Abstract
Background
Blood centers (BCs) rely on schools and businesses. Shelter‐in‐place orders closed them. This study determined how COVID‐19 affected donation habits.
Study Design and Methods
Two periods were reviewed (May 1 through June 30, 2018 vs 2019 [control] and 2019 vs 2020 [study–COVID period]). These donations were reviewed: first‐time, repeat (donation ≤ 2 years), and lapsed (no donation > 2 years); sex; age; ethnicity; and ABO blood groups at high school and college drives. Testing all donors for SARS‐CoV‐2 antibodies started May 18, 2020.
Results
In the study period donations significantly increased (control P = .683, study P ≤ .0001) and comparing sex (control male P = .716, female P = .657; study male P = .004, female P ≤ .0001). In the study period there was a significant decrease in Hispanic (P = .001) and African American (P < .0001) donations also seen among high school and college drives and an increase in Caucasian (P < .0001) donations. There was a significant increase in first‐time (P < .0001) and lapsed donors (P < .0001) in the study period vs control (first‐time P = .087, lapsed P = .308) and a significant decrease in donors not more than 30 years (study 16‐20 P < .0001, 21‐30 P < .0001). There was a significant increase in all blood types in the study period (P < .0001) and in donations after implementation of SARS‐CoV‐2 antibody testing (P = .001).
Conclusions
Significant changes occurred in donation habits in the study vs the control periods. These included increased total donations, comparing sexes, first‐time and lapsed donors, all blood types, and Caucasian donations. Significant decreases were seen in Hispanic and African American donations and those not more than 30 years old.
Keywords: donors, blood center operations, blood management
Abbreviations
- BC(s)
blood center(s)
- CCP
COVID‐19 convalescent plasma
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 or SARS‐CoV‐2 causes “coronavirus disease 2019” (COVID‐19). 1 The World Health Organization (WHO) declared the outbreak of COVID‐19 to be a pandemic on March 11, 2020. 2 COVID‐19 convalescent plasma (CCP) contains antibodies to SARS‐CoV‐2. The U.S. Food and Drug Administration (FDA) issued a guidance to allow for CCP administration to patients with COVID‐19. 1 Use of CCP has been studied in outbreaks of other respiratory infections, including the 2003 SARS‐CoV‐1 epidemic, the 2009 to 2010 H1N1 influenza virus pandemic, and the 2012 MERS‐CoV epidemic. 3 , 4 , 5 During this study donors' plasma that tested positive for antibodies to SARS‐CoV‐2 was used as CCP. Data from the Expanded Access Program of 4330 patients found in the nonintubated patients, either at 7 days or at 28 days, there was a significant decrease in mortality if the patient was less than or equal to 80 years of age and treated with CCP within 3 days of presentation. There was a positive effect of a higher‐titer SARS‐CoV‐2 antibody in reducing mortality. 6 , 7
Blood centers (BCs) rely on collections at schools and businesses. Shelter‐in‐place orders issued in 2020 due to COVID‐19 closed these facilities. Additional donor campaigns were conducted to ensure adequate blood inventory to supply contracted hospitals during the pandemic. To increase the pool of donors for CCP, testing for antibodies to SARS‐CoV‐2 for all allogenic donations was implemented at this BC on May 18, 2020. This study was conducted to determine how COVID‐19 affected donation habits .
