Abstract
Bacterial exopolysaccharides (EPSs) are important alternatives to plant polysaccharides in fermented products and exhibit antioxidant activity, which is particularly desirable for functional foods. This study evaluated the use of spent media wastewater (SMW) derived from kimchi fermentation for the production of an EPS and analyzed the characterization and antioxidant activity of the resulting EPS. The EPS concentration and conversion yields of sequential purification were 7.7–9.0 g/L and 38.6–45.1%, respectively. Fourier transform infrared spectra and NMR spectra indicated that the EPS was a linear glucan with α-(1 → 6) linkages. The EPS also exhibited thermal tolerance to high temperatures. In vitro antioxidant activity analyses indicated the scavenging activity on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals, thiobarbituric acid reactance (TBAR), and ferric ion reducing antioxidant power (FRAP) values of 71.6–79.1, 28.2–33.0%, and 0.04–0.05 mM FeCl3, respectively. These results reveal that the EPS extracted from SMW has potential as a thermally tolerant, nontoxic, and natural antioxidant for industrial applications.
1. Introduction
Lactic acid bacteria (LAB) are food-grade microorganisms that are widely applied in a variety of fermentation industries.1 There is growing interest in the industrial applications of LAB, for example, as an important starter culture; however, the quality of fermented foods depends on the specific LAB species involved in the process.2 The demand for fermented products has increased significantly in recent years, which has led to an increase in the generation of spent media wastewater (SMW) from fermentation industries. Industrial waste is typically disposed off into the environment, causing substantial environmental problems. However, SMW is rich in polysaccharides that can be utilized in the sustainable production of commercially valuable products.3
LAB can produce natural polysaccharides with an enormous structural diversity. Bacterial exopolysaccharides (EPSs), which are synthesized or secreted into the extracellular matrix of LAB, are attached to the cell surface or released into the surrounding environment. An EPS comprises several monosaccharides linked by glycosidic bonds that blend into various structural compositions.4 EPSs have attracted attention in many scientific fields, with a particular focus on the optimization of production processes and functional properties. EPSs possess enormous functional applications in cosmetology, food additives, and pharmacology. The structure and character of bacterial EPSs mainly depend on the strains and fermentation conditions. Compared to other natural polysaccharides, EPSs require shorter production time and a much simpler extraction process. Recent studies have examined the potential health benefits of EPSs and their industrial applications.5 In the food industry, EPSs are regarded as alternatives to plant polysaccharides because they contribute to the texture modification, taste recognition, and stability of fermented foods. Furthermore, EPSs exhibit biological activities, especially antioxidant activity, which is desirable for functional foods and other industries.6
Reactive oxygen species (ROS) play important roles in maintaining the immune system and the redox balance, such as apoptosis, cell signaling, and ion transportation.7 However, excessive ROS can cause damage to the body through a variety of pathological effects, such as cancer, diabetes, and atherosclerosis.8,9 Lipid oxidation is an important reaction in both biological and food systems and is considered a deteriorative reaction in food containing lipids.10 Many studies have attempted to prevent lipid oxidation in food products, and adding antioxidants during food processing is one of the most effective strategies.11−13 Antioxidants can prevent lipid oxidation through various mechanisms, including scavenging free radicals, decomposing lipid peroxides, and preventing the formation of peroxides.14 Although synthetic antioxidants, i.e., butylated hydroxyanisole (BHT) and butylated hydroxytoluene (BHA), have a strong radical-scavenging ability, harmful effects and toxicity limit their application in the food industry.15 Therefore, it is of substantial interest to find natural, harmless, and nontoxic antioxidants instead of chemical antioxidants.
Leuconostoc mesenteroides is the dominant LAB present during early kimchi fermentation and has been commercialized for kimchi starters because of its beneficial effects on sensory characteristics.16 After cultivation, the cells are harvested for the kimchi starter and the fermentation liquid is discarded as industrial waste. In this study, an EPS is extracted and purified from the fermentation liquid produced after kimchi starter cultivation, and its physiochemical properties are characterized. Moreover, the production rate and conversion yield of EPS are calculated based on the initial fermentation media. Furthermore, the scavenging activity on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals, ferric ion reducing antioxidant power (FRAP), and thiobarbituric acid reactance (TBAR) are analyzed to determine the in vitro antioxidant activity of EPS.
2. Results and Discussion
2.1. Production and Purification of EPS
Glucose and sucrose are commonly used as carbon sources for the cultivation and production of bacterial EPSs.17 Considering that the fermentation media account for 30% of the total cost of EPS production, research is focused on identifying cheaper substrates such as agricultural waste, industrial waste, and byproducts.18 However, low-cost waste substrates exhibit negative effects—i.e., different polymers or unexpected byproducts may be synthesized owing to the presence of contaminants and the different metabolic pathways that might be followed by different nutrient compositions.1 SMW, which is discarded after the cultivation of the kimchi starter, does not require an additional carbon source for EPS production.
In this study, the production of EPS and the conversion of sucrose to EPS were calculated according to the sucrose concentration (20 g/L) in the fermentation media. The crude exopolysaccharide (cEPS) may have contained byproducts such as proteins or peptides during fermentation. Since these impurities were removed through purification, the concentration of EPS decreased from 9.0 g/L in cEPS to 7.7g/L in purified exopolysaccharide (pEPS). The conversion yields of cEPS and pEPS were 45.1 and 38.6%, respectively (Table 1).
Table 1. Exopolysaccharide (EPS) Production from Spent Media Wastewater (SMW)a.
Abbreviations: cEPS, crude EPS; pEPS, purified EPS.
Values represent the average of three replicates.
Conversion yield was calculated based on the sucrose concentration in the fermentation media.
