Abstract
BACKGROUND & AIMS:
We aimed to compare the effectiveness of single- vs multiple-strain probiotics in a network meta-analysis of randomized trials.
METHODS:
We searched MEDLINE, Embase, Science Citation Index Expanded, CINAHL, Scopus, Cochrane CENTRAL, BIOSIS Previews, and Google Scholar through January 1, 2019, for studies of single-strain and multistrain probiotic formulations on the outcomes of preterm, low-birth-weight neonates. We used a frequentist approach for network meta-analysis and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to assess the certainty of evidence. Primary outcomes included all-cause mortality, severe necrotizing enterocolitis (NEC) (Bell stage II or more), and culture-proven sepsis.
RESULTS:
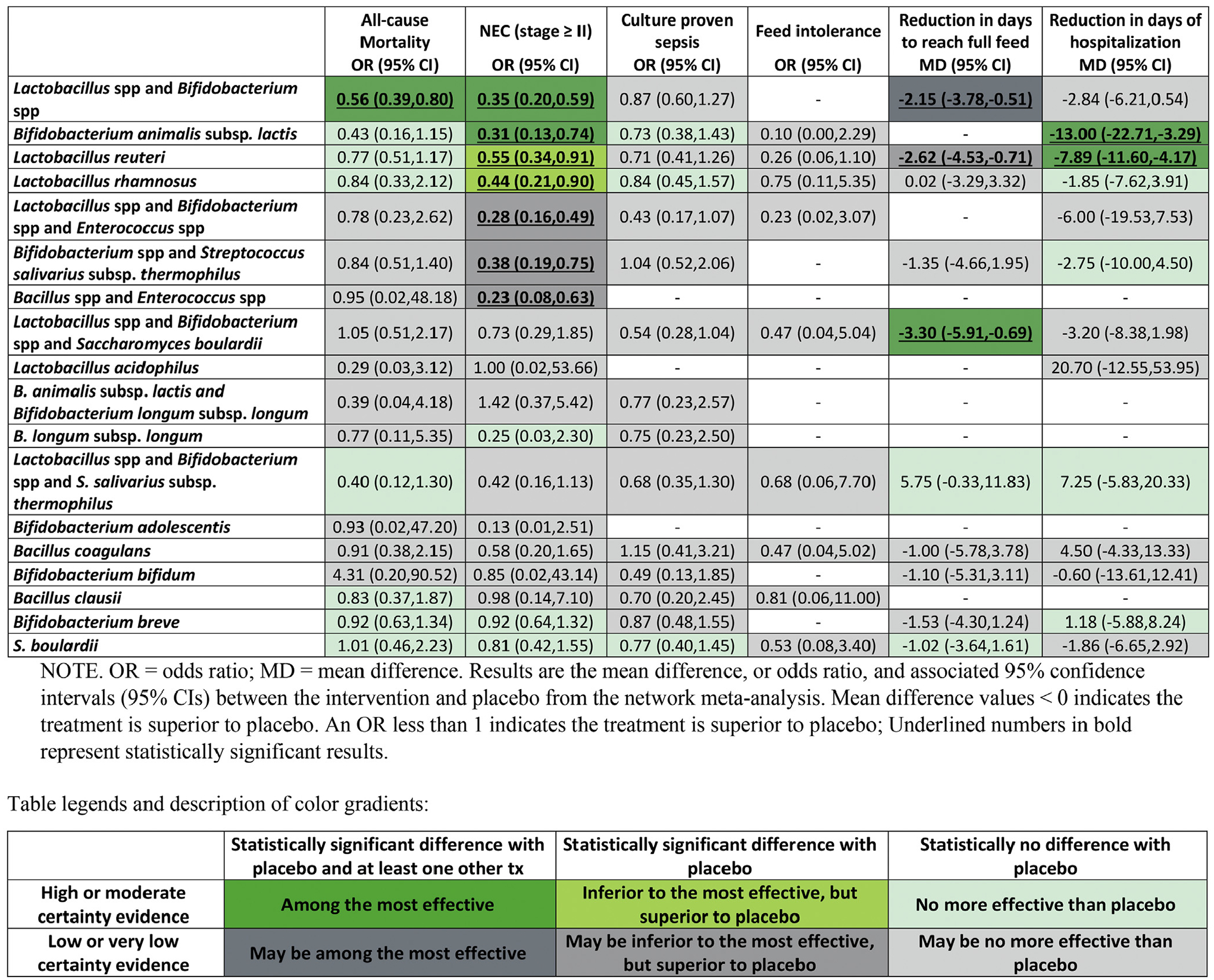
We analyzed data from 63 trials involving 15,712 preterm infants. Compared with placebo, a combination of 1 or more Lactobacillus species (spp) and 1 or more Bifidobacterium spp was the only intervention with moderate- or high-quality evidence of reduced all-cause mortality (odds ratio [OR], 0.56; 95% confidence interval [CI], 0.39–0.80). Among interventions with moderate- or high-quality evidence for efficacy compared with placebo, combinations of 1 or more Lactobacillus spp and 1 or more Bifidobacterium spp, Bifidobacterium animalis subspecies lactis, Lactobacillus reuteri, or Lactobacillus rhamnosus significantly reduced severe NEC (OR, 0.35 [95% CI, 0.20–0.59]; OR, 0.31 [95% CI, 0.13–0.74]; OR, 0.55 [95% CI, 0.34–0.91]; and OR, 0.44 [95% CI, 0.21–0.90], respectively). There was moderate- or high-quality evidence that combinations of 1 or more Lactobacillus spp and 1 or more Bifidobacterium spp and Saccharomyces boulardii reduced the number of days to reach full feeding (mean reduction of 3.30 days [95% CI, reduction of 5.91–0.69 days]). There was moderate- or high-quality evidence that, compared with placebo, the single-species product B animalis subsp lactis or L reuteri significantly reduced duration of hospitalization (mean reduction of 13.00 days [95% CI, reduction of 22.71–3.29 days] and mean reduction of 7.89 days [95% CI, reduction of 11.60–4.17 days], respectively).
CONCLUSIONS:
In a systematic review and network meta-analysis of studies to determine the effects of single-strain and multistrain probiotic formulations on outcomes of preterm, low-birth-weight neonates, we found moderate to high evidence for the superiority of combinations of 1 or more Lactobacillus spp and 1 or more Bifidobacterium spp vs single- and other multiple-strain probiotic treatments. The combinations of Bacillus spp and Enterococcus spp, and 1 or more Bifidobacterium spp and Streptococcus salivarius subsp thermophilus, might produce the largest reduction in NEC development. Further trials are needed.
Keywords: Commensal, Microbiota, Supplement, Newborn
Preterm birth, defined as birth before 37 weeks of gestation, affects nearly 10% of pregnancies and is the leading cause of perinatal morbidity and mortality in the United States.1 Preterm infants are at increased risk of sepsis, death, and lifelong neurodevelopmental and cognitive impairment.2 The most significant acquired disease of the gastrointestinal tract in preterm infants is necrotizing enterocolitis (NEC), which is characterized by bowel necrosis in variable depths and locations but most often affects the terminal ileum and proximal colon.3 Survivors are at increased risk of short bowel syndrome, parenteral nutrition–associated liver disease, pulmonary hypertension, and developmental delay.
The mechanisms by which NEC develops are poorly understood. Numerous studies report fecal microbiota alterations in preterm infants who have NEC and in those who go on to develop NEC4; however, it remains unclear whether these gut microbiota alterations contribute to or simply result from the pathogenesis of NEC. Dozens of trials of microbiome-targeting therapies have tested various agents for their ability to prevent morbidity and mortality in preterm infants.
Probiotics are live microbes which, when consumed in adequate amounts, confer a health benefit on the host.5 Probiotics might reduce the risk of sepsis and NEC by multiple mechanisms, including suppression of inflammation through the nuclear factor-κB signaling pathway, up-regulation of host anti-inflammatory genes, alleviation of hypoxemic injury, production of short-chain fatty acids to lower intestinal pH and support intestinal epithelial cell function, suppression of pathogenic bacterial growth including Enterobacteriaceae via niche exclusion and antimicrobial metabolites, strengthening intestinal barrier function, and regulation of host immunity.6,7 A 2014 Cochrane review8 concluded that probiotics prevent severe NEC and all-cause mortality in preterm infants, although the most effective formulations have yet to be identified. To build on this growing evidence base, we performed a network meta-analysis (NMA) to assess the relative effectiveness of various single-strain and multistrain probiotic formulations for critical clinical outcomes among preterm, low-birth-weight neonates.
