Abstract
The epithelial-mesenchymal transition (EMT) is usually considered the central mechanism of podocyte injury that eventually leads to proteinuria. We used an in vitro TGF-β1 induced podocyte EMT model and an in vivo rat focal segmental glomerulosclerosis (FSGS) model to uncover the mechanism underlying the protective effect of triptolide (TP) on podocytes. We found that TP could reverse the podocyte EMT process and upregulate the expression of TET2 in the TGF-β1-induced podocyte injury model. Bisulfite amplicon sequencing (BSAS) showed TP could alter the methylation status at some specific sites of the medium CpG density region in the promoters of NEPH1 and nephrin, two main markers of the podocyte slit diaphragm. Knockdown of TET2 with shRNA lentivirus (Lv) leads to high methylation of the promoters of NEPH1 and nephrin such that their expression can not return to normal levels, even after treatment with TP. In vivo, we found that TP could protect against podocyte injury in the FSGS rat and increase TET2 expression. These results suggested TET2-mediated DNA demethylation may be partly involved in podocyte injury. We believe these findings can help uncover a novel molecular mechanism of TP in alleviating podocyte-associated glomerular diseases.
Keywords: Triptolide, TET2, demethylation, podocyte, EMT
Introduction
The prevention and treatment of proteinuria has been the primary focus of research on glomerular disease. Podocyte injury is the key to the cause of proteinuria [1]. The epithelial-mesenchymal transition (EMT) is usually considered the major mechanism of podocyte injury [2-4]. EMT is a tightly regulated process by which epithelial cells lose their hallmark epithelial characteristics and gain the features of mesenchymal cells [5]. For podocytes, expression of slit diaphragm genes nephrin and NEPH1 is downregulated during this process. Therefore, understanding the fundamental process of podocyte EMT has become an important breakthrough in the study of proteinuria.
Traditional Chinese medicine has the advantages of providing multiple links and targets in the treatment of diseases. Studies have shown that traditional Chinese medicines have good clinical effects in the prevention and treatment of nephropathy. Emerging evidence from clinical and animal experiments has suggested that triptolide (TP) possesses antiproteinuric effects and has been used for the treatment of various podocytopathies [6-9]. In our previous study, we found TP could inhibit the phenotypic abnormalities of podocytes induced by transforming growth factor beta 1 (TGF-β1) [10]. However, the underlying mechanism still remains to be explored.
The methylation or demethylation of cytosines is a widely studied epigenetic modification that influences a number of biological processes [11]. Several studies have indicated that methylation processes are also involved in regulating the phenotype and function of podocytes [9,12]. Notably, it is reported that ten-eleven translocation (TET) proteins are not only associated with various natural differentiation processes, but also involved in experimentally induced cell differentiation [13]. Therefore, we proposed a preliminary hypothesis that TET-mediated DNA demethylation may also be involved in processes related to podocyte EMT.
To test this hypothesis, we developed an in vitro podocyte EMT model using TGF-β1 induction and established an in vivo FSGS rat model. We then explored the effect of TP on the expression of TET proteins in these models. In addition, we examined the methylation status of the promoters of key molecules contributing to podocyte phenotypes. We anticipate that the results of this study can provide novel targets and an effective means to modulate podocytes for the prevention and treatment of chronic kidney disease.
Materials and methods
Reagents
TP (C20H24O6, molecular weight 360) was purchased from the Chinese National Institute for Control of Pharmaceutical and Biological Products (Beijing, China). Recombinant human TGF-β1 protein (10 µg) was purchased from R&D Systems (Minneapolis, MN, USA).
Cell culture and TP intervention
Human conditionally immortalized glomerular podocytes (HPCs) were cultured at 33°C in complete RPMI 1640 medium containing 10% fetal bovine serum (Gibco, Gaithersburg, MD, USA) and insulin-transferrin-selenium (Gibco). They were differentiated by incubating at 37°C for 10-14 days. When the differentiated cells grew to 70-80% confluence, they were starved in serum-free medium for 24 h, followed by treatment with medium containing either TGF-β1 (5 ng/mL) or TP. For the CCK8 assay, cells subjected to starvation-treated were treated with different concentrations (0.3125, 0.625, 1.25, 2.5, 5, and 10 nM) of TP for 24 h. Then medium was substituted for CCK-8 working solution, and incubated at the cell incubator for 1-4 h. The optical density was detected at 450 nm using a Microplate Reader. In addition, podocytes were pretreated with TP for 30 min before adding TGF-β1 in this experiment.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from HPCs using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized using a Primescript™ RT reagent kit (TaKaRa, Kusatsu, Japan). qRT-PCR was performed on an Applied Biosystems® 7500 Fast RT-PCR system (Thermo Fisher Scientific, Waltham, MA, USA) using TB Green® Premix Ex Taq™ (Takara). Relative mRNA expression levels were normalized to those of GAPDH. The primer sequences used in this study are shown in Table 1.
Table 1.
