Abstract
Background
In March 2020, the Food and Drug Administration (FDA) approved use of COVID‐19 convalescent plasma (CCP) as an investigational new drug for treatment of COVID‐19. Since then, collection of CCP from COVID‐19–recovered patients has been implemented in donor centers nationwide. Childrenʼs Hospital Colorado rapidly put into practice a CCP collection protocol, necessitating development and implementation of assays to evaluate SARS‐CoV‐2 antibodies in CCP units.
Study Design and Methods
We evaluated 87 units of CCP collected from 36 donors over two to four sequential donations using both antigen‐binding assays for SARS‐CoV‐2 nucleoprotein and spike antigens and a live virus focus reduction neutralization test (FRNT50).
Results
Our data show that the majority of donors (83%) had a FRNT50 titer of at least 80, and 61% had a titer of at least 160, which met the FDAʼs criteria for acceptable CCP units. Additionally, our data indicate that analysis of antibodies to a single SARS‐CoV‐2 antigen is likely to miss a percentage of seroconverters; however, these individuals tend to have neutralizing antibody titers of less than 80. There was considerable variability in the short‐term, sustained antibody response, measured by neutralizing antibody titers, among our donor population.
Conclusion
The correlation of neutralizing activity and antigen‐binding assays is necessary to qualify CCP for therapeutic use. Since SARS‐CoV‐2 antibody levels decline in a percentage of donors, and such a decline is not detectable by current qualitative assays implemented in many laboratories, robust, quantitative assays are necessary to evaluate CCP units best suited for therapeutic infusion in COVID‐19 patients.
Keywords: blood component preparations, FFP transfusion, Regulatory and QA
Abbreviations
- CCP
COVID‐19 convalescent plasma
- CHCO
Childrenʼs Hospital of Colorado
- RBD
receptor‐binding domain
- RT
room temperature
1. INTRODUCTION
The Food and Drug Administration (FDA) authorized use of COVID‐19 convalescent plasma (CCP) as an investigational new drug for the treatment of COVID‐19. 1 Initially, CCP donors were accepted only with a confirmed SARS‐CoV‐2–positive polymerase chain reaction (PCR) and were required to be symptom‐free for at least 14 days before donation, be SARS‐CoV‐2 PCR negative upon subsequent testing, and meet all other blood donation eligibility requirements. 2 The FDA recently issued an emergency use authorization that further defines eligibility and testing requirements 3 .
Early in the COVID‐19 crisis in the United States, Childrenʼs Hospital of Colorado (CHCO) rapidly implemented a protocol for collection of CCP, with our first collection on March 31, 2020, with 548 units collected to date. Due to limited testing availability, an initial challenge was finding donors who met the SARS‐CoV‐2 PCR–positive test and other criteria. Although guidelines state that plasma can be collected no more frequently than every 28 days, the FDA did allow more frequent CCP collection at the discretion of the donation center medical director (P. Marks, personal correspondence, April 16, 2020). 4 This exception improved collections by allowing for an earlier return of CCP donors who had previously been successfully screened and tested. Because coagulation factors such as fibrinogen are normally replaced in a donorʼs plasma within 1 week, 5 we chose to collect CCP from donors as frequently as every 7 days. However, the impact on the donorsʼ SARS‐CoV‐2 antibody levels with this frequency of donation, or any frequency, is still being determined, as is the pattern of decline or retention of antibodies to SARS‐CoV‐2.
A number of assays for detection of SARS‐CoV‐2 antibodies, including enzyme‐linked immunosorbent assay (ELISA), high‐throughput immunoassay platforms, and rapid detection lateral flow assays became available in late March, enabling relatively rapid screening of CCP for presence of SARS‐CoV‐2 antibodies, commonly to either the SARS‐CoV‐2 nucleocapsid (N) or spike (S1) antigens, such as the S1 and receptor‐binding domain (RBD) of the S1 protein. 6 , 7 However, FDA recommendations for the investigational new drug protocol state that CCP units intended for transfusion into COVID‐19 patients should have a neutralizing antibody titer of at least 80, and preferably 160. 2 This guidance posed challenges for laboratories that were screening CCP for SARS‐CoV‐2 antibodies with antigen‐binding assays, many of which were primarily qualitative in nature, and do not provide information regarding potential for SARS‐CoV‐2 neutralization. Therefore, a comparison of antigen‐binding assays with virus neutralizing antibody titer is increasingly important to enable triage of CCP units and to develop criteria for suitability for transfusion into patients with COVID‐19.
We compared two ELISA assays, both currently implemented in clinical laboratories for clinical diagnostics and for screening of CCP, with a SARS‐CoV‐2 virus neutralization assay in our CCP donor population. We have additionally examined the persistence of SARS‐CoV‐2 antibodies using the neutralizing antibody assay in repeat CCP donors, who had been symptom‐free for a minimum of 14 days and had a repeat negative COVID‐19 PCR before their first donation. These data contribute to our understanding of the neutralizing antibody response to SARS‐CoV‐2, its correlation with development of N and S1 binding antibodies, and the persistence of the neutralizing antibody response and ultimately strengthen the criteria for CCP donors and analysis of CCP as a therapeutic for COVID‐19.
2. MATERIALS AND METHODS
2.1. CCP donors
SARS‐CoV‐2 PCR–positive individuals who were eligible to donate plasma according to FDA criteria for CCP donors were enrolled under the CHCO CCP donor program. Aliquots of plasma and serum were stored at −80°C until analysis.
2.2. SARS‐CoV‐2 IgG ELISA
Two commercial ELISAs, CE‐marked Epitope Diagnostics Inc. (EDI, San Diego, CA) and Euroimmun (CE‐marked and FDA EUA approved, Lubeck, Germany) were utilized in this study. Both of the ELISAs report results qualitatively, based on a single dilution. The EDI ELISA utilizes a SARS‐CoV‐2 recombinant N protein as the coating antigen. Positive and negative assay controls and samples were diluted 1 in 100 with the kit‐specific COVID‐19 immunoglobulin (Ig)G sample diluent and added to the wells, followed by a 30‐minute incubation at room temperature (RT). Plates were washed five times using the kit‐specific wash buffer and anti‐human IgG horseradish peroxidase (HRP)‐conjugated detection antibody was added, followed by a 30‐minute incubation. Plates were washed five times, and the signal was developed using tetramethylbenzidine. Absorbance was read at 450 nm within 10 minutes of halting the reaction.
