Key Points
Question
Is topical calcineurin inhibitor (TCI) use associated with an increased risk of cancer?
Findings
This systematic review and meta-analysis of 11 studies revealed no association between TCI use and risk of cancer overall or skin cancer. Lymphoma risk was elevated with TCI use.
Meaning
Although this study found a positive association between TCI use and lymphoma, the low absolute risk of lymphoma makes the potential increased risk attributable to TCI use for any individual patient very small.
This systematic review and meta-analysis evaluates the association between use of topical calcineurin inhibitors and risk of cancer in patients with atopic dermatitis.
Abstract
Importance
Topical calcineurin inhibitors (TCIs) are commonly used as second-line treatment for atopic dermatitis. In 2006, the US Food and Drug Administration issued a black box warning against TCI use, citing data from case reports and animal studies indicating a potential risk of cancer.
Objective
To evaluate the association between TCI use and risk of malignant neoplasms compared with nonactive and active comparator groups.
Data Sources
Electronic searches were conducted in MEDLINE via Ovid, Embase via Ovid, and Web of Science from database inception to August 21, 2020.
Study Selection
Observational studies investigating the association between treatment with TCIs (ie, tacrolimus and pimecrolimus) and the development of cancer with nonactive or active comparators were included. The population of interest was not limited to any specific disease state, age, or sex. All articles were assessed independently and in duplicate by 2 reviewers. Risk of bias was assessed using the Newcastle-Ottawa scale. Of 2464 nonduplicate records retrieved from the search, 11 studies met the inclusion criteria.
Data Extraction and Synthesis
Data extraction was conducted independently by 2 reviewers according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Random-effects meta-analyses were used to derive pooled relative risk (RR) estimates. Data were analyzed from July 25 to October 25, 2020.
Main Outcomes and Measures
Risk of cancer overall and risk of specific cancer types (lymphoma, melanoma, and keratinocyte carcinoma).
Results
Eight unique cohort studies (408 366 treated participants [55.1% female], 1 764 313 nonactive comparator controls, and 1 067 280 controls using topical corticosteroids) and 3 unique case-control studies (3898 cases [55.0% male] and 14 026 cancer-free controls [52.4% male]) were included. There was no association between TCI use and cancer overall compared with nonactive comparators (RR, 1.03; 95% CI, 0.92-1.16). Lymphoma risk was elevated with TCI use with both nonactive (RR, 1.86; 95% CI, 1.39-2.49) and topical corticosteroid comparators (RR, 1.35; 95% CI, 1.13-1.61). No significant association was found between TCI use and increased skin cancer (melanoma and keratinocyte carcinoma).
Conclusions and Relevance
The findings of this systematic review and meta-analysis suggest an association between TCI use and risk of lymphoma but not other cancers. Combined with the low absolute risk of lymphoma, the potential increased risk attributable to TCI use for any individual patient is likely very small.
Introduction
Topical calcineurin inhibitors (TCIs), tacrolimus ointment and pimecrolimus cream, are indicated as second-line treatment of atopic dermatitis when first-line topical corticosteroids (TCSs) are ineffective or contraindicated. The US Food and Drug Administration issued a black box warning in 2006 indicating a potentially elevated risk of cancer with TCI use based on the findings of case reports (primarily of lymphomas and skin cancers), animal carcinogenicity studies, and studies on systemic tacrolimus use in organ transplant recipients.1,2,3,4,5,6 Of the more than 6.7 million patients receiving TCI treatment, the black box warning was based on approximately 25 case reports, without systematic analysis supporting causation between TCI use and malignant neoplasms.4
Given the long potential latency for cancer development, postmarketing studies with long follow-up are necessary to determine whether there is an association between TCIs and cancer. A clinically important association would indicate the need for caution with TCI use, particularly for patients with chronic atopic dermatitis requiring long-term topical anti-inflammatory therapy. Conversely, if there is no clinically meaningful association, unwarranted worry stemming from regulatory safety warnings could lead to nonadherence and undertreatment of atopic dermatitis.7 In the 15 years since the US Food and Drug Administration issued their warning, several observational studies investigating the risk of malignant neoplasms with TCI use have been conducted,8,9 but even individual large studies with rare outcomes may fail to accurately identify the true association. We conducted this systematic review and meta-analysis of observational studies to determine the association between treatment with TCIs and the risk of cancer, and specifically the risk of lymphoma, keratinocyte carcinoma, and melanoma.
Methods
We prospectively registered our study protocol in PROSPERO.10 Research ethics board approval was not sought for this project because it did not involve participant-level data. We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline11 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline.12
Data Sources and Searches
We searched MEDLINE via Ovid, Embase via Ovid, and Web of Science from inception to August 21, 2020, using a search strategy with terms for cancer, topical, and calcineurin inhibitors (detailed search strategy in eTables 1-3 in the Supplement). The search strategy was reviewed by a librarian with expertise in systematic reviews. We originally planned to restrict our search to English-language articles, but our final search was performed without language restriction. We manually searched the citations of included studies and relevant review articles for additional studies that were not included in our electronic searches.
Study Selection
We included observational studies investigating the association between treatment with TCIs (tacrolimus or pimecrolimus) and the incidence of cancer or cancer mortality. The population of interest was not limited to any specific disease state (eg, atopic dermatitis), age, or sex. Studies were included in the meta-analyses if they reported an effect estimate for the risk of cancer in individuals receiving TCIs compared with a control group (including active and nonactive comparators). Conference abstracts and unpublished studies were excluded. Outcomes were any cancer, when studies reported the risk of cancer overall, and the specific cancer types lymphoma (including systemic and cutaneous lymphoma), melanoma, and keratinocyte carcinoma.
Data Extraction and Quality Assessment
Two investigators (M.L. and J.W.Z.) screened titles, abstracts, and full texts independently and in duplicate. Discrepancies were resolved by consulting the senior author (A.M.D.). The following data were extracted independently and in duplicate by 2 investigators (M.L. and J.W.Z.) using a standardized form: study characteristics (author, year, study design, country, participant source, inclusion and exclusion criteria, method for identifying cancer, and follow-up period), population characteristics (number of participants, age, sex, and purpose of treatment), treatment factors (type of calcineurin inhibitor, strength, dosing schedule, other concurrent treatment, and duration and amount of use), and outcomes (type of cancer, number and proportion of participants, effect estimate if reported, and factors adjusted for). After protocol registration, we also decided to extract effect estimates on the risk of cancer stratified by TCI strength, cumulative dose, and frequency of use.
We assessed the quality of included studies using the Newcastle-Ottawa scale for cohort and case-control studies.13 Funnel plots were generated and visually inspected to evaluate risk of publication bias.