2. MATERIALS AND METHODS
Two time periods were reviewed (May 1 through June 30, 2018 vs 2019 [control], May 1 through June 30, 2019 vs 2020 [study–COVID period]). The following were reviewed for traditional blood donations: first‐time donors, lapsed donors (no donation in 2 years or more), repeat donors (previous donation within 2 years), change in sex and ethnicity, and ABO groups. Convalescent plasma donations were not included in the analysis. Ethnicity was limited by our blood establishment computer system (RSA, Ft. Lauderdale, Florida) to the following categories: African American, American Indian, Asian, Caucasian, Hispanic, Indian (from India), Native Hawaiian or other Pacific Islander, unknown, and not provided. Donors had the option to choose one category at the time of registration. Sex was binary with options of male and female; however, a donor could choose not to answer this category. For purposes of this study, age was divided into the following categories: 16 to 20, 21 to 30, 31 to 40, 41 to 50, 51 to 60, 61 to 70, and greater than 71 years and age not provided. Blood drives held at high schools and college campuses were compared for total donations to see if there were any changes in the top three ethnicities that presented (African American, Hispanic, and Caucasian). A comparison of total donations was performed before (May 1 through May 17, 2020) and after (May 18 through May 31, 2020) implementation of SARS‐CoV‐2 testing. For the donor demographics, two‐sample t‐test was used to determine statistical significance (Minitab, State College, PA). The study protocol was approved by an intuitional review board (Adverra Pro00038077) and all donors signed an informed consent form.
3. RESULTS
During the time period evaluated there were 264 593 donations. In 2020 there was a significant increase in total donations (2019‐2020 P = <.0001 vs 2018‐2019 P = .683) and by sex (2019‐2020 male P = .004, female P = <.0001 vs 2018‐2019 male P = .716, female P = .657; Table 1, Figure 1A).
TABLE 1.
Total donations by category for the time periods of May 1 through June 30 in 2018, 2019, and 2020
| Year | Males a | Females a | African American | Hispanic | Caucasian | American Indian | Indian | Asian | First‐time | Repeat | Lapsed | 16‐20 y | 21‐30 y | Mean age (y) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | 43 645 | 38 126 | 7076 | 14 102 | 56 994 | 263 | 402 | 1292 | 16 202 | 54 878 | 10 695 | 6003 | 12 276 | 45.08 |
| 2019 | 42 961 | 37 333 | 6780 | 13 672 | 55 927 | 310 | 422 | 1295 | 15 216 | 53 888 | 11 192 | 4858 | 11 830 | 45.84 |
| 2020 | 47 745 | 54 768 | 3555 | 11 076 | 83 931 | 221 | 317 | 1339 | 30 745 | 52 999 | 18 778 | 2547 | 9036 | 50.07 |