Several previous studies have examined the optimal conditions to produce EPSs; the EPS concentration and sucrose–EPS conversion yield obtained in this study were either similar to or greater than those observed in previous studies. For example, EPS production from molasses medium (17.5% sucrose) produced 5.4–5.5 g dextran with a conversion yield of approximately 31%.19 Another study reported that the fermentation of L. mesenteroides after optimization produced approximately 4.9 g/L of EPS with a conversion yield of 48.9%.20
2.2. Chemical Composition and Structural Properties
EPSs produced by LAB can be classified into homosaccharides and heterosaccharides.21 Homopolysaccharides are composed of one type of carbohydrate such as fructose or glucose, whereas heterosaccharides contain two or more monosaccharides, mainly glucose, galactose, fructose, and mannose in different ratios (Table 2). L. mesenteroides is used to produce a glucose polymer known as dextran or alternan.20
Table 2. Monosaccharide Composition of Microbial Exopolysaccharides (EPSs) and Related Antioxidant Activitiesa.
| EPS-producing microorganisms | monosaccharides | antioxidant activity | references |
|---|---|---|---|
| Enterococcus faecium K1 | mannose, glucose, galactose | higher scavenging of hydroxyl and DPPH | Bhat and Bajaj22 |
| Weissella cibaria SJ14 | mannose, glucose, galactose, arabinose, xylose, rhamnose | higher scavenging of DPPH | Zhu et al.23 |
| Weissella confuse | galactose, mannose, glucose, fructose, rhamnose, arabinose, xylose, ribose | higher scavenging of DPPH, H2O2 radicals | Adebayo-tayo et al.24 |
| Lactobacillus delbrueckii ssp. | galactose, glucose | higher scavenging of superoxide, hydroxyl, DPPH radicals | Tang et al.25 |
| Lactobacillus plantarum KX041 | arabinose, mannose, glucose, galactose | higher scavenging of ABTS, DPPH | Wang et al.15 |
| L. plantarum ZDY2013 | galactose, xylose | higher scavenging of hydroxyl radical | Zhang et al.26 |
| L. mesenteroides WiKim32 | glucose | higher scavenging of ABTS, DPPH, TBARS | this study |
Abbreviations: DPPH, 1,1-diphenyl-2-picrylhydrazyl; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); and TBARS, thiobarbituric acid reactive substances.
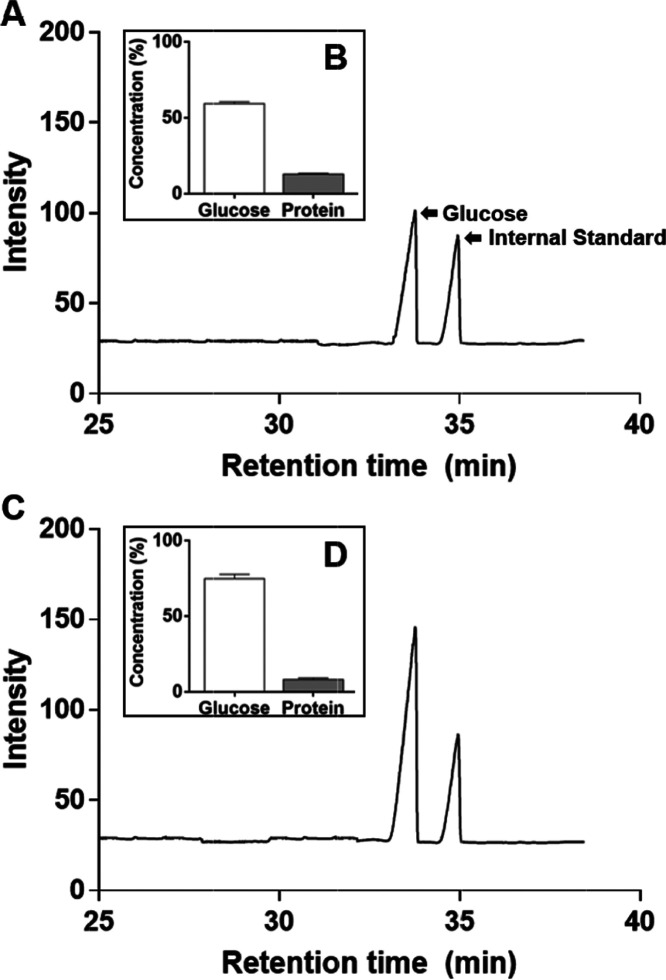
The carbohydrate of the EPS produced from L. mesenteroides WiKim32 contained glucose (Figure 1). No other monosugar was detected (Figure 1A,C). With purification, the glucose content of EPS increased from 59.6% (cEPS) (Figure 1B) to 75.1% (pEPS) (Figure 1D). EPS carbohydrates were affected differently by the purification. Moreover, the protein content decreased from 13.2 to 8.5%, suggesting that the EPS may be a protein-bound polysaccharide.
Figure 1.
Chromatogram and chemical composition of crude exopolysaccharide (cEPS) (A, B) and purified exopolysaccharide (pEPS) (C, D).
This is because the EPS is trapped within the protein matrix and tightly bound to the cell when polysaccharides are layered on the bacterial surface with glycoproteins.27 In previous studies, the EPS separated from L. mesenteroides DRP105 contained 1.85% protein, indicating that it may be a proteoglycan.28
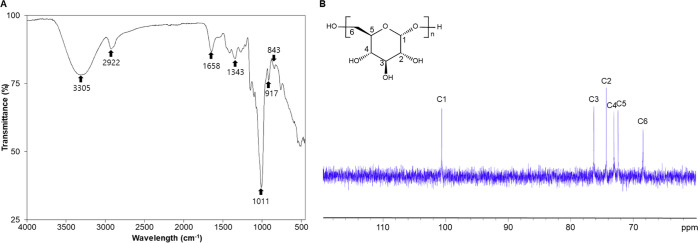
As shown via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), the protein concentrations of whole protein extracts of L. mesenteroides WiKim32 differed between cEPS and pEPS (Figure 2). Several intense cEPS protein bands were observed when compared to those of pEPS; however, these were not observed in dextran. The Fourier transform infrared (FTIR) spectrum of EPS is presented in Figure 3A. UV spectra of pEPS showed a peak at 260 nm, supporting the presence of proteins after the purification process (Figure S1 in the Supporting Information). The band at 3305 cm–1 corresponded to the hydroxyl (OH) group,29 and the band at 2922 cm–1 was attributed to asymmetrical and symmetrical C–H.30 The absorption peak at 1658 cm–1 was a polysaccharide and attributed to C=O stretching.31 The intense peak at 1343 cm–1 was severed carboxyl or carboxylate groups.32 The peak at 1011 cm–1 confirmed that the EPS contained α-glycosidic linkages,33 and the absorption at 917 and 843 cm–1 indicated a glucosyl residue with α-pyranose.34 The absence of a band at 890 cm–1 revealed the absence of a β-glycosidic linkage in the EPS.35 The FTIR spectra of the EPS from L. mesenteroides WiKim32 exhibited similar properties to those from L. pseudomesenteroides(17) and L. citreum.36 FTIR spectral analysis of L. mesenteroides WiKim32 EPS indicated the α-configuration and α-glucosidic polysaccharide.
Figure 2.
