.051 (0.579-1.751) | Hser et al27;Neumann et al28; Otiashvili et al29; Potter et al30 |
| From buprenorphine | 1.609 (1.002-2.420) | Hser et al27; Neumann et al28; Otiashvili et al29; Lee et al31; Ruger et al32; Tanum et al33; Potter et al30 |
| From naltrexone | 1.588 (1.095-2.172) | Lee et al31; Ruger et al32; Tanum et al33; Krupitsky et al34; Krupitsky et al35; Jarvis et al36 |
| Hazard rate ratio for treatment discontinuation | ||
| Psychotherapy | 0.986 (0.772-1.240) | Ling et al37; McLellan et al38; Schwartz et al39; Tetrault et al40; Gruber et el41; Gu et al42; Fiellin et al43; Weiss et al23 |
| Contingency management | 0.594 (0.437-0.787) | Ling et al37; Chen et al44; DeFulio et al45; Dunn et al46; Hser et al47 |
| Psychotherapy combined with contingency management | 0.549 (0.356-0.732) | Ling et al37 |
| Other transitions | ||
| Reentry into treatment (from out of treatment >1 mo) | 0.426 (0.367-0.489) | Krebs et al16 |
| Becoming abstinent and leaving treatment | 0.316 (0.296-0.337) | Krebs et al16 |
| Becoming abstinent, from out of treatment | 0.0791 (0.00401-0.1551) | Estimated |
| Relapse from abstinence <1 yb | 0.379 (0.331-0.430) | Krebs et al16 |
| Relapse from abstinence, ≥10 y | 0.019 (0.00394-0.0342) | Krebs et al16 |
| Rate of initiating IDU | 0.031 (0.020-0.043) | Carlson et al48 |
| Cost, $c | ||
| Annual background health care costs | ||
| Baseline, male aged 30 yd | 2246 | Meara et al49; Liu et al50 |
| Excess cost for OUD | ||
| Out of treatment | 7176 (6476-7911) | Baser et al4 |
| In treatmente | 5748 (2990-8659) | Baser et al51 |
| Annual criminal justice costs, patient aged 30 yf | ||
| Out of treatment | 38 960 (35 365– 42 575) | Krebs et al52 |
| Out of treatment (IDU) | 59 554 (55 612– 63 514) | Krebs et al52 |
| Receiving treatment | 8790 (7886-9700) | Krebs et al52 |
| Receiving treatment (IDU) | 19 665 (18 004-21 334) | Krebs et al52 |
| Abstinent | 5105 (4636-5577) | Krebs et al52 |
| Abstinent (IDU) | 7801 (7282-8322) | Krebs et al52 |
| Annual treatment costs | ||
| Methadone | 6979 (6298-7694) | US Department of Defense21 |
| Buprenorphine | 6370 (5749-7023) | US Department of Defense21 |
| Extended-release naltrexone | 15 032 (13 566-16 572) | US Department of Defense21 |
| Psychotherapy | 4296 (3877-4736) | US Department of Defense21 |
| Contingency management | 3385 (3055-3732) | VA datag |
| Naloxone cost, per initial provision or refill | 71 (64-78) | VA data |
| Health care cost per overdose | ||
| Without naloxone | 2580 (1117-5108) | Coffin et al15 |
| With naloxone | 2063 (884-4116) | Coffin et al15 |
| Quality-of-life multipliers for health states | ||
| Out of treatment | ||
| Month 1 | 0.670 (0.660-0.680) | Krebs et al16; Nosyk et al53 |
| Out of treatment (IDU) | ||
| Month 1 | 0.660 (0.640-0.680) | Krebs et al16; Nosyk et al53 |
| Out of treatment | ||
| Month >1 | 0.670 (0.660-0.680) | Krebs et al16; Nosyk et al53 |
| Out of treatment (IDU) | ||
| Month >1 | 0.660 (0.640-0.680) | Krebs et al16; Nosyk et al53 |
| Induction | ||
| Into treatment | 0.725 (0.700-0.750) | Krebs et al16; Nosyk et al53 |
| Into treatment (IDU) | 0.710 (0.700-0.720) | Krebs et al16; Nosyk et al53 |
| Receiving treatment | 0.725 (0.700-0.750) | Krebs et al16; Nosyk et al53 |
| Receiving treatment (IDU) | 0.710 (0.700-0.720) | Krebs et al16; Nosyk et al53 |
| Abstinence | ||
| First yearh | 0.725 (0.700-0.750) | Krebs et al16; Fryback et al54 |
| Abstinence (IDU) | ||
| First year | 0.710 (0.700-0.720) | Krebs et al16; Fryback et al54 |
| Abstinence | ||
| Year ≥10 | 0.984 (0.970-0.996) | Calculated |
| Abstinence (IDU) | ||
| Year ≥10 | 0.983 (0.969-0.996) | Calculated |
Abbreviations: CDC, US Centers for Disease Control and Prevention; IDU, injection drug use; OUD, opioid use disorder.
For calculations, see the eMethods and eFigure 27 in the Supplement.
Rates of relapse from abstinence from years 2 to 9 of abstinence were linear interpolations of the year 1 and year 10 values.
All costs were updated to 2019 currency using the Consumer Price Index.
Baseline health care costs were age- and sex-specific.
Estimated based on the (conservative) assumption that patients with OUD who are receiving treatment incur 20% higher health care costs on average than those not receiving treatment because of increased access to health care.
Criminal justice costs for other ages were calculated based on the study by Krebs et al.16
See the eMethods and eTable 9 in the Supplement.
Utility values for years 2 through 9 were linear interpolations of the year 1 and year ≥10 utility values.
Interventions
We considered 3 medications for MAT: methadone, oral buprenorphine, and injectable extended-release naltrexone. These medications reduce risk of overdose and death25,26,55 and improve quality of life.16,53 We performed a network meta-analysis to estimate the hazard rate ratio for MAT discontinuation for methadone and buprenorphine compared with naltrexone (eMethods, eFigure 3, and eTable 4 in the Supplement).
We considered psychotherapy (additional treatment beyond routine MAT counseling) and CM as potential MAT add-ons. Both psychotherapy23,37,38,39,40,41,42,43 and CM37,44,45,46,47,56 can extend the time an individual continues taking MAT. We assumed that the effectiveness of psychotherapy would be similar to that for routine individual counseling sessions.23,37,38,39,40,41,42,43 We performed a network meta-analysis to estimate the hazard rate ratio for MAT discontinuation because of CM, psychotherapy, and their combination (eMethods, eFigure 4, and eTables 5-7 in the Supplement).
Finally, we considered OEND for individual patients. Naloxone is an emergency treatment for overdose rather than for OUD per se.15 We assumed OEND could be delivered alone or as an add-on to MAT.
eTable 8 in the Supplement summarizes the direct associations of each intervention with outcomes as captured by our model. Interventions also have indirect associations with outcomes that the model captures (eg, psychotherapy reduces the MAT discontinuation rate, which then leads to reduced mortality).