Primers used for gene expression analyses
| Primer Name | Sequence (5’ to 3’) | Application |
|---|---|---|
| Human-TET1-F | AAAAGGAAACGCAATAAGGA | Real-time PCR |
| Human-TET1-R | GTAGGGTGCCCATTGTATGA | Real-time PCR |
| Human-TET2-F | TCCTGGTGGCAGCTCTGAACG | Real-time PCR |
| Human-TET2-R | TGGTGGTGGTGGTGTGGTAGTG | Real-time PCR |
| Human-TET3-F | CCAGAACGCTGTGATCGTCA | Real-time PCR |
| Human-TET3-R | ACAACCAAAGGAGAAGGAGGC | Real-time PCR |
| Human-Nephrin-F | GGAGTATGAGTGCCAGGTCG | Real-time PCR |
| Human-Nephrin-R | GAGAATGGTGATGTCAGGTGC | Real-time PCR |
| Human-NEPH1-F | GGACATAGGGCGTGTCTTCAC | Real-time PCR |
| Human-NEPH1-R | CTCGTGGGCGTCTTCAATCA | Real-time PCR |
| Human-Vimentin-F | TTGCCGTTGAAGCTGCTAACTACC | Real-time PCR |
| Human-Vimentin-R | AATCCTGCTCTCCTCGCCTTCC | Real-time PCR |
| Human-Fibronection-F | CCGACTGTGGACCAAGTTGATGAC | Real-time PCR |
| Human-Fibronection-R | GTGCTGCTACCTTCTACTGATGGC | Real-time PCR |
| Human-α-SMA-F | TCCCTTGAGAAGAGTTACGAGTT | Real-time PCR |
| Human-α-SMA-R | CATGATGCTGTTGTAGGTGGTT | Real-time PCR |
| Rat-TET2-F | CTTCTCTTGGCAGCTCCGAACAG | Real-time PCR |
| Rat-TET2-R | GTGATGGTGGCGTCATGGTAGTG | Real-time PCR |
| Rat-Nephrin-F | GTTGGTGGTCTTCTGCTGCTCTC | Real-time PCR |
| Rat-Nephrin-F | CTTCTGCTGTGCTAACCGTGGAG | Real-time PCR |
| Rat-NEPH1-F | GGGCTGGCTCTGGGTATGGG | Real-time PCR |
| Rat-NEPH1-R | GGCACTCATAGGAAGCGTCATCAG | Real-time PCR |
| GAPDH-F | ACCACAGTCCATGCCATCAC | Real-time PCR |
| GAPDH-R | TCCACCACCCTGTTGCTGTA | Real-time PCR |
F, forward primer; R, reverse primer.
Western blotting
Total cell extracts or kidney tissues were harvested in RIPA buffer (Beyotime, Haimen, China) containing a cocktail of protease inhibitors. Lysates were incubated on ice for 30 min and then clarified via centrifugation at 10,000 × g for 10 min at 4°C. Total protein concentrations were measured using a bicinchoninic acid protein assay kit (Beyotime). Protein lysates (~50 μg protein/lane) were separated by 6 or 10% SDS-PAGE gels under reducing conditions and then transferred to a PVDF membrane. The membrane was blocked with 5% skimmed milk and incubated with the following primary antibodies: TET2 (Abcam, Cambridge, UK, ab94580; EMD Millipore, Billerica, MA, USA, ABE364), TET1 (GeneTex, Irvine, CA, USA, GTX627420), TET3 (GeneTex, GTX121452-S), nephrin (Abcam, ab58968), NEPH1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA, sc-373787), vimentin (Abcam, ab92547), fibronection (Abcam, ab32419), α-smooth muscle actin (SMA) (Biologicals, Centennial, CO, USA, NBP2-33006), and GAPDH (ProteinTech, Rosemont, IL, USA, 60,004-1-Ig). Afterward, the membranes were washed and incubated with infrared-labeled anti-rabbit/mouse IgG Ab (1:15,000). Finally, the signal was detected using an Odyssey CLx imaging system (LI-COR, Lincoln, NE, USA).
Knockdown of TET2 with shRNA lentivirus (Lv)
Lvs carrying the TET2 shRNA (target site: 5’-AAGGAGCAAACACGAGATCTT-3’) or the corresponding control Lv were purchased from GeneChem (Shanghai, China). For the interference experiment, HPCs were seeded in six-well plates at 2.5 × 105 cells/well. They were infected with TET2 or control Lv according to the manufacturer’s instructions, and then cultured at 37°C for 72 h. Before the TET2 knockdown podocytes were used, silencing of TET2 expression was verified using qRT-PCR and western blotting. All assays were performed 72-96 h after transfection.
Bisulfite amplicon sequencing (BSAS)
BSAS was conducted at Genechem as previously described [14]. Briefly, total DNA was obtained from podocytes using the DNA Extraction Kit (Omega Bio-Tek, Norcross, CA, USA) and analyzed on a Nanodrop spectrophotometer. DNA (500 ng) was treated with sodium bisulfate using the EZ DNA Methylation-Gold kit (Zymo, Research, Irvine, CA, USA) according to the manufacturer’s instructions. During the assay, all unmethylated cytosines were converted to uracil, but the methylated cytosines were not affected. Bisulfate-modified DNA was amplified via PCR with the BSAS primers shown in Table 2. The amplified PCR products were purified and used to generate next generation sequencing libraries using the VAHTSTM Turbo DNA Library Prep Kit for Illumina® (ND102-0102). The denatured and diluted libraries were sequenced on a MiSeq benchtop sequencer (Illumina, San Diego, CA, USA). Next, the next generation sequencing data and quantification of methylation were analyzed.
Table 2.