The Euroimmun ELISA assay utilizes the S1 domain, including the RBD of the SARS‐CoV‐2 S1 protein. 8 For this assay, a kit‐specific calibrator, positive and negative controls and samples, were diluted 1 in 101 with the kit‐specific dilution buffer and added to precoated wells. Following a 1‐hour incubation at 37°C, plates were washed three times with kit‐specific wash buffer. Anti‐human IgG‐HRP–conjugated detection antibody was added and plates were incubated for 30 minutes at 37°C followed by three washes. Tetramethylbenzidine was added and absorbance read at 450 nm within 10 minutes of halting the reaction.
2.3. Interpretation of ELISA results
2.3.1. EDI ELISA
Positive, negative, and borderline results were calculated based on the average optical density (OD) value for the negative control assayed in triplicate for the specific assay. The positive and negative cutoff values were calculated using the formula positive cutoff = 1.1 × (xNC + 0.18) and negative cutoff = 0.9 × (xNC + 0.18), where xNC is the average OD450 of triplicate negative control OD450 values. Samples that had OD values between positive and negative cutoff values were reported as borderline.
2.3.2. Euroimmun ELISA
The ratio of the sample OD450 values to the calibrator OD450 values was calculated for all samples and controls. Samples with a ratio of greater than 0.8 were reported as negative, samples with a ratio of greater than 1.1 were reported as positive, and ratios between 0.8 and 1.1 were reported as borderline.
2.4. Focus reduction neutralization test
Vero E6 cells (ATCC, Manassas, VA) were seeded in 96‐well plates. Serum samples were heat inactivated and serially diluted (2‐fold, starting at 1:10) in DMEM (ThermoFisher, Pittsburgh, PA) plus 1% fetal bovine serum (FBS) in 96‐well plates. Approximately 100 focus‐forming units of SARS‐CoV‐2 USA‐WA1/2020 9 (deposited by the Centers for Disease Control and Prevention and obtained through BEI Resources, NIAID, NIH) was added to each well and the serum plus virus mixture was incubated for 1 hour at 37°C. At the end of 1 hour, medium was removed from cells and the serum sample plus virus mixture was added for 1 hour at 37°C. After 1 hour, samples were removed and cells were overlaid with 1% methylcellulose (MilliporeSigma, St. Louis, MO) in MEM (ThermoFisher)/2% FBS and incubated 30 hours at 37°C. Cells were fixed with 4% paraformaldehyde (Acros Organics, Pittsburgh, PA) and probed with 1 μg/mL of an anti‐SARS‐CoV S1 monoclonal antibody (CR3022, Absolute Antibody, Boston, MA) in perm wash (1× PBS/0.1% saponin/0.1% bovine serum albumin) for 2 hours at RT. After being washed, cells were incubated with HRP‐conjugated goat anti‐human IgG (Southern Biotech, Birmingham, AL, 1:1000) for 1.5 hours at RT. After washing, SARS‐CoV‐2–positive foci were visualized with TrueBlue substrate (ThermoFisher) and counted using a CTL Biospot analyzer and Biospot software (Cellular Technology Ltd, Shaker Heights, OH). The focus reduction neutralization test (FRNT50) titer was calculated relative to a virus only control (no serum) set at 100%, using computer software (GraphPad Prism 8, GraphPad, La Jolla, CA) default nonlinear curve fit constrained between 0 and 100%. The coefficient of variation for the FRNT50 data reported in this study is approximately 20%. Importantly, in validation studies, the FRNT assay did not detect neutralizing activity in 50 known negative samples, including prepandemic sera and sera obtained from PCR‐confirmed cases of hCoV‐OC43, hCoV‐NL63, and other respiratory pathogens. In repeat analyses, the FRNT50 value obtained from the same sample was between 1.2‐ and 2.4‐fold different.
2.5. Statistical analysis
Data were analyzed with computer software (GraphPad Prism Version 8; and Microsoft Excel 2016, Microsoft Corp., Redmond, WA). For OD450 values and S1‐RBD ratios, the mean and 95% confidence intervals (CIs) were calculated using GraphPad Prismʼs statistical analysis package. Significant differences between groups were calculated using Welchʼs test for unequal variances. The difference between groups was considered significant when P < .05.
3. RESULTS
3.1. Characterization of donor serum samples
Eighty‐seven samples from 36 CCP donors from the CHCO Blood Donor Center were included in this study. All plasma donors made an initial donation 12 to 42 days after a positive SARS‐CoV‐2 PCR result, with a minimum symptom‐free period of 14 days from diagnosis and again at the intervals shown in Table 1. Of the 36 donors, 24 donated plasma twice; nine donors, three times; and three donors, four times. The intervals between sequential plasma donations ranged from 7 to 24 days.
TABLE 1.