Outcomes
We measured health care and criminal justice costs. Health care costs included age-, sex-, and OUD state–specific costs of health care, nonfatal and fatal overdoses, and treatment. For individuals who become abstinent and leave treatment, we assumed that their health care costs diminish over time, eventually approaching that of individuals without OUD. For MAT, we included drug-specific ongoing costs associated with the drug and its administration based on US federal data.21 For psychotherapy, we assumed that patients have a 1-hour visit per week at a cost of $83 per visit (eMethods in the Supplement). We assumed that CM consisted of two 15-minute sessions per week with a mean weekly cost of $65 for personnel, drug screening, and incentive payments, based on VA data (eTable 9 in the Supplement). We assumed that naloxone cost $71 per use, based on VA estimates. Multiple studies have found reduced criminal justice costs for individuals taking MAT52,57,58,59,60; costs were specific to the patient’s age and health state.52
For each health state, we assigned a quality-of-life multiplier based on published estimates (Table 1). For individuals who are out of treatment and abstinent, we assumed that their quality of life increases over time, eventually approaching that of individuals without OUD.
For each treatment option, we calculated the mean number of fatal and nonfatal overdoses and total deaths over 5 years for a cohort of 100 000 individuals with OUD and expected lifetime total costs and QALYs. We identified nondominated options and calculated incremental cost-effectiveness ratios. We used a health care sector perspective for costs and QALYs as well as a limited societal perspective that additionally included criminal justice costs and discounted all values to the present at 3% annually.61 The eMethods in the Supplement describes model validation (eFigures 5-10 and eTable 10 in the Supplement) and the sensitivity analyses we performed.
VA-Specific Analysis
We performed an additional analysis focused specifically on VA. We simulated a cohort of individuals from the VA population diagnosed with OUD (eMethods and eTable 11 in the Supplement). We weighted outcomes according to the distribution of age and sex for a cohort of VA patients diagnosed with OUD in fiscal year 2017 (eTable 12 and eFigure 1B in the Supplement). We obtained data on VA treatment costs (eTable 12 in the Supplement) from a micro-costing study we performed using VA data (eMethods, eTable 9, and eTables 13-17 in the Supplement). We assumed that intervention effectiveness parameters were the same as in the base case analysis. We validated the model by comparing model projections with VA data (eTable 10 in the Supplement).
Results
Base Case Analysis
Population Outcomes
In the absence of treatment, 42 717 overdoses (4132 fatal, 38 585 nonfatal) and 12 660 deaths were estimated to occur in the cohort of 100 000 patients over 5 years, and 11.58 discounted lifetime QALYs were estimated to be experienced per person (Table 2).
Table 2. Outcomes for Each Treatment Option, Modeled on a General US Cohort of Individuals With OUD.
| Treatment option | Over 5 y, per 100 000 individuals with OUD, mean (95% CrI) | Lifetime, per person discounted, mean (95% CrI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Overdose | Deathsb | QALYs | Cost in $1000a | |||||
| Fatal | Nonfatal | Total | Health care | Criminal justice | Total | |||
| No treatment | 4132 (3171-5275) | 38 585 (15 642-87 416) | 42 717 (19 406-91 833) | 12 660 (10 936-14 666) | 11.58 (10.06-12.93) | 256 (239-274) | 357 (279-452) | 613 (529-714) |
| OEND | 3399 (1882-4827) | 39 496 (16 374-88 555) | 42 895 (19 482-92 199) | 11 963 (10 012-14 115) | 11.80 (10.25-13.18) | 261 (242-281) | 363 (283-462) | 624 (537-732) |
| MAT with methadone | ||||||||
| Methadone only | 3690 (2502-6473) | 34 451 (12 849-85 051) | 38 141 (15 839-90 293) | 11 905 (9583-17 631) | 12.60 (10.95-13.77) | 272 (236-302) | 244 (189-307) | 516 (442-588) |
| Methadone + CM | 3318 (2224-5935) | 30 970 (11 463-77 093) | 34 287 (14 122-81 921) | 11 176 (9028-16 570) | 13.03 (11.47-14.13) | 292 (254-325) | 216 (167-274) | 508 (440-574) |
| Methadone + PT | 3675 (2478-6463) | 34 304 (12 800-84 848) | 37 979 (15 766-89 971) | 11 874 (9537-17 594) | 12.62 (10.96-13.80) | 285 (247-317) | 243 (187-307) | 528 (453-600) |
| Methadone + OEND | 3118 (1643-5808) | 36 457 (13 601-91 523) | 39 575 (16 115-95 878) | 11 285 (8904-16 673) | 12.80 (11.19-13.98) | 277 (241-307) | 247 (192-313) | 524 (450-599) |
| Methadone + CM + OEND | 2805 (1465-5317) | 32 803 (12 120-83 131) | 35 608 (14 363-87 290) | 10 617 (8428-15 665) | 13.22 (11.70-14.32) | 296 (258-330) | 219 (169-279) | 515 (447-583) |
| Methadone + CM + PT | 3263 (2183-5853) | 30 457 (11 269-75 976) | 33 720 (13 859-80 650) | 11 069 (8941-16 388) | 13.10 (11.55-14.18) | 309 (269-345) | 212 (164-269) | 521 (454-587) |
| Methadone + OEND + PT | 3105 (1634-5794) | 36 302 (13 519-91 220) | 39 407 (16 031-95 695) | 11 257 (8872-16 648) | 12.82 (11.20-14.00) | 290 (252-323) | 246 (190-313) | 536 (461-612) |
| Methadone + CM + OEND + PT | 2759 (1441-5245) | 32 264 (11 905-81 822) | 35 023 (14 102-85 996) | 10 519 (8357-15 498) | 13.28 (11.78-14.37) | 314 (274-350) | 215 (166-274) | 529 (461-596) |
| MAT with buprenorphine | ||||||||
| Buprenorphine only | 3227 (2508-4081) | 30 136 (12 273-68 084) | 33 363 (15 229-71 522) | 10 894 (9588-12 410) | 12.65 (11.54-13.67) | 274 (253-297) | 275 (218-342) | 549 (486-621) |
| Buprenorphine + CM | 2893 (2235-3683) | 27 012 (10 987-61 210) | 29 905 (13 624-64 314) | 10 249 (9042-11 668) | 13.07 (12.04-14.02) | 291 (266-318) | 245 (193-307) | 536 (477-603) |
| Buprenorphine + PT | 3213 (2487-4080) | 30 005 (12 212-67 906) | 33 218 (15 130-71 327) | 10 866 (9545-12 408) | 12.66 (11.55-13.70) | 285 (262-310) | 274 (216-342) | 559 (495-632) |