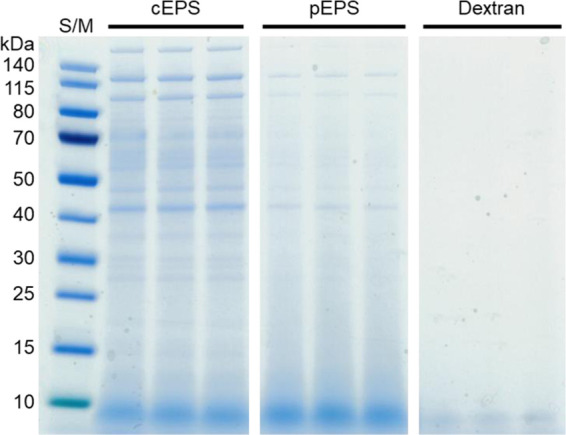
Protein weight distribution of the exopolysaccharide (EPS) on SDS-PAGE (S/M: protein ladder).
Figure 3.
Structural analysis. (A) Fourier transform infrared (FTIR) spectrum and (B) 13C NMR spectrum of the purified exopolysaccharide (EPS) from L. mesenteroides WiKim32.
NMR spectroscopy is a commonly used tool in the structural analysis of complex compounds. The 13C NMR spectrum of EPS, which included ring carbons (50–85 ppm) and anomeric carbon regions (95–110 ppm), is presented in Figure 3B. The anomeric carbon peak at δ 100.6 ppm was attributed to the C1 α-glucopyranosyl repeating unit. The clusters of resonances around the peaks at δ 74.3, δ 76.3, δ 73.0, and δ 72.4 ppm were attributed to C-2, C-3, C-4, and C-5, respectively. No additional peaks appeared within the region of δ 78–85 ppm, indicating the absence of branched linkages. The absorption peak at δ 68.4 ppm was attributed to C-6 of glucose in the α-(1 → 6) linkage polymer.
These results indicate that the EPS from L. mesenteroides WiKim32 is a highly linear dextran with α-(1 → 6) glycosidic linkages (Figure S2 in the Supporting Information) and without branch linkages according to FTIR and NMR results. These structural properties were similar to those of the EPS from L. pseudomesenteroides,17L. pseudomesenteroides YF32,36 and L. mesenteroides TDS2-19.37
2.3. Transmission Electron Microscopy (TEM)
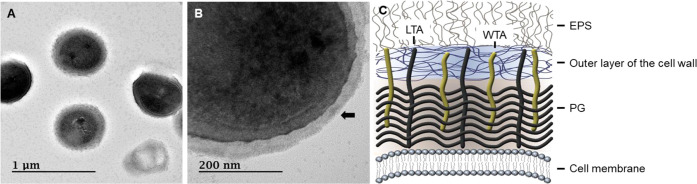
Transmission electron microscopy (TEM) is an essential tool for investigating nanostructural and spatial aspects in biology. L. mesenteroides WiKim32 cells exhibited well-defined cytoplasmic contents and an intact cell wall (Figure 4). An EPS thickness of 37.2 ± 3.4 nm was observed, covering the cell surfaces (Figure 4B). Generally, the cell walls of LAB are formed by four structural models: (i) cell wall made of peptidoglycan, lipoteichoic acids (LTA), and polysaccharides; (ii) EPS attached to the outer surface; (iii) peptidoglycan-containing layer enveloped by the surface layer protein; and (iv) polymer layer cross-linked with the surface layer protein.2 The cell wall structure of L. mesenteroides WiKim32 according to these models is shown in Figure 4C. The functional properties of EPSs have attracted increasing interest in health-related applications, for example, as natural antioxidants.38,39
Figure 4.
Transmission electron microscopy (TEM) images of L. mesenteroides WiKim32. (A) Bacterial cells after cultivation. (B) Layer at the surface of the cell wall represents the exopolysaccharide (EPS) (black arrow). (C) Structural models of the L. mesenteroides WiKim32 cell wall. The bacterial cell envelops the peptidoglycan layer (PG), wall teichoic acids (WTA), lipoteichoic acids (LTA), and the outer layer of the cell wall. The EPS is attached to the cell wall. Scale bars are 1 μm (left) and 200 nm (middle).
2.4. Thermodynamic Behavior
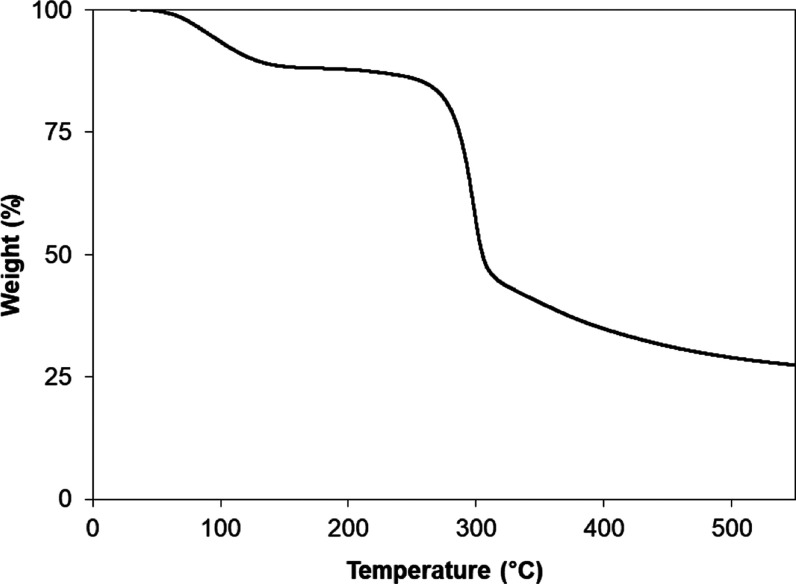
Thermogravimetric analysis (TGA) of EPS was conducted for weight loss in the temperature range 30–550 °C (Figure 5). The results showed that the EPS degraded in three stages. The first stage corresponds to the moisture drying region (at 30–147 °C) with a 12% weight loss. This behavior is similar to the decomposition of the EPS from L. plantarum KF5.40 The EPS remained stable and exhibited thermal tolerance when the temperature increased from 147 to 260 °C. The final stage was 260–320 °C, during which a major loss of mass (42%) occurred due to the depolymerization of EPS. Based on the TGA results, the thermal behavior of dextran was similar to that of EPS, which was due to the similar structure and composition of EPS, derived from L. mesenteroides WiKim32, and commercial dextran (data not shown). These thermodynamic results indicate that EPS has a relatively high degradation temperature; this thermal property can be applied in various industries to improve the thermal tolerance of products.41
Figure 5.