Interval (days) between CCP collection for individual donors
| Donor ID | Interval between positive PCR result and initial donation | Interval between initial and second donation | Interval between second and third donation | Interval between third and fourth donation |
|---|---|---|---|---|
| 001‐D | 33 | 7 | ||
| 002‐D | 36 | 7 | ||
| 003‐D | 28 | 8 | 17 | |
| 005‐D | 29 | 7 | 9 | |
| 006‐D | 29 | 7 | 9 | |
| 007‐D | 42 | 8 | 6 | 8 |
| 008‐D | 20 | 19 | ||
| 009‐D | 18 | 12 | 8 | 7 |
| 010‐D | 33 | 18 | ||
| 011‐D | 30 | 7 | ||
| 012‐D | 19 | 13 | 7 | |
| 013‐D | 31 | 8 | 14 | |
| 014‐D | 25 | 13 | 11 | |
| 015‐D | 22 | 11 | 7 | 7 |
| 016‐D | 36 | 8 | 9 | |
| 018‐D | 27 | 15 | ||
| 019‐D | 26 | 8 | ||
| 020‐D | 22 | 11 | 7 | |
| 021‐D | 32 | 8 | ||
| 022‐D | 24 | 11 | ||
| 023‐D | 31 | 8 | ||
| 024‐D | 33 | 12 | ||
| 025‐D | 29 | 12 | ||
| 026‐D | 27 | 8 | ||
| 027‐D | 26 | 20 | ||
| 028‐D | 12 | 14 | ||
| 029‐D | 36 | 5 | ||
| 030‐D | 23 | 18 | ||
| 031‐D | 28 | 11 | ||
| 032‐D | 24 | 14 | ||
| 033‐D | 22 | 24 | ||
| 034‐D | 29 | 12 | ||
| 035‐D | 36 | 9 | ||
| 036‐D | 24 | 10 | ||
| 037‐D | 26 | 9 | 13 |
3.2. Comparison of N and S1‐RBD antibody detection with virus‐neutralizing activity
To determine if qualitative IgG antibody detection by ELISA, whether against the N or the S1‐RBD antigen, correlated with virus‐neutralizing activity, samples were analyzed for the presence of anti‐N IgG and anti‐S1‐RBD IgG using ELISA and neutralizing activity using a live virus focus reduction assay. Samples with a neutralizing antibody titer of at least 80 had a positive or borderline‐positive result for both N and S1‐RBD IgG antibodies, with the exception of sample 018‐D (neutralizing titer of 85), which was positive for N and negative for S1‐RBD and sample 023‐D (neutralizing titer of 91) which was negative for N and was not analyzed for S1‐RBD antibodies (Table 2). More variability between anti‐N and anti‐S1‐RBD IgG ELISA results was noted for samples with lower neutralizing antibody titers, particularly for the five samples that had neutralizing titers of less than 40. These samples were negative for anti‐N IgG; four of the five samples were either negative or borderline‐positive for anti‐S1‐RBD IgG and one was positive. Of note, donor 019‐D (Samples 1 and 2) remained persistently negative for anti‐N IgG, had a marginal increase in anti‐S1‐RBD IgG on the second CCP donation, and had very low FRNT50 titers.
TABLE 2.
Correlation of FRNT50 reciprocal titers and anti‐N IgG or anti‐S1‐RBD IgG qualitative results [Color table can be viewed at wileyonlinelibrary.com]
| FRNT50 | Anti‐N | Anti‐S1‐RBD | FRNT50 | Anti‐N | Anti‐S1‐RBD | FRNT50 | Anti‐N | Anti‐S1‐RBD | FRNT50 | Anti‐N | Anti‐S1‐RBD | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.00 | Neg | Neg | 26 | 100.93 | Pos | Pos | 51 | 257.63 | Pos | Pos | 76 | 628.06 | Pos | Pos |
| 2 | 13.90 | Neg | Borderline | 27 | 103.75 | Pos | Pos | 52 | 260.02 | Pos | Pos | 77 | 717.79 | Pos | Pos |
| 3 | 22.86 | Neg | Neg | 28 | 115.08 | Pos | Pos | 53 | 263.63 | Pos | Pos | 78 | 722.77 | Pos | Pos |
| 4 | 34.28 | Neg | ND | 29 | 123.31 | Pos | Pos | 54 | 307.61 | Pos | Pos | 79 | 744.73 | Pos | Pos |
| 5 | 35.1 | Neg | Pos | 30 | 129.4 | Pos | Pos | 55 | 309.74 | Pos | Pos | 80 | 820.4 | Pos | Pos |
| 6 | 39.08 | Neg | Borderline | 31 | 129.4 | Pos | Pos | 56 | 314.8 | Pos | Pos | 81 | 1025.65 | Pos | Pos |
| 7 | 43.05 | Neg | Borderline | 32 | 132.4 | Pos | Pos | 57 | 317.69 | Pos | Pos | 82 | 1047.13 | Pos | Pos |
| 8 | 43.2 | Neg | Pos | 33 | 135.21 | Pos | Pos | 58 | 327.34 | Pos | Pos | 83 | 1122.02 | Pos | Pos |
| 9 | 44.16 | Pos | Neg | 34 | 149.97 | Pos | Pos | 59 | 338.06 | Pos | Pos | 84 | 1177.61 | Pos | Pos |
| 10 | 45.81 | Pos | Pos | 35 | 163.7 | Pos | Pos | 60 | 361.41 | Pos | Pos | 85 | 1273.50 | Pos | Pos |
| 11 | 48.75 | Neg | Pos | 36 | 164.44 | Pos | Pos | 61 | 363.08 | Pos | Pos | 86 | 1936.42 | Pos | Pos |
| 12 | 64.1 | Pos | Pos | 37 | 172.58 | Borderline | Pos | 62 | 372.39 | Pos | Pos | 87 | 2301.44 | Pos | Pos |
| 13 | 66.07 | Pos | Borderline | 38 | 179.06 | Pos | Pos | 63 | 375.84 | Pos | Pos | ||||
| 14 | 69.50 | Pos | Pos | 39 | 180.72 | Pos | Pos | 64 | 439.54 | Pos | Pos | ||||
| 15 | 69.50 | Pos | Pos | 40 | 198.15 | Pos | Pos | 65 | 451.86 | Borderline | Pos | ||||
| 16 | 81.66 | Pos | Pos | 41 | 200.45 | Pos | Pos | 66 | 453.94 | Pos | Pos | ||||
| 17 | 81.85 | Pos | Pos | 42 | 204.2 | Pos | Pos | 67 | 476.43 | Pos | Pos | ||||
| 18 | 85.11 | Pos | Neg | 43 | 206.54 | Pos | Pos | 68 | 480.84 | Pos | Pos | ||||
| 19 | 85.31 | Pos | Pos | 44 | 217.27 | Borderline | Pos | 69 | 493.17 | Pos | Pos | ||||
| 20 | 85.5 | Pos | Pos | 45 | 225.42 | Pos | Pos | 70 | 500.03 | Pos | Pos | ||||
| 21 | 90.57 | Pos | Pos | 46 | 227.51 | Pos | Pos | 71 | 509.33 | Pos | Pos | ||||
| 22 | 90.99 | Pos | Pos | 47 | 232.81 | Pos | Pos | 72 | 537.03 | Pos | Pos | ||||
| 23 | 91.41 | Neg | ND d | 48 | 244.34 | Pos | Pos | 73 | 542.00 | Pos | Pos | ||||
| 24 | 92.7 | Pos | Pos | 49 | 248.9 | Pos | Pos | 74 | 559.76 | Pos | Pos | ||||
| 25 | 94.62 | Pos | Pos | 50 | 254.10 | Pos | Pos | 75 | 574.12 | Pos | Pos |
Abbreviation: ND, not done.