| Buprenorphine + OEND | 2653 (1474-3737) | 30 826 (12 836-68 927) | 33 479 (15 275-71 784) | 10 345 (8841-11 987) | 12.84 (11.72-13.88) | 279 (256-303) | 279 (221-348) | 558 (492-634) |
| Buprenorphine + CM + OEND | 2378 (1318-3368) | 27 624 (11 481-61 910) | 30 001 (13 665-64 522) | 9755 (8376-11 279) | 13.24 (12.20-14.21) | 295 (269-323) | 249 (195-312) | 544 (482-614) |
| Buprenorphine + CM + PT | 2841 (2189-3621) | 26 530 (10 768-60 134) | 29 371 (13 367-63 185) | 10 149 (8949-11 551) | 13.13 (12.10-14.08) | 306 (279-337) | 241 (189-302) | 547 (488-613) |
| Buprenorphine + OEND + PT | 2642 (1467-3731) | 30 691 (12 752-68 760) | 33 333 (15 185-71 578) | 10 320 (8808-11 979) | 12.85 (11.71-13.90) | 289 (265-316) | 278 (219-348) | 567 (500-644) |
| Buprenorphine + CM + OEND + PT | 2335 (1292-3310) | 27 129 (11 256-60 855) | 29 464 (13 406-63 367) | 9664 (8301-11 169) | 13.31 (12.27-14.27) | 311 (282-342) | 244 (191-307) | 555 (493-623) |
| MAT with naltrexone | ||||||||
| Naltrexone only | 3226 (2523-4059) | 30 131 (12 298-68 135) | 33 357 (15 275-71 581) | 10 892 (9617-12 367) | 12.65 (11.58-13.65) | 295 (271-321) | 275 (220-339) | 570 (506-642) |
| Naltrexone + CM | 2890 (2250-3655) | 26 991 (11 017-61 068) | 29 881 (13 660-64 122) | 10 244 (9075-11 616) | 13.07 (12.08-14.01) | 319 (290-350) | 245 (195-304) | 564 (504-630) |
| Naltrexone + PT | 3212 (2502-4056) | 29 998 (12 236-67 832) | 33 210 (15 184-71 233) | 10 865 (9574-12 365) | 12.67 (11.58-13.68) | 306 (280-335) | 274 (218-339) | 580 (515-652) |
| Naltrexone + OEND | 2653 (1480-3720) | 30 820 (12 862-68 956) | 33 473 (15 324-71 826) | 10 344 (8863-11 945) | 12.84 (11.75-13.87) | 300 (274-327) | 279 (223-345) | 579 (512-654) |
| Naltrexone + CM + OEND | 2376 (1322-3347) | 27 601 (11 525-61 729) | 29 977 (13 706-64 306) | 9751 (8401-11 230) | 13.25 (12.24-14.20) | 322 (293-355) | 249 (197-309) | 571 (509-641) |
| Naltrexone + CM + PT | 2839 (2202-3593) | 26 506 (10 798-60 048) | 29 345 (13 404-63 072) | 10 144 (8981-11 498) | 13.13 (12.15-14.07) | 335 (304-370) | 241 (190-299) | 576 (516-641) |
| Naltrexone + OEND + PT | 2641 (1471-3715) | 30 684 (12 777-68 698) | 33 325 (15 236-71 465) | 10 319 (8827-11 941) | 12.86 (11.75-13.89) | 310 (283-341) | 278 (221-345) | 588 (521-665) |
| Naltrexone + CM + OEND + PT | 2333 (1297-3289) | 27 105 (11 288-60 686) | 29 438 (13 441-63 271) | 9660 (8322-11 121) | 13.31 (12.31-14.25) | 339 (307-376) | 244 (192-304) | 583 (521-652) |
Abbreviations: CM, contingency management; CrI, credible interval; MAT, medication-assisted treatment; OEND, overdose education and naloxone distribution; OUD, opioid use disorder; PT, psychotherapy; QALY, quality-adjusted life-year.
Currency is reported in 2019 values.
From all causes.
Fatal and Nonfatal Overdose
MAT with methadone was associated with a reduction in overdoses by 10.7% (from 42 717 with no treatment to 38 141). MAT with buprenorphine or naltrexone was associated with a reduction in overdoses by 22.0% (from 42 717 to 33 363 and 33 357, respectively). Total overdoses decreased the most when MAT was combined with CM and psychotherapy: for methadone, buprenorphine, and naltrexone, these reductions were 21.0% (from 42 717 to 33 720), 31.2% (from 42 717 to to 29 371), and 31.4% (from 42 717 to 29 325), respectively. OEND alone was associated with an increase in total overdoses slightly (from 42 717 to 42 895) because of its role in averting fatal overdoses (17.8% reduction from 4132 to 3399); individuals who survive an overdose can overdose again in the future, leading to more nonfatal overdoses (2.4% increase from 38 585 to 39 496).
Mortality
Total deaths decreased for all treatment options compared with no treatment. MAT with methadone was associated with a reduction in deaths by 6.0% (from 12 660 to 11 905). MAT with buprenorphine or naltrexone was associated with a reduction in deaths by 13.9% (from 12 660 to 10 894 and 10 892, respectively). Methadone’s association with death was lower because it is associated with higher mortality during the first month of treatment.26,55,62,63 The greatest reduction in deaths occurred when MAT was combined with CM, OEND, and psychotherapy: deaths were associated with a decrease of 16.9% for methadone (from 12 660 to 10 519) and of 23.7% for buprenorphine and naltrexone (from 12 660 to 9664 and 9660, respectively).
Lifetime Outcomes
Discounted per-person QALYs increased for all treatment options compared with no treatment (Table 2). OEND alone increased per-person QALYs by 0.22 (to 11.80). MAT alone increased per-person QALYs by 1.02 to 1.07 (to 12.60 for methadone and to 12.65 for buprenorphine and naltrexone). QALY gains are similar for the 3 forms of MAT because although individuals entering methadone treatment have higher initial mortality, retention while taking methadone is greater than while taking buprenorphine and naltrexone.27,28,29,31,32,33,34,35,36,64 The greatest QALY increase occurred for MAT with CM, OEND, and psychotherapy: approximately 1.7 QALYs gained per person for all 3 forms of MAT.
Cost-Effectiveness Analysis
When we included only health care costs, methadone alone was the first intervention on the efficient frontier, costing $16 000/QALY gained compared with no treatment, followed by methadone with OEND ($22 000/QALY gained), then by buprenorphine with OEND and CM ($42 000/QALY gained), and then by buprenorphine with OEND, CM, and psychotherapy ($250 000/QALY gained) (Figure 3A).
Figure 3. Cost-effectiveness Analysis for US Population with Opioid Use Disorder.
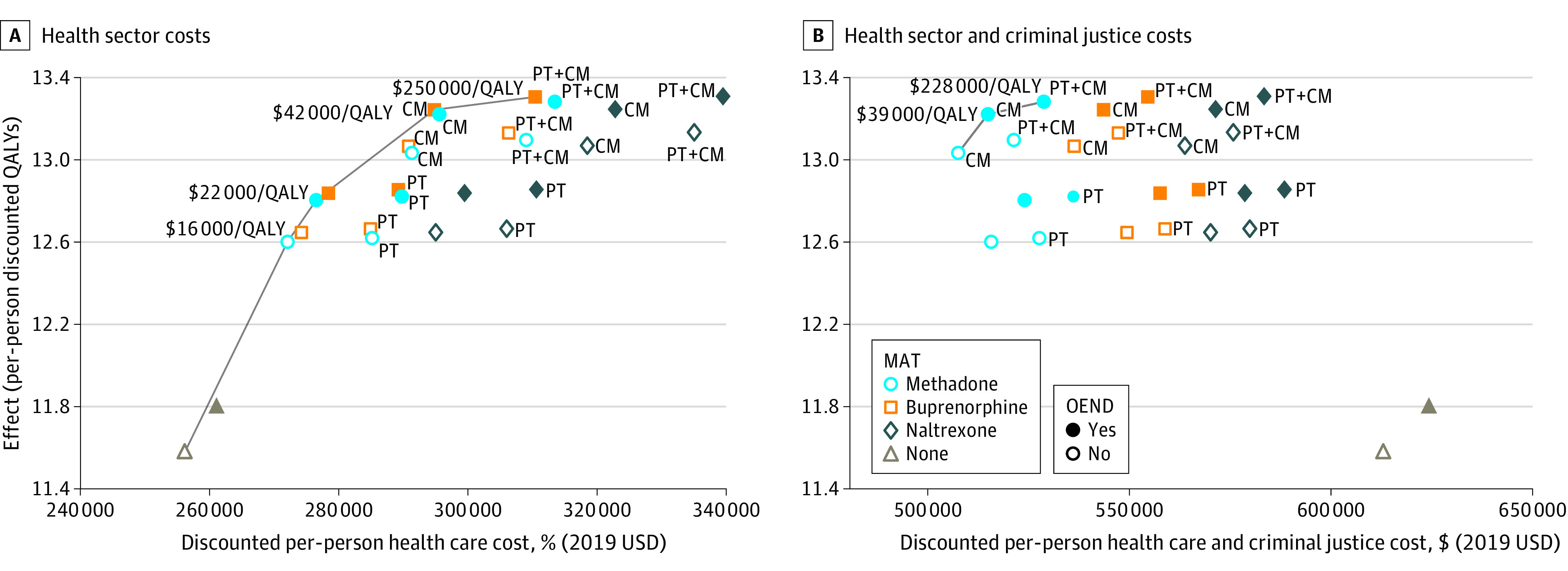
Results are presented for health sector costs (A) and for health sector and criminal justice costs (B). Currency is reported in 2019 values. CM indicates contingency management; OEND, overdose education and naloxone distribution; MAT, medication-assisted treatment; PT, psychotherapy; QALY, quality-adjusted life-year.