Primers used for BSAS analyses
| Primer Name | Sequence (5’ to 3’) | Application |
|---|---|---|
| Human-NEPH1-F | TTTYGTTGGAATTGAGATGG | BSAS |
| Human-NEPH1-R | AAAACTAATATCCCTCAACCCATA | BSAS |
| Human-NEPH1-F | GGGAGGTGGGATTTAGTTTT | BSAS |
| Human-NEPH1-R | ACCCAAATTATTTCCTCTAACTA | BSAS |
| Human-Nephrin-F | GGATTTTGTTAGAGGTAAGGGAT | BSAS |
| Human-Nephrin-R | AACTTAAAACCCAAACTCATAAATCCTA | BSAS |
F, forward primer; R, reverse primer.
Animal study
Clean-grade male Sprague Dawley rats (160-180 g) were used to establish the focal segmental glomerulosclerosis (FSGS) model. Rats were purchased from the Zhejiang Institute of Traditional Chinese Medicine (animal qualification certificate number: SCXK [Shanghai] 2013-0016) and housed under standard conditions as described previously [15]. The FSGS model was established using uninephrectomy and repeated injection of doxorubicin according to our previous report [15]. FSGS rats were allocated randomly into the model and TP groups. Intervention with TP started after the proteinuria occurred and FSGS rats were gavaged with triptolide at a dose of 200 μg/kg/d. Control rats were treated with an equivalent volume of normal saline. After treatment for eight consecutive weeks, serum and the remaining kidneys were harvested for biochemical and molecular analyses as previous described [15]. The animal protocols were reviewed and approved by the ethics committee of the Zhejiang Institute of Traditional Chinese Medicine.
Statistical analysis
All of the values are presented as mean ± SD. The data shown were analyzed for significance via Student’s t-test or a one-way analysis of variance using SPSS 20.0 software (IBM, Chicago, IL, USA). A p-value of less than 0.05 was considered a statistically significant.
Results
Effect of TP on podocyte EMT model
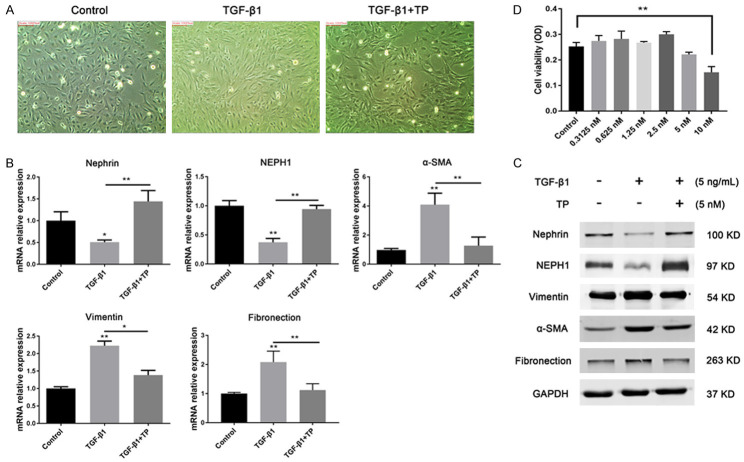
To explore the effect of TP on the podocyte EMT process, the TGF-β1 (5 ng/mL)-induced podocyte EMT model was established. As shown in Figure 1A, the podocytes in the control group showed a small arborized shape with characteristic foot processes. However, cells treated with TGF-β1 assumed an elongated fibroblast-like morphology. Moreover, both RT-PCR and western blotting results showed significantly decreased expression of nephrin and NEPH1 in TGF-β1-treated cells compared with the control group. In contrast, the expression of mesenchymal markers including vimentin, α-SMA, and fibronection increased markedly (Figure 1B, 1C). Taken together, these results demonstrated that the in vitro podocyte EMT model was successfully established. Based on the CCK-8 results, we chose 5 nM as the optimal intervention concentration for TP (Figure 1D). As expected, TGF-β1-induced cells tended to return to their normal epithelial morphology after TP treatment. Correspondingly, the levels of the podocyte markers nephrin and NEPH1 increased significantly in the TP intervention group compared with the group treated with TGF-β1 only, whereas the expression of vimentin, α-SMA, and fibronection was markedly reduced. These results indicate that TP can reverse the podocyte EMT process induced by TGF-β1.
Figure 1.
Effect of TP on the podocyte EMT. A. Morphological changes of podocytes were analyzed under a light microscope. Original magnification, × 200. D. Cell counting kit-8 analysis of the cytotoxicity of TP on podocytes. **P < 0.01. B. Relative mRNA expression levels of the podocyte markers were determined via qRT-PCR. Three independent experiments were analyzed. Data are presented as mean ± SD. *P < 0.05; **P < 0.01. C. Protein expression levels of the podocyte markers were detected using western blotting.
Effect of TP on TET family dioxygenases
It is well known that TET family proteins play key roles in various differentiation processes [16]. Therefore, the expression of TET family dioxygenases (1-3) was evaluated based on the above-mentioned podocyte EMT model. The results indicated that the expression of TET2 was significantly reduced in the TGF-β1-treated group compared with the control group. After TP treatment, the expression of TET2 was increased significantly (Figure 2). However, no obvious changes were observed in the expression of TET1 and TET3. Therefore, we speculate that TP may selectively regulate the expression of TET2 in the process of reversing the podocyte EMT.
Figure 2.