To determine whether OD450 values for anti‐N IgG antibodies or the ratio for anti‐S1‐RBD IgG was predictive of neutralizing antibody titers, we compared the numerical values associated with a positive or negative ELISA result with the corresponding neutralizing antibody titer (Figure 1). Samples with a neutralizing antibody titer of less than 80 had the lowest OD450 values for N or ratios for S1‐RBD. An increase in OD450 value or an increase in S1‐RBD ratio independently correlated with a significant increase in neutralizing antibody titers (Figure 1A,B). However, given the wide variability among OD450 values and ratios within each group, we performed a three‐way comparison of the data to examine whether there was a relationship between the level of positivity for anti‐N IgG, anti‐S1‐RBD IgG, and neutralizing antibody titers (Figure 2). Two of the samples with the highest neutralizing antibody titers, both from donor 001‐D, had the highest ratios for anti‐S1‐RBD IgG, and although they were strongly positive for anti‐N IgG antibodies, they did not have the highest OD450 values in this sample set. In general, samples with both low anti‐N IgG and anti‐S1‐RBD IgG numerical values correlated well with low or minimal neutralizing activity. Five samples with neutralizing titers between 1000 and 1500 had varying levels of positivity for anti‐N IgG and anti‐S1‐RBD IgG, and three samples with the highest OD450 values for anti‐N IgG antibodies had neutralizing antibody titers in the range of 200 (Figure 2).
FIGURE 1.
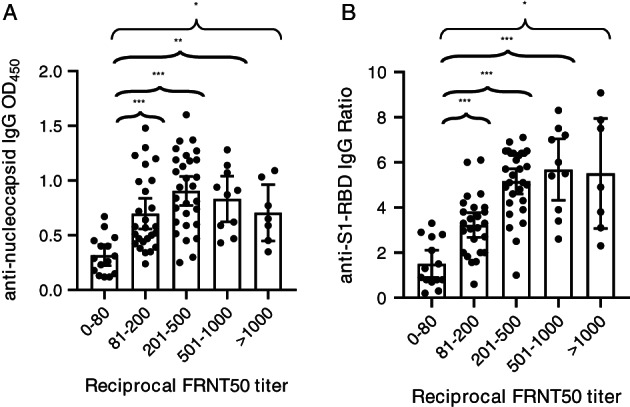
Comparison of reciprocal FRNT50 titer with either anti‐N IgG (A) or anti‐S1‐RBD IgG (B). Data are the mean and 95% CIs for each group of FRNT50 titers. N = 86 for FRNT50 vs anti‐N antibodies and N = 84 for FRNT50 vs anti‐S1‐RBD antibodies. *P < .05, **P < .001, and ***P < .0001 using Welchʼs test for unequal variance
FIGURE 2.
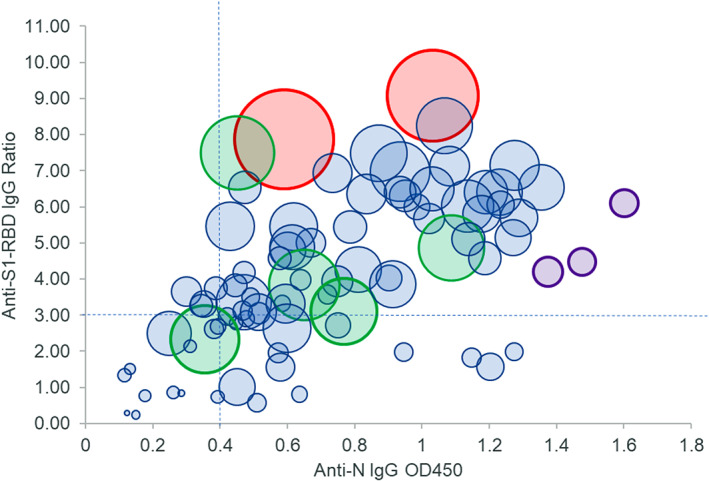
Comparison of anti‐N IgG (OD450, x‐axis), anti‐S1‐RBD IgG (ratio, y‐axis), and FRNT50 reciprocal titer (relative size of bubble, larger bubbles correspond to higher titers). Eighty‐five samples were compared for correlation among anti‐N, anti‐S1‐RBD, and neutralizing antibody titers. Two samples with the highest FRNT50 titers (red circles) also had the highest S1‐RBD ratios and moderately high levels of anti‐N1. Three samples with the highest anti‐N OD450 values had FRNT50 values of approximately 200 (purple circles), while five samples (green circles) had FRNT50 values of approximately 1000 but had varying levels of anti‐N and anti‐S1‐RBD. Dashed lines indicate cutoff values for OD450 and ratios above which 90% of FRNT50 values were 80 or greater [Color figure can be viewed at wileyonlinelibrary.com]
As these ELISAs were performed using a single dilution of serum, it is possible that the numerical values obtained are not in the linear range and, therefore, these results affect correlations with neutralizing antibody titers as the FRNT50 is a quantitative assay. Nevertheless, an anti‐N IgG OD450 of 0.4 and above correlated well with a neutralizing titer of at least 80 in 90% of the samples, and an anti‐S1‐RBD IgG ratio of 3.0 and above correlated with at least 80 neutralizing antibody titer in 82% of the samples (Table 3 and Figure 2). Only three samples with a neutralizing titer of at least 80 had an anti‐N IgG OD450 less than 0.4 and an anti‐S1‐RBD IgG ratio less than 3.0. In general, when the anti‐N IgG OD450 value was less than 0.4, the anti‐S1‐RBD IgG ratio was more than 3.0 and vice versa, indicating that a combination of the two assays accurately captured 96% of CCP samples with at least 80 neutralizing activity. Additionally, specificity of the anti‐S1‐RBD IgG ratio was greater for neutralizing titers, as 93% of samples with less than 80 neutralizing activity had less than 3.0 anti‐S1‐RBD IgG ratios, whereas more than one‐quarter (27%) of these samples had anti‐N IgG OD450 values of more than 0.4 (Table 3).