When criminal justice costs were included, all forms of MAT were cost-saving compared with no treatment (Figure 3B). This is because individuals receiving treatment and individuals who become abstinent due to treatment have significantly lower criminal justice costs than untreated individuals with OUD. The net present per-person cost savings are on the order of $100 000 for methadone, $60 000 for buprenorphine, and $40 000 for extended-release naltrexone.
While the no-treatment option is dominated, it is important to assess the relative cost-effectiveness of the other treatment options. For MAT with methadone, the addition of CM dominated methadone alone, further adding OEND cost $39 000/QALY gained and further adding psychotherapy cost $228 000/QALY gained (Figure 3B). If methadone is not available, for MAT with buprenorphine, the addition of CM increased cost savings and QALYs (eFigure 11B in the Supplement), adding OEND cost $41 000/QALY gained and further adding psychotherapy cost $176 000/QALY gained. For MAT with naltrexone, the addition of CM increased cost savings and QALYs, adding OEND cost $43 000/QALY gained, and further adding psychotherapy cost $192 000/QALY gained (eFigure 12B in the Supplement).
OEND alone cost $51 000/QALY gained compared with no treatment. Because naloxone keeps people alive longer but does not reduce individuals’ criminal justice costs, total costs modestly increase when OEND is provided without MAT.
VA Analysis
A key difference from the base case findings for our VA-specific analysis (eTable 18 and eFigures 13-15 in the Supplement) was that per-person QALY gains from treatment were lower: 0.17 QALYs gained for OEND and 0.83 to 0.87 QALYs gained for MAT. This is because the population of VA patients with OUD is older on average than the general US population with OUD and comprises a greater proportion of male individuals (eFigure 1 in the Supplement).
Despite differences in the magnitude of the health outcomes for the VA population, cost-effectiveness results are similar. Buprenorphine alone cost $13 000/QALY gained, increasing to $23 000/QALY gained when combined with OEND, to $41 000/QALY gained when further adding CM, and to $279 000/QALY gained when further adding psychotherapy (eFigure 13A in the Supplement). MAT was cost saving when criminal justice costs were considered (eFigure 13B in the Supplement). The net present per-person cost savings are slightly lower than for the general population, on the order of $80 000 for methadone, $50 000 for buprenorphine, and $40 000 for extended-release naltrexone, because the VA population with OUD is older than the general population with OUD and such individuals have lower criminal justice costs.
Sensitivity Analysis
We performed sensitivity analysis on MAT discontinuation rates, mortality while receiving MAT, mortality out of treatment, rate at which MAT induces abstinence and individuals leave care, and rate of relapse while out of care (eTables 19-25 in the Supplement). If mortality for individuals receiving MAT is 25% higher and/or treatment discontinuation in MAT is 25% higher than we assumed in the base case, MAT is still cost saving (eFigures 16-18 in the Supplement). MAT is similarly cost saving if there is 25% higher mortality for individuals not receiving treatment (eFigure 19 in the Supplement), if patients never discontinue treatment and maintain abstinence (eFigure 20 in the Supplement), if there is no direct relapse from MAT (eFigure 21 in the Supplement), or if there is no direct relapse from MAT and no relapse after an individual becomes abstinent and leaves treatment (eFigure 22 in the Supplement).
If mortality in the first month following treatment discontinuation is 3 times higher than in subsequent months, as suggested by some studies55,65,66 (the base case assumed no such increase), the ordering of treatment options on the efficient frontier is unchanged from the base case (eFigure 23 and eTable 26 in the Supplement). Methadone is cost saving if it reduces criminal justice costs by at least 10%; for buprenorphine and naltrexone, these values are 23% and 42%, respectively.
In probabilistic sensitivity analysis, in 100% of simulations (across all willingness-to-pay thresholds), no treatment and OEND alone were dominated; MAT was cost saving compared with no treatment; and naltrexone was dominated by methadone or buprenorphine with CM (eFigure 24 in the Supplement). At a willingness to pay of $100 000/QALY gained, methadone combined with CM and OEND was preferred in 67% of simulations, adding psychotherapy was preferred in 15% of simulations, and buprenorphine combined with CM and OEND was preferred in 12% of simulations.
We report results of additional sensitivity analyses in eFigures 25 and 26 in the Supplement as well as the expected value of partial perfect information for all model parameters (eMethods in the Supplement).
Discussion
Our analysis suggests that MAT for OUD with the addition of CM, psychotherapy, and OEND is likely to generate significant health benefits while being cost saving compared with no treatment when costs from the health care and criminal justice systems are considered. OUD is a serious, potentially deadly disorder, and its treatment would merit financial investment whether such treatment were cost saving or not.67 Very few medical interventions for less stigmatized conditions are cost saving when compared with no treatment, nor are they expected to be. However, the fact that OUD treatment is cost saving further strengthens the case for providing it. We find that MAT is cost-effective from the health care perspective and cost saving when criminal justice costs are included.68 These results are robust over a broad range of sensitivity analyses. MAT was associated with approximately 1.0 additional QALYs per person and associated with savings of approximately $15 000 to $90 000 in lifetime costs per person. Methadone and buprenorphine generated the largest savings and health benefits. Naltrexone is also a cost-effective choice that should be available to patients because many will decline methadone and buprenorphine.
Our analysis showed that OEND and CM are cost-effective add-ons to MAT but that the further addition of psychotherapy may not always be cost-effective. We assumed that some counseling is provided with MAT, so these findings only apply to additional psychotherapy implemented when CM is already in place. We assumed that psychotherapy would have a modest association with treatment outcomes, consistent with existing studies.23,37,38,39,40,41,42,43 Psychotherapy can be implemented in a variety of ways37,38,39 and with a broad range of costs. We showed that psychotherapy could be cost-effective if it cost less than $42 per week. Group counseling would be less expensive than the base psychotherapy cost we assumed, with potentially different effectiveness and would be important to model in future studies.