Data Synthesis and Analysis
Data were analyzed from July 25 to October 25, 2020. We used random-effects models to account for potential heterogeneity between studies. We intended to pool cohort studies and case-control studies separately, and ultimately only conducted meta-analyses of cohort studies because there were insufficient case-control studies to pool for each outcome. Primary analyses were performed using the inverse variance method, pooling study-reported effect estimates for the risk of cancers with TCIs for a summary relative risk (RR) estimate. Adjusted effect estimates were used preferentially when available. Incidence ratios, odds ratios, and hazard ratios with corresponding SEs were considered approximate measures of RR and pooled as previously described,14,15,16,17,18 given an underlying assumption of the rarity of outcome events. We used the I2 statistic to assess statistical heterogeneity across studies in the pooled analysis.
We performed subgroup analyses separating topical tacrolimus and pimecrolimus and for studies limited to children. Secondary analyses using the Mantel-Haenszel method were performed if included studies reported zero groups. We performed subsequent sensitivity analyses removing zero groups from the initial secondary analyses. Quantitative analyses were conducted using Review Manager, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration).19 Unpaired 2-sided P < .05 indicated statistical significance.
Results
Description of Included Studies
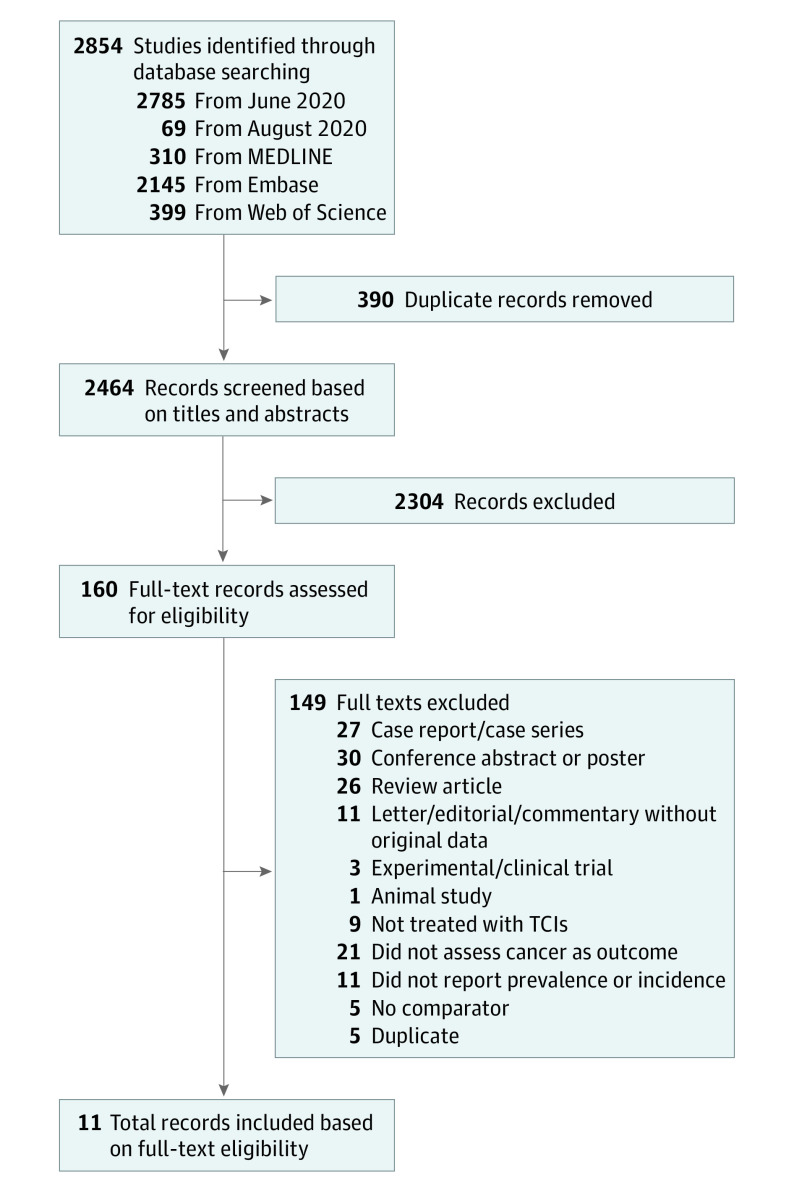
We retrieved 2854 records from our literature search. After removing 390 duplicate records, we assessed 2464 titles and abstracts and excluded 2304. We assessed full texts of 160 studies for eligibility. We ultimately included 8 cohort studies8,9,20,21,22,23,24,25 and 3 case-control studies26,27,28 (Figure 1). Six studies8,22,23,24,27,28 were conducted in the United States, 3 studies9,21,26 were based in Europe (Denmark and United Kingdom), 1 study20 was conducted in Asia (Singapore), and 1 study25 included participants from centers across North America and Europe.
Figure 1. Study Selection Methods.
TCIs indicates topical calcineurin inhibitors.
Five studies8,9,20,23,24 included a nonactive comparator or untreated control group. Two studies22,25 used expected or standardized incidence rates from the Surveillance, Epidemiology, and End Results program of the National Cancer Institute as a comparator. Four studies9,21,23,24 included an active comparator group treated with TCSs.
Participant Characteristics
A total of 408 366 participants were treated with TCIs in cohort studies, with a mean age of 17.1 years and a mean percentage of female participants of 55.1% and male participants of 44.9%. Of these participants, 151 772 were treated with tacrolimus and 214 640 with pimecrolimus. In the 5 studies8,9,20,23,24 with a nonactive comparator group, a total of 1 764 313 untreated controls were reported. Of the 4 studies using a TCS comparator,9,21,23,24 a total of 1 067 280 TCS-treated participants were reported. Four cohort studies9,21,22,25 included a children-only group, with 93 120 children included. A detailed overview of the characteristics of included cohort studies and participants treated with TCIs can be found in the Table. Of the 3 case-control studies,26,27,28 3898 participants with cancer and 14 026 cancer-free controls were reported, with a mean of 45.0% female and 55.0% male cases and 47.6% female and 52.4% male controls (eTable 4 in the Supplement).