15 sex not provided.
FIGURE 1.
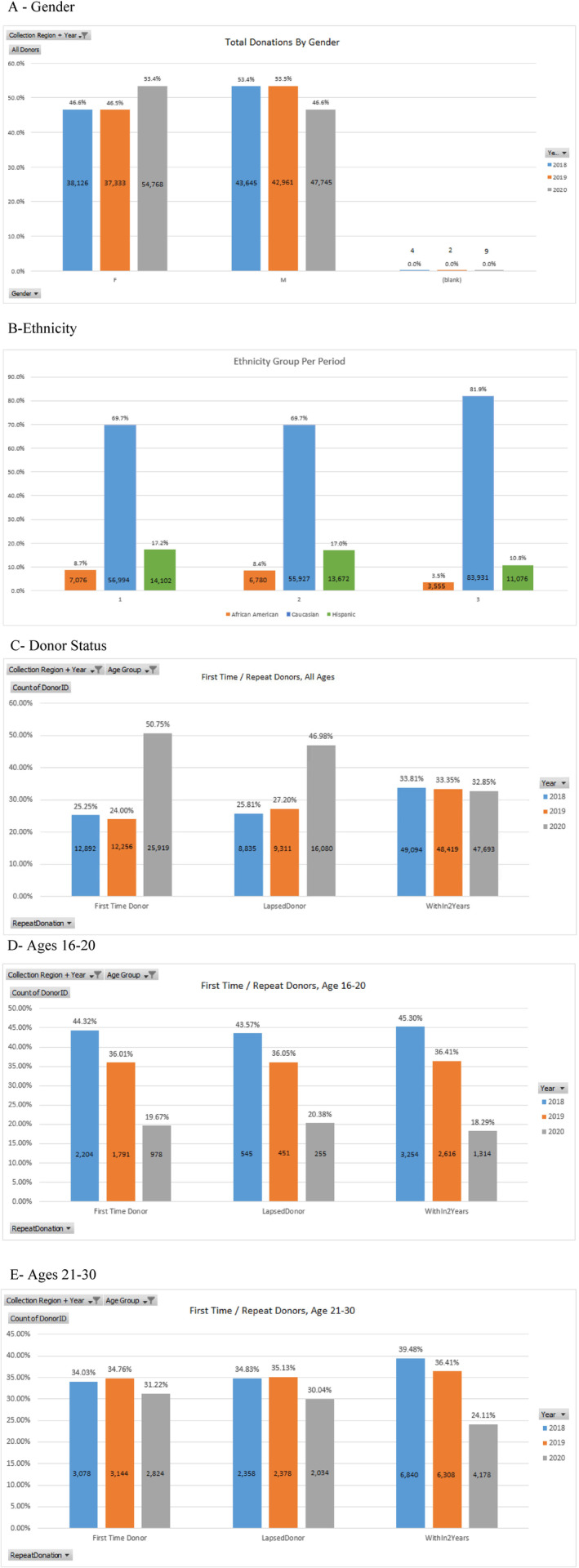
Changes in donations stratified by (A) sex, (B), ethnicity, (C) donor status, (D) ages 16 to 20 years, and (E) ages 21 to 30 [Color figure can be viewed at wileyonlinelibrary.com]
In 2019 to 2020 for the three largest ethnicities there was a significant decrease in Hispanic (P = .001) and African American (P < .0001) donations. There was also a significant increase in Caucasian (P < .0001) donations. This difference among the three ethnic groups was nonsignificant in 2018 to 2019 (African American P = .415, Hispanic P = .620, Caucasian P = .685; Table 1, Figure 1B). Two additional ethnic groups with much smaller donation numbers saw significant increases in donations in 2019 to 2020 (American Indian P = .004, Indian P = .022) compared with 2018 to 2019 (American Indian P = .177, Indian P = .721). No significance change was seen in those of Asian ethnicity during either period (2019‐2020 P = .962 vs 2018‐2019 P = .504).
There was a significant increase in first‐time (P < .0001) and lapsed donors (P < .0001) in 2019 to 2020 compared with 2018 to 2019 first‐time (P = .087) and lapsed donors (P = .308) for traditional blood donations. This was not observed in repeat donors (2018‐2019 P = .730, 2019‐2020 P = .705; (Table 1, Figure 1C).
The mean donor age increased significantly in 2019 to 2020 (P = .024) compared to 2018 to 2019 (P = .904). In 2019 to 2020 there was a significant decrease in the number of donors not more than 30 years old (2019‐2020 16‐20 P < .0001, 21‐30 P < .0001 vs 2018‐2019 16‐20 P = .053, 21‐30 P = .464). There was a significant increase in donations of all age groups over age 30 years in 2019‐20 (P = <.0001) compared with 2018 to 2019 (31‐40 P = .843, 41‐50 P = .905, 51‐60 P = .921, 61‐70 P = .684, 71 and older P = .528; Table 1, Figure 1D, E).
There was also a significant increase in donations of all blood types in 2020 (19‐20 all blood types P < .0001). This was not seen in the previous time period (18‐19 O+ P = .621, O– P = .724, A+ P = .774, A– P = .986, B+ P = .176, B– P = .665, AB+ P = .840, AB– P = .395).
For blood drives held at high schools and college campuses, when comparing the study period there was a significant decrease in total donations (P = .005) when compared to the control period (P = .607; Figure 2A). African American (P < .001) and Hispanic students (P < .001) significantly decreased during the study period when compared to the control period (African American P = .453, Hispanic P = .253). For the Caucasian donors the decrease was not significant in the study (P = .067) and control (P = .464) periods (Figure 2B).
FIGURE 2.