Thermogravimetric analysis (TGA) curve of the purified exopolysaccharide (EPS) from spent media wastewater of L. mesenteroides WiKim32.
2.5. Antioxidant Activity
2.5.1. DPPH Radical-Scavenging Activity
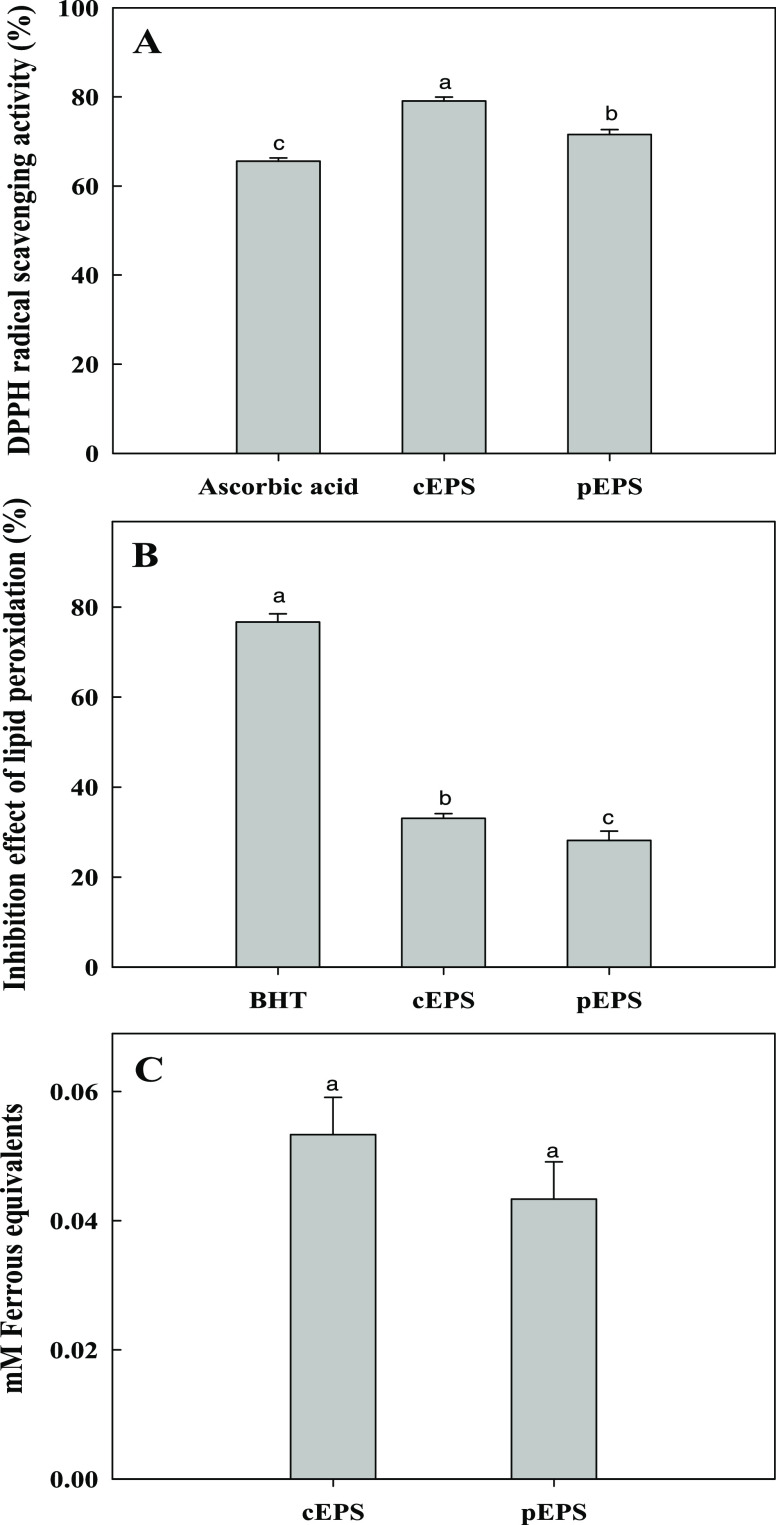
DPPH is a commonly used compound in the assay for evaluating the free-radical-scavenging capacities of antioxidants. During the assay, the antioxidants reduced the stable DPPH radical and the nonradical form of DPPH-H. The effect of antioxidants on DPPH is closely related to its hydrogen donation ability. The results indicate that cEPS and pEPS influence free-radical scavenging (Figure 6A). The DPPH radical-scavenging activity differed for the materials tested (F = 159.0; df = 2, 6; P < 0.001).
Figure 6.
Antioxidant activity of cEPS and pEPS: (A) radical-scavenging capacity of cEPS and pEPS, (B) inhibition effect of lipid peroxidation, and (C) ferrous chelating ability.
The radical-scavenging activities of cEPS and pEPS were 79.1 and 71.6% at 10 mg/mL, respectively, indicating that the scavenging activity of cEPS was higher than that of pEPS. This is because other antioxidant components, such as proteins, amino acids, and microelements, are present in the cEPS.42 These components may exhibit some interactions and may have synergistic effects on the antioxidant activity.43
2.5.2. TBAR Assay
Lipid peroxidation may occur during oxidative damage to cell structures and the toxicity process, leading to cell death. Oxidative alteration of polyunsaturated fatty acids generates multiple degradation products by lipid peroxidation.44 In this study, the FeCl2–H2O2 system was adopted to induce lipid peroxidation. The lipid peroxidation inhibition differed for each material (F = 734.9; df = 2, 6; P < 0.001) (Figure 6B). The inhibition effects of BHT, cEPS, and pEPS were 76.6, 33, and 28.2%, respectively, at a concentration of 2.5 mg/mL (Figure 5B). These results indicate that EPS has moderate inhibitory effects on lipid peroxidation compared with BHT. The inhibition of lipid peroxidation may be attributed to their scavenging abilities on hydroxyl radicals and the H2O2 produced by the FeCl2–H2O2 reaction.45 Another study reported that polysaccharides exhibit inhibitory effects on lipid peroxidation because of their metal ion chelating properties.46
2.5.3. FRAP Assay
The FRAP assay is a simple and direct method of measuring the antioxidative ability of a substance required to directly reduce an oxidant. The reducing potential of antioxidants is related to the ability of electron donation to break the free-radical chain reactions.47 In this study, the FRAP assay was conducted to evaluate the antioxidant power of EPS.