Table. Characteristics of Included Cohort Studies and Participants Treated With TCIs.
| Source | Participant source (location) | Method of identifying cancer | Condition | Treatment | Exposed cohort | Follow-up time, y | |||
|---|---|---|---|---|---|---|---|---|---|
| No. | Age, mean (SD), y | Female, % | |||||||
| Asgari et al,24 2020 | KPNC health plan members (aged ≥40 y) with AD or dermatitis from 01/01/2002 through 12/31/2013 (US) | KPNC electronic pathology database; codes reviewed by dermatologist blinded to exposure | AD and dermatitis | TCI | 7033 | NA | 70.6 | Mean, 7.7a | |
| Cai et al,20 2016 | All patients with atopic and endogenous eczema seen at the National Skin Centre from 01/2004 to 12/2012 (Singapore) | Singapore Cancer Registry | Atopic and endogenous eczema | Tacrolimus | 3020 | 24.4 (18.3) | 48.8 | Mean, 3.8 | |
| Pimecrolimus | 1279 | 18.9 (17.3) | 52.6 | Mean, 4.9 | |||||
| Castellsague et al,21 2018 | PHARMO Database Network in the Netherlands, the Danish and Swedish national health registers, and the CPRD in the UK, from 2002 to 2011 (Europe) | (1) Cancer registries in Denmark, Sweden, and CPRD; (2) Dutch National Pathology Registry; (3) Primary care records in CPRD | AD | Tacrolimus | 86 075 | NA | 58.7 | Mean range, 2.2-4.2 | |
| Pimecrolimus | 60 257 | NA | 58.6 | Mean range, 2.8-6.5 | |||||
| Deleuran et al,9 2016 | Danish Cancer Registry exposed to treatment from 2002 to 2009 (Denmark) | Danish Cancer Registry | AD | TCI | 34 921 | NA | NA | NA | |
| Hui et al,8 2009 | Kaiser Permanente (California) health care consortium member with a diagnosis of AD or eczema from 01/2001 to 12/2004 (US) | KPNC and Kaiser Permanente Southern California Cancer Registry | AD and eczema | Tacrolimus | 15 966 | 34.1 (23.5) | 60.4 | Median, 2.4 | |
| Pimecrolimus | 26 784 | 29.7 (23.8) | 62.8 | Median, 1.9 | |||||
| Margolis et al,22 2015 | PEER study from start in 2004 to 05/2014 (US) | Study participants queried every 6 mo | AD | Pimecrolimus | 7457 | 7.2 (4.0) | 53.2 | 3.6a | |
| Paller et al,25 2020 | Children enrolled in the APPLES study beginning in 05/2005 and terminating on 01/31/2019 (multinational) | Families responded twice yearly to questionnaire or telephone interview regarding child’s AD therapy | AD | Tacrolimus | 7954 | 7.1 (NA) | 52.4 | Approximately 10 | |
| Schneeweiss et al,23 2009 | Health insurance claims database affiliated with Ingenix Research Data Mart from 01/01/2002 to 06/30/2006 (US) | Claims data and medical records adjudicated by oncologists | Dermatitis (seborrheic dermatitis, AD, contact dermatitis, sunburn) | Tacrolimus | 38 757 | NA | 57 | Mean, 1.5 | |
| Pimecrolimus | 118 863 | NA | 58 | Mean, 1.5 | |||||
Abbreviations: AD, atopic dermatitis; APPLES, A Prospective Pediatric Longitudinal Evaluation to Assess the Long-Term Safety of Tacrolimus Ointment for the Treatment of Atopic Dermatitis; CPRD, Clinical Practice Research Datalink; KPNC, Kaiser Permanente Northern California; NA, not available; PEER, Pediatric Eczema Elective Registry; TCI, topical calcineurin inhibitor.
Calculated from person-years.
Quality Assessment of Studies
A summary of scoring distribution for quality assessment using the Newcastle-Ottawa scale for nonrandomized studies can be found in eTable 5 in the Supplement. One study22 was assigned 3 stars, indicating a high or unclear risk of bias, owing to insufficient description of study cohorts and self-report of malignant neoplasm; 6 studies8,9,21,23,25,28 were assigned 4 to 6 stars, indicating a moderate risk of bias; and 4 studies20,24,26,27 were assigned 7 to 9 stars, indicating a low risk of bias.
Risk of Any Cancer With TCI Treatment in Cohort Studies
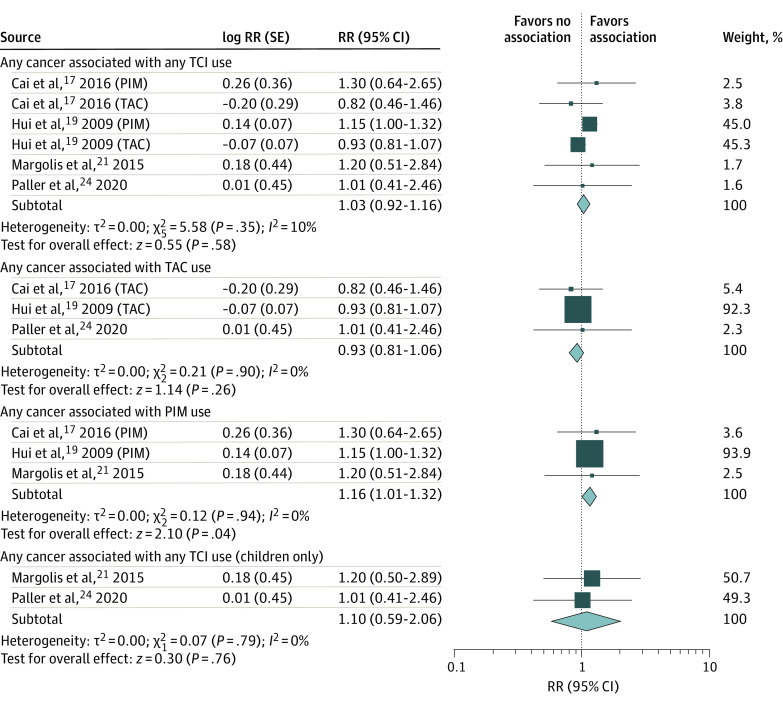
Four studies8,20,22,25 reported effect estimates for risk of any cancer with TCI treatment compared with untreated cohorts (nonactive comparator). Primary analysis using the inverse variance method yielded a pooled RR of 1.03 (95% CI, 0.92-1.16; I2 = 10%) (eFigure 1 in the Supplement). Heterogeneity was decreased (I2 = 0%) when sensitivity analyses were performed with participants treated with tacrolimus8,20,25 (RR, 0.93; 95% CI, 0.81-1.06) and pimecrolimus8,20,22 (RR, 1.16; 95% CI, 1.01-1.32). Two studies22,25 reported risk of any cancer in child-only cohorts, with a pooled RR of 1.10 (95% CI, 0.59-2.06, I2 = 0%) (Figure 2). No studies reported overall risk of any cancer with TCI compared with TCS treatment.