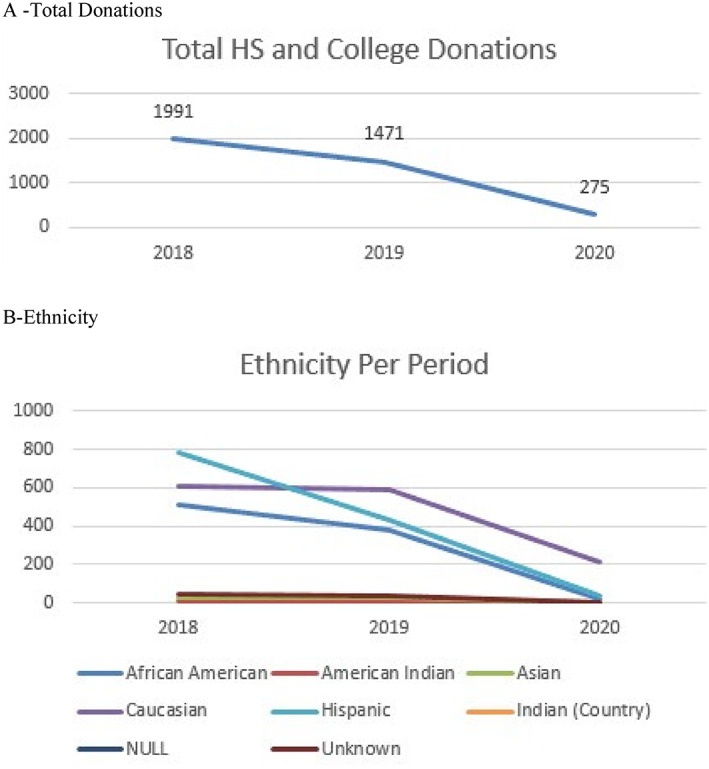
Changes in donations at blood drives held at high schools and college campuses stratified by (A) total donations and (B) ethnicity. Null and unknown mean that the ethnicity was not reported [Color figure can be viewed at wileyonlinelibrary.com]
The addition of antibody testing for SARS‐CoV‐2 significantly increased donations. From May 1, 2020, through May 17, 2020, the mean daily number of donations was 2759 compared with May 18, 2020, through May 31, 2020, where the mean daily number of donations was 3476 (P = .001; Figure 3).
FIGURE 3.
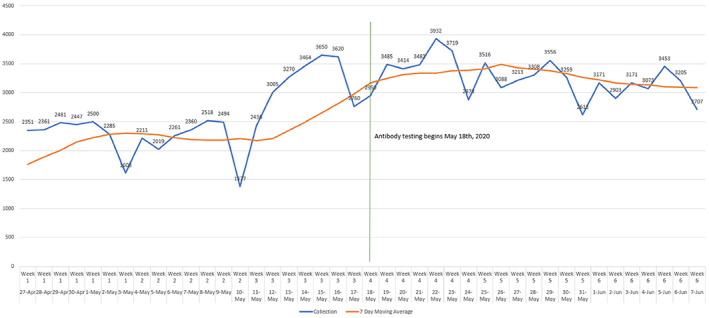
Donations significantly increased (P = .001) once SARS‐CoV‐2 antibody testing was initiated on May 18, 2020 [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
To maintain adequate inventory U.S. BCs have increasingly relied on young donors as part of the overall collection pool. Data from the 2017 National Blood Collection and Utilization Survey (NBCUS) showed that donations stratified by age had those 16 to 18 and 19 to 24 years comprising 12.3% and 10.1% of all those presenting or almost one in every four donations. 8 At the beginning of the SARS‐CoV‐2 pandemic many educational facilities where collections for these donors occurred quickly closed or moved to virtual learning platforms that resulted in cancellation of numerous blood drives and a decrease in available BC inventory. The pandemic also caused other challenges. Donors may not have been able to donate due to illness and employees may have been too ill to work. Also, a general fear of gathering in public spaces such as BC affected collections. 9
Significant changes were seen in donation habits at one BC in 2020 when compared to the control groups of the same period in the previous 2 years (2018, 2019). Two actions were taken by the BC to boost inventory. First, for traditional blood donations (eg, whole blood, plateletpheresis), additional donor campaigns were initiated. These campaigns directed donors to BC fixed locations as well as mobile drives. Because the blood supply was considered to be an essential service, donors needed to be informed that blood donation was exempted from the current shelter‐in‐place orders. Second, there was a great need to rapidly implement collection and develop an inventory of CCP, and because the FDA required titer testing to be performed on CCP collections to confirm efficacy, the BC made the decision to implement testing of all allogeneic donations for SARS‐CoV‐2 antibodies on May 18, 2020. The plan was to convert those with reactive test results that met the FDA requirements from traditional to CCP donors. The antibody testing significantly increased donations in the 2 weeks after implementation. During this time U.S. Surgeon General along with many other public service announcements were made encourage those who had recovered from COVID‐19 to donate CCP. The BC communications team gathered stories with more than a dozen of the initial CCP donors and posted on social and traditional news media. In addition, the BC created videos that took viewers behind the scenes to show the efforts taking place to collect, test, and distribute CCP. A BC public