Based on the calibration curve of FeCl3, the FRAP values for cEPS and pEPS were calculated as 0.05 mM FeCl3 and 0.04 mM FeCl3, respectively (Figure 5C). This indicates that EPS may act as an electron donor and react with free radicals, which can terminate the free-radical chain reaction for stable products. EPS also showed scavenging abilities on hydroxyl radicals and metal ion chelating activities. Therefore, the lipid peroxidation inhibition effects of EPS may be attributed to the Fe2+ chelating ability of polysaccharides.44
2.6. Overall Mass Balance
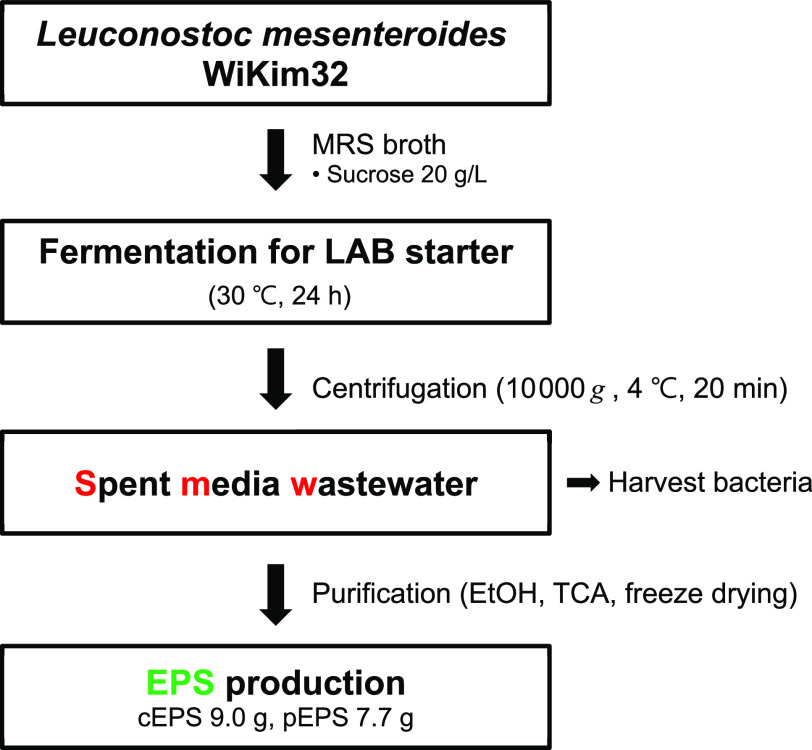
An overall mass balance diagram describing the bacterial fermentation, harvest, and extraction of EPS is shown in Figure 7. L. mesenteroides WiKim32 was fermented for the kimchi starter at 30 °C for 24 h. The bacterial cells were harvested, and the supernatant was collected by centrifugation. Through extraction and purification using ethanol and TCA, 9.0 g of cEPS and 7.7 g of pEPS were obtained with conversion yields of 45.1 and 38.6% per 20 g sucrose, respectively. These results suggest that SMW is a potential resource for EPS production.
Figure 7.
Overall mass balance.
3. Conclusions
This study revealed the potential antioxidant activities of an EPS obtained from SMW derived from the kimchi fermentation process. The EPS concentration and sucrose–EPS conversion yield were similar to or greater than the optimized values. The obtained EPS exhibited structural properties and thermal tolerance similar to dextran. In addition, the functional and antioxidant properties of EPS were examined, revealing DPPH radical-scavenging activity, a lipid peroxidation inhibition effect, and ferrous chelating ability. Further pharmacological research on EPS is required to exploit it as a natural alternative to commercial antioxidants. Moreover, SMW raises the potential for more economic EPS production.
4. Materials and Methods
4.1. EPS Production and Isolation
4.1.1. Microorganism Cultivation
L. mesenteroides WiKim32 (KFCC11639P), developed as a kimchi fermentation starter, was used in this study. The bacteria were cultured in an MRS medium (peptone 10 g/L, beef extract 10 g/L, yeast extract 5 g/L, glucose 20 g/L, Tween 80 1 g/L, ammonium citrate 2 g/L, sodium acetate 5 g/L, magnesium sulfate 0.1 g/L, manganese sulfate 0.05 g/L, and dipotassium phosphate 2 g/L) at 30 °C for 24 h. Then, the cultures were stored at −80 °C (MDF4V; Panasonic, Tokyo, Japan) in the presence of 20% glycerol as a cryoprotectant until further use. To produce the kimchi starter, the freeze-dried cells were cultured in 10 L of modified MRS broth (glucose was replaced by sucrose 20 g/L) and harvested when they reached the midexponential phase of growth (OD 600 nm of 0.6). After cultivation, the cells were collected (10 000g for 20 min at 4 °C) and prepared to produce the kimchi fermentation starter.
4.1.2. Purification of EPS
The EPS was isolated and purified following the method of Adesulu-Dahunsi et al.11 with slight modifications. After cell cultivation and collection, SMW was heated at 100 °C for 10 min to inactivate the enzymes, and the debris was removed by centrifugation (10 000g for 20 min at 4 °C). Three volumes of 95% (v/v) cold ethanol were added to the supernatant and stored at −4 °C overnight and then centrifuged (10 000g for 20 min at 4 °C). Crude EPS (cEPS) was obtained after ethanol precipitation and lyophilization. To remove the protein, cEPS was dissolved in distilled water and deproteinized using 4% (v/v) trichloroacetic acid (TCA). cEPS was reprecipitated with three volumes of 95% (v/v) cold ethanol and dissolved in distilled water. Finally, dialysis was conducted twice daily with distilled water for 2 days at 4 °C using dialysis cassettes (Slide-A-Lyzer 10K MWCO, Thermo Scientific, Waltham, MA) and then lyophilized. The final residual was termed pure EPS (pEPS).