Figure 2. Risk of Any Cancer With Topical Calcineurin Inhibitor (TCI) Treatment and Nonactive Comparator in Cohort Studies.
Relative risks (RRs) were calculated using random-effects inverse variance method. Marker size indicates weight; diamond size indicates heterogeneity. PIM indicates pimecrolimus; TAC, tacrolimus.
Risk of Lymphoma With TCI Treatment in Cohort Studies
Nonactive Comparator
Five studies8,9,20,22,23 reported effect estimates for risk of lymphoma. Primary analysis using the inverse variance method showed a pooled RR of 1.86 (95% CI, 1.39-2.49; I2 = 27%) (eFigure 1 in the Supplement). With subgroup analysis of participants treated with tacrolimus,8,20,23 pooled RR and heterogeneity increased (RR, 2.20; 95% CI, 0.96-5.07; I2 = 70%). Subgroup analysis of participants treated with pimecrolimus8,20,22,23 resulted in a decreased pooled RR and heterogeneity (RR, 1.82; 95% CI, 1.27-2.60; I2 = 0%) (Figure 3A).
Figure 3. Risk of Lymphoma With Topical Calcineurin Inhibitor (TCI) Treatment in Cohort Studies.
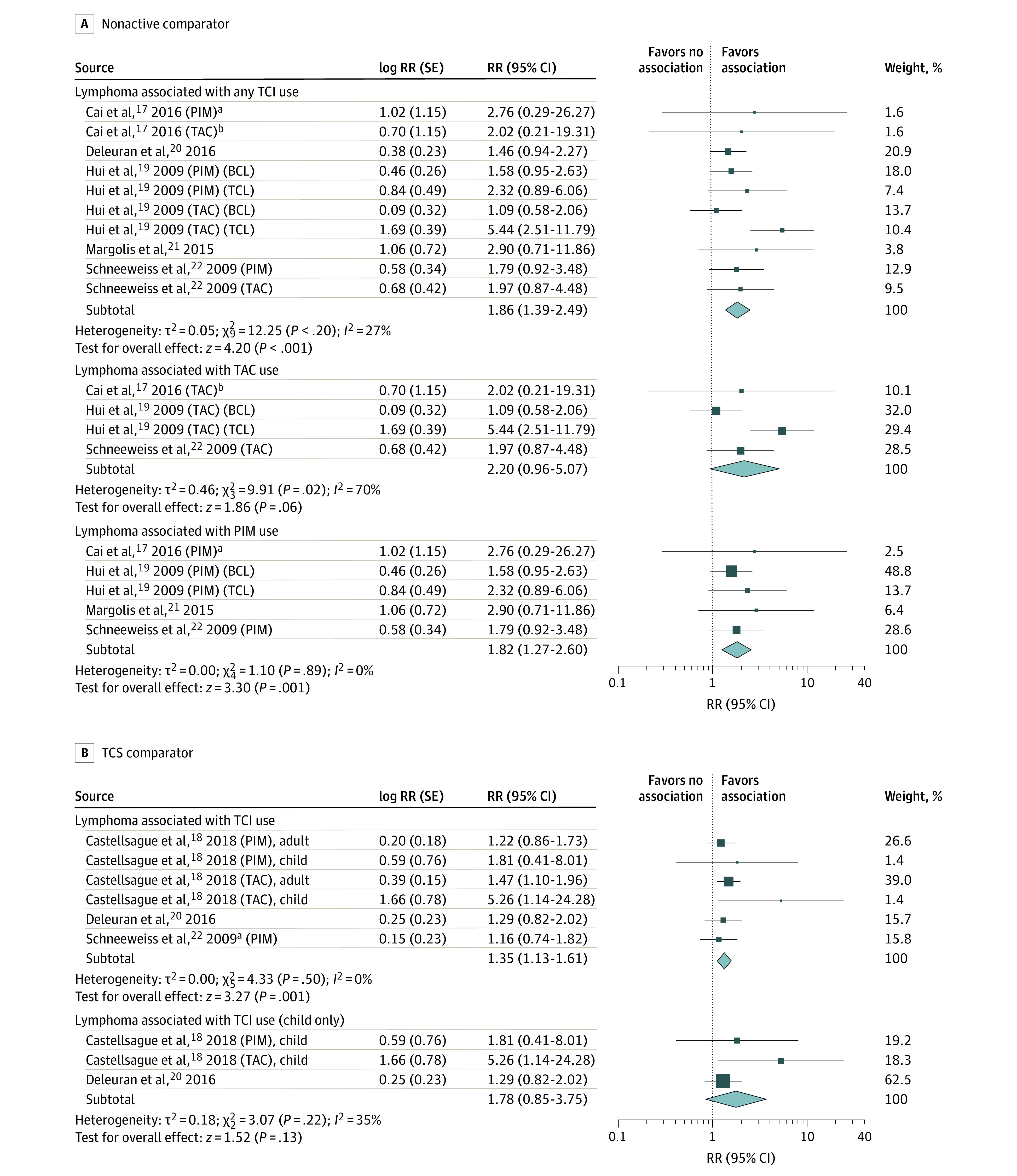
Results are stratified by B-cell lymphoma (BCL) and T-cell lymphoma (TCL) (A) and participant age (B). Relative risks (RRs) were calculated using random-effects inverse variance method. Marker size indicates weight; diamond size indicates heterogeneity. PIM indicates pimecrolimus; TAC, tacrolimus; TCS, topical corticosteroid.
aOther lymphoid and histiocytic tissue.
bLymphosarcoma and reticulosarcoma.
To reduce the possibility of a participant being included in both groups with B-cell lymphoma and T-cell lymphoma as reported in the study by Hui et al,8 we performed subgroup analysis excluding groups with T-cell lymphoma (RR, 1.56; 95% CI, 1.21-2.00; I2 = 0%) and excluding groups with B-cell lymphoma (RR, 2.16; 95% CI, 1.52-3.06; I2 = 21%). One study20 reported zero groups for incidence of lymphoma; thus, a secondary analysis using the Mantel-Haenszel method to include outcomes from zero groups was performed. Meta-analysis of dichotomous outcomes found a similar risk of 1.87 (95% CI, 1.31-2.67; I2 = 44%). Likewise, sensitivity analysis removing zero groups from secondary analysis yielded similar risk with increased heterogeneity (RR, 1.88; 95% CI, 1.29-2.75; I2 = 56%) (eFigure 1 in the Supplement).