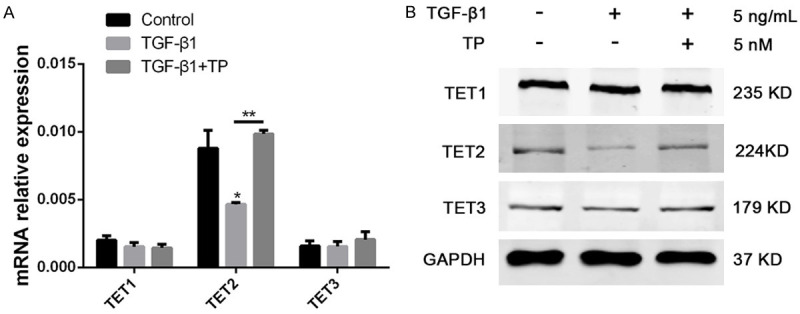
Effect of TP on the expression of TET1-3. A. The relative mRNA levels of TET1, TET2, and TET3 in the podocytes with different treatments. Three independent experiments were analyzed. Quantitative data are provided as means ± SD. **P < 0.01. B. Protein levels of TET1, TET2, and TET3 in podocytes with different treatments.
Effect of TP on promoter methylation of podocyte markers
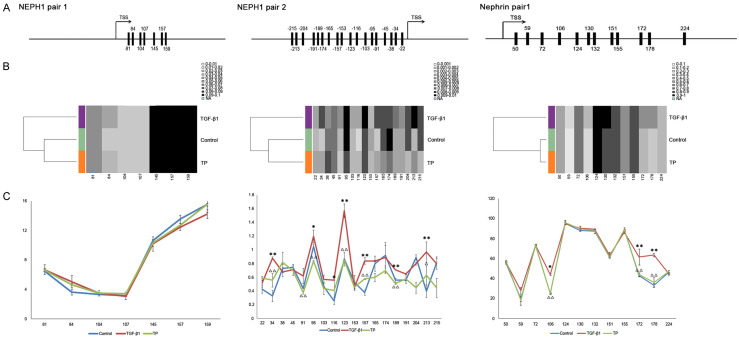
The methylation status of nephrin and NEPH1 was further analyzed via BSAS. Promoter sequences of the NEPH1 and nephrin genes were obtained from -1500 to 1500 relative to the transcriptional start site (Figure S1). Previous studies have showed that intermediate CpG promoters have a close relationship with gene expression [17]. Two intermediate CpG promoter regions of NEPH1 (positions -215 to -22 and 81 to 159) and one of the nephrin promoter region (positions 50 to 224), were predicted to be involved in regulating gene expression, using the online software MethPrimer (Figure 3A). BSAS results indicated that the promoter region of nephrin was hypermethylated, whereas the 5-methylcytosine (5mC) level in the NEPH1 promoter region was low (Figure 3B). Notably, the methylation status in all promoter regions changed in the TGF-β1 group, and the alterations frequently occurred at several specific sites such as +34, +95, +116, +123, +157, +189, and +213 bp in the NEPH1 promoters and the +106, +172, and +178 bp sites in the nephrin promoter (Figure 3C). As expected, the methylation levels at these sites decreased significantly following TP intervention (Figure 3C). These results suggest that TP may regulate 5mC at specific CpG sites in the NEPH1 and nephrin promoters.
Figure 3.
Site-specific 5mC status in the promoters of NEPH1 and nephrin. A. Sequence information of CpG in NEPH1 and nephrin promoters. TSS: transcriptional start site. B. Methylation levels of NEPH1 and nephrin were analyzed via BSAS. Dendrograms showing the unsupervised clustering of the data are presented using Methylation plotter, a web tool for dynamic visualization of DNA methylation data. The different groups are highlighted with different colors. C. Methylation percentages of individual CpG sites in the promoters of NEPH1 and nephrin. *P < 0.05 vs. TGF-β1 group. **P < 0.01 vs. TGF-β1 group. ΔP < 0.05 TP group vs. TGF-β1 group. ΔΔP < 0.01 TP group vs. TGF-β1 group.
Knockdown of TET2 inhibits reversal of the podocyte EMT by TP
To determine the role of TET2 in reversing the podocyte EMT by TP, we knocked down TET2 using a shRNA Lv in the podocytes prior to the TP intervention. qRT-PCR analysis showed that the shRNA Lv2 can significantly reduce the TET2 mRNA level compared with the scrambled control (Figure 4A). Moreover, the shRNA Lv2 markedly mitigated the protein expression of TET2 (Figure 4B).
Figure 4.
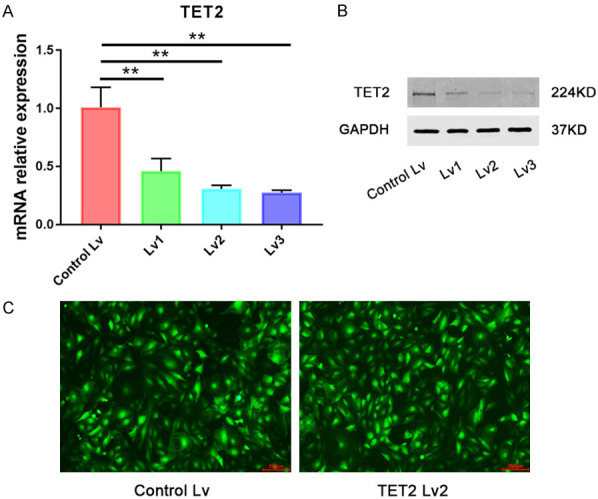
Effect of Lentivirus-mediated shRNA interference on TET2 expression. A. Relative mRNA levels of TET2 in podocytes treated with the Lvs carrying the TET2 shRNA. Three independent experiments were analyzed. Quantitative data are provided as mean ± SD. **P < 0.01. B. Protein level of TET2 in podocytes treated with the Lvs targeting TET2. C. Efficacy of the Lv infection was detected in podocytes using GFP fluorescence under a fluorescence microscope (Olympus IX53). After 72 h of infection with the control (Lvc) or targeting Lv of TET2 (Lv2), most podocytes strongly expressed GFP, thereby demonstrating that both viruses have high infective efficacy. Scale bars, 200 μm.