Our findings highlight the importance of providing MAT. Although we find modest differences in cost-effectiveness among treatment options, the costs of different treatments and availability will vary among settings, and therefore cost-effectiveness will also vary. Clinicians should aim to provide whatever MAT is feasible in their setting and to supplement it with CM. Following the model of the Affordable Care Act,69 policy makers can support these efforts by establishing these treatments as permanent, adequately remunerated benefits in public insurance programs (eg, Medicaid and Medicare) and by enforcing parity regulations that require its coverage in private insurance programs.70 Expanding CM would also be facilitated by exempting the small rewards it provides to patients from antikickback provisions in state and federal law.71
Our VA-specific analyses result in similar findings and conclusions. VA is the largest provider of substance use treatment in the nation, and therefore this setting has particular importance. VA has been a national leader in providing substance use treatment, but even in VA settings a significant proportion of Veterans with OUD do not receive MAT.72,73 Our analyses indicate that provision of MAT is highly cost-effective for the VA, considering only health care costs, and would be cost saving when criminal justice costs are included.
Limitations
Our study has several limitations. Model parameter values were informed by available data, which was limited in some cases. More refined data on treatment costs and effectiveness would improve our model estimates, although the interventions are still likely cost saving if the significant criminal justice costs are averted when individuals receive treatment for OUD. We did not find data that were suitable for traditional model calibration exercises because of selection bias into treatment in the available data. However, we compared our outcomes with available relevant data, and our main conclusions are robust in extensive sensitivity analyses. We did not consider all available forms of MAT (eg, extended-release buprenorphine). We simplified certain pathways in our analysis; for example, we did not explicitly model incarceration (but did include criminal justice costs) nor did we model treatment retention and outcomes as a function of an individual’s treatment history. We did not model incremental improvements in quality of life with sustained abstinence from illicit opioids while receiving treatment, nor diminishing excess health care costs or diminishing discontinuation rates for individuals receiving long-term pharmacotherapy; inclusion of these factors would make MAT appear more favorable than we estimated. We did not include the significant costs associated with lost productivity1,2 nor costs associated with social services (eg, child welfare services) or housing (eg, homelessness) due to uncertainty about such costs; inclusion of these costs would make the treatment interventions look more favorable. This may be particularly true of psychotherapy, which more directly targets the psychosocial effects of OUD and associated comorbidities. Finally, we focused our analysis on MAT with several add-on interventions. A more comprehensive analysis could consider additional evidence-based interventions for associated comorbidities, particularly those that aim to reduce morbidity for people who inject drugs, including HIV screening and treatment, HIV pre-exposure prophylaxis, syringe and needle exchange programs, vaccination for hepatitis B, and screening for hepatitis C infection.
Conclusions
A large fraction of individuals with OUD in the US do not receive any form of MAT,19 and this has been exacerbated by COVID-19.6,7 In our cost-effectiveness analysis, MAT, with or without OEND, CM, and psychotherapy, was associated with significant health benefits and cost saving. Policy makers and many members of Congress have proposed expanding access to MAT and OEND.74,75 Our results indicate that such a policy, especially if it included CM, would generate significant societal cost savings and, more importantly, save numerous lives.
eMethods.
eFigure 1. Age and sex distribution of patients with opioid use disorder (OUD)
eFigure 2. Model schematic showing notation for all compartments and parameters
eFigure 3. Results of network meta-analysis: hazard rate ratio (HR) for discontinuation rate from different forms of MAT compared to naltrexone
eFigure 4. Results of network meta-analysis: hazard rate ratio (HR) for discontinuation rate from MAT for contingency management, psychotherapy, and both compared to no add-on
eFigure 5. Model-projected survival curves for 30-year-old males
eFigure 6. Model projected fatal overdoses curves for 30-year-old males
eFigure 7. Model projected non-fatal overdoses curves for 30-year-old males
eFigure 8. Model projected time in treatment for 30-year-old males
eFigure 9. Model projected healthcare cost for 30-year-old males
eFigure 10. Model-projected survival curves for males aged 30, 40, 50, 60, 70, and 80 years old
eFigure 11. Results of cost-effectiveness analysis for U.S. population with OUD, assuming methadone is not available
eFigure 12. Results of cost-effectiveness analysis for U.S. population with OUD, assuming methadone and buprenorphine are not available
eFigure 13. Results of cost-effectiveness analysis for VA patient population with OUD
eFigure 14. Results of cost-effectiveness analysis for VA patient population with OUD, assuming methadone is not available
eFigure 15. Results of cost-effectiveness analysis for VA patient population with OUD, assuming methadone and buprenorphine are not available
eFigure 16. Results of cost-effectiveness analysis for a U.S. population with OUD, assuming 25% higher rate of discontinuation from MAT
eFigure 17. Results of sensitivity analysis for a U.S. population with OUD, assuming 25% higher mortality rate on MAT
eFigure 18. Results of sensitivity analysis assuming 25% higher rate of discontinuation from MAT and 25% higher mortality rate on MAT
eFigure 19. Results of sensitivity analysis assuming 25% higher mortality rate out of treatment
eFigure 20. Results of sensitivity analysis for a U.S. population with OUD, assuming MAT never leads to abstinence
eFigure 21. Results of sensitivity analysis for a U.S. population with OUD, assuming no direct relapse from MAT
eFigure 22. Results of sensitivity analysis for a U.S. population with OUD, assuming no direct relapse from MAT and no relapse after becoming abstinent and leaving treatment
eFigure 23. Results of sensitivity analysis for a U.S. population with OUD, assuming three times higher mortality in the first month following treatment discontinuation relative to subsequent months
eFigure 24. Cost-effectiveness acceptability curve for U.S. population with OUD, limited societal perspective
eFigure 25. Threshold analysis on effectiveness of psychotherapy (PT) and contingency management (CM) at a willingness to pay of $100,000/QALY, limited societal perspective
eFigure 26. Threshold analysis on effectiveness of contingency management (CM) and effectiveness of adding psychotherapy (PT) to CM at a willingness to pay of $100,000/QALY, limited societal perspective
eFigure 27. Probability of surviving an overdose with and without naloxone
eTable 1. Parameter Distributions
eTable 2. Impact Inventory Analysis
eTable 3. CHEERS (Consolidated Health Economic Evaluation Reporting System) Checklist
eTable 4. Summary of Clinical Trials Evaluating the Relative Treatment Discontinuation Rates for Methadone, Buprenorphine, and Naltrexone
eTable 5. Summary of Clinical Trials Evaluating the Effectiveness of Psychotherapy in Reducing Treatment Discontinuation
eTable 6. Summary of Clinical Trials Evaluating the Effectiveness of Contingency Management in Reducing Treatment Discontinuation
eTable 7. Summary of Clinical Trial Evaluating the Effectiveness of Psychotherapy Combined with Contingency Management
eTable 8. Mean Direct Effects of Interventions
eTable 9. Estimated Cost of Contingency Management at the VA ($2019)
eTable 10. Model-projected Outcomes and Targets for Model Validation
eTable 11. Inclusion Criteria for Opioid Overdose (Initial, Non-Assault)
eTable 12. Demographics and Distribution of Treatment Costs for Veterans Health Administration (VA) patients (US $2019) with Opioid Use Disorder
eTable 13. Exclusion Criteria Used in Treatment Cost Estimation
eTable 14. Estimated Cost of Psychotherapy at the VA ($2019)
eTable 15. Estimated Cost of Methadone Maintenance Treatment at the VA ($2019)
eTable 16. Estimated Cost of Buprenorphine Treatment at the VA ($2019)
eTable 17. Estimated Cost of Long-Lasting Injectable Naltrexone Treatment at the VA ($2019)
eTable 18. Results: Mean Outcomes and 95% Credible Interval for Each Treatment Option, Modeled for a Cohort of Veterans Health Administration (VA) Patients with Opioid Use Disorder
eTable 19. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming 25% Higher Rate of Discontinuation from MAT
eTable 20. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming 25% Higher Mortality on MAT
eTable 21. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming a 25% Higher Rate of Discontinuation from MAT and 25% Higher Mortality on MAT
eTable 22. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming a 25% Higher Mortality Rate Out of Treatment
eTable 23. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming that MAT Never Leads to Abstinence
eTable 24. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming No Direct Relapse from MAT
eTable 25. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming No Direct Relapse from MAT and No Relapse After Becoming Abstinent and Leaving Treatment
eTable 26. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming Three Times Higher Mortality in the First Month Following Treatment Discontinuation Relative to Subsequent Months
eReferences.