TCS Comparator
Three studies9,21,23 reported effect estimates for risk of lymphoma in TCI-treated compared with TCS-treated groups. The pooled RR in the primary analysis was 1.35 (95% CI, 1.13-1.61; I2 = 0%) (eFigure 2 in the Supplement), for an elevated risk of 35% for TCIs. Two studies9,21 reported effect estimates for children-only groups (RR, 1.78; 95% CI, 0.85-3.75; I2 = 35%) (Figure 3B). Secondary analysis was not performed because no zero groups in lymphoma incidence were reported.
Risk of Skin Cancer With TCI Treatment in Cohort Studies
Nonactive Comparator
Four studies8,9,20,24 reported effect estimates for risk of skin cancer (melanoma and keratinocyte carcinoma). Primary analysis found a pooled RR of 0.72 (95% CI, 0.44-1.18; I2 = 57%) for risk of all skin cancer (eFigure 1 in the Supplement). There was a lower risk of melanoma (RR, 0.54; 95% CI, 0.33-0.86) and no association with keratinocyte carcinoma (RR, 1.03; 95% CI, 0.93-1.15) (Figure 4A).
Figure 4. Risk of Melanoma and Keratinocyte Carcinoma After Topical Calcineurin Inhibitor (TCI) Treatment.
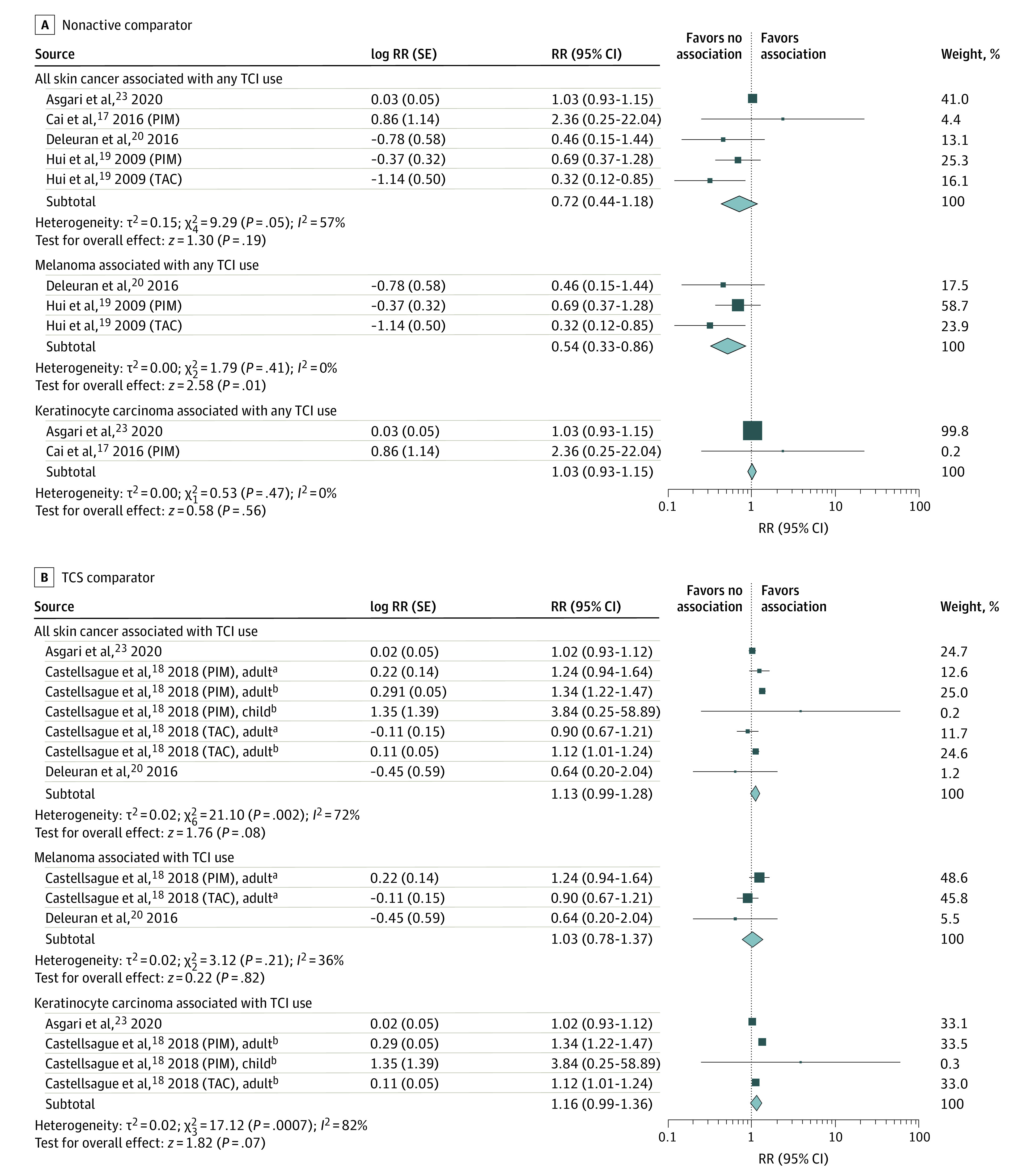
Relative risks (RRs) were calculated using random-effects inverse variance method. Marker size indicates weight; diamond size indicates heterogeneity. PIM indicates pimecrolimus; TAC, tacrolimus; TCS, topical corticosteroid.
aIndicates melanoma.
bIndicates keratinocyte carcinoma.
Secondary analysis including zero groups for risk of all skin cancer yielded a similar overall effect with substantial heterogeneity (RR, 0.70; 95% CI, 0.30-1.61; I2 = 81%). Heterogeneity was reduced with subgroup analysis of melanoma (RR, 0.60; 95% CI, 0.26-1.38; I2 = 51%) and keratinocyte carcinoma (RR, 0.67; 95% CI, 0.18-2.46; I2 = 55%). Heterogeneity was further reduced (I2 = 0%) with removal of zero groups in subsequent sensitivity analyses for both melanoma and keratinocyte carcinoma (eFigure 1 in the Supplement).
TCS Comparator
Three studies9,21,24 reported effect estimates for risk of skin cancer (melanoma and keratinocyte carcinoma) with TCIs vs TCSs, with a pooled RR of 1.13 (95% CI, 0.99-1.28; I2 = 72%) for all skin cancer. Subgroup analysis yielded a pooled RR of 1.03 (95% CI, 0.78-1.37) for melanoma and 1.16 (95% CI, 0.99-1.36) for keratinocyte carcinoma (eFigure 2 in the Supplement). Secondary analysis including zero groups resulted in a pooled RR of 1.04 (95% CI, 0.87-1.24; I2 = 78%) for all skin cancer, and subgroup analyses resulted in risks of 1.02 (95% CI, 0.84-1.24) for melanoma and 1.05 (95% CI, 0.83-1.33) for keratinocyte carcinoma (eFigure 2 in the Supplement).