TABLE 3.
Correlation of more than 80 or less than 80 neutralizing antibody titers with anti‐N or anti‐S1‐RBD level of positivity
| Neutralizing titer | Anti‐S1‐RBD IgG < 3.0 | Anti‐S1‐RBD IgG > 3.0 | Total anti‐N IgG > 0.4 |
|---|---|---|---|
| ≥80 | |||
| Anti‐N IgG < 0.4 | 3 (4.2%) | 4 (5.6%) | |
| Anti‐N IgG > 0.4 | 10 (14.1%) | 54 (76.1%) | 90.2% |
| Total anti‐S1‐RBD IgG > 3.0 | 81.7% | ||
| <80 | |||
| Anti‐N IgG < 0.4 | 10 (71.4%) | 0 (0%) | |
| Anti‐N IgG > 0.4 | 3 (21.4%) | 1 (7%) | 28.4% |
| Total anti‐S1‐RBD IgG > 3.0 | 7% |
As recommended by the FDA, CCP units eligible for therapeutic use for COVID‐19 patients are expected to have a neutralizing antibody titer of at least 80 and preferably at lesat 160. Of the 87 samples tested in this study, 72 of 87 (82.7%) had a neutralizing titer of at least 80 and 53 of 87 (60.9%) had a titer of at least 160 (Table 2 and Figure 3A).
FIGURE 3.
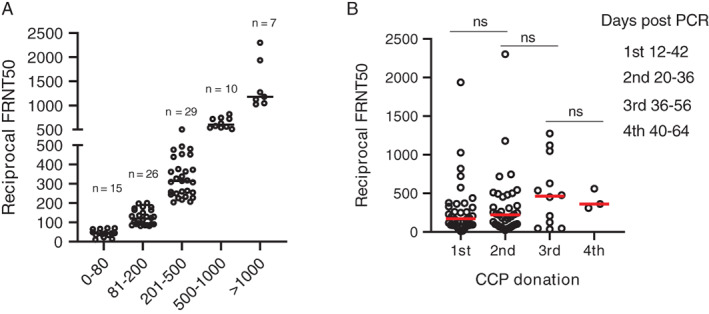
FRNT50 titers in CCP donors (A) and FRNT50 titers grouped by sequential CCP donations (B). Eighty‐seven samples were analyzed for FRNT50 titers. Data are grouped by FRNT50 titer and the number of samples in each group is indicated (A). FRNT50 titers correlated with CCP collection time point. The difference between groups was considered significant (P < .05) using Welchʼs test for unequal variances. ns, not significant [Color figure can be viewed at wileyonlinelibrary.com]
Neutralizing antibody titers were, in general, between 80 and 500 for the majority of samples tested (52%). Approximately 20% had titers greater than 500 and very few (7 of the 87 tested) had neutralizing antibody titers of more than 1000 (Figure 3A).
3.3. Sustainability of the antibody response
Because analysis of N and S1‐RBD IgG antibodies by single‐dilution ELISA is qualitative at best, analyzed the robustness and sustainability of the SARS‐CoV‐2 antibody response by analyzing neutralizing antibody titers in sequential samples from the 36 donors included in this study. Neutralizing antibody titers at the time of initial donation varied significantly from less than 10 to almost 2000 (Figure 3B). Aggregate analysis of plasma samples at the time of initial donation or between 7 and 24 days after initial collection showed an increase from baseline at the time of the second donation and an average greater increase at the third donation; however, these increases were not significant (Figure 3B). Although the mean neutralizing antibody titer appeared to decrease at the time of the fourth donation, there were too few samples to ensure significance at this time point. Given that the considerable variability of SARS‐CoV‐2 neutralizing antibody titers between donors may also be confounded by the interval between donations and the initial neutralizing antibody titer, we analyzed longevity of neutralizing antibody responses for individual donors. The majority of individuals donated two plasma units 7 to 24 days apart (Figure 4A,B). Only one donor (001‐D) had a baseline titer of more than 1500, which increased to more than 2000 at the time of the second donation (Figure 4A). Of the 24 donors who donated plasma twice, neutralizing antibody titers decreased in seven (Figure 4B), increased slightly (<2‐fold) or remained relatively unchanged in 12, and increased between 2‐ to 6‐fold in five donors (Figure 4C). Of the 12 donors with three or four sequential donations (Figure 5A,B), antibody titers increased or remained relatively unchanged from the initial plasma donation for seven donors and declined in the remaining five. Donor 015‐D had the greatest decline in titers, from a 5‐fold increase at the second and third donation to a 2‐fold increase over baseline at the fourth donation. In general, for the seven donors who had an increase in neutralizing antibody titer over time, the fold increase in titer was moderate, with the exception of donor 013‐D who had a 12‐fold increase (from 90 to 1122). Overall, 24 of 36 (67%) and 12 of 36 (33%) donors had either sustained or declined neutralizing antibody titers, respectively, during the observation period compared with their individual baseline titers.
FIGURE 4.