The expression of podocyte markers was then evaluated. The results showed that the levels of nephrin and NEPH1 mRNA and protein decreased significantly in the TET2 knockdown group compared with the scrambled Lv group, after treatment with TP (Figure 5A and 5B). No significant difference in α-SMA expression was observed. Next, methylation levels of the promoter regions of NEPH1 and nephrin were detected. Compared with the scrambled control group, 5mC levels at specific sites (+34, +91, +95, +123, +157, +189, and +213 bp in the NEPH1 promoter and +106, +172, and +178 bp sites in the nephrin promoter) increased markedly in the TET2 Lv group (Figure 5C). These methylation sites were consistently altered at specific sites, comparing with the TGF-β1 alone and TP groups. Taken together, these results indicate that TP may reverse podocyte EMT process partially through the TET2-mediated demethylation of specific sites in promoters of NEPH1 and nephrin.
Figure 5.
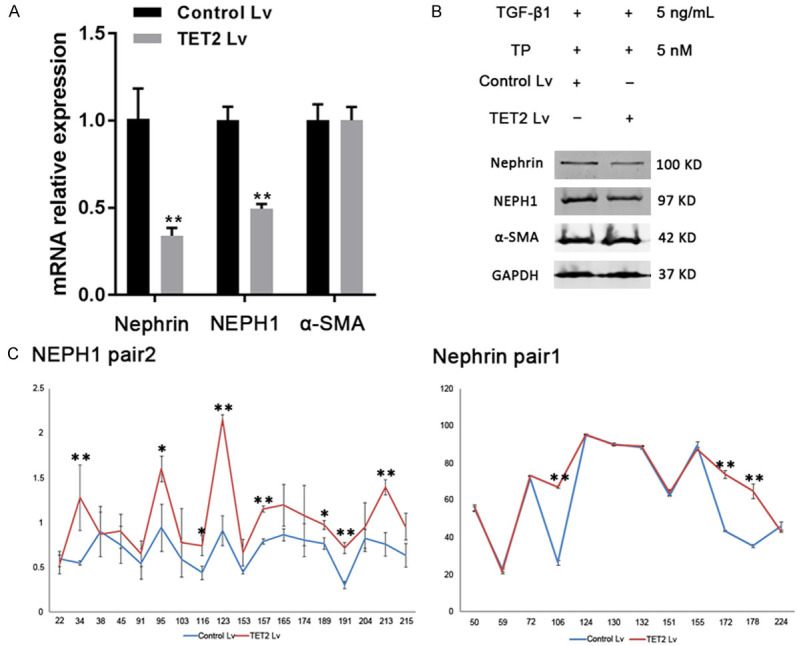
Effect of TET2 on the expression of podocyte markers. A. Relative mRNA levels of nephrin, NEPH1, and α-SMA in podocytes treated with the Lv. Three independent experiments were analyzed. Quantitative data are provided as the mean ± SD. **P < 0.01. B. Protein levels of nephrin, NEPH1, and α-SMA in podocytes treated with the Lv. C. Methylation percentages of individual CpG sites in the promoters of NEPH1 and nephrin. *P < 0.05 and **P < 0.01 vs. the control Lv group.
TET2 is involved in protecting the podocyte injury in the FSGS rat model
To confirm the in vitro data, an FSGS rat model was established. The urinary protein level in the model group was much higher than that in the control group (Figure 6A). Serum creatinine (Scr) and urea nitrogen (BUN) levels also increased significantly. After TP intervention, urinary protein, Scr, and BUN levels were significantly reduced compared with the untreated FSGS group (Figure 6A). Meanwhile, damage to the podocyte foot processes was observed using TEM. Compared with the control rats, the foot processes were diffusely effaced, became flat, and even disappeared in the FSGS group (Figure 6B). These lesions were significantly alleviated after TP intervention (Figure 6B). These results indicate that TP can protect against podocyte injury in FSGS rats.
Figure 6.