Materials and Methods
We produced this NMA as a secondary analysis of an unpublished systematic review and protocol based on the protocol registered with PROSPERO (CRD42018085566).9,10 The results of this analysis inform the “American Gastroenterological Association Institute Technical Review on the Role of Probiotics in the Management of Gastrointestinal Disorders.”11
Search Strategy and Selection Criteria
Detailed methods have been published elsewhere.9,10 In brief, we conducted searches for relevant randomized controlled trials (RCTs) in MEDLINE, Embase, Science Citation Index Expanded and Social Sciences Citation Index, CINAHL, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), BIOSIS Previews, and Google Scholar from inception through January 1, 2019 (see the “Search Strategies” section in the Supplementary Materials). No language restrictions were imposed. We reviewed reference lists from eligible trials and related reviews for additional eligible RCTs.
Pairs of reviewers independently screened the titles and abstracts of all identified studies and, subsequently, assessed the eligibility of the full texts of potentially eligible studies. Reviewers resolved discrepancies through discussion, or, if needed, by adjudication from a third reviewer (BS). We selected RCTs featuring single- or multiple-strain probiotics (defined as living bacteria) for the prevention of morbidity or mortality in preterm (gestational age, <37 weeks) and/or low birth weight (birth weight, <2500 g) infants. We excluded studies that enrolled term infants or included both term and preterm infants, unless data for preterm infants were reported separately or >80% of infants were preterm. We also excluded studies that enrolled infants once they achieved full enteral feed or enrolled infants with early-onset sepsis, feed intolerance, or NEC.
Data Abstraction and Risk-of-Bias Assessment
Pairs of reviewers assessed the risk of bias and extracted the following data, independently and in duplicate: (1) general study information (author’s name, publication year, country of origin, and funding source), (2) study population details (sample size; mean gestational age; birth weight; percentage of caesarean births; and percentage of infants fed exclusively with mother’s, donor’s, or formula milk), (3) details of the intervention and comparison (eg, probiotics species and strains, dosage, time of initiation, and duration of therapy), and (4) outcomes (severe NEC [stage II or greater based on the Bell criteria],12,13 all-cause mortality, culture-proven sepsis, duration of hospitalization, time to establish full enteral feeds (days), and feed intolerance).
We used a modified Cochrane risk-of-bias instrument14,15 that addresses the following issues: random sequence generation, allocation concealment, blinding of study participants (in the case of our study, infants’ parents), blinding of health care providers, blinding of data collectors and outcome assessors/adjudicators, and incomplete outcome data (studies with loss to follow-up of 5% or more of randomized infants were considered at high risk of bias).
Data Synthesis and Statistical Methods
For each direct paired comparison, we calculated the odds ratios (ORs) and associated 95% confidence intervals (CIs) for dichotomous outcomes.10 For continuous outcomes, we calculated weighted mean differences with corresponding 95% CIs. We used the median baseline risk from the control arm of eligible trials to calculate the risk difference using MAGICapp. We used methods described by the Cochrane Handbook16 and Hozo et al.17 to estimate the mean and standard deviation where median, range, and sample size were reported and to impute the standard deviation if the standard error or deviation for the differences were not reported. We merged the following comparisons into a single intervention node called placebo: infants receiving formula, parental nutrition, control, or no treatment, and infants receiving placebo.
Initially, we performed conventional pairwise meta-analysis using a DerSimonian-Laird random-effects model for all comparisons with 2 RCTs or more. We assessed heterogeneity between RCTs for each direct comparison with visual inspection of the forest plots and the I2 statistic. We then performed frequentist random-effects NMA under a consistency model using the methodology of multivariate meta-analysis, assuming a common heterogeneity parameter.18,19 We used the commands and syntax provided in the network suite prepared by Chaimani and Salanti20 and White et al.18,19 We did not perform NMA when there were fewer than 10 studies for an outcome or when the number of studies was less than number of interventions (nodes) because of concerns with the model fitness. We investigated small study effect using the Harbord test for binary outcomes and the Egger test for continuous outcomes in all direct comparisons with at least 10 RCTs.21
We evaluated the presence of incoherence (also called inconsistency) by comparing direct evidence with indirect evidence using the node-splitting method.22,23 We also confirmed the coherence assumption in the entire network using the design-by-treatment model (global test) as described by Higgins et al.24 We estimated ranking probabilities using the surface under the cumulative ranking curve (SUCRA), mean ranks, and rankograms. We used Stata, version 15.1 (StataCorp, College Station, TX) for data preparations and analyses.
Assessing the Certainty of Evidence
We rated the certainty of evidence for each network estimate using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.25–27 Initially, we rated the certainty of each direct comparison; according to established GRADE guidance, the starting point for certainty across the body of RCTs is high but may be rated down based on limitations in risk of bias, imprecision, inconsistency, indirectness, and publication bias.25 Then, we rated the certainty of the indirect evidence, with a focus on the dominant lowest-order loop.26 We rated the certainty of indirect evidence as the lowest certainty of the contributing direct comparisons. Finally, we rated the certainty of network estimates. We considered the relative contribution of direct and indirect evidence to the network estimate when rating the certainty. We considered rating down the certainty in the network estimate if there was incoherence between the indirect and direct estimates or an imprecise treatment effect.26,27
Summary of Results
To optimize the interpretation of NMA findings, we applied a novel approach in which we categorized the interventions—from the most effective to the least effective—based on the effect estimates obtained from the NMA and their associated certainty of evidence. For each outcome, we created groups of interventions as follows.
Group 1: the reference intervention (placebo) and interventions with no evidence of difference compared to placebo (ie, 95% CI includes the null value), which we refer to as among the least effective.
Group 2: interventions superior to placebo but not superior to any other of the intervention(s) superior to placebo, referred to as inferior to the most effective, but superior to the least effective.
Group 3, interventions that proved superior to at least 1 intervention in group 2, which we call among the most effective.
We then divided all 3 categories into 2 categories: those with moderate- or high-certainty evidence relative to placebo and those with low- or very-low-certainty evidence relative to placebo.
Results
Additional results can be found in the Supplementary Figures 1–15 and Supplementary Tables 1–12.
Description of the Evidence
We identified 7562 records through our literature search, of which we included 96 publications from 87 studies. We excluded 16 studies of prebiotics and synbiotics (eg, studies not reporting probiotic and placebo arms) and 8 studies that did not report any of our target outcomes,28–35 leaving 63 eligible RCTs involving 15,712 infants36–98 (see the “References to Trials Included in the Network Meta-analysis” section of the Supplementary Materials). We did not exclude multiarm trials (ie, trials with >2 arms) of prebiotics or synbiotics if they had a probiotic arm and a placebo/control/no treatment arm. Figure 1 provides details of the study selection process.
Figure 1.