4.2. Analytical Methodology
4.2.1. Monosugar Composition and Protein
Monosaccharide compositions were analyzed for their neutral sugar content using gas chromatography (GC). The neutral sugar composition was measured with alditol acetates containing myo-inositol as an internal standard. Samples were analyzed with GC (7890A, Agilent Technology, Palo Alto, CA) using a DB-225 capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness; J&W, Rancho Cordova, CA). The chromatograph was operated with He at an injector temperature of 220 °C and a flame ionization detector at 250 °C; the oven temperature programming was set to 100 °C for 1.5 min, which was then increased by 5 °C/min up to 220 °C.48 The protein concentration was measured using the Lowry method, with bovine serum albumin (BSA) as a protein standard. Proteins in the EPS were separated on 4–12% Bis–Tris NuPage gels (Invitrogen, Carlsbad, CA) in a Mini-Gel tank (Thermo Fisher Scientific, Waltham, MA) at 220 V for 30 min. Gels were stained with SimplyBlue SafeStain (Thermo Fisher Scientific). Based on sucrose and EPS concentrations, the conversion yields were calculated using the following formula
where Csucrose is the amount of sucrose in the fermentation media and CEPS is the amount of cEPS or pEPS. Ultraviolet–visible (UV–vis) spectroscopy analyses were conducted using a Nanophotometer UV–vis spectrophotometer NP80 (Implen, Munchen, Germany). The sample was prepared in distilled water (1 mg/mL) for UV measurements in a wavelength range of 200–900 nm.
4.2.2. Structural Characterization
The morphologies of the bacterial cells were examined using a transmission electron microscope (JEM-1400; JEOL, Tokyo, Japan). Samples were fixed in 2% (v/v) glutaraldehyde (Merck, Darmstadt, Germany), dehydrated to 30, 50, and 70% (v/v) twice in absolute ethanol, and then sectioned using an ultramicrotome with a diamond knife.2 The EPS thickness was determined using Gatan digital micrograph software. The average diameter was measured at different regions of each cell for 16 bacteria. A Fourier transform infrared (FTIR) spectrum of the lyophilized sample was obtained with the ATR technique using a PerkinElmer Spectrum 400 (PerkinElmer, Inc., Shelton, CT). The spectrum was scanned in the range of 4000–400 cm–1 using four scans with a resolution of 4 cm–1. NMR spectra were recorded on a Varian Unity Inova 500 MHz spectrometer (Varian NMR Systems, Palo Alto, CA), operating at 125 MHz for 13C NMR. The data were determined and processed using TopSpin 4.0 software. Thermogravimetric analysis (TGA) was performed using Mettler Toledo equipment (Mettler Toledo, Schwarzenbach, Switzerland). To determine the carbonization temperature, the samples were analyzed in the range of 30–550 °C under a nitrogen atmosphere.
4.3. Antioxidant Activity Assay
4.3.1. DPPH Radical-Scavenging Assay
The scavenging activity on DPPH free radicals was assayed according to the method by Shimada et al.49 with slight modifications. Samples were dissolved in distilled water to a concentration of 1% (10 mg/mL). One milliliter of the sample was added to the same volume of 0.2 mM DPPH solution. The reaction mixture was mixed vigorously and incubated in the dark at 25 °C for 30 min. The absorbance was measured at 517 nm with ascorbic acid as the reference (50 μg/mL). The scavenging activity was defined as follows
4.3.2. Thiobarbituric Acid Reactive Substances Assay
The inhibition rate of lipid peroxidation was evaluated using the thiobarbituric acid (TBA) method from Chen et al.,50 which is based on estimating the inhibition of linoleic acid peroxidation by samples. Briefly, 4% TCA, 0.8% TBA, and 0.4% ascorbic acid solutions were added to the samples and incubated at 100 °C for 10 min. The absorbance of the supernatant was measured at 532 nm with BHT as the reference (3 mM). The inhibition rate of lipid peroxidation was calculated using the following formula
4.3.3. Ferric Reducing Antioxidant Power Assay
A ferric reducing antioxidant power (FRAP) assay was performed using a commercially available assay kit (Abcam, ab234626, Caliph, Ml). Briefly, the total volume of the reaction mixture was 200 μL, and the reaction mixture comprised 152 μL of FRAP assay buffer, 19 μL of FRAP probe, 19 μL of FeCl3 solution, and 10 μL of the sample. The microplates containing the reaction mixtures were kept in the dark at 37 °C for 60 min. The increase in absorbance of the mixtures was measured at 594 nm using a microplate reader. The antioxidant capacity was calculated using a ferrous iron standard curve, and the results were expressed as Fe2+ equivalents (μM).
4.4. Statistical Analysis
Data are presented as the mean of three independent experiments. Data were analyzed using IBM SPSS Statistics for Windows, Version 19 (IBM Corp., Armonk, NY). Analysis of variance followed by Tukey’s honestly significant difference test was used to determine significant differences between treatments at P < 0.05.
Acknowledgments
This research was supported by the World Institute of Kimchi (grant number KE2102-1, KE2102-1-1), funded by the Ministry of Science and ICT, Republic of Korea.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c06095.
UV spectra of EPS in the range 200–900 nm (Figure S1); and Fourier transform infrared spectrum of commercial dextran (Figure S2) (PDF)
Author Contributions
I.S.C. and H.W.P. designed the study. I.S.C., S.H.K., M.E.L., H.M.K., J.E.Y., S.-G.J., and J.-C.K. performed the experiments. K.H.L., J.Y.C., and J.-C.K. contributed to data interpretation. I.S.C. and H.W.P. wrote the main manuscript. All authors read, reviewed, and approved the final manuscript.
The authors declare no competing financial interest.
This paper was published ASAP on March 18, 2021, with a misspelling in the title. The corrected version was posted March 30, 2021.