Pooled analysis for all skin cancer in children-only cohorts resulted in a pooled RR of 1.01 (95% CI, 0.22-4.62; I2 = 29%).9,21 Secondary analysis including zero groups resulted in a decreased association (RR, 0.74; 95% CI, 0.31-1.78; I2 = 0%).
Association Between Cancer and TCI Treatment in Case-Control Studies
We included 3 case-control studies26,27,28 and a fourth nested case-control analysis within the cohort study by Schneeweiss et al23 (eTable 6 in the Supplement). The case-control study by Arellano et al27 and the nested case-control analysis by Schneeweiss et al23 examined lymphoma, with both having very wide CIs overlapping the null when comparing TCI-exposed with unexposed groups and exposure to different combinations of TCIs with each other. One study28 examining the risk of keratinocyte carcinoma reported significantly decreased odds of keratinocyte carcinoma with tacrolimus exposure (odds ratio, 0.43; 95% CI, 0.30-0.60). The remaining case-control study26 did not report effect estimates because no cases of lymphoma were found in any groups aside from the control group, where 3 cases were reported.
Cancer Risk by TCI Dose Response
One cohort study reported hazard ratios for risk of keratinocyte carcinoma with a nonactive comparator, stratified by dose of tacrolimus (0.03% vs 0.1%) and frequency of application.24 The adjusted hazard ratios for risk of keratinocyte carcinoma by dose were 0.93 (95% CI, 0.65-1.33) for low-dose (0.03%) tacrolimus and 0.97 (95% CI, 0.84-1.12) for high-dose (0.1%) tacrolimus. Risk of keratinocyte carcinoma by frequency of application was 0.58 (95% CI, 0.39-0.86) for low frequency and 0.92 (95% CI, 0.82-1.02) for high frequency for any TCI.24
Two cohort studies21,23 reported effect estimates for risk of lymphoma, stratified by cumulative amount used, in comparison with TCS-treated groups. Schneeweiss et al23 examined only pimecrolimus in comparison with a group treated with 60 g or less of TCSs and reported propensity score–adjusted odds ratios for pimecrolimus doses of 1.36 (95% CI, 0.68-2.70) for 60 g or less, 2.98 (95% CI, 0.85-10.5) for 61 to 100 g, and 4.17 (95% CI, 1.06-16.4) for more than 100 g. Castellsague et al21 reported adjusted incidence rate ratios of 1.40 (95% CI, 1.00-1.97) for tacrolimus doses of 0.05 g or less, 1.06 (95% CI, 0.59-1.92) for doses of more than 0.05 to 0.10 g, and 2.27 (95% CI, 1.39-3.69) for doses of more than 0.10 g of tacrolimus. The corresponding estimates for pimecrolimus were 0.89 (95% CI, 0.57-1.37) for doses of 0.05 g or less, 2.25 (95% CI, 1.19-4.26) for doses of more than 0.5 to 1.0 g, and 2.55 (95% CI, 1.27-5.12) for doses of more than 1.0 g.
One case-control study28 reported odds ratios for risk of keratinocyte carcinoma, stratified by number of tubes of topical medication used, where risk decreased as the number of tubes used increased. The corresponding odds ratios for pimecrolimus were 0.49 (95% CI, 0.36-0.68) for less than 1 tube, 0.40 (95% CI, 0.24-0.67) for 1 to 2 tubes, and 0.37 (95% CI, 0.19-0.74) for at least 3 tubes; for tacrolimus, 0.36 (95% CI, 0.25-0.54) for less than 1 tube, 0.33 (95% CI, 0.19-0.55) for 1 to 2 tubes, and 0.18 (95% CI, 0.08-0.42) for at least 3 tubes.28
Risk of Publication Bias Across Studies
Funnel plots were generated based on primary analysis of reported effect estimates in the inverse variance method (eFigures 3-7 in the Supplement). Although most plots did not suggest publication bias, interpretation was unclear owing to the small number of studies.29
Discussion
In this systematic review and meta-analysis, we found that TCI use was not associated with increased risk of cancer overall. Use of TCIs was associated with elevated risk of lymphoma compared with nonactive comparators and TCSs. Risk of skin cancer did not appear elevated with TCI use, and in 1 case-control study there were reduced odds of keratinocyte carcinoma associated with TCI use.
Several mechanisms have been proposed for the development of malignant neoplasms after calcineurin inhibitor use. The immunosuppressive effects of calcineurin inhibitors leading to decreased surveillance of cancerous cells may contribute to tumor promotion. In addition, evidence for direct tumor induction by calcineurin inhibitors exists.30,31,32 Systemic penetration of topical preparations is low,8,33 so although biologically plausible, the overall risk of malignant neoplasms attributable to these topical anti-inflammatory agents is likely low.
Only 2 cases of solid tumors were reported in 25 000 patients treated with pimecrolimus, and no lymphoma in almost 10 000 patients treated with tacrolimus in clinical trials.33 From their approval to 2010, the rate of lymphoma in those prescribed TCIs reported in the US Food and Drug Administration’s adverse event reporting system was lower than the rate seen in the general population.33,34,35,36 A 2015 systematic review37 examining the risk of lymphoma associated with TCIs found that risk ratios were elevated for both topical tacrolimus and pimecrolimus use, but the review included only 2 cohort studies and resulted in wide CIs. We added 3 new studies with nonactive comparators,9,20,22 which increased the precision of our effect estimates. Many individual studies did not show statistically significant associations with lymphoma, but there was a consistently positive direction of association as reflected in low I2 values, and 2 studies21,23 found increased risk of lymphoma with higher cumulative TCI exposure. The association with lymphoma was stronger in studies with a nonactive comparator, as opposed to those that compared TCI and TCS use, indicating that some of the association is likely a result of confounding by indication. Lymphoma is rare, with an annual worldwide incidence of 1.35 per 100 000 in children and 9.88 per 100 000 in adults.38 The 35% increased relative risk found in our study for TCIs compared with TCSs would therefore result in estimated numbers needed to harm for lymphoma of more than 200 000 in children and almost 30 000 in adults.