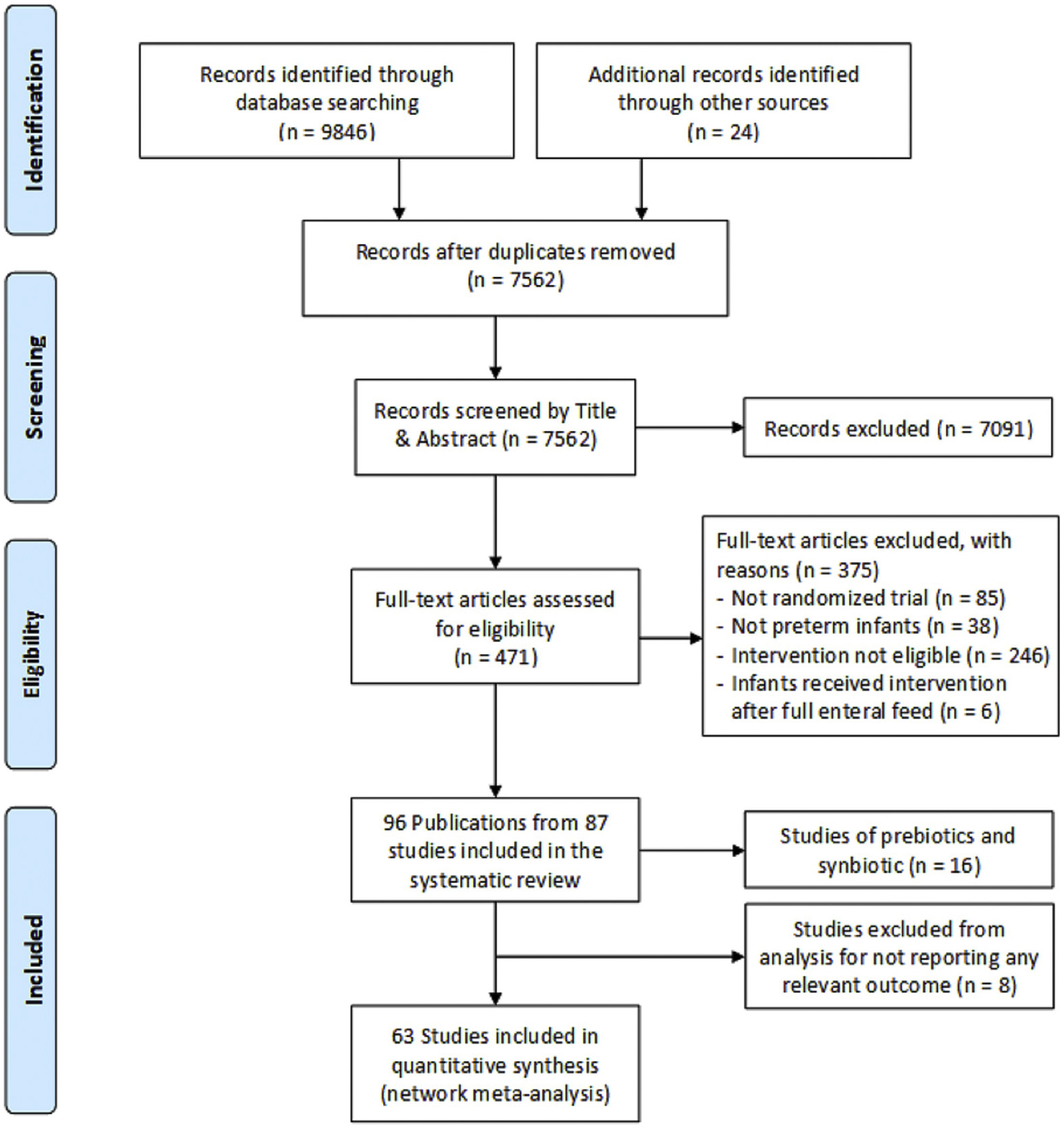
Flow diagram for study selection.
Across the included trials, the median of the average birth weight was 1204 g (interquartile range, 1062–1433), and the median of the average gestational age was 30.1 weeks (IQR, 28.7–31.3). The “Characteristics of Participants of Trials Included in the Network Meta-analysis” section in the Supplementary Materials summarizes the characteristics of infants included in the eligible trials.
The majority of multiple-strain probiotic products contained Lactobacillus species (spp) together with Bifidobacterium spp (28 of 32 studies). Of the 31 studies of a single-strain probiotic, 15 studies used a product containing Lactobacillus spp. In the Supplementary Materials, the “Treatment Characteristics of Trials and Outcomes Included in the Network Meta-analysis” section presents the characteristics of the treatments used, and the “Networks of Treatment Comparisons” section presents the networks of eligible treatment comparisons for each outcome.
Of the 63 studies, 39 were judged to be at low risk of bias for sequence generation and allocation concealment, 44 studies proved to be at low risk of bias for blinding of infants’ parents/caregivers and study personnel, 35 proved to be at low risk of bias for masking of outcome assessments, and 57 proved to be at low risk of bias in terms of missing participant outcome data. The “Summary of Risk-of-Bias Assessments for Included Trials” section of the Supplementary Materials provides additional details.
All-Cause Mortality
All-cause mortality was reported in 52 studies involving 14,003 infants (Supplementary Figure 1). Of the 22 available direct comparisons, in 10 comparisons, 2 or more studies were available for conventional pairwise meta-analysis in which the I2 was 0 in 7 comparisons and <50% for the remaining comparisons (Supplementary Table 1). The results of all direct comparisons proved similar to the NMA estimates (Supplementary Table 7). We did not observe any statistically significant global or loop-specific incoherence (Supplementary Figure 6).
High-certainty evidence showed that combinations of Lactobacillus spp and Bifidobacterium spp reduced all-cause mortality (OR, 0.56; 95% CI, 0.39–0.80; high certainty; risk difference [RD], −2.2%; 95% CI, −3.1 to −0.1) compared to placebo (Figure 2 and Table 1). No other statistically significant difference was identified between the remainder of the treatments and placebo (Figure 2 and Table 1 and Supplementary Table 1). Table 2 provides details of active treatment probiotic combinations and strains showing effectiveness compared to placebo. Supplementary Table 12 and Supplementary Figure 11 provide details of rankings and SUCRA values (see the “SUCRA and Cumulative Probability Plots” section in the Supplementary Materials).
Figure 2.
NMA results sorted based on GRADE certainty of evidence and treatment effectiveness for the comparisons of active treatments vs placebo for each outcome.
Table 1.
NMA Results Sorted Based on GRADE Certainty of Evidence for the Comparisons of Active Treatments vs Placebo for Binary Outcomes
| Outcome | Certainty of evidence | Classification | Intervention | OR (95% CI) vs placebo | RD per 1000 (95% CI) |
|---|---|---|---|---|---|
| All-cause mortality | High (moderate to high) | Among the most effective | Lactobacillus spp and Bifidobacterium spp | 0.56 (0.39–0.80) | 22 fewer (31 fewer to 10 fewer) |
| Among the least effective | Lactobacillus spp, Bifidobacterium spp, and S salivarius subsp thermophilus | 0.40 (0.12–1.30) | 30 fewer (45 fewer to 14 more) | ||
| B animalis subsp lactis | 0.43 (0.16–1.15) | 29 fewer (43 fewer to 7 more) | |||
| L reuteri | 0.77 (0.51–1.17) | 11 fewer (25 fewer to 8 more) | |||
| L rhamnosus | 0.84 (0.33–2.12) | 8 fewer (34 fewer to 52 more) | |||
| B clausii | 0.83 (0.37–1.87) | 8 fewer (32 fewer to 41 more) | |||
| B breve | 0.92 (0.63–1.34) | 4 fewer (18 fewer to 16 more) | |||
| S boulardii | 1.01 (0.46–2.23) | 0 fewer (27 fewer to 57 more) | |||
| Low (low to very low) | May be among the least effective | L acidophilus | 0.29 (0.03–3.12) | 36 fewer (50 fewer to 93 more) | |
| B animalis subsp lactis and B longum subsp longum | 0.39 (0.04–4.18) | 31 fewer (49 fewer to 134 more) | |||
| B longum subsp longum | 0.77 (0.11–5.35) | 11 fewer (46 fewer to 174 more) | |||
| Lactobacillus spp, Bifidobacterium spp, and Enterococcus spp | 0.78 (0.23–2.62) | 11 fewer (39 fewer to 73 more) | |||
| Bifidobacterium spp and S salivarius subsp thermophilus | 0.84 (0.51–1.40) | 8 fewer (25 fewer to 19 more) | |||
| B coagulans | 0.91 (0.38–2.15) | 4 fewer (31 fewer to 53 more) | |||
| B adolescentis | 0.93 (0.02–47.20) | 2 fewer (50 fewer to 672 more) | |||
| Bacillus spp and Enterococcus spp | 0.95 (0.02–48.18) | 2 fewer (50 fewer to 672 more) | |||
| Lactobacillus spp, Bifidobacterium spp, and S boulardii | 1.05 (0.51–2.17) | 2 more (25 fewer to 54 more) | |||
| B bifidum | 4.31 (0.20–90.52) | 3 fewer (50 fewer to 668 more) | |||
| NEC (stage II or more) | High (moderate to high) | Among the most effective | B animalis subsp lactis | 0.31 (0.13–0.74) | 44 fewer (56 fewer to 16 fewer) |
| Lactobacillus spp and Bifidobacterium spp | 0.35 (0.20–0.59) | 41 fewer (51 fewer to 26 fewer) | |||
| Inferior to the most effective/superior to the least effective | L rhamnosus | 0.44 (0.21–0.90) | 35 fewer (50 fewer to 5 fewer) | ||
| L reuteri | 0.55 (0.34–0.91) | 28 fewer (42 fewer to 5 fewer) | |||
| Among the least effective | B longum subsp longum | 0.25 (0.03–2.30) | 48 fewer (63 fewer to 73 more) | ||