service announcement aired on local television stations and cable outlets that brought additional awareness to the need for more CCP donors. All materials were also displayed on the BC website and CCP donor registration page. These actions increased the public's awareness of a potential blood and CCP shortage combined with interest of SARS‐CoV‐2 antibody status may explain, at least in part, the significant increase in total donations as well as first‐time and lapsed donors and among all blood types. The significant decrease in donors age 30 years and younger may be caused, at least in part, by the closure or transition to virtual platforms of educational facilities. The significant decrease in African American and Hispanic donors could not be exclusively linked to the decrease in high school and college campus collections.
A limitations of this study was that it involved only one BC. Categories of ethnicities were self‐identified by the donor and there was not an option to choose multiple ethnicities. Sex choices were binary (male and female) or simply not to provide. There was not a means to capture additional categories.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Gammon RR, Prichard AB, Gannett MS, Yordanov B. The effect of COVID‐19 on blood donation habits. Transfusion. 2021;61:1134–1140. 10.1111/trf.16278
REFERENCES
- 1.Investigational COVID‐19 Convalescent Plasma: Guidance for Industry. Center for Biologics Evaluation and Research, U.S. Food and Drug Administration. Updated September 2, 2020. Accessed October 1, 2020. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/investigational‐covid‐19‐convalescent‐plasma.
- 2. WHO Director‐General's opening remarks at the media briefing on COVID‐19 ‐ March 11, 2020. World Health Organization. 2020. Accessed October 1, 2020. https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19‐11‐march‐2020.
- 3. Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leider JP, Brunker PA, Ness PM. Convalescent transfusion for pandemic influenza: Preparing blood banks for a new plasma product? Transfusion. 2010;50(6):1384–1398. 10.1111/j.1537-2995.2010.02590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arabi YM, Hajeer AH, Luke T, et al. Feasibility of using convalescent plasma immunotherapy for MERS‐CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22(9):1554–1561. 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marks P, Verdun N, Carayiannis. COVID‐19 Convalescent Plasma, the Regulatory Landscape. Presented at AABB 2020 Annual Meeting.
- 7. Joyner MJ, Senefeld JW, Klassen SA, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID‐19: Initial three‐month experience. MedRxiv. 2020. 10.1101/2020.08.12.20169359v1. [DOI] [Google Scholar]
- 8. Sapiano MR, Jones JM, Savinkina AA, Haass KA, Berger JJ, Basavaraju SV. Supplemental findings of the 2017 national blood collection and utilization survey. Transfusion. 2020;60:S17–S37. 10.1111/trf.15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gammon RR, Rosenbaum L, Cooke R, et al. Maintaining adequate donations and a sustainable blood supply‐lessons learned. Transfusion. 2020;61:294–302. 10.1111/trf.16145. [DOI] [PMC free article] [PubMed] [Google Scholar]