References
- 1.Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906. doi: 10.1097/MLR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ballestros K, Echko M, et al. ; US Burden of Disease Collaborators . The state of US health, 1990-2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444-1472. doi: 10.1001/jama.2018.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Data Archive . National survey on drug use and health 2018 (NSDUH-2018-DS0001). Accessed June 14, 2020. https://www.datafiles.samhsa.gov/study-dataset/national-survey-drug-use-and-health-2018-nsduh-2018-ds0001-nid18758
- 4.Baser O, Xie L, Mardekian J, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the veterans health administration. Pain Pract. 2014;14(5):437-445. doi: 10.1111/papr.12097 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . Provisional drug overdose death counts. Accessed August 5, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- 6.Centers for Disease Control and Prevention . Increase in fatal drug overdoses across the United States driven by synthetic opioids before and during the COVID-19 pandemic. https://emergency.cdc.gov/han/2020/han00438.asp
- 7.Alexander GC, Stoller KB, Haffajee RL, Saloner B. An epidemic in the midst of a pandemic: opioid use disorder and COVID-19. Ann Intern Med. 2020;173(1):57-58. doi: 10.7326/M20-1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitt AL, Humphreys K, Brandeau ML. Modeling health benefits and harms of public policy responses to the US opioid epidemic. Am J Public Health. 2018;108(10):1394-1400. doi: 10.2105/AJPH.2018.304590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard CL, Owens DK, Goldhaber-Fiebert JD, Brandeau ML. Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: a model-based analysis. PLoS Med. 2017;14(5):e1002312. doi: 10.1371/journal.pmed.1002312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett PG, Zaric GS, Brandeau ML. The cost-effectiveness of buprenorphine maintenance therapy for opiate addiction in the United States. Addiction. 2001;96(9):1267-1278. doi: 10.1046/j.1360-0443.2001.96912676.x [DOI] [PubMed] [Google Scholar]
- 11.Zaric GS, Barnett PG, Brandeau ML. HIV transmission and the cost-effectiveness of methadone maintenance. Am J Public Health. 2000;90(7):1100-1111. doi: 10.2105/AJPH.90.7.1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langham S, Wright A, Kenworthy J, Grieve R, Dunlop WCN. Cost-effectiveness of take-home naloxone for the prevention of overdose fatalities among heroin users in the United Kingdom. Value Health. 2018;21(4):407-415. doi: 10.1016/j.jval.2017.07.014 [DOI] [PubMed] [Google Scholar]
- 13.Getty CA, Morande A, Lynskey M, Weaver T, Metrebian N. Mobile telephone-delivered contingency management interventions promoting behaviour change in individuals with substance use disorders: a meta-analysis. Addiction. 2019;114(11):1915-1925. doi: 10.1111/add.14725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsden J, Stillwell G, James K, et al. Efficacy and cost-effectiveness of an adjunctive personalised psychosocial intervention in treatment-resistant maintenance opioid agonist therapy: a pragmatic, open-label, randomised controlled trial. Lancet Psychiatry. 2019;6(5):391-402. doi: 10.1016/S2215-0366(19)30097-5 [DOI] [PubMed] [Google Scholar]
- 15.Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1-9. doi: 10.7326/0003-4819-158-1-201301010-00003 [DOI] [PubMed] [Google Scholar]
- 16.Krebs E, Enns B, Evans E, et al. Cost-effectiveness of publicly funded treatment of opioid use disorder in California. Ann Intern Med. 2018;168(1):10-19. doi: 10.7326/M17-0611 [DOI] [PubMed] [Google Scholar]
- 17.Murphy SM, McCollister KE, Leff JA, et al. Cost-effectiveness of buprenorphine-naloxone versus extended-release naltrexone to prevent opioid relapse. Ann Intern Med. 2019;170(2):90-98. doi: 10.7326/M18-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy SM, Polsky D. Economic evaluations of opioid use disorder interventions. Pharmacoeconomics. 2016;34(9):863-887. doi: 10.1007/s40273-016-0400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82. doi: 10.1016/j.drugalcdep.2018.12.030 [DOI] [PubMed] [Google Scholar]
- 20.National Institute on Drug Abuse . Effective treatments for opioid addiction. Accessed Feb 4, 2021. https://www.drugabuse.gov/publications/effective-treatments-opioid-addiction
- 21.Office of the Secretary, Department of Defense (DoD) . TRICARE; mental health and substance use disorder treatment: final rule. Fed Regist. 2016;81(171):61067-61098. [PubMed] [Google Scholar]
- 22.US Census Bureau . MDAT. Accessed February 17, 2021. https://data.census.gov/mdat/#/search?ds=ACSPUMS5Y2018&cv=SEX&rv=AGEP&wt=PWGTP
- 23.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238-1246. doi: 10.1001/archgenpsychiatry.2011.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arias E. United States life tables, 2017. Natl Vital Stat Rep. 2019;68(7):1-66. [PubMed] [Google Scholar]
- 25.Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi: 10.1038/s41380-018-0094-5 [DOI] [PubMed] [Google Scholar]
- 26.Kelty E, Joyce D, Hulse G. A retrospective cohort study of mortality rates in patients with an opioid use disorder treated with implant naltrexone, oral methadone or sublingual buprenorphine. Am J Drug Alcohol Abuse. 2019;45(3):285-291. doi: 10.1080/00952990.2018.1545131 [DOI] [PubMed] [Google Scholar]
- 27.Hser YI, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79-87. doi: 10.1111/add.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann AM, Blondell RD, Jaanimägi U, et al. A preliminary study comparing methadone and buprenorphine in patients with chronic pain and coexistent opioid addiction. J Addict Dis. 2013;32(1):68-78. doi: 10.1080/10550887.2012.759872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otiashvili D, Piralishvili G, Sikharulidze Z, Kamkamidze G, Poole S, Woody GE. Methadone and buprenorphine-naloxone are effective in reducing illicit buprenorphine and other opioid use, and reducing HIV risk behavior: outcomes of a randomized trial. Drug Alcohol Depend. 2013;133(2):376-382. doi: 10.1016/j.drugalcdep.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potter JS, Marino EN, Hillhouse MP, et al. Buprenorphine/naloxone and methadone maintenance treatment outcomes for opioid analgesic, heroin, and combined users: findings from starting treatment with agonist replacement therapies (START). J Stud Alcohol Drugs. 2013;74(4):605-613. doi: 10.15288/jsad.2013.74.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi: 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruger JP, Chawarski M, Mazlan M, Ng N, Schottenfeld R. Cost-effectiveness of buprenorphine and naltrexone treatments for heroin dependence in Malaysia. PLoS One. 2012;7(12):e50673. doi: 10.1371/journal.pone.0050673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205. doi: 10.1001/jamapsychiatry.2017.3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krupitsky E, Zvartau E, Blokhina E, et al. Naltrexone with or without guanfacine for preventing relapse to opiate addiction in St. Petersburg, Russia. Drug Alcohol Depend. 2013;132(3):674-680. doi: 10.1016/j.drugalcdep.2013.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506-1513. doi: 10.1016/S0140-6736(11)60358-9 [DOI] [PubMed] [Google Scholar]