Supplementary Material
References
- Freitas F.; Alves V. D.; Reis M. A. M. Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. 10.1016/j.tibtech.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Choi I. S.; Ko S. H.; Kim H. M.; Chun H. H.; Lee K. H.; Yang J. E.; Jeong S.; Park H. W. Shelf-life extension of freeze-dried Lactobacillus brevis WiKim0069 using supercooling pretreatment. LWT - Food Sci. Technol. 2019, 112, 108230 10.1016/j.lwt.2019.05.128. [DOI] [Google Scholar]
- Guo Y.; Pan D.; Li H.; Sun Y.; Zeng X.; Yan B. Antioxidant and immunomodulatory activity of selenium exopolysaccharide produced by Lactococcus lactis subsp lactis. Food Chem. 2013, 138, 84–89. 10.1016/j.foodchem.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Rehm B. H. A. Bacterial polymers: biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010, 8, 578–592. 10.1038/nrmicro2354. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Coda R.; Shi Q.; Tuomainen P.; Katina K.; Tenkanen M. Exopolysaccharides production during the fermentation of soybean and fava bean flours by Leuconostoc mesenteroides DSM 20343. J. Agric. Food Chem. 2017, 65, 2805–2815. 10.1021/acs.jafc.6b05495. [DOI] [PubMed] [Google Scholar]
- Badel S.; Bernardi T.; Michaud P. New perspectives for Lactobacilli exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66. 10.1016/j.biotechadv.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Barnham K. J.; Masters C. L.; Bush A. I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discovery 2004, 3, 205–214. 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Liu J.; Luo J. G.; Ye H.; Zeng X. X. Preparation, antioxidant and antitumor activities in vitro of different derivatives of levan from endophytic bacterium Paenibacillus polymyxa EJS-3. Food Chem. Toxicol. 2012, 50, 767–772. 10.1016/j.fct.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Yang J.; Xiong Y. Comparative time-course of lipid and myofibrillar protein oxidation in different biphasic systems under hydroxyl radical stress. Food Chem. 2018, 243, 231–238. 10.1016/j.foodchem.2017.09.146. [DOI] [PubMed] [Google Scholar]
- Chaiyasit W.; Elisa R. J.; McClements D. J.; Decker E. A. Role of physical structures in bulk oils on lipid oxidation. Crit. Rev. Food. Sci. Nutr. 2007, 47, 299–317. 10.1080/10408390600754248. [DOI] [PubMed] [Google Scholar]
- Adesulu-Dahunsi A. T.; Sanni A. I.; Jeyaram K. Production, characterization and in vitro antioxidant activities of exopolysaccharide from Weissella cibaria GA44. LWT - Food Sci. Technol. 2018, 87, 432–442. 10.1016/j.lwt.2017.09.013. [DOI] [Google Scholar]
- Alamed J.; Chaiyasit W.; McClements D. J.; Decker E. A. Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 2009, 57, 2969–2976. 10.1021/jf803436c. [DOI] [PubMed] [Google Scholar]
- Phonsatta N.; Deetae P.; Luangpituksa P.; Grajeda-Iglesias C.; Figueroa-Espinoza M. C.; Lecomte J.; Villeneuve P.; Decker E. A.; Visessanguan W.; Panya A. Comparison of antioxidant evaluation assays for investigating antioxidative activity of gallic acid and its alkyl esters in different food matrices. J. Agric. Food Chem. 2017, 65, 7509–7518. 10.1021/acs.jafc.7b02503. [DOI] [PubMed] [Google Scholar]
- Brewer M. S. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- Wang X.; Shao C.; Liu L.; Guo X.; Xu Y.; Lü X. Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2017, 103, 1173–1184. 10.1016/j.ijbiomac.2017.05.118. [DOI] [PubMed] [Google Scholar]
- Lee M.; Song J. H.; Shim W. B.; Chang J. Y. DNAzyme-based quantitative loop-mediated isothermal amplification for strain-specific detection of starter kimchi fermented with Leuconostoc mesenteroides WiKim32. Food Chem. 2020, 333, 127343 10.1016/j.foodchem.2020.127343. [DOI] [PubMed] [Google Scholar]
- Du R.; Qiao X.; Zhao F.; Song Q.; Zhou Q.; Wang Y.; Pan L.; Han Y.; Zhou Z. Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohydr. Polym. 2018, 198, 529–536. 10.1016/j.carbpol.2018.06.116. [DOI] [PubMed] [Google Scholar]
- Freitas F.; Torres C. A. V.; Reis M. A. M. Engineering aspects of microbial exopolysaccharide production. Bioresour. Technol. 2017, 245, 1674–1683. 10.1016/j.biortech.2017.05.092. [DOI] [PubMed] [Google Scholar]
- Vedyashkina T. A.; Revin V. V.; Gogotov I. N. Optimizing the conditions of dextran synthesis by the bacterium Leuconostoc mesenteroides grown in a molasses-containing medium. Appl. Biochem. Microbiol. 2005, 41, 361–364. 10.1007/s10438-005-0061-1. [DOI] [PubMed] [Google Scholar]
- Aman A.; Siddiqui N. N.; Qader S. A. U. Characterization and potential applications of high molecular weight dextran produced by Leuconostoc mesenteroides AA1. Carbohydr. Polym. 2012, 87, 910–915. 10.1016/j.carbpol.2011.08.094. [DOI] [PubMed] [Google Scholar]
- Welman A. D.; Maddox I. S. Exopolysaccharides from lactic acid bacteria: perspectives and challenges. Trend Biotechnol. 2003, 21, 269–274. 10.1016/S0167-7799(03)00107-0. [DOI] [PubMed] [Google Scholar]
- Bhat B.; Bajaj B. K. Hypocholesterolemic and bioactive potential of exopolysaccharide from a probiotic Enterococcus faecium K1 isolated from kalarei. Bioresour. Technol. 2018, 254, 264–267. 10.1016/j.biortech.2018.01.078. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Wang C.; Jia S.; Wang B.; Zhou K.; Chen S.; Yang Y.; Liu S. Purification, characterization and antioxidant activity of the exopolysaccharide from Weissella cibaria SJ14 isolated from Sichuan paocai. Int. J. Biol. Macromol. 2018, 115, 820–828. 10.1016/j.ijbiomac.2018.04.067. [DOI] [PubMed] [Google Scholar]
- Adebayo-tayo B.; Ishola R.; Oyewunmi T. Characterization, antioxidant and immunomodulatory potential on exopolysaccharide produced by wild type and mutant Weissella confusa strains. Biotechnol. Rep. 2018, 19, e00271 10.1016/j.btre.2018.e00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.; Dong M.; Wang W.; Han S.; Rui X.; Chen X.; Jiang M.; Zhang Q.; Wu J.; Li W. Structural characterization and antioxidant property of released exopolysaccharides from Lactobacillus delbrueckii ssp. bulgaricus SRFM-1. Carbohydr. Polym. 2017, 173, 654–666. 10.1016/j.carbpol.2017.06.039. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Liu Z.; Tao X.; Wei H. Characterization and sulfated modification of an exopolysaccharide from Lactobacillus plantarum ZDY2013 and its biological activities. Carbohydr. Polym. 2016, 153, 25–33. 10.1016/j.carbpol.2016.07.084. [DOI] [PubMed] [Google Scholar]