Limitations
Some included studies were small with relatively short follow-up periods, which limit their ability to determine the risk of malignant neoplasm induction with long latency periods. Although we examined lymphoma as a single entity in our primary analysis, it represents a heterogeneous group of diseases, which could bias our results toward the null if a true association exists for only one or some lymphoma subtypes. Our meta-analyses could have double-counted participants, for instance, those taking both tacrolimus and pimecrolimus in the same study; we performed sensitivity analyses to account for this possibility, and the results remained robust. Given the observational design of the included studies, unmeasured confounding limits interpretation of association and causation. Atopic dermatitis itself may be associated with increased risk of lymphoma37,39 and keratinocyte carcinoma.40 In addition, there may be a severity gradient with worse skin disease (and associated increased systemic inflammation) associated with further increased risk of cancer. Therefore, our results could be influenced by diagnostic bias and confounding by indication; our analyses examining the risk of malignant neoplasms with TCS comparators among patients with the same underlying clinical diagnosis (atopic dermatitis) mitigate this concern, but these biases are still possible because TCIs may be preferentially prescribed for more severe or refractory dermatitis. Studies have also suggested the possibility of reverse causation and protopathic bias, in which early cases of lymphoma, including cutaneous T-cell lymphoma, may be misdiagnosed as atopic dermatitis and treated with TCIs, leading to overestimation of the effect of TCIs.8,21,37 Three cohort studies8,20,23 used lag times between TCI exposure and lymphoma diagnosis to mitigate protopathic bias, but these lag times were relatively short (5 to 6 months).
Conclusions
Overall, our findings suggest an association between TCI use and risk of lymphoma but with no increased risk of other cancers, including skin cancers. Given that the absolute risk of lymphoma is low, particularly in children, the increase in relative risk translates to a very small increase in the absolute risk of lymphoma for a given patient.
eTable 1. Search Strategy for MEDLINE via Ovid
eTable 2. Search Strategy for Embase via Ovid
eTable 3. Search Strategy for Web of Science
eTable 4. Characteristics of Included Case-Control Studies on TCI Use in Participants With Cancer
eTable 5. Scoring Distribution of Quality Assessment of Studies Using the Newcastle-Ottawa Scale
eFigure 1. Summary of Meta-analyses of Cancer Risk With TCI Treatment and Nonactive Comparator in Cohort Studies
eFigure 2. Summary of Meta-analyses of Cancer Risk With TCI Treatment and TCS Comparator in Cohort Studies
eTable 6. Exposure to TCI Outcomes in Cancer Cases and Cancer-Free Controls in Case-Control Studies
eFigure 3. Funnel Plot for Any Cancer With Nonactive Comparator
eFigure 4. Funnel Plot for Lymphoma With Nonactive Comparator
eFigure 5. Funnel Plot for Skin Cancer With Nonactive Comparator
eFigure 6. Funnel Plot for Lymphoma With TCS Comparator
eFigure 7. Funnel Plot for Skin Cancer With TCS Comparator
eTable 7. Excluded Full-Text Records
References
- 1.Food and Drug Administration, Office of the Commissioner. Minutes of the Pediatric Advisory Committee meeting. February 14, 2005. Accessed August 24, 2020. https://wayback.archive-it.org/7993/20170404062542/https://www.fda.gov/ohrms/dockets/ac/05/minutes/2005-4089m1_Minutes.pdf
- 2.Radovic TC, Kostovic K, Ceovic R, Mokos ZB. Topical calcineurin inhibitors and malignancy risk. Int J Cancer Manag. 2017;10(4):e6173. doi: 10.5812/ijcm.6173 [DOI] [Google Scholar]
- 3.Hanna S, Zip C, Shear NH. What is the risk of harm associated with topical calcineurin nhibitors? J Cutan Med Surg. 2019;23(4_suppl):19S-26S. doi: 10.1177/1203475419857688 [DOI] [PubMed] [Google Scholar]
- 4.Ormerod AD. Topical tacrolimus and pimecrolimus and the risk of cancer: how much cause for concern? Br J Dermatol. 2005;153(4):701-705. doi: 10.1111/j.1365-2133.2005.06899.x [DOI] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration. Information for healthcare professionals: pimecrolimus (marketed as Elidel). Published 2006. Accessed August 24, 2020. https://wayback.archive-it.org/7993/20170112032734/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm153525.htm
- 6.US Food and Drug Administration. FDA approves updated labeling with boxed warning and medication guide for two eczema drugs, Elidel and Protopic. Published 2006. Accessed August 24, 2020. https://wayback.archive-it.org/7993/20171115034557/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm153941.htm
- 7.Drucker AM, Tadrous M. Topical calcineurin inhibitors and skin cancer—another piece of the puzzle. JAMA Dermatol. 2020;156(10):1053-1054. doi: 10.1001/jamadermatol.2020.2239 [DOI] [PubMed] [Google Scholar]
- 8.Hui RL, Lide W, Chan J, Schottinger J, Yoshinaga M, Millares M. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann Pharmacother. 2009;43(12):1956-1963. doi: 10.1345/aph.1M278 [DOI] [PubMed] [Google Scholar]
- 9.Deleuran M, Vestergaard C, Vølund A, Thestrup-Pedersen K. Topical calcineurin inhibitors, topical glucocorticoids and cancer in children: a nationwide study. Acta Derm Venereol. 2016;96(6):834-835. doi: 10.2340/00015555-2392 [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health Research . The association between topical calcineurin inhibitor use and risk of cancer: a systematic review and meta-analysis. CRD42020190452. Accessed August 21, 2020. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=190452
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 13.Wells GS., O’Connell D, Peterson J, Welch V, Losos L, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Health Research Institute. Published 2019. Accessed August 1, 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 14.Eyawo O, Brockman G, Goldsmith CH, et al. Risk of myocardial infarction among people living with HIV: an updated systematic review and meta-analysis. BMJ Open. 2019;9(9):e025874. doi: 10.1136/bmjopen-2018-025874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández MDM, Saulyte J, Inskip HM, Takkouche B. Premenstrual syndrome and alcohol consumption: a systematic review and meta-analysis. BMJ Open. 2018;8(3):e019490. doi: 10.1136/bmjopen-2017-019490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis. 2015;32:138-146. doi: 10.1016/j.ijid.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 17.Bateson D, Butcher BE, Donovan C, et al. Risk of venous thromboembolism in women taking the combined oral contraceptive: a systematic review and meta-analysis. Aust Fam Physician. 2016;45(1):59-64. [PubMed] [Google Scholar]
- 18.Beckett MW, Ardern CI, Rotondi MA. A meta-analysis of prospective studies on the role of physical activity and the prevention of Alzheimer’s disease in older adults. BMC Geriatr. 2015;15(1):9. doi: 10.1186/s12877-015-0007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Review Manager (RevMan) [computer program]. Version 5.3. Nordic Cochrane Centre, Cochrane Collaboration; 2014.