- 36.Jarvis BP, Holtyn AF, Subramaniam S, et al. Extended-release injectable naltrexone for opioid use disorder: a systematic review. Addiction. 2018;113(7):1188-1209. doi: 10.1111/add.14180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling W, Hillhouse M, Ang A, Jenkins J, Fahey J. Comparison of behavioral treatment conditions in buprenorphine maintenance. Addiction. 2013;108(10):1788-1798. doi: 10.1111/add.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269(15):1953-1959. doi: 10.1001/jama.1993.03500150065028 [DOI] [PubMed] [Google Scholar]
- 39.Schwartz RP, Kelly SM, O’Grady KE, Gandhi D, Jaffe JH. Randomized trial of standard methadone treatment compared to initiating methadone without counseling: 12-month findings. Addiction. 2012;107(5):943-952. doi: 10.1111/j.1360-0443.2011.03700.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tetrault JM, Moore BA, Barry DT, et al. Brief versus extended counseling along with buprenorphine/naloxone for HIV-infected opioid dependent patients. J Subst Abuse Treat. 2012;43(4):433-439. doi: 10.1016/j.jsat.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 41.Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug Alcohol Depend. 2008;94(1-3):199-206. doi: 10.1016/j.drugalcdep.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu J, Lau JT, Xu H, et al. A randomized controlled trial to evaluate the relative efficacy of the addition of a psycho-social intervention to standard-of-care services in reducing attrition and improving attendance among first-time users of methadone maintenance treatment in China. AIDS Behav. 2013;17(6):2002-2010. doi: 10.1007/s10461-012-0393-9 [DOI] [PubMed] [Google Scholar]
- 43.Fiellin DA, Barry DT, Sullivan LE, et al. A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine. Am J Med. 2013;126(1):74.e11-7. doi: 10.1016/j.amjmed.2012.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W, Hong Y, Zou X, McLaughlin MM, Xia Y, Ling L. Effectiveness of prize-based contingency management in a methadone maintenance program in China. Drug Alcohol Depend. 2013;133(1):270-274. doi: 10.1016/j.drugalcdep.2013.05.028 [DOI] [PubMed] [Google Scholar]
- 45.DeFulio A, Everly JJ, Leoutsakos JM, et al. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: a randomized controlled trial. Drug Alcohol Depend. 2012;120(1-3):48-54. doi: 10.1016/j.drugalcdep.2011.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunn KE, Defulio A, Everly JJ, et al. Employment-based reinforcement of adherence to oral naltrexone treatment in unemployed injection drug users. Exp Clin Psychopharmacol. 2013;21(1):74-83. doi: 10.1037/a0030743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hser YI, Li J, Jiang H, et al. Effects of a randomized contingency management intervention on opiate abstinence and retention in methadone maintenance treatment in China. Addiction. 2011;106(10):1801-1809. doi: 10.1111/j.1360-0443.2011.03490.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlson RG, Nahhas RW, Martins SS, Daniulaityte R. Predictors of transition to heroin use among initially non-opioid dependent illicit pharmaceutical opioid users: a natural history study. Drug Alcohol Depend. 2016;160:127-134. doi: 10.1016/j.drugalcdep.2015.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meara E, White C, Cutler DM. Trends in medical spending by age, 1963-2000. Health Aff (Millwood). 2004;23(4):176-183. doi: 10.1377/hlthaff.23.4.176 [DOI] [PubMed] [Google Scholar]
- 50.Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156(4):279-290. doi: 10.7326/0003-4819-156-4-201202210-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baser O, Chalk M, Fiellin DA, Gastfriend DR. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(suppl 8):S235-S248. [PubMed] [Google Scholar]
- 52.Krebs E, Urada D, Evans E, Huang D, Hser YI, Nosyk B. The costs of crime during and after publicly funded treatment for opioid use disorders: a population-level study for the state of California. Addiction. 2017;112(5):838-851. doi: 10.1111/add.13729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nosyk B, Bray JW, Wittenberg E, et al. Short term health-related quality of life improvement during opioid agonist treatment. Drug Alcohol Depend. 2015;157:121-128. doi: 10.1016/j.drugalcdep.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45(12):1162-1170. doi: 10.1097/MLR.0b013e31814848f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Everly JJ, DeFulio A, Koffarnus MN, et al. Employment-based reinforcement of adherence to depot naltrexone in unemployed opioid-dependent adults: a randomized controlled trial. Addiction. 2011;106(7):1309-1318. doi: 10.1111/j.1360-0443.2011.03400.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holloway KR, Bennett TH, Farrington DP. The effectiveness of drug treatment programs in reducing criminal behavior: a meta-analysis. Psicothema. 2006;18(3):620-629. [PubMed] [Google Scholar]
- 58.Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: a meta-analysis. Addiction. 1998;93(4):515-532. doi: 10.1046/j.1360-0443.1998.9345157.x [DOI] [PubMed] [Google Scholar]
- 59.Molero Y, Zetterqvist J, Binswanger IA, Hellner C, Larsson H, Fazel S. Medications for alcohol and opioid use disorders and risk of suicidal behavior, accidental overdoses, and crime. Am J Psychiatry. 2018;175(10):970-978. doi: 10.1176/appi.ajp.2018.17101112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun HM, Li XY, Chow EP, et al. Methadone maintenance treatment programme reduces criminal activity and improves social well-being of drug users in China: a systematic review and meta-analysis. BMJ Open. 2015;5(1):e005997. doi: 10.1136/bmjopen-2014-005997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093-1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 62.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend. 2009;105(1-2):9-15. doi: 10.1016/j.drugalcdep.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 63.Hickman M, Steer C, Tilling K, et al. The impact of buprenorphine and methadone on mortality: a primary care cohort study in the United Kingdom. Addiction. 2018;113(8):1461-1476. doi: 10.1111/add.14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coviello DM, Cornish JW, Lynch KG, Alterman AI, O’Brien CP. A randomized trial of oral naltrexone for treating opioid-dependent offenders. Am J Addict. 2010;19(5):422-432. doi: 10.1111/j.1521-0391.2010.00070.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005. doi: 10.1111/add.12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meara E, Frank RG. Spending on substance abuse treatment: how much is enough? Addiction. 2005;100(9):1240-1248. doi: 10.1111/j.1360-0443.2005.01227.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Humphreys K, Wagner TH, Gage M. If substance use disorder treatment more than offsets its costs, why don’t more medical centers want to provide it? a budget impact analysis in the Veterans Health Administration. J Subst Abuse Treat. 2011;41(3):243-251. doi: 10.1016/j.jsat.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 69.Humphreys K, Frank RG. The Affordable Care Act will revolutionize care for substance use disorders in the United States. Addiction. 2014;109(12):1957-1958. doi: 10.1111/add.12606 [DOI] [PubMed] [Google Scholar]