| S boulardii | 0.81 (0.42–1.55) | 12 fewer (37 fewer to 32 more) | |||
| B breve | 0.92 (0.64–1.32) | 5 fewer (22 fewer to 19 more) | |||
| Low (low to very low) | May be among the most effective | Bacillus spp and Enterococcus spp | 0.23 (0.08–0.63) | 49 fewer (59 fewer to 23 fewer) | |
| Lactobacillus spp, Bifidobacterium spp, and Enterococcus spp | 0.28 (0.16–0.49) | 46 fewer (54 fewer to 32 fewer) | |||
| Bifidobacterium spp and S salivarius subsp thermophilus | 0.38 (0.19–0.75) | 39 fewer (52 fewer to 15 fewer) | |||
| May be among the least effective | B adolescentis | 0.13 (0.01–2.51) | 56 fewer (64 fewer to 83 more) | ||
| Lactobacillus spp, Bifidobacterium spp, and S salivarius subsp thermophilus | 0.42 (0.16–1.13) | 37 fewer (54 fewer to 8 more) | |||
| B coagulans | 0.58 (0.20–1.65) | 26 fewer (51 fewer to 38 more) | |||
| Lactobacillus spp, Bifidobacterium spp, and S boulardii | 0.73 (0.29–1.85) | 17 fewer (45 fewer to 49 more) | |||
| B bifidum | 0.85 (0.02–43.14) | 9 fewer (64 fewer to 685 more) | |||
| B clausii | 0.98 (0.14–7.10) | 1 fewer (55 fewer to 265 more) | |||
| L acidophilus | 1.00 (0.02–53.66) | 0 fewer (64 fewer to 723 more) | |||
| B animalis subsp lactis and B longum subsp longum | 1.42 (0.37–5.42) | 25 more (40 fewer to 208 more) | |||
| Culture-proven late onset sepsis | High (moderate to high) | Among the least effective | B animalis subsp lactis | 0.73 (0.38–1.43) | 38 fewer (92 fewer to 54 more) |
| S boulardii | 0.77 (0.40–1.45) | 32 fewer (88 fewer to 56 more) | |||
| L rhamnosus | 0.84 (0.45–1.57) | 22 fewer (80 fewer to 70 more) | |||
| Low (low to very low) | May be among the least effective | Lactobacillus spp, and Bifidobacterium spp, and Enterococcus spp | 0.43 (0.17–1.07) | 84 fewer (127 fewer to 9 more) | |
| Lactobacillus spp, Bifidobacterium spp, and S boulardii | 0.54 (0.28–1.04) | 66 fewer (108 fewer to 5 more) | |||
| B bifidum | 0.49 (0.13–1.85) | 74 fewer (135 fewer to 100 more) | |||
| Lactobacillus spp, Bifidobacterium spp, and S salivarius subsp thermophilus | 0.68 (0.35–1.30) | 45 fewer (97 fewer to 38 more) | |||
| B clausii | 0.70 (0.20–2.45) | 42 fewer (122 fewer to 157 more) | |||
| L reuteri | 0.71 (0.41–1.26) | 41 fewer (87 fewer to 33 more) | |||
| B longum subsp longum | 0.75 (0.23–2.50) | 35 fewer (117 fewer to 162 more) | |||
| B animalis subsp lactis and B longum subsp longum | 0.77 (0.23–2.57) | 32 fewer (117 fewer to 168 more) | |||
| Lactobacillus spp and Bifidobacterium spp | 0.87 (0.60–1.27) | 18 fewer (57 fewer to 35 more) | |||
| B breve | 0.87 (0.48–1.55) | 18 fewer (76 fewer to 67 more) | |||
| Bifidobacterium spp and S salivarius subsp thermophilus | 1.04 (0.52–2.06) | 5 more (69 fewer to 121 more) | |||
| B coagulans | 1.15 (0.41–3.21) | 20 more (87 fewer to 70 more) | |||
| Feed intolerance | Low (low to very low) | May be among the least effective | B animalis subsp lactis | 0.10 (0.00–2.29) | 239 fewer (272 fewer to 190 more) |
| Lactobacillus spp, Bifidobacterium spp, and Enterococcus spp | 0.23 (0.02–3.07) | 195 fewer (268 fewer to 263 more) | |||
| L reuteri | 0.26 (0.06–1.10) | 186 fewer (254 fewer to 19 more) | |||
| B coagulans | 0.47 (0.04–5.02) | 124 fewer (261 fewer to 381 more) | |||
| Lactobacillus spp, Bifidobacterium spp, and S boulardii | 0.47 (0.04–5.04) | 124 fewer (261 fewer to 382 more) | |||
| S boulardii | 0.53 (0.08–3.40) | 108 fewer (246 fewer to 288 more) | |||
| Lactobacillus spp, Bifidobacterium spp, and S salivarius subsp thermophilus | 0.68 (0.06–7.70) | 70 fewer (254 fewer to 470 more) | |||
| L rhamnosus | 0.75 (0.11–5.35) | 54 fewer (236 fewer to 395 more) | |||
| B clausii | 0.81 (0.06–11.00) | 40 fewer (254 fewer to 531 more) |
Table 2.
Detailed Active Treatment Probiotic Combinations and Strains Showing Effectiveness Compared to Placebo
| Probiotic combinations | All-cause mortality | NEC (stage ≥ II) | Reduction in days to reach full feed | Reduction in days of hospitalization |
|---|---|---|---|---|
| Lactobacillus spp and Bifidobacterium spp |
|
|
|
— |
| Bifidobacterium animalis subsp lactis | — |
|
— |
|
| Lactobacillus reuteri | — |
|
|
|
| Lactobacillus rhamnosus | — |
|
— | — |
| Lactobacillus spp, Bifidobacterium spp, and Enterococcus spp | — |
|
— | — |
| Bifidobacterium spp and S salivarius subsp thermophilus | — |
|
— | — |
| Bacillus spp and Enterococcus spp | — |
|
— | — |
| Lactobacillus spp, Bifidobacterium spp, and S boulardii | — | — |
|
— |
Necrotizing Enterocolitis Stage II or Higher
We included 56 RCTs with 12,738 infants involving 19 preventive therapies (Supplementary Figure 2). Of the 22 direct comparisons, 10 involved 2 studies or more. Heterogeneity was substantial (I2 = 53. 6%) for the comparison Bifidobacterium spp and Streptococcus salivarius subspecies (subsp) thermophilus with placebo. The results of all direct comparisons proved similar to the NMA estimates (Supplementary Tables 2 and 8). We did not observe any statistically significant global or loop-specific incoherence (Supplementary Figure 7).
Among the studies with high- or moderate-certainty evidence relative to placebo, combinations of Lactobacillus spp and Bifidobacterium spp (OR, 0.35; 95% CI, 0.20–0.59; high certainty; RD, −4.1%; 95% CI, −5.1 to −2.6), Bifidobacterium animalis subsp lactis (OR, 0.31; 95% CI, 0.13 to 0.74; high certainty; RD, −4.4%; 95% CI, −5.6 to −1.6), Lactobacillus reuteri (OR, 0.55; 95% CI, 0.34–0.91; moderate certainty; RD, −2.8%; 95% CI, −4.2 to −0.5), and Lactobacillus rhamnosus (OR, 0.44; 95% CI, 0.21–0.90; moderate certainty; RD, −3.5%; 95% CI, −5.0 to −0.5) significantly reduced severe NEC (Figure 2 and Table 1).