- 20.Cai SCS, Li W, Tian EAL, Allen JC, Tey HL. Topical calcineurin inhibitors in eczema and cancer association: a cohort study. J Dermatolog Treat. 2016;27(6):531-537. doi: 10.3109/09546634.2016.1163317 [DOI] [PubMed] [Google Scholar]
- 21.Castellsague J, Kuiper JG, Pottegård A, et al. A cohort study on the risk of lymphoma and skin cancer in users of topical tacrolimus, pimecrolimus, and corticosteroids (Joint European Longitudinal Lymphoma and Skin Cancer Evaluation - JOELLE study). Clin Epidemiol. 2018;10:299-310. doi: 10.2147/CLEP.S146442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margolis DJ, Abuabara K, Hoffstad OJ, Wan J, Raimondo D, Bilker WB. Association between malignancy and topical use of pimecrolimus. JAMA Dermatol. 2015;151(6):594-599. doi: 10.1001/jamadermatol.2014.4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneeweiss S, Doherty M, Zhu S, et al. Topical treatments with pimecrolimus, tacrolimus and medium- to high-potency corticosteroids, and risk of lymphoma. Dermatology. 2009;219(1):7-21. doi: 10.1159/000209289 [DOI] [PubMed] [Google Scholar]
- 24.Asgari MM, Tsai AL, Avalos L, Sokil M, Quesenberry CP Jr. Association between topical calcineurin inhibitor use and keratinocyte carcinoma risk among adults with atopic dermatitis. JAMA Dermatol. 2020;156(10):1-8. doi: 10.1001/jamadermatol.2020.2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83(2):375-381. doi: 10.1016/j.jaad.2020.03.075 [DOI] [PubMed] [Google Scholar]
- 26.Arellano FM, Arana A, Wentworth CE, Fernández-Vidaurre C, Schlienger RG, Conde E. Lymphoma among patients with atopic dermatitis and/or treated with topical immunosuppressants in the United Kingdom. J Allergy Clin Immunol. 2009;123(5):1111-1116.e13. doi: 10.1016/j.jaci.2009.02.028 [DOI] [PubMed] [Google Scholar]
- 27.Arellano FM, Wentworth CE, Arana A, Fernández C, Paul CF. Risk of lymphoma following exposure to calcineurin inhibitors and topical steroids in patients with atopic dermatitis. J Invest Dermatol. 2007;127(4):808-816. doi: 10.1038/sj.jid.5700622 [DOI] [PubMed] [Google Scholar]
- 28.Margolis DJ, Hoffstad O, Bilker W. Lack of association between exposure to topical calcineurin inhibitors and skin cancer in adults. Dermatology. 2007;214(4):289-295. doi: 10.1159/000100879 [DOI] [PubMed] [Google Scholar]
- 29.Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597-600. doi: 10.1136/bmj.333.7568.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yarosh DB, Pena AV, Nay SL, Canning MT, Brown DA. Calcineurin inhibitors decrease DNA repair and apoptosis in human keratinocytes following ultraviolet B irradiation. J Invest Dermatol. 2005;125(5):1020-1025. doi: 10.1111/j.0022-202X.2005.23858.x [DOI] [PubMed] [Google Scholar]
- 31.Datta D, Contreras AG, Basu A, et al. Calcineurin inhibitors activate the proto-oncogene Ras and promote protumorigenic signals in renal cancer cells. Cancer Res. 2009;69(23):8902-8909. doi: 10.1158/0008-5472.CAN-09-1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ume AC, Pugh JM, Kemp MG, Williams CR. Calcineurin inhibitor (CNI)–associated skin cancers: new insights on exploring mechanisms by which CNIs downregulate DNA repair machinery. Photodermatol Photoimmunol Photomed. 2020;36(6):433-440. doi: 10.1111/phpp.12600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thaçi D, Salgo R. Malignancy concerns of topical calcineurin inhibitors for atopic dermatitis: facts and controversies. Clin Dermatol. 2010;28(1):52-56. doi: 10.1016/j.clindermatol.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 34.Söderberg KC, Hagmar L, Schwartzbaum J, Feychting M. Allergic conditions and risk of hematological malignancies in adults: a cohort study. BMC Public Health. 2004;4:51. doi: 10.1186/1471-2458-4-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Holford TR, Leaderer B, et al. Prior medical conditions and medication use and risk of non–Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control. 2004;15(4):419-428. doi: 10.1023/B:CACO.0000027506.55846.5d [DOI] [PubMed] [Google Scholar]
- 36.Berger TG, Duvic M, Van Voorhees AS, VanBeek MJ, Frieden IJ; American Academy of Dermatology Association Task Force . The use of topical calcineurin inhibitors in dermatology: safety concerns: report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54(5):818-823. doi: 10.1016/j.jaad.2006.01.054 [DOI] [PubMed] [Google Scholar]
- 37.Legendre L, Barnetche T, Mazereeuw-Hautier J, Meyer N, Murrell D, Paul C. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72(6):992-1002. doi: 10.1016/j.jaad.2015.02.1116 [DOI] [PubMed] [Google Scholar]
- 38.Institute for Health Metrics and Evaluation. Global Health Data Exchange. Accessed January 14, 2021. http://ghdx.healthdata.org/
- 39.Tennis P, Gelfand JM, Rothman KJ. Evaluation of cancer risk related to atopic dermatitis and use of topical calcineurin inhibitors. Br J Dermatol. 2011;165(3):465-473. doi: 10.1111/j.1365-2133.2011.10363.x [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Bierbrier R, Drucker AM, Chan AW. Noncutaneous and cutaneous cancer risk in patients with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156(2):158-171. doi: 10.1001/jamadermatol.2019.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Strategy for MEDLINE via Ovid
eTable 2. Search Strategy for Embase via Ovid
eTable 3. Search Strategy for Web of Science
eTable 4. Characteristics of Included Case-Control Studies on TCI Use in Participants With Cancer
eTable 5. Scoring Distribution of Quality Assessment of Studies Using the Newcastle-Ottawa Scale
eFigure 1. Summary of Meta-analyses of Cancer Risk With TCI Treatment and Nonactive Comparator in Cohort Studies
eFigure 2. Summary of Meta-analyses of Cancer Risk With TCI Treatment and TCS Comparator in Cohort Studies
eTable 6. Exposure to TCI Outcomes in Cancer Cases and Cancer-Free Controls in Case-Control Studies
eFigure 3. Funnel Plot for Any Cancer With Nonactive Comparator
eFigure 4. Funnel Plot for Lymphoma With Nonactive Comparator
eFigure 5. Funnel Plot for Skin Cancer With Nonactive Comparator
eFigure 6. Funnel Plot for Lymphoma With TCS Comparator
eFigure 7. Funnel Plot for Skin Cancer With TCS Comparator
eTable 7. Excluded Full-Text Records