- 70.Davenport S, Gray TJ, Melek SP. Addiction and mental health vs physical health: widening disparities in network use and provider reimbursement. Milliman. Published November 19, 2020. Accessed December 18, 2020. https://www.milliman.com/en/insight/addiction-and-mental-health-vs-physical-health-widening-disparities-in-network-use-and-p
- 71.Glass JE, Nunes EV, Bradley KA. Contingency management: a highly effective treatment for substance use disorders and the legal barriers that stand in its way. Health Affairs Blog. Published March 11, 2020. Accessed February 17, 2021. https://www.healthaffairs.org/do/10.1377/hblog20200305.965186/full/
- 72.US Government Accountability Office . Veterans health care: services for substance use disorders, and efforts to address access issues in rural areas. Published December 2, 2019. Accessed February 17, 2021. https://www.gao.gov/products/GAO-20-35
- 73.Valenstein-Mah H, Hagedorn H, Kay CL, Christopher ML, Gordon AJ. Underutilization of the current clinical capacity to provide buprenorphine treatment for opioid use disorders within the Veterans Health Administration. Subst Abus. 2018;39(3):286-288. doi: 10.1080/08897077.2018.1509251 [DOI] [PubMed] [Google Scholar]
- 74.Joe Biden for president. The Biden plan to end the opioid crisis. Accessed December 17, 2020. https://joebiden.com/opioidcrisis/
- 75.Medicaid Reentry Act, HR 1329, 116th Cong (2019-2020). Accessed February 17, 2021. https://www.congress.gov/bill/116th-congress/house-bill/1329
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Age and sex distribution of patients with opioid use disorder (OUD)
eFigure 2. Model schematic showing notation for all compartments and parameters
eFigure 3. Results of network meta-analysis: hazard rate ratio (HR) for discontinuation rate from different forms of MAT compared to naltrexone
eFigure 4. Results of network meta-analysis: hazard rate ratio (HR) for discontinuation rate from MAT for contingency management, psychotherapy, and both compared to no add-on
eFigure 5. Model-projected survival curves for 30-year-old males
eFigure 6. Model projected fatal overdoses curves for 30-year-old males
eFigure 7. Model projected non-fatal overdoses curves for 30-year-old males
eFigure 8. Model projected time in treatment for 30-year-old males
eFigure 9. Model projected healthcare cost for 30-year-old males
eFigure 10. Model-projected survival curves for males aged 30, 40, 50, 60, 70, and 80 years old
eFigure 11. Results of cost-effectiveness analysis for U.S. population with OUD, assuming methadone is not available
eFigure 12. Results of cost-effectiveness analysis for U.S. population with OUD, assuming methadone and buprenorphine are not available
eFigure 13. Results of cost-effectiveness analysis for VA patient population with OUD
eFigure 14. Results of cost-effectiveness analysis for VA patient population with OUD, assuming methadone is not available
eFigure 15. Results of cost-effectiveness analysis for VA patient population with OUD, assuming methadone and buprenorphine are not available
eFigure 16. Results of cost-effectiveness analysis for a U.S. population with OUD, assuming 25% higher rate of discontinuation from MAT
eFigure 17. Results of sensitivity analysis for a U.S. population with OUD, assuming 25% higher mortality rate on MAT
eFigure 18. Results of sensitivity analysis assuming 25% higher rate of discontinuation from MAT and 25% higher mortality rate on MAT
eFigure 19. Results of sensitivity analysis assuming 25% higher mortality rate out of treatment
eFigure 20. Results of sensitivity analysis for a U.S. population with OUD, assuming MAT never leads to abstinence
eFigure 21. Results of sensitivity analysis for a U.S. population with OUD, assuming no direct relapse from MAT
eFigure 22. Results of sensitivity analysis for a U.S. population with OUD, assuming no direct relapse from MAT and no relapse after becoming abstinent and leaving treatment
eFigure 23. Results of sensitivity analysis for a U.S. population with OUD, assuming three times higher mortality in the first month following treatment discontinuation relative to subsequent months
eFigure 24. Cost-effectiveness acceptability curve for U.S. population with OUD, limited societal perspective
eFigure 25. Threshold analysis on effectiveness of psychotherapy (PT) and contingency management (CM) at a willingness to pay of $100,000/QALY, limited societal perspective
eFigure 26. Threshold analysis on effectiveness of contingency management (CM) and effectiveness of adding psychotherapy (PT) to CM at a willingness to pay of $100,000/QALY, limited societal perspective
eFigure 27. Probability of surviving an overdose with and without naloxone
eTable 1. Parameter Distributions
eTable 2. Impact Inventory Analysis
eTable 3. CHEERS (Consolidated Health Economic Evaluation Reporting System) Checklist
eTable 4. Summary of Clinical Trials Evaluating the Relative Treatment Discontinuation Rates for Methadone, Buprenorphine, and Naltrexone
eTable 5. Summary of Clinical Trials Evaluating the Effectiveness of Psychotherapy in Reducing Treatment Discontinuation
eTable 6. Summary of Clinical Trials Evaluating the Effectiveness of Contingency Management in Reducing Treatment Discontinuation
eTable 7. Summary of Clinical Trial Evaluating the Effectiveness of Psychotherapy Combined with Contingency Management
eTable 8. Mean Direct Effects of Interventions
eTable 9. Estimated Cost of Contingency Management at the VA ($2019)
eTable 10. Model-projected Outcomes and Targets for Model Validation
eTable 11. Inclusion Criteria for Opioid Overdose (Initial, Non-Assault)
eTable 12. Demographics and Distribution of Treatment Costs for Veterans Health Administration (VA) patients (US $2019) with Opioid Use Disorder
eTable 13. Exclusion Criteria Used in Treatment Cost Estimation
eTable 14. Estimated Cost of Psychotherapy at the VA ($2019)
eTable 15. Estimated Cost of Methadone Maintenance Treatment at the VA ($2019)
eTable 16. Estimated Cost of Buprenorphine Treatment at the VA ($2019)
eTable 17. Estimated Cost of Long-Lasting Injectable Naltrexone Treatment at the VA ($2019)
eTable 18. Results: Mean Outcomes and 95% Credible Interval for Each Treatment Option, Modeled for a Cohort of Veterans Health Administration (VA) Patients with Opioid Use Disorder
eTable 19. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming 25% Higher Rate of Discontinuation from MAT
eTable 20. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming 25% Higher Mortality on MAT
eTable 21. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming a 25% Higher Rate of Discontinuation from MAT and 25% Higher Mortality on MAT
eTable 22. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming a 25% Higher Mortality Rate Out of Treatment
eTable 23. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming that MAT Never Leads to Abstinence
eTable 24. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming No Direct Relapse from MAT
eTable 25. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming No Direct Relapse from MAT and No Relapse After Becoming Abstinent and Leaving Treatment
eTable 26. Results of Sensitivity Analysis: Mean Outcomes for Each Treatment Option, Modeled on a Cohort of U.S. Patients with OUD, Assuming Three Times Higher Mortality in the First Month Following Treatment Discontinuation Relative to Subsequent Months
eReferences.