Among the studies with low or very low certainty, the combinations Bacillus spp and Enterococcus spp (OR, 0.23; 95% CI, 0.08–0.63; low certainty; RD, −4.9%; 95% CI, −5.9 to −2.3), Lactobacillus spp and Bifidobacterium spp and Enterococcus spp (OR, 0.28; 95% CI, 0.16–0.49, low certainty; RD, −4.9%; 95% CI, −5.4 to −3.2), and Bifidobacterium spp and S salivarius subsp thermophilus (OR, 0.38; 95% CI, 0.19–0.75; low certainty; RD, −3.9%; 95% CI, −5.2 to −1.5) significantly decreased the likelihood of severe NEC when compared to placebo (Figure 2 and Table 1). Figure 2 and Table 1, show the comparative effectiveness and the certainty for all pairwise comparisons. Supplementary Table 12 and Supplementary Figure 12 provide details of rankings and SUCRA values.
Culture-Proven Late-Onset Sepsis
Culture-proven sepsis was reported in 48 RCTs involving 12,258 infants (Supplementary Figure 3) with 19 direct comparisons. Our analysis did not show statistical evidence of incoherence either in the design-by-treatment interaction model (global test) or loop-specific models (Supplementary Figure 8). Heterogeneity in 5 of the 10 direct comparisons with 2 or more studies was substantial (I2 > 50% for the comparisons of L reuteri; Lactobacillus spp, Bifidobacterium spp, and Saccharomyces boulardii; Lactobacillus spp, Bifidobacterium spp, and Enterococcus spp; Lactobacillus spp and Bifidobacterium spp; and Bifidobacterium spp and S salivarius subsp thermophilus vs placebo) (Supplementary Table 3). The results of NMA were, however, similar to the direct comparisons (Supplementary Table 9).
None of the probiotic products showed statistically significant reduction in the likelihood of culture-proven late-onset sepsis (Figure 2). Three combinations of products—Lactobacillus spp and Bifidobacterium spp; Lactobacillus spp, Bifidobacterium spp, and S boulardii; and Lactobacillus spp, Bifidobacterium spp, Enterococcus spp—showed nonsignificant effects with very-low-certainty evidence toward benefit (Figure 2 and Table 1). Supplementary Table 12 and Supplementary Figure 13 provide details of rankings and SUCRA values.
Feed Intolerance
Feed intolerance was reported in 12 RCTs involving 2963 infants with 10 direct comparisons, of which 2 comparisons had 2 contributing trials and 1 comparison had 3 contributing trials. The design-by-treatment interaction model showed evidence of incoherence. This was limited to the only available closed loop of L reuteri, L rhamnosus, and placebo. Heterogeneity in the comparison of L reuteri vs placebo was substantial (I2 = 87.2) (Supplementary Table 4). because of the observed incoherence and small number of studies, we did not perform NMA. The 2 comparisons of S boulardii vs placebo and L rhamnosus vs placebo were not statistically significant (OR, 0.42; 95% CI, 0.18–1.00; I2 = 26.0%; low certainty and OR, 0.47; 95% CI, 0.10–2.26; I2 = 35.8%; low certainty, respectively); however, L reuteri may result in reduction of feed intolerance compared to placebo (OR, 0.32; 95% CI, 0.12–0.89; I2 = 87.2%; very low certainty) (Figure 2 and Table 1).
Continuous Outcomes
The “GRADE Presentation of Secondary Outcomes” section of the Supplementary Material provides detailed results of NMA of continuous outcomes, and Figure 2 summarizes these results. The 35 studies (7579 infants) that reported time to reach full enteral feed involved 12 interventions in 13 direct comparisons (Supplementary Figure 4). We did not observe any statistically significant global or loop-specific incoherence (Supplementary Figure 9). Of 9 direct comparisons with 2 or more studies, 5 had substantial heterogeneity (I2 > 50%) (Supplementary Table 5).
Among the studies with high- or moderate-certainty evidence relative to placebo, only combinations of Lactobacillus spp and Bifidobacterium spp and S boulardii (mean difference [MD], −3.30; 95% CI, −5.91 to −0.69; moderate certainty) reduced the mean number of days to reach full feeds (Figure 2 and Table 1). Among the studies with low or very low certainty, L reuteri (MD, −2.62; 95% CI, −4.53 to −0.71; low certainty) and combinations of Lactobacillus spp and Bifidobacterium spp (MD, −2.15; 95% CI, −3.78 to −0.51; low certainty) significantly decreased the number of days to reach full enteral feeding compared to placebo (Figure 2 and Table 1). In the comparisons of combinations of Lactobacillus spp and Bifidobacterium spp and Enterococcus spp, the NMA results showed no statistical reduction in number of days to reach full feed compared to placebo, whereas the direct evidence from 2 RCTs showed moderate-certainty evidence of benefit (MD, −2.47; 95% CI, −4.63 to −0.32) (see the “GRADE Presentation of Continuous Outcomes” section of the Supplementary Materials). Supplementary Table 12 and Supplementary Figure 14 provide details of rankings and SUCRA values (see the “SUCRA and Cumulative Probability Plots” section of the Supplementary Materials).
The 31 studies (7539 infants) that reported duration of hospital stay involved 13 interventions with 14 direct comparisons (Supplementary Figure 5). Our analysis did not show statistical evidence of incoherence either in the design-by-treatment interaction model (global test) or loop-specific models (Supplementary Figure 10). Of the 6 direct comparisons involving 2 or more RCTs, 3 had substantial heterogeneity (I2 > 50%) (Supplementary Table 6). Supplementary Table 11 provides the results of all pairwise comparisons. Only the single-strain probiotics B animalis subsp lactis (MD, −13.00; 95% CI, −22.71 to −3.29; moderate certainty) or L reuteri (MD, −7.89; 95% CI, −11.60 to −4.17; moderate certainty) were statistically more effective than placebo in reducing the duration of hospitalization (Figure 2 and “GRADE Presentation of Continuous Outcomes” section of the Supplementary Materials). Supplementary Table 12 and Supplementary Figure 15 provide details of rankings and SUCRA values (see the “SUCRA and Cumulative Probability Plots” section of the Supplementary Materials).
Discussion
In this systematic review and NMA comparing the effectiveness of different probiotic combinations for the prevention of mortality and morbidity in preterm infants, we found, across a number of outcomes, that few single- and multiple-strain probiotics are more effective than placebo, with no difference in the evidence of harms (ie, sepsis). High-certainty evidence indicates that combinations of 1 or more Lactobacillus spp and 1 or more Bifidobacterium spp are best for the prevention of all-cause mortality and stage II NEC (potentially averting 22 deaths and 41 cases of stage II NEC out of 1000 patients), and moderate-certainty evidence indicates that B animalis subsp lactis, L reuteri, and L rhamnosus prevent stage II NEC (averting 44, 28, and 35 cases of stage II NEC out of 1000 patients, respectively) (Figure 2). The combination of 1 or more Lactobacillus spp and 1 or more Bifidobacterium spp and S boulardii best reduces time to full enteral feeding; however, L reuteri, and combinations of Lactobacillus spp and Bifidobacterium spp, may also be effective. Finally, moderate-certainty evidence suggests that B animalis subsp lactis, as well as L reuteri, reduces the duration of hospital stay. Low-certainty evidence suggests that Bacillus spp and Enterococcus spp; Lactobacillus spp, Bifidobacterium spp, and Enterococcus spp; and Bifidobacterium spp and S salivarius subsp thermophilus prevent stage II NEC (averting 49, 46, and 39 cases of stage II NEC out of 1000 patients, respectively). Currently, there is no evidence for benefit of the remaining probiotic formulations. We did not observe any effect modification for birth weight, gestational age, feeding with breast milk, or birth type.
Our review has a number of strengths. To our knowledge, it is the most comprehensive systematic review on this topic to date, including all available literature from English and non-English RCTs for comparative assessments of the effects of prebiotics, probiotics, and synbiotics. The review is based on analyses using sophisticated statistical models that considered both NMA effect estimates and probability rankings. The review uses the GRADE approach for assessing the certainty in the NMA effect estimates and provides an innovative, transparent presentation of our findings. This presentation captures, in a single summary table, the relative performance of each treatment on each outcome, categorized by the certainty of the evidence (Figure 2).