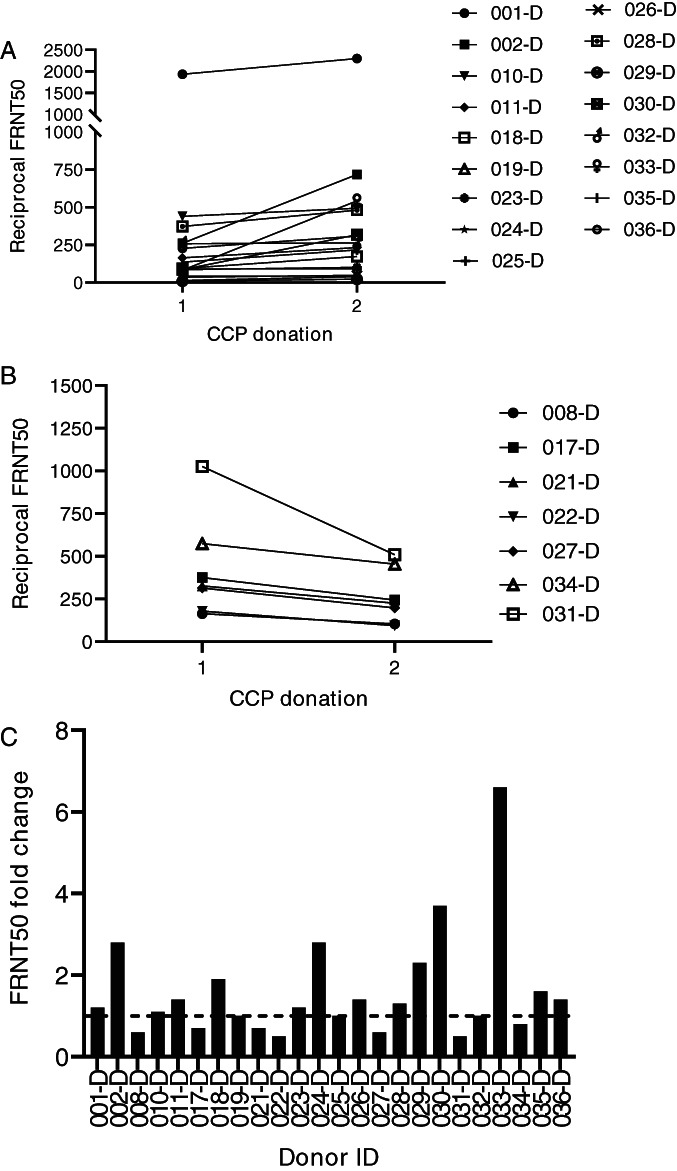
Change in FRNT50 titers over two sequential donations. FRNT50 titer increased or remained relatively unchanged (A, n = 17 donors). FRNT50 titers decreased from the initial to the second donation (B, n = 7). Fold increase from initial donation for donors with two sequential donations (C, n = 24)
FIGURE 5.
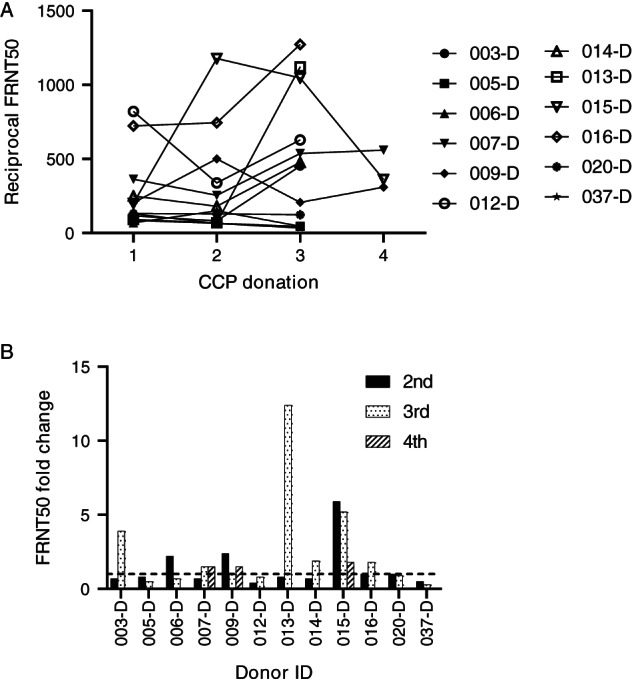
Change in FRNT50 titer over three or four sequential donations. (A) FRNT titers over the course of three or four donations for individual donors. (B) Fold increase from initial donation. n = 12
4. DISCUSSION
Passive transfer of convalescent plasma has been utilized to combat infection with a variety of pathogens including the 1918 H1N1 influenza virus; 10 the 2009 H1N1 influenza virus; 11 and SARS‐CoV‐2–related coronaviruses, MERS and SARS‐CoV. 12 , 13 Transfusion of CCP for the treatment of COVID‐19 received FDA approval for use as an investigational therapeutic in March 2020. 14 Data regarding its efficacy continue to accumulate, and some initial successes have been reported with evidence mounting that higher‐titer CCP administered early in the course of disease (<72 hours) is of the most benefit. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 Because there is a need to meet the growing demand for CCP to combat the COVID‐19 crisis, identifying methods to accurately determine titer and quality of SARS‐CoV‐2 antibodies rapidly is needed.