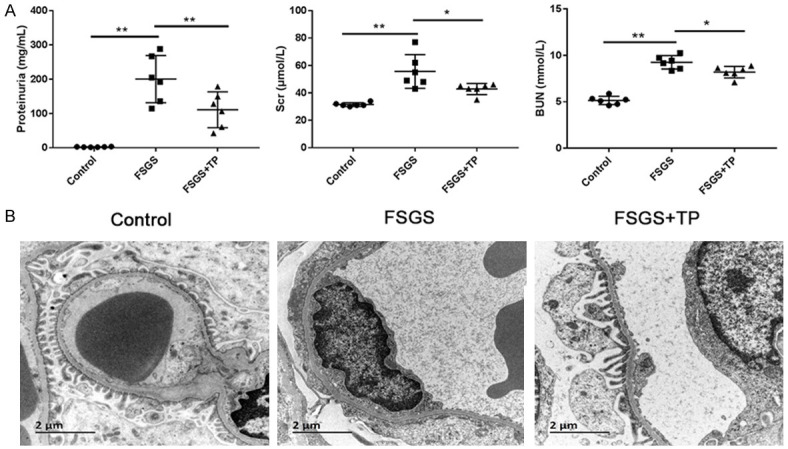
Evaluation of the FSGS rat model. A. Levels of proteinuria (mg/mL), Scr (μmol/L), and BUN (mmol/L) from different groups of rats. Data (n = 6) are presented as mean ± SD. *P < 0.05; **P < 0.01. B. Analysis of podocyte injury by TEM. Representative micrographs in each group are shown. Scale bar 2 μm (bottom left). Original magnification × 10,000, n = 6 animals in each group.
TET2, NEPH1, and nephrin mRNA and protein levels were significantly lower in FSGS than control rats (Figure 7). These results were consistent with human data obtained from the Nephroseq website (nephroseq.org) that provides a comparison of KIRREL (NEPH1), NPHS1 (nephrin), and TET2 levels in normal kidney and kidney of patients with collapsing focal segmental glomerulosclerosis (Figure 7E). However, their expression in the TP intervention group was markedly increased compared with that in FSGS rats (Figure 7). These results provide in vivo evidence that TET2 may be involved in reversal of the podocyte EMT.
Figure 7.
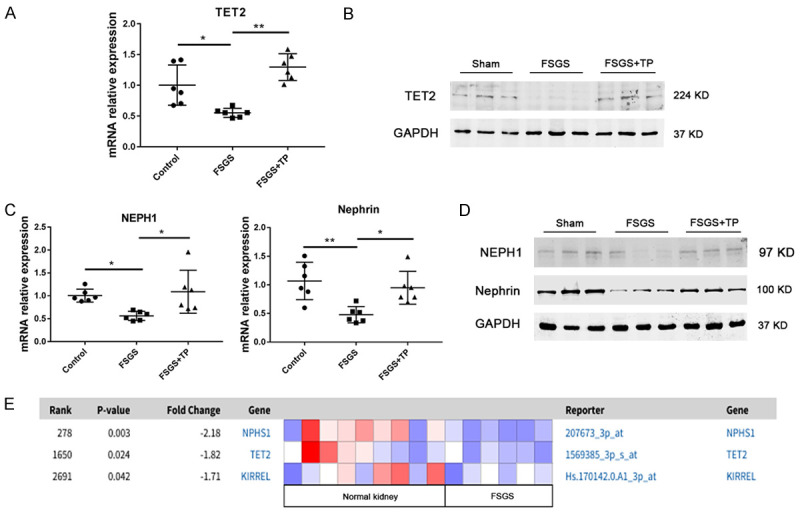
Effect of TET2 in protecting the podocyte injury in the FSGS rat model. A. Relative mRNA levels of TET2 in the rat kidney tissues were detected by qRT-PCR. Quantitative data are shown as mean ± SD. *P < 0.05; **P < 0.01. B. Kidney protein levels of TET2 analyzed using western blotting (three randomly selected samples from each group). C. Relative mRNA levels of nephrin and NEPH1 in rat kidney tissues were detected using qRT-PCR. Quantitative data are shown as the mean ± SD. *P < 0.05; **P < 0.01. D. Kidney protein levels of nephrin and NEPH1 analyzed using western blotting (three randomly selected samples from each group). E. Comparison of KIRREL (NEPH1), NPHS1 (nephrin) and TET2 in collapsing focal segmental glomerulosclerosis vs. normal kidney (log2 median-centered intensity). This photo was obtained from the Nephroseq Main website. Colors are z-scores normalized to depict relative values within rows.
Discussion
Podocyte EMT is regarded as an important pathological mechanism of proteinuria, which is an independent risk factor affecting the prognosis of chronic glomerular diseases [18]. Therefore, blocking the podocyte EMT has become one of the key goals to delay the progression of renal function deterioration.
TP, one of the active components of Tripterygium wilfordii Hook F, was found to have a strong antiproteinuric effect through direct action on podocytes [19]. However, the underlying mechanism remains largely unknown. Recently, growing evidence from different cell lines suggested that TP could inhibit the EMT process via various methods. FangQiong Li et al. discovered TP could inhibit EMT and induce apoptosis in gefitinib-resistant lung cancer cells [20]. Li Liu et al. reported TP could reverse hypoxia-induced EMT and stem-like features in pancreatic cancer by NF-κB downregulation [21]. Mei Xue et al. demonstrated TP could attenuate renal tubular EMT in diabetic kidney disease via the miR-188-5p-mediated PI3K/AKT pathway [22]. These results indicate that the effect of TP intervention may be closely related to the EMT process. Moreover, in our previous study, we also found TP could alter the phenotype of podocytes by upregulating the expression of podocyte markers such as NEPH1 during TGF-β1-induced injury [10]. Hence, further studies to elucidate the manner in which TP affects podocyte EMT should be investigated to determine the exact mechanism whereby TP participates in the reduction of proteinuria.