The most important limitation of our study is that for almost all outcomes, the network of interventions is sparse, with most trials comparing probiotic formulations to placebo (ie, limited direct evidence comparing the effects of different probiotic species/strains with each other). In addition, the limited testing of single- or multiple-strain probiotics (other than Lactobacillus spp and Bifidobacterium spp) may have contributed to the inability to determine, with moderate or high certainty, evidence of benefit, harm, or no effect. Furthermore, it is unclear whether these results might be extrapolated to centers that routinely administer donor human breast milk, because these programs are associated with a decreased baseline risk of NEC.99 Importantly, NEC is difficult to diagnose given its variable clinical presentations, and the modified Bell criteria are quite broad and not always interpreted the same way by different clinicians. NEC risk is also modified by antimicrobial and acid suppression therapy, which often were not reported in the individual trials. Despite these drawbacks inherent to this particular outcome measure, beneficial effects were noted with multiple probiotic formulations.
Many of the trials in the published literature, and thus included in our review, empirically select the most tested probiotics (eg, Lactobacillus spp and Bifidobacterium spp) rather than selecting strains or combinations of strains based on biological plausibility. Although this may be due to many unknowns about the mechanisms causing NEC, what is known about NEC could help researchers make more informed decisions about strains to test. Although different microbial strains within the same genus and species can have different effects on the host, not every published trial describes the specific treatment at the strain level. Furthermore, manufacturers can change both the microbial and nonmicrobial components of commercially available probiotic products over time, and the viability of the live microbes is rarely reported. Although this study differentiated between different species and strains (when reported) of probiotics, there was not enough information given in most trials to extend the examination to nonmicrobial components of the therapies. As our knowledge of microbial functional genomics and strain-level differences continues to deepen, future studies must be as descriptive as possible regarding probiotic formulations and the rationale for their selection. Finally, standard doses and frequencies of probiotic administration have yet to be established, so we chose not to further divide the evidence base according to how the products were administrated.
Recently, 2 strain-specific meta-analyses and an NMA addressed probiotic effectiveness, and their results are consistent with our findings for probiotics. The reviews also addressed possible variability in effectiveness of probiotic strains/species. Athalye-Jape et al examined the effects of L reuteri DSM 17938100 and Bifidobacterium breve M-16V101 in RCTs and nonrandomized studies involving preterm infants and found no significant benefits for B breve M-16V on severe NEC, late-onset sepsis, all-cause mortality, and time to reach full enteral feedings. By contrast, the investigators reported significant reductions in length of hospital stay, time to reach full feedings, and duration of hospitalization, as well as nonsignificant reductions in the incidence of severe NEC and all-cause mortality with L reuteri DSM 17938. Furthermore, an NMA addressing the strain-specific effects of probiotics in 51 RCTs provided evidence that a combination of strains (multiple-strain probiotics) are usually better than any single-strain probiotics, but the paucity of studies addressing particular strains or combinations of strains limited inferences regarding comparative effectiveness.102 These findings were confirmed in a recent systematic review,103 suggesting the continued importance in conducting and reporting on rigorous studies of underreported strains or combinations.
It should also be noted that safety data in the majority of RCTs included in this analysis do not report adverse event and safety outcomes with the same level of rigor that is required in RCTs that test pharmacologic agents. Although this problem pertains to trials of probiotics, which are considered dietary supplements rather than pharmaceuticals, for any clinical condition,104 this concern may be especially relevant to the fragile population included in this review. In 1 recent example, a preterm infant receiving a probiotic died of intestinal mucormycosis resulting from possible contamination in the manufacturing process.105 Although the primary concern of live microbe administration, intestinal barrier translocation leading to sepsis, is decreased by several probiotic formulations, sound clinical judgment should be exercised.
Conclusion
Moderate- to high-certainty evidence shows the superiority of combinations of 1 or more Lactobacillus spp and 1 or more Bifidobacterium spp over alternative single- and multiple-strain probiotic treatments. The 2 combinations of Bacillus spp and Enterococcus spp and of Bifidobacterium spp and S salivarius subsp thermophilus may provide the largest reduction in the development of NEC, but this is supported by only low to very low certainty of evidence. Multicenter and large RCTs should be prioritized to distinguish between the efficacy of single- and multiple-strain probiotics among preterm infants.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the contributions of Joseph Beyene, Martin Offringa, and Philip M. Sherman.
Conflicts of interest
These authors disclose the following: Adam V. Weizman has served on an advisory board for AbbVie, Ferring, Janssen, and Takeda and as a speaker for AbbVie and Janssen. Behnam Sadeghirad received funding from Mitacs Canada and Accelerate Internship, in partnership with Nestlé Canada, to support his graduate student stipend. Mitacs is a national, not-for-profit organization that has designed and delivered research and training programs in Canada working with universities, companies, and both federal and provincial governments. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The remaining authors disclose no conflicts.
Funding
National Institute of Health provided support for Geoffrey Preidis (NIH K08 DK113114) and for Purna Kashyap (NIH DK114007).
Abbreviations used in this paper:
- CI
confidence interval
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- MD
mean difference
- NEC
necrotizing enterocolitis
- NMA
network meta-analysis
- OR
odds ratio
- RCT
randomized controlled trial
- RD
risk difference
- spp
species
- subsp
subspecies
- SUCRA
surface under the cumulative ranking curve
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.05.096.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin JA, Osterman MJK; Centers for Disease Control and Prevention. Preterm births – United States, 2006 and 2010. MMWR Suppl 2013;62:136–138. [PubMed] [Google Scholar]
- 3.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frost BL, Modi BP, Jaksic T, et al. New medical and surgical insights into neonatal necrotizing enterocolitis: a review. JAMA Pediatr 2017;171:83–88. [DOI] [PubMed] [Google Scholar]
- 5.Food and Agriculture Organization of the United Nations, World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Published 2001, Available at: http://www.fao.org/tempref/docrep/fao/meeting/009/y6398e.pdf. Accessed July 27, 2020.
- 6.Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology 2009;136:2015–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel RM, Underwood MA. Probiotics and necrotizing enterocolitis. Semin Pediatr Surg 2018;27:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014;10(4):CD005496. [DOI] [PubMed] [Google Scholar]
- 9.Sadeghirad B, Florez ID, Chang Y, et al. Comparative effectiveness of prophylactic therapies for necrotizing enterocolitis in preterm infants: protocol for a network meta-analysis of randomized trials. Int J Prev Med 2018;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadeghirad B, Florez ID, Morgan RL, et al. Probiotics, prebiotics, and synbiotics for prevention of mortality and morbidity in preterm infants: a systematic review and network meta-analysis of randomized trials (March 18, 2019). Available at: 10.2139/ssrn.3354688. Accessed, July 27, 2020. [DOI]
- 11.Preidis GA, Weizman AV, Kashyap PC, et al. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology 2020;159:708–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh M, Kliegman R. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akl EA, Sun X, Busse JW, et al. Specific instructions for estimating unclearly reported blinding status in randomized trials were reliable and valid. J Clin Epidemiol 2012;65:262–267. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0. The Cochrane Collaboration. Available at: www.handbook.cochrane.org. Updated March 2011. Accessed June 21, 2020.