A variety of serologic assays for analysis of SARS‐CoV‐2 antibodies targeting the immunodominant N and S1 antigens are now available with varying levels of regulatory approval and validation. However, these antigen‐binding assays do not provide information on neutralizing capacity of SARS‐CoV‐2 antibodies. Given that the FDAʼs recommendation for CCP is a neutralizing antibody titer of at least 80 and preferably 160, and that neutralizing antibody assays cannot be easily implemented in most clinical laboratories, it is important to evaluate correlations between antigen‐binding assays and neutralizing antibody titers to triage CCP units for selection of units with adequate neutralizing titers. An analysis of 159 serum samples from healthy, COVID‐19–recovered individuals revealed that while samples with high IgG ELISA titers to the RBD antigen generally correlated with neutralizing activity as determined by a PRNT assay, only half of the individuals tested had more than 160 PRNT50 titer. 23 Our comparison of anti‐N IgG, measured by the EDI assay, and anti‐S1‐RBD IgG, measured by the Euroimmun assay, indicates that an increase in the relative level of positivity of either antibody correlated with an increase in neutralization titer; however, these correlations were mutually exclusive. The level of positivity for S1‐RBD antibodies had a higher correlation with neutralizing activity, as would be expected, since viral entry is mediated by the S1 protein and anti‐N are nonneutralizing. 24 , 25 , 26 This observation is similarly identified by Salazar et al., 27 who recommend that anti‐RBG titers can be correlated to neutralizing antibody activity. SARS‐CoV‐2 N antigen is highly antigenic, 28 and while some of the donor samples were highly positive for anti‐N IgG, they did not have corresponding high neutralizing antibody titers. The drawback of these qualitative assays is that they are based on a single dilution and, therefore, may not measure antibodies in the linear range. Despite this, our data indicate that samples with an anti‐N IgG OD450 of 0.4 by the EDI assay, or a ratio of 3.0 for measurement of anti‐S1‐RBD IgG by the Euroimmun assay, accurately captured the majority of samples with neutralizing titers of at least 80. Furthermore, our data suggest that analysis of antibodies to a single SARS‐CoV‐2 antigen may not be sufficient to be predictive of neutralizing capability.
There is limited information about the peak and decline of SARS‐CoV‐2 antibodies after generation of an antibody response. In a scientific brief released by the World Health Organization, it was stated that there is “currently no evidence that people who have recovered from COVID‐19 and have antibodies are protected from a second infection” and further stated that laboratory tests that detect antibodies to SARS‐CoV‐2, including rapid immunodiagnostic tests, need further validation to determine accuracy and reliability. 29 The presence and longevity of antibodies in an individual may be critical for social and economic recovery, because lasting immunity may be necessary to fully return to work and social activities. In this context, antigen‐binding assays that have been implemented for rapid screening do not provide information regarding the functionality of these antibodies. Such information is typically generated from biologic assays such as the FRNT50, described in our study, that examines the ability of CCP to neutralize viral replication in permissive cell lines. We found that close to 80% of the population of donor samples we tested had a neutralizing antibody titer of at least 80, and 60% at least 160, both of which meet the FDAʼs criteria for eligible CCP units, consistent with other studies. 23
The longevity of the antibody response is critical to protection against reinfection, although such information continues to be gathered. Analysis of the longevity of the antibody response to SARS1 indicates that anti‐SARS1 were detectable 2 to 3 years after infection in one study 30 and, in a second study, detectable for close to 1 year after infection but declined over the course of this observation period. 31
Short‐term studies on the durability of the SARS‐CoV‐2 antibody response have provided variable results. Rapid decay of anti‐SARS‐CoV‐2 IgG was noted in 34 convalescent plasma donors with mild illness, raising concern that immunity may not be long lasting, 32 while other studies have suggested that the presence of antibodies remained high over a similar time frame. Antibody strength and longevity seem to correlate with the severity of illness, 35 and while some studies indicate that antibody titers remain stable over time, 36 others suggest that antibody titers may quickly wane, and concerns for reinfection, particularly with mild or no symptoms, are not unwarranted. 32 , 33 , 34 , 37 For example, one reported case found that a SARS‐CoV‐2 confirmed infected patientʼs IgG antibodies became undetectable by Day 80. 38 Waning antibody levels in repeat CCP donors may have a significant impact on the amount and quality of the SARS‐CoV‐2 antibodies that are transfused as part of CCP therapy and may impact the availability of high‐titer CCP. The Mayo Expanded Access Protocol, a large study of 35 322 patients who received CCP, reported that a higher antibody titer was correlated with reduced mortality. 20 As a result, the FDAʼs recently issued EUA has established a cutoff value for a high antibody titer on the FDA approved Ortho VITROS SARS‐CoV‐2 IgG platform. 3 This incites the question of the adequacy of the minimum threshold for CCP treatment previously established by the FDA, and may impact future collections if the threshold is further increased for therapeutic efficacy or physicians will only accept “high‐titer” units for their patients.
Our data suggest that a majority of donors (67%) had a neutralizing antibody response that was either sustained or increased over the short period of approximately 3 weeks to 2 months after a positive SARS‐CoV‐2 PCR result, and a smaller percentage (33%) showed a decrease in neutralizing antibody titer over sequential donations. Notably, repeat donations did not appear to affect antibody titer for the majority (67%) of donors. A drawback of our data set is that for the majority of donors, we were able to test only two time points (7‐24 days apart) after a positive SARS‐CoV‐2 PCR test, making it challenging to comment on longer‐term sustainability of the response. The longevity of the SARS‐CoV‐2 antibody response and the level of protection it will provide against reinfection is yet to be determined, as is the impact of the cellular immune response beyond IgG antibody. Although SARS‐CoV‐2 neutralizing antibodies are critical for control of infection, two X‐linked agammaglobulinemia patients who had a complete lack of B cells recovered from COVID‐19 without developing severe symptoms, therefore suggesting that the cellular immune response plays a significant protective role. 39 Further elucidation of the respective roles of antibody and cellular responses to SARS‐CoV‐2 is required to determine their contribution to protection against infection.
FUNDING INFORMATION
This study was funded by the Department of Pediatrics, the Department of Immunology and Microbiology, University of Colorado School of Medicine and Childrenʼs Hospital, Colorado.
CONFLICT OF INTEREST
K.A. provides consulting for Terumo BCT. The other authors declare no potential conflict of interest.
Annen K, Morrison TE, DomBourian MG, et al. Presence and short‐term persistence of SARS‐CoV‐2 neutralizing antibodies in COVID‐19 convalescent plasma donors. Transfusion. 2021;61:1148–1159. 10.1111/trf.16261
Kyle Annen and Thomas E. Morrison are co‐first authors.
REFERENCES
- 1. U.S. Food & Drug Administration . Recommendations for investigational COVID‐19 convalescent plasma. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma. Published November 16, 2020. Accessed 02 12, 2021.