DNA methylation is an important epigenetic modification that plays a central role in organisms [23]. Few studies have explored the role of methylation in podocyte EMT. Previous literature reported the transcription factor KLF4 is highly expressed in normal kidney tissue, and its decrease can cause proteinuria [12]. Another study showed the expression of methyltransferase DNMT1 increased markedly in diabetic kidneys and in in vitro-cultured podocytes incubated with high glucose [24]. In addition, the methylation of cytosine was determined to be a dynamic reversible process with the discovery of TET protein families. TET proteins are dioxygenases that encode a family of Fe(II)- and 2-oxoglutarate-dependent proteins [25]. They participate in 5mC hydroxylation, resulting in DNA demethylation [25,26]. Alterations in TET protein function has been linked to several biological processes such as cancer, embryonic stem cell self-renewal, and zygotic cell epigenetic reprogramming [27-30]. Notably, TET proteins were also observed to influence a variety of cell differentiation processes, providing new insights into their functions in transcription, gene expression, and enhancer activity [13,31,32]. So far, studies clarifying the relationship between the TET proteins and podocyte EMT have been scarce.
In our study, we found that TET2 was highly expressed in normal podocytes and its expression was decreased in FSGS rats and the TGF-β1-induced podocyte EMT process. In addition, compared with control kidneys, the expression of TET2 was also decreased in patients with collapsing FSGS. These results indicated that TET2 may be involved in podocyte injury. Interestingly, following TP treatment, TET2 expression was increased significantly, suggesting that TET2 may also be linked to the protective effect of TP on podocytes. To verify our hypothesis, BSAS was used to detect the methylation level of podocyte markers. Among the identified podocyte markers, nephrin and NEPH1 were chosen as the candidates in our study for the following reasons. First, they are the main proteins of the podocyte slit diaphragm and co-localize at the podocyte foot process intercellular junctions and form cis hetero-oligomers [33,34]. Second, these proteins are likely to have CpG islands in their promoters based on our searches of the online software, MethPrimer. Finally, a previous study has demonstrated the protomer of nephrin was related to epigenetic regulation [12]. Our BSAS results showed the methylation level in the TGF-β1 group increased mainly at several specific sites in nephrin and NEPH1 promoter regions. Correspondingly, the methylation levels were reduced in the TP intervention group. Moreover, the methylation at some specific sites increased significantly with the Lv targeting of TET2 compared with the scrambled Lv group. These results demonstrated that TET2 indeed played a vital role in the podocyte EMT process by selectively regulating the methylation status of specific sites.
Additionally, we conducted experiments in FSGS rats to confirm the results from in vitro cultured podocytes. TET2 was decreased significantly in the FSGS rat and had an obvious increase following TP intervention. In addition, NEPH1 and nephrin expression synchronized with TET2 expression. Due to technical difficulties, the methylation status of the NEPH1 and Nephrin promoters was not detected in isolated podocytes from rat kidney.
In conclusion, TET2-mediated podocyte slit diaphragm gene demethylation may be partly involved in the podocyte EMT process. TP may protect against podocyte injury by regulating the methylation status of specific sites in the NEPH1 and nephrin promoters. It is anticipated that the results of this study may provide new insights into the mechanism underlying the protective effect of TP on podocytes.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81803911 and 81673913).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 2.Reidy K, Susztak K. Epithelial-mesenchymal transition and podocyte loss in diabetic kidney disease. Am J Kidney Dis. 2009;54:590–593. doi: 10.1053/j.ajkd.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi Y, Iwano M, Suzuki D, Nakatani K, Kimura K, Harada K, Kubo A, Akai Y, Toyoda M, Kanauchi M. Epithelial-mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis. 2009;54:653–664. doi: 10.1053/j.ajkd.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Kang YS, Li Y, Dai C, Kiss LP, Wu C, Liu Y. Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int. 2010;78:363–373. doi: 10.1038/ki.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ying Q, Wu G. Molecular mechanisms involved in podocyte EMT and concomitant diabetic kidney diseases: an update. Ren Fail. 2017;39:474–483. doi: 10.1080/0886022X.2017.1313164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng CX, Chen ZH, Zeng CH, Qin WS, Li LS, Liu ZH. Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Kidney Int. 2008;74:596–612. doi: 10.1038/ki.2008.203. [DOI] [PubMed] [Google Scholar]
- 7.Ma R, Liu LQ, Liu XM, Wang Y, Jiang W, Xu L. Triptolide markedly attenuates albuminuria and podocyte injury in an animal model of diabetic nephropathy. Exp Ther Med. 2013;6:649–656. doi: 10.3892/etm.2013.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ZH, Qin WS, Zeng CH, Zheng CX, Hong YM, Lu YZ, Li LS, Liu ZH. Triptolide reduces proteinuria in experimental membranous nephropathy and protects against C5b-9-induced podocyte injury in vitro. Kidney Int. 2010;77:974–988. doi: 10.1038/ki.2010.41. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Zhang L, Hou Q, Zhu X, Chen Z, Liu Z. Triptolide attenuates proteinuria and podocyte apoptosis via inhibition of NF-κB/GADD45B. Sci Rep. 2018;8:10843. doi: 10.1038/s41598-018-29203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang RC, Zhu XL, Zhang HQ, Li WD. Effect of triptolide on phenotypic changes of podocytes induced by TGF-β1. Chin Arch Trad Chin Med. 2013;31:2750–2753. [Google Scholar]