- 17.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White IR. Network meta-analysis. Stata J 2015;15:951–985. [Google Scholar]
- 19.White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012;3:111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata J 2015;15:905–950. [Google Scholar]
- 21.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443–3457. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 2006;101:447–459. [Google Scholar]
- 24.Higgins JP, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puhan MA, Schunemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
- 27.Brignardello-Petersen R, Bonner A, Alexander PE, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 2018;93:36–44. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Shimizu T, Hosaka A, et al. Effects of Bifidobacterium breve supplementation on intestinal flora of low birth weight infants. Pediatr Int 2004;46:509–515. [DOI] [PubMed] [Google Scholar]
- 29.Coleta E, Gheonea M, Sarbu M. Oral supplementation with probiotics in premature infants-a randomised clinical trial. Intensive Care Med 2013;39:S113. [Google Scholar]
- 30.Koksal N, Varal I, Ozkan H, et al. Effect of probiotic support on feeding intolerance and mortality at preterm infants. J Perinat Med 2015;43:1035. [Google Scholar]
- 31.Kapiki A, Costalos C, Oikonomidou C, et al. The effect of a fructo-oligosaccharide supplemented formula on gut flora of preterm infants. Early Hum Dev 2007;83:335–339. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Shoji H, Sato H, et al. Effects of oral administration of Bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. J Pediatr Gastroenterol Nutr 2007;44:252–257. [DOI] [PubMed] [Google Scholar]
- 33.Zeber-Lubecka N, Kulecka M, Ambrozkiewicz F. Effect of Saccharomyces boulardii and mode of delivery on the early development of the gut microbial community in preterm infants. PLoS One 2016;11(2):e0150306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Indrio F, Riezzo G, Raimondi F, et al. The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J Pediatr 2008;152:801–806. [DOI] [PubMed] [Google Scholar]
- 35.Partty A, Luoto R, Kalliomaki M, et al. Effects of early prebiotic and probiotic supplementation on development of gut microbiota and fussing and crying in preterm infants: a randomized, double-blind, placebo-controlled trial. J Pediatr 2013;163:1272–1277. [DOI] [PubMed] [Google Scholar]
- 36.Al-Hosni M, Duenas M, Hawk M, et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. J Perinatol 2012;32:253–259. [DOI] [PubMed] [Google Scholar]
- 37.Arora S, Khurana MS, Saini R. To study the role of probiotics in the prevention of necrotizing enterocolitis in preterm neonates. Int J Contemp Pediatrics 2017;4:1792–1797. [Google Scholar]
- 38.Bin-Nun A, Bromiker R, Wilschanski M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 2005;147:192–196. [DOI] [PubMed] [Google Scholar]
- 39.Braga TD, da Silva GAP, de Lira PIC, de Carvalho Lima M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 2011;93:81–86. [DOI] [PubMed] [Google Scholar]
- 40.Chowdhury T, Ali MM, Hossain MM, et al. Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: a double-blind randomized controlled trial. J Coll Physicians Surg Pak 2016;26:770–774. [PubMed] [Google Scholar]
- 41.Chrzanowska-Liszewska D, Seliga-Siwecka J, Kornacka MK. The effect of Lactobacillus rhamnosus GG supplemented enteral feeding on the microbiotic flora of preterm infants-double blinded randomized control trial. Early Hum Dev 2012;88:57–60. [DOI] [PubMed] [Google Scholar]
- 42.Costalos C, Skouteri V, Gounaris A, et al. Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum Dev 2003;74:89–96. [DOI] [PubMed] [Google Scholar]
- 43.Costeloe K, Hardy P, Juszczak E, et al. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 2016;387(10019):649–660. [DOI] [PubMed] [Google Scholar]
- 44.Dani C, Biadaioli R, Bertini G, et al. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 2002;82:103–108. [DOI] [PubMed] [Google Scholar]
- 45.Dashti AS, Afjeh SA, Basiry A, et al. Prophylactic probiotics for prevention of necrotizing enterocolitis (NEC) in low birth weight neonates. Arch Pediatr Infect Dis 2014;2:174–179. [Google Scholar]
- 46.Demirel G, Erdeve O, Celik IH, et al. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled study. Acta Paediatr 2013;102(12):e560–e565. [DOI] [PubMed] [Google Scholar]
- 47.Deng J, Chen K. Early minimal feeding combined with probiotics to prevent necrotizing enterocolitis in preterm infant. Chin J Modern Drug Appl 2010;4:13–14. [Google Scholar]
- 48.Di M, Li X. Effects of Bifidobacterium supplementation for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled trial. Zhong Guo She Qu Yi Shi 2010;231:69. [Google Scholar]
- 49.Dilli D, Aydin B, Fettah ND, et al. The ProPre-Save Study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J Pediatr 2015;166:545–551. [DOI] [PubMed] [Google Scholar]
- 50.Dongol Singh S, Klobassa D, Resch B, et al. Placebo controlled introduction of prophylactic supplementation of probiotics to decrease the incidence of necrotizing enterocolitis at Dhulikhel Hospital in Nepal. Kathmandu Univ Med J 2017;60:319–323. [PubMed] [Google Scholar]
- 51.Dutta S, Ray P, Narang A. comparison of stool colonization in premature infants by three dose regimes of a probiotic combination: a randomized controlled trial. Am J Perinatol 2015;32:733–740. [DOI] [PubMed] [Google Scholar]
- 52.Fernández-Carrocera LA, Solis-Herrera A, Cabanillas-Ayón M, et al. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2013;98:F5–F9. [DOI] [PubMed] [Google Scholar]
- 53.Fujii T, Ohtsuka Y, Lee T, et al. Bifidobacterium breve enhances transforming growth factor β1 signaling by regulating Smad7 expression in preterm infants. J Pediatr Gastroenterol Nutr 2006;43:83–88. [DOI] [PubMed] [Google Scholar]
- 54.Hariharan D, Balasubramanian L, Kannappan V, Veluswami G. Probiotic supplementation in VLBW pre-term infants improves feeding tolerance and reduces risk of gram negative sepsis. J Pediatr Gastroenterol Nutr 2016;62:655. [Google Scholar]
- 55.Hays S, Jacquot A, Gauthier H, et al. Probiotics and growth in preterm infants: a randomized controlled trial, PREMAPRO study. Clin Nutr 2016;35:802–811. [DOI] [PubMed] [Google Scholar]
- 56.Hernández-Enríquez NP, Rosas-Sumano AB, Monzoy-Ventre MA, Galicia-Flores L. Lactobacillus reuteri DSM 17938 en la prevención de enterocolitis necrosante en recién nacidos prematuros. Estudio piloto de eficacia y seguridad. Rev Mex Pediatr 2016;83:37–43. [Google Scholar]
- 57.Hikaru H, Koichi K, Yayoi Y, et al. Bifidobacteria prevents preterm infants from developing infection and sepsis. Int J Probiotics Prebiotics 2010;5:33–36. [Google Scholar]
- 58.Hua XT, Tang J, Mu DZ. Effect of oral administration of probiotics on intestinal colonization with drug-resistant bacteria in preterm infants [Chinese]. Chin J Contemp Pediatr 2014;16:606–609. [PubMed] [Google Scholar]
- 59.Huang B, Yang H, Huang X. Probiotics supplementation for prevention of necrotizing enterocolitis in very low-birth-weight neonates: a randomized, controlled trial. J Guangdong Med Coll 2009;27:37–39. [Google Scholar]
- 60.Indrio F, Riezzo G, Tafuri S, et al. Probiotic supplementation in preterm: feeding intolerance and hospital cost. Nutrients 2017;9(9):965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacobs SE, Tobin JM, Opie GF, et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 2013;132:1055–1062. [DOI] [PubMed] [Google Scholar]
- 62.Kanic Z, Micetic Turk D, Burja S, et al. Influence of a combination of probiotics on bacterial infections in very low birthweight newborns. Wien Klin Wochenschr 2015;127:210–215. [DOI] [PubMed] [Google Scholar]
- 63.Ke D, Su Z, Li L. Effects of bifido supplement for prevention of necrotizing enterocolitis in preterm infants: a randomized controlled trial. Chin Pediatr Emerg Med 2008;12:69–71. [Google Scholar]
- 64.Kitajima H, Sumida Y, Tanaka R, et al. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 1997;76(2):F101–F107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin HC, Hsu CH, Chen HL, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 2008;122:693–700. [DOI] [PubMed] [Google Scholar]