- Ruas-Madiedo P.; de los Reyes-Gavilán C. G. Methods for the screening, isolation and characterization of exopolysaccharides produced by lactic acid bacteria. J. Dairy Sci. 2015, 88, 843–856. 10.3168/jds.S0022-0302(05)72750-8. [DOI] [PubMed] [Google Scholar]
- Xing H. W.; Du R. P.; Zhao F. K.; Han Y.; Xiao H. Z.; Zhou Z. J. Optimization, chain conformation and characterization of exopolysaccharide isolated from Leuconostoc mesenteroides DRP105. Int. J. Biol. Macromol. 2018, 112, 1208–1216. 10.1016/j.ijbiomac.2018.02.068. [DOI] [PubMed] [Google Scholar]
- He Y. L.; Ye M.; Du Z. Z.; Wang H. Y.; Wu Y. N.; Yang L. Purification, characterization and promoting effect on wound healing of an exopolysaccharide from Lachnum YM405. Carbohydr. Polym. 2014, 105, 169–176. 10.1016/j.carbpol.2014.01.080. [DOI] [PubMed] [Google Scholar]
- Sun Y. X.; Liu J. C.; Yang X. D.; Kennedy J. F. Purification, structural analysis and hydroxyl radical-scavenging capacity of a polysaccharide from the fruiting bodies of Russula virescens. Process Biochem. 2010, 45, 874–879. 10.1016/j.procbio.2010.02.007. [DOI] [Google Scholar]
- Chen Z.; Shi J.; Yang X.; Nan B.; Liu Y.; Wang Z. Chemical and physical characteristics and antioxidant activities of the exopolysaccharide produced by Tibetan kefir grains during milk fermentation. Int. Dairy J. 2015, 43, 15–21. 10.1016/j.idairyj.2014.10.004. [DOI] [Google Scholar]
- Shang N.; Xu R. H.; Li P. L. Structure characterization of an exopolysaccharide produced by Bifidobacterium animalis RH. Carbohydr. Polym. 2013, 91, 128–134. 10.1016/j.carbpol.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Saravanan C.; Shetty P. K. H. Isolation and characterization of exopolysaccharide from Leuconostoc lactis KC117496 isolated from idli batter. Int. J. Biol. Macromol. 2016, 90, 100–106. 10.1016/j.ijbiomac.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Shao L.; Wu Z. J.; Zhang H.; Chen W.; Ai L. Z.; Guo B. H. Partial characterization and immunostimulatory activity of exopolysaccharides from Lactobacillus rhamnosus KF5. Carbohydr. Polym. 2014, 107, 51–56. 10.1016/j.carbpol.2014.02.037. [DOI] [PubMed] [Google Scholar]
- Coimbra M. A.; Gonçalves F.; Barros A. S.; Delgadillo I. Fourier transform infrared spectroscopy and chemometric analysis of white wine polysaccharide extracts. J. Agric. Food Chem. 2002, 50, 3405–3411. 10.1021/jf020074p. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Feng F.; Zhou Q.; Zhao F.; Du R.; Zhou Z.; Han Y. Isolation, purification and characterization of exopolysaccharide produced by Leuconostoc pseudomesenteroides YF32 from soybean paste. Int. J. Biol. Macromol. 2018, 114, 529–535. 10.1016/j.ijbiomac.2018.03.162. [DOI] [PubMed] [Google Scholar]
- Du R.; Xing H.; Yang Y.; Jiang H.; Zhou Z.; Han Y. Optimization, purification and structural characterization of a dextran produced by Leuconostoc pseudomesenteroides isolated from Chinese sauerkraut. Carbohydr. Polym. 2017, 174, 409–416. 10.1016/j.carbpol.2017.06.084. [DOI] [PubMed] [Google Scholar]
- Arun J.; Selvakumar S.; Sathishkumar R.; Moovendhan M.; Ananthan G.; Maruthiah T.; Palavesam A. In vitro antioxidant activities of an exopolysaccharide from a salt pan bacterium Halolactibacillus miurensis. Carbohydr. Polym. 2017, 155, 400–406. 10.1016/j.carbpol.2016.08.085. [DOI] [PubMed] [Google Scholar]
- Wang J.; Salem D. R.; Sani R. K. Extremophilic exopolysaccharides: A review and new perspectives on engineering strategies and applications. Carbohydr. Polym. 2019, 205, 8–26. 10.1016/j.carbpol.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Wang H.; Gao X. D.; Zhou G. C.; Cai L.; Yao W. B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 2008, 106, 888–895. 10.1016/j.foodchem.2007.05.068. [DOI] [Google Scholar]
- Wang B.; Song Q.; Zhao F.; Xiao H.; Zhou Z.; Han Y. Purification and characterization of dextran produced by Leuconostoc pseudomesenteroides PC as a potential exopolysaccharide suitable for food applications. Process Biochem. 2019, 87, 187–195. 10.1016/j.procbio.2019.08.020. [DOI] [Google Scholar]
- Li W.; Ji J.; Chen X.; Jiang M.; Rui X.; Dong M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014, 102, 351–359. 10.1016/j.carbpol.2013.11.053. [DOI] [PubMed] [Google Scholar]
- Marco M. L.; Heeney D.; Binda S.; Cifelli C. J.; Cotter P. D.; Foligne B.; Ganzle M.; Kort R.; Pasin G.; Pihlanto A.; Smid E. J.; Hutkins R. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Liu J.; Luo J. G.; Ye H.; Sun Y.; Lu Z. X.; Zeng X. X. In vitro and in vivo antioxidant activity of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2010, 82, 1278–1283. 10.1016/j.carbpol.2010.07.008. [DOI] [Google Scholar]
- Wang Y.; Li C.; Liu P.; Ahmed Z.; Xiao P.; Bai X. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet kefir. Carbohydr. Polym. 2010, 82, 895–903. 10.1016/j.carbpol.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Chun-hui L.; Wang C.; Xu Z.; Wang Y. Isolation, chemical characterization and antioxidant activities of two polysaccharides from the gel and the skin of Aloe barbadensis Miller irrigated with sea water. Process Biochem. 2007, 42, 961–970. 10.1016/j.procbio.2007.03.004. [DOI] [Google Scholar]
- Benzie I. F. F.; Strain J. J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Choi I. S.; Cho E. J.; Moon J.-H.; Bae H.-J. Onion skin waste as a valorization resource for the by-products quercetin and biosugar. Food Chem. 2015, 188, 537–542. 10.1016/j.foodchem.2015.05.028. [DOI] [PubMed] [Google Scholar]
- Shimada K.; Fujikawa K.; Yahara K.; Nakamura T. Antioxidative properties of xanthan on autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. 10.1021/jf00018a005. [DOI] [Google Scholar]
- Chen Q.; Kong B.; Sun Q.; Dong F.; Liu Q. Antioxidant potential of a unique LAB culture isolated from Harbin dry sausage: in vitro and in a sausage model. Meat Sci. 2015, 110, 180–188. 10.1016/j.meatsci.2015.07.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.