- 11.Chen ZX, Riggs AD. DNA methylation and demethylation in mammals. J Biol Chem. 2011;286:18347–18353. doi: 10.1074/jbc.R110.205286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi K, Sasamura H, Nakamura M, Azegami T, Itoh H. KLF4-dependent epigenetic remodeling modulates podocyte phenotypes and attenuates proteinuria. J Clin Invest. 2014;124:2523–2537. doi: 10.1172/JCI69557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott-Browne JP, Lio CJ, Rao A. TET proteins in natural and induced differentiation. Curr Opin Genet Dev. 2017;46:202–208. doi: 10.1016/j.gde.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masser DR, Berg AS, Freeman WM. Focused, high accuracy 5-methylcytosine quantitation with base resolution by benchtop next-generation sequencing. Epigenetics Chromatin. 2013;6:33. doi: 10.1186/1756-8935-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan F, Yang RC, Shi YP, Tang YW, Wang YJ. The protective effect of Phellinus linteus decoction on podocyte injury in the kidney of FSGS rats. BMC Complement Altern Med. 2019;19:272. doi: 10.1186/s12906-019-2705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross SE, Bogdanovic O. TET enzymes, DNA demethylation and pluripotency. Biochem Soc Trans. 2019;47:875–885. doi: 10.1042/BST20180606. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Wu F, Tan L, Kong L, Xiong L, Deng J, Barbera AJ, Zheng L, Zhang H, Huang S, Min J, Nicholson T, Chen T, Xu G, Shi Y, Zhang K, Shi YG. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol Cell. 2011;42:451–464. doi: 10.1016/j.molcel.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrović D, Stojimirović B. Proteinuria as a risk factor for the progression of chronic renal disease. Vojnosanit Pregl. 2008;65:552–558. doi: 10.2298/vsp0807552p. [DOI] [PubMed] [Google Scholar]
- 19.Chen BJ. Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma. 2001;42:253–265. doi: 10.3109/10428190109064582. [DOI] [PubMed] [Google Scholar]
- 20.Li F, Cui H, Jin X, Gong X, Wang W, Wang J. Triptolide inhibits epithelial-mesenchymal transition and induces apoptosis in gefitinib-resistant lung cancer cells. Oncol Rep. 2020;43:1569–1579. doi: 10.3892/or.2020.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Salnikov AV, Bauer N, Aleksandrowicz E, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Schemmer P, Werner J. Triptolide reverses hypoxia-induced epithelial-mesenchymal transition and stem-like features in pancreatic cancer by NF-κB downregulation. Int J Cancer. 2014;134:2489–2503. doi: 10.1002/ijc.28583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue M, Cheng Y, Han F, Chang Y, Yang Y, Li X, Chen L, Lu Y, Sun B, Chen L. Triptolide attenuates renal tubular epithelial-mesenchymal transition via the miR-188-5p-mediated PI3K/AKT pathway in diabetic kidney disease. Int J Biol Sci. 2018;14:1545–1557. doi: 10.7150/ijbs.24032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulis M, Esteller M. DNA methylation and cancer. Adv Genet. 2010;70:27–56. doi: 10.1016/B978-0-12-380866-0.60002-2. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Zhang Q, Liu S, Chen Y, Li R, Lin T, Yu C, Zhang H, Huang Z, Zhao X, Tan X, Li Z, Ye Z, Ma J, Zhang B, Wang W, Shi W, Liang X. DNA methyltransferase 1 may be a therapy target for attenuating diabetic nephropathy and podocyte injury. Kidney Int. 2017;92:140–153. doi: 10.1016/j.kint.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen KD, Helin K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016;30:733–750. doi: 10.1101/gad.276568.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi S, Hong K, Liu R, Shen L, Inoue A, Diep D, Zhang K, Zhang Y. Tet1 controls meiosis by regulating meiotic gene expression. Nature. 2012;492:443–447. doi: 10.1038/nature11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ficz G, Branco MR, Seisenberger S, Santos F, Krueger F, Hore TA, Marques CJ, Andrews S, Reik W. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature. 2011;473:398–402. doi: 10.1038/nature10008. [DOI] [PubMed] [Google Scholar]
- 31.Ko M, Bandukwala HS, An J, Lamperti ED, Thompson EC, Hastie R, Tsangaratou A, Rajewsky K, Koralov SB, Rao A. Ten-Eleven-Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc Natl Acad Sci U S A. 2011;108:14566–14571. doi: 10.1073/pnas.1112317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kallin EM, Rodríguez-Ubreva J, Christensen J, Cimmino L, Aifantis I, Helin K, Ballestar E, Graf T. Tet2 facilitates the derepression of myeloid target genes during CEBPα-induced transdifferentiation of pre-B cells. Mol Cell. 2012;48:266–276. doi: 10.1016/j.molcel.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barletta GM, Kovari IA, Verma RK, Kerjaschki D, Holzman LB. Nephrin and neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem. 2003;278:19266–19271. doi: 10.1074/jbc.M301279200. [DOI] [PubMed] [Google Scholar]
- 34.Verma R, Venkatareddy M, Kalinowski A, Li T, Kukla J, Mollin A, Cara-Fuentes G, Patel SR, Garg P. Nephrin is necessary for podocyte recovery following injury in an adult mature glomerulus. PLoS One. 2018;13:e0198013. doi: 10.1371/journal.pone.0198013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.