- 66.Lin H-C, Su B-H, Chen A-C, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005;115:1–4. [DOI] [PubMed] [Google Scholar]
- 67.Manzoni P, Meyer M, Stolfi I, et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: a randomized clinical trial. Early Hum Dev 2014;90(Suppl 1):S60–S65. [DOI] [PubMed] [Google Scholar]
- 68.Mihatsch WA, Vossbeck S, Eikmanns B, et al. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology 2010;98:156–163. [DOI] [PubMed] [Google Scholar]
- 69.Millar MR, Bacon C, Smith SL, et al. Enteral feeding of premature-infants with Lactobacillus GG. Arch Dis Child 1993;69:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohan R, Koebnick C, Schildt J, et al. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr Res 2008;64:418–422. [DOI] [PubMed] [Google Scholar]
- 71.Oncel MY, Sari FN, Arayici S, et al. Lactobacillus reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2014;99(2):F110–F115. [DOI] [PubMed] [Google Scholar]
- 72.Patole S, Keil AD, Chang A, et al. Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates - a randomised double blind placebo controlled trial. PLoS One 2014;9(3):e89511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Punnahitananda S, Thaithumyanon P, Soongsawang K. Nosocomial infection and necrotizing enterocolitis in preterm neonates treated with Lactobacillus acidophilus and Bifidobacterium infantis in a neonatal intensive care unit: a randomized controlled study. In: Paper presented at: 14th Congress of the Federation of Asia Oceania Perinatal Societies; October 1–5, 2006 Bangkok, Thailand. 2006. [Google Scholar]
- 74.Qiao LX, Zhu WY, Zhang HY, et al. Effect of early administration of probiotics on gut microflora and feeding in pre-term infants: a randomized controlled trial. J Matern Fetal Neonatal Med 2017;30:13–16. [DOI] [PubMed] [Google Scholar]
- 75.Ren B Preventive effect of Bifidobacterium tetravaccine tablets in premature infants with necrotizing enterocolitis. J Pediatr Pharm 2010;16:24–25. [Google Scholar]
- 76.Reuman PD, Duckworth DH, Smith KL, et al. Lack of effect of Lactobacillus on gastrointestinal bacterial colonization in premature infants. Pediatr Infect Dis 1986;5:663–668. [DOI] [PubMed] [Google Scholar]
- 77.Rojas MA, Lozano JM, Rojas MX, et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 2012;130(5):e1113–e1120. [DOI] [PubMed] [Google Scholar]
- 78.Romeo MG, Romeo DM, Trovato L, et al. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late-onset sepsis and neurological outcome. J Perinatol 2011;31:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rouge C, Piloquet H, Butel MJ, et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2009;89:1828–1835. [DOI] [PubMed] [Google Scholar]
- 80.Roy A, Chaudhuri J, Sarkar D, et al. Role of enteric supplementation of probiotics on late-onset sepsis by Candida species in preterm low birth weight neonates: a randomized, double blind, placebo-controlled trial. N Am J Med Sci 2014;6:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sadowska-Krawczenko I, Korbal P, Polak A, et al. Lactobacillus rhamnosus ATC A07FA for preventing necrotizing enterocolitis in very-low-birth-weight preterm infants: a randomized controlled trial (preliminary results) [Polish]. Pediatr Pol 2012;87:139–145. [Google Scholar]
- 82.Saengtawesin V, Tangpolkaiwalsak R, Kanjanapattankul W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J Med Assoc Thai 2014;97(Suppl 6):S20–S25. [PubMed] [Google Scholar]
- 83.Samanta M, Sarkar M, Ghosh P, et al. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr 2009;55:128–131. [DOI] [PubMed] [Google Scholar]
- 84.Sari FN, Dizdar EA, Oguz S, et al. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr 2011;65:434–439. [DOI] [PubMed] [Google Scholar]
- 85.Serce O, Benzer D, Gursoy T, et al. Efficacy of Saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: a randomised controlled trial. Early Hum Dev 2013;89:1033–1036. [DOI] [PubMed] [Google Scholar]
- 86.Shadkam MN, Jalalizadeh F, Nasiriani K. Effects of probiotic Lactobacillus reuteri (DSM 17938) on the incidence of necrotizing enterocolitis in very low birth weight premature infants. Iran J Neonatol 2015;6:15–20. [Google Scholar]
- 87.Shashidhar A, Suman Rao PN, Nesargi S, et al. Probiotics for promoting feed tolerance in very low birth weight neonates - a randomized controlled trial. Indian Pediatr 2017;54:363–367. [DOI] [PubMed] [Google Scholar]
- 88.Sinha A, Gupta SS, Chellani H, et al. Role of probiotics VSL#3 in prevention of suspected sepsis in low birth-weight infants in India: a randomised controlled trial. BMJ Open 2015;5(7):e006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stratiki Z, Costalos C, Sevastiadou S, et al. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev 2007;83:575–579. [DOI] [PubMed] [Google Scholar]
- 90.Tewari VV, Dubey SK, Gupta G. Bacillus clausii for prevention of late-onset sepsis in preterm infants: a randomized controlled trial. J Trop Pediatr 2015;61:377–384. [DOI] [PubMed] [Google Scholar]
- 91.Totsu S, Yamasaki C, Terahara M, et al. Bifidobacterium and enteral feeding in preterm infants: cluster-randomized trial. Pediatr Int 2014;56:714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van Niekerk E, Kirsten GF, Nel DG, Blaauw R. Probiotics, feeding tolerance, and growth: a comparison between HIV-exposed and unexposed very low birth weight infants. Nutrition 2014;30:645–653. [DOI] [PubMed] [Google Scholar]
- 93.Van Niekerk E, Nel DG, Blaauw R, Kirsten GF. Probiotics reduce necrotizing enterocolitis severity in HIV-exposed premature infants. J Trop Pediatr 2015;61:155–164. [DOI] [PubMed] [Google Scholar]
- 94.Wejryd E, Marchini G, Frimmel V, et al. Probiotics promoted head growth in extremely low birthweight infants in a double-blind placebo-controlled trial. Acta Paediatr 2019;108:62–69. [DOI] [PubMed] [Google Scholar]
- 95.Xiao-yuan Z, Lian-qiao LI, Xuan-xuan GAO, Li-duan SU. Relative factors of neonatal necrotizing enterocolitis and preventive effect of microeco-preparation. J Appl Clin Pediatr 2007;22:1392–1393. [Google Scholar]
- 96.Xu L, Wang Y, Fu J, et al. A double-blinded randomized trial on growth and feeding tolerance with Saccharomyces boulardii CNCM I-745 in formula-fed preterm infants. J Pediatr (Rio J) 2016;92:296–301. [DOI] [PubMed] [Google Scholar]
- 97.Yang S, Haiying Y, Bin G, et al. The clinical application value of endangered preterm infants given earlier amounts of micro feedings and adding probiotics. J Pediatr Pharm 2011;17:21–24. [Google Scholar]
- 98.Zhou N The observation of effect of probiotics in the prevention of neonatal necrotizing enterocolitis. Chin J Ethnomed Ethnopharm 2012;21:81. [Google Scholar]
- 99.Kantorowska A, Wei JC, Cohen RS, et al. Impact of donor milk availability on breast milk use and necrotizing enterocolitis rates. Pediatrics 2016;137(3):e20153123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Athalye-Jape G, Rao S, Patole S. Lactobacillus reuteri DSM 17938 as a probiotic for preterm neonates: a strain-specific systematic review. J Parenter Enteral Nutr 2016;40:783–794. [DOI] [PubMed] [Google Scholar]
- 101.Athalye-Jape G, Rao S, Simmer K, et al. Bifidobacterium breve M-16V as a probiotic for preterm infants: a strain-specific systematic review. J Parenter Enteral Nutr 2018;42:677–688. [DOI] [PubMed] [Google Scholar]
- 102.van den Akker CHP, van Goudoever JB, Szajewska H, et al. Probiotics for preterm infants: a strain-specific systematic review and network meta-analysis. J Pediatr Gastroenterol Nutr 2018;67:103–122. [DOI] [PubMed] [Google Scholar]
- 103.Deshpande G, Jape G, Rao S, et al. Benefits of probiotics in preterm neonates in low-income and medium-income countries: a systematic review of randomised controlled trials. BMJ Open 2017;7(12):e017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bafeta A, Koh M, Riveros C, et al. Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Ann Intern Med 2018;169:240–247. [DOI] [PubMed] [Google Scholar]
- 105.Vallabhaneni S, Walker TA, Lockhart SR, et al. Fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morb Mortal Wkly Rep 2015;64(6):155–156. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