- 2. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research . Investigational COVID‐19 convalescent plasma: guidance for industry. https://www.fda.gov/media/136798/download. Document issued November 16, 2020. Accessed 08 23, 2020.
- 3. U.S. Food & Drug Administration . FDA news release: FDA issues emergency use authorization for convalescent plasma as potential promising COVID‐19 treatment, another achievement in administration's fight against pandemic. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment. Released August 23, 2020. Accessed 03 30, 2020.
- 4. U.S. Food & Drug Administration . CFR – code of federal regulations title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=630.3. Page current as of April 1, 2020. Accessed 11 10, 2020.
- 5. Ciszewski TS, Ralston S, Acteson D, Wasi S, Strong SJ. Protein levels and plasmapheresis intensity. Transfus Med. 1993;3(1):59–65. [DOI] [PubMed] [Google Scholar]
- 6. Kohmer N, Westhaus S, Ruhl C, Ciesek S, Rabenau HF. Clinical performance of different SARS‐CoV‐2 IgG antibody tests. J Med Virol. 2020;92:2243–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kruttgen A, Cornelissen CG, Dreher M, Hornef M, Imohl M, Kleines M. Comparison of four new commercial serologic assays for determination of SARS‐CoV‐2 IgG. J Clin Virol. 2020;128:104394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harcourt J, Tamin A, Lu X, et al. Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States. Emerg Infect Dis. 2020;26(6):1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta‐analysis: Convalescent blood products for Spanish influenza pneumonia: A future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. [DOI] [PubMed] [Google Scholar]
- 11. Hung IF, To KK , Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soo YO, Cheng Y, Wong R, et al. Retrospective comparison of convalescent plasma with continuing high‐dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ko JH, Seok H, Cho SY, et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: A single centre experience. Antivir Ther. 2018;23(7):617–622. [DOI] [PubMed] [Google Scholar]
- 14. U.S. Food & Drug Administration . Coronavirus (COVID‐19) update: daily roundup, March 24, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-march-24-2020. Released March 24, 2020. Accessed 03 24, 2020.
- 15. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salazar E, Christensen PA, Graviss EA, et al. Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID‐19) patients transfused early with convalescent plasma containing high‐titer anti‐severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spike protein IgG. Am J Pathol. 2021;191(1):90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID‐19 patients in Wuhan, China. J Med Virol. 2020;92(10):1890–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeng QL, Yu ZJ, Gou JJ, et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020;222(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joyner MJ, Senefeld JW, Klassen SA, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID‐19: Initial three‐month experience. medRxiv. 2020. 10.1101/2020.08.12.20169359. [DOI] [Google Scholar]
- 21. Luetkens T, Metcalf R, Planelles V, et al. Successful transfer of anti–SARS‐CoV‐2 immunity using convalescent plasma in an MM patient with hypogammaglobulinemia and COVID‐19. Blood Adv. 2020;4(19):4864–4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salazar E, Christensen PA, Graviss EA, et al. Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID‐19) patients transfused early with convalescent plasma containing high‐titer anti‐severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spike protein IgG. Am J Pathol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee WT, Girardin RC, Dupuis AP, et al. Neutralizing antibody responses in COVID‐19 convalescent sera. J Infect Dis. 2021;223(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS‐CoV‐2. Nature. 2020;581(7807):221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buchholz UJ, Bukreyev A, Yang L, et al. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci U S A. 2004;101(26):9804–9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salazar E, Kuchipudi SV, Christensen PA, et al. Convalescent plasma anti–SARS‐CoV‐2 spike protein ectodomain and receptor‐binding domain IgG correlate with virus neutralization. J Clin Invest. 2020;130(12):6728–6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salazar E, Kuchipudi SV, Christensen PA, et al. Relationship between anti‐spike protein antibody titers and SARS‐CoV‐2 in vitro virus neutralization in convalescent plasma. bioRxiv. 2020. 10.1101/2020.06.08.138990. [DOI] [Google Scholar]
- 28. Burbelo PD, Riedo FX, Morishima C, et al. Sensitivity in detection of antibodies to Nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. J Infect Dis. 2020;222(2):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Health Organization . “Immunity passports” in the context of COVID‐19: scientific brief. https://www.who.int/news-room/commentaries/detail/immunity-passports-in-the-context-of-covid-19. Published April 24, 2020. Accessed 24 24, 2020.
- 30. Wu LP, Wang NC, Chang YH, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13(10):1562–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Woo PC, Lau SK, Wong BH, et al. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin Diagn Lab Immunol. 2004;11(4):665–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, et al. Rapid decay of anti–SARS‐CoV‐2 antibodies in persons with mild Covid‐19. N Engl J Med. 2020;383(11):1085–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bölke E, Matuschek C, Fischer JC. Loss of anti–SARS‐CoV‐2 antibodies in mild Covid‐19. N Engl J Med. 2020;383(17):1694–1695. [DOI] [PubMed] [Google Scholar]
- 34. Terpos E, Politou M, Sergentanis TN, et al. Rapid reduction of anti‐Sars‐Cov‐2 antibodies in convalescent plasma donors; results of a phase 2 clinical study. Blood. 2020;136(suppl 1):1–2.32430499 [Google Scholar]
- 35. Klein S, Pekosz A, Park HS, et al. Sex, age, and hospitalization drive antibody responses in a COVID‐19 convalescent plasma donor population. medRxiv. 2020. 10.1101/2020.06.26.20139063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS‐CoV‐2 infection persist for months. Science. 2020;370(6521):1227–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS‐CoV‐2 infection in humans. Nat Microbiol. 2020;5(12):1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu A, Wang W, Zhao X, et al. Disappearance of antibodies to SARS‐CoV‐2 in a ‐COVID‐19 patient after recovery. Clin Microbiol Infect. 2020;26:1703–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Soresina A, Moratto D, Chiarini M, et al. Two X‐linked agammaglobulinemia patients develop pneumonia as COVID‐19 manifestation but recover. Pediatr Allergy Immunol. 2020;31(5):565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]


